- 1Cardiovascular Department, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Cardiovascular Disease Center, Xiyuan Hospital, National Clinical Research Center for Chinese Medicine Cardiology, Beijing, China
- 3Graduate School, Beijing University of Chinese Medicine, Beijing, China
Introduction: Heart failure with preserved ejection fraction (HFpEF) is a widespread public health issue worldwide. Despite recent advances in pharmacologic treatments and the introduction of new diagnostic approaches, HFpEF remains underdiagnosed and under-recognized in clinical practice. Traditional Chinese medicine (TCM) may offer a potentially effective treatment for HFpEF. Nevertheless, few clinical trials employ rigorous research methodologies to evaluate the efficacy and safety of TCM in treating HFpEF. Consequently, we propose to assess the hypothesis that patients with HFpEF may benefit from Fuzheng Yangxin Granule (FZYX) and evaluate its safety in a rigorously designed clinical trial.
Methods: This multicenter, double-blind, randomized controlled trial will be conducted across seven tertiary hospitals in China. We will enroll 150 participants aged 18–80 years with confirmed HFpEF (Qi-Yin deficiency and blood stasis syndrome) meeting inclusion criteria. Participants will be randomly assigned (1:1) to the FZYX group or the placebo group, with both groups receiving standardized Western medical therapy according to the National Heart Failure Guideline 2023. The 12-week intervention phase will be followed by 40-week safety follow-up. The primary outcome will be maximal peak oxygen uptake (peak VO2). Secondary outcomes will include composite endpoint events, all-cause mortality, 6-minute walking distance (6MWD), New York Heart Association (NYHA) functional class, serum N-terminal pro-B-type natriuretic peptide (NT-proBNP), echocardiographic variables, Minnesota Living with Heart Failure Questionnaire (MLHFQ) score, TCM syndrome scores, and the FRAIL scale.
Discussion: The objective of this study is to evaluate the efficacy and safety of FZYX in treating HFpEF (Qi-Yin deficiency and blood stasis syndrome), thereby providing a high-quality, reliable evidence-based foundation for clinical practice.
Clinical Trial Registration: China Clinical Trial Registry (ChiCTR2400087293), Registered on July 24, 2024.
1 Introduction
Heart failure with preserved ejection fraction (HFpEF) is not merely a single pathological diagnosis but a complex clinical syndrome characterized by a left ventricular ejection fraction (LVEF) ≥ 50%, along with symptoms such as dyspnea and weakness (1). HFpEF is estimated to account for approximately 50% of all heart failure cases (2), with a 5-year survival rate of 35%–40% following the first hospitalization (3). And a 5-year mortality rate comparable to that of heart failure with reduced ejection fraction (HFrEF) (4).
Due to the heterogeneous pathophysiological mechanisms of HFpEF, evidence-based treatment and diagnosis rely primarily on clinical symptoms, signs, and evidence of structural and/or functional cardiac abnormalities. Despite significant efforts by scholars to develop treatments for HFpEF, translating preclinical experimental medicine into clinical practice remains a challenge. For example, trials targeting the renin-angiotensin system (RAS) [PEP-CHF trial (5), I-PRESERVE study (6), CHARM-Preserved study (7)], mineralocorticoid receptor antagonists (MRA) [TOPCAT study (8)], beta-blockers [OPTIMIZE-HF study (9)], and digitalis [DIG-PEF study (10)] have been conducted. None of these trials have demonstrated that conventional heart failure treatments effectively reduce mortality, readmission rates, or improve prognosis in HFpEF. These include trials investigating nitrate drugs [NEAT-HFPEF study (11)], inhaled nitrites [INDIE-HFpEF study (12)], soluble guanylate cyclase agonists [DILATE-1 study (13)], phosphodiesterase-5 inhibitors [RELAX study (14)], SGLT2 inhibitors [CANVAS study (15), DECLARE-TIMI 58 trial (16), EMPEROR-Preserved study (17)], and angiotensin receptor neprilysin inhibitors (ARNI) [PARAGON-HF trial (18)].
Most of these trials did not yield satisfactory results; however, the EMPEROR-Preserved study (17) is the first successful clinical trial for HFpEF. The study found that Empagliflozin significantly reduced the incidence of the composite endpoint of cardiovascular death or heart failure hospitalization (HHF) compared to placebo. The PARAGON-HF trial (18) did not show a statistically significant difference in primary endpoint events (cardiovascular death and heart failure hospitalization) compared to valsartan (P = 0.0585). Additionally, a trend of benefit was observed in two subgroups: women (HR = 0.73, 95% CI: 0.59–0.90) and LVEF ≤ 57% (HR = 0.78, 95% CI: 0.64–0.95). The 2023 Focused Update of the 2021 ESC Heart Failure Guidelines classified SGLT2 inhibitors as a class 1A recommendation (19). Enhancing the precision of treatment strategies through the development of specific drugs for HFpEF remains a crucial area of ongoing research.
