- 1Nanyang City Center Hospital, Nanyang, China
- 2Nanyang Second General Hospital, Nanyang, China
- 3Shuguang Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Introduction: The presence of abdominal aortic calcification (AAC) is strongly linked to the development of atherosclerosis and the incidence of morbidity and mortality related to cardiovascular diseases (CVD). Urinary albumin creatinine ratio (UACR) was found related with the increased risk of CVD. The aim of this study is to explore the relationship between the UACR and severe AAC (SAAC).
Methods and Results: This study included a total of 2,379 individuals aged over 40 years, and their information was obtained from the National Health and Nutrition Examination Survey conducted (NHANES) in 2013–2014. The measurement of AAC was conducted through dual-energy x-ray absorptiometry and assessed using the Kauppila scoring system. SAAC was characterized by a Kauppila score of 6 or higher. Multivariate regression models were used to analyze the relationship between UACR level and SAAC, with covariate adjustment. In the completely adapted model, the top third subgroup exhibits increased likelihood of SAAC (odds ratio 1.50; 95%CI: 0.98, 2.29; p = 0.030) in contrast to the bottom third subgroup. The subgroup analyses revealed a more pronounced correlation among the older participants (p-value for interaction = 0.013).
Discussion: In the United States, SAAC was more likely to occur in adults who had a higher probability of UACR. The use of UACR has the potential to be a valuable method for forecasting the likelihood of SAAC.
1 Introduction
Cardiovascular disease is a highly fatal illness globally, and atherosclerosis is recognized as a major contributor to cardiovascular diseases (CVD) (1). Abdominal aortic calcification (AAC) serves as an indicator of atherosclerosis and can forecast future morbidity and mortality related to blood vessel health (2–4). A prior investigation discovered that AAC was linked to CVD fatality, and AAC proved to be a more potent prognosticator for overall mortality compared to coronary artery calcium (5). In their study, Namuun Ganbaatar and colleagues found that there was a correlation between the progression of coronary artery calcium and the presence of albuminuria, which was determined by an albumin-to-creatinine ratio exceeding 30 mg/g (6).
Patients may find 24-hour urinary protein collection troublesome and unreliable due to issues with insufficient or excessive collection and laboratory treatment methods, even though it is considered the gold standard for urinary albumin excretion (7). In clinical practice, the urinary albumin creatinine ratio (UACR) is frequently employed as a substitute. Multiple studies have found that UACR is associated with CVD (8, 9), chronic kidney disease (CKD) (10), hypertension (11) and other diseases. There is a wealth of evidence indicating that microalbuminuria (UACR, 30–300 mg/g) and macroalbuminuria (UACR, >300 mg/g) are linked to the advancement of end-stage renal disease (10). Additionally, a study tracking Korean men for 5 years revealed that having high normal albuminuria (UACR <30 mg/g) was a predictor of an elevated likelihood of developing diabetes (12). In the general population, there is a significant association between proteinuria and dyslipidemia (13). Additionally, a direct link has been observed between UACR within the normal range and overall mortality in the general population (8, 14).
However, no studies have been reported on the relationship between UACR and AAC. Our objective was to examine if there is a correlation between normal UACR levels and AAC in the US population during 2013–2014, considering that most individuals in the National Health and Nutrition Examination Survey (NHANES) had UACR within the normal range.
2 Methods
2.1 Study population and design
The study utilized data from NHANES 2013–2014, a survey that employs sampling design to investigate the health and nutrition of individuals in the United States. NHANES covers five key areas, namely demographic information, physical measurements, examinations, laboratory tests, and questionnaires. In the NHANES study conducted in 2013–2014, a grand total of 10,175 individuals took part. Among them, 7,035 individuals did not have AAC data, 23 individuals lacked UACR data, and 370 individuals lacked covariate data. Additionally, 368 individuals with UACR ≥ 30 mg/g were excluded, leaving a final count of 2,379 individuals included in the study. The data inclusion process is shown in Figure 1. All of them signed informed consent forms.
