- 1School of Physical Education and Health, Chongqing College of International Business and Economics, Chongqing, China
- 2Physical Education Institute, Chongqing College of Humanities, Science & Technology, Chongqing, China
- 3Southwest University Hospital, Southwest University, Chongqing, China
Cardiovascular diseases (CVDs) remain a leading cause of morbidity and mortality worldwide, despite advances in prevention and therapy. Emerging evidence highlights the central role of epigenetic modifications and non-coding RNAs including microRNAs (miRNAs) and long non-coding RNAs (lncRNAs) in the regulation of gene expression networks underlying cardiovascular homeostasis and disease. Concurrently, physical exercise has been recognized not only as a preventive and therapeutic strategy for CVDs but also as a potent modulator of epigenetic landscapes. This review explores the mechanistic links between aerobic exercise and epigenetic modulation, focusing on how structured physical activity influences the expression and function of miRNAs and lncRNAs, as well as chromatin remodeling processes in cardiovascular tissues. We provide a comprehensive overview of aerobic exercise-responsive non-coding RNAs implicated in vascular inflammation, endothelial function, cardiac remodeling, myocardial infarction, and atherosclerosis. Additionally, we discuss aerobic exercise-induced changes in DNA methylation and histone modification patterns that contribute to transcriptional reprogramming and long-term cardiovascular benefits. Finally, the review evaluates the translational potential of targeting aerobic exercise-regulated epigenetic factors for early diagnosis, risk stratification, and personalized therapies in CVD management. Understanding the molecular underpinnings of cardioepigenetic responses to exercise opens promising avenues for precision cardiovascular medicine and integrative therapeutic strategies.
1 Introduction
Cardiovascular diseases (CVDs) are still the most important causes of global morbidity and mortality, responsible for a high proportion of healthcare problems and afflicting millions of people every year (1). Increasing incidence of CVDs is closely linked with a range of risk factors, including both modifiable lifestyle-related behaviors (such as physical inactivity, poor diet, smoking, and alcohol consumption) and intermediate clinical conditions (such as obesity, type 2 diabetes, and hypertension) that are influenced by those behaviors (2, 3). In the wake of the emerging public health crisis, non-pharmacological interventions, and especially habitual physical exercise, particularly aerobic training, have garnered significant attention due to their profound cardioprotective effects (4). Research has reported that exercise exerts a positive impact on cardiovascular health via multifaceted mechanisms, such as the enhancement in cardiac function, the reduction in systemic inflammation, and the minimization of oxidative stress (5). Although the physiological effects of exercise are well documented, the molecular foundations of its cardioprotective effects are a subject of keen research. Recent studies have progressively concentrated on the function of non-coding RNAs (ncRNAs), such as microRNAs (miRNAs) and long non-coding RNAs (lncRNAs), as essential regulators of gene expression within the setting of cardiovascular adaptation to exercise (6). Such ncRNAs are presently understood to be key mediators of cellular mechanisms like angiogenesis, apoptosis, fibrosis, and hypertrophy, which are all inherent components of cardiovascular homeostasis (7, 8). For instance, some miRNAs, such as miR-1, miR-133, and miR-206, have been shown to modulate cardiac remodeling and function in response to exercise. Similarly, lncRNAs, through mechanisms involving interaction with chromatin, proteins, and other RNAs, have been shown to modulate gene expression networks that impact cardiovascular health (9). The modulation of these non-coding RNAs following physical exercise highlights their value as biomarkers and therapeutic targets for cardiovascular disease prevention and therapy.
Besides ncRNAs, other major epigenetic modifications include DNA methylation and histone modification have emerged as another leading way through which exercise exerts its cardioprotective effects (10–12). It has been demonstrated that exercise modifies the DNA methylation pattern, especially genes implicated in inflammation, oxidative stress, and metabolic regulation (13–15). Analogously, exercise-promoted changes in histone acetylation and methylation have been associated with the activation of cardioprotective gene programs (16). These epigenetic modifications provide not only a mechanistic basis for the long-term benefits of exercise but also point to a possibility of using exercise as a means to modulate gene expression in a heritable yet reversible manner (17). The present review aims to update the knowledge of the cardioprotective role of physical exercise, focusing particular attention on miRNAs, lncRNAs, and epigenetic modifications contributing to this function. In this review, we focus on the molecular pathways underlying cardiovascular adaptations induced by aerobic exercise by integrating the latest findings in this rapidly evolving field. We highlight the role of non-coding RNAs (miRNAs and lncRNAs) and epigenetic modifications as key mediators of these adaptations, emphasizing their potential as biomarkers and therapeutic targets. A deeper understanding of how aerobic exercise modulates these molecular mechanisms may pave the way for innovative strategies in the prevention and management of CVD, addressing one of the foremost global health challenges.
2 miRNAs and cardioprotection via exercise
2.1 Overview of miRNAs
miRNAs are small, non-coding RNA molecules that control gene expression after transcription by binding to target messenger RNAs (mRNAs) to suppress their translation or promote degradation (8). In the cardiovascular system, miRNAs play crucial roles in various processes, from cardiac development, hypertrophy, fibrosis, and angiogenesis. Dysregulation of specific miRNAs has been implicated in the pathogenesis of cardiovascular disease, including heart failure, myocardial infarction (MI), and atherosclerosis (18). With the potential to modulate multiple pathways simultaneously, miRNAs have been identified as key regulators of cardiac homeostasis (19). Exercise has been shown to influence miRNA expression patterns, suggesting that these molecules contribute to the molecular adaptations that underlie exercise-induced cardioprotection (20).
2.2 Exercise-induced miRNA changes in cardiovascular health
Exercise training has been demonstrated to induce profound and active alterations in the expression levels of miRNAs that are crucial for the regulation of heart function and assisting the body in coping with stress (21). Exercise-induced alteration in miRNA expression is implicated in important biological processes, including cardiac remodeling, inflammation, oxidative stress, and endothelial function, all of which are critical for maintaining cardiovascular health (22). A number of studies have found that certain miRNAs increase or decrease in response to exercise, showing their involvement in the cardiovascular system's adaptation. For example, aerobic exercise upregulates cardiac miR-126 expression, an established miRNA found in endothelial cells (23), as demonstrated in streptozotocin-induced diabetic male Wistar rats (8–10 weeks old) subjected to treadmill running (60 min/day, 5 days/week for 8 weeks), resulting in enhanced vascular endothelial growth factor (VEGF) signaling and angiogenesis in cardiac tissue (24). This illustrates how important miR-126 is in allowing exercise to improve the health of the heart, specifically by affecting the creation of new blood vessels and vascular repair mechanisms.
Analogously, Fathi et al. showed that 14 weeks of endurance training (treadmill running at ∼75% VO2max) in male rats led to physiological cardiac hypertrophy, along with a significant upregulation of miR-1 and miR-133. These changes were associated with improved cardiac function and regulation of apoptosis- and growth-related genes, suggesting a role for these miRNAs in exercise-induced cardiac adaptation (25). Additionally, miR-21, implicated in cardiac hypertrophy and fibrosis, has been modulated by an acute exhaustive exercise in ways that reduce pathological remodeling and enhance survival of cardiomyocytes in chronic heart failure patients (26). These miRNA alterations collectively enhance cardiovascular adaptation by promoting cardiac function, supporting vascular regeneration, and reducing the risk of MI and heart failure.
The mechanisms underlying exercise-induced changes in miRNA expression are complex and involve multiple levels of regulation, including modulation of the miRNA biogenesis machinery. Key proteins in this process Drosha, Dicer, and Argonaute (AGO) orchestrate the sequential processing of primary miRNA transcripts (pri-miRNAs) into mature, functional miRNAs (20). Endurance training has been shown to upregulate Dicer and AGO2 expression in metabolically active tissues such as adipose and skeletal muscle, primarily via activation of AMP-activated protein kinase (AMPK), a central energy sensor responsive to increased energy demand during exercise (27). Concurrently, reactive oxygen species (ROS) generated during aerobic activity play a dual regulatory role; at moderate levels, they enhance the expression of Drosha, Dicer, and Exportin-5, promoting efficient miRNA processing, whereas excessive ROS may impair Dicer and AGO2 stability (28). These effects facilitate the maturation of cardioprotective miRNAs such as miR-1 and miR-133a, which contribute to improved cardiac function and stress adaptation in response to endurance exercise.
These molecular changes are further orchestrated by several upstream signaling pathways that integrate physiological stimuli such as mechanical stress, metabolic shifts, and redox changes. For instance, PGC-1α pathway, activated by aerobic training, co-regulates miRNA programs; notably, it modulates miR-696, which targets PGC-1α and influences muscle glucose metabolism (29). Given that NF-κB is a known transcriptional activator of miR-146a and miR-155 in inflammatory contexts, and that these miRNAs increase following endurance exercise, it is plausible that NF-κB contributes to their upregulation during exercise-induced immune modulation (30, 31).
Wu (32) collectively, these signaling cascades including PGC-1α and NF-κB integrate mechanical, metabolic, and oxidative stimuli to finely regulate miRNA biogenesis and expression in a tissue-specific manner, orchestrating cardiovascular adaptation to exercise.
The mechanisms by which miRNAs stimulated by exercise mediate cardioprotection are broadly categorized according to their effects on cardiomyocyte growth and survival, mitochondrial function and energy metabolism, anti-inflammatory responses, and endothelial function (Table 1). Collectively, these findings highlight the major role of miRNAs in regulating the cardiovascular effects of exercise and their potential as therapeutic targets for CVD.
2.2.1 Cardiomyocyte growth and survival
Cardiac hypertrophy is an adaptive response to endurance exercise, characterized by physiological changes such as cardiomyocyte growth, increased mitochondrial density, and enhanced cardiac reserve (41). This type of hypertrophy, or physiological hypertrophy, is delineated from pathological hypertrophy, which entails maladaptive processes such as fibrosis, apoptosis, and reduced contractility, with the frequent progression to heart failure (42). Exercise-mediated miRNAs have important roles as cardiomyocyte survival and growth regulators with a protective role against pathological remodeling and hypertrophy. For instance, miR-1, whose expression is decreased by exercise (43), inhibits major regulators of cardiac hypertrophy, including calmodulin and insulin-like growth factor 1 (IGF-1), to suppress maladaptive growth (35). Similarly, exercise-upregulated miR-133a suppresses pro-hypertrophic signaling pathways like RhoA and Cdc42 to maintain cardiomyocyte homeostasis (36). Some other miRNAs like miR-17-3p are upregulated during exercise-induced hypertrophy, as observed in adult male C57BL/6 mice (8 weeks old) after 4 weeks of treadmill exercise, where miR-17-3p contributed to physiological cardiac growth via modulation of TIMP3 (Tissue inhibitor of metalloproteinases-3) and the phosphoinositide 3-kinase (PI3K)/Akt pathway (44, 45). Additionally, miR-222 has been shown to regulate cardiomyocyte proliferation by targeting HIPK1 (46), while miR-29c and miR-1 play roles in reducing collagen expression and pathological alterations in conditions like obesity-induced hypertrophy. Aerobic exercise training has been demonstrated to restore the levels of these miRNAs, thus preventing pathological cardiac changes and dysfunction (47, 48). Together, these observations underscore the significance of miRNAs in mediating the salutary effects of exercise on cardiac adaptation, and their potential as therapeutic targets for preventing pathological remodeling in CVDs.
