Abstract
The prevalence of obesity worldwide has increased in recent decades not only among adults, but also in children and adolescents. This phenomenon contributes to an increased risk of cardiovascular diseases (CVD), also after the adjustment for conventional risk factors such as hypertension, diabetes and dyslipidemia. Indeed, obesity contributes to the development of insulin resistance, endothelial dysfunction, sympathetic nervous system activation, increased vascular resistance and inflammatory and prothrombotic state which promote the incidence of major cardiovascular events. On the basis of this evidence, in 2021 obesity has been acknowledged as a definite pathological identity and identified as a recurrent, chronic non-communicable disease. Therapeutic strategies for the pharmacological treatment of obesity include the combination of naltrexone and bupropione and the lipase inhibitor orlistat and they have been recently implemented with the glucagon like peptide-1 receptor agonists semaglutide and liraglutide, which have produced positive and sustained effects on body weight reduction. If drug interventions are not effective, bariatric surgery may be considered, representing an efficacious treatment option for extreme obesity or obesity with comorbidities. The present executive paper is aimed to increase knowledge on the relationships between obesity and CVD, to raise the perception of this condition which is currently insufficient and to support the clinical practice management.
Introduction
In the last decades the prevalence of obesity [defined as body mass index (BMI) ≥ 30 Kg/m2] has enormously increased, becoming a real epidemic which involves hundred millions of people worldwide. The Global Burden of Disease Obesity Collaborations estimated that in 2017 about 603.7 million of adults were affected by obesity, doubling as compared to the early ‘80s (1). These numbers, although often underestimated, become even more impressive when overweight subjects are considered, reaching 3 billions people worldwide (2). Moreover, the World Health Organization has estimated that 18% of males and 21% of females will be obese by 2025, with 40% of these individuals reaching BMI levels ≥25 Kg/m2. This phenomenon is worringly widespreading also among children and adolescents irrespective of age, accounting for 40 and 340 million of obese and overweight subjects, respectively, with a 47% increase in the last thirty years (3).
Although a large body of evidence lends support to the pathophysiological link between obesity and cardiovascular (CV) diseases (CVD), obesity has been considered for a long time a minor risk factor or even a simple amplifier of the recognized role of the other well established CV risk factors, such as hypertension, diabetes and dyslipidemias. Only in 2021 obesity has been finally acknowledged as a definite pathological identity and identified as a recurrent, chronic non-communicable disease (4).
The Italian Society of Cardiovascular Prevention (SIPREC) with the active contributions of experts from different Italian Scientific Societies (see Appendix) has recently produced an updated multisciplinary consensus document to analyze and discuss evidence about the role of obesity in the development of CV events as well as the available pharmacological and non-pharmacological strategies to manage this condition. The purpose of this consensus document was to provide an integrated tool to afford all the main aspects of the cause-effect relationship between obesity and CVD.
In the present executive paper we provide a more synthetic companion document addressed to treat physicians and particularly to Cardiology specialists with the aim to increase knowledge on the relationships between obesity and CVD, to raise the perception of this condition which is currently insufficient and to support the clinical practice management.
Pathophysiological mechanisms of cardiovascular diseases in obesity
Different pathophysiological mechanisms are involved in the development of CVD in obese patients (Table 1).
Table 1
| Condition | Involved mechanisms |
|---|---|
| Hypertension | Endothelial dysfunction, reduced NO, increased levels of endothelial growth factor, plasminogen-1 and thromboxane A2 increasing peripheral vascular resistance and arterial stiffness |
| Atherosclerosis | Visceral obesity associated to a rapid progression of coronary calcifications and to a greater vulnerability of atherosclerotic plaques |
| Heart failure | Neurohormonal imbalance, increased production of ROS, inflammatory mediators, leptin, resistin, visfatin and adipsin and reduced synthesis of adiponectin |
| Diabetes | Activation of NF-κB, p38 and MAPK pathways, increased levels of IFN γ, TNFα, MCP-1, IL-1β and IL-6 and adipokines leading to insulin resistance and of concomitant metabolic alterations |
| Venous thromboembolism | Elevated levels of prothrombotic molecules including Factor VII, fibrinogen and tissue factor and increased expression of plasminogen activator inhibitor-1 (PAI-1) |
| Pulmonary hypertension | Elevated levels of cytokines, TNF-α, and interleukins, IFN γ, insulin resistance and oxidative stress |
Obesity and cardiovascular diseases: summary of pathophysiological mechanisms.
See the text for abbreviations.
First of all, it is today well recognized that the expansion of the visceral adipose tissue is associated to a dysregulation of adipokines secretion, of mitochondrial function and of lipid and glucose metabolism. Concomitant development of insulin resistance, endothelial dysfunction, sympathetic nervous system activation, increased vascular resistance and inflammatory and prothrombotic state which are promoted by obesity may definitely contribute to a higher susceptibility to CVD (5) (Figure 1).