HFpEF is a significant public health concern, and relying solely on modern medical treatments is insufficient for effective management. As the understanding of HFpEF in traditional Chinese medicine (TCM) grows, numerous scholars have conducted extensive studies on TCM treatment protocols and clinical efficacy evaluations for HFpEF. Their findings indicate that TCM offers distinctive advantages in improving clinical symptoms, cardiac function, and quality of life in HFpEF patients (20). A 2018 meta-analysis of 17 randomized controlled trials (RCTs) reported that TCM combined with western medicine effectively improved exercise tolerance and quality of life in HFpEF patients (21). The Chinese medicine QishenYiqi pill has been shown to improve cardiac function and inhibit myocardial fibrosis in HFpEF mice by reducing microvascular endothelial inflammation and activating the NO-cGMP-PKG pathway (22). Therefore, TCM's advantages can be fully utilized to intervene at various stages of HFpEF onset and progression, addressing multiple targets and pathways.
Although numerous clinical trials have been conducted to assess the efficacy and safety of TCM since the first RCT was published in 1982 (23), most current studies are small-scale, low-quality trials, case reports, or clinical experience reports. Additionally, the reliability of study results, report completeness, and their relevance for guiding clinical practice have been questioned (24). The evidence supporting TCM diagnosis and treatment remains widely unaccepted, limiting its broader promotion and application.
According to the Guidelines for Diagnosis and Treatment of Chronic Heart Failure in Traditional Chinese Medicine (2022) (25), the TCM syndrome types for HFpEF include Qi deficiency with blood stasis, Qi-Yin deficiency with blood stasis, and Yang-Qi deficiency with blood stasis. Through long-term treatment and observation of HFpEF patients, we found that they exhibit deficiencies in Qi, blood, Yin, and Yang, along with blood stasis, characterized by symptoms like palpitations, shortness of breath, weakness, exertional sweating, spontaneous or nocturnal sweating, cyanosis of the lips and tongue, and a thin, astringent, intermittent, or weak pulse. Therefore, Qi-Yin deficiency with blood stasis is the most common pattern, prompting us to develop the Fuzheng Yangxin Granule (FZYX). We plan to conduct a rigorously designed RCT to evaluate the efficacy and safety of FZYX in treating HFpEF with Qi-Yin deficiency and blood stasis syndrome.
2 Methods and analysis
2.1 Study design
This study is designed as a randomized, double-blind, placebo-controlled, parallel-group, multicenter trial. The study design follows the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Guidelines (26). Seven tertiary hospitals across different regions of China are participating in this trial. A total of 150 stable HFpEF patients meeting the inclusion and exclusion criteria will be randomized 1:1 into either the experimental or control group. The overall design of the trial is presented in Figure 1.
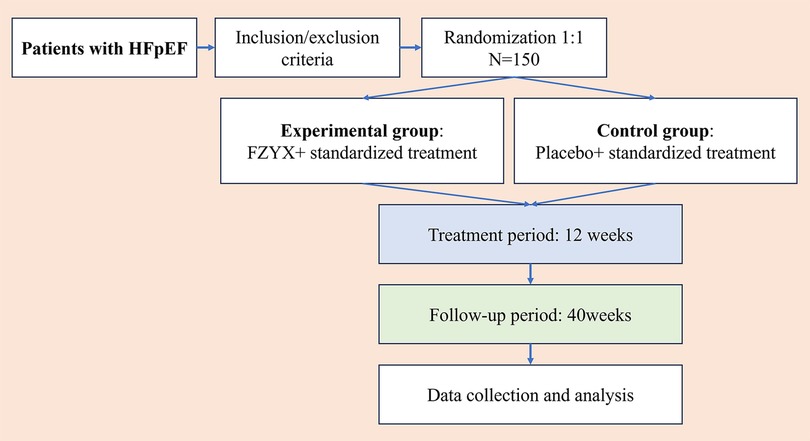
Figure 1. Flow chart of the trial. HFpEF, heart failure with preserved ejection fraction; FZYX, Fuzheng Yangxin Granule.
