Abstract
Background:
Dual anti-platelet therapy (DAPT) after coronary artery bypass graft surgery (CABG) has drawn a lot of controversy. This study aimed to explore the effects of ticagrelor combined with aspirin (compared with aspirin combined with clopidogrel) on the patency of saphenous vein graft (SVG) after off-pump CABG.
Methods:
This was a prospective, randomized, double-blinded clinical controlled trial. Participants were first given aspirin (100 mg/d) within 12 h after off-pump CABG, followed by P2Y12 receptor antagonist (Orally, 75 mg/time of clopidogrel, once daily, for Group C and 90 mg/time of ticagrelor, twice daily, for Group T) within 24 h after off-pump CABG for one year. Computed tomography angiography (CTA) was conducted for all patients. The incidence of major adverse cardiac events(MACE), death, stroke, hemorrhage, left ventricular diameter (LVD), and left ventricular ejection fraction (LVEF) of the participants was assessed one year after off-pump CABG based on a 12-month follow-up.
Results:
A total of 73 participants completed the follow-up, and 219 bypass grafts, including 146 SVGs, were conducted. Notably, 11 bypass grafts (SVGs) were exposed to occlusion (9 in Group C and 2 in Group T). The overall occlusion rates of bypass grafts and SVGs of Groups C and T were significantly different (9/114 vs. 2/105, P = 0.043, 9/76 vs. 2/70, P = 0.04). Moreover, multivariate binary Logistic regression demonstrated that ticagrelor + aspirin anti-platelet therapy could reduce the stenosis risk of bypass grafts (OR = 0.195, 95% CI = 0.039-0.978, P = 0.047).
Conclusions:
Compared with clopidogrel, ticagrelor may reduce the occlusion rate of vein grafts after CABG.
Clinical Trial Registration:
[https://www.chictr.org/], identifier [ChiCTR1900022390].
Background
Coronary artery bypass graft surgery (CABG) is widely used for coronary heart disease treatment (1). Several patients have undergone off-pump CABG due to the development of clinical technology (2). Bypass vessel transplantation mainly involves the combination of the left internal mammary artery (LIMA) and the great saphenous vein (3).
Figure 1
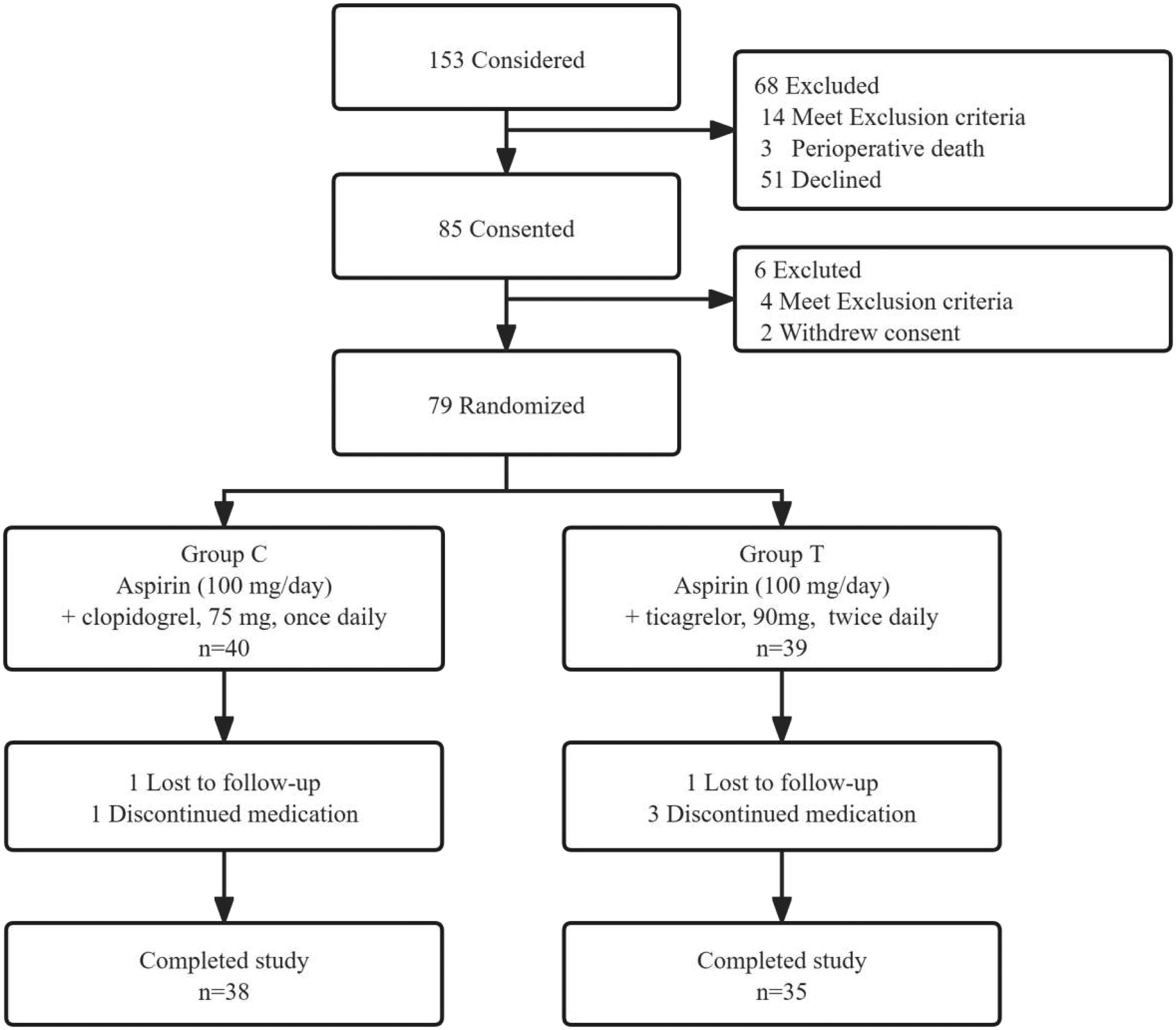
Flow chart of the study.
