- 1Ramsay Générale de Santé, Institut Cardiovasculaire Paris Sud, Interventional Cardiology Department, Massy, France
- 2Ramsay Générale de Santé, Institut Cardiovasculaire Paris Sud, Vascular Surgery Department, Massy, France
- 3Division of Cardiology and Department of Radiology, Centres for Heart Valve Innovation and for Cardiovascular Innovation, St Paul’s and Vancouver General Hospitals, University of British Columbia, Vancouver, BC, Canada
Transcatheter aortic valve replacement (TAVR) indications recently extended to lower surgical risk patients with longer life expectancy. Commissural alignment (CA) is one of the emerging concepts and is becoming one of the cornerstones of the TAVR procedure in a patient with increased longevity. Indeed, CA may improve transcatheter heart valve (THV) hemodynamics, future coronary access, and repeatability. The definition of CA has been recently standardized by the ALIGN-TAVR consortium using a four-tier scale based on CT analysis. Progress has been made during the index TAVR procedure to optimize CA, especially with self-expandable platforms. Indeed, specific delivery catheter orientation, THV rotation, and computed-tomography-derived views have been proposed to achieve a reasonable degree of CA. Recent data demonstrate feasibility, safety, and a significant reduction in coronary overlap using these techniques, especially with self-expandable platforms. This review provides an overview of THV CA including assessment methods, alignment techniques during the index TAVR procedure with different THV platforms, the clinical impact of commissural misalignment, and challenging situations for CA.
Introduction
Transcatheter aortic valve replacement (TAVR) is a well-established therapeutic option for severe aortic stenosis. According to current guidelines, TAVR indications can be extended to lower-surgical-risk patients with longer life expectancy (1). Thus, new concepts for TAVR procedure improvement have emerged as they may impact long-term outcomes in younger TAVR populations (2). Optimal transcatheter heart valve (THV) hemodynamics, future coronary access, and repeatability have been of primary importance (3, 4). Similarly to surgical bioprosthetic valves, THVs may eventually degenerate, and repeat intervention may be required. In some patients, repeat procedures might be associated with a high risk of coronary obstruction due to anatomical and/or device considerations. Commissural alignment (CA), routinely achieved with surgical aortic valve replacement, may improve future coronary access (5, 6). Moreover, CA may improve THV hemodynamics and reduce the risk of hypoattenuated leaflet thickening (HALT) (5, 6).
Specific THV and delivery catheter orientations have been proposed during the index TAVR procedure to improve CA. Recent research demonstrates that preprocedural planning is key to achieving CA, demonstrating feasibility, safety, and advantages in reducing coronary overlap. CA feasibility and benefits are currently better documented for self-expandable devices but may be similar to balloon-expandable THVs (6–9).
This review provides an overview of THV CA including assessment methods, alignment techniques during the index TAVR procedure with different THV platforms, clinical impact of commissural misalignment, and challenging situations for CA.
Commissural alignment with current THV platforms
Self-expandable THVs
Evolut platform
The commissural alignment technique during the index TAVR procedure has been first described with the Evolut (Medtronic, Minneapolis, Minnesota) platform by inserting the delivery catheter with the flush port positioned at 3 O’clock and then orientating the hat marker (90° counterclockwise away from the C-paddle corresponding to one of the THV commissures) in the outer curvature of the descending aorta before final position (Figures 1, 2). These maneuvers aim to orientate the hat marker at the outer curvature or the center front in the 3-cusp coplanar view (right coronary cusp-centered view) and the center front in the right and left coronary cusp overlap view (Figures 1, 2) (10). This technique could be performed in 85.9% of patients (n = 64) in the ALIGN-ACCESS study (7).
