Abstract
Vasovagal syncope (VVS) is the most common cause of transient loss of consciousness in children and adolescents, accounting for 60%–80% of syncope cases. This review synthesizes current evidence on pediatric VVS, focusing on advances in diagnosis, management, and long-term outcomes. Through a comprehensive literature search of studies published between 2001 and 2024, we analyzed epidemiological patterns, pathophysiological mechanisms, diagnostic approaches, management strategies, and prognostic factors. Recent diagnostic advances include implantable loop recorders and smartphone applications, which have improved diagnostic accuracy. Management has evolved toward individualized approaches, emphasizing non-pharmacological interventions (hydration, salt supplementation, physical counterpressure maneuvers) as first-line treatment, with medications such as midodrine and fludrocortisone showing variable efficacy in refractory cases. Long-term studies indicate that while most children experience improvement over time, 33%–50% have recurrent episodes within three years, with factors such as lower mean arterial pressure, higher urine specific gravity, younger age, family history of syncope, and lower body mass index associated with increased recurrence risk. Though generally benign, VVS can significantly impact quality of life and carries substantial psychosocial consequences. Future research should focus on developing predictive models for recurrence risk and exploring personalized treatment approaches to improve outcomes.
1 Introduction
Vasovagal syncope (VVS) represents the predominant etiology of transient loss of consciousness in pediatric and adolescent populations, accounting for approximately 60%–80% of syncope cases (1–3). Characterized by an abrupt, temporary loss of consciousness resulting from global cerebral hypoperfusion, VVS manifests as a consequence of an exaggerated autonomic response to various triggers, precipitating bradycardia and/or peripheral vasodilation (4).
Despite its prevalence, the diagnosis and management of pediatric VVS present significant challenges. The clinical presentation varies considerably, and the differential diagnosis encompasses a spectrum of conditions from benign neurocardiogenic syndromes to potentially life-threatening cardiac conditions (5). Recent advances in diagnostic modalities and management strategies have improved outcomes, yet significant knowledge gaps persist regarding long-term prognosis and optimal treatment approaches for refractory cases (6, 7).
This review aims to synthesize current evidence on pediatric VVS, highlighting recent advances in diagnosis and management while identifying knowledge gaps that should guide future research.
2 Methods
We conducted a comprehensive literature search following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Electronic databases including PubMed/MEDLINE, EMBASE, and the Cochrane Library were searched for articles published between January 2001 and March 2024. The search strategy employed keywords and Medical Subject Headings (MeSH) terms including “vasovagal syncope,” “neurocardiogenic syncope,” “pediatric,” “children,” “adolescents,” “diagnosis,” “management,” and “outcomes” in various combinations.
Inclusion criteria were: (1) studies focusing on VVS in pediatric populations (age ≤18 years); (2) publications in English; (3) studies addressing aspects of diagnosis, management, or outcomes. We prioritized original research, systematic reviews, meta-analyses, and evidence-based clinical guidelines. After initial screening titles and abstracts, relevant articles underwent full-text review by two independent reviewers, with disagreements resolved through consensus discussion.
Due to the heterogeneity of study designs and outcome measures, we employed a narrative synthesis approach, organizing evidence according to key themes including epidemiology, pathophysiology, diagnosis, management, and long-term outcomes, with emphasis on pediatric-specific considerations and recent advances.
3 Results
3.1 Literature search results
The literature search process and results are summarized in Figure 1. Initial database searches yielded 1,245 potentially relevant citations. After removing duplicates (n = 215), 1,030 articles were screened based on titles and abstracts. Of these, 728 were excluded for not meeting inclusion criteria. The remaining 302 articles underwent full-text review, resulting in 175 articles being excluded for various reasons (not specific to pediatric population, not focused on VVS, or being case reports with limited generalizability). Ultimately, 127 articles were included in this comprehensive review.
