- 1Department of Cardiovascular Surgery, Qilu Hospital of Shandong University, Jinan, China
- 2Institute of Thoracoscopy in Cardiac Surgery, Shandong University
Objective: The main purpose of this study was to evaluate the safety and efficacy of Castor single-branched stent-graft combined with fenestrated technique in treatment of thoracic aortic disease (TAD) with unfavorable proximal landing area (PLZ) and isolated left vertebral artery (ILVA).
Methods: From January 2018 to March 2022, 8 patients with TAD (6 patients with type B aortic dissections, 1 patient with type B intramural hematomas, and 1 patient with thoracic aortic aneurysm) underwent thoracic endovascular aortic repair with fenestrated Castor stent-graft due to the existence of ILVA and unfavorable PLZ. Demographic characteristics, surgical details, postoperative complications, follow-up and postoperative CTA imaging results were collected and analyzed.
Results: The primary technical success rate was 100%. The mean operation time was 115 min (range, 70–180 min). All the left subclavian arteries (LSAs) and ILVAs of the eight patients were revascularized by fenestrated Castor stent-grafts. During the follow-up period, no deaths and complications were observed. No internal leakage, aortic rupture, retrograde type A dissection were found on computed tomography angiography. All of the LSAs and ILVAs maintained patency without stenosis.
Conclusion: Castor single-branched stent-graft implantation combined with fenestration technique may be safe and feasible for TAD patients with ILVA and unfavorable PLZ.
Introduction
Thoracic endovascular aortic repair (TEVAR) has been increasingly used for the treatment of various thoracic aortic disease (TAD), including type B aortic dissection (TBAD), intramural hematoma (IMH), penetrating ulcer, thoracic aortic aneurysm, traumatic aortic injury due to its advantages of high safety, less trauma and fewer postoperative complications (1). However, the success of TEVAR largely depends on the sealing of the proximal landing zone (PLZ), which requires that the length of the PLZ is at least 2.0 cm (2). Therefore, for patients with unfavorable PLZ, the proximal stent-graft inevitably needs to cover LSA. Isolated left vertebral artery (ILVA) directly originates from the aortic arch, usually between the left common carotid artery and left subclavian artery (LSA) (3). It is the second most common anatomical variant in the variation of the superior aortic trunk, with an incidence of 0.79%–8% (3–7). TAD with unfavorable PLZ and ILVA is rare. If the circle of Willis is incomplete and the LSA and ILVA are handled improperly, it may lead to an increased risk of postoperative complications such as subclavian steal syndrome, left upper limb ischemia and stroke (8,9).
The stented elephant trunk technique has been reported to treat complicated TBAD with ILVA and unfavorable PLZ (10), but high technical difficulty and mortality rate restrict its application. In addition, TEVAR combined with carotid-subclavian bypass (CSbp) and ILVA transposition was also widely used for treating this special condition, but the trauma was massive (11,12). Fortunately, methods for reconstructing supra-arch branches in TEVAR have developed rapidly, including chimney technique, periscope technique, fenestrated technique and single-branched stent-graft implantation. With these techniques, an adequate proximal landing area and supra-arch branches reconstruction could be realized. In this study, we shared our experience of using Castor single-branched stent-graft combined with fenestrated technique in treatment of TAD patients with ILVA and unfavorable PLZ, which may reduce the complexity and difficulty of surgery. We hope that our experience can provide an additional choice for cardiovascular surgeons to deal with this supra-arch variation, which may be less invasive, safe and effective.
Materials and methods
This is a single-center observational study. From January 2018 to March 2022, a total of 8 TAD patients with ILVA underwent TEVAR in our center (Figure 1). In this small series, there were 6 patients with TBAD, 1 patient with type B IMH, and 1 patient with thoracic aortic aneurysm. As the opening of LSA was involved by TAD in the eight patients, Castor single-branched stent-graft was applied to obtain adequate PLZ and reconstruct LSA. To reserve the ILVA, Castor single-branched stent-graft was fenestrated prior to implantation. Demographic characteristics, comorbidities, surgical details, postoperative conditions and follow-up results were collected and analyzed. This study was approved by the Ethics Committee of Shandong University Qilu Hospital. Because this is a retrospective study and the data are anonymous, we waived informed consent for this study.

