- Institute for Pathophysiology, West German Heart and Vascular Center, University of Duisburg-Essen, Essen, Germany
Introduction: Diazoxide is a powerful cardioprotective agent that activates mitochondrial ATP-dependent K-channels and stimulates mitochondrial respiration. Diazoxide reduced infarct size in isolated rodent heart preparations and upon pretreatment in juvenile pigs with coronary occlusion/reperfusion. We aimed to study the use of diazoxide in a more realistic adult pig model of reperfused acute myocardial infarction when diazoxide was administered just before reperfusion.
Methods and results: In a first approach, we pretreated anaesthetised adult Göttingen minipigs with 7 mg kg−1 diazoxide (n = 5) or placebo (n = 5) intravenously over 10 min and subjected them to 60 min coronary occlusion and 180 min reperfusion; blood pressure was maintained by use of an aortic snare. The primary endpoint was infarct size (triphenyl tetrazolium chloride staining) as a fraction of area at risk; no-reflow area (thioflavin-S staining) was the secondary endpoint. In a second approach, diazoxide (n = 5) was given from 50 to 60 min coronary occlusion, and blood pressure was not maintained. There was a significant reduction in infarct size (22% ± 11% of area at risk with diazoxide pretreatment vs. 47% ± 11% with placebo) and area of no-reflow (14% ± 14% of infarct size with diazoxide pretreatment vs. 46% ± 20% with placebo). With diazoxide from 50 to 60 min coronary occlusion, however, there was marked hypotension, and infarct size (44% ± 7%) and area of no-reflow were not reduced (35% ± 25%).
Conclusions: Cardioprotection by diazoxide pretreatment was confirmed in adult pigs with reperfused acute myocardial infarction but is not feasible when diazoxide is administered in a more realistic scenario before reperfusion and causes hypotension.
Introduction
Cardioprotection is characterised by reduced infarct size and reduced coronary microvascular obstruction (1). Infarct size (2) and coronary microvascular obstruction (3) are major determinants of prognosis in patients with reperfused acute myocardial infarction. Despite a myriad of preclinical studies that demonstrated reduced infarct size and reduced coronary microvascular obstruction by mechanical or pharmacological cardioprotective interventions, the translation of cardioprotection to patient benefit has been largely disappointing so far (4–6). The lack of translation has been attributed to two major factors: (1) The recruitment of low-risk patients into cardioprotection trials in whom it was difficult to show a further reduction in mortality and heart failure development (7, 8); (2) The typical presence of advanced age, comorbidities, and co-medications in patients which were, however, not present in most preclinical studies but interfere with the signal transduction of cardioprotection (9). The signal transduction of cardioprotection by ischaemic conditioning and many pharmacological agents comprises an action on the sarcolemma and its channels and receptors, an activation of cytosolic protein kinase cascades, and ultimately an action on mitochondria and their function (10). To reduce an interference of comorbidities and co-medications with cardioprotective signal transduction to a minimum, it appears prudent to target its most distal signalling step, i.e., the mitochondria. Many mitochondrial ion channels have been targeted successfully in preclinical studies, and this action resulted in cardioprotection (11). However, phase III clinical trials targeting mitochondrial sites such as the permeability transition pore with cyclosporine A (12, 13) or other less defined sites (14) have not been successful, despite promising proof-of-concept trials (15).
Diazoxide is a cardioprotective agent that targets the mitochondria, and it has not yet been clinically tested. Diazoxide was initially identified as an opener of mitochondrial potassium channels in isolated bovine cardiac mitochondria (16). It was first demonstrated to reduce cell death in isolated rabbit cardiomyocytes undergoing simulated ischaemia (17). Diazoxide reduced infarct size in isolated, saline-perfused rabbit hearts undergoing regional ischaemia/reperfusion (I/R), and the mechanism of action was related to opening of mitochondrial ATP-dependent potassium channels (KATP) and the release of reactive oxygen species (ROS) (18). There appears to be also an action of diazoxide on the mitochondrial respiratory chain, which is independent of the activation of KATP; in isolated rat heart mitochondria, diazoxide stimulates mitochondrial respiration and ROS formation at complex III but inhibits ROS formation at complex I during reverse electron transfer as it occurs during early reperfusion (19, 20). Also, diazoxide interacts with Connexin 43 to release ROS (21, 22). These ROS that were released from the mitochondria in response to diazoxide must be considered as cardioprotective signals. These ROS might act to oxidise protein kinases that are then not only upstream of mitochondria in the cardioprotective signal transduction but also downstream (23). In fact, diazoxide might signal through oxidation of a protein tyrosine kinase since its cardioprotective effect is abrogated with genistein (18). Oxidation of protein kinase A—not, however, in response to diazoxide—has been demonstrated to reduce infarct size by prevention of lysosomal loss of calcium into the cytosol and detrimental calcium overload (24). Also, the mitochondrial ROS downregulates L-type calcium channels, thus possibly contributing to attenuated calcium overload (25). Diazoxide’s cardioprotective actions are antagonised by the non-selective KATP antagonist glibenclamide (18, 26) and the mitochondrial-selective KATP antagonist 5-hydroxydecanoate (18, 22). The cardioprotective properties of diazoxide were then related to a temporally and spatially restricted ROS release from mitochondria and in close interaction with mitochondrial Connexin 43. Diazoxide has also demonstrated cardioprotective properties in large animal models. Pretreatment with diazoxide reduced infarct size in sexually immature Yorkshire pigs (27) and creatine kinase-MB release in female piglets (28). Also, the addition of diazoxide to a cardioplegic solution attenuated stunning in pig hearts following global I/R (29, 30). However, in adult pigs when regional ischaemia was followed by cardioplegic arrest before reperfusion, diazoxide decreased infarct size in one study when added to cardioplegia (31) but not in another study with pretreatment of diazoxide before regional ischaemia (32); thus, the protection by diazoxide does not appear to be a robust finding. Also, diazoxide acts on vascular smooth muscle cells and induces profound vasodilation. When given systemically, diazoxide markedly reduces blood pressure, and this clinical indication is approved. A marked hypotension below the coronary autoregulatory range would possibly be counterproductive and promote ischaemia (33), when diazoxide is given systemically in a clinically realistic scenario of cardioprotection, i.e., during coronary occlusion and before reperfusion. The present study, therefore, attempted to: (1) confirm the cardioprotective effect of diazoxide pretreatment in sexually mature minipigs at stable haemodynamic conditions; such confirmation is not trivial since, e.g., infarct size reduction with ischaemic preconditioning was effective in Göttingen minipigs but not in Ossabaw minipigs (34); and (2) to see whether diazoxide would still exert cardioprotection when given just before reperfusion and when it is associated with profound hypotension.
