Abstract
Background:
Atrial fibrillation (AF) is associated with cardiac structural and functional remodeling. We investigated the left atrial (LA) and left ventricular (LV) changes in AF subtypes by using two-dimensional echocardiography strain techniques.
Methods:
The study population consisted of 102 subjects with sinus rhythm (control group) and 463 patients with AF, among which 284 patients had paroxysmal AF (PAF) and 179 patients had persistent AF (PerAF). A speckle tracking automatic functional imaging software was used to perform the strain analysis.
Results:
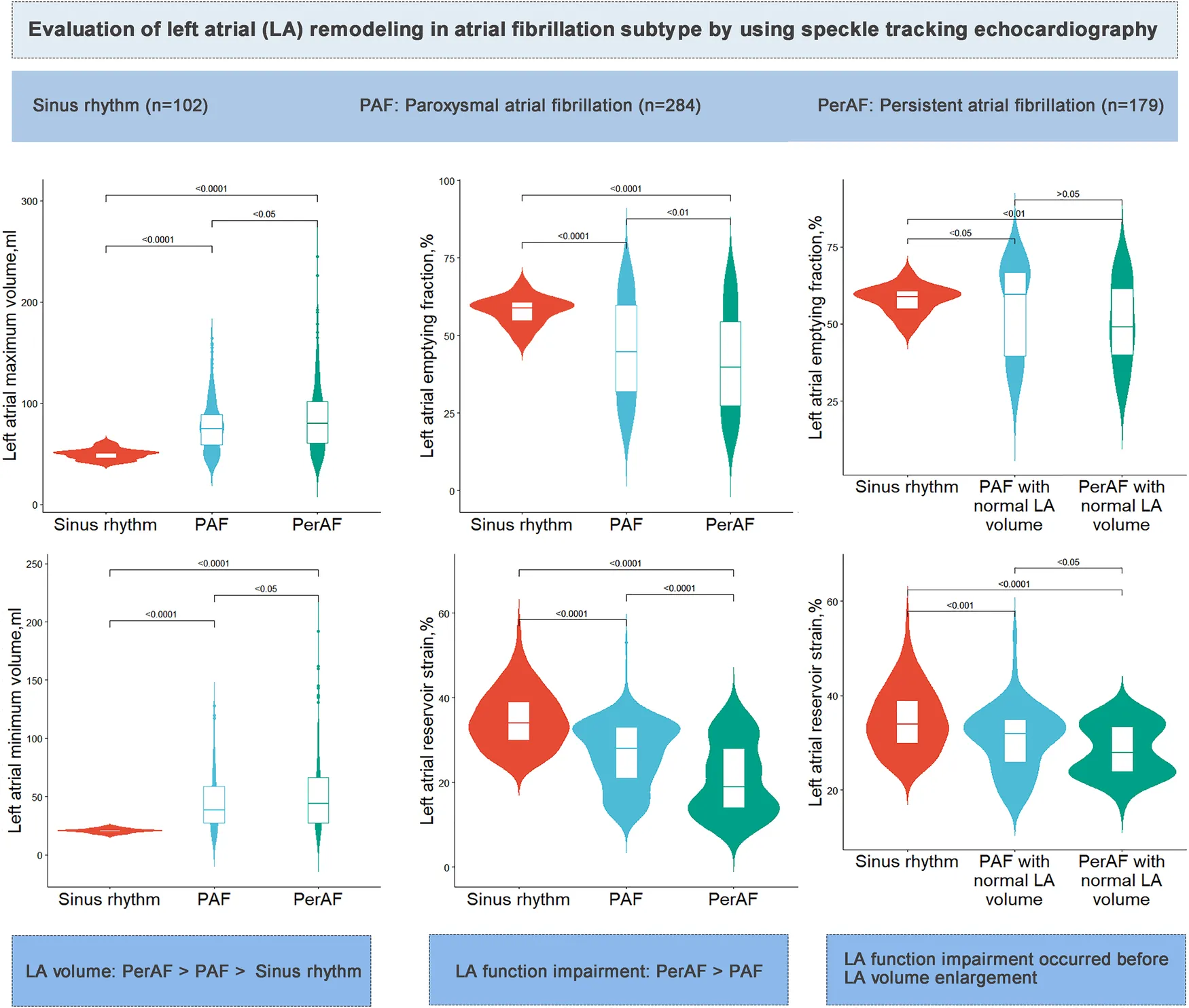
Patients with AF had dilated LA maximum and minimum volume, decreased LA reservoir strain, lower LV ejection fraction (LVEF), and impaired global longitudinal strain (GLS) compared to those of the sinus rhythm control group. In patients with PerAF, the LA maximum and minimum volumes were larger, and the LA reservoir strain [PAF vs. PerAF, 28% (21,33) vs. 19% (14, 28), P < 0.05], LVEF, and absolute GLS value (PAF vs. PerAF, −16.9 ± 3.3 vs. −14.1 ± 3.5%) were lower than those in patients with PAF. Patients with AF regardless of LA enlargement had decreased LA reservoir strain and lower LVEF and absolute GLS value than those in the sinus rhythm control group.
Conclusion:
Compared with those with normal sinus rhythm, patients with AF had dilated LA volume and impaired LA function, which were further worsened in patients with PerAF than those in patients with PAF. LA functional impairment occurred before LA enlargement. Left atrioventricular remodeling happened across different stages of AF development.
1. Introduction
Atrial fibrillation (AF) is a common cardiac arrhythmia that is becoming increasingly prevalent due to the aging population (1, 2). Despite efforts to understand its pathophysiology and improve treatments, identifying the underlying causes of AF in individual patients remains challenging (3, 4).