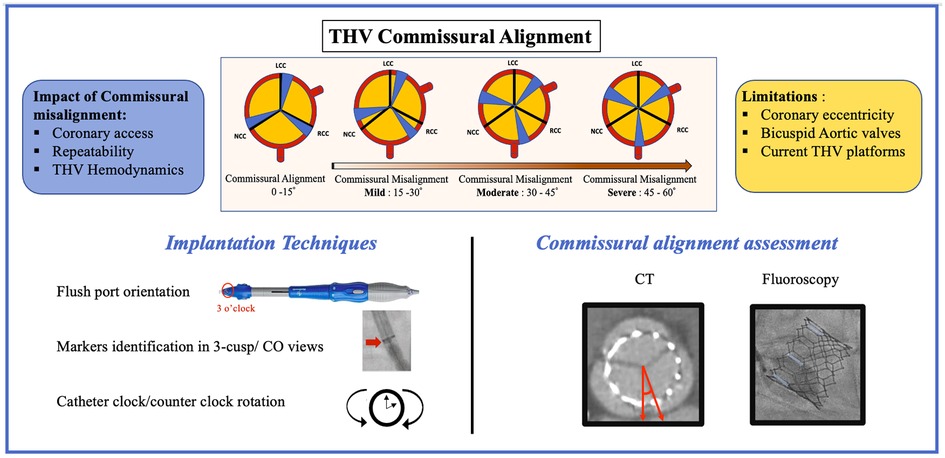
Figure 1. THV commissural alignment: classification, clinical impact, limitations, implantation techniques, and commissural alignment assessment with imaging. CT, computed tomography; CO, cusp overlap; THV, transcatheter heart valve.
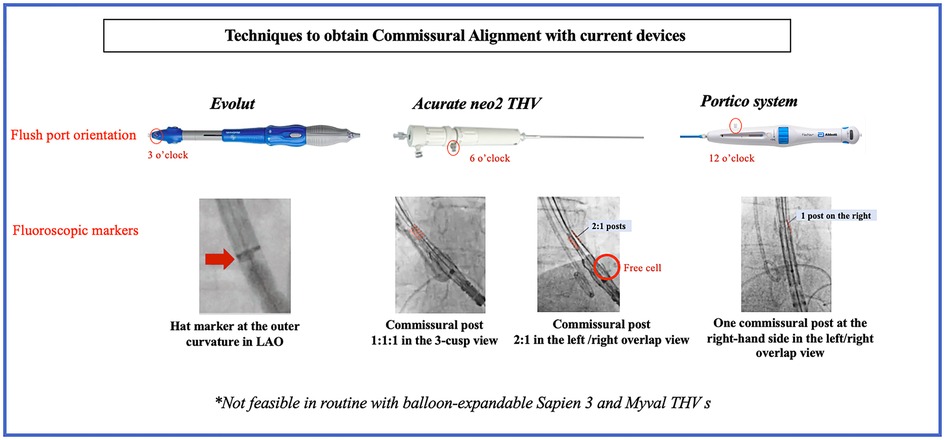
Figure 2. Techniques to obtain commissural alignment with current devices. LAO, left anterior oblique; THV, transcatheter heart valve.
Acurate neo2 THV
The delivery catheter is inserted with the flush port at 6 O’clock with the Acurate neo2 (Acn2) valve (Boston Scientific Corporation, Natick, MA, USA). The THV commissures can be identified using the posts on the stabilizing arches and/or the free cells located on the lower crown. The valve is then checked in the 3-cusp view and rotated 5–10 mm above the annulus plane to achieve the desired position. The Acn2 commissural post configuration should be 1-1-1 in the 3-cusp view (clockwise or counterclockwise maneuvers allow to achieve a 1-1-1 configuration if the initial position is 2–1 or 1–2) and 2-1 in the cusp-overlap view (Figure 2) (11, 12). Valve alignment was achieved successfully in 88.5% of patients (n = 26) in the ALIGN-ACCESS study (7).
Portico system
To optimize CA with the Portico (Abbott Structural Heart) THV, the flush port of the delivery system was inserted at 12 O'clock. The commissural posts can be identified halfway between the THV inflow and outflow. Both the 3-cusp and the right/left cusp-overlap views were used to identify and position one commissural post at the right-hand side in the cusp-overlap view allowing for CA (Figure 2) (11).
Balloon-expandable THVs
Sapien 3 THV
As opposed to self-expandable THVs for which specific techniques have been proposed to achieve CA, CA with the balloon-expandable SAPIEN 3 (S3) (Edwards Lifesciences, Irvine, CA, USA) THV is not currently feasible. Only one study assessed different crimping positions with this THV and failed to demonstrate any crimping position allowing to achieve CA (7).
Myval THV
One study has recently shown the feasibility of CA using the Myval balloon-expandable THV in 10 patients using an in silico simulation with correct alignment in all patients with the in vivo procedures demonstrating correct alignment in six of the 10 patients and mild misalignment in the remaining four patients. In this study, the authors simulated the orientation of the THV in an in silico model and then adapted the THV crimping to achieve the predicted angle for CA using pre-TAVR computed tomography (CT) for a tailored approach (13).
