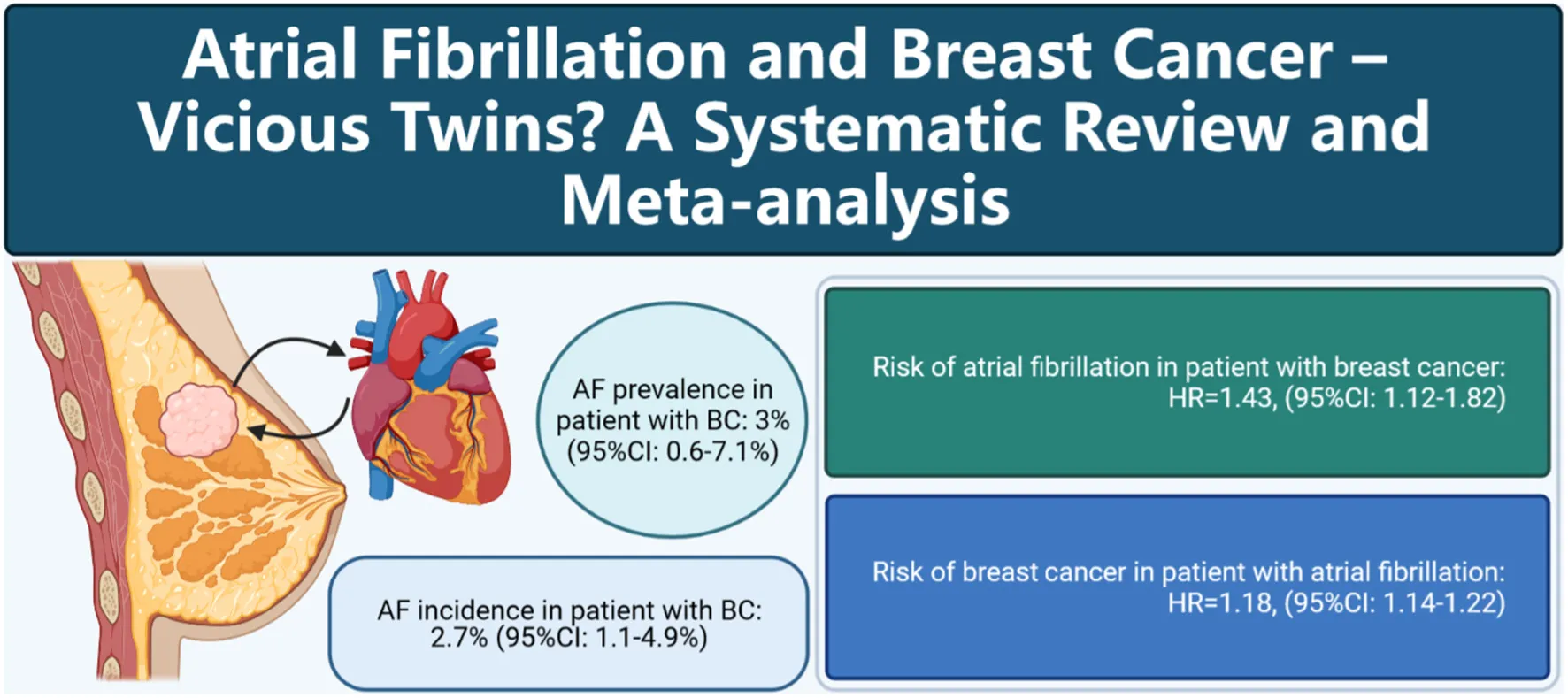
Abstract
Background:
Epidemiological studies suggest a bidirectional association between atrial fibrillation and breast cancer. This study aimed to conduct a meta-analysis to elucidate the prevalence of atrial fibrillation among breast cancer patients, and the bidirectional association between atrial fibrillation and breast cancer.
Methods:
PubMed, the Cochrane Library, and Embase were searched to identify studies reporting the prevalence, incidence, and bidirectional association between atrial fibrillation and breast cancer. The study was registered with PROSPERO (CRD42022313251). Levels of evidence and recommendations were assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE).
Results:
Twenty-three studies (17 retrospective cohort studies, 5 case-control studies and 1 cross-sectional study) involving 8,537,551 participants were included. Among patients with breast cancer, the prevalence of atrial fibrillation was 3% (11 studies; 95% CI: 0.6 to 7.1%) and the incidence was 2.7% (6 studies; 95% CI: 1.1 to 4.9%). Breast cancer was associated with increased risk of atrial fibrillation (5 studies; hazard ratio [HR]: 1.43, 95% CI: 1.12 to 1.82, I2 = 98%). Atrial fibrillation was also significantly associated elevated risk of breast cancer (5 studies HR: 1.18, 95% CI: 1.14 to 1.22, I2 = 0%). Grade assessment shown low certainty of the evidence for the risk of atrial fibrillation and moderate certainty of the evidence for the risk of breast cancer.
Conclusion:
Atrial fibrillation is not uncommon in patients with breast cancer and vice versa. There is a bidirectional association between atrial fibrillation (low certainty) and breast cancer (moderate certainty).
Cartoon depicting the association between atrial fibrillation and breast cancer.
Introduction
Breast cancer is the most frequent cancer amongst women worldwide, accounting for about 24.5 percentage of all cancers (1). Thanks to advances in treatment, the 5-year survival rate for breast cancer patients has significantly increased to 89% (2). However, cardiovascular disease is increasingly becoming a barrier to optimal outcomes for breast cancer patients (3).
Atrial fibrillation, the most common arrhythmia, is an important cause of embolism and death from cardiovascular diseases, with a global prevalence of approximately 2–4%. Age is a strong risk factor for the incidence of atrial fibrillation. The prevalence of atrial fibrillation tends to increase with the increase in human life expectancy (4, 5). One study showed that malignant neoplasms (23.1%) were the most common cause of death in patients with atrial fibrillation (6).
Recently, many epidemiological studies have shown an increased risk of new-onset atrial fibrillation in women with breast cancer (7–15), while other studies have shown an increased risk of breast cancer in women with atrial fibrillation (13, 16–20). It is important to note that these studies have not yielded consistent results. The incidence and prevalence of atrial fibrillation in breast cancer are unknown, and it is unclear whether there is a bidirectional association between atrial fibrillation and breast cancer. Thus, we conducted a systematic review and meta-analysis to elucidate the prevalence and incidence of atrial fibrillation among breast cancer patients and the association between atrial fibrillation and breast cancer.
Methods
Protocol registration
The study was registered in the PROSPERO (International prospective register of systematic reviews. https://www.crd.york.ac.uk/PROSPERO/ -registration number- CRD42022313251). This meta-analysis was performed according to the PRISMA 2021 guideline for systematic review and meta-analysis (21) (Supplementary Table S1).
Search strategy
We systematically searched the PubMed, EMBASE, Cochrane Library online database up to February 25, 2022 with no language restriction. The gray literatures, published in American College of Cardiology (ACC)/American Heart Association (AHA), European Society of Cardiology (ESC) were also reviewed. The search MeSH items and keywords were as follows: (“Breast Neoplasms” [Mesh] OR “breast cancer” OR “breast cancers” OR “breast tumors” OR “breast tumors” OR “mammary Cancer” OR “mammary cancers” OR “mammary gland cancers” OR “mammary gland cancer”) AND (“Atrial Fibrillation” [Mesh] OR “atrial fibrillations” OR “auricular fibrillation”) OR (“Mortality” [Mesh] OR “Mortalities” OR “Case Fatality Rate” OR “Case Fatality Rates” OR “Death Rate” OR “Death Rates”). The detailed search strategy is shown in Supplementary Table S2.
