Abstract
In 2015, the United Nations set important targets to reduce premature cardiovascular disease (CVD) deaths by 33% by 2030. Africa disproportionately bears the brunt of CVD burden and has one of the highest risks of dying from non-communicable diseases (NCDs) worldwide. There is currently an epidemiological transition on the continent, where NCDs is projected to outpace communicable diseases within the current decade. Unchecked increases in CVD risk factors have contributed to the growing burden of three major CVDs—hypertension, cardiomyopathies, and atherosclerotic diseases- leading to devastating rates of stroke and heart failure. The highest age standardized disability-adjusted life years (DALYs) due to hypertensive heart disease (HHD) were recorded in Africa. The contributory causes of heart failure are changing—whilst HHD and cardiomyopathies still dominate, ischemic heart disease is rapidly becoming a significant contributor, whilst rheumatic heart disease (RHD) has shown a gradual decline. In a continent where health systems are traditionally geared toward addressing communicable diseases, several gaps exist to adequately meet the growing demand imposed by CVDs. Among these, high-quality research to inform interventions, underfunded health systems with high out-of-pocket costs, limited accessibility and affordability of essential medicines, CVD preventive services, and skill shortages. Overall, the African continent progress toward a third reduction in premature mortality come 2030 is lagging behind. More can be done in the arena of effective policy implementation for risk factor reduction and CVD prevention, increasing health financing and focusing on strengthening primary health care services for prevention and treatment of CVDs, whilst ensuring availability and affordability of quality medicines. Further, investing in systematic country data collection and research outputs will improve the accuracy of the burden of disease data and inform policy adoption on interventions. This review summarizes the current CVD burden, important gaps in cardiovascular medicine in Africa, and further highlights priority areas where efforts could be intensified in the next decade with potential to improve the current rate of progress toward achieving a 33% reduction in CVD mortality.
Introduction
Approximately three decades ago, conditions such as hypertension and atherosclerotic heart diseases were rare in Africa, and communicable diseases were the major causes of death (1). Historically prioritized on the World Health Organization’s (WHO) agenda, efforts to curb infections such as HIV, tuberculosis and malaria have been fruitful with remarkable declines in the burden of communicable, maternal, neonatal and nutritional (CMNN) diseases since 2005 (2). Unfortunately, over this time, non-communicable diseases (NCDs), in particular cardiovascular diseases (CVDs), have shown an unparalleled rise. Surpassing HIV/AIDS, malaria and other enteric infections in the top 10 causes of death, CVD jumped from the 6th to the 2nd leading cause of death in sub-Saharan Africa (SSA) between 1990 and 2019 (2). The underlying drivers have been marked increases in major risk factors, such as hypertension and diabetes (3), risking to offset the substantial health gains made with communicable diseases. The current status is such that the age standardized DALYs due to CMNNs are almost at par with NCDs (4, 5). The epidemiological shift behind this trend has been heavily influenced by urbanization, influencing the nutritional and activity transitions (6).
Given its’ CVD burden, Africa faces several challenges including scarcity of high-quality data, financial constraints, competing priorities, limited skill sets, as well as diagnostic and management challenges (7). Recent and much needed increases in global and regional efforts to curb CVDs represent steps in the right direction and will need to be matched by surges in funding. The WHO Global Action Plan for the prevention and Control of NCDs has set targets for a 25% decrease in premature CVD mortality by 2025 and a third reduction for premature NCD mortality by 2030, covered by sustainable development goal (SDG) 3.4 (8, 9). Further, there are regional efforts such as Pan-African Society of Cardiology (PASCAR) goal to achieve a 25% reduction in hypertension prevalence in Africa (10). To date, modeling projections estimate a 48 and 52% increase for women and men in mean number of premature deaths due to CVDs by 2025 in SSA, provided continuation of current risk factor trends (11). Given the numerous constraints the continent faces, it is important to re-focus and invest in targeted interventions where the most gains can be made.
Burden of cardiovascular disease in Africa
CVDs are the leading cause of morbidity and mortality worldwide, contributing to about a third of all deaths (4). Globally, cardiovascular-related deaths have steadily increased by over a third from just over 12 million in 1990 to 18.6 million in 2019 (4). In Africa, CVDs are the largest contributor to the total NCD burden, accounting for 38.3% of NCD deaths and 22.9 million DALYs (5, 12). Contrary to several high-income countries (HIC) which recorded reductions in cardiovascular deaths (11), Africa has registered close to a 50% increase in the CVDs burden within the last three decades (2, 5). Aside from increases in major risk factors, this increase in CVD burden on the continent has been attributed to population growth and aging, demonstrated by a general downward trend in age-standardized mortality rates (4, 5, 11). While increases in mortality are seen in all SSA regions, the Western and Eastern regions have shown a steeper rise in CVD mortality (Figure 1, line B, C). These effects of population growth and aging on CVD incidence could considerably be mitigated by reducing the mean population blood pressure, with low-income countries (LIC) countries standing to gain the most benefits (13).
FIGURE 1
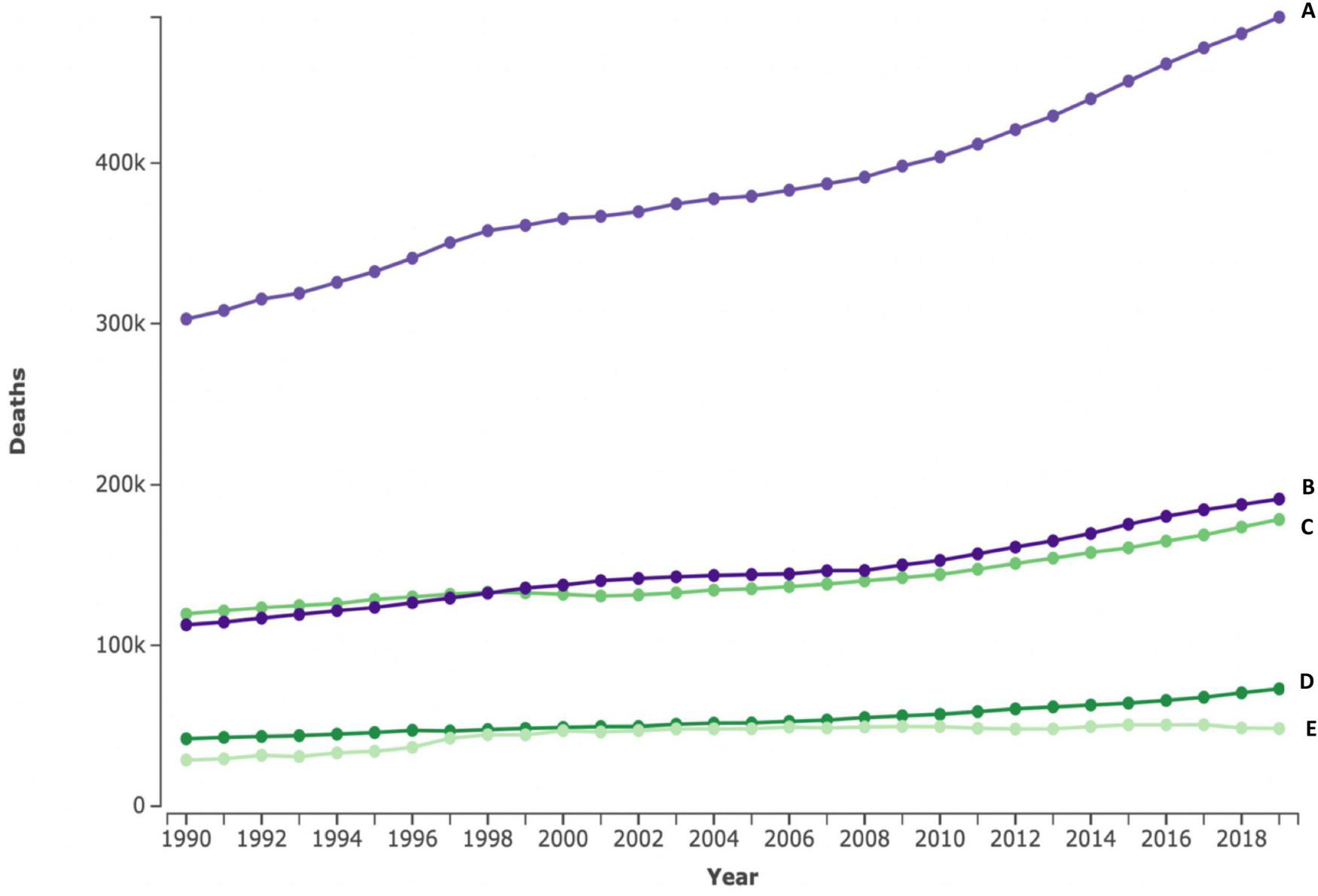
Absolute number of cardiovascular deaths in < 70 years for four SSA regions between 1990 and 2019. The top line represents the four Sub-Saharan African regions combined (A), Western (B), eastern (C), central (D), and southern (E) numbers in 100.000s. Figures extracted from the Institute for Health Metrics and Evaluation (IHME) (2).
Drivers of cardiovascular mortality and morbidity in SSA are considerably different compared to HICs. Although ischemic heart disease (IHD) is identified as the leading cause of cardiovascular mortality globally (nearly 50% of all CVD deaths) (5), stroke (specifically hypertensive stroke), has historically been the greatest contributor to CVD mortality in Africa (12). Nevertheless, the rise in IHD is a major cause for concern (6), with recent modeling studies suggesting that IHD is responsible for the bulk of CVD burden on the continent (5). The THESUS study, a multicenter trial on heart failure (HF) in SSA, showed that the leading contributory causes to HF were hypertension, rheumatic heart disease (RHD), and cardiomyopathies (14). Similar results have been replicated in other centers (15).
Population-based studies in Africa demonstrate a high prevalence of cardiometabolic risk factors with 10-year absolute risk scores ranging 12.5–15.3% for men (16). Hypertension, diabetes and obesity were the most reported risk factors in a scoping review on current NCD research in SSA (17). Of concern, are the high rates of normal weight with central obesity associated with a higher risk of CVDs and mortality compared with high BMI without central obesity (18). Despite all these, Africa is still battling with endemic, and still neglected, CVDs such as RHD and endomyocardial fibrosis (EMF). Although declining, these diseases continue to contribute a substantial portion of the CVD burden in some of its regions (19, 20).
More than half of CVD deaths in Africa are categorized as premature mortalities, occurring between the ages of 30 and 70 years (5, 21, 22). The resulting large DALYs affecting the most productive age group culminates in serious social and economic consequences to the household, community, and nation at large (23). On average, Africa still has low health expenditure (averaged at 103 US$ per capita in 2016), with several countries still below the minimum recommended $44 per capita (8). This, combined with the lack of universal health coverage in most countries, necessitates high out-of-pocket costs for individuals, with resultant impoverishment and inequity in health care access (24).
Risk factors for cardiovascular disease in Africa
The surge in CVD in SSA over the past decade is attributed to a rise in risk factors (11), largely driven by rapid urbanization (6). Four major cardiovascular risk factors—hypertension, high body mass index (BMI), high blood glucose and tobacco smoking- contribute to half of the global burden of disease (25). Hypertension is the leading contributor to stroke, ischemic, and hypertensive heart disease (HHD) burden. Figure 2 shows the burden of CVDs in four African regions and the metabolic risk factors attributing to these CVDs. The largest contribution of risk factors are seen for ischemic heart disease, stroke, and HHD, with high blood pressure having the highest share than any other risk factor, consistently across the four regions. The INTERHEART multicenter study further demonstrated the importance of nine potentially modifiable risk factors (including smoking, hypertension, diabetes, raised ApoB/ApoA1 ratio and abdominal obesity) that accounted for over 90% of all myocardial infarction (MI) risk (3).
FIGURE 2
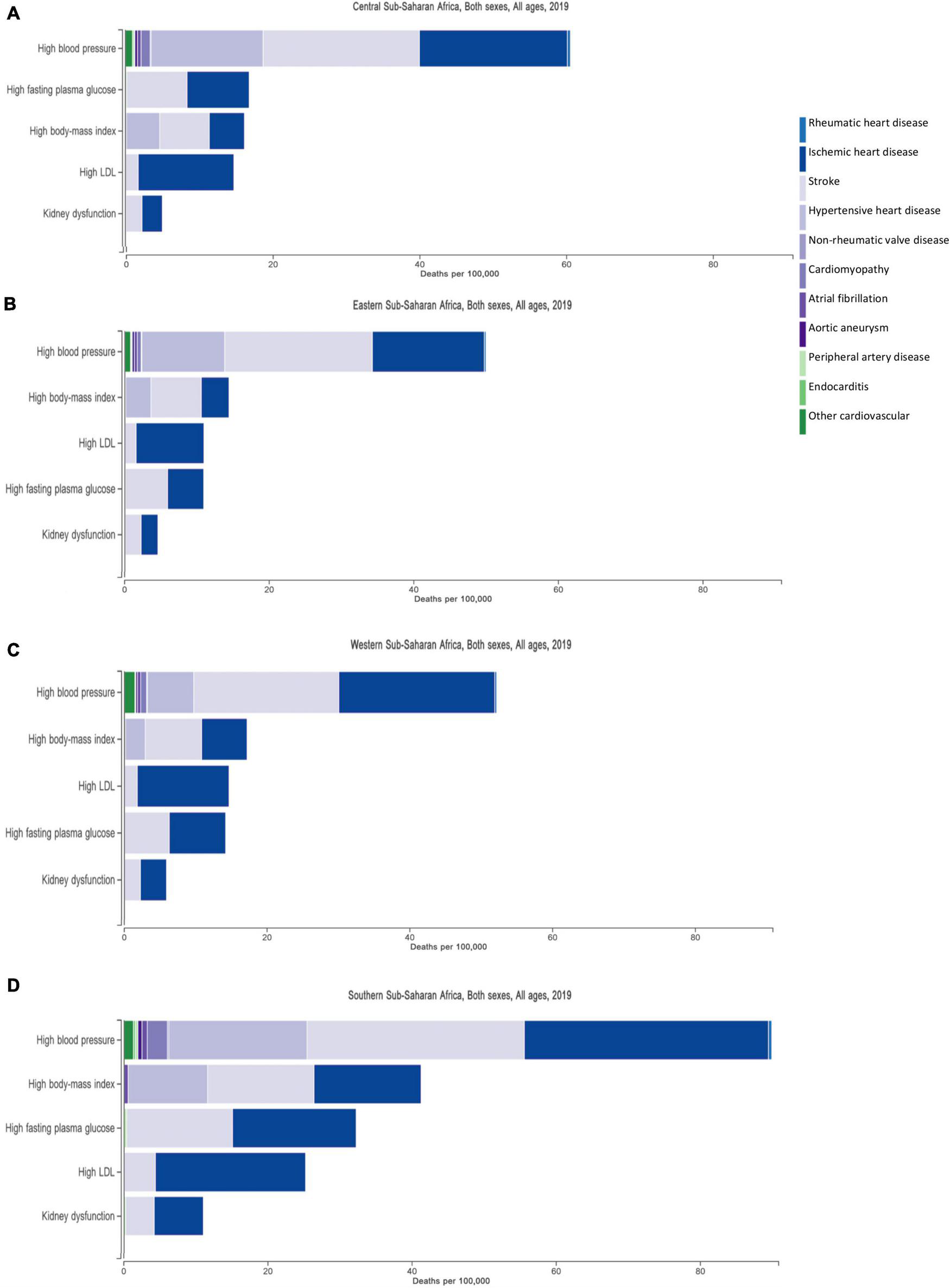
Metabolic risk factors and their contribution to the burden of cardiovascular disease in four regions in Africa, —Central (A), Eastern (B), Western (C), and Southern (D) Sub-Saharan Africa. The three largest contribution of risk factors are illustrated by the three prominent bars making up ischemic heart disease, stroke and hypertensive heart disease.
