- 1Xuzhou Clinical College of Xuzhou Medical University, Division of Cardiology, Xuzhou Central Hospital, Xuzhou, Jiangsu, China
- 2Division of Cardiology, Wuxi People's Hospital Affiliated to Nanjing Medical University, Wuxi, Jiangsu, China
Objective: The aim of this study was to investigate the impact of preprocedural anticoagulation on the incidence of silent cerebral embolisms (SCEs) assessed by magnetic resonance imaging (MRI) after catheter ablation of atrial fibrillation (AF) in patients with low thromboembolic risk.
Methods and results: A total of 141 patients with AF who were identified with low thromboembolic risk based on CHA2DS2-VASc score (0 or 1 for males and 1 or 2 for females) were enrolled in this study. According to whether or not oral anticoagulants (OACs) had been administered for more than 3 weeks prior to the procedure, patients were divided into the anticoagulation group (n = 49) and the non-anticoagulation group (n = 92). Pulmonary veins were isolated by utilizing irrigated-tip ablation catheters under the guidance of the Carto system. A cerebral MRI was performed 24 to 48 h after ablation to detect any new-onset SCEs. The incidences of SCEs were compared between the two groups. SCEs were detected in 25 (17.7%) patients. The incidence of SCEs was significantly higher in the non-anticoagulation group compared with the anticoagulation group [22/92 [23.9%] vs. 3/49 [6.1%], P = 0.002]. Multivariate logistic regression analysis showed that the preprocedural application of OACs for more than 3 weeks was the only independent protective factor of SCEs after AF ablation.
Conclusion: AF ablation carried a substantial risk of SCEs even in patients with low thromboembolic risk. Preprocedural anticoagulation for more than 3 weeks can significantly reduce the incidence of SCEs after ablation in AF patients.
Introduction
Atrial fibrillation (AF) is one of the most prevalent cardiac arrhythmias and an important cause of stroke (1, 2). Catheter ablation has evolved into an effective therapeutic strategy for patients with symptomatic AF (3). Cerebral embolism is one of the most crucial complications following AF ablation. Although symptomatic strokes are rare (4), silent cerebral embolisms (SCEs) are relatively common, ranging from 10% to 40% in different reports (5–8). SCEs have been verified to cause cognitive impairment and dementia potentially and should warrant cautious attention (9, 10).
Studies have shown that an uninterrupted periprocedural anticoagulation strategy can reduce strokes and SCEs (11–13). Accordingly, the guidelines recommend continuous anticoagulant therapy before ablation (14). On the other hand, some studies have confirmed that electroconversion was safe following thrombus exclusion using transesophageal echocardiography (TEE) in patients with AF who have not received conventional anticoagulant treatment for over three weeks (15, 16). Moreover, other studies have demonstrated that catheter ablation without standardized use of oral anticoagulants (OACs) does not increase the incidence of thromboembolism for patients with low thromboembolic risk (17). Therefore, the guidelines also suggest using TEE to exclude left atrial (LA) thrombus as an alternative to conventional anticoagulation, especially for low-risk patients (18). However, there is currently insufficient research to determine whether the absence of receiving standardized anticoagulant therapy prior to ablation procedures can increase the occurrences of SCEs in low-risk AF patients. This study aims to investigate the impact of continuous use of OACs before AF ablation on the incidences of postprocedural SCEs in patients with a CHA2DS2-VASC score of 0–1 for males and 1–2 for females.
