Abstract
Congenital absence of the pericardium (CAP) is a rare cardiac anomaly with an estimated prevalence of <1:10,000. CAP results from premature atrophy of the left common cardinal vein during embryogenesis, leading to pericardial defects. In this report, the case of a 39-year-old male with recurrent left-sided chest tightness who was initially misdiagnosed with pulmonary embolism (PE) is presented. Anticoagulation failed to resolve symptoms, prompting advanced imaging and multidisciplinary team review, which confirmed CAP. Conservative management was chosen because of mild symptoms and low herniation risk. This case underscores the diagnostic complexity of CAP and highlights the role of advanced imaging in differentiating CAP from PE. Clinicians should consider CAP in patients with nonspecific cardiac symptoms and imaging findings of cardiac displacement or abnormal mobility.
Introduction
CAP is a rare congenital anomaly linked to abnormal embryonic pericardial development (1). Approximately 70% of cases involve partial or complete left-sided defects, whereas bilateral or right-sided defects account for <1% of cases (2). Up to 50% of patients are asymptomatic, with CAP often incidentally detected during imaging (3). Symptomatic patients may present with chest discomfort, dyspnoea, or palpitations due to cardiac displacement or coronary compression. Life-threatening complications (e.g., cardiac herniation) are rare but possible (2). Imaging examination is necessary for confirmation of the diagnosis; chest x-ray and echocardiography findings may indicate cardiac displacement, whereas CT angiography or MRI confirms pericardial defects (4). Management ranges from monitoring for asymptomatic patients to surgical repair for high-risk patients.
Here, a case of CAP initially misdiagnosed as PE is presented. We analyse critical diagnostic and management decisions and review embryology, imaging features, and therapeutic advances to improve the clinical recognition of CAP.
Case report