The prevalence of CVD risk factors in Africa has been reported in several heterogeneous studies. The following paragraphs focus on selected classical risk factors with high potential to impact CVD burden in Africa in the foreseeable future (11).
High blood pressure
Hypertension (HTN) is described as one of the leading health challenges on the African continent, with an estimated average prevalence of 27% (26). The reported prevalence of hypertension in Africa varies, with documented rates ranging from 15 to 70%, and a pooled prevalence of 30% in one systematic review (27). Although hypertension is a major problem in all regions of the continent, some regions have reported much higher prevalence. The ETHNA study, looking at hypertension in North African countries found a prevalence of 45.4% (28). However, higher figures have been reported in some populations in South Africa (SA), where hypertension estimates were over 50% (29). Even much higher figures documented in those > 50 years from the Study of Global Ageing and Adult Health (SAGE) SA data—and noted to be comparable to much older people in high income countries (16). On its own, hypertension accounts for a large population attributable risk (PAR), necessitating a special task force for sustained efforts toward its regional control by PASCAR (10, 11).
High blood sugar
Twenty-four million people are estimated to be living with diabetes on the African continent (30), a 126% increase in both total and age-standardized DALYs between 1990 and 2017 (4). The risk of CVD increases with increasing fasting blood glucose (FBG) levels, even prior to levels sufficient for a diabetes diagnosis, at which point the risk of CVD increases 2–3 times (31). Rates of diabetes exhibit regional variations across the continent, with the highest prevalence recorded in North Africa (32). Within SSA, the southern region had double the burden of diabetes and kidney disease compared to other regions (4). Screening rates for diabetes are still poor with 50–60% of cases undiagnosed (30, 33). The current trends and potential burden of high blood sugar is a call for health systems on the continent to invest in programs that will enable population-wide prevention, early detection, and ultimately management of diabetes (32, 34). These efforts will be important to offset the enormous health system costs and catastrophic medical expenses associated with diabetes management including the high burden of associated vascular complications (34).
Obesity
Obesity has been described as a “ticking time bomb” on the African continent, where rates continue to increase rapidly (35). Obesity, a result of energy imbalance between food consumed and energy spent in the form of physical activity, is uniquely more prevalent in women than men in Africa (30). According to WHO estimates, obesity rates range between 13.6 and 31% among 10 African countries with a high-obesity burden (36). High waist to hip ratio, an important predictor of CVD risk, is particularly problematic in some parts of the continent, reaching 61.6 and 73.4% for males and females (16). Obesity prevalence is highest in Northern (Egypt, Libya) and Southern Africa (South Africa, Namibia), with the lowest rates concentrated in the Saharan “central belt” (Ethiopia, Eritrea), amongst the poorest countries (36). Furthermore, variations exist in prevalence within settings, where higher rates are observed in urban compared to the rural populations (29), the result of urbanization and associated transitions.
Tobacco smoking
Although the African region reported the lowest prevalence rates for tobacco smoking (estimated at 18.5%), the rate of decline is low relative to other regions (37). Several outliers exist, with four of the five global countries worldwide that were reported by WHO to have increases in tobacco use recorded in Africa (Egypt, Niger, Congo, and Lesotho), the fifth being Oman, in the Eastern Mediterranean region (37). It is worthwhile to note important data limitations with most of these countries is weak surveillance systems, presenting major problems with tobacco measurements (37). This has contributed to marked variations in the prevalence of tobacco smoking, overall ranging between 4 and 40% (38). Questions on tobacco use during survey data collection are new to many countries and lack of and/or poor data quality are documented major factors affecting WHO trends and country estimates (37). An important target under the United Nations’ Sustainable Development Goals (SDGs) relates to the control of tobacco smoking through global targets, that aims for a 30% relative reduction in prevalence of current tobacco use in those 15 years or older (39). The WHO Framework Convention of Tobacco Control (FCTC) outlines several policies important for achieving this target (38). Overall, 51 African countries have adopted several control strategies, making great strides, but many are lagging on several implementation measures such as tobacco tax excise duty (40). Raising the price of tobacco products through specific tax increases has been shown to be one of the most cost-effective policies to reduce smoking (41). For example, the MPOWER tobacco control measures advocated by WHO have resulted in reduction of initiation rates as well as increasing rates of stoppage in implementing countries (37). Despite this, Africa on average has the lowest tax to retail price ratio (41). Achieving these targets will require concerted efforts toward implementation of the control policies stipulated under FCTC that have worked well in other regions (42).
Overview of cardiovascular pathologies in Africa
Heart failure in Africa
Heart failure (HF) is a complex clinical syndrome resulting from a structural or functional impairment of ventricular function (43). It is the end result of a broad spectrum of cardiac pathologies, including genetic and systemic causes (44). Worldwide, the number of HF cases is over 64 million, collectively responsible for approximately 10 million years lived with disability (YLD) (44).
There are several factors that make HF particularly important in the African context. Foremost, acute HF is the leading cause of admission in specialized cardiology centers (14, 45). Secondly, HF in SSA commonly affects younger age groups, often presenting in late stages with an exceptionally high morbidity and mortality (15, 46). The rates of re-hospitalization and overall 1-year mortality are remarkably high, ranging between 22 and 58% (22, 45, 47). Thirdly, HF etiologies differ between high- and low- income countries (39, 48). While in HICs the main contributor to HF development is coronary heart disease, in SSA, the main contributors to HF are HHD, followed by cardiomyopathies and RHD, together accounting for three quarters of the causes of HF on the continent (15, 45).
In a systemic review of heart failure in SSA, the contribution of IHD to heart failure etiologies on the continent was small, at 7.2% (45). Historically, the relatively low rates of IHD in the midst of abundant risk factors are thought to be explained in part by limited diagnostic capabilities for confirmation (49, 50). Nonetheless, there’s a rapidly rising trend in IHD within Africa and important regional differences should not be overlooked (6, 45). The INTER-CHF study recorded a substantial increase in HF resulting from IHD within five African countries (Figure 3). Important etiologies of HF which are more prevalent on the continent compared to other regions include infectious and inflammatory causes, such as tuberculous pericarditis (51), as well as nutritional deficiencies (52).
FIGURE 3
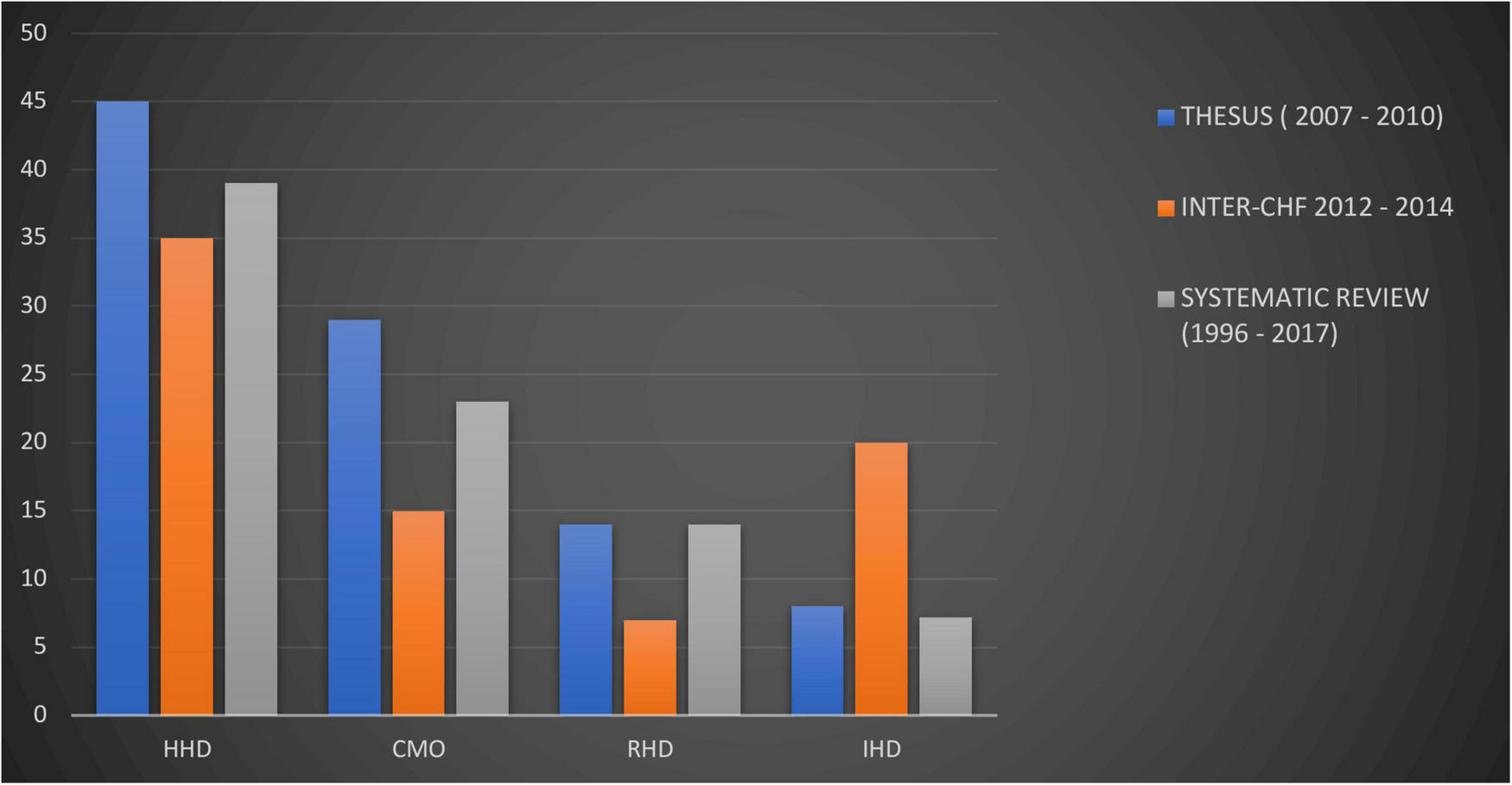
Trends in heart failure etiologies in Africa derived from heart failure cohorts from two different time spans (Thesus-HF and INTER-CHF Africa) and a systematic review (15, 45, 47). HHD, hypertensive heart disease; DCM, dilated cardiomyopathy; RHD, rheumatic heart disease; IHD, ischemic heart disease. CMO compiled as a combination of reported cardiomyopathies, idiopathic dilated, peri-partum, HIV-associated.
HF management in Africa is still challenged by lack of basic supportive investigations such as electrocardiograms, chest x-ray, and echocardiography, which are available in less than 50% of diagnosing centers (45, 53). The use of cardiac biomarkers and advanced invasive and non-invasive imaging is severely limited (53). Secondly, the use of evidence-based, guideline directed therapies such as beta-blockers and angiotensin receptor neprilysin inhibitors (ARNI), have been sub-optimal (15, 47). Thirdly, there is limited availability of device treatment for advanced heart failure, available only to the minority who can afford it (54).
The high rate of preventable heart failure should draw attention to prevention as an important area of focus for the continent. Other efforts should include investing in high quality epidemiological research -including therapeutic interventions, prioritizing early diagnosis, and optimizing treatments according to evidence-based medicine. Further, innovative strategies such as tech-enabled ways to enhance patient follow-up and promote adherence may lead to improved outcomes.
Atherosclerotic cardiovascular diseases
Sub-Saharan Africa has witnessed a substantial increase of atherosclerotic cardiovascular diseases (ASCVD) over the last three decades. According to the Global Burden of Disease (GBD) estimates, the highest contribution to CVD burden on the continent is attributed to atherosclerotic diseases, with 71.4, 37.7, and 154% increases in the burden (all age DALYs) of ischemic heart disease, stroke and peripheral artery disease since 1990 (5) (Table 1). While the figures from the GBD may be an overestimate due to imprecise data from poor country registration and surveillance leading to a reliance and limited data for statistical modeling tools, verbal autopsy and hospital data are also beginning to support the growing contribution of atherosclerotic disease to the CVD burden (55, 56). Hypertension is the most common risk factor driving this burden which, combined with other highly prevalent and poorly treated risk factors such as diabetes, obesity and dyslipidemia, are responsible for the increasing ASCVD burden (11, 57).