2.2.2 Mitochondrial function and energy metabolism
Mitochondrial dysfunction and impaired energy metabolism are central to the pathogenesis of many CVDs (49). miRNAs that are regulated by exercise, specifically miR-499 and miR-208a, have been shown to enhance mitochondrial biogenesis and function by targeting genes involved in oxidative phosphorylation and mitochondrial dynamics (37). For example, miR-499 not only modulates the expression of dynamin-related protein 1 (Drp1), a key regulator of mitochondrial fission, but also represses calcineurin and fission protein 1 (Fis1), thereby promoting mitochondrial fusion over fission. This shift enhances mitochondrial network integrity and bioenergetic efficiency in cardiomyocytes (23). Additionally, miR-499 suppresses the expression of Sox6, a transcriptional repressor of slow-twitch muscle fiber genes and mitochondrial enzymes, thereby favoring oxidative metabolism and endurance capacity (50). In line with this, Fathi et al. found that 14 weeks of endurance training significantly increased miR-499 and decreased Sox6 expression in soleus (slow-twitch) muscle of Wistar rats. Together, these data underscore miR-499/Sox6-driven mitochondrial and fiber-type remodeling as key mechanisms of exercise adaptation (51). miR-208a, predominantly expressed in cardiac muscle, plays a crucial role in mitochondrial homeostasis indirectly through the Thyroid hormone receptor–associated protein 1 (THRAP1) and MED13 (Mediator Complex Subunit 13) signaling axis (38). Moreover, miR-208a can influence metabolic substrate utilization in the heart by suppressing GATA4, a transcription factor involved in mitochondrial gene regulation (52). Collectively, both miR-499 and miR-208a coordinate mitochondrial remodeling and oxidative metabolism in response to physical activity, contributing to the improved metabolic flexibility and resilience of the myocardium during exercise.
2.2.3 Anti-inflammatory effects
Chronic inflammation is a significant contributor to the development of cardiovascular disease (53), and exercise-induced miRNAs have a key function in the regulation of inflammatory processes. For example, miR-146a, which is upregulated by moderate-intensity aerobic exercise in elderly human participants with rheumatoid arthritis (mean age ∼65 years), contributes to anti-inflammatory responses through targeting IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6), key adaptor molecules in the Toll-like receptor (TLR) and Nuclear factor kappa B (NF-κB) signaling pathways By inhibiting these targets, miR-146a reduces the nuclear translocation of NF-κB, leading to decreased transcription of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α), IL-6 (Interleukin 6), and IL-1β. This has been confirmed by observed reductions in serum levels of these cytokines following exercise in miR-146a-upregulated models (39). Similarly, miR-155, another inflammation-associated miRNA, displays context-dependent effects (40). In chronic inflammation settings, such as atherosclerosis, exercise-induced downregulation of miR-155 attenuates macrophage M1 polarization by relieving inhibition of SOCS1 (Suppressor of Cytokine Signaling 1), thereby favoring a shift toward the anti-inflammatory M2 phenotype. This switch is associated with reduced expression of iNOS (inducible nitric oxide synthetase), IL-12, and other pro-inflammatory markers, and increased expression of arginase-1 and IL-10, markers of tissue repair and resolution of inflammation (54, 55). Additionally, miR-21, upregulated by moderate-intensity aerobic training, modulates the PTEN/AKT signaling pathway (56), leading to enhanced activation of eNOS (Endothelial Nitric Oxide Synthase) and suppression of vascular inflammation (57). miR-21 also indirectly inhibits NF-κB activation by targeting PDCD4, a tumor suppressor and pro-inflammatory gene (58), contributing to lower levels of circulating CRP (c-reactive protein) and adhesion molecules such as VCAM-1 (vascular cell adhesion molecule-1) and ICAM-1 (intercellular adhesion molecules) in exercise-conditioned individuals (59, 60). Taken together, these miRNAs mediate anti-inflammatory effects of exercise through targeted suppression of upstream regulators in pro-inflammatory cascades and favorable modulation of systemic inflammatory biomarkers. Their consistent changes post-exercise highlights their potential as therapeutic targets for inflammation-driven CVDs.
2.2.4 Endothelial function and angiogenesis
The endothelium is essential for the maintenance of vascular homeostasis (61), and exercise-induced miRNAs have been found to improve endothelial function as well as induce angiogenesis. For instance, exercise-induced upregulated miR-126 targets negative regulators of the VEGF pathway, such as sprouty-related EVH1 domain-containing protein 1 (SPRED1) and phosphoinositide-3-kinase regulatory subunit 2 (PIK3R2), to promote endothelial cell proliferation and angiogenesis (33). Similarly, miR-210, another exercise-responsive miRNA, was found to promote endothelial cell survival under hypoxic conditions in vitro, using human umbilical vein endothelial cells (HUVECs), where it inhibited apoptosis and mitochondrial dysfunction through targeting PDK1 (3-Phosphoinositide-dependent protein kinase-1) (34). These miRNAs, collectively, increase blood perfusion and vascular health, thereby reducing the risk of ischemic complications and other vascular diseases (62, 63).
3 Long non-coding RNAs in exercise-mediated cardioprotection
3.1 Overview of lncRNAs
lncRNAs are a class of non-coding RNA molecules longer than 200 nucleotides that regulate gene expression at the transcriptional, post-transcriptional, and epigenetic levels. Unlike miRNAs, which primarily suppress gene expression, lncRNAs can act as enhancers, repressors, or scaffolds, modulating multiple signaling pathways (64). In the cardiovascular system, lncRNAs play significant roles in cardiac development, fibrosis, hypertrophy, and vascular function. The dysregulation of some lncRNAs has been involved in various CVDs such as heart failure, atherosclerosis, and MI. Increasing evidence suggests that exercise regulates the expression of lncRNAs, and this is involved in its cardioprotective effect through influencing gene regulatory networks in cardiac remodeling, inflammation, and metabolism (65).
3.2 Exercise-induced lncRNA expression changes
Physical activity has emerged as a potent modulator of lncRNA expression, with significant implications for cardiovascular health (66). Exercise-induced changes in lncRNA expression play key roles in processes such as cardiac hypertrophy, fibrosis, mitochondrial function, and angiogenesis (66). These effects are mediated by complex molecular interactions involving chromatin-modifying complexes, transcription factors, miRNAs, and RNA-binding proteins.
Several lncRNAs have been empirically shown to respond to exercise stimuli in the heart, with evidence from both animal and human models.
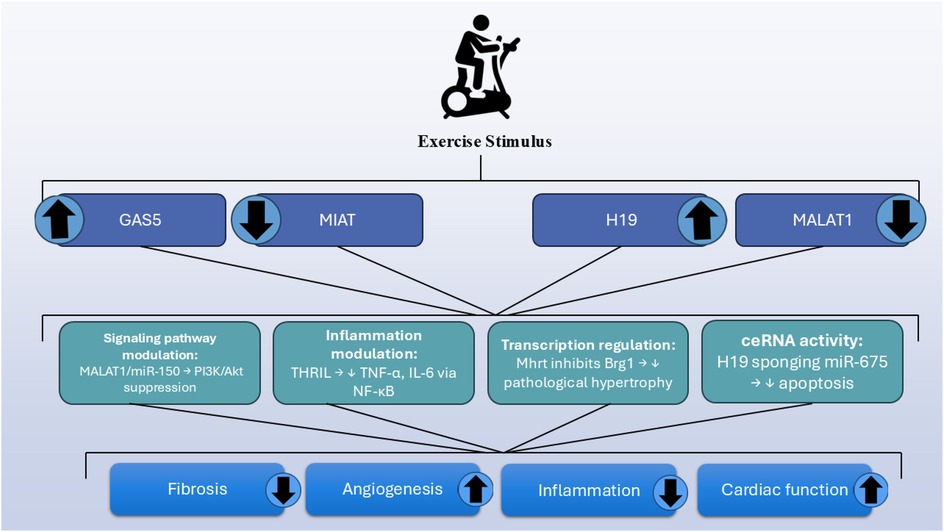
Metastasis-Associated Lung Adenocarcinoma Transcript 1 (MALAT1) was found to be downregulated in a rat model of chronic heart failure subjected to 6 weeks of aerobic treadmill training (5 days/week), accompanied by improved cardiac function and suppression of the PI3K/Akt pathway (67). Likewise, the lncRNA Growth Arrest-Specific Transcript 5 (GAS5) was upregulated in Wistar rats after MI following high-intensity aerobic exercise (66, 68). Given that GAS5 is known to regulate the miR-217/SIRT1 pathway a mechanism implicated in both cancer and fibrosis, this provides a plausible molecular link through which exercise may attenuate cardiac remodeling (69). Exercise-induced expression of lncRNA H19 has been demonstrated to contribute to cardioprotection following MI (70). Mechanistically, H19 exerts its protective effects through multiple pathways: it acts as a competing endogenous RNA for miR-675-5p, thereby promoting angiogenesis, inhibiting cardiomyocyte apoptosis, and improving cardiac function (71). Additionally, H19 directly binds to miR-103/107, suppressing their activity and enhancing the expression of Fas-associated protein with death domain (FADD), which helps prevent oxidative stress-induced cardiomyocyte necrosis (72). It also alleviates myocardial injury via miR-139/Sox8 signaling and activates autophagy to reduce infarct size and enhance cardiac performance (73, 74). These findings highlight lncRNA H19 as a key mediator of the beneficial effects of exercise on post-infarction cardiac remodeling. Importantly, Mhrt779 (Myosin heavy chain-associated RNA transcript), a cardiac-specific lncRNA, was significantly upregulated in C57BL/6 mice following 3 weeks of swimming exercise, and its elevated expression persisted one week post-training. This lncRNA interacts with Brg1 (Brahma-related gene 1) to inhibit maladaptive cardiac remodeling, contributing to the “antihypertrophic memory” observed in trained hearts (75). Exercise training also inhibits MIAT, a pro-fibrotic lncRNA that promotes fibrosis by sequestering microRNA-24 (miRNA-24) and activating the Transforming Growth Factor-beta (TGF-β) pathway (76).