Selection criteria
Eligible studies were as follows: (1) clinical trials or observational studies (cohort, case–control, or case-cohort or nest-case control) reported the prevalence or the incidence of atrial fibrillation in patients with breast cancer. (2) studies assessed the association between atrial fibrillation and breast cancer expressing with Relative Risk (RR)/HR and corresponding 95% CI or that can be calculated. For the incidence of atrial fibrillation or cancer, we only included cohort studies or the case cohort designed data. (3) studies assessed the association between atrial fibrillation and mortality in patients with breast cancers. Two authors (X-L and X.D-Y) independently reviewed the articles for eligibility. Any discrepancies were discussed until consensus.
Accordingly, studies that met the following criteria were excluded: (1) Studies that specifically looked at the influence of breast cancer-related treatments (e.g., radiotherapy chemotherapy and adjuvant therapy) on atrial fibrillation, as anticancer treatment was linked to atrial fibrillation incident by the previous studies (22–31). (2) Reviews, meta-analyses, practice guidelines, patents, cases, replies, comments, or editorial; (3) after contacting the author, the data needed for the article still cannot be obtained.
Data extraction and quality assessment
The information was extracted from each study, including first author, publication year, country, mean age, study design, data source, follow-up, prevalence or incidence of atrial fibrillation or breast cancer, atrial fibrillation diagnosis, breast cancer diagnosis, sample size, any subgroup, effect size, and adjustments. Three author (X.D-Y and X-L Z.Q-T) extracted data. For studies that reported the prevalence of atrial fibrillation or breast cancer, the Joanna Briggs Institute (JBI) critical appraisal checklist was used to assess the study quality. The Newcastle-Ottawa quality scale (NOS) was used to quantify the quality of studies reporting association between breast cancer and atrial fibrillation. A score of JBI > 16 or NOS > 6 was regarded as acceptable quality.
Statistical analysis
We used a random-effect model to summarize the estimates and 95% confidence intervals (CIs). For incidence of atrial fibrillation or breast cancer, the exact binomial (Clopper–Pearson) method was used to calculate 95%CI (32). Estimates were standardized using the Freeman–Tukey double arcsine transformation (33). For the effect of atrial fibrillation on mortality in breast cancer patients, estimates from each study were consolidated by the method of DerSimonian and Laird (34), which designated the weight of each study based on its variance. If a study reported results separately for subgroups (e.g., age), we combined the subgroup-specific estimates using a random-effect so that each study was represented only once in the forest plot. If one study reported one more effect size, we used the most informative one. In the association outcomes, considering the difference in study design, the evidence of cohort and case-control studies was pooled separately. In cohort studies, we treat standardized incidence ratios (SIRs) as equivalent to RR.
Heterogeneity was evaluated using the Higgins I-squared (I2) statistic (25, 50, and 75% represent low, moderate, and high heterogeneity, respectively) (35). Publication bias was addressed by Funel plot, and Egger's and Begg's test, with the results considered to be significant when P < 0.10 (36). For the purpose of appraising the robustness and reliability of the primary study outcomes, we also carried out sensitivity analyses by omitting each study in turn. Subgroup analysis was defined as mean age (< 65 vs. ≥65 years), region (America, Europe, and Asia), sample size (< 5,000 vs. ≥5,000), case (< 50 vs. ≥50) if enough studies were available (N > 10) considering the statistical power according to the guidelines. For the association of atrial fibrillation with breast cancer, an additional subgroup was stratified by hormone therapy. The statistical analysis was performed by the RevMan software, version 5.4.1 (The Cochrane Collaboration, Nordic Cochrane Center Copenhagen, Denmark) and Stata software, Version 16.0 (Stata Corp LP, College Station, Texas, USA). P < 0.05 double-sided was considered statistically significant. We evaluated the quality of evidence for each outcome using the GRADE method (37, 38). Two authors separately assessed the quality of evidence for each outcome. We used GRADEpro GDT to provide evidence profile tables. We present the results of the outcomes as described in the outcome metric type section, footnotes are used to indicate reasons for degradation or escalation of the quality of evidence.
Results
Literature search
As shown in Figure 1, an initial online database search resulted in 1,352 abstracts. After excluding 166 duplicated records and 1,128 animal studies, in vitro studies or irrelevant studies based on the screening of titles/abstracts, 58 reports remained for full-text review. Thirty-six full-length studies were excluded for the following reasons: (1) reviews or editorials (n = 10); (2) did not include the target population (n = 12); or (3) did not include the target outcomes (n = 13). The excluded studies are listed in Supplementary Table S3. Finally, 23 studies were included in this meta-analysis.
Figure 1
Study characteristics and quality
Table 1 summarizes the main characteristics of the included studies. Seventeen were retrospective cohort studies (7, 9–11, 13, 14, 31, 39, 40, 44–46), 5 were case-control studies (8, 12, 15, 41, 43), and 1 was a cross-sectional study (42). The follow-up time ranged from 0.24 to 19.1 years. Seventeen studies reported the prevalence or incidence of atrial fibrillation (7–12, 14, 15, 39–47), 5 studies reported an association between atrial fibrillation and breast cancer (16–20), 5 studies reported an association between breast cancer and atrial fibrillation (7, 10, 11, 13, 14) and 2 studies reported an association between atrial fibrillation and all-cause mortality in patients with breast cancer (11, 41). Overall, these studies were published between 2008 and 2021, the sample sizes ranged from 86 to 1,122,100 with a total of 8,537,551 individuals, nine studies were from North America (the US and Canada) (7, 8, 11, 15, 16, 20, 39–41), 7 studies were from Europe (9, 10, 12, 18, 19, 44, 46), and 7 studies were from Asia (13, 14, 17, 42, 43, 45, 47). The mean age varied from 51.9 to 78 years. Sixteen studies defined atrial fibrillation according to the International Classification of Diseases criteria (7, 10, 11, 13–15, 17–19, 39–41, 43, 44, 46, 47), and 7 studies were based on electrocardiogram reports (8, 9, 12, 16, 20, 42, 45). All studies defined breast cancer from medical records according to the International Classification of Diseases (7, 8, 10, 11, 31, 39–43, 45–47). Among the studies reporting atrial fibrillation and breast cancer, four studies reported crude risk estimates (9, 12, 17, 18), and the other adjustments varied considerably. Ten studies adjusted for significant confounding factors (7, 8, 10, 11, 13–16, 19, 20), including age, sex, smoking, alcohol consumption, hypertension, diabetes, and heart failure, and 5 studies adjusted for confounders associated with anticancer therapy, including radiation therapy, chemotherapy, surgery, therapy after lymph node surgery, and hormone replacement therapy (11, 16, 19, 20, 41).
