- 1Department of Cardiology, Beijing Jishuitan Hospital, Capital Medical University, Beijing, China
- 2Peking University Health Science Center, Beijing, China
- 3Department of Pulmonary and Critical Care Medicine, Beijing Jishuitan Hospital, Capital Medical University, Beijing, China
Background: Venous thromboembolism (VTE) is influenced by both genetic and acquired risk factors, with protein S (PS) deficiency recognized as a well-established inherited thrombophilia. Introduction: We report the case of a 32-year-old male patient presenting with mesenteric venous thrombosis and pulmonary embolism caused by a missense mutation in PROS1 during the COVID-19 pandemic.
Methods: The patient presented with pleuritic chest pain and low-grade fever 15 days after a confirmed COVID-19 infection. Despite initial treatment with glucocorticoids and a macrolide antibiotic, his symptoms worsened and his D-dimer level increased. CT pulmonary angiography confirmed an acute pulmonary embolism.
Results: Clinical history revealed a prior episode of mesenteric vein thrombosis and multiple acquired risk factors, including obesity, sedentariness, COVID-19 infection, glucocorticoid treatment, inflammatory response (elevated CRP and serum ferritin levels), and metabolic abnormalities (non-alcoholic fatty liver disease, hyperuricemia, and hyperlipidemia). Laboratory testing showed decreased PS activity, and genetic sequencing identified a heterozygous missense mutation in PROS1, c.683G>A (p.Cys228Tyr). The patient was treated with low-molecular-weight heparin (LMWH) followed by rivaroxaban. Discussion: No recurrence of VTE of bleeding events was observed during a one-year follow-up, suggesting effective management of thrombosis in the context of both inherited and acquired prothrombotic conditions.
Introduction
Thrombophilia is an inherited or acquired medical condition characterized by an increased tendency towards abnormal blood clotting or thrombosis (1). Inherited thrombophilia is caused by various genetic mutations, including deficiencies in the natural anticoagulant proteins antithrombin, protein C, and protein S (PS), as well as the factor V Leiden and prothrombin gene mutation G20210Ac. Common acquired factors—such as advanced age, obesity, pregnancy, acute infection, inflammation, surgery, trauma, sedentariness, immobilization, and hormone-based contraceptives—may combine with inherited factors to increase the overall risk of thrombosis (2). As a vitamin K-dependent single-stranded glycoprotein, PS plays a key role in the anticoagulation pathway, primarily by serving as a cofactor for activated protein C to promote inactivation of factor Va and factor VIIIa (3). PS deficiency is an autosomal dominant trait, with an estimated prevalence of 0.03%–0.13% in the general Caucasian population (4). Patients with PS deficiency are at increased risk for venous thromboembolism (VTE), including deep vein thrombosis or pulmonary embolism (5). We report the case of a 32-year-old male patient who presented with mesenteric venous thrombosis and pulmonary embolism caused by a heterozygous missense mutation in PROS1, c.683G>A (p.Cys228Tyr) during the COVID-19 pandemic.
