- Department of Endodontics, Advanced Educational Program in Endodontics, School of Dentistry, The University of Alabama at Birmingham, Birmingham, AL, United States
Introduction: An attempt to determine the association of a large array of inflammatory proteins in pulpitis with precise measurement of clinical signs and symptoms, and to correlate these findings with levels in peripheral blood has not been reported. Such an analysis could serve to identify key clinical findings and potential biomarkers to predict the prognosis of vital pulp therapy. The aim of this study was to undertake a preliminary, proof-of-concept study to correlate the levels of key inflammatory mediators in cariously exposed dental pulp of adults with reversible or irreversible pulpitis, and no apical periodontitis, with a panel of subjective and objective diagnostic clinical findings as well as the status of the pulp upon exposure. Pulpal and peripheral blood inflammatory mediators were also compared.
Methods: Dental pulp and peripheral blood were sampled. The Luminex technology was used to assess the expression of a panel of 45 inflammatory proteins to determine their association with clinical signs and symptoms of reversible or irreversible pulpitis.
Results: Data from three pulpal and three peripheral blood samples were used for the analysis. The correlation of levels of the 45 proteins in the inflamed dental pulp and peripheral blood was 0.87. The pulp had significantly higher levels of these proteins collectively than peripheral blood (t-test, p = 0.047). The following proteins had correlated at a level of ≥0.8 with the duration of pain with cold: MMP-12, MMP-9, RANTES, MIP-2, MCP-1, MMP-2, MMP-1, and P-Selectin. Relatively high correlations (0.5-0.75) were also present between these proteins and presenting pain level.
Conclusions: Several pulpal proteins correlated well with spontaneous and evoked pain parameters. Peripheral blood may not be necessary in future similar studies. Finally, additional data is needed to identify candidate proteins to be investigated as potential markers of truly irreversible pulp inflammation.
Introduction
Accurate diagnosis of the condition of the dental pulp allows the practitioner to perform effective treatment and advise the patient on the expected prognosis. According to a systematic review on the diagnostic accuracy of patient symptoms and clinical tests that are used to determine the condition of the dental pulp, there was lack of data and evidence from the literature to achieve this goal (1). Moreover, research in this area has focused primarily on the distinction between vital and necrotic pulp (1, 2), rather than identify the exact condition of the inflamed pulp and whether it could survive vital pulp therapy (VPT). However, patients with extensive caries or deep restorations that has minimal or no symptoms frequently undergo extensive restorative work with the uncertainty of whether the pulp will survive the procedures (3). At the same time, patients with moderate to severe pulpal symptoms, and no apical periodontitis, frequently receive endodontic treatment, although it has long been known that pulpotomy is effective in reducing pulpal symptoms (4–6), and may be equivalent or better in this respect than endodontic therapy (7).
Several older studies have described that the signs and symptoms of the inflamed pulp do not correlate with the histologic findings (8–12). In a more recent study (13), the clinical diagnosis of normal pulp/reversible pulpitis matched the histologic diagnosis in 96.6% of the time and of irreversible pulpitis 84.4%, respectively. The authors also noted that the inflammation and bacterial infection was often localized to the coronal pulp when the tooth responded as irreversible pulpitis. However, in that study, blinded histologic evaluation of all cases together was not reported, and an exhaustive list of signs and symptoms was provided for all symptomatic irreversible cases, presumably with different findings in different cases. Cases with asymptomatic irreversible pulpitis were not shown, but are known from another study to constitute up to 40% of pulpitis cases (3). In addition, the cases that were shown to illustrate irreversible pulpitis seemed to have the inflammation and necrosis localized to the pulp chamber. The prospective correlation of pulp status with exact patient symptoms using specific metrics, precise and timed responses to different pulp sensibility testing, as well as exact measurement of mechanical allodynia to rule out apical periodontitis (14, 15) has not been reported.
Recent data have contemplated the use of molecular mediator to identify the pulpal status (16, 17). The correlation of normal pulp, reversible and symptomatic irreversible pulpitis with protein (18), genetic (19) or epigenetic (20, 21) analyses has revealed a multitude of factors that can be used as biological markers of the pulp status. However, these studies still rely on symptoms to define the diagnostic condition when the symptoms are not necessarily predictive of eventual survival of the pulp. Moreover, the expression of proteins in the pulp represents the functional status of these proteins and can be used to establish the condition of inflammation more accurately than gene expression, at the time of sampling. The determination of levels of specific proteins may eventually give rise to the development of point-of-care tests that help the clinician in predicting the long-term prognosis of treatment. Studies are starting to emerge, in which molecular biomarkers at the protein level, such as matrix metalloprotein-9 (MMP-9) (22, 23) are sampled and correlated with the outcome of VPT. Only then, can the definitive diagnosis of the pulp at the time of treatment be known, and the correlation of the outcome of treatment with molecular markers at the time of treatment be determined.