However, some patients may experience ischemic attack after CABG treatment, mainly due to SVG stenosis/occlusion. About 26.6%, 25.0%, and 1.5% of target vessels in patients undergoing PCI are bypass grafts after CABG, great SVG, and arterial grafts, respectively (4). Therefore, SVG patency should be improved to enhance cardiovascular surgery.
Studies have shown that the activated platelet system is associated with occlusion of bypass grafts. Notably, studies have shown that aspirin, a traditional anti-platelet drug, can improve the patency of bypass grafts after CABG surgery (5). Moreover, studies have shown that P2Y12 receptor antagonist combined with aspirin has synergistic anti-platelet effects. As a result, the dual anti-platelet therapy (DAPT) of aspirin combined with P2Y12 receptor antagonist has been widely applied in secondary prevention after CABG surgery (6). Clopidogrel, a classic P2Y12 receptor antagonist, has been used for many years clinically. However, ticagrelor, as an alternative P2Y12 receptor antagonist, has led to some controversies regarding the development of DAPT strategies.
Ticagrelor has a faster onset and better efficacy than Clopidogrel. Besides, several PLATO trials have shown that Ticagrelor has better efficacy than Clopidogrel (7, 8). Saw et al. found that DAPT involving ticagrelor can better maintain the patency of SVG than aspirin alone (9). Subsequent DACAB trials also showed similar findings through multi-center clinical trials (10). Nevertheless, the Popular-CABG trials found that the DAPT involving ticagrelor cannot maintain better patency of SVG compared with aspirin (11).
The American Heart Association suggested that the DAPT containing ticagrelor combined with aspirin may be superior to clopidogrel combined with aspirin (Class IIa, Evidence Level B) for secondary prevention after CABG surgery (7). Although the European guidelines do not recommend using ticagrelor for DAPT in stable CAD patients after CABG, they suggest performing DAPT under safe conditions (12).
This randomized controlled trial aimed to verify whether ticagrelor combined with aspirin can improve the patency of saphenous vein graft (SVG) after off-pump CABG (compared with aspirin combined with clopidogrel).
Methods
Study design and ethics approval
This was a single-center, prospective, randomized, double-blinded clinical controlled trial, following the principles of the Helsinki Declaration and all applicable laws and regulations (13).
Only patients who received simple CABG were included in this study. The patients were randomly divided into two groups: the aspirin + ticagrelor group and the aspirin + clopidogrel group. The patients provided written informed consent before participation. The study was approved by the Ethics Committee of Dalian Central Hospital (Ethics approval number: YN-2019-018-01) and registered at the Clinical Trial Center in China (Registration no. ChiCTR1900022390. Date of first registration: 09/04/2019). The study follows the CONSORT guidelines for reporting randomised clinical trials.
Clinical setting and participants
Only patients aged 18–80 years who underwent selective simple off-pump CABG at our center with intraoperative bypass grafts of LIMA-left anterior descending coronary artery (LAD), SVG-left circumflex artery (LCX), and SVG-right coronary artery (RCA) were enrolled in this study. Exclusion criteria included: (1) patients who did not receive CABG; (2) patients who underwent other cardiac surgeries at the same period; (3) patients who received emergency CABG; (4) patients with contraindications to clopidogrel or ticagrelor; (5) patients with sinus bradycardia; (6) patients simultaneously receiving liver enzyme potent inducer or inhibitor treatment; (7) patients who required kidney replacement therapy.
All patients underwent transthoracic echocardiography upon admission for evaluation of the structure and function of the left ventricle. The patients received aspirin until the day of CABG surgery. Notably, all patients had signed informed consent before CABG surgery. All patients survived after the CABG surgery, and no contraindications to aspirin/ticagrelor/clopidogrel were observed. Patients were randomized using the random-number table system of the Clinical Trial Center in China. The patients were randomly assigned to the experimental group (Group T) and control group (Group C). The age, gender, height, weight, BMI, coronary heart disease status at admission, previous PCI history, surgical history, hypertension history, diabetes history, smoking history, drinking history, left ventricular diameter and left ventricular ejection fraction (LVEF) were also recorded.
