Abstract
Introduction:
Heart valves have long been regarded as uncomplicated, avascular, and passive structures. However, we hypothesise that their structure and function are complex. Therefore, we have reviewed the available literature to gain a profound understanding of their cellular composition and (patho)physiological behaviour.
Methods:
A systematic search for articles related to the anatomy, histology, and physiology of heart valves was conducted using PubMed and Google Scholar, as well as a manual search of journals and websites. All publications were screened by title and abstract, and potentially eligible articles were reviewed in full text to assess their relevance.
Results:
Cardiac valves comprise a complex, three-layered structure composed of an intricate network of cells. Valvular endothelial cells cover the atrial and ventricular sides of the valves. Valvular endothelial cells are morphologically and functionally distinct from vascular endothelial cells and play a crucial role in maintaining valve function. The three-valve layers, lamina fibrosa, spongiosa, and ventricularis, exhibit distinct biomechanical properties due to their varying extracellular matrix components and valvular interstitial cells. Valvular interstitial cells can be divided into four subtypes, each exhibiting specific cellular functions essential for normal valve physiology. However, pathological stimuli can cause aberrant activation of the valvular interstitial cells, leading to valve calcification and stenosis.
Conclusion:
The intricate interplay of cellular components within cardiac valves is vital for maintaining normal valve function and structural integrity, but also contributes to valve pathology. A holistic understanding of heart valves, integrating cellular, molecular, and neural perspectives, is needed in the future.
1 Introduction
Valvular heart disease is a significant contributor to cardiovascular morbidity and mortality on a global scale (1). The disease burden associated with valvular heart disease is expected to rise in the upcoming decades due to an ageing population and the growing prevalence of multiple risk factors, including hypertension and diabetes. Medication can help alleviate symptoms and delay the progression of the disease (2–4). However, as the disease advances, surgical intervention is often required to repair or replace a damaged heart valve (2–4). While heart valves were previously believed to be passive structures that do not play an active role in maintaining function, tissue integrity, and durability, recent scientific evidence suggests that these structures are far more complex than previously thought. Although the opening and closure of valves are triggered by changes in transvalvular pressure during the cardiac cycle, valves are not entirely passive structures; their active role in cardiovascular physiology and pathophysiology is more complex than previously assumed (5). Therefore, it is crucial to gain a deep understanding of the physiology and structure of heart valves to enhance treatment and improve outcomes for the large number of patients with valvular heart disease.
Regardless of anatomical position, heart valves are composed of leaflets attached to a fibrous ring. Each leaflet comprises a thick extracellular matrix organised in three distinct layers—lamina fibrosa, spongiosa, and ventricularis, as shown in Figure 1. During the early stages of embryonic heart development, the heart begins to form as a tube consisting of two layers: the endocardium and the myocardium, separated by an extracellular matrix known as cardiac jelly (6). Endothelial cells from the endocardium are activated by TGF-β2 and TGF-β3, transforming into a subset of mesenchymal cells that can migrate into the cardiac jelly and develop into mature valves. This process, known as endothelial-to-mesenchymal transformation (7, 8), is crucial to the unique matrix compositions in the three distinct valve layers, which give them their distinct mechanical properties. The lamina atrialis is abundant in elastin, an essential component that facilitates tissue recoil during systolic and diastolic conformation. Lamina spongiosa is the softest layer due to the large amounts of proteoglycans and numerous cells, including the valve interstitial cells (VICs). Finally, the lamina fibrosa comprises a dense network of anisotropically arranged collagen bundles, providing strength and flexibility to the valve areas exposed to the maximum diastolic stress, such as the edges and the “belly” (9). The three distinct layers of the heart valve—the fibrosa, spongiosa, and ventricularis—work synchronously to facilitate the smooth opening and closing of the valve.
Figure 1

A depiction of the histological structure of the mitral valve with three distinct layers: the lamina atrialis, lamina spongiosa, and lamina fibrosa, along with the cellular elements comprising each layer. aVIC, activated myofibroblast-like valve interstitial cells; VEC, valve endothelial cell, qVIC, quiescent fibroblast-like valve interstitial cell.
Valve endothelial cells (VECs) are a distinct population of endothelial cells that cover the inside of the heart and its valves, possessing unique properties not observed elsewhere in the vasculature (10). In addition, VICs have been discovered in the lamina spongiosa, and their structure and function are far more complex than previously believed. The discovery of these diverse cell types in heart valves raises the question of whether the cellular components of cardiac valves are more intricate than previously thought. Additionally, a recent study has identified the presence of distinct nerve terminals in heart valves and a close association of varicose nerve fibres with endothelial, fibroblast, and smooth muscle cells (11), suggesting that neural involvement may play a role in regulating valvular function.
Changes in the cellular components of the heart valve are associated with valvular heart disease. Alterations of the extracellular matrix in the cardiac valves can be caused by inflammation resulting from various stressors, such as hyperlipidemia and smoking (10). This inflammatory process can lead to the accumulation of lipids, which stimulate interstitial cells to adopt a matrix pro-remodelling phenotype, potentially causing tissue calcification (12), altering the overall mechanical properties of the valve (13). Given the complex processes involved in health and disease, a thorough understanding of the individual components that contribute to cardiac valve diseases is crucial.
Heart valve pathologies are often treated with surgical interventions that focus on implanting a passive artificial material to secure immediate valve functionality. However, this approach overlooks the importance of the complex valve structures and functions. To minimise the complications associated with biological and mechanical valve replacement, a more comprehensive approach is necessary in the future. This review aims to discuss the intricacies of heart valves and provide a more sophisticated understanding of their cellular structure and unique function. This knowledge is particularly relevant for developing tissue-engineered heart valves, which aim to create a native replica encompassing a distinct cellular structure and unique function.
2 Materials and methods
This literature review was conducted using a systematic approach. PubMed and Google Scholar were searched for relevant articles from May 1, 2024, to July 9, 2024, with the language restriction set to English.