Although these techniques have been described to optimize CA, some limitations should be considered. First, iliofemoral tortuosity, aorta tortuosity, and vertical aortic annulus may not allow achieving CA; second, these techniques were only evaluated using a transfemoral approach; and finally, excessive catheter manipulation and rotation may increase the potential risk of embolic events and vascular complications.
Imaging for THV commissural alignment assessment
Computed tomography
CT is the gold standard for CA evaluation with direct measurement of the angle between the THV commissural post and native aortic valve commissure. The commissural offset is directly measured using an angle tool with the limbs aligned with the native commissure between the NCC and LCC and with the closest THV commissure. CA has been defined by the ALIGN-TAVR (Alignment of Transcatheter Aortic-Valve Neo-Commissures) consortium according to the commissural offset using a four-tier scale, as follows: aligned (0°–15°), mildly misaligned (15°–30°), moderately misaligned (30°–45°), and severely misaligned (45°–60°) (Figure 1) (6). However, although CT is the gold standard, this requires additional contrast injection and irradiation and may only be used as a postprocedural evaluation tool.
CT-based simulations
CT-based simulations have been shown to be feasible with the Acn2 THV to predict CA based on pre-TAVR CT and using a 3D printed model for in vitro simulations of patient-specific rotation of the delivery system on the basis of patient-specific anatomy (14). CT-based simulations may also allow defining a crimping position for balloon-expandable THVs to achieve CA (12). CT-based simulations allow us to plan for the procedure and predict CA prior the TAVR procedure. However, CT-based simulations require dedicated software and may not be routinely used.
Fluoroscopy
Several fluoroscopic methods have been described to assess for CA. Fluoroscopic methods may help to optimize for CA during the TAVR procedure and to assess for CA immediately following the procedure, avoiding post-TAVR CT.
One of the first described fluoroscopic methods was based on a CT-fluoroscopic overlay (15). Although this method demonstrated a good correlation with post-TAVR CT, this required identical patient positioning on the CT table during the angiographic procedure and CT/fluoroscopy coregistration, which might be challenging in some cases.
More recently, methods based on CT-derived fluoroscopic views have been proposed using both the 3-cusp view and cusp overlap views to align one of the THV commissural posts with the isolated native commissure (Figure 1) (11). Ultimately, although these methods are reproducible, they only provide a qualitative assessment of CA. Quantitative assessment of CA with S3 has been demonstrated by Akodad et al. using trigonometry to calculate the commissural offset between the posterior commissure (between the LCC and NCC) and the closest THV commissure (commissural offset angle θ = arcsine projected distance of commissural strut to centerline/projected radius). This method was shown to have an excellent correlation with post-TAVR CT (16). Although promising, all these fluoroscopic methods require a standardized methodology for reproducibility.
Clinical impact of commissural misalignment
Coronary access
In addition to tall frame THV design with supra-annular leaflets and high implants, severe commissural misalignment is a well-known independent predictor of unsuccessful selective coronary access following TAVR with a supra-annular THV (6, 17). Although coronary access might be less challenging in short-frame THVs, post-TAVR CT studies have demonstrated that around one-third of S3 THV stent frames extend beyond the coronary ostia (7). The RE-ACCESS (Reobtain Coronary Ostia Cannulation Beyond Transcatheter Aortic Valve Stent) prospective study found an overall 7.7% rate of unsuccessful selective coronary cannulation, almost exclusively following implantation of Evolut R or Evolut Pro supra-annular THVs. In addition, high THV implant and THV oversizing were associated with unsuccessful cannulation (18). However, as demonstrated in the ALIGN-ACCESS (TAVR with Commissural Alignment Followed by Coronary Access) study, the CA of supra-annular THVs, which can be achieved with the Evolut platforms, improves the feasibility of selective coronary access (7). Challenging coronary access might be even more frequent in valve-in-valve and redo-TAVR procedures, especially in the case of commissural misalignment and initial high implant of the initial THV, increasing the neo-skirt height (6, 19, 20).
