Abstract
Sudden cardiac death (SCD) is a leading cause of mortality, comprising approximately half of all deaths from cardiovascular disease. In the US, the majority of SCD (85%) occurs in patients with ischemic cardiomyopathy (ICM) and a subset in patients with non-ischemic cardiomyopathy (NICM), who tend to be younger and whose risk of mortality is less clearly delineated than in ischemic cardiomyopathies. The conventional means of SCD risk stratification has been the determination of the ejection fraction (EF), typically via echocardiography, which is currently a means of determining candidacy for primary prevention in the form of implantable cardiac defibrillators (ICDs). Advanced cardiac imaging methods such as cardiac magnetic resonance imaging (CMR), single-photon emission computerized tomography (SPECT) and positron emission tomography (PET), and computed tomography (CT) have emerged as promising and non-invasive means of risk stratification for sudden death through their characterization of the underlying myocardial substrate that predisposes to SCD. Late gadolinium enhancement (LGE) on CMR detects myocardial scar, which can inform ICD decision-making. Overall scar burden, region-specific scar burden, and scar heterogeneity have all been studied in risk stratification. PET and SPECT are nuclear methods that determine myocardial viability and innervation, as well as inflammation. CT can be used for assessment of myocardial fat and its association with reentrant circuits. Emerging methodologies include the development of “virtual hearts” using complex electrophysiologic modeling derived from CMR to attempt to predict arrhythmic susceptibility. Recent developments have paired novel machine learning (ML) algorithms with established imaging techniques to improve predictive performance. The use of advanced imaging to augment risk stratification for sudden death is increasingly well-established and may soon have an expanded role in clinical decision-making. ML could help shift this paradigm further by advancing variable discovery and data analysis.
Introduction
Sudden cardiac death is an unexpected death from a cardiac cause within a short period (typically an hour or less) from symptom onset or, if unwitnessed, within 24 h of last being seen alive (1, 2). While the incidence of SCD has gradually declined over the past decades, the annual incidence is ~200,000–400,000 cases per year (the extensive range being attributable to the uncertainty of the cause of some deaths), amounting to around 15–20% of all deaths (3). Among patients with known cardiovascular disease (CVD), which represents about half of cases of SCD, ventricular arrhythmia (VA) is the leading mechanism of SCD in both ischemic cardiomyopathy (ICM) and non-ischemic cardiomyopathy (NICM). In ICM, obstructive coronary artery disease (CAD) leads to myocardial scarring, and regions of heterogeneous conduction serve as substrates for initiating ventricular tachycardia (VT). NICM encompasses diverse cardiac conditions that can result in scar formation or fibrosis, which along with electrophysiological remodeling, can result in VT or ventricular fibrillation (VF). The entity of ischemia but no CAD (INOCA) has also been described and remains an area of active investigation. Thus, far in clinical practice, primary prevention and risk reduction of SCD is accomplished with medical therapy and the implantable cardiac defibrillator (ICD), with slightly different approaches for patients with previously detected VA or resuscitated arrest. Guidelines for primary prevention ICD in both ICM and NICM are driven by clinical symptoms of heart failure and decreased ejection fraction (EF) <35% as derived by imaging (4). However, more useful prognostic data can be obtained from imaging than EF alone (5, 6).
Efforts to improve risk stratification for SCD, or even to reclassify risk assigned by EF, have been undertaken across all cardiac imaging modalities. Herein, we focus on the role of advanced imaging, namely cardiac magnetic resonance imaging (CMR), single-photon emission computer tomography (SPECT), and positron emission tomography (PET), and its application to risk stratification for SCD (Figure 1). We highlight unique techniques of each modality and their limitations, with particular attention to the patient subgroups that inform the utility and pathophysiology of each method (Table 1). For each study, we distinguish endpoints that are related though not interchangeable: while studies with SCD were emphasized in this review, we mention when cardiac death, all-cause mortality, or VA were the primary endpoint as they too are informative. We also describe current and future applications of machine learning (ML) in advanced imaging for SCD, from image acquisition to model construction.
Figure 1
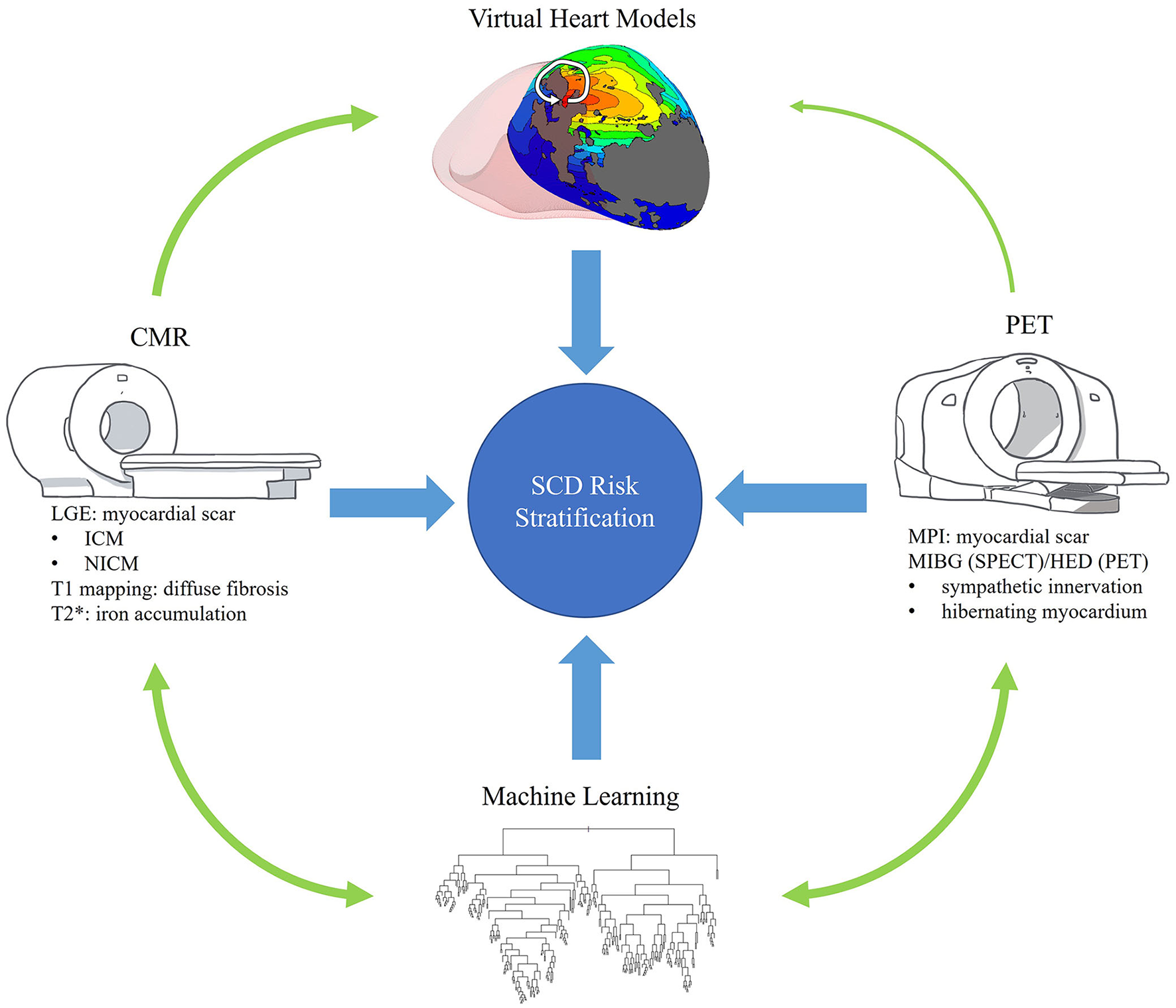
Overview. This review seeks to describe the role of advanced imaging in the risk stratification of SCD and its interplay with promising technologies such as machine learning and personalized virtual heart models. This figure visually summarizes these goals and highlights some of the content to be covered.
Table 1
| Modality | Sequences | Characteristics studied | Evaluation of structure | Evaluation of function | Tissue characterization | Cost |
|---|---|---|---|---|---|---|
| Echocardiography | 2D | • LVEF • Strain |
++ | ++ | + | Low |
| CMR | LGE T1 T2* |
• Strain • Scar • Diffuse fibrosis |
+++ | +++ | +++ | Moderate |
| SPECT | MIBG | • Scar • Viability • Innervation |
+ | + | ++ | High |
| PET | FDG HED |
• Scar • Viability • Innervation |
++ | + | ++ | High |
Comparison of imaging modalities described in this review.
+ for fair, ++ for good, and +++ for excellent.
