- 1Division of Renal Medicine, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden
- 2Department of Nephrology, Lund University, Skåne University Hospital, Malmö, Sweden
- 3Department of Forensic Genetics and Forensic Toxicology, National Board of Forensic Medicine, Linköping, Sweden
- 4Division of Endocrinology and Metabolism, Department of Medicine III, Medical University of Vienna, Vienna, Austria
- 5Clinical and Experimental Neuroscience, Institutes for Aging Research and Bio-Health Research of Murcia, School of Medicine, University of Murcia, Murcia, Spain
- 6Faculty of Nursing, University of Alberta, Edmonton, AB, Canada
- 7Cardiovascular and Stroke Strategic Clinical Network, Alberta Health Services, Edmonton, AB, Canada
- 8Department of Translational Medicine, University of Ferrara, Ferrara, Italy
- 9University Center for Studies on Gender Medicine, University of Ferrara, Ferrara, Italy
- 10Division of Clinical Epidemiology, Research Institute of McGill University Health Centre, McGill University, Montreal, QC, Canada
Cardio-pulmonary diseases, which were once regarded as a man's illness, have been one of the leading causes of morbidity and mortality for both men and women in many countries in recent years. Both gender and sex influence the functional and structural changes in the human body and therefore play an important role in disease clinical manifestation, treatment choice, and/or response to treatment and prognosis of health outcomes. The gender dimension integrates sex and gender analysis in health sciences and medical research, however, it is still relatively overlooked suggesting the need for empowerment in the medical research community. Latest advances in the field of cardiovascular research have provided supportive evidence that the application of biological variables of sex has led to the understanding that heart disease in females may have different pathophysiology compared to males, particularly in younger adults. It has also resulted in new diagnostic techniques and a better understanding of symptomatology, while gender analysis has informed more appropriate risk stratification and prevention strategies. The existing knowledge in the pulmonary field shows the higher prevalence of pulmonary disorders among females, however, the role of gender as a socio-cultural construct has yet to be explored for the implementation of targeted interventions. The purpose of this review is to introduce the concept of gender dimension and its importance for the cardiopulmonary continuum with a focus on shared pathophysiology and disease presentation in addition to interrelation with chronic kidney disease. The review presents basic knowledge of what gender dimension means, and the application of sex and gender aspects in cardiovascular medicine with a specific focus on early pulmonary development, pulmonary hypertension, and chronic obstructive pulmonary disease (COPD). Early vascular aging and inflammation have been presented as a potential pathophysiological link, with further interactions between the cardiopulmonary continuum and chronic kidney disease. Finally, implications for potential future research have been provided to increase the impact of gender dimension on research excellence that would add value to everybody, foster toward precision medicine and ultimately improve human health.
Introduction
Non-communicable diseases (NCD) are a growing concern worldwide due to the numerous medical conditions associated with several slow and progressive chronic illnesses involving various organ systems of the body. The term NCD has been expanded to include a broad variety of health issues which include cardiac, pulmonary, renal, gastrointestinal, hepatic, endocrine, and neurologic disorders (1). The human disease process is a complex interaction of different organ systems that has been termed organ-system or organ-body crosstalk (2). This cross-talk between organs contributes to metabolic balance or imbalance such that acute or chronic dysregulations in one organ system may result in dysfunction or failure in another organ (3). With the increasing global burden of cardio-respiratory disorders in terms of mortality and morbidity, a greater effort should thus be made to further expand our knowledge of the interconnections between the vascular system, heart, lung, and kidney. By envisioning a common soil to describe the interconnections between these organ systems, researchers and clinicians worldwide can ensure a comprehensive approach to clinical research endeavors and ultimately enhanced and personalized patient treatment strategies.
This paper, will attempt to address the sex and gender aspects in connection to their relevance in the implementation of contemporary day research. Indeed, as indicated by the European Commission, integrating sex and gender aspects in the research and innovation content presents the concept of gender dimension, which is related to one of the goals of enhancing gender equality balance. The gender dimension takes into account the biological characteristics of both females and males referred to as sex, while the gender domain includes the evolving social and cultural features of women, men, and gender-diverse people.
The current understanding of the cardio-pulmonary continuum in relation to cardiovascular disease, pulmonary hypertension (PH), chronic obstructive pulmonary disease (COPD) in respect to the overlapping factors observed in inflammaging, early vascular aging process as well as cardio-pulmonary-renal interaction will be discussed in relation to research findings according to sex and gender perspectives.
Importance of sex as a biological variable
Sex pertains to the biological, physiological, and physical differences in genes, hormones, reproductive organs, body fat composition, etc. in males and females. Sex and gender influence disease susceptibility, clinical manifestation, illness severity, treatment choice, therapy responsiveness, health-seeking habits, and prognosis (4). Biological sex and gender factors interact, such that sex for example can influence health by affecting behavior. Estrogen, for example, tends to accelerate the metabolism of nicotine in women, and sex-related changes in nicotine metabolism might alter smoking habits including responsiveness to treatment (5, 6). Differences in metabolism may explain why males respond better to nicotine replacement therapy like patches and gum than women, and men tend to be more susceptible to nicotine's addictive pharmacologic effects (6).
Another female-specific factor that has an impact on health, is the fluctuation of female hormones during the menstrual cycle which can affect mood symptoms (7) and physical performance (8). Pregnancy disorders such as preeclampsia or gestational diabetes, as well as the physical structure of a woman's circulatory system being smaller and more elaborately branched than males, impose a greater risk for future occurrence of cardiovascular disease (CVD) (6, 9). During pregnancy, women are also at an increased risk of bone mass deterioration thus increased bone fragility and osteoporosis (10). The use of hormonal contraceptives in women of reproductive age with migraine may increase the risk for ischemic stroke (11). Women with the polycystic ovarian syndrome (PCOS) have been noted to have emotional disturbance, weight problems, infertility, acne and hirsutism which can thus affect their quality of life (12). Biological sex characteristics and gendered socio-cultural and socioeconomic factors are interlinked and can influence health outcomes in different illness spectra. In ischemic heart illness, a sex difference in clinical presentation is also observed. For example, pain located between the shoulder blades, shortness of breath, nausea, or vomiting, are more frequent in women. Men are more likely to have main vessel obstructive coronary artery disease, while women have coronary microvascular dysfunction leading to the progression of myocardial ischemia (13).
In preclinical and clinical research, an understanding of sex as a biological variable is vital for our comprehension of the basic processes that contribute to disease probability and resilience, especially in circumstances when variations in sex are recognized (14). In experimental studies, including samples from both males and females is important, and sex-disaggregated analysis should be constantly performed. Insufficient inclusion of male or female cell lines and study animals in experiments, disregarding sex-specific data analysis, may contribute to poor reproducibility of biomedical preclinical studies (15). The difference between males and females in respect to illness prevalence, manifestation, and even treatment response may lie in the foundation of genetic influence which begins at conception. The fundamental chromosomal difference of embryos carrying the XX or XY chromosomes results in the variations of the molecular building blocks making up the male and female cells. The idea of asymmetric inheritance is thought to be the source of females' evolutionary advantage in various animal species. For starters, males have only one copy of the X-chromosome, but females have two copies, so any potentially harmful alleles may be obscured by normal alleles on the second X chromosome (16). Another theory based on asymmetric inheritance proposes that there is the optimal selection for compatibility with the female nuclear genome because mitochondria are maternally inherited, and as a result, any hostile male genes would be deleterious to male life expectancy (16). However, biparental inheritance of mitochondrial DNA has been reported in exceptional cases (17), this paper has been highly debated but still worth consideration.
Gender domain
In studies involving human populations, it is also necessary to consider the socio-cultural complexity consisting of gender relations, gender identity, and gender roles. Both sex and gender impact health and disease, and it is therefore important to appreciate both aspects and their interaction. In daily clinical practice, the physician's principal goal is to provide the best and equal health care to all patients, regardless of ethnicity, background, or socioeconomic level. Every patient is unique, and physicians must recognize and value this uniqueness to achieve the best possible health outcomes. Individuals' health experiences will be determined by their biological sex and gender, which confers cultural and social factors that may operate simultaneously. Being a man or a woman varies between cultures, which is why gender is a distinct factor in health. At times, clinicians, scientists, and service consumers are not always conscious of the consequences of sex or gender perspective in clinical practice or academic research.
The gender dimension rationale is integrating sex and gender analysis which enhances the quality of research. It is applying sex-disaggregated analysis in various interdisciplinary areas, and the perspective on gender equilibrium is relevant as it leads to achieving balance and improving the quality of research, innovation, creativity, and excellence (Figure 1). By incorporating sex and gender analysis into experimental design, the scientific discovery would advance in developing better treatment for various diseases and increase social equality and benefits for human health (18). Gender dimension in research denotes also that gender is included in the study design and taken into consideration throughout the research process without necessarily being the primary focus of analysis (19), but nevertheless, be applied wherever practical and relevant.
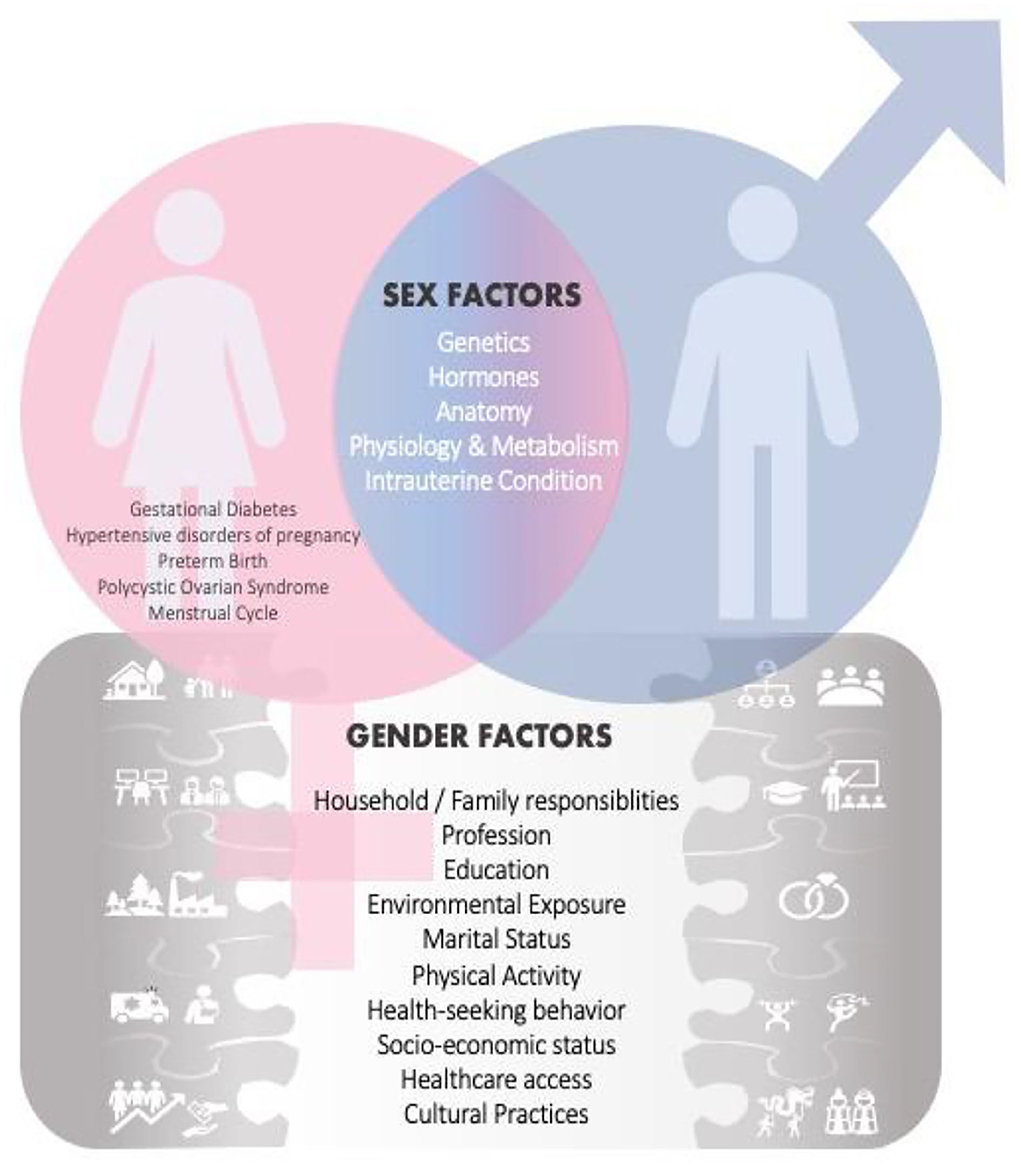
Figure 1. Sex and Gender Factors influence research and health outcomes. In addition to the usual shared sex factors among males and females, there are also sex related factors [i.e., gestational diabetes, hypertensive disorders of pregnancy, polycystic ovarian syndrome (PCOS), menstrual cycle] specific only to women. This female-specific factor places a heavier burden on certain diseases in women. Gender factors are also introduced, and those may have an effect on health and disease outcomes.
Males and females are similar in many ways, but they differ significantly in terms of biology and behavior. Gender, a social construct described only in humans refers to attitudes and behaviors associated with what is considered masculine or feminine. In different societies, men, women, boys, and girls are defined by socially and culturally formed norms, values, roles, and expectations (19, 20). There are four domains that comprise the concept of gender (Figure 2), (a) gender roles are societal behavioral norms that influence people's everyday activities, expectations, and experiences such as nutrition, smoking, stress, and physical exercise and activity have an impact on health and disease vulnerability; (b) gender identity refers to how a person identifies themselves, and how that perception influences feelings and behaviors; (c) gender relations denotes how people treat or interrelate with one other according to their assigned gender; (d) institutionalized gender refers to the power or control of the balance between men and women in society's political and social institutions, and establishes societal norms defining, perpetuating, and justifying the disparities in women's and men's potentials and opportunities (13, 20).