Atrial remodeling is a critical feature of AF, and speckle tracking echocardiography has emerged as an excellent approach for evaluating this process (5, 6). This imaging modality provides a non-invasive assessment of left atrial (LA) strain, which is inversely related to LA wall fibrosis and AF burden (6, 7). LA mechanics differ between AF subtypes of paroxysmal AF (PAF) and persistent AF (PerAF), and these characteristics influence the clinical interpretation of these measures (8). According to Kuppahally's report (9), PerAF had more fibrosis and lower midseptal and midlateral LA strains than PAF. However, only minimal differences in LA remodeling were found between PAF and PerAF in another study (7). Therefore, the differences in LA remodeling between AF subtypes remain debatable. In addition, LA size and function may vary at different stages of AF development. Kojima et al. (10) found that LA functional impairment was observed before LA enlargement in patients with PAF. However, this finding was based on traditional velocity vector imaging and in a relatively small number of subjects, particularly in patients with PAF. There is still a lack of enough evidence on the relationship between LA structural and functional remodeling.
In addition to irregular atrial electrical activity, AF is also characterized by generating irregular activations of the ventricle (11). Mechanical LA remodeling can further damage the active contribution to ventricular filling, resulting in reduced LV function (12). It has been reported that immediate hemodynamic changes caused by AF may contribute to decreased cardiac output and acute heart failure (13). A recent study found that patients with PerAF had significantly reduced LV ejection fraction (LVEF) than that in patients with PAF (8). Although global longitudinal strain (GLS) is a widely used LV strain parameter that provides prognostic information (14), a few studies have externally validated GLS in AF patients.
Thus, this study aimed to evaluate the AF-induced changes in LA mechanics, clarify the association of LA structural and functional remodeling in AF subtypes, and explore the left atrioventricular functional coupling across different stages of AF progression by using speckle tracking echocardiography.
2. Methods
2.1. Study population
From October 2021 to September 2022, we conducted a prospective study, which enrolled 527 patients diagnosed with AF and 102 healthy subjects with sinus rhythm. The determination of AF subtype and sinus rhythm was based on the 2020 European Society of Cardiology (ESC) guidelines. A standard 12-lead ECG recording or a single-lead ECG tracing of ≥30 s, showing heart rhythm with no discernible repeating P waves and irregular RR intervals (when atrioventricular conduction is not impaired), is diagnostic of clinical AF. PAF was defined as terminated spontaneously or with intervention within 7 days of onset. PerAF was defined as continuously sustained beyond 7 days, with episodes terminated by cardioversion (drugs or electrical cardioversion) after ≥7 days (1). The exclusion criteria comprised coronary artery disease (n = 26), organic valvular disease (n = 5), LVEF < 40% (n = 9), previous cardiac surgery (n = 6), other heart diseases or other serious non-cardiac diseases (n = 11), and suboptimal echocardiographic image quality (n = 7). After the application of the exclusion criteria, a total of 463 patients with AF were included in the analysis, among which 284 patients had PAF and 179 patients had PerAF. The Tongji Hospital Ethics Committee approved the study with approval number TJ-IRB20220621, and all participants provided their informed consent before participating in the study.
2.2. Clinical data
At the initiation of the study, we obtained baseline characteristics and clinical data for all participants. Heart rate was determined based on the findings of the standard 12-lead electrocardiogram. Symptoms were scored by European Heart Rhythm Association (EHRA) class (1). We calculated the CHA2DS2-VASc score based on clinical data (1).
2.3. Conventional transthoracic echocardiography
Transthoracic echocardiography was performed using GE Vivid E95 ultrasound equipment (GE Vingmed Ultrasound, Horten, Norway) with an M5Sc transducer (1.7–3.3 MHz) and a frame rate of 70–80 frame/s. According to the prevailing recommendations, M-mode, two-dimensional, color, pulsed, and continuous-wave Doppler data were acquired on standard views adjusting depth, sector width, and gain settings, as required. For participants with sinus rhythm, three cardiac cycles were stored for each image, while for those with AF, at least 10 were saved. All echocardiographic parameters were analyzed with an index beat (preceding RR/pre-preceding RR close to 1) in AF cases, as recommended (15). The internal diameters were measured in accordance with the quantitative method suggested by the American Society of Echocardiography (16). LV relative wall thickness (RWT) was calculated as RWT = 2 × LV posterior wall thickness/LV end-diastolic dimension. LV volumes, LVEF, and LA volumes were measured using the biplane Simpson method. All images were digitally stored for offline analysis.
2.4. Speckle tracking automatic functional imaging
Strain analysis was conducted using a commercial speckle tracking automatic functional imaging (AFI) software (EchoPAC version 2.4, GE Vingmed Ultrasound) (17). This software automatically tracked frame-to-frame speckle changes in two-dimensional images to assess LA and LV strain.
LA function consists of three components, namely, reservoir, conduit, and active pump. The total function of the LA is best reflected by reservoir strain corresponding to LA early diastole with maximum relaxation of its wall, algebraically positive. LA strain was evaluated using AFI-LA methods by the R-wave gating from the apical two- and four-chamber views. During the processing, the LA endocardium surface is manually traced by a point-and-click approach. The epicardial surface tracing is automatically generated by the system in order to obtain a region of interest (ROI). The ROI definition usually starts with delineating the endocardial contour, which should be drawn from the mitral annulus on one side, extrapolates across the pulmonary vein and/or LA appendage orifices, and ends at the mitral annulus on the opposite side. The ROI can be manually adjusted in width and shape, and then the software automatically tracks the quality for each segment and gives the peak LA reservoir strain and strain curves (18) (Figure 1).
Figure 1

Representative cases. Left atrial strain curves of subjects with sinus rhythm (A), paroxysmal atrial fibrillation without left atrial enlargement (B), and persistent atrial fibrillation without (C) and with (D) left atrial volume enlargement. From A to D, the LA reservoir strain gradually decreased. LA, left atrial; LAVmax, left atrial maximal volume.