There is currently a major shift in practice, in which VPT using tricalcium silicates has become very popular, as it was shown to be an effective treatment modality for adult patients (7, 24–27) and even for symptomatic cases (28). However, in order to proceed with clinical trials on VPT the appropriate seminal molecular markers need to be identified in the preliminary studies. Measuring a small number of highly expressed biological markers could help in differentiating between different inflammation stages and accurately predict the pulpal diagnosis and response to conservative treatment (17, 18). There are dozens of inflammatory mediators that may correlate with true irreversible pulpitis (1, 17). It is not known which one or combination of these mediators will be associated with the best prediction of pulp survival, following VPT. Furthermore, considering that ELISA is the most common assay for protein analysis, a relatively large sample of tissue would be needed to run these ELISA assays for many proteins, which is not feasible for studies on the dental pulp, in which the pulp will be preserved. The Luminex protein assay system is a relatively new technology that enables the multiplex screening of a large number of proteins simultaneously. Previous studies on the use of this system to examine pulpitis had limited use and a cross-sectional approach (29, 30).
Most studies in which pulpal mediators were assayed in a manner that would preserve the rest of the pulp sampled only pulpal blood. In VPT studies, investigators would be careful not to disrupt the remaining pulp as the intent is to preserve its vitality. Hence, it is not known how the levels of inflammatory mediators in these samples may compare with those in peripheral blood.
Therefore, the purpose of this proof of concept methodology proposal, with pilot data, was to correlate the levels of 45 key inflammatory mediators in cariously exposed dental pulp of adults with reversible or irreversible pulpitis, and no apical periodontitis, with a panel of subjective and objective diagnostic clinical findings as well as the status of the pulp upon exposure. An additional aim was to determine the correlation of the levels of pulpal mediators in these cases with their levels in peripheral blood.
Materials and Methods
The clinical procedures for this study were approved by the IRB (protocol #17-2658) at the University of North Carolina at Chapel Hill. Patients seeking treatment in the Endodontics clinic, and who fit the inclusion and exclusion criteria were included in the study. Teeth were included if they were clinically diagnosed with reversible or irreversible pulpitis, with no previous pulp therapy and with fully developed roots. Teeth were excluded if they had recent trauma; nonvital pulps; periapical pathosis defined as either radiographic evidence of periapical lesion, swelling in the periapical area, were sensitive to percussion or have a pain threshold lower than 50 Newtons on the bite fork device as compared to contralateral control teeth (Figure 1). The bite force device was fabricated using a piezoresistive force transducer Flexiforce B201 (Tekscan, Boston, MA, USA) (31) in a 3D printed enclosure that is covered by a plastic barrier when used in a patient's mouth. The Elf software that registers the force for this device was calibrated for use in the 100-600 N range by an Instron machine, according to manufacturer guidelines. The concept of this testing is based on older data by Khan et al. (14, 32).
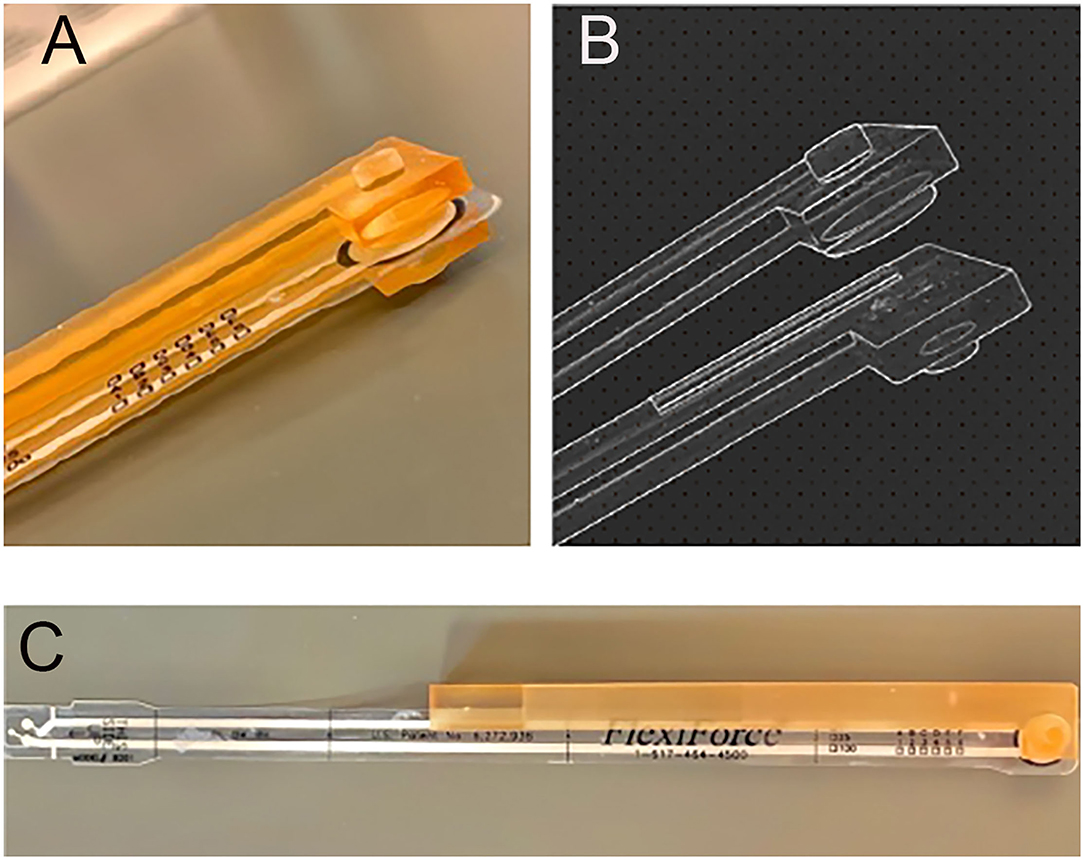
Figure 1. 3D-printed intraoral apparatus covering a FlexiForce (Tekscan Inc., MA, USA) pressure sensor designed and developed to measure bite force value. (A) Photograph of biting area showing protuberance to focus force on one tooth. (B) Detail from planning file. (C) Overview showing the encapsulation of the FlexiForce sensor.