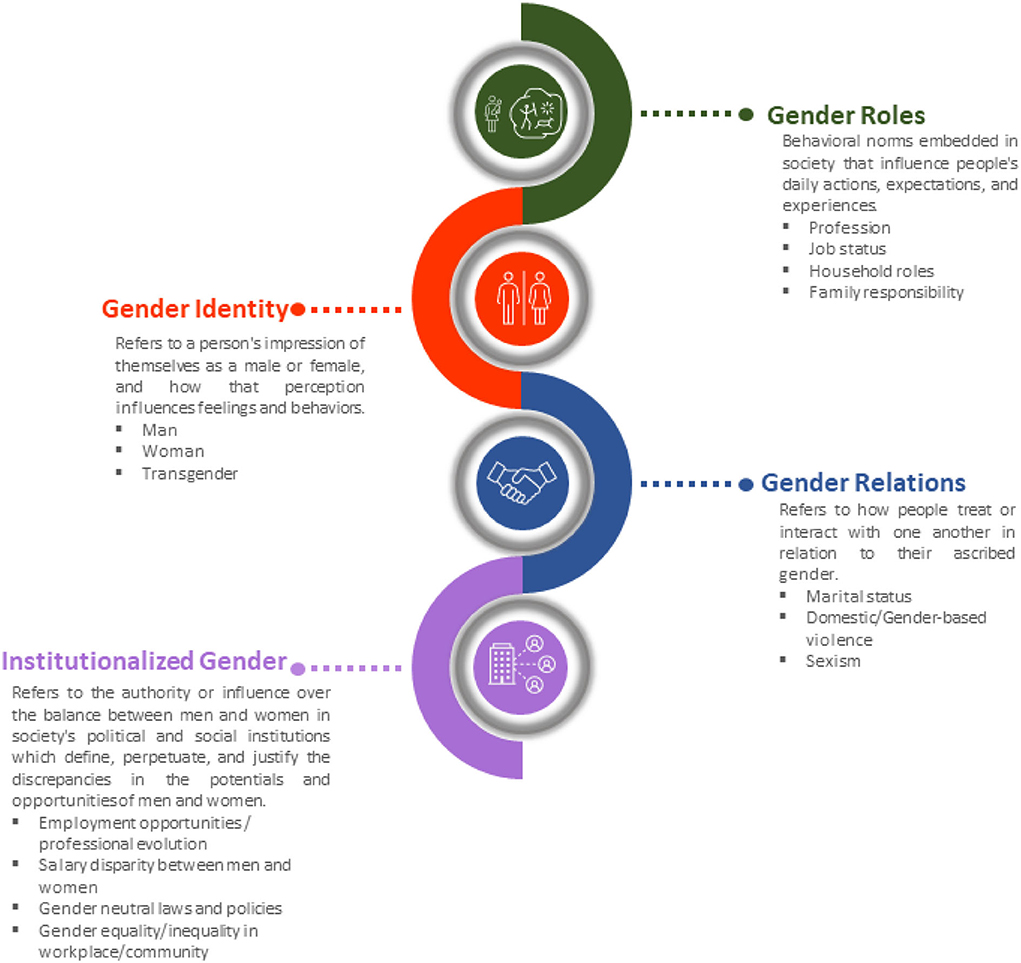
Figure 2. The four gender domain constructs: gender role, gender identity, gender relations, institutionalized gender refers to a person's characteristics or qualities in relation to culturally determined norms.
Gender factors such as stress, environmental exposures, lifestyle choices, or poor nutrition can affect biological parameters and thus health (21). The gender-related characteristics of men and women may have a distinct effect on health, such that bias can exist in the diagnosis and treatment of disease (22, 23). Being regarded as male or female may also elicit distinct reactions from physicians and other medical personnel who may formulate gender-biased diagnoses and management (24). Furthermore, the outcome of health may also be influenced by differences in coping techniques (25) and help-seeking behavior among men and women (26).
Gender medicine advocates for all sexes' needs for better health and healthcare. Thus, doctors and scientists may need to focus on certain areas of medicine or study where data on men and women are limited (21). Sex and gender differences must be addressed, especially in health as a “one-size fits all” approach is less beneficial when it comes to the concept, promotion, and benefit of individualized healthcare measures.
As part of the Gender Outcomes International Group: to Further Well-being Development (GOING-FWD) initiative, is a transatlantic collaboration of a multidisciplinary team of researchers from Canada, Sweden, Austria, Spain, and Italy created a standard five-step technique (4) to identify gender-related characteristics and examine their relevance to research outcomes (Figure 3). This multistep approach aims to aid researchers in identifying gender-related factors and how to create a research plan on the core dataset when different variables in datasets are merged. In addition, the GOING-FWD consortium is also an advocate for promoting a patient-partner organization in research, wherein interactions are built on cooperation and mutual respect in all aspects of information dissemination, offering valuable inputs and participation in public forum meetings and other relevant research activities.
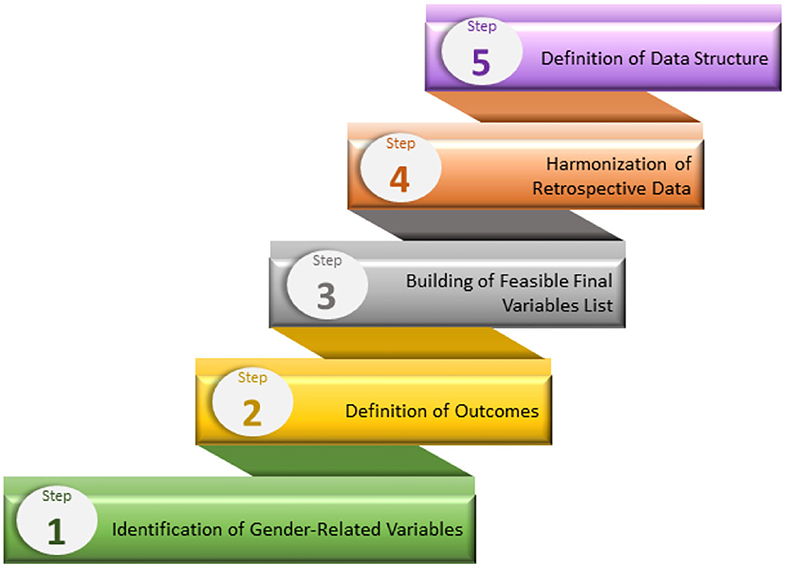
Figure 3. The GOING-FWD multistep approach for identifying and including gender characteristics in research.
The cardio-pulmonary continuum under sex and gender lenses
Sex and gender in cardiovascular disease
Effects of sex
Cardiovascular disease (CVD), a commonly age-associated ailment oftentimes accompanied by metabolic disturbances, is the leading cause of morbidity and mortality in men and women in industrialized nations (27, 28). The sex differences in the increased risk, pathogenesis, and progression of CVD have been attributed to the sex-related changes in sex hormones, vascular biology, and other cellular changes (29). Males tend to develop CVD at a younger age than women due to the cardioprotective role of estrogen in females (28). This sex difference exists until menopause when the incidence of CVD in females quickly approaches and surpasses that of males correlating with the decline of female sex hormones that are associated with menopause (30). Women after menopause have a 10-fold increase in CVD risk, compared to a 4.6-fold increase in men when comparing the same ages groups (30).
Many symptoms associated with CVD include aberrant vascular control, blood pressure dysregulation, poor tissue perfusion, capillary edema, and inflammatory cell recruitment that could lead to hypertension and atherosclerosis (31). Significant elements in CVD progression are vascular inflammation and endothelial dysfunction (31, 32). Estrogens are important regulators of vasomotor tone. The endothelial cell membrane contains binding sites for estradiol leading to the activation of endothelial nitric oxide synthase (eNOS), which promotes the production of nitric oxide (NO) that causes vascular relaxation and endothelial-dependent vasodilation (28). The decrease of estradiol vasodilatory effects along with unopposed vasoconstriction are responsible for the higher deterioration of endothelial function and related increase in CVD in women postmenopause (28, 33, 34).
While traditional and non-traditional risk factors play a role in the CVD process in both men and women, there are additional female-specific risk factors during the reproductive age that may increase a woman's risk for future CVD, in conditions such as hypertensive disorders of pregnancy, gestational diabetes, premature birth, miscarriage, premature menopause, and PCOS (35). Although premenopausal women have been reported to have lower rates of CVD than age-matched males, inflammation-induced endothelial dysfunction is protected by the female sex because of estrogen resulting in faster resolution of inflammation (31). However, premenopausal women with excess androgens, such as those with PCOS, demonstrate higher levels of inflammatory molecules, develop mild hypertension, oxidative stress, and NF-kB activation, and exhibit features of metabolic syndrome (28, 36).
Understanding the consequences of cardiovascular pathology among males and females is important, nevertheless, transgender persons must also be taken into account, as it has been noted that gender-affirming therapy (GHT) used to align an individual's traits with their gender identity may increase the risk of CVD (37). According to a review by Connelly et al. (37), GHT may increase the risk of CVD due to (a) elevation of BP in both transgender males and females, (b) possible alteration of the thrombotic phenotype, especially in transgender females, or (c) modification in cardiometabolic parameters. Further research is required to comprehend the long-term consequence of GHT and the influence of GHT on CVD risks and occurrences. Regarding the transgender and CVD aspect, it is also essential to recognize that the distribution of the influence of sex and gender on CVD risk in this population may not be easily distinguishable at times owing to the complex and combined impacts of biological, social and cultural aspects.
Effects of gender
Gender may interact with biological sex and other social variables such as race, socio-economic status, or behavior to alter cardiovascular health. Indeed the way sex and gender intersect with determinants—such as our income, education, social and physical environments, genetics, and personal health practices—will create varied experiences and health outcomes across populations. (21). A recent study examining the effect of long work hour exposure found that working ≥10 h daily for >10 years was significantly associated with ischemic heart disease (IHD) in men (38). Fadel et al. (38) explained that long work hours may encourage harmful habits associated with an increased risk of CVD, such as smoking, poor nutrition, and inactivity. Another is that chronic psychosocial stress or job shifts may result in activating the autonomic nervous or immune system which may increase the risk for CVD (38). According to Lee et al. (39) sleep deprivation also increases the risk of CVD by reducing productivity and immune function which can lead to social concerns with friends and family. The same group further noted that lack of sleep has been associated with depressive symptoms in older women and death in older men. Sleep quality, duration, and CVD seem to be influenced by sex, and women have been noted to suffer more from insomnia than males due to hormonal changes affecting the circadian rhythm (39).
The quality of preventive care received by a population from its community also has an impact on CVD status. As shown by population-based research in Canada, female immigrants had equivalent or higher overall quality of primary CVD preventive care than males (40). This finding was explained by the possibility that all residents including immigrants are included in the adequate hospital coverage for necessary medical treatments, disease screening, and physician prescriptions. The same group also observed that compared to males, immigrant females from Africa compared to those who came from Caribbean countries used more lipid-lowering and antihyperglycemic drugs. This may in part be due to differences in health-seeking behaviors, customary healthcare practices, and possible inequities in the healthcare system from the country of origin. The social vulnerability has a negative impact on health such that those who are at an increased socioeconomic disadvantage have elevated CVD morbidity and mortality (41).
High education has been linked to lower mortality from all causes, CVD, and coronary heart disease in both sexes (42). Additionally, individuals at high risk for CVD, marital status is independently associated with CVD outcomes, with increased mortality in the unmarried group (43). Age-adjusted mortality from all causes is greater among single people than among married people, both in men and women (42). Furthermore, it has been discovered that individuals who are divorced or separated have a higher risk of myocardial infarction (MI) and cardiovascular mortality than those who are married (43–45). Having a spouse may enhance (modulate) men's lifestyle and treatment compliance thus improving CVD outcome (45). A population-based study in Finland with 302,885 male and female participants showed that men living alone (unmarried, divorced, or widowed) had a higher fatality rate for MI. While in women, cohabitation (living with a non-marital partner) but not marriage was related to a higher fatality (46). This suggests that there is a protective effect on cardiac health associated with living with a marital partner owing to the emotional support from partners. Though cohabitation may offer the same support mechanism as marriage, it may also present greater break-up rates, implying less dedication, stability, and permanence than marriage (46). In addition, a comprehensive understanding of the impacts of marriage or cohabitation on the outcomes of CVD must likewise be considered among same-sex couples, but this may be hampered by a lack of data or studies on same-sex partners and marital quality.
Concerns about CVD must also be addressed in light of the unique medical needs of transgender people (those who were born with a sex that differs from their current gender identification). CVD is one of the health risk factors that transgender people suffer significant variances in prevalence and incidence as well as disparities in outcomes (47). Due to an increase in social pressures, health disparities, and low socioeconomic levels, the transgender group has been reported to have a greater prevalence of behavioral and CVD risk factors compared to the cisgender population (48). The Behavioral Risk Factor Surveillance System (BRFSS) study reported that transgender men have increased odds of having MI when compared to cisgender women and cisgender men, respectively, whereas transgender women had increased odds of having MI when compared to cisgender women but were not significantly different from cisgender men (48). Transgender individuals' increased risk of MI could also be attributed to socioeconomic factors such as depression, stress, unemployment, and poverty, which are often the difficulties faced by the transgender population (49). Numerous transgender individuals are marginalized by stigma, discrimination, violence, and poor health. Accessing proper health care, whether specific to their gender requirements or of a more general character, is frequently difficult for them (50). Challenges to inadequate medical training and clinical guidelines may hamper meeting the unique health care needs of transgender people (47). The existing lack of gender identification in data and minimal research on the transgender community may also limit the application of research results to transgender people.
Atherosclerosis
Atherosclerosis is the leading cause of CVD and is a progressive inflammatory disease of the vessel wall, which starts a multitude of biochemical and histologic events that are characterized by cholesterol deposition in the arterial wall resulting in atherosclerotic plaque development and subsequent rupture (51). Atherosclerosis plaque development and rupture may cause CVD events including myocardial infarction and stroke (52). Damaged endothelium permits cholesterol-containing low density lipoprotein (LDL) particles to accumulate in the artery wall, triggering an inflammatory reaction that fails to resolve (53). Inside the lipid necrotic core of the atheroma, monocytes develop into macrophages, which subsequently become foam cells. Inflammasomes in macrophages generate interleukin-1 (IL-1), interleukin-18 (IL-18), and other pro-inflammatory cytokines, which are chemotactic toward other inflammatory cells, including B-cells and T-cells, which are significant drivers of atherosclerosis (53). And, because women may have distinct sex-related conditions, such as PCOS, preeclampsia, gestational diabetes, and menopause, they are more likely to develop atherosclerotic cardiovascular disease (9). Carotid artery intima-media thickening (IMT), which is an established marker of atherosclerosis is noted to be increased in women with PCOS, and the PCOS-IMT connection is partly related to associated risk factors and weight and fat distribution (9).