Case presentation
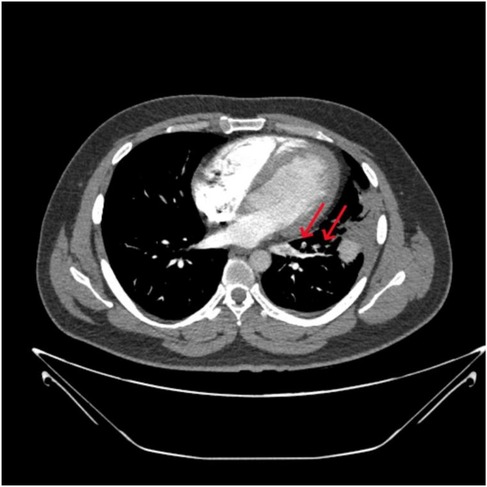
A 32-year-old male patient was admitted to our hospital with a 15-day history of pleuritic chest pain and low-grade fever, both of which worsened with exertion and deep breathing. Initial chest CT imaging raised suspicion of an infection in the left upper lobe, and empirical treatment was initiated with quinolone antibiotics and methylprednisolone. However, over the following 10 days, the patient's chest pain and wheezing progressively worsened. On presentation, the patient had stable vital signs, no signs of hemodynamic instability, an oxygen saturation of 97% on room air, and a simplified Pulmonary Embolism Severity Index (sPESI) score of 0. CT pulmonary angiography (CTPA) revealed a pulmonary embolism in the lingular segment of the left upper lobe and the anteromedial basal segment of the left lower lobe (Figure 1). D-dimer was elevated at 5.09 mg/L FEU, although no thrombosis was detected in the lower extremity veins. Coagulation tests on admission revealed thrombin time of 21.8 s↑, fibrin degradation products of 735 mg/dl↑, and D-dimer of 5.09 mg/L FEU↑. Thrombophilia screening showed decreased PS activity (53.3%↓), while antithrombin III and protein C activities were within normal limits. Tests for lupus anticoagulant, anti-cardiolipin antibody, anti-phosphatidylserine/prothrombin antibodies, anti-nuclear antibodies, and anti-neutrophil cytoplasmic antibodies were all negative. Liver and lipid panels showed elevated alanine aminotransferase (82 IU/L↑), aspartate aminotransferase (68 IU/L↑), and gamma-glutamyl transpeptidase (97 IU/L↑). Total cholesterol and triglyceride levels were 5.69 mmol/L↑ and 2.21 mmol/L↑, respectively. High-sensitivity C-reactive protein was elevated at 17.65 mg/L. Fungal and bacterial infection markers, including 1,3-β-D-glucan test (G test), galactomannan test (GM test), and procalcitonin test (PCT) were all negative, ruling out invasive infections.
The patient's medical history included a three-year history of nonalcoholic fatty liver disease, a prior episode of mesenteric vein thrombosis one year earlier, and a confirmed COVID-19 infection within the preceding month. There was no known family history of VTE. The patient was overweight (BMI 29.4 kg/m²) and regularly smoked and consumed alcohol.
The patient was diagnosed with acute pulmonary embolism (low-risk, according to the ESC guidelines) and started on low-molecular-weight heparin (LMWH) 8,000 IU every 12 h. Given the concurrent diagnosis of community-acquired pneumonia, cefoperazone-sulbactam was administered alongside hepatoprotective agents. Both the patient's symptoms and D-dimer levels significantly improved (Figure 2). LMWH was subsequently transitioned to rivaroxaban 15 mg twice daily for 3 weeks, followed by 20 mg once daily. Following the standard anticoagulation therapy, rivaroxaban was continued at a reduced dose of 10 mg once daily for extended preventive anticoagulation (6). No venous thromboembolism or bleeding events were reported during the 1-year follow-up period.
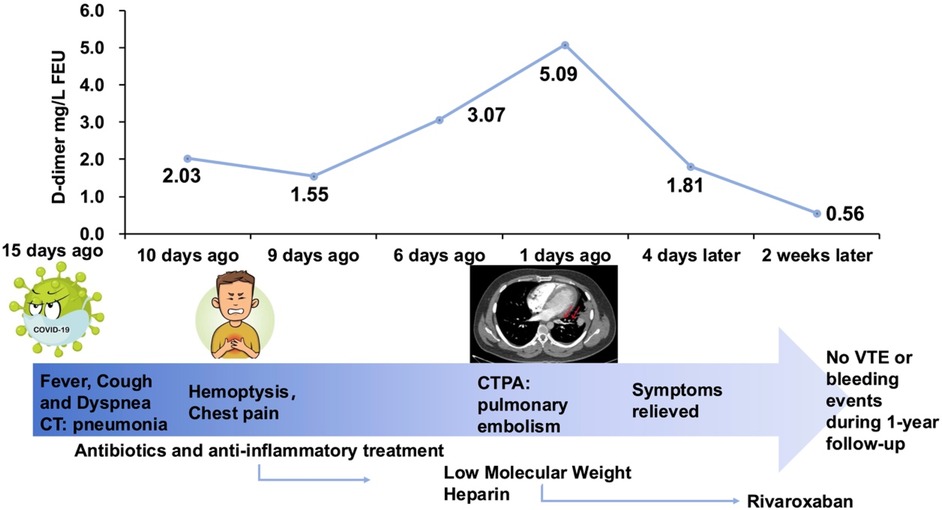
Figure 2. Summary timeline of symptoms, interventions, key diagnostics and outcomes. FEU, fibrinogen equivalent unit; CT, computed tomography; CTPA, computer tomography pulmonary angiography; VTE, venous thromboembolism.
