Abstract
Background:
The optimal cannulation strategy for acute type A aortic dissection (ATAAD) surgery via a minimally invasive approach remains a topic of debate. This study aimed to compare the feasibility and safety of different aortic arch branch cannulation techniques using a single upper hemisternotomy.
Methods:
A retrospective analysis was performed on 207 patients with ATAAD who underwent total arch replacement combined with frozen elephant trunk techniques between December 2019 and July 2023. Patients were categorized into four groups based on the cannulation site: IA group (innominate artery, n = 174), LCA group (left carotid artery, n = 21), RSA group (right subclavian artery, n = 5), and RCA group (right carotid artery, n = 7). Perioperative outcomes, including mortality, complications, and operative times, were compared using appropriate statistical methods.
Results:
A total of 207 patients were included and categorized into four groups based on the site of arterial cannulation: IA (n = 174), LCA (n = 21), RSA (n = 5), and RCA (n = 7). Baseline characteristics, including age and preoperative comorbidities, were comparable across the groups. Intraoperative metrics, such as cross-clamp time, circulatory arrest time, selective cerebral perfusion time, and cardiopulmonary bypass (CPB) time, showed no statistically significant differences. Although the CPB time was numerically shorter in the IA group, this difference was not significant (p > 0.05). Perioperative mortality occurred in 25 patients (12.1%), with no statistically significant differences among the groups (IA: 12.6%, LCA: 0%, RSA: 20.0%, RCA: 28.6%; p > 0.05). Postoperative clinical outcomes, including ventilator support duration, ICU stay, and hospital length of stay, were also similar across all groups.
Conclusion:
Aortic arch branch cannulation is a feasible and safe arterial perfusion strategy for ATAAD surgery via a minimally invasive single upper hemisternotomy. Among the options, the innominate artery demonstrated favorable outcomes and was not inferior to other arch vessels, and may be considered a suitable first choice when feasible.
Introduction
Acute Type A aortic dissection (ATAAD) is a life-threatening cardiovascular emergency with an annual incidence of approximately 5.2 cases per million, characterized by high morbidity and mortality rates (1, 2). If untreated, the 48-h mortality rate for ATAAD exceeds 50%, underscoring the urgency of timely surgical intervention (3). Current standard surgical treatments typically involve total aortic arch replacement combined with frozen elephant trunk techniques, aimed at reducing complications and improving survival outcomes (4).
Arterial cannulation plays a pivotal role in ATAAD surgery, facilitating arterial perfusion and cerebral protection during circulatory arrest. Conventional strategies include femoral artery, axillary artery, and central cannulation, each with distinct advantages and limitations (5, 6). In recent years, innovative techniques such as apical and innominate artery cannulation have been explored to address the shortcomings of traditional methods (7).
The advent of minimally invasive surgery (MIS) has further transformed the management of ATAAD. Single upper hemisternotomy, utilizing J-shaped, L-shaped, V-shaped, or inverted T-shaped incisions, has emerged as a preferred approach for MIS due to reduced surgical trauma, shorter recovery times, and improved cosmetic outcomes (8, 9). However, common cannulation strategies in MIS, such as axillary and femoral artery cannulation, often require additional incisions, increasing the risk of infection and compromising aesthetic benefits. Wire-guided central cannulation, while safer in traditional settings, poses challenges for selective cerebral perfusion in minimally invasive contexts (10).
Cannulation via aortic arch branches offers a promising alternative. This approach eliminates the need for additional incisions, provides antegrade blood flow, and facilitates effective selective cerebral perfusion, making it particularly suited for MIS in ATAAD (3). Since 2017, our center has accumulated substantial experience with aortic arch branch cannulation in minimally invasive ATAAD surgeries, allowing for a comprehensive evaluation of its feasibility and safety under these conditions (11, 12).
This study aims to compare the outcomes of different aortic arch branch cannulation strategies in ATAAD surgery performed via a minimally invasive single upper hemisternotomy approach. By analyzing perioperative outcomes, we seek to determine the optimal cannulation strategy for this challenging clinical scenario.
Methods
Clinical data collection
A retrospective analysis was performed on patients who underwent minimally invasive surgery for acute Type A aortic dissection (ATAAD) at the General Hospital of the Northern Theater Command between December 2019 and July 2023. Perioperative clinical data were collected according to predefined inclusion and exclusion criteria, and patients were categorized based on the site of arterial cannulation.
Inclusion criteria included the confirmation of acute Stanford Type A aortic dissection involving the aortic arch using computed tomography angiography (CTA). Patients were excluded if they met any of the following conditions: (1) preoperative neurological complications such as cerebral hemorrhage or stroke; (2) preoperative malperfusion syndrome (4); or (3) concomitant diseases, including coronary heart disease, mitral valve disease, or congenital heart disease, requiring simultaneous surgical treatment through full sternotomy.
This study was approved by the hospital's ethics committee [Ethical Review K (2022)210], and informed consent was obtained from all patients.
