- 1Department of Cardiology, Heping Hospital Affiliated to Changzhi Medical College, Changzhi, China
- 2Department of Day-Surgery, The First Affiliated Hospital of China Medical University, Shenyang, China
- 3Department of Cardiology, Shenzhen Luohu Hospital Group Luohu People’s Hospital (The Third Affiliated Hospital of Shenzhen University), Shenzhen, China
Background: Previous studies have indicated that the soluble suppression of tumorigenicity 2 protein (sST2) is associated with new-onset atrial fibrillation (NOAF) in patients diagnosed with coronary artery disease (CAD). However, the predictive value of sST2 in patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) has not been well studied.
Methods: A total of 580 patients with STEMI undergoing primary PCI were consecutively recruited between January 2021 and January 2023. These patients were then categorized into two groups: the NOAF group and the no NOAF groups based on the presence of NOAF during admission. The concentration of sST2 in blood samples was measured in all patients. The clinical data from the two groups were prospectively analyzed to investigate the predictive factors of NOAF in patients with STEMI undergoing primary PCI.
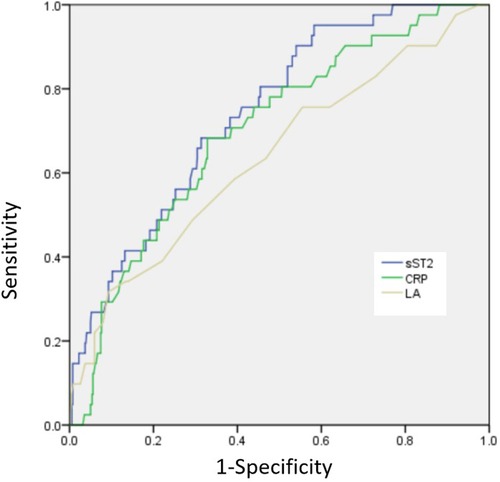
Results: A total of 41 (7.1%) patients developed NOAF. The presence of NOAF has been found to be associated with various factors, including age, diabetes mellitus, hypertension, the left atrial (LA) diameter, N-terminal pro-brain natriuretic peptide, C-reactive protein (CRP), sST2, a Killip class of ≥2, and a final TIMI flow grade of <3. After including multiple factors, it was observed that LA diameter, CRP, sST2, a Killip class of ≥2, and a final TIMI flow grade of <3 remained significant risk factors for developing NOAF. The receiver operating characteristic (ROC) curve showed the following findings: (1) when the LA diameter exceeded 38.5 mm, the sensitivity and specificity values were observed to be 67.2% and 68.2%, respectively, and the area under the ROC curve (AUC) was 0.683 [95% confidence interval (CI): 0.545–0.732; p = 0.003]; (2) when the CRP level exceeded 8.59, the sensitivity and specificity values were observed to be 68.6% and 69.2%, respectively, and the AUC was 0.713 (95% CI: 0.621–0.778; p < 0.001); and (3) when the sST2 value exceeded 53.3, the sensitivity and specificity values were 79.2% and 68.7%, respectively, and the AUC was 0.799 (95% CI: 0.675–0.865; p < 0.001).
Conclusion: sST2 has been identified as an independent predictor of NOAF in patients with STEMI undergoing PCI.
1. Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias encountered in clinical practice. In fact, AF is prevalent in patients diagnosed with acute myocardial infarction (AMI). The incidence rate of new-onset AF (NOAF) ranges from 4.5% to 9.2% in patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing primary percutaneous coronary intervention (PCI) (1–3). NOAF following STEMI is associated with an increased risk of in-hospital and long-term mortality (4, 5). With the coexistence of other cardiovascular risk factors, NOAF is associated with a higher risk of experiencing a nonfatal infarction (5), heart failure (6, 7), malignant arrhythmias (8), and stroke (4, 9). In addition, patients diagnosed with NOAF exhibit a higher tendency for experiencing a poor quality of life and incurring higher healthcare costs (10).