The patients received aspirin (100 mg/day) within 12 h after CABG surgery, followed by P2Y12 receptor antagonist (ticagrelor/clopidogrel, orally) within 24 h after surgery, for 1 year. Patients in the experimental group received ticagrelor, 90 mg, twice daily, while those in Group C received clopidogrel, 75 mg, once daily.
Other heart medications were determined by the therapists. Patients were assigned unique random codes via the Resman system program from the Chinese Clinical Trial Registry Center. Randomization was performed using a computer-generated random number method, and the allocation results were sealed in opaque envelopes by the research coordinator for secure preservation. The research drugs were distributed by cardiologists based on random coding. The study coordinators, patients, physicians, and computed tomography angiography (CTA) interpreters were blinded to drug distribution.
The patients were monitored daily during hospitalization. Moreover, the patients were followed up at 3, 6, and 12 months after discharge. Cardiac CTA and echocardiography examinations were performed one year after surgery.
Variables and measurements
Patency indicators of bypass grafts
The 64-detector rows of coronary artery CTA were re-examined after one year of surgery to evaluate the patency of coronary artery bypass grafts. Technician conducting CTA examination was blinded to the anti-platelet drugs (only aware of the patient's CABG postoperative condition). The degree of coronary artery stenosis was defined based on the FitzGibbon grading, where A and B grades were defined as the patent bypass grafts (14).
Major adverse cardiac events (MACE)
MACE was defined as cardiac death, recurrent myocardial infarction, new-onset agina, and cardiac shock and were observed during the follow-up (15).
Hemorrhage incidence
The main hemorrhage incidents were defined as life-threatening hemorrhage incidents (fatal hemorrhage, intracranial hemorrhage, hemorrhage requiring surgical intervention, hemorrhage leading to severe hypotension, and hemorrhage requiring venous vasoactive drugs), fundus bleeding, hemoglobin reduction exceeding 5 g/d, or blood transfusion exceeding 2 U during the treatment (due to hemorrhage incidents). Minor bleeding was defined as a bleeding event that did not lead to the main hemorrhage incidents (16).
Left ventricular structure and function
The LVEF value and the left ventricular end-diastolic diameter were assessed via cardiac ultrasound at the admission and at the end of follow-up.
Other indicators
Other indicators included incidences of death and stroke during follow-up.
Statistical analysis
Sample size estimation: PASS 15.0 was used for sample size estimation. Literature has reported that the patency rate of venous grafts in patients taking clopidogrel and aspirin was 91.6% three months post-CABG. However, approximately six months post-surgery, the patency rate of venous grafts decreased to around 87% (17, 18). Based on the literature we have reviewed and our clinical observations, we estimate that the patency rate of vein bypass grafts in Group C will be 80% one year post-CABG. Based on relevant studies in recent years, we have found that patients treated with ticagrelor and aspirin have a patency rate of over 90% for vein grafts three months to one year after CABG (11). Moreover, with the advancement of surgical techniques, the patency rate of vein grafts is further improving (19). Therefore, the vein graft patency of Group T after one year of surgery was estimated to be 95%. The sample size of 146 bypass grafts was required to detect an absolute difference of 5% with 80% statistical power and 0.05 bilateral a. Notably, 73 patients were needed for random assignment.
Statistical analysis: the general information and follow-up results of patients were imported into Office 365. SPSS 26.0 was used for all statistical analyses. Measurement data were expressed as mean ± standard deviation while counting data were expressed as frequency and percentage. The t-test and χ2 test were used for inter-group comparison of measurement data and inter-group comparison of counting data, respectively. The counting data with an expected frequency of less than 5 underwent Fisher's exact test. Univariate analysis was conducted to evaluate the impact of antiplatelet therapy strategies, gender, age, height, weight, body mass index (BMI), systolic and diastolic blood pressure, heart rate, respiratory rate, smoking history, hypertension history, diabetes history, percutaneous coronary intervention (PCI) history, surgical history, left ventricular end-diastolic diameter (LVD) at admission, and left ventricular ejection fraction (LVEF) at admission on venous graft patency. Subsequently, multivariate logistic regression analysis was performed to determine the independent effects of these factors on venous graft patency. All tests were conducted bilaterally, with P < 0.05 indicating a statistically significant difference.
Results
Participants, descriptive data, and outcome data
Patient recruitment started from April 2019 to April 2021. but was terminated prematurely due to coronavirus disease 2019 (COVID-19). A total of 153 patients underwent CABG alone in our center and received bypass grafts of LIMA-LAD, SVG-LCX, and SVG-RCA. Finally, 85 patients participated in this study, of which six were excluded after CABG due to contraindications or withdrawal of the consent form. The remaining 79 patients were randomly assigned to Groups C and T. Four patients discontinued medication within three months after surgery due to condition changes. Moreover, two patients were lost during follow-up, and 73 patients completed the follow-up (Figure 1). The general parameters collected from the patients included gender, age, height, weight, body mass index (BMI), systolic and diastolic blood pressure upon admission, resting heart rate, respiratory rate, coronary heart disease status at admission (including stable angina pectoris, unstable angina pectoris, or non-ST-segment elevation myocardial infarction), history of previous surgeries, prior percutaneous coronary intervention (PCI) history, presence of hypertension, preoperative left ventricular internal diameter, and preoperative left ventricular ejection fraction. Notably, the baseline characteristics were not significantly different between the two groups (Table 1).