2.1 Search strategy and study collection
A systematic approach was used to assess all publications. Starting with the controlled subject heading “heart valve.” Controlled subject headings were used to search for data on the following terms: “cytology,” “physiology,” “histology,” “structure,” and “pathophysiology.” Subsequently, a second search was performed using the search words “endothelial cells,” “interstitial cells,” “endothelial-to-mesenchymal transition,” “VIC quiescent,” “VIC active,” “osteoblast-like,” “neurons,” which were related to the findings in the main articles. All articles were screened by title and abstract, and potentially eligible articles were read in full text to evaluate their relevancy. In addition to database searches, relevant articles were manually searched in journals and websites, and reference searches were conducted in the bibliographies of these articles.
2.2 Eligibility criteria
Inclusion criteria: articles published in peer-reviewed journals; studies reporting original data relevant to heart valve biology, structure, function, or biomechanics; publications focusing on endothelial cells, valvular interstitial cells, or their transitions and phenotypes; studies employing either in vivo, in vitro or computational models; human, porcine, sheep or rodent valve tissue; full-text articles. Exclusion criteria: articles not published in English; review articles; conference abstracts; editorials with no primary data; studies focusing on cell types unrelated to cardiac valves; valve tissue from species other than human, porcine, sheep, or rodent; articles with insufficient methodological details to assess quality.
2.3 Data collection
In each study, the following information was gathered: author, country of study, year of publication, journal of publication, and study design.
3 Results
We identified 2.596 studies. After screening for title and abstract and removing duplicate studies, 78 studies were considered eligible for full-text assessment. Forty-eight articles were used to synthesise this review (Figure 2).
Figure 2
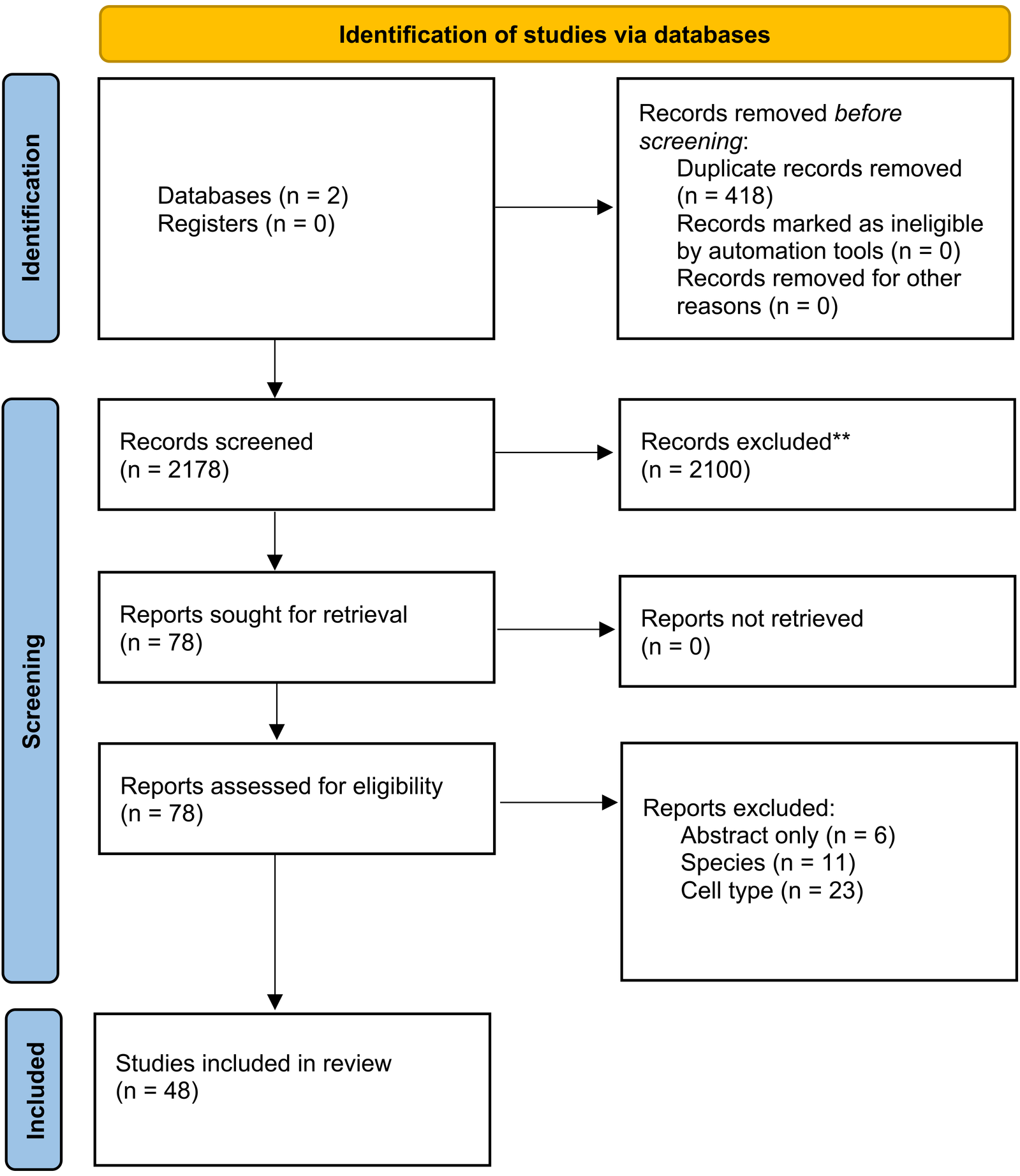
PRISMA flow chart used for study selection.
3.1 Valvular endothelial cells
VECs cover the entire surface of the valve and act as a barrier between the blood components and the underlying valve tissue. These cells exhibit unique morphological characteristics that differ from vascular endothelial cells. Under static in vitro conditions, VECs assume a more stellate pattern than vascular endothelial cells, reflected by a lower shape index (0.78 ± 0.08 vs. 0.84 ± 0.07) (14). Vascular endothelial cells exhibit an elongated structure and alignment in high-shear stress conditions, parallel to their axis. Interestingly, a study by Miragoli et al. demonstrated that vascular endothelial cells on the aortic aspect of the aortic valve cusps showed greater alignment than those on the ventricular surface of the valve, which experiences higher shear stress (15). This suggests that the morphology and alignment of VECs may be more influenced by pressure stress than shear stress. Although canine aortic VECs and cultured porcine aortic VECs orient themselves perpendicular to the flow direction rather than parallel (14, 16), human-cultured aortic VECs do not, indicating that VEC alignment may vary based on the species (17).