Methods
Unless otherwise specified, materials were obtained from Sigma-Aldrich (Deisenhofen, Germany).
The experimental protocols were approved by the Bioethical Committee of the district of Düsseldorf (G1868/21) and conform to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. We followed the ARRIVE 2.0 guidelines (35, 36) and the AVMA guidelines. A full description of the intended experimental design and analysis has not been published in a preclinical registry. Experiments in pigs were performed between June 2022 and February 2023.
Experimental preparation
Validity of animal species and model selection: This in vivo experimental model in pigs replicates many aspects of the human reperfused myocardial infarction, notably its temporal and spatial development (37). Female Göttingen minipigs were purchased from Ellegaard, Dalmose, Denmark. We have recently demonstrated that infarct size and cardioprotection by ischaemic preconditioning are not sex-dependent in our model (38). Pigs were fed with standard chow (twice 300 g/day, #V4133. ssniff, Soest, Germany), had access to water ad libitum, and were kept in tiled rooms (∼2 m2/pig) with straw-bedding at 12 h/12 h light/dark cycles. There was a daily visual inspection of pigs’ health by animal caretakers and veterinarians. All behavioural abnormalities were recorded and monitored; only inconspicuous, apparently healthy pigs were included in the experiment. Pigs were 17 ± 5 months and weighed 36 ± 9 kg. Pigs were sedated with flunitrazepam (i.m.: 0.4 mg kg−1). Anaesthesia was induced with etomidate (i.v.: 0.3 mg kg−1, Hypnomidate, Voorschoten, The Netherlands) and sufentanil (i.v.: 1 µg kg−1, Sufentanil-hameln, Hameln, Germany). Anaesthesia was maintained with isoflurane (2%, TEVA, Eastbourne, United Kingdom) during artificial ventilation with room air (tidal volume: 8–10 ml kg−1, respiratory rate: 10–16 breaths min−1, inspiratory peak pressure 18–25 cm H2O, positive end-expiratory pressure: 5–7 cm H2O). Muscle relaxation during electrosurgery was induced with a single bolus of rocuronium (i.v.: 0.6 mg kg−1, B. Braun, Melsungen, Germany). This anaesthetic regimen is identical to that used in our institution for patients undergoing surgical coronary revascularisation (39). The pigs were placed on a heated table and covered with heated blankets to keep the oesophageal temperature between 36.0 and 38.0°C. ECG-lead II was continuously recorded using a single-channel, calibrated amplifier. A midline cervical incision was performed. The left jugular vein was cannulated for volume replacement and intravenous drug administration, and the right common carotid artery was cannulated to measure arterial pressure. The heart was exposed by a left lateral thoracotomy and instrumented with a micromanometer (DPT-6000, Codan-PVB, Forsting, Germany) in the left ventricle to measure left ventricular pressure (LVP; LabChart 8, Lab Chart, AD Instruments Pty LTD, New South Wales, Australia) and a Teflon catheter in the left atrium for the injection of coloured microspheres (40). Atrial pacing electrodes were sutured to the right atrium for eventual pacing with pulses of 1 V amplitude and 0.002 s duration (LabChart 8). The distal aortic arch was cannulated to withdraw the reference sample for regional blood flow measurement. The left anterior descending coronary artery (LAD) was dissected and prepared distal to its second diagonal branch for later coronary occlusion. The proximal descending aorta was equipped with a ribbon (“Bühner-Band”, Covetus GmbH, Düsseldorf, Germany), which could be manually tightened to maintain LVP.
Regional myocardial blood flow
Coloured microspheres were recovered from transmural myocardial samples taken from the central area at risk by digestion with 4 mol L−1 KOH and subsequent filtration (8 µm pore size, Pieper Filter, Bad Zwischenahn, Germany). Fluorescent dye was resolved from microspheres and quantified in a spectrophotometer (F-7100, Hitachi High-Tech, Krefeld, Germany). Blood flow was calculated as blood flow per tissue mass.
Area at risk, infarct size, and no-reflow
Thirty millilitres of warm 4% thioflavin-S solution (Morphisto, Frankfurt, Germany) was filtered through a 0.2 µm syringe-filter to remove particulate debris and slowly infused into the left atrium to demarcate non-perfused areas of the left ventricle after 180 min reperfusion (41, 42). Thereafter, the LAD was re-occluded at the same location as for the index ischaemia, and 5 ml blue dye (Patentblau V, Guerbet GmbH, Sulzbach, Germany) was quickly injected into the left atrium to delineate the area at risk as remaining unstained. The heart was quickly removed from the chest, rinsed with cold saline, and cut into five slices perpendicular to the ventricular long axis. The tissue slices were examined under ultraviolet light (340–360 nm, VL-UVA 135.11, Vilber Louramat, Eberhardzell, Germany). Areas without yellow-green fluorescence (thioflavin-S-negative) were encircled by incisions. After documenting the slices using a digital camera, the slice shape, the thioflavin-S-negative areas, and the demarcated area at risk were transferred to a transparent film. Thereafter, infarcted tissue was demarcated by triphenyl tetrazolium chloride (TTC) staining (1% dissolved in 90 mmol L−1 sodium phosphate buffer containing 8% dextran, Roth, Karlsruhe, Germany). The TTC-stained slices were again photographed, and transferred together with the tissue areas that remained unstained by TTC to the same transparent film that was used to document the area at risk and the no-reflow areas. Particular care was taken to proper realign the slices using “landmarks,” such as the position of papillary muscles and the incisions surrounding the no-reflow areas.
The transparent films were scanned and analysed using digital planimetry. The following areas were calculated and averaged for both sides of each slice: total area of the left ventricle, the area at risk, the area of TTC-negative tissue (infarcted), and the area of thioflavin-S-negative tissue within the infarcted tissue (no-reflow). Using the slice weight for normalisation, the tissue masses for the area at risk, the no-reflow area, and for the infarcted area were calculated. In addition, the area at risk was calculated as a fraction of the left ventricle, infarct size was calculated as a fraction of the area at risk, and the area of no-reflow was calculated as a fraction of infarct size.
Protocols
Pigs were randomised (using sealed envelopes) to an I/R diazoxide pretreatment or diazoxide before reperfusion protocol, respectively.