Effects of sex
Sex differences have been observed in terms of atherosclerotic plaque development and pathophysiology. Phenotypically, in coronary artery disease, it has been observed that females develop atherosclerotic plaques that are less prone to rupture and of a smaller size when compared to males (54). In addition, in carotid artery disease, an age-specific difference was observed in the likelihood of intraplaque hemorrhage (IPH) being more prevalent in males within younger age groups. However, this sex difference in IPH dissipates in increasing age groups indicative of post-menopause (55). Whilst sex differences in plaque phenotype and size are evident, inflammation within the atherosclerotic plaque may be more relevant in assessing sex-specific mechanisms correlating with CVD mortality (56). Atherosclerotic plaques from males have been found to have significantly increased levels of inflammatory infiltrates, i.e., macrophages, when compared to plaques from age-matched females (57, 58). Interestingly, inflammation-related metabolic activity, assessed by 18F-fluorodeoxyglucose (FDG) uptake during positive emission tomography, has been observed at significantly increased levels in the carotid and femoral arteries of males in a cohort of patients undergoing asymptomatic carotid artery screening compared to females, and comparing the metabolically active subjects men were two-fold more likely to have carotid stenosis (59). This indicates that females have less macrophage infiltration and a lower inflammatory contribution within atherosclerotic plaques compared to males, which may be independent of clinical manifestation and CVD risk profile.
Effects of gender
Lifestyle habit such as smoking is a substantial risk factor for the development of atherosclerosis, and female smokers are more likely than male smokers to have heart attack (9). Passive smoking or exposure to noxious environmental tobacco smoke may exacerbate risk factors in metabolic syndrome, vascular inflammation, thrombus formation and atherosclerosis (9). According to Amiri et al. (60), there is a dose-dependent connection between CVD and smoking, therefore males who smoke more than women are at higher risk of CVD. However, smoking behaviors and reporting discrepancies between men and women may vary by culture and country. There may be a difference in reporting of smoking habits between men and women in more conservative nations like the Middle East, where smoking is seen as a social shame for women (60).
Low socioeconomic status has been linked to an increased prevalence of atherosclerosis as determined by common carotid IMT and carotid stenosis (61). According to the Swedish study (61), women with less education had a higher risk of carotid stenosis than women with more education, this pattern was weakly observed in men. It was also noted that women in manual occupations also had a higher risk of carotid stenosis than women in non-manual occupations, whereas males had no such risk. Furthermore, there was no association between IMT and occupational status in men, whereas there was in women (61). Rosvall's data suggest that both sexes have atherosclerosis and that there may be sex variations in the mechanisms linking socioeconomic position to atherosclerosis.
Gender factors also influence the acceptability and efficacy of invasive and/or non-invasive cardiovascular diagnostics and medical intervention as strategies to address cardiovascular disease (62). Based on an Australian study, gender differences exist in lifestyle adherence among patients who underwent percutaneous coronary intervention for coronary artery disease (63). Authors noted that women are less likely to be physically active, less likely to attend cardiac rehabilitation, and are less likely to comply with statin therapy compared to men possibly due to a higher occurrence of myalgia among females. Disparities in health behavior, in addition to sociocultural norms imposed by society, contribute to behaviors that may steer the development of premature vascular aging phenotype (please see later chapter) include: risk-taking behaviors such as smoking, poor nutrition, lack of physical activity, stress, noxious environmental factors, socioeconomic disadvantage, and handicap or stigma (62).
Sex and gender in lung diseases
Pulmonary hypertension
Definition of pulmonary hypertension (PH) is based on increased mean pulmonary arterial pressure of 20 mmHg or greater. PH is a multifactorial condition and is classified into 5 different subgroups: group 1—pulmonary arterial hypertension (PAH), group 2—PH as a result of left-sided heart disease, group 3—a chronic lung disease caused PH, group 4—chronic thromboembolic PH, group 5—multifactorial/ unclear etiology PH (64). Here we will mainly discuss sex differences in PAH (group 1).
PAH represents pulmonary artery remodeling and dysfunction, and increased pulmonary vascular resistance (64, 65) with predominance among women (65). Increased pulmonary arterial pressure in PAH is polyetiological and includes chronic inflammation, oxidative stress, hormonal disturbances, endothelial dysfunction, and phenotypic switching of pulmonary vascular cells (66). The negative outcome of PAH is right heart failure.
Effects of sex
The knowledge that women are more prone to develop PAH is based on data acquired from different national PAH registries (67–73). Meanwhile men with PAH have worse clinical outcomes for 2 and 5 years after PAH diagnosis compared with women as REVEAL (the Registry to Evaluate Early and Long-term PAH Disease Management) reports, and especially at the age of 60 and greater (74). The role of sex hormones in the prevalence and pathogenesis of PAH has been explicitly defined by previous works (65, 75). Indeed, women compared with men who caries BMPR2 (bone morphogenetic protein receptor type II) mutation—inherited as an autosomal dominant trait—have at least two-fold higher risk to get PAH (76, 77). Though the risk for all-cause mortality has been shown to be sex independent (77). Beyond the role of BMPR2 mutation, reduced expression of normal BMPR2 allele could lead to higher PAH predisposition in women (78). Therefore, the interplay between estrogen and BMPR2 signaling (79) advocates for female predominance in PAH manifestation.
It's important to understand that estrogens have a rather contradicting effect on vasoconstriction, vascular smooth cell proliferation and fibrosis—a hallmark of PAH. Estrogens trigger vasodilatation of pulmonary vasculature through NO pathway and have an anti-inflammatory effect on lung tissue (80). Furthermore, the cardioprotective properties of estrogens explain the lower incidence of right heart failure in females (80), however, the possible adverse estrogen influence on pulmonary vasculature in early life must be elucidated by future studies while increasing evidence that environmental chemicals and pollutants with estrogenic features may affect intrauterine development and trigger disease development later in life (81).
Some subgroups of PAH have worse survival, e.g., Porto-pulmonary hypertension (POPH) as compared to idiopathic PAH. POPH is the third most common cause of PAH and is related to liver diseases (82).
Effects of gender
Pulmonary Hypertension Association Registry (83) reported that patients suffering from POPH (51% females) as compared to idiopathic PAH (75% females) have lower socioeconomic status because they are more likely to be unemployed, have lower education and lower-income further indirectly suggesting that gender-sensitive environment could have a significant impact on the disease outcome. In support, Wu et al. (84) showed that a lower socioeconomic score determines a worse outcome and higher risk for mortality in idiopathic PAH, where the study population was up to 80% consisting of females. Hopefully, further investigations would address more in detail the importance of sex and gender aspects in those analyses.
Chronic obstructive pulmonary disease
Effects of sex
Chronic obstructive pulmonary disease (COPD) is recognized as a disease with a chronic inflammatory state that is characterized by progressive infiltration of innate and adaptive inflammatory leukocytes in the lungs. In addition, C-reactive protein (CRP), necrosis factor-alpha (TNFα), interleukin-6 (IL-6), pulmonary and activation-regulated chemokine (PARC), vascular endothelial growth factor (VEGF), and tumor matrix metalloproteinase 9 (MMP-9), have been linked to the development of COPD (85). COPD patients have higher vascular leakage than healthy patients, VEGF regulates the formation of new arteries, and men have higher levels of VEGF and IL-6 than women in COPD patients (85). COPD is reported to be of two primary types: chronic asthmatic bronchitis which has more female preponderance, and emphysema which is more frequently noted in males (86). Women are less likely than males to die after being admitted to the hospital for COPD, and if they would need artificial ventilation, their survival rate is lower than those of males (87). Due to cyclical hormonal swings, women may perceive variation of symptoms, even noting diminished respiratory function differently than men thus may experience and report shortness of breath more than males, which is a symptom of impaired ventilatory performance and a COPD and emphysema clinical feature (87).
Effects of gender
COPD was once thought to be a condition primarily affecting elderly males but sex difference in COPD exists with regards to susceptibility, smoking status and exposure, presence of co-morbidities, and prevailing underdiagnosis in women (88). In recent years, the incidence of COPD in women has risen, thus narrowing the gap between men and women (89). Possible reasons include women's increased tobacco use and exposure, longer life expectancy among females, and occupational and non-occupational exposure (85, 90). Socio-economic and socio-cultural factors may contribute to the sex discrepancy in COPD leading to gender differences in respiratory symptom awareness, presentation, and diagnosis. For example, Townsend et al. (91) reported that almost 80% of those diagnosed with COPD were non-smoker females which may relate to second-hand exposure in women from smoker male partners.
In addition, the greater risk for hospitalization of women with COPD may likewise be due to women's increased tendencies to seek medical help compared to men (91). Increasing patient awareness of COPD also improves self-management efficiency. One research has reported (92) that females lacked knowledge on how to execute a good action plan and what to do in an emergency. Insufficient patient information may lead therefore to higher exacerbations and emergency visits.
Sex and gender-aspects during preconception, early life factors and lung development
Both the intrinsic and extrinsic environmental factors during pre-conception, intrauterine, and postnatal stages play a role in respiratory health's vulnerability to future potential adult respiratory disorders (93, 94). Suboptimal environmental factors during a key time of lung development may affect a person's respiratory health due to altered lung structure, and function, as well as maladaptive responses to hazardous agents which may result in impaired lung function in adulthood (95).
For example, cigarette smoke exposure, preterm birth and nutrition, birth weight, and early exposure to viruses and allergens are all possible environmental variables that can affect the likelihood of respiratory illness later in life (95). Prenatal and perinatal exposure to air pollutants can result in various unfavorable outcomes like preterm birth and low birth weight (LBW) (96). Developmental defects in lung function early in life are associated with future lung diseases and impaired respiratory function that can extend to adolescence and adulthood (96). Males and females may experience varied impacts to their respiratory health from poor intrauterine conditions and exposure to hazardous substances according to an extensive review by Harding's group (93). Maternal smoking impairs the lung function of offspring due to the influence of nicotine that can rapidly cross the placenta and affect the pulmonary fibroblasts' role in alveolarization (93). Other smoking-related impacts include permanent reduction of energy metabolism in the lungs, as well as increased primitive alveoli size and volume, which reduces gas exchange (93). Airway resistance is higher in female fetuses exposed to maternal smoking, this smoke exposure “masculinizes” the female fetal airways, causing lower airflow rates comparable to males (97). Cigarette smoke exposure during pregnancy and as well as during breastfeeding can increase airway hyperresponsiveness and neutrophil penetration into the lungs, as well as alter the immunological response in the offspring, according to studies in mice (98).
Male and female differences in lung development, susceptibility, pathogenesis, morbidity, and mortality to certain respiratory disorders may be influenced by genetic, hormonal, and environmental variables before and during the neonatal period (99). Androgen and estrogen receptors are expressed in the human lung (99, 100), and male lung maturation typically lags throughout the fetal period due to the delayed production of surfactants secondary to the inhibitory effect of Mullerian-inhibiting hormone from male fetal testes-derived Sertoli cells (101). Compared to females, surfactant production is stimulated by estrogen that favors lung maturation (85, 99, 102). Also, a more abundant surfactant in the lungs of female neonates encourages patency of tiny airways and airspaces contributing to better flow rate and lesser airway resistance (91, 99). Airway development in girls is proportionate to lung parenchymal growth compared to boys wherein airway maturity trails behind parenchymal development causing a disparity between airway and lung size which increases airway resistance (97, 101).
Boys and girls have the same number of alveoli per unit area and alveolar volume, but boys have larger lungs than girls (91, 99). Males have lower forced expiratory flows than females due to more smooth muscle and broader airways, and airway resistance grows quicker in males than in females as they mature (97). The difference in pubertal patterns of lung development has been observed between sexes, as noted in asthma there is male predominance until puberty after which there is a switch resulting in higher incidence in females during adulthood (91, 101).
Preterm-born neonates when compared to those born at term are associated with increased respiratory problems, higher frequency of airflow blockage, gas entrapment, and impaired lung gas exchange (85, 103). Preterm birth has been linked with increased respiratory symptoms notably respiratory disease syndrome (RDS) and bronchopulmonary dysplasia (BPD) which explains the significant share of the morbidity and mortality among prematurely born infants (104). RDS is characterized by surfactant deficiency (105), while BPD is exhibited by a halted alveolarization process and abnormal development in pulmonary vasculature in preterm infants (85).
Sex disparities in morbidity and mortality between prematurely born male and female neonates exist, with males having a significant disadvantage compared to females born at comparable gestational age (106). Male babies are more likely than female neonates to have a higher risk of developing RDS and dying from it (99). Females have a lower incidence of RDS than males regardless of race or ethnicity (107). Before the introduction of glucocorticoids and surfactant therapy, RDS was a major cause of neonatal mortality among male neonates (85). The type of prenatal glucocorticoid (dexamethasone vs. betamethasone) used to prevent RDS was reported to exhibit a sex-specific result (108). Despite the use of mechanical ventilators, prenatal corticosteroids, and surfactants to minimize morbidity and death in very low birth weight newborns, there are still variations in outcomes that favor females compared to males (109). Prematurely born infants who develop BPD can develop long-term consequences such as recurrent or severe respiratory problems (110), pulmonary arterial hypertension (85), and poor neurodevelopmental outcomes particularly in low-birth-weight male neonates (111).
As mentioned earlier, one factor for the increased risk for RDS associated with males can be accounted from the hormonal influence during fetal lung development. Aside from early surfactant production, another advantage of preterm females is the stronger Na+ transport driven alveolar fluid clearance (AFC) due to higher mRNA expression of epithelial Na+ transport channel (ENaC) and Na, K-ATPase, which may be facilitated by improved sensitivity to female sex hormones through higher estrogen receptor-β (ER-β) and progesterone receptor (PR) expression (112).
Sex hormones regulate lung function maturation, with androgens acting as inhibitors while estrogens as stimulators. The placenta produces estrogen, whereas the fetal testes secrete testosterone (113). Estrogens' positive regulatory function in lung development is particularly prominent during the latter stages of lung development namely the saccular to the alveolar stage (102). Increased estrogen concentrations in female fetuses boost estrogen receptor activation, which upregulates genes that influence morphological, functional lung development, and surfactant homeostasis (102). Animal studies using male and female mice have shown that there is direct transcriptional regulation of granulocyte-macrophage colony-stimulating factor (GM-CSF) and platelet-derived growth factor-alpha (PDGF-α) by estrogens via ER-β receptor (114). Patrone's findings suggest a biological explanation for the observed sex disparities in adult lung alveolar shape and identify estrogen receptors in lung development and homeostasis, although further studies are needed to understand lung development and estrogen receptors and their role after birth. Estrogen receptors ER-α and ER-β are necessary for alveoli maintenance according to studies in female mice (115). The expression of ER-β protein in the human bronchial epithelial cells is double that of ER-α (116), and in knockout ER-α and ER-β mice, both types of ER are essential for the alveolar unit development (117). ER-α facilitates appropriate lung differentiation, which results in normal alveoli per surface area numbers, while ER-β, regulates the growth of the extracellular matrix resulting in the lung's flexible tissue recoil pressure (117). Further studies are important to understand the complexity of the hormone environment interaction with hormone receptors after birth and whether it has an impact on the development of lung disease.