Materials and methods
Patient characteristics
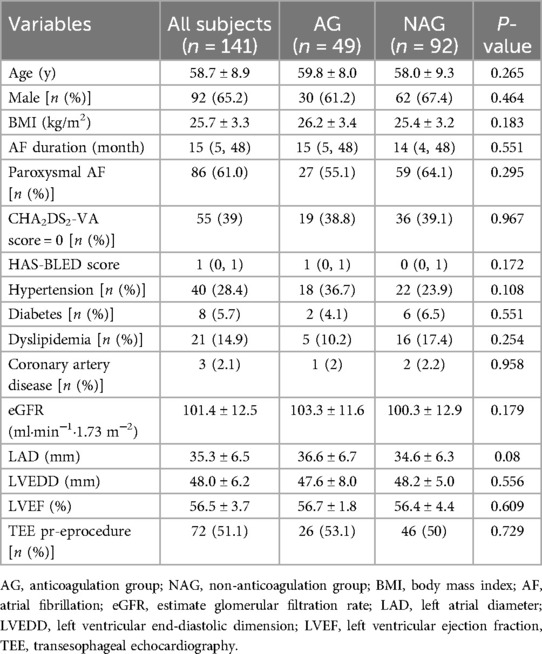
AF patients with low thromboembolic risk who underwent their first-time catheter ablation in Xuzhou Central Hospital from December 2019 to July 2022 were enrolled in this study. The inclusion criteria were as follows: documented paroxysmal or persistent symptomatic non-valvular AF, aged between 18 and 80 years, with CHA2DS2-VASc score (excluding female sex) of 0 or 1. The exclusion criteria included: (1) history of AF catheter ablation or percutaneous left atrial appendage (LAA) occlusion; (2) history of stroke or transient ischemic attacks (TIA); (3) existence of neurological impairment or intracardiac thrombus; (3) contraindications for magnetic resonance imaging (MRI) examination: the presence of cardiac pacemaker, implantable cardioverter defibrillator, or other metal implants that are not compatible with MRI, or severe claustrophobia. Patients who received new oral anticoagulants (NOACs) or warfarin for more than 3 weeks prior to ablation were included in the anticoagulation group. In this group of patients, OACs were uninterruptedly administered on the day of the procedure. Patients not receiving preprocedural anticoagulation were included in the non-anticoagulation group (Figure 1). Antiarrhythmic medications were discontinued at least five half-lives before the procedure. There was no pharmacological or electrical cardioversion in 1 month before PVI in all enrolled patients. Informed consent was signed by all participants prior to study enrollment. The study protocol was reviewed and approved by the ethics committees of Xuzhou Central Hospital.
Electrophysiological study and catheter ablation
TEE or contrast-enhanced computed tomography (CT) was performed on each patient within 24 h before procedure to confirm the absence of LA thrombus. The ablation protocol was the same as we previously reported (19). Local anaesthesia were administered for all procedures. After double transseptal punctures, two 8.5F SL1 sheaths (St. Jude Medical, St. Paul, MN) were advanced into the LA. A circular mapping catheter (Lasso, Biosense-Webster, Diamond Bar, CA) was introduced through a transseptal sheath to record pulmonary vein (PV) potentials. An irrigated contact force–sensing catheter (ThermoCool SmartTouch, Biosense Webster, Diamond Bar, CA) was used for mapping and ablation. The ablation procedure was performed under the guidance of an electro-anatomic mapping system (CARTO 3, Biosense Webster, Irvine, CA). For all patients, PV isolation (PVI) was the primary ablation strategy. Linear point-by-point lesions were placed in the PV antra with the endpoint of electrical isolation. Radiofrequency energy was delivered continuously while moving the catheter tip every 20–30 s, with a maximum power of 30 W on the posterior wall and 35 W on the anterior wall. The temperature of the radiofrequency energy was limited to 43°C, with irrigation rates of 17 to 30 ml/min. If AF persisted after complete PVI, sinus rhythm (SR) was restored by electrical cardioversion. PV-LA conduction block was confirmed again during SR.
Anticoagulation during procedure
Baseline activated clotting time (ACT) was measured at the beginning of the procedure. A bolus of unfractionated heparin was injected immediately after the transseptal puncture. Initial heparin dosing was determined according to the baseline ACT and the patient's body weight. ACT was monitored every 20–30 min during the procedure. The target ACT value was ≥300 s in all patients. Once ACT was less than 300 s, a supplemental heparin bolus was given to keep ACT between 300 and 350 s. The total amount of heparin consumption and the mean ACT during the whole procedure were calculated.
Cerebral magnetic resonance imaging
All patients underwent cerebral MRI at 3.0 Tesla (Philips Medical Systems) 24–48 h after the ablation procedure. Acute SCEs were defined as focal hyperintense lesions on diffusion-weighted (DW) imaging with corresponding hypointense lesions on the apparent diffusion coefficient (ADC) map in a typical vascular pattern, while patients had no neurological disorders. Imaging data were analyzed by two radiologists with at least two years of work experience and blinded to the patient's relevant clinical data. Neurological physical examinations were performed by the physicians both before and after the ablation procedure.
Postablation care and follow-up
All patients were required to take OACs for at least 2 months after the procedure. Antiarrhythmic drugs were also given during the first 2–3 months. All patients received regular telephone and clinic follow-up to confirm whether there were complications or arrhythmia recurrences.