Ischaemia/reperfusion
When the heart rate at baseline was <95 min−1, atrial pacing was performed. After stabilisation for at least 30 min, systemic haemodynamics and regional myocardial blood flow were measured. After intravenous infusion of unfractionated heparin (2,500 IU, Heparin-Natrium-ratiopharm, Ulm, Germany), the LAD was occluded distal to its second diagonal branch using a microvascular clamp (TKL-1, BIOVERR, Hergiswil, Switzerland). Heparin (2,500 IU) was again given at 25 and 55 min coronary occlusion. At 5 and 55 min coronary occlusion, systemic haemodynamics and regional myocardial blood flow were measured again. Reperfusion was induced after 60 min coronary occlusion by quick removal of the vascular clamp and visually confirmed by the reappearance of red colour on the surface of the reperfused myocardium. Systemic haemodynamics were again measured at 10 and 180 min reperfusion and regional myocardial blood flow at 10 and 180 min reperfusion. Ventricular fibrillation during ischaemia or reperfusion, as identified from the continuous lead II ECG recording, was immediately terminated by intra-thoracic defibrillation (up to 50 Ws; 6/4 ms biphasic pulse; Zoll R Series Monitor & Defibrillator, Zoll Medical Cooperation, Chelmsford, MA, United States). We did not use any antiarrhythmic or inotropic agents during resuscitation since they might interfere with the infarction process and/or cardioprotection. At the end of the experiment, pigs were euthanised by intracardiac injection of 20 ml potassium chloride (1 mol L−1).
Diazoxide pretreatment + I/R
The experimental protocol was identical to that of I/R, except that diazoxide (7 mg kg−1 i.v. over 10 min in the solvent solution of 10 ml NaCl, B. Braun with 20% 1 mol L−1 NaOH, pH = 11.7) was administered after baseline measurements before ischaemia. The dose of diazoxide was determined with reference to prior studies (27, 28) and to preliminary experiments in our preparation. During the administration of diazoxide, LVP was maintained through adjustment of the aortic snare.
Diazoxide before reperfusion + I/R
The experimental protocol was identical to that of I/R, except that diazoxide (7 mg kg−1 i.v. over 10 min in solvent solution of 10 ml NaCl, B. Braun with 20% 1 mol L−1 NaOH, pH = 11.7) was administered after 50 min coronary occlusion over 10 min just before reperfusion. In this set of experiments, LVP was not adjusted with the aortic snare.
In the I/R experiments, intravenous 10 ml of the solvent solution over 10 min did not induce a haemodynamic response, and the infarct size data were well in line with our prior data in this model in the absence of such solvent solution (38).
Data and statistical analysis
The investigator who quantitatively assessed haemodynamics, regional myocardial blood flow, infarct size, and area of no-reflow was blinded to the protocol. Explorative statistics were performed on all data to test for normal distribution (Shapiro–Wilk test) and to identify potential outliers. Data are presented as mean ± SD; individual data on infarct size and no-reflow are also presented as scatterplots. Statistical package SAS 9.4 (Cary, NC, United States) was used to analyse haemodynamic and regional myocardial blood flow data by two-way analysis of variance (protocol, time) for repeated measures. Data on area at risk, infarct size, area of no-reflow, and episodes with fibrillation/defibrillation were analysed by one-way analysis of variance. When analysis of variance indicated a significant main effect or interaction, Fisher’s least square difference (LSD) tests were used for comparison of single mean values. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology.
Results
Haemodynamics
At baseline, heart rate, LVP, regional myocardial blood flow, and area at risk were not different between the three groups of I/R without or with diazoxide pretreatment or diazoxide just before reperfusion, respectively (Table 1). With the onset of ischaemia, regional myocardial blood flow was decreased markedly and not different between groups. LVP progressively decreased and heart rate increased. The intravenous administration of diazoxide during 50–60 min ischaemia aggravated the decrease in LVP pressure further, and this decrease persisted into early reperfusion. Regional myocardial blood flow during reperfusion recovered from I/R, displayed reactive hyperaemia in the group with diazoxide pretreatment, and remained depressed in the group with diazoxide before reperfusion. At the end of 180 min reperfusion, there were again no differences in heart rate and LVP between the three groups, but some increase in regional myocardial blood flow in the diazoxide groups persisted. There was no significant difference in ventricular fibrillation/defibrillation episodes between the three groups (Table 1).
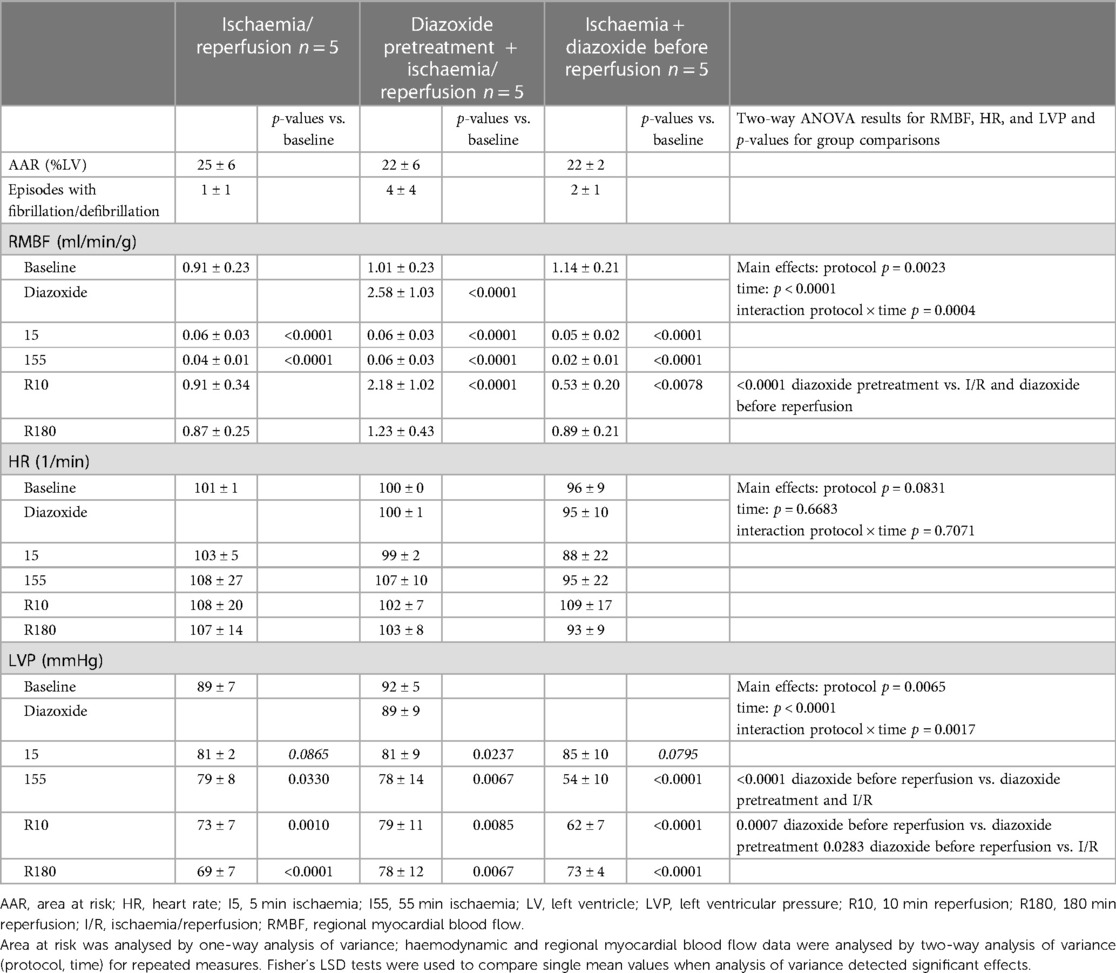
Table 1. Area at risk, regional myocardial blood flow, heart rate, and left ventricular pressure in pigs undergoing ischaemia/reperfusion without and with diazoxide pretreatment or diazoxide before reperfusion.