Inflammageing, a common pathway for understanding sex and gender based aspects of CVD and lung diseases
Effects of sex
The human body typically has its core physiological defense mechanisms against foreign pathogens or tissue injuries. However, the complicated reaction triggered by a shift from normal physiological body function to non-resolving pathological processes such as the continuous and protracted presence of inflammation can be harmful to health (118). The term “inflamm-aging” or” inflammageing” is a term coined by Claudio Franceschi, it is defined as an age-related disorder characterized by increased levels of inflammatory markers in the circulation such as IL-1β, IL-6, TNF-α (119) and hsCRP (high-sensitivity CRP) (120). These pro-inflammatory mediators in the blood increase the risk for chronic illnesses, disability, frailty, and death (53, 121). Activation of the NLRP3 (NOD-, LRR- and pyrin domain-containing protein 3) inflammasome, oxidative stress induced by malfunctioning mitochondria, dysregulated immune cells, and chronic infections are all possible pathways of inflammageing (53). Inflammageing has also been linked to other conditions such as CKD (122, 123), atherosclerosis, diabetes, cancer, depression, dementia (118) as well as lung changes and intestinal dysbiosis that occur with advancing age (119). Inflammation has been reported to be a most probable causative risk factor as well as a pathogenic process for CVD such that inflammation influences CVD, multimorbidity, and frailty by suppressing growth factors, increasing catabolism, and disrupting homeostatic signaling (53).
One factor that may play an important role in inflammation is the sex-associated hormonal change resulting in the observed disparities in inflammation between males and females. In SARS-CoV-2 infection, females are protected against viral infections due to X-chromosomes and sex hormones which regulate adaptive and innate immunity (124). COVID-19 is associated with a greater risk of serious illness and death in men as women are more protected due to estrogen which increases innate and humoral immune responses thus lessening the severity and fatality of COVID-19 (124). A previous study (120) found that women had a stronger association than males in IL-6 and hsCRP hs-CRP levels and that the 51–60 age range appeared to be a pivotal point for this increase. Women's estrogen-mediated regulation of inflammatory biomarkers changes with age, and there are enhanced ER-β expression and pro-inflammatory effects of estrogen that occurs with aging (32). Novella et al. (32) reported that endogenous estrogen depletion contributes to postmenopausal women's age-related escalation in CVD risk. Thus, the cardiovascular protective benefits of estrogen medication propose that the benefits of estrogen in preventing CVD may emerge only when therapy commences before the detrimental effects of aging on the vasculature are evident (32). After age 65, disparities between sexes also become more pronounced, with men exhibiting more innate and pro-inflammatory activity and less adaptive immunity (125). Bupp et al. (126) noted that the aging process is associated with changes in the immune system's function, that inflammaging may cause dysregulation of innate immune cells further adding to inflammatory processes. Men and women have quite distinct immune systems, and that aging may confer a sex-specific effect on the immune system's cellular composition and function which in turn affect age- and sex-associated changes in the frequency and process of autoimmunity, cancer, vaccination efficiency and cancer immunotherapy (126). Immunosenescence is marked by weakened immune capabilities, and inflammaging results in a shortage of anti-inflammatory mediators (127). Thus, immunosenescence and inflammaging when combined play a critical role in the decay of the immune system to combat diseases such as SARS-CoV-2 infection thus resulting in severe COVID-19 in elderly patients (127).
Effects of gender
Aside from molecular or cellular processes, gendered variables such as human behavior can also potentially confer a protective or damaging effect on health. For example, physical exercise or active lifestyle behavior has been reported to influence inflammation by inducing an anti-inflammatory effect by reducing toll-like receptor (TLR) expression on monocytes and changing the phenotype of macrophages in adipose tissue (128). In the young population, gender differences are observed in exercise behaviors, motivations for exercise, and quality of life (129). Inflammaging increases the susceptibility to poor prognosis due to the dysfunctional systemic immune response, which is manifested as a cytokine storm in SARS-CoV-2 infection. According to Padilha de Lima et al. (130), in the COVID-19 situation, strategies that promote an overall healthy lifestyle should be emphasized for both sexes. These strategies include healthier dietary modification, increased physical activity, as decreased physical activity has a detrimental effect on muscle quality and poor nutritional habits which can accelerate immunosenescence and inflammation (130), and in the longer term accelerate arterial stiffening (131).
Women reported having a higher quality of life than men, and they tend to exercise more for weight loss and toning, while men exercised more for fun (129). However, for older people, the pattern and level of physical activity may be limited by the risk or occurrence of injury particularly falling. In a study by Stahl and Albert (132) older females were more prone to being frequent fallers in comparison to older males but did not seem to have an effect on their participation in leisure activities or domestic work, while older males who experienced frequent falls resulted in decreased recreational/ leisure activities and household labor. The authors explained that women participated in relatively little leisure activity which may account for why falling had no effect on their activity pattern. Furthermore, the difference between males and females may be attributed to their gender role expectations for household chores such as females performing more caregiving activities (132). Physical activity is a significant and controllable risk factor for severe COVID-19 outcomes, according to Sallis et al. (133). When compared to individuals who were persistently inactive, those who engaged in some physical activity had a lower chance of hospitalization and mortality, indicating that any level of physical activity in both women and men might help minimize the risk of dying from COVID-19 (133).
Early vascular aging: Sex and gender
Early vascular aging (EVA) is a progressing concept that undiagnosed arterial stiffness that may contribute to an earlier onset of CVD and subsequently an increase in mortality (134). The combination of various damaging stressors to the artery wall, as well as the time spent exposed, might accelerate the normal vascular aging process. EVA is characterized by an increased peripheral vascular resistance due to a decreased elasticity of the arteries (52). Another pathophysiological link to CVD is endothelial dysfunction which could further impair vasodilation resulting in arteriosclerosis phenotype. Endothelial cells in the microvasculature play a role in immunological responses, inflammation, thrombosis, remodeling, and arteriolar vessel tone modulation (135). Vascular endothelial cell damage associated with atherosclerosis releases antigens that initiate and prolong inflammatory responses, and senescent cells are commonly seen in considerable amounts in atherosclerotic plaques (53).
EVA related endothelial senescence and dysfunction may cause pulmonary artery remodeling (136). Evidence from animal studies shows that senescent cells in lung vasculature fuel the progression of pulmonary artery hypertension (137), however the precise outcome of those investigations could not be related to a specific sex, as the sex-disaggregated analysis was not performed. Additionally to these findings, premature microvascular damage due to endothelial dysfunction could contribute to the interrelationship between pulmonary function/diseases and higher risk for CVD (138, 139). According to an investigation of patients with COPD (71% male study group), patients with COPD have a decreased vasodilatory response when compared to controls, which correlates to increased cardiovascular morbidity (139).
The appearance of EVA has been identified in different lung conditions. Increased cardio-ankle vascular index (CAVI), a proxy of arterial stiffness, was associated with pulmonary age in hypertensive patients (140). The other study observed borderline differences in magnetic resonance acquired systemic pulse wave velocity in COPD as compared to healthy controls (141). The early signs of crosstalk between systemic vasculature and lung function are already prevalent in childhood as seen in an investigation of 8-year old children—a study population composed of 50% females—observed a higher carotid augmentation index as well as lower lung volumes (142).
As for the sex differences, at least two studies (143, 144) have shown that males in comparison to females have stiffer arteries, assessed by carotid-femoral pulse wave velocity (cfPWV), which could help to predict the loss of lung function in male subjects. The Swedish CArdioPulmonary bioImage Study (SCAPIS) (145) observed the reduction in PWV by 1-SD increase in Forced Expiratory Volume 1 sec (FEV1) and diffusing capacity for carbon monoxide only in males but not in females from healthy populations.
Thus, resolving the sex and gender issues in early vascular aging and pulmonary health may provide novel targets for modifying vascular remodeling and developing arterial destiffening as well as targets for slowing lung fibrosis.
In a female-specific condition such as PCOS and in the presence of lower lung function (146) development of EVA could be linked with increased CVD later in life (147). This supports the suggestion that metabolic syndrome is encompassed by a combination of factors responsible for EVA such as obesity, hyperglycemia, dyslipidemia, and hypertension (148, 149) further presenting a higher female predominance (150). Aside from that, other mechanisms, e.g., increased plasma visfatin concentrations, have been shown to be associated with CVD in PCOS as compared to healthy obese females (151).
Lungs and vascular tree share similar structural properties, though, the interaction between systemic and pulmonary vascular dysfunction are not fully understood (141). The theory that inflammaging drives these two processes in COPD seems to be plausible, and telomere attrition may be a missing detail in this puzzle. Indeed, telomere shortening has been linked to cellular senescence and CVD (152, 153) as well as pulmonary diseases (154). Telomeres are positioned at the ends of the chromosomes which are essential for chromosomal maintenance. When cell divides telomeres replicate incompletely and subsequently cause DNA damage. Telomere lengths have been noted to be sex-specific, with males being born with shorter telomeres than females (155). Males are programmed to have faster telomere shortening as compared to women (156). Given that women live longer than males, telomeres have been proposed as the causative explanation for the disparity (155). However, telomere attrition in both sexes in combination with other factors, such as oxidative stress or inflammation, triggers cellular senescence (152). The combination of the above-mentioned cellular processes, including variables such as lifestyle choices, physical exercise, and food, might alter vascular repair mechanisms, and likewise affect telomere length (157). Reduced cell damage and endothelial dysfunction, and longer telomeres have been linked to a healthy lifestyle which includes eating a diet rich in fruits and vegetables, particularly the Mediterranean diet, exercising regularly, maintaining a low body mass, and avoiding smoking which are important factors in lowering the risk of cardiovascular disease (158). In a Spanish population study, it was observed that adherence to the Mediterranean diet or consumption of a healthy diet is influenced by occupational socioeconomic strata, particularly younger women who are in danger of social exclusion (159). The authors however could not conclude whether the greater cost of particular Mediterranean diet staples such as fish and fruit is the major reason for the disparity in adherence. Further studies are warranted indeed to assess those gender-sensitive aspects in the aging process.
Cardio-pulmonary-renal interactions
Organ cross-talk is a concept used to describe and explain various health conditions. It helps to understand complex pathogenetic pathways behind multi-organ failure. The interplay between heart and kidney has been recently clarified and classified into 5 different subtypes mainly stressing bidirectional interactions (160). Interestingly, the fifth type defines simultaneous acute dysfunction of both heart and kidney in systemic conditions such as sepsis. As for pulmonary-renal syndrome, it often represents a respiratory failure—diffuse alveolar hemorrhage or acute respiratory distress syndrome (ARDS)—in combination with renal failure—glomerulonephritis or acute kidney injury without present hematuria (161). The most common causes that account for 68.5–95% of all cases are anti-glomerular basement membrane (anti-GBM) disease antineutrophil cytoplasm antibody (ANCA)—associated vasculitis (162, 163). Both diseases have well-defined sex differences in prevalence and outcomes (164) with clear predominance among men.
Unfortunately, the Cardio-Pulmonary-Renal interaction is not well investigated considering its burden of mortality. This is also valid for the absence of indications of sex and gender perspectives. In the light of extended pandemics with SARS-CoV-2 infection, the importance of ARDS in the Cardio-Pulmonary-Renal continuum is worth commenting. Indeed, ARDS is a pro-inflammatory condition (165) associated with critical illness due to determining alterations in the alveolar-capillary barrier with following need for mechanical ventilation. Therefore, here some aspects of sex differences could be of interest. For example; a large observational study (166) showed that shorter women were more likely to receive lower tidal ventilation and that the female sex was associated with higher mortality in severe ARDS. The iatrogenic trauma to the lungs affects pulmonary microcirculation that furtherly leads to increased right ventricular afterload and impaired cardiac output. Finally, it may result in reduced renal flow and acute ischemic kidney failure (167).
The sex differences in immune response (168) might play an important role in different ARDS presentations alongside insufficient ventilation regimes and admission to the intensive care unit. The pathogenesis of ARDS involves mechanisms related to innate and adaptive immunity (169) that consequently will affect target organs such as the heart and kidney. Alveolar epithelial cells, macrophages, and dendritic cells are the first barriers responding to diverse triggers and resulting in cellular response and damage (169), most probably in a sex-specific way, due supportive evidence of sex-specific phenotypes of these cells as seen in animal and human studies (170). For example, females may have a thicker airway wall most likely due to greater protein processing and its phosphorylation in the endoplasmic reticulum in addition to active protein folding (170).
Phelps et al. (171) investigated the mechanisms behind changes in alveolar macrophage activity in the presence or absence of Surfactant Protein-A (SP-A). The SP-A modulates host-defense lung function, including its potential to act as an opsonin assisting in the clearance of different pathogens by alveolar macrophages. Proteomic analysis demonstrated an increase in numerous alveolar macrophage protein spots, suggesting that SP-A may have unique features in females vs. males, since several of the discovered proteins affected estrogen activity (171). Under infectious conditions, SP-A enhances alveolar macrophages immune response which may explain why males could have higher incidence of bacterial pneumonia (172).
At this stage it is also worth noticing the importance of interaction between PH and CKD, and if sex per se could have an effect on both conditions' development. Indeed, PH and CKD could share underlying causes such as autoimmune connective tissue diseases seen predominantly in females and immunodeficiency virus-associated disease in males (173). However, neither a retrospective study of Chinese subjects (174) nor a report from the USA (175) had suggested the predominance of one sex for increased risk in PH in CKD. An additional study reported that in CKD stages 3 to 5 there is a 1.4-fold higher risk for PH (50% male subjects) (176), further indicating increased mortality from PH in males with CKD. Yet, further studies with identification of sex and gender aspects in the disease development and organ interaction are of utmost importance for the combination of PH with CKD.
There are a few literature reports addressing the relationship between COPD and CKD, as it is perceived that CVD is the most important co-morbid that exists in COPD patients. However, further attention must also be placed on the link between between CKD and COPD. The prevalence of CKD and COPD is observed to increase with age and are likewise correlated with atherosclerotic disease, and CKD prevalence in COPD patients ranges from 20–30% (177). CKD and COPD patients share common risk factors such as hypertensive disorders and diabetes (178). According to the German COPD and Systemic Consequences—Comorbidities Network (COSYCONET), poor outcomes may be mediated by CKD's impact on clinical symptoms, functional status, and capacity for exercise. CKD and COPD's share common systemic symptoms like malnutrition, muscle wasting, anemia, osteoporosis, and cardiovascular disease, which all have a negative impact on patients (179). COPD which has been defined as the pulmonary component of systemic endothelial disease wherein the inflammageing process concurrently impact several organs, resulting in a condition of multicomorbidity, without a clear evidence which illness occurred first (180). The inflammatory condition and response observed in both CKD and COPD may exacerbate the symptoms in a bidirectional process. Studies on the repercussions of COPD on CKD or vice versa are still limited. One report showed that females (178) with COPD had 93% higher risk of developing CKD as compared to 43% increased risk in males. Another study has also supported the higher incidence of CKD in females with COPD (181). However, the incidence of end-stage renal disease (182) in subjects with COPD is similar between the sexes. Further studies are warranted to understand both sex or gender-related influence on both conditions and their interaction.