Statistical analysis
SPSS software (Version 23.0; IBM, Armnok, NY, USA) was used for statistical analysis, and the difference was considered statistically significant at P value < 0.05. Variables in normal distribution were presented as mean ± SD and analyzed via Student's t-test, while the non-normally distributed variables were presented as median (interquartile range) and analyzed via Mann–Whitney U-test. Categorical variables were presented as numbers (percentages) and compared using the χ2 test. Univariate and multivariate logistical regression analyses were performed to evaluate the predictors of SCEs. Factors with P-values of <0.05 in the univariate analysis were included in a multivariate logistic regression model to identify independent predictors.
Results
Patient baseline characteristics and procedural variables
A total of 141 AF patients were enrolled in this study. Of these, 49 patients belonged to the anticoagulation group and 92 to the non-anticoagulation group. There were no significant differences between the two groups at the baseline characteristics (Table 1). With regard to the procedural variables, the ACT before the first heparin bolus was significantly longer, and the total amount of heparin consumption was less in the anticoagulation group than in the non-anticoagulation group. Whereas the procedure time, the mean ACT and the proportion of receiving electrical cardioversion were equivalent in both groups (Table 2).
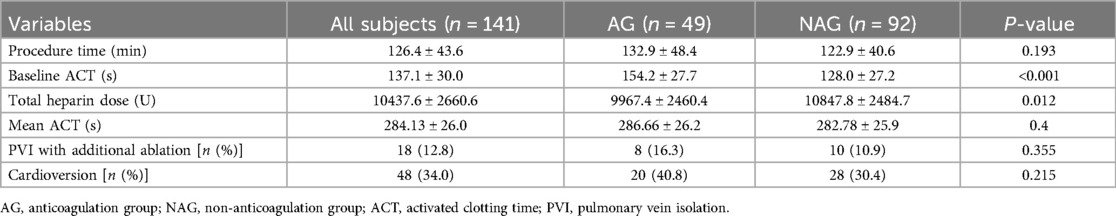
Table 2. Comparison of procedural variables between the anticoagulation group and non-anticoagulation group.
The incidence of SCEs and other complications after AF ablation
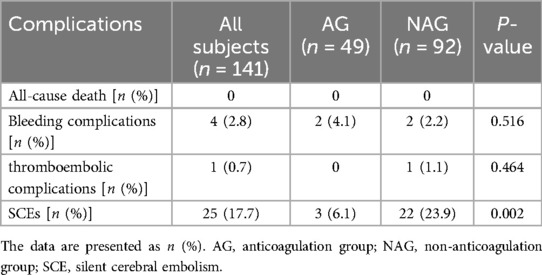
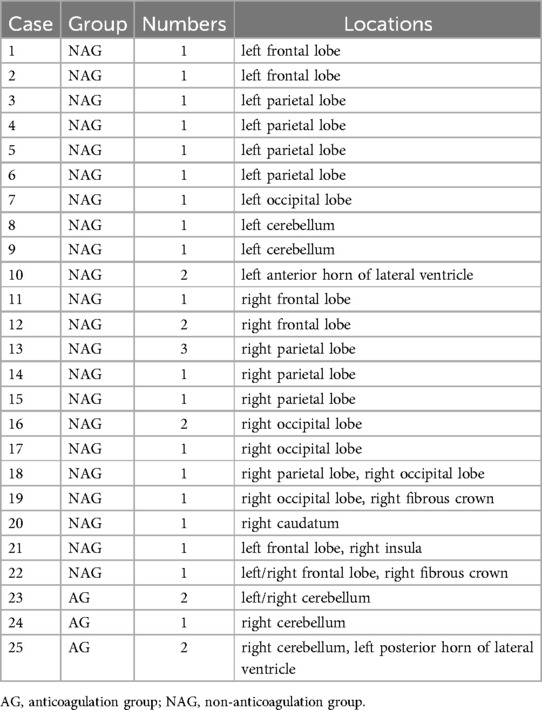
Bleeding events occurred in two patients of the anticoagulation group, including one case of gastrointestinal bleeding and another case of arteriovenous fistula, while two patients experienced inguinal hematomas in the non-anticoagulation group. All patients with bleeding events remained hemodynamically stable and did not require surgical intervention. One 37-year-old male patient with the CHA2DS2-VASc score of 1 in the non-anticoagulation group experienced a symptomatic stroke in the supply area of the right medial cerebral artery, which caused persistent weakness and difficulty in moving the left limbs the second day after the procedure. The total occurrences of complications were similar between the two groups (Table 3). New on-set SCEs were detected by MRI in 25 (17.7%) patients overall after the procedure. Most lesions were solitary and mainly located in the lobes of the cortex, including frontal lobe, parietal lobe, and occipital lobe. The prevalences between the bilateral hemispheres were comparable (Table 4). The incidence of SCEs was significantly higher in the non-anticoagulation group compared to the anticoagulation group (23.9% vs. 6.1%, P = 0.002) (Table 3).
