- Department of Orthodontics, University at Buffalo, Buffalo, NY, United States
Background: Functional Movement Disorder (FMD) is a neurological condition involving involuntary movements without structural brain or nerve damage. It can significantly affect the craniofacial region, disrupting facial and oral motor functions and complicating dental and orthodontic care. This narrative review outlines the clinical presentation of FMD, emphasizing its relevance in orthodontics and offering a clinical management guide.
Findings: A systematic approach is proposed, detailing strategies from the initial screening visit through active treatment, retention, and post-retention stages. Key strategies include using fixed appliances for better control, scheduling shorter visits to reduce symptom aggravation, and incorporating distraction techniques. Collaborative care with neurologists, psychiatrists, psychologists, physical therapists, and dental professionals is vital, addressing both motor and psychological factors.
Conclusion and relevance: Specialized training, improved diagnostic methods, and customized treatment plans are crucial for managing FMD in orthodontics. These efforts are necessary to optimize care and outcomes for affected patients.
Introduction
Functional Neurological Disorder (FND) is a condition that presents with a variety of clinical manifestations, including weakness, sensory changes, involuntary movements, gait disturbance, dissociative episodes and speech problems (1). It is described as a multi-network disorder involving abnormalities within and across brain circuits which are responsible for sending and receiving signals across the nervous system (2). FND often resembles organic neurological diseases such as stroke, epilepsy, or multiple sclerosis, but unlike those conditions, FND symptoms arise from disruptions in brain network function, rather than anatomical abnormalities (3, 4).
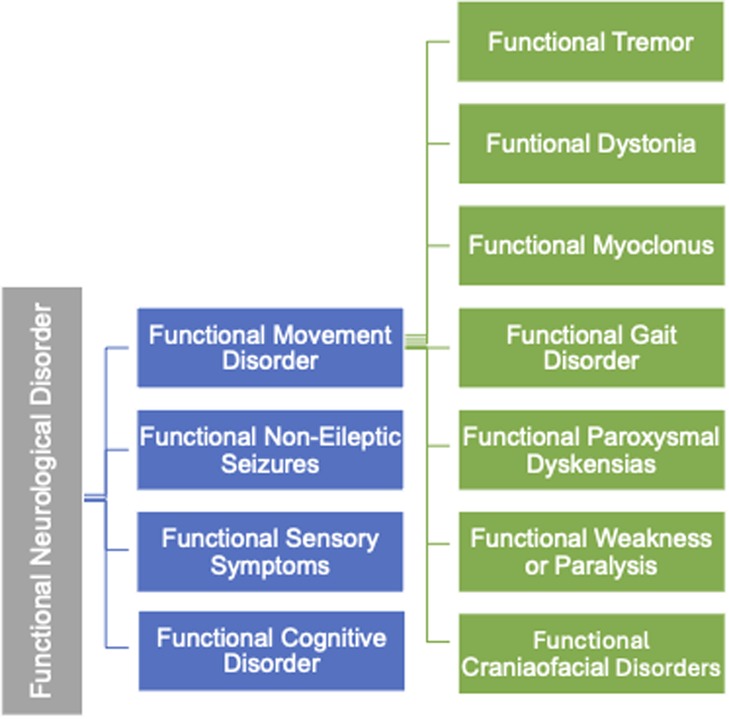
The variability in clinical manifestations has led to the identification of distinct subtypes within FND, one of which is Functional Movement Disorder (FMD) (5). FMD is a condition where patients experience abnormal, involuntary movements without underlying structural damage to the brain or nervous system. The classification of FMD is illustrated in Figure 1.
Functional movement disorder
Initially, FMD was classified as a psychogenic disorder and placed under the broader category of conversion disorders. Conversion disorders (CD) are loss or distortion of neurological function that cannot be fully explained by a known organic neurological disease (6). However, modern research has revealed that the FMD disorder is influenced by a combination of neurobiological, psychological, and environmental factors (7). This paradigm shift has led to updates in diagnostic criteria, which no longer require the identification of psychological triggers, though they may play a role in symptom onset (8).
In addition to motor symptoms, the diagnostic criteria for FMD now emphasize sensory processing and the influence of emotional and environmental factors in triggering or intensify symptoms (9). This shift has fostered a more holistic definition of FMD, recognizing its multifactorial nature by integrating both psychological and neurological component. FMD can severely impact patients’ quality of life, often resulting in substantial disability, similar to that observed in organic neurological disorders (10). This narrative review discusses FMD by reviewing its clinical presentation, prevalence, and etiology, management. It also serves as a clinical guide for orthodontists in managing patients affected by FMD within the orthodontic practice.
Clinical features
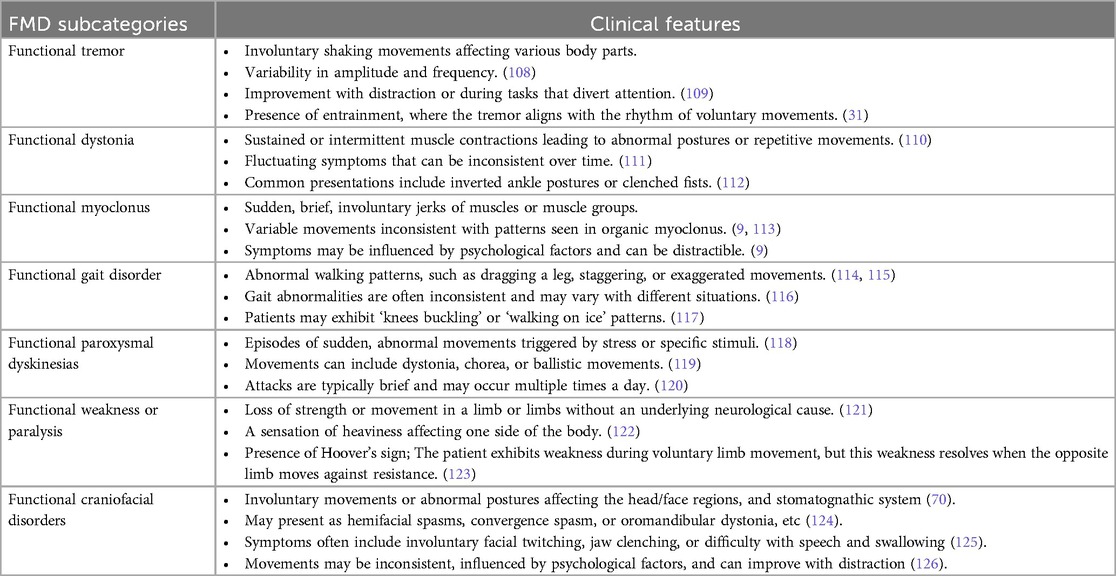
The clinical presentation of FMD is highly variable. Common motor symptoms of FMD include tremors, dystonia, myoclonus, gait disturbances jerks, and paresis (11). One of the key characteristics of FMD is its abrupt onset, often triggered by physical or psychological stressors (12). Table 1 summarizes the clinical features of FMD.
Functional dystonia is characterized by unusual postures or abnormal muscle contractions, but unlike organic dystonia, these symptoms can change over time or even reverse spontaneously. Symptoms often improve when patients are distracted, but they may worsen when attention is focused on the movement. For example, during a neurological examination, a patient's tremor might decrease or disappear when they are asked to perform a cognitive task, such as counting backwards (13).
Patients with FMD may experience a range of non-motor symptoms, including sensory disturbances, cognitive difficulties such as memory loss, and dissociative experiences (14).
Emotional factors, such as anxiety and depression, are frequently linked to FMD, complicating its clinical presentation. Psychiatric comorbidities can intensify symptoms, leading to a more severe and chronic expression of the disorder (15).