Table 1
| References, country | Sample of size, N | AF diagnosis | BC diagnosis | Study design follow up (year) | Mean age (years) | AF cases, N | New-onset AF case, N | Outcomes reported | Adjustments |
|---|---|---|---|---|---|---|---|---|---|
| Epidemiology | |||||||||
| Abdel-Qadir et al. (7, 39), Canada | 90104 | ICD | ICD | Retrospective cohort 10 | 61 | 1,713 | NR | Prevalence of AF | |
| Apte et al. (8), USA | 1872 | ECG | ICD | Case-control | NR | 54 | NR | Prevalence of AF | Age, hypertension, diabetes, BMI, and male sex |
| Boerman et al. (9), Netherland | 350 | ECG | ICD | Retrospective cohort 10 | 63 | NR | 11 | Incidence of AF | |
| Ezaz et al. (40), USA | 1664 | ICD | ICD | Retrospective cohort 3 | 73.6 | 90 | NR | Prevalence of AF | |
| Han et al. (41), USA | 305300 | ICD | ICD | Case-control | NR | 10,741 | NR | Prevalence of AF mortality | Age, race, gender, income, insurance type, year, hospital region, hospital type, hospital bed size, primary tumor site, multiple metastatic sites (≥2), major operating room procedure, chemotherapy, long- term anticoagulants, metastatic sites, coronary artery disease, prior stroke, acquired immune deficiency syndrome (AIDS), alcohol abuse, deficiency anemias, rheumatoid arthritis, chronic blood loss anemia, congestive heart failure, chronic pulmonary disease, coagulopathy, depression, uncomplicated diabetes, diabetes with chronic complications, drug abuse, hypertension, hypothyroidism, obesity, lymphoma, fluid and electrolyte disorders, other neurological disorders, paralysis, peripheral vascular disorders, psychoses, pulmonary circulation disorders, renal failure, ulcer disease, valvular disease, weight loss and liver disease |
| Li et al. (42), China | 5677 | ECG | ICD | Cross-sectional | NR | 93 | NR | Prevalence of AF | |
| Lin et al. (43), China Taiwan | 1188 | ICD | ICD | Case-control | 75.8 | 40 | NR | Prevalence of AF | |
| Mery et al. (44), France | 682 | ICD | ICD | Retrospective cohort 5 | 62.5 | NR | 8 | Incidence of AF | |
| Guzzetti et al. (12), Italy | 502 | ECG | ICD | Case-control | 60.5 | 10 | NR | Prevalence of AF | Ps for age |
| Okura et al. (45), Japan | 2699 | ECG | ICD | Retrospective cohort 10 | NR | 20 | NR | Prevalence of AF | |
| Ording et al. (46), Denmark | 62376 | ICD | ICD | Retrospective cohort 5 | 62.3 | 567 | NR | Prevalence of AF | |
| Yamashita et al. (47), Japan | 86 | ICD | ICD | Retrospective cohort | 58.8 | 2 | NR | Prevalence of AF | |
| Zubair et al. (15), USA | 7734604 | ICD | ICD | Case-control | NR | 1,122,100 | NR | Prevalence of AF | Age, DM, hypertension, CAD, obesity, congestive heart failure valve disorder, thyrotoxicosis, hypothyroidism, collagen vascular disease, and chronic pulmonary disease |
| BC to AF | |||||||||
| Abdel-Qadir et al. (7, 39), Canada | 68113 | ICD | Pathology ICD | Retrospective cohort 10 | 60 | NR | 5,040 | Incidence of AF risk of AF | Age, year of cohort entry, rural residence, ischemic heart disease, heart failure, diabetes, hypertension, peripheral vascular disease, stroke, chronic obstructive pulmonary disease, and chronic kidney disease, number of family physician, specialist claims (updated annually) |
| D'Souza et al. (10), Denmark | 74155 | ICD | ICD | Retrospective cohort 3 | 62 | NR | 987 | Incidence of AF risk of AF | Hypertension, ischemic heart disease, heart failure, diabetes mellitus, thyroid disease, chronic kidney failure, peripheral arterial disease, chronic obstructive pulmonary disease, chronic liver disease |
| Guha et al. (11), USA | 85423 | ICD | ICD | Retrospective cohort 1 | 78 | NR | 2,993 | Incidence of AF risk of AF mortality | Age, race, Hispanic, registry, urban, poverty, laterality, grade, AJCC stage, surgical, lymph node surgery therapy, radiation therapy, breast tumor subtype based on combination receptor status, hypertension, Diabetes, obesity, history of ischemic stroke/transient ischemic attack, hyperlipidemia, history of congestive heart failure, history of myocardial infarction, history of lung disease, smoking, history of depression, history of anemia, anthracycline vs. not, Her2inhib vs. not, cyclophosphamide vs not, taxanes vs. not, platinum compounds vs. not, hormonal therapy vs. not (only who have part D), beta-blockers, angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARBs), spironolactone/eplerenone vs. not |
| Saliba et al. (13), Israel* | 11220 | ICD | ICD | Retrospective cohort 0.24 | NR | NR | NR | Risk of AF risk of BC | Age, sex, smoking, alcohol consumption, physical activity, education, medications use (aspirin, statins, and anticoagulants), and comorbidities (hypertension, diabetes, congestive heart failure, and cardiovascular disease) |
| Yun et al. (14), Korea | 81066 | ICD | ICD | Retrospective cohort 4.5 | 51.9 | NR | 1,212 | Incidence of AF risk of AF | Age, sex, smoking, drinking, regular exercise, socioeconomic status, DM, hypertension, dyslipidemia, BMI, and CKD |
| AF to BC | |||||||||
| Conen et al. (16), USA | NR | ECG | ICD | Retrospective cohort 19.1 | NR | NR | NR | Risk of BC | Age, randomized treatment assignment, educational level, race/ethnicity, and height at study entry and time-dependent measures for BMI, hypertension, hypercholesterolemia, DM, smoking status, number of cigarettes smoked per day, alcohol consumption, physical activity, hormone replacement therapy use, presence of a recent cancer screening test, and incident non-fatal cardiovascular events (CHF, MI, or stroke). |
| Hung et al. (17), China Taiwan | NR | ICD | ICD | Retrospective cohort 3.1 | NR | NR | NR | Risk of BC | |
| Ostenfeld et al. (18), Denmark | NR | ICD | ICD | Retrospective cohort 3.4 | NR | NR | NR | Risk of BC | |
| Vinter et al. (19), Denmark | NR | ICD | ICD | Retrospective cohort 16.7 | NR | NR | NR | Risk of BC | Age, BMI, cumulative alcohol consumption, smoking duration, tobacco consumption, hypertension, hypercholesterolemia, DM, physical activity, hormone treatment (women only), educational level, education length, smoking status, and Healthy Nordic Food Index |
| Wassertheil-Smoller et al. (20), USA | 4,376 | ECG self-reported | ICD | Retrospective cohort 15.3 | NR | NR | NR | Risk of BC | Age, race, educational level, income, marital status, physical activity level, parity, age at menopause, hormone therapy use, hysterectomy, diabetes, and history of cardiovascular disease (MI, stroke, transient ischemic attack, angina, or revascularization) |
Characteristics of included studies in this meta-analysis.
AF, atrial fibrillation; BC, breast cancer; BMI, body mass index; CAD, coronary artery disease; CHF, chronic heart failure; CKD, chronic kidney disease; DM, diabetes mellitus; ECG, electrocardiogram; HR, hazard ratio; ICD, International Classification of Diseases; ICPC, International Classification of Primary Care; MI, myocardial infarction; NR, not reported; OR, odds ratio;
“BC to AF” and “AF to BC”.