Comparisons of clinical and procedural characteristics between patients with and without SCEs
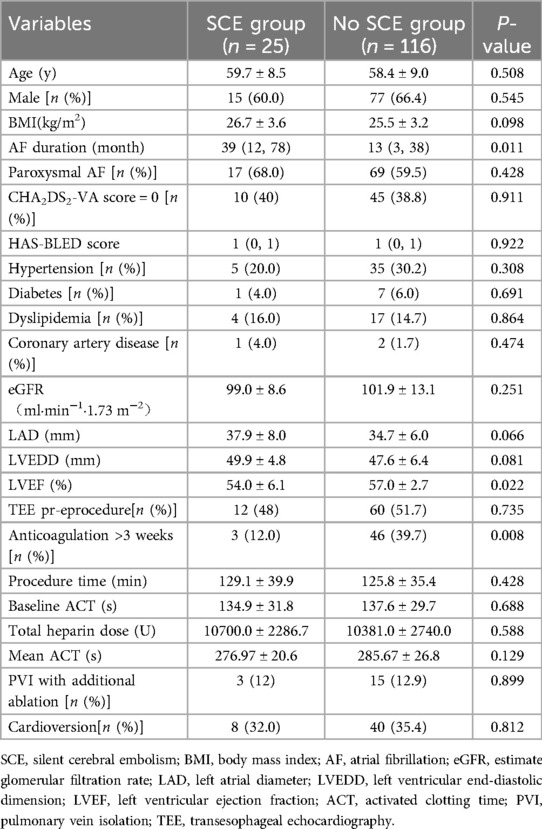
Patients with SCEs had longer AF durations and lower left ventricular ejection fraction (LVEF) compared to those without SCEs. There were no differences in the other characteristics. The details were shown in Table 5.
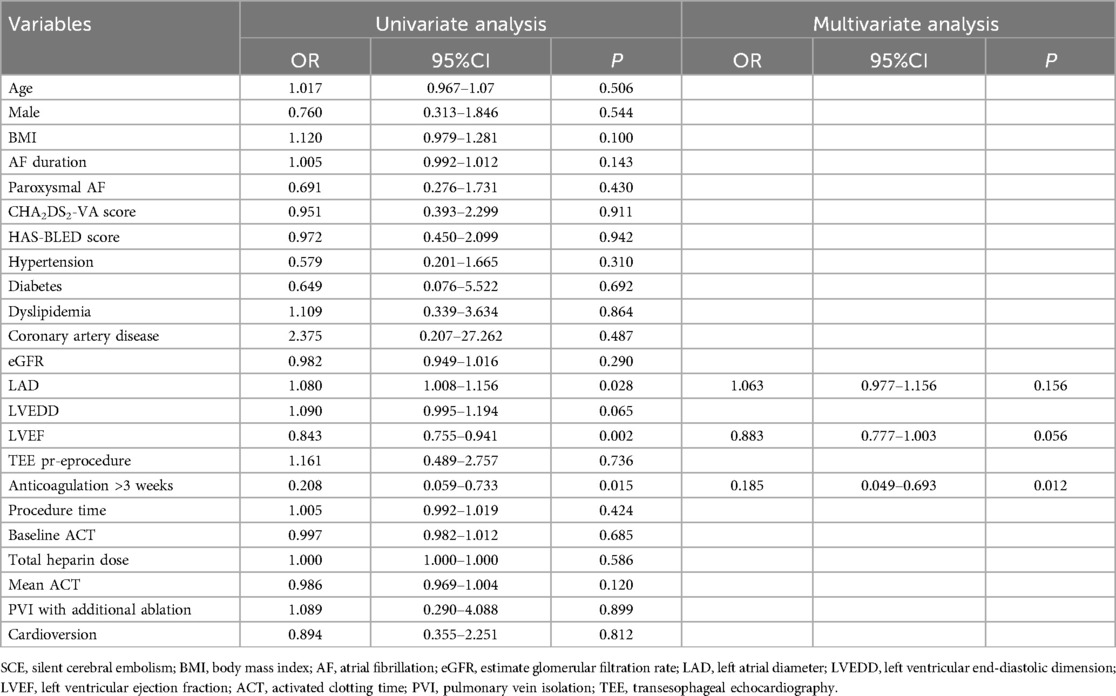
Predictors of SCEs after AF ablation
Univariate logistic regression analysis revealed that left atrial diameter (LAD), LVEF, and whether receiving anticoagulation prior to the procedure were associated with the occurrences of SCEs. In a multivariate regression analysis, receiving anticoagulation for more than 3 weeks prior to the procedure was identified as the only independent protective factor for SCEs [odds ratio [OR] 0.185, 95% confidence interval [CI] 0.049–0.693, P = 0.012] (Table 6).