LV strain was measured using the AFI method from the apical two-, three-, and four-chamber views (19). The software analyzed the myocardial motion by tracking frame-to-frame speckle changes. When necessary, automatic endocardial recognition was manually adjusted to ensure correct “anchorage” of the algorithm to the mitral annulus, exclude papillary muscles and chordae from tracking, and correctly include the LV apex. The ROI was eventually adjusted to ensure tracking of the whole myocardial thickness. LV outflow pulsed Doppler was used to time end systole. The segmental strain curves in apical view and 18-segment bull's-eye diagrams related to strain parameters were automatically displayed. GLS was calculated as the average value of the peak systolic strain in 18 LV myocardial segments. All strain measurements were conducted in accordance with the EACVI/ASE/Industry Task Force guidelines (17, 18).
2.5. Statistical analysis
All statistical analyses were conducted using R version 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria). Normally distributed continuous data were expressed as mean ± standard deviation, while non-normally distributed data were expressed as median and interquartile range (IQR). Normality distribution was checked using the Shapiro–Wilk test and Q–Q plots. The differences among groups were analyzed using the one-way analysis of variation (ANOVA) for normally distributed data with the Bonferroni correction for pairwise comparisons between the two groups. The Kruskal–Wallis rank sum test was used for non-normally distributed data, and the all-pairwise method was used for further pairwise comparisons between the two groups. Categorical data were presented as percentages and analyzed using the χ2-test or Fisher's exact test as appropriate. A receiver operator characteristic (ROC) was performed to obtain the areas under the curve (AUC) and 95% confidence intervals (CIs). A two-tailed P-value < 0.05 was considered a statistically significant difference.
3. Results
3.1. Clinical and echocardiographic characteristics stratified by controls and AF subtype
Patient characteristics stratified by controls and AF subtype are summarized in Table 1 and Figure 2. Compared with the sinus rhythm group, both AF groups showed higher CHA2DS2-VASc score, blood pressure, and heart rate, with a higher proportion of hypertension and EHRA classes 3–4. Moreover, both AF groups had greater LA dimensions, dilated LA maximum and minimum volumes, and volume indexes than those in the sinus rhythm group. In addition to LA structure, LA function was impaired in both AF groups, showing decreased LA emptying fraction (LAEF) and impaired LA reservoir strain than sinus rhythm (Graphical Abstract).
Table 1
| Variables | Sinus rhythm (n = 102) | PAF (n = 284) | PerAF (n = 179) | P-value |
|---|---|---|---|---|
| Clinical characteristics | ||||
| Age, years | 64 ± 10 | 63 ± 12 | 64 ± 11 | 0.84 |
| Male, n (%) | 65 (64) | 187 (66) | 113 (63) | 0.82 |
| Body mass index, kg/m2 | 23.4 ± 2.5 | 24.1 ± 3.6 | 24.1 ± 3.3 | 0.14 |
| Systolic blood pressure, mm Hg | 121 ± 8 | 131 ± 20a | 130 ± 19a | <0.001 |
| Diastolic blood pressure, mm Hg | 74 ± 9 | 81 ± 13a | 80 ± 12a | <0.001 |
| Heart rate, bpm | 72 ± 9 | 81 ± 17a | 78 ± 15a | <0.001 |
| CHA2DS2-VASc score | 0.4 ± 0.8 | 0.9 ± 0.4a | 0.9 ± 0.5a | <0.001 |
| EHRA classes 3–4, n (%) | 0 (0.0) | 61 (22)a | 44 (25)a | <0.001 |
| Current smoking, n (%) | 10 (10) | 44 (16) | 31 (17) | 0.23 |
| Hypertension, n (%) | 10 (10) | 120 (42)a | 76 (43)a | <0.001 |
| Diabetes, n (%) | 8 (8) | 30 (11) | 21 (12) | 0.59 |
| Dyslipidemia, n (%) | 3 (3) | 24 (9) | 12 (7) | 0.17 |
| Medication | ||||
| Antiarrhythmic medication, n (%) | 134 (47) | 84 (47) | 0.84 | |
| Beta-blockers, n (%) | 98 (35) | 61 (34) | 0.97 | |
| Calcium blocker, n (%) | 33 (12) | 21 (12) | 0.92 | |
| Anticoagulation, n (%) | 185 (65) | 114 (64) | 0.83 | |
| Echocardiographic parameters | ||||
| LA dimension, mm | 32 ± 2 | 42 ± 7a | 43 ± 7a | <0.001 |
| LA volume—min, ml | 21 (20, 22) | 39 (27, 59)a | 44 (28, 67)a,b | <0.001 |
| LA volume—max, ml | 50 ± 5 | 77 ± 25a | 86 ± 36a,b | <0.001 |
| LA volume index—min, ml/m2 | 13 (12, 13) | 20 (13, 31)a | 23 (14, 38)a,b | <0.001 |
| LA volume index—max, ml/m2 | 30 ± 3 | 45 ± 15a | 51 ± 22a,b | <0.001 |
| LAEF, % | 58 ± 4 | 46 ± 16a | 41 ± 16a,b | <0.001 |
| LA reservoir strain, % | 34 (30, 39) | 28 (21, 33)a | 19 (14, 28)a,b | <0.001 |
| E/e’ | 9.9 ± 1.9 | 11.1 ± 4.6a | 12.1 ± 4.4a,b | <0.001 |
| LV end-diastolic dimension, mm | 47 ± 8 | 46 ± 9 | 48 ± 3 | 0.134 |
| LVEDV, ml | 86 ± 15 | 92 ± 24a | 98 ± 37a | 0.001 |
| LVESV, ml | 31 (29, 35) | 37 (32, 48)a | 42 (33, 54)a,b | <0.001 |
| LVEDV index, ml/m2 | 52 ± 9 | 54 ± 16a | 59 ± 23a,b | 0.008 |
| LVESV index, ml/m2 | 19 (17, 22) | 22 (18, 28)a | 25 (19, 32)a,b | <0.001 |
| RWT | 0.45 ± 0.08 | 0.47 ± 0.13 | 0.49 ± 0.12 | 0.092 |
| LV mass index, g/m2 | 88 (72, 92) | 92 (80, 103) | 90 (74, 108) | 0.069 |
| LVEF, % | 62 ± 3 | 55 ± 9a | 53 ± 8a,b | <0.001 |
| GLS, -% | 18.3 ± 2.1 | 16.9 ± 3.3a | 14.1 ± 3.5a,b | <0.001 |
Clinical and echocardiographic characteristics in controls (sinus rhythm) and atrial fibrillation subtype.
