Abstract
Heart failure (HF) and chronic obstructive pulmonary disease (COPD) are the leading global epidemiological, clinical, social, and economic burden. Due to similar risk factors and overlapping pathophysiological pathways, the coexistence of these two diseases is common. People with severe COPD and advanced chronic HF (CHF) develop similar symptoms that aggravate if evoking mechanisms overlap. The coexistence of COPD and CHF limits the quality of life (QoL) and worsens symptom burden and mortality, more than if only one of them is present. Both conditions progress despite optimal, guidelines directed treatment, frequently exacerbate, and have a similar or worse prognosis in comparison with many malignant diseases. Palliative care (PC) is effective in QoL improvement of people with CHF and COPD and may be a valuable addition to standard treatment. The current guidelines for the management of HF and COPD emphasize the importance of early integration of PC parallel to disease-modifying therapies in people with advanced forms of both conditions. The number of patients with HF and COPD requiring PC is high and will grow in future decades necessitating further attention to research and knowledge translation in this field of practice. Care pathways for people living with concomitant HF and COPD have not been published so far. It can be hypothesized that overlapping of symptoms and similarity in disease trajectories allow to draw a model of care which will address symptoms and problems caused by either condition.
Introduction
Cardiovascular diseases (CVD) and respiratory diseases are the leading causes of morbidity and mortality in developed countries (1, 2). Among them, chronic heart failure (CHF) and chronic obstructive pulmonary disease (COPD) are regarded as the most common. Both diseases may cause serious clinical, social, and economic burden (1, 2). Heart failure (HF) affects up to 4% of the global population, and the number of new cases is constantly rising (1–3). The causes of this phenomenon are associated mostly with aging of the society and, paradoxically, with the improved survival in HF and acute coronary syndromes being one of common causes of HF (1). COPD is currently the third leading cause of death worldwide (2, 4). As smoking, which is a major risk factor for the development of COPD, as well as for coronary artery disease, is still widespread in the population, particularly of low and moderate incomes, an upward trend in the number of new cases of COPD is still predicted (2, 4). CHF and COPD having similar risk factors coexist in about 30% (range 9–52%) (2, 5, 6). If CHF and COPD coexist, the function of many organs and systems is affected more profoundly, the symptoms overlap, the quality of life (QoL) is limited more seriously, and the mortality risk is higher, particularly in the elderly (1, 2, 7). CHF increases mortality risk in people with COPD and CVD being the most common cause of death among this population, but the influence of COPD on all-cause mortality in the HF has not been proven so far (8, 9). The management of people living with CHF and COPD needs to address similar problems and symptoms and to prevent recurrence of exacerbations of these diseases (1, 5, 6). The guidelines recommend implementation of disease-specific management for HF and COPD even in the most advanced stages of disease. Interestingly, most of them recommend care provided by multidisciplinary team, including palliative care (PC), if needed (1–3, 10, 11).
Palliative care is an active, holistic approach that focuses on improvement of QoL of people living with life-threatening diseases that relieve health-related suffering (12, 13). Nowadays, it is dedicated to all suffering from a disease that does not fully respond to disease-specific management. It should be provided alongside the whole trajectory of living with the disease, regardless of diagnosis, prognosis, and risk of death (1–3, 10, 14–16). The core principle of PC is facilitating effective collaboration between the ill person, her or his family, and healthcare professionals from all disciplines and specialties involved in the care with the goal to empower the person living with a disease to achieve best possible QoL, according to personal values, to strengthen autonomy, and to secure maintaining dignity (17). PC acknowledges the relatives, often being informal caregivers, as subjects who need (PC) support, with the goal to maintain their QoL and facilitate their ability to care (11, 18). PC goes beyond alleviation of symptoms and perceives addressing psychological, spiritual, and social needs as integral aspects of care (1–3, 10, 14–16). Many scientific societies recommend that PC should be integrated in the care for people living with CHF and COPD as soon as the needs emerge. (1–3, 10, 14–16).
Triggers for the implementation of palliative care for people living with advanced chronic heart failure and severe chronic obstructive pulmonary disease
The current guidelines recommend PC as a key component of multidisciplinary approach to people living with CHF and COPD, which should be applied based on needs, along with disease-specific management, regardless of the stage of these diseases and the expected survival (1–3, 11). Needs should be assessed as often as it is necessary, for example, in the case of significant changes in the course of the underlying disease, in general health, or in factors related to the person or his family, optimally using validates tools (1–3) (Table 1). The Needs Assessment Tool: Progressive Disease (NAT:PD) has been validated in cancer, HF, and interstitial lung disease and recently has been proposed as applicable in any progressive disease (19). It contains prompts to assess the needs and wellbeing of the ill person, the ability to care of relatives, and their wellbeing. The last prompt is dedicated to the consideration who will address existing needs (3, 20). Alternatively, other available validated scales assessing the presence and the severity of symptoms can be used to recognize indication for PC involvement (1–3). The most widely used tools are Numeric Rating Scale (NRS) and Edmonton Symptom Assessment Scale (ESAS) being in fact a list of NRS for nine most common and one self-determined symptoms, the Integrated Palliative Care Outcome Scale (IPOS) (3).
TABLE 1
| HF | COPD |
| • De novo HF with severe symptoms refractory to treatment • Symptoms of advanced CHF (NYHA IV) • Qualification for CIEDs • Qualification for TAVI • Eligibility for valve replacement surgery • Qualified for HTX or MCS | • De novo COPD with severe symptoms refractory to treatment • Symptoms of advanced COPD (category D, bronchial obturation GOLD 3/4, mMRC grade 3/4) • Eligibility for lung transplantation • Initiation of long-term oxygen therapy • Initiation of home non-invasive ventilation |
| • Progressive worsening of CHF and COPD with severe symptoms refractory to treatment • Care dependence and poor self-management • Frequent, recurrent exacerbations of CHF and/or COPD • Cachexia • Inability to attend cardiopulmonary rehabilitation • Survived cardiopulmonary resuscitation • Eligibility for heart and lung transplantation | |
The most important changes in the course of HF and COPD initiating the assessment of PC needs; based on: (1–3, 8, 11, 13, 132).
CIEDs, cardiovascular electrical devices; COPD, chronic heart failure; GOLD, global initiative for chronic obstructive lung disease; HF, heart failure; HTX, heart transplantation; MCS, mechanical cardiac support; MRC, medical research council; NYHA, New York heart association; TAVI, transcatheter aortic valve replacement.
The involvement of PC in care for people with heart and/or lung disease is less common than with cancer (18, 21, 22). The EPICTER study has shown that only 15% of patients hospitalized with advanced HF (23% of all HF hospitalizations) receive PC, but mostly only those with symptomatic cancer (as concomitant disease) in the last hours of life, after standard therapy has been exhausted (21, 23). The main causes for late PC involvement are depicted in Table 2 (3, 22–25). After the needs have been assessed, PC can be implemented stepwise. This process is presented below, with particular emphasis on the interdisciplinary approach in this group of patients (Figure 1).
TABLE 2
| Cause | Solution proposal |
| • Uncertain prognosis of COPD and CHF | • Collaboration in a multidisciplinary cardiopulmonary team to optimize standard care and choose the optimal time to start PC • Implementing of needs based model of triggering PC involvement |
| • Underestimation of the PC needs | • Regular assessment of the PC’s needs using available scales, both in primary and secondary care |
| • Person’s fear of talking about PC and end of life | • Improving awareness on PC principles in the society • Providing the patient with psychological, spiritual and social support in the scope adjusted to the needs based model of PC provision |
| • Insufficient physician’s communication skills on end-of-life related topics | • Training in clinical communication skills in non-PC specialists |
| • Incorrect perception of the PC as a solely care for the dying, lack of PC education | • Palliative care education and training programs for healthcare professionals |
| • Insufficient cooperation between PC specialists and other healthcare providers | • Meetings in a multidisciplinary group, including cardiologists, pneumonologists and PC specialists aimed at implementing an integrated PC |
The most common causes of insufficient PC involvement in people living with COPD and CHF; based on (3, 16, 20–23).
CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; PC, palliative care.
FIGURE 1
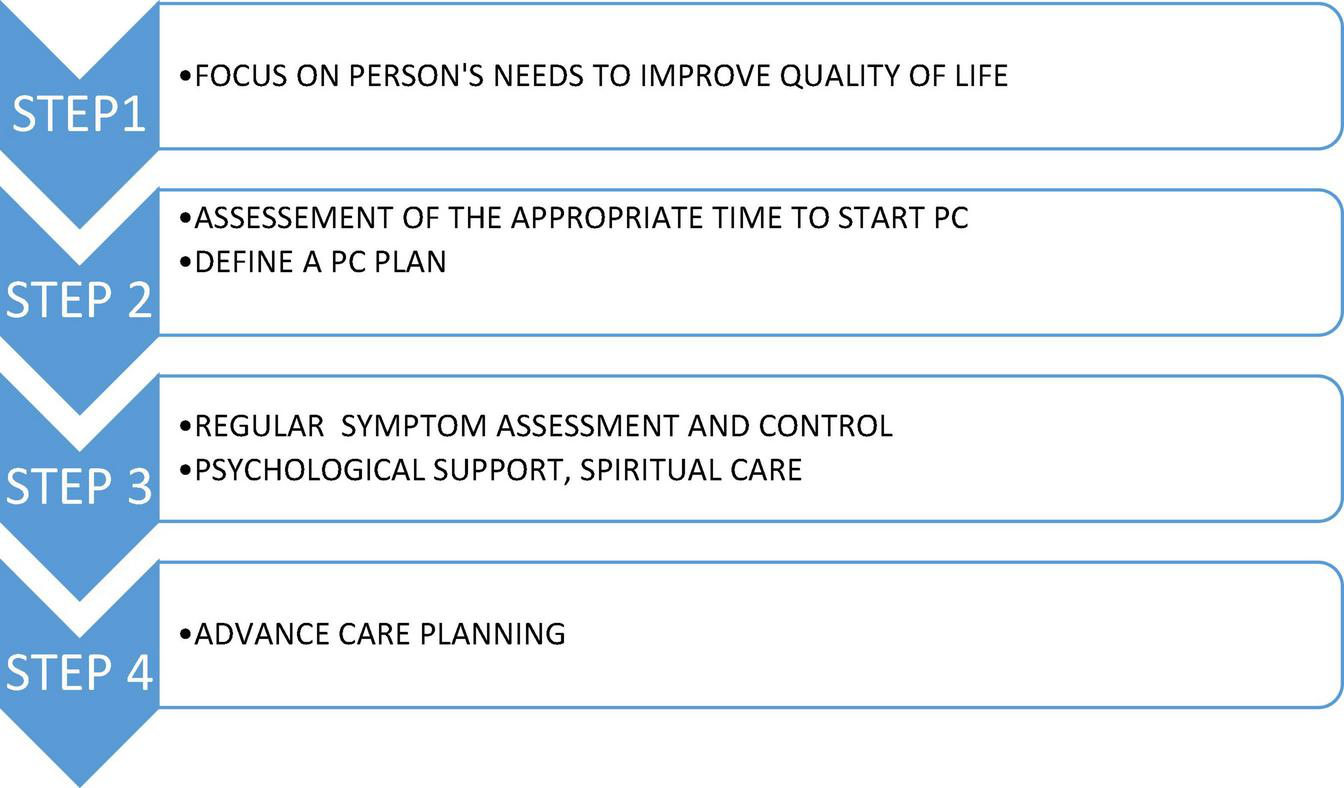
Steps in PC in people living with CHF and COPD; based on (1–3). CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease.
