- 1School of Dental Medicine, Stony Brook University, Stony Brook, NY, United States
- 2Bureau of Chronic Disease Evaluation and Research CSP Data Unit, New York State Department of Health (NYSDOH), Albany, NY, United States
- 3Bureau of Outcomes Research and Evaluation, Office of Quality and Patient Safety, NYSDOH, Albany, NY, United States
- 4Department of Biostatistics, Columbia University Mailman School of Public Health, New York, NY, United States
- 5Consultant to the NYSDOH, Albany, NY, United States
- 6Bureau of Outcomes Research and Evaluation, Office of Quality and Patient Safety, NYSDOH, Albany, NY, United States
- 7Department of Periodontics and Endodontics, University at Buffalo School of Dental Medicine, Buffalo, NY, United States
- 8New York University College of Dentistry, New York, NY, United States
Introduction: Preventive dental services have been associated with improved health outcomes. This study expands on previous observations by examining the relationship between oral healthcare and healthcare outcomes and costs in a publicly insured population with diabetes.
Methods: Utilization of dental services, healthcare outcomes and costs were evaluated for New York State Medicaid members with a diagnosis of diabetes mellitus (DM), ages 42 to 64, who were continuously enrolled between July 1, 2012 and June 30, 2015. Utilization of dental services focused on preventive dental care (PDC) and extractions and endodontic treatment (both indicative of advanced dental infection). Data were analyzed using regression models with propensity score weighting to control for potential confounding.
Results: Receipt of PDC was associated with lower utilization rates and costs compared to members who did not access dental services. The most pronounced average cost difference was observed for inpatient admissions at $823 per year for members who had at least one PDC without extraction or endodontic treatment. Each additional PDC visit received was associated with an 11% lower rate of inpatient admissions and lower average inpatient costs by $407 per member. The need for a dental extraction or endodontic therapy was associated with relatively higher rates and costs.
Conclusions: These findings demonstrate an association between PDC and improved healthcare outcome rates and lower average costs among members with DM and suggest a general health benefit associated with the provision of preventive dental care for persons with DM.
Introduction
Noncommunicable chronic diseases (NCDs) are the major cause of morbidity and mortality across the globe (1). A primary contributor to the onset and progression of NCDs is systemic inflammation (2). Certain oral diseases, specifically periodontitis and endodontic infections, are associated with an intense local inflammatory response and have been identified as risk factors for several NCDs (3–6). More than 50 such associations have been reported, with the strongest observed for cardiovascular disease and diabetes mellitus (4, 7, 8). The mechanisms underlying these associations are related primarily to oral infection eliciting a local, chronic host inflammatory response with production of pro-inflammatory cytokines and other mediators, which ultimately gain access to the circulatory system and contribute to the systemic inflammatory burden (9). In addition, Gram-negative bacteria that characterize the periodontal microflora can gain access to the circulation and contribute directly or indirectly to these associations (10).
Preventive dental care, specifically periodontal therapy, has been shown to reduce the incidence of dental disease and improve oral health-related quality of life (11–13). Further, treatment of periodontitis has been shown to improve outcomes associated with NCDs, usually via evaluation of surrogate markers for progression of disease, including glycated hemoglobin (HbA1c) for diabetes mellitus (DM) and endothelial function for cardiovascular disease (14, 15). Subsequently, population studies using commercial insurance data have examined the effect of dental care, specifically conservative periodontal procedures that remove the precipitating dental biofilm, on healthcare costs and clinical outcomes (16). Here we report for the first time on the relationship between the use of specific dental services and emergency department (ED) visits and costs, inpatient admissions (IP) and costs, pharmacy costs and total adjusted healthcare costs, for members with DM enrolled in the New York State (NYS) Medicaid program.
The relationship of DM to oral disease has been extensively studied. DM is the only recognized chronic disease that is considered a risk factor for periodontitis, and periodontitis has been associated with poor glycemic control in patients with DM (17). Further, treatment of periodontitis is associated with improved glycemic control (18). In addition, many other oral disorders have been associated with DM, including increased tooth loss, Candida infection and dry mouth (19). Considering the close relationship between oral disease and DM, the aim of this study was to evaluate if utilization of preventive dental care was associated with health outcomes, including utilization and cost, among NYS Medicaid members with DM.