Discussion
The main findings of our study are as follows: (1) 17.7% of AF patients with CHA2DS2-VASc score (excluding female sex) of 0 or 1 undergoing PVI had newly visible SCEs on post-procedural MRI; (2) In these patients, anticoagulation therapy for more than 3 weeks before the procedure was associated with a lower incidence of SCEs.
The CHA2DS2-VASc score has been shown to reliably predict stroke risk in patients with AF and recommended for determining whether oral anticoagulation is warranted (20, 21). Patients with a CHA2DS2-VASc score of 0–1 (excluding female sex) are judged to have a low to intermediate risk of stroke. Although current guidelines do not mandate anticoagulation treatment for these patients (18), there still exists controversy on whether anticoagulation is necessary, and conflicting results have been obtained from various studies. Mobley et al.'s research indicated that AF patients with low CHA2DS2-VASc score are still at a substantially increased risk of thromboembolic events (22). Friberg et al. (23) reported that AF patients with a CHA2DS2-VASc score of 1 have a relatively low risk of ischemic stroke, and these patients do not benefit from routine OAC administration. An observational study by Komen et al. (24) suggested that NOACs treatment might be associated with positive net clinical benefits for patients at low risk for stroke (one non-sex-related CHA2DS2-VASc point). It is important to note that the CHA2DS2-VASc score has certain limitations as it does not consider many factors related to stroke risk, such as duration and load of AF, LA size, renal function, coagulation parameters, etc. (25, 26). Therefore, for patients judged to be at low risk of stroke based on the CHA2DS2-VASc score, a more comprehensive evaluation should be conducted when deciding whether to receive anticoagulant therapy and further research is needed (27).
Many previous studies have indicated that adequate anticoagulation therapy is highly effective in mitigating the risk of thromboembolic complications after AF ablation (11). There are also studies confirming that uninterrupted periprocedural use of OACs can reduce the occurrence of SCEs (12, 13). Based on these findings, current guidelines recommend using OACs for at least three weeks before the ablation procedure as a Class I indication. Meanwhile, excluding LA thrombus by TEE is also recommended as an alternative strategy, especially for low thromboembolic risk patients (18). However, to the best of our knowledge, there have been no studies focusing on the impact of periprocedural anticoagulation management on postprocedural thromboembolism and SCEs in low-risk patients. This pilot study revealed an association between the occurrence of SCEs and periprocedural use of OACs, suggesting that even for patients with low thromboembolic risk, anticoagulation therapy for at least three weeks before AF ablation may be beneficial.
There are several possible reasons for the occurrence of SCE. Firstly, undetected pre-existing LA/LAA microthrombi could be dislodged during the procedure. Although TEE is considered as the gold standard for diagnosing LA thrombus, its sensitivity does not reach 100%, particularly when LAA cannot be adequately visualized (28). Previous studies have confirmed that LA thrombi detected by intracardiac echocardiography were vanished after ≥4 weeks of continuous direct oral anticoagulants (DOACs) therapy (29), suggesting that preprocedural anticoagulation treatment may reduce the occurrence of SCEs caused by intraprocedural microthrombus dislodgment. Secondly, SCEs might have existed before ablation. Due to the lack of preprocedural MR imaging in this study, the existing SCEs could not be ruled out. Herm et al.'s study (30) found that even in low- to medium-risk patients, the detection rate of SCEs was 8%. Thirdly, the occurrence of SCEs was related to ablation or catheter manipulation. Endothelial damage and coagulation system activation caused by ablation, air embolism occurring during sheath insertion, and gas bubbles forming during ablation were all possible causes of cerebral embolism and SCEs (4, 7). Furthermore, the correlation between intraprocedural ACT value and the occurrence of SCEs has received attention. Wazni et al.'s study (31) displayed that maintaining ACT at 300–350 s for patients undergoing AF ablation could significantly reduce periprocedural embolic events compared to maintaining ACT at 250–300 s. Scaglione et al. (32) reported that there was no SCEs occurred for intraprocedural ACT >320 s. Doi et al. (33) indicated that minimum ACT ≤260 s was an independent predictor of SCEs. In the present study, the average ACT value was 284.13 s, which may contribute to the relatively high incidence of SCEs observed in this cohort. Further research is required to determine whether increasing the ACT value during the procedure will have an impact on the outcomes. Another study conducted by Harada M et al. (34) demonstrated that compared with patients without SCEs, those with SCEs had lower baseline ACT before heparin injection and a longer time to reach optimal ACT. In our study, even though the mean ACT level was equivalent between both groups, the anticoagulation group had a higher baseline ACT at the beginning of the procedure. This may be one of the reasons account for the lower incidence of SCEs in this group.