Table 1
| Parameters | Group C (n = 38) | Group T (n = 35) | Statistical value | P |
|---|---|---|---|---|
| Male (cases) | 26 | 28 | 1.269a | 0.260 |
| Female (cases) | 12 | 7 | – | – |
| Age (years) | 65.13 ± 7.85 | 66.05 ± 7.57 | 0.512b | 0.611 |
| Height (cm) | 169.89 ± 8.24 | 169.34 ± 7.51 | 0.298b | 0.767 |
| Weigh (kg) | 71.87 ± 10.30 | 71.03 ± 9.03 | 0.369b | 0.713 |
| BMI | 24.87 ± 2.87 | 24.77 ± 2.69 | 0.153b | 0.879 |
| Systolic Blood Pressure (mmHg) | 140.00 ± 21.95 | 136.80 ± 21.86 | 0.624b | 0.535 |
| Diastolic Blood Pressure (mmHg) | 80.53 ± 9.87 | 77.77 ± 11.40 | 0.902b | 0.370 |
| Respiratory Rate | 18.74 ± 2.33 | 19.03 ± 2.42 | 0.524b | 0.602 |
| Resting Heart Rate | 75.42 ± 15.57 | 77.77 ± 11.40 | 0.763b | 0.448 |
| Presentation | – | – | 0.259a | 0.875 |
| Stable angina | 12 | 13 | – | – |
| Unstable angina | 21 | 18 | – | – |
| NSTEMI | 5 | 4 | – | – |
| Previous surgical history | 15 | 13 | 0.042a | 0.838 |
| Previous PCI | 5 | 3 | c | 0.712 |
| Smoke(cases) | 23 | 15 | 2.779b | 0.131 |
| Diabetes (cases) | 14 | 18 | 1.574b | 0.210 |
| Hypertension (cases) | 25 | 25 | 0.268b | 0.604 |
| Left ventricular internal diameter | 51.53 ± 6.08 | 51.74 ± 7.57 | 0.135b | 0.893 |
| Left ventricular ejection fraction | 54.55 ± 11.62 | 53.91 ± 13.91 | 0.213b | 0.832 |
Comparison in general parameters between groups C and T.
χ2 test.
Student's t test.
Fisher's exact test.
Main results
A total of 219 bypass grafts were evaluated, of which 73 grafts were LIMA-LAD. Moreover, 146 SVGs were detected, of which 73 grafts were SVG-LCX, and 73 grafts were SVG-RCA. CTA examination revealed that 11 bypass grafts (SVGs) were exposed to occlusion. Nine bypass grafts in Group C, including six SVG-LCX and three SVG-RCA, were exposed to occlusion. Two bypass grafts in Group T (SVG-RCA) were exposed to occlusion. The total occlusion rate of bypass grafts and SVGs were significantly different between Groups C and T (9/114 vs. 2/105 P = 0.043, 9/76 vs. 2/70, respectively P = 0.04) (Table 2).
Table 2
| Parameters | Group C | Group T | Statistical value | P |
|---|---|---|---|---|
| Total graft occlusion | 9 | 2 | 4.111a | 0.043a |
| SVG graft occlusion | 9 | 2 | 4.223a | 0.04a |
| SVG-LCX | 6 | 2 | b | 0.264 |
| SVG-RCA | 3 | 0 | b | 0.241 |
Graft occlusion and stenosis rates according to randomised treatment.
χ2 test.
Fisher's exact test.
One patient in Group C developed a stroke during the follow-up. Moreover, 16 patients had hemorrhage incidents in Group T, of which two had major bleeding events. Both of the 2 patients with major bleeding events had gastrointestinal bleeding. The incidence of minor bleeding incidents was significantly higher in Group T than in Group C (4/38 vs. 10/35 P = 0.05). Seven patients developed MACE, of which one patient in Group C developed NSTEMI, and all other patients developed new-onset angina. Notably, MACE was not significantly different between the two groups (5/38 vs. 2/35 P = 0.432) (Table 3).
Table 3
| Parameters | Group C (n = 38) | Group T (n = 35) | Statistical value | P |
|---|---|---|---|---|
| Minor Bleeding | 4 | 10 | 3.827a | 0.05 |
| Major Bleeding | 0 | 2 | b | 0.226 |
| MACE | 5 | 2 | b | 0.432 |
| Angina | 4 | 2 | b | 0.676 |
| NSTEMI | 1 | 0 | b | 1.000 |
| Stroke | 1 | 0 | b | 1.000 |
Clinical events between groups C and T.
χ2 test.
Fisher's exact test.
Nonetheless, LVD and LVEF of cardiac ultrasound were not significantly different between Groups C and T (Table 4).