2.2 Sample size estimation
The primary efficacy index of this trial is peak oxygen uptake (peak VO2), as demonstrated in previous related studies (27–29). It is anticipated that the peak oxygen uptake after treatment will be 16 ml/kg/min in the control group and 17.6 ml/kg/min in the experimental group, with a standard deviation of 3.1 ml/kg/min between the two groups. The sample size was calculated using PASS 15.0. The sample size was estimated by comparing the means of two samples in a completely randomized design, using a two-sided test with a significance level of α = 0.05 and power (1-β) of 0.80. With a 1:1 allocation between the experimental and control groups, the sample size for each group was estimated to be 60 participants. Considering a potential 20% attrition rate, 75 participants were included in each group, resulting in a total of 150 participants.
2.3 Inclusion criteria
(1) Participants aged 18–80 years, irrespective of gender.
(2) Participants diagnosed with Qi-Yin deficiency and blood stasis syndrome, as defined by Traditional Chinese Medicine (TCM) principles: Patients must present with at least two of three cardinal symptoms (shortness of breath/dyspnea, fatigue, or palpitations) combined with two of four secondary symptoms (persistent thirst/dry pharynx, spontaneous daytime sweating exacerbated by activity or night sweating ceasing upon awakening, heat sensation in palms/soles, or cyanotic facial/lip discoloration). Characteristic tongue manifestations include dark red or purplish coloration with ecchymosis/petechiae/varicose sublingual veins, thin body with scanty, absent, peeled, or fissured coating. Pulse findings must demonstrate thready-rapid-weak characteristics or irregular-intermittent rhythm. Syndrome confirmation requires concurrent fulfillment of symptom criteria and corresponding tongue-pulse presentations.
(3) New York Heart Association (NYHA) functional class II-III with hemodynamic stability.
(4) Meeting the diagnostic criteria for HFpEF as outlined in the National Heart Failure Guideline 2023 (30).
(a) Epidemiological and population characteristics of HFpEF patients;
(b) Signs and/or symptoms of heart failure;
(c) LVEF ≥ 50%;
(d) Objective evidence of cardiac structural and/or functional abnormalities consistent with left ventricular diastolic dysfunction and/or elevated left ventricular filling pressures. This includes: (i) E/e’ >15, (ii) Septal e’ <7 cm/s or Lateral e’ <10 cm/s, (iii) tricuspid regurgitant velocity >2.8 m/s or estimated PASP > 35 mmHg, (iv) elevated LAVI (sinus rhythm: LAVI >34 ml/m2, atrial fibrillation: LAVI > 40 ml/m2), and (v) elevated natriuretic peptide levels.
(5) Voluntary participation, with participants providing informed consent after understanding the study details.
2.4 Exclusion criteria
(1) Any prior echocardiographic LVEF measurement <40%.
(2) Uncontrolled hypertension, defined as resting systolic blood pressure ≥180 mmHg and/or diastolic blood pressure ≥110 mmHg, confirmed at two separate examinations before randomization.
(3) ALT, AST, or bilirubin levels exceeding 3 times the upper limit of normal (not due to heart failure), glomerular filtration rate <15 ml/min/1.73 m2, and blood potassium >5.5 mmol/L.
(4) Within 3 months of: acute coronary syndrome, stroke, transient ischemic attack, cardiac, carotid, or other major vascular surgery, PCI, carotid angioplasty, coronary artery bypass grafting, or any non-cardiac condition that may impair exercise capacity or be exacerbated by strenuous exercise (e.g., infection, liver/kidney failure, thyrotoxicosis).
(5) Patients with severe primary diseases affecting the liver, kidneys, hematopoietic, nervous, or endocrine systems, tumors, or psychiatric disorders.
(6) Life expectancy of less than one year.
(7) Known allergies to any trial medications.
(8) Participation in other drug trials within the last month.
(9) Patients currently using Chinese medicine or patent medicines containing ingredients similar to FZYX.
(10) Pregnancy (confirmed by a positive test if needed), lactation, or women of childbearing age not using effective contraception.
(11) Patients deemed unable to complete or comply with the study requirements, per investigator's judgement.
2.5 Randomization and blinding
A computer-generated blocked randomization scheme will be implemented using permuted blocks of 6 participants (3 FZYX:3 placebo) to ensure balanced allocation throughout recruitment. An independent statistician will generate the randomization sequence with consecutive numbering from 001 to 150 assigned to pre-packaged, identical medication bags. Participants will be sequentially enrolled and allocated medications strictly according to their entry order, with each bag containing either FZYX or placebo labeled only with the corresponding randomization code. The allocation list will remain securely encrypted in a password-protected file accessible solely to the independent statistician until database lock. This double-blind design ensures concealment from participants, investigators, outcome assessors, and data analysts throughout the trial duration.