Surgical procedure
All patients included in this study underwent minimally invasive surgery via a single upper hemisternotomy approach for the repair of the proximal ascending aorta, total aortic arch replacement, and deployment of a frozen elephant trunk. The surgical technique has been described in detail in prior research and is summarized as follows:
An incision was made from the sternal notch to the fourth intercostal space. The sternum was sawed vertically either to the right (resulting in a J-shaped incision) or to the left (resulting in an L-shaped incision) (9). The aortic arch branches—including the innominate artery (IA), left carotid artery (LCA), and left subclavian artery (LSA)—were carefully dissected and examined. The site for arterial cannulation was determined based on whether these vessels were affected by the dissection (Figure 1).
Figure 1
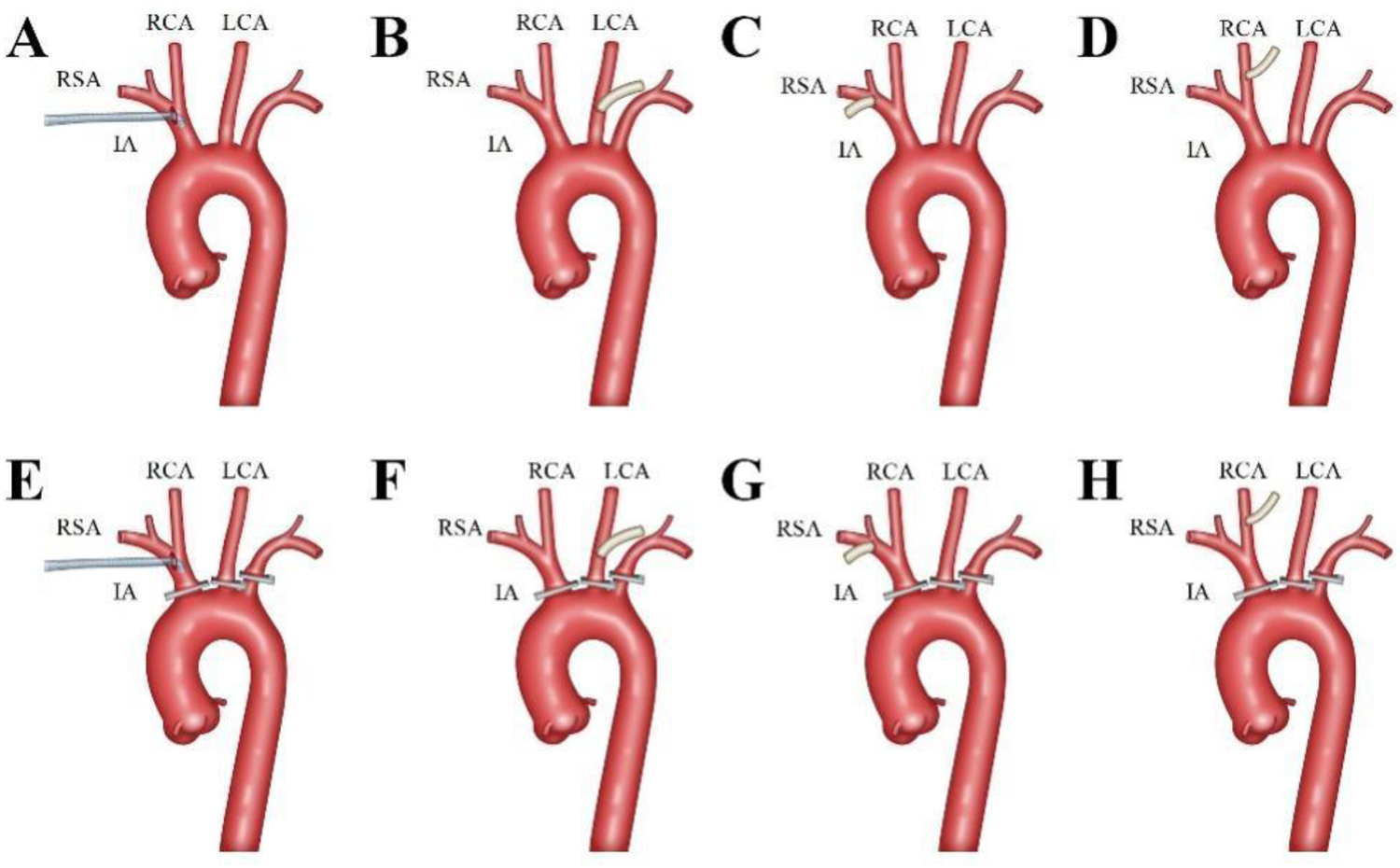
Aortic Arch Branches Cannulation Strategy. The innominate artery was the preferred cannulation site, with an 18Fr or 20Fr right angle arterial cannula (Longlaifu, Changzhou, China) inserted directly with its opening towards the heart (A,E). The left carotid artery (LCA), right subclavian artery (RSA), and right carotid artery (RCA) were sequentially chosen as cannulation sites, with end-to-side anastomosis of a 0.8 cm diameter synthetic vessel to the LCA (B,F), RSA (C,G), and RCA (D,H), respectively.