Study limitations
This study had several limitations. Firstly, this was only a preliminary experience in a single center with a relatively small sample size limited to Chinese patients. Secondly, it was a non-randomized study. There was a risk of selection bias due to whether taking OACs before ablation was at the discretion of the treating physician. Prospective randomized studies involving larger patient populations are required to further determine the impact of anticoagulation strategies on the occurrence of SCEs associated with AF ablation in patients with low thromboembolic risk.
Conclusions
Even in patients with low thromboembolic risk, there are still substantial risks of SCEs after AF ablation, and continuous anticoagulation therapies before the procedures are associated with lower incidences of SCEs. These findings suggests additional investigations into clinical decision-making for patients with AF at low thromboembolic risk on future preprocedural anticoagulation strategies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Biomedical Research Ethics Review Committee of Xuzhou central hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MW: Data curation, Methodology, Writing – original draft, Conceptualization. WD: Data curation, Methodology, Writing – original draft. Y-lF: Data curation, Methodology, Writing – original draft. HY: Data curation, Investigation, Writing – original draft. Q-sD: Data curation, Resources, Writing – original draft. X-jL: Resources, Validation, Writing – review & editing. S-jL: Supervision, Writing – review & editing. R-xW: Conceptualization, Methodology, Writing – review & editing. BH: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Xuzhou science and technology project (Grant No. KC21155), Xuzhou leading talent training project (Grant No. XWRCHT20210032).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. (2014) 129:837–47. doi: 10.1161/circulationaha.113.005119
2. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart disease and stroke statistics-2022 update: a report from the American heart association. Circulation. (2022) 145:e153–639. doi: 10.1161/cir.0000000000001052
3. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Updated worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circ Arrhythm Electrophysiol. (2010) 3:32–8. doi: 10.1161/circep.109.859116
4. Haeusler KG, Kirchhof P, Endres M. Left atrial catheter ablation and ischemic stroke. Stroke. (2012) 43:265–70. doi: 10.1161/strokeaha.111.627067
5. Schrickel JW, Lickfett L, Lewalter T, Mittman-Braun E, Selbach S, Strach K, et al. Incidence and predictors of silent cerebral embolism during pulmonary vein catheter ablation for atrial fibrillation. Europace. (2010) 12:52–7. doi: 10.1093/europace/eup350
6. Gaita F, Caponi D, Pianelli M, Scaglione M, Toso E, Cesarani F, et al. Radiofrequency catheter ablation of atrial fibrillation: a cause of silent thromboembolism? Magnetic resonance imaging assessment of cerebral thromboembolism in patients undergoing ablation of atrial fibrillation. Circulation. (2010) 122:1667–73. doi: 10.1161/CIRCULATIONAHA.110.937953
7. Haeusler KG, Koch L, Herm J, Kopp UA, Heuschmann PU, Endres M, et al. 3 Tesla MRI-detected brain lesions after pulmonary vein isolation for atrial fibrillation: results of the MACPAF study. J Cardiovasc Electrophysiol. (2013) 24:14–21. doi: 10.1111/j.1540-8167.2012.02420.x
8. Calvert P, Kollias G, Pürerfellner H, Narasimhan C, Osorio J, Lip GYH, et al. Silent cerebral lesions following catheter ablation for atrial fibrillation: a state-of-the-art review. Europace. (2023) 25:1–8. doi: 10.1093/europace/euad151
9. Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. (2003) 348:1215–22. doi: 10.1056/NEJMoa022066