Table 4
| Parameters | Group C (n = 38) | Group T (n = 35) | Statistical value | P |
|---|---|---|---|---|
| Left ventricular internal diameter | 50.79 ± 4.45 | 50.40 ± 7.52 | 0.266 | 0.791 |
| Left ventricular ejection fraction | 57.05 ± 8.75 | 55.46 ± 10.50 | 0.707 | 0.482 |
Left ventricular diameter and left ventricular ejection fraction 1 year after surgery.
The univariate predictive factors for occlusion of bypass grafts are shown in Table 5. Ticagrelor + aspirin DAPT use predicted a significantly lower graft occlusion(OR 0.035, 95% CI 0.002–0.570, p = 0.018). The predictive factors of vein graft patency were analyzed using multivariate binary Logistic regression via stepwise regression analysis. Finally, the regression model suggested that ticagrelor + aspirin DAPT could reduce the stenosis risk of bypass grafts (OR = 0.195, 95% CI = 0.039-0.978, P = 0.047).
Table 5
| Parameters | OR | 95%CI | P |
|---|---|---|---|
| Ticagrelor | 0.195 | 0.039–0.978 | 0.047 |
| Male | 0.997 | 0.02–49.35 | 0.999 |
| Age | 0.972 | 0.842–1.122 | 0.695 |
| BMI | 10.312 | 0.074–1430.476 | 0.354 |
| Height (cm) | 1.74 | 0.39–7.76 | 0.468 |
| Weigh (kg) | 0.461 | 0.083–2.563 | 0.376 |
| Systolic Blood Pressure (mmHg) | 0.994 | 0.94–1.052 | 0.844 |
| Diastolic Blood Pressure (mmHg) | 0.865 | 0.721–1.039 | 0.122 |
| Resting Heart Rate | 1.018 | 0.946–1.094 | 0.637 |
| Respiratory Rate | 1.043 | 0.642–1.694 | 0.864 |
| Left Ventricular Internal Diameter | 1.274 | 0.99–1.639 | 0.06 |
| Left Ventricular Ejection Fraction | 1.096 | 0.971–1.237 | 0.136 |
| Previous surgical history | 1.191 | 0.155–9.128 | 0.867 |
| Previous PCI | 0 | 0 | 0.999 |
| Smoke (cases) | 0.417 | 0.109–1.59 | 0.2 |
| Hypertension (cases) | 0.741 | 0.078–7.048 | 0.794 |
| Diabetes (cases) | 1.78 | 0.184–17.219 | 0.618 |
Univariate predictors of graft occlusion.
Discussion
SVG occlusion depends on the following three pathophysiological processes after CABG surgery: thrombosis and technical failure are the main mechanisms in the first week and month after CABG, followed by intimal hyperplasia from one month to one year, and atherosclerosis one year later.
Intimal hyperplasia of SVG is an adaptive mechanism for high arterial pressure (“arterialization”). The activation of the platelet system plays an essential role in the arterialization process (20). Activated platelets secrete various cytokines (interleukin-1 and interleukin-6) and growth factors (platelet-derived growth factor and transforming growth factor β) that promote smooth muscle cell (SMC) proliferation. Meanwhile, coagulation activation leads to thrombin formation, thus depositing polymeric fibrin. Thrombin directly or indirectly stimulates SMC proliferation through platelets-secreted platelet derived growth factor (PDGF). Neo-endothelium forms a layer of platelets and fibrin from the edge of the damaged area. The SMC in the inner layer undergoes phenotypic regulation from a static contraction state to a synthesis stage, similar to fibroblast migration towards the endometrium. The intima further thickens by secreting an extracellular matrix containing elastin, collagen, glycoprotein, and proteoglycan. Highly proliferative fibroblasts migrate from the adventitia to the endometrium and differentiate into myofibroblasts, promoting intima thickening. This process starts at the anastomotic site of the transplanted blood vessels and extends to the entire SVG over time (21).
As a result, aspirin combined with clopidogrel/ticagrelor DAPT has been widely used as a routine secondary preventive medication after CABG surgery. Several studies (since 1980s) have shown that aspirin can improve SVG patency (22). For instance, a meta-analysis with 25,728 patients showed that clopidogrel combined with aspirin can lower the incidence of early SVG failure [hazard ratio; 0.59 (95% CI, 0.43–0.82); P = 0.02] and a 30-day mortality rate (0.8% vs. 1.9%; P < 0.0001) (23). Compared with aspirin alone, CRYSSA trial showed that aspirin combined with clopidogrel can significantly reduce the occlusion rate of 12-month SVG in off-pump CABG patients (24). Furthermore, DACAB trial showed that ticagrelor + aspirin can increase SVG patency (ticagrelor + aspirin = 88.7% and aspirin alone = 76.5%, P < 0.001) one year after CABG surgery (10). However, 75.8% of patients in the DACAB trial received off-pump CABG, indicating that the results may mainly apply to off-pump CABG patients.