Although PC for those sub-populations exists, there is a lack of guidelines for the PC management of people living with COPD and coexisting CHF. This manuscript merges the available general and specific for either condition PC.
Main clinical problems in people living with heart failure and chronic obstructive pulmonary disease
The disease-specific management should be continuously optimized according to current guidelines, even in the most advanced stages of the disease and in people receiving care with the main focus on symptom relief. The applicability of this treatment and adjustment of treatment goals, according to given situation, is mandatory (1–3) (Figure 2). It should be emphasized that patients with COPD and CHF present many similar symptoms and experience many common problems that may overlap, constituting a therapeutic challenge for cardiologists and pneumologists (1–3, 5, 6) (Figure 3).
FIGURE 2
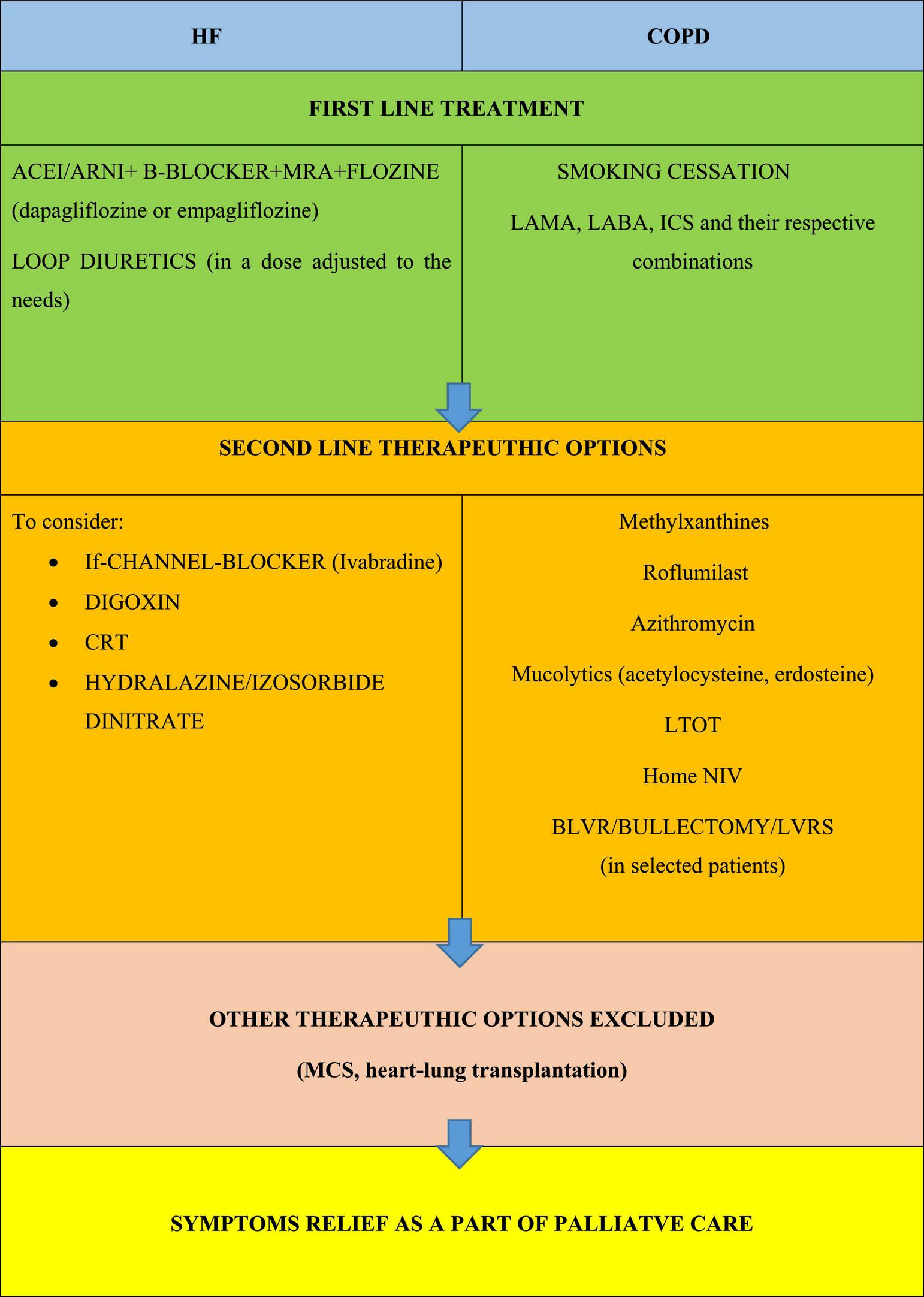
Principles of optimizing the treatment of the underlying disease in people living with COPD and HF; based on (1–3). ACE, angiotensin converting enzyme inhibitor; ARNI, angiotensin receptor neprilysin inhibitor; BLVR, bronchoscopic lung volume reduction; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronisation therapy; CS, corticosteroids; HF, heart failure; ICS, inhaled glucocorticoids; LABA, long acting B2 agonist; LAMA, long acting muscarinic antagonist; LTOT, long term oxygen therapy; LVRS, lung volume reduction surgery; MCS, mechanical cardiac support; MRA, mineralocorticoid receptor antagonist; NIV, non-invasive ventilation; PDE-4, phosphodiesterase-4; SABA, short acting β agonist.
FIGURE 3
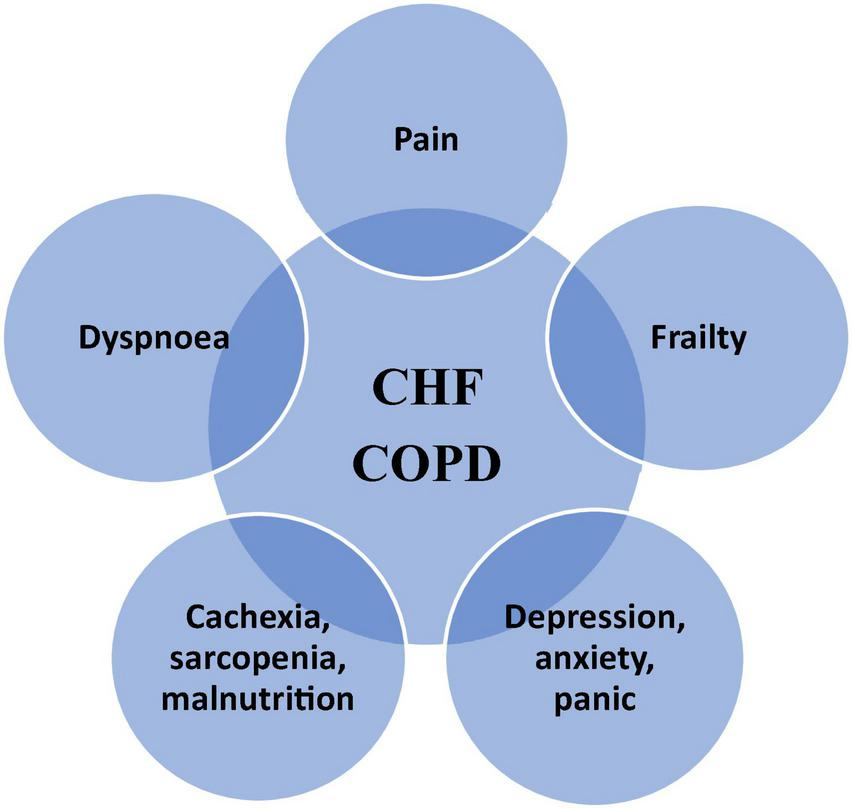
The most common clinical problems overlapping in CHD and COPD; adapted from (1–3). PC, palliative care.
The symptomatic management of most common symptoms experienced by people with advanced CHF and severe COPD is discussed below.
Breathlessness
Breathlessness is ubiquitously present in people living with CHF or COPD, beginning from very early stages of disease till the phase of dying. Disease-specific treatment alleviates it, but does not eliminate it completely, especially in advanced disease, when it is present all the time, despite optimal cardiological or pneumological management (1–3). The term “breathlessness” is commonly used to call the difficulties in breathing; however, people experiencing it use several descriptors to express their experience (e.g., tightness in the chest, air hunger, shortness of breath, shallow respiration, breathlessness, difficulty in breathing, heavy breathing, and feeling lack of air). It is regarded that this plurality of names describes the plurality of pathophysiological processes evoking breathlessness (1–3). Even if the factors causing breathlessness in heart and lung disease differ in less advanced stages, as the diseases progress, they increasingly overlap or even become common (1–3, 14, 26) (Figure 4).