According to the JBI critical appraisal checklist, 17 studies (7–12, 14, 15, 39–47) that reported the prevalence of atrial fibrillation met at least six of the nine criteria, indicating that the articles used a rigorous approach (Supplementary Table S4). Based on the NOS, all 13 studies (7–11, 13–20) involving the association of atrial fibrillation and breast cancer were considered moderate to high quality, with a score range of 6–9, and one study in which the control group included hospital-based non-tumor patients rather than community-derived controls and adjusted for confounders had a score of 5 (12) (Supplementary Table S5).
Atrial fibrillation and breast cancer
Epidemiology of atrial fibrillation in patients with breast cancer
Ten studies (8, 12, 15, 39–42, 45–47) with 8,204,884 participants reported the prevalence of atrial fibrillation among patients with breast cancer. The pooled prevalence of atrial fibrillation was 3% (95% CI: 0.6 to 7.1%), with high heterogeneity (I2 = 99.99%) (Figure 2A).
Figure 2
In the subgroup analysis, the prevalence of atrial fibrillation (3%) was highest among breast cancer patients in the Americas (5%), followed by Asia (1.7%), and Europe (0.9%) (P for subgroup difference = 0.028) (Figure 3B). Older (age ≥ 65) patients with breast cancer (8.6%) showed a higher prevalence of atrial fibrillation than younger (age < 65) patients with breast cancer (1.9%) (Figure 3A). Moreover, the prevalence of atrial fibrillation was higher among breast cancer patients (3.5%) in studies with larger sample sizes (≥5,000) than among breast cancer patients (2.6%) in studies with smaller sample sizes (< 5,000) (Figure 3C). The prevalence of atrial fibrillation was higher among breast cancer patients (3.7%) in studies with a greater number of atrial fibrillation cases (≥50) than among breast cancer patients (1.9%) in studies with a smaller number of atrial fibrillation cases (< 50) (Figure 3D). However, there were no significant differences regarding age (P = 0.15), sample size (P = 0.76) or number of atrial fibrillation cases (P = 0.47) (Table 2).
Figure 3
Table 2
| Items | Number of studies | Proportion (95%CI) | P | P*h (%) | P# | ||
|---|---|---|---|---|---|---|---|
| Result of primary analysis | 11 | 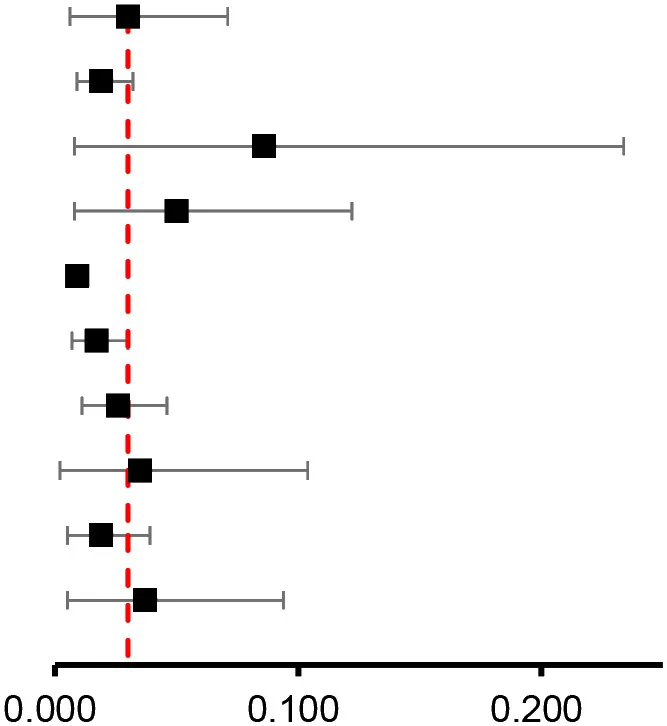 | 0.030 (0.006–0.071) | ||||
| Mean age | < 65 years | 5 | 0.019 (0.009–0.032) | < 0.001 | 99.8 | 0.15 | |
| ≥65 years | 3 | 0.086 (0.008–0.234) | 0.007 | - | - | ||
| Region | America | 5 | 0.050 (0.008–0.122) | < 0.001 | 99.9 | 0.028 | |
| Europe | 2 | 0.009 (0.008–0.009) | - | - | - | ||
| Asia | 4 | 0.017 (0.007–0.030) | < 0.001 | 91.1 | - | ||
| Sample size | < 5,000 | 6 | 0.026 (0.011–0.046) | < 0.001 | 95 | 0.76 | |
| ≥5,000 | 5 | 0.035 (0.002–0.104) | < 0.001 | 99.9 | - | ||
| Cases | < 50 | 4 | 0.019 (0.005–0.039) | < 0.001 | 91.2 | 0.47 | |
| ≥50 | 7 | 0.037 (0.005–0.094) | 0.002 | 99.9 | - | ||
Subgroup analysis of prevalence of atrial fibrillation in patient with breast cancer.
P for within-group heterogeneity,
P for subgroup difference.
Six studies (7, 9–11, 14, 44) with a total of 309,789 participants reported the incidence of atrial fibrillation among patients with breast cancer. The pooled incidence of atrial fibrillation was 2.7% (95% CI: 1.1 to 4.9%) with high heterogeneity (I2 = 99.90%) (Figure 2B).
Bidirectional association between breast cancer and atrial fibrillation
A total of five articles (7, 10, 11, 13, 14) reported the risk of new-onset atrial fibrillation among breast cancer patients. As shown in Figure 4A, the risk of atrial fibrillation was significantly increased in breast cancer patients compared with non-breast cancer patients in pooled cohort studies (HR: 1.43, 95% CI: 1.12 to 1.82, I2 = 98%).
Figure 4
Five articles (16–20) reported the incidence of breast cancer among patients with atrial fibrillation. As shown in Figure 4B, the incidence of breast cancer was significantly increased in patients with atrial fibrillation in pooled cohort studies (HR: 1.18, 95% CI: 1.14 to 1.22, I2 = 0%).
According to the predefined criteria, subgroup analysis was not performed due to the limited number of studies.
Publication bias and sensitivity analysis
As shown in Supplementary Figure S1, funnel plots, Egger's test (both P > 0.1), and Begg's test (both P > 0.1) showed no significant publication bias in risk of atrial fibrillation, risk of breast cancer and incidence of atrial fibrillation, even though funnel plots are not recommended when the number of articles included is limited (N < 10). However, funnel plots, Egger's (p < 0.05), and Begg's tests (p < 0.05) for the prevalence of atrial fibrillation suggest publication bias. Sensitivity analysis by omitting each study indicated that our results were stable and reliable, with a range from 1.31 (95% CI: 1.01 to 1.69) to 1.56 (95% CI: 1.32 to 1.83) for the risk of atrial fibrillation and 1.16 (95% CI: 1.12 to 1.21) to 1.21 (95% CI: 1.15 to 1.29) for the risk of breast cancer (Supplementary Figure S2). For the risk of atrial fibrillation, the results remained stable when we used the fixed effects analysis model (HR: 1.28, 95% CI: 1.25 to 1.31) (Supplementary Figure S3).