Infarct size and no-reflow area
Infarct size with I/R was 47% ± 11% of area at risk, and area of no-reflow was 46% ± 20% of infarct size; thus, both were very close to our prior data in this model (38). Diazoxide pretreatment with maintained haemodynamics (aortic snare) reduced both infarct size and area of no-reflow markedly (Figures 1, 2). However, when diazoxide was given systemically and induced hypotension, infarct size and area of no-reflow were no longer reduced. In one pig, there was marked hypotension into early reperfusion (LVP: 42 mmHg at 55 min ischaemia and 53 mmHg at 10 min reperfusion), associated with a large area of no-reflow, which resulted in the lack of TTC washout such that infarct size could no longer be clearly identified as TTC-negative (Figure 3).
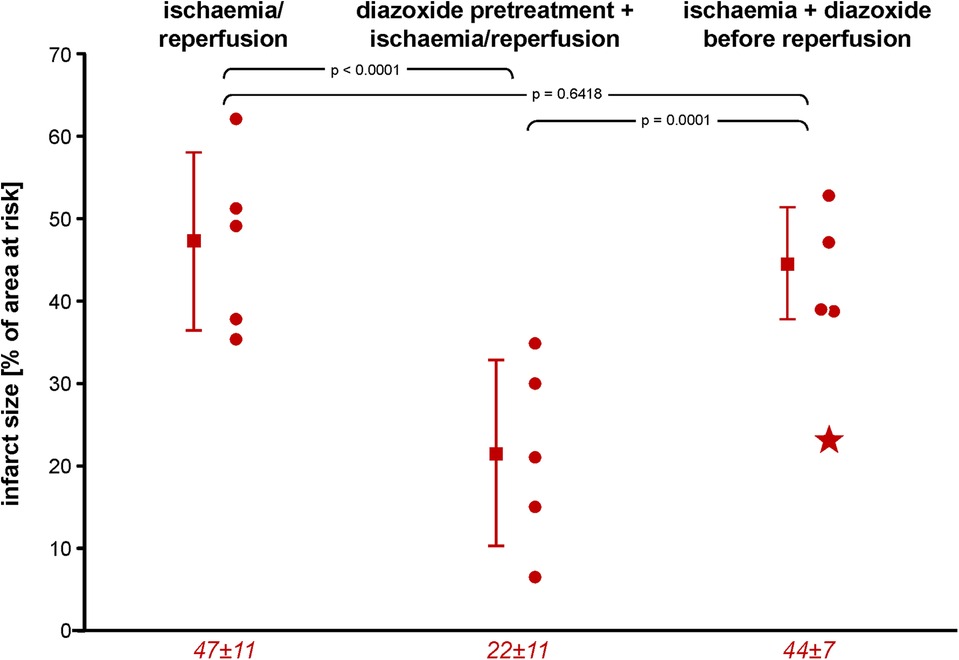
Figure 1. Infarct size following ischaemia/reperfusion without and with diazoxide pretreatment or diazoxide before reperfusion, respectively; individual data (circles) and means (squares) with SD; mean ± SD are also given as numerical data for ischaemia/reperfusion (n = 5), pretreatment with diazoxide + ischaemia/reperfusion (n = 5), and diazoxide before reperfusion (n = 4). The asterisk in the scatter plot of data with diazoxide before reperfusion indicates one pig with persistent TTC-positive staining within an area of no-reflow, as indicated by the negative thioflavin-S staining. Data were analysed by one-way analysis of variance and Fisher’s LSD post-hoc tests. LSD, least square difference; TTC, triphenyl tetrazolium chloride.
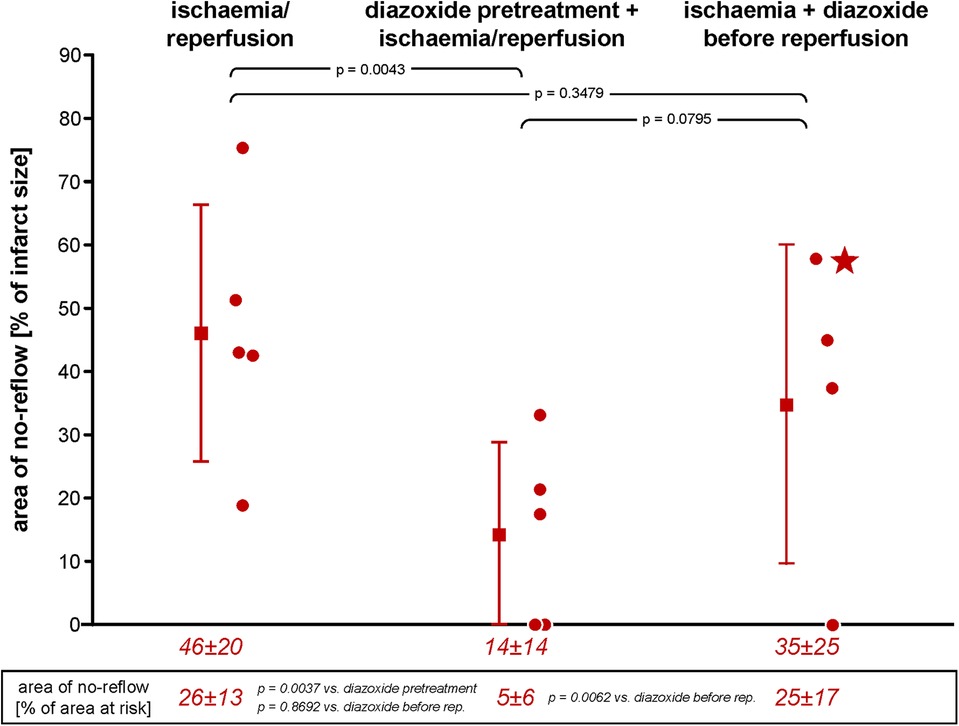
Figure 2. Area of no-reflow following ischaemia/reperfusion without and with diazoxide pretreatment or diazoxide before reperfusion, respectively; individual data (circles) and means (squares) with SD; mean ± SD are also given as numerical data for ischaemia/reperfusion (n = 5), pretreatment with diazoxide + ischaemia/reperfusion (n = 5), and diazoxide before reperfusion (n = 4). The asterisk in the scatter plot of data diazoxide before reperfusion indicates a pig with persistent TTC-positive staining within an area of no-reflow, as indicated by the negative thioflavin-S staining. Data were analysed by one-way analysis of variance and LSD post-hoc tests. LSD, least square difference; TTC, triphenyl tetrazolium chloride.