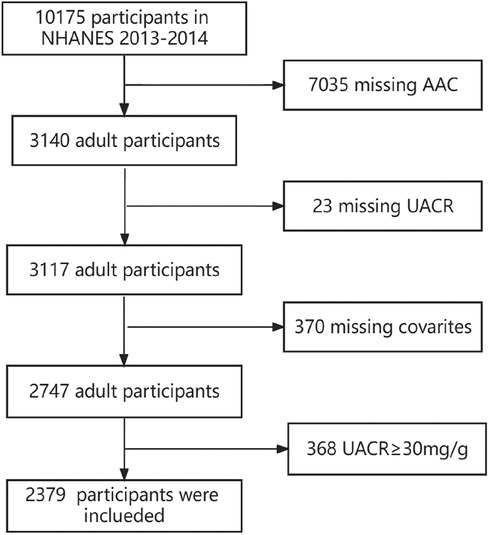
Figure 1. Participant flowchart. NHANES: National Health and Nutrition Examination Survey. AAC: abdominal aortic calcification. UACR: urine albumin creatinine ratio.
2.2 AAC measurement
As previously reported (15–17), AAC was accurately assessed through a lateral scan of the lumbar spine (vertebrae L1-L4) using dual-energy x-ray absorptiometry (DXA). To measure the degree of AAC, the Kauppila scoring system was utilized (15–17). The AAC-24 scale, which is the accepted technique for evaluating AAC on lateral plain films of the lumbar spine, utilizes a 24-point system (18). This scoring method divides the anterior and posterior aortic walls into four segments, aligning with the regions in front of the lumbar vertebrae L1-L4. Within these 8 segments, aortic calcification is identified visually as either a dispersed white stippling that extends to the anterior and/or posterior aortic walls or as white linear calcification on the anterior and/or posterior walls. The scoring for aortic calcification was as follows: a score of “0” indicated the absence of calcification, a score of “1” indicated calcification of one-third or less of the aortic wall in that segment, a score of “2” indicated calcification of more than one-third but less than two-thirds, and a score of “3” indicated calcification of more than two-thirds. Anterior and posterior aortic walls were scored separately, resulting in a range of “0”–“6” for each vertebral level and “0”–“24” for the total. In view of the previous research (16, 17), severe AAC (SAAC) was characterized as more than 5 score.
2.3 UACR
Samples of urine were prepared, kept in suitable frozen (−30°C) conditions, and sent to the University of Minnesota in Minneapolis, MN for examination.
2.4 Covariates
The covariates consisted of the following variables: gender (male or female), age, race (Mexican American, Other Hispanic, Non-Hispanic White, Non-Hispanic Black or Other Race), body mass index (BMI), waist circumference, smoking status (participants who had smoked at least 100 cigarettes in their lifetime were considered smokers), alcohol consumption (participants who had consumed at least 12 alcoholic drinks per year were considered drinkers), levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine (Scr), serum uric acid, total calcium,phosphorus,25OHD3, albumin, alkaline phosphatase(ALP), hemoglobin (Hb), hemoglobin A1c (HbA1c), total cholesterol (TC), and diabetes status (participants who were told by a doctor that they have diabetes), hypertension status (participants who were informed by a doctor or other health professional that they have high blood pressure), and systemic immune-inflammationindex [SII, which is calculated as P multiplied by N divided by L, where P represents platelet count, N represents neutrophil count, and L represents lymphocyte count (109/L)]. The SII is closely associated with cardiovascular death and all-cause death according to previous studies (19, 20). The estimated glomerular filtration rate (eGFR) was calculated using the equation from the Modification of Diet in Renal Disease (MDRD) study (21).
2.5 Statistical analysis
The analysis was conducted using R Statistical Software (Version 4.2.2, http://www.R-project.org, The R Foundation) and the Free Statistics analysis platform (Version 1.8, Beijing, China). A P-value less than 0.05 was considered statistically significant. The UACR was categorized into three groups, with the first group serving as the reference. The initial explanations, categorized by AAC stratification (without SAAC and SAAC), were presented as a ratio for categorical factors, average ± standard deviation or median for continuous factors. Multivariate logistic regression models were used to explore the independent relationship between UACR and ACC in three different models. The adjusted regression model Ⅰ incorporated variables such as age, gender, and race. The adjusted regression model Ⅱ incorporated variables such as age, sex, race, diabetes, high blood pressure, alcohol consumption, smoking, body mass index (BMI), and waist circumference. In the adjusted regression model Ⅲ, besides the above variable, HbA1c, Hb, albumin, AST, ALT, ALP, total cholesterol, uric acid, and serum creatinine, total calcium, phosphorus, 25OHD3, SII and eGFR were analysed. Stratified Logistic regression models were utilized to perform the subgroup analyses. The likelihood ratio test was employed to examine interaction among subgroups.