Figure 1
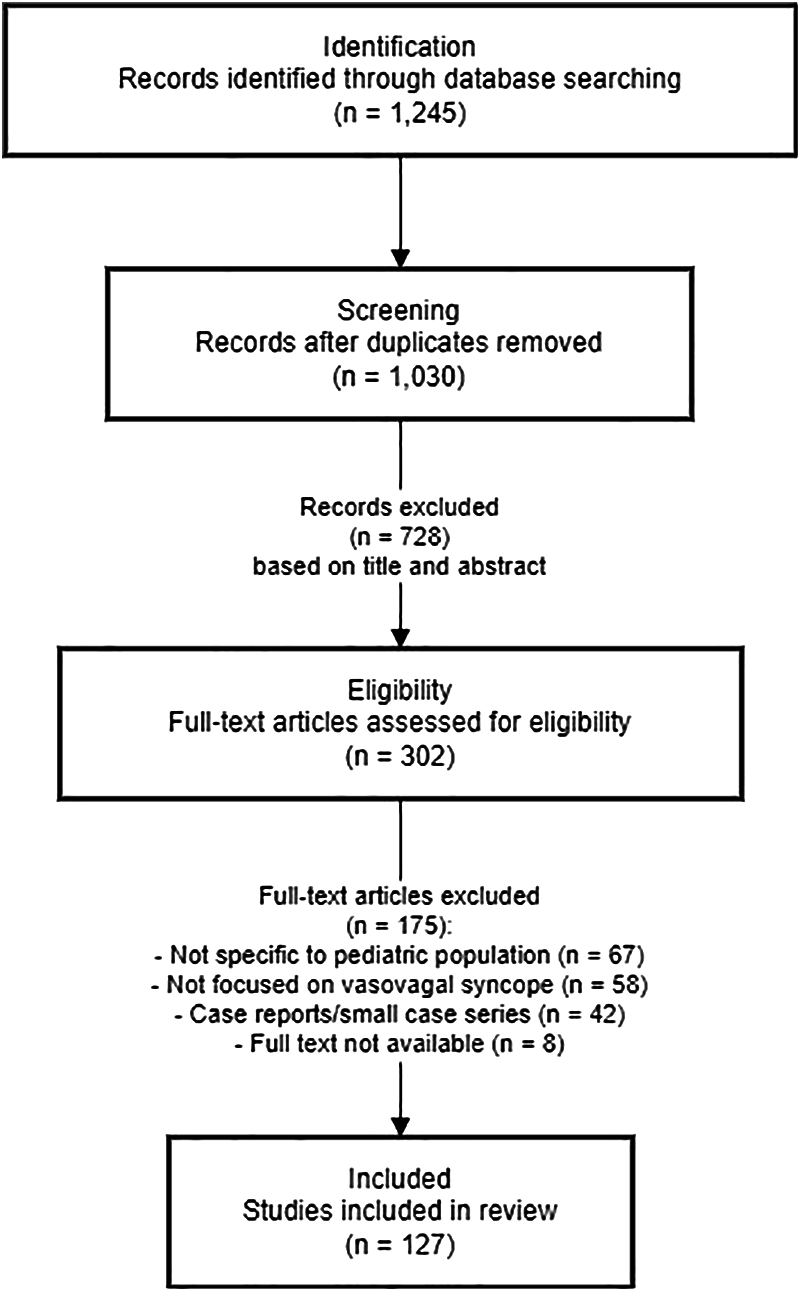
Flow diagram of literature search and selection process.
3.2 Epidemiological patterns of pediatric VVS
Our analysis of epidemiological studies revealed significant patterns in the prevalence and distribution of VVS in children and adolescents. VVS accounts for 60%–80% of syncope cases in pediatric populations (1–3), with syncope representing 1%–3% of emergency department visits and having an overall incidence of 0.1%–0.5% in the pediatric population (8).
Age-specific patterns show that the incidence of VVS increases dramatically during adolescence, peaking between ages 15–19 years (2). Population-based studies demonstrate a notable gender disparity, with a cumulative incidence of 41% for females and 28% for males (9). Female sex is consistently identified as a significant risk factor, with girls being 1.5–2 times more likely to experience VVS than boys (10, 11).
Additional predisposing factors include family history of syncope, low body mass index, anxiety disorders and orthostatic intolerance (12, 13). Environmental triggers such as dehydration, prolonged standing, and exposure to hot environments are commonly identified (4), while a sedentary lifestyle and poor physical conditioning may increase susceptibility (14).
The recurrence rate of VVS in children and adolescents is substantial. Studies report that 33%–50% of patients experience at least one recurrent episode within three years of the initial event (15). However, the frequency and severity of episodes tend to decrease over time, with many patients experiencing improvement by early adulthood (16).
Despite its generally benign nature, VVS carries a significant economic burden, estimated at several hundred million dollars annually in the United States (17), reflecting costs associated with emergency department visits, hospitalizations, and diagnostic evaluations.
3.3 Pathophysiological mechanisms underlying pediatric VVS
The pathophysiology of VVS involves complex interactions between cardiovascular, neurological, and neuroendocrine systems. The fundamental process involves a sudden, inappropriate decrease in systemic blood pressure and/or heart rate, leading to transient global cerebral hypoperfusion (18).
The classic model centers on the Bezold-Jarisch reflex, a cardio-inhibitory response triggered by mechanoreceptors (C-fibers) in the left ventricle (19). In susceptible individuals, various stimuli such as prolonged standing or emotional stress lead to excessive venous pooling and reduced venous return. This results in vigorous contraction of an underfilled left ventricle, activating mechanoreceptors and initiating the reflex (18).
The activated reflex causes paradoxical bradycardia and vasodilation through increased parasympathetic activity and withdrawal of sympathetic tone (20). This response is mediated by the nucleus tractus solitarius in the brainstem, which receives afferent signals from mechanoreceptors and modulates efferent autonomic outflow (21).
Recent research has highlighted the role of impaired baroreflex sensitivity in VVS. Studies have demonstrated that children with recurrent VVS exhibit reduced baroreflex sensitivity compared to healthy controls, suggesting dysfunction in blood pressure homeostasis (22).
Neuroendocrine factors also contribute to VVS pathophysiology. Elevated levels of epinephrine have been observed during syncopal episodes, potentially exacerbating vasodilation (23). Alterations in serotonergic neurotransmission have been implicated in modulating central autonomic responses (24).