Two recent randomized controlled trials have also shown that the efficacy of off-pump CABG and on-pump CABG is not significantly different (2, 3). Extracorporeal circulation may impact the coagulation and platelet function of patients. To the best of our knowledge, this is the first clinical study to compare the effects of different DAPT strategies on SVG after off-pump CABG surgery. Patient recruitment was originally expected to be completed before April 2021. However, nearly half of the patients who met the recruitment criteria refused to participate due to various factors, such as unwillingness to sign informed consent and cooperate with follow-up. Additionally, COVID-19 affected patient recruitment and follow-up, and thus the study was completed much earlier. Nonetheless, this single-center, prospective, randomized, double-blind clinical controlled trial indicated that aspirin combined with ticagrelor DAPT after CABG can better maintain the patency of vein grafts one year after surgery. The findings indicated that ticagrelor may be a uni- and multivariate predictor for reducing occlusion of vein grafts.
Clopidogrel is a precursor drug metabolized by cytochrome P450 and an irreversible P2Y12 inhibitor. Clopidogrel can inhibit diphosphate-induced platelet aggregation, selectively inhibit platelet cyclooxygenase-1, and interrupt the formation of thromboxane A2 (17). Unlike clopidogrel, Ticagrelor reversibly binds directly, and requires two steps of cyp1-dependent metabolism (25), indicating that ticagrelor has a more substantial anti-platelet effect. Some scholars have in recent years conducted research on the effectiveness of aspirin combined with clopidogrel/ticagrelor DAPT strategies through randomized controlled trials with 147 CABG patients and shown that ticagrelor combined with clopidogrel does not significantly affect the patency of SVG (91.0% vs. 89.9% P = 0.751) (26). However, 69.4% of patients underwent on-pump CABG, indicating that the conclusions are not applicable to simple off-pump CABG patients (26). Additionally, more extensive sample studies are needed for further verification since this was a single-center study.
Previous PLATO studies showed that ticagrelor has a better therapeutic effect on acute coronary syndrome than clopidogrel. Furthermore, PLATO studies indicated that ticagrelor can significantly reduce the incidence of main cardiovascular death, myocardial infarction, and other serious adverse cardiovascular events during the one-year follow-up (9.8% vs. 11.7%, P < 0.001) (27). Ticagrelor also significantly reduced cardiovascular event mortality in the CABG subgroup evaluation (4.1% vs. 7.9%, p < 0.01) (8). Notably, increased postoperative LVD and decreased LVEF may be predictive factors for the prognosis (28). In this study, ticagrelor did not significantly impact postoperative LVD and LVEF, possibly due t small sample size. Moreover, there was low overall incidence of MACE events during the one-year follow-up, necessitaing further studies for validation.
Compared with aspirin alone, DATP can increase the risk of hemorrhage incidents (29). However, some scholars have shown that ticagrelor may have a higher risk of bleeding than clopidogrel (30). Overall, ticagrelor is usually limited to minor bleeding risk events, consistent with previous studies (31).
Limitation
This was a single-center study. Although we enrolled participants according to the pre-determined sample size, the rate of venous graft stenosis/occlusion in Group C was lower than anticipated. In the initial design phase, we anticipated this possibility and planned to include more paticipants to ensure adequate statistical power. However, the recruitment was prematurely discontinued due to COVID-19. Consequently, further validation of these findings may require a larger-scale study. Additionally, this study did not perform clopidogrel genotype testing, patient genotype analysis, or gene-related analyses.
Conclusion
Compared with clopidogrel, ticagrelor treatment after CABG can significantly reduce the occlusion rate of grafts. Furthermore, postoperative ticagrelor combined with aspirin DAPT can be a predictive factor for improving the patency of vein grafts.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: the study was registered at the Clinical Trial Center in China (Registration no. ChiCTR1900022390. Date of first registration: 09/04/2019).
Ethics statement
The studies involving humans were approved by Ethics Committee of Dalian Central Hospital (Ethics approval number: YN-2019-018-01). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
WW: Formal analysis, Writing – original draft, Writing – review & editing. QC: Data curation, Formal analysis, Writing – review & editing. FG: Conceptualization, Writing – original draft. XH: Data curation, Writing – original draft. YG: Data curation, Writing – review & editing. LS: Writing – original draft, Data curation, Investigation. WL: Writing – original draft, Investigation. WF: Writing – review & editing, Investigation. LZ: Formal analysis, Writing – review & editing. CX: Writing – review & editing. XZ: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Key R&D Program of China (sub-project) (2023YFB3810102), the Liaoning Province Doctoral Research Start-up Fund Project (2022-BS-357), the Dalian Science and Technology Innovation Fund for Basic Application Project in the Field of Life and Health (2023JJ12SN03), the Dalian Medical Science Research Program (2211002).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, body mass index; CABG, coronary artery bypass graft surgery; COVID-19, coronavirus disease 2019; CTA, computed tomography angiography; DAPT, dual anti-platelet therapy; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LIMA, left internal mammary artery; LVD, left ventricular diameter; LVEF, left ventricular diameter; MACE, major adverse cardiac events; PCI, percutaneous transluminal coronary intervention; PDGF, platelet derived growth factor; RCA, right coronary artery; SMC, smooth muscle cell; SVG, saphenous vein graft.