Prevalence
The true prevalence of FMD remains difficult to estimate due to factors such as underdiagnosis and misdiagnosis. Recent studies suggest that FMD accounts for 2%–20% of patients seen in movement disorder clinics, although this varies based on location and diagnostic criteria applied (16). Epidemiological studies have consistently shown that FMD is more prevalent in women, with a female-to-male ratio of approximately 4:1 (17). The prevalence of FMD phenotypes varies depending on the specific presentation and population. In Western countries, studies report that functional tremor is the most common phenotype, accounting for approximately 36% of cases, followed by functional dystonia at around 34% (18).
FMD typically presents in midlife, between the ages of 35 and 45 years, though this likely underestimates cases in pediatric and elderly populations (19). In pediatric populations, FMD may emerge after acute stressors, such as school-related pressures or family conflicts, while in older adults, it may be misdiagnosed as age-related conditions like Parkinsonism (20). Socioeconomic factors also contribute to the prevalence of FMD, with higher rates observed among individuals from lower socioeconomic backgrounds (21).
Etiology
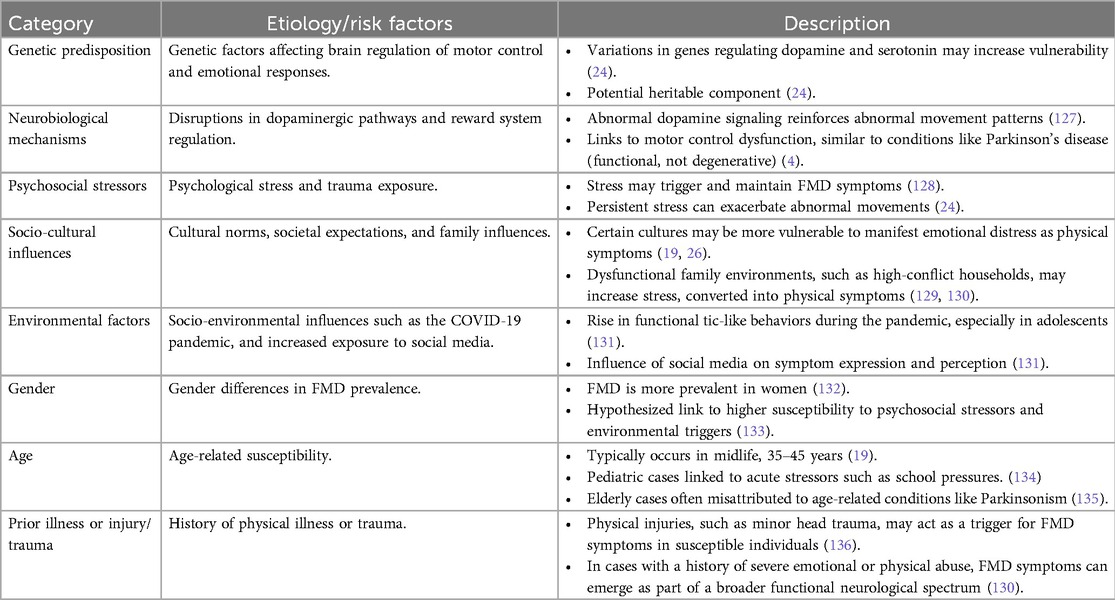
The etiology of FMD is complex, involving interactions between several contributing factors, including genetic predispositions, alterations in brain networks, and psychosocial stressors (22).
An emerging area of interest is the role of genetic predisposition. Studies suggest that certain individuals may be more susceptible to FMD due to genetic factors that affect the brain's regulation of motor control and emotional responses (23). For example, variations in genes associated with neurotransmitter systems, particularly those regulating dopamine and serotonin, may increase vulnerability to abnormal movement patterns when exposed to stress or trauma. These findings point to a potential heritable component in FMD, though further research is needed to clarify the specific genetic factors involved (24).
Neurobiological findings also emphasize disruptions in the brain's reward system, particularly involving the dopaminergic pathways. Abnormal dopamine signaling may lead to the reinforcement of abnormal movement patterns, contributing to the persistence of symptoms even after the initial stressor has passed (25).
Social and cultural factors can play a significant role in the development of FMD. Research indicates that societal expectations, cultural norms, and familial influences may shape how symptoms are expressed and perceived (19, 26). In some cultures, certain types of emotional distress may be more likely to manifest as physical symptoms like abnormal movements, reflecting a form of somatization (27). A recent study highlights that prevalence of FMD has increased in recent years, particularly in young individuals, possibly due to socio-environmental factors (28). For instance, the COVID-19 pandemic and increased exposure to social media platforms have been linked to the rise in cases of functional tic-like behaviors, especially in adolescents (29). This suggests that sociocultural and psychological factors may significantly influence the presentation and prevalence of FMD in specific populations. A summary of etiology and risk factors associated with FMD are found on Table 2.
Diagnosis
The diagnosis of FMD is based on recognizing positive clinical signs, rather than through a process of exclusion (30). A key diagnostic feature is symptom inconsistency, where abnormal movement patterns vary over time in terms of amplitude, frequency, and distribution (31, 32). Additionally, distractibility is an important indicator; abnormal movements often resolve when the patient's attention is directed elsewhere (33).
Functional neuroimaging, particularly fMRI, has become a key tool in diagnosing FMD. Studies have revealed abnormalities in brain regions associated with motor control, most notably the premotor cortex and thalamus, where FMD patients show reduced volume and connectivity (34).
Beyond the premotor cortex and thalamus, research has identified altered activity in regions such as the supplementary motor area (SMA) and basal ganglia, both of which are critical for voluntary motor control (35). Additionally, hyperactivity in emotional processing centers, including the amygdala and cingulate cortex, has been observed in FMD patients, linking emotional dysregulation to movement dysfunction. The increased functional connectivity between motor regions and the emotion-processing areas suggests that FMD arises from a combined disruption of motor and emotional networks (36).
Electrophysiological testing, including electroencephalogram (EEG) and electromyography (EMG), plays an essential role in diagnosing FMD and distinguishing it from other neurological conditions. These tests assess the electrical activity of muscles and nerves, offering valuable insights into movement abnormalities (37). For example, in cases of psychogenic tremor, EMG can detect co-activation of antagonist muscles—a pattern uncommon in organic movement disorders but characteristic of FMD. This simultaneous contraction of opposing muscles results in inefficient or erratic movement, pointing to a functional, rather than structural, abnormality (38).
Management
Management for FMD incorporates a multidisciplinary approach, blending physical, psychological, neuromodulation, and pharmacological therapies (39).
Physiotherapy plays a pivotal role in the management of FMD. The core components of effective intervention include gaining a comprehensive understanding of the patient's symptoms, assessing the impact on daily function, evaluating the patient's perception and confidence in the established diagnosis, and collaboratively setting clear goals for physiotherapy (40). Research has shown that targeted physiotherapy can be effective in providing sustained symptom relief. In one study, an intensive short-term rehabilitation program led to a 73.5% improvement rate (41).
Occupational therapy, with its holistic approach that addresses physical, mental, and social determinants of health, is also well-suited to assist patients with FMD in maximizing functional outcomes. As in physiotherapy, occupational therapy interventions should be tailored to meet the specific goals of FMD treatment (42).
Neuromodulation therapy represents an exciting and innovative approach for treating complex and challenging cases of FMD, particularly those for whom evidence-based treatment options remain limited. As a novel therapeutic strategy, neuromodulation has the potential to influence key brain networks, positioning it as a promising candidate for addressing the needs of these patients (43).