Cardiac magnetic resonance imaging
Ventricular geometry and function
Chamber geometry has long been known to reflect the role of remodeling in CVD and, in turn, is associated with outcomes including VA (7, 8). Cardiac MRI has been described as the gold standard for structural and functional quantification of cardiac chambers (9, 10). Using CMR, geometry-based approaches to SCD risk stratification have emphasized these attributes, and measurements based on wall thickness have been found to distinguish patients with ICM who develop SCD (11). From a clinical standpoint, studies have suggested that at-risk patients may be inadvertently excluded from ICD as echocardiography overestimates EF when compared with CMR (12, 13). While every imaging modality is to some extent operator dependent, it is perhaps most prominently described in echocardiography as affecting interpretation (14). This highlights that LVEF thresholds may need to be individualized for different modalities, and CMR may be preferable to TTE for therapeutic decision-making in patients with intermediate-range LVEF (15). That said, EF < 35% as a selection criterion for primary prevention ICDs has routinely been critiqued for its low sensitivity and specificity in predicting VA and SCD. Furthermore, patients meeting these criteria encompass only 13% of all suffering SCD (16).
Role of LGE in ICM and CAD
CMR with LGE has developed as a means of characterizing myocardial tissue, and its value in prognosticating cardiovascular outcomes, including SCD, has been widely reviewed and increasingly well-established (17, 18). This has been demonstrated across a wide variety of phenotypes in both patients with ICM and NICM. In ICM, heterogeneous scar, also termed gray zone, is an independent predictor of VA and SCD in a number of studies of patients with ICD or undergoing ICD implantation (19–21). A recent retrospective study among 979 patients with CAD and majority EF with > 35% showed that gray zone mass was more strongly associated with SCD than LVEF (22). The same group showed that in a mixed ICM/NICM population with ICD and cardiac resynchronization therapy, the absence of myocardial fibrosis on visual assessment virtually excluded patients at risk of VA and SCD over 7 years follow-up and among those with scar, gray zone extent added predictive value and improved net reclassification (23). Likewise, a prospective study in a mixed population of ICM and NICM patients undergoing primary prevention ICD demonstrated that the combination of low gray zone mass and low high-sensitivity C-reactive protein identified a subgroup with very low risk for VA (24). In a similar population (mixed ICM/NICM with ICD), an analysis incorporating random survival forests for model construction showed LV scar mass as well as gray zone mass by LGE were top predictors for VA and SCD (25). The same study suggested a hierarchy of risk wherein no scar was less risky than scar, larger total/core scar had higher risk than smaller scar, and larger gray zone had higher risk than smaller gray zone for the same core scar size. Additional applications of LGE, beyond presence and extent, have also been developed, including LV entropy, a measure of the distribution of pixel intensity across the myocardium (26, 27). Amongst patients with CAD and ICDs, entropy was a significant predictor for VA and SCD (27, 28). A mechanistically-minded approach in LGE has been identifying conducting channels, a nidus for VT as identified in CAD (29). A study in a mixed ICM/NICM population showed that the presence and mass of these channels were associated with the risk of VA and appropriate primary prevention ICD therapy (30).
Role of LGE in NICM
In NICM, the role of LGE for SCD risk stratification has been frequently studied in patients with dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM) (31–33).
LGE has been used to identify higher-risk patients who fall outside the EF criterion for primary prevention ICD (34). In a multivariate model for SCD in NICM, LGE had incremental prognostic value over clinical measures, whereas EF did not (35). Among HCM patients, LGE extent has been favorably compared with clinical risk models and, in some cases, exceeded the performance of these models (36–38). To define an LGE cut-off for risk stratification, various thresholds of LGE have been described. In HCM, LGE extent >10% identified patients with SCD rates up to an order of magnitude greater than predicted with clinical risk score (39). A further analysis using serial LGE imaging in DCM illustrated that amongst patients with fibrosis progression, the majority had minimal change in EF (<5%), ergo identifying a high-risk cohort for all-cause mortality not captured by LVEF alone (40). In a case series among athletes, LGE pattern, specifically involving the lateral LV wall, was also noted to correlate with increased risk of malignant arrhythmias (41). As in ICM, LV entropy in DCM patients with ICD significantly improved a clinical model for VA, although there was only one instance of SCD among these (26). Despite these advances and its prominence in the literature, LGE has yet to be included in clinical guidelines. A likely rationale for this is that there has yet to be a completed randomized control trial using LGE for risk stratification, though several are enrolling and underway (18, 31).
Proposed diagnostic guidelines for arrhythmogenic cardiomyopathies, which carry profound and inherent risk for VA and SCD, have included LGE patterns among other criteria (42). In arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D) specifically, abnormal CMR findings including LGE were associated with increased VA (43). Advances in CMR may allow for improved RV assessment facilitating earlier disease detection and ergo risk stratification (44). Similarly in myocarditis, LGE presence and extent have been associated with increased risk of major adverse cardiac events, including VA and SCD (45, 46).
Shortcomings of LGE and alternative CMR sequences
While LGE is perhaps the most widely studied CMR method for SCD risk stratification, other CMR sequences have also been investigated, which may address certain shortcomings of LGE. An intrinsic limitation of gadolinium administration is toxicity, particularly among patients with renal insufficiency, though the degree of risk is controversial (47, 48). Fortunately, the most feared complication, nephrogenic systemic fibrosis, is exceedingly rare with modern gadolinium agents; a recent consensus statement from the American College of Radiology and the National Kidney Foundation leaves the decision for gadolinium administration in renal impairment to the clinician (49). LGE-CMR furthermore depends on several factors such as the timing of contrast injection and selection of scan parameters that can impact the interpretation of intensity values on imaging. Another limitation of LGE for risk stratification of SCD is that a subset of patients with NICM who develop VF may not have LGE on CMR (50). Several alternative measures derived from CMR have been proposed that may address some of these limitations, including native T1 mapping and extracellular volume (ECV), which reflect diffuse fibrosis, a characteristic not captured by LGE (51, 52). In a prospective study of T1 mapping and LGE assessment in participants receiving ICDs, native T1 was independently associated with VA, although performed more poorly than LGE in reclassifying participants to a low-risk group (53). Among patients with DCM, native T1 was predictive of death independent of LGE, which was present only in 27% of the study population (54). Another study in HCM patients without LGE at CMR showed an association of native T1 with SCD, though it was limited by the small number of patients (n = 5) reaching this endpoint (55). Less well studied is T2* mapping, which combines spin-spin relaxation (T2) with magnetic field inhomogeneity to detect field distortions from the presence of materials such as iron (56). As such, it was traditionally applied to identify myocardial iron accumulation in iron storage diseases and considered arrhythmogenic in those populations (57). More recently, it has been suggested T2* may add to the assessment of fibrosis, although this has not yet been well-studied (58, 59). Thus, far, a small study of the association of T2* with VA in patients with HCM was negative (60). Yet another sequence, T2-weighted short-tau inversion recovery (T2w-STIR) has been used to assess myocardial edema in survivors of cardiac arrest. Presence of edema, hypothesized to represent a transient arrhythmogenic substrate, has been associated with fewer ICD shocks (61).
Innovative uses of CMR for personalized virtual heart models to predict VA and SCD risk
A more recent development in SCD risk stratification is the use of advanced imaging to build electrophysiologic virtual heart models, which can be used to simulate arrhythmias in silico (62). These 3D computational models entail a biophysical approach from the cell-scale to the organ-scale and are specific to each patient's disease and resultant remodeling. Perhaps uniquely among risk stratification methodologies, virtual hearts evaluate how triggers from different locations will interact with the substrate to initiate VA, potentially providing mechanistic insights into how and VAs can develop in the patient heart (63). This approach was initially demonstrated in patients with ICM and ICDs; wherein virtual hearts were superior to clinical risk factors in predicting VA (64). This work has also been extended in a small study of patients not meeting ICD implantation criteria, distinguishing patients with VT history from those without (65). These models make use of complex patterns of imaging data such as distribution and degree of fibrosis, as well as a fusion of different pulse sequences as was done with T1 mapping in HCM (66). It is worth noting these studies have all been retrospective thus far regarding event prediction. However, virtual heart technology has recently been used to prospectively identify VT ablation targets in a small cohort, extending the utility of CMR from predicting SCD risk to guiding therapy (67).