Figure 1
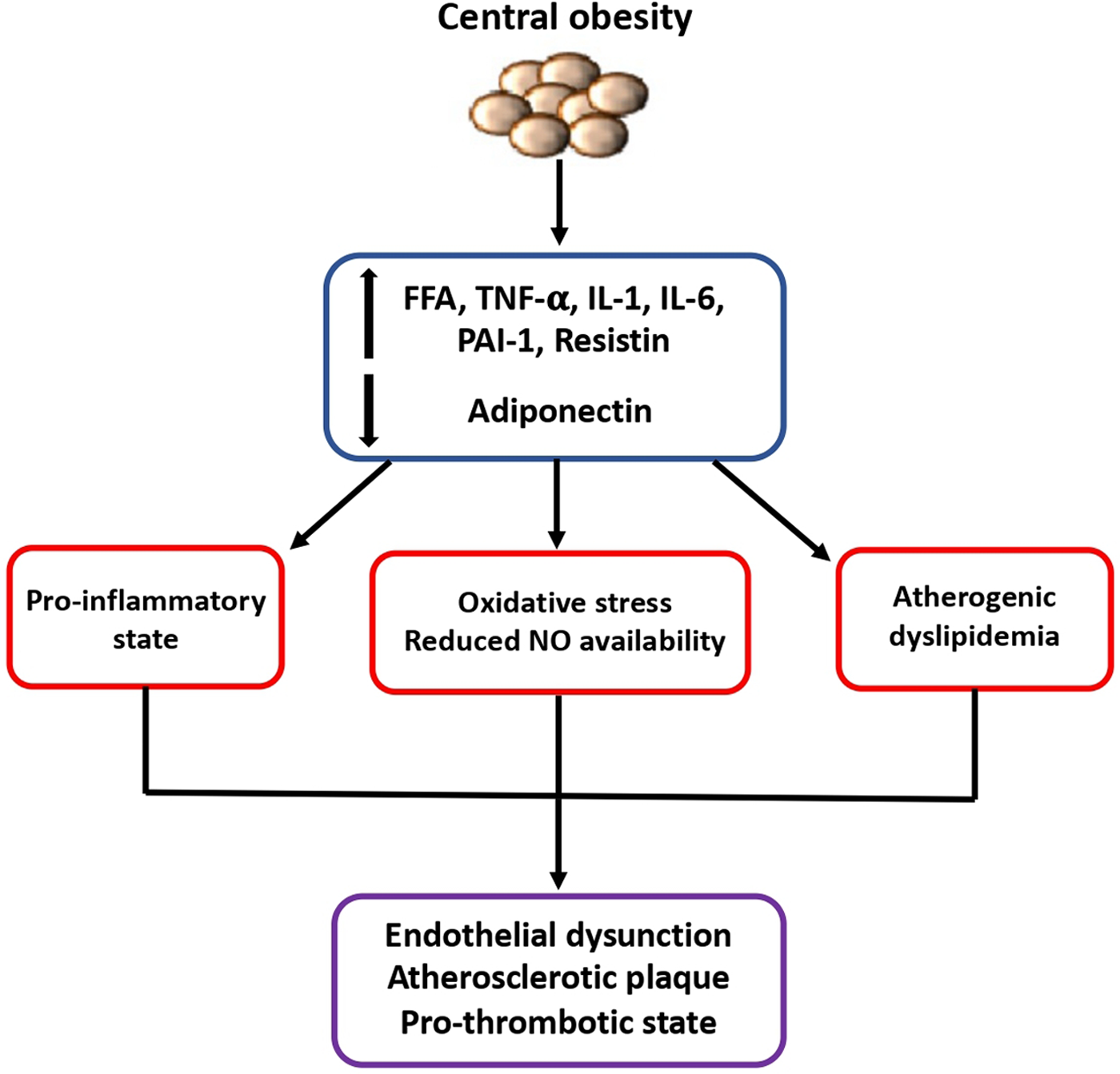
Mechanisms of higher susceptibility to cardiovascular diseases in obese patients. The expansion of visceral adipose tissue is associated to a dysregulated secretion of adipokines and other inflammatory cytokines which contribute to the development of insulin resistance, endothelial dysfunction, and prothrombotic state, finally leading to increased susceptibility to cardiovascular diseases. FFA, free fatty acid; IL-1, interleukin-1; IL-6, interleukin-6; NO, nitric oxide; PAI-1, plasminogen activator inhibitor-1; TNF, tumor necrosis factor.
Obesity and diabetes
Intracellular lipid accumulation promotes the expression of toll-like-receptor-4 (TLR4) in adipocyte cells and resident macrophages, favoring the activation of NF-κB, p38 and MAPK pathways, thus increasing the production of reactive oxygen species (ROS) and the secretion of inflammatory cytokines (6). Indeed, in inflammed adipose tissue T-lymphocytes express increased interferon- γ (IFN γ) levels which stimulate the production of other inflammatory cytokines including tumor necrosis factor-α (TNFα), monocyte chemoattractant protein-1 (MCP-1), interleukins 1β and 6 (IL-1β and IL-6) that recruit monocytes and mature macrophages in a vicious circle leading to hypoxia and cellular death (7, 8). Moreover, dysregulated adipose tissue produces different adipokines, including leptin, resistin, lipocalin 2, adiponectin, apelin and fibroblast growth factor-21 (FGF-21), which play an important role in the development of insulin resistance and of concomitant metabolic alterations in obese subjects (9). Insulin resistance, in turn, promotes the development and progression of the metabolic syndrome which by itself perpetuates and worsens the obesity status.
As a consequence, type 2 diabetes is a frequent associated condition accounting for 40% of obese patients. The Diabetes Prevention Study that investigated the effects of lifestyle modification on the risk of developing diabetes demonstrated that a 5% decrease of baseline body weight was associated to a 60% reduction in the risk of new-onset diabetes compared to subjects who did not achieve a weight loss (10). Consistently, in the Diabetes Prevention Program a 7% body weight reduction was associated to a 58% lower risk of developing diabetes at 4-year follow-up (11).
Obesity and hypertension
Obesity causes microvascular damage corresponding to capillary number reduction and endothelial dysfunction, contributing to ROS secretion, free fatty acid (FFA) release, increased vascular resistances and hypertension (12).
A study conducted on 3,216 subjects has shown that 44% of hypertensive subjects were obese, whereas this percentage was only 11% among normotensive individuals (13).
Other studies demonstrated a linear relationship between body weight and blood pressure (BP) levels, corresponding to a 20%–30% increase in the risk of hypertension for each 5% rise in body weight (14). Among the proposed pathophysiological mechanisms, the increased levels of circulating FFA, angiotensin-II and leptin have been shown to play an important role (15). Obesity is indeed characterized by an increase in heart rate and in tubular sodium and water reabsorption, resulting in volume overload and increased BP levels (16). Moreover, the inflammatory status is associated with endothelial dysfunction, reduced nitric oxide production and increase in angiogenetic factors such as endothelial growth factor, plasminogen-1 and thromboxane A2, which contribute to increased peripheral vascular resistance, arterial stiffness and hypertension (17–19). Several studies have demonstrated that hypertension is a principal mediator of CV sequalae of obesity (20). Otherwise, a weight loss of 8 Kg is associated to a reduction of left ventricular wall thickness in mildly obese patients with hypertension (21).
Obesity and atherosclerotic disease
A large body of evidence supports the association between obesity and development of major CV events, including myocardial infarction (MI), heart failure (HF) and sudden cardiac death (22–24).
In obese patients the development of atherosclerosis starts earlier and has a quicker progression than in individuals with normal body weight. Pathological studies have also shown that visceral obesity is associated to a greater vulnerability of coronary plaques (22).