Data are presented as mean ± SD or median (interquartile range) for continuous variables and count (%) for categorical variables. PAF, paroxysmal atrial fibrillation; PerAF, persistent atrial fibrillation; CHA2DS2VASc, history of congestive heart failure, hypertension, diabetes mellitus, stroke/transient ischemic attack/prior thromboembolism, vascular disease, age, and sex; EHRA, European Heart Rhythm Association; LA, left atrial; min: minimum; max: maximum; LAEF, left atrial emptying fraction; E/e’, mitral inflow peak early diastolic velocity/mitral annular peak early diastolic velocity; LV, left ventricular; RWT, relative wall thickness; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain.
P < 0.05 vs. the sinus rhythm group.
P < 0.05 vs. the paroxysmal atrial fibrillation group.
Figure 2

Violin plots for comparisons of echocardiographic characteristics between controls and atrial fibrillation subtype. (A) LAEF, (B) LA reservoir strain, (C) LVEF, and (D) GLS. PAF, paroxysmal atrial fibrillation; PerAF, persistent atrial fibrillation; LAEF, left atrial emptying fraction; LA, left atrial; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain. Wider sections of the violin plot represent a higher distribution probability, the thin line in the center white box represents the median, and the white box in the center of the violin represents the interquartile range.
In terms of LV, both AF groups showed greater LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV), higher mitral inflow peak early diastolic velocity/mitral annular peak early diastolic velocity (E/e'), and impaired LV systolic function compared to those in the sinus rhythm group.
The PerAF group showed dilated LA maximum and minimum volume, decreased LAEF, and impaired LA reservoir strain than those in the PAF group. A higher LVESV, LVESV index, and E/e' and lower LVEF and absolute GLS value were also shown in the PerAF group than those in the PAF group.
3.2. Cardiac structure and function remodeling in AF patients
To gain insight into the association between LA size and function, both AF groups were dichotomized into groups according to the recommended LA maximum volume index (≤34 ml/m2) (20). There were 70 patients with PAF and normal LA maximum volume index [PAF EL (−) group], 214 patients with PAF and dilated LA maximum volume index [PAF EL (+) group], 39 patients with PerAF and normal LA maximum volume index [PerAF EL (−) group], and 140 patients with PerAF and dilated LA maximum volume index [PerAF EL (+) group]. Compared with the sinus rhythm group, patients with AF regardless of LA enlargement had significantly lower LAEF, LA reservoir strain, LVEF, and GLS, indicating that impairment of LA and LV function occurred before LA enlargement (Graphical Abstract, Table 2 and Figure 3).
Table 2
| Variables | Sinus rhythm (n = 102) | PAF EL (−) (n = 70) | PAF EL (+) (n = 214) | PerAF EL (−) (n = 39) | PerAF EL (+) (n = 140) | P-value |
|---|---|---|---|---|---|---|
| LAEF, % | 58 ± 4 | 55 ± 15a | 43 ± 16a,b | 51 ± 13a,c | 39 ± 16a,b,c,d | <0.001 |
| LA reservoir strain, % | 34 (30, 39) | 32 (26, 35)a | 27 (19, 32)a,b | 28 (24, 34)a,b,c | 16 (12, 24)a,b,c,d | <0.001 |
| LVEDV, ml | 86 ± 15 | 87 ± 15 | 94 ± 26a,b | 89 ± 22c | 101 ± 39a,b,c,d | <0.001 |
| LVESV, ml | 31 (29, 35) | 36 (33, 43)a | 38 (32, 50)a | 38 (33, 46)a | 44 (33, 56)a,b | <0.001 |
| LVEDV index, ml/m2 | 52 ± 9 | 52 ± 12 | 55 ± 17a,b | 55 ± 15a,b | 60 ± 24a,b,c,d | 0.008 |
| LVESV index, ml/m2 | 19 (17, 22) | 21 (18, 26)a | 22 (18, 29)a | 24 (20, 29)a,b,c | 26 (19, 33)a,b,c,d | <0.001 |
| E/e’ | 9.9 ± 1.9 | 8.7 ± 2.8a | 11.9 ± 4.8a,b | 11.4 ± 4.4b | 12.4 ± 4.4a,b | <0.001 |
| LVEF, % | 62 ± 3 | 56 ± 7a | 55 ± 10a | 56 ± 8a | 53 ± 7a,b,c,d | <0.001 |
| GLS, -% | 18.3 ± 2.1 | 17.3 ± 3.2a | 16.8 ± 3.3a | 15.6 ± 3.2a,b,c | 13.7 ± 3.5a,b,c,d | <0.001 |
Echocardiographic characteristics stratified according to atrial fibrillation subgroups with or without left atrial enlargement.
Data are presented as mean ± SD or median (interquartile range) for continuous variables. PAF, paroxysmal atrial fibrillation; PerAF, persistent atrial fibrillation; EL, enlargement; LAEF, left atrial emptying fraction; LA, left atrial; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; E/e’, mitral inflow peak early velocity/mitral annular peak early velocity; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain.
P < 0.05 vs. the sinus rhythm group.
P < 0.05 vs. PAF EL (−) group.
P < 0.05 vs. PAF EL (+) group.
P < 0.05 vs. PerAF EL (−) group.
Figure 3

Violin plots for comparison of echocardiographic characteristics between controls and atrial fibrillation subgroup with or without left atrial volume enlargement. (A) LAEF, (B) LA reservoir strain, (C) LVEF, and (D) GLS. PAF, paroxysmal atrial fibrillation; PerAF, persistent atrial fibrillation; EL, enlargement; LAEF, left atrial emptying fraction; LA, left atrial; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain.