An increased number of dendritic cells and the inflammatory response has been identified in female mice model of bronchial asthma (183) which may explain possible underlying causes for the higher prevalence of asthma in women. Hereby inflammation per se (184) as well as pro-apoptotic pathways, may contribute to progression toward Cardio-Pulmonary-Renal syndrome in a sex-specific manner, however gendered-specific variables and their impact have yet to be explored in future studies. Bronchial asthma also has an effect on other chronic disorders, particularly those affecting the cardiovascular and glucose metabolic systems. In a Chinese population study, individuals with bronchial asthma (60% female participants) may be at a higher risk of developing CKD, that there is a 9.6% prevalence of CKD in the group with chronic asthma than the group without persistent asthma (185).
According to an extensive review by Hussain-Syed's group (167), understanding the intricate biological transmission and response mechanism between the heart, lung and kidney relationship can serve to better investigate the pathophysiology, epidemiology, diagnosis and therapeutic management of various diseases. A dysfunction in each organ may grow gradually until a cumulative effect ensues or individuals with disease in one organ may die from complications of the other organ before the complete collapse of the first organ (167). Each defective organ is capable of initiating and perpetuating mutual harm through various sex-specific cell signaling feedback or physiologic mechanisms while numerous bouts of acute or chronic decompensatory processes may result in corresponding end-organ-damage. Gender specific variables on the other hand may impact the development of a particular organ disease concurrently, and health outcomes are strongly influenced by human behavior which varies according to societal roles, identity, and other cultural interactions.
Future directions—targets for intervensions
Biomedical scientists, researchers, and clinicians must have a good grasp of the concepts of sex and gender in research and be able to address all of the important biological and social elements to obtain a scientifically sound result that is valid for human health. There is an important need to further raise awareness in conducting sex and gender-balanced approach in scientific study, and likewise in the use of the “sex” and “gender” terminologies appropriately.
Discussed in some papers are guides (4, 20, 186, 187) for researchers to aid in integrating sex and gender in various phases of research. Incorporating sex and gender into research and innovation contributes to the advancement of scientific knowledge. Consideration of the needs of men, women, boys, girls, and other gender varied factors can improve the quality of disseminated information as well as increase the societal applicability of research findings (188). For example, the approach utilized by Pelletier and the group (189), is to use gender-related scoring in research to produce a femininity or masculinity score that is used to evaluate the recurrence of an acute coronary syndrome.
A sex-informed framework especially in the study of humans considers sex variations in anatomy and physiology, as well as insights on critical times of prenatal, childhood, pubertal development, reproductive window and aging (187). When designing research, it is also critical to evaluate if the investigator has gender preconceptions that may influence the selection of study participants (190). The current situation in the European Union, National Institutes of Health in the United States as well as the Canadian Institutes of Health Research seems to be optimistic as a growing number of professional organizations, funding agencies, philanthropic groups, editors of peer-reviewed journals, academic institutions, ethics boards, health care systems and industry are refining policies and making concerted efforts to integrate sex and gender into biomedical research (191).
Gender study in the laboratory using cell and animal models is somewhat more complicated due to the normally controlled environmental conditions and other practical limitations, and gender is intrinsically a cultural construct in human society that cannot be simply equated with social dynamics among non-human subjects (186). Therefore, it is equally important to disclose and justify the use of single-sex experimental models in research as it may prompt a discourse about the ramifications of certain developments or study outcomes.
The close interconnected nature and many overlapping components addressing the issues of sex and gender pose a challenge for researchers to address them separately. At times, a variety of gender-related variables may inhibit or exacerbate inherent biological inequalities in health (4). The interaction between sex and gender may influence the probability of acquiring certain illnesses, symptoms and prognosis, and response to treatment (4). Currently, several articles (4, 192, 193) have been published to provide a practical guide for researchers on how to integrate sex and gender into their studies.
Resolving the sex and gender issues in methodologies or research designs may provide novel insights that are crucial for the generation of new ideas or hypotheses, as well as increasing the relevance and practical application of results and their reproducibility. It cannot be overemphasized that research must move toward sex and gender-balanced research. Equal representation of both sexes including the social constructs that accompany any study population of interest would further enhance the advancement of targeted precision medicine in improving human health and corresponding therapeutic management. Efficient analysis of disparities between males and females would pave the way to further identify gaps in information and comprehend how and why the differences occur in order to create effective strategies to remedy them.
Finally in respect to human diagnosis and therapy, it is known that males and females have a propensity for increased burden in certain disease areas. However, in the majority of cases, males and females tend to be subjected to the same medical management as most clinical trials have been skewed toward males, and underpowered in female participants. Therefore, we must recognize the importance and need for a system where both sexes get balanced representations to get equally safe treatments. In the era of modern medicine, clinicians must have a clear and comprehensive awareness of the necessity of providing customized medicine to both sexes (194). As noted by Tannenbaum and Day (194) comparable drug trial findings across age and sex groups from improved research designs, data collecting, selection of biomarkers and analytic methods will undeniably improve risk-benefit profiling for the identification of new age-or sex-specific pharmacological targets.
Author contributions
KK contributed to the conceptualization of this review paper together with LH, AL-C, and LW for review layout. LH, AL-C, LW, and KK drafted the manuscript. AK-W, M-TH, CN, VR, LP, PS, and KK critically reviewed the manuscript. All authors read and approved the final manuscript.
Funding
The review was supported by the Njurfonden (Swedish Renal Foundation) (PS and KK), Hjärt lungfonden (PS), CIMED (PS), ALF (PS), and Swedish Research Council (Vetenskapsrådet 2018-00932 GOING-FWD) (KK).
Conflict of interest
Author PS serves on the Scientific Advisory Boards of Baxter, Astra Zeneca, and REATA.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AFC, Alveolar fluid clearance; ANCA, Antineutrophil cytoplasm antibody; anti-GBM, Anti-glomerular basement membrane; ARDS, Acute respiratory distress syndrome; BMPR2, Bone morphogenetic protein receptor type II; BPD, Bronchopulmonary dysplasia; CAVI, Cardio-ankle vascular index; cfPWV, Carotid-femoral pulse wave velocity; CKD, Chronic Kidney Disease; COPD, Chronic Obstructive Pulmonary Disease; COSYCONET, COPD and Systemic Consequences – Comorbidities Network, ; CRP, C-reactive protein; CVD, Cardiovascular Disease; ER-β, Estrogen receptor-beta; ENaC, Epithelial Na+ transport channel; EVA, Early vascular aging; FDG, Fluorodeoxyglucose; FEV1, Forced Expiratory Volume 1 s; GM-CSF, Granulocyte-macrophage colony-stimulating factor; hsCRP, high-sensitivity CRP; IHD, Ischemic Heart Disease; IL, Interleukin; IPH, Intraplaque hemorrhage; IMT, Intima-media thickening; LBW, Low birth weight; LDL, Low density lipoprotein; MMP-9, Matrix metalloproteinase 9; MI, Myocardial infarction; NCD, Non-communicable disease; NO, Nitric oxide; PCOS, Polycystic ovarian syndrome; PAH, Pulmonary arterial hypertension; PARC, pulmonary and activation-regulated chemokine; PDGF-α, Platelet-derived growth factor-alpha; PH, Pulmonary Hypertension; POPH, Porto-pulmonary hypertension; PR, Progesterone receptor; RDS, Respiratory disease syndrome; REVEAL, Registry to Evaluate Early and Long-term PAH Disease Management; SP-A, Surfactant Protein-A; SCAPIS, Swedish CArdioPulmonary bioImage Study; TLR, Toll-like receptor; TNFα, Tumor ne necrosis factor-alpha; VEGF, Vascular endothelial growth factor.
References
1. Budreviciute A, Damiati S, Sabir DK, Onder K, Schuller-Goetzburg P, Plakys G, et al. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health. (2020) 8:788. doi: 10.3389/fpubh.2020.574111
2. Oishi Y, Manabe I. Organ system crosstalk in cardiometabolic disease in the age of multimorbidity. Front Cardiovasc Med. (2020) 7:64. doi: 10.3389/fcvm.2020.00064
3. Armutcu F. Organ crosstalk: the potent roles of inflammation and fibrotic changes in the course of organ interactions. Inflam Res. (2019) 68:825–39. doi: 10.1007/s00011-019-01271-7
4. Raparelli V, Norris CM, Bender U, Herrero MT, Kautzky-Willer A, Kublickiene K, et al. Identification and inclusion of gender factors in retrospective cohort studies: the GOING-FWD framework. BMJ Glob Health. (2021) 6:e005413. doi: 10.1136/bmjgh-2021-005413
5. Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Therap. (2006) 79:480–8. doi: 10.1016/j.clpt.2006.01.008
6. National Institutes of Health Office of Research on Women's Health. How SEX and GENDER Influence Health and Disease. (2005). Available online at: https://orwh.od.nih.gov/sites/orwh/files/docs/SexGenderInfographic_11x17_508.pdf (accessed March 24, 2022).
7. Lorenz TK, Gesselman AN, Vitzthum VJ. Variance in mood symptoms across menstrual cycles: implications for premenstrual dysphoric disorder. Womens Reprod Health. (2017) 4:77–88. doi: 10.1080/23293691.2017.1326248
8. Julian R, Hecksteden A, Fullagar HHK, Meyer T. The effects of menstrual cycle phase on physical performance in female soccer players. PLoS ONE. (2017) 12:e0173951. doi: 10.1371/journal.pone.0173951
9. Vakhtangadze T, Tak RS, Singh U, Baig MS, Bezsonov E. Gender differences in atherosclerotic vascular disease: from lipids to clinical outcomes. Front Cardiovasc Med. (2021) 8:707889. doi: 10.3389/fcvm.2021.707889
10. Sanz-Salvador L, García-Pérez MA, Tarín JJ, Cano A. Bone metabolic changes during pregnancy: A period of vulnerability to osteoporosis and fracture. Eur J Endocrinol. (2015) 172:R53–65. doi: 10.1530/EJE-14-0424
11. Sacco S, Merki-Feld GS, Ægidius KL, Bitzer J, Canonico M, Kurth T, et al. Hormonal contraceptives and risk of ischemic stroke in women with migraine: a consensus statement from the European Headache Federation (EHF) and the European Society of Contraception and Reproductive Health (ESC). J Headache Pain. (2017) 18:1–20. doi: 10.1186/s10194-017-0815-1
12. Barnard L, Ferriday D, Guenther N, Strauss B, Balen AH, Dye L. Quality of life and psychological well being in polycystic ovary syndrome. Hum Reprod. (2007) 22:2279–86. doi: 10.1093/humrep/dem108
13. Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, et al. Sex and gender: modifiers of health, disease, and medicine. Lancet. (2020) 396:565–82. doi: 10.1016/S0140-6736(20)31561-0
14. Bale TL, Epperson CN. Sex as a biological variable: who, what, when, why, and how. Neuropsychopharmacology. (2016) 42:386–96. doi: 10.1038/npp.2016.215
15. Clayton JA, Collins FS. NIH to balance sex in cell and animal studies. Nature. (2014) 509:282–3. doi: 10.1038/509282a
16. Austad SN, Fischer KE. Sex differences in lifespan. Cell Metab. (2016) 23:1022–33. doi: 10.1016/j.cmet.2016.05.019
17. Luo S, Valencia CA, Zhang J, Lee NC, Slone J, Gui B, et al. Biparental inheritance of mitochondrial DNA in Humans. Proc Natl Acad Sci USA. (2018) 115:13039–44. doi: 10.1073/pnas.1810946115
18. Tannenbaum C, Ellis RP, Eyssel F, Zou J, Schiebinger L. Sex and gender analysis improves science and engineering. Nature. (2019) 575:137–46. doi: 10.1038/s41586-019-1657-6
19. Korsvik TR, Rustad, LM,. What is the Gender Dimension in Research? Case Studies in Interdisciplinary Research. (2018). Available online at: https://kjonnsforskning.no/sites/default/files/what_is_the_gender_dimension_roggkorsvik_kilden_genderresearch.no_.pdf (accessed January 13, 2022).