In a meta-analysis of 6 studies, which included 1,593 obese subjects with documented coronary artery disease, increased mortality risk was associated to the excess of visceral obesity, defined as augmented waist circumference and waist-hip ratio, rather than to BMI (25). In the Health, Aging and Body composition study, visceral obesity was associated to an increased incidence of MI in women aged between 70 and 79 years at a 4.6-year follow-up (26). The CARDIA (Coronary Artery Risk Development in Young Adults) study has demonstrated a significant relationship between the time of exposure to excessive visceral adiposity and the presence and progression of coronary calcifications (27).
Other studies have shown that the accumulation of ectopic cardiac fat at pericardial and epicardial levels might be involved in the development of coronary atherosclerosis (28, 29). In the Multi-Ethnic Study of Atherosclerosis pericardial fat has emerged as a predictive factor for coronary events with an additive role to other traditional risk factors (30).
A 10 kg increase in body weight is associated with a 12% higher risk of coronary artery disease, worsened by the frequent coexistence of microvascular dysfunction (21). In a meta-analysis conducted on 300,000 subjects within the total of 18,000 registered acute coronary events, these were significantly more frequent among obese and overweight individuals (31).
In addition, for each kg/m2 of BMI above the normal range the risk of ischemic and hemorrhagic stroke increases by 4% and 6% respectively (21).
Obesity and heart failure
Obesity contributes to the development of atrial and ventricular remodeling, systolic and diastolic dysfunction and to an increase in ventricular filling pressures and pulmonary pressures which result in subclinical organ damage and then may progress to overt HF (21–23). Tachycardia, excess blood volume and increased vascular resistance and cardiac overload are common CV “finger-prints” in obese subjects. Moreover, increased fibrosis, reduced conduction velocity, epicardial fat and fatty infiltration represent detrimental factors to atrial and ventricular electrophysiologcal properties which may promote a proarrhythmic substrate (32). These morphological and functional changes often lead to atrial fibrillation which per se represents another patho-physiological mechanism of HF as well as a condition predisposing to stroke (23) (Figure 2).
Figure 2
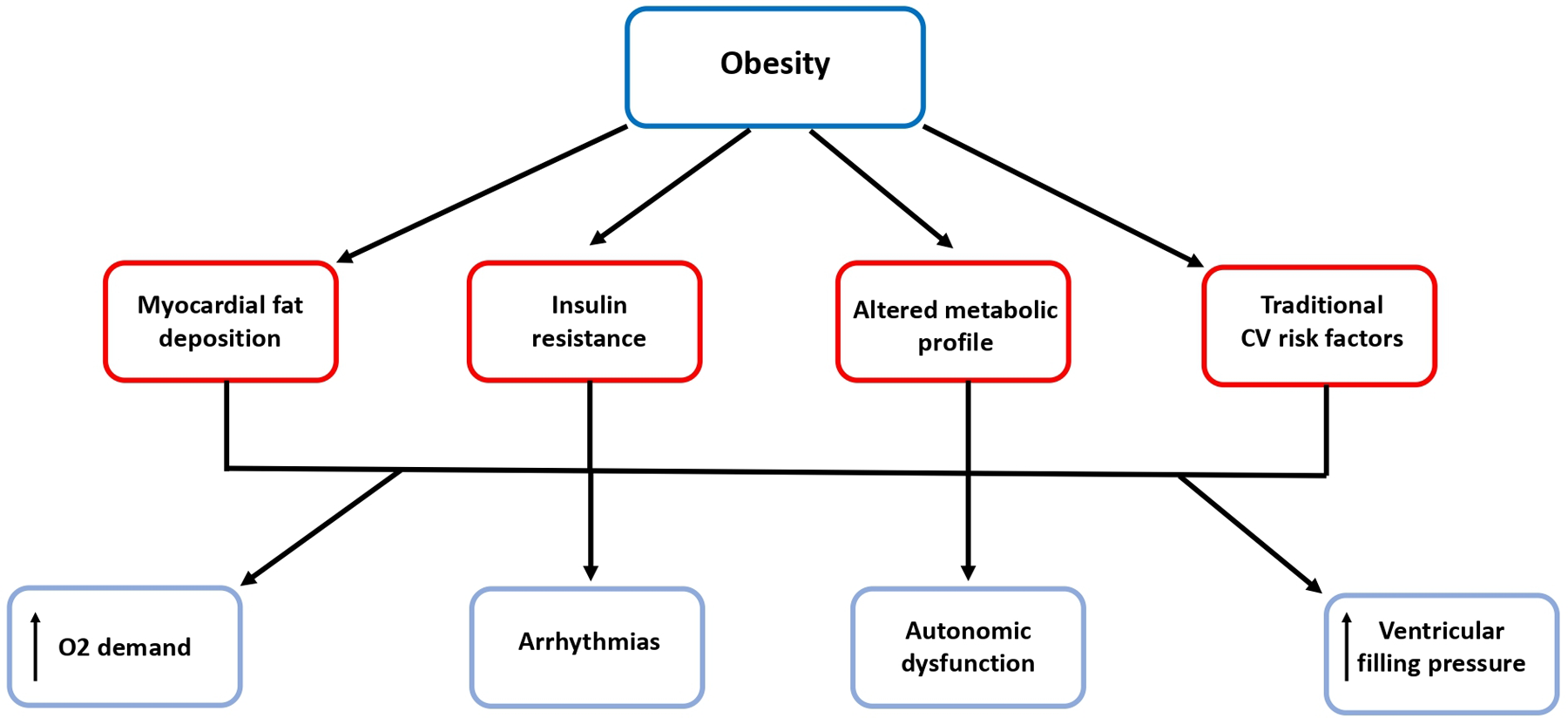
Obesity predisposes to arrhythmias and heart failure. Beside the relationship with traditional risk factors, obesity is associated with myocardial fat deposition, insulin resistance and altered metabolic profile, which contribute to the development of atrial and ventricular remodeling, systolic and diastolic dysfunction and to increased ventricular filling pressures and finally to arrhythmias and overt HF. CV, cardiovascular; O2, oxygen.
A key role in the pathogenesis of HF is carried out by the neurohormonal imbalance consisting in the hyperactivation of the sympathetic nervous and renin angiotensin aldosterone systems, by the increased production of ROS, inflammatory mediators including IL-6, TNF-α, C-reactive protein, leptin, resistin, visfatin and adipsin and by the reduced synthesis of adiponectin (33).
