Abstract
Introduction:
Hypertrophic cardiomyopathy (HCM) is a common genetic cardiac disease marked by abnormal ventricular hypertrophy. Recent studies have highlighted that left atrial (LA) remodelling—including dilation, fibrosis, and functional impairment—plays a key role in disease progression and prognosis, notably increasing the risk of atrial fibrillation (AF) and stroke.
Material and methods:
This review article systematically examines published clinical, imaging, and interventional studies. The analysis focuses on identifying the determinants of atrial myopathy, its relationship with diastolic dysfunction and left ventricular outflow tract obstruction (LVOTO), and the effects of therapeutic interventions such as septal reduction therapy and cardiac myosin inhibitors.
Results:
The findings reveal that LA remodelling in HCM is characterized by increased LA volume, reduced atrial strain, and prolonged conduction times—all of which are strongly linked to the onset and recurrence of AF. Moreover, interventions that reduce LVOTO (e.g., surgical myectomy) have been shown to induce LA reverse remodelling and improve diastolic parameters. Emerging therapies, like cardiac myosin inhibitors, also improve LV function but present complex effects on atrial performance, with some evidence suggesting a reduction in atrial strain that warrants further investigation.
Conclusion:
Atrial remodelling is a significant marker of disease severity in HCM and an important independent predictor of adverse outcomes, including AF and cardioembolic events. Early detection through comprehensive multimodal imaging and timely therapeutic intervention can potentially mitigate these risks, making atrial myopathy both a critical prognostic factor and a promising therapeutic target.
Graphical Abstract
Highlights
- •
Hypertrophic cardiomyopathy not only affects the left ventricle but also causes structural and functional changes in the left atrium, leading to atrial myopathy, a key marker of disease severity.
- •
Atrial myopathy significantly increases the risk of atrial fibrillation, which in turn raises the likelihood of stroke and worsens overall prognosis.
- •
Left atrium size and function are strong predictors of cardiovascular events, emphasizing the need for early intervention to reduce morbidity and mortality.
- •
ECG, echocardiography and cardiac magnetic resonance are crucial for early detection of atrial dysfunction.
- •
Surgical myectomy promotes reverse atrial remodelling by reducing left ventricular outflow tract obstruction and mitral regurgitation. Cardiac myosin inhibitors may offer similar benefits, but their effect on atrial remodelling is controversial due to potential negative inotropic effects on atrial function.
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiomyopathy, with a prevalence of approximately 1:500 in the general population (1). The disease exhibits autosomal dominant mendelian inheritance in about half of the cases, with variable penetrance; over 500 mutations have been described in 13 genes, primarily encoding for proteins of the cardiac sarcomere (2–4). HCM is characterized by abnormal hypertrophy of the left ventricular wall and is defined by the presence of unexplained left ventricular (LV) wall hypertrophy ≥15 mm, or ≥13 mm in the presence of family history or ECG abnormalities (4). Most HCM phenotypes present with asymmetric hypertrophy, primarily localized in the basal anterior septum, which can lead to systolic anterior motion (SAM) of the mitral valve leaflets, resulting in mitral regurgitation (MR) and dynamic LV outflow tract obstruction (LVOTO). Another hallmark feature of HCM is impaired diastolic function, characterized by reduced ventricular compliance and elevated LV filling pressures. These abnormalities lead to diminished exercise capacity and may ultimately progress to overt heart failure and increased risk of mortality. Indeed sudden cardiac death (SCD) is the most feared complication, particularly among individuals in the second and third decades of life, which is why several clinical predictors have been validated to identify high-risk patients who may benefit from primary prevention implantable cardioverter-defibrillator (ICD) therapy (5). Unfortunately, SCD may be the first manifestation of the disease in otherwise healthy and asymptomatic individuals, including athletes (6). While HCM is the most frequent cause of SCD in young individuals and can lead to functional disability due to heart failure (HF) and stroke, in most cases it does not cause significant symptoms or a major reduction in life expectancy. However, about 25% of cases may progress to “adverse remodelling” due to the progressive development of fibrosis, leading to thinning of the ventricular walls and a gradual reduction in the LV ejection fraction (EF). In a minority of patients, this can lead to relevant LV dysfunction with EF < 50% (7, 8). Although HCM has traditionally been considered a disease that primarily, if not exclusively, affects the LV, it is important to emphasize that it can also cause significant changes in the function and structure of the left atrium (LA), leading to a true “atrial myopathy”. This condition has been identified as a marker of disease severity and an important independent prognostic factor for cardiovascular events (9–11). Furthermore, it is the main contributor to the onset of clinically significant arrhythmias, such as atrial fibrillation (AF), which significantly increases the risk of stroke in HCM patients compared to the general population (12). Therefore, this review aims to examine the underlying mechanisms and consequences of atrial myopathy, exploring its prognostic value and proposing possible preventive strategies, with the goal of highlighting an aspect of HCM that is often overlooked.
Prevalence and determinants of left atrial remodelling
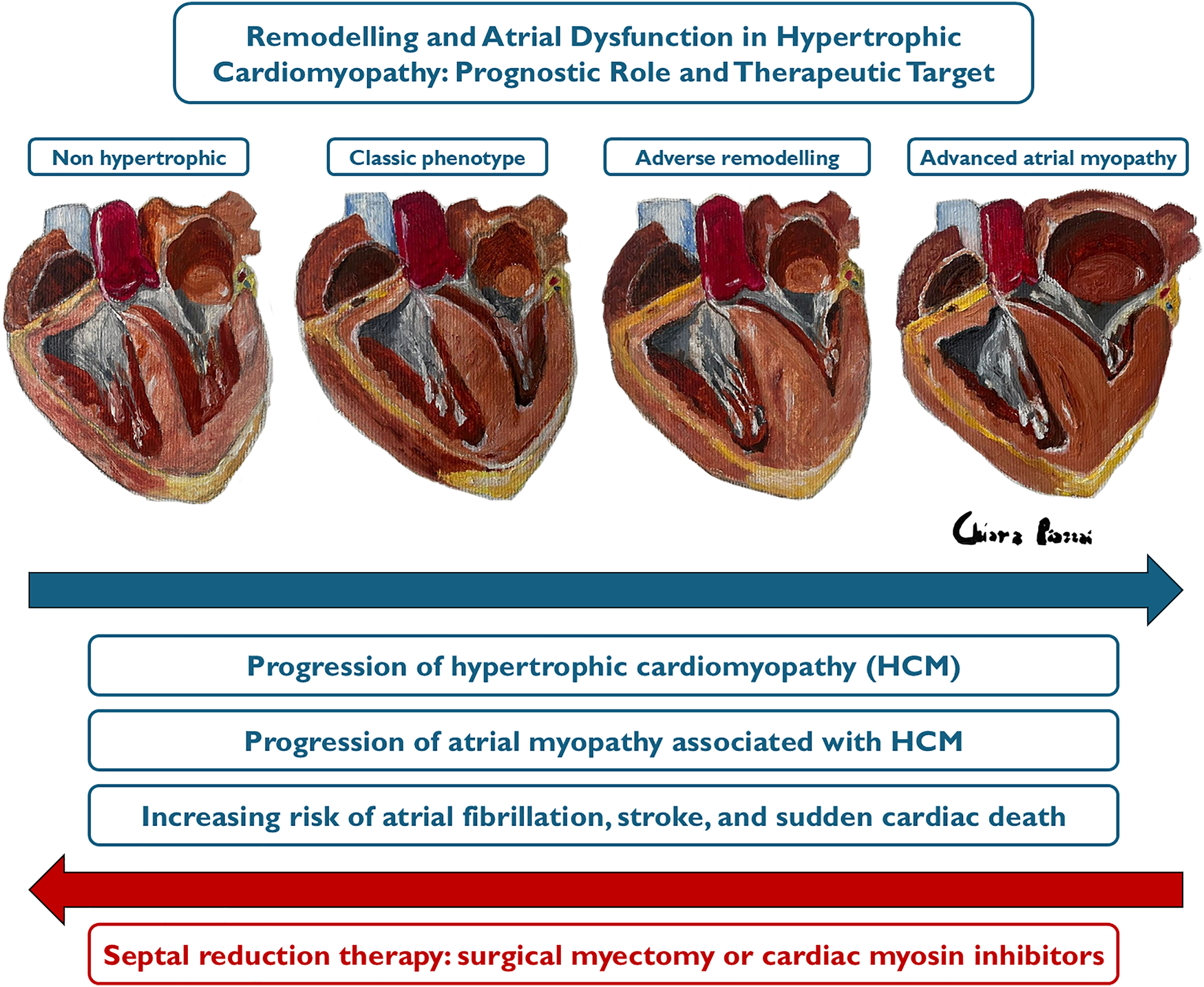
The size of the LA serves as a barometer for chronic left atrial pressure elevation, which results from the combination of diastolic dysfunction, MR, and atrial arrhythmias (13). In fact, prolonged exposure to MR, LVOTO, and LV remodelling, leading to high ventricular filling pressures and diastolic dysfunction, results in increased LA afterload, causing its adverse remodelling. This is characterized by atrial complex changes in contractility, electrophysiology and chamber architecture, which define the condition known as atrial myopathy (Figure 1), where ageing, oxidative stress and stretch, and inflammation lead to fibrosis, electrical remodelling and pro-thrombotic state, determining a close interplay among AF and stroke (Graphical Abstract) (14, 15).
Figure 1
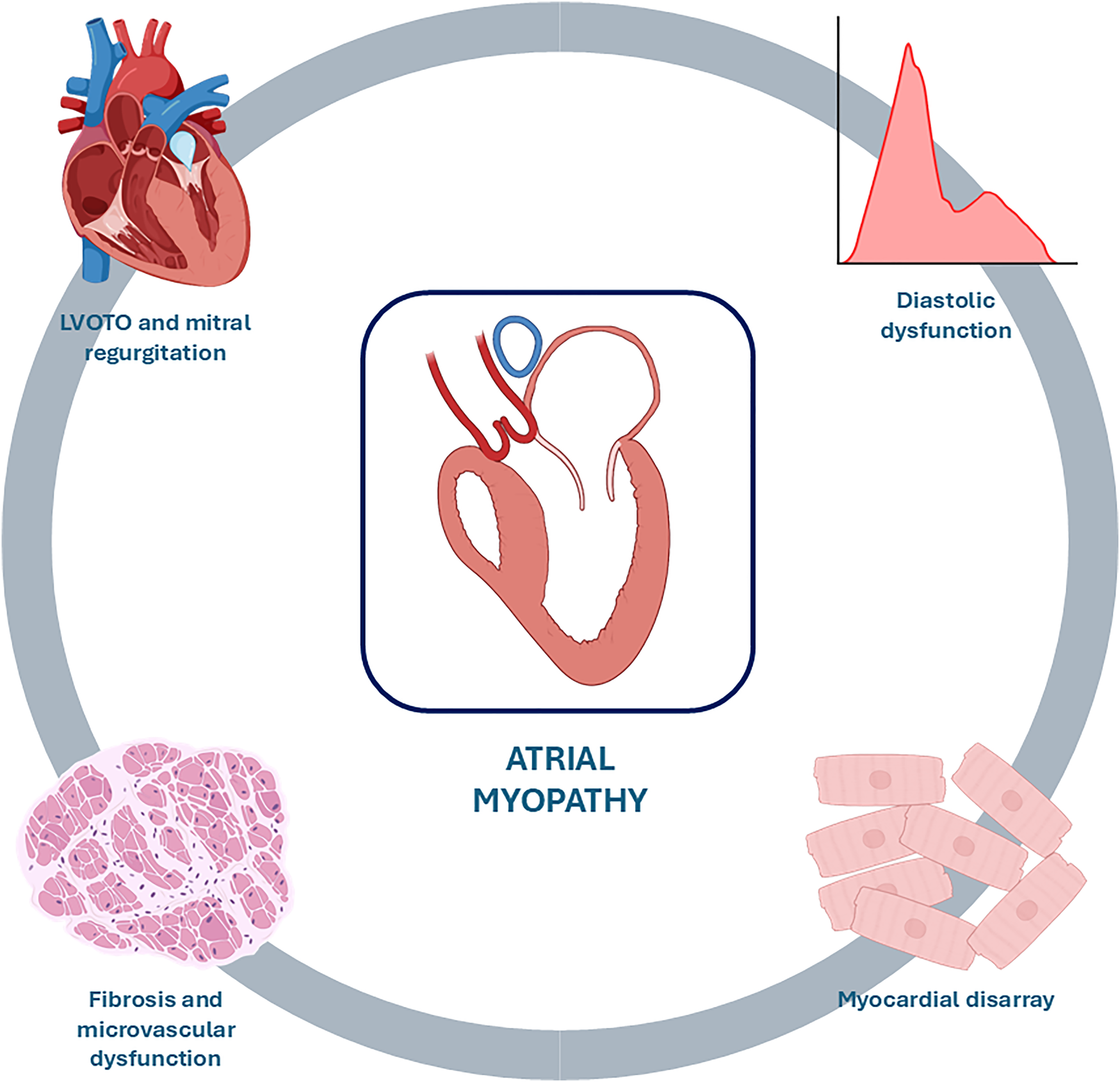
Causes of atrial myopathy. Prolonged exposure to mitral regurgitation, LVOTO, and myocardial disarray with resulting fibrosis, microvascular dysfunction, and diastolic dysfunction leads to adverse remodelling of the LA characterized by dilation and functional deterioration, a condition known as atrial myopathy. LVOTO, left ventricular outflow tract obstruction.