3 Results
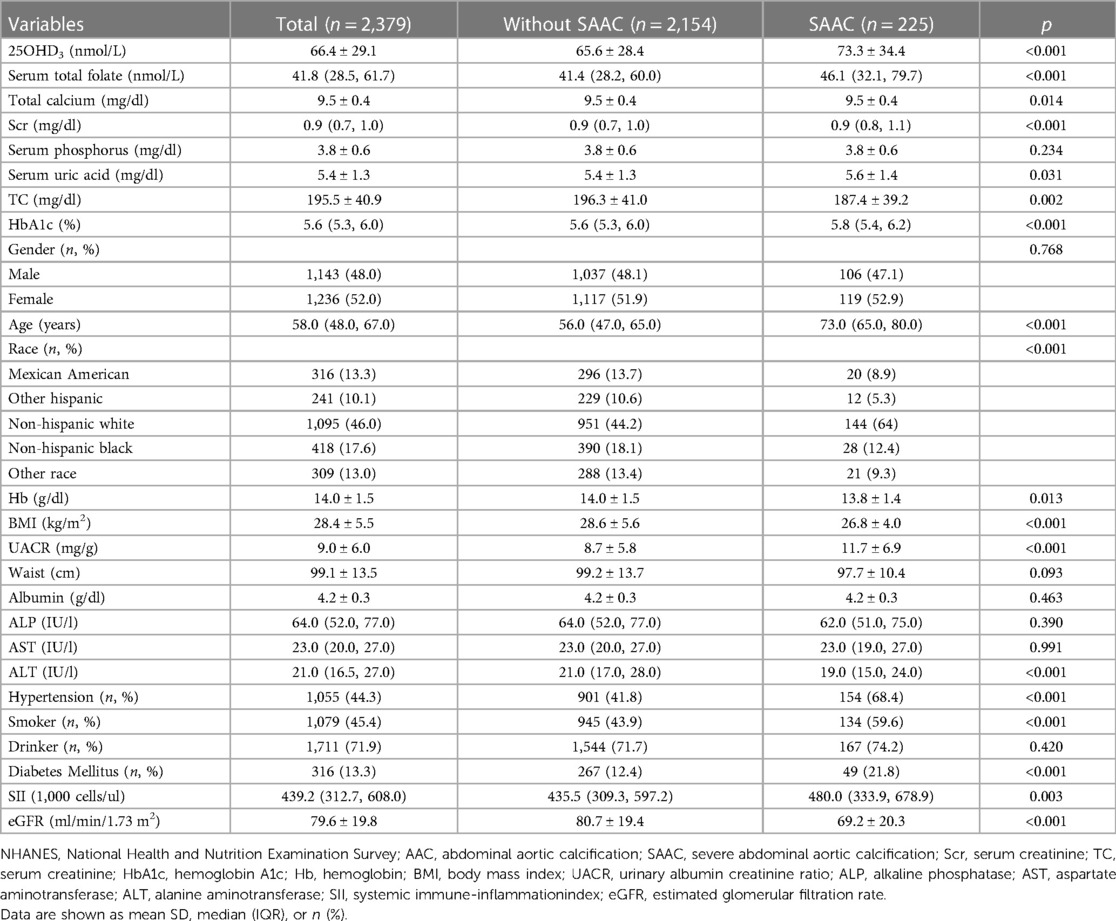
The baseline characteristics were shown in Table 1. Total 2,379 participants were separated into SAAC group (225, 9.5%) and without SAAC group (2,154). The average age was 58 years. Participants with SAAC appeared to be older, Caucasian compared to those without SAAC. Also there was a significant difference in BMI, 25OHD3, serum total folate, total calcium, serum uric acid, Scr, TC, HbA1c, ALT, Hb, SII, eGFR among the two groups. The participants in SAAC group had the lowest levels of TC, Hb, BMI, ALT, eGFR; had the highest levels of 25OHD3, folate, Scr, serum uric acid, HbA1c, UACR; had the higher prevalence of hypertension, DM and smoking.