2.6 Intervention
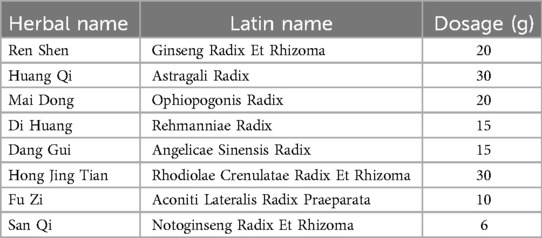
Following randomization, participants will receive either FZYX or matched placebo orally (2 sachets three times daily) for 12 weeks, with both preparations manufactured under Good Manufacturing Practice (GMP) standards at Xiyuan Hospital's pharmaceutical facility. The investigational products will be distributed through a serialized tracking system managed by the principal investigator, with documentation of batch numbers, dispensing dates, and return quantities. All participants will concurrently receive guideline-directed standardized medical therapy including: (1) sodium-glucose cotransporter-2 inhibitors (SGLT2i: empagliflozin/dapagliflozin) for HFpEF management; (2) diuretics for fluid-overloaded patients (NYHA II-IV); (3) angiotensin receptor-neprilysin inhibitors (ARNI: sacubitril/valsartan) indicated for symptomatic females [any left ventricular ejection fraction (LVEF)] or males (LVEF <55%–60%); (4) mineralocorticoid receptor antagonists (MRA: spironolactone) indicated for symptomatic females (any LVEF) or males (LVEF <55%–60%); (5) stable regimens for comorbidities (e.g., hypertension/diabetes). FZYX's herbal composition (see Table 1) and placebo share identical organoleptic properties through standardized production protocols.
2.7 Study visits and follow-up
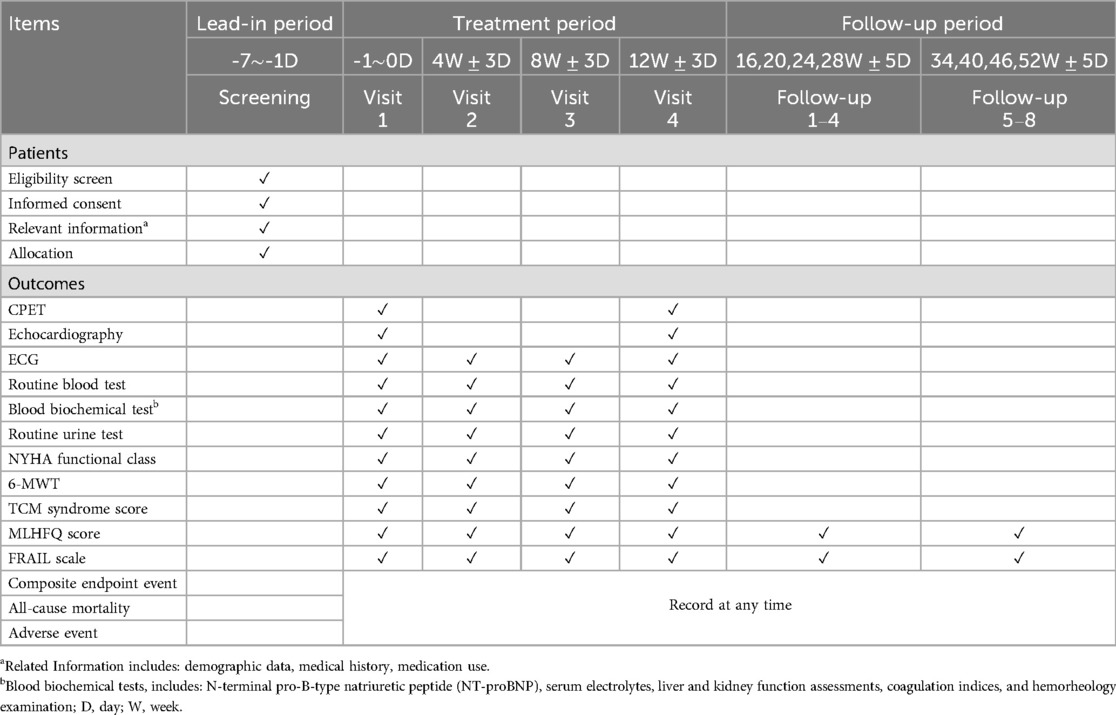
The investigator will obtain informed consent from each participant before initiating any protocol-related procedures. Eligible participants will be contacted by telephone and scheduled to attend the trial center within one week (Visit 1). During Visit 1 and 4, the investigator will collect relevant information, including demographic data, medical history, medication use, and conduct a physical examination. Participants will then be randomized into either the experimental or control group. In this trial, participants will take FZYX or placebo three times a day for 12 weeks. All participants will undergo scheduled testing and evaluations, including cardiopulmonary exercise testing (CPET), echocardiography, 12-lead electrocardiogram (ECG), routine blood tests, routine urine tests, and blood biochemical tests, including N-terminal pro-B-type natriuretic peptide (NT-proBNP), serum electrolytes, liver and kidney function assessments, coagulation indices, and hemorheology examination. In Visits 2 and 3, participants will undergo the same tests and evaluations as in Visit 1, except for echocardiography and CPET. Follow-up visits 1–8 will consist of telephone follow-ups to record Minnesota Living with Heart Failure Questionnaire (MLHFQ) scores, FRAIL scale scores, all-cause mortality, and composite endpoint events. The detailed follow-up schedule is shown in Table 2.