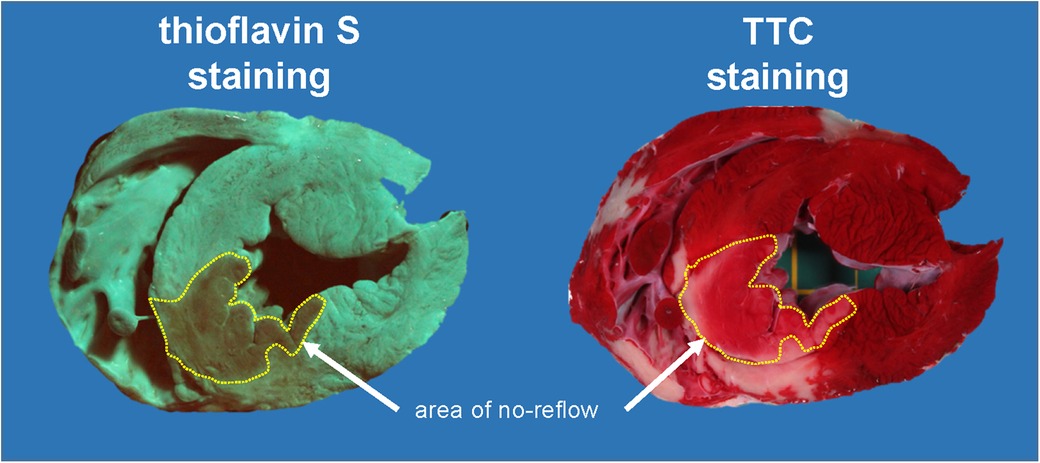
Figure 3. Original heart slice after demarcation of areas of no-reflow with thioflavin-S and after demarcation of avital tissue by TTC staining. The thioflavin-S-negative stained area (no-reflow) is indicated in both images. Almost the entire area of no-reflow is stained TTC positive. This pig had profound hypotension with intravenous diazoxide given over 10 min just before reperfusion (left ventricular pressure: 42 mmHg at 55 min ischaemia and 53 mmHg at 10 min reperfusion). TTC, triphenyl tetrazolium chloride.
Discussion
The present study confirms the well-established fact that diazoxide is a powerful cardioprotective agent. In the present study, fibrillation/defibrillation episodes were not significantly different between control ischaemia/reperfusion, diazoxide pretreatment and diazoxide treatment at reperfusion, and if anything increased by diazoxide, thus excluding a primary antiarrhythmic action of diazoxide. However, diazoxide pretreatment before coronary occlusion and reperfusion and with maintenance of heart rate and LVP reduced infarct size, confirming in an adult pig model prior data from juvenile pig models of reperfused acute myocardial infarction (27, 28). Of note—and this is novel—cardioprotection was also seen in the coronary circulation (43), since the area of no-reflow was reduced by diazoxide pretreatment. Our study, however, did not only aim to confirm cardioprotection by diazoxide in adult rather than juvenile pigs and protection not only of cardiomyocytes but also of the coronary circulation, but our study also aimed for the translational potential of diazoxide. We, therefore, in a second approach, administered diazoxide in a more realistic scenario as would occur in a clinical setting of acute myocardial infarction, i.e., before reperfusion and without compensation of the marked hypotension that diazoxide induces upon systemic administration. Diazoxide before reperfusion and in the presence of marked hypotension, which added to the decrease in LVP inflicted by the coronary occlusion per se, no longer reduced infarct size and area of no-reflow. In fact, in a single extreme case, the loss of coronary perfusion pressure caused such pronounced microvascular obstruction that the washout of NADH oxidase and reducing NADH equivalents was delayed to an extent that TTC staining remained positive. These data clearly indicate that systemic administration of diazoxide before reperfusion could not induce cardioprotection, but in fact rather caused haemodynamic complications. Apart from the deleterious role of severe hypotension per se, the decrease in perfusion pressure and further decrease in regional myocardial blood flow will also have decreased the diazoxide delivery to the ischaemic myocardium. Theoretically then, one would attempt intracoronary administration of diazoxide before or at reperfusion. Unfortunately, however, diazoxide is poorly soluble (product information Sigma-Aldrich GCY/NSB 12/03) (44), requiring either a pH > 10 in saline (present study), high volume of ethanol or methanol, or use of toxic solvents such as dimethyl sulfoxide (45, 46) or dimethylformamide (47), and thus clearly precluding an intracoronary infusion of diazoxide into a reperfused myocardial region, which is anyway at jeopardy. Chronic systemic treatment with diazoxide would, apart from hypotension, is faced with further problems, in that its cardioprotective efficacy might be impaired by high cholesterol levels (26), and that itself through ROS-triggered ROS release from mitochondria and NADPH oxidase might induce nitrate tolerance (48–52).
We must, therefore, conclude that diazoxide is a powerful cardioprotectant with an action on mitochondrial KATP and respiration, but unfortunately it is not feasible for translation into a realistic clinical setting of acute myocardial infarction (Figure 4). Mechanistically, our study emphasises the pivotal importance of adequate coronary perfusion pressure above the autoregulatory range (33), which dominates during ischaemia and reperfusion above the potential protection by diazoxide. Gentle reperfusion over the first 30 min of reperfusion is protective, but perfusion pressure must be restored to above the lower limit of the autoregulatory range (53). Unfortunately, we did not measure aortic/coronary perfusion pressure in the present study. However, even LVP at 10 min reperfusion was still around 60 mmHg, and therefore coronary perfusion pressure was definitely less than 50 mmHg, which is the lower limit of coronary autoregulation in an anesthetised open-chest preparation. Our study also demonstrated that left ventricular unloading is not cardioprotective unless coronary perfusion pressure is preserved. Also, whereas gentle reperfusion may attenuate injury and reduce infarct size (53), perfusion pressure during such gentle reperfusion must still be above the lower limit of the autoregulatory range. Strategically, our study again emphasises that cardioprotective research must progress from reductionist models to realistic scenarios if it really aims for translation (54).
