- Department of Preventive Dentistry, Periodontology and Implant Biology, School of Dentistry, Faculty of Health Sciences, Aristotle University of Thessaloniki, Thessaloniki, Greece
Aim: A pioneer periodontal surgical approach employing the closed surgical technique (CST) in combination with the tissue-engineered biocomplex aimed to gain access to the osseous defect and improve soft tissue architecture.
Methods: The CST was applied in four systemically healthy periodontitis patients/defects who were followed for 12 months. It avoids papilla dissection and retraction of flaps in an open manner; thus, suturing is not required. It is designed for the reconstruction of residual isolated periodontal defects and is best indicated in the aesthetic region. It can be applied as a sole treatment approach to facilitate deep instrumentation of the defect, or it could be combined with subgingival application of regenerative materials. Hereby, the biocomplex was applied containing autologous alveolar bone marrow mesenchymal stem cells, seeded into collagen scaffolds, enriched with autologous fibrin/platelet lysate.
Results: The CST minimized postoperative discomfort and led to improved treatment outcomes with probing pocket depth reduction [average (SD)] of 24.4% (18.5), gain in clinical attachment levels of 25.8% (20.1), and evidence of remineralisation of the alveolar crest.
Conclusion: The CST is a tissue-friendly operation and facilitates subgingival application of biological agents via tunneling the soft tissues. However, surgical experience is required for nontraumatic manipulation of the gingival tissues during “closed” retraction of the flaps.
Clinical trial registration: Registered with Clinicaltrials.gov; ClinicalTrials.gov ID: NCT02449005.
Introduction
Shifts in nonsurgical periodontal therapy and periodontal surgery toward minimally invasive treatment techniques, papilla preservation flaps and single flaps aim to promote periodontal wound healing, while reducing morbidity (1–7). Minimally invasive procedures can be applied at intrabony defects in conjunction with grafting materials, i.e., bone grafts, membrane barriers, and biological agents (8), although the latter have been shown to offer no significant additional improvement to that already achieved by surgery alone (9, 10). Despite good clinical outcomes, the clinical effectiveness of minimally invasive surgical techniques over traditional periodontal surgery of infrabony defects could not be systemically evaluated due to paucity of data in the literature (11) and thus remains unclear. Guided tissue regeneration (GTR) utilizing resorbable barriers and an enamel matrix derivative (EMD), which comprise the gold standard for the surgical treatment of deep intrabony defects, led to improved clinical outcomes over access flap (12), although having small additional effects (13).
These data indicate that there is still need for advancements in the design of surgical applications to promote periodontal regeneration while reducing cost and postoperative complications i.e., patient morbidity and incidence of membrane exposure during GTR. Developments in periodontal regeneration comprise applications of tissue engineering and cell therapies based on evidence derived from preclinical and clinical studies (14). Recently, a proof of principle randomized controlled study that employed a novel surgical methodology preserving the soft tissue wall of intrabony defects by the minimal access flap (MAF) surgery in combination with a tissue-engineered “biocomplex” was assessed for its efficacy (15). The biocomplex comprised autologous mesenchymal stem cells expanded ex vivo from alveolar bone marrow biopsies. The cells enriched with autologous fibrin/platelet lysate (aFPL) were seeded into collagen scaffolds. The MAF technique sought to retain the full thickness of gingival flaps by not intentionally excising the underlying granulation tissue to achieve primary wound closure and promote regeneration. Periodontal surgery ultimately leads to inflammation control and, thus, granulation tissue turns into mature connective tissue minimizing gingival recession. Interestingly, equivalence of treatment outcomes between the cell-based therapy and the MAF surgery alone was demonstrated in the study's cohort of patients and clinical setting (15). These data highlighted the merits of surgical flap procedures that are tissue-friendly and trigger the intrinsic healing potential of tissues. That study suggested the use of the biocomplex in selected cases having a more complicated anatomic configuration (15). As a continuation of the previous study, a new “closed” surgical technique (CST) was designed to improve tissue architecture while diminishing tissue trauma by avoiding dissection of the papilla and retraction of flaps in an open manner. That was achieved by tunneling the gingival tissues under the papilla, and no suturing was required to stabilize the flaps. The closed tunnel technique (16) has been widely used in plastic-aesthetic periodontal surgery and, if modified, it could also be applied for the treatment of intrabony defects and offer wound stability. The CST was combined with the biocomplex application for the reconstruction of interdental periodontal defects focusing on the preservation of the soft tissue volume and architecture. The current report aimed to describe for the first time the application of CST in the augmentation of an interdental papilla and in the management of periodontal osseous defects. Treatment outcomes were followed over 12 months.
Materials and methods
Ethical approval
The clinical applications were conducted at the Postgraduate Periodontics Clinic, Aristotle University of Thessaloniki (AUTh), between 2016 and 2017. The study protocol was approved by the Dental School Ethics Committee, AUTh (322/15-4-2013), and all four participants signed an informed consent form and were followed for 12 months.
Patient selection
Four interdental periodontal defects of systemically healthy nonsmokers were treated by the CST 6 months following cause-related periodontal treatment and were closely followed for 12 months (1 week, 2 weeks, 6 weeks, 3 months, 6 months, 9 months, 12 months). During the study period, clinical and radiographic examinations were performed at baseline (before anesthesia) and 12 months (Table 1). These cases had at least one periodontal defect with probing pocket depth (PPD) and clinical attachment levels (CAL) ≥5 mm and were all located in the anterior zone. The defects had an intrabony component <3 mm (cases #1–4) and a wide angle between the vertical axis of the root and the interdental bone wall (cases #2–4) (Table 1). Exclusion criteria comprised concurrent illness, poorly controlled systemic disease, drug-induced gingival hyperplasia, bone metabolic diseases, disorders compromising wound healing, bisphosphonate medication, anti-inflammatory drugs, immunosuppression/radiation, alcohol intake, pregnancy/lactation, poor compliance during cause-related periodontal treatment, and compromised oral hygiene (full-mouth Plaque Index >30%).