TABLE 1
| 1990 (′000) | 2017 (′000) | % Change 1990–2017 | |
| Cardiovascular diseases | 15 565.2 (14 490.3–16 567.9) | 22 860.8 (21 507.2–24 304.8) | 46.9% |
| Rheumatic heart disease | 973.2 (822.8–1129.2) | 1036.7 (866.3–1228.8) | 6.5% |
| Ischemic heart disease | 4928.7 (4483.3–5383.4) | 8449.7 (7813.7–9248.5) | 71.4% |
| Stroke | 5904.4 (5431.4–6399.4) | 8129.9 (7579.9–8673.7) | 37.7% |
| Hypertensive heart disease | 1057.5 (701.9–1352.7) | 1597.5 (1033.8–2056.3) | 51.1% |
| Non-rheumatic valvular heart disease | 99.2 (80.8–127.7) | 162.2 (141.9–188.5) | 38.9% |
| Cardiomyopathy and myocarditis | 818.6 (645.5–987.3) | 994.0 (867.2–1118.5) | 21.4% |
| Atrial fibrillation and flutter | 113.8 (92.5–138.4) | 234.8 (190.3–283.8) | 106.4% |
| Aortic aneurysm | 136.6 (102.5–175.7) | 184.4 (151.5–213.1) | 35.0% |
| Peripheral vascular disease | 25.8 (15.9–38.9) | 65.5 (41.7–91.0) | 154.0% |
| Endocarditis | 401.6 (276.9–579.4) | 446.2 (359.7–548.7) | 11.1% |
| Other cardiovascular and circulatory diseases | 1105.8 (851.0–1638.7) | 1559.9 (1238.4–2200.0) | 41.1% |
All-age total DALYs in 1990 and 2017 and percentage change from 1990 to 2017 from CVD in sub-Saharan Africa [GBD].
Age-standardized and all-age DALY rates and total DALYs in 1990 and 2017, and percentage change from 1990 to 2017. Gouda et al. (5). [cited 2022/05/26]; p.e1379. Available from: https://www.thelancet.com/action/showFullTableHTML?isHtml=true&tableId=tbl1&pii=S2214-109X%2819%2930374-2.
Coronary artery disease
Coronary artery disease (CAD) is the leading cause of death globally, responsible for over 50% of all cardiovascular deaths (58). Over the past three decades, there has been a substantial increase in CAD prevalence, previously responsible for only 6% of CVDs in the early 1990’s (59). Current estimates suggest IHD is responsible for close to 40% of all age- total cardiovascular DALYs (5). CAD in Africa affects the younger, more productive population and therefore has substantial impact on the economy of African nations (60). In the INTERHEART study, African patients presenting to hospital with acute coronary syndrome were significantly younger than those from the rest of the world (52 ± 12 years vs. 57 ± 12 years, respectively), and had higher levels of hypertension, diabetes, smoking, and depression (57).
African countries face several challenges with regards to CAD. Apart from poor recognition of angina symptoms (61), there is sub-optimal diagnosis and treatment of CAD and its risk factors. Lack of access to and variable quality of essential medications to treat CAD also compounds the burden (62). Secondly, delayed hospital presentation after symptom onset hampers life-saving emergency care (10, 63, 64). Thirdly, given limited access to fibrinolytic therapies and barely existent percutaneous coronary intervention services, emergent coronary revascularization remains extremely challenging and unaffordable to many. Together, this translates into high mortality for patients presenting to health units in the region (65).
Stroke
As with CAD, there has been a substantial decline in global, age-standardized stroke mortality rates over the last two decades, possibly as a result of improved stroke care. However, this decline in incidence has not been reflected in Africa (66). The annual incidence rate of stroke in Africa, 316 per 100,000 individuals, is striking. Africa also records the highest case-fatality rates for stroke at 30 days in the world, ranging from 16.2 to 46% (67).
Inadequate control of stroke risk factors, poor recognition of the urgency of symptoms leading to delayed presentation, lack of timely diagnosis and fibrinolytic services, and the dearth of post-stroke rehabilitation services are major drivers of stroke burden in SSA (67, 68). It is also possible that the difference in stroke sub-types in Africa contributes to this burden, where a relatively larger fraction of intracerebral hemorrhage occurs in the younger population and portends worse outcomes (68). Within the atherosclerotic stroke subtypes, there is a considerable proportion of small vessel disease even among younger age groups, highlighting poor risk control and perhaps suggesting that vascular dementia may be particularly troublesome in the region (69).
Improving outcome for atherosclerotic cardiovascular disease in Africa
Moving forward, investment in cost-effective ways for the prevention and treatment of risk factors for atherosclerotic disease is paramount. Systematic screening, improving access to medication, and implementing standardized guideline-directed clinical management may prove vital to control efforts (8). Investing in population-level interventions such as promoting physical activity, reducing levels of salt intake, and increasing fruit and vegetable intake through policies in different sectors would have wider impacts in reducing risk and disease burden (10, 70). Further financing emergency care for these conditions is needed, integrating pre-hospital emergency services with the rest of the health system to better ensure linkage with specialized centers. Early fibrinolysis is worth developing for both acute coronary syndrome and acute stroke, given the paucity of catheterization laboratories for emergent endovascular procedures. Finally, there is evidence that the development of multi-disciplinary specialized care centers to manage post-MI and stroke patients leads to improved outcomes (71). Multi-disciplinary specialized care centers have been successfully implemented in some parts of the African region (72) and it is important that such efforts are scaled up throughout the continent.
Hypertension
Hypertension is the number one driver of CVD in Africa. According to recent estimates, the African region has the highest prevalence of hypertension in the world (26), as well as the greatest rise in prevalence since 1990 (4). Africa also has the highest age standardized DALYs secondary to HHD, particularly central SSA (4). Behavioral risk factors as a result of urbanization, aging, social stress, and poor access to health care have been the main drivers (11).
Dubbed the “silent killer,” a result of the initial asymptomatic nature of the disease, many present for the first time to health care facilities with fatal complications. Hypertension is responsible for more than half of total cardiovascular related deaths, being the main driver of IHD, HHD, and stroke burden on the continent (2, 11; Figure 2). In a 3-year prospective study in Tanzania, hypertension-related deaths accounted for an in-hospital mortality of 20% (73). These were largely attributed to hypertensive stroke (53.2%), followed by hypertensive heart failure (27.1%), hypertensive emergencies (17.5%), and hypertensive renal disease (2.2%) (73).
The benefits of adequately controlled blood pressure are well-known, with reported reductions in cardiovascular mortality as large as 38.0 and 30.4% among females and males (74). Of critical importance is the large burden of undiagnosed, untreated, and uncontrolled hypertension (27). A pooled analysis of data from SSA revealed that only 27% of those with hypertension were aware of their status, and just 18% of those with hypertension were receiving treatment (27). Even among treated cases, poor control is common (10). Figure 4 depicts this cascade of hypertension from prevalence, awareness, diagnosis, and control (27–18–7%), demonstrating that a very small percentage of the total population is actually treated and controlled (27). Important to note that there was significant heterogeneity in these parameters across the studies pooled, mostly explained by variations in the mean age of participants and study designs (27).
FIGURE 4
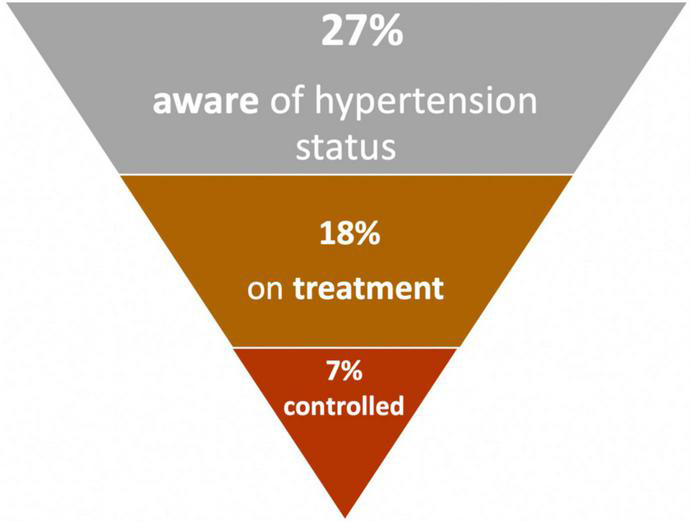
Prevalence, awareness, treatment and control of hypertension in Africa (27).
The need for hypertension control in Africa is undoubtedly one of the highest priorities. Integrating care widely in primary health facilities by equipping them with tools and quality, accessible medications to manage hypertension in concert with other cardiovascular risk factors should be a priority (75). One approach would be to leverage the existent and widespread HIV care network. Employing task-shifting to provide guideline directed lifestyle and pharmacological management, whilst gearing efforts toward universal health coverage are approaches that may prove beneficial (76). The HEARTS (Healthy-lifestyle counseling, Evidence-based treatment protocols, Access to essential medicines and technology, Risk-based CVD management, Team-based care, and Systems for monitoring) technical package is an example of a scalable strategy to improve HTN control at the primary care level, adopted by a few countries on the continent (77). Likewise, population-wide interventions for addressing behavioral risk factors such as high salt intake, physical inactivity, cigarette smoking, and poor dietary habits should be active on the policy agenda. In modeling studies, salt reduction contributed to at least 15% of the reduction seen in the probability of dying from CVDs (13). Policies targeted at whole populations such as mandatory reformulation will be important to achieve significant reductions in salt consumption among other interventions (78). The strides to successful implementation of salt reduction policies are well demonstrated by the South Africa’s mandatory salt reduction policy that recorded a reduction in salt intake of 1.2 g/day within 2 years of implementation (79). Results and follow-up from large clinical trials in the future will be important informants on whether this translates into reduction of disease burden and the extent thereof.
Human immunodeficiency virus and cardiovascular disease morbidity in sub-Saharan Africa
Sub-Saharan Africa (SSA) has the highest burden of HIV in the world, accounting for more than two thirds of the global HIV burden (3). Remarkable strides have been gained in managing chronic HIV in SSA–with increased access to anti-retroviral therapy (ART), viral suppression has resulted in less HIV-defining illnesses and a rise in non-AIDS defining illnesses. The drivers are multi-factorial, however, chronic HIV infection is a well-recognized risk factor for CVD, including myocardial infarction (80), heart failure (81), and stroke (82). SSA accounts for half of the global burden of DALYs lost due to HIV-related CVD, and the PAR for HIV-associated CVD reaches 15% in parts of the continent (83).
In high-income countries, people living with HIV (PLHIV) and hypertension have higher risks of cardiovascular events and all-cause mortality compared to HIV-uninfected persons with hypertension or PLHIV with normal blood pressure (84). Absolute differences in risk may be magnified in SSA because of poor access to hypertension care and treatment. In addition, the cardiovascular risk prediction model used in the general population have not proved accurate in predicting CVD risk related to HIV (85). Despite the high prevalence of traditional risk factors amongst a Ugandan population with HIV, a low prevalence of coronary calcium score was recorded in this population (86). Research is needed to highlight the parameters that better predict risk in PLHIV. The ongoing Ndlovu study comparing a cohort of HIV negative, and HIV positive patients will give further insights on risk prediction (87).
Integrating CVD care into HIV programs is key to mitigating the growing burden of CVD in HIV populations in Africa. As an example, PLHIV attending primary health care facilities in rural South Africa received better care for both hypertension and diabetes (88). But in general, many HIV programs in Africa are not keen on screening for CVDs and their risk factors, which delays early detection and institution of appropriate measures to slow disease progression (89, 90). The “double care” imposed on HIV/CVD patients translates into increased expenditure and worse outcomes as limitations in infrastructure compromise optimum, holistic patient care. There is need to look beyond only the HIV-set program targets to overall patient wellbeing (91, 92). As a continent, several lessons can be learned on integration of HIV and CVD care, from existing programs in countries such as South Africa, Malawi, Kenya and Swaziland (93). Opportunities exist for developing national health policies that recognize the need for care integration, as well as efficient coordination between policymakers, researchers and implementers in leveraging on successful HIV programs (93).
Cardiomyopathies
Cardiomyopathies are defined as disorders in which the heart muscle is structurally and functionally abnormal, in the absence of CAD, hypertension, valvular disease, and congenital heart disease (CHD) sufficient to cause the observed myocardial abnormality (94). They are classified as dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy (RCM), arrhythmogenic right ventricular cardiomyopathy (ARVC), and unclassified cardiomyopathies. All cardiomyopathy phenotypes have been reported in the African population with significant contribution (20–30%) to heart failure in adults (95). In particular, DCM and endomyocardial fibrosis (EMF) are endemic in SSA (96).
Dilated cardiomyopathy
DCM is defined by the presence of left ventricular dilatation and left ventricular systolic dysfunction in the absence of abnormal loading conditions (hypertension, valve disease) or coronary artery disease sufficient to cause global systolic impairment (94). DCM is the most prevalent of cardiomyopathies and one of the commonest causes of heart failure in black Africans, only second to hypertension (97). Over the past 50 years, the prevalence of DCM in some regions of the African continent has greatly increased from 10 to 17% (96) to approximately 35% of all cardiac diseases (98). An ongoing systematic review on risk factors and prevalence of DCM in SSA will give an insight on the possible factors underlying these trends (99).
Two thirds of patients with DCM have poor prognostic factors and die within 5 years of their first symptoms (100). The causes for DCM are largely unknown, hence commonly referred to as idiopathic and generally accepted that the disease probably represents a final common expression of myocardial damage that could be provoked by multiple insults. The causative factors that have been examined in Africans include infections and myocarditis, autoimmune mechanisms, iron overload and other metabolic factors, genetic factors, alcohol and nutritional deficiencies, chemotherapy, and pregnancy (96).
Other cardiomyopathies
A number of other cardiomyopathies contribute to CVDs in Africa, albeit much less common than DCM. Hypertrophic cardiomyopathy (HCM) was hardly diagnosed in Africa prior to widespread availability of echocardiography (97). Current reports for HCM have documented a prevalence of 0.2–2% (97, 101, 102), with some reports suggesting a higher prevalence in people of African descent compared to Caucasians (103). Limited availability of intra-cardiac device (ICD) implantation and other invasive treatment ultimately have influenced the poor outcomes of patients with HCM in Africa.
Endomyocardial fibrosis (EMF), an endemic cardiomyopathy, is believed to be the most common primary form of restrictive cardiomyopathy in Africa, elaborated on later in this review. Other rare forms of cardiomyopathies which have been reported in Africa include ARVC and NCLV (non-compaction of the left ventricle). The diagnosis of ARVC is still a challenge in SSA countries. The only available data on this condition comes from a single center study in South Africa, where 12 cases were reported in 6 years (104). NCLV is characterized by prominent left ventricular trabeculae and deep inter-trabecular recesses (94). It is not as rare as once believed, with documented reports from different African countries since 2006 (105).