Transcranial magnetic stimulation (TMS) has long been studied for its ability to noninvasively assess cortical excitability and connectivity (44). Repetitive TMS (rTMS), in particular, has shown potential to produce lasting neuromodulatory effects through mechanisms like long-term potentiation. A recent study involving 33 FMD patients compared TMS applied over the motor cortex contralateral to symptoms against TMS over spinal roots in a control group (45). The observed symptom improvement suggests that nonspecific factors, including behavioral changes or placebo effects, may contribute to the therapeutic response.
Recently, Intermittent Theta Burst Stimulation (iTBS), a form of transcranial magnetic stimulation, has emerged as a potential treatment for FMD. iTBS is a non-invasive brain stimulation technique that involves delivering short bursts of high-frequency magnetic pulses, used to modulate cortical activity in various neurological disorders, including FMD, resulting in reduction of symptoms (46).
The pharmaceutical approach for managing FMD has evolved to encompass a diverse array of agents that target both the symptomatic and underlying neurophysiological abnormalities. By employing various medications, clinicians can tailor interventions to alleviate pain and modulate muscle activity, while improving functional outcomes (47).
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) are commonly prescribed for pain related to muscular, temporomandibular joint (TMJ) issues, migraines, and trauma (48, 49). They work by inhibiting prostaglandin synthesis, reducing inflammation and pain, but may also slow tooth movement at high doses by affecting bone resorption (50–52).
Selective Serotonin Reuptake Inhibitors (SSRIs) are used to treat depressive and anxiety-related disorders, including those linked to movement issues (53). However, they may alter muscle tone and reduce osteoblastic activity, potentially compromising dental enamel and orthodontic tooth movement (54–57).
Botulinum Toxin (BoNT-A) is utilized for a range of hyperkinetic movement disorders such as cervical dystonia, hemifacial spasm, and tics (58, 59). It reduces muscle hyperactivity in the head and neck but may impact craniofacial growth and mandibular development, especially in children (60–63).
Lastly, dopaminergic and anticholinergic agents, used in conditions like Parkinson's disease, dystonia, and tardive dyskinesia, help improve muscle coordination and oral hygiene (64–67). However, they can also cause xerostomia, increasing the risk for dental caries and tooth structure loss (68, 69).
FMD in the craniofacial region
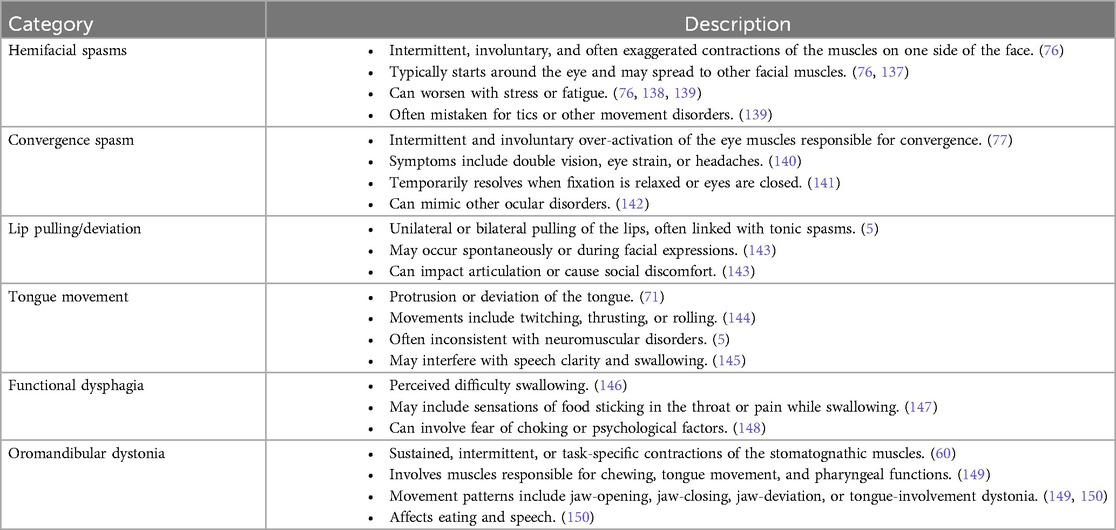
In the craniofacial region, FMD commonly impacts the face, eyes, and stomatognathic system, which encompasses the jaws, tongue, lips, palate, teeth and associated soft tissues (70). The symptoms can vary from involuntary facial movements (hemifacial spasm), abnormal eye movements (convergence spasm), and irregular jaw and tongue movements (oromandibular dystonia) (71). Table 3 presents a summary of the craniofacial characteristics of FMD. Studies suggest that FMD in the craniofacial region may be caused by disruptions in the striatothalamocortical circuits, which are responsible for motor control (72).
Hemifacial spasm involves involuntary contractions, or twitching of the muscles on one side of the face (73). The spasms in FMD may appear intermittently and are often inconsistent in terms of frequency and intensity (74). The condition is often triggered by fatigue, anxiety, stress, and symptoms may persist during sleep (75). It can also be accompanied by pain, and in chronic cases, may lead to the development of ipsilateral facial weakness (76).
Convergence spasm has been reported as the most frequent functional eye movement disorder (71, 77). It involves excessive, involuntary contraction of the muscles responsible for eye convergence, resulting in the eyes turning inward with an inability to focus properly, often leading to blurred or double vision (78). It is often misdiagnosed because it can mimic other conditions originating from organic causes, such as abducens palsy (which occurs when the sixth cranial nerve—abducens nerve, is damaged or is not functioning properly) (77).
Oromandibular dystonia (OMD) is a condition that affects the muscles of the jaw, mouth and tongue, causing abnormal jaw clenching, and involuntary mouth and tongue movements, often leading to difficulties in speaking or swallowing (79). OMD are often suppressed during sleep but may intensify with stress, emotional distress, or fatigue (80). OMD can be caused by chronic exposure to antipsychotic drugs, which is referred to as tardive OMD (81). Dopamine receptor blocking agents are the most common drug group implicated in the causation of this condition (82). Also, oromandibular-facial trauma, dental procedures and parotid gland surgery have been reported to exacerbate OMD (83). In clinical practice, botulinum toxin (BoNT) injection is considered to be the most effective treatment for OMD, supported by various small and large scale studies (84, 85).
Orthodontic implications
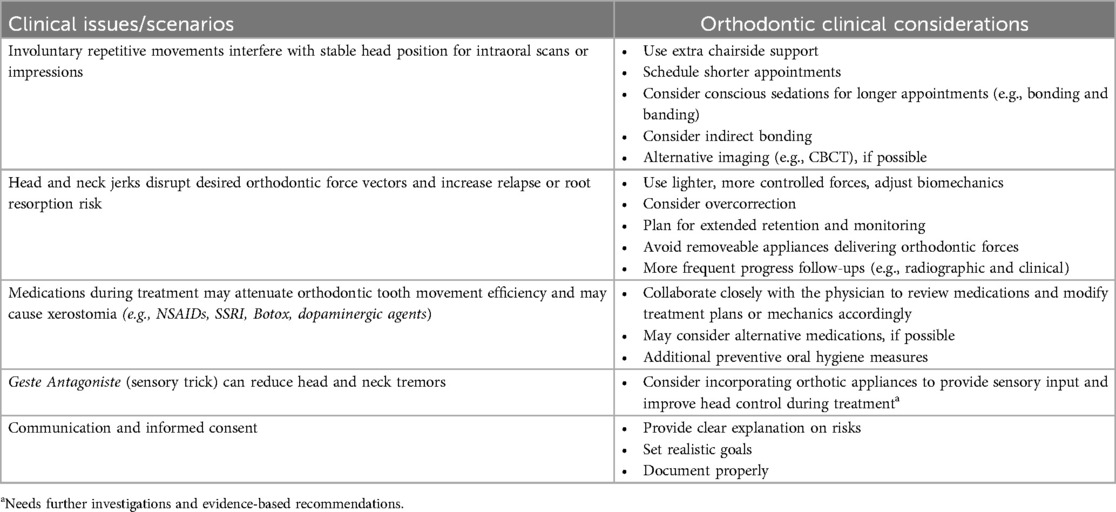
FMD can have profound implications in the dental and orthodontic settings, particularly because of the complex interactions between facial muscles and oral structures, which can interfere with routine dental and orthodontic procedures impacting treatment planning, biomechanics and retention protocols and follow ups significantly (60, 86).