Methods
A retrospective cohort analysis was conducted using NYS Medicaid administrative claim and encounter data from July 1, 2012, to June 30, 2015. In the United States, there are two large government-sponsored health insurance programs. Medicare is federally funded and is primarily for persons 65 years of age and older. Coverage includes hospitalizations, physician office visits and prescription medications. Medicaid is a joint federal and state health insurance program for persons with limited income. Individual states vary in terms of eligibility requirements and what services are covered. This is especially true for dental services. New York State has relatively robust dental coverage.
The eligible population included adults 42–64 years of age as of June 30, 2015, who remained enrolled in Medicaid throughout the study period. Those dually eligible for Medicare at any point in the study period were excluded as Medicare claim and encounter data were not available to the authors. Pregnant women and residents of nursing homes or other institutionalized settings were also excluded. The 2015 cutoff was chosen due to the introduction of ICD-10 codes in October of that year. The study protocol was submitted to the NYS Department of Health Institutional Review Board which determined the study did not qualify as research involving human subjects.
Procedure codes for dental services from July 1, 2012, to June 30, 2014, were used to assign members to dental utilization groups. Members with DM that received any dental care1, any preventive dental care2 (PDC), preventive dental care without an extraction and/or endodontic treatment3 (PDC without Ext/Endo), preventive dental care and an extraction and/or endodontic treatment (PDC with Ext/Endo), and an extraction and/or endodontic treatment without preventive dental care (Ext/Endo without PDC) were compared to members who did not receive dental services. Tooth extraction and endodontic treatment are surrogate indicators of severe dental infection, affecting the oral mucosal tissue as well as the alveolar bone in which the teeth are housed. Assignment to the dental treatment groups was not mutually exclusive.
Outcomes in year 3 (July 1, 2014, to June 30, 2015) included rate of all-cause ED visits, rate of all-cause IP, and the average cost per member of (1) ED, (2) IP, (3) pharmacy, and (4) adjusted total healthcare (total costs minus dental costs). Costs were defined as the total amount paid across Medicaid fee-for-service (FFS) and Medicaid managed care (MMC) programs.
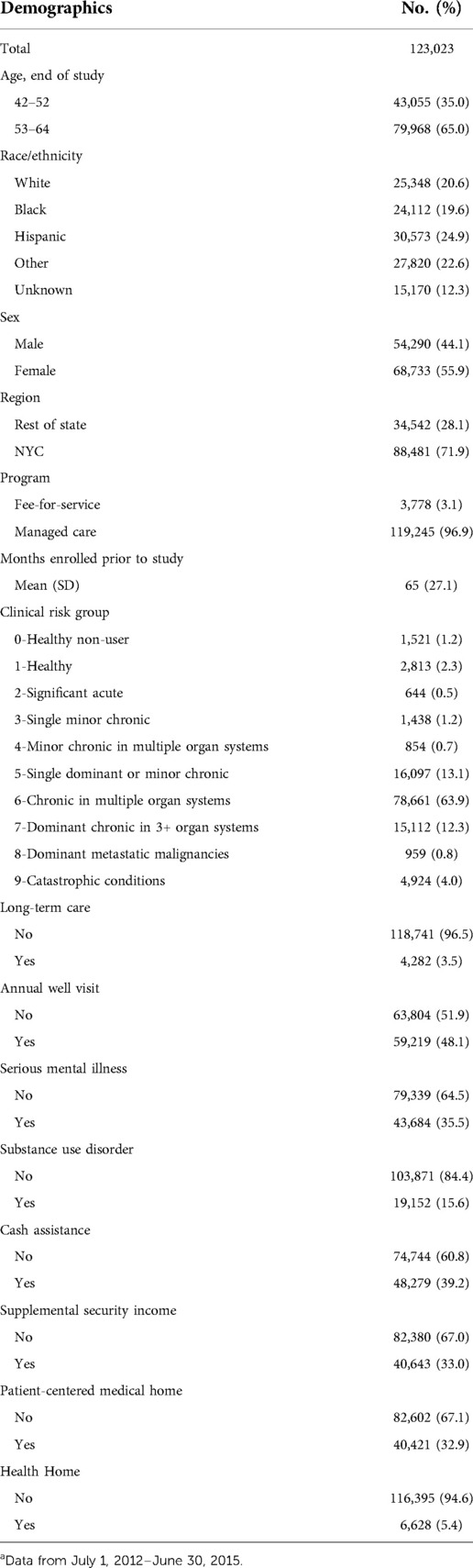
Demographic variables included age group; race/ethnicity; sex; region of residence; type of Medicaid coverage program4 (MMC or FFS); receipt of cash assistance from NYS; and receipt of supplemental security income (SSI). Cash assistance and SSI serve as proxies for socioeconomic status and disability, respectively, pursuant to eligibility criteria. Other covariates included the number of months of Medicaid enrollment prior to July 1, 2012, eligibility for long-term care (LTC)5 services, obtainment of an annual well-visit6 as a proxy for medical engagement, attribution to a Patient Centered Medical Home (PCMH) recognized by the National Committee for Quality Assurance (NCQA®), enrollment in an NYS Health Home, and underlying health status. PCMHs provide coordinated and integrated care while those in Health Homes have chronic and/or behavioral health needs. Both groups may have better access to dental care and different healthcare utilization and costs. Health status was based on clinical risk group (CRG) assignments. Using