Overall, investments into further research will be informative in enhancing our understanding of the etiology and management of cardiomyopathies in Africa. Lack of confirmatory diagnostic facilities, i.e., endomyocardial biopsy, cardiac magnetic resonance imaging, and genetic studies have contributed to under-diagnosis of some cardiomyopathies in Africa; however, the rising use of echocardiography has increased suspicion and may result in increased reporting in the coming years.
Rheumatic fever and rheumatic heart disease
RHD results from recurrent episodes of acute rheumatic fever (ARF), a long-term complication of Group A streptococcal (GAS) infection. ARF is thought to be an autoimmune-mediated response to interactions between the body and certain components of the GAS bacterial cell wall protein. RHD is currently estimated to affect approximately forty million people worldwide, the majority of whom are children and young adults in sub-Saharan Africa (4). About 395,000 children are thought to die from RHD annually, largely contributing to premature CVD mortality, with an average age at death of 28 years (106). Women of childbearing age suffer the highest burden of RHD (106, 107).
A combination of factors including health system factors, poor health seeking behavior, and evolving virulence of the causative agent have resulted in the persistence of GAS and its long term sequelae, RHD, in SSA (107). The burden of RHD is further driven by poor recognition, diagnosis and treatment of acute GAS infection and missed opportunities to identify RHD in its early, asymptomatic stage (latent RHD). Consequently, most cases present to hospitals with severe valve disease either requiring expensive surgery that is often not accessible to most, or when the benefit-risk ratio from surgery is unfavorable.
Given its long and extended life cycle, RHD does offer several opportunities for life saving interventions. Primordial prevention involves a multi-pronged approach at the government level that targets improvements in living conditions of the population including improving housing standards to prevent overcrowding, fostering good hygiene practices, and promoting education. Primary prevention aims at preventing the rheumatic process from starting and targets prompt detection, treatment, and follow up of GAS pharyngitis. Although several interventions have been implemented in Cuba and Nicaragua, this approach requires near overhauling of the health system and may not be cost effective (108). Secondary prevention that targets reducing recurrent episodes of ARF and thus RHD progression is by far the most effective intervention to date. A registry-based approach based on monthly injectable benzathine penicillin (BPG) has been shown to be both effective and affordable (108). Tertiary prevention through catheter-based therapies and valve surgery programs is still a much-needed part of RHD case management in SSA, but insufficient access and associated financial constraints of these therapies leave this as an area needing further investment in many countries.
Important questions remain regarding RHD: (1) How can health systems in LMIC improve access to primary prevention services? (2) In patients with ARF or RHD, which delivery systems can help improve adherence to BPG medication? Are depot, long term preparations of penicillin feasible? (3) Does ARF present differently in Africa and are health care workers adequately equipped to diagnose, manage and perform its surveillance? And (4) What are the immunological processes that underlie progression from ARF to clinical RHD? Several important research works are on-going examining some of the aforementioned aspects of RHD management. The landmark GOAL trial showed that BPG prevented progression of latent RHD in children who were administered monthly BPG (109). Further, studies are looking at GAS molecular biology and ARF immunology using omics technology which could shed light on our understanding of the disease continuum and future pathways for RHD elimination.
Cardiac arrhythmias
The CVD epidemic in Africa includes and/or is a harbinger for cardiac arrhythmias and their related health impact (110). The spectrum of arrhythmias in SSA has been recently described in systematic reviews (111, 112), however, the available data still remains quite limited (113). Overall, arrhythmias remain a neglected field of cardiology in Africa (114).
Chief among the cardiac arrhythmia maladies is atrial fibrillation (AF), noted to have a higher prevalence in the general population than previously thought (104). A recent systematic review has summarized the epidemiology of AF in Africa where the prevalence ranged between 6.7 and 34.8% in patients with ischemic stroke, 9.5 and 46.8% in those with RHD, and between 5 and 31.5% in patients with dilated cardiomyopathy (115). The main risk factors for AF were hypertension, valvular heart disease, and cardiomyopathy. Complications of AF included heart failure in about two thirds and stroke in 10–15% of cases (115). The use of anticoagulation for stroke prevention is challenging, given its use is inconsistent and largely suboptimal (115). Overall, the management of AF is associated with exorbitant costs, contributing to the challenges (115, 116).
Likewise, data on the epidemiology and management of supraventricular arrhythmias, ventricular arrhythmias, brady-arrhythmias and sudden cardiac arrest in Africa remain scant (111). The Africa Heart Rhythm Association (AFHRA) (117) has described most of what we know about the epidemiology and access to care of cardiac arrhythmias in Africa (112, 114). Major gaps described include the low rates of cardiac ICD insertions and rarity of invasive arrhythmia treatment centers in SSA (54, 111).
Overall, there is a growing need for appropriate arrhythmia care in Africa, an area that remains largely unmet because of complex multi-level challenges (112). Locally relevant solutions, such as “warfarin care bundles” designed to address anticoagulation challenges have been put forward (118). Since its inception, AFHRA is taking leadership in expanding cardiac arrhythmia research and increasing access to care. Multifaceted strategies have been implemented, with particular emphasis on personnel training through fellowship programs (119) and focusing on preventive care (112, 114). For cardiac pacing, the use of reconditioned pacemakers might be a short-term solution to the cost-related challenges that limit access to device therapy in Africa (120).
Neglected diseases specific to Africa: Endomyocardial fibrosis
Endomyocardial fibrosis (EMF) is the most common form of restrictive cardiomyopathy globally (121). Since its discovery in Uganda in 1938, EMF has been described in several parts of the world with majority of cases clustered in the tropical regions of Africa, Southeast Asia, and Latin America. Nearly half of the published cases globally originate from SSA (122–126; Figure 5). In endemic areas, EMF largely affects children and young adults living in conditions of poverty and contributes to premature cardiovascular morbidity and mortality (124).
FIGURE 5
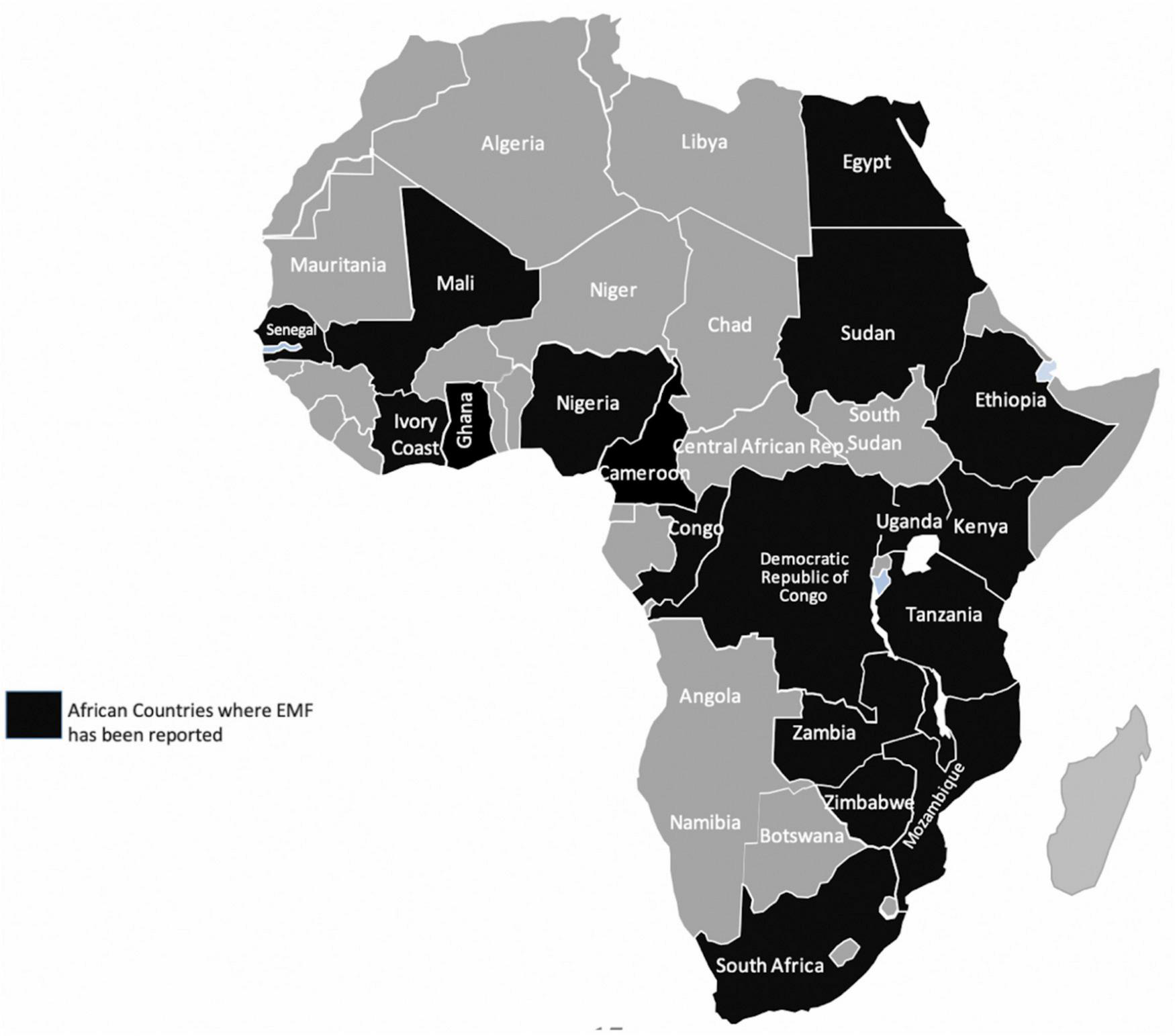
Countries in Africa where Endomyocardial fibrosis (EMF) has been described previously. Data extracted from Mocumbi et al. (126).
The etiopathogenetic mechanisms underlying the disease still remain debatable, in part due to lack of investment in research. Environmental (hyperesinophilia from helminths and other tropical infections, geochemical toxins and diet), socioeconomic, and genetic factors are all thought to play a role (122, 125–127). Focal or diffuse endocardial thickening resulting from fibro-collagen tissue deposition, typically involving the ventricular apices and atrioventricular (AV) valves, is characteristic of the disease and this results in varying degrees of AV valve regurgitation and subsequent heart failure. Both acute and chronic phases of the disease are recognized (122, 128).
Advanced EMF carries a poor prognosis. Medical therapy is directed toward treatment of heart failure symptoms. Death often results from refractory heart failure, arrhythmias and thromboembolism. Corrective surgery, which has been shown to improve survival and quality of life (122, 129), is virtually inaccessible for the vast majority of EMF patients in Africa. Recent reports suggest a decline in EMF prevalence in once endemic areas in Africa (130–132) and India (133), most likely related to improvement in socioeconomic conditions. These declines likely represent reduction of symptomatic cases, and it’s unclear whether asymptomatic disease still persists in previously endemic communities. Epidemiologic studies to assess community burden of EMF in Africa have only been undertaken in Mozambique (52, 134). Recently, echocardiography-based EMF severity staging criteria have been proposed but these are yet to be validated (52). More targeted community screening for EMF to understand the true burden of asymptomatic EMF in Africa and prioritize treatment pathways and surgery for those affected is needed.
Cardiovascular disease in pregnancy
In patients with diagnosed and undiagnosed CVD, pregnancy and labor pose remarkable physiological and hemodynamic stress and portends significant negative consequences on both maternal and fetal outcomes. Globally, 1–4% of pregnancies are complicated by maternal CVDs (excluding hypertension) (135). On its own, hypertension occurs in 10% of all pregnancies, while pre-eclampsia complicates 2–8% of all pregnancies and is responsible for the highest maternal mortality (136, 137).
A systematic review on antenatal heart disease burden in South Africa showed a prevalence of 0.6% (138). Whereas the pattern of CVD in pregnancy in developed countries consists mostly of congenital heart disease, the spectrum of antenatal heart disease in Africa is dominated by RHD (accounting for 88–90%, and predominantly of mitral valve disease), followed by cardiomyopathies and congenital heart diseases (138). Mitral stenosis, prosthetic valves, and cardiomyopathies portend poor outcomes (138, 139). Peripartum cardiomyopathy (PPCMP) has been reported to affect 17.4% of mothers presenting with heart failure in the peripartum period and carries a high maternal mortality (140). Marked differences exists in the prevalence of PPCMP across the continent; whereas 1 in 96 deliveries were reported in Nigeria, the prevalence of 1 in 1,000 deliveries reported in South Africa was more than ten times less (141, 142).
Challenges still exist in the management of patients with heart disease in pregnancy. African women in general are under social pressure to bear children and will take the risk despite the severity of disease (143). Secondly, there is limited awareness and access to preconception counseling, with many seeking healthcare after or at the end of the first trimester whilst on contraindicated chronic cardiac medications and warfarin anticoagulation (144). Moreover, there is still limited access to surgical procedures for most women in the childbearing age (145). Finally, anticoagulation for AF, often accompanying mitral stenosis, is another entity that is even more challenging in pregnancy in addition to general challenges discussed above. Monitoring of warfarin is difficult, while heparin is less affordable (146).
Improving outcomes of pregnant mothers with CVD requires an integrated and context- specific approach that engages the patient (understanding the patient needs), and involves a multi-disciplinary team headed by an obstetrician and cardiologist. Joint obstetric-cardiac care has been shown to improve both maternal and fetal outcomes (147). Sustained efforts, including resources and advocacy for RHD screening to allow early diagnosis and timely interventions could improve outcomes.
Congenital heart disease
Congenital heart disease accounts for one third of all birth defects (148). The global birth prevalence rates of CHD show significant geographic variation, with African rates of 1.9–2.3 per 1,000 births being much lower than the global rates of 9.1–9.4 per 1,000 births (148, 149). The lower prevalence rates and the under-representation of critical CHD phenotypes such as ductal-dependent lesions seen in Africa is generally thought to be an under-estimation due to lack of accurate epidemiological data and reduced survival (149–152). Dramatic advances in diagnostic and treatment options have seen 85% of all children born with CHD in high-income countries now survive into childhood (153). This includes 80% survival for complex lesions, such as truncus arteriosus and transposition of great arteries, and 95% of those with simple defects, like ventricular septal defects, likely reaching adulthood (153–155). Unfortunately, children in Africa generally have worse prognosis. Whilst there was a global decline in mortality from CHD, SSA recorded a tremendous increase in mortality between the years 1990–2017 (150). The southern region of SSA was the only region that registered a decline in CHD mortality rates (150).