20. Tadiri CP, Raparelli V, Abrahamowicz M, Kautzy-Willer A, Kublickiene K, Herrero MT, et al. Methods for prospectively incorporating gender into health sciences research. J Clin Epidemiol. (2021) 129:191–7. doi: 10.1016/j.jclinepi.2020.08.018
21. Regitz-Zagrosek V. Sex and gender differences in health. Sci Soc Series Sex Sci EMBO Rep. (2012) 13:596–603. doi: 10.1038/embor.2012.87
22. Beery TA. Gender bias in the diagnosis and treatment of coronary artery disease. Heart Lung. (1995) 24:427–35. doi: 10.1016/S0147-9563(95)80020-4
23. Abuful A, Gidron Y, Henkin Y. Physicians' attitudes toward preventive therapy for coronary artery disease: is there a gender bias? Clin Cardiol. (2005) 28:389. doi: 10.1002/clc.4960280809
24. Regitz-Zagrosek V. Sex and gender differences in heart failure. Int J Heart Failure. (2020) 2:157–81. doi: 10.36628/ijhf.2020.0004
25. Meléndez JC, Mayordomo T, Sancho P, Tomás JM. Coping strategies: gender differences and development throughout life span. Span J Psychol. (2012) 15:1089–98. doi: 10.5209/rev_SJOP.2012.v15.n3.39399
26. Stain N, Unsworth B. Gender bias and help seeking in coronary artery disease. Br J Cardiac Nurs. (2013) 8:382–7. doi: 10.12968/bjca.2013.8.8.382
27. Hyun KK, Millett ERC, Redfern J, Brieger D, Peters SAE, Woodward M. Sex differences in the assessment of cardiovascular risk in primary health care: a systematic review. Heart Lung Circul. (2019) 28:1535–48. doi: 10.1016/j.hlc.2019.04.005
28. Stanhewicz AE, Wenner MM, Stachenfeld NS. Sex differences in endothelial function important to vascular health and overall cardiovascular disease risk across the lifespan. Am J Physiol Heart Circul Physiol. (2018) 315:H1569–88. doi: 10.1152/ajpheart.00396.2018
29. Miller VM. Sex-based differences in vascular function. Women Health. (2010) 6:737–52. doi: 10.2217/WHE.10.53
30. Mathur P, Ostadal B, Romeo F, Mehta JL. Gender-related differences in atherosclerosis. Cardiovasc Drugs Therapy. (2015) 29:319–27. doi: 10.1007/s10557-015-6596-3
31. Rathod KS, Kapil V, Velmurugan S, Khambata RS, Siddique U, Khan S, et al. Accelerated resolution of inflammation underlies sex differences in inflammatory responses in humans. J Clin Invest. (2017) 127:169–82. doi: 10.1172/JCI89429
32. Novella S, Heras M, Hermenegildo C, Dantas AP. Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler Thromb Vasc Biol. (2012) 32:2035–42. doi: 10.1161/ATVBAHA.112.250308
33. Kublickiene K, Svedas E, Landgren BM, Crisby M, Nahar N, Nisell H, et al. Small artery endothelial dysfunction in postmenopausal women: in vitro function, morphology, and modification by estrogen and selective estrogen receptor modulators. J Clin Endocrinol Metabolism. (2005) 90:6113–22. doi: 10.1210/jc.2005-0419
34. Kublickiene K, Fu XD, Svedas E, Landgren BM, Genazzani AR, Simoncini T. Effects in postmenopausal women of estradiol and medroxyprogesterone alone and combined on resistance artery function and endothelial morphology and movement. J Clin Endocrinol Metab. (2008) 93:1874–83. doi: 10.1210/jc.2007-2651
35. Cho L, Davis M, Elgendy I, Epps K, Lindley KJ, Mehta PK, et al. Summary of updated recommendations for primary prevention of cardiovascular disease in women of-the-art review. J Am Coll Cardiol. (2020) 75:2602–18. doi: 10.1016/j.jacc.2020.03.060
36. Marciniak A, Nawrocka-Rutkowska J, Brodowska A, Wiśniewska B, Starczewski A. Cardiovascular system diseases in patients with polycystic ovary syndrome—the role of inflammation process in this pathology and possibility of early diagnosis and prevention. Ann Agric Environ Med. (2016) 23:537–41. doi: 10.5604/12321966.1226842
37. Connelly PJ, Freel EM, Perry C, Ewan J, Touyz RM, Currie G, et al. Gender-affirming hormone therapy, vascular health and cardiovascular disease in transgender adults. Hypertension. (2019) 74:1266–74. doi: 10.1161/HYPERTENSIONAHA.119.13080
38. Fadel M, Li J, Sembajwe G, Gagliardi D, Pico F, Ozguler A, et al. Cumulative exposure to long working hours and occurrence of ischemic heart disease: evidence from the constances cohort at inception. J Am Heart Assoc. 9:e015753. (2020). doi: 10.1161/JAHA.119.015753
39. Lee S, Mu CX, Wallace ML, Andel R, Almeida DM, Buxton OM, et al. Sleep health composites are associated with the risk of heart disease across sex and race. Sci Rep. (2022) 12:2023. doi: 10.1038/s41598-022-05203-0
40. Vyas MV, Yu AYX, Chu A, Fang J, Austin PC, Kapral MK, et al. Immigration status and sex differences in primary cardiovascular disease prevention: a retrospective study of 5 million adults. J Am Heart Assoc. (2021) 10:22635. doi: 10.1161/JAHA.121.022635
41. Barrington DS, Powell-Wiley TM. Social determinants of cardiovascular health in an era of rising social disadvantage. Circul Cardiovasc Qual Outcomes. (2022) 15:e008704. doi: 10.1161/CIRCOUTCOMES.121.008704
42. Malyutina S, Bobak M, Simonova G, Gafarov V, Nikitin Y, Marmot M. Education, marital status, and total and cardiovascular mortality in Novosibirsk, Russia: a prospective cohort study. Ann Epidemiol. (2004) 14:244–9. doi: 10.1016/S1047-2797(03)00133-9
43. Schultz WM, Hayek SS, Tahhan AS, Ko YA, Sandesara P, Awad M, et al. Marital status and outcomes in patients with cardiovascular disease. J Am Heart Assoc. (2017) 6:e005890. doi: 10.1161/JAHA.117.005890
44. Raparelli V, Proietti M, Basili S. Explanatory power of gender relations in cardiovascular outcomes: the missing piece of the puzzle. Heart. (2018) 104:1900–1. doi: 10.1136/heartjnl-2018-313469
45. Wong CW, Kwok CS, Narain A, Gulati M, Mihalidou AS, Wu P, et al. Marital status and risk of cardiovascular diseases: a systematic review and meta-analysis. Heart. (2018) 104:1937–48. doi: 10.1136/heartjnl-2018-313005
46. Kilpi F, Konttinen H, Silventoinen K, Martikainen P. Living arrangements as determinants of myocardial infarction incidence and survival: a prospective register study of over 300,000 Finnish men and women. Social Sci Med. (2015) 133:93–100. doi: 10.1016/j.socscimed.2015.03.054
47. Streed CG, Harfouch O, Marvel F, Blumenthal RS, Martin SS, Mukherjee M. Cardiovascular disease among transgender adults receiving hormone therapy: a narrative review. Ann Intern Med. (2017) 167:256–67. doi: 10.7326/M17-0577
48. Alzahrani T, Nguyen T, Ryan A, Dwairy A, McCaffrey J, Yunus R, et al. Cardiovascular disease risk factors and myocardial infarction in the transgender population. Circul Cardiovasc Qual Outcomes. (2019) 12:e005597. doi: 10.1161/CIRCOUTCOMES.119.005597
49. Chan PS. Invisible gender in medical research. Circul Cardiovasc Qual Outcomes. (2019) 12:e005694. doi: 10.1161/CIRCOUTCOMES.119.005694
50. Winter S, Diamond M, Green J, Karasic D, Reed T, Whittle S, et al. Transgender people: health at the margins of society. Lancet. (2016) 388:390–400. doi: 10.1016/S0140-6736(16)00683-8
51. Alfaddagh A, Martin SS, Leucker TM, Michos ED, Blaha MJ, Lowenstein CJ, et al. Inflammation and cardiovascular disease: From mechanisms to therapeutics. Am J Prev Cardiol. (2020) 4:100130. doi: 10.1016/j.ajpc.2020.100130
52. Nilsson PM. Hemodynamic aging as the consequence of structural changes associated with early vascular aging (EVA). Aging Dis. (2014) 5:109–13. doi: 10.14336/ad.2014.0500109
53. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty HHS Public Access. Nat Rev Cardiol. (2018) 15:505–22. doi: 10.1038/s41569-018-0064-2
54. Lansky AJ, Ng VG, Maehara A, Weisz G, Lerman A, Mintz GS, et al. Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. JACC Cardiovasc Imag. (2012) 5:S62–72. doi: 10.1016/j.jcmg.2012.02.003
55. Singh N, Moody AR, Zhang B, Kaminski I, Kapur K, Chiu S, et al. Age-specific sex differences in magnetic resonance imaging-depicted carotid intraplaque hemorrhage. Stroke. (2017) 48:2129–35. doi: 10.1161/STROKEAHA.117.017877
56. Man JJ, Beckman JA, Jaffe IZ. Sex as a biological variable in atherosclerosis. Circ Res. (2020) 126:1297–319. doi: 10.1161/CIRCRESAHA.120.315930
57. Yuan XM, Ward LJ, Forssell C, Siraj N, Li W. Carotid atheroma from men has significantly higher levels of inflammation and iron metabolism enabled by macrophages. Stroke. (2018) 49:419–25. doi: 10.1161/STROKEAHA.117.018724
58. Hellings WE, Pasterkamp G, Verhoeven BAN, de Kleijn DPV, de Vries JPPM, Seldenrijk KA, et al. Gender-associated differences in plaque phenotype of patients undergoing carotid endarterectomy. J Vasc Surg. (2007) 45:289–96. doi: 10.1016/j.jvs.2006.09.051
59. Kaneko K, Kawasaki T, Masunari S, Yoshida T, Omagari J. Determinants of extraaortic arterial 18F-FDG accumulation in asymptomatic cohorts: sex differences in the association with cardiovascular risk factors and coronary artery stenosis. J Nucl Med. (2013) 54:564–70. doi: 10.2967/jnumed.112.111930
60. Amiri P, Mohammadzadeh-Naziri K, Abbasi B, Cheraghi L, Jalali-Farahani S, Momenan AA, et al. Smoking habits and incidence of cardiovascular diseases in men and women: findings of a 12 year follow up among an urban Eastern-Mediterranean population. BMC Public Health. (2019) 19:1042. doi: 10.1186/s12889-019-7390-0
61. Rosvall M, Östergren PO, Hedblad B, Isacsson SO, Janzon L, Berglund G. Occupational status, educational level, and the prevalence of carotid atherosclerosis in a general population sample of middle-aged Swedish men and women: results from the Malmö Diet and Cancer Study. Am J Epidemiol. (2000) 152:334–46. doi: 10.1093/aje/152.4.334
62. Seeland U, Nemcsik J, Lønnebakken MT, Kublickiene K, Schluchter H, Park C, et al. Sex and gender aspects in vascular ageing—focus on epidemiology, pathophysiology, and outcomes. Heart Lung Circul. (2021) 30:1637–46. doi: 10.1016/j.hlc.2021.07.006
63. Perera S, Aslam A, Stehli J, Kaye D, Layland J, Nicholls SJ, et al. Gender differences in healthy lifestyle adherence following percutaneous coronary intervention for coronary artery disease. Heart Lung Circul. (2021) 30:37–40. doi: 10.1016/j.hlc.2020.06.024
64. Mandras SA, Mehta HS, Vaidya A. Pulmonary hypertension: a brief guide for clinicians. Mayo Clinic Proc. (2020) 95:1978–88. doi: 10.1016/j.mayocp.2020.04.039
65. Morris H, Denver N, Gaw R, Labazi H, Mair K, MacLean MR. Sex differences in pulmonary hypertension. Clin Chest Med. (2021) 42:217–28. doi: 10.1016/j.ccm.2020.10.005
66. Thenappan T, Ormiston ML, Ryan JJ, Archer SL. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ. (2018) 360:j5492. doi: 10.1136/bmj.j5492
67. McGoon MD, Miller DP, REVEAL. a contemporary US pulmonary arterial hypertension registry. Eur Respir Rev. (2012) 21:8–18. doi: 10.1183/09059180.00008211
68. Escribano-Subias P, Blanco I, López-Meseguer M, Lopez-Guarch CJ, Roman A, Morales P, et al. Survival in pulmonary hypertension in Spain: insights from the Spanish registry. Eur Respir J. (2012) 40:596–603. doi: 10.1183/09031936.00101211
69. Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. (2006) 173:1023–30. doi: 10.1164/rccm.200510-1668OC
70. Jansa P, Jarkovsky J, Al-Hiti H, Popelova J, Ambroz D, Zatocil T, et al. Epidemiology and long-term survival of pulmonary arterial hypertension in the Czech Republic: a retrospective analysis of a nationwide registry. BMC Pulm Med. (2014) 14:45. doi: 10.1186/1471-2466-14-45
71. Skride A, Sablinskis K, Lejnieks A, Rudzitis A, Lang I. Characteristics and survival data from Latvian pulmonary hypertension registry: comparison of prospective pulmonary hypertension registries in Europe. Pulm Circ. (2018) 8:1–9. doi: 10.1177/2045894018780521
72. Rådegran G, Kjellström B, Ekmehag B, Larsen F, Rundqvist B, Blomquist SB, et al. Characteristics and survival of adult Swedish PAH and CTEPH patients 2000–2014. Scand Cardiovasc J. (2016) 50:243–50. doi: 10.1080/14017431.2016.1185532