the 3M™ Clinical Risk Grouping software, members were assigned to CRGs based on their history of diagnoses, procedures, and prescriptions (20). A member's first available CRG assignment during the study period was used for the adjustment. Additionally, serious mental illness (SMI) was identified using Episode Diagnostic Categories (EDCs) from 3M's CRG algorithms in combination with diagnosis codes, and substance use disorders (SUD)7 were identified using diagnostic and procedural code data. Members with DM were also identified using EDCs for the study period8.
All-cause ED and IP rates were compared among dental utilization groups using a multivariable negative binomial regression model. ED, IP, and adjusted total costs were analyzed using a linear model. Pharmacy costs were examined using a multivariable marginal zero-inflated Poisson model for cohorts with a severe inflation at value 0. Otherwise, pharmacy costs were examined with a multivariable negative binomial regression model.
To minimize confounding, multivariable logistic regression models were used to generate propensity scores for each member within a dental utilization category based on the independent variables found in Table 1. Variables were selected using a stepwise approach and retained if significant (p ≤ 0.05). Propensity scores were assigned to quartiles then entered in models as an independent variable to adjust for the associations between dental utilization category and outcomes.
In addition, year 3 outcomes were analyzed for a possible inverse incremental association between the number of PDC visits in the previous 2 years (the program allows a maximum of 2 PDC visits in each year, for a maximum of 4 visits in the first two years of the study). The inverse incremental association for ED and IP rates was analyzed using a multivariable negative binomial model while for costs for ED, IP, and adjusted total healthcare a multivariable linear model was used. For pharmacy costs, a multivariable normal distribution regression model was applied. A p value of 0.05 was considered statistically significant in all models. All analyses were conducted using SAS version 9.4. This study followed RECORD guidelines (https://record-statement.org/checklist.php).
Results
A total cohort of 518,689 members met the inclusion criteria. Of these, 123,023 had received a diagnosis of DM. The majority of members with DM were aged 53–64, from a diverse set of racial/ethnic groups, female, residents of NYC, and enrolled in MMC (Table 1). Nearly two-thirds of members were assigned to CRG 6, indicating chronic disease in multiple organ systems.
More than half of the members (54.7%) received dental care in the first two years of the study (Table 2). Of those members receiving dental care, 73.5% received at least one PDC service. Approximately one-third of the members with a PDC also received Ext/Endo during the first two years of observation. Further, almost 12% received Ext/End without a PDC visit.
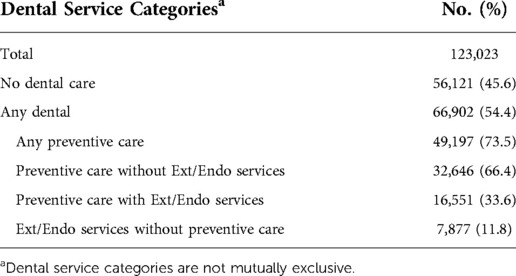
Table 2. Dental service categories for members with diabetes mellitus in the first two years of study.
As indicated by the adjusted rate ratio (ARR), significantly lower ED rates were found for cohorts with PDC (Table 3), particularly among members who received PDC without Ext/Endo (ARR = 0.96). In contrast, those with Ext/Endo without PDC had a significantly higher ED visit rate (ARR = 1.13). All dental service cohorts had significantly lower IP rates except members who received Ext/Endo services without PDC where no difference was observed compared to members who did not receive dental care.