In a rat model of MI induced, Farsangi et al. investigated the impact of a moderate-intensity aerobic exercise protocol (50 min/day, 5 days/week for 4 weeks) on the expression of MI-associated long non-coding RNAs. Their findings showed that exercise significantly decreased the elevated expression of the pro-fibrotic lncRNA MIAT, restored the reduced expression of H19 to near-normal levels, and increased the expression of GAS5. These molecular alterations were accompanied by reduced cardiac fibrosis and apoptosis, as well as improved hemodynamic parameters and contractility indices. This study provides direct experimental evidence that aerobic exercise modulates specific lncRNAs involved in post-infarction remodeling in the heart of Wistar rats (68).
These empirical examples underline that lncRNA regulation by exercise is both tissue- and model-dependent, and that specific transcripts exhibit robust expression changes in the heart in response to regular aerobic activity. Such findings strengthen the rationale for targeting exercise-responsive lncRNAs in future cardiovascular therapies (Figure 1). In a chronic heart failure model using adult male Sprague-Dawley rats, six weeks of aerobic treadmill training (5 days/week) improved cardiac function by downregulating the lncRNA MALAT1 and upregulating miR-150-5p, thereby modulating the PI3K/Akt signaling pathway. These findings suggest that aerobic exercise exerts therapeutic effects in chronic heart failure through the MALAT1/miR-150-5p/PI3K/Akt regulatory axis (67). These findings emphasize the central function of exercise-induced lncRNA regulation in cardiovascular adaptation and protection against disease and shed new light on the molecular mechanisms of the cardioprotective action of exercise and pave the way for new therapeutic approaches targeting lncRNAs for cardiovascular disease.
Aerobic training of exercise modulates the expression of miRNAs and lncRNAs, which are associated with improved cardiovascular health. Treadmill exercise enhances cardiac function and prevents left ventricular remodeling in heart failure by suppressing MALAT1 and upregulating miRNA-150. Aerobic exercise further reduces cardiomyocyte apoptosis and fibrosis in MI models by restoring the expression of lncRNAs such as H19, MIAT, and GAS5. Exercise also suppresses MIAT, a fibrotic pro-fibrotic lncRNA that promotes fibrosis by sequestering miRNA-24 and activating the TGF-β pathway.
3.3 Mechanisms of lncRNA-mediated cardioprotection
LncRNAs exert their cardioprotective effects through a variety of mechanisms, many of which are enhanced or modulated by physical activity. These mechanisms include acting as competing endogenous RNAs (ceRNAs), interacting with chromatin modifiers, regulating transcription factors, and modulating inflammatory responses (Table 2) (77). By integrating these diverse functions, lncRNAs play a pivotal role in mediating the beneficial effects of exercise on heart health, making them promising candidates for novel therapeutic interventions.
3.3.1 ceRNA activity
One of the key mechanisms by which lncRNAs mediate cardioprotection is by acting as ceRNAs or molecular sponges. LncRNAs can bind miRNAs and inhibit their interaction with their target mRNAs, thereby modulating gene expression. For example, the lncRNA H19 has been shown to act as a sponge for miR-675, a miRNA involved in the regulation of cardiomyocyte hypertrophy and apoptosis. By sequestering miR-675, H19 promotes cardiomyocyte survival and reduces pathological remodeling. Similarly, the lncRNA CHRF (Cardiac Hypertrophy-Related Factor) functions as a ceRNA for miR-489, which targets genes involved in cardiac hypertrophy. Exercise-induced upregulation of CHRF has been associated with reduced hypertrophy and improved cardiac function (77, 78).
3.3.2 Regulation of transcription factors
LncRNAs have been shown to play a significant role in the regulation of transcription factors involved in cardiovascular health directly or indirectly. For instance, the lncRNA Mhrt interacts with the chromatin-remodeling factor Brg1, which is involved in pathological hypertrophy. The interaction of Mhrt inhibits Brg1 binding to pro-hypertrophic gene promoters, thereby preventing maladaptive cardiac remodeling (80). Similarly, the lncRNA Mhrt779 has been shown to be upregulated following three weeks of swimming exercise in adult male C57BL/6 mice (8–10 weeks old), with its levels remaining elevated even one week after exercise cessation. This prolonged expression of Mhrt779 was associated with exercise-induced hypertrophic preconditioning and increased resistance to pathological cardiac hypertrophy in murine models. Mhrt779 accomplishes this through interaction with Brg1 and modulating the HDAC2/AKT/GSK3β pathway, reinforcing further the roles of lncRNAs as mediators for exercise-induced heart protective effects (75).
3.3.3 Modulation of inflammatory responses
Among the main causatives of the progress of cardiovascular illnesses is persistent inflammation, and lncRNAs play a fundamental role in the regulation of inflammatory response to physical exercise (7). As an example, the THRIL (TNFα and Heterogeneous Nuclear Ribonucleoprotein L-Related Immunoregulatory LncRNA) lncRNA regulates inflammatory cytokine TNFα and IL-6 gene expression through its interaction with hnRNPL, a protein involved in mRNA splicing and stability (81). Moreover, THRIL has also been demonstrated to be regulated after exercise stress, such as in a half marathon race, reflecting its involvement in exercise-induced inflammation regulation. Aside from its mechanism of action within the NF-κB pathway, THRIL is most probably to influence other pathways involved in the regulation of inflammation as well, further contributing to its role in exercise, inflammation, and cardiovascular health interaction (79). By regulating these pathways, exercise-induced lncRNAs contribute to the reduction of chronic inflammation and its detrimental effects on cardiovascular health.
3.3.4 Therapeutic implications
The discovery of exercise-responsive lncRNAs and their cardioprotective functions has provided new possibilities for the development of lncRNA-based therapies. For instance, synthetic lncRNA mimics or inhibitors may be employed to regulate the expression of particular lncRNAs in patients with CVDs, thus improving cardiac function, decreasing inflammation, and promoting vascular health. In addition, circulating lncRNAs, which are stable in biofluids such as blood, can serve as biomarkers for monitoring the cardiovascular adaptations to exercise and guiding personalized therapeutic strategies. In conclusion, lncRNAs play a central role in mediating the cardioprotective effects of exercise through diverse mechanisms, including ceRNA activity, chromatin remodeling, regulation of transcription factors, and modulation of inflammatory responses. A deeper understanding of these mechanisms may pave the way for innovative therapeutic strategies that harness the power of lncRNAs to prevent and treat CVDs, ultimately improving outcomes for patients worldwide (Table 2).
4 Epigenetic modifications and their role in exercise-induced cardioprotection
4.1 Overview of epigenetics in cardiovascular health
Epigenetics refers to heritable gene expression changes that occur without alterations in the DNA sequence. These modifications include DNA methylation, histone modifications, and non-coding RNA interactions, all of which play crucial roles in regulating gene activity in response to environmental stimuli, including exercise (82). In the cardiovascular system, epigenetic regulation influences key processes such as cardiac remodeling, inflammation, oxidative stress, and endothelial function. Epigenetic process dysregulation has been implicated in the pathogenesis of cardiovascular disease such as atherosclerosis, heart failure, and hypertension (83). Exercise has been shown to induce beneficial epigenetic modifications that promote cardioprotection, highlighting the potential for targeting epigenetic pathways in cardiovascular disease prevention and treatment (Table 3).
4.2 Exercise and DNA methylation
DNA methylation, which involves the addition of a methyl group to cytosine residues in CpG islands, is a critical epigenetic process to regulate gene expression. Aberrant DNA methylation patterns have been associated with CVDs, contributing to inflammation, vascular dysfunction, and metabolic dysregulation (83). Regular physical activity has been shown to modify DNA methylation patterns in genes related to cardiovascular health, leading to favorable gene expression changes (91). While exercise-regulated DNA methylation in cardiac tissue has not yet been fully documented, the evidence shows that exercise-induced DNA methylation in tissues other than cardiac tissue, such as skeletal muscle and adipose tissue, plays a major role in providing anti-inflammatory effects and metabolic remodeling (92). Such epigenetic changes lead to the decreased prevalence of cardiac disease, which is indicative of the possible systemic impact of exercise on cardiovascular health. Exercise-induced DNA methylation has been recognized as a primary mechanism in the reduction of inflammation, improvement of metabolic health, and lowering of cardiovascular disease risk. Research indicates that exercise increases the methylation of ASC (apoptosis-associated speck-like protein), a gene involved in inflammasome activation, leading to reduced interleukin-1β production and improved outcomes in heart failure (85, 86). Exercise also promotes hypermethylation of Kruppel-like factor 14 (KLF14) and src homology 2 domain-containing transforming protein C1, which are associated with anti-inflammatory effects and healthy vascular aging, respectively (87, 88). Exercise induces hypomethylation of PGC-1α in skeletal muscle, a central controller of mitochondrial biogenesis and energy metabolism (84), while also modulating the methylation of PDK4 and PPARD, genes essential for glucose and fatty acid metabolism (92). Similarly, exercise regulates the methylation of NRF1 and NR4A1, transcription factors linked to insulin sensitivity and mitochondrial function (93). In adipose tissue, exercise upregulates methylation of RALBP1, a gene implicated in metabolic syndrome (94). Collectively, these findings nominate exercise-evoked DNA methylation as a vital element for improving cardiometabolic health and an emerging therapeutic application for prevention against cardiac and metabolic diseases (Figure 2).
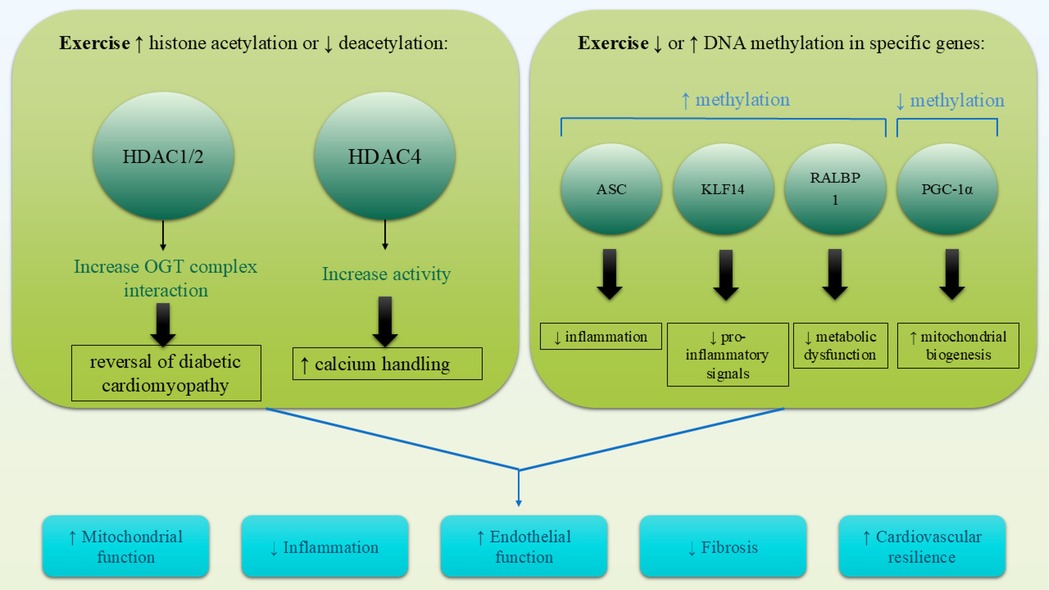
Figure 2. Exercise induces cardioprotective epigenetic modifications through alterations in DNA methylation and histone modifications. Hypomethylation of genes like PGC-1α enhances mitochondrial biogenesis, while hypermethylation of ASC and KLF14 reduces inflammation. Simultaneously, exercise promotes histone acetylation (e.g., PGC-1α, VEGF) and reduces HDAC activity (e.g., HDAC4, HDAC1/2), leading to improved gene expression profiles involved in cardiac repair, energy metabolism, and vascular health.