Nuclear imaging
Overview
Nuclear medicine presents another advanced imaging modality that has been applied for risk stratification of SCD (68). Single-photon emission computer tomography (SPECT) is commonly used in cardiology for myocardial perfusion imaging (MPI) to assess coronary patency (69). Among patients with CAD, composite scores of fixed and reversible perfusion defects determined via SPECT are independently associated with SCD, including patients with EF > 35% (70, 71). These results were also reproduced in patients without significant CAD (72). Positron emission tomography (PET) provides another method of MPI, with the degree of myocardial abnormalities, such as scar, ischemia, or hibernating myocardium, associated with cardiac death (although data on PET MPI and SCD specifically is sparse) (73, 74). Both PET and SPECT can be used to characterize myocardial scar, a similar substrate to what is assessed by LGE. In an unadjusted analysis, scar extent by SPECT was associated with SCD (75). A larger study using PET among patients with EF <35% showed that scar alone, and not reversible ischemia, was significantly associated with ICD firing and SCD (76). Interestingly in the study above, the scar alone was not a significant predictor for SCD after adjustment, although it is worth noting participants in this study had a considerably higher EF (70). Combining MPI with imaging of cardiac inflammation using (18) F-fluorodeoxyglucose (FDG) has identified higher risk NICM patients independent of LVEF and clinical markers (77). Other myocardial features associated with SCD that PET and SPECT can image include sympathetic innervation and hibernating myocardium (78–80).
Means of measuring sympathetic innervation and its significance
Assessment of innervation is conducted with radiolabeled catecholamines, most commonly iodine-123-labeled metaiodobenzylguanidine (MIBG) in SPECT though several tracers have also been studied for use with PET, most notably 11C-hydroxyephedrine (HED). Globally decreased uptake of these tracers is thought to reflect the increased sympathetic tone, which can trigger malignant arrhythmias (81). However, regional reductions in uptake can be seen in sympathetic denervation caused by myocardial ischemia and are prevalent in CAD (82). Studies, primarily undertaken amongst patients with ICM, have demonstrated both global and regional approaches for risk stratification of SCD. A prospective study of patients with HFrEF showed that reduced global uptake of MIBG was significantly associated with potentially lethal arrhythmias and cardiac death (83). Using a different measure of reuptake, another group demonstrated that abnormal MIBG washout in HFrEF was associated with SCD specifically (though this group excluded the use of beta-blockers, perhaps limiting the generalizability of the study) (84). Using PET with HED, a study among ICM patients showed regionally reduced uptake; specifically, the volume of denervated tissue was associated with SCD (85). This was similarly demonstrated with regional MIBG washout being associated with SCD (86). The role of sympathetic denervation in HFpEF and NICM continues to be explored, and studies have shown decreased MIBG uptake is associated with increased mortality and readmission. However, the relationship with SCD is unclear (87–89).
Hibernating myocardium
Conceptually, hibernating myocardium describes regions of viable tissue with chronically reduced function and resting perfusion caused by chronic ischemia or recurrent (90). This is thought to be an arrhythmogenic substrate and has been associated with VA in porcine models (91, 92). In studies of patients with ischemic cardiomyopathy, the extent of hibernating myocardium has been associated with all-cause mortality and composite cardiac deaths (93, 94). However, in the aforementioned study of PET with HED in ICM, hibernating myocardium was not associated with SCD and was only rarely identified (85). It has been suggested that modern revascularization strategies and medical therapy may diminish the role of hibernating myocardium for risk stratification of SCD (95).
Future potential of hybrid imaging
It is worth briefly noting that hybrid PET/MRI scanners have become commercially available relatively recently (since 2010) and brought with them a unique set of technical challenges as well as clinical possibilities (96). Thus far, PET/MRI has been applied in similar roles as its constituent modalities—in perfusion and viability studies (97). An example comparing PET and CMR in the same patient, albeit not using a hybrid scanner, demonstrates the utility of each modality. PET/MRI has in particular been investigated for diagnosis of cardiac sarcoidosis and proof-of-concept demonstrated; it is speculated that the increased quality of data gathered with PET/MRI in sarcoid patients may 1 day be used for identification of those at greater risk for VA and death (98–101). However, the role of PET/MRI in risk stratification remains hypothetical, and no study has yet described the association of any PET/MRI measure with SCD (96).
Computed tomography
CT is presently a more accessible and generally lower cost modality than those previously mentioned. It provides higher spatial resolution and for certain purposes better temporal resolution as well. CT is perhaps most prominently used in cardiology for non-invasive assessment of the coronary arteries, the presence (or absence) of which is diagnostically significant and certainly a significant predictor of both VA and SCD (102). In terms of tissue characterization however, CT lags behind CMR, PET, and SPECT though developments are being made rapidly to improve this (103). It is also worth noting that there are patients in whom CMR may be contraindicated due to implants or foreign objects, or where ICD-related artifact is too limiting, in whom CT may provide a necessary alternative for risk stratification in the future.
CT has been used to detect fibrosis, such as by iodinated contrast enhancement (104). Scar qualification/quantification by CT has been favorably compared with LGE-CMR (105). A group recently used CT to map wall thinning to identify potential VT isthmuses in post-MI patients with a VT history, yielding 100% sensitivity when compared with gold-standard EP studies although with 50% positive predictive value (106). Similarly, regions of myocardial fat deposition as characterized by CT have been associated with VT circuit sites in patients with history of VA (107). CT has also been used to identify areas of lipomatous metaplasia after infarction which are associated with VA in experimental models (108). As with the previously mentioned modalities, CT has also been used for development of virtual heart models (109).
The emerging role of machine learning and artificial intelligence
Machine learning in image acquisition
In recent years, machine learning techniques have seen increasing integration within medicine, particularly as applied in imaging (110). This has specifically included applications across numerous modalities in cardiology, including CMR and nuclear imaging (111). The value of ML in the risk stratification of SCD can be appreciated at multiple stages of the imaging pipeline (Table 2). ML has been used to enhance image processing on a granular level, such as by voxel denoising. In time-intensive modalities such as MRI, this has been shown to allow for a significant reduction of acquisition time while preserving quantitative metrics such as demonstrated in brain imaging of cerebral blood flow (112). On a more experimental level, ML has been applied in low-field MRI to address reconstruction using noisy data, which may increase CMR availability in less resource-rich environments (124, 125). Finally, as an analog in PET, the clinical utility of dynamic scans required for more rapid imaging and low count protocols minimizing radiation exposure in young patients is limited by poorer image quality (113). While hardware advances provide one means of addressing this challenge, a likely more cost-effective pathway is the utilization of ML to improve the quality of the reconstructed image (126). In parallel to improving image quality, ML has been used to lower the computational burden of contemporary post-processing techniques such as scatter correction in PET (114, 115). Another forward-looking application of ML is in attenuation and scatter correction for PET-only, SPECT-only, or hybrid PET/MRI imaging, as this is conventionally accomplished with simultaneous CT (116). This may increase the accessibility and capability of advanced imaging and allow for a broader range of patients to undergo risk stratification with these modalities.
Table 2
| Imaging steps | Role of ML | Examples of applications |
|---|---|---|
| Acquisition | • Increasing acquisition speed • Decreasing radiation exposure |
• Voxel denoising in CMR (112) • Implementing low count (radiation) protocols for PET (113) |
| Processing | • Reducing computation burden • Automating labor-intensive analysis • Standardization |
• Scatter correction (114, 115) • Synthetic CT (applied to PET, SPECT, and PET/MRI) (116) • Automated scar quantification (117) • Common algorithm for segmentation across multiple centers (118) |
| Feature extraction | • Generating novel features, texture analysis • Investigation of hypothetical markers |
• ML-derived scar heterogeneity (119) • Generating hundreds of features from individual sequences (120) |
| Model construction | • Advanced analytics • Dimension reduction; identifying significant markers • Data synthesis |
• Applying random survival forests (121) • Combining non-simultaneous PET, MRI modalities (122) • Unified analysis using EMR (123) |
Incorporation of machine learning into advanced imaging.
Machine learning in image processing
Perhaps more widely reported are clinician-facing advances in image analysis and interpretation using ML, described by the overarching term “computer vision” to include any automated interpretation of images (127). ML-based algorithms have been available in commercial software for segmentation for some time now. They are widely used for automated quantification of structure and function, for example, ventricular volumes and EF (128). Some of these data are, as previously mentioned, used in models for the prediction of SCD. However, automation has extended to tissue characterization as well. LGE segmentation, when performed manually, can be a labor-intensive process requiring significant training to mitigate subjectivity and inter-operator variability (129).
Nonetheless, manual segmentation has previously been considered the gold standard for accuracy, especially when compared with techniques based on older ML algorithms (130). More recent studies have demonstrated the feasibility of novel approaches, such as deep convolutional neural networks, for automated scar quantification approaching manual segmentation in subsets of patients such as those with ICM and HCM (117, 131). This has also shown promise in multicenter datasets, potentially addressing the challenge of practice standardization (118, 132).