Examination of the patients followed a systematic approach, guided by a secure online form, developed for this study, and depicted in Figure 2. The information collected from each patient were: pain status on the day of visit, severity of pain using a Visual Analog Scale, worst pain level experienced with the affected tooth, onset of pain (spontaneous, with biting or cold or hot), duration (lingering) and quality (sharp, throbbing, etc.) of pain, and medication (type, dose, and duration) taken for the pain and their effectiveness. In addition, a brief health history and a detailed history of the dental problem were obtained.
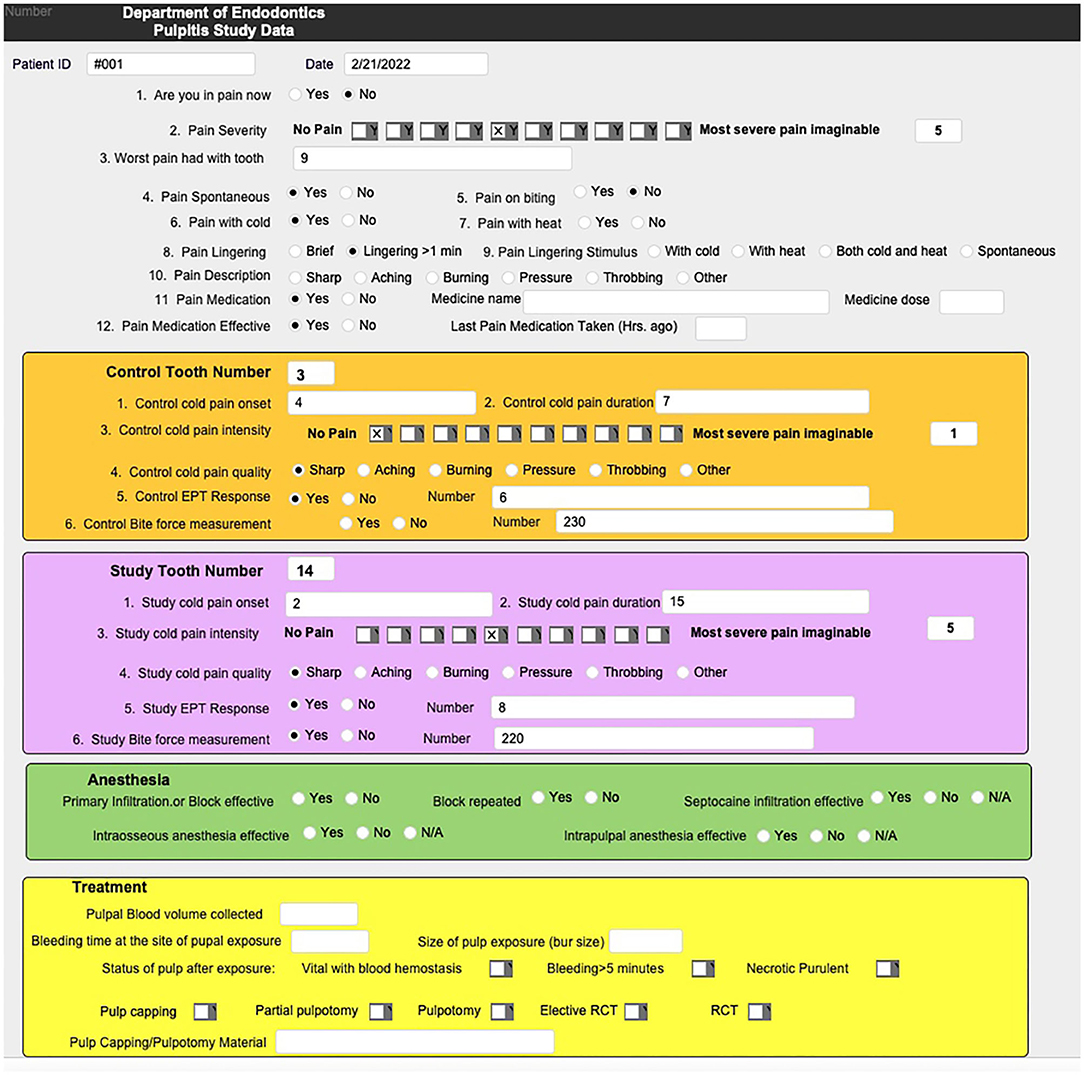
Figure 2. Online database questionnaire was developed using a Filemaker Pro database and used for the collection of systematic clinical signs and symptoms for each patient enrolled in the study.