A 39-year-old man presented with two months of intermittent left-sided chest tightness (5-minute episodes, 2–3 times/month). Prior coronary angiography findings were normal, whereas CT pulmonary angiography findings suggested left lower lobar PE. Right heart catheterization revealed normal pulmonary pressure. Despite therapeutic rivaroxaban (15 mg twice daily) for 4 weeks, his symptoms intermittently persisted. Physical examination revealed a laterally displaced apical impulse (6th intercostal space, midclavicular line). Vital signs and laboratory results (NT-proBNP, CBC, and renal/hepatic function indicators) were unremarkable. Electrocardiography revealed sinus rhythm with incomplete right bundle branch block and T-wave abnormalities (Figure 1A). Echocardiography revealed right ventricular enlargement (52 mm; normal 20–42 mm) and mild mitral regurgitation. Chest x-ray revealed left ventricular elongation (“Snoopy sign”; Figure 1B).
Figure 1
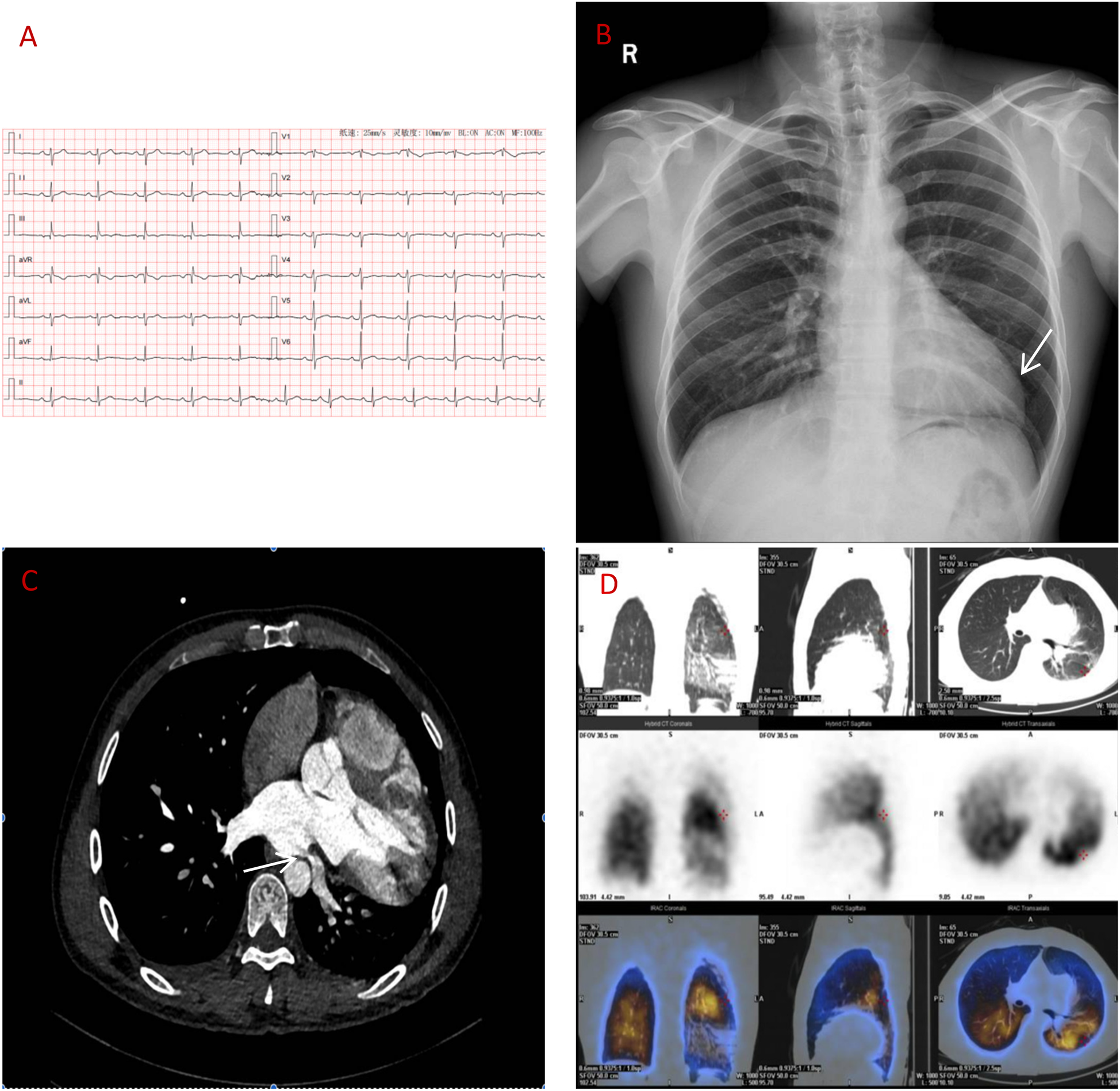
(A) ECG: sinus rhythm, incomplete right bundle branch block. (B) Chest x-ray: elongated left ventricular contour. (C) CT angiography: left atrial compression of the left lower pulmonary vein (over 90%). (D) Lung perfusion scan: low perfusion in the left lower lobe.
Despite initial imaging suggesting PE, persistent symptoms and negative D-dimer levels contradict typical PE progression. Repeat CT angiography revealed right ventricular enlargement and mild left lower pulmonary vein narrowing (Figure 1C). A lung perfusion scan revealed a small wedge-shaped perfusion defect in the dorsal segment of the left lower lobe, suggesting pulmonary embolism (Figure 1D). Right heart catheterization revealed normal pulmonary artery pressure and vascular resistance. Pulmonary angiography revealed no filling defects or branch truncations in the right pulmonary artery (Pulmonary flow grade 3) or left pulmonary artery. However, delayed contrast flow in the left lower pulmonary artery and venous return (Pulmonary flow grade 2) provided insufficient evidence for pulmonary embolism (5). A multidisciplinary team review revealed leftward cardiac rotation, discontinuity of the left pericardium (Figure 2), and left atrial compression of the lower pulmonary vein, which were consistent with CAP. Given the mild symptoms and low degree of herniation risk, conservative management with follow-up was chosen. At 3-month follow-up (July 2025), the patient remains asymptomatic. This case uniquely highlights pulmonary venous compression due to cardiac displacement-a previously unreported manifestation of CAP-suggesting its clinical variability.