Machine learning for feature extraction
Sequential to obtaining imaging measures is its interpretation and application to predicting cardiovascular endpoints. Techniques such as texture analysis may be applied to pre-existing LGE segmentation to obtain features beyond quantitative scar burden, such as measures of heterogeneity and shape, which have been proven valuable in conventional workflows (119, 133). Numerous extracted features and varying ML models have been evaluated for this role; in other words, ML can be used to identify novel predictors and implement novel prediction methods (134, 135). However, these extracted features are typically unrecognizable to the human eye, especially when obtained from composite, multistep analyses necessitating further selection to identify covariates with the strongest predictive value (119, 136). For example, in a cohort of patients with DCM, principal component analysis was applied across ventricular geometric models to derive shape-based features, which were integrated into a score that was shown to be independently associated with composite VA and SCD (137). Another study generated 608 features from LGE and T2 sequences in Takotsubo patients to predict outcomes, including MI and death (120). ML was also used for dimensionality reduction in this study, which interestingly resulted in all LGE-derived features being discarded. Along these lines, a recent study used an unsupervised, deep learning approach on cine CMR among ICM patients to derive cardiac features that were then used as inputs in a separate deep neural network that successfully predicted VA risk (138). The inverse approach, using ML to generate pre-defined features, is also appreciable in recent literature. For example, ML-derived measures based on LGE, such as scar complexity, were associated with VA in a cohort where entropy was not a significant predictor (139).
Innovating the role of advanced imaging with machine learning
In the future, machine learning may be integrated into the risk stratification workflow from the point of image acquisition to patient-facing risk prediction models, and there are studies demonstrating this in principle. One example is incorporating manual measurements and segmentation with using ML for feature extraction and statistical model-building (134). Sophisticated virtual heart simulations incorporating both MRI and PET data, then using ML to synthesize imaging and clinical data for SCD prediction, have been shown to outperform existing risk models (122). This approach is not specific to VA or SCD outcomes, and it has also been used to predict improvement in EF after cardiac resynchronization therapy (140). Because of the sheer volume of data available to the clinician from the electronic medical record and conglomerate imaging, it seems increasingly likely that ML will play a central role in the fusion of data of different sources and risk modeling (123, 141–143).
Conclusion
As described in this review, there are numerous promising applications of advanced imaging to identify patients at risk of SCD. Some of the above techniques and sequences may likely be incorporated into clinical guidelines and ultimately into regular practice in the coming years. Machine learning may enable advances democratizing advanced imaging for at-risk patients, as has been achieved with CT screening in smoking. Physicians and cardiologists of the future will likely have a wide variety of complementary imaging modalities and analytic tools to identify SCD risk amongst different patient populations optimally.
Funding
The authors acknowledge funding from NIH (NIH/NHLBI R01HL103812 and R01HL132181 to KW and R01HL142496 and R01HL124893 to NT), Leducq Foundation (NT), and AHA (Predoctoral Fellowship to Esu).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Author contributions
EX and JC contributed to the conception, tables, and design of the manuscript. EX, ESu, ESa, and JC contributed to creation and editing of figures. All authors agree to be accountable for the content of the work. All authors contributed to the drafting, editing, and review of the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors would like to thank Steven Xie for providing illustrations of the scanners in Figure 1.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Zipes D Wellens HJ . Sudden Cardiac Death. Berlin: Springer (2000). p. 621-45.
2.
Adabag AS Luepker RV Roger VL Gersh BJ . Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. (2010) 7:216–25. 10.1038/nrcardio.2010.3
3.
Kuriachan VP Sumner GL Mitchell LB . Sudden cardiac death. Curr Probl Cardiol. (2015) 40:133–200. 10.1016/j.cpcardiol.2015.01.002
4.
Al-Khatib SM Stevenson WG Ackerman MJ Bryant WJ Callans DJ Curtis AB et al . 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. (2018) 72:e91–e220.
5.
Bertini M Schalij MJ Bax JJ Delgado V . Emerging role of multimodality imaging to evaluate patients at risk for sudden cardiac death. Circ Cardiovasc Imaging. (2012) 5:525–35. 10.1161/CIRCIMAGING.110.961532
6.
Deyell MW Krahn AD Goldberger JJ . Sudden cardiac death risk stratification. Circ Res. (2015) 116:1907–18. 10.1161/CIRCRESAHA.116.304493
7.
Mann DL Bristow MR . Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. (2005) 111:2837–49. 10.1161/CIRCULATIONAHA.104.500546
8.
Konstam MA Kramer DG Patel AR Maron MS Udelson JE . Left Ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. (2011) 4:98–108. 10.1016/j.jcmg.2010.10.008
9.
Seraphim A Knott KD Augusto J Bhuva AN Manisty C Moon JC . Quantitative cardiac MRI. J Magn Reson Imaging. (2020) 51:693–711. 10.1002/jmri.26789
10.
Lorenz CH Walker ES Morgan VL Klein SS Graham TP . Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. (1999) 1:7–21. 10.3109/10976649909080829
11.
Vadakkumpadan F Trayanova N Wu KC . Image-based left ventricular shape analysis for sudden cardiac death risk stratification. Heart Rhythm. (2014) 11:1693–700. 10.1016/j.hrthm.2014.05.018
12.
De Haan S De Boer K Commandeur J Beek A van Rossum A Allaart C . Assessment of left ventricular ejection fraction in patients eligible for ICD therapy: discrepancy between cardiac magnetic resonance imaging and 2D echocardiography. Neth Heart J. (2014) 22:449–55. 10.1007/s12471-014-0594-0
13.
Pontone G Guaricci AI Andreini D Solbiati A Guglielmo M Mushtaq S et al . Prognostic benefit of cardiac magnetic resonance over transthoracic echocardiography for the assessment of ischemic and nonischemic dilated cardiomyopathy patients referred for the evaluation of primary prevention implantable cardioverter–defibrillator therapy. Circ Cardiovasc Imaging. (2016) 9:e004956. 10.1161/CIRCIMAGING.115.004956
14.
Alsharqi M Woodward W Mumith J Markham D Upton R Leeson P . Artificial intelligence and echocardiography. Echo Res Pract. (2018) 5:R115–R25. 10.1530/ERP-18-0056
15.
Wu KC Calkins H . Powerlessness of a number: why left ventricular ejection fraction matters less for sudden cardiac death risk assessment. Circ Cardiovasc Imaging. (2016) 9:e005519. 10.1161/CIRCIMAGING.116.005519
16.
Wellens HJ Schwartz PJ Lindemans FW Buxton AE Goldberger JJ Hohnloser SH et al . Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. (2014) 35:1642–51. 10.1093/eurheartj/ehu176
17.
Wu KC Weiss RG Thiemann DR Kitagawa K Schmidt A Dalal D et al . Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. (2008) 51:2414–21. 10.1016/j.jacc.2008.03.018
18.
Wu KC . Sudden cardiac death substrate imaged by magnetic resonance imaging: from investigational tool to clinical applications. Circ Cardiovasc Imaging. (2017) 10:e005461. 10.1161/CIRCIMAGING.116.005461
19.
Schmidt A Azevedo CF Cheng A Gupta SN Bluemke DA Foo TK et al . Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. (2007) 115:2006–14. 10.1161/CIRCULATIONAHA.106.653568
20.
Roes SD Borleffs CJW Geest RJvd Westenberg JJM Marsan NA Kaandorp TAM et al . Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. (2009) 2:183–90. 10.1161/CIRCIMAGING.108.826529
21.
Zeidan-Shwiri T Yang Y Lashevsky I Kadmon E Kagal D Dick A et al . Magnetic resonance estimates of the extent and heterogeneity of scar tissue in ICD patients with ischemic cardiomyopathy predict ventricular arrhythmia. Heart Rhythm. (2015) 12:802–8. 10.1016/j.hrthm.2015.01.007
22.
Zegard A Okafor O de Bono J Kalla M Lencioni M Marshall H et al . Myocardial fibrosis as a predictor of sudden death in patients with coronary artery disease. J Am Coll Cardiol. (2021) 77:29–41. 10.1016/j.jacc.2020.10.046
23.
Leyva F Zegard A Okafor O Foley P Umar F Taylor RJ et al . Myocardial fibrosis predicts ventricular arrhythmias and sudden death after cardiac electronic device implantation. J Am Coll Cardiol. (2022) 79:665–78. 10.1016/j.jacc.2021.11.050
24.
Wu KC Gerstenblith G Guallar E Marine JE Dalal D Cheng A et al . Combined cardiac magnetic resonance imaging and C-reactive protein levels identify a cohort at low risk for defibrillator firings and death. Circ Cardiovasc imaging. (2012) 5:178–86. 10.1161/CIRCIMAGING.111.968024
25.
Wu KC Wongvibulsin S Tao S Ashikaga H Stillabower M Dickfeld TM et al . Baseline and dynamic risk predictors of appropriate implantable cardioverter defibrillator therapy. J Am Heart Assoc. (2020) 9:e017002-e. 10.1161/JAHA.120.017002
26.
Muthalaly RG Kwong RY John RM van der Geest RJ Tao Q Schaeffer B et al . Left ventricular entropy is a novel predictor of arrhythmic events in patients with dilated cardiomyopathy receiving defibrillators for primary prevention. JACC Cardiovasc Imaging. (2019) 12:1177–84. 10.1016/j.jcmg.2018.07.003
27.