Genetic factors may predispose certain individuals to VVS, with familial clustering observed and potential genetic loci identified (13). Age-related differences in autonomic function, particularly during puberty, may explain the increased prevalence in adolescents. Puberty is associated with significant changes in cardiovascular autonomic regulation, including alterations in baroreflex sensitivity and sympathovagal balance (25).
3.4 Advances in diagnostic approaches for pediatric VVS
The diagnosis of VVS in children requires a comprehensive approach, combining careful history-taking, physical examination, and selective use of diagnostic tests. The primary goal is to differentiate VVS from other potentially life-threatening causes of syncope (26).
A detailed clinical history remains the cornerstone of VVS diagnosis. Key elements include identifying precipitating factors such as prolonged standing or emotional stress (4); recognizing prodromal symptoms like lightheadedness, nausea, and visual changes (27); characterizing the event, including duration and rapid recovery (28); and assessing post-syncopal such as fatigue and nausea (29).
Physical examination should include comprehensive cardiovascular assessment (heart rate, blood pressure, orthostatic measurements) and neurological examination to exclude other etiologies (5).
While diagnosis is often based on clinical presentation, several diagnostic tests may be employed. A 12-lead ECG is recommended for all children presenting with syncope to rule out cardiac causes such as long QT syndrome or Brugada syndrome (2). Echocardiography may be indicated when structural heart disease is suspected based on history, examination, or ECG findings (30).
The Head-Up Tilt Table Test (HUTT) is considered the gold standard for diagnosing VVS, with a sensitivity of 60%–70% and specificity of 90%–95% in pediatric populations (31, 32). The test involves passive standing on a tilt table at a 60–70 degree angle for up to 45 min, with continuous monitoring of heart rate and blood pressure. A positive test is defined as the reproduction of syncope or pre-syncope associated with hypotension and/or bradycardia (33).
Recent technological advances have expanded the diagnostic arsenal. Implantable loop recorders (ILRs) allow for long-term cardiac monitoring in cases of recurrent, unexplained syncope. A study by Placidi et al. demonstrated the effectiveness of miniaturized ILRs in pediatric patients, enabling correlation of symptoms with cardiac rhythm (34). Exercise stress testing may be useful in cases of exertion-related syncope (35).
Smartphone-based ECG applications represent another significant advancement, providing non-invasive, cost-effective means of arrhythmia detection (36). These technologies facilitate ambulatory monitoring and may improve diagnostic yield in cases where conventional monitoring is inconclusive.
Diagnostic challenges in pediatric VVS include variability in presentation across different age groups (37), difficulty obtaining clear history from younger children (38), and symptom overlap with other conditions, particularly anxiety disorders (39). Careful consideration of differential diagnoses, including cardiac syncope, neurological causes such as seizures, orthostatic hypotension, and psychogenic pseudosyncope, is essential (40, 41).
3.5 Evolution of management strategies for pediatric VVS
Management of VVS in pediatric patients has evolved toward a multifaceted, individualized approach based on clinical presentation, episode frequency, and impact on quality of life (26, 42).
Non-pharmacological interventions form the cornerstone of VVS management. Comprehensive patient and family education is essential, focusing on trigger identification and avoidance (43). Optimizing hydration (>2 L/day) and salt intake (>6 g/day) has shown effectiveness, particularly in patients with orthostatic intolerance (44). Physical counterpressure maneuvers, involving isometric muscle contractions to augment venous return during prodromal symptoms, have demonstrated efficacy in reducing syncope recurrence (45).
Structured exercise programs focusing on aerobic conditioning and lower limb strength training have shown promise. A randomized controlled trial reported that yoga as an adjunctive therapy significantly reduced syncopal and presyncopal events and improved quality of life compared to standard therapy alone (46). Orthostatic training (tilt training), involving progressively prolonged periods of upright posture, has also demonstrated effectiveness. Clinical experience suggests that these interventions pose no harm and may improve outcomes and reduce the frequency of episodes (47).
Pharmacological interventions are typically reserved for patients with frequent, severe episodes or those refractory to non-pharmacological measures. Fludrocortisone, a mineralocorticoid promoting sodium and water retention, has been studied in children with VVS. However, the multicenter Prevention of Syncope Trial (POST) 2 did not meet its primary objective of demonstrating significant reduction in syncope recurrence compared to placebo (48).
Midodrine, an α1-adrenergic agonist increasing peripheral vascular resistance, has shown more promise. A randomized controlled study in children with VVS demonstrated that the addition of midodrine hydrochloride to conventional therapy significantly reduced syncope recurrence rates compared to conventional therapy alone, with a significantly lower recurrence rate during follow-up periods of at least 6 months (49). Beta-blockers, while commonly prescribed, have demonstrated limited efficacy. A randomized controlled trial comparing metoprolol to conventional treatment in children and adolescents found no significant difference in syncope recurrence (50).