Figure 1. Preoperative CTA. (A–C) in a patient with thoracic aortic aneurysm, the ILVA (the yellow arrow) and aortic aneurysm (the orange arrow) are evident on the cross-sectional and three-dimensional reconstruction images; (D–F) in a patient with type B intramural hematoma, the ILVA (the blue arrow) and intramural hematoma (the green arrow) can be clearly seen from the cross-sectional and three-dimensional reconstruction images.
TEVAR procedure
The Castor stent-graft used in this study was the first single-branched stent-graft in China (9). It was with an integrated design of the main body and branch. All TEVAR procedures were performed under general anesthesia in the hybrid operating room by two experienced surgeons. The distance between LSA and ILVA, diameters of aortic zone 2, distal landing zone and the location of LSA and ILVA opening were obtained from preoperative computed tomography angiography (CTA). Usually, the right femoral artery (RFA) was chosen as the main approach. The details of the operation were as follows: first, the proximal part of Castor single-branched stent-graft was partially released from the sheath on the operation table. According to the aortic parameters obtained for preoperative CTA, including the relative location of LSA and ILVA, the size of ILVA opening, a fenestration was performed at an appropriate position (Figure 2A). The fenestration should be sutured firmly and circularly to achieve a smooth edge. The sutures around the stent-graft were tightened to avoid its deformation. The stent-graft was reinstalled and returned to the delivery sheath. Next, the RFA was exposed and two 6-F sheaths were inserted percutaneously into the left brachial artery (LBA) and left femoral artery (LFA), respectively. A 5-F pigtail catheter with a guidewire (catheter A) was pushed through the true lumen from the LFA to the ascending aorta. Angiography was subsequently performed to show the situation of the supra-arch branches and confirm the measurements obtained from CTA (Figure 2B). Then, another 5-F catheter (catheter B) was introduced from the LBA to the RFA through the true lumen along with a guidewire, and it was drawn out from the RFA and placed externally as a traction catheter. The third pigtail catheter with a guidewire (catheter C) was led from RFA to the ascending aorta and replaced with a super stiff guidewire. The traction wire of the branch was threaded into catheter B from RFA to LBA. Thereafter, the main body of the Castor single-branched stent-graft was delivered to the thoracic aorta along the super stiff guidewire, while the catheter B and the traction wire of the branch were moved simultaneously with the main body of delivery system. The position of the stent-graft was constantly adjusted to ensure the branch stent-graft was in good alignment with the LSA. Afterwards. the outer sheath and the soft sheath were removed, and the branch stent-graft was dragged into the LSA by pulling the traction wire. Finally, the main and branch grafts were released by pulling the trigger wire and the traction wire, respectively. Angiography was performed immediately to evaluate the stent-graft position, endoleak, LSA and ILVA perfusion (Figure 2C). All patients were given aspirin (100 mg/day) after operation to prevent thrombosis of the single-branched stent at least 6 months.
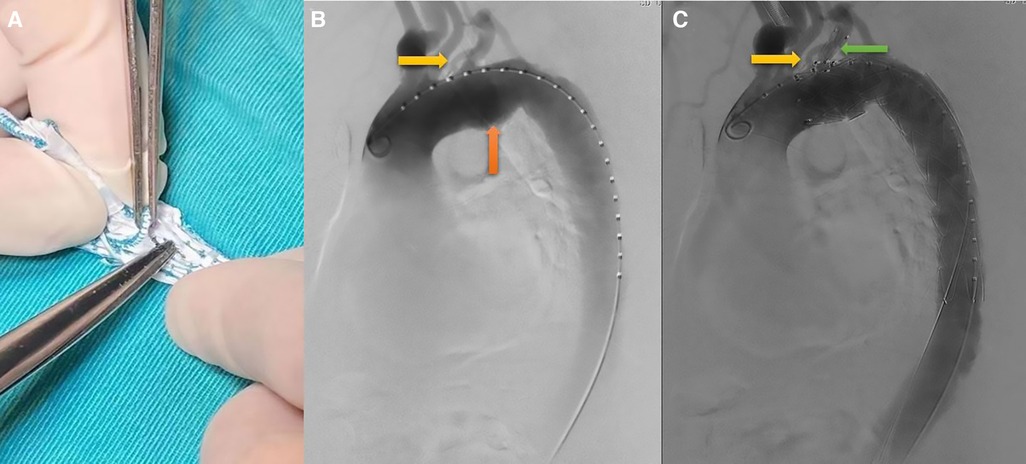
Figure 2. TEVAR procedure. (A) on-the-table fenestration; (B) preoperative angiography showed that isolated left vertebral artery (the yellow arrow) and aortic aneurysm (the orange arrow); (C) preoperative angiography showed that both ILVA (the yellow arrow) and LSA (the green arrow) were reconstructed.