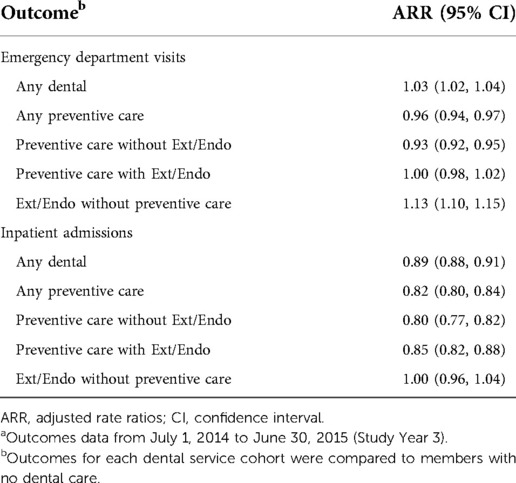
Table 3. All-cause ED visit and inpatient admission adjusted rate ratios for members with diabetes mellitus.a
Adjusted average ED costs per member were significantly higher for members with DM who received Ext/Endo without PDC (Table 4). Lower adjusted average costs for IP admissions were found for members who received any dental care (−$590.67), any PDC (−$776.31), PDC without Ext/Endo (−$823.02) and PDC with Ext/Endo (−$722.62) but not for members who received Ext/Endo without PDC. Observed average pharmacy cost differences, though significant, were small for all dental service cohorts. Adjusted analyses also found significantly lower total adjusted healthcare costs for members with DM who received any dental care, any PDC, and PDC without Ext/Endo, ranging from −$538.84 to −$983.88 compared to members receiving no dental services. No differences were seen among the Ext/Endo without PDC cohort. For both IP and total adjusted healthcare average costs, the lower costs were most pronounced for members receiving PDC without Ext/Endo.

Table 4. Adjusted differences (or adjusted relative risks) in average cost per member with diabetes Mellitus by outcome.a
Incremental reductions in risks and cost associations were observed for each additional PDC visit obtained in the first two years of the study. Specifically, there was a significant 4% lower ARR for ED and an even lower ARR (11%) for IP (Table 5). Additional PDC visits received were associated with small but significantly lower ED costs. On average, IP costs were also lower for members with each additional PDC (−$407.58) as well as total adjusted healthcare cost (−$665.74). There were no observed differences in pharmacy costs.
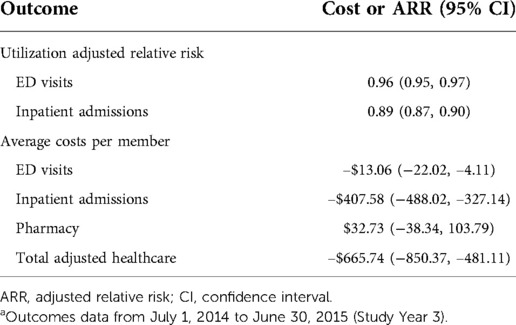
Table 5. Adjusted relative risk or average costs for each additional preventive dental care visit received in the first two study years.a
Discussion
A primary purpose of preventive dental care is to remove the biofilm that elicits the inflammatory and immune response that characterizes periodontal disease. Adherence to a regular schedule of professional preventive care visits has been associated with a reduction in the occurrence and progression of periodontal disease and improved health outcomes including a reduction in the risk of incident type 2 DM (21, 22). In addition, a study from Sweden followed persons referred for periodontal treatment for a mean of 21.7 years and found that a poor response to retreatment was associated with a 39% increased risk of developing DM (23).
This study examined the relationship between the utilization of dental care services and healthcare outcomes and costs for NYS Medicaid members with DM. The use of PDC was associated with a lower risk of ED and IP, while extraction or endodontic treatment, both indicative of an advanced dental infection, were generally associated with higher ED/IP utilization and healthcare costs among members with DM. The beneficial effects of PDC were most pronounced for IP which account for approximately two-thirds of the healthcare costs for persons with NCDs (24).
We found lower ED/IP utilization and costs for members with DM who received PDC compared to members with DM who did not receive dental care, with greater reductions as the number of preventive care visits increased. In an earlier publication from this analysis, we observed improved healthcare outcomes in the entire Medicaid population ages 42–64 years, albeit to a lesser degree (25). As an example, the effect of an additional PDC visit was associated with a reduction in the relative risk of 3% for an ED visit and a 9% reduction in IP. For members with DM, these percentages were 4% and 11%, respectively, demonstrating a larger percentage reduction in health benefits among members with DM. A comparison between the entire cohort and members with DM suggests that the improved outcomes observed for the entire cohort may be driven by members with NCDs who utilize preventive dental services. Therefore, a targeted preventive dental benefit for individuals with DM is one possible approach to improve health outcomes and reduce healthcare costs in a high-risk population.