Upon pulp exposure, 15-20 μl of blood was collected from the exposed pulp (using 2 x 10μl microcapillary tubes) along with 5 ml of peripheral blood from the antecubital region from each patient. The volume of the blood sample was precisely determined by measuring the penetration depth of the blood in the microcapillary tube and calculated in relation to the maximal volume capacity of the microcapillary tube. The blood obtained from the exposed dental pulp was transferred immediately from the microcapillary tubes into BD p100 tube (BD Biosciences, San Jose, CA) containing 2 ml PBS solution and stored temporarily at 4°C. These tubes minimize pre-analytical variability in plasma protein analysis. They are spray coated with K2EDTA anticoagulant and proprietary protease inhibitor cocktail, specifically formulated for stabilization of human plasma proteins at the point of collection. Samples were centrifuged at 1,520 rpm for 10 min within 2 h of sample collection, and then transferred to −80°C without freeze-thaw until analyzed.
Collected samples were tested on the Luminex assay platform. R&D Luminex Assay Customization Kit (R&D Systems, Minneapolis, USA) was used to assess 45 human protein biomarkers (Figure 3). Four combinations of analytes were multiplexed together, based on company standards: a 2-plex of G-CSF and IL-23, a 4-plex of RANTES, MMP-2, MMP-9 and MPO, a 1-plex of TIM-1 and a 33-plex of the remaining analytes. The assays were performed in 96-well solid plate following the manufacturer's instructions with the quality controls and standards added in duplicates. Plates were washed, and the quality controls and standards were added into the wells in duplicates. The sample and microsphere beads coated with monoclonal antibodies against target proteins were added in the wells and incubated for 2 h. The plates were washed twice. A mixture of biotinylated secondary antibodies was added into each well and incubated for 30 min. After plates were washed twice, SAPE (Streptavidin Phycoerythrin, fluorescent reporter molecule) was added into each well and incubated for 30 min. After plates were washed twice, the beads with reading buffer were immediately analyzed in the Luminex 200TM instrument (Luminex Corporation, Austin, TX, USA). The report was analyzed with ProcartaPlex Analyst Software 1.0 (Affymetrix, Thermo Fisher Scientific Inc.).
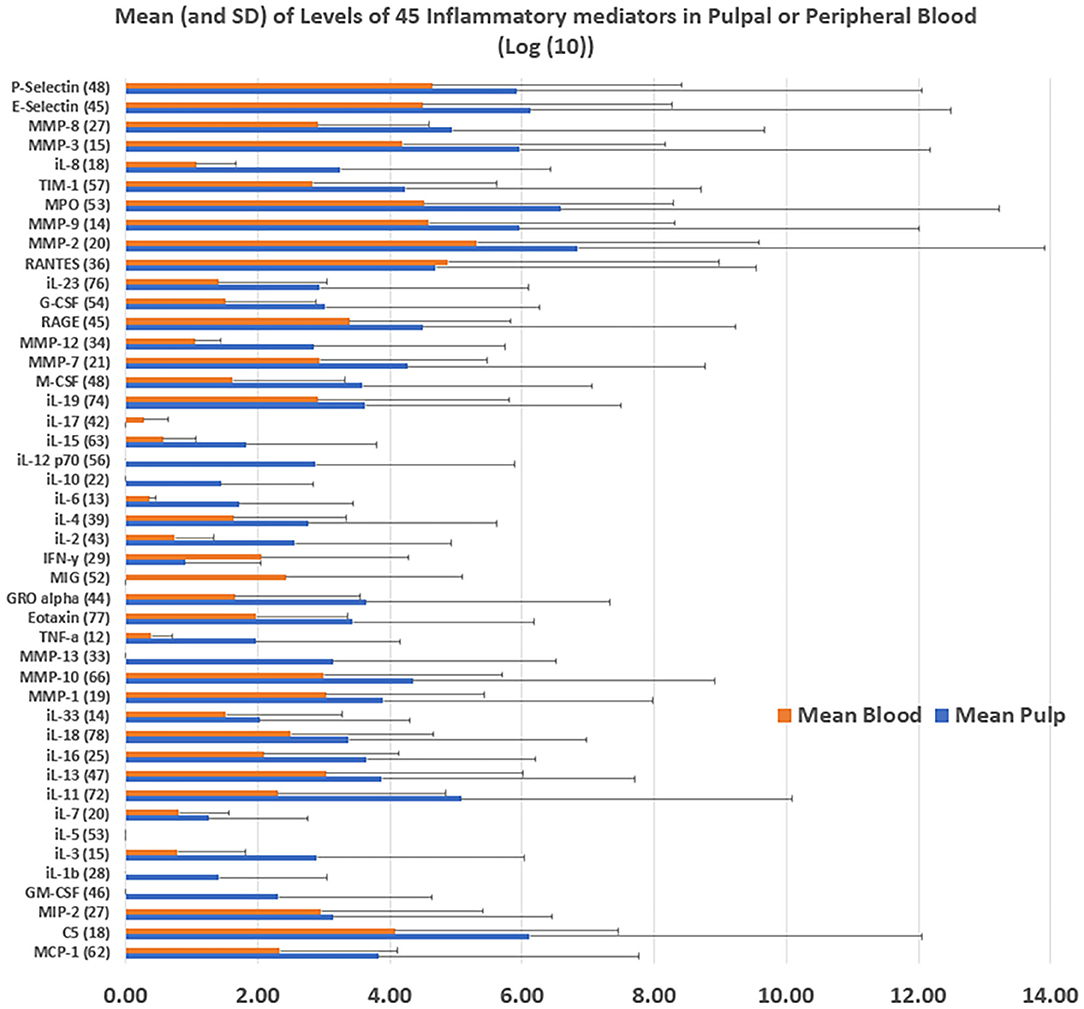
Figure 3. Mean levels of 45 inflammatory proteins in pulpal or peripheral blood [Log (10)] were calculated from the average of expression levels from the collected pulpal and peripheral blood of three subjects enrolled in the study.