Mitral regurgitation and LVOTO
The primary phenotypic expression of obstructive HCM manifests through the characteristic elongation of the mitral valve leaflets, LV hypertrophy, and systolic hyper dynamism. These factors contribute to the phenomenon known as SAM of the mitral valve leaflets, in which one or both leaflets come into contact with the interventricular septum during ventricular systole, causing obstruction of normal blood flow in the left ventricular outflow tract (LVOT) towards the aorta (16). Additionally, the increased intraventricular pressure due to LVOTO can cause functional mitral insufficiency, with a regurgitant jet typically affecting the lateral and posterior wall of the LA. The severity of the regurgitation and the degree of the outflow gradient are dynamic and can be influenced by conditions that alter inotropism (e.g., physical exercise or the use of positive inotropic drugs), modify preload (e.g., dehydration, Valsalva manoeuvre, or change from squatting to standing position), or reduce afterload (e.g., physical exercise, vasodilator drugs, or septic states) (17). Symptoms associated with obstruction and mitral regurgitation include dyspnoea, chest pain, reduced exercise tolerance, presyncope, and syncope. LVOTO is not only an important pathophysiological component of HCM and one of the main determinants of atrial myopathy, but it is also associated with a worse prognosis compared to non-obstructive forms of HCM. In this context, a study involving 1,101 patients with HCM demonstrated that patients with LVOTO have a significantly higher risk of cardiovascular death, cardioembolic stroke, and progression to NYHA class III or IV (18).
Diastolic dysfunction
A large proportion of patients with HCM experience HF symptoms despite normal systolic function and the absence of LVOTO and mitral insufficiency, identifying a phenotype of HF with preserved EF (HFpEF). This underscores the importance of myocardial relaxation and LV filling in generating symptoms in this disease (19). Indeed, in patients with HCM, diastolic dysfunction is an important component of the disease pathophysiology (20). The grading of diastolic dysfunction is crucial for the evaluation of patients with HCM and HFpEF, since the nature and extent of the disturbed physiology are able to explain patient's symptoms; hence, this grading should be assessed through the LV filling velocities (mitral E/A ratio) and the level of LV filling pressures [according to E/e' ratio, peak tricuspid regurgitation (TR) velocity, indexed left atrial volume (LAVi) and peak atrial longitudinal strain (PALS)] (21).
The origin of diastolic dysfunction lies primarily in cellular alterations, such as myocyte disarray, which are also associated with a state of hypercontractility and an inability to achieve complete relaxation. These microstructural alterations underlie myocardial hypertrophy, microvascular dysfunction, and fibrosis, factors known to increase myocardial wall stress in HCM patients. This causes an increase in LV filling pressures and LA afterload, resulting in elevated pressure in the LA to compensate for the reduced compliance of the LV (22). This process gradually leads to left atrial myopathy.
Correlation with atrial fibrillation and cardioembolic stroke
AF is the most common sustained supraventricular arrhythmia in patients with HCM, with a clinical incidence of 2%–4% per year and a prevalence ranging from 19% to 30% in various cohorts, far exceeding rates in the general population of similar age (23). The prevalence of AF in HCM increases with disease progression, from subclinical phenotypes to end-stage disease, with a cumulative incidence over 40% in patients with LV dysfunction (24, 25). Moreover, up to 25% of patients with HCM, who are cardiac implantable electronic devices (CIEDs) carriers, experience paroxysmal and clinically silent episodes of AF (26, 27).
Due to the rigidity and reduced compliance of the LV, the contribution of atrial systole during diastole is typically increased in HCM patients compared to healthy individuals, both in obstructive and non-obstructive forms (28). Therefore, impairment of atrial function and the onset of AF leads to a reduction or loss of atrial systole, significantly influencing the risk of HF (25, 29). Patients with HCM and AF experience HF symptoms approximately 5 times more frequently than the general population, leading to increased rates of hospitalization, cardiovascular mortality, and a general deterioration in quality of life (25, 30, 31).
Thanks to the greater awareness of this condition, however, HCM is often diagnosed at an earlier stage, often before AF develops (32). Therefore, it is essential to identify patients with HCM who are at high risk for developing AF. LA size is a strong predictor for the development of AF, but even so, patients with HCM can develop AF in the absence of LA dilation, and conversely, not all patients with LA dilation will develop AF (12). Therefore, given the clinical importance and relevance of early AF diagnosis in HCM patients, several clinical risk stratification algorithms for developing AF have been proposed. Recently, a predictive model for the development of AF over the next 5 years (Hypertrophic Cardiomyopathy Atrial Fibrillation, HCM-AF Score, Table 1) has been validated in a large cohort of HCM patients, categorizing patients into three risk groups (low, intermediate, and high) (33). This score incorporates 4 variables (LA diameter, age, age at HCM diagnosis and presence of HF symptoms) and has been proven to perform better than other existing non-HCM specific tools (CHARGE-AF, HARMS2-AF, C2HEST, CHA2DS2-VA) for prediction of AF in patients with HCM. The authors of this study recommend increased attention to patients at high risk for AF, justifying a multi-level strategy that involves careful rhythm monitoring through periodic 48-hour Holter ECG or implantable loop recorder (ILR) implantation, and patient education regarding signs and symptoms of AF. In the largest external validation study to date, retrospectively conducted on 602 HCM patients without prior AF and followed for a median of approximately three years, the HCM-AF score demonstrated strong discriminatory power, maintaining consistent predictive accuracy across diverse subgroups, including White and non-White patients, obese and non-obese individuals, and gene-positive vs. gene-elusive patients. These findings reinforce the use of the HCM-AF score for personalized AF risk stratification in HCM. Accordingly, the 2024 AHA/ACC guidelines recommend validated tools such as the HCM-AF score for individualized AF risk assessment (34). Looking ahead, advances in artificial intelligence may drive a paradigm shift in risk prediction, as emerging machine learning models incorporating numerous variables show encouraging potential for even more precise assessment (35, 36).
Table 1
| Clinical variable | Value | Score |
|---|---|---|
| LA anteroposterior diameter (mm) | 24–29 | +8 |
| 30–35 | +10 | |
| 36–41 | +12 | |
| 42–47 | +14 | |
| 48–53 | +16 | |
| 54–59 | +18 | |
| 60–65 | +20 | |
| Age at the time of the visit (years) | 0–9 | +0 |
| 10–19 | +3 | |
| 20–29 | +6 | |
| 30–39 | +9 | |
| 40–49 | +12 | |
| 50–59 | +15 | |
| 60–69 | +18 | |
| 70–79 | +21 | |
| Age at the diagnosis of HCM (years) | 0–9 | +0 |
| 10–19 | −2 | |
| 20–29 | −4 | |
| 30–39 | −6 | |
| 40–49 | −8 | |
| 50–59 | −10 | |
| 60–69 | −12 | |
| 70–79 | −14 | |
| HF symptoms | Yes | +3 |
| No | +0 |
HCM-AF score for assessing the risk of atrial fibrillation in hypertrophic cardiomyopathy patients (33).
HCM-AF Score ≤17: low risk (<5% at 5 years).
HCM-AF Score 18–21: intermediate risk (5%–10% at 5 years).
HCM-AF Score ≥22: high risk (>10% at 5 years).
LA, left atrium; HCM, hypertrophic cardiomyopathy; AF, atrial fibrillation; HF, heart failure.
Anticoagulation therapy
A large number of studies have shown that AF is associated with a dramatically higher risk of stroke in patients with HCM compared to healthy individuals (25, 30, 37). For this reason, according to the most recent European Society of Cardiology guidelines on Cardiomyopathies, anticoagulation therapy should be initiated in HCM patients diagnosed with AF, regardless of the CHA2DS2-VASc score (Class I, Level of Evidence B) (4).
However, it should be considered that the changes described in atrial structure and function lead to reduced intra-atrial flow, resulting in blood stasis, thereby triggering the Virchow triad, even in the presence of stable sinus rhythm (38). Indeed, recent studies have suggested that the incidence of ischemic cardioembolic stroke in patients with HCM and LA dilation may be similar between patients in sinus rhythm and those with AF, raising the question of whether patients with extreme remodelling and dysfunction of the LA should receive prophylactic anticoagulation even when in sinus rhythm (39). This phenomenon has been observed in various clinical scenarios and is a feature shared by other cardiomyopathies with hypertrophic characteristics, such as Fabry disease and cardiac amyloidosis, where atrial thrombi are widely documented even in patients maintaining sinus rhythm (40–42). From a therapeutic standpoint, although both vitamin K antagonists and direct oral anticoagulants are equally effective for thromboprophylaxis, the latter maintain a better safety profile and improved tolerability, making them the preferred option, unless contraindicated (43, 44). The role of left atrial appendage closure in HCM remains unknown.
Antiarrhythmic therapy
Due to the clinical and hemodynamic impact of AF onset in patients with HCM, maintaining sinus rhythm is an important therapeutic goal. However, current antiarrhythmic therapy has significant limitations in terms of efficacy and safety, as adequate rhythm control can only be achieved in a minority of treated individuals (42% at 3 years of follow-up) (45).
Amiodarone is generally safe in the HCM population and may reduce the arrhythmic burden (45, 46). QT interval prolongation is rare, and proarrhythmic consequences are highly limited (45). However, long-term treatment can lead to hepatic, thyroid, and pulmonary toxicity, resulting in worse prognosis and treatment discontinuation (45). These issues are particularly important in this clinical context because most patients with HCM have a long-life expectancy.
Disopyramide is an older class IA antiarrhythmic drug commonly used to reduce LVOTO in HCM patients (46). It may represent a reasonable choice for rhythm control, although its overall efficacy is modest (35% after 3 years), due to its negative inotropic effect that facilitates the reduction of LVOT gradient (45). However, a significant proportion of patients on this treatment require discontinuation due to its anticholinergic effects.
Class IC antiarrhythmic drugs (such as flecainide and propafenone) are widely used in the general population for the prevention of AF recurrences. A small observational study of 48 patients with obstructive HCM showed a good safety profile in these patients treated with flecainide, with moderate improvement in obstruction and symptomatic status (47). The authors did not report any data on the efficacy of suppressing atrial or ventricular arrhythmias, and the small sample size did not allow for definitive conclusions. Therefore, their use is not yet indicated in HCM patients.