The relationship of non-surgical periodontal treatment to health outcomes for persons with DM has been reported previously. (26–28). Jeffcoat et al. found reductions in both total cost and hospitalizations for persons with type 2 DM, coronary artery disease, cerebral vascular disease, and pregnancy conditions (but not rheumatoid arthritis), ranging from 11% to 74% (26). Nasseh et al. examined the relationship of periodontal therapy to both healthcare costs and utilization for patients with DM, finding reductions over two years for total DM-related healthcare costs (−$408) and total healthcare costs (−$1799) (27). Another study (21) analyzed Dutch insurance data for the effect of periodontal treatment on healthcare costs for persons with DM. Total healthcare costs in the year following receipt of periodontal services were significantly reduced as compared to costs among those who did not receive such services.
While it is reasonable to postulate that individuals who receive regular dental care may also lead healthy lifestyles, evidence also indicates that removal of dental biofilm and non-surgical periodontal therapy can also have important health benefits. Studies have indicated that conservative periodontal therapy is associated with a reduction in serum markers of inflammation (29–31). Receipt of non-surgical periodontal therapy resulted in a significant decrease in glycated hemoglobin, a surrogate marker of progression of diabetes (32, 33).
In this study, we observed Medicaid members with DM who required Ext/Endo without PDC services had increased utilization and costs compared to those who did not receive dental care. In adults, most tooth extractions are related directly or indirectly to oral infection, including caries (46%), periodontal disease (32%), failed endodontic treatment (7%) and fracture of the tooth root (4%) (34). Similar findings were reported in another study, where periodontal disease and/or caries accounted for two-thirds of extractions of adult teeth (35).
There are a number of strengths of this study. This is the first report to evaluate these relationships for a large publicly insured population with a heavy oral disease and systemic disease burden. Furthermore, studies cited above (26–28) adjusted for a limited number of potential confounders, while this analysis allowed for adjustment for 15 epidemiologic, demographic, healthcare and financial variables, and compared members with DM who did and did not receive dental care. In addition, unlike the studies cited above, here the effect of surrogate markers of advanced oral infection (tooth extraction and endodontic therapy) on health outcomes was also considered. Further, our analysis identified an inverse incremental association between PDC visits and both health outcomes and costs.
There are limitations of this study, including the absence of data regarding smoking habits, weight, and physical activity. In addition, dental claims and encounter data do not contain diagnostic codes to identify the nature of the oral disease. Clinical dental services rely upon clinical procedure codes, and while dental diagnostic codes to identify the nature of the oral disease have been developed (36, 37), they are not widely used or reported.
One practical consideration in this field of investigation is the decision by several dental insurance companies to provide enhanced preventive dental care visits to individuals with certain chronic diseases including DM. This decision was based on their data, similar to what is reported here, suggesting improved health outcomes for insured individuals with one or more chronic diseases who received preventive dental care (38).
Conclusions
The relationship between DM and oral diseases has been extensively studied, and DM remains the only chronic disease that is a risk factor for periodontitis (39, 40). This study and similar studies noted earlier (26–28) emphasize the importance of interprofessional care, including oral healthcare, for persons with DM (41).
Further, this study provides evidence to support the inclusion of preventive dental care in a comprehensive healthcare plan for adults, here demonstrated for persons with DM. In addition to improving oral health and oral health-related quality of life (42, 43), the provision of such services is associated with reduced utilization of medical services and reduced healthcare costs. At a time when achieving such outcomes is a national concern, this finding offers a novel approach for populations with a heavy disease burden.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Author contributions
IBL: contributed to conception, design, data interpretation, drafting and critical revision of the manuscript. KPM: contributed to conception, design, data acquisition and interpretation, and critical revision of the manuscript. PMD: contributed to data interpretation, performed statistical analysis, drafting and critical revision of the manuscript. BC: contributed to data interpretation, performed statistical analysis and drafting of the manuscript. VLW: contributed to design, data interpretation, drafting and critical revision of the manuscript. JMM: contributed to design, data interpretation, and critical revision of the manuscript. AP: contributed to data interpretation and critical revision of the manuscript. YX: contributed to data acquisition, interpretation, and performed statistical analysis. SNA: contributed to conception, design, and data interpretation. MCA: contributed to conception, design, data interpretation, and critical revision of the manuscript. No financial disclosures were reported by the authors of this paper. All authors contributed to the article and approved the submitted version.