Definition and main results
Primary technical success was defined as successful stent deployment without conversion to open surgery or death, patency of LSA with ILVA without significant stenosis, and no obvious signs of endoleak. Postoperative stroke was determined by brain CT or MRI scan. The main manifestations of spinal cord ischemia were distal weakness of lower limbs and intermittent claudication. The main symptoms of left upper limb ischemia were pulseless of left arm, intermittent claudication of left arm and cold shoulder sensation.
The follow-up results were obtained by telephone interview and at outpatient clinic visits. Primary outcomes during the follow-up included early (<30 days) survival, late survival, freedom from aortic-related mortality, the patency of LSA and ILVA. Furthermore, endoleak, retrograde type A dissection, aortic rupture, stent-graft fracture and displacement were also evaluated from postoperative CTA.
Statistical analysis
Statistical analysis SPSS 26.0 (IBM, Armonk, New York, USA) was used for statistical analysis. Continuous variables are presented as mean ± SD and range. Categorical variables were presented as frequencies and percentages.
Results
Demographics and clinical characteristics
From January 2018 to March 2022, a total of 8 patients with TAD and ILVA underwent TEVAR in our center. Six (75.0%) patients were male, and the median age was 62.5 years old (range, 41 to 75 years old). The most common comorbidities are hypertension (62.5%). Four (50.0%) patients had smoking history, and 2 (25.0%) patients were diagnosed with COPD. According to preoperative CTA, there were 1 (12.5%) case of left vertebral artery dominant, 4 (50.0%) cases of right vertebral artery dominant, and 3 (37.5%) cases of symmetrical vertebral artery dominant. Additional details of patient characteristics were listed in Table 1.
Details of the procedure
TEVAR was performed in all patients, and the technical success rate was 100%. For each patient, the length of stent-graft main body was 200 mm and the length of the branch part was 25 mm. The median proximal stent-graft diameter was 34 mm (range, 30–36 mm). The median expansion ratio of proximal stents was 12.5% (range, 6.3%–15.4%). The median operation time was 115 min (range, 70–180 min). All the LASs and ILVAs were reconstructed. There was no endoleak during surgery.
Early and late outcomes
The median duration of hospital stay was 8 days (range, 3–13 days). No postoperative complications were observed in the eight patients, including stoke, spinal cord ischemia, left upper limb ischemia, AKI and puncture complication. There were no in-hospital deaths.
The median duration of follow-up was 48 months (range, 6–72 months). No neurological complications were observed during follow-up. The long-term survival rate was 100%. All of the LSAs and ILVAs maintained patency without stenosis (Figure 3). In addition, no endoleak, aortic rupture, and retrograde type A aortic dissection were observed in the eight patients. None of them required further intervention. More details were shown in Table 2.