Recognizing the symptoms
Dental professionals should be well-prepared and adequately trained to recognize and differentiate the symptoms of FMD, allowing them to develop and implement tailored treatment plans for these patients. Overlapping symptoms, such as jaw pain, restricted mouth opening, and chewing difficulties are common in both temporomandibular disorders (TMD) and OMD. However, TMD is typically characterized by localized joint pain, often accompanied by clicking or popping sounds during joint movement. It arises primarily from mechanical or structural disturbances affecting the TMJ and associated musculature, frequently linked to parafunctional habits like bruxism (87). Conversely, OMD is a functional movement disorder involving dysfunction in motor control pathways, particularly within the basal ganglia (88). It is marked by involuntary, sustained muscle contractions and abnormal movements, with less emphasis on joint dysfunction.
A distinguishing feature of OMD is the presence of a sensory trick, or geste antagoniste, where simple tactile stimuli, such as touching the chin, can momentarily suppress the dystonic movement (89). This phenomenon is absent in TMD and serves as a valuable clinical indicator to differentiate it from OMD. Additionally, while TMD is predominantly pain-driven, OMD patients primarily experience uncontrollable movements, with discomfort emerging as a secondary consequence of muscle fatigue or joint stress. Importantly, these conditions may coexist or influence one another. The repetitive, involuntary jaw movements in OMD can impose abnormal forces on the TMJ, leading to secondary TMD symptoms such as joint pain or disc displacement (90). On the other hand, chronic TMD-related pain and dysfunction may increase muscle tension and, in susceptible individuals, trigger or exacerbate dystonic patterns (91). Unlike TMD, which follows predictable biomechanical patterns of pain and movement restriction, FMD symptoms may fluctuate with psychological stressors and often lack a clear anatomical basis. The hallmark of FMD includes task-specific manifestations that can transiently improve with distraction or simple maneuvers, further distinguishing them from structural TMJ disorders (92, 93).
Patient management
FMD is often associated with heightened pain sensitivity, making routine orthodontic adjustments more uncomfortable for patients. It can also cause changes in occlusion due to involuntary muscle contractions, spasms, and abnormal tongue movements, leading to dental misalignment such as open bites, cross bites, and shifting teeth. Parafunctional habits like bruxism and tongue thrusting may further cause enamel wear, tooth mobility, and malocclusion.
To effectively accommodate patients with FMD, dental professionals should begin by establishing clear treatment goals and setting realistic expectations. It is essential to explain any potential risks and complexities in a calm, reassuring manner, while also maintaining thorough and accurate documentation.
During treatment, consider using shorter appointment durations to help prevent fatigue and reduce the risk of overstimulation, both of which can exacerbate functional symptoms. Scheduled breaks can further improve patient tolerance and comfort. Additionally, incorporating distraction techniques such as light, reassuring conversation, calming background music, or sensory tools like stress balls may help reduce anxiety and support a more relaxed experience. Additionally, the use of nitrous oxide sedation during appointments can be an effective option when other pain management techniques prove insufficient.
Orthodontic mechanics
The sustained or repetitive contractions of the jaw muscles make it difficult for patients to maintain a stable jaw posture during dental treatment (5).
Abrupt head movements and spasms commonly associated with FMD may compromise traditional impression-taking, often requiring multiple attempts due to movement-induced errors. To mitigate this challenge, clinicians may consider alternative modalities, such as intra-oral scanning and cone-beam computed tomography, which offers reduced acquisition time and improved accuracy (94, 95).
Involuntary tongue movements, jaw spasms, and other motor disturbances can dislodge wires, accelerate appliance wear, and require frequent adjustments or replacements, thereby prolonging treatment duration and increasing the risk of dental complications. Similarly, extraoral appliances, such as reverse pull headgear, maybe poorly tolerated by FMD patients. Involuntary movements can destabilize these appliances, causing discomfort and placing excessive stress on dental and craniofacial structures, potentially compromising treatment outcomes.
Given these challenges, fixed appliances may be more suitable for FMD patients, as they provide greater stability and are less affected by involuntary movement-related disruptions.
Indirect bonding techniques can provide significant advantages for FMD patients by reducing chair time during bonding procedures. This approach lowers the risk of accidental events, such as self-inflicted oral injuries or broken brackets from involuntary jaw movements.
Orthodontists should frequently reassess occlusal stability and consider adjustments like occlusal splints. Splints may exert therapeutic effects by modulating sensory signals transmitted via the trigeminal nerve to the sensory trigeminal nucleus (96–99). This nucleus spans from the cervical spinal cord to the mesencephalon and interfaces with reticular interneurons across various levels of the central nervous system. The structural and functional integration of this trigeminal complex may explain the observed overlap between sensory tricks and the use of oral appliances in managing OMD (96). However, the precise mechanisms by which the trigeminal nucleus modulates dystonic movements remain poorly understood. While oral appliances show promise as an adjunct to medical treatments, further research is necessary to clarify their role and optimize their design.
Overall, careful appliance selection and regular monitoring are critical to ensure optimal care and minimize side effects.
Orthodontic tooth movement
Physiologic orthodontic tooth movement relies on forces of appropriate magnitude and vector to induce bone remodeling on both the compression and tension sides of the periodontal ligament (100). Any disruption in this process can impair orthodontic mechanics and result in imprecise tooth movement. In patients with OMD, unbalanced forces from peri-oral muscles and the masseteric apparatus may counteract the vector-specific forces applied by orthodontic appliances.
Furthermore, high-frequency tremors and unpredictable repetitive forces resulting from orofacial tics may introduce uncontrolled micro forces on teeth, potentially accelerating root resorption in susceptible patients, further complicating orthodontic treatment (101, 102).
Retention and relapse
Retention is important for patients with FMD, as involuntary muscle forces can lead to relapse even after achieving satisfactory occlusion during orthodontic treatment (103).
Fixed lingual retainers are commonly utilized to counteract the undesirable forces associated with muscle hyperactivity, providing continuous stabilization of the dental arches.
Pharmacologic approaches, such as botulinum toxin (BoNT-A) injections, have shown promise in reducing abnormal muscular activity, which can help improve stability after active orthodontic treatment (104).
These interventions may be beneficial during the retention phase, as they can mitigate the impact of involuntary muscle forces. By integrating appropriate retention strategies and considering adjunctive therapies like BoNT-A, orthodontists can enhance treatment stability and minimize the risk of relapse in FMD patients.
Effects of medications for managing FMD on orthodontic treatment
Medications used to manage FMD can significantly influence orthodontic treatment outcomes by affecting tooth movement, dentoalveolar and craniofacial growth, retention, and relapse. These effects often result from mechanisms such as altered bone remodeling, muscle activity regulation, and oral health changes like xerostomia and dental attrition (105).
Some medications may slow tooth movement by inhibiting bone turnover, while others can compromise retention by increasing the risk of enamel wear or muscle hyperactivity (106). Understanding these interactions is crucial for orthodontists to make adjustments to treatment plans, minimize complications, and ensure long-term stability, especially in patients requiring prolonged medication use. Table 4 summarizes various medication classes used in managing FMD and their effects on OTM, dentoalveolar growth, retention, and relapse.