The excess of visceral adiposity causes an increased degradation of natriuretic peptides (NPs) (34), which are produced by the heart in conditions of volume and pressure overload and exert different protective functions in HF with natriuretic, diuretic, anti-fibrotic and anti-remodeling actions counterbalancing the detrimental neurohormonal hyperactivation. NPs also promote lipolysis and synthesis of adiponectin (35).
In obese subjects HF develops about 10 years earlier than in those with normal body weight. The risk of HF is increased respectively by 70% after 20% and 90% after 30 years lived in a condition of obesity (36).
A sub-analysis of the CHARM study showed that the 75% of patients affected by HF before an age of 40 years were obese or overweight (37).
Among patients affected by HF the proportions of obese and overweight subjects range between 32% and 49% and 31%–40%, respectively, and a 10% of HF cases is directly referable to obesity (21).
In addition, every 2 years lived in a condition of obesity the risk of CV mortality is increased by 7% (38). Consistently, data from the Framingham Heart Study have shown a linear relationship between the duration of obesity and mortality irrespective of BMI and concomitant risk factors. The incidence of sudden cardiac death is 40-fold higher in obese subjects as a consequence of increased electrical irritability, ion channel remodeling, reduction of connexin proteins (39) and impaired sympathetic-vagal balance and of more frequent and complex ventricular arrhythmias even in the absence of an overt HF condition (35).
Obesity and obstructive sleep apnea syndrome
Obesity represents one of the most important reversible risk factors for obstructive sleep apnea syndrome (OSAS), accounting for 41% and 58% of total and moderate-to-severe cases, respectively (40).
OSAS is characterized by repeated episodes of upper airway obstruction during sleep, resulting in repetitive hypoxemia and intermittent pauses in breathing causing oxygen desaturation, arousal from sleep and excessive daytime sleepiness (41). The prevalence of OSAS is three-fold higher among obese subjects because of airways narrowing due to fat accumulation, increased mechanical loading of the respiratory system and reduced functional residual capacity (42). As a consequence, OSAS may contribute to increase the obesity-related risk of hypertension, stroke, CVD and sudden death (43).
Obesity, venous thromboembolism and pulmonary hypertension
Obesity has been associated with an increased risk of venous thromboembolism (VTE), consisting in pulmonary embolism and deep venous thrombosis (44). Different factors contribute to the development of VTE in obese patients, including elevated levels of prothrombotic molecules such as Factor VII, fibrinogen and tissue factor, an increased expression in visceral fat of plasminogen activator inhibitor-1 (PAI-1) resulting in impaired fibrinolysis, reduced venous return and physical activity (45, 46). Moreover, obese patients are more prone to develop pulmonary hypertension after a first PE (47). In such a context, systemic and local inflammation with elevated levels of cytokines, TNF-α, and interleukins, IFN γ, insulin resistance and oxidative stress play a role in exacerbating the vascular remodeling process involved in pulmonary hypertension (47).
Obesity and outcomes of COVID-19: the lessons learned
In the last 3 years the coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has dramatically changed the priorities and the use of available resources by the national healthcare systems (48, 49).
A bidirectional link exists between obesity and COVID-19. Due to the lockdown measures introduced in the early phases of the pandemic aimed at reducing SARS-CoV-2 transmission a significant increase in the incidence of obesity has been documented, this phenomenon being described as “covibesity” (50). On the other hand, a large body of evidence has shown that obesity is a determinant factor for the severity of COVID-19.
In a retrospective study conducted on 124 patients who were admitted to intensive care unit (ICU) for severe COVID-19 the 75.8% were obese (51). In another study on 3,615 patients, obesity has emerged as a fundamental prognostic factor for a severe course of COVID-19. Patients aged <60 years with a BMI >30 kg/m² had a 2-fold higher risk of being hospitalized and to develop a severe disease compared to those with normal body weight. In a prospective cohort study that enrolled 7 million individuals has demonstrated that a BMI >23 kg/m² is associated to a worse prognosis in patients affected by severe COVID-19, particularly in those aged <40 years and Black people. A linear relationship has been detected between BMI increase and the risk of hospitalizations, mortality and ICU admission. The risk of being admitted to ICU was 4-fold higher in patients with severe obesity (BMI >35 kg/m²) (52).
The hypothesized pathophysiological mechanisms include the secretion of adipokines, chemokines and cytokines, the impaired qualitative and quantitative response of immune cells in the adipose tissue, with a significant reduction of Th2 and Treg cells, of M2 macrophages and an increase of pro-inflammatory T CD8+ and M1 macrophages (53).
For these reasons and particularly in view of the high toll paid by obese patients during the course of SARS-COV-2 infection, as well as during the course of other viral or bacterial infections, it appears reasonable to consider the obese as a frail population which needs specific measures of prevention and care.
Pharmacological and non-pharmacological strategies
Lifestyle changes represent the first step to achieve and maintain an effective body weight reduction.
Energy restriction is the cornerstone of weight loss, particularly when associated to physical activity. With this aim, several types of dietetic strategies may be suggested consisting in hypocaloric diets, Mediterranean diet, high-protein diets to preserve lean muscle mass and enhance satiety, low or very low carbohydrate diets, moderate carbohydrate diets and low-fat diets, intermittent fasting or time-restricted eating diets. Among the proposed strategies, the benefits of the Mediterranean diet tend to persist over time without an increased risk of ketogenesis (54, 55).
However, this type of intervention is often not sufficient and additional pharmacological and not-pharmacological measures are required (54).
For many years bariatric/metabolic surgery (BMS), defined as the procedures inducing loss of body weight throughout the modification of gastrointestinal physiology, has represented the only available and more effective strategy. BMI ≥40 kg/m2 or BMI ≥35 kg/m2 with comorbidities are considered the threshold for surgery by international guidelines. The dominant procedures are sleeve gastrectomy and roux-en-Y gastric bypass, accounting for approximately 90% of all operations performed worldwide with efficacious mid- and long-term outcomes. Other approaches include biliopancreatic diversion with duodenal switch, one-anastomosis gastric bypass and the less invasive adjustable gastric banding (56). BMS procedures produce a 14%–25% body weight reduction associated to a significant lower risk of hypertension, diabetes, non-alcoholic fatty liver disease and mortality. However, BMS remains an underused tool, prescribed only to the 1%–2% of subjects who may benefit, probably due to inadequate information and support from healthcare systems and to the risks associated with surgery (57, 58).