2.8 Outcome measures
2.8.1 Primary outcome
The primary evaluation measure is peak VO2.
2.8.2 Secondary outcomes
(1) All-cause mortality;
(2) Composite endpoint events (treatment abandoned due to worsening heart failure, successful resuscitation after cardiac arrest, malignant arrhythmia, nonfatal stroke, heart failure hospitalization);
(3) 6-minute walking distance (6MWD);
(4) NYHA functional class;
(5) Serum NT-proBNP levels;
(6) Echocardiography: LVEF, E/e', and others;
(7) TCM syndrome scores;
(8) Minnesota Living with Heart Failure Questionnaire (MLHFQ) scores;
(9) Blood rheology (fibrinogen, whole blood viscosity, platelet aggregation test, erythrocyte sedimentation rate, and others);
(10) FRAIL scale.
2.9 Safety assessment and adverse events report
Detailed observation and reporting of all types of drug-related allergic reactions (including rash), gastrointestinal reactions (such as nausea, vomiting, diarrhea), neuropsychiatric disorders (including insomnia, headache, dizziness), and abnormal laboratory test results. Laboratory tests include routine blood tests (white blood cell count, red blood cell count, hemoglobin, platelets), routine urine tests (urine leukocytes, urine protein, occult blood, urine glucose), liver function tests (alanine transaminase, aspartate transaminase, total bilirubin, gamma-glutamyl transferase, alkaline phosphatase), renal function tests (serum creatinine, blood urea nitrogen), serum electrolytes (serum potassium, sodium, chloride), and 12-lead ECG. Any serious adverse events occurring during the trial must be immediately reported to the principal investigator and the ethics committee. Additionally, a “Serious Adverse Event” form must be completed.
2.10 Statistical collection and analysis
Study data will be collected at each center using standardized paper-based Case Report Forms (CRFs). Participants will be provided with a private space to complete the quality of life form to address privacy concerns. Two-tailed tests will be applied with P < 0.05 as the significance threshold. No adjustments for multiple testing will be performed for secondary endpoints, as they are exploratory. Analyses will be conducted using SPSS 23.0 and R 4.3.1. Primary outcome: Analyzed using ANCOVA adjusted for baseline peak VO₂, age, and sex. Secondary continuous outcomes: Assessed via mixed-effects models for repeated measures. Non-normally distributed data will use bootstrap CIs (10,000 replications). Binary/categorical data: Analyzed using chi-squared or Fisher's exact test. Ordinal outcomes: Evaluated via proportional odds models.
2.11 Study monitoring and quality assurance
The study will adhere to the clinical trial protocol, the Declaration of Helsinki, and applicable Chinese clinical research regulations. Investigators will design CRFs to document data related to trial participants. All investigators will receive standardized training to ensure a comprehensive understanding of the clinical trial before its initiation. Investigators will ensure that all reported trial data are accurate, complete, and verifiable against source documents. A dedicated monitor will supervise the entire trial process, regularly verifying that the trial is conducted and documented according to the protocol, standard operating procedures, and applicable regulations.
3 Discussion
HFpEF, a highly heterogeneous clinical syndrome, has become a significant type of heart failure globally. The pathophysiology of HFpEF is associated with several factors, including chronic systemic inflammation, microvascular dysfunction, metabolic disorders, myocardial fibrosis, epicardial adipose tissue accumulation, and neuroendocrine system activation (31). Moreover, non-cardiovascular comorbidities, such as chronic kidney disease, diabetes mellitus, and obesity, are more prevalent in this population (32). The prevalence of HFpEF is expected to rise in the coming years, driven by population aging and the increasing prevalence of comorbidities associated with the condition. The complex pathophysiology and heterogeneity of clinical phenotypes in HFpEF are key factors contributing to the failure of many clinical trials, making it a challenging condition to treat.