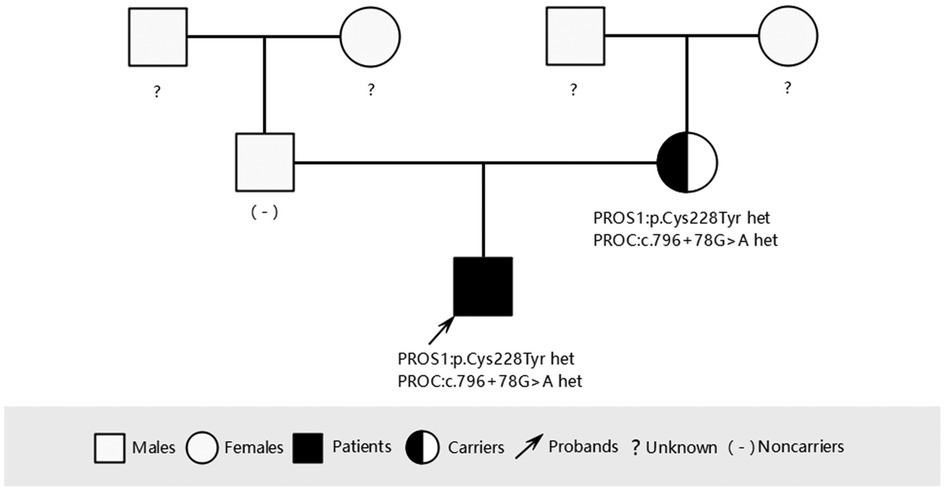
To investigate the cause of the thromboembolism, thrombophilia screening was performed. PS activity was markedly reduced (53.3%; male reference range: 63.5%–149%). Genetic analysis was conducted to identify potential genetic causes of PS deficiency. A combination of single-gene Sanger sequencing and next-generation sequencing (NGS), including whole-exome sequencing (WES), was employed, achieving 99.93% genome coverage with an average coverage depth of 112×. Genes encoding anticoagulant proteins, coagulation factors, and fibrinolytic regulators were analyzed. A rare heterozygous mutation in the PROS1 gene (c.683G>A; p.Cys228Tyr) was identified (Figure 3a). This variant was present in both the patient and his mother but absent in his father (Figures 3b, 4).
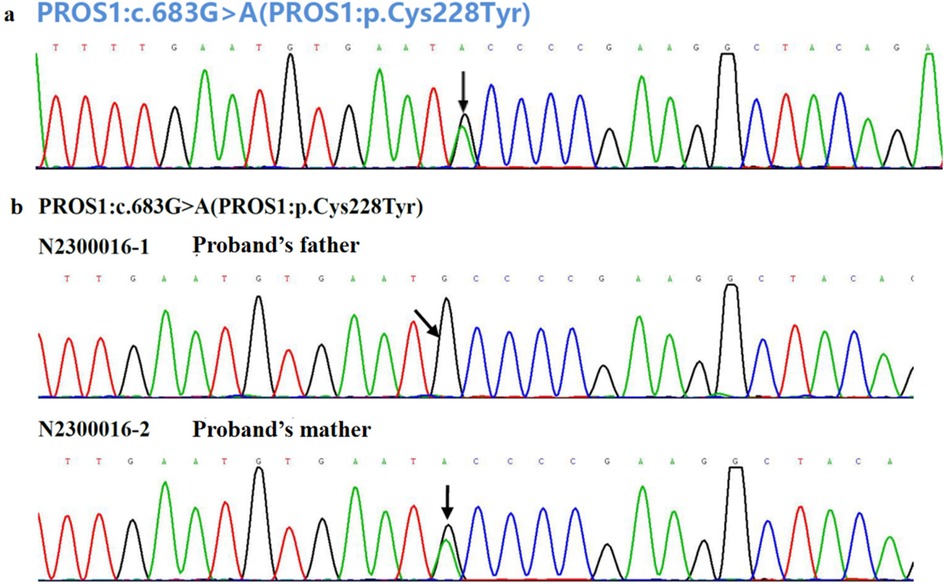
Figure 3. (a) Genetic analysis for the causal mutation of the patient with VTE and (b) his parents. The black arrow indicates the base pair substitution site.