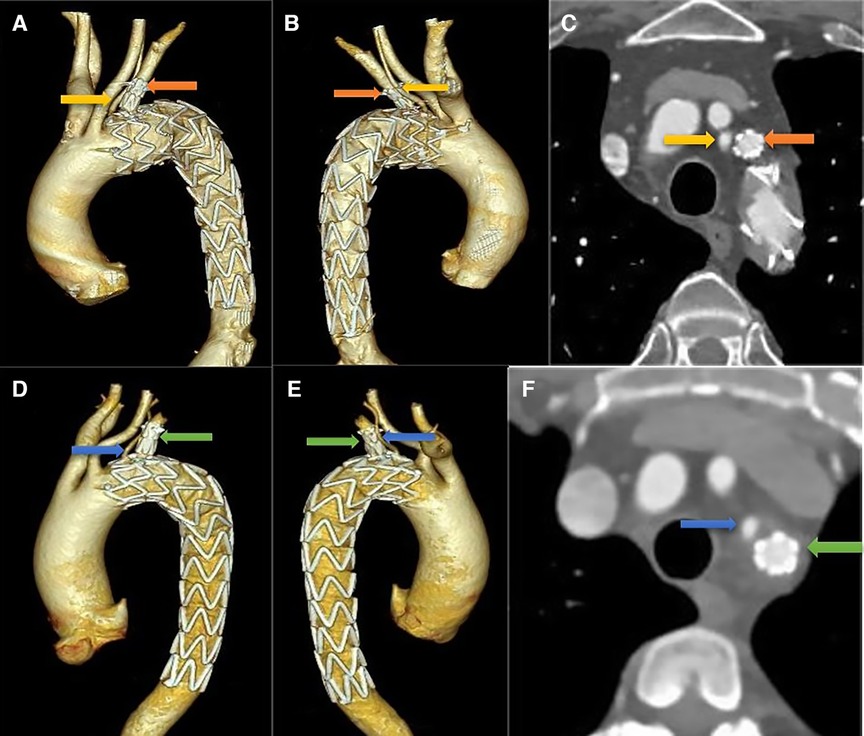
Figure 3. Follow-up CTA imaging. (A–C) in a patient with thoracic aortic aneurysm, both ILVA and LSA maintained patency without stenosis on three-dimensional reconstruction and cross-sectional images; (D–F) the same results can be seen in a patient with type B intramural hematoma.
Discussion
ILVA has been increasingly recognized as the second most common aortic arch branch variation, with an incidence of 0.79%–8% in general population (3–7). In patients with TBAD, the frequency of this anomaly is 3.6%, suggesting that ILVA may be related to the occurrence of TBAD (13). However, there were no clear guidelines whether ILVA should be reconstructed during surgery. In the most previous studies, the authors stated that the ILVA should be preserved (14,15). The left vertebral artery is an important component of the vertebrobasilar artery, which accounts for two fifths of the blood flow of the posterior cerebral artery (16). The posterior cerebral artery is the basic component of the Willis circle. It has been reported that the frequency of a complete Willis circle was 42% in the Western population. However, the frequency of a complete Willis circle in Chinese population was only 27% (17,18). Coverage of ILVA may increase the risk of spinal cord injury and postoperative stroke. However, some surgeons did not reconstruct the ILVA regularly (13). In their view, the ILVA was not necessarily reconstructed if it was with hypoplasia or thin size. Considering a high incidence of an incomplete circle of Willis in the Chinese population and the absence of cerebral artery CTA in an emergency, we regularly reconstructed the ILVA.
Several studies have reported the results of different surgical procedures for thoracic aortic disease and ILVA (10–12), including open surgery, hybrid surgery and total TEVAR. Zhu et al. (10) reported that seven patients with TBAD and ILVA underwent the stented elephant trunk procedure (open surgery). No deaths and postoperative complications were observed in the early term. Yang et al. (12) reviewed thirteen patients with thoracic aortic disease and ILVA using TEVAR with ILVA transposition and carotid-subclavian bypass (hybrid surgery). No deaths were observed in a mean follow-up of 22 months (range, 13–29 months). No complications were observed during the follow-up, including neurologic deficits, bypass occlusion and ILVA stenosis. Open surgery and hybrid surgery had shown satisfactory outcomes in treating the patients with thoracic aortic disease and ILVA. However, considering the massive trauma of these surgical methods, we attempted a less invasive approach, which is total TEVAR.
Total TEVAR for the thoracic aortic disease has been the mainstream treatment for its encouraging outcomes and less invasiveness. A length of 2.0 cm PLZ is essential for the success of total TEVAR. However, total TEVAR of thoracic aortic disease with ILVA and unfavorable PLZ was rarely reported. Ding et al. (13) reported 9 TBAD patients with ILVA treated with zone-2 TEVAR combined with LSA chimney technique. In order not to cover ILVA, the chimney stents were all bare stents. Neurologic deficits and chimney stent occlusions were not observed during surgery and follow-up, but complications such as immediate type I endoleak, type II endoleak and occlusion of the ILVA origin occurred. In our study, Castor single-branched stent-graft implantation combined with fenestration technique was used for treating patients with thoracic disease and ILVA. During the follow-up, all of the LSAs and ILVAs maintained patency without stenosis, no endoleak, aortic rupture and retrograde type A aortic dissection were observed in the eight patients. Based on excellent results, Castor single-branched stent-graft combined with fenestrated technique may be safe and feasible for repairing thoracic aortic disease with ILVA and insufficient PLZ.