Figure 2
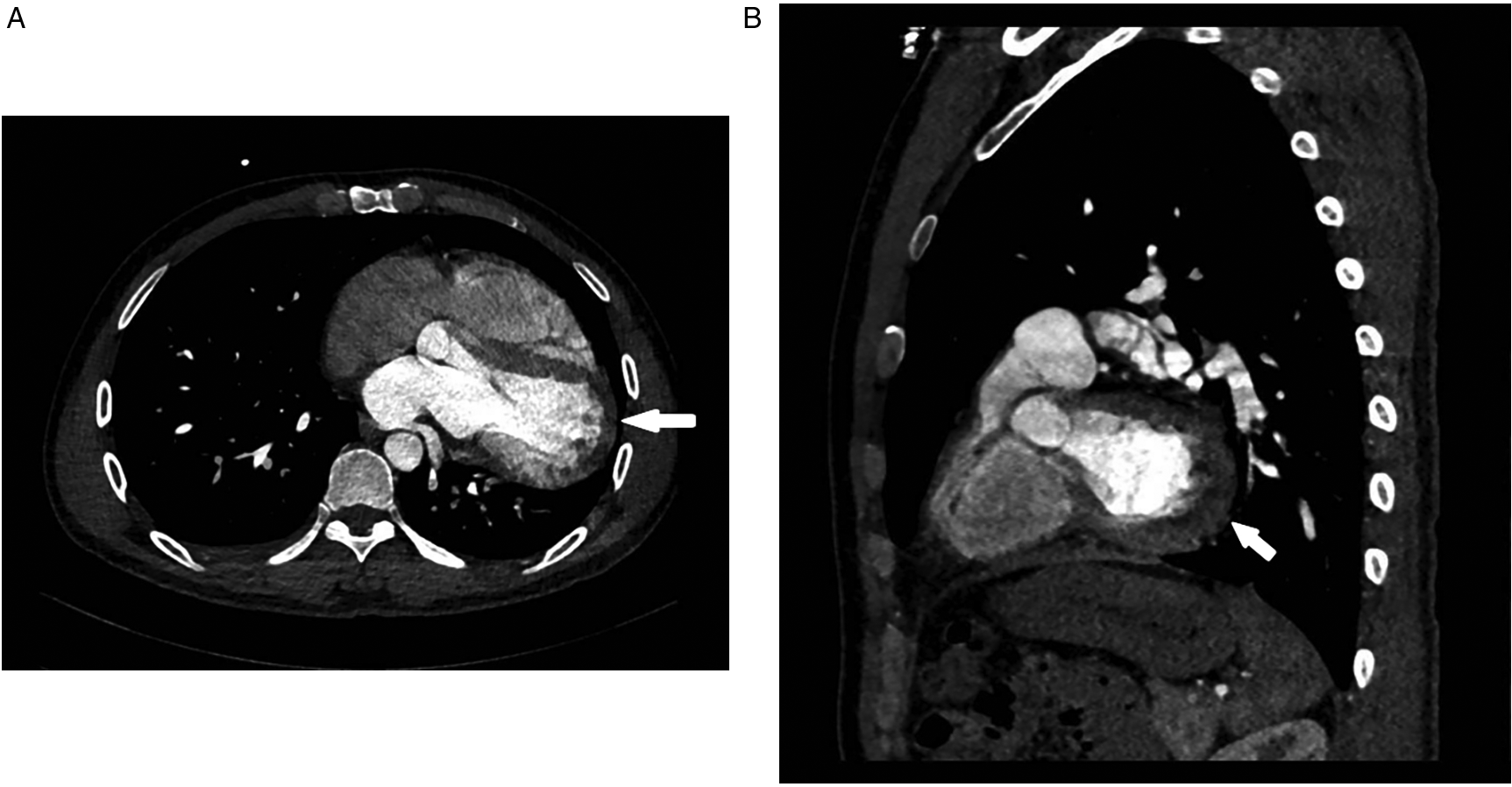
Ct angiography [axial (A), sagittal (B) planes]: absence of the left pericardium.
Discussion
CAP arises from abnormal embryological pericardial development, primarily due to premature regression of the left common cardinal vein (1). The clinical heterogeneity of CAP likely reflects anatomical variations. While 30%–50% of patients remain asymptomatic, common presentations include nonspecific chest pain, dyspnoea, or palpitations. Prior studies emphasize complications such as cardiac displacement, chest pain, coronary compression, and sudden death (6–9); in recent reports aortic dissection (10), ventricular fibrillation (11), and Kommerell diverticulum are described (12). This case uniquely demonstrates left atrial compression of the lower pulmonary vein due to cardiac displacement—a direct consequence of CAP. Although advanced PE management strategies (e.g., catheter-directed thrombolysis) and inflammatory contributors to thrombogenesis are critical in true PE (13), such therapies remain irrelevant here, given the definitive exclusion of thrombotic disease by pulmonary angiography and persistently negative D-dimer. This mechanistic contrast—anatomical compression vs. inflammatory thrombogenesis—underscores CAP's potential to haemodynamically mimic PE while demanding divergent management.