Patient perspective
The patient's symptoms improved following diagnosis, and an appropriate therapeutic plan was established. During hospitalization, the patient expressed comfort and reassurance after engaging in shared decision-making with the medical team. He also acknowledged the valuable support provided by both the healthcare provider and family members, which contributed to his rehabilitation and clinical outcomes during the 1-year follow-up period.
Discussion and conclusion
In this study, we report the case of a young, overweight male with multiple acquired thrombotic risk factors, including pneumonia, COVID-19 infection, glucocorticoid use, inflammatory activation, and metabolic disorders, who presented with mesenteric venous thrombosis and recurrent VTE. Considering the reduced PS activity and normal findings in other thrombophilia-related tests (including lupus anticoagulants, antiphospholipid antibodies, and tumor biomarkers), genetic screening was performed for inherited thrombophilia. A heterozygous missense mutation in PROS1 (c.683G>A, p.Cys228Tyr) was identified, which has not been reported previously. The patient was treated with enoxaparin, followed by rivaroxaban, and experienced no adverse events during a 1-year follow-up.
PS is an anticoagulant and its deficiency increases the risk of VTE. PS deficiency occurs in approximately 2% of patients with unselected VTE and in 1%–13% of patients overall (7). In a Han Chinese population-based case-control study, 8.5% (51/603) of patients with VTE exhibited PS activity deficiency (8). Acquired PS deficiency may result from warfarin use, pregnancy, hepatic dysfunction, nephrotic syndrome, chronic infections (e.g., HIV), or disseminated intravascular coagulation (9). Hereditary PS deficiency—an autosomal dominant disorder—results from mutations in the PROS1 gene (located at 3q11.2), which comprises 15 exons and spans over 80 kb of genomic DNA. More than 200 mutations in PROS1 gene have been reported in patients with PS deficiency, approximately 50% of which are point mutations (10). Missense mutations primarily cause quantitative PS deficiency through defective synthesis, stability, or secretion of mutated proteins. In this case, we identified a missense mutation in PROS1 (c.683G>A, p.Cys228Tyr), which likely represents a causal variant for the loss of PS function and contributes to an increased risk of VTEs, such as pulmonary embolism and mesenteric venous thrombosis. Most patients with hereditary thrombophilia experience their first thrombotic episode before the age of 45 (7), consistent with this case. Additionally, the patient's pulmonary embolism likely resulted from recurrent venous thrombosis, a recognized feature in patients with antithrombin, protein C, or PS deficiency (11). The patient harbored a heterozygous missense variant in the PROS1 gene: c.683G>A (p.Cys228Tyr). This variant was not identified in major population databases, including the 1,000 Genomes Project, ESP6500, and ExAC, and was also absent in both thrombotic cases and controls within the BGI local cohort. In-silico pathogenicity prediction tools uniformly indicated damaging effects: SIFT “D,” PolyPhen-2 “D,” MutationTaster_pred “D,” VEST4 score 0.960, REVEL score 0.959, with aggregate predictions of “5D/1H”. Regarding the biochemical impact and conservative analysis, the mutation results in the substitution of cysteine with tyrosine—both polar, uncharged amino acids—at a site highly conserved across vertebrate orthologs. This supports the likelihood of deleterious structural or functional consequences. The variant was not identified in ClinVar and Human Gene Mutation Database (HGMD) databases. However, neighboring residues are also implicated in PS deficiency; c.701A>G (p.Tyr234Cys) is a known pathogenic variant associated with autosomal recessive PS deficiency, while both c.682T>A (p.Cys228Ser) and c.684C>G (p.Cys228Trp) are classified as pathogenic or likely pathogenic in HGMD. Considering the variant's extreme rarity, consistent in-silico deleterious predictions of pathogenicity, strong evolutionary conservation, and proximity to established pathogenic variants, c.683G>A (p.Cys228Tyr) is classified as a likely pathogenic (Class B) mutation, according to the current guidelines. Nonetheless, the absence of functional assays and segregation data precludes a definitive pathogenic classification.