Analysis: Luminex data for pulpal and peripheral blood on each of the 45 analytes was an average of duplicate experiments. The mean data for all analytes in the pulpal blood and those in peripheral blood were compared using paired t-test. Eighteen preoperative data points were available for each tooth treated (Figure 2). Of these, continuous data was available for Pain Level, Worst pain level, Pain with cold and Cold Pain Duration. These values were correlated with levels of inflammatory proteins.
Results
Data for three pulpal and three peripheral blood samples from four patients were available for analysis. A peripheral blood sample could not be obtained from one patient after multiple trials, and on another patient who was diagnosed preoperatively with symptomatic irreversible pulpitis, there was evidence of partial necrosis upon access to the pulp and so the sample was excluded. All patients had bite force measurements on the molars treated that were <50 N in magnitude compared to their contralateral molar. Three patients (including the case with partial necrosis) received non-surgical root canal treatment and one case received vital pulp therapy. The detailed history and responses to the questionnaire depicted in Figure 2 was collected for the patients, and the data was correlated with protein analysis.
The correlation of levels of the 45 proteins in the inflamed dental pulp and peripheral blood was 0.87. The pulp had higher levels of protein than the peripheral blood for 41 of 45 mediators tested, and had significantly higher levels of all proteins collectively than peripheral blood (t-test, p = 0.047) (Figure 3). The following proteins correlated at a level of ≥0.8 with the duration of pain with cold: MMP-12, MMP-9, RANTES, MIP-2, MCP-1, MMP-2, MMP-1, and P-Selectin. Relatively high correlations (0.5-0.75) were also present between these proteins and presenting pain level (Figure 4).
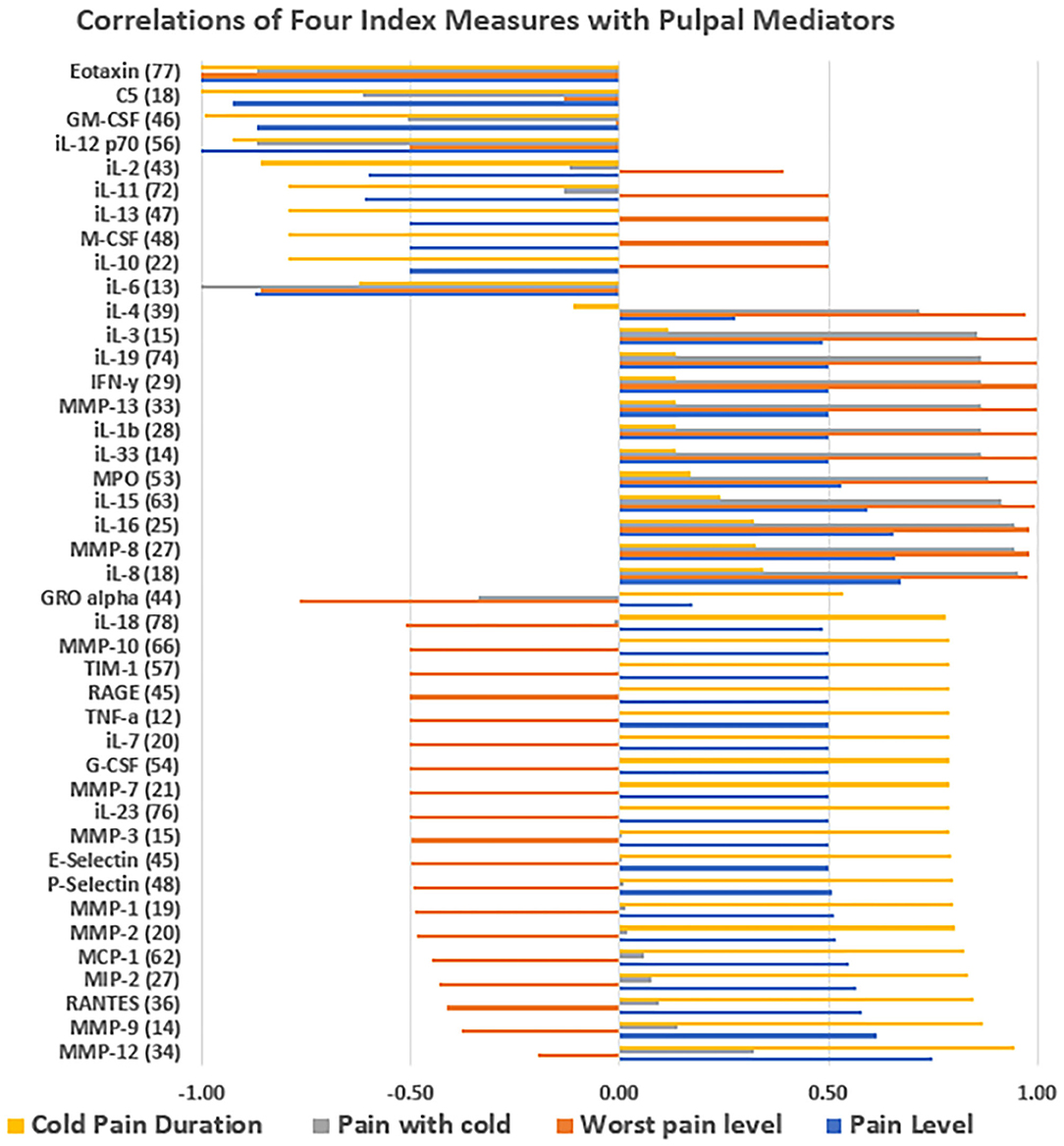
Figure 4. Correlation of four index measures with pulpal mediators: the correlation between the average level of expressed protein mediators from three subjects enrolled in the study and four indices including the severity of pain level on the day of visit, worst pain level experienced since the initial onset of pain, severity of pain with cold testing and duration of pain with cold testing. The X-axis shows positive or negative correlation level.
Discussion
This preliminary study was intended to define a protocol that would allow the registry of several important signs and symptoms of pulpitis without apical periodontitis, the clinical finding upon access preparation and the levels of a relatively large number of inflammatory mediators at the protein level in pulpal and peripheral blood. This protocol may be used to undertake studies to define associations among these variables with larger sample sizes. More importantly, this protocol may be used to run longitudinal studies in which the outcomes of VPT are determined. In these long-term studies, the intent would be to define potential chair-side biomarkers at the time of treatment, as well as exact signs and symptoms, that could predict the prognosis of VPT. If associations are found between the molecular mediators and