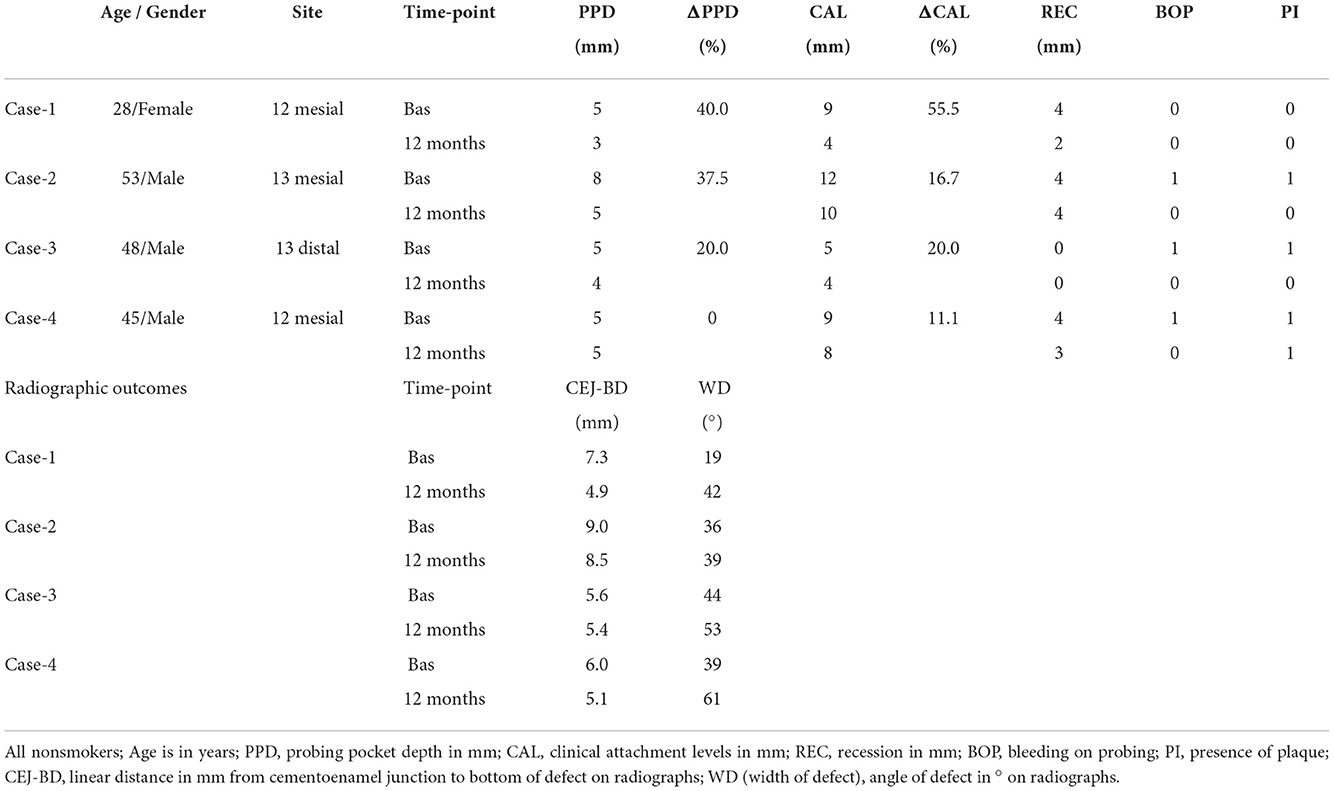
Table 1. Baseline characteristics of the participants and clinical/radiographic data between baseline (before anesthesia) and 12 months.
Surgical procedures
The “closed” surgical technique was designed for periodontal defects at or wider than 2 mm. Local anesthesia (1 cartridge of Lignospan® standard, Septodont, France) avoided papilla infiltration. Strictly intra-sulcular incisions (mini-scalpel blade #67, Hu-Friedy, Chicago, IL, USA) dissected the periodontal attachment of the defect-associated tooth extending to the adjacent tooth (mid-buccal to mid-lingual) to facilitate soft tissue handling (Figure 1). The interdental papilla was not severed by the scalpel and was left intact. Full-thickness gingival flaps including the interdental papilla were detached from the alveolar bone in a “closed” manner using sharp periodontal elevators (TKN1, TKN2, PH26M, Hu-Friedy). Gingival tissues of the adjacent teeth associated with the defect were tunneled including the papilla on the buccal and lingual aspects. In essence, the papilla and the gingival flaps were mobilized from the osseous surface in the buccal-lingual direction to allow full access to the root surface and the underlying osseous defect. Care was taken not to thin the tissues by intentionally removing the soft tissue wall that lined the defect. Therefore, the granulation tissue was left in situ attached onto the retracted flaps, but any loose tissue that remained within the osseous defect was thoroughly removed. Microsurgical instruments and ×3.5 magnification loupes were used to avoid tissue perforation or damage of the papilla during tunneling and “closed” retraction of the flaps. Flaps were raised beyond the mucogingival line only if required to gain access. Manual (mini-five Gracey-curette, Hu-Friedy) and power-driven (PS, Perio Slim, EMS) root instrumentation was meticulously performed to the bottom of the defect while reflecting the soft tissues in a tension-free manner with an elevator. The handling of the interdental papilla was performed with extra caution to preserve tissue integrity during instrumentation. Repeated saline irrigation and drying with cotton pellets allowed visual assessment of the narrow operation field. Subsequently, the biocomplex was extemporaneously prepared as previously described (15), comprising autologous clinical-grade alveolar bone marrow mesenchymal stem cells (a-BMMSCs), seeded into collagen scaffolds (Parasorb® fleece, Resorba, Germany), enriched with aFPL was gently placed within the osseous defect. Flaps were repositioned in their original position by gentle pressure with sterile wet gauzes for 30 s. A plastic syringe (NIPRO Medical Corporation, Bridgewater, NJ, USA) was used to apply aFPL cross-linked by 1M CaCl2 (15) and seal the opening of the periodontal pocket. Soft tissue adaptation was achieved in absence of suturing.