Interestingly, although the same PROS1 mutation was identified in the patient's mother, she had no documented history of venous thrombosis, suggesting that additional environmental or physiological triggers were necessary for thrombus formation. In addition to well-known risk factors such as inflammation, surgery, and trauma, COVID-19 might have acted as a triggering event for this patient. Notably, COVID-19 enhances thrombin production and increases the von Willebrand factor and factor V levels, resulting in a hypercoagulable state (12). Additional modifiable lifestyle-related risk factors for VTE include obesity, sedentariness, and immobilization. Therefore, both the patient and his mother were educated to avoid potential thrombotic risk factors.
Anticoagulation is considered the primary therapy for acute pulmonary embolism. Anticoagulants include parenteral anticoagulation, vitamin K antagonists (VKA), and direct oral anticoagulants (DOACs) (13). While conventional treatment of VTE is effective and relatively safe, it poses several limitations, including the requirement for parenteral administration of heparin and frequent monitoring of VKA (14). DOACs, which have shown comparable efficacy and improved safety profiles relative to VKA for pulmonary embolism (15) and VTE (16), offer several advantages over conventional therapy. These include fixed dosing with predictable pharmacokinetics, no requirement for laboratory monitoring, and minimal food and drug interactions, all of which may improve medication adherence (17, 18). A prospective cohort study demonstrated DOACs' association with lower 2-year VTE recurrence post-discontinuation compared to heparin/VKA in patients with inherited thrombophilia and VTE. Additionally, this study showed significantly higher overall bleeding rates with DOACs than with heparin/VKA (6). In this case, the patient received continued rivaroxaban treatment after the initial therapy, as the patient's clinical profile suggested a low bleeding tendency and the benefits from extended anticoagulation outweighed the risk of bleeding.
In short, inherited PS deficiency due to a heterozygous missense mutation in PROS1 (p.Cys228Tyr) represented the genetic basis in this patient, and long-term anticoagulant therapy was beneficial.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
JH: Writing – original draft, Writing – review & editing. YZ: Data curation, Writing – review & editing. HY: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. WL: Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Beijing Jishuitan Hospital Nova Program (XKXX202206), Beijing Jishuitan Hospital Youth Fund (QN202311), Young Elite Scientists Sponsorship Program by BAST, Beijing Natural Science Foundation (7232078) and Capital's Funds for Health Improvement and Research (2024-2-2073).
Acknowledgments
We thank patients for providing clinical examination data. We appreciate Nana Zhao of BestNovo (Beijing) Medical Laboratory Co., Ltd for the technical support and analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PS, protein S; LMWH, low-molecular-weight heparin; VTE, venous thromboembolism; VKA, vitamin K antagonists
References
1. Mannucci PM, Franchini M. The real value of thrombophilia markers in identifying patients at high risk of venous thromboembolism. Expert Rev Hematol. (2014) 7(6):757–65. doi: 10.1586/17474086.2014.960385
2. Koupenova M, Kehrel BE, Corkrey HA, Freedman JE. Thrombosis and platelets: an update. Eur Heart J. (2017) 38(11):785–91. doi: 10.1093/eurheartj/ehw550
3. Esmon CT. The regulation of natural anticoagulant pathways. Science. (1987) 235(4794):1348–52. doi: 10.1126/science.3029867