In patients with AMI, an inflammatory response occurs in the infarcted area, which is accompanied by myocardial dysfunction and mechanical stress. This process further facilitates the release of a soluble form of ST2 (sST2). As a member of the interleukin-1 receptor family, sST2 is associated with inflammatory responses and developing tissue fibrosis (11). The presence of inflammation and cardiac stress has been proven to induce structural and electrical remodeling and increase the risk of developing AF (12, 13). However, as a biomarker for fibrosis and inflammation, sST2 is not fully involved in the onset and development of AF, specifically in patients with STEMI undergoing primary PCI. Nortamo et al. (14) demonstrated that sST2 and high-sensitivity C-reactive protein (hs-CRP) exhibit a good predictive capacity for the occurrence of NOAF in patients with coronary artery disease (CAD), which may slightly improve the discrimination of the risk model. Chen et al. (15) suggested that sST2 is an independent predictor of NOAF in patients with AMI and can improve the accuracy of the AF risk model. Cardiac remodeling occurs in patients with STEMI undergoing PCI, and sST2 has been proven to be involved in the regulatory processes associated with cardiac remodeling. Furthermore, sST2 has demonstrated use in assessing the risk of adverse cardiac remodeling and the subsequent onset of heart failure in these individuals (16). Nevertheless, there is a lack of data on the relationship between sST2 and the risk of NOAF in patients with STEMI undergoing primary PCI.
Given the limited efforts in the prediction of NOAF in patients with STEMI undergoing primary PCI and the significant association between baseline sST2 levels and cardiac remodeling, we hypothesized that sST2 levels would be associated with the occurrence of NOAF in patients with STEMI undergoing primary PCI and may be useful to improve the prediction accuracy of NOAF in this patient population.
2. Methods
2.1. Study population
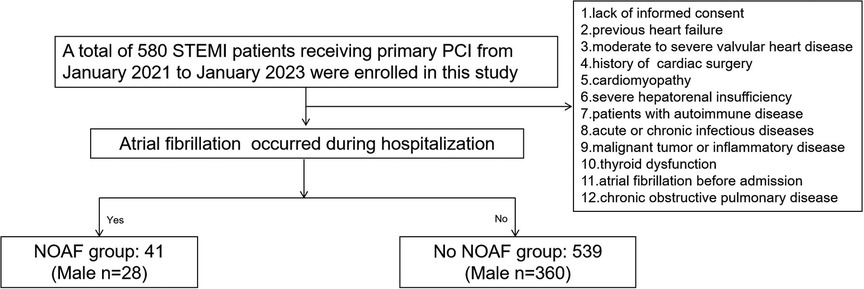
The study consecutively enrolled 612 patients diagnosed with STEMI undergoing PCI at Heping Hospital affiliated with Changzhi Medical College and Luohu People's Hospital from January 2021 to January 2023. The diagnosis of all patients was conducted in accordance with the criteria for STEMI established by the European Society of Cardiology (17). A primary PCI was performed on individuals who developed symptoms within 24 h. The exclusion criteria for this study include the following: (1) refusal to provide informed consent; (2) previous history of heart failure; (3) the presence of moderate to severe valvular heart disease; (4) prior history of cardiac surgery; (5) presence of cardiomyopathy; (6) severe hepatorenal insufficiency; (7) autoimmune disease; (8) presence of acute or chronic infectious diseases; (9) malignant tumor or inflammatory disease; (10) thyroid dysfunction; (11) history of AF prior to admission; and (12) a diagnosis of chronic obstructive pulmonary disease. Ultimately, a total of 580 participants were included in this study (Figure 1). This study was approved by the ethics committee of Heping Hospital, affiliated with Changzhi Medical College and Luohu People's Hospital, and was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided informed consent prior to their inclusion in the study.
2.2. Clinical and laboratory data assessments
Following the hospitalization period, several clinical baseline data were recorded, including demographic characteristics, previous medical history, medication history, and echocardiographic and laboratory characteristics. Blood samples were collected via the median cubital vein after admission for laboratory analysis. The concentration of sST2 in blood samples was determined by fluorescent immunoassay and chromatography method employing a dry immunity analyzer (A2000, Emmy Medical Technology Co., Ltd., Guangxi, China). During admission, all patients were subjected to electrocardiogram (ECG) monitoring in the coronary care unit (CCU) for the first 3 days. In addition, 12-lead ECGs were conducted twice daily throughout the hospitalization period. For cases of suspected AF or to address a complaint of palpitations or arrhythmia, a 12-lead ECG was performed to confirm the presence of NOAF.