The arrangement of VECs can significantly impact compliance. Miragoli et al. demonstrated that VECs on the ventricular side of the aortic valve are notably less compliant than those on the aortic side, which are more aligned along a single axis. This difference in compliance was observed even when VECs from the two surfaces of the aortic valve were selectively cultured. Hence, the differences in compliance cannot be solely attributed to differences in the stiffness of the underlying tissue. Additionally, the fact that these differences were observed in cultured cells not exposed to flow suggests that the difference in compliance is not solely due to different surface flow patterns. Instead, Miragoli et al. proposed that epigenetic pathways regulating gene expression, such as NO type III, may confer the differences to the VECs on either side of the valve, which determine their mechanical properties (15).
The transformation of endothelial cells to mesenchymal cells (endothelial-to-mesenchymal transformation) plays a significant role during embryonic development. Pioneering research has demonstrated that during embryonic development, the transition of endocardial cells into cardiomyocytes initiates biosynthetic processes, culminating in the formation of cardiac cushion mesenchyme and cardiac valves (18, 19). Endothelial-to-mesenchymal transformation can also occur in adulthood and can be induced by factors such as oxidative stress, fatty acid oxidation, hypoxia, hyperglycemia, and shear stress (20). Furthermore, it has been demonstrated that endothelial-to-mesenchymal transformation in heart valves can be potentiated by exposure to cyclic mechanical strain (21). VECs in adult heart valves can undergo endothelial-to-mesenchymal transformation (22, 23) and may play a role in the pathogenesis of cardiovascular disease in adulthood, such as calcific aortic valve disease (22, 24, 25). VEC dysfunction has been identified as an essential factor in the early stages of calcific aortic valve disease. This dysfunction has been linked to the recruitment of immune cells (26), disruption of protective nitric oxide signalling (27, 28), and the ability of VECs to change their characteristics and express proteins that contribute to calcification (29). These findings suggest that dysfunctional VECs may play a significant role in the development of valvular disease.
Nevertheless, VICs have been shown to play a significant role in regulating VECs. Hjortnaes et al. found that VICs have an inhibitory effect on endothelial-to-mesenchymal transformation and VEC osteogenesis. However, VECs did not inhibit VIC mineralisation. Time course analysis led the authors to conclude that endothelial-to-mesenchymal transformation precedes VEC osteogenesis. This study highlights the importance of VEC-VIC interactions in maintaining valve homeostasis (22).
It has also been suggested that endothelial-to-mesenchymal transformation plays a role in the pathogenesis of mitral valve disease. Mitral VECs have been observed to differentiate into mesenchymal cells, with some demonstrating osteogenic potential in vitro (29, 30). It has, therefore, been proposed that endothelial-to-mesenchymal transformation may contribute to the renewal of VICs. Moreover, the VECs may also exhibit osteogenic characteristics under pathological conditions and could induce valvular calcification (29, 30). A related study has explored the potential role of endothelial-to-mesenchymal transformation in the mitral valve changes associated with valve prolapse. This study identified elevated circulating osteoprotegerin levels in patients undergoing mitral valve replacement due to valve prolapse. Using cultured VECs isolated from the pathological mitral valves, they later found that osteoprotegerin, primarily associated with bone metabolism, can induce the endothelial-to-mesenchymal transformation process. Notably, the study also revealed a novel discovery: that mitral valve endothelial cells undergoing endothelial-to-mesenchymal transformation were capable of producing and secreting osteoprotegerin. This finding suggests the existence of an autocrine pathogenic mechanism for endothelial-to-mesenchymal transformation (31).
3.2 Valvular interstitial cells
VICs are the most prevalent cells in the heart valves and are found in all three layers of the cusps. Rippel et al. determined the densest concentrations of VICs to be in the lamina fibrosa (32). A study by Butcher et al. revealed that VICs possess contractile properties and the ability to synthesise matrix components (33). Based on these findings, the authors suggested that VICs play a crucial role in maintaining the leaflet environment (33). Various VIC phenotypes have been identified in diseased human heart valves, each exhibiting a specific set of cellular functions essential in normal valve physiology and pathological processes. Figure 3 schematically shows the four phenotypes.