The clinical need for HFpEF is significant and unmet, with TCM research showing promise. Given its unique framework and extensive experience, TCM warrants further exploration for HFpEF treatment. Qi-Yin deficiency with blood stasis is the most common TCM syndrome in HFpEF. FZYX, suited for this syndrome, consists of Ren Shen, Huang Qi, Mai Dong, Di Huang, Dang Gui, Hong Jing Tian, Fu Zi, and San Qi. In this formula, Ren Shen and Huang Qi benefit Qi; Mai Dong, Di Huang, and Dang Gui nourish Yin and promote fluids; Fu Zi (in small amounts) warms Yang and benefits Qi; Dang Gui and San Qi invigorate blood and remove stasis.
In our clinical practice (33–36), we studied TCM prescriptions like FZYX combined with hemofiltration in heart failure patients with diuretic resistance. Studies showed that patients receiving hemofiltration with TCM had lower rates of hypokalemia, hyponatremia, and hypotension compared to those without TCM. Additionally, heart function improved more significantly in patients treated with TCM. These positive results in treating severe heart failure suggest promising applications for TCM in broader heart failure treatment. In a previous experiment, we found that FZYX improves cardiac function and protects cardiomyocytes by regulating STAT3 expression and inhibiting apoptosis in a heart failure rat model (37).
Previous studies of TCM for HFpEF have encountered three major issues. First, many face methodological flaws. The current RCT designs for TCM in HFpEF lack rigor, with improper execution of randomization, blinding, allocation concealment, and reporting biases, all of which undermine study credibility. Secondly, some trials rely solely on modern diagnoses, neglecting TCM symptom selection, which hinders subject “homogenization” and impacts clinical efficacy. Modern medicine classifies by disease, while TCM uses symptom differentiation. Integrating both is essential for standardizing research subjects. Third, hard endpoints like cardiovascular death and rehospitalization are often overlooked (38). Prognostic indicators require long observation periods and significant resources. In contrast, easier-to-detect, shorter-term indicators are commonly used (39). Yet, given HFpEF's severity, more trials should focus on mortality as the primary endpoint.
This study has three key strengths justifying the trial. First, the protocol follows SPIRIT guidelines, ensuring high-quality clinical evidence. Second, patient diagnoses combine modern HFpEF criteria with TCM's Qi-Yin deficiency and blood stasis syndrome, integrating disease and syndrome. Third, the study evaluates long-term prognostic outcomes in addition to objective HFpEF tests. In conclusion, this multicenter, randomized controlled trial evaluates FZYX for HFpEF treatment, aiming to provide high-quality, evidence-based support for its efficacy and safety. Additionally, this study integrates Chinese and Western medicine, potentially guiding alternative treatment strategies.
Ethics statement
The Ethics Committee of Xiyuan Hospital, China Academy of Chinese Medicine, approved this trial protocol on May 21, 2024 (2024XLA083-1). Any deviations from the protocol require prior approval from the Ethics Committee. The trial is registered in the Chinese Clinical Trials Registry (ChiCTR2400087293). The investigator must provide all subjects with a detailed explanation of the study and obtain informed consent. Subjects will be given sufficient time to make an informed decision regarding their participation in the trial.