2.3. Interventional procedures
The radial artery was the preferred access site for all individuals. However, in certain patients, access was determined by the operators. PCI was performed following the guidelines for revascularization. The infarction-related artery was determined by at least two cardiologists. Angiographic and interventional procedure characteristics were recorded and analyzed. Written informed consent was obtained from all patients before the procedure.
2.4. Statistical analysis
The statistical analysis was performed using SPSS version 20.0 (IBM, USA). Normally distributed continuous variables were presented as the mean ± standard deviation and compared between groups using the Student's t-test. Data that were not normally distributed were expressed as medians and compared between groups using the Mann–Whitney U test. Categorical data were presented as rates or percentages and subjected to analysis using the chi-square test or Fisher's exact test. Univariable regression was performed to analyze the factors associated with NOAF, and multivariable logistic regression analysis was performed to determine the predictors of NOAF. The receiver operating characteristic (ROC) curve was used to evaluate the accuracy, sensitivity, and specificity of the left atrial (LA) diameter, sST2, and CRP in distinguishing NOAF from STEMI patients undergoing primary PCI, as well as the optimal cutoff value for predicting NOAF. All tests were two-sided, and p-values < 0.05 were considered significant.
3. Results
3.1. Baseline and clinical characteristics
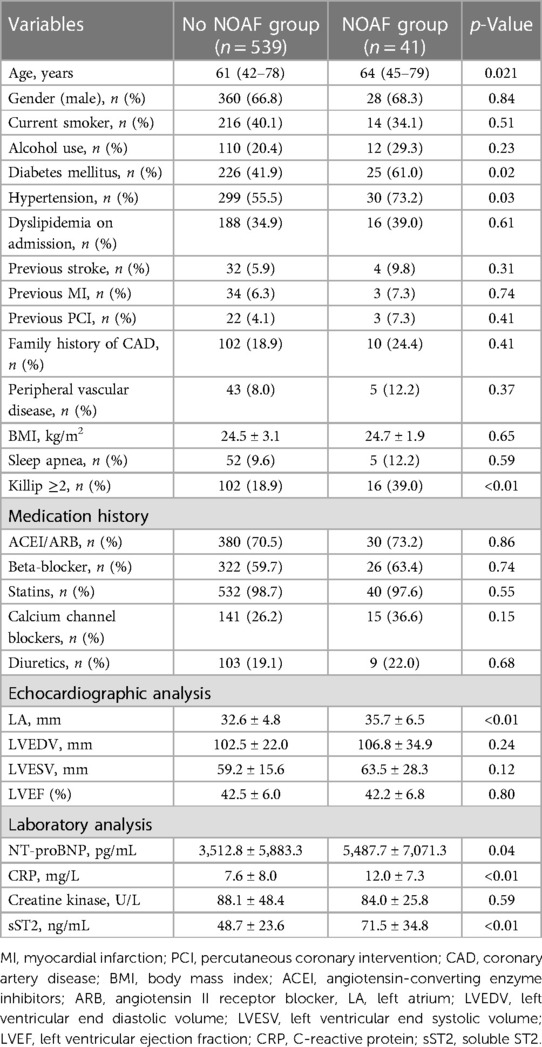
A total of 580 patients with STEMI undergoing PCI were included in this study. Among this group, the occurrence of NOAF was observed in 7.1% (41/580) of the cases. Baseline characteristics, medications received in the hospital, and ECG and laboratory parameters are shown in Table 1. No significant differences were observed between patients with and without NOAF in terms of gender, current smoking status, alcohol use, dyslipidemia, stroke, previous history of PCI and AMI, family history of CAD, peripheral vascular disease, body mass index, or sleep apnea (p > 0.05). However, patients with NOAF tended to be older and exhibited a higher prevalence of comorbidities such as diabetes mellitus, hypertension, and cardiac dysfunction classified as Killip ≥2 (p < 0.05; Table 1). There were no differences in medications received during hospitalization, including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, statins, calcium channel blockers, and diuretics (Table 1). There were also no differences in terms of left ventricular end diastolic volume, left ventricular end systolic volume, or left ventricular ejection fraction. However, the diameter of the LA in patients with NOAF was significantly larger than that in patients without NOAF (32.6 ± 4.8 vs. 35.7 ± 6.5; p < 0.01; Table 1). In addition to creatine kinase, there were significant differences in clinical variables, including N-terminal pro-brain natriuretic peptide (NT-proBNP), CRP, and sST2, between patients with and without NOAF (p < 0.05; Table 1).