In the last few years different pharmacological strategies have been introduced in clinical practice.
Liraglutide, a glucagon-like peptide-1 receptor agonist (GLP1-RA) already approved for the treatment of type 2 diabetes has been recently introduced at the dosage of 3 mg for obese subjects with associated comorbidities such as hypertension, dyslipidemia and obstructive sleep apnea syndrome (59–63). Beside the glucose-lowering effect related to the stimulation of insulin secretion, liraglutide slows gastric emptying and increases hypothalamic sense of satiety, stimulating pro-opiomelanocortin (POMC) neurons (64). In the SCALE Obesity and Prediabetes the loss of body weight produced by the treatment with liraglutide has been maintained for 3 years and has been associated to a 80% reduction of the risk of developing diabetes and to an improvement of metabolic profile (65). Moreover, a body weight loss of 7.8 Kg was associated with a 12.5 mmHg reduction of systolic BP (65).
The association of naltrexone, used for the treatment of depression and nicotine addiction, with bupropion, used for addiction to opioids and alcohol, has been demonstrated to reduce body weight by acting on hypothalamic nucleus arcuatus and on the dopaminergic mesolimbic system (66–70). In particular, bupropion stimulates POMC neurons to release α-melanocyte-stimulating hormone, whereas naltrexone blocks the negative feedback produced by the action of β-endorphins on POMC neurons. As a consequence, naltrexone enhances the action of bupropion increasing energetic expenditure and reducing appetite (71).
Semaglutide, another long lasting GLP1-RA, has been recently approved at the dosage of 2.4 mg. The STEP (Semaglutide Treatment Effect in People with obesity) study and its sub-analyses have generated great enthusiasm due to the greater loss of body weight compared to previously experimented drugs (72–76). Treatment with semaglutide produced a 5% reduction of body weight in >90% of subjects and a 20% reduction in about 35%, these results being comparable to those achieved with BMS. Consistently, patients who received semaglutide improved their BP control and their exercise performance (72–76). The Semaglutide Effects on Heart Disease and Stroke in Patients with Overweight or Obesity (SELECT) study is testing the superiority of semaglutide 2.4 mg subcutaneously once weekly compared to placebo in preventing major adverse cardiovascular events in patients with established CVD and overweight or obesity but without diabetes. As such, SELECT has the potential for advancing new approaches to CVD risk reduction while targeting obesity (77).
Growth differentiation factor 15 (GDF15), a distant member of the transforming growth factor-β, has been demonstrated to bind glial cell-derived neurotrophic factor family receptor alpha-like (GFRAL) reducing the intake of high-fat diets in animal models, to recruit the receptor tyrosine kinase (RET) contributing to weight loss and to improve glycemic control (78). On the basis of these evidence, long-acting analogues of GDF15 are currently under investigation and might represent a future interesting therapeutic option for obese patients.
Conclusions
In this document the SIPREC Committee aimed to provide an extended update of the role of obesity not only as an amplifier of traditional risk factors, but also as an independent complex chronic and recurrent condition whose pathophysiological aspects needs further studies and whose management deserves specific and targeted pharmacological and non-pharmacological strategies.
The clinical appraisal of obesity must increase, as it is deserved by a chronic disease which carries a heavy burden of CV and metabolic consequences. More structured lifestyle advice and new available medications should be systematically prescribed whenever appropriate and as early as possible in the clinical practice to reduce the consequent disease burden and the metabolic and CV sequelae of this condition.
Our auspices are to promote an increasing interest in the medical community and the adoption of early and effective tailored treatment strategies to fight this emerging disease.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
MV and GG contributed to the design, revision and draft of the work and approved the version to be published. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
AfshinAForouzanfarMHReitsmaMBSurPEstepKLeeAet alGBD 2015 Obesity collaborators. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377:13–27. 10.1056/NEJMoa1614362
2.
MaffetonePBRivera-DominguezILaursenPB. Overfat and underfat: new terms and definitions long overdue. Front Public Health. (2017) 4:e00279. 10.3389/fpubh.2016.00279
3.
TalukdarDSeenivasanSCameronAJSacksG. The association between national income and adult obesity prevalence: empirical insights into temporal patterns and moderators of the association using 40 years of data across 147 countries. PLoS One. (2020) 15(5):e0232236. 10.1371/journal.pone.0232236
4.
BurkiT. European Commission classifies obesity as a chronic disease. Lancet Diabetes Endocrinol. (2021) 9(7):418. 10.1016/S2213-8587(21)00145-5
5.
ChaitAden HartighLJ. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. (2020) 7:22. 10.3389/fcvm.2020.00022
6.
LiBLeungJCKChanLYYYiuWHTangSCW. A global perspective on the crosstalk between saturated fatty acids and toll-like receptor 4 in the etiology of inflammation and insulin resistance. Prog Lipid Res. (2020) 77:101020. 10.1016/j.plipres.2019.101020
7.
KojtaIChaciskaMBlachnio-ZabielskaA. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients. (2020) 12(5):1305. 10.3390/nu12051305
8.
LongoMZatteraleFNaderiJParrilloLFormisanoPRacitiGAet alAdipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. (2019) 20(9):2358. 10.3390/ijms20092358
9.
UnamunoXGómez-AmbrosiJRodríguezABecerrilSFrühbeckGCatalánV. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Invest. (2018) 48(9):e12997. 10.1111/eci.12997
10.
TuomilehtoJLindströmJErikssonJGValleTTHämäläinenHIlanne-ParikkaPet alPrevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. (2001) 344(18):1343–50. 10.1056/NEJM200105033441801
11.
KnowlerWCBarrett-ConnorEFowlerSEHammanRFLachinJMWalkerEAet alReduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. (2002) 346(6):393–403. 10.1056/NEJMoa012512
12.
JonkAMHoubenAJde JonghRTSernèEHSchaperNCStehouwerCD. Microvascular dysfunction in obesity: a potential mechanism in the pathogenesis of obesity-associated insulin resistance and hypertension. Physiology (Bethesda). (2007) 22:252–60. 10.1152/physiol.00012.2007
13.
KotsisVStabouliSBouldinMLowAToumanidisSZakopoulosNImpact of obesity on 24-hour ambulatory blood pressure and hypertension. Hypertension. (2005) 45:602–7. 10.1161/01.HYP.0000158261.86674.8e
14.