The innominate artery was the preferred site for cannulation. A right-angled arterial cannula (18Fr or 20Fr, Longlaifu, Changzhou, China) was inserted directly, with its opening directed toward the heart. When the IA was involved in the dissection, alternative sites were selected in the following order: LCA, right subclavian artery (RSA), and right carotid artery (RCA). For these alternative sites, end-to-side anastomosis was performed using a synthetic vessel with a diameter of 0.8 cm (Maquet, La Ciotat Cedex, France), which was connected to the cardiopulmonary bypass (CPB) circuit via a connector.
Venous drainage was achieved through cannulation of the right atrium, and a left atrial drainage tube was placed through the right upper pulmonary vein. After CPB initiation, gradual cooling was employed. Following aortic clamping and cardiac arrest, the proximal ascending aorta was repaired. Selective bilateral antegrade cerebral perfusion was provided using a pump-controlled system (13).
When the target nasopharyngeal temperature was reached, either lower body circulatory arrest (28°C) (11) or brief circulatory arrest followed by lower body perfusion (31°C) (12) was utilized for descending aorta procedures. The surgery concluded with routine total aortic arch replacement, involving sequential anastomoses of the LCA, proximal ascending aorta, LSA, and IA.
Statistical analysis
All statistical analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables with a normal distribution are presented as mean ± standard deviation (SD) and were compared using one-way analysis of variance (ANOVA). Non-normally distributed continuous variables are expressed as medians with interquartile ranges (IQR, P25-P75) and were analyzed using the Kruskal–Wallis test. Categorical variables are presented as counts and percentages, and group comparisons were conducted using Fisher's exact test. A two-tailed p-value of <0.05 was considered statistically significant.
Results
Patient inclusion and grouping
The study initially included 213 patients. Six cases were excluded: two due to preoperative neurological complications, one due to preoperative malperfusion syndrome, two due to the need for concurrent mitral valve surgery, and one due to intraoperative discovery of coronary involvement requiring coronary artery bypass grafting. Consequently, 207 patients were included in the final analysis (Figure 2).
Figure 2
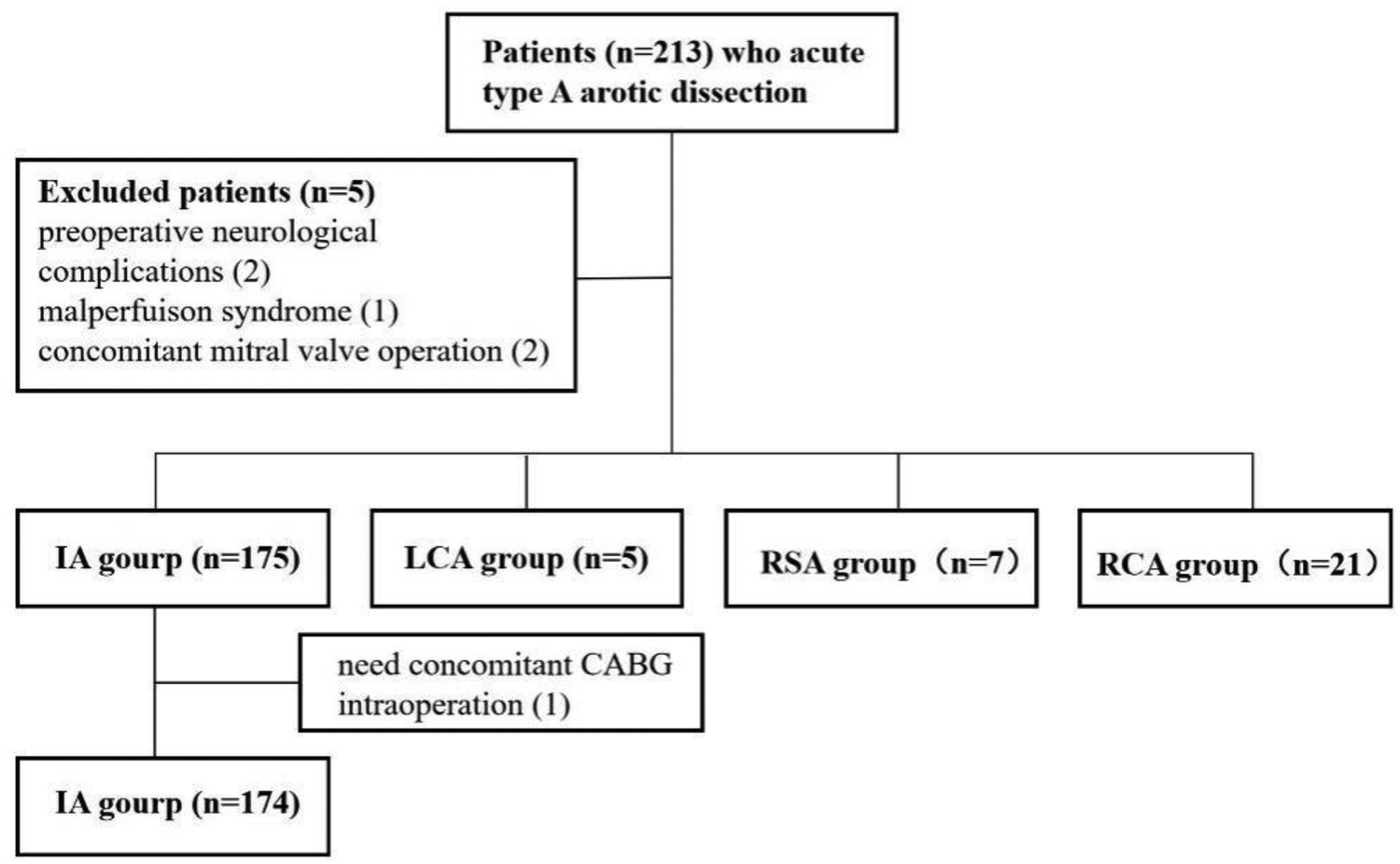
Patients enrolled based on inclusion and exclusion criteria.