Physical examination, ECG, chest x-ray, and echocardiography are necessary for CAP diagnosis, with confirmation through cardiac CT angiography or MRI. Physical findings typically include leftward displacement of the apical impulse and splitting of the second heart sound, likely owing to cardiac rotation and increased pulmonary artery flow. ECG typically reveals right axis deviation, incomplete or complete right bundle branch block, and poor R-wave progression in precordial leads due to vagal stimulation (2). ST-segment elevation may occur if coronary arteries are compressed by herniated myocardium. Chest x-ray often reveals leftward cardiac rotation, elongated left ventricular contour (“Snoopy sign”), and increased lucency of the aortopulmonary window. However, these findings lack specificity; the absence of tracheal deviation despite cardiac rotation is a distinctive feature (14). Echocardiographic clues include: (1) abnormal acoustic windows, (2) right ventricular enlargement, (3) hypermobile cardiac motion, and (4) abnormal septal movement (15). Right ventricular enlargement may result from increased ventricular compliance due to pericardial absence or cardiac displacement. Cardiac CT angiography or MRI is definitive: CT angiography visualizes pericardial discontinuity and cardiac displacement, whereas MRI dynamically assesses cardiac motion. Combined use increases diagnostic accuracy (4).
There are no standardized treatment guidelines for CAP. Asymptomatic patients are managed conservatively with regular monitoring (e.g., echocardiography or CT angiography every 1–2 years; prompt CT angiography/MRI if new murmurs or worsening symptoms arise) and avoidance of strenuous activity to prevent cardiac herniation (16). Surgical intervention is indicated for patients with the following (17): ① high risk of herniation (defect >3 cm), ② refractory chest pain, or ③ coexisting cardiac anomalies requiring repair. The procedures included pericardioplasty or pericardiectomy.
Conclusion
This case underscores the importance of integrating advanced imaging and multidisciplinary evaluation to differentiate CAP from PE, particularly when anticoagulation fails. Pulmonary venous compression represents a novel mechanism of CAP-related symptoms, warranting inclusion in diagnostic guidelines.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
GJ: Writing – original draft, Formal analysis, Resources, Visualization, Funding acquisition, Project administration, Data curation, Methodology, Supervision, Writing – review & editing, Software, Conceptualization, Investigation, Validation. YF: Methodology, Formal analysis, Supervision, Writing – original draft, Project administration, Software, Writing – review & editing, Data curation, Investigation, Resources, Conceptualization, Visualization, Funding acquisition, Validation. LC: Data curation, Formal analysis, Writing – review & editing, Project administration, Writing – original draft, Validation, Methodology, Visualization, Investigation, Supervision, Conceptualization, Software, Resources, Funding acquisition. LM: Supervision, Methodology, Investigation, Writing – review & editing, Validation, Data curation, Conceptualization, Software, Visualization, Formal analysis, Writing – original draft, Resources, Funding acquisition, Project administration. JX: Validation, Conceptualization, Methodology, Supervision, Data curation, Investigation, Writing – original draft, Writing – review & editing, Funding acquisition, Visualization, Project administration, Formal analysis, Software, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study is supported by Research on the effect of USP11 on structural remodeling of mouse heart failure (Grant number XJ2023003901).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Amado-EscañuelaMGJiménez López-GuarchCSolís MartínJ. A “heart” within a heart: partial absence of the pericardium. Rev Esp Cardiol (Engl Ed). (2020) 73(12):1062. 10.1016/j.rec.2020.05.014
2.
JaglanALeybishkisBTajikAJ. Topsy-turvy heart: a case of congenital absence of the pericardium. Eur Heart J Cardiovasc Imaging. (2020) 21(3):269. 10.1093/ehjci/jez315
3.
SchicchiN. Congenital absence of pericardium: the swinging heart. J Imaging. (2024) 10(8):199. 10.3390/jimaging10080199
4.
WilsonSRKronzonIMachnickiSCRuizCE. A constrained heart: a case of sudden onset unrelenting chest pain. Circulation. (2014) 130(18):1625–31. 10.1161/CIRCULATIONAHA.114.011410
5.