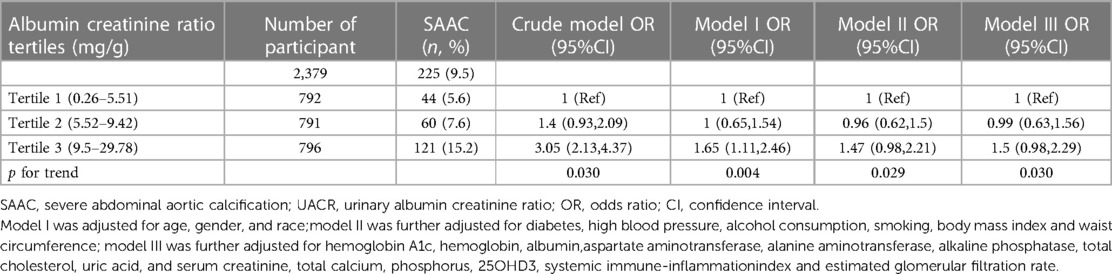
Table 2 shows the logistic regression analysis between different UACR levels and the SAAC. From the Tertile 1 to Tertile 3 level, the prevalence of the SAAC was gradually rised. The risk of SAAC increased as the UACR rose in both the crude model and the adjusted models I and III. In Model III, the odds ratios (ORs) for participants in the second and third tertiles of UACR were 0.99 [95% confidence interval (CI) 0.63, 1.56] and 1.5 (95% CI: 0.98, 2.29), respectively, when compared to those in the first tertile. The p-value for trend was 0.03.
Figure 2 displayed the subgroup analyses conducted to assess the stability of the correlation between UACR and SAAC. Only in the age group, we observed the significant difference between the <60 years and the older age (P = 0.013). The association was consistent in the gender, BMI, eGFR, Race, hypertension, DM. There was a nearly statistically significant distinction observed between the eGFR<90 mL/min/1.73 m2 and ≥90 mL/min/1.73 m2 (P = 0.065).
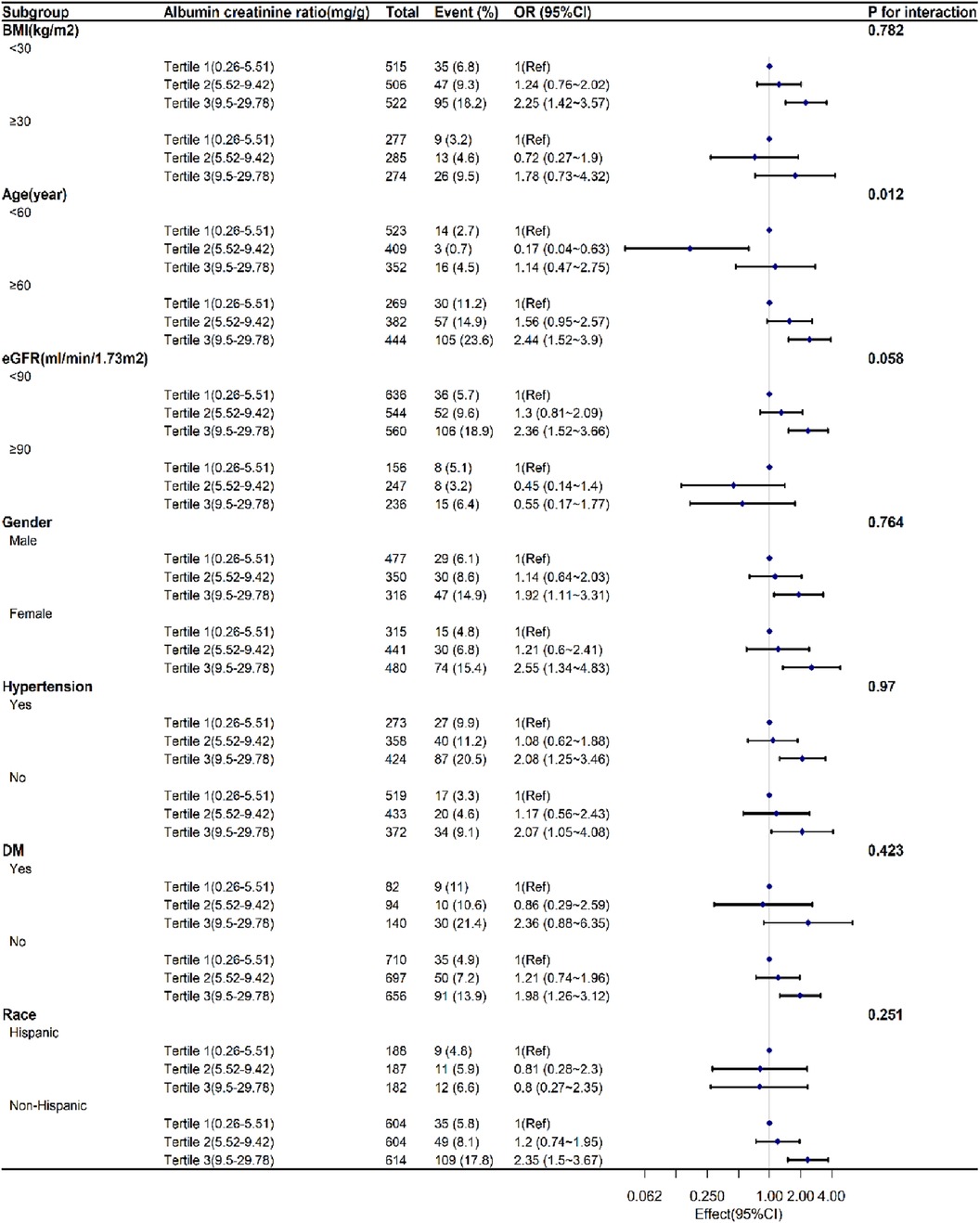
Figure 2. Subgroup analyses exploring the correlation between UACR levels and SAAC.accounting for factors such as age, sex, race, diabetes, high blood pressure, alcohol consumption, tobacco use, body mass index, waist circumference, glycated hemoglobin, hemoglobin, albumin, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, overall cholesterol, uric acid, and blood creatinine levels, as well as total calcium, phosphorus, 25-hydroxyvitamin D3, systemic immune-inflammation index, and estimated glomerular filtration rate.UACR:urinary albumin creatinine ratio. SAAC:severe abdominal aortic calcification. OR:Odds Ratio. CI:confidence interval. BMI:body mass index. eGFR:estimated glomerular filtration rate. DM: diabetes mellitus.
4 Discussion
The objective of our research was to uncover the correlation between the typical UACR range and the SAAC. Our investigation revealed a direct association between the UACR level and the likelihood of experiencing SAAC.