3.2. Angiographic and procedural characteristics of the studied patients
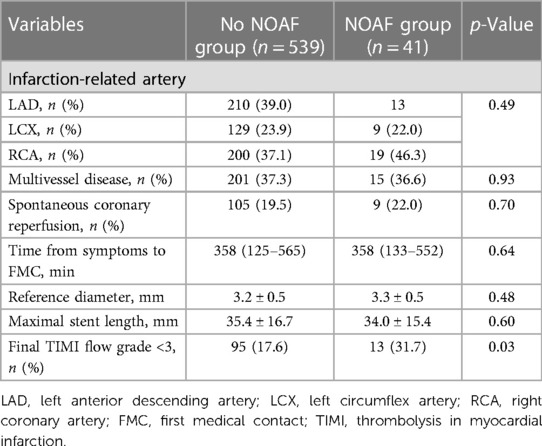
The angiographic and procedural characteristics of the studied patients are shown in Table 2. No significant differences were observed in relation to infarction-related arteries, multivessel disease, spontaneous coronary reperfusion, time from symptom onset to initial medical contact, reference diameter, or maximal stent length. However, patients with NOAF exhibited a higher proportion of final TIMI flow grade of <3 (31.7% vs. 17.6%; p = 0.03; Table 2).
3.3. Association of the markers with the risk of NOAF
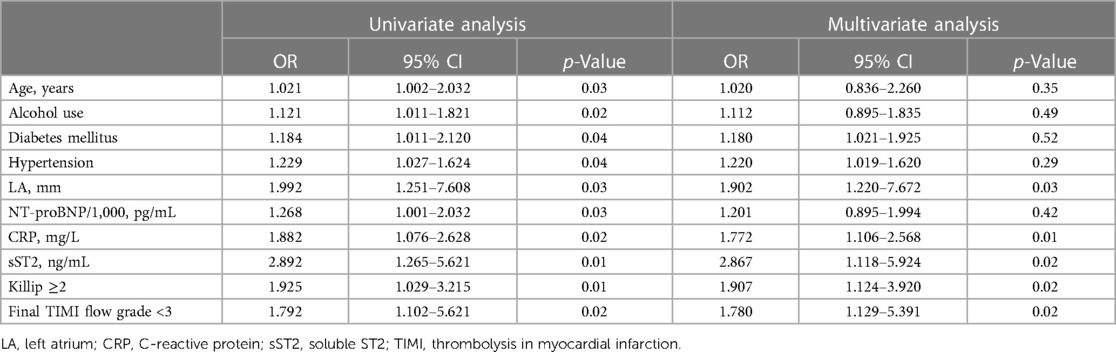
The presence of NOAF was found to be associated with several factors, including age, diabetes mellitus, hypertension, LA diameter, NT-proBNP levels, CRP levels, sST2 levels, Killip class of ≥2, and a final TIMI flow grade of <3. After including multiple factors, it was found that LA diameter, CRP, sST2, Killip class of ≥2, and a final TIMI flow grade of <3 remained significant risk factors for developing NOAF (Table 3). The ROC curve showed the following findings: (1) when the LA diameter exceeded 38.5 mm, the sensitivity and specificity values were observed to be 67.2% and 68.2%, respectively, and the area under the ROC curve (AUC) was 0.683 (95% confidence interval [CI]: 0.545–0.732; p = 0.003); (2) when the CRP level exceeded 8.59, the sensitivity and specificity values were 68.6% and 69.2%, respectively, and the AUC was 0.713 (95% CI: 0.621–0.778, p<0.001); and (3) when the sST2 value exceeded 53.3, the sensitivity and specificity values were 79.2% and 68.7%, respectively, and the AUC was 0.799 (95% CI: 0.675–0.865; p < 0.001; Figure 2).
