- 1Department of Cardiology, Henan Provincial People's Hospital, People's Hospital of Zhengzhou University, Zhengzhou, China
- 2Microbiome Laboratory, Henan Provincial People's Hospital, People's Hospital of Zhengzhou University, Zhengzhou, China
- 3Department of Radiology, Henan Provincial People's Hospital, People's Hospital of Zhengzhou University, Zhengzhou, China
- 4Department of Cardiology, Dalian Medical University, Dalian, China
- 5Department of Cardiology, Second Affiliated Hospital of Kunming Medical University, Kunming, China
Objectives: Little is known about the clinical prognosis of gasdermin D (GSDMD) in patients with ST-elevation myocardial infarction (STEMI). The purpose of this study was to investigate the association of GSDMD with microvascular injury, infarction size (IS), left ventricular ejection fraction (LVEF), and major adverse cardiac events (MACEs), in STEMI patients with primary percutaneous coronary intervention (pPCI).
Methods: We retrospectively analyzed 120 prospectively enrolled STEMI patients (median age 53 years, 80% men) treated with pPCI between 2020 and 2021 who underwent serum GSDMD assessment and cardiac magnetic resonance (CMR) within 48 h post-reperfusion; CMR was also performed at one year follow-up.
Results: Microvascular obstruction was observed in 37 patients (31%). GSDMD concentrations ≧ median (13 ng/L) in patients were associated with a higher risk of microvascular obstruction and IMH (46% vs. 19%, P = 0.003; 31% vs. 13%, P = 0.02, respectively), as well as with a lower LVEF both in the acute phase after infarction (35% vs. 54%, P < 0.001) and in the chronic phase (42% vs. 56%, P < 0.001), larger IS in the acute (32% vs. 15%, P < 0.001) and in the chronic phases (26% vs. 11%, P < 0.001), and larger left ventricular volumes (119 ± 20 vs. 98 ± 14, P = 0.003) by CMR. Univariable and multivariable Cox regression analysis results showed that patients with GSDMD concentrations ≧ median (13 ng/L) had a higher incidence of MACE (P < 0.05).
Conclusions: High GSDMD concentrations in STEMI patients are associated with microvascular injury (including MVO and IMH), which is a powerful MACE predictor. Nevertheless, the therapeutic implications of this relation need further research.
1. Introduction
STEMI is the most acute manifestation of acute coronary syndrome and is associated with considerable morbidity and mortality (1). Although pPCI is the most effective approach to rescue viable myocardium, reduce infarction size, and improve cardiac function, the existence of microvascular post-reperfusion dysfunction adversely affects patient prognosis. Microvascular dysfunction [microvascular obstruction (MVO) and intramyocardial hemorrhage (IMH)] due to myocardial ischemia-reperfusion (MI/R) injury is associated with a poorer cardiac function and prognosis (2–4). Little is known about the pathophysiological processes of MVO and IMH, but inflammation has been considered to be a pivotal factor of this process, through the involvement of monocytes, neutrophils and cytokines (5, 6). In addition, the excessive inflammation after myocardial ischemia reperfusion injury could result in cell death.
Gasdermin D (GSDMD) was recently identified as a mediator of pyroptosis-inflammatory cell death triggered by cytosolic sensing of invasive infection and danger signals (7, 8). Upon activation, GSDMD forms cell membrane pores, inducing the release of pro-inflammatory cytokines and damaging the integrity of the cell membrane (9). Accumulating evidence has shown that GSDMD-mediated pyroptosis plays a pivotal role in a large number of diseases (10, 11). Our previous animal experiments indicated that GSDMD-mediated pyroptosis is involved in post-reperfusion microvascular injury (12, 13). However, whether GSDMD-medicated pyroptosis may exert its detrimental effects in STEMI patients by facilitating microvascular dysfunction remains unknown.
Cardiac magnetic resonance (CMR) imaging has been increasingly used for assessment of myocardial function, myocardial edema, infarction size (IS), and microvascular damage with high accuracy and within a single examination (14). The recent availability of multiparametric mapping CMR has provided novel insights into the pathophysiology underlying post-STEMI MVO and IMH (2, 15). Earlier research indicated that the presence of MVO and IMH was associated with higher concentrations of inflammatory factors and myocardial necrosis (15). Yet, new parametric mapping techniques enable the accurate quantification of myocardial necrosis and microvascular damage based on changes in T1, T2, and T2*(star) relaxation times and extracellular volume (ECV) (14). In addition, T2* mapping is currently considered the best method for IMH extent quantification (16, 17). Therefore, the relationship between GSDMD and T2* mapping or other CMR characteristics is a problem deserving research attention.
In the present work, CMR was used to confirm the presence of MVO and IMH, and to study myocardial function, IS, left ventricular volume. The objective of this study was to investigate the association between GSDMD in serum post-reperfusion and microvascular injury confirmed by CMR, and investigate the prognostic value of GSDMD as well as CMR parameters in post-reperfusion STEMI patients.
2. Material and methods
2.1. Study population
The present study is a retrospective, observational analysis of 120 consecutive STEMI patients enrolled between June 2020 and May 2021 in Henan Provincial People's Hospital. The follow-up duration was one year. All patients had been treated with pPCI within 12 h after symptoms onset and had undergone CMR in the early post-infarction phase (within two days from symptoms onset). Exclusion criteria were chronic MI, acute myocarditis, Killip-IV heart failure, an estimated glomerular filtration rate <30 ml/min/1.73 m2, and contraindications to CMR (presence of ferromagnetic implants, aneurysm clips, significant claustrophobia, severe contrast agent allergy to gadolinium, and hemodynamic instability). Blood samples of GSDMD, CRP, IL-6, and IL-1β were obtained via peripheral venipuncture 24 ± 12 h post-PCI. All patients gave informed written consent to study protocols approved by the ethics committee of our hospital.
2.2. Follow-up and outcome
All patients were enrolled between June 2020 and May 2021 and were followed up until the time to an event or, in the case of no event, April 2022.The primary clinical endpoint [major adverse cardiac events (MACE)] was defined as a composite of all cause death, non-fatal reinfarction, the occurrence of new heart failure and stroke (18, 19). Additionally, we compared risk stratification based on the median GSDMD levels in patients with STEMI (i.e., low-to-intermediate risk [<13 ng/L] and high risk [≧13 ng/L]).