Androulakis AFA Zeppenfeld K Paiman EHM Piers SRD Wijnmaalen AP Siebelink HJ et al . Entropy as a novel measure of myocardial tissue heterogeneity for prediction of ventricular arrhythmias and mortality in post-infarct patients. JACC Clin Electrophysiol. (2019) 5:480–9. 10.1016/j.jacep.2018.12.005
28.
Gould J Porter B Claridge S Chen Z Sieniewicz BJ Sidhu BS et al . Mean entropy predicts implantable cardioverter-defibrillator therapy using cardiac magnetic resonance texture analysis of scar heterogeneity. Heart Rhythm. (2019) 16:1242–50. 10.1016/j.hrthm.2019.03.001
29.
Perez-David E Arenal Á Rubio-Guivernau JL. Castillo Rd Atea L Arbelo E et al . Noninvasive identification of ventricular tachycardia-related conducting channels using contrast-enhanced magnetic resonance imaging in patients with chronic myocardial infarction. J Am Coll Cardiol. (2011) 57:184–94. 10.1016/j.jacc.2010.07.043
30.
Sánchez-Somonte P Quinto L Garre P Zaraket F Alarcón F Borràs R et al . Scar channels in cardiac magnetic resonance to predict appropriate therapies in primary prevention. Heart Rhythm. (2021) 18:1336–43. 10.1016/j.hrthm.2021.04.017
31.
Ganesan AN Gunton J Nucifora G McGavigan AD Selvanayagam JB . Impact of late gadolinium enhancement on mortality, sudden death and major adverse cardiovascular events in ischemic and nonischemic cardiomyopathy: a systematic review and meta-analysis. Int J Cardiol. (2018) 254:230–7. 10.1016/j.ijcard.2017.10.094
32.
Di Marco A Anguera I Schmitt M Klem I Neilan TG White JA et al . Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: systematic review and meta-analysis. JACC: Heart Failure. (2017) 5:28–38. 10.1016/j.jchf.2016.09.017
33.
Kuruvilla S Adenaw N Katwal AB Lipinski MJ Kramer CM Salerno M . Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging. (2014) 7:250–8. 10.1161/CIRCIMAGING.113.001144
34.
Halliday BP Gulati A Ali A Guha K Newsome S Arzanauskaite M et al . Association between midwall late gadolinium enhancement and sudden cardiac death in patients with dilated cardiomyopathy and mild and moderate left ventricular systolic dysfunction. Circulation. (2017) 135:2106–15. 10.1161/CIRCULATIONAHA.116.026910
35.
Klem I Klein M Khan M Yang EY Nabi F Ivanov A et al . Relationship of LVEF and myocardial scar to long-term mortality risk and mode of death in patients with nonischemic cardiomyopathy. Circulation. (2021) 143:1343–58. 10.1161/CIRCULATIONAHA.120.048477
36.
Greulich S Seitz A Herter D Günther F Probst S Bekeredjian R et al . Long-term risk of sudden cardiac death in hypertrophic cardiomyopathy: a cardiac magnetic resonance outcome study. Eur Heart J Cardiovasc Imaging. (2021) 22:732–41. 10.1093/ehjci/jeaa423
37.
Rowin EJ Maron MS Adler A Albano AJ Varnava AM Spears D et al . Importance of newer cardiac magnetic resonance-based risk markers for sudden death prevention in hypertrophic cardiomyopathy: An international multicenter study. Heart rhythm. (2021). 10.1016/j.hrthm.2021.12.017
38.
O'Mahony C Jichi F Pavlou M Monserrat L Anastasakis A Rapezzi C et al . A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. (2014) 35:2010–20. 10.1093/eurheartj/eht439
39.
Todiere G Nugara C Gentile G Negri F Bianco F Falletta C et al . Prognostic role of late gadolinium enhancement in patients with hypertrophic cardiomyopathy and low-to-intermediate sudden cardiac death risk score. Am J Cardiol. (2019) 124:1286–92. 10.1016/j.amjcard.2019.07.023
40.
Mandawat A Chattranukulchai P Mandawat A Blood AJ Ambati S Hayes B et al . Progression of myocardial fibrosis in nonischemic DCM and association with mortality and heart failure outcomes. JACC Cardiovasc Imaging. (2021) 14:1338–50. 10.1016/j.jcmg.2020.11.006
41.
Zorzi A Perazzolo Marra M Rigato I De Lazzari M Susana A Niero A et al . Nonischemic left ventricular scar as a substrate of life-threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol. (2016) 9:e004229. 10.1161/CIRCEP.116.004229
42.
Corrado D Marra MP Zorzi A Beffagna G Cipriani A De Lazzari M et al . Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol. (2020) 319:106–14. 10.1016/j.ijcard.2020.06.005
43.
Deac M Alpendurada F Fanaie F Vimal R Carpenter J-P Dawson A et al . Prognostic value of cardiovascular magnetic resonance in patients with suspected arrhythmogenic right ventricular cardiomyopathy. Int J Cardiol. (2013) 168:3514–21. 10.1016/j.ijcard.2013.04.208
44.
Gandjbakhch E Redheuil A Pousset F Charron P Frank R . Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia. J Am Coll Cardiol. (2018) 72:784–804. 10.1016/j.jacc.2018.05.065
45.
Gräni C Eichhorn C Bière L Murthy VL Agarwal V Kaneko K et al . Prognostic value of cardiac magnetic resonance tissue characterization in risk stratifying patients with suspected myocarditis. J Am Coll Cardiol. (2017) 70:1964–76. 10.1016/j.jacc.2017.08.050
46.
Georgiopoulos G Figliozzi S Sanguineti F Aquaro GD di Bella G Stamatelopoulos K et al . Prognostic impact of late gadolinium enhancement by cardiovascular magnetic resonance in myocarditis: a systematic review and meta-analysis. Circ Cardiovasc Imaging. (2021) 14:e011492. 10.1161/CIRCIMAGING.120.011492
47.
Pasquini L Napolitano A Visconti E Longo D Romano A Tomà P et al . Gadolinium-based contrast agent-related toxicities. CNS Drugs. (2018) 32:229–40. 10.1007/s40263-018-0500-1
48.
Schieda N Blaichman JI Costa AF Glikstein R Hurrell C James M et al . Gadolinium-based contrast agents in kidney disease: a comprehensive review and clinical practice guideline issued by the Canadian Association of Radiologists. Can J Kidney Health Dis. (2018) 5:2054358118778573. 10.1177/2054358118778573
49.
Weinreb JC Rodby RA Yee J Wang CL Fine D McDonald RJ et al . Use of intravenous gadolinium-based contrast media in patients with kidney disease: consensus statements from the American College of Radiology and the National Kidney Foundation. Kidney Med. (2021) 3:142–50. 10.1016/j.xkme.2020.10.001
50.
Voskoboinik A Wong MCG Elliott JK Costello BT Prabhu S Mariani JA et al . Absence of late gadolinium enhancement on cardiac magnetic resonance imaging in ventricular fibrillation and nonischemic cardiomyopathy. Pacing Clin Electrophysiol. (2018) 41:1109–15. 10.1111/pace.13426
51.
Kammerlander Andreas A Marzluf Beatrice A Zotter-Tufaro C Aschauer S Duca F Bachmann A et al . T1 Mapping by CMR imaging. JACC Cardiovasc Imaging. (2016) 9:14–23. 10.1016/j.jcmg.2015.11.002
52.
Messroghli DR Moon JC Ferreira VM Grosse-Wortmann L He T Kellman P et al . Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. (2017) 19:1–24. 10.1186/s12968-017-0389-8
53.
Chen Z Sohal M Voigt T Sammut E Tobon-Gomez C Child N et al . Myocardial tissue characterization by cardiac magnetic resonance imaging using T1 mapping predicts ventricular arrhythmia in ischemic and non-ischemic cardiomyopathy patients with implantable cardioverter-defibrillators. Heart Rhythm. (2015) 12:792–801. 10.1016/j.hrthm.2014.12.020
54.
Puntmann VO Carr-White G Jabbour A Yu C-Y Gebker R Kelle S et al . T1-mapping and outcome in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. (2016) 9:40–50. 10.1016/j.jcmg.2015.12.001
55.
Xu J Zhuang B Sirajuddin A Li S Huang J Yin G et al . MRI T1 mapping in hypertrophic cardiomyopathy: evaluation in patients without late gadolinium enhancement and hemodynamic obstruction. Radiology. (2020) 294:275–86. 10.1148/radiol.2019190651
56.