Catheter ablation is a more effective rhythm control strategy than antiarrhythmic therapy in the general population (48, 49). However, in patients with HCM, the efficacy of ablation is significantly reduced, with an increased incidence of AF recurrences (50–53). In this context, a recent study demonstrated that HCM is associated with a three-fold increase in the risk of AF recurrence following catheter ablation [hazard ratio (HR) 3.07, p < 0.001], compared to individuals without any form of cardiomyopathy (54). This is due to the persistence of mitral regurgitation, LVOTO, diastolic dysfunction, and resultant atrial myopathy, even after the procedure. Indeed, the success rate of a single catheter ablation is approximately 40% at 3–5 years, and around 50% after multiple procedures (55). It should be noted that the success rate of ablation varies depending on the timing: catheter ablation is more effective in younger, less symptomatic individuals with less atrial remodelling, and those with paroxysmal rather than persistent AF (55, 56). Procedurally, standard pulmonary vein isolation (PVI) is performed only in patients with paroxysmal AF, while in cases of persistent AF or extensive pathological atrial substrate, non-pulmonary triggers such as the posterior wall, roof, and mitral isthmus are typically targeted (57). One study reported similar efficacy between cryoablation and radiofrequency techniques, while data on emerging electroporation ablation are still lacking (58).
Electroanatomic mapping can be an excellent indicator of atrial health as it allows the assessment of any fibrotic areas, defined as low-voltage regions. In particular, a study by Professor Efremidis' group highlighted that patients with HCM and AF have significantly larger fibrotic areas compared to HCM patients in sinus rhythm and healthy individuals with AF. Additionally, the study found that an area of low voltage (≤0.25 mV) occupying more than 14.1% of the total LA surface was the best and only predictor of AF recurrence after catheter ablation, with excellent sensitivity and specificity (100%) (59).
Surgical ablation is a viable option in patients with HCM undergoing septal myectomy (60). Surgeons can perform classical PVI along with additional lesions aimed at the left atrial appendage, mitral isthmus, and roof and posterior wall segments. Surgical ablation is undoubtedly the most effective technique in HCM patients, as it has been shown that 75%-80% of patients are free from AF recurrence at follow-up (60). However, this outcome may be attributed not only to the direct effect of surgical ablation but also to the concomitant reduction in LVOTO and/or correction of MR, factors that significantly influence the atrial substrate.
Evidence from multimodal imaging and prognostic significance
The size of the LA is an independent risk factor for adverse outcomes in HCM patients, and an increased LA volume is associated with a higher risk of cardiovascular mortality, AF, thromboembolic stroke, and hospitalizations (10, 12, 31, 61, 62). In a review of 427 patients with HCM, Hiemstra et al. found that a LAVi ≥34 ml/m2 was predictive of an increased risk of all-cause mortality, heart transplantation, SCD and the use of an ICD (63). Specifically, in this review, LA dilation was shown to be an independent and strong predictor of SCD. For this reason, the LA anteroposterior diameter is an important parameter in the validated HCM-SCD Risk Score, the main risk score that guides the indication for ICD implantation in primary prevention in HCM patients (5). Therefore, timely detection of LA remodelling is a primary goal in the management of HCM to reduce mortality, prevent the onset of AF and cardioembolic stroke, and improve symptoms of HF (64). However, LA dilation in HCM represents an indicator of overt atrial myopathy (Figure 2), emphasizing the need for the development of new parameters capable of predicting the increase in LA size and its worsening in function (Figure 3) (65). Insights into this issue can be drawn from studies on LA function in athletes, which reveal that atrial size alone is insufficient to provide mechanistic information about atrial health. Notably, an increase in atrial size does not inherently indicate atrial dysfunction (66). Indeed, in athletes, despite significant atrial enlargement, the atria typically maintain normal reservoir function, myocardial stiffness, and dynamic adaptability to varying loading conditions. Consequently, the assessment of atrial function, rather than size alone, is crucial for accurately evaluating atrial myopathy (66).
Figure 2
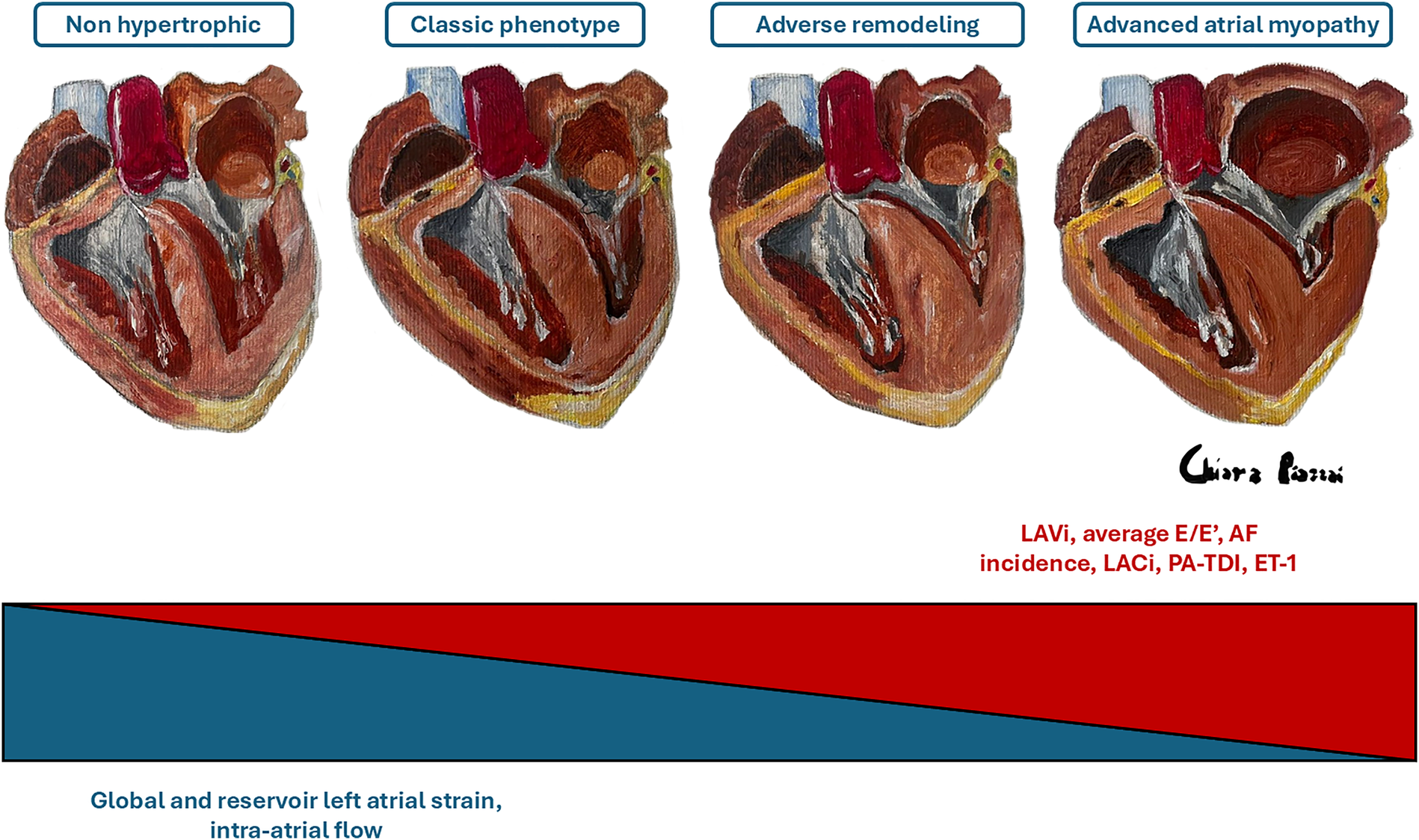
Evolution of atrial myopathy. The figure schematizes the main parameters that increase (in red) or decrease (in blue) as the severity of atrial myopathy progresses. ET-1, endothelin-1; AF, atrial fibrillation; LACi, left atrioventricular coupling index; PA-TDI, total atrial conduction time; LAVi, indexed left atrial volume. Dr. Chiara Piazzai, Four Hearts on Canvas, Oil and Turpentine, 2024.
Figure 3
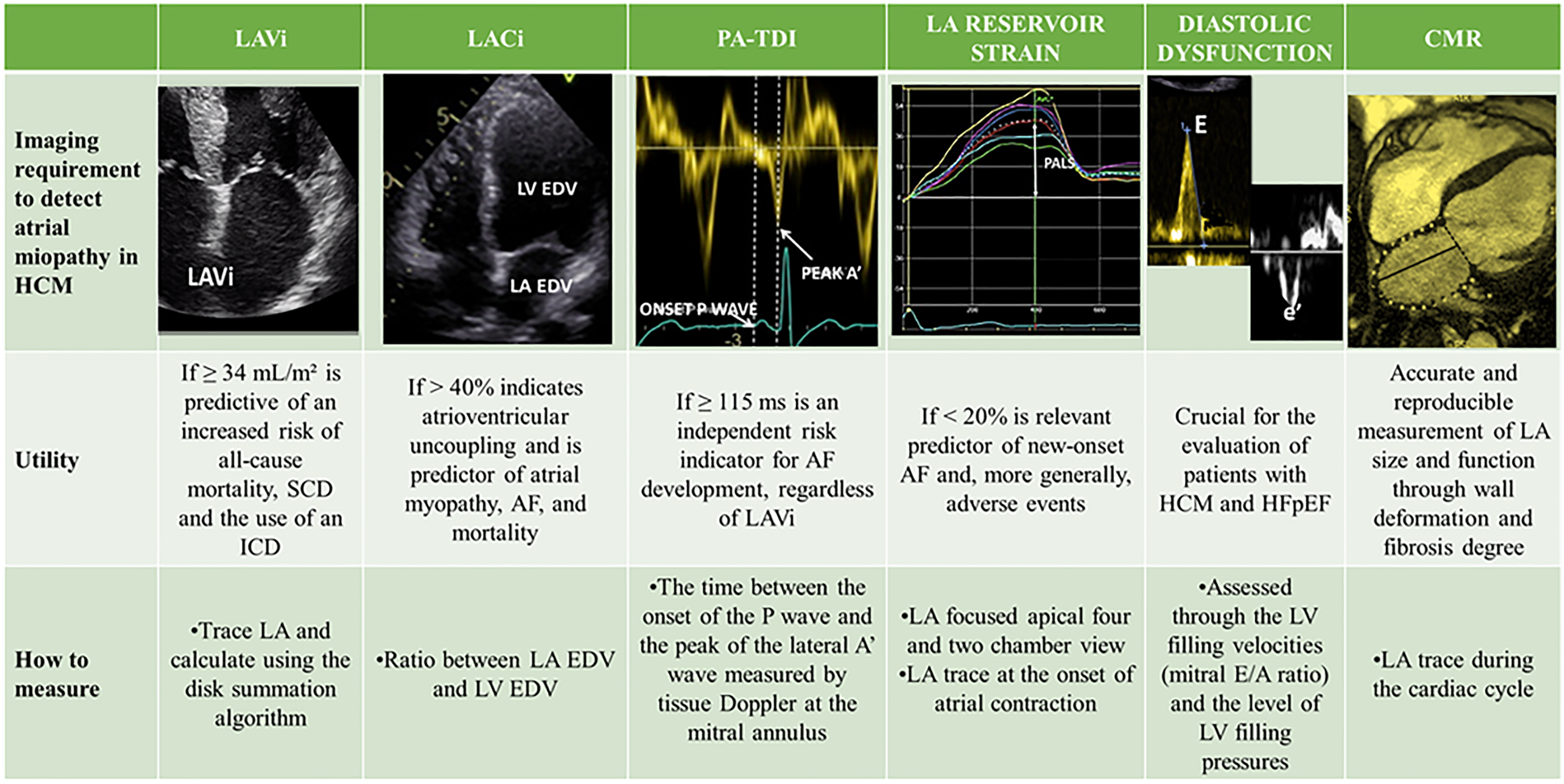
Multimodal imaging of atrial myopathy. AF, atrial fibrillation; CMR, cardiac magnetic resonance; EDV, end-diastolic volume; HCM, hypertrophic cardiomyopathy; HFpEF, heart failure with preserved ejection fraction; LA, left atrium; LACi, left atrioventricular coupling index; LAVi, indexed left atrial volume; LV, left ventricle; PA-TDI, total atrial conduction time; PALS, peak atrial longitudinal strain; SCD, sudden cardiac death.