In patients with PAF, LAEF and LA reservoir strain were lower in patients with LA enlargement than those in patients without LA enlargement. Similarly, in patients with PerAF, LAEF and LA reservoir strain were lower in patients with LA enlargement than those in patients without LA enlargement. These results demonstrated that LA function was further impaired as LA volume expands (Table 2 and Figure 3).
Compared with the PAF EL (+) group, LAEF and LA reservoir strain were lower in the PerAF EL (+) group while higher in the PerAF EL (−) group. Patients with PerAF regardless of LA dilation had higher LVESV index and impaired GLS than those in the PAF EL (+) group. These results demonstrated that as AF progressed, LV systolic function significantly decreased while LA function was also related to the presence of LA enlargement (Table 2 and Figure 3).
To further explore the association between LA structure and function, according to the cutoff of LA reservoir strain obtained from ROC, the LA myocardium was divided into compliant (LA reservoir strain ≥24%) and stiff (LA reservoir strain < 24%). Table 3 and Figure 4 list the prevalence of LA anatomical remodeling and functional impairment according to AF subtype. The PAF group most frequently had large yet compliant LA, whereas the PerAF group most frequently had large and stiff LA (P < 0.001). Notably, there were 32 (18.4%) PerAF patients with large but compliant LA and 14 (4.9%) PAF patients and 15 (8.4%) PerAF patients with small but stiff LA, which demonstrated that LA function might be normal even if the size was enlarged, whereas the size might be normal even if the function was impaired.
Table 3
| PAF (n = 284) | PerAF (n = 179) | P-value | |
|---|---|---|---|
| Small and compliant LA | 56 (19.7) | 24 (13.4) | <0.001 |
| Small but stiff LA | 14 (4.9) | 15 (8.4) | <0.001 |
| Large but compliant LA | 123 (43.3) | 32 (18.4) | <0.001 |
| Large and stiff LA | 91 (32.0) | 107 (59.8) | <0.001 |
Left atrial anatomic remodeling and functional impairment according to the type of atrial fibrillation.
PAF, paroxysmal atrial fibrillation; PerAF, persistent atrial fibrillation; LA, left atrial.
Figure 4

The distribution prevalence of left atrial anatomical remodeling and functional impairment according to atrial fibrillation subtype. (A) paroxysmal atrial fibrillation and (B) persistent atrial fibrillation. LA, left atrium; LAVimax, left atrial maximal volume index.
3.3. Evaluation of echocardiographic parameters in the detection of AF
ROC curve analyses showed that LA reservoir strain had a relatively higher diagnostic value than LAEF not only in distinguishing between AF and sinus rhythm (AUC: LA reservoir strain vs. LAEF 0.82 vs. 0.75) but also in distinguishing between PAF and PerAF (AUC: LA reservoir strain vs. LAEF 0.70 vs. 0.57) (Figure 5).
Figure 5

Receiver operating characteristic curves of LA function parameters to identify atrial fibrillation in all patients (A) and persistent atrial fibrillation in all atrial fibrillation patients. (B) AUC, area under the curve; LAEF, left atrial emptying fraction; LA, left atrial.
4. Discussion
The present study, which was conducted on 463 patients with AF and 102 subjects with sinus rhythm, identified several important findings. (1) Compared with sinus rhythm, both PAF and PerAF were associated with larger LA and LV volumes, as well as impaired LA and LV function. (2) PerAF had larger LA maximum and minimum volume and more impaired LA and LV function than PAF (3) The impairment of LA and LV function occurred before LA enlargement in AF. (4) The impairment of LA function was significantly aggravated when LA volume was enlarged in AF. (5) As AF progressed, LV systolic function significantly decreased while LA function varied depending on the presence of LA enlargement.
4.1. AF-induced changes in LA detected by echocardiography
Consistent with previous studies (21, 22), our study confirmed that AF was significantly associated with LA anatomical and functional remodeling. Additionally, our findings confirmed on a larger scale that AF transition from paroxysmal to persistent was often characterized by advancing atrial structural and functional remodeling, which are in accordance with the findings of Olsen et al. (8).
Among the LA function parameters, our report showed that LA reservoir strain had a higher value than LAEF in distinguishing not only between AF and sinus rhythm but also between PAF and PerAF. Raman et al. (23) had similar results with the magnetic resonance feature-tracking strain, which illustrated that LA reservoir strain was a major predictor of the onset of AF in patients with hypertrophic cardiomyopathy. It might be because the LA reservoir function was the most important feature of AF since it respectively reflected the compliance and loading conditions of LA. The development of AF evolves from sole rhythm disturbance to complex cardiomyopathy (24). Experimental studies have shown that the atrial remodeling of AF is characterized by the presence of predominantly interstitial fibrosis, which impacts atrial compliance (25, 26). Additionally, interstitial fibrosis promotes replacement fibrosis, resulting in impaired contractile function of LA cardiomyocytes. Therefore, a comprehensive assessment of LA volume and reservoir strain may provide additional insight into LA remodeling caused by AF.
4.2. LA volume and function remodeling were not always concordant in AF
In line with the study previously mentioned (10), the present report showed that LA volume and function were not always concordant in AF. LA function remodeling has been already impaired before LA volume enlargement, whereas even with enlarged LA volume, LA function impairment may not be apparent. The study also highlighted that large yet compliant atria were more prevalent in PAF, which may indicate a more advanced stage of disease with atrial enlargement yet without impaired strain. It is reported that this condition could have a greater likelihood of successful AF ablation (27).
The study also indicated that LA function was severely impaired when LA volume was enlarged in AF. This could be because the increased LA volume can increase wall stress, triggering myocyte hypertrophy and fibrosis (28). In addition, LA reservoir strain was higher in PerAF without dilated LA volume than that in PAF with dilated LA, suggesting that LA reservoir strain was not only associated with the AF subtype but also related to the LA volume enlargement.