Patients were categorized based on the site of arterial cannulation into four groups: IA (n = 174), LCA (n = 21), RSA (n = 5), and RCA (n = 7). Baseline characteristics were comparable across all groups, with no statistically significant differences (Table 1).
Table 1
| Indicators | IA group (n = 174) | LCA group (n = 5) | RSA group (n = 7) | RCA group (n = 21) | P-value |
|---|---|---|---|---|---|
| Age (years) | 51.0 (43.0, 59.0) | 52.0 (46.5, 58.5) | 54.0 (45.0, 62.0) | 55.0 (45.5, 64.5) | 0.732 |
| Male [n (%)] | 128 (73.6) | 3 (60.0) | 5 (71.4) | 15 (71.4) | 0.851 |
| Weight (kg) | 79.0 (68.0, 90.0) | 62.0 (58.0, 72.5) | 85.0 (65.0, 95.0) | 72.5 (65.0, 89.0) | 0.070 |
| Hypertension [n (%)] | 5 (2.9) | 0 (0.0) | 0 (0.0) | 1 (4.8) | 0.652 |
| Diabetes [n (%)] | 83 (48.0) | 0 (0.0) | 4 (57.1) | 10 (47.6) | 0.193 |
| Smoking History [n (%)] | 7 (4.0) | 0 (0.0) | 0 (0.0) | 2 (9.5) | 0.567 |
| LVEF (%) | 59.0 (57.0, 60.0) | 59.0 (56.5, 60.5) | 58.0 (57.0, 60.0) | 58.0 (57.0, 60.0) | 0.913 |
| Preoperative ALT (U/L) | 20.9 (14.6, 33.7) | 12.8 (11.6, 57.5) | 35.3 (17.4, 43.8) | 23.8 (15.3, 27.5) | 0.434 |
| Preoperative (AST) (U/L) | 20.2 (15.1, 27.6) | 25.4 (17.2, 49.6) | 25.4 (22.3, 44.1) | 19.4 (17.4, 32.3) | 0.103 |
| Preoperative UN (mmol/L) | 6.0 (4.7, 7.6) | 8.2 (4.9, 8.9) | 6.0 (3.9, 6.3) | 5.8 (4.3, 7.3) | 0.415 |
| Preoperative Cr (mmol/L) | 68.9 (57.3, 89.2) | 62.1 (44.8, 85.1) | 56.7 (48.8, 73.7) | 66.3 (47.0, 77.9) | 0.267 |
Preoperative factors of patients.
LVEF, left ventricular ejection fraction; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UN, urea nitrogen; Cr, creatinine.
Intraoperative data
Intraoperative data are summarized in Table 2. There were no significant differences among the four groups in terms of CPB time, aortic clamping time, circulatory arrest time, selective cerebral perfusion time, or nadir temperatures (both nasopharyngeal and rectal).
Table 2
| Indicators | IA group (n = 174) | LCA group (n = 5) | RSA group (n = 7) | RCA group (n = 21) | P-value |
|---|---|---|---|---|---|
| Aortic valve repair [n (%)] | 82 (47.1) | 4 (80.0) | 5 (71.4) | 12 (57.1) | 0.274 |
| Aortic valve replacement [n (%)] | 6 (3.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Bentall procedure [n (%)] | 20 (11.5) | 1 (20.0) | 0 (0.0) | 4 (19.0) | 0.417 |
| Wheat procedure [n (%)] | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| David procedure [n (%)] | 4 (2.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Ascending Aorta replacement [n (%)] | 136 (78.2) | 4 (80.0) | 6 (85.7) | 16 (76.2) | 0.969 |
| Total arch + ETS [n (%)] | 174 (100.0) | 5 (100.0) | 7 (100.0) | 20 (95.2) | 0.159 |
| Bilateral cerebral perfusion [n (%)] | 163 (93.7) | 5 (100.0) | 6 (85.7) | 20 (95.2) | 0.619 |
| CPB time (min) | 155.0 (137.0,180.0) | 170.0 (156.0,188.5) | 163.0 (134.0,179.0) | 171.0 (158.5,196.5) | 0.072 |
| CA time (min) | 6.0 (5.0,12.0) | 11.0 (4.5,15.0) | 6.0 (4.0,7.0) | 7.0 (5.0,13.0) | 0.330 |
| ACC time (min) | 88.5 (74.0,100.3) | 97.0 (91.5,117.0) | 96.0 (87.0,109.0) | 86.0 (59.0,105.5) | 0.280 |
| SCP time (min) | 30.0 (25.0, 36.3) | 28.0 (23.5, 49.0) | 32.0 (31.0, 39.0) | 30.0 (25.0, 44.5) | 0.560 |
| MNT (℃) | 29.5 (28.0, 30.4) | 29.4 (28.1, 30.2) | 31.6 (29.5, 32.3) | 28.8 (27.6, 30.5) | 0.069 |
| MRT (℃) | 31.0 (30.0, 32.0) | 30.9 (30.5, 31.9) | 31.0 (30.0, 32.0) | 31.1 (30.9, 31.9) | 0.589 |
Intraoperative factors of patients.