4.3 Exercise and histone modifications
Histone modifications, including acetylation, methylation, and phosphorylation, influence chromatin structure and gene transcription. Histone acetylation, mediated by histone acetyltransferases (HATs), generally promotes gene expression, while histone deacetylation, facilitated by histone deacetylases (HDACs), leads to gene repression (95). Exercise has been shown to modulate histone modifications in a manner that supports cardiovascular health, such as increasing histone acetylation in genes involved in angiogenesis and mitochondrial biogenesis (96). Additionally, reductions in HDAC activity caused by exercise have been linked to improved cardiac function, reduced fibrosis, and enhanced endothelial homeostasis. For example, exercise-induced fragments of HDAC4 avert heart failure through the regulation of the hexosamine biosynthetic pathway, protein O-GlcNAcylation, and calcium handling (89). Additionally, HDAC1 and HDAC2 contribute to exercise-induced cardioprotection, with evidence showing that exercise promotes the interaction between the mSin3A/HDAC1/HDAC2 complex and O-GlcNAc transferase (OGT), reversing diabetic heart pathology (90). These findings suggest that exercise-induced histone modifications are cardioprotective in heart failure, diabetic cardiomyopathy, and obesity-related cardiac dysfunction. Further studies are needed to elucidate the exact mechanisms of HDACs and investigate other histone modifications, such as methylation, phosphorylation, and ubiquitination, of exercise-induced cardioprotection. Through the regulation of histone modifications, physical training creates a more favorable epigenetic environment conducive to long-term cardiovascular protection.
4.4 Epigenetic memory: persistence or reversibility of exercise-induced marks
A critical question in cardioepigenetics is whether exercise-induced epigenetic changes are transient or capable of persisting after cessation of physical activity. Accumulating evidence suggests that while some epigenetic modifications are short-lived and require continued exercise stimuli, others can endure for extended periods, creating what is now referred to as epigenetic memory.
One of the most well-characterized examples is DNA hypomethylation of the PGC-1α promoter, a master regulator of mitochondrial biogenesis. In both human and rodent models, this epigenetic mark has been shown to remain at least partially reduced for several days to weeks following exercise cessation, supporting prolonged oxidative adaptations even during inactivity (97). In a landmark human study using muscle biopsies before and after a 7-week resistance training program, followed by a detraining period and then retraining, researchers identified over 18,000 CpG sites that remained hypomethylated even after inactivity. Notably, genes such as AXIN1, GRIK2, CAMK4, and TRAF1 retained their epigenetic changes, suggesting that prior training leaves a molecular imprint that may facilitate future adaptations (98). On the other hand, histone modifications such as phosphorylation (e.g., H3S10ph) and acetylation (e.g., H3K9ac), while critical for acute transcriptional responses to exercise, tend to be more labile and transient, often reverting to baseline shortly after exercise cessation (99). However, the persistence of these modifications appears to be tissue-specific, dose-dependent, and individualized, influenced by factors such as exercise intensity, age, sex, and genetic background. Some modifications regress quickly upon inactivity, highlighting the importance of exercise regularity in maintaining beneficial epigenetic states (100).
Collectively, these findings suggest that exercise can induce both reversible and persistent epigenetic reprogramming, with DNA methylation-based changes playing a pivotal role in long-term cardiovascular adaptations. This has important implications for the design of personalized exercise programs and the development of “epigenetic dosing” models aimed at maximizing health benefits through sustained molecular signaling.
5 Exercise modality, intensity, and their impact on cardiovascular miRNA regulation
Both aerobic and resistance exercise have been shown to modulate the expression of cardiovascular-related miRNAs, but their regulatory effects differ in terms of mechanism, target pathways, and outcomes. Aerobic training, especially when performed at moderate to high intensities for sustained durations (e.g., ≥30 min per session, ≥3 times per week), commonly upregulates miRNAs associated with endothelial function, angiogenesis, mitochondrial biogenesis, and cardioprotection such as miR-126, miR-133a, and miR-210. These effects are often mediated through improved oxidative metabolism and hemodynamic stress adaptation (101). Studies have demonstrated that regular aerobic and high-intensity exercise can increase levels of miR-126 in both the bloodstream and specific tissues, promoting angiogenesis and endothelial function (24).
In contrast, resistance training (RT), typically involving high-intensity, short-duration bouts of mechanical loading, more prominently influences miRNAs related to muscle remodeling, inflammation, and cellular survival (102). For instance, RT has been associated with increased expression of miR-21, miR-222, miR-208b, and miR-499, which are involved in pathways regulating cardiac contractility, fibrosis inhibition, and mitochondrial function (103–105). Moreover, preliminary evidence suggests that the intensity and volume of resistance training may influence the profile of miRNA expression, although direct comparative studies between different loading schemes remain limited (106). Furthermore, combined aerobic and resistance exercise may induce adaptive changes in circulating miRNAs that reflect both metabolic and structural cardiovascular remodeling. For instance, in patients with HFrEF undergoing 15 weeks of combined training, specific plasma miRNAs (e.g., miR-146a, miR-23a) were modulated and associated with VO₂peak improvements and cardiac remodeling markers (107).
Altogether, these findings emphasize that the type, intensity, and duration of exercise are key modulators of miRNA and lncRNA expression patterns, and support the concept of personalized exercise prescriptions to maximize cardiovascular epigenetic benefits.
6 Interplay between miRNAs, lncRNAs, and epigenetics
Exercise-induced cardioprotection is coordinated by a highly interactive network of miRNA-lncRNA-epigenetic alteration interactions but not by distinct molecular processes. These factors co-operate to modulate gene expression, govern cell signaling pathways, and trigger adaptive responses in the cardiovascular system (21). Through the creation of a dynamic feedback loop, they increase cardiac resilience, reduce inflammation, and improve vascular function. For instance, lncRNAs can act as molecular sponges for miRNAs, sequestering them and preventing their interaction with target mRNAs. This interaction fine-tunes the regulatory effects of miRNAs on gene expression (108). Conversely, epigenetic changes can also impact the expression of both miRNAs and lncRNAs, creating a bidirectional network of regulation. For instance, DNA methylation and histone acetylation can control miRNA expression such as miR-1 and miR-133, which influence gene regulation regarding cardiac remodeling and function (108). The combined effects of miRNAs, lncRNAs, and epigenetic modifications enhance cardiac resilience by promoting adaptive hypertrophy, reducing fibrosis, and improving mitochondrial function. These molecular changes also attenuate chronic inflammation and oxidative stress, which are key drivers of cardiovascular disease (17). For example, exercise-induced upregulation of miR-126 and lncRNA H19 promotes angiogenesis and endothelial repair, while epigenetic modifications such as histone acetylation of antioxidant genes enhance cellular resistance to oxidative damage (68, 109). Together, these mechanisms create a protective molecular environment that supports long-term cardiovascular health.
7 Future directions and research gaps
The discovery of exercise-induced miRNAs and their cardioprotective mechanisms has opened new avenues for therapeutic strategies aimed at mimicking the molecular benefits of physical activity. Synthetic miRNA mimics (e.g., miR-126, miR-133a, miR-146a) and inhibitors (e.g., anti-miR-1 or anti-miR-21) hold potential for modulating key pathways involved in inflammation, fibrosis, apoptosis, and mitochondrial function (110). However, despite encouraging preclinical data, the clinical translation of miRNA-based therapies remains highly challenging. Most findings are derived from animal models, and only a limited number of human trials have progressed to advanced phases. Key limitations include the lack of safe, efficient, and tissue-specific delivery systems, as well as the risk of off-target effects and immune activation due to non-physiological delivery methods. Moreover, the pleiotropic nature of miRNAs regulating multiple mRNA targets raises concerns about unintended downstream consequences (111, 112).
Among the few miRNA-based interventions tested in humans, miR-132-3p inhibition has shown promising translational potential. A first-in-human phase Ib study in heart failure patients demonstrated favorable tolerability and preliminary efficacy, following robust preclinical data in rodent and porcine models (113, 114). Among the promising therapeutic targets, miR-155 inhibition shows strong anti-inflammatory effects in preclinical cardiac inflammation models by modulating macrophage activity and NF-κB signaling. The antimiR-155 drug cobomarsen reached phase I clinical trials for lymphoma but was discontinued in phase II for strategic reasons ( (115, 116). miR-92a-3p is highly expressed in endothelial cells and plays a critical role in vascular and myocardial injury. AntimiR-92a (MRG-110) has demonstrated pro-angiogenic and tissue repair effects in preclinical models, including mice and pigs. Early clinical trials in healthy volunteers have assessed its safety and pharmacodynamics via intravenous and intradermal administration, marking important steps toward therapeutic application (116, 117).
Long-term safety, pharmacokinetics, and chronic administration effects remain largely unexplored, particularly with respect to their interaction with endogenous regulatory networks. Considering these limitations, future research must focus on several critical priorities: conducting well-controlled, longitudinal human studies to validate animal-based findings; improving delivery technologies (e.g., nanoparticle- or exosome-based carriers); and deepening our mechanistic understanding of miRNA–lncRNA–epigenetic interplay. Furthermore, standardizing exercise intervention protocols and characterizing the influence of variables such as age, sex, comorbidities, and exercise dose on molecular responses are essential steps toward clinical applicability.