Although there are several papers demonstrating the impact of AF on LA remodeling as well as the utility of LA size and function on AF recurrence prediction (29–31), our study shows that patients with PerAF had a higher degree of LA anatomical remodeling and functional impairment than that in patients with PAF, reflecting different stages of the disease. Moreover, LA reservoir strain could be a useful indicator in estimating cardiac function remodeling induced by AF even before LA volume dilation and showed better discriminative value than LAEF to separate AF from sinus rhythm patients. This is important to facilitate appropriate clinical management decisions, because both anatomical remodeling and functional impairment could have implications for the success of AF ablation. Therefore, the added value of LA strain detected by speckle tracking echocardiography allows effective triaging of the AF patient, suggesting that it should be implemented in the systematic evaluation of AF patients before ablation. The ability to identify mild disease before morphological changes provides a basis for risk stratification before surgery, particularly in patients with non-dilated LA, and allows more selective prophylactic therapy for postoperative complications. According to Ma et al. (32), LA strain could be of great use in identifying patients with a high risk of AF recurrence after catheter ablation. It is reported that LA strain predicted the incidence of post-operative AF independently of LA dilation in severe aortic stenosis (33). It has also been proven to provide a diagnostic role of thrombotic risk assessment for non-valvular AF patients planned for electrical cardioversion (34). Additionally, patients who have suffered from specific cardiomyopathies and valve diseases such as myocardial infarction are at risk of developing AF, which may lead to a prognostic value of LA strain as a significant predictor of incident AF (35). Nevertheless, there are several limitations of LA strain including the lack of a “universal” definition of normal ranges and the impact of vendors/segmentation on data reproducibility. Although LA strain consists of three phases, i.e., reservoir, conduit, and pump, in the case of normal diastolic function, the relative contribution of the particular LA phases into the LV filling is as follows: reservoir, 40%; conduit, 35%; and pump 25% (36). The impaired phasic function of the LA was described in many cardiovascular diseases. However, in patients with PerAF at the time of the echocardiographic exam, it is impossible to measure the LA pump strain. Further studies are needed to focus on LA strain analysis, especially the reservoir strain, with a large sample to analyze its value in clinical practice.
4.3. Left atrioventricular functional coupling across different stages of AF progression
In our study, LV systolic and diastolic function was significantly impaired in AF than sinus rhythm, which was consistent with the study by Ross Agner et al. (37), who reported that GLS was significantly impaired in AF compared to sinus rhythm controls independent of age, sex, heart rate, LVEF, and LV mass. Our study also suggested that subclinical alterations in LV function may have preceded the deterioration of LV volume dilation.
We found that patients with AF had significantly higher heart rates compared to those in patients with sinus rhythm. However, the fact that myocardial oxygen consumption increases and myocardial efficiency decreases with increased heartbeat is well known. Literature on the relationship between heart rate and strain measurements showed that GLS in normal subjects with high and low heart rates was similar (38). All strain and strain rate variables in the longitudinal, circumferential, and radial directions were not significantly different between pacing rates (39).
Previous studies have shown that atrioventricular coupling is a dynamic and time-variant process in AF (40). On the one hand, AF can lead to progressive ventricular remodeling through tachycardia and irregular ventricular rhythm (41). On the other hand, LV fibrosis in patients with AF may reflect the same process in the LV that leads to atrial fibrosis, which may be associated with a pathologic myocardial process that triggers AF recurrence (40). It is reported that LA reservoir function is closely associated with heart failure (42). AF-associated ventricular remodeling results in myocardial fibrosis, chamber dilation, and mitral and tricuspid regurgitation, all of which contribute to damaging LV diastolic as well as systolic function (43).
5. Limitations
There were several limitations in the present study. First, software available on the market used different algorithms of strain analysis with possible consequent biases in comparison between studies. Second, the number of samples, especially sinus rhythm controls (n = 102) and PerAF patients without LA dilation (n = 39), was small, and more patients would be required to provide a robust conclusion in this aspect. Third, there was a lack of comparison with other imaging techniques such as three-dimensional echocardiography and cardiac magnetic resonance imaging. Forth, this is a transversal study, and we cannot conclude that LA strain detects an early disease but, rather, that LA strain diagnoses a mild disease. Additionally, there are various factors affecting LA strain. These factors change simultaneously in most cases, making it difficult to assess the net influence of each factor. Through the transversal study, we cannot determine whether the causes of the decrease of LA function and increase of LA volume in the population of patients with AF are the morpho-pathological changes at the level of the LA caused by the presence of AF or if they are the cause of AF. These confounding factors might also have biased the results. A prospective study in a larger patient population is required to clarify the influence of these confounding factors on LA strain in patients with AF and validate the predictive value of LA strain on long-term outcomes.
6. Conclusions
Compared with the normal sinus rhythm group, patients with AF had dilated LA volume and impaired LA function, which were further worsened in patients with PerAF than those in patients with PAF. LA volume and function remodeling were not always concordant in AF, and LA function impairment could occur before LA volume enlargement. Left atrioventricular remodeling happened across different stages of AF development, and patients with AF had significantly more impaired GLS than the normal sinus rhythm group.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Tongji Hospital Ethics Committee, with approval number TJ-IRB20220621. The patients/participants provided their written informed consent to participate in this study.
Author contributions
YD, YL, and HL conceived and designed the study. All authors collected the clinical and echocardiographic data. SL, YD, YL, and HL performed the statistical analysis and interpretation of data. SL, YD, YL, and HL wrote the manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank the study participants and referring technicians for their participation in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
HindricksGPotparaTDagresNArbeloEBaxJJBlomstrom-LundqvistCet al2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. (2021) 42(5):373–498. 10.1093/eurheartj/ehaa612
2.
KornejJBorschelCSBenjaminEJSchnabelRB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. (2020) 127(1):4–20. 10.1161/CIRCRESAHA.120.316340
3.
BrundelBAiXHillsMTKuipersMFLipGYHde GrootNMS. Atrial fibrillation. Nat Rev Dis Primers. (2022) 8(1):21. 10.1038/s41572-022-00347-9
4.
WijesurendraRSCasadeiB. Mechanisms of atrial fibrillation. Heart. (2019) 105(24):1860–7. 10.1136/heartjnl-2018-314267
5.