Repeatability
Redo-TAVR might be an increasingly performed procedure as the age at the first TAVR procedure tends to decrease in a lower-risk population with longer life expectancy. The risks of coronary obstruction and challenging coronary access following redo-TAVR are well documented. Indeed, a recent CT simulation study has demonstrated that around one-third of patients have a high risk of coronary obstruction following TAVR, regardless of the THV type, with this risk being increased if the failed THV is a self-expandable platform (4). The chimney technique is a well-known bailout strategy to prevent or treat coronary obstruction (21). Leaflet modifications techniques including bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction (BASILICA) may also decrease the risk of acute coronary obstruction and facilitate future coronary access in patients undergoing valve-in-valve or redo-TAVR identified at high risk for coronary occlusion (22). However, these techniques are only feasible in the case of acceptable CA and may be irrelevant in the case of overlap between the THV or bioprosthetic valve commissural posts and the coronary ostia. Moreover, if the coronary ostia are located too close to the failed THV commissural post, this may lead to unsuccessful BASILICA with coronary obstruction (6).
THV hemodynamics
Although future coronary access is the main concern when considering commissural misalignment, other concerns have been raised, especially the impact of commissural misalignment on THV function, durability, and risk of HALT (6). Indeed, significant commissural misalignment may lead to non-physiological flow with increased leaflet stress and blood stagnation inside the sinuses of Valsalva and eventually increased thrombogenicity and early THV deterioration (6, 23). A bench study assessing the impact of commissural misalignment on THV hydrodynamics using the Sapien XT device failed to demonstrate any impact of commissural misalignment on THV hydrodynamics but showed impaired fluid flow patterns in the case of THV misalignment (24). Another bench study with the Acurate neo THV in a valve-in-valve configuration demonstrated that THV commissural misalignment was associated with a higher incidence of paravalvular leakage and impaired leaflet motion due to interaction with the leaflets of the surgical valve (25). Recently, an association between severe commissural misalignment and an early increase in gradient following an S3 THV implant have been suggested (26). Data from the RESOLVE registry among 324 patients showed that significant commissural misalignment defined as commissural offset > 30° was observed in 52.8% of patients but was not associated with significant paravalvular leakage, intravalvular leakage, increase in the mean gradient >20 mmHg, or HALT initially. However, significant commissural misalignment was associated with twice as much significant increase in THV mean gradient (>50% relative increase) from discharge to 1 month (17.6% vs. 8.26%; p = 0.038) compared with commissural alignment <30° and with a linear relationship (1.3-fold risk of a significant increase in mean gradient every 10° of commissural misalignment) (26). A significantly higher rate of mild valvular regurgitation has been recently described in patients with at least moderate commissural misalignment (27). The incidence of HALT was higher with the S3 THV in the case of commissural misalignment (40% vs. 28% in case of CA) in a recent report (28). Although unfavorable THV hemodynamics and blood stagnation have been advocated as potentially increasing the risk of reduced THV durability, no clear evidence exists, and further investigations are required.
Discussion
Commissural alignment is becoming one of the cornerstones in TAVR when considering the lifetime management of aortic stenosis in younger populations. Progress was made aligning commissures with most of the available THV platforms to date, and assessment of CA using CT and fluoroscopy has now shown to be accurate. However, although CA represents one step in the right direction for TAVR procedure optimization, a certain number of limits remain (Figure 1).
Limitations
Coronary eccentricity
Although CA appears to be a reasonable goal to achieve, it might not always reflect alignment between the THV and coronary ostium. Indeed, coronary eccentricity with the coronary ostium not being centered in the corresponding cusp has been described recently and is more frequent with the right coronary artery than the left main, which may lead to coronary overlap despite adequate commissural alignment (29, 30). New approaches have been proposed using CT-derived (coronary ostial overlap) views to better understand the relationship between the THV commissures and coronary ostia (30) with promising results. Moreover, leaflet modification techniques may be more efficient in the case of coronary alignment than commissural alignment (29, 30).
Bicuspid aortic valve
A recent CT study has shown that cusp asymmetry was more frequent in patients with type 1 bicuspid aortic valves (69%) compared with tricuspid aortic valves (17.5%), the dominant cusp being the NCC in the majority of bicuspid cases (30). Thus, CA might be more challenging with bicuspid aortic valves, especially in the case of left-right cusps fusion. Depending on the type of bicuspid valve, CA might be achieved using the same techniques as for tricuspid valves, or advanced catheter techniques may be used based on pre-TAVR CT analysis. Coronary alignment rather than CA might be more accurate, especially in bicuspid anatomies. Patient-specific simulation may be useful to avoid overlap between THV commissures and coronary ostia.