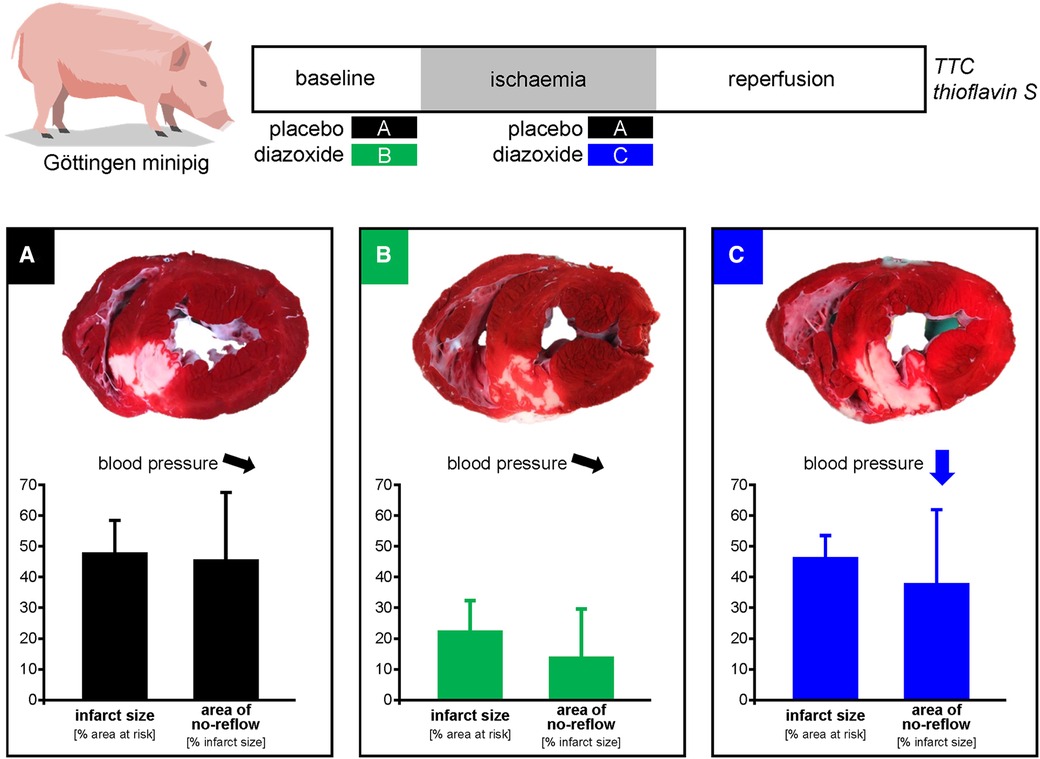
Figure 4. Schematic summary. Diazoxide activates mitochondrial ATP-dependent K-channels and stimulates respiration. Experiments in juvenile pigs had reported infarct size reduction by pretreatment with intravenous diazoxide. We now analysed the use of diazoxide in adult minipigs and in a more realistic scenario of administration before reperfusion. In comparison to placebo (A), diazoxide pretreatment (B) reduced infarct size and area of no-reflow, but diazoxide before reperfusion (C) caused hypotension and did not reduce infarct size and no-reflow. Thus, cardioprotection with diazoxide is not feasible in a clinical scenario of acute myocardial infarction with administration before reperfusion.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by the Bioethical Committee of the district of Düsseldorf (G1868/21).
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was supported by the German Research Foundation (SFB 1116 B08 to GH and PK) and the European COST ACTION in CARDIOPROTECTION (CA16225 to GH and IG16225 to GH and PK).
Acknowledgments
We thank Kerstin Abu Hamed and Anita van de Sand for their excellent technical assistance.
Conflict of interest
The reviewer RS declared a past co-authorship with the author GH to the handling editor.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. (2020) 17:773–89. doi: 10.1038/s41569-020-0403-y
2. Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, et al. Relationship between infarct size and outcomes following primary PCI: patient-level analysis from 10 randomized trials. J Am Coll Cardiol. (2016) 67:1674–83. doi: 10.1016/j.jacc.2016.01.069
3. de Waha S, Patel MR, Granger CB, Ohman EM, Maehara A, Eitel I, et al. Relationship between microvascular obstruction and adverse events following primary percutaneous coronary intervention for ST-segment elevation myocardial infarction: an individual patient data pooled analysis from seven randomized trials. Eur Heart J. (2017) 38:3502–10. doi: 10.1093/eurheartj/ehx414
4. Heusch G, Rassaf T. Time to give up on cardioprotection? A critical appraisal of clinical studies on ischemic pre-, post-, and remote conditioning. Circ Res. (2016) 119:676–95. doi: 10.1161/CIRCRESAHA.116.308736
5. Heusch G. Critical issues for the translation of cardioprotection. Circ Res. (2017) 120:1477–86. doi: 10.1161/CIRCRESAHA.117.310820
6. Heusch G, Bøtker EH, Ferdinandy P, Schulz R. Primordial non-responsiveness—a novel obstacle to cardioprotection. Eur Heart J. (2023). doi: 10.1093/eurheartj/ehad160. [Epub ahead of print]36943315
7. Heusch G, Gersh BJ. Is cardioprotection salvageable? Circulation. (2020) 141:415–7. doi: 10.1161/CIRCULATIONAHA.119.044176
8. Hausenloy DJ, Bøtker HE. Why did remote ischaemic conditioning not improve clinical outcomes in acute myocardial infarction in the CONDI-2/ERIC-PPCI trial? Cardiovasc Res. (2019) 115:e161–3. doi: 10.1093/cvr/cvz242
9. Ferdinandy P, Andreadou I, Baxter GF, Bøtker HE, Davidson SM, Dobrev D, et al. Interaction of cardiovascular nonmodifiable risk factors, comorbidities and comedications with ischemia/reperfusion injury and cardioprotection by pharmacological treatments and ischemic conditioning. Pharmacol Rev. (2023) 75:159–216. doi: 10.1124/pharmrev.121.000348
10. Heusch G. Molecular basis of cardioprotection: signal transduction in ischemic pre-, post-, and remote conditioning. Circ Res. (2015) 116:674–99. doi: 10.1161/CIRCRESAHA.116.305348
11. Hausenloy DJ, Schulz R, Girao H, Kwak BR, De Stefani D, Rizzuto R, et al. Mitochondrial ion channels as targets for cardioprotection. J Cell Mol Med. (2020) 24:7102–14. doi: 10.1111/jcmm.15341
12. Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. (2015) 373:1021–103. doi: 10.1056/NEJMoa1505489
13. Ottani F, Latini R, Staszewsky L, La Vecchia L, Locuratolo N, Sicuro M, et al. Cyclosporine A in reperfused myocardial infarction. The multicenter, contolled, open-label CYCLE trial. J Am Coll Cardiol. (2016) 67:365–74. doi: 10.1016/j.jacc.2015.10.081
14. Atar D, Arheden H, Berdeaux A, Bonnet JL, Carlsson M, Clemmensen P, et al. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results. Eur Heart J. (2015) 36:112–9. doi: 10.1093/eurheartj/ehu331
15. Bøtker HE, Cabrera-Fuentes HA, Ruiz-Meana M, Heusch G, Ovize M. Translational issues for mitoprotective agents as adjunct to reperfusion therapy in patients with ST-segment elevation myocardial infarction. J Cell Mol Med. (2020) 24:2717–29. doi: 10.1111/jcmm.14953
16. Garlid KD, Paucek P, Yarov-Yarovoy V, Sun X, Schindler PA. The mitochondrial KATP channel as a receptor for potassium channel openers. J Biol Chem. (1996) 271:8796–9. doi: 10.1074/jbc.271.15.8796
17. Liu Y, Sato T, O’Rourke B, Marban E. Mitochondrial ATP-dependent potassium channels. Novel effectors of cardioprotection? Circulation. (1998) 97:2463–9. doi: 10.1161/01.CIR.97.24.2463