According to the current guidelines, the UACR concentration above 30 mg/g was considered to be clinically meaningful albuminuria (22). However, the normal range albuminuria was found associated with an increased CVD risk (23), hypertension (24–26), CKD (27) and atherosclerosis (28). In a nondiabetic population with normal-range UACR and eGFR, Aiko Okubo and colleagues discovered that high-normal albuminuria and hypertension were linked to the development of CKD (27). A comprehensive study of a significant population indicated that mild albuminuria (less than 30 mg/g) is linked to mortality from all causes and cardiovascular diseases (29). According to research conducted in Hong Kong, it was discovered that even when the UACR reached levels as low as 1–1.4 mg/mmol (8.4–11.76 mg/g), there was a notable rise in the likelihood of CVD or mortality in both males and females (30). In a cross-sectional investigation conducted in Korea, it was found that patients with type 2 diabetes who had high normal albuminuria showed a significant correlation with atherosclerotic vascular alterations (31). According to a 2005 publication, the UACR has a strong and autonomous correlation with both the existence and seriousness of atherosclerosis in the overall populace, including individuals with normal levels (28).
While the UACR was acknowledged as a possible contributor to cardiovascular disease, the precise mechanisms behind it are still not fully understood. A possible mechanism is that the UACR is associated with permeability of multiple capillary beds and endothelial dysfunction (32). Other study found that low-grade inflammation is related with the UACR (33, 34). The development of increased UACR, endothelial dysfunction, and chronic inflammation occur simultaneously and progress over time (35). Vascular calcification can be facilitated by chronic inflammation (36, 37).
In older participants, the subgroup analysis revealed a stronger correlation between UACR and SAAC. The process of getting older may cause dysregulation of the immune system, which can lead to chronic inflammation at a low level (38). This, in turn, increases the vulnerability to chronic illness, disability, frailty, and early mortality (39). Besides, a stronger connection was found in the eGFR below the 90 ml/min/1.73 m2. Individuals suffering from CKD and end-stage renal disease experience notable cardiovascular morbidity and mortality, which can be attributed, at least in part, to the occurrence of vascular calcification (40). These reminds us that the UACR should be concerned in older and CKD patients.