Role of ECG
Due to its low cost and ease of use, significant attention has been given in the literature to the identification of electrocardiographic parameters capable of predicting the onset of atrial remodelling and AF in patients with HCM. Among these parameters, P wave duration has emerged as the most reliable. In a study conducted by our group on 110 HCM patients in sinus rhythm, it was shown that a P wave duration greater than 140 ms was associated with an increased risk of AF, although with suboptimal sensitivity (56%) and specificity (83%) (67).
Role of echocardiography
The LA and LV are directly coupled during ventricular diastole, and in the absence of mitral valve stenosis, their functions and filling pressures are linked. An increase in LA volume relative to LV volume at the end of diastole directly reflects impaired LV compliance. Following this concept, one study demonstrated that values of the left atrioventricular coupling index (LACi, defined as the ratio of LA to LV end-diastolic volumes) greater than 40% are indicative of atrioventricular uncoupling and are better predictors of atrial myopathy, AF, and mortality than simple LA size assessment (68). This suggests that an initial change in LV compliance, geometry, and pressures, leading to a reduction in LV volumes due to worsening hypertrophy and disease progression, could signal functional deterioration of the LA before its overt enlargement.
Significant mitral regurgitation has been shown to be associated with LA myopathy independently of the degree of LV diastolic dysfunction in HCM. Therefore, identification of moderate or greater mitral insufficiency, regardless of LA size, warrants greater attention and further investigation (69).
Moreover, in a study involving a cohort of 208 patients, total atrial conduction time (PA-TDI), a parameter evaluating the relationship between structural and electrical remodelling of the atrial myocardium, was significantly correlated with new-onset AF in HCM patients. PA-TDI is calculated as the time between the onset of the P wave on the ECG and the peak of the lateral A' wave measured by tissue Doppler at the mitral annulus. A PA-TDI value ≥115 ms was found to be an independent risk indicator for AF development, regardless of LAVi (70).
Finally, speckle-tracking echocardiography (STE) has emerged as a valuable tool for the early detection of LA dysfunction, even when LA dimensions are preserved. By measuring myocardial deformation throughout the cardiac cycle, LA strain offers a comprehensive assessment of atrial function. It is typically divided into three components (a) LA reservoir strain, reflecting LA expansion during ventricular systole and isovolumetric relaxation; (b) LA conduit strain, representing passive emptying of the LA during early diastole after mitral valve opening; (c) LA booster strain, indicating active LA contraction in late diastole (71). A decline in global LA strain and PALS, expression of maximal deformation at the end of the reservoir phase, often precedes the LA enlargement. Notably, reduced LA strain may be one of the earliest detectable abnormalities in carriers of HCM-related gene mutations, even before phenotypic expression of the disease (72). LAS not only correlates well with volumetric analysis of LA but also has the ability to detect atrial fibrosis and the development of AF as well as prevalence of MACE in patients diagnosed with HCM (73). Interestingly, a reduced global PALS was found to be the strongest predictor of invasively assessed severe LA fibrosis at cardiac biopsy in patients with end-stage HF, explaining the high burden of AF in these patients (74). Moreover, LA reservoir strain has been identified as a strong predictor of both new-onset AF and its recurrence following catheter ablation. Lack of significant improvement in LA strain after the procedure is associated with a higher likelihood of arrhythmia recurrence (75, 76). Finally, recent evidences suggest that a reduction in global LA strain <23.4% or in reservoir strain <20% are relevant predictors of new-onset AF and, more generally, adverse events, independent of LA size (73, 77, 78). As suggested by a recent meta-analysis, integrating LA strain into current risk prediction models could significantly improve individualized care and guide medical or procedural strategies in patients with HCM (73). Beyond the atrium, STE is also instrumental in evaluating LV mechanics, providing early markers of systolic and diastolic dysfunction. In a study of 56 patients with non-obstructive HCM, reduced longitudinal, circumferential, and radial LV strain values were detected before a decline in LVEF, highlighting the sensitivity of STE for subclinical myocardial dysfunction (79). Furthermore, the STE of the LV provides valuable insights regarding the AF risk in HCM patients. Indeed, in a prospective cohort of 250 HCM patients without prior AF, a global longitudinal LV strain value >–14% was a significant predictor of new-onset AF, underlining the role of LV mechanics in arrhythmia risk stratification. In conclusion, In conclusion, STE is a powerful, non-invasive imaging modality that allows simultaneous assessment of LA and LV function. It provides sensitive, reproducible, and clinically meaningful data that enhance both diagnostic evaluation and risk stratification.
Role of cardiac magnetic resonance
Cardiac magnetic resonance (CMR) is an excellent tool as it allows highly accurate and reproducible measurement of LA size and function by assessing wall deformation and fibrosis degree, which is also fundamental to predict response to ablation therapy in AF patients (80–84).
Additionally, a novel CMR technique, 4D flow, has proven more effective than transoesophageal echocardiography in detecting blood stasis within the LA, a critical parameter for thrombus formation (85). However, there are currently no studies demonstrating an increased thromboembolic risk in HCM patients with reduced CMR intra-atrial flow. Although several CMR studies have been conducted in HCM patients, none have yet identified earlier and more reliable predictors of atrial myopathy and AF than LA enlargement or dysfunction. Future studies should also aim to compare reproducibility of LA strain measurements using CMR based techniques to standardize normal and abnormal values for LA strain in the HCM population.
Role of genetics
In addition to morphological parameters, non-classical factors, such as genetic variants causing HCM, may play a crucial role in increasing the incidence of atrial myopathy and AF. Sarcomeric mutations have been shown to be associated with a higher prevalence and earlier onset of AF when compared to non-familial forms of HCM (86–88). Supporting this finding, it has been demonstrated that HCM patients with sarcomeric mutations, specifically in MYH7 and MYBPC3, exhibit a higher prevalence of low-voltage areas in the LA on electroanatomical mapping, indicative of LA scarring that may contribute to the AF burden, compared to genotype-negative HCM patients (6.1% vs. 2.3%; p < 0.001) (89). A study involving over 1,000 HCM patients, followed for an average of about 7 years, revealed that the incidence of AF in patients with sarcomeric mutations was approximately 19%, regardless of echocardiographic or clinical parameters. Notably, the presence of likely pathogenic or pathogenic mutations in the MYH7 gene had the highest incidence of AF after adjusting for age, sex, proband status, left atrial size, maximal wall thickness, and peak pressure gradient (HR 1.7, p = 0.009) compared to other HCM-related genotypes (90). A previously published analysis reported a peak AF prevalence of 47% in a family of 15 individuals with HCM caused by the Arg663His missense variant in the MYH7 gene (91). This represents a significantly higher incidence than in any other previously described HCM cohort (67).
However, numerous studies suggest that among sarcomeric mutations, those affecting genes encoding thin filament proteins are associated with a higher likelihood of advanced diastolic dysfunction and HF symptoms compared to those affecting thick filament proteins. In fact, a triphasic diastolic filling pattern of the LV is particularly common in HCM patients with this subclass of sarcomeric mutations, reflecting deep diastolic dysfunction that may predispose to LA remodelling and AF development (92). In this context, the missense variant Met228Thr in the ACTN2 gene, which encodes the α-actinin-2 protein, has been associated with familial mid-apical HCM and early-onset AF and atrioventricular blocks (93).
Finally, although less frequent and less widely studied, mutations in non-sarcomeric genes may increase the risk of AF and could therefore be considered potential modifiers of the HCM phenotype. In this regard, Orenes-Piñero et al. identified the 344T>C polymorphism in the CYP11B2 gene as an independent predictor of AF development in HCM patients (94). This polymorphism is associated with increased activation of the renin-angiotensin-aldosterone system, resulting in elevated serum aldosterone levels that may promote fibrosis and atrial remodelling.
Table 2 provides a comprehensive overview of all known genetic predispositions to early-onset AF reported in the literature to date.
Table 2
| Gene | Mutation | Frequency/HR | Characteristics |
|---|---|---|---|
| Sarcomeric genes | |||
| MYH7 (90) | Pathogenic or likely pathogenic | HR 1.72; p = 0.009, vs. MYBPC3 mutations | Obstructive HCM and early-onset AF |
| MYH7 (91) | c.12601G>A (p.Arg663His) missense mutation (autosomal dominant inheritance) | 47% (7/15 members of the family) vs. 13% (p = 0.006) reported in HCM population with unknown genotypes and a similar age distribution | Obstructive HCM and early-onset AF |
| MYH7 or MYBPC3 (89) | Pathogenic or likely pathogenic | 6.1% low-voltage areas in the LA at electroanatomical mapping (vs. 2.3% in genotype-negative HCM; p < 0.001) | Obstructive HCM |
| MYH6 (88) | Pathogenic or likely pathogenic | / | Early-onset AF and lower risk of mortality because it is predominantly expressed in the atria with a more isolated atrial involvement compared with those with an MYH7 variant |
| TTN (117, 118) | Pathogenic or likely pathogenic, truncating variant | HR 1.77; p < 0.001, vs. healthy people | Early-onset AF |
| MYL4 (119) | c.234delC frameshift deletion (autosomal recessive inheritance) | 100% (8/8 members of the family) | Early-onset AF |
| TNNT2 (120, 121) | c.835C > T (p.Gln279Ter) nonsense mutation (autosomal dominant inheritance) | 12% (1/8 members of the family) | Early-onset AF |
| ACTN2 (93) | c.683T > C (p.Met228Thr) missense mutation | 36% (4/11 members of the family) with early-onset AF | Mid-apical HCM, early-onset AF, and AV block |
| Other genes | |||
| CYP11B2 (94) | c.344T > C polymorphism | HR 3.31; p = 0.008, vs. healthy people | Early-onset AF due to increased activation of the renin-angiotensin-aldosterone system |
| JPH2 (122) | c.505G > A (p.Glu169Lys) missense mutation | 100% (2/2 members of the family) with early-onset AF | Early-onset AF. Loss-of-function mutations in junctophilin-2 (JPH2) impair its ability to stabilize the sarcoplasmic reticulum, promoting calcium (Ca2+) leak and increasing susceptibility to atrial arrhythmias |
Genetic predisposition to atrial fibrillation (AF).
AF, atrial fibrillation; HCM, hypertrophic cardiomyopathy.
Circulating molecules
A study conducted on 375 HCM patients found that endothelin-1 (ET-1), a circulating protein, is an independent predictor of AF in this population. Specifically, ET-1 levels ≥0.285 pmol/L showed good specificity (75%) and sensitivity (56%) in predicting AF, even in the absence of LA dilation (95).
Finally, inflammation seems to play an important role in the pro-thrombotic state associated with AF (96). However, only a few studies have evaluated the importance of inflammatory biomarkers in directly predicting major clinical outcomes such as stroke and mortality. Therefore, further studies with larger sample sizes and important clinical endpoints are needed to assess the potential clinical significance of inflammatory biomarkers in AF.
Impact of treatment
In light of the discussion above, it is crucial not only to identify atrial remodelling in its early stages to prevent severe complications such as AF, but it is equally important to understand how to halt and, if possible, reverse this process. As previously emphasized, LA remodelling in patients with HCM is mainly correlated with LVOTO, the severity of MR and diastolic dysfunction. Based on this evidence, it is intriguing to speculate that the removal of a triggering factor, such as LVOTO, could lead to subsequent reverse remodelling of the LA, with normalization of both dimensional and functional values. The main therapeutic options currently available are:
- •
Septal reduction therapy, which can be performed through either a surgical procedure (Morrow myectomy) or an interventional procedure (septal alcohol ablation);
- •
More recently, the use of cardiac myosin inhibitors.
These treatments aim to reduce LVOTO in order to significantly improve symptoms and potentially survival. However, at present, these therapies are often considered in symptomatic patients with obstructive HCM who are refractory to first-line medications (
4). A list of studies related to this is available in
Table 3.