2.3. Enzyme-linked immunosorbent assay (ELISA)
GSDMD (ab272463) ELISA kits were purchased from Abcam (Cambridge, UK). The concentration of cytokines was measured by the ELISA kits according to the manufacturers' instructions. The results were normalized by volume for serum samples. All samples were tested at least in triplicate.
2.4. Statistical analysis
All statistical analyses were carried out using SPSS (Statistical Package for the Social Sciences version 26.0, IBM, Armonk, NY, USA). Kolmogorov-Smirnov test was used to test whether the value comes from a normal distribution. The continuous data were expressed as mean ± SD and compared between groups by t-test and the Wilcoxon-Mann-Whitney U-test. Proportions were compared by chi-square test or by Fisher's exact test, as appropriate. The association of GSDMD with LVEF, IS, and microvascular injury (MVO and IMH) was evaluated in linear as well as binary multivariable regression analyses as indicated. For binary logistic regression analysis, LVEF and IS were dichotomized by clinical established cut-off values (LVEF < 50%; IS > 24% of LV; presence of MVO or IMH) MACE-free survival was estimated and depicted by the Kaplan-Meier method. GSDMD as continuous variable was dichotomized for low concentration and high concentration before log-rank test by using optimal cutoff values determined by ROC curve. To guarantee prognostic value of GSDMD for STEMI patients. Univariable and multivariable regression models were developed to investigate the potential independent association between MVO and MACE-free survival. To disclose independent predictors of MACE, all inflammatory biomarkers and CMR parameters were included, GSDMD, IL-1β, IL-6, TNF-α, CRP, CtnI, age, smoking, diabetes, anterior infarct localization as calculated in univariable regression analysis, GSDMD, IL-1β, CtnI were entered in a multivariable regression model. CMR model included the major CMR prognosis markers (LVEF, infarct size, MVO and IMH) according to literature (15). Receiver operating curve (ROC) analysis was performed to evaluate predictors of MACE. A two-side P < 0.05 was considered to indicate statistically significant differences.
3. Results
3.1. Study population and patient characteristics
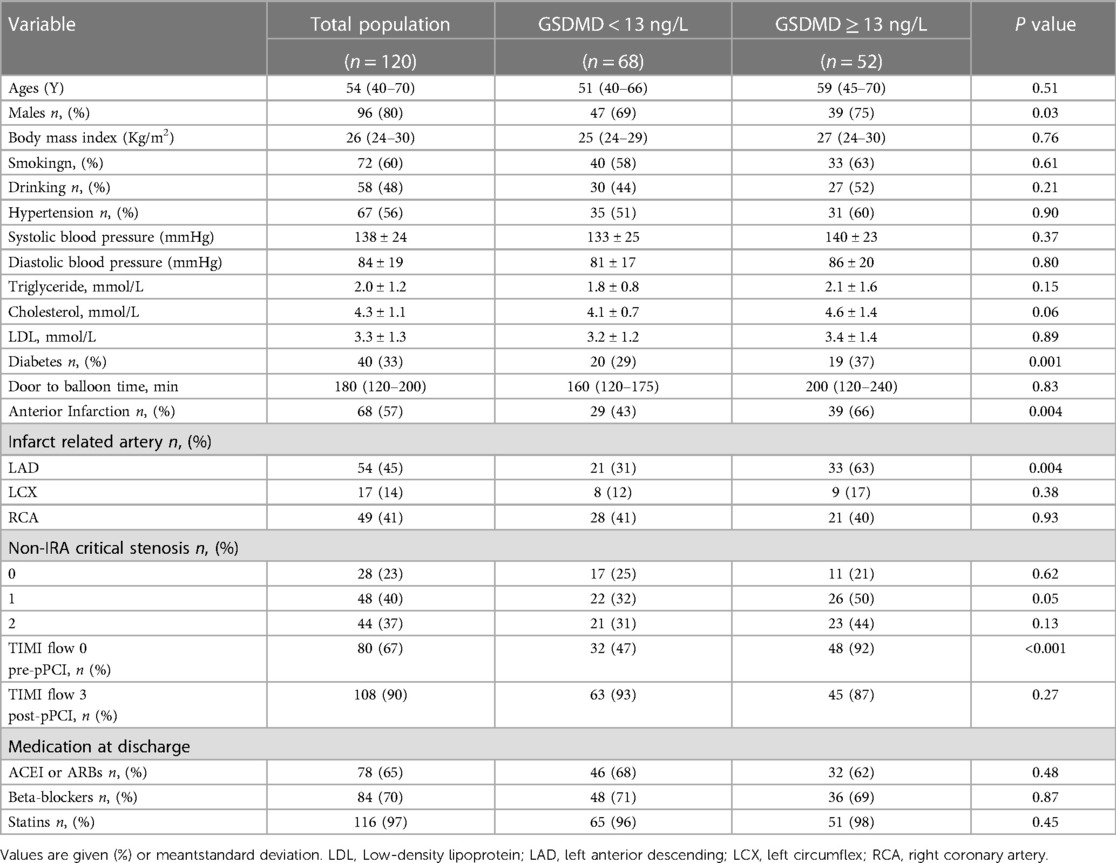
The baseline clinical characteristics of the classified based on a threshold of GSDMD concentrations (<13 ng/L vs. ≧13 ng/L) are presented in Table 1. Patients with GSDMD levels above the median level were more likely to have diabetes (P = 0.001), had more often anterior infarction (P = 0.004), left anterior descending artery (LAD) as an infarction-related artery (P = 0.004), and TIMI flow 0 pre-pPCI (P < 0.001) occurred more often than in patients with GSDMD levels below the median.