VasanRSLarsonMGLeipEPKannelWBLevyD. Assessment of frequency of progression to hypertension in non-hypertensive participants in the framingham heart study: a cohort study. Lancet. (2001) 358(9294):1682–6. 10.1016/S0140-6736(01)06710-1
15.
RahmouniKCorreiaMLHaynesWGMarkAL. Obesity-associated hypertension: new insights into mechanisms. Hypertension. (2005) 45(1):9–14. 10.1161/01.HYP.0000151325.83008.b4
16.
KotsisVNilssonPGrassiGManciaGRedonJLuftFet alNew developments in the pathogenesis of obesity-induced hypertension. J Hypertens. (2015) 33:1499–508. 10.1097/HJH.0000000000000645
17.
AroorRJiaGSowersJR. Cellular mechanisms underlying obesity-induced arterial stiffness. Am J Physiol Integr Comp Physiol. (2018) 314:R387–98. 10.1152/ajpregu.00235.2016
18.
KotsisVStabouliSPapakatsikaSet alMechanisms of obesity-induced hypertension. Hypertens Res. (2010) 33:386–93. 10.1038/hr.2010.9
19.
SeravalleGGrassiG. Obesity and hypertension. Pharmacol Res. (2017) 122:1–7. 10.1016/j.phrs.2017.05.013
20.
KotsisVJordanJMicicDFinerNLeitnerDRToplakHet alObesity and cardiovascular risk: a call for action from the European society of hypertension working group of obesity, diabetes and the high-risk patient and European association for the study of obesity: part A: mechanisms of obesity induced hypertension, diabetes and dyslipidemia and practice guidelines for treatment. J Hypertens. (2018) 36(7):1427–40. 10.1097/HJH.0000000000001730
21.
KoliakiCLiatisSKokkinosA. Obesity and cardiovascular disease: revisiting an old relationship. Metab Clin Exp. (2019) 92:98–107. 10.1016/j.metabol.2018.10.011
22.
LavieCJMilaniRVVenturaHO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. (2009) 53(21):1925–32. 10.1016/j.jacc.2008.12.068
23.
RychterAMRatajczakAEZawadaADobrowolskaAKrela-Kaz'mierczakI. Non-Systematic review of diet and nutritional risk factors of cardiovascular disease in obesity. Nutrients. (2020) 12(3):814. 10.3390/nu12030814
24.
Powell-WileyTMPoirierPBurkeLEDesprésJ-PGordon-LarsenPLavieCJet alObesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. (2021) 143(21):e984–e1010. 10.1161/CIR.0000000000000973
25.
CoutinhoTGoelKCorrêa de SáDKragelundCKanayaAMZellerMet alCentral obesity and survival in subjects with coronary artery disease: a systematic review of the literature and collaborative analysis with individual subject data. J Am Coll Cardiol. (2011) 57:1877–86. 10.1016/j.jacc.2010.11.058
26.
NicklasBJPenninxBWCesariMKritchevskySBNewmanABKanayaAMet alAssociation of visceral adipose tissue with incident myocardial infarction in older men and women: the health, aging and body composition study. Am J Epidemiol. (2004) 160:741–9. 10.1093/aje/kwh281
27.
ReisJPLoriaCMLewisCEPowell-WileyTMWeiGSCarrJJet alAssociation between duration of overall and abdominal obesity beginning in young adulthood and coronary artery calcification in middle age. JAMA. (2013) 310:280–8. 10.1001/jama.2013.7833
28.
NeelandIJPoirierPDesprésJP. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation. (2018) 137:1391–406. 10.1161/CIRCULATIONAHA.117.029617
29.
PichéMEPoirierP. Obesity, ectopic fat and cardiac metabolism. Expert Rev Endocrinol Metab. (2018) 13:213–21. 10.1080/17446651.2018.1500894
30.
ShahRVAndersonADingJBudoffMRiderOPetersenSEet alPericardial, but not hepatic, fat by CT is associated with CV outcomes and structure: the multi-ethnic study of atherosclerosis. JACC Cardiovasc Imaging. (2017) 10(9):1016–27. 10.1016/j.jcmg.2016.10.024
31.
BogersRPBemelmansWJHoogenveenRTBoshuizenHCWoodwardMKnektPet alAssociation of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta-analysis of 21 cohort studies including more than 300.000 persons. Arch Intern Med. (2007) 167:1720–8. 10.1001/archinte.167.16.1720
32.
AbedHSSamuelCSLauDHKellyDJRoyceSGAlasadyMet alObesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. (2013) 10:90–100. 10.1016/j.hrthm.2012.08.043
33.
SavjiNMeijersWCBartzTMBhambhaniVCushmanMNayorMet alThe association of obesity and cardiometabolic traits with incident HFpEF and HFrEF. JACC Heart Fail. (2018) 6(8):701–9. 10.1016/j.jchf.2018.05.018
34.
ClericoAGiannoniAVittoriniSEmdinM. The paradox of low BNP levels in obesity. Heart Fail Rev. (2012) 17(1):81–96. 10.1007/s10741-011-9249-z
35.
VolpeMRubattuSBurnettJJr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J. (2014) 35(7):419–25. 10.1093/eurheartj/eht466
36.
NeelandIJWindersBRAyersCRDasSRChangAYBerryJDet alHigher natriuretic peptide levels associate with a favorable adipose tissue distribution profile. J Am Coll Cardiol. (2013) 62(8):752–60. 10.1016/j.jacc.2013.03.038
37.
KenchaiahSPocockSJWangDFinnPVZornoffLASkaliHet alBody mass index and prognosis in patients with chronic heart failure: insights from the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) program. Circulation. (2007) 116(6):627–36. 10.1161/CIRCULATIONAHA.106.679779
38.
NtinopoulouPNtinopoulouEPapathanasiouIVFradelosECKotsiouORoussasNet alObesity as a risk factor for venous thromboembolism recurrence: a systematic review. Medicina (Kaunas). (2022) 58(9):1290. 10.3390/medicina58091290
39.
PathakRKMahajanRLauDHSandersP. The implications of obesity for cardiac arrhythmia mechanisms and management. Can J Cardiol. (2015) 31:203–10. 10.1016/j.cjca.2014.10.027
40.