ETS, elephant trunk stent; CPB, cardiopulmonary bypass; ACC, aortic cross-clamp; CA, circulatory arrest; SCP, selective cerebral perfusion; MNT, minimum nasopharyngeal temperature; MRT, minimum rectal temperature.
Postoperative clinical outcomes
A total of 25 patients (12.1%) died during the perioperative period. This included 22 cases (12.6%) in the IA group, no deaths in the LCA group, 1 case (20.0%) in the RSA group, and 2 cases (28.6%) in the RCA group. While the RSA and RCA groups had numerically higher mortality rates, Fisher's exact test indicated no statistically significant differences among the groups.
Postoperative clinical metrics, including ventilator support duration, ICU stay, and hospital length of stay, were analyzed. No statistically significant differences were observed among the four groups (Table 3).
Table 3
| Indicators | IA group (n = 174) | LCA group (n = 5) | RSA group (n = 7) | RCA group (n = 21) | P-value |
|---|---|---|---|---|---|
| Ventilation time (h) | 38.08 (18.75, 88.56) | 21.08 (16.83, 1,180.42) | 64.33 (41.00, 83.00) | 22.33 (17.29, 88.25) | 0.611 |
| ICU stay time (h) | 46.46 (39.31, 112.02) | 85.17 (28.54, 1,926.42) | 89.25 (48.75, 161.17) | 45.58 (19.04, 113.04) | 0.301 |
| Reoperation for bleeding [n (%)] | 1 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1.000 |
| Reventilation [n (%)] | 7 (4.0) | 0 (0.0) | 2 (28.6) | 0 (0.0) | 0.073 |
| Postoperative 24 h Drainage (ml) | 200.00 (150.00, 270.00) | 200.00 (185.00, 495.00) | 240.00 (110.00, 350.00) | 180.00 (150.00, 260.00) | 0.660 |
| Hospitalization time (d) | 15.00 (11.00, 19.00) | 11.00 (7.00, 13.00) | 16.00 (10.00, 28.00) | 17.00 (11.00, 19.50) | 0.171 |
| In-hospital deat [n (%)] | 22 (12.6) | 1 (20.0) | 2 (28.6) | 0 (0.0) | 0.080 |
| CRRT [n (%)] | 14 (8.0) | 0 (0.0) | 1 (14.3) | 2 (9.5) | 0.720 |
| Preoperative ALT (U/L) | 25.670 (16.460, 41.478) | 29.590 (14.585, 79.700) | 49.230 (20.920, 170.910) | 31.190 (20.300, 59.475) | 0.285 |
| Preoperative (AST) (U/L) | 36.310 (26.833, 59.825) | 34.830 (25.570, 99.010) | 38.490 (26.320, 151.810) | 46.910 (25.630, 64.560) | 0.836 |
| Preoperative UN (mmol/L) | 13.125 (10.353, 16.065) | 11.740 (8.990, 21.945) | 14.860 (12.630, 19.790) | 11.750 (9.160, 14.870) | 0.174 |
| Preoperative Cr (mmol/L) | 119.455 (82.398, 167.225) | 75.100 (62.410, 237.025) | 172.930 (89.100, 274.180) | 99.270 (64.445, 156.325) | 0.262 |
| Stroke [n (%)] | 13 (7.5) | 1 (20.0) | 1 (14.3) | 1 (4.8) | 0.339 |
| Postoperative transfusion [n (%)] | 111 (63.8) | 3 (60.0) | 3 (42.9) | 12 (57.1) | 0.630 |
Postoperative factors of patients.
ICU, intensive care unit; CRRT, continuous renal replacement therapy; ALT, alanine aminotransferase; AST, aspartate aminotransferase; UN, urea nitrogen; Cr, creatinine.
Discussion
This study suggests that the innominate artery is a suitable and effective site for cannulation in minimally invasive incisions for ATAAD, with the left carotid artery, right carotid artery, and right subclavian artery serving as viable alternatives. While the favorable outcomes observed with innominate artery cannulation support its use, it should be noted that patients in whom this approach was feasible may represent a lower-risk subset, which could introduce selection bias. The use of aortic arch branches as the primary strategy for arterial perfusion eliminates the need for additional incisions, thereby improving cosmetic outcomes and reducing the risk of infection, while maintaining procedural feasibility and safety. Conventional ATAAD surgeries are widely regarded as highly invasive procedures associated with significant bleeding risks (14, 15), making patient survival the foremost priority. Although right axillary and femoral artery cannulation remain common due to their rapid setup, these methods require additional incisions, which can increase complications and compromise cosmetic outcomes. With advancements in minimally invasive techniques, the feasibility of performing ATAAD surgeries through single upper hemisternotomy incisions has been increasingly explored by cardiovascular surgery centers (16, 17). This approach aligns with clinical goals to minimize invasiveness while improving cosmetic outcomes.