Table 3
| Study | Procedure | Patients number | Mean age (years) | Follow up duration | LA diameter (mm) | LAVi (ml/m2) | E wave (cm/s) | E/A | E/e’ | LA strain (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Septal reduction therapy | ||||||||||
| Monteiro et al., 2007 (97) | Myectomy | 150 | 44.5 | 3 years | From 43.6 to 45.2 (P = 0.06) | / | From 95 to 87 (P = 0.008) | From 1.53 to 1.44 (P = 0.30) | / | / |
| Menon et al., 2008 (98) | Myectomy | 32 | 11.6 | / | / | From 52.1 to 33.2 (P < 0.0001) | From 130 to 100 (P = 0.01) | From 1.9 to 1.3 (P < 0.0001) | Mean, From 20.7 to 10.8 (P < 0.001) | / |
| Moravsky et al., 2013 (99) | Myectomy | 66 | 54 | 6–18 months | / | From 48 to 37 (P < 0.05) | From 80 to 78 (P > 0.05) | / | Lateral, from 11 to 8 (P < 0.05) | / |
| Finocchiaro et al., 2016 (101) | Myectomy (31) and alcohol septal ablation (9) | 40 | 50 | 1 years | / | From 63 to 55 (P < 0.001) | From 91 to 92 (P = 0.92) | / | Mean, from 15 to 16 (P = 0.48) | / |
| Cavigli et al., 2018 (123) | Myectomy (71) and alcohol septal ablation (55) | 126 | 53 | 5 years | From 50 to 47 (P = 0.027) | / | / | / | / | / |
| Nguyen et al., 2018 (100) | Myectomy | 656 | 56 | 2 years | / | From 47 to 38 (P < 0.001) | / | From 1.15 to 0.95 (P = 0.002) | Mean, from 11.9 to 9.1 (P < 0.001) | / |
| Ha et al., 2023 (113) | Myectomy | 44 | 52.8 | 1 year | / | From 61.5 to 49.1 (P < 0.001) | From 90 to 80 (P = 0.011) | / | Septal, from 25.7 to 23.0 (P = 0.049) | Global, from 24.4 to 30.5 (P = 0.004) |
| Cardiac myosin inhibitors | ||||||||||
| Ho et al., 2020 (106) | Mavacamten in non-obstructive HCM | 40 | 54 | 24 weeks | / | From 37.3 to 38.7 (P = 0.90) | / | / | Mean, from 14.1 to 11.5 (P = 0.50) | / |
| Hegde et al., 2021 (114) | Mavacamten | 123 | 58.5 | 30 weeks | / | From 40.0 to 32.5 (P < 0.0001) | From 88 to 81.6 (P = 0.06) | / | Mean, from 17.5 to 13.9 (P < 0.0001) | / |
| Saberi et al., 2021 (111) | Mavacamten vs. placebo | 35 | 60 | 30 weeks | / | Reduction of 10.3 (P < 0.001) | / | / | / | / |
| Cremer et al., 2022 (112) | Mavacamten | 56 | 59.8 | 16 weeks | / | From 41.3 to 36.1 (P = 0.005) | / | From 1.4 to 1.3 (P = 0.57) | Mean, from 17.1 to 13.7 (P < 0.001) | / |
| Tian et al., 2023 (108) | Mavacamten | 54 | 52.4 | 30 weeks | / | From 43.3 to 29.5 (P < 0.001) | / | / | / | / |
| Wessly et al., 2023 (115) | Mavacamten | 15 | 70 | 30 days | / | From 39.5 to 35 (P = 0.37) | From 91 to 84 cm/s (P = 0.12) | From 1.00 to 1.10 (P = 0.15) | Mean, from 18 to 15 (P = 0.005) | Reservoir, from 27.5 to 22 (P = 0.012) |
| Desai et al., 2023 (107) | Mavacamten | 56 | 59.8 | 56 weeks | / | From 41.3 to 35.8 (P not specified) | / | / | Septal, from 19.6 to 15.5 (P not specified | / |
Main studies on the impact of LVOTO reduction on atrial myopathy.
HCM, hypertrophic cardiomyopathy.
Septal reduction therapy
A study involving 150 HCM patients undergoing surgical myectomy found a significant improvement in LV filling parameters, with a reduction in the mitral E-wave velocity (from 95 to 87 cm/s, p = 0.008) (97). Menon et al. retrospectively analysed data from a paediatric sample (ages 1–22 years, n = 32) who underwent septal myectomy for obstructive HCM. In this study, LAVi decreased from 52.1 ± 2.2 to 33.2 ± 11.9 ml/m2 (p < 0.001), the E/A ratio decreased from 1.9 ± 0.6 to 1.3 ± 0.5 (p < 0.001), and the tissue Doppler velocity at the medial mitral annulus (E′ medial) increased from 6.2 ± 1.9 to 13 ± 2.6 cm/s (p = 0.043) (98). Moravsky et al., in a study involving 66 patients with obstructive HCM, demonstrated significant reverse remodelling of the LA (LAVi decreased from 48 ± 16 to 37 ± 13 ml/m2, p < 0.05) after myectomy (echocardiographic follow-up of 6–18 months), with symptom relief and a reduction in obstruction (99). These results were confirmed by a large study of 653 patients who underwent surgical myectomy with a 24-month follow-up. This study showed a reduction in LAVi from 48 to 43 ml/m2 after the first year, and to 38 ml/m2 at the end of follow-up (p < 0.001), highlighting that LA reverse remodelling is an ongoing process years after the procedure (100). This large study also noted a significant improvement in diastolic function, with a decrease in the mitral E/A ratio from 1.15 to 0.95 (p = 0.002) and a decrease in mean E/e' ratio from 11.9 to 9.1 (p < 0.001) (100). Finally, it is important to mention a study by Finocchiaro et al., as it analysed a sample of around 40 patients who underwent both surgical myectomy and alcohol septal ablation. Consistent with previous trials, this study demonstrated that septal reduction therapy was associated with significant reverse remodelling of the LA and a major reduction in associated symptoms (101). However, it observed a trend towards greater reduction in LA volume after myectomy compared to alcohol septal ablation. This highlights how myectomy, often associated with mitral valve repair (another important determinant of atrial myopathy) is more effective in reducing filling pressures compared to alcohol ablation (102). In contrast to earlier studies, this one did not observe a significant improvement in diastolic function, particularly in tissue Doppler parameters such as E/e′. This may be due to the different demographic characteristics of the cohort, as the population studied had a longer disease history with more advanced atrial myopathy, associated with more significant LVOTO and MR, where diastolic dysfunction may have become fixed and no longer influenced by factors such as LVOTO.
These studies indicate that LA reverse remodelling is directly related to the reduction of the LVOT gradient and the severity of MR in patients with obstructive HCM. Since LA size reflects LV filling pressures, these results suggest that removal of the obstruction reduces atrial pressures, leading to actual reverse remodelling, particularly in the earlier stages of the disease. Therefore, it is crucial to also assess latent obstruction using stress echocardiography: the detection of positive hemodynamic criteria (exercise-induced hypotension and exercise-induced LVOTO >50 mmHg at peak exercise stress) allows the prediction of future development of progressive HF symptoms and the detection of the disease at an earlier stage, before obstruction becomes manifest (103, 104).
Cardiac myosin inhibitors
In recent years, the pathophysiology of HCM has been progressively clarified, leading to a paradigm shift in treatment. This has marked a transition from an era of symptomatic therapies to one of targeted therapies that can actually modify the natural course of the disease. For HCM, these therapies include cardiac myosin inhibitors such as Mavacamten and Aficamten, small molecules that act directly at the sarcomeric level on the primary pathophysiological alteration underlying the disease, improving cardiomyocyte relaxation. The development of this type of therapy represents a very promising option for patients with HCM, as it has shown substantial hemodynamic, clinical, electrical, and structural improvement in both obstructive and non-obstructive HCM (105–110). These data offer extremely promising prospects regarding the long-term effects of these molecules. Therefore, numerous studies are ongoing to evaluate the impact of these molecules on atrial remodelling. The results obtained so far indicate that treatment with Mavacamten, similar to surgical myectomy, not only improves LVOT gradients but also results in an improvement in diastolic function parameters (111–113). In particular, the largest study to date, involving 251 patients with symptomatic obstructive HCM, showed that patients treated with Mavacamten had a significant reduction in LAVi, from 40 to 32.5 ml/m2 (p < 0.0001), a decrease in the lateral E/e' ratio from 15.0 to 11.2 (p < 0.0001), and a reduction in the degree of MR, thanks to a higher incidence of complete resolution of the mitral SAM (47% increase compared to the placebo group, p < 0.0001) (114).
Although studies demonstrate a positive effect of cardiac myosin inhibitors on atrial remodelling, the incidence of AF in this patient group does not seem to improve as markedly. Some small cohort studies on Mavacamten-treated patients indicate that the typical negative inotropic effect of the drug also occurs at the atrial level, resulting in a reduction of LA strain and of LA reservoir strain, both validated parameters and predictors of AF risk (115, 116). Therefore, further research on larger populations is needed to evaluate the long-term impact of myosin inhibitors on AF incidence.
In conclusion, treatment with cardiac myosin inhibitors induces LA reverse remodelling, leading to improvements in diastolic function and MR, similar to the effects observed with surgical myectomy. However, unlike myectomy, myosin inhibitors have a negative impact on atrial inotropism, as demonstrated by the results of preliminary studies on small cohorts, which show a significant reduction in LA strain. It is still unclear whether the positive effect of reverse atrial remodelling or the negative effect of atrial inotropism predominates in determining the incidence of AF. Further studies based on real-world clinical experience could clarify this issue.
Limitations
While this review highlights the potential role of atrial myopathy in the pathophysiology and clinical course of HCM, it is important to acknowledge several limitations.
First, current evidence does not definitively establish atrial myopathy as a critical prognostic determinant of HCM severity. Although an association between atrial structural and electrical remodelling and adverse outcomes, particularly AF, is consistently observed, causality remains unclear. Furthermore, it is uncertain whether targeting atrial myopathy directly could meaningfully alter the natural history of HCM. Most available studies are observational, and interventional data addressing whether treatment of atrial myopathy improves long-term outcomes in HCM are lacking.
In addition, much of the literature focuses on surrogate markers, such as atrial size or fibrosis on imaging, which may not fully capture the complexity of atrial remodelling. There is also considerable heterogeneity in the definitions and methods used to assess atrial myopathy, making comparisons across studies difficult.
Finally, this review may be limited by publication bias and the predominance of data from tertiary care centres, potentially reducing generalizability.
Future studies are needed to clarify the prognostic significance of atrial myopathy in HCM and to determine whether it represents a meaningful therapeutic target beyond rhythm control in patients with AF.
Conclusions
In patients with HCM, factors such as SAM of the mitral valve, LVOTO, increased LV filling pressures, and genetic predisposition contribute to LA dilation and fibrosis, leading to the development of a true atrial myopathy. This condition has been associated with an increased risk of cardiovascular events, all-cause mortality, and the incidence of AF. Therefore, it is crucial to identify strong predictors of atrial myopathy through the use of multimodal imaging, including ECG, echocardiography and CMR, to detect the early onset of the disease. In this context, LA strain has proven to be a particularly promising non-invasive tool, more sensitive than traditional volumetric analysis, as a significant reduction in LA reservoir strain often precedes the evident enlargement of the LA. From a therapeutic perspective, septal reduction therapies, particularly surgical myectomy, have been shown to be highly effective in halting this progression and promoting LA reverse remodelling, in addition to improving diastolic function. Data regarding the use of myosin modulators are promising but still inconclusive and somewhat discordant.