KonstantinidesSVMeyerGBecattiniCBuenoHPepke-ZabaJ. 2019 ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European respiratory society (ERS). Eur Heart J. (2019) 41(4):543–603. 10.1093/eurheartj/ehz405
6.
RorrisFPTsoutsinosAKanakisM. Absent pericardium causing extreme levoposition in a child. Clin Case Rep. (2025) 13(1):e70047. 10.1002/ccr3.70047
7.
LiXYJiangYLiHWLiuYKBaiJ. Congenital absence of the left pericardium: a case report. BMC Cardiovasc Disord. (2023) 23(1):247. 10.1186/s12872-023-03262-3
8.
ParchakeMBPathakHVidhateSSukhadeveRTumramNKambleR. Sudden death due to lethal strangulation of heart by congenital partial pericardium. Egypt J Forensic Sci. (2016) 6(4):520–3. 10.1016/j.ejfs.2016.06.002
9.
AlyamiBAlharbiAAlhajjiMGendiSHamiraniYS. A case report of congenital absence of the pericardium that was diagnosed by cardiac computed tomography angiogram (CCTA). Radiol Case Rep. (2022) 17(9):3380–4. 10.1016/j.radcr.2022.06.066
10.
NishimotoKUmegakiTOhiraSNakajumaYSoedaTKamibayahiT. Relief of cardiac tamponade by a congenital partial left-sided pericardial defect in a patient with ruptured acute type A aortic dissection: a case report. JA Clinical Reports. (2019) 5:4. 10.1186/s40981-019-0223-4
11.
WangGCLiXRHuangNTianHT. Sudden ventricular fibrillation due to absence of pericardium in left upper lobectomy-a case report. Korean J Anesthesiol. (2024) 77(3):401–4. 10.4097/kja.23625
12.
SaniZASavand-RoomiZVojdanparastMSarafanSSeiflANezafatiP. Congenital partial absence of the pericardium presenting with a rare concurrent abnormality of vascular ring diagnosed by cardiac magnetic resonance imaging. Adv Biomed Res. (2016) 5(1):203. 10.4103/2277-9175.192630
13.
KorosoglouGMouselimisDKoenigEKonstantinidesS. Ultrasound-assisted catheter-directed thrombolysis in a patient with COVID-19 infection and bilateral intermediate-to-high-risk pulmonary embolism: a case report. Eur Heart J Case Rep. (2024) 8(1):ytad628. 10.1093/ehjcr/ytad628
14.
HanJXiangHRidleyWERidleyLJ. Snoopy sign: congenital absence of the left pericardium. J Med Imaging Radiat Oncol. (2018) 62:47. 10.1111/1754-9485.33_12785
15.
IgnaszewskiMBaturinBWaldmanBPriscillaP. Cardiac eclipse: congenital absence of the pericardium manifesting as atypical chest pain. CASE. (2019) 4(2):59–62. 10.1016/j.case.2019.07.005
16.
SharmaRBertacchiJJaafarNPorterfieldJ. A missed diagnosis: a case of partial pericardial defect. Clin Res Cardiol. (2025). 10.1007/s00392-025-02659-8
17.
SonaglioniANicolosiGLTrevisanRLombardoMGrassoEGensiniGFet alThe influence of pectus excavatum on cardiac kinetics and function in otherwise healthy individuals: a systematic review. Int J Cardiol. (2023) 381:10–9. 10.1016/j.ijcard.2023.03.058
Summary
Keywords
congenital absence of the pericardium, pulmonary embolism, congenital heart disease, pericardium, case report
Citation
Junqi G, Fengyou Y, Chaohui L, Mingjian L and Xiaobo J (2025) Case Report: The phantom gap: a case of congenital pericardial absence revealed by advanced imaging. Front. Cardiovasc. Med. 12:1624625. doi: 10.3389/fcvm.2025.1624625
Received
08 May 2025
Accepted
25 July 2025
Published
12 August 2025
Volume
12 - 2025
Edited by
Grigorios Korosoglou, GRN Klinik Weinheim, Germany
Reviewed by
Sorin Giusca, GRN Klinik Weinheim, Germany
Petar Kalaydzhiev, Medical University Sofia, Bulgaria
Updates
Copyright
© 2025 Junqi, Fengyou, Chaohui, Mingjian and Xiaobo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiang Xiaobo jiangxiaobochengdu@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.