Selective serotonin reuptake inhibitors (SSRIs) may modulate central autonomic responses (51), but pediatric data are limited. Invasive interventions, including cardiac pacing with rate-drop response algorithms and catheter ablation of ganglionated plexus (52–54), are rarely indicated in pediatric VVS and reserved for severe, refractory cases (55).
A stepwise approach to management is recommended, beginning with education and lifestyle modifications, progressing to structured exercise programs, and considering pharmacological therapy for frequent or severe cases.
Regular follow-up is essential to assess treatment efficacy and adjust management strategies. Objective measures, such as the Calgary Syncope Symptom Score or quality of life assessments, can be utilized to quantify clinical improvement (56).
Special considerations in pediatric VVS management include addressing the psychological burden through cognitive-behavioral therapy or other psychological interventions (57), and individualized risk stratification for young athletes (58).
Several novel approaches are under investigation for refractory VVS, such as ivabradine (a selective If channel inhibitor) (59). Given the benign nature of VVS, cost-effectiveness should be a consideration in management decisions, with a conservative approach being most cost-effective for initial management (60).
3.6 Long-term outcomes and prognostic factors in pediatric VVS
The prognosis of VVS in children is generally favorable, with most patients experiencing improvement or resolution of symptoms over time. However, natural history and long-term outcomes vary significantly among individuals.
The natural course of pediatric VVS tends toward spontaneous improvement. A study of 29 pediatric patients with neurocardiogenic syncope found that clinical events were greatly reduced in both treated and untreated groups during follow-up, with recurrences becoming unlikely after 24 months (61). This improvement is thought to be related to physiological maturation of the autonomic nervous system and adaptation to orthostatic stress.
Despite the overall favorable prognosis, recurrence rates remain substantial. In a study of 352 children with VVS followed for a median of 22 months, factors associated with an increased risk of recurrence included lower mean arterial pressure in the supine position, higher urine specific gravity, younger age, family history of syncope, and lower body mass index (62). The study developed a prognostic nomogram model incorporating these factors, which showed good predictive ability for 1-year, 2-year, and 3-year recurrence rates.
While not life-threatening, VVS can significantly impact quality of life. A cross-sectional study using the PedsQL™ 4.0 Generic Core Scales found that children with recurrent VVS had lower scores in physical, emotional, and social functioning compared to healthy controls (63). However, with appropriate management and patient education, most children can achieve good symptom control and maintain normal activities (64).
There is no evidence suggesting that childhood VVS is associated with increased cardiovascular morbidity or mortality in adulthood. A large population-based cohort study with a median follow-up of 17 years found no increased risk of major adverse cardiovascular events in individuals with VVS history compared to the general population (65).
The psychological impact of VVS in children and adolescents should not be underestimated. A prospective case-control study found significantly higher rates of psychopathology in VVS patients compared to controls, with 21.3% meeting criteria for major depressive disorder (vs. 2% of controls) and 19.1% diagnosed with social anxiety disorder, generalized anxiety disorder, or conversion disorder (57). Early intervention and psychological support may mitigate these long-term psychosocial effects.
Several factors predict long-term outcomes in pediatric VVS. A higher number of syncopal episodes prior to diagnosis predicts a more protracted course (15, 66), while early improvement with conservative measures suggests favorable long-term outcomes (45).
For young athletes with VVS, prognosis is generally good with appropriate management. Many are able to return to competitive activities after proper diagnosis and treatment (67). Children with a predominantly cardioinhibitory response on tilt testing may have higher risk of recurrent syncope and injury (68), but long-term studies have not demonstrated increased risk of sudden cardiac death in this subgroup (65).
4 Discussion
This comprehensive review highlights significant advances in understanding and managing pediatric VVS, while also identifying persistent challenges and knowledge gaps. Our findings underscore the multifaceted nature of VVS in children and adolescents, requiring individualized diagnostic and management approaches.
The epidemiological data presented confirm that VVS represents a significant health burden in pediatric populations, with a notable peak during adolescence and higher prevalence in females. This gender disparity warrants further investigation into potential hormonal and physiological factors that may contribute to increased susceptibility in girls.
Recent diagnostic advances, including implantable loop recorders and smartphone-based ECG applications, have expanded the toolkit for clinicians evaluating syncope in children. However, these technologies should complement rather than replace thorough clinical assessment. The traditional approach of detailed history-taking and physical examination remains the cornerstone of diagnosis, with technological adjuncts providing valuable confirmatory evidence.
Our review of management strategies reveals a paradigm shift toward non-pharmacological interventions as first-line therapy. The demonstrated efficacy of hydration, salt supplementation, physical counterpressure maneuvers, and structured exercise programs supports a conservative initial approach. The variable efficacy of pharmacological options suggests the need for improved patient selection criteria to identify those most likely to benefit from medication.
The long-term outcome data present a generally reassuring picture, with most children experiencing improvement over time. However, the substantial recurrence rate (33%–50% within three years) and identified risk factors for recurrence highlight the need for prognostic tools to guide monitoring and management intensity. The development of validated prediction models, such as the nomogram described by Sun et al. (62), represents a promising step toward personalized risk stratification.