YoungTPeppardPETaheriS. Excess weight and sleep-disordered breathing. J Appl Physiol. (2005) 99:1592–9. 10.1152/japplphysiol.00587.2005
41.
MussoGCassaderMOlivettiCRosinaFCarboneGGambinoR. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. (2013) 14(5):417–31. 10.1111/obr.12020
42.
TishlerPVLarkinEKSchluchterMDRedlineS. Incidence of sleep-disordered breathing in an urban adult population: the relative importance of risk factors in the development of sleep-disordered breathing. JAMA. (2003) 289(17):2230–7. 10.1001/jama.289.17.2230
43.
YeghiazariansYJneidHTietjensJRRedlineSBrownDLEl-SherifNet alObstructive sleep apnea and cardiovascular disease: a scientific statement from the American heart association. Circulation. (2021) 144(3):e56–67. 10.1161/CIR.0000000000000988
44.
SamadFRufW. Inflammation, obesity, and thrombosis. Blood. (2013) 122(20):3415–22. 10.1182/blood-2013-05-427708
45.
BlokhinIOLentzSR. Mechanisms of thrombosis in obesity. Curr Opin Hematol. (2013) 20(5):437–44. 10.1097/MOH.0b013e3283634443
46.
EichingerSHronGBialonczykCHirshlMMinarEWagnerOet alOverweight, obesity and the risk of recurrent venous thromboembolism. Arch Intern Med. (2008) 168:1678–83. 10.1001/archinte.168.15.1678
47.
AyinapudiKSinghTMotwaniALe JemtelTHOparilS. Obesity and pulmonary hypertension. Curr Hypertens Rep. (2018) 20(12):99. 10.1007/s11906-018-0899-2
48.
VolpeMGalloG. COVID-19 and the forgotten majority. High Blood Press Cardiovasc Prev. (2020) 27(5):339. 10.1007/s40292-020-00407-9
49.
GalloGCalvezVSavoiaC. Hypertension and COVID-19: current evidence and perspectives. High Blood Press Cardiovasc Prev. (2022) 29(2):115–23. 10.1007/s40292-022-00506-9
50.
KhanMAMoverley SmithJE. “Covibesity,” a new pandemic. Obes Med. (2020) 19:100282. 10.1016/j.obmed.2020.100282
51.
SimonnetAChetbounMPoissyJRaverdyVNouletteJDuhamelAet alHigh prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity. (2020) 28:1195–9. 10.1002/oby.22831
52.
LighterJPhillipsMHochmanSSterlingSJohnsonDFrancoisFet alObesity in patients younger than 60 years is a risk factor for COVID-19 hospital admission. Clin Infect Dis. (2020) 71:896–7. 10.1093/cid/ciaa415
53.
GazzarusoCPaolozziEValentiCBrocchettaMNaldaniDGrignaniCet alAssociation between antithrombin and mortality in patients with COVID-19. A possible link with obesity. Nutr Metab Cardiovasc Dis. (2020) 30:1914–9. 10.1016/j.numecd.2020.07.040
54.
VolpeMGalloGModenaMGFerriCDesideriGTocciGet alUpdated recommendations on cardiovascular prevention in 2022: an executive document of the Italian society of cardiovascular prevention. High Blood Press Cardiovasc Prev. (2022) 29(2):91–102. 10.1007/s40292-021-00503-4
55.
FriedMYumukVOppertJMScopinaroNTorresAWeinerRet alInternational federation for surgery of obesity and metabolic disorders-European chapter (IFSO-EC); European association for the study of obesity (EASO); European association for the study of obesity obesity management task force (EASO OMTF). interdisciplinary European guidelines on metabolic and bariatric surgery. Obes Surg. (2014) 24(1):42–55. 10.1007/s11695-013-1079-8
56.
EisenbergDShikoraSAAartsEAminianAAngrisaniLCohenRVet al2022 American society of metabolic and bariatric surgery (ASMBS) and international federation for the surgery of obesity and metabolic disorders (IFSO) indications for metabolic and bariatric surgery. Obes Surg. (2023) 33(1):3–14. 10.1007/s11695-022-06332-1
57.
ChangSHStollCRSongJVarelaJEEagonCJColditzGA. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. (2014) 149(3):275–87. 10.1001/jamasurg.2013.3654
58.
RobertsonAGNWigginsTRobertsonFP. Perioperative mortality in bariatric surgery: meta-analysis. Br J Surg. (2021) 108(8):892–7. 10.1093/bjs/znab245
59.
SantilliFSimeonePGGuagnanoMTLeoMMaccaroneMTDi CastelnuovoAet alEffects of liraglutide on weight loss, fat distribution, and β-cell function in obese subjects with prediabetes or early type 2 diabetes. Diabetes Care. (2017) 40(11):1556–64. 10.2337/dc17-0589
60.
Pi-SunyerXAstrupAFujiokaKGreenwayFHalpernAKrempfMet alA randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. (2015) 373(1):11–22. 10.1056/NEJMoa1411892
61.
le RouxCWAstrupAFujiokaKGreenwayFLauDCWVan GaalLet alL 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double blind trial. Lancet. (2017) 389(10077):1399–409. 10.1016/S0140-6736(17)30069-7
62.
MarsoSPDanielsGHBrown-FrandsenKKristensenPMannJFNauckMAet alLiraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. (2016) 375(4):311–22. 10.1056/NEJMoa1603827
63.
NeelandIJMarsoSPAyersCRLewisBOslicaRFrancisWet alEffects of liraglutide on visceral and ectopic fat in adults with overweight and obesity at high cardiovascular risk: a randomised, double-blind, placebo-controlled, clinical trial. Lancet Diabetes Endocrinol. (2021) 9(9):595–605. 10.1016/S2213-8587(21)00179-0
64.
SecherAJelsingJBaqueroAFHecksher-SørensenJCowleyMADalbøgeLSet alThe arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest. (2014) 124(10):4473–88. 10.1172/JCI75276
65.
DaviesMJBergenstalRBodeBKushnerRFLewinASkjøthTVet al, NN8022-1922 Study Group, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. (2015) 314(7):687–99. 10.1001/jama.2015.9676
66.
GreenwayFLFujiokaKPlodkowskiRAMudaliarSGuttadauriaMEricksonJet alCOR-I Study Group, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England). (2010) 376(9741):595–605. 10.1016/S0140-6736(10)60888-4
67.