Figure 3
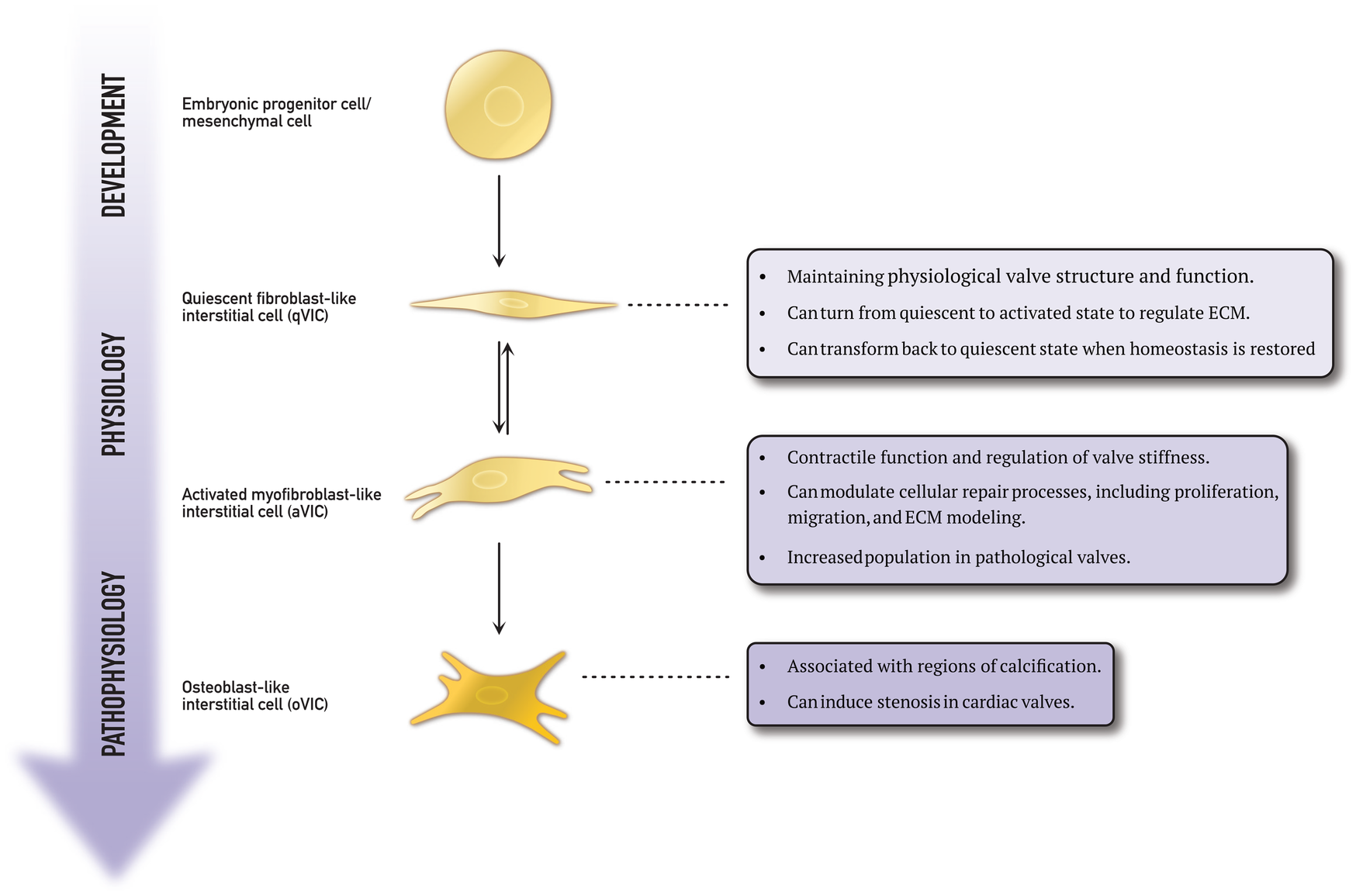
This schematic representation explains the differentiation process of embryonic progenitor cells and mesenchymal cells into various valvular interstitial cell phenotypes.
As previously mentioned, embryonic progenitor and mesenchymal cells play a crucial role during the endothelial-to-mesenchymal transformation during embryogenesis. This process is also implicated in the development of cardiovascular disease (22, 24, 25). Wang et al. discovered a group of VICs expressing markers characteristic of progenitor cells in porcine heart valves (34). These cells were located near the centre of the valve. Progenitor cells are present in many tissues, such as the bone marrow and circulation, and can enter the valve (35, 36). Chen et al. identified a subpopulation of multipotent mesenchymal progenitor cells in aortic VICs (37). The multilineage potential of both freshly isolated and subcultured aortic VICs was evaluated in vitro. The study found that the VICs demonstrated inducibility to osteogenic, adipogenic, chondrogenic, and myofibrogenic lineages. Moreover, a high-frequency putative osteoprogenitor subpopulation was identified within the VIC population. These morphologically distinct cells were shown to self-renew and produce bone matrix from single cells. This study demonstrates the presence of a mesenchymal progenitor cell population within the aortic valve, with the potential to significantly contribute to valve calcification (37).
Quiescent fibroblast-like valvular interstitial cells (qVICs) are dormant in the mature adult heart valve. Their role is to maintain the physiological valve structure and function (38). Once stimulated, qVICs are reprogrammed to differentiate into activated myofibroblast-like VICs (aVICs) to regulate the functional remodelling of the extracellular matrix collagen (25). Rabkin et al. discovered that aVICs can return to quiescence when equilibrium is restored (38). This dynamic transformation between quiescent and active states is vital in sustaining normal valve function. It has been demonstrated that pathological stimuli can also activate qVICs (39). During their differentiation from qVICs to aVICs, the phenotype and morphology change from spindle/tailed to rhomboid cells (40). The qVIC phenotype is known to be unstable and difficult to study (41). However, Roosens et al. attempted to investigate this by creating a 3D in vitro model of aggregated VICs (42). They successfully preserved the quiescent/native state of the VICs by down-regulating α-smooth muscle actin expression. The VIC aggregates were able to produce their own extracellular matrix, resembling native valve composition.
aVICs have been found to express the same contractile proteins and transcription factors as those found in skeletal muscle (43). However, they also express α-smooth muscle actin, a marker commonly found in myofibroblasts (44). In addition to α-smooth muscle actin, aVICs also express other cell markers, such as embryonic non-muscle myosin heavy chain and TGF-β, typically not found in qVICs (40, 45). When activated, VICs have two key functional properties. First, their myogenic structure allows them to contract, and second, they can synthesize extracellular matrix components. The contraction of aVICs has been demonstrated to alter the mechanical properties of valve tissue. El-Hamamsy et al. have observed that increased aVIC contraction leads to an increase in valve stiffness and that various vasoactive agents can enhance intracellular calcium levels and, thus, the degree of aVIC contraction (46).
Additionally, endothelial cells appear to be synthesising these vasoactive agents. Ku et al. discovered that the property of contraction impacts not only the structure of the valve but also the synthesis of collagen (47). They observed that the degree and duration of stretch applied to cells and tissue affect collagen synthesis.