4. Dykes AC, Walker ID, McMahon AD, Islam SI, Tait RC. A study of protein S antigen levels in 3788 healthy volunteers: influence of age, sex and hormone use, and estimate for prevalence of deficiency state. Br J Haematol. (2001) 113(3):636–41. doi: 10.1046/j.1365-2141.2001.02813.x
5. Engesser L, Broekmans AW, Briët E, Brommer EJ, Bertina RM. Hereditary protein S deficiency: clinical manifestations. Ann Intern Med. (1987) 106(5):677–82. doi: 10.7326/0003-4819-106-5-677
6. Campello E, Spiezia L, Simion C, Tormene D, Camporese G, Dalla Valle F, et al. Direct oral anticoagulants in patients with inherited thrombophilia and venous thromboembolism: a prospective cohort study. J Am Heart Assoc. (2020) 9(23):e018917. doi: 10.1161/JAHA.120.018917
7. Seligsohn U, Lubetsky A. Genetic susceptibility to venous thrombosis. N Engl J Med. (2001) 344(16):1222–31. doi: 10.1056/NEJM200104193441607
8. Wu Y, Liu J, Zeng W, Hu B, Hu Y, Tang LV. Protein S deficiency and the risk of venous thromboembolism in the Han Chinese population. Front Cardiovasc Med. (2021) 8:796755. doi: 10.3389/fcvm.2021.796755
9. ten Kate MK, van der Meer J. Protein S deficiency: a clinical perspective. Haemophilia. (2008) 14(6):1222–8. doi: 10.1111/j.1365-2516.2008.01775.x
10. Pintao MC, Garcia AA, Borgel D, Alhenc-Gelas M, Spek CA, de Visser MC, et al. Gross deletions/duplications in PROS1 are relatively common in point mutation-negative hereditary protein S deficiency. Hum Genet. (2009) 126(3):449–56. doi: 10.1007/s00439-009-0687-9
11. van den Belt AG, Sanson BJ, Simioni P, Prandoni P, Büller HR, Girolami A, et al. Recurrence of venous thromboembolism in patients with familial thrombophilia. Arch Intern Med. (1997) 157(19):2227–32. doi: 10.1001/archinte.1997.00440400077009
12. Poor HD. Pulmonary thrombosis and thromboembolism in COVID-19. Chest. (2021) 160(4):1471–80. doi: 10.1016/j.chest.2021.06.016
13. Yuan M, Wen HC, Wang Y, Du J. Precision monitoring of antithrombotic therapy in cardiovascular disease. Cardiovasc Innov Appl. (2024) 9(1):1–19. doi: 10.15212/CVIA.2024.0013
14. Buller HR, Lensing AW, Prins MH, Agnelli G, Cohen A, Gallus AS, et al. A dose-ranging study evaluating once-daily oral administration of the factor Xa inhibitor rivaroxaban in the treatment of patients with acute symptomatic deep vein thrombosis: the Einstein-DVT dose-ranging study. Blood. (2008) 112(6):2242–7. doi: 10.1182/blood-2008-05-160143
15. Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. (2012) 366(14):1287–97. doi: 10.1056/NEJMoa1113572
16. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. (2010) 363(26):2499–510. doi: 10.1056/NEJMoa1007903
17. Zhao Y, Ren H, Xu S. Comparison of warfarin, rivaroxaban, and dabigatran for effectiveness and safety in atrial fibrillation patients with different CHA2DS2-VASc scores: a retrospective cohort study. BMC Cardiovasc Disord. (2024) 24(1):361. doi: 10.1186/s12872-024-04020-9
Keywords: venous thromboembolism, PROS1, protein S deficiency, missense mutation, COVID-19, case-report
Citation: Huang J, Zhang Y, Yu H and Liu W (2025) PROS1 (Cys228Tyr) missense mutation associated with mesenteric and pulmonary venous thromboembolism during the COVID-19 pandemic: a case report. Front. Cardiovasc. Med. 12:1610580. doi: 10.3389/fcvm.2025.1610580
Received: 12 April 2025; Accepted: 4 July 2025;
Published: 23 July 2025.
Edited by:
Luca Spiezia, University of Padua, ItalyReviewed by:
Chiara Simion, University of Padua, ItalyDola Sundeep, Indian Institute of Information Technology Design and Manufacturing, India
Copyright: © 2025 Huang, Zhang, Yu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haixu Yu, eXVoYWl4dTY2MTlAMTYzLmNvbQ==; Wei Liu, bGl1d2VpNTI1QGhvdG1haWwuY29t
 Jingyi Huang
Jingyi Huang Yunjian Zhang3
Yunjian Zhang3 Haixu Yu
Haixu Yu Wei Liu
Wei Liu