ApovianCMAronneLRubinoD, COR-II Study Group, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity. (2013) 21(5):935–43. 10.1002/oby.20309
68.
WaddenTAForeytJPFosterGDet alWeight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity. (2011) 19(1):110–20. 10.1038/oby.2010.147
69.
HollanderPGuptaAKPlodkowskiRGreenwayFBaysHBurnsCet al, COR-Diabetes Study Group, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes Care. (2013) 36(12):4022–9. 10.2337/dc13-0234
70.
NissenSEWolskiKEPrcelaLet alEffect of naltrexone-bupropion on Major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors. JAMA. (2016) 315(10):990–1004. 10.1001/jama.2016.1558
71.
GreenwayFLWhitehouseMJGuttadauriaMAndersonJWAtkinsonRLFujiokaKet alRational design of a combination medication for the treatment of obesity. Obesity. (2009) 17:30–9. 10.1038/oby.2008.461
72.
WildingJPHBatterhamRLCalannaSDaviesMVan GaalLFLingvayIet alOnce-weekly semaglutide in adults with overweight or obesity. N Engl J Med. (2021) 384(11):989–1002. 10.1056/NEJMoa2032183
73.
DaviesMFærchLJeppesenOKPaksereshtAPedersenSDPerreaultLet alSemaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy,placebo-controlled, phase 3 trial. Lancet. (2021) 397(10278):971–84. 10.1016/S0140-6736(21)00213-0
74.
WaddenTABaileyTSBillingsLKDaviesMFriasJPKorolevaAet alSTEP 3 Investigators. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. (2021) 325(14):1403–13. 10.1001/jama.2021.1831
75.
RubinoDAbrahamssonNDaviesMHesseDGreenwayFLJensenCet alSTEP 4 Investigators. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with over weight or obesity: the STEP 4 randomized clinical trial. JAMA. (2021) 325(14):1414–25. 10.1001/jama.2021.3224
76.
MarsoSPBainSCConsoliAEliaschewitzFGJódarELeiterLAet alSemaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. (2016) 375(19):1834–44. 10.1056/NEJMoa1607141
77.
RyanDHLingvayIColhounHMDeanfieldJEmersonSSKahnSEet alSemaglutide effects on cardiovascular outcomes in people with overweight or obesity (SELECT) rationale and design. Am Heart J. (2020) 229:61–9. 10.1016/j.ahj.2020.07.008
78.
WangDDayEATownsendLKDjordjevicDJørgensenSBSteinbergGR. GDF15: emerging biology and therapeutic applications for obesity and cardiometabolic disease. Nat Rev Endocrinol. (2021) 17(10):592–607. 10.1038/s41574-021-00529-7
Appendix
Alessio Basolo and Guido Salvetti, Obesity and Lipodystrophy Center, Endocrinology Unit, University Hospital of Pisa, Pisa, Italy; Simonetta Bellone and Roberta Ricotti, Department of Health Sciences, University of Piemonte Orientale, Novara, Italy; Vanessa Bianconi, Massimo R. Mannarino and Matteo Pirro, Unit of Internal Medicine, Angiology and Arteriosclerosis Diseases, Department of Medicine, University of Perugia, Perugia, Italy; Agostino Consoli, Department of Medicine and Aging Sciences and Centro Scienze dell'Invecchiamento-Medicina Traslazionale (CeSI-MeT), University G. D'Annunzio, Chieti, Italy; Maurizio De Luca, Division of General Surgery, Castelfranco and Montebelluna Hospitals, Treviso, Italy; Rita Del Pinto, Claudio Ferri and Giovambattista Desideri, Department of Life, Health & Environmental Sciences, University of L'Aquila, L'Aquila, Italy; Leonarda Galiuto, Department of Clinical and Molecular Medicine, Sapienza University of Rome, Rome, Italy; Guido Grassi and Gino Seravalle, University of Milano-Bicocca, Milan, Italy; Elisa Lodi and Maria Grazia Modena, University of Modena and Reggio Emilia, Modena, Italy; Maria Chiara Meucci, Department of Cardiovascular and Pulmonary Sciences, Catholic University of the Sacred Heart Rome, Italy; Carmine Morisco and Bruno Trimarco, Department of Advanced Biomedical Sciences, Federico II University of Naples, Naples, Italy; Giulio Nati, General Practitioner; Speranza Rubattu, Department of Clinical and Molecular Medicine, Sapienza University of Rome, Rome, Italy and IRCCS Neuromed, Pozzilli (IS), Italy; Saula Vigili de Kreutzenberg, Department of Medicine-DIMED, University of Padova, Padua, Italy; Roberto Volpe, Health and Safety Unit (SPP), National Research Council (CNR), Rome, Italy.
Italian Society of Cardiology [SIC], Italian Society of Diabetology (SID), Italian Society of Internal Medicine [SIMI], Italian Society of Arterial Hypertension [SIIA], Italian Society for the Study of Atherosclerosis [SISA], Italian Society of Nephrology [SIN], Italian Society of Obesity [SIO], Italian Society of Digital Health and Telemedicine [SIT], Italian Society of Nutraceutics [SINut], Italian Association of Clinical, Preventive and Rehabilitation Cardiology (AICPR), Italian Society of Gerontology and Geriatrics [SIGG], the Mediterrean Diet Foundation [FDM].
Summary
Keywords
obesity, cardiovascular prevention, cardiovascular disease, chronic non-communicable disease, pharmacological treatment
Citation
Volpe M and Gallo G (2023) Obesity and cardiovascular disease: An executive document on pathophysiological and clinical links promoted by the Italian Society of Cardiovascular Prevention (SIPREC). Front. Cardiovasc. Med. 10:1136340. doi: 10.3389/fcvm.2023.1136340
Received
02 January 2023
Accepted
20 February 2023
Published
13 March 2023
Volume
10 - 2023
Edited by
Amit K Dey, National Institutes of Health (NIH), United States
Reviewed by
Kumar Ashish, CarolinaEast Medical Center, United States Anubhav Jain, Michigan State University, United States
Updates
Copyright
© 2023 Volpe and Gallo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Volpe massimo.volpe@uniroma1.it
Specialty Section: This article was submitted to Cardiovascular Epidemiology and Prevention, a section of the journal Frontiers in Cardiovascular Medicine
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.