Quality assessment
The GRADE tool was used to evaluate the quality of evidence of cohort studies. All the included studies were observational cohort studies, so the initial level of evidence was moderate (37). As there was greater heterogeneity in the risk of atrial fibrillation (I2 = 98%), downgrades were given. Ultimately, the risk of atrial fibrillation was evaluated as low certainty, and the risk of breast cancer was evaluated as moderate by the GRADE tool (Supplementary Table S6).
Discussion
Major findings
Our results with a sample size of 8,537,551 individuals showed that the prevalence of atrial fibrillation among breast cancer patients was 3% and that incidence was 2.7%. Patients with breast cancer had an increased risk of atrial fibrillation of 43%, and patients with atrial fibrillation had an increased risk of breast cancer of 18% in pooled cohorts, which first showed a bidirectional association between atrial fibrillation and breast cancer (Graphical Abstract).
Age is a vital risk factor for both atrial fibrillation and breast cancer, and the prevalence of both increases with aging (4, 48). A national retrospective cohort study showed that breast cancer was associated with an increased risk of atrial fibrillation from 6 months to 3 years of follow-up, both in age < 60 (HR: 1.80, 95% CI: 1.38–2.35) and age >60 groups (HR: 1.14, 95% CI: 1.05 to 1.25) after adjustments (10). However, a large case-control study with a sample size of 122,100 showed a reduced risk of atrial fibrillation in the age < 65 group (OR: 0.70, 95% CI: 0.69 to 0.71) and 65–80 group (OR: 0.91, 95% CI: 0.90 to 0.92) but an increased risk of atrial fibrillation in the age >80 group (OR: 1.01, 95% CI: 1.00 to 1.02) (15). Our results showed that the prevalence of atrial fibrillation among elderly individuals (8.6%) was higher than that in those aged < 65 years (1.9%). Studies that included atrial fibrillation in patients with breast cancer adjusted for age, so our results suggest that the increased risk of atrial fibrillation in breast cancer patients may be independent of age.
In addition, the treatment of breast cancer patients, such as surgery, chemotherapy and radiation, can also contribute to atrial fibrillation (49, 50). The most commonly used chemotherapy drugs for breast cancer, such as alkylates, anthracyclines, paclitaxel and trastuzumab have been found to be cardiotoxic and can induce atrial fibrillation in breast cancer patients (51, 52). Moreover, pain medications used by patients with advanced cancer, including non-steroidal anti-inflammatory drugs and opioids, are associated with an increased risk of atrial fibrillation (53, 54). A case-control study involving patients first diagnosed with breast cancer who had not been treated showed a significantly increased risk of atrial fibrillation in these patients compared with non-breast cancer patients (12). To some extent, the increased risk of atrial fibrillation in breast cancer patients may be independent of cancer treatment.
Similarly, drug treatment for patients with atrial fibrillation may also play a role in promoting breast cancer, such as amiodarone, which may be associated with an increased risk of cancer (55). In addition, a retrospective cohort study of 2,116,029 women showed a significantly increased risk of breast cancer among patients currently on digoxin compared with a control group (RR: 1.39, 95% CI: 1.32 to 1.46) (56). It is speculated that this may be related to the estrogen-like effect of digoxin. However, the relationship between cardiac glycosides and breast cancer remains unclear. Recent studies have shown that cardiac glycosides exert antitumor effects through different mechanisms (57, 58). A recent study in the Danish Breast Cancer Cohort confirmed that among 49,312 patients enrolled from 1995 to 2008, the overall risk of breast cancer recurrence did not change significantly in the digoxin group compared with the non-digoxin group (HR: 1.13, 95% CI: 0.88 to 1.46) (59). Although antiarrhythmic drugs may contribute to atrial fibrillation due to estrogenic effects, the risk of breast cancer in patients with atrial fibrillation remains significantly higher after adjusting for hormone replacement therapy (20). Furthermore, our subgroup analysis also showed that the association between atrial fibrillation and breast cancer persists regardless of hormone therapy adjustment (Supplementary Figure S3).
Two articles (1 retrospective cohort study and 1 case-control study) (11, 41) reported the association between atrial fibrillation and all-cause mortality in patients with breast cancer. One retrospective cohort study of 85,423 breast cancer patients showed that new-onset atrial fibrillation was associated with increased all-cause mortality in patients with breast cancer after 1 year of follow-up (HR: 2.15, 95% CI: 1.32 to 3.48) (11). In addition, one case-control study of 2,478,598 patients with metastatic cancer showed that atrial fibrillation was associated with increased all-cause mortality in patients with breast cancer (OR: 1.43, 95% CI: 1.15 to 1.78) (41). Previous study showed an increase in all-cause mortality in patients with atrial fibrillation (11, 41). Guha et al. showed that this increased all-cause mortality was mainly due to increased cardiovascular mortality (11). The most common cause of cardiovascular death is heart failure, followed by embolism and stroke. It is noteworthy that the use of anticoagulants reduced all-cause mortality, either because of fewer embolic events or because anticoagulants hindered breast cancer development (60, 61).
Underlying mechanism
Our study showed a 43% increased risk of atrial fibrillation in patients with breast cancer compared to those without breast cancer. There is accumulating evidence that breast cancer is closely related to the incidence of atrial fibrillation. Several mechanisms have been proposed to explain this association. First, previous studies have confirmed that the increased risk of atrial fibrillation in cancer patients may be related to the systemic inflammatory state caused by cancer. Inflammation has been considered a vital mechanism involved in the initiation and maintenance of atrial fibrillation (62). Inflammatory markers are significantly increased in patients with breast cancer, such as C-reactive protein, tumor necrosis factor-α, interleukin-2, interleukin-6, and interleukin-8 (63). In addition, breast cancer and atrial fibrillation develop through the same inflammatory pathways, such as the NOD-like receptor protein 3 (NLRP-3) inflammasome (62, 64, 65). Second, some studies have also suggested that autonomic dysfunction in cancer patients may contribute to an increased risk of atrial fibrillation. Patients with breast cancer may face long-term physical pain and mental stress, which may increase sympathetic nerve activity and lead to atrial fibrillation (66, 67). However, there is reason to suspect that the medical exposure of cancer patients is far greater than that of non-cancer patients, which may lead to detection bias. However, in a recent study (7), after adjusting for medical exposure, patients with cancer still had a significantly higher risk of atrial fibrillation than those without cancer, suggesting that this detection bias does not hold.
Our study showed an 18% increased risk of breast cancer in patients with atrial fibrillation. The current study can only show the association between breast cancer and atrial fibrillation but cannot prove a causal relationship. It is speculated that the bidirectional relationship between the two diseases may be related to common risk factors, especially the procoagulant state, as thrombin is a drivers of both atrial fibrillation and breast cancer (68). In addition, apoptosis occurs in the development of atrial fibrillation (69), which may lead to the imbalance between proapoptotic and anti-apoptotic factors, thus reducing the apoptosis of cancer cells and promoting the germinal development of cancer (70). However, several studies have shown a significant increase in the risk of cancer after 90 days of atrial fibrillation and a significant decrease after 90 days, although still associated with cancer, suggesting that in addition to the detection bias caused by the medical exposure of atrial fibrillation patients, many atrial fibrillation patients may already have cancer at the time of diagnosis (16, 18, 19). In other words, common risk factors for both breast cancer and atrial fibrillation only may be early signs of occult cancer (16, 18, 19). Finally, although most studies have adjusted for confounding factors, the association may still be the result of residual confounding. Therefore, the mechanism of the association between atrial fibrillation and breast cancer is very complex, and there is no conclusion yet. Further studies are needed to clarify their association.