4. Discussion
This study found that the incidence rate of NOAF in patients with STEMI undergoing PCI was 7.1%. LA, CRP, sST2, a higher proportion of Killip class of ≥2, and a final TIMI flow grade of <3 were independent predictors of NOAF in patients with STEMI undergoing PCI. sST2 may provide valuable information in relation to the discrimination risk associated with NOAF, surpassing the predictive capabilities of LA and CRP.
STEMI is a critical cardiovascular disease with high disability and mortality rates. Early reperfusion therapies were of vital importance for relieving symptoms and improving the prognosis. Primary PCI was the most widely used strategy in the revascularization of STEMI due to its simplicity, convenience, and efficiency. Despite the increasing prevalence of early PCI, AF remains a prevalent condition in patients with STEMI. Previous studies have suggested that the incidence rate of NOAF ranges between 4.5% and 9.2% in patients with STEMI undergoing primary PCI (1–3). NOAF following STEMI is associated with an increased risk of in-hospital and long-term mortality (4, 5). Although atrial fibrosis, inflammation, and cardiac stress have been suggested to increase the risk of AF (12, 13), the pathogenesis and clinical predictive biomarkers of NOAF in patients with STEMI undergoing primary PCI remain unclear.
The occurrence of NOAF in STEMI is still a common yet challenging issue in clinical practice. Further studies are required for the accurate prediction and appropriate management of this problem. Numerous studies have suggested that age, diabetes mellitus, and hypertension are independent risk factors for NOAF in patients with STEMI (2, 3). The present study found that patients with NOAF exhibited a higher mean age compared with those without NOAF. However, after multivariate adjustments, no statistically significant difference in age was observed between the groups. The potential reasons could be attributed to the inclusion of younger individuals and the presence of diverse races, comorbidities, and clinical characteristics, which may have influenced the observed outcomes. In addition, the number of included patients was relatively small. While a notable increase was observed in the incidence of diabetes mellitus and hypertension among patients with NOAF, no statistically significant difference was found after multivariate adjustments. We speculated that the current blood pressure and blood sugar control were not fully evaluated in this study. Consequently, our results may have to be interpreted differently compared with those in previous studies.
The LA diameter and its strain have been reported to be associated with NOAF (14, 18). Nortamo et al. (14) discovered that the LA diameter and NT-proBNP were independent predictors of NOAF in patients with CAD. However, the predictive value of the LA diameter in patients with STEMI undergoing primary PCI is still unknown. The present study found that the LA diameter exhibited a comparable and good predictive capacity in determining the risk of NOAF in patients with STEMI undergoing primary PCI. When the LA diameter exceeded 38.5 mm, the sensitivity and specificity values were 67.2% and 68.2%, respectively. As a biomarker of LA strain, higher NT-proBNP concentrations have been linked to the occurrence of NOAF in general population-based studies (19, 20). However, we found that the association of higher NT-proBNP concentrations with NOAF was lost after multivariate adjustments. Only specific STEMI patients were included in this study, which might have affected the results. Similar to a previous study, we found that a Killip class of ≥2 at admission was an independent predictor of NOAF in patients with STEMI undergoing primary PCI (2, 4, 21). Our study found that a postprocedural TIMI flow grade of <3 was an independent predictor of the occurrence of NOAF, which is similar to that found in a previous study (4). We speculated that patients exhibiting a TIMI flow grade of <3 would be associated with an increased risk of microvascular perfusion, a larger infarction size, and a higher risk of NOAF.
A previous study suggested that inflammation may play an important role in developing AF. Notably, patients with AF have obvious inflammatory changes in their atrial tissues (22). In addition, serum CRP levels are significantly higher in patients with paroxysmal and chronic AF (23). Furthermore, Chen et al. (15) suggested that hs-CRP is an independent predictor of NOAF in patients with STEMI undergoing PCI. CRP, as a biomarker of inflammation, is associated with an increased risk of developing NOAF (2). Similar to previous studies, we discovered that a higher CRP level was a predictive factor for an increased risk of NOAF in patients with STEMI undergoing PCI.