The role of activated VICs in valvular disease has been studied extensively. In particular, Rabkin et al. investigated human mitral valves taken from patients with myxomatous degeneration and compared them to native mitral valves (45). They found that VICs in myxomatous valves exhibited activated myofibroblast-like features and expressed excessive levels of catabolic enzymes. Numerous studies have identified a subtype of VICs called osteoblast-like VICs (oVICs). These cells exhibit a distinct cellular phenotype and produce a more calcified matrix under osteogenic conditions than other VICs (34). oVICs can deposit calcium in the extracellular matrix, leading to stenosis (48).
3.3 Neurons
The primary sensory and autonomic components of the nervous system play a crucial role in regulating the heart valve function. Marron et al. examined the density and distribution of nervous innovation in heart valves using confocal microscopy, histochemical, and quantitative immunohistochemical techniques (11). In the atrioventricular valves, the nerves are situated in the atrial aspect of the valve and extend over the proximal and medial portions of the leaflets. Conversely, the nerve fibres in the arterial valves are situated on the ventricular aspect of the valve. Variations in innervation were also observed within the atrioventricular valves and arterial valves. In the mitral valve, Maroon et al. discovered a two-fold higher nerve density within the anterior mitral valve leaflet compared to the posterior leaflet. Although the arterial valves generally have a similar density of innervation, the noncoronary leaflet of the aortic valve has reduced innervation compared to the two coronary leaflets. The study also revealed that no nerves reached the free edges of the leaflets or the fibrous core in all four heart valves. Finally, Marron et al. discovered that nerve fibres were closely related to VECs and VICs. Similarly, Steele et al. investigated intrinsic cardiac neurons in guinea pig hearts (49). The researchers observed that the most concentrated innervation by axons of intrinsic neurons within the heart occurred in the valves. The findings emphasise the complex and varied innervation patterns of heart valves, underscoring their significance in valve function. A detailed understanding of neural distribution may change the future therapeutic strategies for heart valve diseases.
4 Discussion
This review of primary research articles examines the intricate cellular and neural mechanisms underlying heart valve function. The predominance of in vitro studies using animal tissues underscores the challenges in replicating the complex in vivo environment of human valves. Nonetheless, the transition to in vivo-like studies and 3D modelling represents a significant advancement in closely mimicking physiological conditions. Due to the limited number of studies on human tissue, this review includes studies conducted on both human tissue and tissue from other mammalian species. Thus, the question of whether it is sound to compare cardiac valve tissue across species quickly arises. Although the porcine species are known to share remarkable similarities with their human counterparts in terms of cardiac anatomy and physiology (50), studies have identified differences in the extracellular matrix composition between the two species (51). As such, using animal tissue and cells for research always represents uncertainties due to interspecies differences. However, animal tissue studies remain a viable alternative due to the ethical and practical constraints of using human tissue and cells.
VECs exhibit distinctive morphological characteristics influenced by various mechanical stressors, particularly shear and pressure. Studies, including those by Miragoli et al., reveal that the alignment and compliance of VECs are not solely determined by shear stress but are also influenced by intrinsic factors that epigenetic pathways may regulate (15). Thus, VECs can exhibit epigenetic signatures reflective of their tissue of origin, suggesting a potential for retaining their phenotype. However, the long-term stability of these signatures during extended culture remains uncertain, and endothelial plasticity is known to be influenced by environmental factors (52). In most cases, cultured endothelial cells tend to adopt a more uniform phenotype, highlighting the importance of local signalling and biomechanical cues (53). This plasticity poses both challenges and opportunities in engineering donor-specific, biocompatible heart valve substitutes. Understanding the extent to which VEC phenotypes can be preserved or reprogrammed in engineered scaffolds remains a critical frontier in valvular tissue engineering. This nuanced comprehension of VEC behaviour underscores their vital role in maintaining valve integrity and function. Moreover, the phenomenon of endothelial-to-mesenchymal transformation in VECs during development and adulthood suggests its potential contribution to valve pathology, including calcific aortic valve disease and mitral valve prolapse. The interactions between VECs and VICs are crucial in modulating these processes, indicating that therapeutic interventions targeting these interactions could be beneficial in preventing or treating valvular diseases.
It is well established that the mesenchymal cells are responsible for the embryonic origin of VECs and VICs (6, 7, 36). However, the maintenance of these cells through ageing and the variation in their numbers over time have yet to be adequately investigated. While some studies have focused on the health and disease of the valves, few have investigated valves from different age groups. Anstine et al. studied various cell markers across embryonic to adult stages and found an increased percentage of cells expressing hematopoietic markers (54). This finding suggests an increase in the number of extracardiac cells in the valves as age progresses. However, a better understanding of the changes in the cell population over time is needed.
VICs, the predominant cells in heart valves, are pivotal in preserving and reorganising the extracellular matrix. Their dynamic shift between quiescent and activated states, as well as their potential differentiation into osteoblast-like cells, emphasises their involvement in normal valve function and pathological calcification. Identifying progenitor and mesenchymal cells within the VIC population adds another layer of complexity, suggesting a potential source for regenerative therapies. The potential of VICs in regenerative therapies offers promise for the future of treatment for valve diseases.
Although valvular calcification and atherosclerosis share several pathophysiological mechanisms and risk factors, this overlap has not translated into equivalent therapeutic responses. Notably, statins, while effective in reducing the atherosclerotic burden, have consistently failed to demonstrate a clinical benefit in slowing the progression of calcific aortic stenosis in randomised controlled trials (55). This is despite in vitro studies showing that statins can attenuate osteogenic differentiation and calcification in cultured VICs (56). The reasons for this discrepancy remain unclear but may relate to differences in local microenvironments, disease stage at treatment initiation, or pharmacokinetic limitations in targeting valve tissue. These findings underscore the need for valvular-specific therapeutic strategies rather than extrapolation from vascular disease paradigms.
The intricate innervation of heart valves, detailed by Marron et al. (11) and Steele et al. (49), underscores the influence of the nervous system on valve function. The varying distribution of nerve fibres in different valve regions indicates that innervation may impact the mechanical and functional properties of the valves. Understanding these neural patterns not only presents an intriguing prospect but also holds the potential to lead to innovative therapeutic approaches that capitalise on the nervous system to maintain or restore valve function.