Several important limitations in current evidence merit consideration. First, many studies have relatively short follow-up periods, limiting understanding of very long-term outcomes extending into adulthood. Second, heterogeneity in outcome measures across studies complicates direct comparison of intervention efficacy. Third, most studies have not addressed the impact of comorbidities, particularly psychological conditions, on VVS presentation and management.
Future research should focus on several key areas. Genetic and molecular studies may identify specific markers for VVS susceptibility, enabling targeted therapies. Longitudinal studies with extended follow-up are needed to elucidate the natural history into adulthood. Randomized controlled trials of emerging therapies, particularly in refractory cases, would address significant treatment gaps. Finally, standardization of outcome measures across studies would facilitate meta-analysis and strengthen evidence-based recommendations.
From a clinical perspective, our findings support a stepwise, individualized approach to VVS management, beginning with education and lifestyle modifications before considering pharmacological interventions. The significant psychosocial impact of VVS underscores the importance of addressing both physical and psychological aspects of the condition. Multidisciplinary collaboration among cardiologists, neurologists, psychologists, and primary care providers would optimize comprehensive care.
5 Conclusion
This review synthesizes current evidence on pediatric VVS, highlighting advances in diagnosis, management, and understanding of long-term outcomes. While technological innovations have improved diagnostic capabilities, and evidence increasingly supports non-pharmacological interventions as first-line therapy, significant challenges remain in predicting individual outcomes and managing refractory cases.
VVS in children represents a complex clinical entity requiring individualized assessment and management. Although generally benign with favorable long-term prognosis, its impact on quality of life and potential psychological consequences necessitate comprehensive care approaches.
Future research should focus on developing robust prediction models for recurrence risk, evaluating emerging therapeutic options, and establishing standardized outcome measures to strengthen the evidence base. By bridging the gap between scientific advances and clinical practice, we can continue to improve outcomes for the many children and adolescents affected by VVS.
Statements
Author contributions
WZ: Conceptualization, Investigation, Writing – original draft. XB: Data curation, Investigation, Writing – review & editing. JL: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the Shandong Provincial Hospital Affiliated to Shandong First Medical University and the Shandong Provincial Clinical Research Center for Children's Health and Disease office for their support. We appreciate the assistance of colleagues in the Department of Pediatric Cardiology and the library staff for accessing relevant literature.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Massin MM Bourguignont A Coremans C Comte L Lepage P Gerard P . Syncope in pediatric patients presenting to an emergency department. J Pediatr. (2004) 145:223–8. 10.1016/j.jpeds.2004.01.048
2.
Fischer JWJ Cho CS . Pediatric syncope: cases from the emergency department. Emerg Med Clin North Am. (2010) 28:501–16. 10.1016/j.emc.2010.03.009
3.
Kanjwal K Calkins H . Syncope in children and adolescents. Cardiol Clin. (2015) 33:397–409. 10.1016/j.ccl.2015.04.008
4.
Stewart JM van Dijk JG Balaji S Sutton R . A framework to simplify paediatric syncope diagnosis. Eur J Pediatr. (2023) 182:4771–80. 10.1007/s00431-023-05114-w
5.
Shen WK Sheldon RS Benditt DG Cohen MI Forman DE Goldberger ZD et al 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. Circulation. (2017) 136:e60–e122. 10.1161/CIR.0000000000000499
6.
McIntyre-Patton L Wanderski S Graef D Woessner L Baker R . Randomized trial evaluating the effectiveness of a leg crossing and muscle tensing technique on decreasing vasovagal symptoms among pediatric and young adult patients undergoing peripheral IV catheter insertion. J Pediatr Nurs. (2018) 38:53–6. 10.1016/j.pedn.2017.09.012
7.
Behnoush AH Yazdani K Khalaji A Tavolinejad H Aminorroaya A Jalali A et al Pharmacologic prevention of recurrent vasovagal syncope: a systematic review and network meta-analysis of randomized controlled trials. Heart Rhythm. (2023) 20:448–60. 10.1016/j.hrthm.2022.12.010
8.
Fant C Cohen A . Syncope in pediatric patients: a practical approach to differential diagnosis and management in the emergency department. Pediatr Emerg Med Pract. (2017) 14:1–28.
9.
Ganzeboom KS Mairuhu G Reitsma JB Linzer M Wieling W van Dijk N . Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35–60 years. J Cardiovasc Electrophysiol. (2006) 17:1172–6. 10.1111/j.1540-8167.2006.00595.x
10.
Ganzeboom KS Colman N Reitsma JB Shen WK Wieling W . Prevalence and triggers of syncope in medical students. Am J Cardiol. (2003) 91:1006–8, A1008. 10.1016/s0002-9149(03)00127-9
11.
Anderson JB Czosek RJ Cnota J Meganathan K Knilans TK Heaton PC . Pediatric syncope: national hospital ambulatory medical care survey results. J Emerg Med. (2012) 43:575–83. 10.1016/j.jemermed.2012.01.020
12.
Ojha A Chelimsky TC Chelimsky G . Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr. (2011) 158:20–3. 10.1016/j.jpeds.2010.07.005
13.