Future perspectives
CT-based patient-specific simulation, although not routinely performed, may help to better understand and plan for specific device orientation or crimping position to achieve optimal CA (13, 14). This represents a useful tool, especially for lifetime management in younger patients. Along with TAVR procedure improvement, new TAVR devices with markers aligned with the THV commissures and improved torquability and self-rotation may allow a more accurate commissural alignment prior to deployment, especially for balloon-expandable THVs. Future investigations are still required to assess the impact of commissural misalignment on long-term outcomes following TAVR.
Conclusion
Commissural alignment is becoming an important parameter for procedure optimization in younger patients undergoing TAVR. Although progress has been made to improve CA with the current device and CA understanding during the TAVR procedure, improvement in implant techniques and devices is expected.
Author contributions
MA: conceptualization, data curation, investigation, visualization, and writing—original draft. YL: data curation, data curation. DM: data curation, review. FS: data curation, review. TH: data curation, review. PB: data curation, review. JS: data curation, review. GT: data curation, review. JL: data curation, review. DW: data curation, review. JW: review. BC: validation, review, and editing. All authors contributed to the article and approved the submitted version.
Conflict of interest
MA has received research funding from Medtronic, Biotronik, MUSE Explore, and Federation Française de Cardiologie. DM is supported by the Swiss National Science Foundation (grant no. P2LAP3_199561) and SICPA. PB is a consultant to Edwards Lifesciences and provides CT core lab services to Edwards Lifesciences, Medtronic, Neovasc, Boston, and Abbott, for which no direct compensation is received. JL is supported by a Canadian Research Chair in Advanced Cardiopulmonary Imaging, a consultant for MVRX, Heartflow Inc., and Circle Cardiovascular Imaging, and provides CT core lab services to Edwards Lifesciences, Medtronic, Neovasc, Boston Scientific, and Tendyne Holdings, for which no direct compensation is received. JS has received speaking fees from Edwards Lifesciences and is a consultant to Edwards Lifesciences, Boston Scientific, NVT Medical, and Medtronic. GT is supported by the Fondation Vaudoise de Cardiologie and the SICPA Foundation. DW is a consultant to, and has received research funding from, Edwards Lifesciences and Abbott. JW is a consultant to Edwards Lifesciences and Abbott. BC is a minor shareholder of Colibri and CERC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. (2021) 60(4):727–800. doi: 10.1093/ejcts/ezab389
2. Avvedimento M, Tang GHL. Transcatheter aortic valve replacement (TAVR): recent updates. Prog Cardiovasc Dis. (2021) 69:73–83. doi: 10.1016/j.pcad.2021.11.003
3. Basman C, Pirelli L, Singh VP, Reimers CD, Hemli J, Brinster DR, et al. Lifetime management for aortic stenosis: planning for future therapies. J Cardiol. (2022) 80(3):185–9. doi: 10.1016/j.jjcc.2021.12.010
4. Medranda GA, Soria Jimenez CE, Torguson R, Case BC, Forrestal BJ, Ali SW, et al. Lifetime management of patients with symptomatic severe aortic stenosis: a computed tomography simulation study. EuroIntervention. (2022) 18(5):e407–16. doi: 10.4244/EIJ-D-21-01091
5. Sá MP, Ramlawi B, Sicouri S, Torregrossa G, Al Abri Q, Kempfert J, et al. Lifetime management of aortic valve disease: aligning surgical and transcatheter armamentarium to set the tone for the present and the future. J Card Surg. (2022) 37(1):205–13. doi: 10.1111/jocs.16110