the exact condition of the pulp status, this can enhance our understanding of pulpal inflammation and help identify candidate proteins that may play a critical role in pulp survival. It would also allow transition to clinical trials in which these candidate proteins are examined for their accuracy in predicting the outcome of VPT. Due to advances in biotechnology, these molecular mediators, if validated in clinical trials, could be incorporated into the chair-side diagnostic tools available to the dentist or endodontist. Furthermore, in future, the detection of specific biomarkers in the pulp that are associate with unfavorable prognosis may be an indication to treatment with topical or other anti-inflammatory agents, or biological agents intended to decrease the inflammation by specifically downregulating the expression of these proteins. This would allow pulpal treatment to reach the age of precision medicine.
However, there are limitations to using a small size methods study such as this one before running larger validation studies. These include lack of coverage or the entire spectrum of normal and pulpitis cases, including an incomplete list of inflammatory mediators, missing important signs and symptoms of the disease and inability to do a cluster analysis of the gathered data.
This preliminary research showed that pulpal protein levels was mostly higher than those levels in peripheral blood. This finding is useful in showing that peripheral sampling may not be necessary in pulp sampling studies. This step has mostly been avoided in these experiments in the past, due to difficulty and potential lack of necessity, and this experiment confirms that this is mostly the case.
MMP-9 was highly correlated with duration of pain to cold in this study. This was in agreement with previous studies that have shown that MMP-9 was an important inflammatory biomarker in pulpitis (18, 22, 23, 33). No studies investigated MMP-1, MMP-2, or MMP-12, which were also highly correlated with pain to cold in this study. However, one study reported significantly high correlations of MMP-8 levels and cold sensitivity in reversible and irreversible pulpitis (34). In this study, MMP-8 was highly correlated with worst pain ever (0.98) and pain with cold (0.95), but not as much with duration of pain to cold (0.33). However, no studies could be found to relate the levels of RANTES, MIP-2, MCP-1, MMP-2, MMP-1 and P-Selectin to pulpitis pain to cold or any of the other variable studied.
Several important proinflammatory cytokines and chemokines examined in this study did not show high levels in these pulpitis cases, such as IL-17, IFN-gamma, and MIG (CXCL9), or showed similar levels to peripheral blood, such as RANTES (CCL5) and MIP-2 (CXCL-2). Other important proinflammatory cytokines such as IL-1 beta, IL-2, IL-6 and TNF-alpha or anti-inflammatory cytokines such as IL-4 and IL-10 were markedly elevated in pulpal compared with peripheral blood. These findings need to be further explored in larger studies in order to identify the fluctuations in these inflammatory markers across the inflammation spectrum in pulpitis, as well as how they may, individually or in clusters, predict the survival of the dental pulp following VPT.