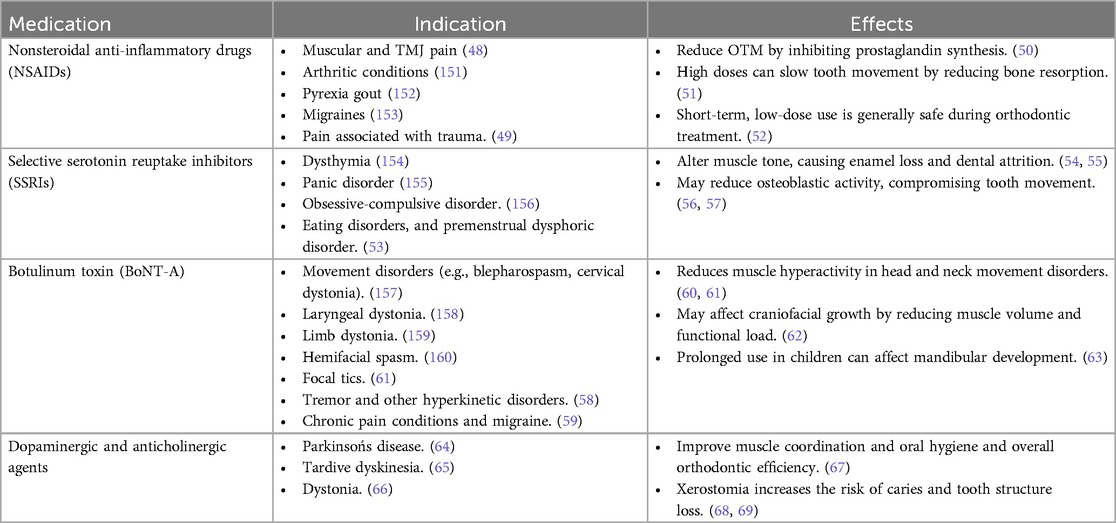
Table 4. Effects of medications for managing FMD on orthodontic tooth movement, mechanics, retention and relapse.
Challenges and future directions
There are many challenges that complicate the recognition of FMD. Most importantly, it's symptoms often overlap with, and may co-exist alongside, known organic conditions such as Parkinson's disease, multiple sclerosis, and organic dystonias, making diagnosis challenging.
The low prevalence of most FMD cases, combined with limited evidence largely derived from case reports and case series, makes it difficult for oral healthcare professionals to recognize and effectively address FMD manifestations. The absence of standardized treatment guidelines and orthodontic-focused research further complicate these challenges.
Moreover, the lack of structural abnormalities complicates FMD diagnosis, and is requiring clinicians to rely heavily on detailed clinical observation and a thorough patient history. Insufficient training in recognizing FMD among oral healthcare professionals often leads to delayed diagnoses and ineffective treatments, which may worsen the patient's condition.
Addressing these challenges requires a comprehensive approach involving advanced diagnostic techniques, innovative appliance designs, interdisciplinary collaboration, and enhanced training for orthodontists, to improve outcomes and patient experiences. Additionally, advancing research into the pathophysiology of FMD is crucial for improving both diagnosis and treatment. Functional neuroimaging (fMRI) techniques have already demonstrated potential in identifying abnormalities within brain networks responsible for motor control, and advanced investigations should prioritize refining these methods (107).
Conclusion
Further research on FMD is essential to provide medical professionals with clearer evidence for improved diagnosis and treatment. In orthodontic care, FMD poses significant challenges as involuntary muscle movements can disrupt procedures, compromise occlusal stability, and impact treatment outcomes. FMD's complex etiology, involving motor abnormalities and psychological factors, requires a multidisciplinary approach that goes beyond symptom management.
Accurate diagnosis, particularly in the craniofacial region where FMD can mimic organic conditions, is crucial to avoid misdiagnosis and compromised care. FMD-related muscle hyperactivity can lead to prolonged treatment durations and increased relapse risk.
Table 5 provides insights into specific orthodontic considerations for patients with FMD. Effective management strategies include the use of fixed appliances for greater stability, shorter appointments to minimize symptom exacerbation, distraction techniques, medications, and retention measures like occlusal splints and fixed retainers. Expanding professional training and awareness can promote early recognition and reduce diagnostic errors, ultimately enhancing treatment outcomes and improving the quality of care for FMD patients in orthodontic practice.
Author contributions
TA: Conceptualization, Methodology, Supervision, Writing – review & editing, Investigation. AZ: Writing – original draft, Resources, Methodology, Data curation, Investigation, Conceptualization, Writing – review & editing. AP: Resources, Writing – review & editing, Methodology.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. McLoughlin C, Hoeritzauer I, Cabreira V, Aybek S, Adams C, Alty J, et al. Functional neurological disorder is a feminist issue. J Neurol Neurosurg Psychiatry. (2023) 94(10):855–62. doi: 10.1136/jnnp-2022-330192
2. Aybek S, Perez DL. Diagnosis and management of functional neurological disorder. Br Med J. (2022) 376:1–19. doi: 10.1136/bmj.o64
3. Sinanović O, Zukić S, Banović S, Sinanović E, Muftić M. Functional neurological disorders. Berlin: Mind, Brain and Education: Springer (2023). p. 129–39.
4. Voon V, Cavanna AE, Coburn K, Sampson S, Reeve A, LaFrance WC Jr. Functional neuroanatomy and neurophysiology of functional neurological disorders (conversion disorder). J Neuropsychiatry Clin Neurosci. (2016) 28(3):168–90. doi: 10.1176/appi.neuropsych.14090217
5. Yoshida K. Clinical characteristics of functional movement disorders in the stomatognathic system. Front Neurol. (2020) 11:123. doi: 10.3389/fneur.2020.00123
6. Atmaca M, Aydin A, Tezcan E, Poyraz AK, Kara B. Volumetric investigation of brain regions in patients with conversion disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2006) 30(4):708–13. doi: 10.1016/j.pnpbp.2006.01.011
7. Mavroudis I, Kazis D, Kamal FZ, Gurzu IL, Ciobica A, Pădurariu M, et al. Understanding functional neurological disorder: recent insights and diagnostic challenges. Int J Mol Sci. (2024) 25(8):4470. doi: 10.3390/ijms25084470
8. Bazydlo S, Eccles FJ. Living with functional movement disorders: a tale of three battles. An interpretative phenomenological analysis. Psychol Health. (2024) 39(8):1130–47. doi: 10.1080/08870446.2022.2130312
9. Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: merging mind and brain. Lancet Neurol. (2012) 11(3):250–60. doi: 10.1016/S1474-4422(11)70310-6
10. Carson A, Stone J, Hibberd C, Murray G, Duncan R, Coleman R, et al. Disability, distress and unemployment in neurology outpatients with symptoms ‘unexplained by organic disease’. J Neurol Neurosurg Psychiatry. (2011) 82(7):810–3. doi: 10.1136/jnnp.2010.220640
11. Kola S, LaFaver K. Updates in functional movement disorders: from pathophysiology to treatment advances. Curr Neurol Neurosci Rep. (2022) 22(5):305–11. doi: 10.1007/s11910-022-01192-9
12. Pareés I, Kojovic M, Pires C, Rubio-Agusti I, Saifee TA, Sadnicka A, et al. Physical precipitating factors in functional movement disorders. J Neurol Sci. (2014) 338(1–2):174–7. doi: 10.1016/j.jns.2013.12.046
13. Simani L, Roozbeh M, Rostami M, Pakdaman H, Ramezani M, Asadollahi M. Attention and inhibitory control deficits in patients with genetic generalized epilepsy and psychogenic nonepileptic seizure. Epilepsy Behav. (2020) 102:106672. doi: 10.1016/j.yebeh.2019.106672
14. Gilmour GS, Langer LK, Lang AE, MacGillivray L, Lidstone SC. Neuropsychiatric phenotypes in functional movement disorder. CNS Spectr. (2023) 28(6):747–55. doi: 10.1017/S1092852923002353
15. Patron VG, Rustomji Y, Yip C, Jenkins LM. Psychiatric comorbidities in functional neurologic symptom disorder. Pract Neurol (Fort Wash Pa). (2022) 21(3):71.36644502