It is considered that fenestrated or single-branched stent technique may lead to the risk of endoleak due to groove formation (12). However, previous published studies showed that the incidence of endoleak after Castor single-branched stent-graft implantation was low (19,20). Actually, single-branched stent-graft is more consistent with the design concept of anatomic correction. The anchoring effect of its branch part reduces the risk of stent displacement and endoleak (21). In 2004, McWilliams et al. first applied the in-situ fenestration technique of a thoracic stent-graft. to preserve LSA and demonstrated its feasibility (22). Since then, fenestration techniques have been developed continuously, mainly including in-situ fenestration and on-the-table fenestration. Currently, this technique has been applied in clinical practice through several preoperative and intraoperative methods classified as (1) mechanical, such as wires and hollow needles, and (2) physical, including laser and radiofrequency perforation (23). The incidence of endoleak in the early stage of fenestration is 0%–3.2% described in the previous study (24). In fact, in cases of aortic arch disease that involved only the inner curve of the aorta, the selection of on-the-table fenestration technique can help reduce the incidence of endoleak after TEVAR (25). However, in our study, no endoleak of fenestration was observed during surgery and follow-up, which may be due to the small size of fenestration and healthy aortic wall surrounding the ILVA opening. Although the fenestrated technique may affect the long-term durability of the stent-graft, the early and mid-term results are still satisfactory whether in external pre-fenestration or in-situ fenestration (24–26). It is worth mentioning that computational fluid dynamics method based on patient-specific CTA images has been widely applied to evaluate the efficacy of complex TEVAR (27). Quantitative analysis of computational hemodynamics would be conducive to revealing the physiological effect of Castor single-branched stent-graft on the thoracic aortic disease with unfavorable PLZ and ILVA. Therefore, we are considering a related computational hemodynamics study in eight patients in the future.
Of course, our study still has some limitations. First, it is a single-center retrospective study with a small sample size. Second, the follow-up time was relatively short, and the long-term effect still needs further follow-up. Third, our study lacks relevant study on computational fluid dynamics, which needs to be further improved in the future. Finally, we lacked a comparison with open or hybrid surgery. These features and the limited experience with these anatomical variants do not allow us to make definitive recommendations from clinical or technical aspects. However, the surgical approach we have employed has hardly been reported in previous studies. We hope that larger registries or studies will confirm its feasibility and safety in addressing this challenge in the future.
Conclusion
Our limited experience indicates that single-branch stent-graft combined with fenestrated technique may be a safe and feasible treatment for TAD with ILVA and unfavorable PLZ. It can provide surgeons with an attractive choice due to its less invasiveness and good short-term effects. However, the durability and long-term effects of the technique needs to be further evaluated. Future studies with larger sample sizes and longer follow-up are warranted to confirm this finding.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Shandong University Qilu Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
XZ designed the study. ZW prepared the first draft of the paper. ZW and CF were responsible for statistical analysis of the data. ZW and CF designed the methodology for searching. HS, DW, XM, XB and CL performed the data collection. All authors took part in revising it critically for important intellectual content; agreed to submit to the current journal; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Youth Fund Project [grant number 81500367]; and the clinical research program of Shandong University [grant number 2020SDUCRCC031]. All funding from the two funding bodies were used for research design, data collection, analysis and interpretation.