The presence of atrial fibrosis, inflammation, and cardiac stress can lead to structural and electrical remodeling, hence increasing the risk of developing AF (12, 13). AMI triggers an inflammatory response within the infarcted region, which promotes structural changes in the atrium, leading to the occurrence and development of NOAF. As a member of the interleukin-1 receptor family, sST2 is associated with inflammation and developing tissue fibrosis (11). The occurrence of STEMI leads to a significant increase in sST2 levels as a result of myocardial dysfunction and mechanical stress, which may activate IL-6, resulting in a higher level of TNF-α and hs-CRP from inflammatory and endothelial cells (24). This inflammatory reaction may lead to vascular endothelial injury, which in turn promotes the release of TNF-α and hs-CRP (25). This results in vicious cycles of inflammatory response, which may increase the risk of patients with STEMI developing NOAF. In addition, sST2 has been proven to exhibit a strong association with the process of extracellular matrix remodeling and the occurrence of inflammation, which has been suggested to be associated with arrhythmias (26). From another aspect, as STEMI occurs, early myocardial remodeling develops in both the left ventricle and the LA (27). The receptor ST2L plays a key role in preventing hypertrophy and fibrosis in tissues that suffer from mechanical strain. High concentrations of sST2 can prevent this protective effect and perhaps contribute to myocardial remodeling (28), increasing the risk for developing AF. As a biomarker of fibrosis, sST2 may play a potential role in both inflammatory processes and early myocardial remodeling in STEMI. Chen et al. (14) suggested that higher levels of sST2 were an independent predictor of NOAF in patients with AMI. Similar to a previous study, our findings suggest that sST2 is an independent predictor of NOAF in patients with STEMI undergoing PCI. In addition, Ma suggested that an elevated sST2 level may be involved in predicting the risk of emergency admission or heart failure in patients with AF. The exact mechanism of sST2 in AF among patients with STEMI undergoing primary PCI requires further investigation. However, as a promising biomarker for inflammation and fibrosis, sST2 exhibits several advantages over other biomarkers, primarily due to its low biological variability, minimal susceptibility to renal function, and favorable response to effective treatment. Our findings suggest that sST2 may serve as a new predictive biomarker for assessing the risk of developing NOAF in patients with STEMI undergoing primary PCI.
This study had some limitations. Firstly, this was a single-center study with a small sample size, which can lead to selective bias. Considering the paroxysmal and asymptomatic nature of AF, it is possible that AF cases could go undetected. Furthermore, the absence of screening measures to rule out preexisting AF raises the possibility that some of the patients classified as having NOAF may have really had AF prior to their admission for STEMI. Furthermore, the study design does not allow for the exclusion of chronically high sST2 levels, which may indeed contribute to increase the risk of NOAF; this is also a limitation of this study. Secondly, although multivariate analyses were performed, residual covariates may still be present; this may affect the predictive value of sST2. Thirdly, the major adverse cardiovascular and cerebrovascular events (MACCE) that occurred during hospitalization were not reported due to the relatively small sample size in the NOAF group. In addition, both short-term and long-term follow-up was not conducted. Finally, the number of patients included in this study was relatively small, and given the low event rates (40 patients presented with NOAF), the number of variables included in the multivariate analysis is quite large. This may have affected the statistical significance of the study. Future studies including a large sample size and involving multiple centers are needed to validate our conclusions.