As noted, the study showed that several MMPs together with RANTES and P-Selectin correlated with duration of pain with cold and presenting pain level. The ability to evaluate many protein levels simultaneously is an elegant finding of the Luminex system. It has been shown previously with a small number of proteins tested (29, 30), however, the ability to run larger numbers of important proinflammatory and anti-inflammatory proteins together with a large array of clinical findings and use cluster analysis and other multivariable analyses could result in significant advances in this field.
Recognizing the limitations of sample size of this study, it can be concluded that peripheral blood contained less inflammatory mediators than pulpal blood, and therefore, may not be necessary to collect in future similar studies. In addition, several pulpal proteins correlated with spontaneous and evoked pain parameters. Finally, additional data is needed to identify candidate proteins to be investigated as potential markers of truly irreversible pulp inflammation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by University of North Carolina at Chapel Hill. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
AF conceived this research and conducted the study with help from students and staff and wrote the manuscript.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The author wishes to thank Mr. Russell Mallory Kaye, Mr. Dewey Chapa, and Drs. Dan Zhao, Mohamed Akram, Gurmukh Dhaliwal, and Babek Yousefi for their help with the study.
References
1. Mejare IA, Axelsson S, Davidson T, Frisk F, Hakeberg M, Kvist T, et al. Diagnosis of the condition of the dental pulp: a systematic review. Int Endod J. (2012) 45:597–613. doi: 10.1111/j.1365-2591.2012.02016.x
2. Mainkar A, Kim SG. Diagnostic accuracy of 5 dental pulp tests: a systematic review and meta-analysis. J Endod. (2018) 44:694–702. doi: 10.1016/j.joen.2018.01.021
3. Michaelson PL, Holland GR. Is pulpitis painful? Int Endod J. (2002) 35:829–32. doi: 10.1046/j.1365-2591.2002.00579.x
4. Hasselgren G, Reit C. Emergency pulpotomy: pain relieving effect with and without the use of sedative dressings. J Endod. (1989) 15:254–6. doi: 10.1016/S0099-2399(89)80219-5
5. McDougal RA, Delano EO, Caplan D, Sigurdsson A, Trope M. Success of an alternative for interim management of irreversible pulpitis. J Am Dent Assoc. (2004) 135:1707–12. doi: 10.14219/jada.archive.2004.0123
6. Eren B, Onay EO, Ungor M. Assessment of alternative emergency treatments for symptomatic irreversible pulpitis: a randomized clinical trial. Int Endod J. (2017) 51(Suppl 3):e227–37. doi: 10.1111/iej.12851
7. Galani M, Tewari S, Sangwan P, Mittal S, Kumar V, Duhan J. Comparative evaluation of postoperative pain and success rate after pulpotomy and root canal treatment in cariously exposed mature permanent molars: a randomized controlled trial. J Endod. (2017) 43:1953–62. doi: 10.1016/j.joen.2017.08.007
8. Seltzer S, Bender IB, Ziontz M. The dynamics of pulp inflammation: correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg Oral Med Oral Pathol. (1963) 16:846–71 contd. doi: 10.1016/0030-4220(63)90323-2
9. Hasler JE, Mitchell DF. Painless pulpitis. J Am Dent Assoc. (1970) 81:671–7. doi: 10.14219/jada.archive.1970.0293
10. Mitchell DF, Tarplee RE. Painful pulpitis; a clinical and microscopic study. Oral Surg Oral Med Oral Pathol. (1960) 13:1360–70. doi: 10.1016/0030-4220(60)90301-7
11. Langeland K. Management of the inflamed pulp associated with deep carious lesion. J Endod. (1981) 7:169–81. doi: 10.1016/S0099-2399(81)80231-2
12. Dummer PM, Hicks R, Huws D. Clinical signs and symptoms in pulp disease. Int Endod J. (1980) 13:27–35. doi: 10.1111/j.1365-2591.1980.tb00834.x
13. Ricucci D, Loghin S, Siqueira JF Jr. Correlation between clinical and histologic pulp diagnoses. J Endod. (2014) 40:1932–9. doi: 10.1016/j.joen.2014.08.010
14. Khan AA, McCreary B, Owatz CB, Schindler WG, Schwartz SA, Keiser K, et al. The development of a diagnostic instrument for the measurement of mechanical allodynia. J Endod. (2007) 33:663–6. doi: 10.1016/j.joen.2006.06.003
15. Owatz CB, Khan AA, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. The incidence of mechanical allodynia in patients with irreversible pulpitis. J Endod. (2007) 33:552–6. doi: 10.1016/j.joen.2007.01.023
16. Fouad AF, Khan AA, Silva RM, Kang MK. Genetic and epigenetic characterization of pulpal and periapical inflammation. Front Physiol. (2020) 11:21. doi: 10.3389/fphys.2020.00021