Lota AS Gatehouse PD Mohiaddin RH . T2 mapping and T2* imaging in heart failure. Heart Fail Rev. (2017) 22:431–40. 10.1007/s10741-017-9616-5
57.
van der Bijl P Podlesnikar T Bax JJ Delgado V . Sudden Cardiac Death Risk Prediction: The role of cardiac magnetic resonance imaging. Revista Española de Cardiología. (2018) 71:961–70. 10.1016/j.rec.2018.05.019
58.
Triadyaksa P Oudkerk M Sijens PE . Cardiac T2* mapping: techniques and clinical applications. J Magn Reson Imaging. (2020) 52:1340–51. 10.1002/jmri.27023
59.
Gastl M Gotschy A von Spiczak J Polacin M Bönner F Gruner C et al . Cardiovascular magnetic resonance T2* mapping for structural alterations in hypertrophic cardiomyopathy. Eur J Radiol Open. (2019) 6:78–84. 10.1016/j.ejro.2019.01.007
60.
Gastl M Gruner C Labucay K Gotschy A Von Spiczak J Polacin M et al . Cardiovascular magnetic resonance T2* mapping for the assessment of cardiovascular events in hypertrophic cardiomyopathy. Open heart. (2020) 7:e001152. 10.1136/openhrt-2019-001152
61.
Zorzi A Mattesi G Baldi E Toniolo M Guerra F Cauti FM et al . Prognostic role of myocardial edema as evidenced by early cardiac magnetic resonance in survivors of out-of-hospital cardiac arrest: a multicenter study. J Am Heart Assoc. (2021) 10:e021861. 10.1161/JAHA.121.021861
62.
Trayanova NA Pashakhanloo F Wu KC Halperin HR . Imaging-based simulations for predicting sudden death and guiding ventricular tachycardia ablation. Circ Arrhythm Electrophysiol. (2017) 10:e004743. 10.1161/CIRCEP.117.004743
63.
Sung E Etoz S Zhang Y Trayanova NA . Whole-heart ventricular arrhythmia modeling moving forward: Mechanistic insights and translational applications. Biophy Rev. (2021) 2:031304. 10.1063/5.0058050
64.
Arevalo HJ Vadakkumpadan F Guallar E Jebb A Malamas P Wu KC et al . Arrhythmia risk stratification of patients after myocardial infarction using personalized heart models. Nat Commun. (2016) 7:1–8. 10.1038/ncomms11437
65.
Deng D Arevalo HJ Prakosa A Callans DJ Trayanova NA . A feasibility study of arrhythmia risk prediction in patients with myocardial infarction and preserved ejection fraction. EP Europace. (2016) 18(Suppl. 4):iv60–6. 10.1093/europace/euw351
66.
O'Hara RP Binka E Prakosa A Zimmerman SL Cartoski MJ Abraham MR et al . Personalized computational heart models with T1-mapped fibrotic remodeling predict sudden death risk in patients with hypertrophic cardiomyopathy. Elife. (2022) 11:e73325. 10.7554/eLife.73325
67.
Prakosa A Arevalo HJ Deng D Boyle PM Nikolov PP Ashikaga H et al . Personalized virtual-heart technology for guiding the ablation of infarct-related ventricular tachycardia. Nat Biomed Eng. (2018) 2:732–40. 10.1038/s41551-018-0282-2
68.
Juneau D Erthal F Chow BJW Redpath C Ruddy TD Knuuti J et al . The role of nuclear cardiac imaging in risk stratification of sudden cardiac death. J Nucl Cardiol. (2016) 23:1380–98. 10.1007/s12350-016-0599-8
69.
Mariani G Bruselli L Kuwert T Kim EE Flotats A Israel O et al . A review on the clinical uses of SPECT/CT. Eur J Nucl Med Mol Imaging. (2010) 37:1959–85. 10.1007/s00259-010-1390-8
70.
Piccini JP Horton JR Shaw LK Al-Khatib SM Lee KL Iskandrian AE et al . Single-photon emission computed tomography myocardial perfusion defects are associated with an increased risk of all-cause death, cardiovascular death, and sudden cardiac death. Circ Cardiovasc Imaging. (2008) 1:180–8. 10.1161/CIRCIMAGING.108.776484
71.
Piccini Jonathan P Starr Aijing Z Horton John R Shaw Linda K Lee Kerry L Al-Khatib Sana M et al . Single-photon emission computed tomography myocardial perfusion imaging and the risk of sudden cardiac death in patients with coronary disease and left ventricular ejection fraction >35%. J Am Coll Cardiol. (2010) 56:206–14. 10.1016/j.jacc.2010.01.061
72.
Adamu U Knollmann D Almutairi B Alrawashdeh W Deserno V Vogt F et al . Stress/rest myocardial perfusion scintigraphy in patients without significant coronary artery disease. J Nucl Cardiol. (2010) 17:38–44. 10.1007/s12350-009-9133-6
73.
Dorbala S Di Carli Marcelo F Beanlands Rob S Merhige Michael E Williams Brent A Veledar E et al . Prognostic value of stress myocardial perfusion positron emission tomography. J Am Coll Cardiol. (2013) 61:176–84. 10.1016/j.jacc.2012.09.043
74.
Dorbala S Di Carli MF . Cardiac PET perfusion: prognosis, risk stratification, and clinical management. Semin Nucl Med. (2014) 44:344–57. 10.1053/j.semnuclmed.2014.05.003
75.
Morishima I Sone T Tsuboi H Mukawa H Uesugi M Morikawa S et al . Risk stratification of patients with prior myocardial infarction and advanced left ventricular dysfunction by gated myocardial perfusion SPECT imaging. J Nucl Cardiol. (2008) 15:631–7. 10.1016/j.nuclcard.2008.03.009
76.
Gupta A Harrington M Albert Christine M Bajaj Navkaranbir S Hainer J Morgan V et al . Myocardial scar but not ischemia is associated with defibrillator shocks and sudden cardiac death in stable patients with reduced left ventricular ejection fraction. JACC Clin Electrophysiol. (2018) 4:1200–10. 10.1016/j.jacep.2018.06.002
77.
Blankstein R Osborne M Naya M Waller A Kim CK Murthy VL et al . Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. (2014) 63:329–36. 10.1016/j.jacc.2013.09.022
78.
Zelt JG deKemp RA Rotstein BH Nair GM Narula J Ahmadi A et al . Nuclear imaging of the cardiac sympathetic nervous system: a disease-specific interpretation in heart failure. JACC Cardiovas Imaging. (2020) 13:1036–54. 10.1016/j.jcmg.2019.01.042
79.
Canty JM Jr Suzuki G Banas MD Verheyen F Borgers M Fallavollita JA . Hibernating myocardium: chronically adapted to ischemia but vulnerable to sudden death. Circ Res. (2004) 94:1142–9. 10.1161/01.RES.0000125628.57672.CF
80.
Popescu CE Cuzzocrea M Monaco L Caobelli F . Assessment of myocardial sympathetic innervation by PET in patients with heart failure: a review of the most recent advances and future perspectives. Clin Transl Imaging. (2018) 6:459–70. 10.1007/s40336-018-0293-8
81.
Fukuda K Kanazawa H Aizawa Y Ardell JL Shivkumar K . Cardiac innervation and sudden cardiac death. Circ Res. (2015) 116:2005–19. 10.1161/CIRCRESAHA.116.304679
82.
Malhotra S Fernandez SF Fallavollita JA Canty JM . Prognostic significance of imaging myocardial sympathetic innervation. Curr Cardiol Rep. (2015) 17:62. 10.1007/s11886-015-0613-9
83.
Jacobson AF Senior R Cerqueira MD Wong ND Thomas GS Lopez VA et al . Myocardial iodine-123 meta-iodobenzylguanidine imaging and cardiac events in heart failure: results of the prospective ADMIRE-HF (AdreView Myocardial Imaging for Risk Evaluation in Heart Failure) study. J Am Coll Cardiol. (2010) 55:2212–21. 10.1016/j.jacc.2010.01.014
84.
Tamaki S Yamada T Okuyama Y Morita T Sanada S Tsukamoto Y et al . Cardiac Iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. (2009) 53:426–35. 10.1016/j.jacc.2008.10.025
85.
Fallavollita James A Heavey Brendan M Luisi Andrew J Michalek Suzanne M Baldwa S Mashtare Terry L et al . Regional myocardial sympathetic denervation predicts the risk of sudden cardiac arrest in ischemic cardiomyopathy. J Am Coll Cardiol. (2014) 63:141–9. 10.1016/j.jacc.2013.07.096
86.
Yamamoto H Yamada T Tamaki S Morita T Furukawa Y Iwasaki Y et al . Prediction of sudden cardiac death in patients with chronic heart failure by regional washout rate in cardiac MIBG SPECT imaging. J Nucl Cardiol. (2019) 26:109–17. 10.1007/s12350-017-0913-0
87.