This retrospective study of 207 ATAAD cases demonstrated the feasibility of using aortic arch branches as cannulation sites for arterial perfusion. Specifically, 174 cases utilized innominate artery (IA) cannulation, 21 used left carotid artery (LCA) cannulation, 5 adopted right subclavian artery (RSA) cannulation, and 7 employed right carotid artery (RCA) cannulation. These findings highlight the suitability of aortic arch branches for arterial perfusion in the majority of ATAAD surgeries, with IA cannulation being the preferred choice, followed by LCA, RSA, and RCA.
The IA group showed shorter cardiopulmonary bypass (CPB) and aortic clamping times compared to the other groups, although the differences were not statistically significant. This can be attributed to the direct cannulation technique used in the IA group, whereas the other groups required synthetic vessel anastomosis. Additionally, the severity of dissection involvement in the IA observed in other groups may have contributed to the lack of statistical significance. While these results suggest that cannulation site selection has limited impact on overall surgical outcomes, the study supports the feasibility and practicality of using aortic arch branches as arterial perfusion sites in minimally invasive ATAAD surgeries.
The selection of arterial perfusion cannulation is guided by several critical considerations, as outlined in reference (11). These include: (1) assessment of aortic arch branch involvement using preoperative computed tomography angiography (CTA); (2) intraoperative evaluation of the degree of branch involvement; (3) determination of branch diameter; (4) suitability for selective cerebral perfusion; and (5) ease of exposure and cannulation of the target vessel. Based on these criteria, the innominate artery is identified as the optimal site for arterial perfusion.
The innominate artery offers several distinct advantages: (1) it has the largest diameter among the aortic arch branches, ensuring adequate flow; (2) it provides straightforward exposure and facilitates direct cannulation; and (3) it supports the direct execution of selective cerebral perfusion. From our experience, a right-angled arterial cannula, oriented toward the heart, is preferred for direct insertion. Orienting the cannula toward the brain can result in excessive perfusion, known as luxury perfusion, which may have adverse effects. Given the relatively narrower diameter of the innominate artery compared to the ascending aorta, an 18Fr or 20Fr right-angled cannula is typically chosen based on intraoperative findings. Special attention is given to avoiding the use of an oversized cannula, which could occlude the innominate artery and compromise cerebral blood supply.
In cases where the innominate artery is affected or unsuitable for cannulation, alternative sites, including the left common carotid artery (LCA), right subclavian artery (RSA), and right carotid artery (RCA), are considered. Due to their smaller diameters, direct cannulation of these vessels is often impractical. Instead, synthetic vessels are utilized for end-to-side anastomosis, enabling connection to the cardiopulmonary bypass (CPB) system through a connector. This approach effectively achieves the desired arterial perfusion while addressing the limitations of smaller vessel diameters.
The most significant advantage of utilizing aortic arch branches as conduits for arterial perfusion lies in eliminating the need for additional surgical incisions (18). Compared to femoral artery cannulation, this approach offers several benefits: (1) mitigation of retrograde dissection expansion caused by retrograde perfusion; (2) prevention of blood supply to the false lumen, thereby avoiding retrograde blood flow that could dislodge atherosclerotic plaques and reduce the risk of stroke; (3) avoidance of lower limb ischemia resulting from cannulation; and (4) facilitation of antegrade selective cerebral perfusion, which is essential for optimal surgical outcomes (19, 20).
Similarly, when compared to axillary artery cannulation, aortic arch branch cannulation provides additional advantages (6, 21): (1) avoidance of the complex axillary artery dissection procedure, thereby reducing the risk of complications such as brachial plexus nerve injury; and (2) prevention of catastrophic complications associated with vascular anomalies, such as misdiagnosis of an aberrant right subclavian artery.
However, aortic arch branch cannulation is not without limitations. The restricted diameter of the target vessels may limit cannulation options and result in constrained flow rates. Nonetheless, during the cooling phase of cardiopulmonary bypass (CPB), the demand for high flow rates decreases, and this limitation becomes less critical. During the rewarming phase, a four-branch synthetic vessel is used for perfusion, ensuring adequate flow to meet metabolic demands.
Our clinical experience confirms that during total arch replacement performed through a minimally invasive single upper hemisternotomy, the simultaneous application of pump-controlled selective cerebral perfusion (13) and antegrade lower body perfusion (22) is both safe and feasible. Despite occupying a portion of the surgical field, these procedures can be effectively conducted through the minimally invasive incision, demonstrating the practicality of this approach in achieving successful surgical outcomes.