17. Rechenberg DK, Galicia JC, Peters OA. Biological markers for pulpal inflammation: a systematic review. PLoS ONE. (2016) 11:e0167289. doi: 10.1371/journal.pone.0167289
18. Mente J, Petrovic J, Gehrig H, Rampf S, Michel A, Schurz A, et al. A prospective clinical pilot study on the level of matrix metalloproteinase-9 in dental pulpal blood as a marker for the state of inflammation in the pulp tissue. J Endod. (2016) 42:190–7. doi: 10.1016/j.joen.2015.10.020
19. Galicia JC, Henson BR, Parker JS, Khan AA. Gene expression profile of pulpitis. Genes Immun. (2016) 17:239–43. doi: 10.1038/gene.2016.14
20. Galicia JC, Naqvi AR, Ko CC, Nares S, Khan AA. MiRNA-181a regulates toll-like receptor agonist-induced inflammatory response in human fibroblasts. Genes Immun. (2014) 15:333–7. doi: 10.1038/gene.2014.24
21. Galicia J, Khan AA. Non-coding RNAs in endodontic disease. Semin Cell Dev Biol. (2021) 124:82–4. doi: 10.1016/j.semcdb.2021.07.006
22. Sharma R, Kumar V, Logani A, Chawla A, Mir RA, Sharma S, et al. Association between concentration of active MMP-9 in pulpal blood and pulpotomy outcome in permanent mature teeth with irreversible pulpitis - a preliminary study. Int Endod J. (2021) 54:479–89. doi: 10.1111/iej.13437
23. Ballal NV, Duncan HF, Wiedemeier DB, Rai N, Jalan P, Bhat V, et al. MMP-9 levels and NaOCl lavage in randomized trial on direct pulp capping. J Dent Res. (2021) 101:414–9. doi: 10.1177/00220345211046874
24. Asgary S, Eghbal MJ, Fazlyab M, Baghban AA, Ghoddusi J. Five-year results of vital pulp therapy in permanent molars with irreversible pulpitis: a non-inferiority multicenter randomized clinical trial. Clin Oral Investig. (2015) 19:335–41. doi: 10.1007/s00784-014-1244-z
25. Kang CM, Sun Y, Song JS, Pang NS, Roh BD, Lee CY, et al. A randomized controlled trial of various MTA materials for partial pulpotomy in permanent teeth. J Dent. (2017) 60:8–13. doi: 10.1016/j.jdent.2016.07.015
26. Kundzina R, Stangvaltaite L, Eriksen HM, Kerosuo E. Capping carious exposures in adults: a randomized controlled trial investigating mineral trioxide aggregate versus calcium hydroxide. Int Endod J. (2017) 50:924–32. doi: 10.1111/iej.12719
27. Linsuwanont P, Wimonsutthikul K, Pothimoke U, Santiwong B. Treatment outcomes of mineral trioxide aggregate pulpotomy in vital permanent teeth with carious pulp exposure: the retrospective study. J Endod. (2017) 43:225–30. doi: 10.1016/j.joen.2016.10.027
28. Taha NA, Al-Khatib H. 4-year follow-up of full pulpotomy in symptomatic mature permanent teeth with carious pulp exposure using a stainproof calcium silicate-based material. J Endod. (2021) 48:87–95. doi: 10.1016/j.joen.2021.09.008
29. Ghattas Ayoub C, Aminoshariae A, Bakkar M, Ghosh S, Bonfield T, Demko C, et al. Comparison of IL-1beta, TNF-alpha, hBD-2, and hBD-3 expression in the dental pulp of smokers versus nonsmokers. J Endod. (2017) 43:2009–13. doi: 10.1016/j.joen.2017.08.017
30. Brizuela C, Meza G, Mercade M, Inostroza C, Chaparro A, Bravo I, et al. Inflammatory biomarkers in dentinal fluid as an approach to molecular diagnostics in pulpitis. Int Endod J. (2020) 53:1181–91. doi: 10.1111/iej.13343
31. Testa M, Di Marco A, Pertusio R, Van Roy P, Cattrysse E, Roatta S. A validation study of a new instrument for low cost bite force measurement. J Electromyogr Kinesiol. (2016) 30:243–8. doi: 10.1016/j.jelekin.2016.08.005
32. Khan AA, Owatz CB, Schindler WG, Schwartz SA, Keiser K, Hargreaves KM. Measurement of mechanical allodynia and local anesthetic efficacy in patients with irreversible pulpitis and acute periradicular periodontitis. J Endod. (2007) 33:796–9. doi: 10.1016/j.joen.2007.01.021
33. Al-Natour B, Rankin R, McKenna R, McMillan H, Zhang SD, About I, et al. Identification and validation of novel biomarkers and therapeutics for pulpitis using connectivity mapping. Int Endod J. (2021) 54:1571–80. doi: 10.1111/iej.13547
Keywords: molecular mediators, Luminex®, pulpitis, vital pulp therapy (VPT), diagnostic test, Endodontics, pain, inflammation
Citation: Fouad AF (2022) Molecular Characterization of Irreversible Pulpitis: A Protocol Proposal and Preliminary Data. Front. Dent. Med. 3:867414. doi: 10.3389/fdmed.2022.867414
Received: 01 February 2022; Accepted: 21 April 2022;
Published: 10 May 2022.
Edited by:
Paul Roy Cooper, University of Otago, New ZealandReviewed by:
Prasanna Neelakantan, The University of Hong Kong, Hong Kong SAR, ChinaIkhlas El karim, Queen's University Belfast, United Kingdom
Copyright © 2022 Fouad. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ashraf F. Fouad, YWZvdWFkQHVhYi5lZHU=
 Ashraf F. Fouad
Ashraf F. Fouad