Statements
Author contributions
CP: Writing – review & editing, Conceptualization, Methodology. AP: Formal analysis, Writing – review & editing, Methodology, Conceptualization, Software, Writing – original draft, Investigation, Data curation. AS: Data curation, Writing – review & editing, Formal analysis. FD: Validation, Supervision, Visualization, Writing – review & editing. IO: Visualization, Resources, Validation, Conceptualization, Supervision, Writing – review & editing. MC: Writing – review & editing, Visualization, Validation, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AF, atrial fibrillation; CMR, cardiac magnetic resonance; ET-1, endothelin-1; EF, ejection fraction; HCM, hypertrophic cardiomyopathy; HF, heart failure; LA, left atrium; LACi, left atrioventricular coupling index; LAVi, indexed left atrial volume; LV, left ventricle; LVOT, left ventricular outflow tract; LVOTO, left ventricular outflow tract obstruction; MR, mitral regurgitation; PA-TDI, total atrial conduction time; SAM, systolic anterior motion; SCD, sudden cardiac death.
References
1.
MaronBJMaronMS. Hypertrophic cardiomyopathy. Lancet. (2013) 381:242–55. 10.1016/S0140-6736(12)60397-3
2.
AlcalaiRSeidmanJGSeidmanCE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol. (2008) 19:104–10. 10.1111/j.1540-8167.2007.00965.x
3.
TeekakirikulPZhuWHuangHCFungE. Hypertrophic cardiomyopathy: an overview of genetics and management. Biomolecules. (2019) 9:1–11. 10.3390/biom9120878
4.
ArbeloEProtonotariosAGimenoJRArbustiniEBarriales-VillaRBassoCet alESC Guidelines for the management of cardiomyopathies: developed by the task force on the management of cardiomyopathies of the European Society of Cardiology (ESC). Eur Heart J. (2023) 44:3503–626. 10.1093/eurheartj/ehad194
5.
O'MahonyCJichiFPavlouMMonserratLAnastasakisARapezziCet alA novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J. (2014) 35:2010–20. 10.1093/eurheartj/eht439
6.
FinocchiaroGPapadakisMTanzarellaGDhutiaHMilesCTomeMet alSudden death can be the first manifestation of hypertrophic cardiomyopathy: data from a United Kingdom pathology registry. JACC Clin Electrophysiol. (2019) 5:252–4. 10.1016/j.jacep.2018.11.004
7.
OlivottoICecchiFPoggesiCYacoubMH. Patterns of disease progression in hypertrophic cardiomyopathy an individualized approach to clinical staging. Circ Heart Fail. (2012) 5:535–46. 10.1161/CIRCHEARTFAILURE.112.967026
8.
MarstrandPHanLDaySMOlivottoIAshleyEAMichelsMet alHypertrophic cardiomyopathy with left ventricular systolic dysfunction: insights from the SHaRe registry. Circulation. (2020) 141:1371–83. 10.1161/CIRCULATIONAHA.119.044366
9.
YangHWooAMonakierDJamorskiMFedwickKWigleEDet alEnlarged left atrial volume in hypertrophic cardiomyopathy: a marker for disease severity. J Am Soc Echocardiogr. (2005) 18:1074–82. 10.1016/j.echo.2005.06.011
10.
LosiM-ABetocchiSBarbatiGParisiVTocchettiC-GPastoreFet alPrognostic significance of left atrial volume dilatation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. (2009) 22:76–81. 10.1016/j.echo.2008.11.001
11.
EssayaghBResseguierNMichelNCasaltaA-CRenardSDonghiVet alLeft atrial dysfunction as marker of poor outcome in patients with hypertrophic cardiomyopathy. Arch Cardiovasc Dis. (2021) 114:96–104. 10.1016/j.acvd.2020.06.004
12.
LosiMAMondaELombardiRLioncinoMCancielloGRubinoMet alPrediction of incident atrial fibrillation in hypertrophic cardiomyopathy. Int J Cardiol. (2024) 395:1–5. 10.1016/j.ijcard.2023.131575
13.
GeskeJBOmmenSRGershBJ. Hypertrophic cardiomyopathy: clinical update. JACC Heart Fail. (2018) 6:364–75. 10.1016/j.jchf.2018.02.010
14.
ShenMJAroraRJalifeJ. Atrial myopathy. JACC Basic Transl Sci. (2019) 4:640–54. 10.1016/j.jacbts.2019.05.005
15.
GoetteACorradiDDobrevDAguinagaLCabreraJ-AChughSSet alAtrial cardiomyopathy revisited—evolution of a concept. A clinical consensus statement of the European heart rhythm association (EHRA) of the ESC, the heart rhythm society (HRS), the Asian Pacific heart rhythm association (APHRS), and the Latin American heart rhythm society (LAHRS). Europace. (2024) 26:1–31. 10.1093/europace/euae204
16.
MaronMSOlivottoIZenovichAGLinkMSPandianNGKuvinJTet alHypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. (2006) 114:2232–9. 10.1161/CIRCULATIONAHA.106.644682
17.
ArgiròAZampieriMMarchiAdel FrancoAPàlinkàsEDBiagioniGet alApprocci terapeutici nella cardiomiopatia ipertrofica: dal controllo dei sintomi alla terapia di precisione. G Ital Cardiol. (2023) 24:792–9. 10.1714/4100.40979
18.
MaronMSOlivottoIBetocchiSCaseySALesserJRLosiMAet alEffect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. (2003) 348:295–303. 10.1056/NEJMoa021332
19.
SachdevVShizukudaYBrennemanCLBirdsallCWWaclawiwMAAraiAEet alLeft atrial volumetric remodeling is predictive of functional capacity in nonobstructive hypertrophic cardiomyopathy. Am Heart J. (2005) 149:730–6. 10.1016/j.ahj.2004.07.017
20.
CarassoSYangHWooAJamorskiMWigleEDRakowskiH. Diastolic myocardial mechanics in hypertrophic cardiomyopathy. J Am Soc Echocardiogr. (2010) 23:164–71. 10.1016/j.echo.2009.11.022
21.
DiniFLCameliMStefaniniAAboumarieHSLisiMLindqvistPet alEchocardiography in the assessment of heart failure patients. Diagnostics. (2024) 14:1–22. 10.3390/diagnostics14232730
22.
PakPHMaughanWLBaughmanKLKassDA. Marked discordance between dynamic and passive diastolic pressure-volume relations in idiopathic hypertrophic cardiomyopathy. Circulation. (1996) 94:52–60. 10.1161/01.CIR.94.1.52
23.
GuttmannOPRahmanMSO'MahonyCAnastasakisAElliottPM. Atrial fibrillation and thromboembolism in patients with hypertrophic cardiomyopathy: systematic review. Heart. (2014) 100:465–72. 10.1136/heartjnl-2013-304276
24.
SiontisKCGeskeJBOngKNishimuraRAOmmenSRGershBJ. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical correlations, and mortality in a large high-risk population. J Am Heart Assoc. (2014) 3:1–8. 10.1161/JAHA.114.001002
25.
RowinEJHausvaterALinkMSAbtPGionfriddoWWangWet alClinical profile and consequences of atrial fibrillation in hypertrophic cardiomyopathy. Circulation. (2017) 136:2420–36. 10.1161/CIRCULATIONAHA.117.029267
26.
RowinEJOrfanosAEstesNAMWangWLinkMSMaronMSet alOccurrence and natural history of clinically silent episodes of atrial fibrillation in hypertrophic cardiomyopathy. Am J Cardiol. (2017) 119:1862–5. 10.1016/j.amjcard.2017.02.040
27.
van VelzenHGTheunsDAMJYapS-CMichelsMSchinkelAFL. Incidence of device-detected atrial fibrillation and long-term outcomes in patients with hypertrophic cardiomyopathy. Am J Cardiol. (2017) 119:100–5. 10.1016/j.amjcard.2016.08.092
28.
BonowROFrederickTMBacharachSLGreenMVGoosePWMaronBJet alAtrial systole and left ventricular filling in hypertrophic cardiomyopathy: effect of verapamil. Am J Cardiol. (1983) 51:1386–91. 10.1016/0002-9149(83)90317-x
29.
GlancyDLO'BrienKPGoldHKEpsteinSE. Atrial fibrillation in patients with idiopathic hypertrophic subaortic stenosis. Br Heart J. (1970) 32:652–9. 10.1136/hrt.32.5.652
30.
OlivottoICecchiFCaseySADolaraATraverseJHMaronBJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. Circulation. (2001) 104:2517–24. 10.1161/hc4601.097997
31.
CiabattiMFumagalliCBeltramiMVigniniEMartineseLTomberliAet alPrevalence, causes and predictors of cardiovascular hospitalization in patients with hypertrophic cardiomyopathy. Int J Cardiol. (2020) 318:94–100. 10.1016/j.ijcard.2020.07.036
32.
CanepaMFumagalliCTiniGVincent-TompkinsJDaySMAshleyEAet alTemporal trend of age at diagnosis in hypertrophic cardiomyopathy: an analysis of the international sarcomeric human cardiomyopathy registry. Circ Heart Fail. (2020) 13:E007230. 10.1161/CIRCHEARTFAILURE.120.007230
33.
CarrickRTMaronMSAdlerAWesslerBHossSChanRHet alDevelopment and validation of a clinical predictive model for identifying hypertrophic cardiomyopathy patients at risk for atrial fibrillation: the HCM-AF score. Circ Arrhythm Electrophysiol. (2021) 14:E009796. 10.1161/CIRCEP.120.009796
34.
OmmenSRHoCYAsifIMBalajiSBurkeMADaySMet al2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology joint committee on clinical practice guidelines. J Am Coll Cardiol. (2024) 83:2324–405. 10.1016/j.jacc.2024.02.014
35.
BhattacharyaMLuD-YVentoulisIGreenlandGVYalcinHGuanYet alMachine learning methods for identifying atrial fibrillation cases and their predictors in patients with hypertrophic cardiomyopathy: the HCM-AF-risk model. CJC Open. (2021) 3:801–13. 10.1016/j.cjco.2021.01.016
36.
LuRLumishHSHasegawaKMaurerMSReillyMPWeinerSDet alPrediction of new-onset atrial fibrillation in patients with hypertrophic cardiomyopathy using machine learning. Eur J Heart Fail. (2025) 27:275–84. 10.1002/ejhf.3546
37.
FurlanAJCraciunARRajuNRHartN. Cerebrovascular complications associated with idiopathic hypertrophic subaortic stenosis. Stroke. (1984) 15:282–4. 10.1161/01.str.15.2.282
38.
WatsonTShantsilaELipGY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. (2009) 373:155–66. 10.1016/S0140-6736(09)60040-4
39.
FumagalliCBonanniFBeltramiMRuggieriRZocchiCTassettiLet alIncidence of stroke in patients with hypertrophic cardiomyopathy in stable sinus rhythm during long-term monitoring. Int J Cardiol. (2023) 381:70–5. 10.1016/j.ijcard.2023.04.008
40.
BernardiniACamporealeAPieroniMPieruzziFFigliozziSLusardiPet alAtrial dysfunction assessed by cardiac magnetic resonance as an early marker of fabry cardiomyopathy. JACC Cardiovasc Imaging. (2020) 13:2262–4. 10.1016/j.jcmg.2020.05.011
41.
CappelliFTiniGRussoDEmdinMDel FrancoAVergaroGet alArterial thrombo-embolic events in cardiac amyloidosis: a look beyond atrial fibrillation. Amyloid. (2021) 28:12–8. 10.1080/13506129.2020.1798922
42.
MoroniATondiLMilaniVPieroniMPieruzziFBevilacquaFet alLeft atrial remodeling in hypertrophic cardiomyopathy and Fabry disease: a CMR-based head-to-head comparison and outcome analysis. Int J Cardiol. (2023) 393:1–8. 10.1016/j.ijcard.2023.131357
43.
DominguezFClimentVZorioERipoll-VeraTSalazar-MendiguchíaJGarcía-PinillaJMet alDirect oral anticoagulants in patients with hypertrophic cardiomyopathy and atrial fibrillation. Int J Cardiol. (2017) 248:232–8. 10.1016/j.ijcard.2017.08.010
44.
JungHYangP-SJangEYuHTKimT-HUhmJ-Set alEffectiveness and safety of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation with hypertrophic cardiomyopathy: a nationwide cohort study. Chest. (2019) 155:354–63. 10.1016/j.chest.2018.11.009
45.