Matveeva N Titov B Bazyleva E Pevzner A Favorova O . Towards understanding the genetic nature of vasovagal syncope. Int J Mol Sci. (2021) 22:10316. 10.3390/ijms221910316
14.
Koene RJ Adkisson WO Benditt DG . Syncope and the risk of sudden cardiac death: evaluation, management, and prevention. J Arrhythm. (2017) 33:533–44. 10.1016/j.joa.2017.07.005
15.
Kouakam C Vaksmann G Pachy E Lacroix D Rey C Kacet S . Long-term follow-up of children and adolescents with syncope; predictor of syncope recurrence. Eur Heart J. (2001) 22:1618–25. 10.1053/euhj.2000.2577
16.
Sheldon RS Sheldon AG Connolly SJ Morillo CA Klingenheben T Krahn AD et al Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. (2006) 17:49–54. 10.1111/j.1540-8167.2005.00267.x
17.
Sun BC Emond JA Camargo CA Jr . Direct medical costs of syncope-related hospitalizations in the United States. Am J Cardiol. (2005) 95:668–71. 10.1016/j.amjcard.2004.11.013
18.
Martone AM Parrini I Ciciarello F Galluzzo V Cacciatore S Massaro C et al Recent advances and future directions in syncope management: a comprehensive narrative review. J Clin Med. (2024) 13:727. 10.3390/jcm13030727
19.
Li HX Gao L Yuan Y . Advance in the understanding of vasovagal syncope in children and adolescents. World J Pediatr. (2021) 17:58–62. 10.1007/s12519-020-00367-z
20.
van Dijk JG Wieling W . Pathophysiological basis of syncope and neurological conditions that mimic syncope. Prog Cardiovasc Dis. (2013) 55:345–56. 10.1016/j.pcad.2012.10.016
21.
Iwase S Nishimura N Mano T . Role of sympathetic nerve activity in the process of fainting. Front Physiol. (2014) 5:343. 10.3389/fphys.2014.00343
22.
Alnoor MS Varner HK Butler IJ Lankford JE Zhu L Numan MT . Arterial baroreceptor physiology: differences between normal subjects and pediatric patients with postural tachycardia and neurocardiogenic syncope. Pediatr Cardiol. (2022) 43:1011–9. 10.1007/s00246-022-02815-1
23.
Stewart JM Medow MS Sutton R Visintainer P Jardine DL Wieling W . Mechanisms of vasovagal syncope in the young: reduced systemic vascular resistance versus reduced cardiac output. J Am Heart Assoc. (2017) 6:e004417. 10.1161/JAHA.116.004417
24.
Brindley RL Bauer MB Blakely RD Currie KPM . Serotonin and serotonin transporters in the adrenal medulla: a potential hub for modulation of the sympathetic stress response. ACS Chem Neurosci. (2017) 8:943–54. 10.1021/acschemneuro.7b00026
25.
Stewart JM . A new guideline for diagnosis and treatment of syncope in children and adolescents that stimulates further thought and discussion. Sci Bull (Beijing). (2018) 63:1527–8. 10.1016/j.scib.2018.09.020
26.
Brignole M Moya A de Lange FJ Deharo JC Elliott PM Fanciulli A et al 2018 ESC guidelines for the diagnosis and management of syncope. Kardiol Pol. (2018) 76:1119–98. 10.5603/KP.2018.0161
27.
Sheldon RS Grubb BP 2nd Olshansky B Shen WK Calkins H Brignole M et al 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. (2015) 12:e41–63. 10.1016/j.hrthm.2015.03.029
28.
Liao Y Du J Benditt DG Jin H . Vasovagal syncope or psychogenic pseudosyncope: a major issue in the differential diagnosis of apparent transient loss of consciousness in children. Sci Bull (Beijing). (2022) 67:1618–20. 10.1016/j.scib.2022.07.024
29.
Wu REY Khan FM Hockin BCD Lobban TCA Sanatani S Claydon VE . Faintly tired: a systematic review of fatigue in patients with orthostatic syncope. Clin Auton Res. (2022) 32:185–203. 10.1007/s10286-022-00868-z
30.
Strickberger SA Benson DW Biaggioni I Callans DJ Cohen MI Ellenbogen KA et al AHA/ACCF scientific statement on the evaluation of syncope: from the American Heart Association councils on clinical cardiology, cardiovascular nursing, cardiovascular disease in the young, and stroke, and the quality of care and outcomes research interdisciplinary working group; and the American College of Cardiology foundation: in collaboration with the heart rhythm society: endorsed by the American autonomic society. Circulation. (2006) 113:316–27. 10.1161/CIRCULATIONAHA.105.170274
31.
Macedo P Leite LR Asirvatham SJ Hachul DT Dos Santos-Neto LL Shen W-K . Head up tilt testing: an appraisal of its current role in the management of patients with syncope. J Atr Fibrillation. (2011) 4:333. 10.4022/jafib.333
32.
Teodorovich N Swissa M . Tilt table test today - state of the art. World J Cardiol. (2016) 8:277–82. 10.4330/wjc.v8.i3.277
33.