7.1 Clinical applications of exercise-induced epigenetic discoveries
Recent advances in exercise epigenetics have opened promising avenues for clinical translation, particularly in the realm of precision cardiovascular medicine. Epigenetic alterations such as changes in DNA methylation, histone modifications, and non-coding RNA expression represent dynamic, modifiable markers that can serve as both diagnostic tools and therapeutic targets (118). For instance, specific exercise-regulated miRNAs, such as miR-126 and miR-146a, as well as lncRNAs like H19 and GAS5, have shown promise as circulating biomarkers of cardiovascular stress and vascular inflammation. Their stability in blood and detectability by qPCR make them suitable for liquid biopsy approaches to assess cardiovascular risk or training response (119). In parallel, the development of epigenetic drugs targeting enzymes like DNA methyltransferases (DNMTs), histone deacetylases (HDACs), or RNA-based therapeutics (e.g., miRNA mimics/inhibitors) offers potential for mimicking or enhancing the beneficial molecular effects of exercise particularly in individuals unable to engage in regular physical activity due to advanced disease, disability, or aging. Although these strategies remain largely experimental, their integration into clinical protocols may complement conventional rehabilitation or preventive measures (120).
Integration of individualized epigenetic profiling into clinical practice may enable personalized exercise prescriptions. By assessing baseline epigenetic signatures such as DNA methylation patterns in genes regulating inflammation, mitochondrial function, and vascular homeostasis clinicians could tailor the type, intensity, and duration of exercise to achieve maximal benefit (121, 122). As technologies such as next-generation sequencing and machine learning evolve, the prospect of epigenetically guided exercise interventions is becoming increasingly feasible (123, 124).
These clinical applications position epigenetic discoveries not only as mechanistic insights but also as transformative tools for individualized prevention and treatment of cardiovascular disease.
7.2 Expanded future perspectives: standardization, biomarkers, and environmental interactions
To advance the clinical utility of exercise epigenetics, future research must prioritize the standardization of exercise protocols in both animal and human models. Currently, the heterogeneity in exercise modalities (e.g., aerobic, resistance, HIIT), intensities (e.g., moderate vs. vigorous), durations, and frequencies limits the comparability of studies and hinders meta-analysis. whereas aerobic exercise has been shown to increase cardiac expression of lncRNAs like MALAT1 and Mhrt, associated with cardiomyocyte survival (67), resistance-type protocols such as swimming in rodents have been reported to upregulate the cardiac lncRNA Mhrt779, mediating physiological antihypertrophic remodeling via the Brg1/Hdac2/p-Akt/p-GSK3β axis (75, 125).
Similarly, cardiac miRNAs such as miR-126 (angiogenesis) and miR-222 (cell cycle and cardiomyocyte growth) are differentially regulated depending on training load and modality: in diabetic rats, interval training elicited significantly greater myocardial expression of miR-126 and miR-222 compared to continuous exercise (126); in humans, both serial and integrated concurrent exercise sessions induced acute increases in circulating miR-126 and miR-222, underscoring their sensitivity to exercise stimulus ( (127).
Standardized protocols will thus be essential to define molecular thresholds for cardioprotective effects, especially in relation to non-coding RNA regulation such as miRNAs and lncRNAs. Moreover, epigenetic modifications particularly DNA methylation of promoters such as PGC-1α, a key regulator of mitochondrial biogenesis are emerging as critical mediators of exercise-induced cardiovascular adaptation and warrant standardized investigation (128).
Another emerging area involves exploring the modulatory effects of lifestyle and environmental factors, including diet, sleep quality, chronic stress, circadian disruption, and pollution exposure, on the cardiac epigenome (129, 130). These variables can enhance or suppress the molecular benefits of exercise by interfering with chromatin remodeling or non-coding RNA expression. For instance, sleep deprivation alters circulating miRNA profiles, indicating its potential to blunt ncRNA-mediated exercise responses (131–133), while antioxidant-rich diets and polyphenols have been shown to modulate lncRNA expression in cardiovascular contexts (134). Integrative multi-omic profiling and systems biology approaches will be essential to dissect these interactions and move toward personalized exercise interventions.
In summary, by addressing these key priorities protocol standardization, biomarker discovery, and environmental interaction mapping future research can bridge the gap between experimental findings and clinical translation, ultimately contributing to precision cardiovascular medicine grounded in epigenetic regulation.
7.3 Technical and bioethical challenges for lncRNA-based therapies
Despite the growing evidence supporting the role of lncRNAs in cardiovascular adaptation to exercise, the clinical translation of lncRNA-based therapies remains particularly challenging. Delivery challenges are especially pronounced due to the structural complexity and diverse modes of action of lncRNAs, which often interact with chromatin, RNA, or proteins in a context-specific manner (135). Additionally, the low sequence conservation of lncRNAs across species complicates the extrapolation of preclinical findings to human physiology (65).
From a bioethical standpoint, manipulating lncRNAs raises concerns about unpredictable off-target effects, long-term epigenetic alterations, and the persistence of synthetic molecules in human tissues (136). These risks are particularly relevant in otherwise healthy individuals where preventive interventions may be considered. Careful ethical scrutiny and regulatory oversight are therefore essential, especially when genome-editing tools or chronic systemic delivery methods are involved (66).
Looking ahead, non-pharmacological approaches that harness endogenous regulatory mechanisms—such as personalized exercise prescriptions informed by individual lncRNA expression profiles—may offer safer and more physiologically harmonious alternatives (137). Emerging liquid biopsy platforms may allow clinicians to monitor circulating lncRNAs in real time, enabling dynamic optimization of training intensity, duration, and modality (138). Furthermore, integrating multi-omics profiling with machine learning could help identify patient subgroups most likely to benefit from lncRNA-targeted strategies, paving the way for precision exercise-based cardiovascular medicine (139).
8 Conclusions
The coordinated interaction between miRNAs, lncRNAs, and epigenetic modulation in exercise-induced cardioprotection is an exciting area with extensive therapeutic potential. By elucidating the complex crosstalk of these molecular regulators, researchers can develop new interventions for cardiovascular disease prevention and therapy. Future studies must focus on bridging existing knowledge gaps and translating preclinical findings into the clinic, ultimately improving cardiovascular outcomes for patients worldwide.
Author contributions
SY: Data curation, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. QY: Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. RQ: Conceptualization, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mensah GA, Fuster V, Murray CJL, Roth GA. Global burden of cardiovascular diseases and risks, 1990–2022. J Am Coll Cardiol. (2023) 82:2350–473. doi: 10.1016/j.jacc.2023.11.007
2. Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, et al. Social determinants of risk and outcomes for cardiovascular disease. Circulation. (2015) 132:873–98. doi: 10.1161/CIR.0000000000000228
3. Piché M-E, Tchernof A, Després J-P. Obesity phenotypes, diabetes, and cardiovascular diseases. Circ Res. (2020) 126:1477–500. doi: 10.1161/CIRCRESAHA.120.316101
4. Gomes MJ, Pagan LU, Okoshi MP. Non-pharmacological treatment of cardiovascular disease|importance of physical exercise. Arq Bras Cardiol. (2019) 113:9–10. doi: 10.5935/abc.20190118
5. Franklin BA, Jae SY. Physical activity, cardiorespiratory fitness and atherosclerotic cardiovascular disease: part 1. Pulse. (2024) 12:113–25. doi: 10.1159/000541165
6. Zhang Y, He N, Feng B, Ye H. Exercise mediates heart protection via non-coding RNAs. Front Cell Dev Biol. (2020) 8:182. doi: 10.3389/fcell.2020.00182
7. Xie X, Huang M, Ma S, Xin Q, Wang Y, Hu L, et al. The role of long non-coding RNAs in cardiovascular diseases: a comprehensive review. Non-Coding RNA Res. (2025) 11:158–87. doi: 10.1016/j.ncrna.2024.12.009
8. Mokarram P, Niknam M, Sadeghdoust M, Aligolighasemabadi F, Siri M, Dastghaib S, et al. PIWI Interacting RNAs perspectives: a new avenues in future cancer investigations. Bioengineered. (2021) 12:10401–19. doi: 10.1080/21655979.2021.1997078
9. Sanchis-Gomar F, Arnau-Moyano M, Daimiel L, Lippi G, Leischik R, Vallecillo N, et al. Circulating microRNAs fluctuations in exercise-induced cardiac remodeling: a systematic review. Am J Transl Res. (2021) 13:13298–309.35035676
10. Jiang H, Jia D, Zhang B, Yang W, Dong Z, Sun X, et al. Exercise improves cardiac function and glucose metabolism in mice with experimental myocardial infarction through inhibiting HDAC4 and upregulating GLUT1 expression. Basic Res Cardiol. (2020) 115:28. doi: 10.1007/s00395-020-0787-1
11. Denham J, O'Brien BJ, Marques FZ, Charchar FJ. Changes in the leukocyte methylome and its effect on cardiovascular-related genes after exercise. J Appl Physiol. (2015) 118:475–88. doi: 10.1152/japplphysiol.00878.2014
12. Zhang Y, Chen Y, Xu Z, Wu Y, Zhang Y, Shi L. Chronic exercise mediates epigenetic suppression of L-type Ca2+ channel and BKCa channel in mesenteric arteries of hypertensive rats. J Hypertens. (2020) 38:1763–76. doi: 10.1097/HJH.0000000000002457
13. Dimauro I, Paronetto MP, Caporossi D. Exercise, redox homeostasis and the epigenetic landscape. Redox Biol. (2020) 35:101477. doi: 10.1016/j.redox.2020.101477
14. Damal Villivalam S, Ebert SM, Lim HW, Kim J, You D, Jung BC, et al. A necessary role of DNMT3A in endurance exercise by suppressing ALDH1L1-mediated oxidative stress. EMBO J. (2021) 40:e106491. doi: 10.15252/embj.2020106491
15. Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance? Exerc Immunol Rev. (2006) 12:6–33.17201070
16. Wu G, Zhang X, Gao F. The epigenetic landscape of exercise in cardiac health and disease. J Sport Health Sci. (2021) 10:648–59. doi: 10.1016/j.jshs.2020.12.003
17. Sun Y, Peng Z, Liang H. Role of physical activity in cardiovascular disease prevention: impact of epigenetic modifications. Front Cardiovasc Med. (2025) 12:182. doi: 10.3389/fcvm.2025.1511222
18. Sessa F, Salerno M, Esposito M, Cocimano G, Pomara C. miRNA dysregulation in cardiovascular diseases: current opinion and future perspectives. Int J Mol Sci. (2023) 24:5192.36982265
19. Wronska A. The role of microRNA in the development, diagnosis, and treatment of cardiovascular disease: recent developments. J Pharmacol Exp Ther. (2023) 384:123–32. doi: 10.1124/jpet.121.001152
20. Silva GJJ, Bye A, el Azzouzi H, Wisløff U. MicroRNAs as important regulators of exercise adaptation. Prog Cardiovasc Dis. (2017) 60:130–51. doi: 10.1016/j.pcad.2017.06.003
21. Gao J, Song J, Yan Y, Gokulnath P, Vulugundam G, Li G, et al. Exercise training-induced MicroRNA alterations with protective effects in cardiovascular diseases. Rev Cardiovasc Med. (2023) 24:251. doi: 10.31083/j.rcm2409251
22. Masoumi-Ardakani Y, Najafipour H, Nasri HR, Aminizadeh S, Jafari S, Safi Z. Moderate endurance training and MitoQ improve cardiovascular function, oxidative stress, and inflammation in hypertensive individuals: the role of miR-21 and miR-222: a randomized, double-blind, clinical trial. Cell J. (2022) 24:577–85. doi: 10.22074/cellj.2022.8089
23. Ono K. Chapter 10—MicroRNA-linked heart disease and therapeutic potential. In: Laurence J, editor. Translating MicroRNAs to the Clinic. Boston: Academic Press (2017). p. 259–81.