3.2. GSDMD and cardiac magnetic resonance parameters
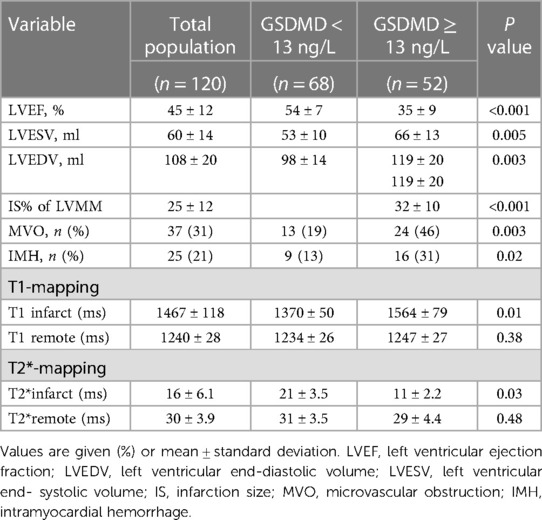
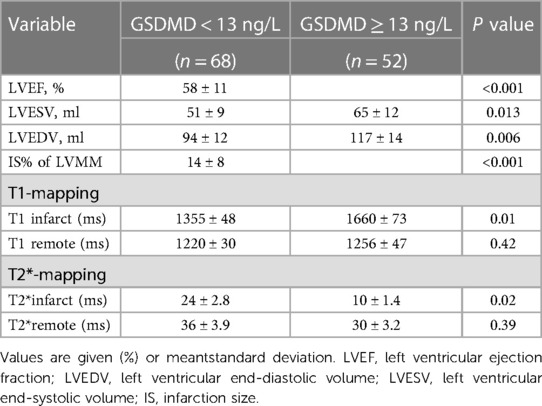
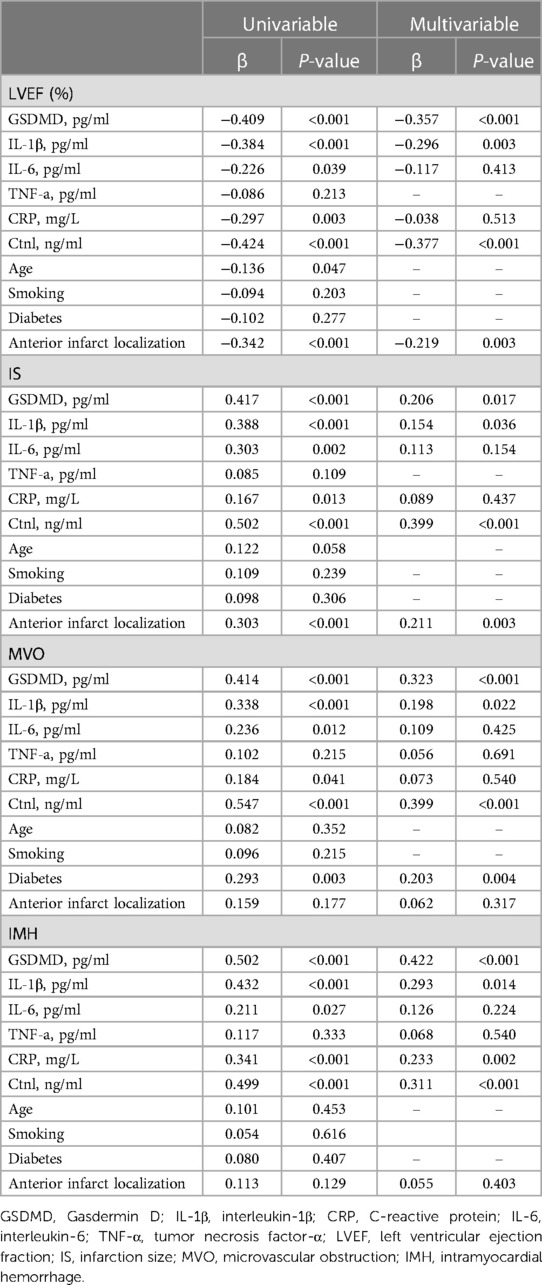
Baseline CMR characteristics and their relationship with GSDMD levels are presented in Table 2 and Table 3. Out of 134 STEMI patients enrolled, 120 (90%) had complete CMR data at the follow up examination and a median CMR time of 24 h (Figure 1). A higher risk of microvascular obstruction and IMH (46% vs. 19%, P = 0.003; 31% vs. 13%, P = 0.02, respectively) was found in patients with high GSDMD concentrations (≧13 ng/L). Furthermore, patients with high GSDMD concentrations (≧13 ng/L) showed a lower LVEF both in the acute phase after infarction (35% vs. 54%, P < 0.001) (Figure 2) and in the chronic phase (42% vs. 56%, P < 0.001). Patients presenting GSDMD concentrations ≧ median also were significantly associated with larger IS in the acute (32% vs. 15%, P < 0.001) and in the chronic phases (26% vs. 11%, P < 0.001) (Figure 2). In addition, increased GSDMD levels (≧13 ng/L) were associated with more dilated LVESV (66 ± 13 vs. 53 ± 10, P = 0.005) as well as LVEDV (119 ± 20 vs. 98 ± 14, P = 0.003) (Figure 3).