10. Kühne M, Krisai P, Coslovsky M, Rodondi N, Müller A, Beer JH, et al. Silent brain infarcts impact on cognitive function in atrial fibrillation. Eur Heart J. (2022) 43:2127–35. doi: 10.1093/eurheartj/ehac020
11. Di Biase L, Burkhardt JD, Santangeli P, Mohanty P, Sanchez JE, Horton R, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the role of coumadin in preventing thromboembolism in atrial fibrillation (AF) patients undergoing catheter ablation (COMPARE) randomized trial. Circulation. (2014) 129:2638–44. doi: 10.1161/circulationaha.113.006426
12. Di Biase L, Gaita F, Toso E, Santangeli P, Mohanty P, Rutledge N, et al. Does periprocedural anticoagulation management of atrial fibrillation affect the prevalence of silent thromboembolic lesion detected by diffusion cerebral magnetic resonance imaging in patients undergoing radiofrequency atrial fibrillation ablation with open irrigated catheters? Results from a prospective multicenter study. Heart Rhythm. (2014) 11:791–8. doi: 10.1016/j.hrthm.2014.03.003
13. Nagao T, Suzuki H, Matsunaga S, Nishikawa Y, Harada K, Mamiya K, et al. Impact of periprocedural anticoagulation therapy on the incidence of silent stroke after atrial fibrillation ablation in patients receiving direct oral anticoagulants: uninterrupted vs. interrupted by one dose strategy. Europace. (2019) 21:590–7. doi: 10.1093/europace/euy224
14. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J. (2021) 42:373–498. doi: 10.1093/eurheartj/ehaa612
15. Roijer A, Eskilsson J, Olsson B. Transoesophageal echocardiography-guided cardioversion of atrial fibrillation or flutter. Selection of a low-risk group for immediate cardioversion. Eur Heart J. (2000) 21:837–47. doi: 10.1053/euhj.1999.1869
16. Klein AL, Grimm RA, Murray RD, Apperson-Hansen C, Asinger RW, Black IW, et al. Use of transesophageal echocardiography to guide cardioversion in patients with atrial fibrillation. N Engl J Med. (2001) 344:1411–20. doi: 10.1056/nejm200105103441901
17. Duytschaever M, Berte B, Acena M, De Meyer G, Bun SS, Van Heuverswyn F, et al. Catheter ablation of atrial fibrillation in patients at low thrombo-embolic risk: efficacy and safety of a simplified periprocedural anticoagulation strategy. J Cardiovasc Electrophysiol. (2013) 24:855–60. doi: 10.1111/jce.12148
18. Van Gelder IC, Rienstra M, Bunting KV, Casado-Arroyo R, Caso V, Crijns HJGM, et al. 2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2024) 45:3314–414. doi: 10.1093/eurheartj/ehae176
19. Zheng J, Wang M, Tang QF, Xue F, Li KL, Dang SP, et al. Atrial fibrillation ablation using robotic magnetic navigation reduces the incidence of silent cerebral embolism. Front Cardiovasc Med. (2021) 8:777355. doi: 10.3389/fcvm.2021.777355
20. Leif Friberg MR, Lip GYH. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish atrial fibrillation cohort study. Eur Heart J. (2012) 33:1500–10. doi: 10.1093/eurheartj/ehr488
21. Kim TH, Yang PS, Kim D, Yu HT, Uhm JS, Kim JY, et al. CHA(2)DS(2)-VASc score for identifying truly low-risk atrial fibrillation for stroke: a Korean nationwide cohort study. Stroke. (2017) 48:2984–90. doi: 10.1161/strokeaha.117.018551
22. Mobley AR, Subramanian A, Champsi A, Wang X, Myles P, McGreavy P, et al. Thromboembolic events and vascular dementia in patients with atrial fibrillation and low apparent stroke risk. Nat Med. (2024) 30:2288–94. doi: 10.1038/s41591-024-03049-9
23. Friberg L, Skeppholm M, Terént A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol. (2015) 65:225–32. doi: 10.1016/j.jacc.2014.10.052
24. Komen JJ, Pottegård A, Mantel-Teeuwisse AK, Forslund T, Hjemdahl P, Wettermark B, et al. Oral anticoagulants in patients with atrial fibrillation at low stroke risk: a multicentre observational study. Eur Heart J. (2022) 43(37):3528–38. doi: 10.1093/eurheartj/ehac111