Aikawa T Naya M Obara M Oyama-Manabe N Manabe O Magota K et al . Regional interaction between myocardial sympathetic denervation, contractile dysfunction, and fibrosis in heart failure with preserved ejection fraction: 11C-hydroxyephedrine PET study. Eur J Nucl Med Mol Imaging. (2017) 44:1897–905. 10.1007/s00259-017-3760-y
88.
Katoh S Shishido T Kutsuzawa D Arimoto T Netsu S Funayama A et al . Iodine-123-metaiodobenzylguanidine imaging can predict future cardiac events in heart failure patients with preserved ejection fraction. Ann Nucl Med. (2010) 24:679–86. 10.1007/s12149-010-0409-3
89.
Seo M Yamada T Tamaki S Watanabe T Morita T Furukawa Y et al . Prognostic significance of cardiac 123I-MIBG SPECT imaging in heart failure patients with preserved ejection fraction. JACC Cardiovasc Imaging. (2021). 10.1016/j.jcmg.2021.08.003
90.
Kloner RA . Stunned and hibernating myocardium: where are we nearly 4 decades later?J Am Heart Assoc. (2020) 9:e015502. 10.1161/JAHA.119.015502
91.
Canty JM Fallavollita JA . Hibernating myocardium. J Nucl Cardiol. (2005) 12:104–19. 10.1016/j.nuclcard.2004.11.003
92.
Fernandez SF Ovchinnikov V Canty JM Jr Fallavollita JA . Hibernating myocardium results in partial sympathetic denervation and nerve sprouting. Am J Physiol Heart Circ Physiol. (2013) 304:H318–H27. 10.1152/ajpheart.00810.2011
93.
Uebleis C Hellweger S Laubender RP Becker A Sohn H-Y Lehner S et al . The amount of dysfunctional but viable myocardium predicts long-term survival in patients with ischemic cardiomyopathy and left ventricular dysfunction. Int J Cardiovasc Imaging. (2013) 29:1645–53. 10.1007/s10554-013-0254-2
94.
Desideri A Cortigiani L Christen Alejandra I Coscarelli S Gregori D Zanco P et al . The extent of perfusion–F18-fluorodeoxyglucose positron emission tomography mismatch determines mortality in medically treated patients with chronic ischemic left ventricular dysfunction. J Am Coll Cardiol. (2005) 46:1264–9. 10.1016/j.jacc.2005.06.057
95.
Malhotra S Canty John M . Structural and physiological imaging to predict the risk of lethal ventricular arrhythmias and sudden death. JACC Cardiovasc Imaging. (2019) 12:2049–64. 10.1016/j.jcmg.2019.05.034
96.
Nensa F Bamberg F Rischpler C Menezes L Poeppel TD la Fougère C et al . Hybrid cardiac imaging using PET/MRI: a joint position statement by the European Society of Cardiovascular Radiology (ESCR) and the European Association of Nuclear Medicine (EANM). Eur Radiol. (2018) 28:4086–101. 10.1007/s00330-017-5008-4
97.
Rischpler C Siebermair J Kessler L Quick HH Umutlu L Rassaf T et al . editors. Cardiac PET/MRI: current clinical status and future perspectives. Semin Nucl Med. (2020) 50:260–9. 10.1053/j.semnuclmed.2020.02.012
98.
Hanneman K Kadoch M Guo HH Jamali M Quon A Iagaru A et al . Initial experience with simultaneous 18F-FDG PET/MRI in the evaluation of cardiac sarcoidosis and myocarditis. Clin Nucl Med. (2017) 42:e328–e34. 10.1097/RLU.0000000000001669
99.
Vita T Okada DR Veillet-Chowdhury M Bravo PE Mullins E Hulten E et al . Complementary value of cardiac magnetic resonance imaging and positron emission tomography/computed tomography in the assessment of cardiac sarcoidosis. Circ Cardiovasc Imaging. (2018) 11:e007030. 10.1161/CIRCIMAGING.117.007030
100.
Schneider S Batrice A Rischpler C Eiber M Ibrahim T Nekolla SG . Utility of multimodal cardiac imaging with PET/MRI in cardiac sarcoidosis: implications for diagnosis, monitoring and treatment. Eur Heart J. (2014) 35:312. 10.1093/eurheartj/eht335
101.
Wisenberg G Thiessen J Pavlovsky W Butler J Wilk B Prato F . Same day comparison of PET/CT and PET/MR in patients with cardiac sarcoidosis. J Nucl Cardiol. (2020) 27:2118–29. 10.1007/s12350-018-01578-8
102.
Sparrow P Merchant N Provost Y Doyle D Nguyen E Paul N . Cardiac MRI and CT features of inheritable and congenital conditions associated with sudden cardiac death. Eur Radiol. (2009) 19:259–70. 10.1007/s00330-008-1169-5
103.
Mahida S Sacher F Dubois R Sermesant M Bogun F Haïssaguerre M et al . Cardiac imaging in patients with ventricular tachycardia. Circulation. (2017) 136:2491–507. 10.1161/CIRCULATIONAHA.117.029349
104.
Shiozaki AA Senra T Arteaga E Martinelli Filho M Pita CG Ávila LFR et al . Myocardial fibrosis detected by cardiac CT predicts ventricular fibrillation/ventricular tachycardia events in patients with hypertrophic cardiomyopathy. J Cardiovasc Comput Tomogr. (2013) 7:173–81. 10.1016/j.jcct.2013.04.002
105.
Langer C Lutz M Eden M Lüdde M Hohnhorst M Gierloff C et al . Hypertrophic cardiomyopathy in cardiac CT: a validation study on the detection of intramyocardial fibrosis in consecutive patients. Int J Cardiovasc Imaging. (2014) 30:659–67. 10.1007/s10554-013-0358-8
106.
Takigawa M Duchateau J Sacher F Martin R Vlachos K Kitamura T et al . Are wall thickness channels defined by computed tomography predictive of isthmuses of postinfarction ventricular tachycardia?Heart Rhythm. (2019) 16:1661–8. 10.1016/j.hrthm.2019.06.012
107.
Sasaki T Calkins H Miller CF Zviman MM Zipunnikov V Arai T et al . New insight into scar-related ventricular tachycardia circuits in ischemic cardiomyopathy: fat deposition after myocardial infarction on computed tomography–a pilot study. Heart Rhythm. (2015) 12:1508–18. 10.1016/j.hrthm.2015.03.041
108.
Mordi I Radjenovic A Stanton T Gardner RS McPhaden A Carrick D et al . Prevalence and prognostic significance of lipomatous metaplasia in patients with prior myocardial infarction. JACC Cardiovasc Imaging. (2015) 8:1111–2. 10.1016/j.jcmg.2014.07.024
109.
Sung E Prakosa A Aronis KN Zhou S Zimmerman SL Tandri H et al . Personalized digital-heart technology for ventricular tachycardia ablation targeting in hearts with infiltrating adiposity. Circ Arrhythm Electrophysiol. (2020) 13:e008912. 10.1161/CIRCEP.120.008912
110.
Giger ML . Machine learning in medical imaging. J Am Coll Radiol. (2018) 15:512–20. 10.1016/j.jacr.2017.12.028
111.
Quer G Arnaout R Henne M Arnaout R . Machine learning and the future of cardiovascular care: JACC state-of-the-art review. J Am Coll Cardiol. (2021) 77:300–13. 10.1016/j.jacc.2020.11.030
112.
Xie D Li Y Yang H Bai L Wang T Zhou F et al . Denoising arterial spin labeling perfusion MRI with deep machine learning. Magn Reson Imaging. (2020) 68:95–105. 10.1016/j.mri.2020.01.005
113.
Wang T Lei Y Fu Y Curran WJ Liu T Nye JA et al . Machine learning in quantitative PET: A review of attenuation correction and low-count image reconstruction methods. Physica Medica. (2020) 76:294–306. 10.1016/j.ejmp.2020.07.028
114.
Gong K Berg E Cherry SR Qi J . Machine learning in PET: from photon detection to quantitative image reconstruction. Proc IEEE. (2019) 108:51–68. 10.1109/JPROC.2019.2936809
115.
Xiang H Lim H Fessler JA Dewaraja YK . A deep neural network for fast and accurate scatter estimation in quantitative SPECT/CT under challenging scatter conditions. Eur J Nucl Med Mol Imaging. (2020) 47:2956. 10.1007/s00259-020-04840-9
116.
Arabi H AkhavanAllaf A Sanaat A Shiri I Zaidi H . The promise of artificial intelligence and deep learning in PET and SPECT imaging. Physica Medica. (2021) 83:122–37. 10.1016/j.ejmp.2021.03.008
117.
Fahmy AS Rausch J Neisius U Chan RH Maron MS Appelbaum E et al . Automated cardiac MR scar quantification in hypertrophic cardiomyopathy using deep convolutional neural networks. JACC Cardiovasc Imaging. (2018) 11:1917–8. 10.1016/j.jcmg.2018.04.030
118.