24. Dastah S, Tofighi A, Tolouei Azar J, Alivand M. Aerobic exercise leads to upregulation of mir-126 and angiogenic signaling in the heart tissue of diabetic rats. Gene Rep. (2020) 21:100914. doi: 10.1016/j.genrep.2020.100914
25. Fathi M, Gharakhanlou R, Rezaei R. The changes of heart miR-1 and miR-133 expressions following physiological hypertrophy due to endurance training. Cell J. (2020) 22:133–40. doi: 10.22074/cellj.2020.7014
26. Xu T, Zhou Q, Che L, Das S, Wang L, Jiang J, et al. Circulating miR-21, miR-378, and miR-940 increase in response to an acute exhaustive exercise in chronic heart failure patients. Oncotarget. (2016) 7:12414. doi: 10.18632/oncotarget.6966
27. Brandão BB, Madsen S, Rabiee A, Oliverio M, Ruiz GP, Ferrucci DL, et al. Dynamic changes in DICER levels in adipose tissue control metabolic adaptations to exercise. Proc Natl Acad Sci U S A. (2020) 117:23932–41. doi: 10.1073/pnas.2011243117
28. Li J, Wang Z, Li C, Song Y, Wang Y, Bo H, et al. Impact of exercise and aging on mitochondrial homeostasis in skeletal muscle: roles of ROS and epigenetics. Cells. (2022) 11:2086. doi: 10.3390/cells11132086
29. Aoi W, Naito Y, Mizushima K, Takanami Y, Kawai Y, Ichikawa H, et al. The microRNA miR-696 regulates PGC-1α in mouse skeletal muscle in response to physical activity. Am J Physiol Endocrinol Metab. (2010) 298:E799–806. doi: 10.1152/ajpendo.00448.2009
30. Li D, Wang P, Wei W, Wang C, Zhong Y, Lv L, et al. Serum MicroRNA expression patterns in subjects after the 5-km exercise are strongly associated with cardiovascular adaptation. Front Physiol. (2021) 12:755656. doi: 10.3389/fphys.2021.755656
31. Taganov K, Boldin M, Chang K-J, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. (2006) 103:12481–6. doi: 10.1073/pnas.0605298103
33. Ma Y, Liu H, Wang Y, Xuan J, Gao X, Ding H, et al. Roles of physical exercise-induced MiR-126 in cardiovascular health of type 2 diabetes. Diabetol Metab Syndr. (2022) 14:169. doi: 10.1186/s13098-022-00942-6
34. Li Y, Yang C, Zhang L, Yang P. MicroRNA-210 induces endothelial cell apoptosis by directly targeting PDK1 in the setting of atherosclerosis. Cell Mol Biol Lett. (2017) 22:3. doi: 10.1186/s11658-017-0033-5
35. Chistiakov DA, Orekhov AN, Bobryshev YV. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J Mol Cell Cardiol. (2016) 94:107–21. doi: 10.1016/j.yjmcc.2016.03.015
36. Li N, Zhou H, Tang Q. miR-133: a suppressor of cardiac remodeling? Front Pharmacol. (2018) 9:903. doi: 10.3389/fphar.2018.00903
37. Liu J, Liang X, Zhou D, Lai L, Xiao L, Liu L, et al. Coupling of mitochondrial function and skeletal muscle fiber type by a miR-499/Fnip1/AMPK circuit. EMBO Mol Med. (2016) 8:1212–28. doi: 10.15252/emmm.201606372
38. Zhang X-T, Xu M-G. Potential link between microRNA-208 and cardiovascular diseases. J Xiangya Med. (2021) 6:1–11. doi: 10.21037/jxym-21-8
39. Liao Z, Zheng R, Shao G. Mechanisms and application strategies of miRNA-146a regulating inflammation and fibrosis at molecular and cellular levels (review). Int J Mol Med. (2023) 51:7. doi: 10.3892/ijmm.2022.5210
40. Santos M, Cirino M, Tirapelli V, Celani M, Lellis J, Thomazini J, et al. The role of exercise in the expression of caspase 3 and microRNAs miR-138 and miR-155 associated with apoptosis in the cerebellum of rats submitted to cerebral ischemia.
41. Chen H, Chen C, Spanos M, Li G, Lu R, Bei Y, et al. Exercise training maintains cardiovascular health: signaling pathways involved and potential therapeutics. Signal Transduct Target Ther. (2022) 7:306. doi: 10.1038/s41392-022-01153-1
42. Bei Y, Tao L, Cretoiu D, Cretoiu SM, Xiao J. MicroRNAs mediate beneficial effects of exercise in heart. Exerc Cardiovasc Dis Prev Treat. (2017) 2:261–80. doi: 10.1007/978-981-10-4304-8_15
43. Bahrami F, Fathi M, Ahmadvand H, Pajohi N. Endurance training changes the expression of miR-1 and miR-133 and predicted genes in slow and fast twitch muscles. Arch Gerontol Geriatr. (2023) 108:104929. doi: 10.1016/j.archger.2023.104929
44. Shi J, Bei Y, Kong X, Liu X, Lei Z, Xu T, et al. miR-17-3p contributes to exercise-induced cardiac growth and protects against myocardial ischemia-reperfusion injury. Theranostics. (2017) 7:664. doi: 10.7150/thno.15162
45. Correia IM, da Silva Rodrigues G, Noronha NY, Watanabe LM, Luciano de Almeida M, Sobrinho ACDS, et al. Older postmenopausal women with lower lean mass have hypermethylated sites in the PI3K-akt pathway. Front Physiol. (2023) 14:11. doi: 10.3389/fphys.2023.1150821
46. Vujic A, Lerchenmüller C, Wu T-D, Guillermier C, Rabolli CP, Gonzalez E, et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. (2018) 9:1659. doi: 10.1038/s41467-018-04083-1
47. Silveira AC, Fernandes T, Soci ÚP, Gomes JL, Barretti DL, Mota GG, et al. Exercise training restores cardiac microRNA-1 and microRNA-29c to nonpathological levels in obese rats. Oxid Med Cell Longevity. (2017) 2017:1549014. doi: 10.1155/2017/1549014
48. Soci UPR, Fernandes T, Hashimoto NY, Mota GF, Amadeu MA, Rosa KT, et al. MicroRNAs 29 are involved in the improvement of ventricular compliance promoted by aerobic exercise training in rats. Physiol Genomics. (2011) 43:665–73. doi: 10.1152/physiolgenomics.00145.2010
49. Yang HM. Mitochondrial dysfunction in cardiovascular diseases. Int J Mol Sci. (2025) 26:1917. doi: 10.3390/ijms26051917
50. Han X, Wang S, Yong Z, Zhang X, Wang X, You P. Effect of miR-499-5p/SOX6 axis on atrial fibrosis in rats with atrial fibrillation. Open Med. (2023) 18:20230654. doi: 10.1515/med-2023-0654
51. Fathi M. The effect of endurance activity on miR-499 and sox6 genes expression in fast and slow twitch skeletal muscles (2017).