Funding
This research was supported in part by the John A. Hartford Foundation, the DentaQuest Foundation, and the Santa Fe Group.
Acknowledgements
Thanks are expressed to Mrs. Cynthia Rubiera for her help with the preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1. ^Identified using Current Dental Terminology (CDT) codes
2. ^Preventive Dental care defined as having record of receiving prophylaxis (D1110), maintenance (D4910) or non-surgical procedures (D4341 or D4342)
3. ^Extraction and/or Endodontic Treatment defined as having received endodontic procedures (D3310 – D3999), or extractions (D7140-D7250)
4. ^Based on the majority of enrollment months, not continuous enrollment
5. ^Evidence of eligibility for non-institutional LTC services, home healthcare, ICF/DD services, or home- and community-based services for 4 consecutive months during the study
6. ^CPT codes: 99385, 99386, 99387, 99395, 99396, 99397
7. ^From the NYSDOH Medicaid Clinical Datamart based on qualification for Identification of Alcohol and Other Drugs (IAD) HEDIS measure for calendar years 2012, 2013, and 2014.
8. ^Diabetes EDC codes and descriptions: 424, diabetes; 427, diabetes juvenile onset; 428, diabetes with circulatory complication
References
1. Bauer UE, Briss PA, Goodman RA, Bowman BA. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. (2014) 384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6
2. Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25(12):1822–32. doi: 10.1038/s41591-019-0675-0
3. Caplan DJ, Chasen JB, Krall EA, Cai J, Kang S, Garcia RI, et al. Lesions of endodontic origin and risk of coronary heart disease. J Dent Res. (2006) 85(11):996–1000. doi: 10.1177/154405910608501104
4. Tonetti MS, Van Dyke TE. Working group 1 of the joint EAW. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Clin Periodontol. (2013) 40(Suppl 14):S24–9. doi: 10.1902/jop.2013.1340019
5. Petersen J, Glassl EM, Nasseri P, Crismani A, Luger AK, Schoenherr E, et al. The association of chronic apical periodontitis and endodontic therapy with atherosclerosis. Clin Oral Investig. (2014) 18(7):1813–23. doi: 10.1007/s00784-013-1156-3
6. Aoyama N, Suzuki JI, Kobayashi N, Hanatani T, Ashigaki N, Yoshida A, et al. Periodontitis deteriorates peripheral arterial disease in Japanese population via enhanced systemic inflammation. Heart Vessels. (2017) 32(11):1314–9. doi: 10.1007/s00380-017-1003-6
7. Nguyen ATM, Akhter R, Garde S, Scott C, Twigg SM, Colagiuri S, et al. The association of periodontal disease with the complications of diabetes mellitus. A systematic review. Diabetes Res Clin Pract. (2020) 165:108244. doi: 10.1016/j.diabres.2020.108244
8. Beck JD, Papapanou PN, Philips KH, Offenbacher S. Periodontal medicine: 100 years of progress. J Dent Res. (2019) 98(10):1053–62. doi: 10.1177/0022034519846113
9. Schenkein HA, Papapanou PN, Genco R, Sanz M. Mechanisms underlying the association between periodontitis and atherosclerotic disease. Periodontol. (2000) 83(1):90–106. doi: 10.1111/prd.12304
10. Demmer RT, Breskin A, Rosenbaum M, Zuk A, LeDuc C, Leibel R, et al. The subgingival microbiome, systemic inflammation and insulin resistance: the oral infections, glucose intolerance and insulin resistance study. J Clin Periodontol. (2017) 44(3):255–65. doi: 10.1111/jcpe.12664
11. Axelsson P, Nystrom B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. J Clin Periodontol. (2004) 31(9):749–57. doi: 10.1111/j.1600-051X.2004.00563.x
12. Schlosser R, Hebbes T. Effect of a dedicated oral care program on periodontal status of medically compromised patients at the Toronto Rehabilitation Institute Dental Clinic. Gen Dent. (2016) 64(4):e5–e9.27367641