Tan MP Duncan GW Parry SW . Head-up tilt table testing: a state-of-the-art review. Minerva Med. (2009) 100:329–38.
34.
Placidi S Drago F Milioni M Verticelli L Tamburri I Silvetti MS et al Miniaturized implantable loop recorder in small patients: an effective approach to the evaluation of subjects at risk of sudden death. Pacing Clin Electrophysiol. (2016) 39:669–74. 10.1111/pace.12866
35.
Bogossian H Alhanafi D Kloppe A Höltgen R Mijic D . Stress testing: a relevant examination in rhythmology. Herzschrittmacherther Elektrophysiol. (2023) 34:333–8. 10.1007/s00399-023-00967-y
36.
Haberman ZC Jahn RT Bose R Tun H Shinbane JS Doshi RN et al Wireless smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol. (2015) 26:520–6. 10.1111/jce.12634
37.
Longo S Legramante JM Rizza S Federici M . Vasovagal syncope: an overview of pathophysiological mechanisms. Eur J Intern Med. (2023) 112:6–14. 10.1016/j.ejim.2023.03.025
38.
Villafane J Miller JR Glickstein J Johnson JN Wagner J Snyder CS et al Loss of consciousness in the young child. Pediatr Cardiol. (2021) 42:234–54. 10.1007/s00246-020-02498-6
39.
Donmez YN Giray D Epcacan S Yalcin SS . Comorbidity of behavioral problems and parental acceptance-rejection in children diagnosed with chest discomfort, palpitations, vasovagal syncope, and underlying heart disease: a multiple case-control study. BMC Psychiatry. (2024) 24:70. 10.1186/s12888-024-05527-3
40.
Task Force for the Diagnosis and Management of Syncope; European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA); Heart Failure Association (HFA); Heart Rhythm Society (HRS); Moya A, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. (2009) 30:2631–71. 10.1093/eurheartj/ehp298
41.
Raj V Rowe AA Fleisch SB Paranjape SY Arain AM Nicolson SE . Psychogenic pseudosyncope: diagnosis and management. Auton Neurosci. (2014) 184:66–72. 10.1016/j.autneu.2014.05.003
42.
Ali M Pachon Maetos JC Kichloo A Masudi S Grubb BP Kanjwal K . Management strategies for vasovagal syncope. Pacing Clin Electrophysiol. (2021) 44:2100–8. 10.1111/pace.14402
43.
van Dijk JG Sheldon R Sutton R . Making certain that noninvasive therapy for vasovagal syncope has failed before proceeding to invasive interventions. Europace. (2024) 26:euae081. 10.1093/europace/euae081
44.
Alharbi A Shah M Gupta M Rejent K Mahmoud M Alsughayer A et al The efficacy of non-pharmacological and non-pacing therapies in preventing vasovagal syncope: tilt training, physical counter pressure maneuvers, and yoga - a systematic review and meta-analysis. Auton Neurosci. (2024) 251:103144. 10.1016/j.autneu.2023.103144
45.
van Dijk N Quartieri F Blanc JJ Garcia-Civera R Brignole M Moya A et al Effectiveness of physical counterpressure maneuvers in preventing vasovagal syncope: the physical counterpressure manoeuvres trial (PC-trial). J Am Coll Cardiol. (2006) 48:1652–7. 10.1016/j.jacc.2006.06.059
46.
Sharma G Ramakumar V Sharique M Bhatia R Naik N Mohanty S et al Effect of yoga on clinical outcomes and quality of life in patients with vasovagal syncope (LIVE-yoga). JACC Clinical Electrophysiology. (2022) 8:141–9. 10.1016/j.jacep.2021.09.007
47.
Ballantyne BA Letourneau-Shesaf S Raj SR . Management of vasovagal syncope. Auton Neurosci. (2021) 236:102904. 10.1016/j.autneu.2021.102904
48.
Salim MA Di Sessa TG . Effectiveness of fludrocortisone and salt in preventing syncope recurrence in children: a double-blind, placebo-controlled, randomized trial. J Am Coll Cardiol. (2005) 45:484–8. 10.1016/j.jacc.2004.11.033
49.
Liu X-Y Wang C Wu L-J Hu C-Y Lin P Li M-X et al Efficacy of midodrine hydrochloride in the treatment of children with vasovagal syncope. Zhonghua Yi Xue Za Zhi. (2009) 89:1951–4.
50.
Zhang Q Jin H Wang L Chen J Tang C Du J . Randomized comparison of metoprolol versus conventional treatment in preventing recurrence of vasovagal syncope in children and adolescents. Med Sci Monit. (2008) 14:CR199–203.
51.
Flevari P Leftheriotis D Repasos E Katsaras D Katsimardos A Lekakis J . Fluoxetine vs. placebo for the treatment of recurrent vasovagal syncope with anxiety sensitivity. Europace. (2017) 19:127–31. 10.1093/europace/euw153
52.
Brignole M Menozzi C Moya A Andresen D Blanc JJ Krahn AD et al Pacemaker therapy in patients with neurally mediated syncope and documented asystole: third international study on syncope of uncertain etiology (ISSUE-3): a randomized trial. Circulation. (2012) 125:2566–71. 10.1161/CIRCULATIONAHA.111.082313
53.