Acknowledgments
The authors would like to thank the patients who participated in this study, along with colleagues and medical staff who took part in patient care.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Czerny M, Pacini D, Aboyans V, Al-Attar N, Eggebrecht H, Evangelista A, et al. Current options and recommendations for the use of thoracic endovascular aortic repair in acute and chronic thoracic aortic disease: an expert consensus document of the European society for cardiology (ESC) working group of cardiovascular surgery, the ESC working group on aorta and peripheral vascular diseases, the European association of percutaneous cardiovascular interventions (EAPCI) of the ESC and the European association for cardio-thoracic surgery (EACTS). Eur J Cardiothorac Surg. (2021) 59(1):65–73. doi: 10.1093/ejcts/ezaa268
2. Yoon WJ, Mell MW. Outcome comparison of thoracic endovascular aortic repair performed outside versus inside proximal landing zone length recommendation. J Vasc Surg. (2020) 72(6):1883–90. doi: 10.1016/j.jvs.2020.03.033
3. Lazaridis N, Piagkou M, Loukas M, Piperaki ET, Totlis T, Noussios G, et al. A systematic classification of the vertebral artery variable origin: clinical and surgical implications. Surg Radiol Anat. (2018) 40(7):779–97. doi: 10.1007/s00276-018-1987-3
4. Magklara EP, Pantelia ET, Solia E, Panagouli E, Piagkou M, Mazarakis A, et al. Vertebral artery variations revised: origin, course, branches and embryonic development. Folia Morphol (Warsz). (2021) 80(1):1–12. doi: 10.5603/FM.a2020.0022
5. Popieluszko P, Henry BM, Hsieh WC, Saganiak K, Pękala PA, et al. A systematic review and meta-analysis of variations in branching patterns of the adult aortic arch. J Vasc Surg. (2018) 68(1):298–306.e10. doi: 10.1016/j.jvs.2017.06.097
6. Tsiouris C, Lazaridis N, Piagkou M, Hsieh WC, Saganiak K, Pękala PA, et al. The left-sided aortic arch variants: prevalence meta-analysis of imaging studies. Surg Radiol Anat. (2022) 44(5):673–88. doi: 10.1007/s00276-022-02945-4
7. Woraputtaporn W, Ananteerakul T, Iamsaard S, Namking M. Incidence of vertebral artery of aortic arch origin, its level of entry into transverse foramen, length, diameter and clinical significance. Anat Sci Int. (2019) 94(4):275–9. doi: 10.1007/s12565-019-00482-6
8. Saremi F, Hassani C, Lin LM, Lee C, Wilcox AG, Fleischman F, et al. Image predictors of treatment outcome after thoracic aortic dissection repair. Radiographics. (2018) 38(7):1949–72. doi: 10.1148/rg.2018180025
9. Fang C, Wang C, Liu K, Pang X. Early outcomes of left subclavian artery revascularization using castor single-branched stent-graft in the treatment of type B aortic dissection or intramural hematoma. Ann Thorac Cardiovasc Surg. (2021) 27(4):251–9. doi: 10.5761/atcs.oa.20-00166
10. Zhu JM, Qi RD, Liu YM, Zheng J, Xing XY, Sun LZ. Repair of complicated type B dissection with an isolated left vertebral artery using the stented elephant trunk technique. Eur J Cardiothorac Surg. (2016) 49(3):778–82. doi: 10.1093/ejcts/ezv242
11. Piffaretti G, Gelpi G, Tadiello M, Ferrarese S, Socrate AM, Tozzi M, et al. Transposition of the left vertebral artery during endovascular stent-graft repair of the aortic arch. J Thorac Cardiovasc Surg. (2020) 159(6):2189–2198.e1. doi: 10.1016/j.jtcvs.2019.06.011
12. Yang G, Chen H, Sun G, Lou W, Chen X, Zhang L. Transposition of isolated left vertebral artery in hybrid thoracic endovascular aortic repair. Front Cardiovasc Med. (2021) 8:783656. doi: 10.3389/fcvm.2021.783656
13. Ding H, Zhu Y, Wang H, Luo S, Liu Y, Huang W, et al. Management of type B aortic dissection with an isolated left vertebral artery. J Vasc Surg. (2019) 70(4):1065–71. doi: 10.1016/j.jvs.2018.11.052
14. Ad N, Lee P, Cox JL. Type A aortic dissection with associated anomaly of the carotid and vertebral vessels. J Thorac Cardiovasc Surg. (2002) 123(3):570–1. doi: 10.1067/mtc.2002.121673