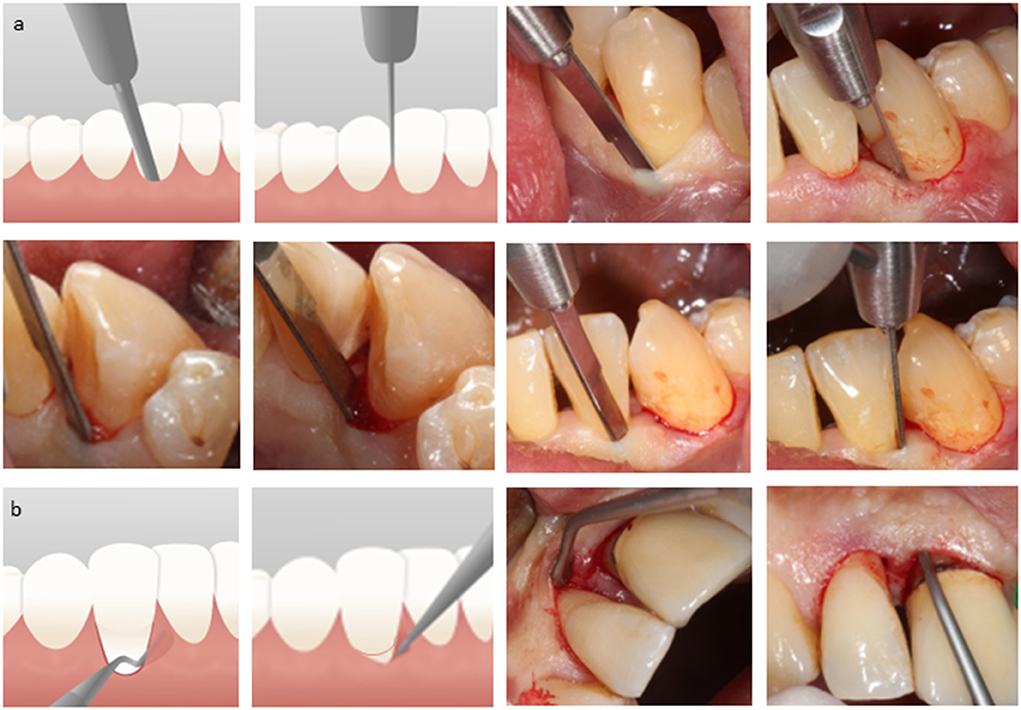
Figure 1. Closed surgical technique: (a) strictly intrasulcular incisions; dissection of the periodontal attachment of the adjacent teeth related to the interdental defect leaving the interdental papilla intact; a microsurgical blade is used (b) full-thickness gingival flaps are prepared vertically and horizontally by sharp elevators; flaps are retracted from the alveolar bone in a “closed” manner to obtain access to the root surface and the osseous defect; the papilla is not dissected; gingival tissues including the papilla are mobilized via tunneling interdentally (buccal-lingual direction) using dedicated elevators; granulation tissue is not intentionally excised; retraction of flaps does not extend beyond the mucogingival line, unless required to obtain access; following careful instrumentation, the site is irrigated with saline and flaps are repositioned without suturing.
Biocomplex description and assembly
An osseous biopsy of ~3 ml in volume was collected in a standardized manner from the maxillary tuberosity or an implant-recipient site in the maxilla under local anesthesia (Lignospan® standard, Septodont, France) as previously described (15) utilizing a rotary trephine bur (TRE02, Biomet 3i, FL, USA) and/or a periodontal chisel (CO1, Hu-Friedy, Chicago, IL, USA). Each sample was irrigated with saline and placed in sterile 50-ml falcon tubes (Corning, NY, USA) containing 15 ml of α-MEM culture medium, supplemented with antimicrobial/antifungal agents (50 μg/ml gentamycin/2.5 mg/ml Amphotericn-B; Invitrogen, Karlsruhe, Germany) and was transported on cool packs to a compatible—Good Manufacturing Practice (c-GMP)—authorized facility (http://www.biohellenika.gr) within 2 h. Additionally, 20 ml of blood were drawn from the antecubital vein of each subject for the preparation of aFPL. From biopsy uptake to chair-side assembly of the biocomplex and transplantation, a time-interval on average of 3 weeks was required. The quality control criteria for cell release and the detailed preclinical protocol are demonstrated in a previous report (15). Five million a-BMMSCs enriched with aFPL at a total volume of 100 μl were delivered to the clinic in an insulin syringe having a cap, and the content was evenly loaded onto 1.0 ×1.0 ×0.5 cm of collagen fleece. The construct was left 5 min to rest before fibrin was cross-linked by 100 mM CaCl2 (Sigma-Aldrich). Before the biocomplex solidified (<10 min), it was carefully trimmed by scissors to be adjusted into the osseous defect via the retracted gingival tissues.
Fibrin/platelet lysate
Twenty ml of blood was transferred into a 50-ml falcon tube (Corning) having 2.8 ml CPD (Citrate Phosphate Dextrose, Sigma-Aldrich, Steinheim, Germany) as anticoagulant. After the determination of platelet counts (≥133,000 platelets/μl), the blood sample was centrifuged (270 g, 7 min, 20°C) and plasma (devoid of leukocytes) was separated and stored in another falcon tube. The sample was centrifuged (1,000 g, 5 min, 20°C) and platelet-rich plasma (PRP, containing 1,000,000 platelets/μl) was separated from the supernatant platelet-poor plasma (PPP). Both preparations were further processed to obtain PRP lysates by freeze/thaw cycles as a source of thrombin and fibrinogen from PPP by cryoprecipitation/10% ethanol. Then, both preparations were homogenized following a standard protocol (Biohellenika), and by addition of 1M CaCl2, thrombin was activated to convert soluble fibrinogen into fibrin.