References
1.
LiuJFanRLiC-lLiuY-qLiuD-hLiWet alPredictive value of left ventricular dyssynchrony for short-term outcomes in three-vessel disease patients undergoing coronary artery bypass grafting with preserved or mildly reduced left ventricular ejection fraction. Front Cardiovasc Med. (2022) 9:1036780. 10.3389/fcvm.2022.1036780
2.
DiegelerABörgermannJKappertUHilkerMDoenstTBöningAet alFive-year outcome after off-pump or on-pump coronary artery bypass grafting in elderly patients. Circulation. (2019) 139(16):1865–71. 10.1161/CIRCULATIONAHA.118.035857
3.
LamyADevereauxPJPrabhakaranDTaggartDPHuSStrakaZet alFive-year outcomes after off-pump or on-pump coronary-artery bypass grafting. N Engl J Med. (2016) 375(24):2359–68. 10.1056/NEJMoa1601564
4.
XenogiannisIZenatiMBhattDLRaoSVRodés-CabauJGoldmanSet alSaphenous vein graft failure: from pathophysiology to prevention and treatment strategies. Circulation. (2021) 144(9):728–45. 10.1161/CIRCULATIONAHA.120.052163
5.
BrilakisESO’DonnellCIPennyWArmstrongEJTsaiTMaddoxTMet alPercutaneous coronary intervention in native coronary arteries versus bypass grafts in patients with prior coronary artery bypass graft surgery. JACC: Cardiovasc Interv. (2016) 9(9):884–93. 10.1016/j.jcin.2016.01.034
6.
AntonopoulosASOdutayoAOikonomouEKTrivellaMPetrouMCollinsGSet alDevelopment of a risk score for early saphenous vein graft failure: an individual patient data meta-analysis. J Thorac Cardiovasc Surg. (2020) 160(1):116–127.e114. 10.1016/j.jtcvs.2019.07.086
7.
KulikARuelMJneidHFergusonTBHiratzkaLFIkonomidisJSet alSecondary prevention after coronary artery bypass graft surgery. Circulation. (2015) 131(10):927–64. 10.1161/CIR.0000000000000182
8.
HeldCÅsenbladNBassandJPBeckerRCCannonCPClaeysMJet alTicagrelor versus clopidogrel in patients with acute coronary syndromes undergoing coronary artery bypass surgery. J Am Coll Cardiol. (2011) 57(6):672–84. 10.1016/j.jacc.2010.10.029
9.
SawJWongGCMayoJBernsteinVManciniGBJYeJet alTicagrelor and aspirin for the prevention of cardiovascular events after coronary artery bypass graft surgery. Heart. (2016) 102(10):763–9. 10.1136/heartjnl-2015-308691
10.
ZhaoQZhuYXuZChengZMeiJChenXet alEffect of ticagrelor plus aspirin, ticagrelor alone, or aspirin alone on saphenous vein graft patency 1 year after coronary artery bypass grafting. JAMA. (2018) 319(16):1677–86. 10.1001/jama.2018.3197
11.
WillemsenLMJanssenPWAPeperJSoliman-HamadMAvan StratenAHMKleinPet alEffect of adding ticagrelor to standard aspirin on saphenous vein graft patency in patients undergoing coronary artery bypass grafting (POPular CABG). Circulation. (2020) 142(19):1799–807. 10.1161/CIRCULATIONAHA.120.050749
12.
NeumannF-JSousa-UvaMAhlssonAAlfonsoFBanningAPBenedettoUet al2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. (2019) 40(2):87–165. 10.1093/eurheartj/ehy394
13.
Association WM. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 320(20):2191–4. 10.1001/jama.2013.281053
14.
FitzgibbonGMKafkaHPLeachAJKeonWJHooperGDBurtonJR. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5, 065 grafts related to survival and reoperation in 1, 388 patients during 25 years. J Am Coll Cardiol. (1996) 28(3):616–26. 10.1016/0735-1097(96)00206-9
15.
HicksKATchengJEBozkurtBChaitmanBRCutlipDEFarbAet al2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials. J Am Coll Cardiol. (2015) 66(4):403–69. 10.1016/j.jacc.2014.12.018
16.
MehranRRaoSVBhattDLGibsonCMCaixetaAEikelboomJet alStandardized bleeding definitions for cardiovascular clinical trials. Circulation. (2011) 123(23):2736–47. 10.1161/CIRCULATIONAHA.110.009449
17.
GaoGZhengZPiYLuBLuJHuS. Aspirin plus clopidogrel therapy increases early venous graft patency after coronary artery bypass surgery a single-center, randomized, controlled trial. J Am Coll Cardiol. (2010) 56:1639–43. 10.1016/j.jacc.2010.03.104
18.
IbrahimKTjomslandOHalvorsenDWisethRWahbaAKarevoldAet alEffect of clopidogrel on midterm graft patency following off-pump coronary revascularization surgery. Heart Surg Forum. (2006) 9(6):E581–856. 10.1532/HSF98.20061034
19.