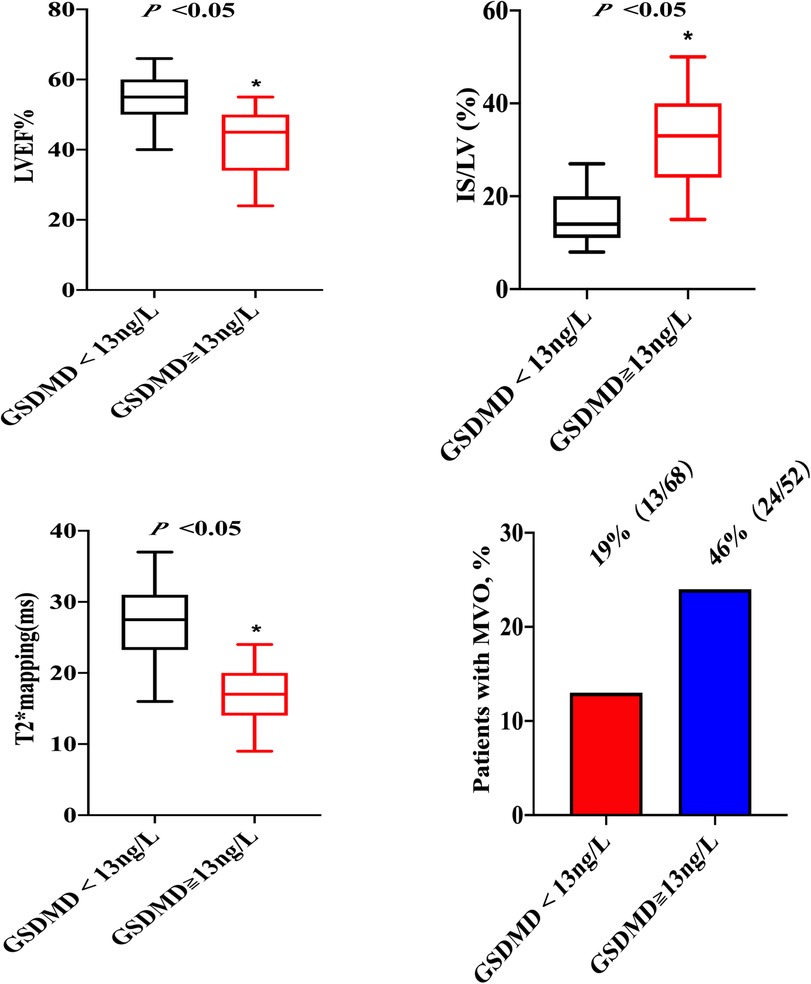
Figure 2. Association of GSDMD with LVEF, IS, T2*maps and MVO. LVEF, left ventricular ejection fraction; IS, infarction size; MVO, microvascular obstruction.
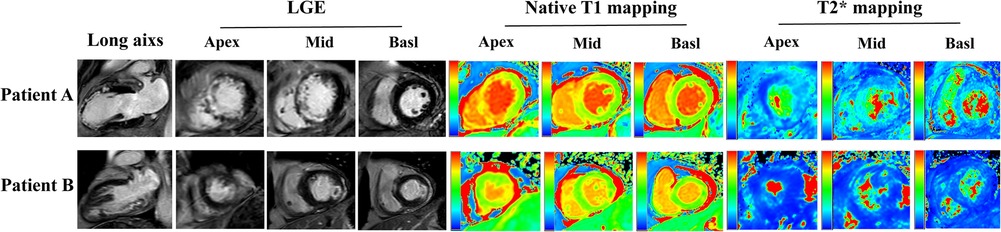
Figure 3. CMR images from two patients with STEMI treated by PPCI. Short axis slices in the basal, middle and apical region and long axis long axis two-chamber heart are showed. Patient A: A 57-year-old patient with anterior STEMI treated by PPCI and with GSDMD concentrations ≧ median (13 ng/L). The CMR images on day 2 showing transmural infarction in the anterior wall with MVO, and the Native T1 and T2* maps represent myocardial damage and intramyocardial hemorrhage. Patient B: A 49-year-old patient with lateral STEMI treated by pPCI and with GSDMD concentrations< median (13ng/L). The CMR images on day 2 manifesting infarction in the lateral wall with MVO, and the Native T1 and T2* maps represent myocardial damage and intramyocardial hemorrhage.
In multiple linear regression analysis, GSDMD was significantly related to baseline LVEF (β = −0.409, P < 0.001), IS (β = 0.417, P < 0.001), MVO (β = 0.414, P < 0.001), and IMH (β = 0.502, P < 0.001). Four models were evaluated where either baseline LVEF, IS, MVO or IMH were regarded as the dependent variable, and GSDMD, IL-1β, CtnI, diabetes, CRP and anterior infarction localization as independent variables. In all four models, plasma GSDMD levels were significantly correlated with LVEF (β = −0.357, P < 0.001), IS (β = 0.206, P = 0.017), MVO (β = 0.323, P < 0.001) as well as IMH (β = 0.422, P < 0.001, Table 4). All parameters included in multivariable analysis showed a VIF of <2.
In binary logistic regression analysis, GSDMD emerged as independent predictor of baseline LVEF [odds ratio (OR): 1.06, 95% CI 0.90–2.23, P < 0.01], IS (OR:3.29, 95% CI 1.42–5.40, P < 0.01), MVO (OR: 1.20, 95% CI 1.30–2.17, P < 0.01), and IMH (OR: 1.73, 95% CI 1.17–5.69, P < 0.01).
3.3. Association between GSDMD and native T1 and T2* mapping
On native T1 maps, the mean T1 value of the infarcted was significantly higher than that of the remote myocardium (1467 ± 118 ms vs. 1240 ± 28 ms, P < 0.05) (Figure 3, Table 2). Meanwhile, the mean T1 values of infarcted myocardium were higher in patients with high GSDMD concentrations (≧ 13 ng/L) both in the acute and chronic phase after infarction (1564 ± 79 ms vs. 1370 ± 50 ms, P = 0.01; 1660 ± 73 ms vs. 1335 ± 48 ms, P = 0.01) (Figure 3, Table 3). On T2* maps, the mean T2* values of infarcted myocardium were significantly lower than that of the remote area (16 ± 6.1 ms vs. 30 ± 3.9 ms, P < 0.05). Moreover, the mean T2* values of infarcted myocardium were lower in patients with high GSDMD concentrations (≧13 ng/L) both in the acute and chronic phase (11 ± 2.2 ms vs. 21 ± 3.5 ms, P = 0.03; 10 ± 1.4 ms vs. 24 ± 2.8 ms, P = 0.02) (Figures 2, 3, Table 3).