Sutton R de Jong JSY Stewart JM Fedorowski A de Lange FJ . Pacing in vasovagal syncope: physiology, pacemaker sensors, and recent clinical trials-precise patient selection and measurable benefit. Heart Rhythm. (2020) 17:821–8. 10.1016/j.hrthm.2020.01.029
54.
Debruyne P Rossenbacker T Janssens L Collienne C Ector J Haemers P et al Durable physiological changes and decreased syncope burden 12 months after unifocal right-sided ablation under computed tomographic guidance in patients with neurally mediated syncope or functional Sinus node dysfunction. Circ Arrhythm Electrophysiol. (2021) 14:e009747. 10.1161/CIRCEP.120.009747
55.
Li H Shao W Yu X Gao L Yuan Y . Efficacy of catheter ablation in ganglionated plexus for malignant vasovagal syncope children. Cardiol Young. (2024) 34:1571–6. 10.1017/S1047951124000659
56.
Oliveira PML Silva RMFL Tonelli HAF Meira ZMA Mota CCC . Clinical and autonomic profile, and modified calgary score for children and adolescents with presumed vasovagal syncope submitted to the tilt test. Arq Bras Cardiol. (2023) 120:e20220543. 10.36660/abc.20220543
57.
Kara A Dogan MT . The psychopathology, depression, and anxiety levels of children and adolescents with vasovagal syncope: a case-control study. J Nerv Ment Dis. (2021) 209:547–51. 10.1097/NMD.0000000000001334
58.
Maron BJ Zipes DP Kovacs RJ . Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: preamble, principles, and general considerations: a scientific statement from the American Heart Association and American College of Cardiology. J Am Coll Cardiol. (2015) 66:2343–9. 10.1016/j.jacc.2015.09.032
59.
Towheed A Nesheiwat Z Mangi MA Karabin B Grubb BP . Ivabradine in children with postural orthostatic tachycardia syndrome: a retrospective study. Cardiol Young. (2020) 30:975–9. 10.1017/S1047951120001341
60.
Coffin ST Raj SR . Non-invasive management of vasovagal syncope. Auton Neurosci. (2014) 184:27–32. 10.1016/j.autneu.2014.06.004
61.
Biffi M Boriani G Bronzetti G Frabetti L Picchio FM Branzi A . Neurocardiogenic syncope in selected pediatric patients–natural history during long-term follow-up and effect of prophylactic pharmacological therapy. Cardiovasc Drugs Ther. (2001) 15:161–7. 10.1023/a:1011179014084
62.
Sun R Kang Y Zhang M Wang H Shi L Li X . Development of prognostic nomogram model to predict syncope recurrence in children with vasovagal syncope. Front Cardiovasc Med. (2023) 10:1099115. 10.3389/fcvm.2023.1099115
63.
Anderson JB Czosek RJ Knilans TK Marino BS . The effect of paediatric syncope on health-related quality of life. Cardiol Young. (2012) 22:583–8. 10.1017/S1047951112000133
64.
Wang C Liao Y Wang S Tian H Huang M Dong X-Y et al Guidelines for the diagnosis and treatment of neurally mediated syncope in children and adolescents (revised 2024). World J Pediatrics. (2024) 20:983–1002. 10.1007/s12519-024-00819-w
65.
Soteriades ES Evans JC Larson MG Chen MH Chen L Benjamin EJ et al Incidence and prognosis of syncope. N Engl J Med. (2002) 347:878–85. 10.1056/NEJMoa012407
66.
Barón-Esquivias G Errázquin F Pedrote A Cayuela A Gómez S Aguilera A et al Long-term outcome of patients with vasovagal syncope. Am Heart J. (2004) 147:883–9. 10.1016/j.ahj.2003.11.022
67.
Gielerak G Szyfner K . Physical training improves orthostatic tolerance. Patterns of physical activity that are useful in prevention of vasovagal syncope. Kardiol Pol. (2006) 64:316–21.
68.
Wang S Peng Y Zou R Liao D Yan J Chen D et al Relationship between hemodynamic type and syncopal symptoms in pediatric vasovagal syncope. Eur J Pediatr. (2024) 183:179–84. 10.1007/s00431-023-05278-5
Summary
Keywords
vasovagal syncope, pediatric, diagnosis, management, outcomes
Citation
Zhu W, Bian X and Lv J (2025) Advances in diagnosis, management, and long-term outcomes of pediatric vasovagal syncope: a comprehensive review. Front. Cardiovasc. Med. 12:1481749. doi: 10.3389/fcvm.2025.1481749
Received
16 August 2024
Accepted
10 April 2025
Published
25 April 2025
Volume
12 - 2025
Edited by
Robert Sheldon, University of Calgary, Canada
Reviewed by
Marija Vavlukis, Ss. Cyril and Methodius University in Skopje, North Macedonia
Updates
Copyright
© 2025 Zhu, Bian and Lv.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Jianli Lv drjllv@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.