Treatment outcomes assessment
Subjects were followed over a period of 12 months and were assessed clinically using a manual periodontal probe (Hu-Friedy XP-23/QW) to the nearest millimeter parallel to the long axis for bleeding on probing (BOP), plaque index (PI), recession (REC), PPD and CAL. Radiographic examination followed a previous methodology (15). In brief, standardized periapical digital radiographs (RadioVisioGraphy; Trophy Radiology S.A., Paris, France) were obtained using the long-cone paralleling technique at 10 cm distance between the x-ray head and the digital sensor. To standardize the exposure geometry, a customized bite-block was adjusted on the sensor holder and stored at room temperature for reuse. The landmarks assessed on each radiograph were the CEJ (cementoenamel junction) and the BD (bottom of defect). The width of the defect and the distance CEJ-BD were linearly assessed with reference to a 5-mm metal bar attached onto the sensor using the VixWin™ Platinum|Gendex software. In addition, at baseline (day of surgery) and 12 months, radiographs were digitally registered and pairwise subtracted as previously described (17). In brief, the 12-month radiograph was subtracted from that taken at baseline using a digital image-processing software (EIKONA Subtraction Radiography) (17). To correct the geometrical distortions of the x-rays before their subtraction, several pairs of user-defined landmark points that correspond to identical anatomical elements on the two digital radiographs were selected and images were superimposed after eliminating any brightness and contrast differences that might exist between them.
Clinical cases
Four clinical cases of interdental periodontal defects are reported here to describe the CST. Case-1 had no clinical signs of periodontitis in the rest of her dentition but demonstrated facial flaring of the #12 and a diastema in-between the lateral and central incisor due to a previous periodontal abscess as self-reported (Figure 2). The defect-associated papilla had negative architecture and gingival recession was associated with the interproximal surfaces of the two implicated incisors. The CST was applied, and following thorough subgingival root instrumentation and gentle osseous curettage, the site was irrigated before the next step. The biocomplex was then transplanted into the bottom of the osseous defect and was submerged by the flaps and the interdental papilla which remained intact during surgical manipulation of the tissues. Composite resin (Tetric EvoCeram; Ivoclar Vivadent AG, Liechtenstein) was used to create a contact point between the two incisors to drive the soft tissue healing and hold in place the cross-linked aFPL that covered the surgical site as a film. At 3 months, a second application of a-BMMSCs was applied. Five million a-BMMSCs were suspended in autologous platelet-rich plasma (PRP) lysate at a total volume of 100 μl and were delivered to the clinic in an insulin syringe having a cap instead of a needle. The cell preparation was then injected (23G, 19 mm; NIPRO) within the gingiva at the base of the defect-associated papilla on the buccal and lingual aspects and the patient was closely followed for 12 months. At each follow-up visit, the composite resin was carefully recontoured interdentally (Hawe™ Finishing and Polishing Strips; Kerr, Switzerland) to allow space for the gingival tissues to grow. This was repeated until macroscopic evidence of positive soft tissue architecture was achieved, approximately at 6 months.
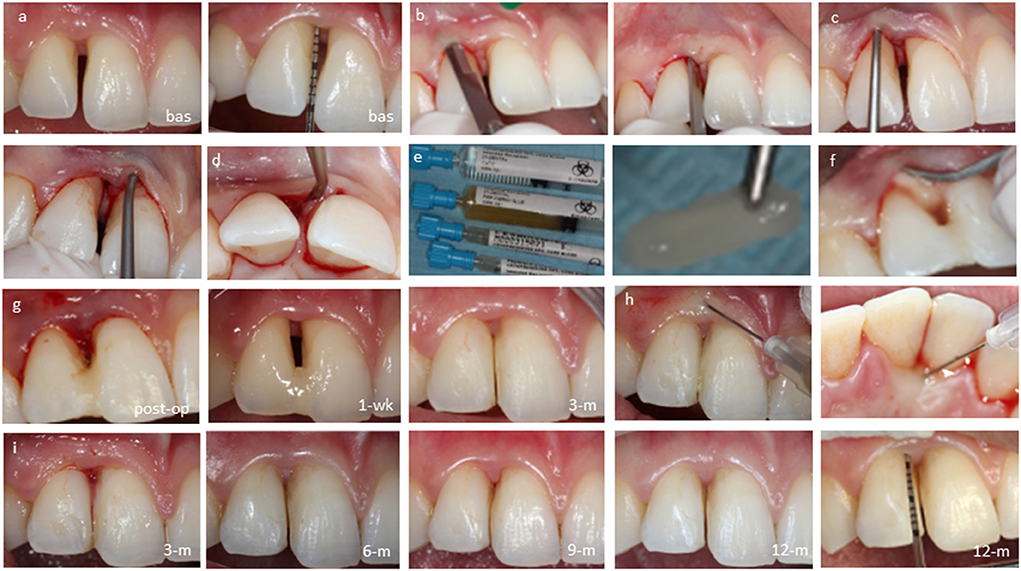
Figure 2. Case-1, #12 mesial: (a) baseline status before anesthesia; (b) intrasulcular dissection of the periodontal attachment; (c) full-thickness gingival flaps retracted from the alveolar bone in a closed manner; tunneled papilla (d) soft tissue retraction to obtain access to the osseous defect; (e) tissue-engineered biocomplex assembled chairside; (f) subgingival application of the biocomplex; (g) immediately postoperative with cross-linked autologous fibrin platelet lysate in place at 1-week and 3-month follow-up; (h) second application at 3 months of autologous clinical-grade alveolar bone marrow mesenchymal stem cells suspended in autologous platelet-rich plasma lysate in an injectable solution; (i) immediately postoperative at 3 months and at 6, 9, and 12 months.
Three additional interdental periodontal defects in the anterior region were treated by the CST (Cases: #2–4, Figures 3–5, respectively). In case 2, the test tooth had mobility Grade-II and was splinted preoperatively. Following the CST application, thorough mechanical instrumentation was performed to the bottom of the osseous defect taking care not to sever the gingival flaps. The sites were irrigated with saline to remove any debris before the biocomplex was carefully placed into the defect. No sutures were employed to stabilize the flaps and treatment outcomes were assessed clinically and radiographically over 12 months.