18. Pain T, Yang XM, Critz SD, Yue Y, Nakano A, Liu GS, et al. Opening of mitochondrial K(ATP) channels triggers the preconditioned state by generating free radicals. Circ Res. (2000) 87:460–6. doi: 10.1161/01.res.87.6.460
19. Dröse S, Brandt U, Hanley PJ. K+-independent actions of diazoxide question the role of inner membrane KATP channels in mitochondrial cytoprotective signaling. J Biol Chem. (2006) 281:23733–9. doi: 10.1074/jbc.M602570200
20. Dröse S, Hanley PJ, Brandt U. Ambivalent effects of diazoxide on mitochondrial ROS production at respiratory chain complexes I and III. Biochim Biophys Acta. (2009) 1790:558–65. doi: 10.1016/j.bbagen.2009.01.011
21. Heinzel FR, Luo Y, Li X, Boengler K, Buechert A, García-Dorado D, et al. Impairment of diazoxide-induced formation of reactive oxygen species and loss of cardioprotection in connexin 43 deficient mice. Circ Res. (2005) 97:583–6. doi: 10.1161/01.RES.0000181171.65293.65
22. He H, Li N, Zhao Z, Han F, Wang X, Zeng Y. Ischemic postconditioning improves the expression of cellular membrane connexin 43 and attenuates the reperfusion injury in rat acute myocardial infarction. Biomed Rep. (2015) 3:668–74. doi: 10.3892/br.2015.485
23. Andreadou I, Daiber A, Baxter GF, Brizzi MF, Di Lisa F, Kaludercic N, et al. Influence of cardiometabolic comorbidities on myocardial function, infarction, and cardioprotection: role of cardiac redox signaling. Free Radic Biol Med. (2021) 166:33–52. doi: 10.1016/j.freeradbiomed.2021.02.012
24. Simon JN, Vrellaku B, Monterisi S, Chu SM, Rawlings N, Lomas O, et al. Oxidation of protein kinase A regulatory subunit PKARIalpha protects against myocardial ischemia-reperfusion injury by inhibiting lysosomal-triggered calcium release. Circulation. (2021) 143:449–65. doi: 10.1161/CIRCULATIONAHA.120.046761
25. Gonzalez G, Zaldivar D, Carrillo E, Hernandez A, Garcia M, Sanchez J. Pharmacological preconditioning by diazoxide downregulates cardiac L-type Ca(2+) channels. Br J Pharmacol. (2010) 161:1172–85. doi: 10.1111/j.1476-5381.2010.00960.x
26. Csonka C, Kupai K, Bencsik P, Gorbe A, Paloczi J, Zvara A, et al. Cholesterol-enriched diet inhibits cardioprotection by ATP-sensitive K+ channel activators cromakalim and diazoxide. Am J Physiol Heart Circ Physiol. (2014) 306:H405–13. doi: 10.1152/ajpheart.00257.2013
27. Schwartz LM, Welch TS, Crago MS. Cardioprotection by multiple preconditioning cycles does not require mitochondrial KATP channels in pigs. Am J Physiol Heart Circ Physiol. (2002) 283:H1538–44. doi: 10.1152/ajpheart.00040.2002
28. Sarja HE, Anttila T, Mustonen C, Honkanen HP, Herajarvi J, Haapanen H, et al. Diazoxide attenuates ischemic myocardial injury in a porcine model. Heart Surg Forum. (2017) 20:E153–61. doi: 10.1532/hsf.1790
29. Suarez-Pierre A, Lui C, Zhou X, Kearney S, Jones M, Wang J, et al. Diazoxide preserves myocardial function in a swine model of hypothermic cardioplegic arrest and prolonged global ischemia. J Thorac Cardiovasc Surg. (2020) 163:e385–400. doi: 10.1016/j.jtcvs.2020.08.069
30. Velez AK, Etchill E, Giuliano K, Kearney S, Jones M, Wang J, et al. ATP-sensitive potassium channel opener diazoxide reduces myocardial stunning in a porcine regional with subsequent global ischemia model. J Am Heart Assoc. (2022) 11:e026304. doi: 10.1161/JAHA.122.026304
31. Wakiyama H, Cowan DB, Toyoda Y, Federman M, Levitsky S, McCully JD. Selective opening of mitochondrial ATP-sensitive potassium channels during surgically induced myocardial ischemia decreases necrosis and apoptosis. Eur J Cardiothorac Surg. (2002) 21:424–33. doi: 10.1016/s1010-7940(01)01156-3
32. Davies JE, Digerness SB, Killingsworth CR, Zaragoza C, Katholi CR, Justice RK, et al. Multiple treatment approach to limit cardiac ischemia-reperfusion injury. Ann Thorac Surg. (2005) 80:1408–16. doi: 10.1016/j.athoracsur.2005.04.022
33. Heusch G. Myocardial ischemia: lack of coronary blood flow, myocardial oxygen supply-demand imbalance, or what? Am J Physiol Heart Circ Physiol. (2019) 316:H1439–46. doi: 10.1152/ajpheart.00139.2019
34. Kleinbongard P, Lieder HR, Skyschally A, Alloosh M, Gödecke A, Rahmann S, et al. Non-responsiveness to cardioprotection by ischaemic preconditioning in Ossabaw minipigs with genetic predisposition to, but without the phenotype of the metabolic syndrome. Basic Res Cardiol. (2022) 117:58. doi: 10.1007/s00395-022-00965-0
35. Percie du Sert N, Ahluwalia A, Alam S, Avey MT, Baker M, Browne WJ, et al. Reporting animal research: explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. (2020) 18:e3000411. doi: 10.1371/journal.pbio.3000411
36. Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. PLoS Biol. (2020) 18:e3000410. doi: 10.1371/journal.pbio.3000410