5. Conclusion
The incidence rate of NOAF in patients diagnosed with STEMI undergoing primary PCI was found to be 7.1%. As a promising biomarker for inflammation and fibrosis, sST2 has been identified as an independent predictor of NOAF in patients with STEMI undergoing PCI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
T-tZ and YW acquired the data, performed the statistical analyses, and drafted the manuscript. X-yP and Y-bY conceived the study, participated in its design and coordination, helped draft the manuscript, and revised the manuscript critically for important intellectual content. T-jP revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rene AG, Généreux P, Ezekowitz M, Kirtane AJ, Xu K, Mehran R, et al. Impact of atrial fibrillation in patients with ST-elevation myocardial infarction treated with percutaneous coronary intervention (from the HORIZONS-AMI [harmonizing outcomes with revascularization and stents in acute myocardial infarction] trial). Am J Cardiol. (2014) 113:236–42. doi: 10.1016/j.amjcard.2013.09.016
2. Selçuk M, Çınar T, Şaylık F, Akbulut T, Asal S, Çiçek V, et al. Predictive value of uric acid/albumin ratio for the prediction of new-onset atrial fibrillation in patients with ST-elevation myocardial infarction. Rev Invest Clin. (2022) 74:156–64. doi: 10.24875/RIC.22000072
3. Khalfallah M, Elsheikh A. Incidence, predictors, and outcomes of new-onset atrial fibrillation in patients with ST-elevation myocardial infarction. Ann Noninvasive Electrocardiol. (2020) 25:e12746. doi: 10.1111/anec.12746
4. Mrdovic I, Savic L, Krljanac G, Perunicic J, Asanin M, Lasica R, et al. Incidence, predictors, and 30-day outcomes of new-onset atrial fibrillation after primary percutaneous coronary intervention: insight into the RISK-PCI trial. Coron Artery Dis. (2012) 23:1–8. doi: 10.1097/MCA.0b013e32834df552
5. Wi J, Shin DH, Kim JS, Kim BK, Ko YG, Choi D, et al. Transient new-onset atrial fibrillation is associated with poor clinical outcomes in patients with acute myocardial infarction. Circ J. (2016) 80:1615–23. doi: 10.1253/circj.CJ-15-1250
6. Rathore SS, Berger AK, Weinfurt KP, Schulman KA, Oetgen WJ, Gersh BJ, et al. Acute myocardial infarction complicated by atrial fibrillation in the elderly: prevalence and outcomes. Circulation. (2000) 101:969–74. doi: 10.1161/01.cir.101.9.969
7. Lopes RD, Elliott LE, White HD, Hochman JS, Van de Werf F, Ardissino D, et al. Antithrombotic therapy and outcomes of patients with atrial fibrillation following primary percutaneous coronary intervention: results from the APEX-AMI trial. Eur Heart J. (2009) 30:2019–28. doi: 10.1093/eurheartj/ehp213
8. Ruwald AC, Bloch Thomsen PE, Gang U, Jørgensen RM, Huikuri HV, Jons C. New-onset atrial fibrillation predicts malignant arrhythmias in post-myocardial infarction patients–a cardiac arrhythmias and risk stratification after acute myocardial infarction (CARISMA) substudy. Am Heart J. (2013) 166:855–863.e3. doi: 10.1016/j.ahj.2013.08.017
9. Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. (2007) 69:546–54. doi: 10.1212/01.wnl.0000267275.68538.8d
10. Kornej J, Apostolakis S, Bollmann A, Lip GY. The emerging role of biomarkers in atrial fibrillation. Can J Cardiol. (2013) 29:1181–93. doi: 10.1016/j.cjca.2013.04.016
11. Gerber Y, Weston SA, Enriquez-Sarano M, Jaffe AS, Manemann SM, Jiang R, et al. Contemporary risk stratification after myocardial infarction in the community: performance of scores and incremental value of soluble suppression of tumorigenicity-2. J Am Heart Assoc. (2017) 6:e005958. doi: 10.1161/JAHA.117.005958
12. Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. (2008) 51:802–9. doi: 10.1016/j.jacc.2007.09.064
13. Sonmez O, Ertem FU, Vatankulu MA, Erdogan E, Tasal A, Kucukbuzcu S, et al. Novel fibro-inflammation markers in assessing left atrial remodeling in non-valvular atrial fibrillation. Med Sci Monit. (2014) 20:463–70. doi: 10.12659/MSM.890635