3.4. GSDMD and clinical outcome
The GRACE 3.0 score has been validated for risk stratification in patients with non-ST-segment elevation acute coronary syndromes (NSTE-ACS) (20). However, the GSDMD to predict MACE in STEMI patients was unclear. MACE data after a follow-up of one-year following STEMI were available for 120 (100%) patients. Of them, 17 (14%) STEMI patients experienced MACE, including five (4%) nonfatal MI, six new congestive heart failures (5%), four unstable angina pectoris requiring revascularization (3%), and two ventricular arrhythmias (2%) occurred. In ROC analysis, circulating GSDMD significantly predicted MACE (AUC = 0.89, 95% CI 0.83–0.96, P = 0.02) (Figure 4). Kaplan-Meier curve analysis indicated that patients having GSDMD concentrations > median had a significantly higher MACE-free survival (hazard ratio: 3.22, [95% CI, 1.29–9.49], P = 0.008), and there has no significance between patients with GSDMD concentrations ≧ median (13 ng/L) and high risk [>3%; >140 points] (Figure 5). After adjusting for baseline characteristics and clinical predictors, there was a 9% higher risk of MACE (adjusted hazard ratio [adj HR] 1.10, 95% CI 1.13–1.27), 12% higher risk of nonfatal MI (adj HR 1.15, 95% CI 1.07–1.31), and a 16% higher risk of congestive heart failures or ventricular arrhythmias (adj HR 1.08, 95% CI 1.01–1.24) for higher GSDMD concentrations (≧13 ng/L) patients.
Figure 4. Receiver operating characteristic curve for the prediction of death in hospital in STEMI patients.
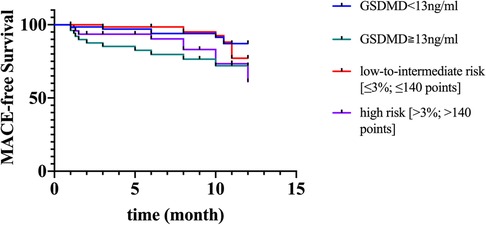
Figure 5. Kaplan-Meier curves suggesting the risk of MACE in relation to GSDMD concentrations compared to GRACE score. MACE, major adverse cardiac events; GSDMD, gasdermin D.
In ROC analysis, circulating GSDMD significantly predicted microvascular injury (AUC = 0.95, 95% CI 0.93–0.99, P = 0.0001). Furthermore, for the prediction of MACE, a cut-off value of 13.835 was set for risk assessment. Patients having high GSDMD concentration showed a significantly higher MACE-free survival, as determined by Kaplan-Meier curve analysis (Figure 6).
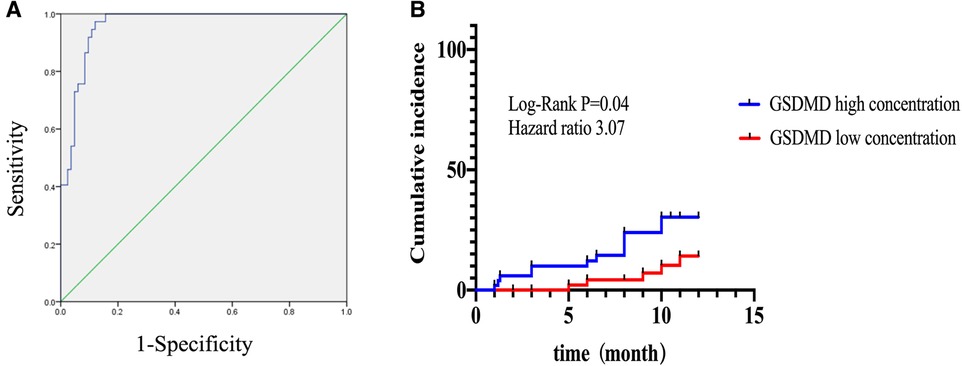
Figure 6. Kaplan-Meier curves according to GSDMD optimal cutoff values for predicting MACE. (A) Receiver operating characteristic curve for the prediction optimal cutoff values of GSDMD and for prediction microvascular dysfunction diagnosis. (B) High concentration of GSDMD correlated with poor outcomes in STEMI patients.
3.5. CMR and clinical outcome
During the follow-up period, the occurrence of MACE was significantly higher in patients with higher GSDMD concentrations (21% vs. 8%, P < 0.001). In univariable analysis, presence of MVO, presence of IMH, IS, LVEF, EDV and ESV were significantly associated with the occurrence of MACE (hazard ratio 5.93, [95% CI, 2.33–15.09], P < 0.001; (hazard ratio: 4.82, [95% CI, 1.78–13.00], P = 0.002; (hazard ratio: 4.92, [95% CI, 2.02–11.98], P < 0.001; hazard ratio: 4.96, [95% CI, 1.83–13.48], P = 0.002; (hazard ratio: 4.06, [95% CI, 1.52–10.28], P = 0.003; and hazard ratio: 4.10 [95% CI, 1.61–10.42], P = 0.003; respectively). In the multivariable Cox regression analysis, the presence of MVO and IMH were independent predictors of a higher incidence of MACE (hazard ratio: 5.35, [95% CI, 1.35–10.27]; P = 0.02 and hazard ratio: 3.97, [95% CI, 1.59–8.76], P = 0.03; respectively) (Supplementary Table S1).
4. Discussion
The main findings of our study were as follows: (1) The increased levels of the GSDMD in serum were associated with failed microvascular reperfusion (including MVO and IMH), larger IS, and worse myocardial function; (2) MVO and IMH were independently associated with MACE at one-year post-STEMI. The significant association persisted after adjustment for potential biomarkers, such as IL-6, IL-1β, CRP; (3) Higher levels of circulating GSDMD were associated with a higher incidence of MVO and IMH and lower MACE-free survival. Therefore, GSDMD has a promising role for GSDMD as a prognostic biomarker for more serious outcomes in the early post-STEMI phase and could also serve as a potential target for future clinical cardio-protection trials.
While the timely restoration of the epicardial coronary artery to the ischemic myocardium by PCI is the primary approach to preserve myocardial viability, it also contributes to I/R injury not only to cardiomyocytes, but also to various myocardial components, including the microvasculature. The presence of MVO and IMH after STEMI is associated with a worse outcome (21). Accumulating evidence has revealed that inflammation and cell death are the hallmarks of post-reperfusion microvascular injury. However, the complex pathophysiology of this process has not been fully elucidated so far.
Inflammation, traditional biomarkers such as C-reactive protein (CRP), Interleukin-6 (IL-6), and interleukin-1β (IL-1β), has been associated with cardiovascular events. IL-6 is an inflammatory cytokine that mediates both immune and inflammatory responses. A body of evidence previously highlighted that IL-6 increases substantially after AMI and is involved in the pathophysiological process of microvascular injury (22). Consistent with the previous result, although a significant association between elevated IL-6 and microvascular injury has been found, this observation was not confirmed in multivariable regression analysis in our present study.