FIGURE 4
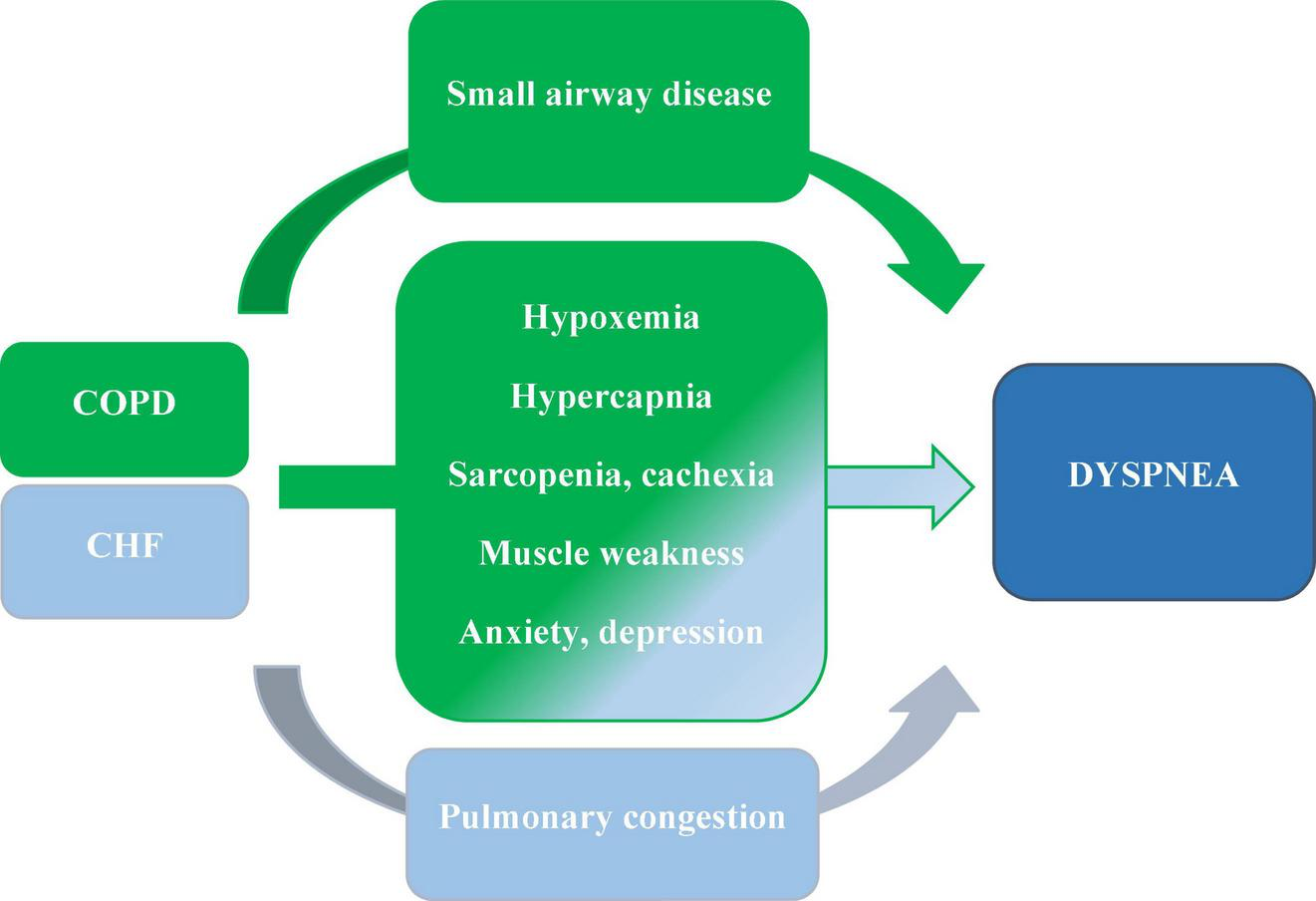
The major causes of dyspnea in people living with COPD and CHF coexistence; based on (1–3, 30).
The first step in alleviating breathlessness is optimization of treatment of the underlying disease or concomitant disease that can additionally aggravate it (like pneumonia, hydrothorax, or anemia). For many years, the intensification of β-blocker therapy in patients with HF and coexisting COPD has been controversial. Nevertheless, according to the current ESC and GOLD guidelines, these patients should be treated for HF in the same way as patients without COPD, including optimization of β-blocker treatment. Recent studies showed that β-blockers, particularly cardioselective ones, are safe in the treatment of COPD patients, reduce all-cause mortality, and might contribute to the reduction of COPD-related hospitalizations (27–29). On the contrary, long-acting anticholinergics and β2-agonists needed for COPD can evoke tachycardia, undesired in CHF. Optimization of treatment of each disease requires close monitoring of the function of the other one (30).
Improvement of the burden caused by breathlessness requires implementation of non-pharmacological management which may be especially successful if the breathlessness is of moderate severity (14, 26). In COPD, dyspnea exceeding point 2 on mMRC scale is an indication for general and pulmonary rehabilitation. In alleviation of chronic breathlessness training, the breathing technique adapted to the needs and possibilities of physical activity, cooling the face with a hand-hold fan directed at the triangle between mouth, nose, and cheeks, spraying a cold water on this area, psychological support, and education of patients and their relatives are considered as potentially helpful (1–3, 14, 26, 31). In COPD neuromuscular electrical stimulation and chest wall vibration, stimulation of mucus removal can be tried (2, 26). Oxygen can improve breathlessness in the majority of patients with COPD, sometimes even without documented hypoxemia, and in some patients with HF, predominately those with hypoxia (1–3).
The pharmacological, palliative treatment of chronic, refractory dyspnea is based on low doses of opioids (i.e., significantly lower, as used for pain management), mainly morphine. They have been studied predominantly in COPD and in cancer. Their efficacy and safety in HF are still not well-proven; however, they are recommended in cardiological guidelines (1–3, 14, 26, 32, 33). Morphine significantly alleviates breathlessness in about 50% of treated patients. The most commonly suggested initial dose is 10 mg/day p.o. that, if needed, can be up titrated gradually by 10 mg/day steps, every 7–10 days, to maximal 30 mg/day. Some experts recommend starting with much lower dose, what can be reasonable in elderly, cachectic, fragile, affected with comorbidities, with organ insufficiency (3, 34). The side effects, seen sometimes even with low doses of opioids, include constipation, usually transient nausea and vomiting, sedation/drowsiness, and addiction (1–3, 26). The most feared is respiratory depression that can be successfully prevented by proper dose titration and meticulous monitoring of symptoms and side effects as well as changes in clinical status and co-medication. The fear of opioid side effects (respiratory depression and addiction) together with lack of skills in their prescribing by non-palliative medical specialties is the most frequent cause of undertreatment of breathlessness (33). Benzodiazepines are commonly used for breathlessness alleviation, despite the lack of proven benefits and known harms of side effects. High doses can cause drowsiness and somnolence, especially in the elderly. The risk of addiction should not be neglected. Myorelaxant properties can impair respiratory muscle function and increase the risk of falls (1–3). Benzodiazepines may be considered as second- or third-line treatment of acute dyspnea in case of ineffectiveness of other therapeutic options, particularly in the presence of severe anxiety or in the dying (1–3). Mirtazapine is waking growing interest as a therapeutic option for people suffering for refractory breathlessness, especially those with concomitant anxiety, but randomized trials are underway (35).
Pain
Pain is as common in people living with CHF and COPD as in people living with cancer, but its intensity is lower (36). That is why non-pharmacological management and non-opioids are more often sufficient, as in people with cancer. If the pain is severe, the addition of opioids should be considered. The non-steroidal anti-inflammatory drugs (NSAIDs) are contraindicated in people with HF, unless in very selected situation, the antiphlogistic effect is indispensable, as additionally to general sided effects (like gastrointestinal bleeding), they increase the risk of HF-related hospitalizations, renal function worsening, major atherothrombotic events, and death (1–3). The only exception among NSAIDs is acetylsalicylic acid (ASA), used at a dose of 75–100 mg/day as secondary prophylaxis in atherosclerosis (i.e., coronary artery disease). There are not known specific contraindications for their use in people with COPD, but administration of NSAIDs, including ASA, has been shown to increase risk of new atrial fibrillation or bleeding (37, 38). The randomized ENABLE-CHF-PC trial has shown that PC telehealth significantly improves pain intensity and its interference with daily life, but not QoL or mood (39).
Sarcopenia and cachexia
Sarcopenia is a metabolic syndrome characterized by muscle loss, leading to diminished muscle strength, and performance, resulting in reduced mobility (40, 41). If sarcopenia is accompanied by unintended loss of more than 5% edema-free body weight within 12 months, cachexia should be diagnosed. Both conditions are seen in people living with advanced chronic diseases, with a prevalence 5–15% in advanced COPD and HF. The prevalence of sarcopenia, due to lack of universal clinical criteria, is more difficult to ascertain. Some authors suggest that it can affect 27% patients with COPD, with the prevalence growing with the advancement of the disease (42, 43). Among those living with HF, the prevalence of sarcopenia is 20% higher than in the age-matched healthy probands (44). It is associated with adverse outcomes, including falls, dysfunction, weakness, and death (42, 45). The most important factors leading to sarcopenia and cachexia are inadequate protein intake, malabsorption due to gut edema and hypoperfusion, metabolic imbalance, and physical inactivity (46, 47).
Nutritional screening should be performed in each patient with CHF and COPD using on of established tools: Nutritional Risk Screening 2002 (NRS 2002), Subjective Global Assessment (SGA), Mini Nutritional Assessment (MNA), Malnutrition Universal Screening Tool (MUST), Short Nutritional Assessment Questionnaire (SNAQ), for cachexia and SARC-F, SARC-calF test, and Mini Sarcopenia Risk Assessment (MSRA) for sarcopenia (48–55). Monitoring of weight is not a sensitive screening method as fluid retention can mask the loss of dry body weight and malnutrition. Useful for muscle function assessment is the handgrip strength measurement (56). Alternatively, body composition analysis (BIA), dual-energy X-ray absorptiometry (DXA), or muscle ultrasound may be used (47). BIA is a safe, reliable, inexpensive, and widely available tool (57). The main limitation of BIA and DXA in PC CHF/COPD patients is body water accumulation which may negatively affect the results (58).
Aerobic exercise and dietary interventions are suggested to prevent sarcopenia/cachexia, but there are no large studies in people with advanced CHF and COPD. The hypercaloric and hyperproteic supplementation of Dietary Approaches to Stop Hypertension (DASH) or Mediterranean diets are recommended, but this has not been evaluated in randomized studies (47). Participation in rehabilitation programs lead to improvements in exercise capacity and QoL (59).
Exacerbations
Acute exacerbations of COPD (AECOPD) and acute decompensations of CHF (ADCHF) are the common cause of (re-)hospitalizations (1, 2). Nearly 20% of COPD patients and about 25% of CHF patients are re-hospitalized within 1 month (1, 2, 18, 24, 60). Each ADCHF and AECOPD are a potential life-threatening condition and significantly increase the risk of all-cause mortality and re-hospitalization (1, 2). Undoubtedly, the optimization of guidelines directed management for COPD and CHF is crucial for the prevention of disease exacerbations (1, 2). Preliminary reports suggest that PC provided as an element of holistic approach may also additionally contribute to reduction of the rate of exacerbation-related readmissions (21, 24, 61–65).