16. Gilmour GS, Lidstone SC, Lang AE. The diagnosis of functional movement disorder. Pract Neurol. (2022):40–53.
17. Peckham EL, Hallett M. Psychogenic movement disorders. Neurol Clin. (2009) 27(3):801–19. doi: 10.1016/j.ncl.2009.04.008
18. Kara BY, Gurlen NO, Dogu O. Epidemiology of Functional Movement Disorders. Hoboken, NJ USA: Movement Disorder: Wiley (2022). p. S288–S288.
19. Lidstone SC, Costa-Parke M, Robinson EJ, Ercoli T, Stone J. Functional movement disorder gender, age and phenotype study: a systematic review and individual patient meta-analysis of 4905 cases. J Neurol Neurosurg Psychiatry. (2022) 93(6):609–16. doi: 10.1136/jnnp-2021-328462
20. Larsh T, Wilson J, Mackenzie KM, O'Malley JA. Diagnosis and Initial Treatment of Functional Movement Disorders in Children. Amsterdam: Seminars in pediatric neurology: Elsevier (2022). p. 100953.
22. Lindstone S. Functional Movement Disorders. U: UpToDate, Hurtig HI, Eichler AF ed UpToDate. Waltham, MA: UpToDate (2023).
23. Spagnolo PA, Norato G, Maurer CW, Goldman D, Hodgkinson C, Horovitz S, et al. Effects of TPH2 gene variation and childhood trauma on the clinical and circuit-level phenotype of functional movement disorders. J Neurol Neurosurg Psychiatry. (2020) 91(8):814–21. doi: 10.1136/jnnp-2019-322636
24. Baizabal-Carvallo JF, Hallett M, Jankovic J. Pathogenesis and pathophysiology of functional (psychogenic) movement disorders. Neurobiol Dis. (2019) 127:32–44. doi: 10.1016/j.nbd.2019.02.013
25. Fasano A, Espay AJ, Morgante F. Finding gaps and building bridges in movement disorders. Expert Rev Neurother. (2012) 12(7):781–4. doi: 10.1586/ern.12.58
26. Munhoz RP, Zavala JA, Becker N, Teive HA. Cross-cultural influences on psychogenic movement disorders–a comparative review with a Brazilian series of 83 cases. Clin Neurol Neurosurg. (2011) 113(2):115–8. doi: 10.1016/j.clineuro.2010.10.004
27. Xie X-Y, Lin G-z, Huang Q, Li CB, Hallett M, Voon V, et al. Opinions and clinical practice of functional movement disorders: a nationwide survey of clinicians in China. BMC Neurol. (2021) 21:1–10. doi: 10.1186/s12883-021-02474-4
28. Hull M, Parnes M. Tics and TikTok: functional tics spread through social media. Mov Disord Clin Pract. (2021) 8(8):1248–52. doi: 10.1002/mdc3.13267
29. Hull M, Parnes M, Jankovic J. Increased incidence of functional (psychogenic) movement disorders in children and adults amid the COVID-19 pandemic: a cross-sectional study. Neurol Clin Pract. (2021) 11(5):e686–90. doi: 10.1212/CPJ.0000000000001082
30. Gupta A, Lang AE. Psychogenic movement disorders. Curr Opin Neurol. (2009) 22(4):430–6. doi: 10.1097/WCO.0b013e32832dc169
31. Roper LS, Saifee TA, Parees I, Rickards H, Edwards MJ. How to use the entrainment test in the diagnosis of functional tremor. Pract Neurol. (2013) 13(6):396–8. doi: 10.1136/practneurol-2013-000549
32. Barbey A, Aybek S. Functional movement disorders. Curr Opin Neurol. (2017) 30(4):427–34. doi: 10.1097/WCO.0000000000000464
33. Huys A-CM, Bhatia KP, Edwards MJ, Haggard P. The flip side of distractibility—executive dysfunction in functional movement disorders. Front Neurol. (2020) 11:969. doi: 10.3389/fneur.2020.00969
34. Nicholson T, Aybek S, Kempton M, Daly EM, Murphy DG, David AS, et al. A structural MRI study of motor conversion disorder: evidence of reduction in thalamic volume. J Neurol Neurosurg Psychiatry. (2014) 85(2):227–9. doi: 10.1136/jnnp-2013-305012
35. Voon V, Brezing C, Gallea C, Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov Disord. (2011) 26(13):2396–403. doi: 10.1002/mds.23890
36. Voon V, Brezing C, Gallea C, Ameli R, Roelofs K, LaFrance WC Jr, et al. Emotional stimuli and motor conversion disorder. Brain. (2010) 133(5):1526–36. doi: 10.1093/brain/awq054
37. Kamble N, Pal PK. Electrophysiology in functional movement disorders: an update. Tremor Other Hyperkinet Mov. (2023) 13:1–15. doi: 10.5334/tohm.793
38. Deuschl G, Köster B, Lücking CH, Scheidt C. Diagnostic and pathophysiological aspects of psychogenic tremors. Mov Disord. (1998) 13(2):294–302. doi: 10.1002/mds.870130216
39. Gilmour GS, Nielsen G, Teodoro T, Yogarajah M, Coebergh JA, Dilley MD, et al. Management of functional neurological disorder. J Neurol. (2020) 267:2164–72. doi: 10.1007/s00415-020-09772-w
40. Nielsen G, Stone J, Matthews A, Brown M, Sparkes C, Farmer R, et al. Physiotherapy for functional motor disorders: a consensus recommendation. J Neurol Neurosurg Psychiatry. (2015) 86(10):1113–9. doi: 10.1136/jnnp-2014-309255
41. Czarnecki K, Thompson JM, Seime R, Geda YE, Duffy JR, Ahlskog JE. Functional movement disorders: successful treatment with a physical therapy rehabilitation protocol. Parkinsonism Relat Disord. (2012) 18(3):247–51. doi: 10.1016/j.parkreldis.2011.10.011
42. Mätzold S, Geritz J, Zeuner KE, Berg D, Paschen S, Hieke J, et al. Functional movement disorders in neurogeriatric inpatients: underdiagnosed, often comorbid to neurodegenerative disorders and treatable. Z Gerontol Geriatr. (2019) 52(4):324–9. doi: 10.1007/s00391-019-01562-y
43. Pollak TA, Nicholson TR, Edwards MJ, David AS. A systematic review of transcranial magnetic stimulation in the treatment of functional (conversion) neurological symptoms. J Neurol Neurosurg Psychiatry. (2014) 85(2):191–7. doi: 10.1136/jnnp-2012-304181
44. Latorre A, Rocchi L, Berardelli A, Bhatia KP, Rothwell JC. The use of transcranial magnetic stimulation as a treatment for movement disorders: a critical review. Mov Disord. (2019) 34(6):769–82. doi: 10.1002/mds.27705
45. Garcin B, Mesrati F, Hubsch C, Mauras T, Iliescu I, Naccache L, et al. Impact of transcranial magnetic stimulation on functional movement disorders: cortical modulation or a behavioral effect? Front Neurol. (2017) 8:338. doi: 10.3389/fneur.2017.00338
46. Spagnolo PA, Parker J, Horovitz S, Hallett M. Corticolimbic modulation via intermittent theta burst stimulation as a novel treatment for functional movement disorder: a proof-of-concept study. Brain Sci. (2021) 11(6):791. doi: 10.3390/brainsci11060791