Several challenges affect CHD care in Africa: (1) prenatal diagnosis of CHD in Africa is rare because of severely limited antenatal screening capacity for CHD (156, 157). (2) Late presentation with cardiac and pulmonary complications is the norm (157, 158). (3) Access to definitive surgical or transcatheter treatment for CHD is severely limited across the African continent with 90% of children with CHD having no access to appropriate surgical care (159, 160), leading to a considerable number of deaths in the neonatal and infancy period (161). (4) Although early surgical outcomes are acceptable (162, 163), long-term follow up data from the continent is poorly described, and (5) The genetic basis for CHD in Africa is still grossly understudied, and the impact of environmental factors are not fully explored (164). Ongoing research such as the PROTEA project by researchers from the University of Cape Town hopes to create an accurately phenotyped and genotyped longitudinal CHD cohort in southern Africa (165).
To improve CHD care, it is important to: (1) Increase funding to strengthen already existing CHD centers through both governmental and non-governmental organizations, (2) support training and mentorship of super-specialists across the CHD spectrum, and (3) increase awareness, promote early neonatal screening, and treatment through universal health insurance.
Africa’s progress toward sustainable development goal 3.4—Cardiovascular disease component
Currently half way between 2015 and 2030, status quo projections indicate that Africa’s overall progress is insufficient to achieve SDG 3.4 by 2030 (166). Data from the years 2010–2016 revealed stagnation in the probability of dying prematurely from four major NCDs, including CVDs in many countries in Africa. At best case scenario, these countries will have to achieve rates of decline as good as the ten best performing countries worldwide—an ambitious target given Africa’s current projections (166). Figure 6 shows the current projections to 2030 in African countries on their progress toward cardiovascular premature mortality reductions from 2015, the baseline year (13). These projections begin from the year 2020. For the majority of countries, the rate of decline in the probability of dying prematurely between 30 and 70 years of age (40q30), has been too slow to reach the 33% reduction target. Many are projected to attain between 10 and 20% reduction. The two lines well above y = 1 (baseline) are Cape Verde (which increased from 6.3% in 2015 to 7.9% in 2030) and Eritrea (which increased from 13% in 2015 to 13.9% in 2030). Comoros and Equatorial Guinea both hover around no change from 2015 to 2030. Conversely, South Africa and Algeria are on track to achieve the target with SA projected to see the largest decrease (from 8.9% in 2015 to 5.6% in 2030, representing a 36.5% relative reduction) and Algeria projected to achieve the 33% relative reduction (where the 40q30 decreased from 9.9% in 2015 to 6.5% in 2030). Below we summarize current gaps and highlight important priorities going forward for the continent.
FIGURE 6
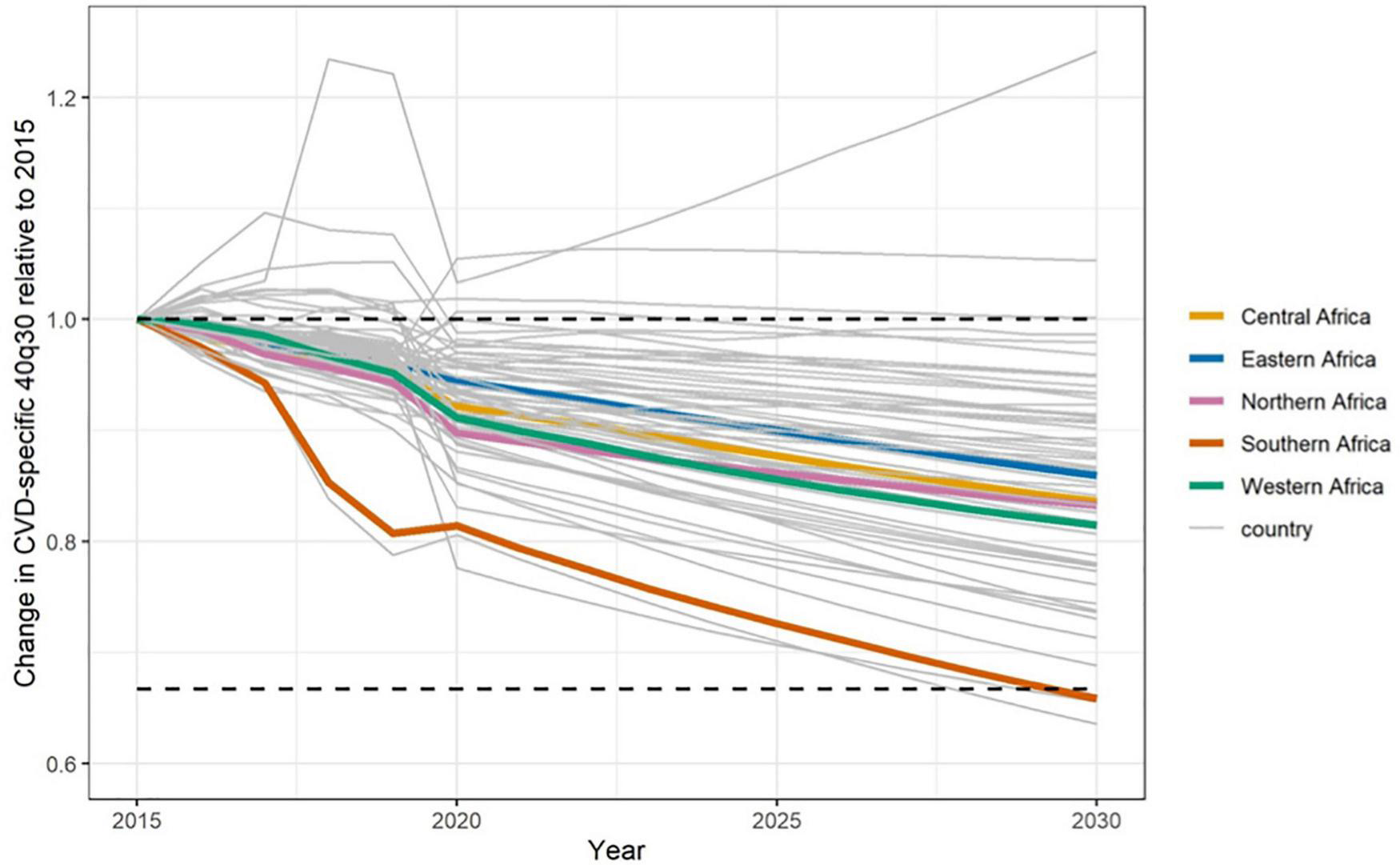
Status quo projections for 40q30 through 2030 for cardiovascular disease in Africa by country and 5 sub-regions (Southern, Central, Eastern, Western and North Africa). The horizontal lines denote baseline 40q30 in 2015 and a third reduction in 40q30 with respect to 2015 baseline rate (13).
Important gaps in cardiovascular health in Africa
- •
There’s inadequate research productivity from Africa which contributes to the widening gap in health outcomes with other countries (167, 168). Importantly, lack of systematic surveillance data covering vital statistics such as causes of mortality within countries hampers disease burden estimates and consequently affects monitoring and evaluation of interventions.
- •
Data on the physician-to-population ratio confirm a persistent human resource crisis in the health sector, which is even more pronounced for sub-specialties (24). Overtime, an increase in medical schools has been marred by faculty shortages and likely reduced quality, poor renumeration, and consequently “brain drain” to other countries (169).
- •
There is a large gap to fill for general access to CVD care, contributory factors include:
- ∘
Low health budget allocation—According to the WHO, most African countries have insufficient health financing systems with high out of pocket expenditures. Almost half of all African countries still have out of pocket expenditures of above 40% (170). In general, health spending in still very low in Africa, with the Central, Western and Eastern African regions documented to have the lowest worldwide (171). Moreover, there is a huge reliance on donor funding among several African countries and relatively less government health service expenditure on primary care (171).
- ∘
Lack of universal health insurance and consequently high out-of-pocket expenditure, sometimes resulting in catastrophic health spending.
- ∘
Inadequate access and long-term affordability of evidenced-based CVD medications, including important drugs for CVD prevention (172).
- ∘
Inadequate facilities for advanced cardiovascular imaging and procedures (54).
- ∘
- •
Low education and health literacy levels (173), affects health choices made, including health-seeking behavior (174) partly contributing to challenges described such as the late presentation of CVDs and subsequently poor prognosis. In terms of risk factors, those with more years of schooling are less likely to be involved in risk behaviors such as excessive drinking and smoking (175). Finally, there is a paucity of cost-effective, integrated and evidenced-based approaches for CVD prevention targeted at whole populations (11) and supported by effective policies and government commitment.
Key priorities
- •
Prevention: identifying and implementing cost effective, preventive strategies targeting both high risk and the general population is the most reliable solution to the rising burden of CVD in Africa. Preventive efforts such as the adoption of the FCTC for tobacco control (176), introducing sugar tax for processed foods (177), and implementing population-based interventions that promote healthy lifestyles have proven effective in several settings. This will require sustained advocacy by in-country expert groups of public health specialists and other policy makers to prioritize these policies in order to improve CVD outcomes.
Hypertension Prevention deserves concerted efforts given its contribution to CVD in Africa. Universal and periodic screening, diagnosis and guideline-directed treatment through strengthening and integration into primary care facilities, task-shifting, and universal availability of effective drugs should be prioritized in most countries. Intensively accelerating control through two population-based interventions—pharmacological guideline-directed treatment of hypertension and “upstream” policies targeted at a reduction in salt consumption could reduce CVD mortality by up to 22% relative to status quo (13). The WHO STEPwise surveillance tool for in-country monitoring of HTN detection and control has been recommended by PASCAR (9).
- •
Increase Research Outputs: high-quality epidemiological and implementation research, as well as dedicated investments toward strengthening vital statistic data and systematic surveillance, will inform practice and contribute towards identifying and evaluating’ sustainable interventions in various settings (70), track progress, and strengthen policy development and adoption.
- •
Improve Training: priorities to improve training should include establishing in-country cardiology training centers and strengthening existing training centers through bench marking and fostering collaboration with strong external cardiology training institutions.
- •
Improve Access: a number of options exists to increase access to cardiovascular care:
- ∘
reducing out-of-pocket expenditure and implementing health financing plans with the objective of increasing equitable and effective access to CVD care (169). Among other factors, health reforms to promote progress towards UHC will involve country commitments to increasing revenue generation and overall government expenditure on health, employing a whole system approach (169). Increasing government expenditure on health by allocating at least 15% of their national budgets to the health sector, including a minimum of US$ 44 per capita for health funding (170).
- ∘
task shifting of preventive cardiovascular care strategies like screening, lifestyle modification counseling, routine, uncomplicated, follow-ups, specialized care linkage, and coordination of NCD support groups (76).
- ∘
investing in infrastructure for high-quality care including acute cardiovascular care is an essential component of reducing cardiovascular mortality (71).
- ∘
incentives for health care workers that aim to enhance service delivery and retain knowledgeable and skilled medical personnel within countries (170).
- ∘
Conclusion
The burden of CVD in Africa is a result of increasing and unchecked risk factors with considerable heterogeneity in risk trends across its regions, that has led to explosive rates of CVD-related morbidity and mortality on the continent. Despite the sustained efforts to combat CVDs on the continent by the different advocacy groups such as WHO, PASCAR, and World Heart Federation, the adoption of effective policies is lagging in many African states. This review has highlighted several gaps in various sectors of CVD care on the continent. Overall, the evidence suggests applying preventive strategies to whole populations that target major risk factors will make important contributions toward reaching set targets by 2030. Specifically, for Africa, intensified and tailored regional efforts need to be channeled to lower blood pressure and other risk factors that should be coupled with continued surveillance and reliable data collection and monitoring programs. Concerted efforts from global, regional, and local experts are needed to increase advocacy for CVDs. Efforts should focus on identifying innovative ways to improve access and service provision at a primary health care level, investing toward universal health coverage, investing in cost-effective preventive approaches that are easily adaptable, and prioritizing research. Given country commitments to strengthen health systems, adopt and implement key metrics targeting awareness, prevention and management of CVDs, significant strides are possible toward changing the current trajectory of CVD burden in Africa.
Statements
Author contributions
EO, JR, DN, and NM contributed to conception and initial outline of the review. NM, DN, TA, WZ, IS, JN, WA, SL, EN, JR, EO, and JK contributed to writing different subsections. JK, EO, EN, SL, DN, and NM contributed to manuscript content and language editing. All authors contributed to manuscript revision, read, and approved the submitted version.
Acknowledgments
We would like to sincerely thank Sarah Pickersgill and David Watkins for their comments and contributions to the 40q30 CVD projections (Figure 6). NM & DN are fellows under the AHA 20SFRN35380042.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
MurrayCJLopezAD. Global and regional cause-of-death patterns in 1990.Bull World Health Organ. (1994) 72:447.
2.
Institute for Health Metrics and Evaluation [IHME].GBD Compare Data Visualization. (2020). Available online at: http://vizhub.healthdata.org/gbd-compare(accessed April 21, 2022).
3.
YusufSHawkenSOunpuuSDansTAvezumALanasFet alEffect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-Control Study.Lancet. (2004) 364:937–52. 10.1016/s0140-6736(04)17018-9
4.
RothGAMensahGAJohnsonCOAddoloratoGAmmiratiEBaddourLMet alGlobal burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study.J Am Coll Cardiol. (2020) 76:2982–3021. 10.1016/j.jacc.2020.11.010
5.
GoudaHNCharlsonFSorsdahlKAhmadzadaSFerrariAJErskineHet alBurden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017.Lancet Global Health. (2019) 7:e1375–87.
6.
TeoKKRafiqT. Cardiovascular risk factors and prevention: a perspective from developing countries.Can J Cardiol. (2021) 37:733–43. 10.1016/j.cjca.2021.02.009
7.