Developing three-dimensional in vitro models, such as organoids, is crucial in understanding the cells in adjacent tissues. However, numerous challenges need to be addressed. For instance, VICs must be preserved and recovered before being studied in an actual valve model (42). To create an accurate 3D model, VICs must form an aggregate to mimic the in vivo microenvironment. In tissue engineering, ongoing exploration of new hypotheses aims to create cardiac valves that closely resemble native valves and can be used in heart surgery. A combination of in vivo and in vitro studies can enhance the understanding of the complexity of cellular components. in vitro studies on the cells in the valves have provided valuable insights into their components; however, these findings should be considered in the context of the cells’ functions, including communication through mediators and cellular pathways. An examination of the subject from multiple perspectives can provide a more comprehensive understanding of the native heart valve, which can contribute to the advancement of research into valve diseases and the development of surgical treatments.
Heart valves are currently treated from a purely mechanical perspective, with little consideration for their biological function. The diseased valve is typically replaced with a passive mechanical structure, but this review has revealed that this approach may be oversimplified. Heart valves are incredibly complex, with a dynamic cellular composition enabling them to perform various functions. To truly understand and treat diseased heart valves, we need a more nuanced approach considering the biological factors at play. Bioscaffolds offer a promising avenue for research, enabling the body to rebuild full valve function with an active, dynamic cellular composition that mimics the complexity of genuine heart valves. In this context, it is noteworthy that heart valves have been proposed to exhibit a degree of immune privilege, attributed to their avascularity, limited regenerative capacity, and low expression of MHC and ABO antigens (57). This property may explain why cryopreserved allografts can function long-term without HLA or ABO matching in the absence of immunosuppression. Even in the setting of cardiac transplant rejection, the valves often remain unaffected, suggesting an immunological distinction from the surrounding myocardial tissue (57). Recognising this phenomenon has important implications for tissue-engineered heart valves, since it raises the prospect of developing constructs that minimise immunogenicity while maintaining long-term functionality.
5 Conclusion
Our understanding of heart valves has advanced significantly, revealing that these structures are far more complex and active than previously thought. The diverse roles of VECs and VICs in maintaining valve integrity, their dynamic interactions, and the implications of endothelial-to-mesenchymal transformation in disease progression underscore the need for more sophisticated therapeutic approaches. Identifying various VIC phenotypes and their contributions to physiological and pathological processes, such as calcification, emphasises the potential for regenerative therapies targeting these cells. Moreover, the emerging recognition of the nervous system's influence on valve function opens new avenues for innovative treatments. Given the limitations of current surgical interventions, which often rely on passive mechanical replacements, a paradigm shift towards approaches that incorporate the dynamic biological functions of heart valves is imperative. Tissue-engineered heart valves, designed to replicate the native structure and function, represent a promising direction for future research and clinical application. A holistic understanding of heart valves, integrating cellular, molecular, and neural perspectives is needed in the future. This comprehensive approach will pave the way for innovative treatments and significantly enhance the management of valvular heart disease.
Statements
Author contributions
JJ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. OH: Data curation, Formal analysis, Investigation, Validation, Writing – review & editing. VP: Resources, Supervision, Writing – review & editing. JH: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Novo Nordisk Foundation [Grant number NNF20OC0065584] and Inge og Asker Larsens Fond.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
aVICs, activated myofibroblast-like valve interstitial cells; oVICs, osteoblast-like valve interstitial cells; qVICs, quiescent fibroblast-like valve interstitial cells; TGF, transforming growth factor; VECs, valve endothelial cells; VICs, valve interstitial cells.
References
1.
Aluru JS Barsouk A Saginala K Rawla P Barsouk A . Valvular heart disease epidemiology. Med Sci (Basel). (2022) 10:32. 10.3390/medsci10020032
2.
Jouan J . Mitral valve repair over five decades. Ann Cardiothorac Surg. (2015) 4:13. 10.3978/j.issn.2225-319X.2015.01.07
3.
Vahanian A Beyersdorf F Praz F Milojevic M Baldus S Bauersachs J et al 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. (2022) 43:561–632. 10.1093/eurheartj/ehab395
4.
Otto CM Nishimura RA Bonow RO Carabello BA Erwin JP III Gentile F et al 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. (2021) 143:35–71. 10.1161/CIR.0000000000000932
5.
El-Hamamsy I Yacoub M Chester A . Neuronal regulation of aortic valve cusps. CVP. (2009) 7:40–6. 10.2174/157016109787354088
6.
Tan H Junor L Price RL Norris RA Potts JD Goodwin RL . Expression and deposition of fibrous extracellular matrix proteins in cardiac valves during chick development. Microsc Microanal. (2011) 17:91–100. 10.1017/S1431927610094365
7.
Boyer AS Ayerinskas II Vincent EB McKinney LA Weeks DL Runyan RB . TGF2 and TGF3 Have Separate and Sequential Activities during Epithelial–Mesenchymal Cell Transformation in the Embryonic Heart (1999).
8.
Armstrong EJ Bischoff J . Heart valve development: endothelial cell signaling and differentiation. Circ Res. (2004) 95:459–70. 10.1161/01.RES.0000141146.95728.da
9.
Garobolo G Ferrari S Rizzi S Barbuto M Bernava G Pesce M . Harnessing mechanosensation in next generation cardiovascular tissue engineering. Biomolecules. (2020) 10:1419. 10.3390/biom10101419
10.
Stewart BF Siscovick D Lind BK Gardin JM Gottdiener JS Smith VE et al Clinical factors associated with calcific aortic valve disease. J Am Coll Cardiol. (1997) 29:630–4. 10.1016/S0735-1097(96)00563-3
11.
Marron K Yacoub MH Polak JM Sheppard MN Fagan D Whitehead BF et al Innervation of human atrioventricular and arterial valves. Circulation. (1996) 94:368–75. 10.1161/01.CIR.94.3.368
12.
Bertazzo S Gentleman E Cloyd KL Chester AH Yacoub MH Stevens MM . Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nature Mater. (2013) 12:576–83. 10.1038/nmat3627
13.