Previous studies indicated that CRP is the most extensively investigated inflammatory marker for coronary heart disease (23). Reindl et al. have been found that the concentrations of CRP were significantly higher in STEMI patients with MVO (24). Our study confirms previous experimental results, CRP were markedly associated with the occurrence of microvascular injury (MVO and IMH). Nevertheless, the relationship of CRP and microvascular obstruction was not verified by multivariable regression analysis.
High-sensitivity cardiac troponin I (hs-cTnI) assays have been recognized for improving diagnostic accuracy in the early detection of AMI and for their potential in risk stratification for patients with AMI. Previous studies demonstrate that risk assessment in STEMI patients could be improved by combination with cTnI (25). Consistent with the previous studies, despite its association with the risk of microvascular injury, this observation was not confirmed in multivariable regression analysis in our present study.
However, whether there are novel biomarkers should be explored. GSDMD mediated-pyroptosis act as a novel proinflammatory programmed cell death as well as release of proinflammatory cytokines IL-1β and IL-18 (8). Recently, roles for pyroptosis in cardiovascular disease are emerging, including myocardial infarction, ischemia-reperfusion injury, atherosclerosis and diabetic cardiomyopathy (26). However, it is not clear whether pyroptosis is associated with microvascular dysfunction following myocardial reperfusion. A recent report from our animal experiment demonstrated that I/R induced pyroptosis was involved in the pathophysiological process of microvascular dysfunction (12). Nevertheless, whether GSDMD mediated-pyroptosis contributes to microvascular dysfunction in STEMI patients is largely unknown. In the present study, we propose circulating GSDMD as a novel microvascular injury predictor post-STEMI. With the present data, relation of GSDMD with both MVO and IMH remained independent from conventional prognostic biomarkers even after adjustment for other established clinical parameters.
IL-1β acts as pro-inflammatory multifunctional cytokine and is one of the main regulators following cardiovascular events. To investigate whether IL-1β is implicated in pathophysiology of microvascular dysfunction following myocardial reperfusion. The present study revealed that IL-1β is an important contributor of microvascular injury (MVO and IMH). Since GSDMD and IL-1β are closely related, it is important to evaluate whether GSDMD provides additional prognostic value above and beyond IL-1β. In our study, we emphasize the pivotal role of GSDMD in the acute setting post infarction since the association of GSDMD with microvascular injury remained independent even after adjustment for IL-1β.
Accumulating evidence has revealed that the presence of microvascular injury (including MVO and IMH) and blood stasis adversely affects early left ventricular remodeling and are independently associated with adverse cardiovascular events (27, 28). Recently, CMR was identified as a crucial noninvasive imaging modality for assessing the cardiac morphology, function, and myocardial necrosis in STEMI patients (14). However, the association between GSDMD and CMR-derived indexes in STEMI patients is not well understood. Our data suggest that increased GSDMD levels in the serum exert negative effects on LVEF and are associated with a larger infarction and left ventricular volume assessed by CMR. However, the implementation of delayed enhancement cardiovascular magnetic resonance imaging (DE-MRI) for the assessment of acute myocardial infarction is partly hampered by methodological and technical challenges. T1 mapping has recently emerged as a promising technique for the quantification of myocardial injury early after STEMI and prediction of recovery (29, 30). In our present study, native T1 mapping could detect myocardial injury in patients with STEMI, with the results are consistent with those of a previous study (29). Interestingly, native T1 values were higher in patients with high GSDMD concentrations (≧13 ng/L), which further indicates that elevated serum GSDMD levels might be involved in the pathophysiological process of ischemia-reperfusion injury in STEMI patients who undergo pPCI.
Clinical studies have also revealed that CMR can be applied as a tool for risk stratification and guide in the management of STEMI patients at risk of MACE (31, 32). In this study, we focused on the direct impact of IS, LVEF, EDV, ESV, the presence of MVO, and IMH on the primary endpoint. Our present findings showed that MVO and IMH are the strongest predictor of the clinical outcome, which remained an independent predictor of MACE in the multivariable Cox regression analysis results after adjustment for other established prognostic risk factors including IS, LVEF, EDV and ESV.
Yet, the pathophysiological mechanisms of the relationship between MVO or IMH and worse outcome are not fully understood. Recent experimental and clinical studies have indicated the presence of a link between IMH and the persistence of inflammation (5, 33). In an animal model of myocardial reperfusion injury, massive inflammatory cells recruitment was detected in the infarction area, accompanied by MVO and IMH (34). In STEMI post-reperfusion patients, the quantification of IMH by T2* imaging found iron depositions, which was associated with an inflammatory reaction (15). Therefore, we aimed to determine whether the increased GSDMD levels in STEMI patients receiving PCI are related to MVO or IMH. Our data showed that patients with high GSDMD concentrations (≧13 ng/L) had lower T2* values in the infarct region, suggesting that GSDMD may be involved in the pathophysiological processes of MVO or IMH, affecting the prognosis of post-reperfusion STEMI patients. In the present study, we confirm the prognostic significance of elevated GSDMD levels, which could have their potential application as a more precise risk marker for STEMI patients undergoing pPCI.
4.1. Study limitations
The study population in this work was relatively small and the duration of the follow-up period was limited to one year after STEMI. Additionally, the number of the investigated events was relatively low. Furthermore, our data assessed the prognostic information of the presence of MVO and IMH but did not evaluate the extent of MVO and IMH. It would thus be important for future studies to elucidate the percentage of MVO in LV. CMR was performed on two days in STEMI patients who undergo pPCI. Yet, the IMH evaluation could have been affected due to its temporal variations in the few days after infarction.
Serum GSDMD levels were measured once, within 48 h post-reperfusion, the median time of GSDMD measurement was 28 h. Therefore, the GSDMD release dynamics over time (pre- and post-PCI) and its potential prognostic information could not be provided. However, our other data suggest that the inflammatory factor IL-1β reaches its peak at 48 h post-reperfusion and this might be the optimal time point of measurement. Nevertheless, the optimal timing of GSDMD measurements remains unknown in our study and needs further research. Moreover, the promising gasdermins family (GSDMC and GSDME) was not investigated in the present study. Thus, future investigations are required to evaluate the association between GSDMC or GSDME and MVO or IMH in STEMI patients receiving pPCI.