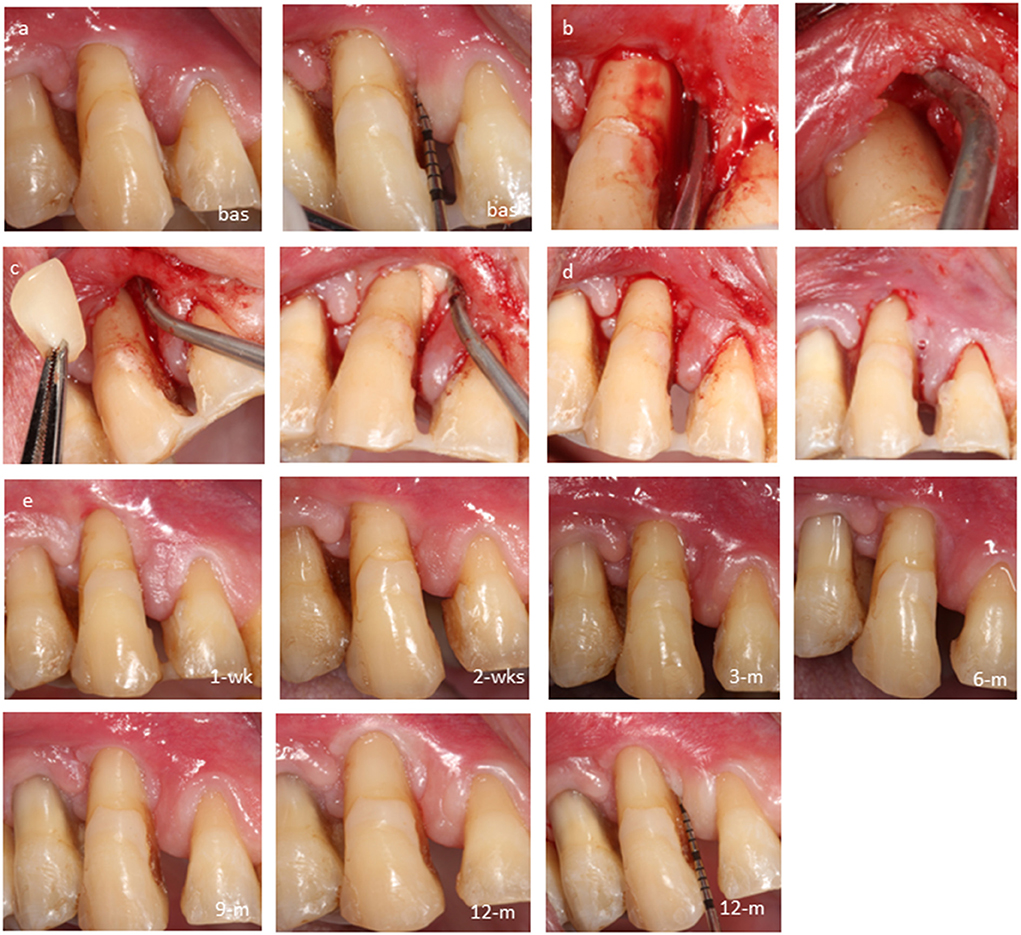
Figure 3. Case-2, #13 mesial: (a) baseline probing before anesthesia, (b) closed retraction of gingival flaps including the papilla to obtain access to the bottom of defect, (c) subgingival application of the biocomplex; (d) immediate postoperative view; cross-linked autologous fibrin platelet lysate by CaCl2 seals the sulcus; (e) follow-ups at 1 week, 2 weeks, 3 months, 6 months, 9 months, and 12 months.
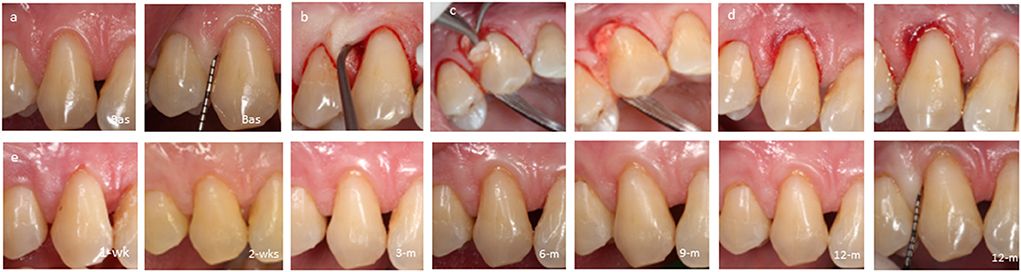
Figure 4. Case-3, #13 distal: (a) baseline probing before anesthesia; (b) closed retraction of flaps; (c) subgingival application of the biocomplex; (d) immediate postoperative view; cross-linked autologous fibrin platelet lysate by CaCl2 seals the sulcus; (e) follow-ups at 1 week, 2 weeks, 3 months, 6 months, 9 months, and 12 months.
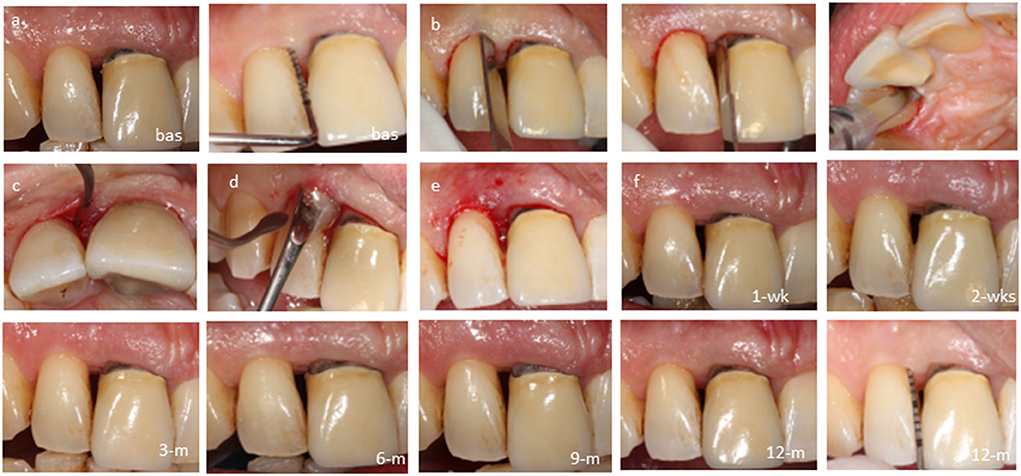
Figure 5. Case-4, #12 mesial: (a) baseline probing before anesthesia; (b) intrasulcular dissection of the periodontal attachment; (c) closed retraction of gingival flaps including the papilla to gain access to the bottom of the defect; (d) biocomplex transplantation via the opening of the sulcus; (e) immediate postoperative view; cross-linked autologous fibrin platelet lysate by CaCl2 seals the sulcus; (f) follow-ups at 1 week, 2 weeks, 3 months, 6 months, 9 months, and 12 months.