15. Qi R, Sun L, Zhu J, Liu Y, Zheng J, Li C, et al. Total arch replacement in patients with aortic dissection with an isolated left vertebral artery. Ann Thorac Surg. (2013) 95(1):36–40. doi: 10.1016/j.athoracsur.2012.07.078
16. Woo EY, Bavaria JE, Pochettino A, Gleason TG, Woo YJ, Velazquez OC, et al. Techniques for preserving vertebral artery perfusion during thoracic aortic stent grafting requiring aortic arch landing. Vasc Endovascular Surg. (2006) 40(5):367–73. doi: 10.1177/1538574406293735
17. Li Q, Li J, Lv F, Li K, Luo T, Xie P. A multidetector CT angiography study of variations in the circle of willis in a Chinese population. J Clin Neurosci. (2011) 18(3):379–83. doi: 10.1016/j.jocn.2010.07.137
18. Sun LZ, Qi RD, Chang Q, Zhu JM, Liu YM, Yu CT, et al. Istotal arch replacement combined with stented elephant trunk implantation justified for patients with chronic stanford type a aortic dissection? J Thorac Cardiovasc Surg. (2009) 138:892–6. doi: 10.1016/j.jtcvs.2009.02.041
19. Jing Z, Lu Q, Feng J, Zhou J, Feng R, Zhao Z, et al. Endovascular repair of aortic dissection involving the left subclavian artery by castor stent graft: a multicentre prospective trial. Eur J Vasc Endovasc Surg. (2020) 60(6):854–61. doi: 10.1016/j.ejvs.2020.08.022
20. Huang B, Jia H, Lai H, Chen Z, Sun Y, Wang C. Outcomes of thoracic endovascular aortic repair for penetrating aortic ulcers involving the left subclavian artery with the castor single-branched stent graft. Eur J Cardiothorac Surg. (2022) 62(2):ezac102. doi: 10.1093/ejcts/ezac102
21. Chen BL, Zhuang XM, Wei MX. Short-term efficacy of unibody single-branched stent in the treatment of lesions involving the left subclavian artery: two-year follow-up outcomes. J Geriatr Cardiol. (2020) 17(2):120–3. doi: 10.11909/j.issn.1671-5411.2020.02.006
22. McWilliams RG, Murphy M, Hartley D, Lawrence-Brown MM, Harris PL. In situ stent-graft fenestration to preserve the left subclavian artery. J Endovasc Ther. (2004) 11(2):170–4. doi: 10.1583/03-1180.1
23. Glorion M, Coscas R, McWilliams RG, Javerliat I, Goëau-Brissonniere O, Coggia M. A comprehensive review of in situ fenestration of aortic endografts. Eur J Vasc Endovasc Surg. (2016) 52(6):787–800. doi: 10.1016/j.ejvs.2016.10.001
24. Chang H, Wang Y, Liu B, Wang W, Li Y. Endovascular repair for acute type B aortic dissection with unfavorable proximal landing zone. Ann Thorac Surg. (2022) 113(2):545–53. doi: 10.1016/j.athoracsur.2021.02.092
25. Shu C, Fan B, Luo M, Li Q, Fang K, Li M, et al. Endovascular treatment for aortic arch pathologies: chimney, on-the-table fenestration, and in-situ fenestration techniques. J Thorac Dis. (2020) 12(4):1437–48. doi: 10.21037/jtd.2020.03.10
26. Luo M, Fang K, Fan B, Li Q, Li M, He H, et al. Midterm results of retrograde in situ needle fenestration during thoracic endovascular aortic repair of aortic arch pathologies. J Endovasc Ther. (2021) 28(1):36–43. doi: 10.1177/1526602820953406
Keywords: isolated left vertebral artery, unfavorable proximal landing zone, castor single-branched stent-graft, fenestrated technique, thoracic aortic disease
Citation: Wang Z, Fang C, Song H, Wei D, Meng X, Bai X, Liu C and Zhao X (2023) Endovascular repair of thoracic aortic disease with isolated left vertebral artery and unfavorable proximal landing zone using fenestrated castor stent-graft. Front. Cardiovasc. Med. 10:1168180. doi: 10.3389/fcvm.2023.1168180
Received: 17 February 2023; Accepted: 11 August 2023;
Published: 24 August 2023.
Edited by:
Gianni Angelini, University of Bristol, United KingdomReviewed by:
Roman Gottardi, University Heart Center Freiburg, GermanyYonghui Qiao, Zhejiang University, China
Kexiang Liu, Jilin University, China
© 2023 Wang, Fang, Song, Wei, Meng, Bai, Liu and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Zhao emhhb3hpbkBlbWFpbC5zZHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Zhenhua Wang1,2,†
Zhenhua Wang1,2,† Changcun Fang
Changcun Fang Chunxiao Liu
Chunxiao Liu Xin Zhao
Xin Zhao