13. Reisine S, Schensul JJ, Salvi A, Grady J, Ha T, Li J. Oral health-related quality of life outcomes in a randomized clinical trial to assess a community-based oral hygiene intervention among adults living in low-income senior housing. Health Qual Life Outcomes. (2021) 19(1):227. doi: 10.1186/s12955-021-01859-w
14. Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. (2007) 356(9):911–20. doi: 10.1056/NEJMoa063186
15. Botero JE, Rodriguez C, Agudelo-Suarez AA. Periodontal treatment and glycaemic control in patients with diabetes and periodontitis: an umbrella review. Aust Dent J. (2016) 61(2):134–48. doi: 10.1111/adj.12413
16. Elani HW, Simon L, Ticku S, Bain PA, Barrow J, Riedy CA. Does providing dental services reduce overall health care costs?: A systematic review of the literature. J Am Dent Assoc. (2018) 149(8):696–703 e2. doi: 10.1016/j.adaj.2018.03.023
17. Stohr J, Barbaresko J, Neuenschwander M, Schlesinger S. Bidirectional association between periodontal disease and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Sci Rep. (2021) 11(1):13686. doi: 10.1038/s41598-021-93062-6
18. Ata-Ali F, Melo M, Cobo T, Nagasawa MA, Shibli JA, Ata-Ali J. Does non-surgical periodontal treatment improve glycemic control? A comprehensive review of meta-analyses. J Int Acad Periodontol. (2020) 22(4):205–22.32980833
19. Verhulst MJL, Loos BG, Gerdes VEA, Teeuw WJ. Evaluating all potential oral complications of diabetes mellitus. Front Endocrinol (Lausanne). (2019) 10:56. doi: 10.3389/fendo.2019.00056
20. 3M. 3MTM Clinical Risk Group Software (2019). Available from: http://www.3m.com.
21. Chang Y, Lee JS, Lee KJ, Woo HG, Song TJ. Improved oral hygiene is associated with decreased risk of new-onset diabetes: a nationwide population-based cohort study. Diabetologia. (2020) 63(5):924–33. doi: 10.1007/s00125-020-05112-9
22. Lindhe J, Nyman S. Long-term maintenance of patients treated for advanced periodontal disease. J Clin Periodontol. (1984) 11(8):504–14. doi: 10.1111/j.1600-051X.1984.tb00902.x
23. Holmlund A, Lind L. Periodontal disease and a poor response to periodontal treatment were associated with an increased risk of incident diabetes: a longitudinal cohort study in Sweden. J Clin Periodontol. (2021) 48(12):1605–12. doi: 10.1111/jcpe.13558
24. Joo JY, Liu MF. Case management effectiveness in reducing hospital use: A systematic review. Int Nurs Rev. (2017) 64(2):296–308. doi: 10.1111/inr.12335
25. Lamster IB, Malloy KP, DiMura PM, Cheng B, Wagner VL, Matson J, et al. Dental services and health outcomes in the New York State Medicaid Program. J Dent Res. (2021) 100(9):928–34. doi: 10.1177/00220345211007448
26. Jeffcoat MK, Jeffcoat RL, Gladowski PA, Bramson JB, Blum JJ. Impact of periodontal therapy on general health: evidence from insurance data for five systemic conditions. Am J Prev Med. (2014) 47(2):166–74. doi: 10.1016/j.amepre.2014.04.001
27. Nasseh K, Vujicic M, Glick M. The relationship between periodontal interventions and healthcare costs and utilization. Evidence from an integrated dental, medical, and pharmacy commercial claims database. Health Econ. (2017) 26(4):519–27. doi: 10.1002/hec.3316
28. Smits KPJ, Listl S, Plachokova AS, Van der Galien O, Kalmus O. Effect of periodontal treatment on diabetes-related healthcare costs: a retrospective study. BMJ Open Diabetes Res Care. (2020) 8(1):e001666. doi: 10.1136/bmjdrc-2020-001666
29. Teeuw WJ, Slot DE, Susanto H, Gerdes VE, Abbas F, D'Aiuto F, et al. Treatment of periodontitis improves the atherosclerotic profile: a systematic review and meta-analysis. J Clin Periodontol. (2014) 41(1):70–9. doi: 10.1111/jcpe.12171
30. Doughty KN, Del Pilar NX, Audette A, Katz DL. Lifestyle medicine and the management of cardiovascular disease. Curr Cardiol Rep. (2017) 19(11):116. doi: 10.1007/s11886-017-0925-z