ZenatiMABhattDLBakaeenFGStockEMBiswasKGazianoJMet alREGROUP trial investigators. Randomized trial of endoscopic or open vein-graft harvesting for coronary-artery bypass. N Engl J Med. (2019) 380(2):132–41. 10.1056/NEJMoa1812390
20.
MehtaRHFergusonTBLopesRDHafleyGEMackMJKouchoukosNTet alSaphenous vein grafts with multiple versus single distal targets in patients undergoing coronary artery bypass surgery. Circulation. (2011) 124(3):280–8. 10.1161/CIRCULATIONAHA.110.991299
21.
GaudinoMDi FrancoAAlexanderJHBakaeenFEgorovaNKurlanskyPet alSex differences in outcomes after coronary artery bypass grafting: a pooled analysis of individual patient data. Eur Heart J. (2022) 43(1):18–28. 10.1093/eurheartj/ehab504
22.
MylesPSSmithJAForbesASilbertBJayarajahMPainterTet alStopping vs. Continuing aspirin before coronary artery surgery. N Engl J Med. (2016) 374(8):728–37. 10.1056/NEJMoa1507688
23.
BhattDLChewDPHirschATRinglebPAHackeWTopolEJ. Superiority of clopidogrel versus aspirin in patients with prior cardiac surgery. Circulation. (2001) 103(3):363–8. 10.1161/01.CIR.103.3.363
24.
MannacioVADi TommasoLAntignanADe AmicisVVosaC. Aspirin plus clopidogrel for optimal platelet inhibition following off-pump coronary artery bypass surgery: results from the CRYSSA (prevention of coronary arteRY bypaSS occlusion after off-pump procedures) randomised study. Heart. (2012) 98(23):1710–5. 10.1136/heartjnl-2012-302449
25.
GimbelMQaderdanKWillemsenLHermanidesRBergmeijerTde VreyEet alClopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet. (2020) 395(10233):1374–81. 10.1016/S0140-6736(20)30325-1
26.
TangYFanXZhangBZhangJXueQXuZet alAspirin plus ticagrelor or clopidogrel on graft patency one year after coronary bypass grafting: a single-center, randomized, controlled trial. J Thorac Dis. (2021) 13(3):1697–705. 10.21037/jtd-20-3407
27.
WallentinLBeckerRCBudajACannonCPEmanuelssonHHeldCet alTicagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J. (2009) 360(11):1045–57. 10.1056/NEJMoa0904327
28.
GlaveckaiteSValevicieneNPalionisDPuronaiteRSerpytisPLauceviciusA. Prediction of long-term segmental and global functional recovery of hibernating myocardium after revascularisation based on low dose dobutamine and late gadolinium enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. (2014) 16(1):83. 10.1186/s12968-014-0083-z
29.
SandnerSRedforsBAngiolilloDJAudisioKFremesSEJanssenPWAet alAssociation of dual anti-platelet therapy with ticagrelor with vein graft failure after coronary artery bypass graft surgery. Jama. (2022) 328(6):554–562. 10.1001/jama.2022.11966
30.
DiNicolantonioJJD'AscenzoFTomekAChatterjeeSNiaziAKBiondi-ZoccaiG. Clopidogrel is safer than ticagrelor in regard to bleeds: a closer look at the PLATO trial. Int J Cardiol. (2013) 168(3):1739–44. 10.1016/j.ijcard.2013.06.135
31.
HanssonECRexiusHDellborgMAlbertssonPJeppssonA. Coronary artery bypass grafting-related bleeding complications in real-life acute coronary syndrome patients treated with clopidogrel or ticagrelor†. Eur J Cardiothorac Surg. (2014) 46(4):699–705. 10.1093/ejcts/ezt662
Summary
Keywords
coronary artery bypass surgery, off-pump, saphenous vein graft, ticagrelor, clopidogrel
Citation
Wang W, Chi Q, Gao F, He X, Gao Y, Shi L, Liu W, Fan W, Zhang L, Xu C and Zhuang X (2025) Ticagrelor combined with aspirin may improve patency of vein graft one year after off-pump coronary artery bypass grafting: a single-center, randomized double-blinded clinical controlled trial. Front. Cardiovasc. Med. 12:1461370. doi: 10.3389/fcvm.2025.1461370
Received
08 July 2024
Accepted
24 April 2025
Published
09 May 2025
Volume
12 - 2025
Edited by
Tommaso Gori, Johannes Gutenberg University Mainz, Germany
Reviewed by
Praveen Varma, Amrita Vishwa Vidyapeetham University, India
Ricardo Adrian Nugraha, Faculty of Medicine Universitas Airlangga—Dr. Soetomo General Hospital, Indonesia
Updates
Copyright
© 2025 Wang, Chi, Gao, He, Gao, Shi, Liu, Fan, Zhang, Xu and Zhuang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xijing Zhuang dlmchcsd@126.com
ORCID Xijing Zhuang orcid.org/0000-0002-0805-6928
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.