14. Nortamo S, Ukkola O, Lepojärvi S, Kenttä T, Kiviniemi A, Junttila J, et al. Association of sST2 and hs-CRP levels with new-onset atrial fibrillation in coronary artery disease. Int J Cardiol. (2017) 248:173–8. doi: 10.1016/j.ijcard.2017.07.022
15. Chen L, Chen W, Shao Y, Zhang M, Li Z, Wang Z, et al. Association of soluble suppression of tumorigenicity 2 with new-onset atrial fibrillation in acute myocardial infarction. Cardiology. (2022) 147:381–8. doi: 10.1159/000524765
16. Tymińska A, Kapłon-Cieślicka A, Ozierański K, Budnik M, Wancerz A, Sypień P, et al. Association of galectin-3 and soluble ST2, and their changes, with echocardiographic parameters and development of heart failure after ST-segment elevation myocardial infarction. Dis Markers. (2019) 2019:9529053. doi: 10.1155/2019/9529053
17. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al; ESC Scientific Document Group. 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. doi: 10.1093/eurheartj/ehx393
18. Svartstein A-SW, Lassen MH, Skaarup KG, Grove GL, Vyff F, Ravnkilde K, et al. Predictive value of left atrial strain in relation to atrial fibrillation following acute myocardial infarction. Int J Cardiol. (2022) 364:52–9. doi: 10.1016/j.ijcard.2022.05.026
19. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. (2004) 350:655–63. doi: 10.1056/NEJMoa031994
20. Kara K, Geisel MH, Möhlenkamp S, Lehmann N, Kälsch H, Bauer M, et al. B-type natriuretic peptide for incident atrial fibrillation—the Heinz Nixdorf Recall study. J Cardiol. (2015) 65:453–8. doi: 10.1016/j.jjcc.2014.08.003
21. Biccirè FG, Pastori D, Torromeo C, Acconcia MC, Capone S, Ferrari I, et al. Acute atrial ischemia associates with early but not late new-onset atrial fibrillation in STEMI patients treated with primary PCI: relationship with in-hospital outcomes. J Cardiol. (2021) 78:368–74. doi: 10.1016/j.jjcc.2021.05.013
22. Engelmann MD, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. (2005) 26:2083–92. doi: 10.1093/eurheartj/ehi350
23. Chung MK, Martin DO, Sprecher D, Wazni O, Kanderian A, Carnes CA, et al. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. (2001) 104:2886–91. doi: 10.1161/hc4901.101760
24. Chirinos JA, Orlenko A, Zhao L, Basso MD, Cvijic ME, Li Z, et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. (2020) 75:1281–95. doi: 10.1016/j.jacc.2019.12.069
25. Del Turco S, Basta G, De Caterina AR, Sbrana S, Paradossi U, Taddei A, et al. Different inflammatory profile in young and elderly STEMI patients undergoing primary percutaneous coronary intervention (PPCI): its influence on no-reflow and mortality. Int J Cardiol. (2019) 290:34–9. doi: 10.1016/j.ijcard.2019.05.002
26. Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, et al. Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. (2009) 2:684–91. doi: 10.1161/CIRCHEARTFAILURE.109.873240
27. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. (2011) 4:98–108. doi: 10.1016/j.jcmg.2010.10.008
Keywords: soluble ST2, new-onset atrial fibrillation, acute ST-segment elevation myocardial infarction, PCI, predictor
Citation: Zhao T-t, Pan T-j, Yang Y-b, Pei X-y and Wang Y (2023) Association of soluble suppression of tumorigenicity 2 protein with new-onset atrial fibrillation in patients with acute ST-segment elevation myocardial infarction undergoing primary PCI. Front. Cardiovasc. Med. 10:1207219. doi: 10.3389/fcvm.2023.1207219
Received: 17 April 2023; Accepted: 6 September 2023;
Published: 21 September 2023.
Edited by:
Faisal Syed, University of North Carolina at Chapel Hill, United StatesReviewed by:
Alina Scridon, George Emil Palade University of Medicine, Pharmacy, Sciences and Technology of Târgu Mureş, RomaniaSvetlana Apostolović, University Clinical Center, Serbia
© 2023 Zhao, Pan, Yang, Pei and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Wang ZHJ3YW5neW9uZzIwMTZAMTYzLmNvbQ==
Abbreviations STEMI, ST-segment elevation myocardial infarction; NOAF, new-onset atrial fibrillation; AMI, acute myocardial infarction; CAD, coronary artery disease.
 Ting-ting Zhao1
Ting-ting Zhao1 Yong Wang
Yong Wang