47. Jankovic J. Medical treatment of dystonia. Mov Disord. (2013) 28(7):1001–12. doi: 10.1002/mds.25552
48. Ghlichloo I, Gerriets V. Nonsteroidal Anti-inflammatory drugs (NSAIDs). Hinxton: Europe PMC (2019).
49. Oyler DR, Parli SE, Bernard AC, Chang PK, Procter LD, Harned ME. Nonopioid management of acute pain associated with trauma: focus on pharmacologic options. J Trauma Acute Care Surg. (2015) 79(3):475–83. doi: 10.1097/TA.0000000000000755
50. Bartzela TN, Maltha JC. Medication Effects on the Rate of Orthodontic Tooth Movement. Berlin: Springer (2016).
51. Gargya I, Singh B, Talnia S. NSAIDS (non-steroidal anti-inflammatory drugs)-their effects and side effects in orthodontic therapy-A review. Dent J Adva Stud. (2017) 5(01):008–13. doi: 10.1055/s-0038-1672075
52. Stabile A, Stuani MBS, Leite-Panissi CRA, Rocha M. Effects of short-term acetaminophen and celecoxib treatment on orthodontic tooth movement and neuronal activation in rat. Brain Res Bull. (2009) 79(6):396–401. doi: 10.1016/j.brainresbull.2009.05.014
53. Masand PS, Gupta S. Selective serotonin-reuptake inhibitors: an update. Harv Rev Psychiatry. (1999) 7(2):69–84. doi: 10.1093/hrp/7.2.69
54. Visco DB, Manhaes-de-Castro R, Chaves WF, Lacerda DC, da Conceição Pereira S, Ferraz-Pereira KN, et al. Selective serotonin reuptake inhibitors affect structure, function and metabolism of skeletal muscle: a systematic review. Pharmacol Res. (2018) 136:194–204. doi: 10.1016/j.phrs.2018.09.004
55. Abed H, Ezzat Y, Alsaadawi L, Almarzouki R, Aboulkhair R, Alqarni A, et al. Negative impacts of psychiatric medications on oral health: a literature review. Cureus. (2023) 15(12):1–9.
56. Hodge JM, Wang Y, Berk M, Collier FM, Fernandes TJ, Constable MJ, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. (2013) 74(1):32–9. doi: 10.1016/j.biopsych.2012.11.003
57. Krishnan V, Zahrowski JJ, Davidovitch Z. The effect of drugs, hormones, and diet on orthodontic tooth movement. Biol Mech Tooth Mov. (2021):199–215. doi: 10.1002/9781119608912.ch14
58. Dreissen YEM, Dijk JM, Gelauff JM, Zoons E, van Poppelen D, Contarino MF, et al. Botulinum neurotoxin treatment in jerky and tremulous functional movement disorders: a double-blind, randomised placebo-controlled trial with an open-label extension. J Neurol Neurosurg Psychiatry. (2019) 90(11):1244–50. doi: 10.1136/jnnp-2018-320071
59. Matak I, Lacković Z. Botulinum toxin A, brain and pain. Prog Neurobiol. (2014) 119:39–59. doi: 10.1016/j.pneurobio.2014.06.001
60. Yoshida K. Botulinum toxin therapy for oromandibular dystonia and other movement disorders in the stomatognathic system. Toxins (Basel). (2022) 14(4):282. doi: 10.3390/toxins14040282
61. Anandan C, Jankovic J. Botulinum toxin in movement disorders: an update. Toxins (Basel). (2021) 13(1):42. doi: 10.3390/toxins13010042
62. Tsai C-Y, Chiu WC, Liao Y-H, Tsai C-M. Effects on craniofacial growth and development of unilateral botulinum neurotoxin injection into the masseter muscle. Am J Orthod Dentofacial Orthop. (2009) 135(2):142.e1–142. e6. doi: 10.1016/j.ajodo.2008.06.020
63. Balanta-Melo J, Toro-Ibacache V, Kupczik K, Buvinic S. Mandibular bone loss after masticatory muscles intervention with botulinum toxin: an approach from basic research to clinical findings. Toxins (Basel). (2019) 11(2):84. doi: 10.3390/toxins11020084
64. Katzenschlager R, Sampaio C, Costa J, Lees A, Group CMD. Anticholinergics for symptomatic management of Parkinson s disease. Cochrane Database Syst Rev. (1996) 2010(1):1–21. doi: 10.1002/14651858.cd003735
65. Klawans H, Rubovits R. Effect of cholinergic and anticholinergic agents on tardive dyskinesia. J Neurol Neurosurg Psychiatry. (1974) 37(8):941–7. doi: 10.1136/jnnp.37.8.941
66. Vanegas-Arroyave N, Caroff SN, Citrome L, Crasta J, McIntyre RS, Meyer JM, et al. An evidence-based update on anticholinergic use for drug-induced movement disorders. CNS Drugs. (2024) 38(4):239–54. doi: 10.1007/s40263-024-01078-z
67. D’Arrigo A, Floro S, Bartesaghi F, Casellato C, Papa GF, Centanni S, et al. Respiratory dysfunction in Parkinson’s disease: a narrative review. ERJ Open Res. (2020) 6(4):1–12. doi: 10.1183/23120541.00165-2020
68. Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. (2003) 9(4):165–76. doi: 10.1034/j.1601-0825.2003.03967.x
69. Erickson LE, Trump B. Oral and Dental Effects of Xerostomia. Xerostomia: CRC Press (2024). p. 51–76.
70. Mishra A, Pandey S. Cranial functional movement disorders: a case series with literature review. Tremor Other Hyperkinet Mov. (2020) 10:1–12. doi: 10.5334/tohm.352
71. Kaski D, Bronstein AM, Edwards MJ, Stone J. Cranial functional (psychogenic) movement disorders. Lancet Neurol. (2015) 14(12):1196–205. doi: 10.1016/S1474-4422(15)00226-4
72. Gandevia S. Roles for perceived voluntary motor commands in motor control. Trends Neurosci. (1987) 10(2):81–5. doi: 10.1016/0166-2236(87)90030-0
73. Baizabal-Carvallo JF, Jankovic J. Distinguishing features of psychogenic (functional) versus organic hemifacial spasm. J Neurol. (2017) 264:359–63. doi: 10.1007/s00415-016-8356-0
74. Kola S, LaFaver K. Functional movement disorder and functional seizures: what have we learned from different subtypes of functional neurological disorders? Epilepsy Behav Rep. (2022) 18:100510. doi: 10.1016/j.ebr.2021.100510
75. Tan NC, Chan LL, Tan EK. Hemifacial spasm and involuntary facial movements. QJM. (2002) 95(8):493–500. doi: 10.1093/qjmed/95.8.493
76. Evidente VGH, Adler CH. Hemifacial spasm and other craniofacial movement disorders. Amsterdam: Mayo Clinic Proceedings: Elsevier (1998). p. 67–71.