MarquezPVFarringtonJL.The Challenge of Non-Communicable Diseases and Road Traffic Injuries in Sub-Saharan Africa: An Overview. (2013). Available online at: https://openknowledge.worldbank.org/handle/10986/16451(accessed March 30, 2022).
8.
World Health Organization [WHO].World Health Statistics 2016: Monitoring Health for the SDGs Sustainable Development Goals.Geneva: World Health Organization (2016).
9.
United Nations [UN].Transforming Our World: The 2030 Agenda for Sustainable Development. (2015). Available online at: https://sdgs.un.org/publications/transforming-our-world-2030-agenda-sustainable-development-17981#(accessed May 2, 2022).
10.
DzudieATwagirumukizaMCornickRAbdou BaSDamascenoARaynerBet alRoadmap to achieve 25% hypertension control in Africa by 2025.Cardiovasc J Afr. (2017) 28:262–73. 10.1016/j.gheart.2017.06.001
11.
SaccoRLRothGAReddyKSArnettDKBonitaRGazianoTAet alThe heart of 25 by 25: achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study from the American heart association and world heart federation.Circulation. (2016) 133:e674–90. 10.1016/j.gheart.2016.04.002
12.
MensahGASampsonUKRothGAForouzanfarMHNaghaviMMurrayCJet alMortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013.Cardiovasc J Afr. (2015) 26:S6. 10.5830/cvja-2015-036
13.
PickersgillSJMsemburiWTCobbLIdeNMoranAESuYet alModeling global 80-80-80 blood pressure targets and cardiovascular outcomes.Nat Med. (2022) 28:1693–9. 10.1038/s41591-022-01890-4
14.
SliwaKDavisonBAMayosiBMDamascenoASaniMOgahOSet alReadmission and death after an acute heart failure event: predictors and outcomes in sub-Saharan Africa: results from the THESUS-HF registry.Eur Heart J. (2013) 34:3151–9. 10.1093/eurheartj/eht393
15.
DamascenoAMayosiBMSaniMOgahOSMondoCOjjiDet alThe causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries: results of the sub-Saharan Africa survey of heart failure.Arch Intern Med. (2012) 172:1386–94. 10.1001/archinternmed.2012.3310
16.
Gómez-OlivéFXMontanaLWagnerRGKabudulaCWRohrJKKahnKet alCohort profile: health and ageing in Africa: a Longitudinal Study of an indepth community in South Africa (HAALSI).Int J Epidemiol. (2018) 47:689–90j. 10.1093/ije/dyx247
17.
MudieKJinMMKendallLAddoJdos-Santos-SilvaIQuintJet alNon-communicable diseases in sub-Saharan Africa: a scoping review of large cohort studies.J Glob Health. (2019) 9:020409. 10.7189/jogh.09.020409
18.
MohamedSFHareguTNKhayeka-WandabwaCMuthuriSKKyobutungiC. Magnitude and predictors of normal-weight central obesity–the AWI-Gen Study findings.Glob Health Action. (2019) 12:1685809. 10.1080/16549716.2019.1685809
19.
ZühlkeLEngelMEKarthikeyanGRangarajanSMackiePCupidoBet alCharacteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the global rheumatic heart disease registry (The REMEDY Study).Eur Heart J. (2015) 36:1115–22. 10.1093/eurheartj/ehu449
20.
MocumbiAOHFerreiraMB. Neglected cardiovascular diseases in Africa: challenges and opportunities.J Am Coll Cardiol. (2010) 55:680–7. 10.1016/j.jacc.2009.09.041
21.
YuyunMFSliwaKKengneAPMocumbiAOBukhmanG. Cardiovascular diseases in sub-Saharan Africa compared to high-income countries: an epidemiological perspective.Global Heart. (2020) 15:15. 10.5334/gh.403
22.
SalomonJAWangHFreemanMKVosTFlaxmanADLopezADet alHealthy life expectancy for 187 countries, 1990–2010: a systematic analysis for the Global Burden Disease Study 2010.Lancet. (2012) 380:2144–62. 10.1016/s0140-6736(12)61690-0
23.
GheorgheAGriffithsUMurphyALegido-QuigleyHLampteyPPerelP. The economic burden of cardiovascular disease and hypertension in low-and middle-income countries: a systematic review.BMC Public Health. (2018) 18:975. 10.1186/s12889-018-5806-x
24.
AgyepongIASewankamboNBinagwahoAColl-SeckAMCorrahTEzehAet alThe path to longer and healthier lives for all Africans by 2030: the lancet commission on the future of health in Sub-Saharan Africa.Lancet. (2017) 390:2803–59. 10.1016/s0140-6736(17)31509-x
25.
MurrayCJAravkinAYZhengPAbbafatiCAbbasKMAbbasi-KangevariMet alGlobal burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019.Lancet. (2020) 396:1223–49. 10.1016/S0140-6736(20)30752-2
26.
World Health Organization [WHO].Hypertension [Fact sheet] [Online]. (2021). Available online at: https://www.who.int/news-room/fact-sheets/detail/hypertension(accessed June 17, 2022).
27.
AtaklteFErqouSKaptogeSTayeBEchouffo-TcheuguiJBKengneAP. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis.Hypertension. (2015) 65:291–8. 10.1161/HYPERTENSIONAHA.114.04394
28.
NejjariCArharbiMChentirM-TBoujnahRKemmouOMegdicheHet alEpidemiological trial of hypertension in North Africa (ETHNA): an International Multicentre Study in Algeria, Morocco and Tunisia.J Hypertens. (2013) 31:49–62. 10.1097/HJH.0b013e32835a6611
29.
WollumAGabertRMcNellanCRDalyJMReddyPBhattPet alIdentifying gaps in the continuum of care for cardiovascular disease and diabetes in two communities in South Africa: baseline findings from the HealthRise project.PLoS One. (2018) 13:e0192603. 10.1371/journal.pone.0192603
30.
ChoNHKiringaJMbanyaJ-COgurstonK.IDF Diabetes Atlas Eight Edition 2017.Brussels: International Diabetes Federation (2017).
31.
CollaborationERF. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies.Lancet. (2010) 375:2215–22. 10.1016/s0140-6736(10)60484-9
32.
DanaeiGFinucaneMMLuYSinghGMCowanMJPaciorekCJet alNational, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants.Lancet. (2011) 378:31–40. 10.1016/S0140-6736(11)60679-X
33.
PastakiaSDPeknyCRManyaraSMFischerL. Diabetes in sub-Saharan Africa–from policy to practice to progress: targeting the existing gaps for future care for diabetes.Diabetes Metab. Syndr Obes. (2017) 10:247. 10.2147/DMSO.S126314
34.
World Health Organization [WHO].Global Report on Diabetes. 2016.Geneva: World Health Organization (2018).
35.
AzeezTA. Obesity in Africa: the challenges of a rising epidemic in the midst of dwindling resources.Obes Med. (2022) 31:100397. 10.1016/j.obmed.2022.100397
36.
World Health Organization [WHO].The Global Health Observatory. Noncommunicable Diseases: Risk factors [Internet]. (2022). Available online at: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/ncd-risk-factors(accessed June 17.2022).
37.
World Health Organization [WHO].WHO Global Report on Trends in Prevalence of Tobacco Use 2000-2025.4th ed. Geneva: World Health Organization (2019).
38.
World Health Organization [WHO].WHO Framework Convention on Tobacco Control.Geneva: World Health Organization (2003). 13 p.
39.
World Health Organization [WHO].Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013-2020.Geneva: World Health Organization (2013).
40.
World Health Organization [WHO].2012 Global Progress Report on Implementation of the WHO Framework Convention on Tobacco Control.Geneva: World Health Organization (2012).
41.
BelcherERossH.Tobacco Use in Africa: Tobacco Control through Prevention.Georgia, ATL: American Cancer Society (2015). 2247 p.
42.
ZatonskiWZatonskiMPrzewozniakK. Health improvement in Poland is contingent on continued extensive tobacco control measures.Ann Agric Environ Med. (2013) 20:405–11.
43.
YancyCWJessupMBozkurtBButlerJCaseyDEDraznerMHet al2013 ACCF/AHA guideline for the management of heart failure: a report of the American college of cardiology foundation/American heart association task force on practice guidelines.J Am Coll Cardiol. (2013) 62:e147–239. 10.1161/cir.0b013e31829e8776
44.
BragazziNLZhongWShuJAbu MuchALotanDGrupperAet alBurden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017.Eur J Prev Cardiol. (2021) 28:1682–90. 10.1093/eurjpc/zwaa147
45.
AgborVNEssoumaMNtusiNANyagaUFBignaJJNoubiapJJ. Heart failure in sub-Saharan Africa: a contemporaneous systematic review and meta-analysis.Int J Cardiol. (2018) 257:207–15. 10.1016/j.ijcard.2017.12.048
46.
DokainishHTeoKZhuJRoyAAlHabibKFElSayedAet alGlobal mortality variations in patients with heart failure: results from the international congestive heart failure (INTER-CHF) prospective Cohort Study.Lancet Glob Health. (2017) 5:e665–72. 10.1016/S2214-109X(17)30196-1
47.
KarayeKMDokainishHElSayedAMondoCDamascenoASliwaKet alClinical profiles and outcomes of heart failure in five African Countries: results from INTER-CHF Study.Glob Heart. (2021) 16:50. 10.5334/gh.940
48.
YusufSRangarajanSTeoKIslamSLiWLiuLet alCardiovascular risk and events in 17 low-, middle-, and high-income countries.N Engl J Med. (2014) 371:818–27. 10.1056/nejmoa1311890
49.
HertzJTKwekaGLBloomfieldGSLimkakengATJr.LoringZTemuGet alPatterns of emergency care for possible acute coronary syndrome among patients with chest pain or shortness of breath at a Tanzanian referral hospital.Glob Heart. (2020) 15:9. 10.5334/gh.402
50.
OnenCL. Epidemiology of ischaemic heart disease in sub-Saharan Africa.Cardiovasc J Afr. (2013) 24:34–42. 10.5830/CVJA-2012-071
51.
MayosiBM. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa.Heart. (2007) 93:1176–83. 10.1136/hrt.2007.127746
52.
MocumbiAOFerreiraMBSidiDYacoubMH. A Population Study of endomyocardial fibrosis in a rural area of Mozambique.N Engl J Med. (2008) 359:43–9. 10.1056/NEJMoa0708629
53.
CarlsonSDuberHCAchanJIkileziGMokdadAHStergachisAet alCapacity for diagnosis and treatment of heart failure in sub-Saharan Africa.Heart. (2017) 103:1874–9. 10.1136/heartjnl-2016-310913
54.
BonnyANgantchaMJeilanMOkelloEKavirajBTalleMAet alStatistics on the use of cardiac electronic devices and interventional electrophysiological procedures in Africa from 2011 to 2016: report of the Pan African Society of Cardiology (PASCAR) Cardiac Arrhythmias and Pacing Task Forces.Europace. (2018) 20:1513–26. 10.1093/europace/eux353
55.
KalyesubulaRMutyabaIRabinTAndia-BiraroIAlupoPKimuliIet alTrends of admissions and case fatality rates among medical in-patients at a tertiary hospital in Uganda; A four-year Retrospective Study.PLoS One. (2019) 14:e0216060. 10.1371/journal.pone.0216060
56.
Le VayJNFraserAByassPTollmanSKahnKD’AmbruosoLet alMortality trends and access to care for cardiovascular diseases in Agincourt, rural South Africa: a mixed-methods analysis of verbal autopsy data.BMJ Open. (2021) 11:e048592. 10.1136/bmjopen-2020-048592
57.
SteynKSliwaKHawkenSCommerfordPOnenCDamascenoAet alRisk factors associated with myocardial infarction in Africa: the Interheart Africa Study.Circulation. (2005) 112:3554–61. 10.1161/circulationaha.105.563452
58.
MonastaLRonfaniLGlobal Causes of Death Collaborators.Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017.Lancet. (2018) 392:1736–88. 10.1016/s0140-6736(18)32203-7
59.
BertrandE. Coronary heart disease in black Africans: an overview.East Afr Med J. (1995) 72:37–41. 10.1016/s0140-6736(14)61682-2
60.
MwitaJCGodmanB. Poverty and cardiovascular diseases in sub-saharan Africa. In: MonyekiKDKemperHCGeditors. Lifestyle and Epidemiology: The Double Burden of Poverty and Cardiovascular Diseases in African Populations.London: Intechopen (2021). 17 p. 10.5772/intechopen.98575
61.
HertzJTMadutDBTeshaRAWilliamGSimmonsRAGalsonSWet alPerceptions of chest pain and healthcare seeking behavior for chest pain in northern Tanzania: a community-based survey.PLoS One. (2019) 14:e0212139. 10.1371/journal.pone.0212139
62.
AntignacMDiopBIDoBN’GuettaRToureIAZabsonrePet alQuality assessment of 7 cardiovascular drugs in 10 sub-Saharan countries: the Seven Study.JAMA Cardiol. (2017) 2:223–5. 10.1001/jamacardio.2016.3851
63.
HertzJTSakitaFMKwekaGLLimkakengATGalsonSWJinnyJYet alAcute myocardial infarction under-diagnosis and mortality in a Tanzanian emergency department: a prospective Observational Study.Am Heart J. (2020) 226:214–21. 10.1016/j.ahj.2020.05.017
64.
YameogoNVSamadoulougouAMillogoGKologoKJKombassereKToguyeniBet alDelays in the management of acute coronary syndromes with ST-ST segment elevation in Ouagadougou and factors associated with an extension of these delays: a Cross-Sectional Study about 43 cases collected in the CHU-Yalgado Ouédraogo.Pan Afr Med J. (2012) 13:90.
65.
YaoHEkouANiamkeyTHounhoui GanSKouaméIAfassinouYet alAcute coronary syndromes in sub-saharan Africa: a 10-year systematic review.J Am Heart Assoc. (2022) 11:e021107. 10.1161/JAHA.120.021107
66.
JohnsonCONguyenMRothGANicholsEAlamTAbateDet alGlobal, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016.Lancet Neurol. (2019) 18:439–58. 10.1016/S1474-4422(19)30034-1
67.
AdoukonouTKossiOFotso MefoPAgbetouMMagneJGbaguidiGet alStroke case fatality in sub-Saharan Africa: systematic review and meta-analysis.Int J Stroke. (2021) 16:902–16. 10.1177/1747493021990945
68.