Comparison with previous studies
Our research is somewhat pioneering. Two previous meta-analyses reported an increased risk of cancer diagnosis within 90 days in patients with newly developed atrial fibrillation, but these studies focused on all malignancies rather than specific tumor subtypes (71, 72). A recent meta-analysis reported the prevalence of atrial fibrillation in breast cancer patients across different types of cancer therapy (73). We first assessed the bidirectional association of atrial fibrillation and breast cancer patients.
Clinical implications
The overall prevalence (3%) of atrial fibrillation among breast cancer patients was similar to that of the general population; however, subgroup analysis showed an increased prevalence of atrial fibrillation among breast cancer patients >65 years of age (8.6%). Given the overall low prevalence of atrial fibrillation among breast cancer patients, screening for atrial fibrillation in breast cancer patients and screening for breast cancer in patients with atrial fibrillation are not currently recommended (4), although the prevalence of atrial fibrillation among breast cancer patients is higher than that among non-breast cancer patients. However, our subgroup analysis showed that the prevalence of atrial fibrillation among patients with breast cancer >65 years of age was four times higher than that among younger breast cancer patients and twice as high as that in the general population >65 years of age (4.4%) (4). Therefore, oncologist must be vigilant about the increased risk of atrial fibrillation in elderly patients with breast cancer.
Study limitations
Our systematic review and meta-analysis had several limitations. First, all the studies included were observational and could not prove causality. However, a bidirectional relationship between breast cancer and atrial fibrillation was found in cohort rather than case-control studies. Most cohort studies were retrospective cohort studies; therefore, reverse causality may still exist, and more prospective studies are needed. Second, most of the studies we included featured female breast cancer patients, and a considerable number of studies included very few male breast cancer patients. Since we could not contact the researchers to obtain the original data, we included these individual male patients. Third, atrial fibrillation is known to contribute significantly to the incidence of stroke. However, none of the studies we included reported an outcome event for stroke; more studies are needed to clarify the outcomes, especially stroke, in patients with atrial fibrillation and breast cancer.
Conclusions
Atrial fibrillation is not uncommon in patients with breast cancer, and there is an increased risk of atrial fibrillation in breast cancer patients and vice versa. In addition, breast cancer patients with atrial fibrillation had increased all-cause mortality. GRADE assessment indicated low certainty for the risk of atrial fibrillation and moderate certainty for the risk of breast cancer. More studies are needed to determine whether screening for breast cancer in patients with atrial fibrillation is necessary.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
RQY contributed to the study concept and design and revised the draft. XDY, QWH, and XL performed the search strategy and contributed to database research, acquisition of data, and statistical analyses. All the authors participated in data analysis, reviewed, and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81960081), the Jiangxi Thousand Talents Plan Project (No. jxsq2019201029), and the 5511 Science and Technology Innovation Talent Program of Jiangxi (No. 20171BCB18004). The source of funding did not have any impact on study design; collection, analysis, and interpretation of data or writing of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1113231/full#supplementary-material
References
1.
SungHFerlayJSiegelRLLaversanneMSoerjomataramIJemalAet al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. 10.3322/caac.21660
2.
RugoHS. Achieving improved survival outcomes in advanced breast cancer. N Engl J Med. (2019) 381:371–2. 10.1056/NEJMe1906236
3.
GuhaADeyAKJneidHIbarzJPAddisonDFradleyM. Atrial Fibrillation in the Era of Emerging Cancer Therapies. Eur Heart J. (2019) 40:3007–10. 10.1093/eurheartj/ehz649
4.
HindricksGPotparaTDagresNArbeloEBaxJJBlomström-LundqvistCet al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. 10.1093/eurheartj/ehab648
5.
PicciniJPHammillBGSinnerMFJensenPNHernandezAFHeckbertSRet al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outc. (2012) 5:85–93. 10.1161/CIRCOUTCOMES.111.962688
6.
AnYOgawaHYamashitaYIshiiMIguchiMMasunagaNet al. Causes of death in Japanese patients with atrial fibrillation: The Fushimi Atrial Fibrillation Registry. Eur Heart J Qual Care Clin Outcomes. (2019) 5:35–42. 10.1093/ehjqcco/qcy033
7.
Abdel-QadirHThavendiranathanPFungKAmirEAustinPCAndersonGSet al. Association of early-stage breast cancer and subsequent chemotherapy with risk of atrial fibrillation. JAMA Netw Open. (2019) 2:e1911838. 10.1001/jamanetworkopen.2019.11838
8.
ApteNDherangePMustafaUYa'qoubLDawsonDHigginbothamKet al. Cancer radiation therapy may be associated with atrial fibrillation. Front Cardiovasc Med. (2021) 8:610915. 10.3389/fcvm.2021.610915
9.
BoermanLMMaassSvan der MeerPGietemaJAMaduroJHHummelYMet al. Long-term outcome of cardiac function in a population-based cohort of breast cancer survivors: A cross-sectional study. Eur J Cancer. (2017) 81:56–65. 10.1016/j.ejca.2017.05.013
10.
D'SouzaMSmedegaardLMadelaireCNielsenDTorp-PedersenCGislasonGet al. Incidence of atrial fibrillation in conjunction with breast cancer. Heart Rhythm. (2019) 16:343–8. 10.1016/j.hrthm.2018.10.017
11.
GuhaAFradleyMGDentSFWeintraubNLLustbergMBAlonsoAet al. Incidence, risk factors, and mortality of atrial fibrillation in breast cancer: a SEER-Medicare analysis. Eur Heart J. (2022) 43:300–12. 10.1093/eurheartj/ehab745
12.
GuzzettiSCostantinoGVernocchiASadaSFundaroC. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern Emerg Med. (2008) 3:227–31. 10.1007/s11739-008-0124-4
13.
SalibaWRennertHSGronichNGruberSBRennertG. Association of atrial fibrillation and cancer: Analysis from two large population-based case-control studies. PLoS ONE. (2018) 13:e0190324. 10.1371/journal.pone.0190324
14.
YunJPChoiEKHanKDParkSHJungJHParkSHet al. Risk of atrial fibrillation according to cancer type: a nationwide population-based study. JACC CardioOncol. (2021) 3:221–32. 10.1016/j.jaccao.2021.03.006
15.
Zubair KhanMGuptaAPatelKAbrahamAFranklinSKimDYet al. Association of atrial fibrillation and various cancer subtypes. J Arrhythm. (2021) 37:1205–14. 10.1002/joa3.12589
16.
ConenDWongJASandhuRKCookNRLeeIMBuringJEet al. Risk of malignant cancer among women with new-onset atrial fibrillation. JAMA Cardiol. (2016) 1:389–96. 10.1001/jamacardio.2016.0280
17.
HungYPHuYWLiuCJinYJChangSLLoLWet al. Risk and predictors of subsequent cancers of patients with newly-diagnosed atrial fibrillation - A nationwide population-based study. Int J Cardiol. (2019) 296:81–6. 10.1016/j.ijcard.2019.08.021
18.