MillerCASMaronMSEstesNAMPriceLLRowinEJMaronBJet alSafety, side effects and relative efficacy of medications for rhythm control of atrial fibrillation in hypertrophic cardiomyopathy. Am J Cardiol. (2019) 123:1859–62. 10.1016/j.amjcard.2019.02.051
46.
AmmiratiEContriRCoppiniRCecchiFFrigerioMOlivottoI. Pharmacological treatment of hypertrophic cardiomyopathy: current practice and novel perspectives. Eur J Heart Fail. (2016) 18:1106–18. 10.1002/ejhf.541
47.
HarukiSMinamiYSuzukiAHagiwaraN. Effects of flecainide on left ventricular pressure gradient and symptoms in obstructive hypertrophic cardiomyopathy: a comparison of flecainide and disopyramide. Heart Vessels. (2015) 30:604–10. 10.1007/s00380-014-0534-3
48.
TzeisSGerstenfeldEPKalmanJSaadEBSepehri ShamlooAAndradeJGet al2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. (2024) 26:1–107. 10.1093/europace/euae043
49.
Van GelderICRienstraMBuntingKVCasado-ArroyoRCasoVCrijnsHJGMet al2024 ESC guidelines for the management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS). Eur Heart J. (2024) 45:3314–414. 10.1093/eurheartj/ehae176
50.
McCreadyJWSmedleyTLambiasePDAhsanSYSegalORRowlandEet alPredictors of recurrence following radiofrequency ablation for persistent atrial fibrillation. Europace. (2011) 13:355–61. 10.1093/europace/euq434
51.
SantangeliPDi BiaseLThemistoclakisSRavieleASchweikertRALakkireddyDet alCatheter ablation of atrial fibrillation in hypertrophic cardiomyopathy long-term outcomes and mechanisms of arrhythmia recurrence. Circ Arrhythm Electrophysiol. (2013) 6:1089–94. 10.1161/CIRCEP.113.000339
52.
ProvidenciaRElliottPPatelKMcCreadyJBabuGSrinivasanNet alCatheter ablation for atrial fibrillation in hypertrophic cardiomyopathy: a systematic review and meta-analysis. Heart. (2016) 102:1533–43. 10.1136/heartjnl-2016-309406
53.
CastagnoDDi DonnaPOlivottoIFronteraACalòLScaglioneMet alTranscatheter ablation for atrial fibrillation in patients with hypertrophic cardiomyopathy: long-term results and clinical outcomes. J Cardiovasc Electrophysiol. (2021) 32:657–66. 10.1111/jce.14880
54.
KassarAChamounNHaykalRChahineYAl YasiriHHensleyTet alImpact of catheter ablation on atrial fibrillation outcomes in various cardiomyopathies: findings from LGE-MRI quantified atrial fibrosis analysis. J Interv Card Electrophysiol. (2025):1–10. 10.1007/s10840-025-02027-6(e-pub ahead of print).
55.
FarazFRehmanMEUSabirBGhaffarAIftikharAMaqsoodAet alEfficacy of catheter ablation for atrial fibrillation in hypertrophic cardiomyopathy: a systematic review and meta-analysis. Curr Probl Cardiol. (2023) 48:1–23. 10.1016/j.cpcardiol.2022.101524
56.
CretaAElliottPEarleyMJDhinojaMFinlayMSportonSet alCatheter ablation of atrial fibrillation in patients with hypertrophic cardiomyopathy: a European observational multicentre study. Europace. (2021) 23:1409–17. 10.1093/europace/euab022
57.
StarekZDi CoriABettsTRClericiGGrasDLyanEet alBaseline left atrial low-voltage area predicts recurrence after pulmonary vein isolation: WAVE-MAP AF results. Europace. (2023) 25:1–11. 10.1093/europace/euad194
58.
KinjoTKimuraMHoriuchiDItohTIshidaYNishizakiKet alComparing cryoballoon and contact-force guided radiofrequency ablation in pulmonary vein isolation for atrial fibrillation in patients with hypertrophic cardiomyopathy. J Interv Card Electrophysiol. (2024) 67:1635–45. 10.1007/s10840-024-01822-x
59.
EfremidisMBazoukisGVlachosKPrappaEAnastasakisAMegarisiotouAet alAtrial substrate characterization in patients with atrial fibrillation and hypertrophic cardiomyopathy: evidence for an extensive fibrotic disease. J Electrocardiol. (2021) 69:87–92. 10.1016/j.jelectrocard.2021.06.001
60.
LapennaEPozzoliADe BonisMLa CannaGNisiTNascimbeneSet alMid-term outcomes of concomitant surgical ablation of atrial fibrillation in patients undergoing cardiac surgery for hypertrophic cardiomyopathy. Eur J Cardiothorac Surg. (2017) 51:1112–8. 10.1093/ejcts/ezx017
61.
TaniTTanabeKOnoMYamaguchiKOkadaMSumidaTet alLeft atrial volume and the risk of paroxysmal atrial fibrillation in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. (2004) 17:644–8. 10.1016/j.echo.2004.02.010
62.
YangW-IShimCYKimYJKimS-ARheeSJChoiE-Yet alLeft atrial volume index: a predictor of adverse outcome in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. (2009) 22:1338–43. 10.1016/j.echo.2009.09.016
63.
HiemstraYLDebonnaire,PBootsmaMvan Zwet,EWDelgado,VSchalijMJet alGlobal longitudinal strain and left atrial volume Index provide incremental prognostic value in patients with hypertrophic cardiomyopathy. Circ Cardiovasc Imaging. (2017) 10:1–9. 10.1161/CIRCIMAGING.116.005706
64.
PhilipsonDJRaderFSiegelRJ. Risk factors for atrial fibrillation in hypertrophic cardiomyopathy. Eur J Prev Cardiol. (2021) 28:658–65. 10.1177/2047487319828474
65.
ChungH. Emerging indicators of left atrial function evaluation considering the unique characteristics of hypertrophic cardiomyopathy. J Cardiovasc Imaging. (2023) 31:49–50. 10.4250/jcvi.2022.0116
66.
D'AscenziFAnselmiFFocardiMMondilloS. Atrial enlargement in the athlete’s heart: assessment of atrial function may help distinguish adaptive from pathologic remodeling. J Am Soc Echocardiogr. (2018) 31:148–57. 10.1016/j.echo.2017.11.009
67.
CecchiFMontereggiAOlivottoIMarconiPDolaraAMaronBJ. Risk for atrial fibrillation in patients with hypertrophic cardiomyopathy assessed by signal averaged P wave duration. Heart. (1997) 78:44–9. 10.1136/hrt.78.1.44
68.
MeucciMCFortuniFGallooXBootsmaMCreaFBaxJJet alLeft atrioventricular coupling index in hypertrophic cardiomyopathy and risk of new-onset atrial fibrillation. Int J Cardiol. (2022) 363:87–93. 10.1016/j.ijcard.2022.06.017
69.
ZegkosTKamperidisVGossiosTNteliosDParcharidouDPapanastasiouCAet alMitral regurgitation impact on left atrial myopathy in hypertrophic cardiomyopathy. Echocardiography. (2022) 39:819–26. 10.1111/echo.15370
70.
TjahjadiCHiemstraYLvan der BijlPPioSMBootsmaMAjmone MarsanNet alAssessment of left atrial electro-mechanical delay to predict atrial fibrillation in hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. (2021) 22:589–96. 10.1093/ehjci/jeaa174
71.
GanGCHFerkhABoydAThomasL. Left atrial function: evaluation by strain analysis. Cardiovasc Diagn Ther. (2018) 8:29–46. 10.21037/cdt.2017.06.08
72.
AlyMFABrouwerWPKleijnSAvan RossumACKampO. Three-dimensional speckle tracking echocardiography for the preclinical diagnosis of hypertrophic cardiomyopathy. Int J Cardiovasc Imaging. (2014) 30:523–33. 10.1007/s10554-014-0364-5
73.
HussainKNsoNTsourdinisGHaiderSMianRSanagalaTet alA systematic review and meta-analysis of left atrial strain in hypertrophic cardiomyopathy and its prognostic utility. Curr Probl Cardiol. (2024) 49:1–9. 10.1016/j.cpcardiol.2023.102146
74.
LisiMMandoliGECameliMPastoreMCRighiniFMBenfariGet alLeft atrial strain by speckle tracking predicts atrial fibrosis in patients undergoing heart transplantation. Eur Heart J Cardiovasc Imaging. (2022) 23:829–35. 10.1093/ehjci/jeab106
75.
RamanBSmillieRWMahmodMChanKArigaRNikolaidouCet alIncremental value of left atrial booster and reservoir strain in predicting atrial fibrillation in patients with hypertrophic cardiomyopathy: a cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. (2021) 23:1–14. 10.1186/s12968-021-00793-6
76.
KhanHRYakupogluHYKralj-HansIHaldarSBahramiTClagueJet alLeft atrial function predicts atrial arrhythmia recurrence following ablation of long-standing persistent atrial fibrillation. Circ Cardiovasc Imaging. (2023) 16:E015352. 10.1161/CIRCIMAGING.123.015352
77.
DebonnairePJoyceEHiemstraYMertensBJAtsmaDESchalijMJet alLeft atrial size and function in hypertrophic cardiomyopathy patients and risk of new-onset atrial fibrillation. Circ Arrhythm Electrophysiol. (2017) 10:1–10. 10.1161/CIRCEP.116.004052
78.
ZegkosTNteliosDParcharidouDKatranasSPanagiotidisTPapanastasiouCAet alThe predictive value of left ventricular and left atrial mechanics for atrial fibrillation and heart failure in hypertrophic cardiomyopathy: a prospective cohort study. Int J Cardiovasc Imaging. (2021) 37:2679–90. 10.1007/s10554-021-02232-0
79.
AbozguiaKNallur-ShivuGPhanTTAhmedIKalraRWeaverRAet alLeft ventricular strain and untwist in hypertrophic cardiomyopathy: relation to exercise capacity. Am Heart J. (2010) 159:825–32. 10.1016/j.ahj.2010.02.002
80.
OakesRSBadgerTJKholmovskiEGAkoumNBurgonNSFishENet alDetection and quantification of left atrial structural remodeling with delayed-enhancement magnetic resonance imaging in patients with atrial fibrillation. Circulation. (2009) 119:1758–67. 10.1161/CIRCULATIONAHA.108.811877
81.
KuppahallySSAkoumNBurgonNSBadgerTJKholmovskiEGVijayakumarSet alLeft atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging. (2010) 3:231–9. 10.1161/CIRCIMAGING.109.865683
82.
MaronBJHaasTSMaronMSLesserJRBrowningJAChanRHet alLeft atrial remodeling in hypertrophic cardiomyopathy and susceptibility markers for atrial fibrillation identified by cardiovascular magnetic resonance. Am J Cardiol. (2014) 113:1394–400. 10.1016/j.amjcard.2013.12.045
83.
SivalokanathanSZghaibTGreenlandGVVasquezNKudchadkarSMKontariEet alHypertrophic cardiomyopathy patients with paroxysmal atrial fibrillation have a high burden of left atrial fibrosis by cardiac magnetic resonance imaging. JACC Clin Electrophysiol. (2019) 5:364–75. 10.1016/j.jacep.2018.10.016
84.
TruongVTPalmerCWolkingSSheetsBYoungMNgoTNMet alNormal left atrial strain and strain rate using cardiac magnetic resonance feature tracking in healthy volunteers. Eur Heart J Cardiovasc Imaging. (2020) 21:446–53. 10.1093/ehjci/jez157
85.
MarklMLeeDCNgJCarrMCarrJGoldbergerJJ. Left atrial 4-dimensional flow magnetic resonance imaging stasis and velocity mapping in patients with atrial fibrillation. Invest Radiol. (2016) 51:147–54. 10.1097/RLI.0000000000000219
86.
MarsigliaJDCCredidioFLde OliveiraTGMReisRFAntunesMdOde AraujoAQet alScreening of MYH7, MYBPC3, and TNNT2 genes in Brazilian patients with hypertrophic cardiomyopathy. Am Heart J. (2013) 166:775–82. 10.1016/j.ahj.2013.07.029
87.