Exercise rehabilitation
Exercise rehabilitation is an essential part of the multidisciplinary approach to people with HF and COPD recommended by ESC guidelines to all patients with HF and by GOLD experts to all patients with COPD who are able to undertake it (1, 2, 66). In population with HF, the rehabilitation improves physical capacity and QoL and reduces the frequency of HF-related hospitalizations (1, 2, 66–68). HF is a cause of only 15% of referral for cardiac rehabilitation, and as high proportion as 20% of patients with HF terminate the rehabilitation programs prematurely (69). Pulmonary rehabilitation, particularly involving aerobic exercises, increases physical capacity, reduces the feeling of dyspnea and fatigue, and significantly improves the QoL in people living with COPD (2, 14, 26, 70, 71). In patients with coexistence of CHF and COPD, exercise rehabilitation is especially needed, because in this group the decrease in cardiopulmonary capacity has a particularly negative impact on physical capacity, the severity of the dominant symptoms (particularly breathlessness), and the QoL (72, 73). The limitation of physical capacity cachexia and sarcopenia resulting in skeletal muscle dysfunction exaggerates breathlessness and fatigue in people with CHF and COPD (1, 2, 66). Exercise rehabilitation improves function of peripheral muscles, physical condition, symptoms of depression, and anxiety in people living with HF and COPD (14, 74, 75). Aerobic exercises or endurance training, inspiratory muscle training, or neuromuscular electrical stimulation (NMES) of lower limb muscles are especially recommended (76). The coexistence of COPD and CHF does not limit benefit of exercise rehabilitation (77). Exercise rehabilitation should be thus considered in all patients with HF and COPD, including severe forms of these diseases (1, 2, 66).
The rehabilitation can be part of an integrated PC in patients with COPD and CHF. Maddocks et al. and Reticker et al. suggest that PC and pulmonary rehabilitation have common goals (14, 78). The choice of the type, intensity, and duration of training should be tailored individually, with emphasis on the patient’s general condition and capabilities, the severity of the underlying disease, and the coexistence of comorbidities. Even in patients with severe HF and COPD, supervised exercise rehabilitation allows for individualization of training while maintaining a high level of safety (1, 2, 66). Telerehabilitation offers remote supervision of rehabilitation specialists (26, 79). Bernocchi et al. showed that with 4-month home telerehabilitation, the improvement with the distance in 6-min walk test (6MWT), the severity of dyspnea, the physical activity, and QoL can be achieved (80). Unfortunately, despite the clear benefits of simultaneous cardiac rehabilitation and pulmonary rehabilitation in COPD and CHF patients, implementation of cardiopulmonary rehabilitation in the form of integration of both programs is still challenging (81, 82).
Psychiatric disorders
Diagnosing of depression in people living with advanced disease can be quite challenging, as anxiety, fatigue, loss of appetite, or insomnia can be caused by diminished physical capacity and mood disorder. Using Patient Health Questionnaire-9 (PHQ-9) can facilitate timely and proper recognition of this comorbidity (83).
Depression is common and affects up to 40% of people living with CHF and/or COPD (84, 85). Depression favors unhealthy living still, smoking, diminished activity, and weight gain (84). It exerts negative impact on the QoL and adherence to treatment and increases the risk of hospitalization and death, including suicides (85–88). For those reason, it is suggested to assess people affected by HF for depression and treat them if required (3). Adequate statements with COPD have not been published so far, but it can be hypothesized that the approach should be similar. The management should consist of non-pharmacological (cognitive behavioral therapy (CBT) and aerobic exercise) and pharmacological interventions (89, 90). Selective serotonin reuptake inhibitors (SSRI) and mirtazapine are considered the first choice (35). Sertraline and escitalopram have been shown to be safe in people with HF and sertraline gave promising results in COPD (91, 92). Citalopram can cause QTc prolongation, especially in higher doses and in older patients, that is why it should be prescribed with caution. SSRIs can cause hyponatremia in the mechanism of syndrome of inappropriate antidiuretic hormone secretion, especially when combined with thiazide diuretics. SSRIs co-administrated with ASA and/or clopidogrel increase the risk of bleeding. Fluvoxamine and fluoxetine increase the concertation of warfarin, but decrease the metabolism of clopidogrel to its active metabolite.
Bupropion, noradrenaline and dopamine reuptake inhibitor (NDRI), is an effective and safe antidepressant registered for smoking cessation, which is particularly significant in COPD patients. Importantly, bupropion is a substrate for CYP2D6 similarly to clopidogrel and can decrease digoxin level; therefore, serum concentration monitoring is required. Most SSRIs, duloxetine, and bupropion inhibit CYP2D6 causing increased exposure of beta-blockers, and their dose reduction might be required. Mirtazapine has an interaction of unknown mechanism with warfarin causing increased risk of bleeding (93). Mirtazapine can also cause somnolence and weight gain which in some cases, however, can be beneficial. Medications used in COPD like beta-agonists used with SSRIs, mirtazapine, and trazodone can induce QT prolongation. Muscarinic antagonists can increase the risk of delirium and symptoms like dry mouth, constipation, and urinary retention which can be also caused by mirtazapine and trazodone (94). SSRI and NDRI—like venlafaxine and duloxetine—can cause hypertension and prolong QT interval. Tricyclic antidepressants and monoamine oxidase inhibitors are contraindicated in HF due to effect on blood pressure and QT prolongation, trazodone although trazodone is useful as a hypnotic agent not causing addiction should not be used in patients with ventricular arrhythmias (84, 93). A good strategy is to “start low, go slow” considering decreased drug metabolism. A new antidepressant strategy is ketamine—NMDA antagonist which turned out to be effective, rapid-acting add-on treatment in resistant depression (TRD). Its intranasal enantiomer es-ketamine has been recently approved by the FDA as an add-on treatment in TRD. Cardiac safety of add-on es-ketamine was evaluated in 1700 patients with TRD (95). Other experimental treatment used in depression and existential distress in patients with life-threatening disease is psilocybin-assisted psychotherapy. An RCT found reduction in the level of depression, suicidal ideation, and hopelessness after single dose of 0.3 mg/kg psilocybin administered in conjunction with psychotherapy (96). The results of recent Omega-3 Supplementation for Co-Morbid Depression and Heart Failure Treatment (OCEAN) trial revealed that high dose omega-3 was associated with improvement in cognitive depressive symptoms, social functioning, and 6MWT in depressed patients with HF (97).
Anxiety
General anxiety disorder and panic disorder are common, especially in COPD patients and in patients with implantable cardioverter defibrillator (ICD) (85, 98). The Hospital Anxiety and Depression Scale (HADS) is a good tool to screen hospitalized patients for anxiety and depression (99, 100). In COPD patients, CBT is recommended as an effective non-pharmacological treatment for anxiety (101). Pharmacotherapy, apart from SSRIs, which are the first-line treatment for anxiety disorders, includes also buspirone (93). BDZ is generally not recommended in elderly and patients with HF due to risk of falls and delirium (102, 103). Alprazolam—short acting benzodiazepine, has an interaction with amiodarone which inhibits its metabolism through CYP3A4 and can cause enhanced alprazolam effects (93).
Insomnia is common and requires efforts to remediate the underlying cause if it is possible. When pharmacotherapy is needed, antidepressants and melatonin can be considered.
Delirium
Delirium is an acute neuro-psychiatric condition caused by global brain dysfunction reaching prevalence up to 40% in palliative setting. The hallmark of delirium is disturbance in attention and awareness (104). Typically, delirium has acute onset and fluctuating course. Delirium is often underdiagnosed or misdiagnosed. A useful screening tool recommended by National Institute for Health and Care Excellence (NICE) is Confusion Assessment Method (CAM) (105, 106). Etiology of delirium is multifactorial, but most common triggering factors are infection, substance withdrawal, electrolyte disturbance, hypoxia, dehydration, anemia, hypo/hyperglycemia, organ dysfunctions, neurological diseases, and medications. Delirium correlates with increased mortality, morbidity, longer hospitalization, and higher costs of treatment. It increases the risk of falls and significantly disturbs the process of communication with the person (107). According to NICE guideline, prevention strategies like avoiding unnecessary catheterization, optimizing sleep conditions, encouraging physical activity, avoiding sensory deprivation (glasses, hearing aids), and using clock and calendar for orientation can be very effective (105). There are data on melatonin as an effective and safe preventing therapy in older patients undergoing surgical procedures (108).
The main goal in treatment should be identifying and cessation of the source of delirium. About 50% cases of delirium in palliative patients can be reversed with good communication at the end of person’s life (107, 109). No medication is registered for delirium treatment, but if the person is distressed or considered risk to themselves or others, low dose and short-term haloperidol or olanzapine can be considered (105, 110).
Spiritual care
Spirituality is the dynamic dimension of human life that relates to the way persons experience, express and/or seek meaning, purpose, and transcendence, and the way they connect to the moment, to self, to others, to nature, to the significant, and/or the sacred (111).
Living with progressive disease confronts people with spiritual issues, that is, why spiritual care is integral part of PC. It supports people in coping with existential questions. As the spirituality, especially in West-European countries goes beyond religiously, spiritual care needs to be provided by, in addition to chaplains and pastoral care workers, all healthcare professionals offering their therapeutic presence. This kind of care is based on relations between the patient, caring team, and patients’ close ones (3). Evidence shows that spiritual counseling improves QoL in patients with HF (112). Spiritual peace along with healthy lifestyle has been shown to predict 5-year mortality better than functional status and comorbidity in people with HF (113).
Advance care planning
Advance care planning (ACP) is a process of preparedness for the decision-making for the future, whereby individuals identify their goals and preferences concerning future care and treatment as well as discuss these goals and preferences with healthcare providers and family (114). It is aimed to ensure medical care the person receives, especially at the end of life, aligns with their preferences (115). During ACP process, the person is invited to reflect her or his personal values and goals, and based on this, to try to foresee what from applicable treatment and care options could be concordant with those values and goals in future, usually in the hypothetical end-of-life situation (116). Open and honest clinical communication helps to get realistic insight in current disease-related situation, the risk of progression, and chances for improvement in case of treatment success but as well for deterioration in case it fails (117, 118). The ACP decisions should be recorded and revised or update if appropriate (119). The outcome of the ACP process can be just being prepared for the moment of decision-making, writing advance directives (AD), sharing the conclusions with family (i.e., advance statement) without preparing any formal document, or asking the treating physician to prepare orders for life-sustaining therapies (Physician Orders for Life-Sustaining Therapies, POLST) (120). The AD can summarize the treatment or care options expected or unwished and/or indicate formal representative who will make medical decisions (surrogate) for the case the person loses decision-making capacity. The appropriate form of expression own will in given country is matter of local law and local traditions (121–123).