Ghanbari F Joyce T Kozerke S Guaricci A Masci P Pavon A et al . Performance of a machine-learning algorithm for fully automatic LGE scar quantification in the large multi-national derivate registry. Eur Heart J Cardiovasc Imaging. (2021) 22 (Suppl. 2):jeab090. 10.1093/ehjci/jeab090.023
119.
Cheng S Fang M Cui C Chen X Yin G Prasad SK et al . LGE-CMR-derived texture features reflect poor prognosis in hypertrophic cardiomyopathy patients with systolic dysfunction: preliminary results. Eur Radiol. (2018) 28:4615–24. 10.1007/s00330-018-5391-5
120.
Mannil M Kato K Manka R von Spiczak J Peters B Cammann VL et al . Prognostic value of texture analysis from cardiac magnetic resonance imaging in patients with Takotsubo syndrome: a machine learning based proof-of-principle approach. Sci Rep. (2020) 10:20537. 10.1038/s41598-020-76432-4
121.
Wongvibulsin S Wu KC Zeger SL . Clinical risk prediction with random forests for survival, longitudinal, and multivariate (RF-SLAM) data analysis. BMC Med Res Methodol. (2020) 20:1–14. 10.1186/s12874-019-0863-0
122.
Shade JK Prakosa A Popescu DM Yu R Okada DR Chrispin J et al . Predicting risk of sudden cardiac death in patients with cardiac sarcoidosis using multimodality imaging and personalized heart modeling in a multivariable classifier. Sci Adv. (2021) 7:eabi8020. 10.1126/sciadv.abi8020
123.
Bhattacharya M Lu D-Y Kudchadkar SM Greenland GV Lingamaneni P Corona-Villalobos CP et al . Identifying ventricular arrhythmias and their predictors by applying machine learning methods to electronic health records in patients with hypertrophic cardiomyopathy (HCM-VAr-Risk Model). Am J Cardiol. (2019) 123:1681–9. 10.1016/j.amjcard.2019.02.022
124.
Koonjoo N Zhu B Bagnall GC Bhutto D Rosen M . Boosting the signal-to-noise of low-field MRI with deep learning image reconstruction. Sci Rep. (2021) 11:1–16. 10.1038/s41598-021-87482-7
125.
Campbell-Washburn AE Ramasawmy R Restivo MC Bhattacharya I Basar B Herzka DA et al . Opportunities in interventional and diagnostic imaging by using high-performance low-field-strength MRI. Radiology. (2019) 293:384–93. 10.1148/radiol.2019190452
126.
Ravishankar S Ye JC Fessler JA . Image reconstruction: from sparsity to data-adaptive methods and machine learning. Proc IEEE. (2019) 108:86–109. 10.1109/JPROC.2019.2936204
127.
Esteva A Chou K Yeung S Naik N Madani A Mottaghi A et al . Deep learning-enabled medical computer vision. NPJ Digit Med. (2021) 4:1–9. 10.1038/s41746-020-00376-2
128.
Leiner T Rueckert D Suinesiaputra A Baeßler B Nezafat R Išgum I et al . Machine learning in cardiovascular magnetic resonance: basic concepts and applications. J Cardiovasc Magn Reson. (2019) 21:1–14. 10.1186/s12968-019-0575-y
129.
McAlindon E Pufulete M Lawton C Angelini GD Bucciarelli-Ducci C . Quantification of infarct size and myocardium at risk: evaluation of different techniques and its implications. Eur Heart J Cardiovasc Imaging. (2015) 16:738–46. 10.1093/ehjci/jev001
130.
Engblom H Tufvesson J Jablonowski R Carlsson M Aletras AH Hoffmann P et al . A new automatic algorithm for quantification of myocardial infarction imaged by late gadolinium enhancement cardiovascular magnetic resonance: experimental validation and comparison to expert delineations in multi-center, multi-vendor patient data. J Cardiovasc Magn Reson. (2016) 18:1–13. 10.1186/s12968-016-0242-5
131.
Popescu DM Abramson HG Yu R Lai C Shade JK Wu KC et al . Anatomically informed deep learning on contrast-enhanced cardiac magnetic resonance imaging for scar segmentation and clinical feature extraction. Cardiovasc Digit Health J. (2021). 10.1016/j.cvdhj.2021.11.007
132.
Fahmy AS Neisius U Chan RH Rowin EJ Manning WJ Maron MS et al . Three-dimensional deep convolutional neural networks for automated myocardial scar quantification in hypertrophic cardiomyopathy: a multicenter multivendor study. Radiology. (2020) 294:52–60. 10.1148/radiol.2019190737
133.
Aronis KN Okada DR Xie E Daimee UA Prakosa A Gilotra NA et al . Spatial dispersion analysis of LGE-CMR for prediction of ventricular arrhythmias in patients with cardiac sarcoidosis. Pacing Clin Electrophysiol. (2021) 44:2067–74. 10.1111/pace.14406
134.
Alis D Guler A Yergin M Asmakutlu O . Assessment of ventricular tachyarrhythmia in patients with hypertrophic cardiomyopathy with machine learning-based texture analysis of late gadolinium enhancement cardiac MRI. Diagn Interv Imaging. (2020) 101:137–46. 10.1016/j.diii.2019.10.005
135.
Fei J-L Pu C-L Xu F-Y Wu Y Hu H-J . Progress in radiomics of common heart disease based on cardiac magnetic resonance imaging. J Mol Clin Med. (2021) 4:29–38. 10.31083/j.jmcm.2021.01.801
136.
Shu S Wang C Hong Z Zhou X Zhang T Peng Q et al . Prognostic value of late enhanced cardiac magnetic resonance imaging derived texture features in dilated cardiomyopathy patients with severely reduced ejection fractions. Front Cardiovasc Med. (2021) 8:766423. 10.3389/fcvm.2021.766423
137.
Balaban G Halliday BP Hammersley D Rinaldi CA Prasad SK Bishop MJ et al . Left ventricular shape predicts arrhythmic risk in fibrotic dilated cardiomyopathy. Europace. (2021). 10.1093/europace/euab306
138.
Krebs J Mansi T Delingette H Lou B Lima JAC Tao S et al . CinE caRdiac magneTic resonAnce to predIct veNTricular arrhYthmia (CERTAINTY). Sci Rep. (2021) 11:22683. 10.1038/s41598-021-02111-7
139.
Okada DR Miller J Chrispin J Prakosa A Trayanova N Jones S et al . Substrate spatial complexity analysis for the prediction of ventricular arrhythmias in patients with ischemic cardiomyopathy. Circ Arrhythm Electrophysiol. (2020) 13:e007975. 10.1161/CIRCEP.119.007975
140.
Khamzin S Dokuchaev A Bazhutina A Chumarnaya T Zubarev S Lyubimtseva T et al . Machine Learning prediction of cardiac resynchronisation therapy response from combination of clinical and model-driven data. Front Physiol. (2021) 2021:2283. 10.1101/2021.09.03.458464
141.
Cuocolo R Perillo T De Rosa E Ugga L Petretta M . Current applications of big data and machine learning in cardiology. J Geriatr Cardiol. (2019) 16:601. 10.11909/j.issn.1671-5411.2019.08.002
142.
Smole T Žunkovič B Pičulin M Kokalj E Robnik-Šikonja M Kukar M et al . A machine learning-based risk stratification model for ventricular tachycardia and heart failure in hypertrophic cardiomyopathy. Comput Biol Med. (2021) 135:104648. 10.1016/j.compbiomed.2021.104648
143.
Trayanova NA Popescu DM Shade JK . Machine learning in arrhythmia and electrophysiology. Circ Res. (2021) 128:544–66. 10.1161/CIRCRESAHA.120.317872
Summary
Keywords
sudden cardiac death (SCD), ventricular arrhythmias, cardiovascular magnetic resonance (CMR), positron emission tomography (PET), single-photon emission computerized tomography (SPECT), computed tomography
Citation
Xie E, Sung E, Saad E, Trayanova N, Wu KC and Chrispin J (2022) Advanced imaging for risk stratification for ventricular arrhythmias and sudden cardiac death. Front. Cardiovasc. Med. 9:884767. doi: 10.3389/fcvm.2022.884767
Received
27 February 2022
Accepted
02 August 2022
Published
22 August 2022
Volume
9 - 2022
Edited by
Shimon Rosenheck, Hebrew University of Jerusalem, Israel
Reviewed by
Filippo Cademartiri, Gabriele Monasterio Tuscany Foundation (CNR), Italy; Paolo Severino, Sapienza University of Rome, Italy; Federico Migliore, University of Padua, Italy
Updates
Copyright
© 2022 Xie, Sung, Saad, Trayanova, Wu and Chrispin.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan Chrispin chrispin@jhmi.edu
This article was submitted to Cardiac Rhythmology, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.