37. Heusch G, Skyschally A, Schulz R. The in-situ pig heart with regional ischemia/reperfusion—ready for translation. J Mol Cell Cardiol. (2011) 50:951–63. doi: 10.1016/j.yjmcc.2011.02.016
38. Kleinbongard P, Lieder H, Skyschally A, Heusch G. No sex-related differences in infarct size, no-reflow and protection by ischaemic preconditioning in Göttingen minipigs. Cardiovasc Res. (2023) 119:561–70. doi: 10.1093/cvr/cvac062
39. Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single-centre randomised, double-blind, controlled trial. Lancet. (2013) 382:597–604. doi: 10.1016/S0140-6736(13)61450-6
40. Kowallik P, Schulz R, Guth BD, Schade A, Paffhausen W, Gross R, et al. Measurement of regional myocardial blood flow with multiple colored microspheres. Circulation. (1991) 83:974–82. doi: 10.1161/01.CIR.83.3.974
41. Skyschally A, Amanakis G, Neuhäuser M, Kleinbongard P, Heusch G. Impact of electrical defibrillation on infarct size and no-reflow in pigs subjected to myocardial ischemia-reperfusion without and with ischemic conditioning. Am J Physiol Heart Circ Physiol. (2017) 313:H871–8. doi: 10.1152/ajpheart.00293.2017
42. Bøtker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol. (2018) 113:39. doi: 10.1007/s00395-018-0696-8
43. Heusch G. The coronary circulation as a target of cardioprotection. Circ Res. (2016) 118:1643–58. doi: 10.1161/CIRCRESAHA.116.308640
44. World Health Organization. Diazoxide (Diazoxidum). In: The International Pharmacopoeia (2016). Sixth Edition. Department of Essential Medicines and Health Products, Human Info NGO (Organization), HumanityCD Ltd, University of Waikato, USA: World Health Organization, 2016.
45. Dudeck O, Jordan O, Hoffmann KT, Okuducu AF, Tesmer K, Kreuzer-Nagy T, et al. Organic solvents as vehicles for precipitating liquid embolics: a comparative angiotoxicity study with superselective injections of swine rete mirabile. Am J Neuroradiol. (2006) 27:1900–6.17032862
46. Chaloupka JC, Vinuela F, Malanum RP, Ji C, Goller DE, Robert J, et al. Technical feasibility and performance studies of a Doppler guide wire for potential neuroendovascular applications. Am J Neuroradiol. (1994) 15:503–7.8197947
47. Kennedy GL Jr., Sherman H. Acute and subchronic toxicity of dimethylformamide and dimethylacetamide following various routes of administration. Drug Chem Toxicol. (1986) 9:147–70. doi: 10.3109/01480548608998272
48. Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JM, Muller J, et al. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid Redox Signal. (2008) 10:1435–47. doi: 10.1089/ars.2007.1969
49. Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. (2010) 1797:897–906. doi: 10.1016/j.bbabio.2010.01.032
50. Kröller-Schön S, Steven S, Kossmann S, Scholz A, Daub S, Oelze M, et al. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid Redox Signal. (2014) 20:247–66. doi: 10.1089/ars.2012.4953
51. Daiber A, Di Lisa F, Oelze M, Kröller-Schön S, Steven S, Schulz E, et al. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br J Pharmacol. (2017) 174:1670–89. doi: 10.1111/bph.13403
52. Bertero E, Heusch G, Münzel T, Maack C. A pathophysiological compass to personalize antianginal drug treatment. Nat Rev Cardiol. (2021) 18:838–52. doi: 10.1038/s41569-021-00573-w
53. Musiolik J, van Caster P, Skyschally A, Boengler K, Gres P, Schulz R, et al. Reduction of infarct size by gentle reperfusion without activation of reperfusion injury salvage kinases in pigs. Cardiovasc Res. (2010) 85:110–7. doi: 10.1093/cvr/cvp271
Keywords: cardioprotection, coronary microvascular obstruction, diazoxide, infarct size, myocardial infarction, reperfusion
Citation: Kleinbongard P, Lieder H, Skyschally A and Heusch G (2023) Diazoxide is a powerful cardioprotectant but is not feasible in a realistic infarct scenario. Front. Cardiovasc. Med. 10:1173462. doi: 10.3389/fcvm.2023.1173462
Received: 24 February 2023; Accepted: 31 March 2023;
Published: 19 April 2023.
Edited by:
Tommaso Gori, Johannes Gutenberg University Mainz, GermanyReviewed by:
Andreas Daiber, Johannes Gutenberg University Mainz, GermanyRainer Schulz, University of Giessen, Germany
Bodo Levkau, Heinrich Heine University of Düsseldorf, Germany
© 2023 Kleinbongard, Lieder, Skyschally and Heusch. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerd Heusch Z2VyZC5oZXVzY2hAdWstZXNzZW4uZGU=
†ORCID Petra Kleinbongard orcid.org/0000-0003-3576-3772 Helmut Lieder orcid.org/0000-0001-8631-3355 Andreas Skyschally orcid.org/0000-0001-8396-0450 Gerd Heusch orcid.org/0000-0001-7078-4160
Specialty Section: This article was submitted to Coronary Artery Disease, a section of the journal Frontiers in Cardiovascular Medicine
 Petra Kleinbongard
Petra Kleinbongard Helmut Lieder
Helmut Lieder Andreas Skyschally
Andreas Skyschally Gerd Heusch
Gerd Heusch