VoigtJUMalaescuGGHaugaaKBadanoL. How to do LA strain. Eur Heart J Cardiovasc Imaging. (2020) 21(7):715–7. 10.1093/ehjci/jeaa091
6.
CameliMMandoliGELoiaconoFSparlaSIardinoEMondilloS. Left atrial strain: a useful index in atrial fibrillation. Int J Cardiol. (2016) 220:208–13. 10.1016/j.ijcard.2016.06.197
7.
HopmanLMulderMJvan der LaanAMDemirkiranABhagirathPvan RossumACet alImpaired left atrial reservoir and conduit strain in patients with atrial fibrillation and extensive left atrial fibrosis. J Cardiovasc Magn Reson. (2021) 23(1):131. 10.1186/s12968-021-00820-6
8.
OlsenFJDarknerSChenXPehrsonSJohannessenAHansenJet alLeft atrial structure and function among different subtypes of atrial fibrillation: an echocardiographic substudy of the AMIO-CAT trial. Eur Heart J Cardiovasc Imaging. (2020) 21(12):1386–94. 10.1093/ehjci/jeaa222
9.
KuppahallySSAkoumNBurgonNSBadgerTJKholmovskiEGVijayakumarSet alLeft atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. (2010) 3(3):231–9. 10.1161/CIRCIMAGING.109.865683
10.
KojimaTKawasakiMTanakaROnoKHiroseTIwamaMet alLeft atrial global and regional function in patients with paroxysmal atrial fibrillation has already been impaired before enlargement of left atrium: velocity vector imaging echocardiography study. Eur Heart J Cardiovasc Imaging. (2012) 13(3):227–34. 10.1093/ejechocard/jer281
11.
AllessieMAusmaJSchottenU. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. (2002) 54(2):230–46. 10.1016/S0008-6363(02)00258-4
12.
LyonAvan MourikMCrutsLHeijmanJBekkersSSchottenUet alBoth beat-to-beat changes in RR-interval and left ventricular filling time determine ventricular function during atrial fibrillation. Europace. (2021) 23(23 Suppl 1):i21–i8. 10.1093/europace/euaa387
13.
VerhaertDVMBrunner-La RoccaHPvan VeldhuisenDJVernooyK. The bidirectional interaction between atrial fibrillation and heart failure: consequences for the management of both diseases. Europace. (2021) 23(23 Suppl 2):ii40–ii5. 10.1093/europace/euaa368
14.
BuntingKVO'ConnorKSteedsRPKotechaD. Cardiac imaging to assess left ventricular systolic function in atrial fibrillation. Am J Cardiol. (2021) 139:40–9. 10.1016/j.amjcard.2020.10.012
15.
KongLYSunLLChenLLLvXLiuF. Value of index beat in evaluating left ventricular systolic and diastolic function in patients with atrial fibrillation: a dual pulsed-wave Doppler study. Ultrasound Med Biol. (2020) 46(2):255–62. 10.1016/j.ultrasmedbio.2019.10.028
16.
LangRMBadanoLPMor-AviVAfilaloJArmstrongAErnandeLet alRecommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. (2015) 28(1):1–39.e14. 10.1016/j.echo.2014.10.003
17.
VoigtJUPedrizzettiGLysyanskyPMarwickTHHouleHBaumannRet alDefinitions for a common standard for 2D speckle tracking echocardiography: consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2015) 16(1):1–11. 10.1093/ehjci/jeu184
18.
BadanoLPKoliasTJMuraruDAbrahamTPAurigemmaGEdvardsenTet alStandardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: a consensus document of the EACVI/ASE/industry task force to standardize deformation imaging. Eur Heart J Cardiovasc Imaging. (2018) 19(6):591–600. 10.1093/ehjci/jey042
19.
NegishiKNegishiTKurosawaKHristovaKPopescuBAVinereanuDet alPractical guidance in echocardiographic assessment of global longitudinal strain. JACC Cardiovasc Imaging. (2015) 8(4):489–92. 10.1016/j.jcmg.2014.06.013
20.
ThomasLMarwickTHPopescuBADonalEBadanoLP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state-of-the-art review. J Am Coll Cardiol. (2019) 73(15):1961–77. 10.1016/j.jacc.2019.01.059
21.
GuptaDKShahAMGiuglianoRPRuffCTAntmanEMGripLTet alLeft atrial structure and function in atrial fibrillation: ENGAGE AF-TIMI 48. Eur Heart J. (2014) 35(22):1457–65. 10.1093/eurheartj/eht500
22.
SchaafMAndrePAltmanMMaucort-BoulchDPlacideJChevalierPet alLeft atrial remodeling assessed by 2D and 3D echocardiography identifies paroxysmal atrial fibrillation. Eur Heart J Cardiovasc Imaging. (2017) 18(1):46–53. 10.1093/ehjci/jew028
23.
RamanBSmillieRWMahmodMChanKArigaRNikolaidouCet alIncremental value of left atrial booster and reservoir strain in predicting atrial fibrillation in patients with hypertrophic cardiomyopathy: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. (2021) 23(1):109. 10.1186/s12968-021-00793-6
24.
SohnsCMarroucheNF. Atrial fibrillation and cardiac fibrosis. Eur Heart J. (2020) 41(10):1123–31. 10.1093/eurheartj/ehz786
25.
Dzeshka MikhailSLip GregoryYHSnezhitskiyVShantsilaE. Cardiac fibrosis in patients with atrial fibrillation. J Am Coll Cardiol. (2015) 66(8):943–59. 10.1016/j.jacc.2015.06.1313
26.
EverettTHOlginJE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. (2007) 4(3, Supplement):S24–S7. 10.1016/j.hrthm.2006.12.040
27.
BaxMAjmone MarsanNDelgadoVBaxJJvan der BijlP. Effect of bi-atrial size and function in patients with paroxysmal or permanent atrial fibrillation. Am J Cardiol. (2022) 183:33–9. 10.1016/j.amjcard.2022.07.024
28.