ButtersAIsbisterJCMediCRajuHTurnerCSyRWet alEpidemiology and clinical characteristics of atrial fibrillation in patients with inherited heart diseases. J Cardiovasc Electrophysiol. (2020) 31:465–73. 10.1111/jce.14346
88.
YonedaZTAndersonKCYeFQuintanaJAO’NeillMJSimsRAet alMortality among patients with early-onset atrial fibrillation and rare variants in cardiomyopathy and arrhythmia genes. JAMA Cardiol. (2022) 7:733–41. 10.1001/jamacardio.2022.0810
89.
HaqIUAkhiyatNAl-ShakarchiNSiontisKCMulpuruSKSugrueAet alAtrial fibrillation substrate and catheter ablation outcomes in MYBPC3- and MYH7-mediated hypertrophic cardiomyopathy. Clin Electrophysiol. (2024) 10:1380–91. 10.1016/J.JACEP.2024.03.026
90.
LeeS-PAshleyEAHomburgerJCaleshuCGreenEMJacobyDet alIncident atrial fibrillation is associated with MYH7 sarcomeric gene variation in hypertrophic cardiomyopathy. Circ Heart Fail. (2018) 11:e005191. 10.1161/CIRCHEARTFAILURE.118.005191
91.
James GruverEFatkinDDoddsGAKissloJMaronBJSeidmanJGet alFamilial hypertrophic cardiomyopathy and atrial fibrillation caused by Arg663His Beta-cardiac myosin heavy chain mutation. Am J Cardiol. (1999) 83:13H–8H. 10.1016/s0002-9149(99)00251-9
92.
CoppiniRHoCYAshleyEDaySFerrantiniCGirolamiFet alClinical phenotype and outcome of hypertrophic cardiomyopathy associated with thin-filament gene mutations. J Am Coll Cardiol. (2014) 64:2589–600. 10.1016/j.jacc.2014.09.059
93.
GirolamiFIasconeMTomberliBBardiSBenelliMMarsegliaGet alNovel α-actinin 2 variant associated with familial hypertrophic cardiomyopathy and juvenile atrial arrhythmias: a massively parallel sequencing study. Circ Cardiovasc Genet. (2014) 7:741–50. 10.1161/CIRCGENETICS.113.000486
94.
Orenes-PineroEHernandez-RomeroDRomero-AniorteAIMartinezMGarcia-HonrubiaACaballeroLet alPrognostic value of two polymorphisms in non-sarcomeric genes for the development of atrial fibrillation in patients with hypertrophic cardiomyopathy. QJM. (2014) 107:613–21. 10.1093/qjmed/hcu046
95.
LiuLWuLZhengLDingLChenGFanXet alAssociations between multiple circulating biomarkers and the presence of atrial fibrillation in hypertrophic cardiomyopathy with or without left ventricular outflow tract obstruction. Int Heart J. (2019) 60:327–35. 10.1536/ihj.18-438
96.
GuoYLipGYHApostolakisS. Inflammation in atrial fibrillation. J Am Coll Cardiol. (2012) 60:2263–70. 10.1016/j.jacc.2012.04.063
97.
MonteiroPFOmmenSRGershBJDearaniJASchaffHVNishimuraRAet alEffects of surgical septal myectomy on left ventricular wall thickness and diastolic filling. Am J Cardiol. (2007) 100:1776–8. 10.1016/j.amjcard.2007.07.031
98.
MenonSCAckermanMJOmmenSRCabalkaAKHaglerDJO'LearyPWet alImpact of septal myectomy on left atrial volume and left ventricular diastolic filling patterns: an echocardiographic study of young patients with obstructive hypertrophic cardiomyopathy. J Am Soc Echocardiogr. (2008) 21:684–8. 10.1016/j.echo.2007.11.006
99.
MoravskyGBruchal-GarbiczBJamorskiMRalph-EdwardsAGrunerCWilliamsLet alMyocardial mechanical remodeling after septal myectomy for severe obstructive hypertrophic cardiomyopathy. J Am Soc Echocardiogr. (2013) 26:893–900. 10.1016/j.echo.2013.05.012
100.
NguyenASchaffHVNishimuraRADearaniJAGeskeJBLahrBDet alDeterminants of reverse remodeling of the left atrium after transaortic myectomy. Ann Thorac Surg. (2018) 106:447–53. 10.1016/j.athoracsur.2018.03.039
101.
FinocchiaroGHaddadFKobayashiYLeeDPavlovicASchnittgerIet alImpact of septal reduction on left atrial size and diastole in hypertrophic cardiomyopathy. Echocardiography. (2016) 33:686–94. 10.1111/echo.13158
102.
Bogachev-ProkophievAAfanasyevAZheleznevSFomenkoMSharifulinRKretovEet alMitral valve repair or replacement in hypertrophic obstructive cardiomyopathy: a prospective randomized study. Interact Cardiovasc Thorac Surg. (2017) 25:356–62. 10.1093/icvts/ivx152
103.
FinocchiaroGHaddadFPavlovicASinagraGSchnittgerIKnowlesJWet alLatent obstruction and left atrial size are predictors of clinical deterioration leading to septal reduction in hypertrophic cardiomyopathy. J Card Fail. (2014) 20:236–43. 10.1016/j.cardfail.2014.01.014
104.
RowinEJMaronBJOlivottoIMaronMS. Role of exercise testing in hypertrophic cardiomyopathy. JACC Cardiovasc Imaging. (2017) 10:1374–86. 10.1016/j.jcmg.2017.07.016
105.
OlivottoIOreziakABarriales-VillaRAbrahamTPMasriAGarcia-PaviaPet alMavacamten for treatment of symptomatic obstructive hypertrophic cardiomyopathy (EXPLORER-HCM): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. (2020) 396:759–69. 10.1016/S0140-6736(20)31792-X
106.
HoCYMealiffeMEBachRGBhattacharyaMChoudhuryLEdelbergJMet alEvaluation of mavacamten in symptomatic patients with nonobstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. (2020) 75:2649–60. 10.1016/j.jacc.2020.03.064
107.
DesaiMYOwensAWolskiKGeskeJBSaberiSWangAet alMavacamten in patients with hypertrophic cardiomyopathy referred for septal reduction: week 56 results from the VALOR-HCM randomized clinical trial. JAMA Cardiol. (2023) 8:968–77. 10.1001/jamacardio.2023.3342
108.
TianZLiLLiXWangJZhangQLiZet alEffect of mavacamten on chinese patients with symptomatic obstructive hypertrophic cardiomyopathy: the EXPLORER-CN randomized clinical trial. JAMA Cardiol. (2023) 8:957–65. 10.1001/jamacardio.2023.3030
109.
LiaoHLLiangYLiangB. Evaluation of mavacamten in patients with hypertrophic cardiomyopathy. J Cardiovasc Med (Hagerstown). (2024) 25:491–8. 10.2459/JCM.0000000000001638
110.
CoatsCJMaronMSAbrahamTPOlivottoILeeMMYAradMet alExercise capacity in patients with obstructive hypertrophic cardiomyopathy: SEQUOIA-HCM baseline characteristics and study design. JACC Heart Fail. (2024) 12:199–215. 10.1016/j.jchf.2023.10.004
111.
SaberiSCardimNYamaniMSchulz-MengerJLiWFloreaVet alMavacamten favorably impacts cardiac structure in obstructive hypertrophic cardiomyopathy: EXPLORER-HCM cardiac magnetic resonance substudy analysis. Circulation. (2021) 143:606–8. 10.1161/CIRCULATIONAHA.120.052359
112.
CremerPCGeskeJBOwensAJaberWAHarbSCSaberiSet alMyosin inhibition and left ventricular diastolic function in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy: insights from the VALOR-HCM study. Circ Cardiovasc Imaging. (2022) 15:E014986. 10.1161/CIRCIMAGING.122.014986
113.
HaKEChoiKLeeHGwakSKimKChoIet alEffects of septal myectomy on left atrial and left ventricular function in obstructive hypertrophic cardiomyopathy. ESC Heart Fail. (2023) 10:2939–47. 10.1002/ehf2.14481
114.
HegdeSMLesterSJSolomonSDMichelsMElliottPMNaguehSFet alEffect of mavacamten on echocardiographic features in symptomatic patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. (2021) 78:2518–32. 10.1016/j.jacc.2021.09.1381
115.
WesslyPLazzaraGEBuerglerJMNaguehSF. Early observations on effects of mavacamten on left atrial function in obstructive hypertrophic cardiomyopathy patients. JACC Cardiovasc Imaging. (2023) 16:1633–4. 10.1016/j.jcmg.2023.06.008
116.
CastrichiniMAlsidawiSGeskeJBNewmanDBArruda-OlsonAMBosJMet alIncidence of newly recognized atrial fibrillation in patients with obstructive hypertrophic cardiomyopathy treated with Mavacamten. Heart Rhythm. (2024) 21:2065–7. 10.1016/j.hrthm.2024.04.055
117.
AhlbergGRefsgaardLLundegaardPRAndreasenLRantheMFLinscheidNet alRare truncating variants in the sarcomeric protein titin associate with familial and early-onset atrial fibrillation. Nat Commun. (2018) 9:1–11. 10.1038/s41467-018-06618-y
118.
VadOBMonfortLMPaludan-MüllerCKahnertKDiederichsenSZAndreasenLet alRare and common genetic variation underlying atrial fibrillation risk. JAMA Cardiol. (2024) 9:732–40. 10.1001/jamacardio.2024.1528
119.
GudbjartssonDFHolmHSulemPMassonGOddssonAMagnussonOTet alA frameshift deletion in the sarcomere gene MYL4 causes early-onset familial atrial fibrillation. Eur Heart J. (2017) 38:27–34. 10.1093/EURHEARTJ/EHW379
120.
GaoGLiuGChenWTongYMaoCLiuJet alA novel nonsense mutation in TNNT2 in a Chinese pedigree with hypertrophic cardiomyopathy: a case report. Medicine. (2020) 99:E21843. 10.1097/MD.0000000000021843
121.
PionerJMVitaleGGentileFScelliniBPiroddiNCerbaiEet alGenotype-driven pathogenesis of atrial fibrillation in hypertrophic cardiomyopathy: the case of different TNNT2 mutations. Front Physiol. (2022) 13:864547. 10.3389/fphys.2022.864547
122.
BeaversDLWangWAtherSVoigtNGarbinoADixitSSet alMutation E169K in junctophilin-2 causes atrial fibrillation due to impaired RyR2 stabilization. J Am Coll Cardiol. (2013) 62:2010–9. 10.1016/j.jacc.2013.06.052
123.
CavigliLFumagalliCMauriziNRossiAArretiniATargettiMet alTiming of invasive septal reduction therapies and outcome of patients with obstructive hypertrophic cardiomyopathy. Int J Cardiol. (2018) 273:155–61. 10.1016/j.ijcard.2018.09.004
Summary
Keywords
hypertrophic cardiomyopathy, atrial myopathy, multimodal imaging, atrial fibrillation, septal reduction therapy, cardiac myosin inhibitors
Citation
Piazzai C, Petrone A, Stefanini A, D’Ascenzi F, Olivotto I and Cameli M (2025) Atrial remodelling and dysfunction in hypertrophic cardiomyopathy: prognostic role and therapeutic target. Front. Cardiovasc. Med. 12:1620313. doi: 10.3389/fcvm.2025.1620313
Received
29 April 2025
Accepted
25 June 2025
Published
08 July 2025
Volume
12 - 2025
Edited by
Ahmed Abuzaid, Providence Alaska Medical Center, United States
Reviewed by
Aadhavi Sridharan, Banner - University Medical Center Tucson, United States
Danuta Szczesna-Cordary, University of Miami, United States
Updates
Copyright
© 2025 Piazzai, Petrone, Stefanini, D’Ascenzi, Olivotto and Cameli.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alessio Petrone alessiopetrone5@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.