25. Szymanski FM, Lip GY, Filipiak KJ, Platek AE, Hrynkiewicz-Szymanska A, Opolski G. Stroke risk factors beyond the CHA₂DS₂-VASc score: can we improve our identification of “high stroke risk” patients with atrial fibrillation? Am J Cardiol. (2015) 116:1781–8. doi: 10.1016/j.amjcard.2015.08.049
26. Kaplan RM, Koehler J, Ziegler PD, Sarkar S, Zweibel S, Passman RS. Stroke risk as a function of atrial fibrillation duration and CHA(2)DS(2)-VASc score. Circulation. (2019) 140:1639–46. doi: 10.1161/circulationaha.119.041303
27. Overvad TF, Nielsen PB, Lip GY. Treatment thresholds for stroke prevention in atrial fibrillation: observations on the CHA2DS2-VASc score. Eur Heart J Cardiovasc Pharmacother. (2017) 3:37–41. doi: 10.1093/ehjcvp/pvw022
28. Ren JF, Marchlinski FE, Supple GE, Hutchinson MD, Garcia FC, Riley MP, et al. Intracardiac echocardiographic diagnosis of thrombus formation in the left atrial appendage: a complementary role to transesophageal echocardiography. Echocardiography. (2013) 30:72–80. doi: 10.1111/j.1540-8175.2012.01819.x
29. Patel K, Natale A, Yang R, Trivedi C, Romero J, Briceno D, et al. Is transesophageal echocardiography necessary in patients undergoing ablation of atrial fibrillation on an uninterrupted direct oral anticoagulant regimen? Results from a prospective multicenter registry. Heart Rhythm. (2020) 17:2093–9. doi: 10.1016/j.hrthm.2020.07.017
30. Herm J, Schurig J, Martinek MR, Höltgen R, Schirdewan A, Kirchhof P, et al. MRI-detected brain lesions in AF patients without further stroke risk factors undergoing ablation—a retrospective analysis of prospective studies. BMC Cardiovasc Disord. (2019) 19:58. doi: 10.1186/s12872-019-1035-1
31. Wazni OM, Rossillo A, Marrouche NF, Saad EB, Martin DO, Bhargava M, et al. Embolic events and char formation during pulmonary vein isolation in patients with atrial fibrillation: impact of different anticoagulation regimens and importance of intracardiac echo imaging. J Cardiovasc Electrophysiol. (2005) 16:576–81. doi: 10.1111/j.1540-8167.2005.40480.x
32. Scaglione M, Blandino A, Raimondo C, Caponi D, Gaita F. Impact of ablation catheter irrigation design on silent cerebral embolism after radiofrequency catheter ablation of atrial fibrillation: results from a pilot study. J Cardiovasc Electrophysiol. (2012) 23:801–5. doi: 10.1111/j.1540-8167.2012.02298.x
33. Doi A, Takagi M, Kakihara J, Hayashi Y, Tatsumi H, Fujimoto K, et al. Incidence and predictors of silent cerebral thromboembolic lesions after catheter ablation for atrial fibrillation in patients treated with direct oral anticoagulants. Heart Vessels (2017) 32(10):1227–35. doi: 10.1007/s00380-017-0985-4
Keywords: atrial fibrillation, anticoagulation, catheter ablation, silent cerebral embolism, low thromboembolic risk
Citation: Wang M, Du W, Fei Y-l, Yang H, Dong Q-s, Li X-j, Li S-j, Wang R-x and Han B (2025) Impact of preprocedural anticoagulation on the incidence of silent cerebral embolisms after catheter ablation of atrial fibrillation in patients with low thromboembolic risk. Front. Cardiovasc. Med. 12:1559347. doi: 10.3389/fcvm.2025.1559347
Received: 12 January 2025; Accepted: 3 April 2025;
Published: 24 April 2025.
Edited by:
Faisal Syed, University of North Carolina at Chapel Hill, United StatesReviewed by:
Zoltán Salló, Semmelweis University, HungaryTobias Uhe, University Hospital Leipzig, Germany
Copyright: © 2025 Wang, Du, Fei, Yang, Dong, Li, Li, Wang and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bing Han, aGJpbmc3NzdAaG90bWFpbC5jb20=; Ru-xing Wang, cnV4aW5nd0BhbGl5dW4uY29t
†These authors have contributed equally to this work
 Meng Wang
Meng Wang Wei Du
Wei Du Ya-lan Fei1
Ya-lan Fei1 Ru-xing Wang
Ru-xing Wang Bing Han
Bing Han