5. Conclusion
The increased serum levels of GSDMD measured at 48 h in STEMI patients treated with pPCI were independently associated with a higher MVO and IMH rates and larger myocardial injury as established by comprehensive CMR imaging. Moreover, the present results emphasize the value of GSDMD as a biomarker for risk stratification, which can serve as a potential therapeutic target for STEMI patients with microvascular post-reperfusion injury.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Clinal experiments were reviewed and approved by the Clinical Medicine Research Center Ethics Committee of the Henan Provincial People's Hospital (approval date 2020, code 158). The patients/participants provided their written informed consent to participate in this study.
Author contributions
YJC and SJD conceived and designed the study; WJS, CQW and SHC collected data; YW and SHZ analyzed the data; YJC, SJD and WJS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported in part by Henan Province Joint Construction Project (grant No. LH-GJ20210046), Henan Province Science and Technology Research Project (grant No. 122102310068). Fundamental Research Program of Yunnan Provincial Department of Science and Technology (Kunming Medical Joint Special Project) (grant No. 2018FE001(-236)).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2023.1138352/full#supplementary-material
References
1. Mozaffarian D, Benjamin E, Go A, Arnett D, Blaha M, Cushman M, et al. Executive summary: heart disease and stroke statistics–2016 update: a report from the American heart association. Circulation. (2016) 133(4):447–54. doi: 10.1161/CIR.0000000000000366
2. de Waha S, Patel M, Granger C, Ohman E, Maehara A, Eitel I, et al. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J. (2017) 38(47):3502–10. doi: 10.1093/eurheartj/ehx414
3. Berry C, Ibáñez B. Intramyocardial hemorrhage: the final frontier for preventing heart failure post-myocardial infarction. J Am Coll Cardiol. (2022) 79(1):49–51. doi: 10.1016/j.jacc.2021.11.002
4. Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol. (2019) 114(6):45. doi: 10.1007/s00395-019-0756-8
5. Ghugre N, Pop M, Thomas R, Newbigging S, Qi X, Barry J, et al. Hemorrhage promotes inflammation and myocardial damage following acute myocardial infarction: insights from a novel preclinical model and cardiovascular magnetic resonance. J Cardiovasc Magn Reson. (2017) 19(1):50. doi: 10.1186/s12968-017-0361-7
6. Annibali G, Scrocca I, Aranzulla T, Meliga E, Maiellaro F, Musumeci G. “No-Reflow” phenomenon: a contemporary review. J Clin Med. (2022) 11(8):2233. doi: 10.3390/jcm11082233
7. Zhang J, Yu Q, Jiang D, Yu K, Yu W, Chi Z, et al. Epithelial gasdermin D shapes the host-microbial interface by driving mucus layer formation. Science Immunol. (2022) 7(68). eabk2092. doi: 10.1126/sciimmunol.abk2092
8. Liu X, Zhang Z, Ruan J, Pan Y, Magupalli V, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. (2016) 535(7610):153–8. doi: 10.1038/nature18629
9. Ding J, Wang K, Liu W, She Y, Sun Q, Shi J, et al. Erratum: pore-forming activity and structural autoinhibition of the gasdermin family. Nature. (2016) 540(7631):150. doi: 10.1038/nature20106
10. Shi H, Gao Y, Dong Z, Yang J, Gao R, Li X, et al. GSDMD-Mediated Cardiomyocyte pyroptosis promotes myocardial I/R injury. Circ Res. (2021) 129(3):383–96. doi: 10.1161/CIRCRESAHA.120.318629
11. Yu P, Zhang X, Liu N, Tang L, Peng C, Chen X. Pyroptosis: mechanisms and diseases. Signal Transduction and Targeted Therapy. (2021) 6(1):128. doi: 10.1038/s41392-021-00507-5
12. Sun W, Lu H, Dong S, Li R, Chu Y, Wang N, et al. Beclin1 controls caspase-4 inflammsome activation and pyroptosis in mouse myocardial reperfusion-induced microvascular injury. Cell Commun and Signaling: CCS. (2021) 19(1):107. doi: 10.1186/s12964-021-00786-z
13. Sun W, Lu H, Lyu L, Yang P, Lin Z, Li L, et al. Gastrodin ameliorates microvascular reperfusion injury-induced pyroptosis by regulating the NLRP3/caspase-1 pathway. J Physiol Biochem. (2019) 75(4):531–47. doi: 10.1007/s13105-019-00702-7
14. Bulluck H, Dharmakumar R, Arai A, Berry C, Hausenloy D. Cardiovascular magnetic resonance in acute ST-segment-elevation myocardial infarction: recent advances, controversies, and future directions. Circulation. (2018) 137(18):1949–64. doi: 10.1161/CIRCULATIONAHA.117.030693
15. Reinstadler S, Stiermaier T, Reindl M, Feistritzer H, Fuernau G, Eitel C, et al. Intramyocardial haemorrhage and prognosis after ST-elevation myocardial infarction. Eur Heart J Cardiovasc Imaging. (2019) 20(2):138–46. doi: 10.1093/ehjci/jey101
16. Ferré-Vallverdú M, Sánchez-Lacuesta E, Plaza-López D, Díez-Gil J, Sepúlveda-Sanchis P, Gil-Cayuela C, et al. Prognostic value and clinical predictors of intramyocardial hemorrhage measured by CMR T2* sequences in STEMI. Int J Cardiovasc Imaging. (2021) 37(5):1735–44. doi: 10.1007/s10554-020-02142-7
17. Messroghli D, Moon J, Ferreira V, Grosse-Wortmann L, He T, Kellman P, et al. Correction to: clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: a consensus statement by the society for cardiovascular magnetic resonance (SCMR) endorsed by the European association for cardiovascular imaging (EACVI). J Cardiovasc Magn Reson. (2018) 20(1):9. doi: 10.1186/s12968-017-0408-9