Author contributions
JC: Writing – original draft. ZY: Writing – original draft, Writing – review & editing. JW: Conceptualization, Data curation, Formal analysis, Visualization, Writing – review & editing. LG: Conceptualization, Data curation, Investigation, Visualization, Writing – review & editing. ZJ: Conceptualization, Formal analysis, Resources, Visualization, Writing – review & editing. FW: Conceptualization, Data curation, Investigation, Writing – review & editing. RB: Conceptualization, Data curation, Investigation, Visualization, Writing – review & editing. XM: Data curation, Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Xiyuan Hospital CACMS Enhancement Fund (NO. XYZX0201-12).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. (2022) 145(18):e876–94. doi: 10.1016/j.jacc.2021.12.011
2. Campbell P, Rutten FH, Lee MM, Hawkins NM, Petrie MC. Heart failure with preserved ejection fraction: everything the clinician needs to know. Lancet. (2024) 403(10431):1083–92. doi: 10.1016/S0140-6736(23)02756-3
3. Withaar C, Lam CSP, Schiattarella GG, De Boer RA, Meems LMG. Heart failure with preserved ejection fraction in humans and mice: embracing clinical complexity in mouse models. Eur Heart J. (2021) 42(43):4420–30. doi: 10.1093/eurheartj/ehab389
4. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. (2017) 70(20):2476–86. doi: 10.1016/j.jacc.2017.08.074
5. Cleland JGF, Tendera M, Adamus J, Freemantle N, Polonski L, Taylor J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. (2006) 27(19):2338–45. doi: 10.1093/eurheartj/ehl250
6. Massie BM, Carson PE, McMurray JJ, Komajda M, McKelvie R, Zile MR, et al. Irbesartan in patients with heart failure and preserved ejection fraction. N Engl J Med. (2008) 359(23):2456–67. doi: 10.1056/NEJMoa0805450
7. Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJV, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet (London, England). (2003) 362(9386):777–81. doi: 10.1016/S0140-6736(03)14285-7
8. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. (2014) 370(15):1383–92. doi: 10.1056/NEJMoa1313731
9. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Coll Cardiol. (2007) 50(8):768–77. doi: 10.1016/j.jacc.2007.04.064
10. Ahmed A, Rich MW, Fleg JL, Zile MR, Young JB, Kitzman DW, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. (2006) 114(5):397–403. doi: 10.1161/CIRCULATIONAHA.106.628347
11. Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. (2015) 373(24):2314–24. doi: 10.1056/NEJMoa1510774
12. Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, et al. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA. (2018) 320(17):1764–73. doi: 10.1001/jama.2018.14852
13. Bonderman D, Pretsch I, Steringer-Mascherbauer R, Jansa P, Rosenkranz S, Tufaro C, et al. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. (2014) 146(5):1274–85. doi: 10.1378/chest.14-0106
14. Redfield MM, Chen HH, Borlaug BA, Semigran MJ, Lee KL, Lewis G, et al. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. (2013) 309(12):1268–77. doi: 10.1001/jama.2013.2024
15. Figtree GA, Rådholm K, Barrett TD, Perkovic V, Mahaffey KW, de Zeeuw D, et al. Effects of canagliflozin on heart failure outcomes associated with preserved and reduced ejection fraction in type 2 diabetes mellitus. Circulation. (2019) 139(22):2591–3. doi: 10.1161/CIRCULATIONAHA.119.040057
16. Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, et al. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation. (2019) 139(22):2528–36. doi: 10.1161/CIRCULATIONAHA.119.040130
17. Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, et al. Empagliflozin in heart failure with a preserved ejection fraction. N Engl J Med. (2021) 385(16):1451–61. doi: 10.1056/NEJMoa2107038
18. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. (2019) 381(17):1609–20. doi: 10.1056/NEJMoa1908655
19. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2023 focused update of the 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2023) 44(37):3627–39. doi: 10.1093/eurheartj/ehad195
20. Fan Y, Yang Z, Wang L, Liu Y, Song Y, Liu Y, et al. Traditional Chinese medicine for heart failure with preserved ejection fraction: clinical evidence and potential mechanisms. Front Pharmacol. (2023) 14:1154167. doi: 10.3389/fphar.2023.1154167
21. Wang J, Yang R, Zhang F, Jia C, Wang P, Liu J, et al. The effect of Chinese herbal medicine on quality of life and exercise tolerance in heart failure with preserved ejection fraction: a systematic review and meta-analysis of randomized controlled trials. Front Physiol. (2018) 9:1420. doi: 10.3389/fphys.2018.01420
22. Huang Y, Zhang K, Liu M, Su J, Qin X, Wang X, et al. An herbal preparation ameliorates heart failure with preserved ejection fraction by alleviating microvascular endothelial inflammation and activating NO-cGMP-PKG pathway. Phytomedicine. (2021) 91:153633. doi: 10.1016/j.phymed.2021.153633
23. Chen K, Qian Z, Zhang W, Guo S, Kou W, Wu X, et al. Effectiveness analysis for double blinded treatment with refined coronary tablets on angina pectoris leaded by coronary heart disease in 112 cases. Chinese J Cardiol. (1982) 10(2):85–9.