The ACP process in people living with HF and COPD should, additionally to general topics like hospital admission, or tube feeding, address disease-specific issues like ventilation, modification of cardiovascular implantable electronic devices (CIEDs), and/or mechanical circulatory support (124). The decision how to proceed with ventricular assist device (VAD) in case of serious advance events like refractory sepsis, cerebral bleeding, or cerebrovascular embolic insult is especially challenging, as most of affected people lose their decision-making capacity in such situations (125).
People with cardiovascular and pulmonary diseases prefer the early initiation of ACP conversations, that is, at the time of diagnosis and at transition points in the follow-up (126, 127). In practice, healthcare providers often initiate ACP process at the advanced stage of illness only (128, 129).
Composition of palliative care team
For the adequate provision of specialized PC services, a qualified multidisciplinary team is required (130). The core team consists of physicians and nurses with specialized training, whereas psychologists, physiotherapists, social workers, and spiritual care workers constitute the extended, multiprofessional PC team. Other important contributors include psychologists, office workers, bereavement counselors, wound management and lymphedema specialists, occupational, art and speech therapists, dietitians, pharmacists, complementary therapists, trainers, and librarians (131). To ensure cohesive continuity of care, all involved including the person living with a disease and her or his family need to create a network (130, 132). Trained volunteers are important members of the therapeutic team, supporting services provided by professionals (133). Close cooperation of cardiology and/or pulmonology team, certified PC nurse, and physician assure achievement of better QoL and symptom relieve in people living with HF and COPD (134, 135).
Organization of palliative care
Every person with needs has a right to get access to PC and pain as well as other symptom relief. To realize this, governments should facilitate integration of PC in healthcare systems and healthcare insurances need to reimburse the service (136, 137). PC can be provided on two levels: PC approach (called as well generic PC) and specialist PC (13). In case of PC approach, all healthcare professionals used to be engaged in the management of the person living with a disease implement basic PC principles in the care they usually provide, in a place the person has been cared for so far (e.g., general hospitals, cardiology or pulmonology units, outpatients clinics, and nursing homes). The specialist PC is provided by healthcare professionals having special training, provision of PC is their main business, this level of care should be provided to people with complex needs, or PC approach has appeared as not enough sufficient. Specialist PC should optimally be served by multiprofessional team, but acceptable can be engagement of physician and nurse with PC expertise cooperating with such a team (138). In many countries, there are also hospital PC support teams, providing an advisory service to hospital staff (139).
Modification of cardiovascular implantable electronic devices activity at the end of life
Growing number of people living with CHF, also those with coexisting COPD, had received one of CIEDs, which, when the end-of-life approaches, can require a special attention to prevent device related suffering and/or providing futile therapies. The family of CIEDs includes antibradycardia pacemakers, cardiac resynchronization therapy (CRT) pacemakers, ICDs, or combination of them (CRT + ICD = CRT-D). They are originally implanted to improve the QoL (antibradycardia pacing and CRT) and/or prevent sudden cardiac death (ICD and CRT-D). Pursuing the main goal of healthcare—assuring the best possible QoL, during the whole life, even its last phase–dying, requires adjustment of all ongoing therapies, including the electrotherapies provided by CIEDs. Modification of CIEDs activities is aimed to prevent unneeded suffering caused by under- or over-treatment. Keeping active the whole devices or their functions that prevent, in unnoticeable for the patient way, bradycardia- or pauses-related symptoms or improve the synchrony of heart contraction, has from a medical perspective never to be questioned. On the contrary, the high voltage antitachycardia therapies (cardioversion/defibrillation), usually painful, and in the dying phase (when the death is not consequence of tachyarrhythmia) futile, should be considered as medically not indicated and discontinued (after receiving patient’s consent). Low-voltage antitachycardia pacing, even if adequate and unnoticeable, can prolog the dying, as terminating of tachycardia, even if potentially lethal, cannot save the life of person dying for otherwise end-stage disease (not for arrhythmia). When impending of the death can be anticipated, the sense of device activity must be evaluated and discussed with affected person. If the death cannot be avoided, and the potential arrhythmia is not the cause, but the mode of deaths, terminating it should be considered as prolonging dying, and not saving life (140–142). The deactivation of shocking function should be performed at the end of life, but the communication on this should happen much earlier, optimally even before implantation of CIED. Decision on modification of CIED activity should be integral part of advance care planning (141, 143). Communicating this is regarded by many clinicians as more challenging than withdrawal from other life-sustaining therapies (144). Clinicians should be educated that ICD deactivation is not a form of euthanasia and their decision should be supported by healthcare provider organizations with policies of management of devices with an option of planned device inactivation (140).
Conclusion
Chronic heart failure and COPD are common, often coexist, and cause similar symptoms that cannot be completely alleviated with optimal guidelines driven disease-specific treatment, especially in advanced stages. The persistent symptoms caused by either condition limit the QoL and are a cause of suffering that can potentially be addressed by PC. The PC should be understood as additional layer of care, provided additionally to the disease-specific treatment, but not its alternative, that should be applied always when the needs emerge, independent of expected length of life/risk of dying. In people affected by HF and COPD, the close cooperation between cardiologists, pneumonologists, palliative medicine specialists, general practitioners, and other specialists, if necessary, is crucial. If the needs are complex or have not been efficiently addressed by multidisciplinary team, the involvement of specialized PC team should be considered.
Statements
Author contributions
AK, MB, PS, AW, IP, LP, PJ, and EJ wrote sections of the manuscript. All authors contributed to conception and design of the study, manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
McDonaghTAMetraMAdamoMGardnerRSBaumbachABöhmMet al2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure.Eur Heart J. (2021) 42:3599–726. 10.1093/eurheartj/ehab368
2.
2022 Gold Reports. Global Initiative for Chronic Obstructive Lung Disease - GOLD. (2022). Available online at: https://goldcopd.org/2022-gold-reports-2/(accessed April 29, 2022).
3.
SobanskiPZAlt-EppingBCurrowDCGoodlinSJGrodzickiTHoggKet alPalliative care for people living with heart failure: European association for palliative care task force expert position statement.Cardiovasc Res. (2020) 116:12–27. 10.1093/cvr/cvz200
4.
WHO. Chronic Obstructive Pulmonary Disease (COPD). (2022). Available online at: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)(accessed February 23, 2022).
5.
GüderGStörkS. COPD and heart failure: differential diagnosis and comorbidity.Herz. (2019) 44:502–8. 10.1007/s00059-019-4814-7
6.
HawkinsNMPetrieMCJhundPSChalmersGWDunnFGMcMurrayJJV. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology.Eur J Heart Fail. (2009) 11:130–9. 10.1093/eurjhf/hfn013
7.
FranceschiCGaragnaniPMorsianiCConteMSantoroAGrignolioAet alThe continuum of aging and age-related diseases: common mechanisms but different rates.Front Med. (2018) 5:61. 10.3389/fmed.2018.00061
8.
AxsonELRagutheeswaranKSundaramVBloomCIBottleACowieMRet alHospitalisation and mortality in patients with comorbid COPD and heart failure: a systematic review and meta-analysis.Respir Res. (2020) 21:54. 10.1186/S12931-020-1312-7
9.
CanepaMStraburzynska-MigajEDrozdzJFernandez-VivancosCPinillaJMGNyolczasNet alCharacteristics, treatments and 1-year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long-Term Registry.Eur J Heart Fail. (2018) 20:100–10. 10.1002/EJHF.964
10.
KidaKDoiSSuzukiN. Palliative care in patients with advanced heart failure.Heart Fail Clin. (2020) 16:243–54. 10.1016/j.hfc.2019.12.006
11.
HillLPrager GellerTBaruahRBeattieJMBoyneJde StoutzNet alIntegration of a palliative approach into heart failure care: a European Society of Cardiology Heart Failure Association position paper.Eur J Heart Fail. (2020) 22:2327–39. 10.1002/EJHF.1994
12.
RadbruchLDe LimaLKnaulFWenkRAliZBhatnagharSet alRedefining palliative care—a new consensus-based definition.J Pain Symptom Manage. (2020) 60:754–64. 10.1016/j.jpainsymman.2020.04.027
13.
PayneSHardingAWilliamsTLingJOstgatheC. Revised recommendations on standards and norms for palliative care in Europe from the European Association for Palliative Care (EAPC): a Delphi study.Palliat Med. (2022) 36:680–97. 10.1177/02692163221074547
14.
MaddocksMLovellNBoothSManWD-CHigginsonIJ. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease.Lancet. (2017) 390:988–1002. 10.1016/S0140-6736(17)32127-X
15.
PantilatSZ. Palliative care for patients with heart failure.JAMA. (2004) 291:2476. 10.1001/jama.291.20.2476
16.
WHO. Palliative Care. (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/palliative-care(accessed February 23, 2022).
17.
JüngerSPayneSBrearleySPloenesVRadbruchL. Consensus building in palliative care: a europe-wide delphi study on common understandings and conceptual differences.J Pain Symptom Manage. (2012) 44:192–205. 10.1016/j.jpainsymman.2011.09.009
18.
SioutaNHeylenAAertgeertsBClementPJanssensWVan CleemputJet alQuality of Life and Quality of Care in patients with advanced Chronic Heart Failure (CHF) and advanced Chronic Obstructive Pulmonary Disease (COPD): implication for palliative care from a prospective observational study.Prog Palliat Care. (2021) 29:11–9. 10.1080/09699260.2020.1831248
19.
NAT. Needs Assessment Tool: Progressive Disease (NAT: PD) User Guide. (2022). Available online at: http://www.newcastle.edu.au/research-centre/cherp/professional-resources(accessed April 7, 2022).
20.
JanssenDJBoyneJCurrowDCScholsJMJohnsonMJLa RoccaH-PB. Timely recognition of palliative care needs of patients with advanced chronic heart failure: a pilot study of a Dutch translation of the Needs Assessment Tool: progressive disease – heart failure (NAT:PD-HF).Eur J Cardiovasc Nurs. (2019) 18:375–88. 10.1177/1474515119831510
21.
NelsonC. Inpatient palliative care consults and the probability of hospital readmission.Perm J. (2011) 15:48–51. 10.7812/TPP/10-142
22.
MeffertCHatamiIXanderCBeckerG. Palliative care needs in COPD patients with or without cancer: an epidemiological study.Eur Respir J. (2015) 46:663–70. 10.1183/09031936.00208614
23.