52. Callis TE, Pandya K, Seok HY, Tang R-H, Tatsuguchi M, Huang Z-P, et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest. (2009) 119:2772–86. doi: 10.1172/JCI36154
53. Henein MY, Vancheri S, Longo G, Vancheri F. The role of inflammation in cardiovascular disease. Int J Mol Sci. (2022) 23:12906. doi: 10.3390/ijms232112906
54. Balchin C, Tan AL, Wilson OJ, McKenna J, Stavropoulos-Kalinoglou A. The role of microRNAs in regulating inflammation and exercise-induced adaptations in rheumatoid arthritis. Rheumatol Adv Pract. (2023) 7:rkac110. doi: 10.1093/rap/rkac110
55. Fitzsimons S, Oggero S, Bruen R, McCarthy C, Strowitzki MJ, Mahon NG, et al. microRNA-155 is decreased during atherosclerosis regression and is increased in urinary extracellular vesicles during atherosclerosis progression. Front Immunol. (2020) 11:576516. doi: 10.3389/fimmu.2020.576516
56. Wang S, Liu Z, Wang J, Ji X, Yao Z, Wang X. Mir-21 promotes osteoclastogenesis through activation of PI3 K/akt signaling by targeting pten in RAW264.7 cells. Mol Med Rep. (2020) 21:1125–32. doi: 10.3892/mmr.2020.10938
57. Li T, Zhong Y, Tang T, Luo J, Cui H, Fan R, et al. Formononetin induces vasorelaxation in rat thoracic aorta via regulation of the PI3K/PTEN/akt signaling pathway. Drug Des Devel Ther. (2018) 12:3675–84. doi: 10.2147/DDDT.S180837
58. Buscaglia LE, Li Y. Apoptosis and the target genes of microRNA-21. Chin J Cancer. (2011) 30:371–80. doi: 10.5732/cjc.30.0371
59. Saadh MJ, Jasim NY, Ahmed MH, Ballal S, Kumar A, Atteri S, et al. Critical roles of miR-21 in promotions angiogenesis: friend or foe? Clin Exp Med. (2025) 25:66. doi: 10.1007/s10238-025-01600-7
60. Toma L, Sanda GM, Niculescu LS, Deleanu M, Sima AV, Stancu CS. Phenolic compounds exerting lipid-regulatory, anti-inflammatory and epigenetic effects as complementary treatments in cardiovascular diseases. Biomolecules. (2020) 10:641. doi: 10.3390/biom10040641
61. Sun H-J, Wu Z-Y, Nie X-W, Bian J-S. Role of endothelial dysfunction in cardiovascular diseases: the link between inflammation and hydrogen sulfide. Front Pharmacol. (2020) 10:1568. doi: 10.3389/fphar.2019.01568
62. Zaccagnini G, Maimone B, Fuschi P, Longo M, Da Silva D, Carrara M, et al. Hypoxia-Induced miR-210 is necessary for vascular regeneration upon acute limb ischemia. Int J Mol Sci. (2019) 21:129. doi: 10.3390/ijms21010129
63. Yu B, Jiang Y, Wang X, Wang S. An integrated hypothesis for miR-126 in vascular disease. Med Res Arch. (2020) 8:2133. doi: 10.18103/mra.v8i5.2133
64. Zhang X, Wang W, Zhu W, Dong J, Cheng Y, Yin Z, et al. Mechanisms and functions of long non-coding RNAs at multiple regulatory levels. Int J Mol Sci. (2019) 20:5573. doi: 10.3390/ijms20225573
65. Jha S, Thasma Loganathbabu VK, Kumaran K, Krishnasamy G, Aruljothi KN. Long non-coding RNAs (lncRNAs) in heart failure: a comprehensive review. Noncoding RNA. (2023) 10:3. doi: 10.3390/ncrna10010003
66. Correia CCM, Rodrigues LF, de Avila Pelozin BR, Oliveira EM, Fernandes T. Long non-coding RNAs in cardiovascular diseases: potential function as biomarkers and therapeutic targets of exercise training. Noncoding RNA. (2021) 7:65. doi: 10.3390/ncrna7040065
67. Hu L, Xu Y-N, Wang Q, Liu M-J, Zhang P, Zhao L-T, et al. Aerobic exercise improves cardiac function in rats with chronic heart failure through inhibition of the long non-coding RNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1). Ann Transl Med. (2021) 9:340. doi: 10.21037/atm-20-8250
68. Farsangi SJ, Rostamzadeh F, Sheikholeslami M, Jafari E, Karimzadeh M. Modulation of the expression of long non-coding RNAs H19, GAS5, and MIAT by endurance exercise in the hearts of rats with myocardial infarction. Cardiovasc Toxicol. (2021) 21:162–8. doi: 10.1007/s12012-020-09607-0
69. Zhang Y-H, Sun T-T, Liu Z-H, Li X, Fan X-F, Han L-P. LncRNA GAS5 restrains ISO-induced cardiac fibrosis by modulating mir-217 regulation of SIRT1. Sci Rep. (2024) 14:7652. doi: 10.1038/s41598-024-58239-9
70. Guo Y, Chen J, Qiu H. Novel mechanisms of exercise-induced cardioprotective factors in myocardial infarction. Front Physiol. (2020) 11:199. doi: 10.3389/fphys.2020.00199
71. Huang P, Wang L, Li Q, Tian X, Xu J, Xu J, et al. Atorvastatin enhances the therapeutic efficacy of mesenchymal stem cells-derived exosomes in acute myocardial infarction via up-regulating long non-coding RNA H19. Cardiovasc Res. (2019) 116:353–67. doi: 10.1093/cvr/cvz139
72. Wang J-X, Zhang X-J, Li Q, Wang K, Wang Y, Jiao J-Q, et al. MicroRNA-103/107 regulate programmed necrosis and myocardial ischemia/reperfusion injury through targeting FADD. Circ Res. (2015) 117:352–63. doi: 10.1161/CIRCRESAHA.117.305781
73. Gong L-C, Xu H-M, Guo G-L, Zhang T, Shi J-W, Chang C. Long non-coding RNA H19 protects H9c2 cells against hypoxia-induced injury by targeting MicroRNA-139. Cell Physiol Biochem. (2017) 44:857–69. doi: 10.1159/000485354
74. Zhou M, Zou YG, Xue YZ, Wang XH, Gao H, Dong HW, et al. Long non-coding RNA H19 protects acute myocardial infarction through activating autophagy in mice. Eur Rev Med Pharmacol Sci. (2018) 22:5647–51. doi: 10.26355/eurrev_201809_15831
75. Lin H, Zhu Y, Zheng C, Hu D, Ma S, Chen L, et al. Antihypertrophic memory after regression of exercise-induced physiological myocardial hypertrophy is mediated by the long noncoding RNA Mhrt779. Circulation. (2021) 143:2277–92. doi: 10.1161/CIRCULATIONAHA.120.047000
76. Qu X, Du Y, Shu Y, Gao M, Sun F, Luo S, et al. MIAT is a pro-fibrotic long non-coding RNA governing cardiac fibrosis in post-infarct myocardium. Sci Rep. (2017) 7:42657. doi: 10.1038/srep42657
77. Collins L, Binder P, Chen H, Wang X. Regulation of long non-coding RNAs and MicroRNAs in heart disease: insight into mechanisms and therapeutic approaches. Front Physiol. (2020) 11:798. doi: 10.3389/fphys.2020.00798
78. Pereira N, Gatto C, de Oliveira EM, Fernandes T. Noncoding RNAs in the cardiovascular system: exercise training effects. In: Valarmathi MT, editor. Muscle Cells-Recent Advances and Future Perspectives. London: IntechOpen (2020). doi: 10.5772/intechopen.86054
79. Gary MA, Tanner EA, Davis AA, McFarlin BK. Combined bead-based multiplex detection of RNA and protein biomarkers: implications for understanding the time course of skeletal muscle injury and repair. Methods. (2019) 158:92–6. doi: 10.1016/j.ymeth.2018.11.012
80. Chang C-P, Han P. Epigenetic and lncRNA regulation of cardiac pathophysiology. Biochim Biophys Acta Mol Cell Res. (2016) 1863:1767–71. doi: 10.1016/j.bbamcr.2016.03.005
81. Li Z, Chao T-C, Chang K-Y, Lin N, Patil VS, Shimizu C, et al. The long noncoding RNA THRIL regulates TNFalpha expression through its interaction with hnRNPL. Proc Natl Acad Sci U S A. (2013) 111:1002–7. doi: 10.1073/pnas.1313768111
82. Ghavami S, Zamani M, Ahmadi M, Erfani M, Dastghaib S, Darbandi M, et al. Epigenetic regulation of autophagy in gastrointestinal cancers. Biochim Biophys Acta Mol Cell Res. (2022) 1868:166512. doi: 10.1016/j.bbadis.2022.166512
83. Shi Y, Zhang H, Huang S, Yin L, Wang F, Luo P, et al. Epigenetic regulation in cardiovascular disease: mechanisms and advances in clinical trials. Signal Transduct Target Ther. (2022) 7:200. doi: 10.1038/s41392-022-01055-2
84. Kärkkäinen O, Tuomainen T, Mutikainen M, Lehtonen M, Ruas JL, Hanhineva K, et al. Heart specific PGC-1α deletion identifies metabolome of cardiac restricted metabolic heart failure. Cardiovasc Res. (2019) 115:107–18. doi: 10.1093/cvr/cvy155
85. Butts B, Butler J, Dunbar SB, Corwin E, Gary RA. Effects of exercise on ASC methylation and IL-1 cytokines in heart failure. Med Sci Sports Exerc. (2018) 50:1757. doi: 10.1249/MSS.0000000000001641
86. Butts B, Gary RA, Dunbar SB, Butler J. Methylation of apoptosis-associated speck-like protein with a caspase recruitment domain and outcomes in heart failure. J Card Fail. (2016) 22:340–6. doi: 10.1016/j.cardfail.2015.12.004
87. Spólnicka M, Pośpiech E, Adamczyk JG, Freire-Aradas A, Pepłońska B, Zbieć-Piekarska R, et al. Modified aging of elite athletes revealed by analysis of epigenetic age markers. Aging. (2018) 10:241. doi: 10.18632/aging.101385
88. Streese L, Suades R, Cosentino F, Hanssen H. Exercise-induced improvement of microvascular phenotype and reprogramming of p66Shc DNA methylation. Eur Heart J. (2019) 40:3948–9. doi: 10.1093/eurheartj/ehz830
89. Lehmann LH, Jebessa ZH, Kreusser MM, Horsch A, He T, Kronlage M, et al. A proteolytic fragment of histone deacetylase 4 protects the heart from failure by regulating the hexosamine biosynthetic pathway. Nat Med. (2018) 24:62–72. doi: 10.1038/nm.4452
90. Cox EJ, Marsh SA. Exercise and diabetes have opposite effects on the assembly and O-GlcNAc modification of the mSin3A/HDAC1/2 complex in the heart. Cardiovasc Diabetol. (2013) 12:1–15. doi: 10.1186/1475-2840-12-1
91. Papaioannou F, Karatzanos E, Chatziandreou I, Philippou A, Nanas S, Dimopoulos S. Epigenetic effects following acute and chronic exercise in cardiovascular disease: a systematic review. Int J Cardiol. (2021) 341:88–95. doi: 10.1016/j.ijcard.2021.07.055
92. Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. (2012) 15:405–11. doi: 10.1016/j.cmet.2012.01.001
93. Kasch J, Kanzleiter I, Saussenthaler S, Schürmann A, Keijer J, van Schothorst E, et al. Insulin sensitivity linked skeletal muscle Nr4a1 DNA methylation is programmed by the maternal diet and modulated by voluntary exercise in mice. J Nutr Biochem. (2018) 57:86–92. doi: 10.1016/j.jnutbio.2018.03.015
94. Chen X-W, Leto D, Chiang S-H, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. (2007) 13:391–404. doi: 10.1016/j.devcel.2007.07.007
95. Delcuve GP, Khan DH, Davie JR. Roles of histone deacetylases in epigenetic regulation: emerging paradigms from studies with inhibitors. Clin Epigenetics. (2012) 4:5. doi: 10.1186/1868-7083-4-5
96. Robinson EL. Chapter 5—Histone modifications in cardiovascular disease initiation and progression. In: Devaux Y, Robinson EL, editors. Epigenetics in Cardiovascular Disease. Amsterdam: Academic Press (2021). p. 77–112.