SarfoFSOvbiageleBGebregziabherMWahabKAkinyemiRAkpaluAet alStroke among young West Africans: evidence from the SIREN (Stroke investigative research and educational network) Large Multisite Case–Control Study.Stroke. (2018) 49:1116–22. 10.1161/strokeaha.118.020783
69.
AkinyemiROOvbiageleBAdenijiOASarfoFSAbd-AllahFAdoukonouTet alStroke in Africa: profile, progress, prospects and priorities.Nat Rev Neurol. (2021) 17:634–56. 10.1038/s41582-021-00542-4
70.
ZhouBPerelPMensahGAEzzatiM. Global epidemiology, health burden and effective interventions for elevated blood pressure and hypertension.Nat Rev Cardiol. (2021) 18:785–802. 10.1038/s41569-021-00559-8
71.
KrukMGageAArsenaultCJordanKLeslieHRoder-DeWanSet alHigh-quality health systems in the Sustainable Development Goals era: time for a revolution.Lancet Glob Health. (2018) 6:e1196–252. 10.1016/s2214-109x(18)30386-3
72.
MagaqaQArianaPPolackS. Examining the availability and accessibility of rehabilitation services in a rural district of South Africa: a Mixed-Methods Study.Int J Environ Res Public Health. (2021) 18:4692. 10.3390/ijerph18094692
73.
PeckRNGreenEMtabajiJMajingeCSmartLRDownsJAet alHypertension-related diseases as a common cause of hospital mortality in Tanzania: a 3-year Prospective Study.J Hypertens. (2013) 31:1806. 10.1097/HJH.0b013e328362bad7
74.
PatelSAWinkelMAliMKNarayanKVMehtaNK. Cardiovascular mortality associated with 5 leading risk factors: national and state preventable fractions estimated from survey data.Ann Intern Med. (2015) 163:245–53. 10.7326/m14-1753
75.
World Health Organization [WHO].A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013.Geneva: World Health Organization (2013).
76.
OgedegbeGGyamfiJPlange-RhuleJSurkisARosenthalDMAirhihenbuwaCet alTask shifting interventions for cardiovascular risk reduction in low-income and middle-income countries: a systematic review of randomised controlled trials.BMJ Open. (2014) 4:e005983. 10.1136/bmjopen-2014-005983
77.
World Health Organization [WHO].HEARTS: Technical Package for Cardiovascular Disease Management in Primary Health Care: Implementation Guide.Geneva: World Health Organization (2018).
78.
HyseniLElliot-GreenALloyd-WilliamsFKypridemosCO’FlahertyMMcGillRet alSystematic review of dietary salt reduction policies: evidence for an effectiveness hierarchy?PLoS One. (2017) 12:e0177535. 10.1371/journal.pone.0177535
79.
Strauss-KrugerMWentzel-ViljoenEWareLJVan ZylTCharltonKEllisSet alEarly evidence for the effectiveness of South Africa’s legislation on salt restriction in foods: the African-PREDICT Study.J Hum Hypertens. (2022):1–8. [Epub ahead of print]. 10.1038/s41371-021-00653-x
80.
FreibergMSChangC-CHKullerLHSkandersonMLowyEKraemerKLet alHIV infection and the risk of acute myocardial infarction.JAMA Intern Med. (2013) 173:614–22. 10.1001/jamainternmed.2013.3728
81.
FreibergMSChangC-CHSkandersonMPattersonOVDuVallSLBrandtCAet alAssociation between HIV infection and the risk of heart failure with reduced ejection fraction and preserved ejection fraction in the antiretroviral therapy era: results from the Veterans Aging Cohort Study.JAMA Cardiol. (2017) 2:536–46. 10.1001/jamacardio.2017.0264
82.
ChowFCReganSFeskeSMeigsJBGrinspoonSKTriantVA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system.J Acquir Immune Defic Syndr. (2012) 60:351. 10.1097/qai.0b013e31825c7f24
83.
ShahASStelzleDLeeKKBeckEJAlamSCliffordSet alGlobal burden of atherosclerotic cardiovascular disease in people living with HIV: systematic review and meta-analysis.Circulation. (2018) 138:1100–12. 10.1161/CIRCULATIONAHA.117.033369
84.
ArmahKAChangC-CHBakerJVRamachandranVSBudoffMJCraneHMet alPrehypertension, hypertension, and the risk of acute myocardial infarction in HIV-infected and-uninfected veterans.Clin Infect Dis. (2014) 58:121–9. 10.1093/cid/cit652
85.
TriantVAPerezJReganSMassaroJMMeigsJBGrinspoonSKet alCardiovascular risk prediction functions underestimate risk in HIV infection.Circulation. (2018) 137:2203–14. 10.1161/circulationaha.117.028975
86.
AlencherryBEremGMirembeGSsinabulyaIYunC-HHungC-Let alCoronary artery calcium, HIV and inflammation in Uganda compared with the USA.Open Heart. (2019) 6:e001046. 10.1136/openhrt-2019-001046
87.
VosATempelmanHDevilléWBarthRWensingAKretzschmarMet alHIV and risk of cardiovascular disease in sub-Saharan Africa: rationale and design of the Ndlovu Cohort Study.Eur J Prev Cardiol. (2017) 24:1043–50. 10.1177/2047487317702039
88.
Manne-GoehlerJMontanaLGómez-OlivéFXRohrJHarlingGWagnerRGet alThe ART advantage: healthcare utilization for diabetes and hypertension in rural South Africa.J Acquir Immune Defic Syndr. (2017) 75:561. 10.1097/qai.0000000000001445
89.
HyleEPBekkerLGMarteyEBHuangMXuAParkerRAet alCardiovascular risk factors among ART-experienced people with HIV in South Africa.J Int AIDS Soc. (2019) 22:e25274. 10.1002/jia2.25274
90.
MutemwaMPeerNDe VilliersAMukasaBMatshaTEMillsEJet alPrevalence, detection, treatment, and control of hypertension in human immunodeficiency virus (HIV)-infected patients attending HIV clinics in the Western Cape Province, South Africa.Medicine. (2018) 97:e12121. 10.1097/MD.0000000000012121
91.
MudduMTusubiraAKNakiryaBNalwogaRSemitalaFCAkitengARet alExploring barriers and facilitators to integrated hypertension-HIV management in Ugandan HIV clinics using the consolidated framework for implementation research (CFIR).Implement Sci Commun. (2020) 1:45. 10.1186/s43058-020-00033-5
92.
MudduMSsinabulyaIKigoziSPSsennyonjoRAyebareFKatwesigyeRet alHypertension care cascade at a large urban HIV clinic in Uganda: a Mixed Methods Study using the Capability, Opportunity, Motivation for Behavior change (COM-B) model.Implement Sci Commun. (2021) 2:121. 10.1186/s43058-021-00223-9
93.
MwagombaBLMAmehSBongominPJumaPAMacKenzieRKKyobutungiCet alOpportunities and challenges for evidence-informed HIV-noncommunicable disease integrated care policies and programs: lessons from Malawi, South Africa, Swaziland and Kenya.AIDS. (2018) 32:S21–32. 10.1097/qad.0000000000001885
94.
ElliottPAnderssonBArbustiniEBilinskaZCecchiFCharronPet alClassification of the cardiomyopathies: a position statement from the European society of cardiology working group on myocardial and pericardial diseases.Eur Heart J. (2008) 29:270–6. 10.1093/eurheartj/ehm342
95.
SliwaKMayosiBM. Recent advances in the epidemiology, pathogenesis and prognosis of acute heart failure and cardiomyopathy in Africa.Heart. (2013) 99:1317–22. 10.1136/heartjnl-2013-303592
96.
SliwaKDamascenoAMayosiBM. Epidemiology and etiology of cardiomyopathy in Africa.Circulation. (2005) 112:3577–83. 10.1161/circulationaha.105.542894
97.
FalaseAOOgahOS. Cardiomyopathies and myocardial disorders in Africa: present status and the way forward.Cardiovasc J Afr. (2012) 23:552–62. 10.5830/cvja-2012-046
98.
SliwaKWilkinsonDHansenCNtyintyaneLTibazarwaKBeckerAet alSpectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a Cohort Study.Lancet. (2008) 371:915–22. 10.1016/s0140-6736(08)60417-1
99.
FundikiraLSChilloPvan LaakeLWMutagaywaRKSchmidtAFKamuhabwaAet alRisk factors and prevalence of dilated cardiomyopathy in Sub-Saharan Africa: protocol for a systematic review.JMIR Res Protoc. (2021) 10:e18229. 10.5334/gh.1166
100.
FreersJMayanja-KizzaHParryE. The heart. In: ParryEGodfreyRMabeyDGillGeditors. Principles of Medicine in Africa Third edition; Textbook of Tropical Surgery.Cambridge: Cambridge University Press (2004).
101.
MaroEJanabiMKaushikR. Clinical and Echocardiographic Study of hypertrophic cardiomyopathy in Tanzania.Trop Doct. (2006) 36:225–7. 10.1258/004947506778604904
102.
MbakwemAOkeDAjuluchukwuJ. Hypertrophic cardiomyopathy in South Western Nigeria: hypertrophic cardiomyopathy.SA Heart. (2009) 6:104–9. 10.24170/6-2-1998
103.
MaronBJTowbinJAThieneGAntzelevitchCCorradoDArnettDet alContemporary definitions and classification of the cardiomyopathies: an American heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention.Circulation. (2006) 113:1807–16. 10.1161/CIRCULATIONAHA.106.174287
104.
MunclingerMJPatelJJMithaAS. Follow-up of patients with arrhythmogenic right ventricular cardiomyopathy dysplasia.S Afr Med J. (2000) 90:61–8.
105.
GayeNDNgaïdéAABahMBBabakaKMbayeAAbdoulK. Non-compaction of left ventricular myocardium in sub-Saharan African adults.Heart Asia. (2017) 9:e010884. 10.1136/heartasia-2017-010884
106.
ZühlkeLKarthikeyanGEngelMERangarajanSMackiePCupido-Katya MauffBet alClinical outcomes in 3343 children and adults with rheumatic heart disease from 14 low-and middle-income countries: two-year follow-up of the global rheumatic heart disease registry (the REMEDY Study).Circulation. (2016) 134:1456–66. 10.1161/CIRCULATIONAHA.116.024769
107.
WatkinsDAJohnsonCOColquhounSMKarthikeyanGBeatonABukhmanGet alGlobal, regional, and national burden of rheumatic heart disease, 1990–2015.N Engl J Med. (2017) 377:713–22. 10.1056/nejmoa1603693
108.
CoatesMMSliwaKWatkinsDAZühlkeLPerelPBertelettiFet alAn investment case for the prevention and management of rheumatic heart disease in the African Union 2021–30: a Modelling Study.Lancet Glob Health. (2021) 9:e957–66. 10.1016/S2214-109X(21)00199-6
109.
BeatonAOkelloERwebemberaJGroblerAEngelmanDAlepereJet alSecondary antibiotic prophylaxis for latent rheumatic heart disease.N Engl J Med. (2022) 386:230–40. 10.1056/NEJMoa2102074
110.
AmegahAK. Tackling the growing burden of cardiovascular diseases in sub-Saharan Africa: need for dietary guidelines.Circulation. (2018) 138:2449–51. 10.1161/CIRCULATIONAHA.118.037367
111.
YuyunMFBonnyANgGASliwaKKengneAPChinAet alA systematic review of the spectrum of cardiac arrhythmias in sub-Saharan Africa.Glob Heart. (2020) 15:37. 10.5334/gh.808
112.
BonnyANgantchaMScholtzWChinANelGAnzouan-KacouJ-Bet alCardiac arrhythmias in Africa: epidemiology, management challenges, and perspectives.J Am Coll Cardiol. (2019) 73:100–9.
113.
MkokoPBahiruEAjijolaOABonnyAChinA. Cardiac arrhythmias in low-and middle-income countries.Cardiovasc Diagn Ther. (2020) 10:350. 10.21037/cdt.2019.09.21
114.
BonnyANgantchaMYuyunMFKarayeKMScholtzWSulimanAet alCardiac arrhythmia services in Africa from 2011 to 2018: the second report from the pan African society of cardiology working group on cardiac arrhythmias and pacing.Europace. (2020) 22:420–33. 10.1093/europace/euz354
115.
NoubiapJJNyagaUF. A review of the epidemiology of atrial fibrillation in sub-Saharan Africa.J Cardiovasc Electrophysiol. (2019) 30:3006–16. 10.1111/jce.14222
116.
PatelPJDeoR. Atrial fibrillation in sub-Saharan Africa: not so black and white?J Cardiovasc Electrophysiol. (2019) 30:3017–9.
117.
JeilanMRwebemberaJAdenHBonnyAAkinyiLAjijolaOAet alThe inaugural meeting of the Africa heart rhythm association (AFHRA).Cardiovasc J Afr. (2020) 31:162–4. 10.5830/cvja-2020-019
118.
MoutonJPBlockmanMSekaggya-WiltshireCSemakulaJWaittCPirmohamedMet alImproving anticoagulation in sub-Saharan Africa: what are the challenges and how can we overcome them?Br J Clin Pharmacol. (2021) 87:3056–68. 10.1111/bcp.14768
119.
RwebemberaJJeilanMAjijolaOATalleMSaniMUKarayeKMet alCardiac pacing training in Africa: endorsed by the Africa heart rhythm association (AFHRA): JACC international.J Am Coll Cardiol. (2020) 76:465–72. 10.1016/j.jacc.2020.04.079
120.
WunderlyKYousefZBonnyAWeatherwaxKJLavanBAllmendingerCet alUsing reconditioned pacemakers to treat bradycardia in Africa.Nat Rev Cardiol. (2018) 15:725–6. 10.1038/s41569-018-0076-y
121.
WilliamsA. Heart disease in the native population of Uganda.East Afr Med J. (1938) 15:229.
122.
GrimaldiAMocumbiAOFreersJLachaudMMirabelMFerreiraBet alTropical endomyocardial fibrosis: natural history, challenges, and perspectives.Circulation. (2016) 133:2503–15. 10.1161/circulationaha.115.021178
123.