18. Hudson M. In suspected AMI, an extended algorithm increased sensitivity and decreased specificity for predicting 30-day MACE. Ann Intern Med. (2020) 172(2). JC11. doi: 10.7326/ACPJ202001210-011
19. Bosco E, Hsueh L, McConeghy K, Gravenstein S, Saade E. Major adverse cardiovascular event definitions used in observational analysis of administrative databases: a systematic review. BMC Med Res Methodol. (2021) 21(1):241. doi: 10.1186/s12874-021-01440-5
20. Wenzl F, Kraler S, Ambler G, Weston C, Herzog S, Räber L, et al. Sex-specific evaluation and redevelopment of the GRACE score in non-ST-segment elevation acute coronary syndromes in populations from the UK and Switzerland: a multinational analysis with external cohort validation. Lancet (London, England). (2022) 400(10354):744–56. doi: 10.1016/S0140-6736(22)01483-0
21. Scarsini R, Shanmuganathan M, De Maria G, Borlotti A, Kotronias R, Burrage M, et al. Coronary microvascular dysfunction assessed by pressure wire and CMR after STEMI predicts long-term outcomes. JACC Cardiovascular Imaging. (2021) 14(10):1948–59. doi: 10.1016/j.jcmg.2021.02.023
22. Tiller C, Reindl M, Holzknecht M, Lechner I, Schwaiger J, Brenner C, et al. Association of plasma interleukin-6 with infarct size, reperfusion injury, and adverse remodelling after ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. (2022) 11(2):113–23. doi: 10.1093/ehjacc/zuab110
23. Reindl M, Reinstadler SJ, Feistritzer H-J, Klug G, Tiller C, Mair J, et al. Relation of inflammatory markers with myocardial and microvascular injury in patients with reperfused ST-elevation myocardial infarction. Eur Heart J: Acute Cardiovasc Care. (2016) 6(7):640–9. doi: 10.1177/2048872616661691
24. Holzknecht M, Tiller C, Reindl M, Lechner I, Troger F, Hosp M, et al. C-reactive protein velocity predicts microvascular pathology after acute ST-elevation myocardial infarction. Int J Cardiol. (2021) 338:30–6. doi: 10.1016/j.ijcard.2021.06.023
25. Khullar N, Buckley A, O'Connor C, Ibrahim A, Ibrahim A, Ahern C, et al. Peak troponin T in STEMI: a predictor of all-cause mortality and left ventricular function. Open Heart. (2022) 9(1):e001863. doi: 10.1136/openhrt-2021-001863
26. Jia C, Chen H, Zhang J, Zhou K, Zhuge Y, Niu C, et al. Role of pyroptosis in cardiovascular diseases. Int Immunopharmacol. (2019) 67:311–8. doi: 10.1016/j.intimp.2018.12.028
27. Rodriguez-Palomares JF, Gavara J, Ferreira-Gonzalez I, Valente F, Rios C, Rodriguez-Garcia J, et al. Prognostic value of initial left ventricular remodeling in patients with reperfused STEMI. JACC Cardiovasc Imaging. (2019) 12(12):2445–56. doi: 10.1016/j.jcmg.2019.02.025
28. Massussi M, Cipriani A, Meneghin S, De la Cruz N, Cecere A, D'Amico G, et al. Prognostic value of left ventricular blood stasis in patients with acute myocardial infarction: a cardiac magnetic resonance study. Int J Cardiol. (2022) 358:128–33. doi: 10.1016/j.ijcard.2022.04.012
29. Liu D, Borlotti A, Viliani D, Jerosch-Herold M, Alkhalil M, De Maria G, et al. CMR Native T1 mapping allows differentiation of reversible versus irreversible myocardial damage in ST-segment-elevation myocardial infarction: an OxAMI study (Oxford acute myocardial infarction). Circ Cardiovasc Imaging. (2017) 10(8):e005986. doi: 10.1161/CIRCIMAGING.116.005986
30. Dall'Armellina E, Piechnik S, Ferreira V, Si Q, Robson M, Francis J, et al. Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction. J Cardiovasc Magn Reson. (2012) 14(15). doi: 10.1186/1532-429X-14-15
31. Bulluck H, Carberry J, Carrick D, McCartney P, Maznyczka A, Greenwood J, et al. A noncontrast CMR risk score for long-term risk stratification in reperfused ST-segment elevation myocardial infarction. JACC Cardiovascular Imaging. (2022) 15(3):431–40. doi: 10.1016/j.jcmg.2021.08.006
32. Reindl M, Reinstadler S. Risk stratification by CMR after STEMI: is it time to drop the gadolinium? JACC Cardiovasc Imaging. (2022) 15(3):441–4. doi: 10.1016/j.jcmg.2021.09.010
33. Carrick D, Haig C, Ahmed N, McEntegart M, Petrie M, Eteiba H, et al. Myocardial hemorrhage after acute reperfused ST-segment-elevation myocardial infarction: relation to microvascular obstruction and prognostic significance. Circ Cardiovasc Imaging. (2016) 9(1):e004148. doi: 10.1161/CIRCIMAGING.115.004148
Keywords: ST-segment elevation myocardial infarction, magnetic resonance imaging, microvascular dysfunction, GSDMD, IMH, MVO
Citation: Sun W, Wang C, Cui S, Wang Y, Zhao S, Lu M, Yang F, Dong S and Chu Y (2023) Association of GSDMD with microvascular-ischemia reperfusion injury after ST-elevation myocardial infarction. Front. Cardiovasc. Med. 10:1138352. doi: 10.3389/fcvm.2023.1138352
Received: 28 February 2023; Accepted: 10 May 2023;
Published: 6 June 2023.
Edited by:
Matteo Pagnesi, ASST Spedali Civili di Brescia, ItalyReviewed by:
Alessandro Villaschi, Humanitas University, ItalyMauro Massussi, University of Brescia, Italy
© 2023 Sun, Wang, Cui, Wang, Zhao, Lu, Yang, Dong and Chu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shujuan Dong aG5zeXp6ZHNqQDE2My5jb20= Yingjie Chu aG5xYmRzbEAxMjYuY29t
†These authors have contributed equally to this work and share first authorship
 Wenjing Sun1,2,†
Wenjing Sun1,2,† Yingjie Chu
Yingjie Chu