There are certain constraints associated with this research. To clarify the causality, it is necessary to conduct prospective research with larger sample sizes as the cross-sectional study design did not allow us to determine a causal relationship. Furthermore, the individuals involved in our study were recruited exclusively from one nation, and their ethnic background may not be applicable to numerous countries across the globe. Third, only individuals aged 40 and above were included in the acquisition of AAC data obtained from NHANES.
5 Conclusion
According to our research, the UACR within the normal range was linked to the likelihood of experiencing SAAC. Identifying patients at risk of AAC is crucial, as emphasized by the current results, which underscore the significance of managing the UACR within the normal range. Nevertheless, additional investigation is still necessary to validate our discoveries.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Review Board of National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XX: Writing – original draft. CL: Writing – original draft. DC: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This work was funded by Key project of the Chinese Hemodialysis Vascular Access Youth Physician Research Project (Number: 20220727).
Acknowledgments
We would like to thank the staff and participants of the NHANES for their contributions to this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Zhang S, Liu Y, Cao Y, Zhang S, Sun J, Wang Y, et al. Targeting the microenvironment of vulnerable atherosclerotic plaques: an emerging diagnosis and therapy strategy for atherosclerosis. Adv Mater. (2022) 34(29):e2110660. doi: 10.1002/adma.202110660
2. Niu Q, Hong Y, Lee CH, Men C, Zhao H, Zuo L. Abdominal aortic calcification can predict all-cause mortality and CV events in dialysis patients: a systematic review and meta-analysis. PLoS One. (2018) 13(9):e0204526. doi: 10.1371/journal.pone.0204526
3. Bartstra JW, Mali WPTM, Spiering W, de Jong PA. Abdominal aortic calcification: from ancient friend to modern foe. Eur J Prev Cardiol. (2021) 28(12):1386–91. doi: 10.1177/2047487320919895
4. Ramírez-Vélez R, García-Hermoso A, Correa-Rodríguez M, Lobelo F, González-Ruiz K, Izquierdo M. Abdominal aortic calcification is associated with decline in handgrip strength in the U.S. Adult population ≥40 years of age. Nutr Metab Cardiovasc Dis. (2021) 31(4):1035–43. doi: 10.1016/j.numecd.2020.11.003
5. Criqui MH, Denenberg JO, McClelland RL, Allison MA, Ix JH, Guerci A, et al. Abdominal aortic calcium, coronary artery calcium, and cardiovascular morbidity and mortality in the multi-ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol. (2014) 34(7):1574–9. doi: 10.1161/ATVBAHA.114.303268
6. Ganbaatar N, Kadota A, Hisamatsu T, Araki SI, Kume S, Fujiyoshi A, et al. Relationship between kidney function and subclinical atherosclerosis progression evaluated by coronary artery calcification. J Atheroscler Thromb. (2022) 29(9):1359–71. doi: 10.5551/jat.63030
7. Toto RD. Microalbuminuria: definition, detection, and clinical significance. J Clin Hypertens (Greenwich). (2004) 6(11 Suppl 3):2–s. doi: 10.1111/j.1524-6175.2004.4064.x
8. Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. (2001) 286(4):421–6. doi: 10.1001/jama.286.4.421
9. Hillege HL, Fidler V, Diercks GF, van Gilst WH, de Zeeuw D, van Veldhuisen DJ, et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation. (2002) 106(14):1777–82. doi: 10.1161/01.CIR.0000031732.78052.81
10. Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, et al. Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int. (2011) 80(1):93–104. doi: 10.1038/ki.2010.531
11. Jessani S, Levey AS, Chaturvedi N, Jafar TH. High normal levels of albuminuria and risk of hypertension in Indo-Asian population. Nephrol Dial Transplant. (2012) 27 Suppl 3(Suppl 3):iii58–64. doi: 10.1093/ndt/gfr200