Fernández-MartinezJRomero-CorreaMSalamanca-BautistaPAramburu-BodasÓFormigaFVázquez-RodríguezPet alPrevalence of advanced heart failure and use of palliative care in admitted patients: findings from the EPICTER study.Int J Cardiol. (2021) 327:125–31. 10.1016/j.ijcard.2020.11.002
24.
MirWAYSiddiquiAHPaulVHabibSReddySGaireSet alPalliative care and chronic obstructive pulmonary disease (COPD) readmissions: a narrative review.Cureus. (2021) 13:e16987. 10.7759/cureus.16987
25.
SioutaNClementPAertgeertsBVan BeekKMentenJ. Professionals’ perceptions and current practices of integrated palliative care in chronic heart failure and chronic obstructive pulmonary disease: a qualitative study in Belgium.BMC Palliat Care. (2018) 17:103. 10.1186/s12904-018-0356-7
26.
AmbrosinoNFracchiaC. Strategies to relieve dyspnoea in patients with advanced chronic respiratory diseases. A narrative review.Pulmonology. (2019) 25:289–98. 10.1016/j.pulmoe.2019.04.002
27.
YangYLXiangZJYangJHWangWJXuZCXiangRL. Association of β-blocker use with survival and pulmonary function in patients with chronic obstructive pulmonary and cardiovascular disease: a systematic review and meta-analysis.Eur Heart J. (2020) 41:4415–22. 10.1093/EURHEARTJ/EHAA793
28.
NielsenAOPedersenLSodeBFDahlM. β-blocker therapy and risk of chronic obstructive pulmonary disease - a danish nationwide study of 1⋅3 million individuals.EClinicalMed. (2019) 7:21–6. 10.1016/J.ECLINM.2019.01.004
29.
BhattSPWellsJMKinneyGLWashkoGRBudoffMKimYIlet alβ-Blockers are associated with a reduction in COPD exacerbations.Thorax. (2016) 71:8–14. 10.1136/THORAXJNL-2015-207251
30.
HawkinsNMPetrieMCMacDonaldMRJhundPSFabbriLMWikstrandJet alHeart failure and chronic obstructive pulmonary disease.J Am Coll Cardiol. (2011) 57:2127–38. 10.1016/j.jacc.2011.02.020
31.
Damps-KonstańskaIKostrzewskiR. Palliative care in patients with chronic obstructive pulmonary disease.Med Paliatywna w Prakt. (2011) 11:91–5.
32.
JohnsonMJCockayneSCurrowDCBellKHicksKFairhurstCet alOral modified release morphine for breathlessness in chronic heart failure: a randomized placebo-controlled trial.ESC Hear Fail. (2019) 6:1149–60. 10.1002/ehf2.12498
33.
MoranTZentnerDWongJPhilipJSmallwoodN. Chronic breathlessness in advanced cardiorespiratory disease: patient perceptions of opioid use.BMJ Support Palliat Care. (2021) [Online ahead of print]. 10.1136/bmjspcare-2020-002853
34.
CurrowDCMcDonaldCOatenSKennyBAllcroftPFrithPet alOnce-daily opioids for chronic dyspnea: a dose increment and pharmacovigilance study.J Pain Symptom Manage. (2011) 42:388–99. 10.1016/j.jpainsymman.2010.11.021
35.
KrajnikMHepgulNWilcockAJassemEBandurskiTTanziSet alDo guidelines influence breathlessness management in advanced lung diseases? A multinational survey of respiratory medicine and palliative care physicians.BMC Pulm Med. (2022) 22:41. 10.1186/S12890-022-01835-0
36.
van Dam van IsseltEFGroenewegen-SipkemaKHSpruit-van EijkMChavannesNHde WaalMWMJanssenDJAet alPain in patients with COPD: a systematic review and meta-analysis.BMJ Open. (2014) 4:e005898. 10.1136/bmjopen-2014-005898
37.
DanelichIMWrightSSLoseJMTefftBJCicciJDReedBN. Safety of nonsteroidal antiinflammatory drugs in patients with cardiovascular disease.Pharmacotherapy. (2015) 35:520–35. 10.1002/PHAR.1584
38.
OlschewskiHCanepaMKovacsG. Pulmonary and cardiac drugs: clinically relevant interactions.Herz. (2019) 44:517–21. 10.1007/S00059-019-4834-3
39.
BakitasMADionne-OdomJNEjemDBWellsRAzueroAStockdillMLet alEffect of an early palliative care telehealth intervention vs usual care on patients with heart failure.JAMA Intern Med. (2020) 180:1203. 10.1001/jamainternmed.2020.2861
40.
Bielecka-DabrowaAEbnerNSantosMRIshidaJHasenfussGHaehlingS. Cachexia, muscle wasting, and frailty in cardiovascular disease.Eur J Heart Fail. (2020) 22:2314–26. 10.1002/ejhf.2011
41.
Cruz-JentoftAJBahatGBauerJBoirieYBruyèreOCederholmTet alSarcopenia: revised European consensus on definition and diagnosis.Age Ageing. (2019) 48:16–31. 10.1093/ageing/afy169
42.
OhnumaTAliMAAdigunR.Anorexia and Cachexia.Treasure Island, FL: StatPearls Publishing (2022).
43.
Sepúlveda-LoyolaWOsadnikCPhuSMoritaAADuqueGProbstVS. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: a systematic review and meta-analysis.J Cachexia Sarcopenia Muscle. (2020) 11:1164–76. 10.1002/jcsm.12600
44.
EmamiASaitohMValentovaMSandekAEvertzREbnerNet alComparison of sarcopenia and cachexia in men with chronic heart failure: results from the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF).Eur J Heart Fail. (2018) 20:1580–7. 10.1002/ejhf.1304
45.
HeNZhangYZhangLZhangSYeH. Relationship between sarcopenia and cardiovascular diseases in the elderly: an overview.Front Cardiovasc Med. (2021) 8:743710. 10.3389/fcvm.2021.743710
46.
SandekABauditzJSwidsinskiABuhnerSWeber-EibelJvon HaehlingSet alAltered intestinal function in patients with chronic heart failure.J Am Coll Cardiol. (2007) 50:1561–9. 10.1016/j.jacc.2007.07.016
47.
Fernández-PomboARodríguez-CarneroGCastroAICantón-BlancoASeoaneLMCasanuevaFFet alRelevance of nutritional assessment and treatment to counteract cardiac cachexia and sarcopenia in chronic heart failure.Clin Nutr. (2021) 40:5141–55. 10.1016/j.clnu.2021.07.027
48.
MalmstromTKMorleyJE. SARC-F: a simple questionnaire to rapidly diagnose sarcopenia.J Am Med Dir Assoc. (2013) 14:531–2. 10.1016/j.jamda.2013.05.018
49.
Barbosa-SilvaTGMenezesAMBBielemannRMMalmstromTKGonzalezMC. Grupo de estudos em composição corporal e nutrição (COCONUT). Enhancing SARC-F: improving sarcopenia screening in the clinical practice.J Am Med Dir Assoc. (2016) 17:1136–41. 10.1016/j.jamda.2016.08.004
50.
YangMHuXXieLZhangLZhouJLinJet alComparing mini sarcopenia risk assessment with SARC-F for screening sarcopenia in community-dwelling older adults.J Am Med Dir Assoc. (2019) 20:53–7. 10.1016/j.jamda.2018.04.012
51.
KondrupJRasmussenHHHambergOStangaZ, Ad Hoc Espen Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials.Clin Nutr. (2003) 22:321–36. 10.1016/s0261-5614(02)00214-5
52.
DetskyASMcLaughlinJRBakerJPJohnstonNWhittakerSMendelsonRet alWhat is subjective global assessment of nutritional status?J Parenter Enter Nutr. (1987) 11:8–13. 10.1177/014860718701100108
53.
VellasBGuigozYGarryPJNourhashemiFBennahumDLauqueSet alThe mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients.Nutrition. (1999) 15:116–22. 10.1016/S0899-9007(98)00171-3
54.
StrattonRJHackstonALongmoreDDixonRPriceSStroudMet alMalnutrition in hospital outpatients and inpatients: prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults.Br J Nutr. (2004) 92:799–808. 10.1079/BJN20041258
55.
KruizengaHMSeidellJCde VetHCWWierdsmaNJvan Bokhorst–de van der SchuerenMAE. Development and validation of a hospital screening tool for malnutrition: the short nutritional assessment questionnaire (SNAQ©).Clin Nutr. (2005) 24:75–82. 10.1016/j.clnu.2004.07.015
56.
CarboneSKirkmanDLGartenRSRodriguez-MiguelezPArteroEGLeeDet alMuscular strength and cardiovascular disease.J Cardiopulm Rehabil Prev. (2020) 40:302–9. 10.1097/HCR.0000000000000525
57.
OreopoulosAEzekowitzJAMcAlisterFAKalantar-ZadehKFonarowGCNorrisCMet alAssociation between direct measures of body composition and prognostic factors in chronic heart failure.Mayo Clin Proc. (2010) 85:609–17. 10.4065/mcp.2010.0103
58.
BuckinxFLandiFCesariMFieldingRAVisserMEngelkeKet alPitfalls in the measurement of muscle mass: a need for a reference standard.J Cachexia Sarcopenia Muscle. (2018) 9:269–78. 10.1002/jcsm.12268
59.
PiepoliMFDavosCFrancisDPCoatsAJS, ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH).BMJ. (2004) 328:189. 10.1136/bmj.37938.645220.EE
60.
MartiCNFonarowGCGheorghiadeMButlerJ. Timing and duration of interventions in clinical trials for patients with hospitalized heart failure.Circ Hear Fail. (2013) 6:1095–101. 10.1161/CIRCHEARTFAILURE.113.000518
61.
GadeGVenohrIConnerDMcGradyKBeaneJRichardsonRHet alImpact of an inpatient palliative care team: a randomized controlled trial.J Palliat Med. (2008) 11:180–90. 10.1089/jpm.2007.0055
62.
O’ConnorNRMoyerMEBehtaMCasarettDJ. The impact of inpatient palliative care consultations on 30-day hospital readmissions.J Palliat Med. (2015) 18:956–61. 10.1089/jpm.2015.0138
63.
WiskarKCeliLAWalleyKRFruhstorferCRushB. Inpatient palliative care referral and 9-month hospital readmission in patients with congestive heart failure: a linked nationwide analysis.J Intern Med. (2017) 282:445–51. 10.1111/joim.12657
64.
BharadwajPHelfenKMDeleonLJThompsonDMWardJRPattersonJet alMaking the case for palliative care at the system level: outcomes data.J Palliat Med. (2016) 19:255–8. 10.1089/jpm.2015.0234
65.