24. Proehl JA, Alexander S, Manton A. Integrity and transparency in reporting clinical trials. J Emerg Nurs. (2017) 43(2):96–7. doi: 10.1016/j.jen.2017.01.009
25. Chinese Association of Traditional Chinese Medicine. Traditional Chinese medicine diagnosis and treatment guidelines for chronic heart failure. J Tradit Chin Med. (2022) 64(7):743–56. doi: 10.13288/j.11-2166/r.2023.07.016
26. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža-Jerić K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158(3):200–7. doi: 10.7326/0003-4819-158-3-201302050-00583
27. Conraads VM, Metra M, Kamp O, De Keulenaer GW, Pieske B, Zamorano J, et al. Effects of the long-term administration of nebivolol on the clinical symptoms, exercise capacity, and left ventricular function of patients with diastolic dysfunction: results of the ELANDD study. Eur J Heart Fail. (2012) 14(2):219–25. doi: 10.1093/eurjhf/hfr161
28. Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the aldo-DHF randomized controlled trial. JAMA. (2013) 309(8):781–91. doi: 10.1001/jama.2013.905
29. Kitzman DW, Hundley WG, Brubaker PH, Morgan TM, Moore JB, Stewart KP, et al. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail. (2010) 3(4):477–85. doi: 10.1161/CIRCHEARTFAILURE.109.898916
30. National Center for Cardiovascular Diseases, China National Heart Failure Society, Chinese Heart Failure Association of Chinese Medical Doctor Association, Editorial Board of Chinese Journal of Heart Failure and Cardiomyopathy. China National heart failure guideline 2023. Chinese J Heart Fail Cardiomyopathy (2023) 7(4):215–311. doi: 10.3760/cma.j.issn.101460-20231209-00052
31. Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. (2023) 81(18):1810–34. doi: 10.1016/j.jacc.2023.01.049
32. Borlaug BA. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol. (2020) 17(9):559–73. doi: 10.1038/s41569-020-0363-2
33. Qi L. Clinical Study of Shenfu Yiqi Qiangxin Decoction Combined with Hemofiltration in the Treatment of Heart Failure Due to Yang Deficiency (dissertation/master’s thesis). Beijing University of Chinese Medicine, Beijing, China (2010).
34. Fan L.Clinical Observation of Modified Shenfu Decoction Combined with Hemofiltration in the Treatment of Heart Failure (dissertation/master’s thesis). Beijing University of Chinese Medicine, Beijing, China. (2012).
35. Wang X. Clinical Observation of the Yiqi Fuzheng Method Combined with Hemofiltration in the Treatment of Heart Failure (dissertation/master’s thesis). Beijing University of Chinese Medicine, Beijing, China (2016).
36. Zheng Y. Clinical and Targeted Metabolomics Study of Traditional Chinese Medicine Combined with Hemofiltration in the Treatment of Heart Failure (dissertation/doctor’s thesis). Beijing University of Chinese Medicine, Beijing, China (2020).
37. Wang A, Zhao W, Yan K, Guo L, Gao F, Chen J, et al. Investigating the cardioprotective effects of Fuzheng Yangxin recipe based on network pharmacology and experimental evaluation. Front Pharmacol. (2022) 13:1004929. doi: 10.3389/fphar.2022.1004929
38. Hao P, Jiang F, Cheng J, Ma L, Zhang Y, Zhao Y. Traditional Chinese medicine for cardiovascular disease: evidence and potential mechanisms. J Am Coll Cardiol. (2017) 69(24):2952–66. doi: 10.1016/j.jacc.2017.04.041
Keywords: heart failure with preserved ejection fraction, Fuzheng Yangxin Granule, traditional Chinese medicine, clinical trial protocol, herbal medicine, randomized controlled trial
Citation: Chen J, Yan Z, Wang J, Guo L, Jiang Z, Wang F, Bai R and Ma X (2025) A multi-center, randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of Fuzheng Yangxin Granule in treating heart failure with preserved ejection fraction (Qi-Yin deficiency and blood stasis syndrome): study protocol. Front. Cardiovasc. Med. 12:1514181. doi: 10.3389/fcvm.2025.1514181
Received: 20 October 2024; Accepted: 10 April 2025;
Published: 29 April 2025.
Edited by:
Lauren Kathryn Truby, University of Texas Southwestern Medical Center, United StatesReviewed by:
Youhua Wang, Shanghai University of Traditional Chinese Medicine, ChinaYunlun Li, Shandong University of Traditional Chinese Medicine, China
Copyright: © 2025 Chen, Yan, Wang, Guo, Jiang, Wang, Bai and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaochang Ma, bWF4aWFvY2hhbmdAeDI2My5uZXQ=
†These authors have contributed equally to this work and share first authorship
 Jingjing Chen
Jingjing Chen Zian Yan1,3,†
Zian Yan1,3,† Ruina Bai
Ruina Bai Xiaochang Ma
Xiaochang Ma