73. Chazova IY, Martynyuk T, Valieva ZS, Gratsianskaya SY, Aleevskaya AM, Zorin A, et al. Clinical and instrumental characteristics of newly diagnosed patients with various forms of pulmonary hypertension according to the Russian National Registry. Biomed Res Int. (2020) 2020:6836973. doi: 10.1155/2020/6836973
74. Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest. (2012) 141:363–73. doi: 10.1378/chest.10-3114
75. Oakland HT, Joseph P. Sex differences and the role of sex hormones in pulmonary hypertension. Clin Chest Med. (2021) 42:457–65. doi: 10.1016/j.ccm.2021.04.006
76. Best DH, Sumner KL, Smith BP, Damjanovich-Colmenares K, Nakayama I, Brown LM, et al. EIF2AK4 mutations in patients diagnosed with pulmonary arterial hypertension. Chest. (2017) 151:821–8. doi: 10.1016/j.chest.2016.11.014
77. Evans JDW, Girerd B, Montani D, Wang XJ, Galiè N, Austin ED, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med. (2016) 4:129–37. doi: 10.1016/S2213-2600(15)00544-5
78. Hamid R, Cogan JD, Hedges LK, Austin E, Phillips JA, Newman JH, et al. Penetrance of pulmonary arterial hypertension is modulated by the expression of normal BMPR2 allele. Hum Mutat. (2009) 30:649–54. doi: 10.1002/humu.20922
79. Austin ED, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ. (2012) 3:6. doi: 10.1186/2042-6410-3-6
80. Martin YN, Pabelick CM. Sex differences in the pulmonary circulation: Implications for pulmonary hypertension. Am J Physiol Heart Circul Physiol. (2014) 306:1253–64. doi: 10.1152/ajpheart.00857.2013
81. Domínguez-López I, Yago-Aragón M, Salas-Huetos A, Tresserra-Rimbau A, Hurtado-Barroso S. Effects of dietary phytoestrogens on hormones throughout a human lifespan: a review. Nutrients. (2020) 12:1–25. doi: 10.3390/nu12082456
82. Krowka MJ, Fallon MB, Kawut SM, Fuhrmann V, Heimbach JK, Ramsay MAE, et al. International liver transplant society practice guidelines: diagnosis and management of hepatopulmonary syndrome and portopulmonary hypertension. Transplantation. (2016) 100:1440–52. doi: 10.1097/TP.0000000000001229
83. DuBrock HM, Burger CD, Bartolome SD, Feldman JP, Ivy DD, Rosenzweig EB, et al. Health disparities and treatment approaches in portopulmonary hypertension and idiopathic pulmonary arterial hypertension: an analysis of the Pulmonary Hypertension Association Registry. Pulm Circ. (2021) 11:1–10. doi: 10.1177/20458940211020913
84. Wu WH, Yang L, Peng FH, Yao J, Zou LL, Liu D, et al. Lower socioeconomic status is associated with worse outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. (2013) 187:303–10. doi: 10.1164/rccm.201207-1290OC
85. Silveyra P, Fuentes N, Rodriguez Bauza DE. Sex and Gender Differences in Lung Disease. Adv Exp Med Biol. (2021) 1304:227. doi: 10.1007/978-3-030-68748-9_14
86. Han MLK, Postma D, Mannino DM, Giardino ND, Buist S, Curtis JL, et al. Gender and chronic obstructive pulmonary disease: Why it matters. Am J Respir Crit Care Med. (2007) 176:1179–84. doi: 10.1164/rccm.200704-553CC
87. Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. (1999) 54:1119–38. doi: 10.1136/thx.54.12.1119
88. Trigueros JA, Riesco JA, Alcázar-Navarrete B, Campuzano A, Pérez J. Clinical features of women with COPD: sex differences in a cross-sectional study in Spain (“The ESPIRAL-ES study”). Int J COPD. (2019) 14:2469–78. doi: 10.2147/COPD.S217921
89. Ntritsos G, Franek J, Belbasis L, Christou MA, Markozannes G, Altman P, et al. Gender-specific estimates of COPD prevalence: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. (2018) 13:1507–14. doi: 10.2147/COPD.S146390
90. Aryal S, Diaz-Guzman E, Mannino DM. Influence of sex on chronic obstructive pulmonary disease risk and treatment outcomes. Int J Chron Obstruct Pulmon Dis. (2014) 9:1145–54. doi: 10.2147/COPD.S54476
91. Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. (2012) 33:1–47. doi: 10.1210/er.2010-0031
92. Wakabayashi R, Motegi T, Kida K. Gender differences in chronic obstructive pulmonary disease using the lung information needs questionnaire. SAGE Open Nurs. (2019) 5:2377960819831462. doi: 10.1177/2377960819831462
93. Harding R, Snibson K, O'reilly M, Maritz G. Early environmental influences on lung development: implications for lung function and respiratory health throughout life. In: Newnham JP, Ross MG, editors. Early Life Origins of Human Health and Disease. Clifton, NJ: Cabi Direct (2009). doi: 10.1159/000221155
94. Azad MB, Moyce BL, Guillemette L, Pascoe CD, Wicklow B, Mcgavock JM, et al. Mini-symposium: maternal diseases effecting the newborn diabetes in pregnancy and lung health in offspring: developmental origins of respiratory disease. Paediatr Respir Rev. (2017) 21:19–26. doi: 10.1016/j.prrv.2016.08.007
95. Carraro S, Scheltema N, Bont L, Baraldi E. Early-life origins of chronic respiratory diseases: understanding and promoting healthy ageing. Eur Respir J. (2014) 44:1682–96. doi: 10.1183/09031936.00084114
96. Kwon EJ, Kim YJ. What is fetal programming? A lifetime health is under the control of in utero health. Obstetr Gynecol Sci. (2017) 60:506. doi: 10.5468/ogs.2017.60.6.506
97. Liptzin DR, Landau LI, Taussig LM. Sex and the lung: observations, hypotheses, and future directions. Pediatr Pulmonol. (2015) 50:1159–69. doi: 10.1002/ppul.23178
98. Janbazacyabar H, van Daal M, Leusink-Muis T, van Ark I, Garssen J, Folkerts G, et al. The effects of maternal smoking on pregnancy and offspring: possible role for EGF? Front Cell Dev Biol. (2021) 9:680902. doi: 10.3389/fcell.2021.680902
99. Carey MA, Card JW, Voltz JW, Arbes SJ, Germolec DR, Korach KS, et al. It's all about sex: male-female differences in lung development and disease. Trends Endocrinol Metab. (2007) 18:308. doi: 10.1016/j.tem.2007.08.003
100. Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y. Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab. (2010) 21:729–38. doi: 10.1016/j.tem.2010.09.001
101. Harris C, Zivanovic S, Lunt A, Calvert S, Bisquera A, Marlow N, et al. Lung function and respiratory outcomes in teenage boys and girls born very prematurely. Pediatr Pulmonol. (2020) 55:682–9. doi: 10.1002/ppul.24631
102. Gortner L, Shen J, Tutdibi E. Sexual dimorphism of neonatal lung development. Klin Padiatr. (2013) 225:64–9. doi: 10.1055/s-0033-1333758
103. Anand D, Stevenson CJ, West CR. Lung function and respiratory health in adolescents of very low birth weight. Arch Dis Child. (2003) 88:135–8. doi: 10.1136/adc.88.2.135
104. Townsel CD, Emmer SF, Campbell WA, Hussain N. Gender differences in respiratory morbidity and mortality of preterm neonates. Front Pediatr. (2017) 5:6. doi: 10.3389/fped.2017.00006
105. Bolton CE, Bush A, Hurst JR, Kotecha S, McGarvey L. Lung consequences in adults born prematurely. Thorax. (2015) 70:574–80. doi: 10.1136/thoraxjnl-2014-206590
106. Ito M, Tamura M, Namba F. Role of sex in morbidity and mortality of very premature neonates. Pediatr Int. (2017) 59:898–905. doi: 10.1111/ped.13320
107. Anadkat JS, Kuzniewicz MW, Chaudhari BP, Cole FS, Hamvas A. Increased risk for respiratory distress among white, male, late preterm and term infants. J Perinatol. (2012) 32:780–5. doi: 10.1038/jp.2011.191
108. Roberge S, Lacasse Y, Tapp S, Tremblay Y, Kari A, Liu J, et al. Role of fetal sex in the outcome of antenatal glucocorticoid treatment to prevent respiratory distress syndrome: systematic review and meta-analysis. J Obstetr Gynaecol Canada. (2011) 33:216–26. doi: 10.1016/S1701-2163(16)34822-8
109. Stevenson DK, Tyson JE, Korones SB, Bauer CR, Stoll BJ, Papile LA, et al. Sex differences in outcomes of very low birthweight infants: the newborn male disadvantage. Arch Dis Child Fetal Neonatal Ed. (2000) 83:F182–5. doi: 10.1136/fn.83.3.F182
110. Ali K, Greenough A. Long-term respiratory outcome of babies born prematurely. Ther Adv Respir Dis. (2012) 6:115–20. doi: 10.1177/1753465812436803
111. Brothwood M, Wolke D, Gamsu HR, Benson J, Cooper D. Prognosis of the very low birthweight baby in relation to gender. Arch Dis Child. (1986) 61:559–64. doi: 10.1136/adc.61.6.559
112. Kaltofen T, Haase M, Thome UH, Laube M. Male sex is associated with a reduced alveolar epithelial sodium transport. PLoS ONE. (2015) 10:e0136178. doi: 10.1371/journal.pone.0136178
113. Lomauro A, Aliverti A. Sex differences in respiratory function. Breathe. (2018) 14:131. doi: 10.1183/20734735.000318
114. Patrone C, Cassel TN, Pettersson K, Piao Y-S, Cheng G, Ciana P, et al. Regulation of postnatal lung development and homeostasis by estrogen receptor β. Mol Cell Biol. (2003) 23:8542–52. doi: 10.1128/MCB.23.23.8542-8552.2003
115. Massaro D, Massaro GDC. Estrogen receptor regulation of pulmonary alveolar dimensions: alveolar sexual dimorphism in mice. Am J Physiol Lung Cellular Mol Physiol. (2006) 290:L1154–9. doi: 10.1152/ajplung.00396.2005
116. Ivanova MM, Mazhawidza W, Dougherty SM, Minna JD, Klinge CM. Activity and intracellular location of estrogen receptors α and β in human bronchial epithelial cells. Mol Cell Endocrinol. (2009) 305:12–21. doi: 10.1016/j.mce.2009.01.021
117. Tam A, Churg A, Wright JL, Zhou S, Kirby M, Coxson HO, et al. Sex differences in airway remodeling in a mouse model of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. (2016) 193:825–34. doi: 10.1164/rccm.201503-0487OC
118. Xia S, Zhang X, Zheng S, Khanabdali R, Kalionis B, Wu J, et al. An update on inflamm-aging: mechanisms, prevention, and treatment. J Immunol Res. (2016) 2016:8426874. doi: 10.1155/2016/8426874
119. Kovacs EJ, Boe DM, Boule LA, Curtis BJ. Inflammaging and the lung. Clin Geriatr Med. (2017) 33:459–71. doi: 10.1016/j.cger.2017.06.002
120. Milan-Mattos JC, Anibal FF, Perseguini NM, Minatel V, Rehder-Santos P, Castro CA, et al. Effects of natural aging and gender on pro-inflammatory markers. Braz J Med Biol Res. (2019) 52:e8392–e92. doi: 10.1590/1414-431x20198392
121. Baylis D, Bartlett DB, Patel HP, Roberts HC. Understanding how we age: insights into inflammaging. Longevity Healthspan. (2013) 2:1–8. doi: 10.1186/2046-2395-2-8
122. Ebert T, Pawelzik SC, Witasp A, Arefin S, Hobson S, Kublickiene K, et al. Inflammation and premature ageing in chronic kidney disease. Toxins (Basel). (2020) 12:227. doi: 10.3390/toxins12040227
123. Stenvinkel P, Chertow GM, Devarajan P, Levin A, Andreoli SP, Bangalore S, et al. Chronic inflammation in chronic kidney disease progression: role of Nrf2. Kidney Int Rep. (2021) 6:1775. doi: 10.1016/j.ekir.2021.04.023
124. Ciarambino T, Para O, Giordano M. Immune system and COVID-19 by sex differences and age. Women's Health. (2021) 17:17455065211022262. doi: 10.1177/17455065211022262
125. Márquez EJ, Chung C han, Marches R, Rossi RJ, Nehar-Belaid D, Eroglu A, et al. Sexual-dimorphism in human immune system aging. Nat Commun. (2020) 11:1–17. doi: 10.1038/s41467-020-14396-9
126. Bupp MRG, Potluri T, Fink AL, Klein SL. The confluence of sex hormones and aging on immunity. Front Immunol. (2018) 9:1269. doi: 10.3389/fimmu.2018.01269
127. Domingues R, Lippi A, Setz C, Outeiro TF, Krisko A. SARS-CoV-2, immunosenescence and inflammaging: partners in the COVID-19 crime. Aging (Albany NY). (2020) 12:18778. doi: 10.18632/aging.103989
128. Flynn MG, Markofski MM, Carrillo AE. Elevated inflammatory status and increased risk of chronic disease in chronological aging: inflamm-aging or inflamm-inactivity? Aging Dis. (2019) 10:147–56. doi: 10.14336/AD.2018.0326
129. Craft BB. Professor of Psychology A, Carroll HA, Faculty A, Kathleen Lustyk MB. Gender differences in exercise habits and quality of life reports: assessing the moderating effects of reasons for exercise HHS public access. Int J Lib Arts Soc Sci. (2014) 2:65–76.