BarkleyJEMcCallAMaslowALSkudlarskaBAChenX. Timing of palliative care consultation and the impact on thirty-day readmissions and inpatient mortality.J Palliat Med. (2019) 22:393–9. 10.1089/jpm.2018.0399
66.
AmbrosettiMAbreuACorràUDavosCHHansenDFrederixIet alSecondary prevention through comprehensive cardiovascular rehabilitation: from knowledge to implementation. 2020 update. A position paper from the Secondary prevention and rehabilitation section of the European association of preventive cardiology.Eur J Prev Cardiol. (2020) 28:460–95. 10.1177/2047487320913379
67.
MerelesDEhlkenNKreuscherSGhofraniSHoeperMMHalankMet alExercise and respiratory training improve exercise capacity and quality of life in patients with severe chronic pulmonary hypertension.Circulation. (2006) 114:1482–9. 10.1161/CIRCULATIONAHA.106.618397
68.
O’ConnorCMWhellanDJLeeKLKeteyianSJCooperLSEllisSJet alEfficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial.JAMA. (2009) 301:1439–50. 10.1001/JAMA.2009.454
69.
BostromJSearcyRWaliaARzucidloJBancoDQuienMet alEarly termination of cardiac rehabilitation is more common with heart failure with reduced ejection fraction than with ischemic heart disease.J Cardiopulm Rehabil Prev. (2020) 40:E26–30. 10.1097/HCR.0000000000000495
70.
PaneroniMSimonelliCVitaccaMAmbrosinoN. Aerobic exercise training in very severe chronic obstructive pulmonary disease: a systematic review and meta-analysis.Am J Phys Med Rehabil. (2017) 96:541–8. 10.1097/PHM.0000000000000667
71.
MakeBJYawnBP. Breathing life into COPD management: ongoing monitoring, pulmonary rehabilitation, and individualized care.Chest. (2018) 154:980–1. 10.1016/J.CHEST.2018.08.1023
72.
Dos SantosPBSimõesRPda GoulartCRoscaniMGMarinhoRSCamargoPFet alEccentric left ventricular hypertrophy and left and right cardiac function in chronic heart failure with or without coexisting COPD: impact on exercise performance.Int J Chron Obstruct Pulmon Dis. (2021) 16:203–14. 10.2147/COPD.S285812
73.
Borghi-SilvaAGarcia-AraújoASWinkermannECarusoFRBassi-DibaiDGoulartCet alExercise-based rehabilitation delivery models in comorbid chronic pulmonary disease and chronic heart failure.Front Cardiovasc Med. (2021) 8:729073. 10.3389/FCVM.2021.729073
74.
NederJAO’DonnellDEE. Heart, lungs, and muscle interplay in worsening activity-related breathlessness in advanced cardiopulmonary disease.Curr Opin Support Palliat Care. (2020) 14:157–66. 10.1097/SPC.0000000000000516
75.
MilaniRVLavieCJ. Impact of cardiac rehabilitation on depression and its associated mortality.Am J Med. (2007) 120:799–806. 10.1016/J.AMJMED.2007.03.026
76.
JonesSManWDCGaoWHigginsonIJWilcockAMaddocksM. Neuromuscular electrical stimulation for muscle weakness in adults with advanced disease.Cochrane database Syst Rev. (2016) 10:CD009419. 10.1002/14651858.CD009419.PUB3
77.
MentzRJSchultePJFlegJLFiuzatMKrausWEPińaILet alClinical characteristics, response to exercise training, and outcomes in patients with heart failure and chronic obstructive pulmonary disease: findings from heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION).Am Heart J. (2013) 165:193–9. 10.1016/J.AHJ.2012.10.029
78.
RetickerALNiciLZuWallackR. Pulmonary rehabilitation and palliative care in COPD: two sides of the same coin?Chron Respir Dis. (2012) 9:107–16. 10.1177/1479972312441379
79.
AmbrosinoNFracchiaC. The role of tele-medicine in patients with respiratory diseases.Expert Rev Respir Med. (2017) 11:893–900. 10.1080/17476348.2017.1383898
80.
BernocchiPVitaccaMLa RovereMTVolterraniMGalliTBarattiDet alHome-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial.Age Age. (2018) 47:82–8. 10.1093/AGEING/AFX146
81.
ManWDCChowdhuryFTaylorRSEvansRADohertyPSinghSJet alBuilding consensus for provision of breathlessness rehabilitation for patients with chronic obstructive pulmonary disease and chronic heart failure.Chron Respir Dis. (2016) 13:229–39. 10.1177/1479972316642363
82.
JonesAVEvansRAManWDCBoltonCEBreenSDohertyPJet alOutcome measures in a combined exercise rehabilitation programme for adults with COPD and chronic heart failure: a preliminary stakeholder consensus event.Chron Respir Dis. (2019) 16:1479973119867952. 10.1177/1479973119867952
83.
HammashMHHallLALennieTAHeoSChungMLLeeKSet alPsychometrics of the PHQ-9 as a measure of depressive symptoms in patients with heart failure.Eur J Cardiovasc Nurs. (2013) 12:446–53. 10.1177/1474515112468068
84.
GhoshRKBallSPrasadVGuptaA. Depression in heart failure: intricate relationship, pathophysiology and most updated evidence of interventions from recent clinical studies.Int J Cardiol. (2016) 224:170–7. 10.1016/j.ijcard.2016.09.063
85.
ZareifopoulosNBellouASpiropoulouASpiropoulosK. Prevalence, contribution to disease burden and management of comorbid depression and anxiety in chronic obstructive pulmonary disease: a narrative review.COPD J Chronic Obstr Pulm Dis. (2019) 16:406–17. 10.1080/15412555.2019.1679102
86.
SbolliMFiuzatMCaniDO’ConnorCM. Depression and heart failure: the lonely comorbidity.Eur J Heart Fail. (2020) 22:2007–17. 10.1002/ejhf.1865
87.
LinC-YHarnodTLinC-LKaoC-H. Suicide attempt and suicidal drug overdose in chronic obstructive pulmonary disease patients with or without depression.Front Psychiatry. (2020) 11:270. 10.3389/fpsyt.2020.00270
88.
KorkmazHKorkmazSÇakarM. Suicide risk in chronic heart failure patients and its association with depression, hopelessness and self esteem.J Clin Neurosci. (2019) 68:51–4. 10.1016/j.jocn.2019.07.062
89.
JeyananthamKKotechaDThankiDDekkerRLaneDA. Effects of cognitive behavioural therapy for depression in heart failure patients: a systematic review and meta-analysis.Heart Fail Rev. (2017) 22:731–41. 10.1007/s10741-017-9640-5
90.
BlumenthalJABabyakMAO’ConnorCKeteyianSLandzbergJHowlettJet alEffects of exercise training on depressive symptoms in patients with chronic heart failure.JAMA. (2012) 308:465–74. 10.1001/jama.2012.8720
91.
AngermannCEGelbrichGStörkSGunoldHEdelmannFWachterRet alEffect of escitalopram on all-cause mortality and hospitalization in patients with heart failure and depression.JAMA. (2016) 315:2683. 10.1001/jama.2016.7635
92.
DoddJWHoggLNolanJJeffordHGrantALordVMet alThe COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study.Thorax. (2011) 66:425–9. 10.1136/thx.2010.156372
93.
PińaILDi PaloKEVenturaHO. Psychopharmacology and cardiovascular disease.J Am Coll Cardiol. (2018) 71:2346–59. 10.1016/j.jacc.2018.03.458
94.
WeissAPorterSRozenbergDO’ConnorELeeTBalterMet alChronic obstructive pulmonary disease: a palliative medicine review of the disease, its therapies, and drug interactions.J Pain Sympt Manage. (2020) 60:135–50. 10.1016/j.jpainsymman.2020.01.009
95.
DohertyTWajsEMelkoteRMillerJSinghJBWeberMA. Cardiac safety of esketamine nasal spray in treatment-resistant depression: results from the clinical development program.CNS Drugs. (2020) 34:299–310. 10.1007/s40263-020-00699-4
96.
RossSAgin-LiebesGLoSZeifmanRJGhazalLBenvilleJet alAcute and sustained reductions in loss of meaning and suicidal ideation following psilocybin-assisted psychotherapy for psychiatric and existential distress in life-threatening cancer.ACS Pharmacol Transl Sci. (2021) 4:553–62. 10.1021/acsptsci.1c00020
97.
JiangWWhellanDJAdamsKFBabyakMABoyleSHWilsonJLet alLong-chain omega-3 fatty acid supplements in depressed heart failure patients.JACC Hear Fail. (2018) 6:833–43. 10.1016/j.jchf.2018.03.011
98.
BergSKHerningMSvendsenJHChristensenAVThygesenLC. The Screen-ICD trial. Screening for anxiety and cognitive therapy intervention for patients with implanted cardioverter defibrillator (ICD): a randomised controlled trial protocol.BMJ Open. (2016) 6:e013186. 10.1136/bmjopen-2016-013186
99.
BergSKHerningMThygesenLCCromhoutPFWagnerMKNielsenKMet alDo patients with ICD who report anxiety symptoms on hospital anxiety and depression scale suffer from anxiety?J Psychosom Res. (2019) 121:100–4. 10.1016/j.jpsychores.2019.03.183
100.
AnnunziataMAMuzzattiBBidoliEFlaibanCBombenFPiccininMet alHospital Anxiety and Depression Scale (HADS) accuracy in cancer patients.Support Care Cancer. (2020) 28:3921–6. 10.1007/s00520-019-05244-8
101.
YohannesAMJunkes-CunhaMSmithJVestboJ. Management of dyspnea and anxiety in chronic obstructive pulmonary disease: a critical review.J Am Med Dir Assoc. (2017) 18:1096.e1–e17. 10.1016/j.jamda.2017.09.007
102.
LeeKPresslerSJTitlerM. Falls in patients with heart failure.J Cardiovasc Nurs. (2016) 31:555–61. 10.1097/JCN.0000000000000292
103.
KawadaKFukudaHKuboTOhtaTIshidaTMorisawaSet alAdded value of anxiolytic benzodiazepines in predictive models on severe delirium in patients with acute decompensated heart failure: a retrospective analysis.PLoS One (2021) 16:e0250372. 10.1371/journal.pone.0250372
104.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM–5). (2022). Available online at: https://www.psychiatry.org/psychiatrists/practice/dsm(accessed February 23, 2022).
105.
NICE. Delirium: Prevention, Diagnosis and Management.London: National Institute for Health and Care Excellence (2019).
106.