12. Park SK, Seo MH, Ryoo JH, Kim MG, Choi JM, Shin H, et al. Urinary albumin excretion within the normal range predicts the development of diabetes in Korean men. Diabetes Res Clin Pract. (2015) 109(2):427–33. doi: 10.1016/j.diabres.2015.05.006
13. Wang YX, Wang AP, Ye YN, Gao ZN, Tang XL, Yan L, et al. Elevated triglycerides rather than other lipid parameters are associated with increased urinary albumin to creatinine ratio in the general population of China: a report from the REACTION study. Cardiovasc Diabetol. (2019) 18(1):57. doi: 10.1186/s12933-019-0863-8
14. Kovesdy CP, Lott EH, Lu JL, Malakauskas SM, Ma JZ, Molnar MZ, et al. Outcomes associated with microalbuminuria: effect modification by chronic kidney disease. J Am Coll Cardiol. (2013) 61(15):1626–33. doi: 10.1016/j.jacc.2012.11.071
15. Aleksova J, Kurniawan S, Vucak-Dzumhur M, Kerr P, Ebeling PR, Milat F, et al. Aortic vascular calcification is inversely associated with the trabecular bone score in patients receiving dialysis. Bone. (2018) 113:118–23. doi: 10.1016/j.bone.2018.05.014
16. Rodríguez AJ, Lewis JR, Scott DS, Kiel DP, Schousboe JT, Ebeling PR, et al. Aortic calcification is associated with five-year decline in handgrip strength in older women. Calcif Tissue Int. (2018) 103(6):589–98. doi: 10.1007/s00223-018-0458-5
17. Lewis JR, Schousboe JT, Lim WH, Wong G, Zhu K, Lim EM, et al. Abdominal aortic calcification identified on lateral spine images from bone densitometers are a marker of generalized atherosclerosis in elderly women. Arterioscler Thromb Vasc Biol. (2016) 36(1):166–73. doi: 10.1161/ATVBAHA.115.306383
18. Pariente-Rodrigo E, Sgaramella GA, García-Velasco P, Hernández-Hernández JL, Landeras-Alvaro R, Olmos-Martínez JM. Reliability of radiologic evaluation of abdominal aortic calcification using the 24-point scale. Radiologia (Panama). (2016) 58(1):46–54. doi: 10.1016/j.rx.2015.03.002
19. Hua X, Duan F, Zhai W, Song C, Jiang C, Wang L, et al. A novel inflammatory-nutritional prognostic scoring system for patients with early-stage breast cancer. J Inflamm Res. (2022) 15:381–94. doi: 10.2147/JIR.S338421
20. Xia Y, Xia C, Wu L, Li Z, Li H, Zhang J. Systemic immune inflammation Index (SII), system inflammation response Index (SIRI) and risk of all-cause mortality and cardiovascular mortality: a 20-year follow-up cohort study of 42,875 US adults. J Clin Med. (2023) 12(3):1128. doi: 10.3390/jcm12031128
21. Wang F, Zheng J. Association between serum alpha-klotho and severe abdominal aortic calcification among civilians in the United States. Nutr Metab Cardiovasc Dis. (2022) 32(6):1485–92. doi: 10.1016/j.numecd.2022.02.017
22. Inker LA, Astor BC, Fox CH, Isakova T, Lash JP, Peralta CA, et al. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for the evaluation and management of CKD. Am J Kidney Dis. (2014) 63(5):713–35. doi: 10.1053/j.ajkd.2014.01.416
23. Inoue K, Streja E, Tsujimoto T, Kobayashi H. Urinary albumin-to-creatinine ratio within normal range and all-cause or cardiovascular mortality among U.S. Adults enrolled in the NHANES during 1999–2015. Ann Epidemiol. (2021) 55:15–23. doi: 10.1016/j.annepidem.2020.12.004
24. Wang TJ, Evans JC, Meigs JB, Rifai N, Fox CS, D'Agostino RB, et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation. (2005) 111(11):1370–6. doi: 10.1161/01.CIR.0000158434.69180.2D
25. Yadav D, Kang DR, Koh SB, Kim JY, Ahn SV. Association between urine albumin-to-creatinine ratio within the normal range and incident hypertension in men and women. Yonsei Med J. (2016) 57(6):1454–60. doi: 10.3349/ymj.2016.57.6.1454
26. Ren F, Li M, Xu H, Qin X, Teng Y. Urine albumin-to-creatinine ratio within the normal range and risk of hypertension in the general population: a meta-analysis. J Clin Hypertens (Greenwich). (2021) 23(7):1284–90. doi: 10.1111/jch.14263