130. Padilha de Lima A, Macedo Rogero M, Araujo Viel T, Garay-Malpartida HM, Aprahamian I, Lima Ribeiro SM. Interplay between inflammaging, frailty and nutrition in COVID-19: preventive and adjuvant treatment perspectives. J Nutr Health Aging 2021 (2021) 26:67–76. doi: 10.1007/s12603-021-1720-5
131. Theofilis P, Oikonomou E, Lazaros G, Vogiatzi G, Mystakidi VC, Goliopoulou A, et al. The association of physical activity with arterial stiffness and inflammation: insight from the “Corinthia” Study. Angiology. (2022) 2022:00033197211065795. doi: 10.1177/00033197211065795
132. Stahl ST, Albert SM. Gender differences in physical activity patterns among older adults who fall ✰ HHS Public Access. Prev Med. (2015) 71:94–100. doi: 10.1016/j.ypmed.2014.12.016
133. Sallis R, Young DR, Tartof SY, Sallis JF, Sall J, Li Q, et al. Physical inactivity is associated with a higher risk for severe COVID-19 outcomes: a study in 48 440 adult patients. Br J Sports Med. (2021) 55:1099–105. doi: 10.1136/bjsports-2021-104080
134. Cunha PG, Boutouyrie P, Nilsson PM, Laurent S. Early vascular ageing (EVA): definitions and clinical applicability. Curr Hypertens Rev. (2017) 13:1–8. doi: 10.2174/1573402113666170413094319
135. Huxley VH, Kemp SS. Sex-specific characteristics of the microcirculation. Adv Exp Med Biol. (2018) 1065:307. doi: 10.1007/978-3-319-77932-4_20
136. Piccari L, Pozo R. del, Blanco I, García-Lucio J, Torralba Y, Tura-Ceide O, et al. Association between systemic and pulmonary vascular dysfunction in COPD. Int J Chron Obstruct Pulmon Dis. (2020) 15:2037–47. doi: 10.2147/COPD.S257679
137. Ramadhiani R, Ikeda K, Miyagawa K, Ryanto GRT, Tamada N, Suzuki Y, et al. Senescent endothelial cells exacerbate pulmonary hypertension through notch-mediated juxtacrine interaction with pulmonary artery smooth muscle cells. Eur Heart J. (2021) 42: ehab724–3419. doi: 10.1093/eurheartj/ehab724.3419
138. Theodorakopoulou MP, Bakaloudi DR, Dipla K, Zafeiridis A, Boutou AK. Vascular endothelial damage in COPD: current functional assessment methods and future perspectives. Exp Rev Resp Med. (2021) 15:1121–33. doi: 10.1080/17476348.2021.1919089
139. Vaes AW, Spruit MA, Theunis J, Goswami N, Vanfleteren LE, Franssen FME, et al. Endothelial function in patients with chronic obstructive pulmonary disease: a systematic review of studies using flow mediated dilatation. Expert Rev Respir Med. (2017) 11:1021–31. doi: 10.1080/17476348.2017.1389277
140. Masugata H, Senda S, Okada H, Murao K, Inukai M, Himoto T, et al. Association between arterial stiffness and pulmonary function in hypertensive patients. Hypertens Res. (2012) 35:388–92. doi: 10.1038/hr.2011.199
141. Weir-McCall JR, Liu-Shiu-Cheong PSK, Struthers AD, Lipworth BJ, Houston JG. Disconnection of pulmonary and systemic arterial stiffness in COPD. Int J Chron Obstruct Pulmon Dis. (2018) 13:1755. doi: 10.2147/COPD.S160077
142. Ayer JG, Belousova EG, Harmer JA, Toelle B, Celermajer DS, Marks GB. Lung function is associated with arterial stiffness in children. PLoS ONE. (2011) 6:e0026303. doi: 10.1371/journal.pone.0026303
143. Zureik M, Benetos A, Neukirch C, Courbon D, Bean K, Thomas F, et al. Reduced pulmonary function is associated with central arterial stiffness in men. Am J Respir Crit Care Med. (2002) 164:2181–5. doi: 10.1164/ajrccm.164.12.2107137
144. Bolton CE, Cockcroft JR, Sabit R, Munnery M, McEniery CM, Wilkinson IB, et al. Lung function in mid-life compared with later life is a stronger predictor of arterial stiffness in men: the Caerphilly Prospective Study. Int J Epidemiol. (2009) 38:867–76. doi: 10.1093/ije/dyn374
145. Zaigham S, Östgren CJ, Persson M, Muhammad IF, Nilsson PM, Wollmer P, et al. The association between carotid-femoral pulse-wave velocity and lung function in the Swedish CArdioPulmonary bioImage study (SCAPIS) cohort. Respir Med. (2021) 185:106504. doi: 10.1016/j.rmed.2021.106504
146. van der Plaat DA, Minelli C, Jarvis DL, Garcia-Aymerich J, Leynaert B, Gómez-Real F. Polycystic ovary syndrome and lung function: a Mendelian randomization study. Am J Obstet Gynecol. (2020) 223:455–7. doi: 10.1016/j.ajog.2020.03.003
147. Meyer C, McGrath BP, Teede HJ. Overweight women with polycystic ovary syndrome have evidence of subclinical cardiovascular disease. J Clin Endocrinol Metab. (2005) 90:5711–6. doi: 10.1210/jc.2005-0011
148. Nilsson PM, Laurent S, Cunha PG, Olsen MH, Rietzschel E, Franco OH, et al. Characteristics of healthy vascular ageing in pooled population-based cohort studies: the global metabolic syndrome and artery research consortium. J Hypertens. (2018) 36:2340–9. doi: 10.1097/HJH.0000000000001824
149. Scuteri A, Laurent S, Cucca F, Cockcroft J, Cunha PG, Mañas LR, et al. The Metabolic Syndrome across Europe: different clusters and risk factors. Eur J Prev Cardiol. (2015) 22:486. doi: 10.1177/2047487314525529
150. Pucci G, Alcidi R, Tap L, Battista F, Mattace-Raso F, Schillaci G. Sex- and gender-related prevalence, cardiovascular risk and therapeutic approach in metabolic syndrome: a review of the literature. Pharmacol Res. (2017) 120:34–42. doi: 10.1016/j.phrs.2017.03.008
151. Dambala K, Paschou SA, Michopoulos A, Siasos G, Goulis DG, Vavilis D, et al. Biomarkers of endothelial dysfunction in women with polycystic ovary syndrome. Angiology. (2019) 70:797–801. doi: 10.1177/0003319719840091
152. Shimizu I, Minamino T. Cellular senescence in cardiac diseases. J Cardiol. (2019) 74:313–9. doi: 10.1016/j.jjcc.2019.05.002
153. Yeh JK, Wang CY. Telomeres and telomerase in cardiovascular diseases. Genes. (2016) 7:7090058. doi: 10.3390/genes7090058
154. Córdoba-Lanús E, Cazorla-Rivero S, Espinoza-Jiménez A, de-Torres JP, Pajares MJ, Aguirre-Jaime A, et al. Telomere shortening and accelerated aging in COPD: findings from the BODE cohort. Respir Res. (2017) 18:59. doi: 10.1186/s12931-017-0547-4
155. Hägg S, Jylhävä J. Sex differences in biological aging with a focus on human studies. ELife. (2021) 10:e63425. doi: 10.7554/eLife.63425
156. Barrett ELB, Richardson DS. Sex differences in telomeres and lifespan. Aging Cell. (2011) 10:913–21. doi: 10.1111/j.1474-9726.2011.00741.x
157. Gomez-Marcos MA, Martinez-Salgado C, Gonzalez-Sarmiento R, Hernandez-Rivas JM, Sanchez-Fernandez PL, Recio-Rodriguez JI, et al. Association between different risk factors and vascular accelerated ageing (EVA study): study protocol for a cross-sectional, descriptive observational study. BMJ Open. (2016) 6:e011031. doi: 10.1136/bmjopen-2016-011031
158. Marín C, Yubero-Serrano EM, López-Miranda J, Pérez-Jiménez F. Endothelial aging associated with oxidative stress can be modulated by a healthy mediterranean diet. Int J Mol Sci. (2013) 14:8869. doi: 10.3390/ijms14058869
159. Álvarez-Fernández C, Romero-Saldaña M, Álvarez-López Á, Molina-Luque R, Molina-Recio G, Vaquero-Abellán M, et al. Adherence to the Mediterranean diet according to occupation-based social classifications and gender. Arch Environ Occup Health. (2020) 76:275–81. doi: 10.1080/19338244.2020.1825210
160. Ronco C, Bellasi A, di Lullo L. Cardiorenal syndrome: an overview. Adv Chronic Kidney Dis. (2018) 25:382–90. doi: 10.1053/j.ackd.2018.08.004
161. Ind PW, Arulkumaran N, Pusey CD, West SC. Pulmonary-renal syndrome: a life threatening but treatable condition. Postgrad Med J. (2013) 89:274–83. doi: 10.1136/postgradmedj-2012-131416
162. Sanders JSF, Rutgers A, Stegeman CA, Kallenberg CGM. Pulmonary: renal syndrome with a focus on anti-GBM disease. Semin Respir Crit Care Med. (2011) 32:328–34. doi: 10.1055/s-0031-1279829
163. Saxena R, Bygren P, Arvastson B, Wieslander J. Circulating autoantibodies as serological markers in the differential diagnosis of pulmonary renal syndrome. J Intern Med. (1995) 238:143–52. doi: 10.1111/j.1365-2796.1995.tb00912.x
164. Tampe D, Korsten P, Ströbel P, Hakroush S, Tampe B. Comprehensive analysis of sex differences at disease manifestation in ANCA-associated glomerulonephritis. Front Immunol. (2021) 12:3916. doi: 10.3389/fimmu.2021.736638
165. Heffernan DS, Dossett LA, Lightfoot MA, Fremont RD, Ware LB, Sawyer RG, et al. Gender and acute respiratory distress syndrome in critically injured adults: a prospective study. J Trauma. (2011) 71:878–85. doi: 10.1097/TA.0b013e31822c0d31
166. McNicholas BA, Madotto F, Pham T, Rezoagli E, Masterson CH, Horie S, et al. Demographics, management and outcome of females and males with acute respiratory distress syndrome in the LUNG SAFE prospective cohort study. Eur Respir J. (2019) 54:1900609. doi: 10.1183/13993003.00609-2019
167. Husain-Syed F, McCullough PA, Birk HW, Renker M, Brocca A, Seeger W, et al. Cardio-pulmonary-renal interactions: a multidisciplinary approach. J Am Coll Cardiol. (2015) 65:2433–48. doi: 10.1016/j.jacc.2015.04.024
168. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. (2016) 16:626–38. doi: 10.1038/nri.2016.90
169. Ju J, Wong M, Leong JY, Lee JH, Albani S, Yeo JG. Insights into the immuno-pathogenesis of acute respiratory distress syndrome. Ann Transl Med. (2019) 7:4–4. doi: 10.21037/atm.2019.09.28
170. Heyder T, Yang M, Kohler M, Merikallio H, Forsslund H, Li C-X, et al. Profiling of airway epithelial proteome reveals sex differences in early stage COPD linked to xenobiotic metabolism and ER stress. Eur Respir J. (2020) 56:3829. doi: 10.1183/13993003.congress-2020.3829
171. Phelps DS, Umstead TM, Floros J. Sex differences in the response of the alveolar macrophage proteome to treatment with exogenous surfactant protein-A. Proteome Sci. (2012) 10:1–15. doi: 10.1186/1477-5956-10-44
172. Wagenvoort GHJ, Sanders EAM, Vlaminckx BJ, de Melker HE, van der Ende A, Knol MJ. Sex differences in invasive pneumococcal disease and the impact of pneumococcal conjugate vaccination in the Netherlands, 2004 to 2015. Eurosurveillance. (2017) 22:30481. doi: 10.2807/1560-7917.ES.2017.22.10.30481
173. Batton KA, Austin CO, Bruno KA, Burger CD, Shapiro BP, Fairweather DL. Sex differences in pulmonary arterial hypertension: role of infection and autoimmunity in the pathogenesis of disease. Biol Sex Diff. (2018) 9:1–14. doi: 10.1186/s13293-018-0176-8
174. Zhang Q, Wang L, Zeng H, Lv Y, Huang Y. Epidemiology and risk factors in CKD patients with pulmonary hypertension: a retrospective study. BMC Nephrol. (2018) 19:70. doi: 10.1186/s12882-018-0866-9
175. Navaneethan SD, Wehbe E, Heresi GA, Gaur V, Minai OA, Arrigain S, et al. Presence and outcomes of kidney disease in patients with pulmonary hypertension. Clin J Am Soc Nephrol. (2014) 9:855–63. doi: 10.2215/CJN.10191013
176. O'Leary JM, Assad TR, Xu M, Birdwell KA, Farber-Eger E, Wells QS, et al. Pulmonary hypertension in patients with chronic kidney disease: invasive hemodynamic etiology and outcomes. Pulm Circ. (2017) 7:674–83. doi: 10.1177/2045893217716108
177. Navaneethan SD, Schold JD, Huang H, Nakhoul G, Jolly SE, Arrigain S, et al. Mortality outcomes of patients with chronic kidney disease and chronic obstructive pulmonary disease. Am J Nephrol. (2016) 43:39–46. doi: 10.1159/000444422
178. Chen CY, Liao KM. Chronic obstructive pulmonary disease is associated with risk of chronic kidney disease: a nationwide case-cohort study. Sci Rep. (2016) 6:1–8. doi: 10.1038/srep25855
179. Trudzinski FC, Alqudrah M, Omlor A, Zewinger S, Fliser D, Speer T, et al. Consequences of chronic kidney disease in chronic obstructive pulmonary disease. Respir Res. (2019) 20:1–9. doi: 10.1186/s12931-019-1107-x
180. Morgan AD, Zakeri R, Quint JK. Defining the relationship between COPD and CVD: what are the implications for clinical practice? Therap Adv Respir Dis. (2018) 12:1753465817750524. doi: 10.1177/1753465817750524
181. Pelaia C, Pastori D, Armentaro G, Miceli S, Cassano V, Barbara K, et al. Predictors of renal function worsening in patients with chronic obstructive pulmonary disease (COPD): a multicenter observational study. Nutrients. (2021) 13:2811. doi: 10.3390/nu13082811
182. Lai CC, Wu CH, Wang YH, Wang CY, Wu VC, Chen L. The association between COPD and outcomes of patients with advanced chronic kidney disease. Int J Chron Obstruct Pulmon Dis. (2018) 13:2899. doi: 10.2147/COPD.S174215
183. Masuda C, Miyasaka T, Kawakami K, Inokuchi J, Kawano T, Dobashi-Okuyama K, et al. Sex-based differences in CD103+ dendritic cells promote female-predominant Th2 cytokine production during allergic asthma. Clin Exp Allergy. (2018) 48:379–93. doi: 10.1111/cea.13081
184. White LE, Hassoun HT. Inflammatory mechanisms of organ crosstalk during ischemic acute kidney injury. Int J Nephrol. (2012) 2012:505197. doi: 10.4061/2012/505197
185. Huang HL, Ho SY Li CH, Chu FY, Ciou LP, Lee HC, et al. Bronchial asthma is associated with increased risk of chronic kidney disease. BMC Pulm Med. (2014) 14:80. doi: 10.1186/1471-2466-14-80
186. Ritz SA, Antle DM, Côté J, Deroy K, Fraleigh N, Messing K, et al. First steps for integrating sex and gender considerations into basic experimental biomedical research. FASEB J. (2014) 28:4–13. doi: 10.1096/fj.13-233395
187. Rich-Edwards JW, Kaiser UB, Chen GL, Manson JAE, Goldstein JM. Sex and gender differences research design for basic, clinical, and population studies: essentials for investigators. Endocr Rev. (2018) 39:424. doi: 10.1210/er.2017-00246
188. Spence JD, Pilote L. Importance of sex and gender in atherosclerosis and cardiovascular disease. Atherosclerosis. (2015) 241:208–10. doi: 10.1016/j.atherosclerosis.2015.04.806
189. Pelletier R, Khan NA, Cox J, Daskalopoulou SS, Eisenberg MJ, Bacon SL, et al. Sex versus gender-related characteristics which predicts outcome after acute coronary syndrome in the young? J Am Coll Cardiol. (2016) 67:127–35. doi: 10.1016/j.jacc.2015.10.067
190. Schiebinger L, Stefanick ML. Gender matters in biological research and medical practice. J Am Coll Cardiol. (2016) 67:136–8. doi: 10.1016/j.jacc.2015.11.029
191. White J, Tannenbaum C, Klinge I, Schiebinger L, Clayton J. The integration of sex and gender considerations into biomedical research: lessons from international funding agencies. J Clin Endocrinol Metab. (2021) 106:3034–48. doi: 10.1210/clinem/dgab434
192. O'neill ZR, Raparelli V, Norris CM, Pilote L, the GOING-FWD Investigators. Demystifying how to incorporate Sex and Gender into cardiovascular research: a practical guide. Can J Cardiol. (2022). doi: 10.1016/j.cjca.2022.05.017
193. Runnels V, Tudiver S, Doull M, Boscoe M. The challenges of including sex/gender analysis in systematic reviews: a qualitative survey. Syst Rev. (2014) 3:33. doi: 10.1186/2046-4053-3-33
Keywords: gender dimension, cardiovascular disease, pulmonary function, chronic kidney disease, gender, sex
Citation: Hernandez L, Laucyte-Cibulskiene A, Ward LJ, Kautzky-Willer A, Herrero M-T, Norris CM, Raparelli V, Pilote L, Stenvinkel P, Kublickiene K and the GOING-FWD Consortium (2022) Gender dimension in cardio-pulmonary continuum. Front. Cardiovasc. Med. 9:916194. doi: 10.3389/fcvm.2022.916194
Received: 08 April 2022; Accepted: 11 July 2022;
Published: 08 August 2022.
Edited by:
Günther Silbernagel, Medical University of Graz, AustriaReviewed by:
Ketul R. Chaudhary, Dalhousie University, CanadaMichihisa Umetani, University of Houston, United States
Copyright © 2022 Hernandez, Laucyte-Cibulskiene, Ward, Kautzky-Willer, Herrero, Norris, Raparelli, Pilote, Stenvinkel, Kublickiene and the GOING-FWD Consortium. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Karolina Kublickiene, a2Fyb2xpbmEua3VibGlja2llbmVAa2kuc2U=
†These authors have contributed equally to this work and share first authorship
 Leah Hernandez
Leah Hernandez Agne Laucyte-Cibulskiene
Agne Laucyte-Cibulskiene Liam J. Ward
Liam J. Ward Alexandra Kautzky-Willer
Alexandra Kautzky-Willer Maria-Trinidad Herrero
Maria-Trinidad Herrero Colleen M. Norris
Colleen M. Norris Valeria Raparelli
Valeria Raparelli Louise Pilote
Louise Pilote Peter Stenvinkel
Peter Stenvinkel Karolina Kublickiene
Karolina Kublickiene