KornejJSeewosterT. Left atrial size and function as markers of AF progression and outcomes. Int J Cardiol. (2019) 294:41. 10.1016/j.ijcard.2019.06.047
29.
Jasic-SzpakEMarwickTHDonalEPrzewlocka-KosmalaMHuynhQGozdzikAet alPrediction of AF in heart failure with preserved ejection fraction: incremental value of left atrial strain. JACC Cardiovasc Imaging. (2021) 14(1):131–44. 10.1016/j.jcmg.2020.07.040
30.
SmisethOABaronTMarinoPNMarwickTHFlachskampfFA. Imaging of the left atrium: pathophysiology insights and clinical utility. Eur Heart J Cardiovasc Imaging. (2022) 23(1):2–13. 10.1093/ehjci/jeab191
31.
MotocAScheirlynckERoosensBLuchianM-LChamelevaHGeversMet alAdditional value of left atrium remodeling assessed by three-dimensional echocardiography for the prediction of atrial fibrillation recurrence after cryoballoon ablation. Int J Cardiovasc Imaging. (2022) 38(5):1103–11. 10.1007/s10554-021-02493-9
32.
MaX-XBoldtL-HZhangY-LZhuM-RHuBParwaniAet alClinical relevance of left atrial strain to predict recurrence of atrial fibrillation after catheter ablation: a meta-analysis. Echocardiography. (2016) 33(5):724–33. 10.1111/echo.13184
33.
Pessoa-AmorimGMancioJVougaLRibeiroJGamaVBettencourtNet alImpaired left atrial strain as a predictor of new-onset atrial fibrillation after aortic valve replacement independently of left atrial size. Rev Esp Cardiol Engl Ed. (2018) 71(6):466–76. 10.1016/j.rec.2017.10.005
34.
SonaglioniALombardoMNicolosiGLRigamontiEAnzàC. Incremental diagnostic role of left atrial strain analysis in thrombotic risk assessment of nonvalvular atrial fibrillation patients planned for electrical cardioversion. Int J Cardiovasc Imaging. (2021) 37(5):1539–50. 10.1007/s10554-020-02127-6
35.
SvartsteinA-SWLassenMHSkaarupKGGroveGLVyffFRavnkildeKet alPredictive value of left atrial strain in relation to atrial fibrillation following acute myocardial infarction. Int J Cardiol. (2022) 364:52–9. 10.1016/j.ijcard.2022.05.026
36.
KupczynskaKMandoliGECameliMKasprzakJD. Left atrial strain—a current clinical perspective. Kardiol Pol. (2021) 79(9):955–64. 10.33963/KP.a2021.0105
37.
Ross AgnerBFKatzMGWilliamsZRDixenUJensenGBSchwarzKQ. Left ventricular systolic function assessed by global longitudinal strain is impaired in atrial fibrillation compared to sinus rhythm. J Atr Fibrillation. (2017) 10(4):1437. 10.4022/jafib.1437eCollection.
38.
YamauchiYTanakaHYokotaSMochizukiYYoshigaiYShirakiHet alEffect of heart rate on left ventricular longitudinal myocardial function in type 2 diabetes mellitus. Cardiovasc Diabetol. (2021) 20(1):87. 10.1186/s12933-021-01278-7
39.
SuzukiRMatsumotoHTeshimaTKoyamaH.Influence of heart rate on myocardial function using two-dimensional speckle-tracking echocardiography in healthy dogs. J Vet Cardiol. (2013) 15(2):139–46. 10.1016/j.jvc.2012.12.004
40.
Sengupta ParthoPNarulaJ. À la mode atrioventricular mechanical coupling. JACC Cardiovasc Imaging. (2014) 7(1):109–11. 10.1016/j.jcmg.2013.12.001
41.
GopinathannairREtheridge SusanPMarchlinski FrancisESpinale FrancisGLakkireddyDOlshanskyB. Arrhythmia-induced cardiomyopathies. J Am Coll Cardiol. (2015) 66(15):1714–28. 10.1016/j.jacc.2015.08.038
42.
ReddyYNVObokataMVerbruggeFHLinGBorlaugBA. Atrial dysfunction in patients with heart failure with preserved ejection fraction and atrial fibrillation. J Am Coll Cardiol. (2020) 76(9):1051–64. 10.1016/j.jacc.2020.07.009
43.
ShantsilaEShantsilaABlannADLipGYH. Left ventricular fibrosis in atrial fibrillation. Am J Cardiol. (2013) 111(7):996–1001. 10.1016/j.amjcard.2012.12.005
Summary
Keywords
atrial fibrillation, speckle tracking echocardiography, left atrial, left ventricular, strain, volume
Citation
Lu S, Liu H, Sun J, Zhang J, Li L, Tang Q, Liu Y and Deng Y (2023) Evaluation of left atrial and ventricular remodeling in atrial fibrillation subtype by using speckle tracking echocardiography. Front. Cardiovasc. Med. 10:1208577. doi: 10.3389/fcvm.2023.1208577
Received
19 April 2023
Accepted
21 July 2023
Published
10 August 2023
Volume
10 - 2023
Edited by
Sanjeev Bhattacharyya, Barts Heart Centre, United Kingdom
Reviewed by
Jennifer Mancio, Guy’s and St Thomas’ NHS Foundation Trust, United Kingdom Diana Ruxandra Florescu, Spitalul Clinic Judetean de Urgentã Craiova, Romania
Updates
Copyright
© 2023 Lu, Liu, Sun, Zhang, Li, Tang, Liu and Deng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Youbin Deng ybdeng2007@hotmail.com
Abbreviations AF, atrial fibrillation; PAF, paroxysmal atrial fibrillation; PerAF, persistent atrial fibrillation; EHRA, European Heart Rhythm Association; LA, left atrial; LV, left ventricular; LAEF, left atrial emptying fraction; LVEF, left ventricular ejection fraction; GLS, global longitudinal strain; E/e', mitral inflow peak early diastolic velocity/mitral annular peak early diastolic velocity; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume.
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.