27. Okubo A, Nakashima A, Doi S, Doi T, Ueno T, Maeda K, et al. High-normal albuminuria is strongly associated with incident chronic kidney disease in a nondiabetic population with normal range of albuminuria and normal kidney function. Clin Exp Nephrol. (2020) 24(5):435–43. doi: 10.1007/s10157-019-01842-2
28. Furtner M, Kiechl S, Mair A, Seppi K, Weger S, Oberhollenzer F, et al. Urinary albumin excretion is independently associated with carotid and femoral artery atherosclerosis in the general population. Eur Heart J. (2005) 26(3):279–87. doi: 10.1093/eurheartj/ehi014
29. Kang M, Kwon S, Lee J, Shin JI, Kim YC, Park JY, et al. Albuminuria within the normal range can predict all-cause mortality and cardiovascular mortality. Kidney360. (2022) 3(1):74–82. doi: 10.34067/KID.0003912021
30. Fung CS, Wan EY, Chan AK, Lam CL. Association of estimated glomerular filtration rate and urine albumin-to-creatinine ratio with incidence of cardiovascular diseases and mortality in Chinese patients with type 2 diabetes mellitus—a population-based retrospective cohort study. BMC Nephrol. (2017) 18(1):47. doi: 10.1186/s12882-017-0468-y
31. Yoon HE, Kim ES, Mo EY, Shin SJ, Moon SD, Han JH. High normal albuminuria is associated with arterial stiffness and carotid atherosclerosis in Korean patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. (2015) 25(8):787–94. doi: 10.1016/j.numecd.2015.03.011
32. Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. (2012) 226(4):562–74. doi: 10.1002/path.3964
33. Zambrano-Galvan G, Rodríguez-Morán M, Simental-Mendía LE, Lazalde B, Reyes-Romero MA, Guerrero-Romero F. C-reactive protein is directly associated with urinary albumin-to-creatinine ratio. Arch Med Res. (2011) 42(6):451–6. doi: 10.1016/j.arcmed.2011.09.009
34. Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Nijpels G, Dekker JM, et al. C-reactive protein and soluble vascular cell adhesion molecule-1 are associated with elevated urinary albumin excretion but do not explain its link with cardiovascular risk. Arterioscler Thromb Vasc Biol. (2002) 22(4):593–8. doi: 10.1161/01.ATV.0000013786.80104.D4
35. Stehouwer CD, Gall MA, Twisk JW, Knudsen E, Emeis JJ, Parving HH. Increased urinary albumin excretion, endothelial dysfunction, and chronic low-grade inflammation in type 2 diabetes: progressive, interrelated, and independently associated with risk of death. Diabetes. (2002) 51(4):1157–65. doi: 10.2337/diabetes.51.4.1157
36. Sage AP, Tintut Y, Demer LL. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. (2010) 7(9):528–36. doi: 10.1038/nrcardio.2010.115
37. Alesutan I, Luong TTD, Schelski N, Masyout J, Hille S, Schneider MP, et al. Circulating uromodulin inhibits vascular calcification by interfering with pro-inflammatory cytokine signalling. Cardiovasc Res. (2021) 117(3):930–41. doi: 10.1093/cvr/cvaa081
38. Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. The origins of age-related proinflammatory state. Blood. (2005) 105(6):2294–9. doi: 10.1182/blood-2004-07-2599
39. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. (2018) 15(9):505–22. doi: 10.1038/s41569-018-0064-2
Keywords: urinary albumin creatinine ratio, abdominal aortic calcification, cross-sectional study, National Health and Nutrition Examination Survey (NHANES), atherosclerosis
Citation: Xue X, Li C and Chen D (2024) A cross-sectional study investigating the relationship between urinary albumin creatinine ratio and abdominal aortic calcification in adults. Front. Cardiovasc. Med. 11:1352921. doi: 10.3389/fcvm.2024.1352921
Received: 9 December 2023; Accepted: 15 February 2024;
Published: 4 March 2024.
Edited by:
Nhat Tu Le, Houston Methodist Research Institute, United StatesReviewed by:
Anahita Mojiri, Houston Methodist Research Institute, United StatesSurbhi Chaudhary, Cornell University, United States
© 2024 Xue, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongping Chen MTM3NjQzNjI1NjlAMTYzLmNvbQ==
 Xian Xue
Xian Xue Chen Li
Chen Li Dongping Chen
Dongping Chen