6. Tang GHL, Amat-Santos IJ, De Backer O, Avvedimento M, Redondo A, Barbanti M, et al. Rationale, definitions, techniques, and outcomes of commissural alignment in TAVR: from the ALIGN-TAVR consortium. JACC Cardiovasc Interv. (2022) 15(15):1497–518. doi: 10.1016/j.jcin.2022.06.001
7. Tarantini G, Nai Fovino L, Scotti A, Massussi M, Cardaioli F, Rodinò G, et al. Coronary access after transcatheter aortic valve replacement with commissural alignment: the ALIGN-ACCESS study. Circ Cardiovasc Interv. (2022) 15(2):e011045. doi: 10.1161/CIRCINTERVENTIONS.121.011045
8. Tang GHL, Sengupta A, Alexis SL, Zaid S, Leipsic JA, Blanke P, et al. Conventional versus modified delivery system technique in commissural alignment from the evolut low-risk CT substudy. Catheter Cardiovasc Interv. (2022) 99(3):924–31. doi: 10.1002/ccd.29973
9. Piazza N, Martucci G, Spaziano M. Commissural or coronary alignment for TAVR?: align what and by how much?. JACC Cardiovasc Interv. (2022) 15(2):147–9. doi: 10.1016/j.jcin.2021.12.012
10. Tang GHL, Zaid S, Fuchs A, Yamabe T, Yazdchi F, Gupta E, et al. Alignment of transcatheter aortic-valve neo-commissures (ALIGN TAVR): impact on final valve orientation and coronary artery overlap. JACC Cardiovasc Interv. (2020) 13(9):1030–42. doi: 10.1016/j.jcin.2020.02.005
11. Bieliauskas G, Wong I, Bajoras V, Wang X, Kofoed KF, De Backer O, et al. Patient-specific implantation technique to obtain neo-commissural alignment with self-expanding transcatheter aortic valves. JACC Cardiovasc Interv. (2021) 14(19):2097–108. doi: 10.1016/j.jcin.2021.06.033
12. Kitamura M, Wilde J, Gohmann R, Majunke N, Gutberlet M, Shibata M, et al. Commissural alignment of the ACURATE neo valve in transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2021) 14(15):1740–2. doi: 10.1016/j.jcin.2021.05.037
13. Santos-Martínez S, Redondo A, González-Bartol E, Barrero A, Sánchez-Luna JP, Revilla-Orodea A, et al. Feasibility of precise commissural and coronary alignment with balloon-expandable TAVI. Rev Esp Cardiol (Engl Ed). (2023) 76(1):19–24. doi: 10.1016/j.recesp.2022.03.005
14. Redondo A, Valencia-Serrano F, Santos-Martínez S, Delgado-Arana JR, Barrero A, Serrador A, et al. Accurate commissural alignment during ACURATE neo TAVI procedure. Proof of concept. Rev Esp Cardiol (Engl Ed). (2022) 75(3):203–12. doi: 10.1016/j.recesp.2021.02.008
15. Spilias N, Sabbak N, Harb SC, Yun JJ, Vargo PR, Unai S, et al. A novel method of assessing commissural alignment for the SAPIEN 3 transcatheter aortic valve. JACC: Cardiovascular Interventions. (2021) 14(11):1269–72. doi: 10.1016/j.jcin.2021.03.059
16. Akodad M, Tzimas G, Meier D, Haugan D, Gibson H, Ringhofer J, et al. Quantification of commissural alignment of balloon-expandable THV on fluoroscopy: a comparison study with post-TAVR CT. JACC Cardiovasc Interv. (2022) 15(23):2374–83. doi: 10.1016/j.jcin.2022.08.006
17. Tarantini G, Tang G, Nai Fovino L, Blackman D, Van Mieghem NM, Kim WK, et al. Management of coronary artery disease in patients undergoing transcatheter aortic valve implantation. A clinical consensus statement from the European Association of Percutaneous Cardiovascular Interventions in collaboration with the ESC working group on cardiovascular surgery. EuroIntervention. (2023) EIJ-D-22–00958. doi: 10.4244/EIJ-D-22-00958
18. Barbanti M, Costa G, Picci A, Criscione E, Reddavid C, Valvo R, et al. Coronary cannulation after transcatheter aortic valve replacement: the RE-ACCESS study. JACC Cardiovasc Interv. (2020) 13(21):2542–55. doi: 10.1016/j.jcin.2020.07.006
19. Meier D, Akodad M, Landes U, Barlow AM, Chatfield AG, Lai A, et al. Coronary access following redo TAVR: impact of THV design, implant technique, and cell misalignment. JACC Cardiovasc Interv. (2022) 15(15):1519–31. doi: 10.1016/j.jcin.2022.05.005