31. Burton WN, Chen CY, Li X, Schultz AB. Association between employee dental claims, health risks, workplace productivity, and preventive services compliance. J Occup Environ Med. (2017) 59(8):721–6. doi: 10.1097/JOM.0000000000001069
32. Madianos PN, Koromantzos PA. An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes. J Clin Periodontol. (2018) 45(2):188–95. doi: 10.1111/jcpe.12836
33. Baeza M, Morales A, Cisterna C, Cavalla F, Jara G, Isamitt Y, et al. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci. (2020) 28:e20190248. doi: 10.1590/1678-7757-2019-0248
34. Chrysanthakopoulos NA. Reasons for extraction of permanent teeth in Greece: a five-year follow-up study. Int Dent J. (2011) 61(1):19–24. doi: 10.1111/j.1875-595X.2011.00004.x
35. Reich E, Hiller KA. Reasons for tooth extraction in the western states of Germany. Community Dent Oral Epidemiol. (1993) 21(6):379–83. doi: 10.1111/j.1600-0528.1993.tb01103.x
36. Kalenderian E, Ramoni RL, White JM, Schoonheim-Klein ME, Stark PC, Kimmes NS, et al. The development of a dental diagnostic terminology. J Dent Educ. (2011) 75(1):68–76. doi: 10.1002/j.0022-0337.2011.75.1.tb05024.x
37. Yansane A, Tokede O, White J, Etolue J, McClellan L, Walji M, et al. Utilization and validity of the dental diagnostic system over time in academic and private practice. JDR Clin Trans Res. (2019) 4(2):143–50. doi: 10.1177/2380084418815150
38. Alfano M. The economic impact of periodontal inflammation. In: Glick M, editor. The oral-systemic health connection: A guide to patient care. 2nd ed. Batavia, IL, USA: Quintessence Publishing Company, Inc (2019).
39. Kocher T, Konig J, Borgnakke WS, Pink C, Meisel P. Periodontal complications of hyperglycemia/diabetes mellitus: epidemiologic complexity and clinical challenge. Periodontol. (2000) 78(1):59–97. doi: 10.1111/prd.12235
40. Polak D, Sanui T, Nishimura F, Shapira L. Diabetes as a risk factor for periodontal disease-plausible mechanisms. Periodontol. (2000) 83(1):46–58. doi: 10.1111/prd.12298
41. Do H, Calache H, Darby I, Lau P. The effectiveness of interprofessional education for the management of diabetes and oral health: a systematic review. J Interprof Care. (2021) 35(3):454–63. doi: 10.1080/13561820.2020.1758046
42. Ferreira MC, Dias-Pereira AC, Branco-de-Almeida LS, Martins CC, Paiva SM. Impact of periodontal disease on quality of life: a systematic review. J Periodontal Res. (2017) 52(4):651–65. doi: 10.1111/jre.12436
Keywords: preventive dental care, health services, oral diseases, diabetes mellitus, hospitilizations
Citation: Lamster IB, Malloy KP, DiMura PM, Cheng B, Wagner VL, Matson JM, Proj A, Xi Y, Abel SN and Alfano MC (2022) Preventive dental care is associated with improved healthcare outcomes and reduced costs for Medicaid members with diabetes. Front. Dent. Med 3:952182. doi: 10.3389/fdmed.2022.952182
Received: 24 May 2022; Accepted: 28 July 2022;
Published: 25 August 2022.
Edited by:
Emily Chu, National Institutes of Health (NIH), United StatesReviewed by:
Natalino Lourenço Neto, University of São Paulo, BrazilMurad Alrashdi, Qassim University, Saudi Arabia
Copyright: © 2022 Lamster, Malloy, DiMura, Cheng, Wagner, Matson, Proj, Xi, Abel and Alfano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ira B. Lamster, aXJhLmxhbXN0ZXJAc3Rvbnlicm9va21lZGljaW5lLmVkdQ==
†Present Address: Yizhao Xi,Statistical Programmer, Data Operations, Novartis, Florham Park, New Jersey, NY, United States
Specialty Section: This article was submitted to Systems Integration, a section of the journal Frontiers in Dental Medicine
 Ira B. Lamster
Ira B. Lamster Kevin P. Malloy2
Kevin P. Malloy2 Philip M. DiMura
Philip M. DiMura Victoria L. Wagner
Victoria L. Wagner Anisa Proj
Anisa Proj Yizhao Xi
Yizhao Xi