RwebemberaJManyilirahWZhuZWNabbaaleJNamuyongaJSsinabulyaIet alPrevalence and characteristics of primary left-sided valve disease in a cohort of 15,000 patients undergoing echocardiography studies in a tertiary hospital in Uganda.BMC Cardiovasc Disord. (2018) 18:82. 10.1186/s12872-018-0813-5
124.
CheloDNguefackFAwaHDMKingueS. Endomyocardial fibrosis in Sub Saharan Africa: the geographical origin, socioeconomic status, and dietary habits of cases reported in Yaounde, Cameroon.Ann Pediatr Cardiol. (2015) 8:202. 10.4103/0974-2069.164693
125.
GallagherJMcDonaldKLedwidgeMWatsonCJ. Heart failure in sub-Saharan Africa.Cardiac Fail Rev. (2018) 4:21. 10.15420/cfr.2018:4:1
126.
MocumbiAOStothardJRCorreia-de-SáPYacoubM. Endomyocardial fibrosis: an update after 70 years.Curr Cardiol Rep. (2019) 21:148. 10.1007/s11886-019-1244-3
127.
BeatonASableCBrownJHoffmanJMungomaMMondoCet alGenetic susceptibility to endomyocardial fibrosis.Glob Cardiol Sci Pract. (2015) 2014:60. 10.5339/gcsp.2014.60
128.
BeatonAMocumbiAO. Diagnosis and management of endomyocardial fibrosis.Cardiol Clin. (2017) 35:87–98. 10.1016/j.ccl.2016.08.005
129.
SchneiderUJenniRTurinaJTurinaMHessO. Long term follow up of patients with endomyocardial fibrosis: effects of surgery.Heart. (1998) 79:362–7. 10.1136/hrt.79.4.362
130.
AkinwusiPOOdeyemiAO. The changing pattern of endomyocardial fibrosis in South-west Nigeria.Clin Med Insights Cardiol. (2012) 6:163–8. 10.4137/cmc.s10141
131.
MocumbiAOH. Recent trends in the epidemiology of endomyocardial fibrosis in Africa.Paediatr Int Child Health. (2012) 32:63–4. 10.1179/204690512X13345408275810
132.
AlikuTORwebemberaJLubegaSWanzhuZLugeroCNamuyongaJet alTrends In annual incidence rates of newly diagnosed endomyocardial fibrosis cases at the uganda heart institute: a fourteen-year review.Front Cardiovasc Med. (2022) 9:841346. 10.3389/fcvm.2022.841346
133.
VijayaraghavanGSivasankaranS. Tropical endomyocardial fibrosis in India: a vanishing disease!Indian J Med Res. (2012) 136:729.
134.
FerreiraBMatsika-ClaquinMHausse-MocumbiASidiDPaquetC. Geographic origin of endomyocardial fibrosis treated at the central hospital of Maputo (Mozambique) between 1987 and 1999.Bull Soc Pathol Exot. (2002) 95:276–9.
135.
Regitz-ZagrosekVRoos-HesselinkJBauersachsJBlomstrom-LundqvistCCifkovaRDe BonesMet al2018 ESC guidelines for the management of cardiovascular diseases during pregnancy: the task force for the management of cardiovascular diseases during pregnancy of the European society of cardiology.Eur Heart J. (2018) 39:3165–241. 10.1093/eurheartj/ehy340
136.
DuleyLeditor. The global impact of pre-eclampsia and eclampsia.Semin Perinatol. (2009) 33:130–7.
137.
MoodleyJ. Maternal deaths due to hypertensive disorders in pregnancy: saving mothers report 2002-2004.Cardiovasc J Afr. (2007) 18:358–61. 10.1016/j.bpobgyn.2007.11.004
138.
WatkinsDASebitloaneMEngelMEMayosiBM. The burden of antenatal heart disease in South Africa: a systematic review.BMC Cardiovasc Disord. (2012) 12:23. 10.1186/1471-2261-12-23
139.
DiaoMKaneANdiayeMBMbayeABodianMDiaMMet alPregnancy in women with heart disease in sub-Saharan Africa.Arch Cardiovasc Dis. (2011) 104:370–4. 10.1016/j.acvd.2011.04.001
140.
NabbaaleJOkelloEKibirigeDSsekitolekoIIsangaJKarungiPet alBurden, predictors and short-term outcomes of peripartum cardiomyopathy in a black African cohort.PLoS One. (2020) 15:e0240837. 10.1371/journal.pone.0240837
141.
KarayeKMIshaqNSa’iduHBalarabeSTalleMIsaMet alIncidence, clinical characteristics, and risk factors of peripartum cardiomyopathy in Nigeria: results from the PEACE Registry.ESC Heart Fail. (2020) 7:236–44. 10.1002/ehf2.12562
142.
DesaiDMoodleyJNaidooD. Peripartum cardiomyopathy: experiences at King Edward VIII Hospital, Durban, South Africa and a review of the literature.Trop Doct. (1995) 25:118–23. 10.1177/004947559502500310
143.
MocumbiAOSliwaK. Women’s cardiovascular health in Africa.Heart. (2012) 98:450–5. 10.1136/heartjnl-2011-301025
144.
UkohaWCMtshaliNGAdepejuL. Current state of preconception care in sub-Saharan Africa: a systematic scoping review.Afr J Prim Health Care Fam Med. (2022) 14:e1–11. 10.4102/phcfm.v14i1.3096
145.
ZhangWOkelloENyakoojoWLwabiPMondoCK. Proportion of patients in the Uganda rheumatic heart disease registry with advanced disease requiring urgent surgical interventions.Afr Health Sci. (2015) 15:1182–8. 10.4314/ahs.v15i4.17
146.
FogertyAE. Challenges of anticoagulation therapy in pregnancy.Curr Treat Options Cardiovasc Med. (2017) 19:76. 10.1007/s11936-017-0575-x
147.
SliwaKLibhaberEElliottCMombergZOsmanAZühlkeLet alSpectrum of cardiac disease in maternity in a low-resource cohort in South Africa.Heart. (2014) 100:1967–74. 10.1136/heartjnl-2014-306199
148.
Van Der LindeDKoningsEESlagerMAWitsenburgMHelbingWATakkenbergJJet alBirth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis.J Am Coll Cardiol. (2011) 58:2241–7. 10.1016/j.jacc.2011.08.025
149.
LiuYChenSZühlkeLBlackGCChoyM-KLiNet alGlobal birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies.Int J Epidemiol. (2019) 48:455–63. 10.1093/ije/dyz009
150.
ZimmermanMSSmithAGCSableCAEchkoMMWilnerLBOlsenHEet alGlobal, regional, and national burden of congenital heart disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017.Lancet Child Adolesc Health. (2020) 4:185–200. 10.1016/S2352-4642(19)30402-X
151.
NamuyongaJLubegaSAlikuTOmaginoJSableCLwabiP. Pattern of congenital heart disease among children presenting to the Uganda Heart Institute, Mulago Hospital: a 7-year review.Afr Health Sci. (2020) 20:745–52. 10.4314/ahs.v20i2.26
152.
AlikuTBeatonALubegaSDewyerAScheelAKamaremboJet alProfile of congenital heart disease and access to definitive care among children seen at gulu regional referral hospital in Northern Uganda: a four-year experience.J Congenit Cardiol. (2021) 5:9. 10.1186/s40949-021-00064-0
153.
MandalenakisZRosengrenASkoglundKLappasGErikssonPDellborgM. Survivorship in children and young adults with congenital heart disease in Sweden.JAMA Intern Med. (2017) 177:224–30. 10.1001/jamainternmed.2016.7765
154.
MarinoBSLipkinPHNewburgerJWPeacockGGerdesMGaynorJWet alNeurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American heart association.Circulation. (2012) 126:1143–72. 10.1161/CIR.0b013e318265ee8a
155.
RaissadatiANieminenHHaukkaJSairanenHJokinenE. Late causes of death after pediatric cardiac surgery: a 60-year Population-Based Study.J Am Coll Cardiol. (2016) 68:487–98. 10.1016/j.jacc.2016.05.038
156.
ZühlkeLMirabelMMarijonE. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities.Heart. (2013) 99:1554–61. 10.1136/heartjnl-2013-303896
157.
AlikuTOLubegaSNamuyongaJMwambuTOketchoMOmaginoJOet alPediatric cardiovascular care in Uganda: current status, challenges, and opportunities for the future.Ann Pediatr Cardiol. (2017) 10:50. 10.4103/0974-2069.197069
158.
ZimmermanMSableCeditors. Congenital heart disease in low-and-middle-income countries: focus on sub-Saharan Africa.Am J Med Genet C Semin Med Genet. (2020) 184:36–46.
159.
NeirottiR. Paediatric cardiac surgery in less privileged parts of the world.Cardiol Young. (2004) 14:341–6. 10.1017/s1047951104003191
160.
VervoortDSwainJDPezzellaATKpodonuJ. Cardiac surgery in low-and middle-income countries: a state-of-the-art review.Ann Thorac Surg. (2021) 111:1394–400. 10.1016/j.athoracsur.2020.05.181
161.
ThakurJSNegiPCAhluwaliaSKSharmaReditors. Integrated community-based screening for cardiovascular diseases of childhood.World Health Forum. (1997) 18:24–7.
162.
AlikuTOLubegaSLwabiPOketchoMOmaginoJOMwambuT. Outcome of patients undergoing open heart surgery at the Uganda heart institute, Mulago hospital complex.Afr Health Sci. (2014) 14:946–53. 10.4314/ahs.v14i4.25
163.
EdwinFKinsleyRHBrinkJMartinGMamorareHColsenP. Late primary arterial switch for transposition of the great arteries with intact ventricular septum in an African population.World J Pediatr Congenit Heart Surg. (2011) 2:237–42. 10.1177/2150135110395335
164.
LaingNKrausSShaboodienGNtusiN. An overview of the genetic basis of cardiovascular disease.S Afr Med J. (2019) 109:364–70. 10.7196/SAMJ.2019.v109i6.14069
165.
AldersleyTLawrensonJHumanPShaboodienGCupidoBComitisGet alPROTEA, A Southern African multicenter congenital heart disease registry and biorepository: rationale, design, and initial results.Front Pediatr. (2021) 9:763060. 10.3389/fped.2021.763060
166.
BennettJEKontisVMathersCDGuillotMRehmJChalkidouKet alNCD countdown 2030: pathways to achieving sustainable development goal target 3.4.Lancet. (2020) 396:918–34. 10.1016/S0140-6736(20)31761-X
167.
MendisSYachDBengoaRNarvaezDZhangX. Research gap in cardiovascular disease in developing countries.Lancet. (2003) 361:2246–7. 10.1016/s0140-6736(03)13753-1
168.
QureshiNQMufarrihSHBloomfieldGSTariqWAlmasAMokdadAHet alDisparities in cardiovascular research output and disease outcomes among high-, middle-and low-income countries–an analysis of global cardiovascular publications over the last decade (2008–2017).Glob Heart. (2021) 16:4. 10.5334/gh.815
169.
MullanFFrehywotSOmaswaFBuchEChenCGreysenSRet alMedical schools in sub-Saharan Africa.Lancet. (2011) 377:1113–21. 10.1016/s0140-6736(10)61961-7:
170.
World Health Organization [WHO].State of Health Financing in the African Region.Geneva: World Health Organization (2013).
171.
World Health Organization [WHO].Global Spending on Health: A World in Transition. Report No.: 9240040374.Geneva: World Health Organization (2019).
172.
KhatibRMcKeeMShannonHChowCRangarajanSTeoKet alAvailability and affordability of cardiovascular disease medicines and their effect on use in high-income, middle-income, and low-income countries: an analysis of the PURE Study data.Lancet. (2016) 387:61–9. 10.1016/S0140-6736(15)00469-9
173.
BoatengDWekesahFBrowneJLAgyemangCAgyei-BaffourPAikinsADet alKnowledge and awareness of and perception towards cardiovascular disease risk in sub-Saharan Africa: a systematic review.PLoS One. (2017) 12:e0189264. 10.1371/journal.pone.0189264
174.
EconomosCDBrownsonRCDeAngelisMAFoersterSBForemanCTGregsonJet alWhat lessons have been learned from other attempts to guide social change?Nutr Rev. (2001) 59:S40–56. 10.1111/j.1753-4887.2001.tb06985.x
175.
CutlerDMLleras-MuneyA. Understanding differences in health behaviors by education.J Health Econ. (2010) 29:1–28. 10.1016/j.jhealeco.2009.10.003
176.
HusainMJEnglishLMRamanandraibeN. An overview of tobacco control and prevention policy status in Africa.Prev Med. (2016) 91:S16–22. 10.1016/j.ypmed.2016.02.017
177.
NakhimovskySSFeiglABAvilaCO’SullivanGMacgregor-SkinnerESprancaM. Taxes on sugar-sweetened beverages to reduce overweight and obesity in middle-income countries: a systematic review.PLoS One. (2016) 11:e0163358. 10.1371/journal.pone.0163358
Summary
Keywords
cardiovascular diseases, cardiovascular medicine, Africa, sub-Saharan Africa (SSA), priorities, gaps, SDG 3.4
Citation
Minja NW, Nakagaayi D, Aliku T, Zhang W, Ssinabulya I, Nabaale J, Amutuhaire W, de Loizaga SR, Ndagire E, Rwebembera J, Okello E and Kayima J (2022) Cardiovascular diseases in Africa in the twenty-first century: Gaps and priorities going forward. Front. Cardiovasc. Med. 9:1008335. doi: 10.3389/fcvm.2022.1008335
Received
31 July 2022
Accepted
24 October 2022
Published
10 November 2022
Volume
9 - 2022
Edited by
Lebo F. Gafane-Matemane, North-West University, South Africa
Reviewed by
Francesc Xavier Gomez-Olive, University of the Witwatersrand, South Africa; Sepiso Kenias Masenga, Mulungushi University, Zambia
Updates
Copyright
© 2022 Minja, Nakagaayi, Aliku, Zhang, Ssinabulya, Nabaale, Amutuhaire, de Loizaga, Ndagire, Rwebembera, Okello and Kayima.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neema W. Minja, neemaminja@gmail.com
†These authors have contributed equally to this work and share first authorship
This article was submitted to General Cardiovascular Medicine, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.