Postoperative care
Postoperative pain and oedema were controlled by 1x oral intake of Ibuprofen, 400 mg. All participants were prescribed with antibiotics (Amoxicillin, 500 mg per 8 h for 5 days) and were instructed to apply chlorhexidine gel (CHX-0.2%) twice daily for 2 weeks. Any postoperative adverse events (i.e., swelling, suppuration, incomplete flap closure/flap dehiscence, discomfort/persistent pain) were recorded at the 1- and 2-week follow-ups and thereafter (6 weeks, 3, 6, 9, 12 months). Tooth brushing using a postsurgical brush resumed at 2 weeks. The use of interdental brushes of one size smaller than the optimal soaked in CHX-0.12% commenced at 4 weeks, while interdental brushes of appropriate size were used after 6 weeks. Patients received oral hygiene reinforcement and supragingival plaque control throughout the study period.
Treatment outcomes
During the 12-month study period, no adverse healing events, pain, or swelling were recorded. At 12 months, recession remained either stable (2/4) or decreased (2/4) (Table 1), leading to an aesthetic outcome. In 3/4 subjects PPD was reduced [average ± SD: 2 ± 1 mm; 32.5 ± 10.9 %], while in one subject there was no change in PPD. Gain in attachment levels was noted in all cases (2.25 ± 1.90 mm; 25.8 ± 20.1 %). Radiographic examination revealed tissue stability (Table 1) and subtraction radiography demonstrated remineralisation of the alveolar osseous contour as indicated by the white regions noted mainly on the crest of the associated sites at 12 months (Figure 6).
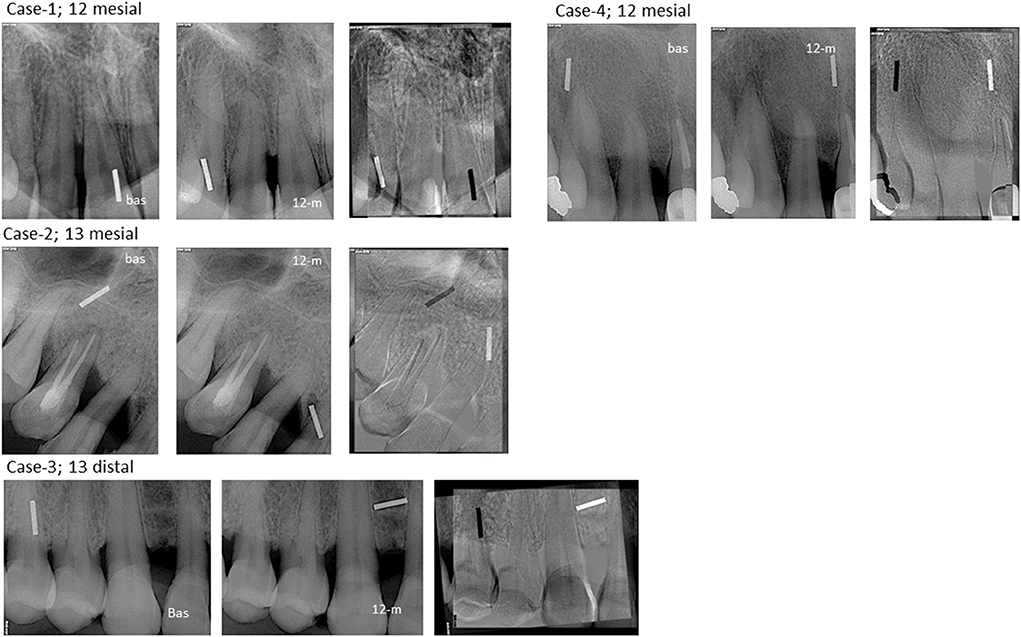
Figure 6. Radiographic assessment of cases #1–4. Regions in which both radiographic images had the same intensity are shown as gray on the digital subtractive image. Regions in which the later radiograph (12-m) is more radiolucent than the earlier one (baseline) are demonstrated as dark regions on the digital subtractive image, whereas regions in which the later radiograph is more radio-opaque than the earlier one, i.e., regions that correspond to bone apposition, are presented as white regions. White regions are evident on the alveolar crest of all cases.
Discussion
Soft tissue architecture and firmness of the gingival tissues improved postoperatively along with clinical attachment levels. Remineralisation of the alveolar crest was radiographically evident at 12 months in all cases. The closed surgical technique was well-tolerated by patients. There are several elements in the CST that may account for an enhanced treatment outcome, and its advantages are comprehensively summarized. The CST is related to (i) wound stability and rich vascularisation of flaps as gingival tissues are not dissected except for their periodontal attachment, (ii) primary wound closure as interdental papillae are left intact to protect the blood clot and reconstructed tissues within the defect, (iii) access to the root surface and to the osseous defect albeit the narrow field of operation for effective subgingival mechanical instrumentation, (iv) ability for application of agents and/or biomaterials in direct contact with the osseous defect and the root surface, (v) minimal tissue trauma during flap detachment from the osseous surface and thus comfort to the patient in absence of postoperative pain and discomfort, (vi) no vascular compromise and/or bacterial contamination of the site as no sutures are employed to stabilize the flaps, (vii) retention of the full volume of the gingival tissues as granulation tissue is not intentionally excised, and (viii) no scar tissue formation. The CST may be applied to treat intrabony defects regardless of depth or width but can also be applied at suprabony defects. On the other hand, it is a sensitive surgical procedure. It requires experience of the operator in soft-tissue handling to avoid severance of the gingival flaps during “closed” retraction, especially when of thin phenotype or when the defect is not isolated or located in the posterior region. The minimum interdental width of 2 mm safeguards the integrity of the papilla during closed retraction and tunneling, especially in thin phenotypes.