Limitations
This study has several inherent limitations: (1) As a retrospective investigation, the study is subject to selection bias, potentially influencing the results. (2) Being a single-center study with a relatively small sample size, the findings may be susceptible to confounding factors and may not be generalizable to broader populations. (3) The limited number of patients in the non-IA groups reduces the statistical power of between-group comparisons. This imbalance reflects institutional practice and is difficult to avoid in a retrospective single-center study, but it realistically represents real-world clinical decision-making. (4) In addition, patients who underwent innominate artery cannulation may have represented a lower-risk cohort with more favorable anatomy, particularly with respect to cerebral malperfusion. This potential selection bias could have influenced the observed clinical outcomes and should be considered when interpreting the results. Future multicenter studies with larger sample sizes are warranted to validate these findings and provide more robust conclusions.
Conclusion
This study demonstrates that aortic arch branch cannulation is a feasible and effective arterial perfusion strategy for acute Type A aortic dissection surgeries performed via a minimally invasive single upper hemisternotomy. This technique eliminates the need for additional incisions, enhances cosmetic outcomes, and maintains procedural safety. Among the aortic arch branches, the innominate artery was associated with favorable surgical outcomes and ease of access, and may be considered a suitable first-line option when feasible, though potential selection bias should be acknowledged.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Ethics Research Board of General Hospital of Northern Theater Command. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LX: Conceptualization, Funding acquisition, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. YL: Conceptualization, Data curation, Formal analysis, Investigation, Software, Writing – original draft. XX: Data curation, Investigation, Methodology, Writing – original draft. ZY: Data curation, Formal analysis, Methodology, Resources, Writing – review & editing. YG: Data curation, Formal analysis, Methodology, Resources, Validation, Writing – review & editing. BW: Data curation, Formal analysis, Investigation, Resources, Writing – review & editing. YL: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. HJ: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by the Independent Research Project of General Hospital of Northern Theater Command (grant ZZKY2024055), National Natural Science Foundation of China for Young Scholars (grant 82100513, 82203909), Science and Technology Foundation of Liaoning Province guided by the central government in 2023 (grant 2023JH6/100100034), Collaborative Program of Liaoning Province for Applied Basic Research in 2023 (grant 2023JH2/101700107) and Natural Science Foundation of Shenyang City (21-173-9-49).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2025.1549736/full#supplementary-material
References
1.
BaueAE. Dissections of the aorta. In: BaueAEGehaASHammondGLLaksHNaunheimKSGlennWWL, editors. Glenn’s Thoracic and Cardiovascular Surgery, 6th edn, vol 2. Stanford, CT: Appleton and Lange (1996). 2273–4.
2.
DebakeyMEMcCollumCHCrawfordE. Dissection and dissecting aneurysms of aorta: twenty year follow up of five hundred and twenty seven patients treated surgically. Surgery. (1982) 92:1118–34.
3.
KaulP. How I do it–sole innominate cannulation for acute type A aortic dissection. J Cardiothorac Surg. (2012) 7:125. 10.1186/1749-8090-7-125
4.
IsselbacherEMPreventzaOHamilton BlackJ3rdAugoustidesJGBeckAWBolenMAet al2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. (2022) 146(24):e334–e482. Epub 20221102.10.1161/cir.0000000000001106. PubMed PMID: ; PubMed Central PMCID: PMCPMC9876736.
5.
SabikJFLytleBWMcCarthyPMCosgroveDM. Axillary artery: an alternative site of arterial cannulation for patients with extensive aortic and peripheral vascular disease. J Thorac Cardiovasc Surg. (1995) 109(5):885–90; discussion 90-1.10.1016/s0022-5223(95)70312-8. PubMed PMID: .
6.
AbeTUsuiA. The cannulation strategy in surgery for acute type A dissection. Gen Thorac Cardiovasc Surg. (2017) 65(1):1–9. 10.1007/s11748-016-0711-7
7.
InoueYMinatoyaKSeikeYOhmuraAUeharaKSasakiHet alEarly results of total arch replacement under partial sternotomy. Gen Thorac Cardiovasc Surg. (2018) 66(6):327–33. Epub 20180329.10.1007/s11748-018-0913-2. PubMed PMID: .
8.
El-Sayed AhmadARisteskiPPapadopoulosNRadwanMMoritzAZiererA. Minimally invasive approach for aortic arch surgery employing the frozen elephant trunk technique. Eur J Cardiothorac Surg. (2016) 50(1):140–4. Epub 20160127.10.1093/ejcts/ezv484. PubMed PMID: .
9.
YangZJiangHLiuYGeYWangH. Both J- and L-shaped upper hemisternotomy approaches are suitable for total arch replacement with frozen elephant trunk in patients with Type A dissection. Front Cardiovasc Med. (2022) 9:998139. Epub 20221110.10.3389/fcvm.2022.998139. PubMed PMID: ; PubMed Central PMCID: PMCPMC9687356.
10.
ReeceTBTribbleCGSmithRLSinghRRStilesBMPeelerBBet alCentral cannulation is safe in acute aortic dissection repair. J Thorac Cardiovasc Surg. (2007) 133(2):428–34. Epub 20061229.10.1016/j.jtcvs.2006.09.059. PubMed. PMID: .
11.