97. Światowy WJ, Drzewiecka H, Kliber M, Sąsiadek M, Karpiński P, Pławski A, et al. Physical activity and DNA methylation in humans. Int J Mol Sci. (2021) 22:12989. doi: 10.3390/ijms222312989
98. Seaborne RA, Strauss J, Cocks M, Shepherd S, O’Brien TD, van Someren KA, et al. Human skeletal muscle possesses an epigenetic memory of hypertrophy. Sci Rep. (2018) 8:1898. doi: 10.1038/s41598-018-20287-3
99. Komar D, Juszczynski P. Rebelled epigenome: histone H3S10 phosphorylation and H3S10 kinases in cancer biology and therapy. Clin Epigenetics. (2020) 12:147. doi: 10.1186/s13148-020-00941-2
100. Noone J, Mucinski JM, DeLany JP, Sparks LM, Goodpaster BH. Understanding the variation in exercise responses to guide personalized physical activity prescriptions. Cell Metab. (2024) 36:702–24. doi: 10.1016/j.cmet.2023.12.025
101. Sapp RM, Shill DD, Roth SM, Hagberg JM. Circulating microRNAs in acute and chronic exercise: more than mere biomarkers. J Appl Physiol. (2017) 122:702–17. doi: 10.1152/japplphysiol.00982.2016
102. Paoli A, Moro T, Marcolin G, Neri M, Bianco A, Palma A, et al. High-Intensity interval resistance training (HIRT) influences resting energy expenditure and respiratory ratio in non-dieting individuals. J Transl Med. (2012) 10:237. doi: 10.1186/1479-5876-10-237
103. Improta Caria AC, Nonaka CKV, Pereira CS, Soares MBP, Macambira SG, Souza BSF. Exercise training-induced changes in MicroRNAs: beneficial regulatory effects in hypertension, type 2 diabetes, and obesity. Int J Mol Sci. (2018) 19:3608. doi: 10.3390/ijms19113608
104. Nielsen S, Åkerström T, Rinnov A, Yfanti C, Scheele C, Pedersen BK, et al. The miRNA plasma signature in response to acute aerobic exercise and endurance training. PLoS One. (2014) 9:e87308. doi: 10.1371/journal.pone.0087308
105. Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. (2015) 21:584–95. doi: 10.1016/j.cmet.2015.02.014
106. Sieland J, Niederer D, Engeroff T, Vogt L, Troidl C, Schmitz-Rixen T, et al. Effects of single bouts of different endurance exercises with different intensities on microRNA biomarkers with and without blood flow restriction: a three-arm, randomized crossover trial. Eur J Appl Physiol. (2021) 121:3243–55. doi: 10.1007/s00421-021-04786-2
107. Witvrouwen I, Gevaert AB, Possemiers N, Ectors B, Stoop T, Goovaerts I, et al. Plasma-Derived microRNAs are influenced by acute and chronic exercise in patients with heart failure with reduced ejection fraction. Front Physiol. (2021) 12:736494. doi: 10.3389/fphys.2021.736494
108. Ballantyne MD, McDonald RA, Baker AH. lncRNA/MicroRNA interactions in the vasculature. Clin Pharmacol Ther. (2016) 99:494–501. doi: 10.1002/cpt.355
109. Uhlemann M, Möbius-Winkler S, Fikenzer S, Adam J, Redlich M, Möhlenkamp S, et al. Circulating microRNA-126 increases after different forms of endurance exercise in healthy adults. Eur J Prev Cardiol. (2014) 21:484–91. doi: 10.1177/2047487312467902
110. Ke S, Cao X, Lu X, Xu J, Zhang C-Y, Xu L, et al. Atheroprotective roles of exercise-regulated microRNAs. Atherosclerosis. (2025) 405:119229. doi: 10.1016/j.atherosclerosis.2025.119229
111. Cloonan N. Re-thinking miRNA-mRNA interactions: intertwining issues confound target discovery. Bioessays. (2015) 37:379–88. doi: 10.1002/bies.201400191
112. Diener C, Keller A, Meese E. The miRNA–target interactions: an underestimated intricacy. Nucleic Acids Res. (2024) 52:1544–57. doi: 10.1093/nar/gkad1142
113. Batkai S, Genschel C, Viereck J, Rump S, Bär C, Borchert T, et al. CDR132l Improves systolic and diastolic function in a large animal model of chronic heart failure. Eur Heart J. (2021) 42:192–201. doi: 10.1093/eurheartj/ehaa791
114. Täubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J. (2021) 42:178–88. doi: 10.1093/eurheartj/ehaa898
115. Anastasiadou E, Seto AG, Beatty X, Hermreck M, Gilles M-E, Stroopinsky D, et al. Cobomarsen, an oligonucleotide inhibitor of miR-155, slows DLBCL tumor cell growth in vitro and in vivo. Clin Cancer Res. (2021) 27:1139–49. doi: 10.1158/1078-0432.CCR-20-3139
116. Laggerbauer B, Engelhardt S. MicroRNAs as therapeutic targets in cardiovascular disease. J Clin Invest. (2022) 132:e159179. doi: 10.1172/JCI159179
117. Abplanalp WT, Fischer A, John D, Zeiher AM, Gosgnach W, Darville H, et al. Efficiency and target derepression of anti-miR-92a: results of a first in human study. Nucleic Acid Ther. (2020) 30:335–45. doi: 10.1089/nat.2020.0871
118. Dai W, Qiao X, Fang Y, Guo R, Bai P, Liu S, et al. Epigenetics-targeted drugs: current paradigms and future challenges. Signal Transduct Target Ther. (2024) 9:332. doi: 10.1038/s41392-024-02039-0
119. Schulte C, Zeller T. microRNA-based diagnostics and therapy in cardiovascular disease-summing up the facts. Cardiovasc Diagn Ther. (2015) 5:17–36. doi: 10.3978/j.issn.2223-3652.2014.12.03
120. Prabhakaran R, Thamarai R, Sivasamy S, Dhandayuthapani S, Batra J, Kamaraj C, et al. Epigenetic frontiers: miRNAs, long non-coding RNAs and nanomaterials are pioneering to cancer therapy. Epigenetics Chromatin. (2024) 17:31. doi: 10.1186/s13072-024-00554-6
121. Voisin S, Eynon N, Yan X, Bishop DJ. Exercise training and DNA methylation in humans. Acta Physiol. (2015) 213:39–59. doi: 10.1111/apha.12414
122. Molla G, Bitew M. Revolutionizing personalized medicine: synergy with multi-omics data generation, main hurdles, and future perspectives. Biomedicines. (2024) 12:2750. doi: 10.3390/biomedicines12122750
123. Satam H, Joshi K, Mangrolia U, Waghoo S, Zaidi G, Rawool S, et al. Next-Generation sequencing technology: current trends and advancements. Biology (Basel). (2023) 12:997. doi: 10.3390/biology12070997
124. Abdi G, Tarighat M, Jain M, Tendulkar R, Tendulkar M, Barwant M. Revolutionizing genomics: exploring the potential of next-generation sequencing. In: Harish Kumar TS, editor. Revolutionizing Genomics: Exploring the Potential of Next-Generation Sequencing. Hershey, PA: IGI Global (2024). p. 1–33.
125. Wu C, Chen X, Yang L, Sun H, Bao S, Li H, et al. Exercise mediates noncoding RNAs in cardiovascular diseases: pathophysiological roles and clinical application. Expert Rev Mol Med. (2025) 27:e2. doi: 10.1017/erm.2024.25
126. Akbari J, Shirvani H, Shamsoddini A, Bazgir B, Samadi M. Investigation of expression of myocardial miR-126, miR-29a and miR-222 as a potential marker in STZ- induced diabetic rats following interval and continuous exercise training. J Diabetes Metab Disord. (2022) 21:189–95. doi: 10.1007/s40200-021-00957-2
127. Brisebois M, Gordon R, Zumbro E, Sokoloski M, Duplanty A, Juma S, et al. Acute effects of serial and integrated concurrent exercise on circulating MicroRNAs -126 and -222 in sedentary adults. Int J Exerc Sci. (2024) 17:1444–60. doi: 10.70252/XFJK8005
128. Lochmann TL, Thomas RR, Bennett JP Jr, Taylor SM. Epigenetic modifications of the PGC-1α promoter during exercise induced expression in mice. PLoS One. (2015) 10:e0129647. doi: 10.1371/journal.pone.0129647
129. Blaustein JR, Quisel MJ, Hamburg NM, Wittkopp S. Environmental impacts on cardiovascular health and biology: an overview. Circ Res. (2024) 134:1048–60. doi: 10.1161/CIRCRESAHA.123.323613
130. Bi F, Gao C, Guo H. Epigenetic regulation of cardiovascular diseases induced by behavioral and environmental risk factors: mechanistic, diagnostic, and therapeutic insights. FASEB Bioadv. (2024) 6:477–502. doi: 10.1096/fba.2024-00080
131. Chennaoui M, Gomez-Merino D, Drogou C, Geoffroy H, Dispersyn G, Langrume C, et al. Effects of exercise on brain and peripheral inflammatory biomarkers induced by total sleep deprivation in rats. J Inflamm. (2015) 12:56. doi: 10.1186/s12950-015-0102-3
132. Zheng TS, Gao XR, Gu C, Ru YN, Xu RP, Yang YH, et al. Long-Term exercise mitigates energy expenditure and inflammatory responses induced by sleep deprivation in mice. Biomolecules. (2025) 15:862. doi: 10.3390/biom15060862
133. Ahangarpour A, Belali R. The role of MicroRNAs in regulation of sleep/wakefulness and their expression changes in the brain following sleep deprivation. Jundishapur J Physiol. (2023) 2(2):e148771.
134. Pant T, Uche N, Juric M, Bosnjak ZJ. Clinical relevance of lncRNA and mitochondrial targeted antioxidants as therapeutic options in regulating oxidative stress and mitochondrial function in vascular complications of diabetes. Antioxidants. (2023) 12:898. doi: 10.3390/antiox12040898
135. Gil-Cabrerizo P, Simon-Yarza T, Garbayo E, Blanco-Prieto MJ. Navigating the landscape of RNA delivery systems in cardiovascular disease therapeutics. Adv Drug Delivery Rev. (2024) 208:115302. doi: 10.1016/j.addr.2024.115302
136. Adjeroh DA, Zhou X, Paschoal AR, Dimitrova N, Derevyanchuk EG, Shkurat TP, et al. Challenges in LncRNA biology: views and opinions. Noncoding RNA. (2024) 10:43. doi: 10.3390/ncrna10040043
137. Kong X, Li F, Wang Y. Emerging roles of long non-coding RNAs in cardiovascular diseases. J Cell Mol Med. (2025) 29:e70453. doi: 10.1111/jcmm.70453
138. Kim N, Chung WY, Cho JY. The role and medical prospects of long non-coding RNAs in cardiovascular disease. Heart Fail Rev. (2023) 28:1437–53. doi: 10.1007/s10741-023-10342-1
Keywords: microRNAs, long non-coding RNAs, epigenetic modifications, cardioprotection, exercise
Citation: Yuan S, Ye Q and Qin R (2025) Cardioepigenetics in action: aerobic exercise-induced modulation of miRNAs, lncRNAs, and chromatin remodeling in cardiovascular disease. Front. Cardiovasc. Med. 12:1579352. doi: 10.3389/fcvm.2025.1579352
Received: 19 February 2025; Accepted: 11 July 2025;
Published: 1 August 2025.
Edited by:
Alex Cleber Improta-Caria, University of São Paulo, BrazilReviewed by:
Guilherme Da Silva Rodrigues, Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo, BrazilLívia Agostini, Universidade Federal de Ouro Preto, Brazil
Copyright: © 2025 Yuan, Ye and Qin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Qin, cWlucmFuMDIxMUAxNjMuY29t
 Shoudu Yuan1
Shoudu Yuan1 Ran Qin
Ran Qin