The CST entails full-thickness gingival flaps via a sulcular incision that dissects the periodontal attachment, and flap retraction does not necessarily extend beyond the mucogingival line. It is indicated for the reconstruction of periodontal defects regardless of the gingival phenotype; however, it is recommended for isolated periodontal defects to better control the handling of tissues with residual inflammation. In this clinical setting, the CST combined with the biocomplex was applied to moderate/deep periodontal pockets with a vertical component that was wide and <3 mm deep, which might have accounted for the lack of robust PPD changes. However, gain in attachment was noted in all cases taking into consideration that soft tissue healing was of outmost importance as all defects were in the aesthetic region with evidence of recession prior to surgery. The attachment gain reported here is in line with previous data reported on regenerative studies (12). Hereby, the CST was used in combination with a tissue-engineered construct—the biocomplex—which possibly reformed the site and regulated the remaining inflammatory infiltrate (15) to drive connective tissue augmentation. The most likely scenario is that the tissue-resident stem cells reconstruct the damaged periodontal tissues as stimulated by the bioactive factors secreted by the engrafted MSCs that have immunomodulatory and trophic properties (18). However, taking the regulatory and economical constraints of cell therapy, as previously discussed, the biocomplex may be best indicated to apply in nonsupporting defects where it may show its greatest healing potential (15). The use of the CST could be alternatively combined with other bioactive agents (i.e., EMD, platelet concentrates, hyaluronic acid) and can be otherwise applied in self-contained defects. However, tissue-friendly and patient-centered surgical techniques that minimize tissue trauma and retain the full volume of tissues and blood supply may trigger the intrinsic healing potential of a wound. Clinical improvements may be obtained in absence of grafting materials (19). Likewise, the flap design described here may be applied without the use of products as it offers wound stability and rich blood supply while it protects blood clot formation by primary wound closure. The efficacy of these applications should be further tested by a randomized clinical study with traditional flap surgery serving as the control.
A tissue-engineering protocol has been previously described for papilla reconstruction employing autologous iliac crest-derived BMMSCs, PRP, and hyaluronic acid in addition to thrombin/CaCl2 to obtain an insoluble gel (20). Prior to polymerization, this gel was injected into the mucosa between two implant-borne central incisors while no flaps were raised during the operation. Subtle improvements of the mucosal papilla were found postoperatively. Interestingly, the most pronounced changes were noted at 6 months in the configuration of the associated prosthesis with wider contact surfaces interproximally that filled up the black triangle in-between the two implant-borne incisors. However, the two protocols are not strictly comparable as the current report describes a novel surgical technique based on “closed” retraction of flaps to manage a periodontal osseous defect associated with a soft tissue defect due to a periodontal abscess. In contrast, the previous work reported on improvements of a black triangle in-between implants placed in resorbed alveolar bone following trauma employing tissue engineering alone (20). The importance of minimizing recession in the treatment of vertical osseous defects has been highlighted in a recent study (21). In that study, soft tissue trauma was lessened during cause-related therapy and the buccal flap was advanced coronally to increase the potential for attachment gain following surgery. In line with current findings, stable or reduced levels of recession are central to the treatment design and indicate optimal wound healing of both the hard and soft tissues.
In this surgical design, vertical releasing incisions were avoided, and flaps were stabilized without employing sutures. While sutures have no negative effect on healing when there is minimal tension and adequate blood supply of the flap, they may interfere with blood circulation, especially in the interdental region and the papilla that is the most coronal part of a flap (22). However, if coronal advancement of the flaps is sought to minimize gingival recession in parallel with the treatment of an intrabony defect, then the CST might be combined with interdental sutures using bonded contact points as an anchorage (23).
Conclusion
This report describes a pioneer periodontal treatment procedure employing a novel surgical technique in combination with the tissue-engineered biocomplex. The CST is designed for the reconstruction of intrabony and suprabony residual periodontal defects. This technique may be best indicated for defects located in the anterior region, where there are aesthetic considerations as it does not dissect the gingival papillae or the attached gingiva. The CST aims to retain and/or improve the initial architecture of the soft tissues, while it allows full access to an osseous defect. It can be applied as a sole treatment approach to facilitate deep instrumentation of an isolated periodontal defect preserving the soft tissue volume, or it could be combined with subgingival application of regenerative materials and/or biological agents. In addition, the CST led to periodontal clinical and radiographic improvements and appeared to be well tolerated by patients who experienced a minimally invasive surgery and thus minimum postoperative discomfort. It should be noted that the limited number of cases reported hereby does not allow us to draw robust conclusions. The efficacy of this novel surgical technique should be assessed vs. the access flap in a randomized clinical trial on defects with a deep intrabony component and report on short- and long-term treatment outcomes in addition to patient centered outcomes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Dental School Ethics Committee, AUTh (322/15-4-2013). The patients/participants provided their written informed consent to participate in this study.
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
The T. Koulourides Research Award partially funded the current study for the cell therapy component.
Acknowledgments
I would like to thank the helpful staff of the laboratory facility of Biohellenika for the clinical-grade alveolar-bone marrow mesenchymal stem cells (a-BMMSCs) preparation, G. Mikrogrorgis for his technical advice on the digital radiograph subtraction analysis, and Chris Ntantos for his technical assistance in designing the schemas of the CST in Figure 1.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Harrel SK, Rees TD. Granulation tissue removal in routine and minimally invasive procedures. Compend Contin Educ Dent. (1995) 16:960, 962, 964 passim.
2. Cortellini P, Prato GP, Tonetti MS. The modified papilla preservation technique. A new surgical approach for interproximal regenerative procedures. J Periodontol. (1995) 66:261–6. doi: 10.1902/jop.1995.66.4.261
3. Cortellini P, Prato GP, Tonetti MS. The simplified papilla preservation flap. A novel surgical approach for the management of soft tissues in regenerative procedures. Int J Periodontics Restorative Dent. (1999) 19:589–99.