JiangHLiuYYangZGeYLiLWangH. Total Arch Replacement via Single Upper-Hemisternotomy Approach in Patients With Type A Dissection. Ann Thorac Surg. (2020) 109(5):1394–9. Epub 20191004.10.1016/j.athoracsur.2019.08.095. PubMed PMID: .
12.
JiangHLiuYYangZGeYDuY. Mild Hypothermic Circulatory Arrest with Lower Body Perfusion for Total Arch Replacement Via Upper Hemisternotomy in Acute Type A Dissection. Heart Surg Forum. (2021) 24(2):E345–e50. Epub 20210401.10.1532/hsf.3729. PubMed PMID: .
13.
LiuYJiangHWangBYangZXiaLWangH. Efficacy of pump-controlled selective antegrade cerebral perfusion in total arch replacement: A propensity-matched analysis. Front Surg. (2022) 9:918461. Epub 20220818.10.3389/fsurg.2022.918461. PubMed PMID: ; PubMed Central PMCID: PMCPMC9433986.
14.
EvangelistaAIsselbacherEMBossoneEGleasonTGEusanioMDSechtemUet alInsights From the International Registry of Acute Aortic Dissection: A 20-Year Experience of Collaborative Clinical Research. Circulation. (2018) 137(17):1846–60. 10.1161/circulationaha.117.031264. PubMed PMID: .
15.
DuanLZhangCChenXWangEYeZDuanYet alMyocardial Priority Promotes Cardiovascular Recovery for Acute Type A Aortic Dissection Combined with Coronary Artery Disease Undergoing Aortic Arch Surgery. J Pers Med. (2023) 13(9). Epub 20230825.10.3390/jpm13091296. PubMed PMID: ; PubMed Central PMCID: PMCPMC10532919.
16.
XieXBDaiXFFangGHQiuZHJiangDBChenLW.Extensive repair of acute type A aortic dissection through a partial upper sternotomy and using complete stent-graft replacement of the arch. J Thorac Cardiovasc Surg. (2022) 164(4):1045–52. Epub 20201024.10.1016/j.jtcvs.2020.10.063. PubMed PMID: .
17.
XiaoCSLiDRenCLWangG. Total Arch Replacement and Frozen Elephant Trunk Implantation for Acute Type A Dissection Using Complete Cerebral Perfusion and Upper Hemisternotomy. Chin Med J (Engl). (2018) 131(17):2130–1. 10.4103/0366-6999.239302. PubMed PMID: ; PubMed Central PMCID: PMCPMC6111687.
18.
PreventzaOBakaeenFGStephensEHTrocciolaSMde la CruzKICoselliJS. Innominate artery cannulation: an alternative to femoral or axillary cannulation for arterial inflow in proximal aortic surgery. J Thorac Cardiovasc Surg. (2013) 145(3 Suppl):S191–6. Epub 20121220.10.1016/j.jtcvs.2012.11.061. PubMed PMID: .
19.
ShimokawaTTakanashiSOzawaNItohT.Management of intraoperative malperfusion syndrome using femoral artery cannulation for repair of acute type A aortic dissection. Ann Thorac Surg. (2008) 85(5):1619–24. 10.1016/j.athoracsur.2008.01.048. PubMed PMID: .
20.
FuscoDSShawRKTranquilliMKopfGSElefteriadesJA. Femoral cannulation is safe for type A dissection repair. Ann Thorac Surg. (2004) 78(4):1285–9; discussion -9.10.1016/j.athoracsur.2004.04.072. PubMed PMID: .
21.
BednarkiewiczMBruschweilerIChristensonJT. Undiagnosed aberrant right subclavian artery: pitfall in aortic arch surgery. Cardiovasc Surg. (2003) 11(1):61–3. 10.1177/096721090301100111
22.
TangZLvYWangBYangZLiuYJiangH. Lower Body Perfusion Reduces the Morbidity of Postoperative Acute Kidney Injury in Type A Dissection: A Propensity-Matched Analysis. Braz J Cardiovasc Surg. (2023) 38(3):346–52. Epub 20230504.10.21470/1678-9741-2022-0190. PubMed PMID: ; PubMed Central PMCID: PMCPMC10159064.
Summary
Keywords
acute type A aortic dissection, minimally invasive surgery, arterial cannulation, aortic arch branch perfusion, single upper hemisternotomy
Citation
Xia L, Lyu Y, Xiao X, Yang Z, Ge Y, Wang B, Liu Y and Jiang H (2025) Optimizing aortic arch branch cannulation in acute type A dissection surgery: a minimally invasive approach. Front. Cardiovasc. Med. 12:1549736. doi: 10.3389/fcvm.2025.1549736
Received
21 December 2024
Accepted
05 May 2025
Published
16 May 2025
Volume
12 - 2025
Edited by
Bernhard Winkler, Vienna Health Association, Austria
Reviewed by
Nicolas Henry Pope, Medical University of South Carolina, United States
Arnaud Van Linden, University Hospital Frankfurt, Germany
Updates
Copyright
© 2025 Xia, Lyu, Xiao, Yang, Ge, Wang, Liu and Jiang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Liu heroliu2000@sina.com Hui Jiang jiangh33471@126.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.