Santoro R Scaini D Severino LU Amadeo F Ferrari S Bernava G et al Activation of human aortic valve interstitial cells by local stiffness involves YAP-dependent transcriptional signaling. Biomaterials. (2018) 181:268–79. 10.1016/j.biomaterials.2018.07.033
14.
Butcher JT Penrod AM García AJ Nerem RM . Unique morphology and focal adhesion development of valvular endothelial cells in static and fluid flow environments. ATVB. (2004) 24:1429–34. 10.1161/01.ATV.0000130462.50769.5a
15.
Miragoli M Yacoub MH El-Hamamsy I Sanchez-Alonso JL Moshkov A Mongkoldhumrongkul N et al Side-specific mechanical properties of valve endothelial cells. Am J Physiol Heart Circ Physiol. (2014) 307:H15–24. 10.1152/ajpheart.00228.2013
16.
Deck JD . Endothelial cell orientation on aortic valve leaflets. Cardiovasc Res. (1986) 20:760–7. 10.1093/cvr/20.10.760
17.
Holliday CJ Ankeny RF Jo H Nerem RM . Discovery of shear- and side-specific mRNAs and miRNAs in human aortic valvular endothelial cells. Am J Physiol Heart Circ Physiol. (2011) 301:H856–867. 10.1152/ajpheart.00117.2011
18.
Markwald RR Fitzharris TP Smith WNA . Structural analysis of endocardial cytodifferentiation. Dev Biol. (1975) 42:160–80. 10.1016/0012-1606(75)90321-8
19.
Markwald RR Fitzharris TP Manasek FJ . Structural development of endocardial cushions. Am J Anat. (1977) 148:85–119. 10.1002/aja.1001480108
20.
Piera-Velazquez S Jimenez SA . Endothelial to mesenchymal transition: role in physiology and in the pathogenesis of human diseases. Physiol Rev. (2019) 99:1281–324. 10.1152/physrev.00021.2018
21.
Balachandran K Alford PW Wylie-Sears J Goss JA Grosberg A Bischoff J et al Cyclic strain induces dual-mode endothelial-mesenchymal transformation of the cardiac valve. Proc Natl Acad Sci USA. (2011) 108:19943–8. 10.1073/pnas.1106954108
22.
Hjortnaes J Shapero K Goettsch C Hutcheson JD Keegan J Kluin J et al Valvular interstitial cells suppress calcification of valvular endothelial cells. Atherosclerosis. (2015) 242:251–60. 10.1016/j.atherosclerosis.2015.07.008
23.
Paranya G Vineberg S Dvorin E Kaushal S Roth SJ Rabkin E et al Aortic valve endothelial cells undergo transforming growth factor-β-mediated and non-transforming growth factor-β-mediated transdifferentiation in vitro. Am J Pathol. (2001) 159:1335–43. 10.1016/S0002-9440(10)62520-5
24.
Anbara T Sharifi M Aboutaleb N . Endothelial to mesenchymal transition in the cardiogenesis and cardiovascular diseases. CCR. (2021) 16:306–14. 10.2174/1573403X15666190808100336
25.
Ma X Zhao D Yuan P Li J Yun Y Cui Y et al Endothelial-to-mesenchymal transition in calcific aortic valve disease. Acta Cardiologica Sinica. (2020) 36:183–94. 10.6515/ACS.202005_36(3).20200213A
26.
Skowasch D Schrempf S Wernert N Steinmetz M Jabs A Tuleta I et al Cells of primarily extravalvular origin in degenerative aortic valves and bioprostheses. Eur Heart J. (2005) 26:2576–80. 10.1093/eurheartj/ehi458
27.
Bosse K Hans CP Zhao N Koenig SN Huang N Guggilam A et al Endothelial nitric oxide signaling regulates Notch1 in aortic valve disease. J Mol Cell Cardiol. (2013) 60:27–35. 10.1016/j.yjmcc.2013.04.001
28.
Richards J El-Hamamsy I Chen S Sarang Z Sarathchandra P Yacoub MH et al Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. Am J Pathol. (2013) 182:1922–31. 10.1016/j.ajpath.2013.01.037
29.
Wylie-Sears J Aikawa E Levine RA Yang J-H Bischoff J . Mitral valve endothelial cells with osteogenic differentiation potential. ATVB. (2011) 31:598–607. 10.1161/ATVBAHA.110.216184
30.
Bischoff J Casanovas G Wylie-Sears J Kim D-H Bartko PE Guerrero JL et al CD45 Expression in mitral valve endothelial cells after myocardial infarction. Circ Res. (2016) 119:1215–25. 10.1161/CIRCRESAHA.116.309598
31.
Songiaa P Branchettic E Parolarid A Myasoedovaa V Ferraric G Alamannia F et al Mitral valve endothelial cells secrete osteoprotegerin during endothelial mesenchymal transition. J Mol Cell Cardiol. (2016) 98:48–57. 10.1016/j.yjmcc.2016.06.061
32.
Rippel RA Ghanbari H Seifalian AM . Tissue-engineered heart valve: future of cardiac surgery. World J Surg. (2012) 36:1581–91. 10.1007/s00268-012-1535-y
33.
Butcher JT Nerem RM . Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. J Heart Valve Dis. (2004) 13:478–85. discussion 485-486.
34.
Wang H Sridhar B Leinwand LA Anseth KS . Characterization of cell subpopulations expressing progenitor cell markers in porcine cardiac valves. PLoS One. (2013) 8:e69667. 10.1371/journal.pone.0069667
35.
Deb A Wang SH Skelding K Miller D Simper D Caplice N . Bone marrow-derived myofibroblasts are present in adult human heart valves. J Heart Valve Dis. (2005) 14:674–8.
36.
Hajdu Z Romeo SJ Fleming PA Markwald RR Visconti RP Drake CJ . Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol. (2011) 51:955–65. 10.1016/j.yjmcc.2011.08.006
37.