InouyeSK. Clarifying confusion: the confusion assessment method.Ann Intern Med. (1990) 113:941. 10.7326/0003-4819-113-12-941
107.
BushSHTierneySLawlorPG. Clinical assessment and management of delirium in the palliative care setting.Drugs. (2017) 77:1623–43. 10.1007/s40265-017-0804-3
108.
CampbellAMAxonDRMartinJRSlackMKMollonLLeeJK. Melatonin for the prevention of postoperative delirium in older adults: a systematic review and meta-analysis.BMC Geriatr. (2019) 19:272. 10.1186/s12877-019-1297-6
109.
LawlorPGGagnonBManciniILPereiraJLHansonJSuarez-AlmazorMEet alOccurrence, causes, and outcome of delirium in patients with advanced cancer.Arch Intern Med. (2000) 160:786. 10.1001/archinte.160.6.786
110.
AgarMBushSH. Delirium at the end of life.Med Clin North Am. (2020) 104:491–501. 10.1016/j.mcna.2020.01.006
111.
Spiritual Care. European Association for Palliative Care, EAPC. (2022). Available online at: https://www.eapcnet.eu/eapc-groups/reference/spiritual-care/(accessed April 11, 2022).
112.
TadwalkarRUdeojiDUWeinerRJAvestruzFLLaChanceDPhanAet alThe beneficial role of spiritual counseling in heart failure patients.J Relig Health. (2014) 53:1575–85. 10.1007/s10943-014-9853-z
113.
ParkCLAldwinCMChounSGeorgeLSureshDPBlissD. Spiritual peace predicts 5-year mortality in congestive heart failure patients.Health Psychol. (2016) 35:203–10. 10.1037/hea0000271
114.
RietjensJACSudoreRLConnollyMvan DeldenJJDrickamerMADrogerMet alDefinition and recommendations for advance care planning: an international consensus supported by the European Association for Palliative Care.Lancet Oncol. (2017) 18:e543–51. 10.1016/S1470-2045(17)30582-X
115.
SudoreRLLumHDYouJJHansonLCMeierDEPantilatSZet alDefining advance care planning for adults: a consensus definition from a multidisciplinary delphi panel.J Pain Symptom Manage. (2017) 53:821–832.e1. 10.1016/j.jpainsymman.2016.12.331
116.
VanderhaeghenBBossuytIMentenJRoberP. What is good advance care planning according to hospitalized palliative patients and their families? An explorative study.J Palliat Care. (2020) 35:236–42. 10.1177/0825859720938583
117.
DingfieldLEKayserJB. Integrating advance care planning into practice.Chest. (2017) 151:1387–93. 10.1016/j.chest.2017.02.024
118.
FahnerJCBeundersAJMvan der HeideARietjensJACVanderschurenMMvan DeldenJJMet alInterventions guiding advance care planning conversations: a systematic review.J Am Med Dir Assoc. (2019) 20:227–48. 10.1016/j.jamda.2018.09.014
119.
JimenezGTanWSVirkAKLowCKCarJHoAHY. Overview of systematic reviews of advance care planning: summary of evidence and global lessons.J Pain Symptom Manage. (2018) 56:436–459.e25. 10.1016/j.jpainsymman.2018.05.016
120.
HickmanSESabatinoCPMossAHNesterJW. The POLST (Physician Orders for Life-Sustaining Treatment) paradigm to improve end-of-life care: potential state legal barriers to implementation.J Law Med Ethics. (2008) 36:119–40. 10.1111/J.1748-720X.2008.00242.X
121.
TsohJPeisahCNarumotoJWongpakaranNWongpakaranTO’NeillNet alComparisons of guardianship laws and surrogate decision-making practices in China, Japan, Thailand and Australia: a review by the Asia Consortium, International Psychogeriatric Association (IPA) capacity taskforce.Int Psychogeriatr. (2015) 27:1029–37. 10.1017/S104161021400266X
122.
VeshiDNeitzkeG. Advance directives in some western european countries: a legal and ethical comparison between Spain, France, England, and Germany.Eur J Health Law. (2015) 22:321–45. 10.1163/15718093-12341368
123.
SzeroczyńskaMCzarkowskiMKrajnikMKrajewskiRPawłowskiLAdamczykAet alInstitution of the health care agent in polish legislation: position of the polish working group on end-of-life ethics.Pol Arch Med Wewn. (2016) 126:313–20. 10.20452/PAMW.3405
124.
DunlaySMSwetzKMMuellerPSRogerVL. Advance directives in community patients with heart failure.Circ Cardiovasc Qual Outcomes. (2012) 5:283–9. 10.1161/CIRCOUTCOMES.112.966036
125.
PakESJonesCAMatherPJ. Ethical challenges in care of patients on mechanical circulatory support at end-of-life.Curr Hear Fail Rep. (2020) 17:153–60. 10.1007/S11897-020-00460-4
126.
HjorthNEHaugenDFSchaufelMA. Advance care planning in life-threatening pulmonary disease: a focus group study.ERJ Open Res. (2018) 4:00101–2017. 10.1183/23120541.00101-2017
127.
KitakataHKohnoTKohsakaSFujisawaDNakanoNShiraishiYet alPreferences on advance care planning and end-of-life care in patients hospitalized for heart failure.ESC Hear Fail. (2021) 8:5102–11. 10.1002/ehf2.13578
128.
TavaresNJarrettNHuntKWilkinsonT. Palliative and end-of-life care conversations in COPD: a systematic literature review.ERJ Open Res. (2017) 3:00068–2016. 10.1183/23120541.00068-2016
129.
MeehanESweeneyCFoleyTLehaneEBurgess KelleherAHallyRMet alAdvance care planning in COPD: guidance development for healthcare professionals.BMJ Support Palliat Care. (2019) [Online ahead of print]. 10.1136/bmjspcare-2019-002002
130.
SioutaNvan BeekKPrestonNHasselaarJHughesSPayneSet alTowards integration of palliative care in patients with chronic heart failure and chronic obstructive pulmonary disease: a systematic literature review of European guidelines and pathways.BMC Palliat Care. (2016) 15:18. 10.1186/s12904-016-0089-4
131.
RadbruchLPayneS, EAPC Board of Directors. White Paper on standards and norms for hospice and palliative care in Europe: part 2 Recommendations from the European Association for Palliative Care.Eur J Palliat Care. (2010) 17: 22–33.
132.
Integrated Palliative Care. The Archived Website of the InSup-C Project.Nijmegen: Radboud University Medical Center (2016).
133.
Voice of Volunteering. The EAPC madrid charter on volunteering in hospice and palliative care.Palliat Med Pract. (2018) 12:127–8.
134.
RogersJGPatelCBMentzRJGrangerBBSteinhauserKEFiuzatMet alPalliative care in heart failure.J Am Coll Cardiol. (2017) 70:331–41. 10.1016/j.jacc.2017.05.030
135.
RoseEKO’ConnorJ. Addressing advance care planning in patients with COPD.Chest. (2021) 161:676–83. 10.1016/J.CHEST.2021.10.037
136.
CentenoCSitteTde LimaLAlsirafySBrueraECallawayMet alWhite paper for global palliative care advocacy: recommendations from a PAL-LIFE expert advisory group of the pontifical academy for life, Vatican city.J Palliat Med. (2018) 21:1389–97. 10.1089/jpm.2018.0248
137.
RadbruchLde LimaLLohmannDGwytherEPayneS. The prague charter: urging governments to relieve suffering and ensure the right to palliative care.Palliat Med. (2013) 27:101–2. 10.1177/0269216312473058
138.
RadbruchLPayneS, Board of Directors of the EACP. White Paper on standards and norms for hospice andpalliative care in Europe: part 1 Recommendations from the European Association for Palliative Care.Eur J Palliat Care. (2009) 16:278–89.
139.
HigginsonIJFinlayIGoodwinDMCookAMHoodKEdwardsAGet alDo hospital-based palliative teams improve care for patients or families at the end of life?J Pain Symptom Manage. (2002) 23:96–106. 10.1016/S0885-3924(01)00406-7
140.
PitcherDSoarJHoggKLinkerNChapmanSBeattieJMet alCardiovascular implanted electronic devices in people towards the end of life, during cardiopulmonary resuscitation and after death: guidance from the Resuscitation Council (UK), British Cardiovascular Society and National Council for Palliative Care.Heart. (2016) 102:A1–17. 10.1136/heartjnl-2016-309721
141.
SchleiferJWShenW-K. Implantable cardioverter-defibrillator implantation, continuation, and deactivation in elderly patients.Curr Geriatr Rep. (2017) 6:279–89. 10.1007/s13670-017-0226-9
142.
Kinch WesterdahlASjöblomJMattiassonA-CRosenqvistMFrykmanV. Implantable cardioverter-defibrillator therapy before death.Circulation. (2014) 129:422–9. 10.1161/CIRCULATIONAHA.113.002648
143.
Gonzalez-JaramilloVGuyerJLuethiNSobanskiPZbindenRRodriguezEet alValidation of the German version of the needs assessment tool: progressive disease-heart failure.Health Qual Life Outcomes. (2021) 19:214. 10.1186/S12955-021-01817-6
144.
KramerDBKesselheimASBrockDWMaiselWH. Ethical and legal views of physicians regarding deactivation of cardiac implantable electrical devices: a quantitative assessment.Hear Rhythm. (2010) 7:1537–42. 10.1016/j.hrthm.2010.07.018
Summary
Keywords
heart failure, chronic heart failure, chronic obstructive pulmonary disease, palliative care, advanced care planning
Citation
Kowalczys A, Bohdan M, Wilkowska A, Pawłowska I, Pawłowski L, Janowiak P, Jassem E, Lelonek M, Gruchała M and Sobański P (2022) Comprehensive care for people living with heart failure and chronic obstructive pulmonary disease—Integration of palliative care with disease-specific care: From guidelines to practice. Front. Cardiovasc. Med. 9:895495. doi: 10.3389/fcvm.2022.895495
Received
13 March 2022
Accepted
22 August 2022
Published
27 September 2022
Volume
9 - 2022
Edited by
Vasundhara Kain, University of South Florida, United States
Reviewed by
Naoki Ishimori, Hokkaido University, Japan; Liliane Lins-Kusterer, Universidade Federal da Bahia, Brazil
Updates
Copyright
© 2022 Kowalczys, Bohdan, Wilkowska, Pawłowska, Pawłowski, Janowiak, Jassem, Lelonek, Gruchała and Sobański.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Kowalczys, anna.roz@gumed.edu.pl
†These authors have contributed equally to this work
This article was submitted to Heart Failure and Transplantation, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.