20. Akodad M, Sellers S, Landes U, Meier D, Tang GHL, Gada H, et al. Balloon-expandable valve for treatment of evolut valve failure: implications on neoskirt height and leaflet overhang. JACC Cardiovasc Interv. (2022) 15(4):368–77. doi: 10.1016/j.jcin.2021.12.021
21. Mercanti F, Rosseel L, Neylon A, Bagur R, Sinning JM, Nickenig G, et al. Chimney stenting for coronary occlusion during TAVR: insights from the chimney registry. JACC Cardiovasc Interv. (2020) 13(6):751–61. doi: 10.1016/j.jcin.2020.01.227
22. Greenbaum AB, Kamioka N, Vavalle JP, Lisko JC, Gleason PT, Paone G, et al. Balloon-assisted BASILICA to facilitate redo-TAVR. JACC Cardiovasc Interv. (2021) 14(5):578. doi: 10.1016/j.jcin.2020.10.056
23. Tang GHL, Zaid S. Commissural (mis)alignment in TAVR and hemodynamic impact: more questions raised than answered. JACC Cardiovasc Interv. (2022) 15(11):1137–9. doi: 10.1016/j.jcin.2022.04.044
24. Salmonsmith JA, Ducci A, Burriesci G. Does transcatheter aortic valve alignment matter? Open Heart. (2019) 6(2):e001132. doi: 10.1136/openhrt-2019-001132
25. Meier D, Akodad M, Chatfield AG, Lutter G, Puehler T, Søndergaard L, et al. Impact of commissural misalignment on hydrodynamic function following valve-in-valve intervention with the ACURATE neo. JACC Cardiovasc Interv. (2022) 15(15):1532–9. doi: 10.1016/j.jcin.2022.05.034
26. Raschpichler M, Flint N, Yoon SH, Kaewkes D, Patel C, Singh C, et al. Commissural alignment after balloon-expandable transcatheter aortic valve replacement is associated with improved hemodynamic outcomes. JACC Cardiovasc Interv. (2022) 15(11):1126–36. doi: 10.1016/j.jcin.2022.04.006
27. Fuchs A, Kofoed KF, Yoon SH, Schaffner Y, Bieliauskas G, Thyregod HG, et al. Commissural alignment of bioprosthetic aortic valve and native aortic valve following surgical and transcatheter aortic valve replacement and its impact on valvular function and coronary filling. JACC Cardiovasc Interv. (2018) 11(17):1733–43. doi: 10.1016/j.jcin.2018.05.043
28. Khan JM, Rogers T, Weissman G, Torguson R, Rodriguez-Weisson FJ, Chezar-Azerrad C, et al. Anatomical characteristics associated with hypoattenuated leaflet thickening in low-risk patients undergoing transcatheter aortic valve replacement. Cardiovasc Revasc Med. (2021) 27:1–6. doi: 10.1016/j.carrev.2020.09.034
29. Redondo A, Baladrón Zorita C, Tchétché D, Santos-Martinez S, Delgado-Arana JR, Barrero A, et al. Commissural versus coronary optimized alignment during transcatheter aortic valve replacement. JACC Cardiovasc Interv. (2022) 15(2):135–46. doi: 10.1016/j.jcin.2021.10.005
Keywords: THV, TAVR, coronary access, redo TAVR, commissural alignment
Citation: Akodad M, Lounes Y, Meier D, Sanguineti F, Hovasse T, Blanke P, Sathananthan J, Tzimas G, Leipsic J, Wood DA, Webb J and Chevalier B (2023) Transcatheter heart valve commissural alignment: an updated review. Front. Cardiovasc. Med. 10:1154556. doi: 10.3389/fcvm.2023.1154556
Received: 30 January 2023; Accepted: 28 March 2023;
Published: 19 April 2023.
Edited by:
Antonino S. Rubino, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Marisa Avvedimento, University of Naples Federico II, Italy© 2023 Akodad, Lounes, Meier, Sanguineti, Hovasse, Blanke, Sathananthan, Tzimas, Leipsic, Wood, Webb and Chevalier. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mariama Akodad bS5ha29kYWRAaWNwcy5jb20uZnI=
Specialty Section: This article was submitted to Structural Interventional Cardiology, a section of the journal Frontiers in Cardiovascular Medicine
 Mariama Akodad
Mariama Akodad Youcef Lounes
Youcef Lounes David Meier
David Meier Francesca Sanguineti1
Francesca Sanguineti1 Thomas Hovasse
Thomas Hovasse Janarthanan Sathananthan
Janarthanan Sathananthan Georgios Tzimas
Georgios Tzimas