OstenfeldEBErichsenRPedersenLFarkasDKWeissNSSørensenHT. Atrial fibrillation as a marker of occult cancer. PLoS ONE. (2014) 9:e102861. 10.1371/journal.pone.0102861
19.
VinterNChristesenAMSFenger-GronMTjonnelandAFrostL. Atrial fibrillation and risk of cancer: a danish population-based cohort study. J Am Heart Assoc. (2018) 7:e009543. 10.1161/JAHA.118.009543
20.
Wassertheil-SmollerSMcGinnAPMartinLRodriguezBLStefanickMLPerezM. The associations of atrial fibrillation with the risks of incident invasive breast and colorectal cancer. Am J Epidemiol. (2017) 185:372–84. 10.1093/aje/kww185
21.
PageMJMcKenzieJEBossuytPMBoutronIHoffmannTCMulrowCDet al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71
22.
GonçalvesABraudACViretFTarpinCCharaffe-JauffretEJacquemierJet al. High-dose alkylating agents with autologous hematopoietic stem cell support and trastuzumab in ERBB2 overexpressing metastatic breast cancer: a feasibility study. Anticancer Res. (2005) 25:663–667.
23.
JacobJBelinLPiergaJYGobillionAVincent-SalomonADendaleRet al. Concurrent administration of trastuzumab with locoregional breast radiotherapy: long-term results of a prospective study. Breast Cancer Res Treat. (2014) 148:345–53. 10.1007/s10549-014-3166-5
24.
KellyHKimmickGDeesECCollichioFGattiLSawyerLet al. Response and cardiac toxicity of trastuzumab given in conjunction with weekly paclitaxel after doxorubicin/cyclophosphamide. Clin Breast Cancer. (2006) 7:237–43. 10.3816/CBC.2006.n.035
25.
KropIESuterTMDangCTDirixLRomieuGZamagniCet al. Feasibility and cardiac safety of trastuzumab emtansine after anthracycline-based chemotherapy as (neo)adjuvant therapy for human epidermal growth factor receptor 2-positive early-stage breast cancer. J Clin Oncol. (2015) 33:1136–42. 10.1200/JCO.2014.58.7782
26.
MeattiniICecchiniSMuntoniCScottiVDe Luca CardilloCMangoniMet al. Cutaneous and cardiac toxicity of concurrent trastuzumab and adjuvant breast radiotherapy: a single institution series. Med Oncol. (2014) 31:891. 10.1007/s12032-014-0891-x
27.
PiergaJYPetitTDelozierTFerreroJMCamponeMGligorovJet al. Neoadjuvant bevacizumab, trastuzumab, and chemotherapy for primary inflammatory HER2-positive breast cancer (BEVERLY-2): an open-label, single-arm phase 2 study. Lancet Oncol. (2012) 13:375–84. 10.1016/S1470-2045(12)70049-9
28.
PolkAShahmarvandNVistisenKVaage-NilsenMLarsenFOSchouMet al. Incidence and risk factors for capecitabine-induced symptomatic cardiotoxicity: a retrospective study of 452 consecutive patients with metastatic breast cancer. BMJ Open. (2016) 6:e012798. 10.1136/bmjopen-2016-012798
29.
WangSYLongJBHurriaAOwusuCSteingartRMGrossCPet al. Cardiovascular events, early discontinuation of trastuzumab, and their impact on survival. Breast Cancer Res Treat. (2014) 146:411–9. 10.1007/s10549-014-3029-0
30.
AgrawalRKAlhassoABarrett-LeePJBlissJMBlissPBloomfieldDet al. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol. (2011) 100:93–100. 10.1016/j.radonc.2011.06.026
31.
HoriguchiHYasunagaHHashimotoHMatsudaS. Incidence of severe adverse events requiring hospital care after trastuzumab infusion for metastatic breast cancer: a nationwide survey using an administrative claim database. Breast J. (2011) 17:683–5.
32.
NewcombeRG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. (1998) 17:857–72. 10.1002/(SICI)1097-0258(19980430)17:8<857::AID-SIM777>3.0.CO;2-E
33.
FreemanMFTukeyJW. Transformations related to the angular and the square root. Ann Mathem Stat. (1950) 21:607–611. 10.1214/aoms/1177729756
34.
DerSimonianRLairdN. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. 10.1016/0197-2456(86)90046-2
35.
LiuXLongCXiongQChenCMaJSuYet al. Association of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with risk of COVID-19, inflammation level, severity, and death in patients with COVID-19: A rapid systematic review and meta-analysis. Clin Cardiol. (2020) 5:23421. 10.1002/clc.23421
36.
Higgins JP, Green, S,. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0. The Cochrane Collaboration, 2017. (2011). Available from https://handbook-5-1.cochrane.org/chapter_10/10_4_3_1_recommendations_on_testing_for_funnel_plot_asymmetry.htm (acessed March 2017).
37.
AtkinsDBestDBrissPAEcclesMFalck-YtterYFlottorpSet al. Grading quality of evidence and strength of recommendations. BMJ. (2004) 328:1490. 10.1136/bmj.328.7454.1490
38.
GuyattGHOxmanADVistGEKunzRFalck-YtterYAlonso-CoelloPet al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336:924–6. 10.1136/bmj.39489.470347.AD
39.
Abdel-QadirHThavendiranathanPAustinPCLeeDSAmirETuJVet al. Development and validation of a multivariable prediction model for major adverse cardiovascular events after early stage breast cancer: a population-based cohort study. Eur Heart J. (2019) 40:3913–20. 10.1093/eurheartj/ehz460
40.
EzazGLongJBGrossCPChenJ. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. (2014) 3:e000472. 10.1161/JAHA.113.000472
41.
HanHChenLLinZWeiXGuoWYuYet al. Prevalence, trends, and outcomes of atrial fibrillation in hospitalized patients with metastatic cancer: findings from a national sample. Cancer Med. (2021) 10:5661–70. 10.1002/cam4.4105
42.
LiQLiuFTangYLeeSLangCBaiLet al. The distribution of cardiovascular-related comorbidities in different adult-onset cancers and related risk factors: analysis of 10 year retrospective data. Front Cardiovasc Med. (2021) 8:695454. 10.3389/fcvm.2021.695454
43.
LinHFLiaoKFChangCMLinCLLaiSWHsuCY. Correlation of the tamoxifen use with the increased risk of deep vein thrombosis and pulmonary embolism in elderly women with breast cancer: A case-control study. Medicine. (2018) 97:e12842. 10.1097/MD.0000000000012842
44.
MeryBFouillouxARowinskiECatella-ChatronJGuichardJBDa CostaAet al. Cardiovascular disease events within 5 years after a diagnosis of breast cancer. BMC Cancer. (2020) 20:337. 10.1186/s12885-020-06838-w
45.
OkuraYTakayamaTOzakiKTanakaHSekiHTakenouchiTet al. Burden of cardiovascular disease in Japanese cancer patients and survivors: a single cancer-center study in Niigata City. Int J Clin Oncol. (2019) 24:196–210. 10.1007/s10147-018-1341-0
46.
OrdingAGHorvath-PuhoEGarneJPNyströmPWVybergMSørensenHTet al. Impact of comorbidity on risk of venous thromboembolism in patients with breast cancer: a Danish population-based cohort study. BMJ Open. (2014) 4:e005082. 10.1136/bmjopen-2014-005082
47.