Chen J-H Yip CYY Sone ED Simmons CA . Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. (2009) 174:1109–19. 10.2353/ajpath.2009.080750
38.
Rabkin-Aikawa E Farber M Aikawa M Schoen FJ . Dynamic and reversible changes of interstitial cell phenotype during remodeling of cardiac valves. J Heart Valve Dis. (2004) 13:841–7.
39.
Liu AC Joag VR Gotlieb AI . The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. (2007) 171:1407–18. 10.2353/ajpath.2007.070251
40.
Alia MS Deba N Wanga X Rahmanb M Christopherb GF Lacerdaa CMR . Correlation between valvular interstitial cell morphology and phenotypes: a novel way to detect activation. Tissue and Cell. (2018) 54:38–46. 10.1016/j.tice.2018.07.004
41.
Schenke-Layland K Riemann I Opitz F Konig K Halbhuber KJ Stock UA . Comparative study of cellular and extracellular matrix composition of native and tissue engineered heart valves. Matrix Biol. (2004) 23:113–25. 10.1016/j.matbio.2004.03.005
42.
Roosens A Puype I Cornelissen R . Scaffold-free high throughput generation of quiescent valvular microtissues. J Mol Cell Cardiol. (2017) 106:45–54. 10.1016/j.yjmcc.2017.03.004
43.
Brand NJ Roy A Hoare G Chester A Yacoub MH . Cultured interstitial cells from human heart valves express both specific skeletal muscle and non-muscle markers. Int J Biochem Cell Biol. (2006) 38:30–42. 10.1016/j.biocel.2005.06.018
44.
Latif N Sarathchandra P Taylor PM Antoniw J Yacoub MH . Localization and pattern of expression of extracellular matrix components in human heart valves. J Heart Valve Dis. (2005) 14:218–27.
45.
Rabkin E Aikawa M Stone JR Fukumoto Y Libby P Schoen FJ . Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation. (2001) 104:2525–32. 10.1161/hc4601.099489
46.
El-Hamamsy I Balachandran K Yacoub MH Stevens LM Sarathchandra P Taylor PM et al Endothelium-Dependent regulation of the mechanical properties of aortic valve cusps. J Am Coll Cardiol. (2009) 53:1448–55. 10.1016/j.jacc.2008.11.056
47.
Ku C-H Johnson PH Batten P Sarathchandra P Chambers RC Taylor PM et al Collagen synthesis by mesenchymal stem cells and aortic valve interstitial cells in response to mechanical stretch. Cardiovasc Res. (2006) 71:548–56. 10.1016/j.cardiores.2006.03.022
48.
Galeone A Brunetti G Oranger A Grecob G Benedetto AD Mori G et al Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int J Cardiol. (2013) 169:296–304. 10.1016/j.ijcard.2013.09.012
49.
Steele PA Gibbins IL Morris JL . Projections of intrinsic cardiac neurons to different targets in the Guinea-pig heart. J Auton Nerv Syst. (1996) 56:191–200. 10.1016/0165-1838(95)00066-6
50.
Lelovas PP Kostomitsopoulos NG Xanthos TT . A comparative anatomic and physiologic overview of the porcine heart. J Am Assoc Lab Anim Sci. (2014) 53:432–8.
51.
Stephens EH Kearney DL Grande-Allen KJ . Insight into pathologic abnormalities in congenital semilunar valve disease based on advances in understanding normal valve microstructure and extracellular matrix. Cardiovasc Pathol. (2012) 21:46–58. 10.1016/j.carpath.2011.01.002
52.
McCann JA Peterson SD Plesniak MW Webster TJ Haberstroh KM . Mechanical and biochemical stimuli alter endothelial cell gene expression. In: Proceedings of the IEEE 31st Annual Northeast Bioengineering Conference, 2005. IEEE; Hoboken, New Jersey, USA (2005). p. 211–2
53.
Craig LE Spelman JP Strandberg JD Zink MC . Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvasc Res. (1998) 55:65–76. 10.1006/mvre.1997.2045
54.
Anstine LJ Horne TE Horwitz EM Lincoln J . Contribution of extra-cardiac cells in murine heart valves is age-dependent. JAHA. (2017) 6:e007097. 10.1161/JAHA.117.007097
55.
Zhao Y Nicoll R He YH Henein MY . The effect of statins therapy in aortic stenosis: meta-analysis comparison data of RCTs and observationals. Data Brief. (2016) 7:357–61. 10.1016/j.dib.2016.02.045
56.
Wu B Elmariah S Kaplan FS Cheng G Mohler ER III . Paradoxical effects of statins on aortic valve myofibroblasts and osteoblasts: implications for End-stage valvular heart disease. ATVB. (2005) 25:592–7. 10.1161/01.ATV.0000154278.01871.64
57.
Hill MA Kwon JH Gerry B Hardy WA Walkowiak OA Kavarana MN et al Immune privilege of heart valves. Front Immunol. (2021) 12:731361. 10.3389/fimmu.2021.731361
Summary
Keywords
valvular heart disease, cardiac valves, cardiac valve physiology, cardiac valve pathophysiology, valve endothelial cells, valve interstitial cells
Citation
Jedrzejczyk JH, Hjertensgaard O, Puelles VG and Hasenkam JM (2025) Unveiling the intricacies of cardiac valve pathophysiology. Front. Cardiovasc. Med. 12:1570271. doi: 10.3389/fcvm.2025.1570271
Received
03 February 2025
Accepted
08 July 2025
Published
24 July 2025
Volume
12 - 2025
Edited by
Marta Scatena, University of Washington, United States
Reviewed by
Antonino S. Rubino, Kore University of Enna, Italy
Richard Mitchell, Brigham and Women's Hospital and Harvard Medical School, United States
Updates
Copyright
© 2025 Jedrzejczyk, Hjertensgaard, Puelles and Hasenkam.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
* Correspondence: Johannes H. Jedrzejczyk johjed@clin.au.dk
ORCID Johannes H. Jedrzejczyk orcid.org/0000-0001-5029-9117 J. Michael Hasenkam orcid.org/0000-0001-7960-1172
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.