4. Cortellini P, Tonetti MS. A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra-bony defects: a novel approach to limit morbidity. J Clin Periodontol. (2007) 34:87–93. doi: 10.1111/j.1600-051X.2006.01020.x
5. Cortellini P, Tonetti MS. Improved wound stability with a modified minimally invasive surgical technique in the regenerative treatment of isolated interdental intrabony defects. J Clin Periodontol. (2009) 36:157–63. doi: 10.1111/j.1600-051X.2008.01352.x
6. Trombelli L, Farina R, Franceschetti G, Calura G. Single-flap approach with buccal access in periodontal reconstructive procedures. J Periodontol. (2009) 80:353–60. doi: 10.1902/jop.2009.080420
7. Ribeiro FV, Casarin RC, Palma MA, Júnior FH, Sallum EA, Casati MZ. Clinical and patient-centered outcomes after minimally invasive non-surgical or surgical approaches for the treatment of intrabony defects: a randomized clinical trial. J Periodontol. (2011) 82:1256–66. doi: 10.1902/jop.2011.100680
8. Harrel SK, Wilson TG, Nunn ME. Prospective assessment of the use of enamel matrix proteins with minimally invasive surgery. J Periodontol. (2005) 76:380–4. doi: 10.1902/jop.2005.76.3.380
9. Cortellini P, Tonetti MS. Clinical and radiographic outcomes of the modified minimally invasive surgical technique with and without regenerative materials: a randomized-controlled trial in intra-bony defects. J Clin Periodontol. (2011) 38:365–73. doi: 10.1111/j.1600-051X.2011.01705.x
10. Trombelli L, Simonelli A, Pramstraller M, Wikesjö UM, Farina R. Single flap approach with and without guided tissue regeneration and a hydroxyapatite biomaterial in the management of intraosseous periodontal defects. J Periodontol. (2010) 81:1256–63. doi: 10.1902/jop.2010.100113
11. Clementini M, Ambrosi A, Cicciarelli V, De Risi V, de Sanctis M. Clinical performance of minimally invasive periodontal surgery in the treatment of infrabony defects: systematic review and meta-analysis. J Clin Periodontol. (2019) 46:1236–53. doi: 10.1111/jcpe.13201
12. Nibali L, Koidou VP, Nieri M, Barbato L, Pagliaro U, Cairo F. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: a systematic review and meta-analysis. J Clin Periodontol. (2020) 47:320–51. doi: 10.1111/jcpe.13237
13. Tu YK, Needleman I, Chambrone L, Lu HK, Faggion CM Jr. A Bayesian network meta-analysis on comparisons of enamel matrix derivatives, guided tissue regeneration and their combination therapies. J Clin Periodontol. (2012) 39:303–14. doi: 10.1111/j.1600-051X.2011.01844.x
14. Iwasaki K, Peng Y, Kanda R, Umeda M, Ishikawa I. Stem cell transplantation and cell-free treatment for periodontal regeneration. Int J Mol Sci. (2022) 23:1011. doi: 10.3390/ijms23031011
15. Apatzidou DA, Bakopoulou AA, Kouzi-Koliakou K, Karagiannis V, Konstantinidis A. A tissue-engineered biocomplex for periodontal reconstruction. A proof-of-principle randomized clinical study. J Clin Periodontol. (2021) 48:1111–25. doi: 10.1111/jcpe.13474
16. Sculean A, Allen EP. The laterally closed tunnel for the treatment of deep isolated mandibular recessions: surgical technique and a report of 24 cases. Int J Periodontics Restorative Dent. (2018) 38:479–87. doi: 10.11607/prd.3680
17. Mikrogeorgis G, Lyroudia K, Molyvdas I, Nikolaidis N, Pitas I. Digital radiograph registration and subtraction: a useful tool for the evaluation of the progress of chronic apical periodontitis. J Endod. (2004) 30:513–7. doi: 10.1097/00004770-200407000-00013
18. Caplan AI. Mesenchymal stem cells: time to change the name! Stem Cells Transl Med. (2017) 6:1445–51. doi: 10.1002/sctm.17-0051
19. Cortellini P, Tonetti MS. Clinical concepts for regenerative therapy in intrabony defects. Periodontol 2000. (2015) 68:282–307. doi: 10.1111/prd.12048
20. Yamada Y, Nakamura S, Ueda M, Ito K. Papilla regeneration by injectable stem cell therapy with regenerative medicine: long-term clinical prognosis. J Tissue Eng Regen Med. (2015) 9:305–9. doi: 10.1002/term.1737
21. Zucchelli G, De Sanctis M. A novel approach to minimizing gingival recession in the treatment of vertical bony defects. J Periodontol. (2008) 79:567–74. doi: 10.1902/jop.2008.070315
22. McLean TN, Smith BA, Morrison EC, Nasjleti CE, Caffesse RG. Vascular changes following mucoperiosteal flap surgery: a fluorescein angiography study in dogs. J Periodontol. (1995) 66:205–10. doi: 10.1902/jop.1995.66.3.205
Keywords: closed flap retraction, tissue engineering, tissue trauma, papilla reconstruction, intrabony defect, soft tissue tunneling, gingival flaps
Citation: Apatzidou DA (2022) A pioneer surgical technique for isolated periodontal defects by “closed” retraction of the papilla: A feasibility study. Front. Dent. Med. 3:956601. doi: 10.3389/fdmed.2022.956601
Received: 30 May 2022; Accepted: 28 June 2022;
Published: 15 July 2022.
Edited by:
Georgios N. Belibasakis, Karolinska Institutet (KI), SwedenReviewed by:
Elena Calciolari, University of Parma, ItalyOleh Andrukhov, University Dental Clinic Vienna, Austria
Copyright © 2022 Apatzidou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danae Anastasia Apatzidou, ZGFwYXR6aWRvdUBkZW50LmF1dGguZ3I=; cGVyaW9hcGF0emlkb3VAeWFob28uZ3I=
 Danae Anastasia Apatzidou
Danae Anastasia Apatzidou