YamashitaKTanakaHHatazawaKTanakaYSumimotoKShonoAet al. Association between clinical risk factors and left ventricular function in patients with breast cancer following chemotherapy. Int J Cardiovasc Imag. (2021) 37:197–205. 10.1007/s10554-020-01976-5
48.
YancikRWesleyMNRiesLAHavlikRJEdwardsBKYatesJW. Effect of age and comorbidity in postmenopausal breast cancer patients aged 55 years and older. Jama. (2001) 285:885–92. 10.1001/jama.285.7.885
49.
YaylaliYTSaricopurAYurtdasMSenolHGokoz-DoguG. Atrial function in patients with breast cancer after treatment with anthracyclines. Arq Bras Cardiol. (2016) 107:411–9. 10.5935/abc.20160146
50.
LeongDPCaronFHillisCDuanAHealeyJSFraserGet al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. (2016) 128:138–40. 10.1182/blood-2016-05-712828
51.
KhouriMGDouglasPSMackeyJRMartinMScottJMScherrer-CrosbieMet al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. (2012) 126:2749–63. 10.1161/CIRCULATIONAHA.112.100560
52.
YuanMTseGZhangZHanXWuWKKLiGet al. The incidence of atrial fibrillation with trastuzumab treatment: A systematic review and meta-analysis. Cardiovasc Therap. (2018) 36:e12475. 10.1111/1755-5922.12475
53.
ChuangSYHsuPFLinFJHuangYWWangGZChangWCet al. Association between nonsteroidal anti-inflammatory drugs and atrial fibrillation among a middle-aged population: a nationwide population-based cohort. Br J Clin Pharmacol. (2018) 84:1290–300. 10.1111/bcp.13558
54.
LeeCWMuoCHLiangJALinMCKaoCH. Atrial fibrillation is associated with morphine treatment in female breast cancer patients: a retrospective population-based time-dependent cohort study. Medicine. (2016) 95:e3102. 10.1097/MD.0000000000003102
55.
FaresWH. Amiodarone and the risk of cancer: a nationwide population-based study. Cancer. (2013) 119:4051. 10.1002/cncr.28266
56.
BiggarRJWohlfahrtJOudinAHjulerTMelbyeM. Digoxin use and the risk of breast cancer in women. J Clin Oncol. (2011) 29:2165–70. 10.1200/JCO.2010.32.8146
57.
HossanMSChanZYCollinsHMShiptonFNButlerMSRahmatullahMet al. Cardiac glycoside cerberin exerts anticancer activity through PI3K/AKT/mTOR signal transduction inhibition. Cancer Lett. (2019) 453:57–73. 10.1016/j.canlet.2019.03.034
58.
ZhangHQianDZTanYSLeeKGaoPRenYRet al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci U S A. (2008) 105:19579–86. 10.1073/pnas.0809763105
59.
BiggarRJAndersenEWKromanNWohlfahrtJMelbyeM. Breast cancer in women using digoxin: tumor characteristics and relapse risk. Breast Cancer Res. (2013) 15:R13. 10.1186/bcr3386
60.
AdamsGNSharmaBKRosenfeldtLFrederickMFlickMJWitteDPet al. Protease-activated receptor-1 impedes prostate and intestinal tumor progression in mice. J Thromb Haemost. (2018) 16:2258–69. 10.1111/jth.14277
61.
DeFeoKHayesCChernickMRynJVGilmourSK. Use of dabigatran etexilate to reduce breast cancer progression. Cancer Biol Ther. (2010) 10:1001–8. 10.4161/cbt.10.10.13236
62.
ScottLLiNDobrevD. Role of inflammatory signaling in atrial fibrillation. Int J Cardiol. (2019) 287:195–200. 10.1016/j.ijcard.2018.10.020
63.
DiakosCICharlesKAMcMillanDCClarkeSJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. (2014) 15:e493–503. 10.1016/S1470-2045(14)70263-3
64.
ErshaidNSharonYDoronHRazYShaniOCohenNet al. NLRP3 inflammasome in fibroblasts links tissue damage with inflammation in breast cancer progression and metastasis. Nat Communic. (2019) 10:4375. 10.1038/s41467-019-12370-8
65.
YaoCVelevaTScottLCaoSLiLChenGet al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. (2018) 138:2227–42. 10.1161/CIRCULATIONAHA.118.035202
66.
LakoskiSGJonesLWKroneRJSteinPKScottJM. Autonomic dysfunction in early breast cancer: Incidence, clinical importance, and underlying mechanisms. Am Heart J. (2015) 170:231–41. 10.1016/j.ahj.2015.05.014
67.
ChangHMOkwuosaTMScarabelliTMoudgilRYehETH. Cardiovascular complications of cancer therapy: best practices in diagnosis, prevention, and management: Part 2. J Am Coll Cardiol. (2017) 70:2552–65. 10.1016/j.jacc.2017.09.1095
68.
MarchettiMGiaccheriniCMasciGVerzeroliCRussoLCelioLet al. Thrombin generation predicts early recurrence in breast cancer patients. J Thromb Haemost. (2020) 18:2220–31. 10.1111/jth.14891
69.
CardinSLiDThorin-TrescasesNLeungTKThorinENattelS. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. (2003) 60:315–25. 10.1016/j.cardiores.2003.08.014
70.
WongRS. Apoptosis in cancer: from pathogenesis to treatment. J Exper Clin Cancer Res. (2011) 30:87. 10.1186/1756-9966-30-87
71.
LateefNKapoorVAhsanMJLatifAAhmedUMirzaMet al. Atrial fibrillation and cancer; understanding the mysterious relationship through a systematic review. J Community Hosp Intern Med Perspect. (2020) 10:127–32. 10.1080/20009666.2020.1726571
72.
ZhangMLiLLZhaoQQPengXDWuKLiXet al. The association of new-onset atrial fibrillation and risk of cancer: a systematic review and meta-analysis. Cardiol Res Pract. (2020) 2020:2372067. 10.22541/au.158981188.83217663
73.
MauroELucaFTettaCPariseOParriniIPariseGet al. Breast cancer and atrial fibrillation. J Clin Med. (2022) 11:1417. 10.3390/jcm11051417
Summary
Keywords
atrial fibrillation, breast cancer, incidence, prevalence, association
Citation
Yao X, Hu Q, Liu X, Ling Q, Leng Y, Zhao H, Yu P, Ma J, Zhao Y, Liu M and Yang R (2023) Atrial fibrillation and breast cancer—Vicious twins? A systematic review and meta-analysis. Front. Cardiovasc. Med. 10:1113231. doi: 10.3389/fcvm.2023.1113231
Received
01 December 2022
Accepted
13 February 2023
Published
10 March 2023
Volume
10 - 2023
Edited by
Yang Liu, Guangdong Provincial People's Hospital, China
Reviewed by
Tong Liu, Tianjin Medical University, China; Yun-Jiu Cheng, Guangdong Academy of Medical Sciences, China
Updates
Copyright
© 2023 Yao, Hu, Liu, Ling, Leng, Zhao, Yu, Ma, Zhao, Liu and Yang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renqiang Yang yangrenqiangcn@163.com
†These authors have contributed equally to this work
This article was submitted to Cardiac Rhythmology, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.