77. Fekete R, Baizabal-Carvallo JF, Ha AD, Davidson A, Jankovic J. Convergence spasm in conversion disorders: prevalence in psychogenic and other movement disorders compared with controls. Journal of neurology. Neurosurgery & Psychiatry. (2012) 83(2):202–4. doi: 10.1136/jnnp-2011-300733
78. Teodoro T, Cunha JM, Abreu LF, Yogarajah M, Edwards MJ. Abnormal eye and cranial movements triggered by examination in people with functional neurological disorder. Neuro-Ophthalmology. (2019) 43(4):240–3. doi: 10.1080/01658107.2018.1536998
79. Gonzalez-Alegre P, Schneider RL, Hoffman H. Clinical, etiological, and therapeutic features of jaw-opening and jaw-closing oromandibular dystonias: a decade of experience at a single treatment center. Tremor and Other Hyperkinetic Movements. (2014) 4:1–6. doi: 10.5334/tohm.194
80. Clark GT, Ram S. Four oral motor disorders: bruxism, dystonia, dyskinesia and drug-induced dystonic extrapyramidal reactions. Dental Clinics. (2007) 51(1):225–43. doi: 10.1016/j.cden.2006.09.002
81. Bakke M, Henriksen T, Biernat HB, Dalager T, Møller E. Interdisciplinary recognizing and managing of drug-induced tardive oromandibular dystonia: two case reports. Clin Case Rep. (2018) 6(11):2150. doi: 10.1002/ccr3.1548
82. Blanchet PJ, Rompré PH, Lavigne GJ, Lamarche C. Oral dyskinesia: a clinical overview. Int J Prosthodont. (2005) 18(1):10–4.15754887
83. Karp BI, Alter K. Botulinum Toxin Treatment of Blepharospasm, Orofacial/oromandibular Dystonia, and Hemifacial Spasm. Seminars in Neurology: Thieme Medical Publishers (2016). p. 084–91.
84. Ameer MA, Bhatti D. Chemodenervation for oromandibular dystonia utilizing botulinum toxins. Cureus. (2021) 13(10):1–5. doi: 10.7759/cureus.18425
85. Yu GLT, Rosales RL. Treatment of oromandibular dystonia using botulinum toxin injections–case series and illustrative muscle targeting. Basal Ganglia. (2018) 13:7–16. doi: 10.1016/j.baga.2018.05.002
86. Gresty MA, Halmagyi GM. Abnormal head movements. J Neurol Neurosurg Psychiatry. (1979) 42(8):705–14. doi: 10.1136/jnnp.42.8.705
87. Haque T. Correlation between temporomandibular joint disorders and bruxism: a systematic review and meta-analysis. Bangladesh J Med Sci. (2024) 23(4):1008–19. doi: 10.3329/bjms.v23i4.76510
88. Handa S, Shaefer JR, Keith DA. Oromandibular dystonia and temporomandibular disorders. J Am Dent Assoc. (2022) 153(9):899–906. doi: 10.1016/j.adaj.2021.07.026
89. Raoofi S, Khorshidi H, Najafi M. Etiology, diagnosis and management of oromandibular dystonia: an update for stomatologists. J Dent. (2017) 18(2):73.
90. Shrivastava M, Ye L. Oromandibular dystonia and temporomandibular disorders—a review on diagnosis and management. J Oral Maxillofac Anesth. (2024) 3:1–10. doi: 10.21037/joma-23-34
91. Sude A, Matsumoto J, Kaimal S, Petersen A, Nixdorf DR. Temporomandibular disorder–related characteristics and treatment outcomes in oromandibular dystonia patients in two different clinical settings: a cross-sectional study. J Oral Rehabil. (2021) 48(5):542–50. doi: 10.1111/joor.13162
92. Frucht L, Perez DL, Callahan J, MacLean J, Song PC, Sharma N, et al. Functional dystonia: differentiation from primary dystonia and multidisciplinary treatments. Front Neurol. (2021) 11:605262. doi: 10.3389/fneur.2020.605262
93. Saraf U, Chandarana M, Divya K, Krishnan S. Oromandibular dystonia–a systematic review. Ann Indian Acad Neurol. (2022) 25(1):26–34. doi: 10.4103/aian.aian_242_21
94. Skomro P, Lietz-Kijak D, Garstka AA, Szczucka L, Gronwald H. The application of intraoral scanners in orthodontic care for adolescents with disabilities. Appl Sci. (2024) 14(8):3344. doi: 10.3390/app14083344
95. Kau CH, Littlefield J, Rainy N, Nguyen JT, Creed B. Evaluation of CBCT digital models and traditional models using the little’s index. Angle Orthodontist. (2010) 80(3):435–9. doi: 10.2319/083109-491.1
96. Yoshida K. Sensory trick splint as a multimodal therapy for oromandibular dystonia. J Prosthodont Res. (2018) 62(2):239–44. doi: 10.1016/j.jpor.2017.09.004
97. De Meyer M, Vereecke L, Bottenberg P, Jacquet W, Sims AB, Santens P. Oral appliances in the treatment of oromandibular dystonia: a systematic review. Acta Neurol Belg. (2020) 120:831–6. doi: 10.1007/s13760-020-01404-4
98. Lane H, Rose LE, Woodbrey M, Arghavani D, Lawrence M, Cavanaugh JT. Exploring the effects of using an oral appliance to reduce movement dysfunction in an individual with Parkinson disease: a single-subject design study. J Neurol Phys Ther. (2017) 41(1):52–8. doi: 10.1097/NPT.0000000000000160
99. Cooper T, Smith L. Dental appliance therapy in pantothenate kinase-associated neurodegeneration: case report. Spec Care Dentist. (2019) 39(1):56–8. doi: 10.1111/scd.12337
100. Henneman S, Von den Hoff J, Maltha J. Mechanobiology of tooth movement. Eur J Orthod. (2008) 30(3):299–306. doi: 10.1093/ejo/cjn020
101. Fan J, Caton JG. Occlusal trauma and excessive occlusal forces: narrative review, case definitions, and diagnostic considerations. J Periodontol. (2018) 89:S214–22. doi: 10.1002/JPER.16-0581
102. Gandhi YR. Oro-mandibular dystonia. Natl J Maxillofac Surg. (2010) 1(2):150–2. doi: 10.4103/0975-5950.79218
103. Littlewood S, Kandasamy S, Huang G. Retention and relapse in clinical practice. Aust Dent J. (2017) 62:51–7. doi: 10.1111/adj.12475
104. Sonone TP, Soni V, Gupta S, Shekatkar YK, Thorat AS, Pol TR. Botox and dermal fillers in orthodontics–a review. J Pharm Bioallied Sci. (2022) 14(Suppl 1):S60–4. doi: 10.4103/jpbs.jpbs_184_22
105. Friedlander AH, Mahler M, Norman KM, Ettinger RL. Parkinson disease: systemic and orofacial manifestations, medical and dental management. J Am Dent Assoc. (2009) 140(6):658–69. doi: 10.14219/jada.archive.2009.0251
106. Rajan R, Sun Y-M. Reevaluating antidepressant selection in patients with bruxism and temporomandibular joint disorder. J Psychiatric Pract. (2017) 23(3):173–9. doi: 10.1097/PRA.0000000000000227
107. Holtbernd F, Eidelberg D. Functional brain networks in movement disorders: recent advances. Curr Opin Neurol. (2012) 25(4):392–401. doi: 10.1097/WCO.0b013e328355aa94
108. Hallett M. Functional (psychogenic) movement disorders–clinical presentations. Parkinsonism Relat Disord. (2016) 22:S149–52. doi: 10.1016/j.parkreldis.2015.08.036
109. Huys A-CM, Haggard P, Bhatia KP, Edwards MJ. Misdirected attentional focus in functional tremor. Brain. (2021) 144(11):3436–50. doi: 10.1093/brain/awab230
110. Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah H. The functional neuroanatomy of dystonia. Neurobiol Dis. (2011) 42(2):185–201. doi: 10.1016/j.nbd.2011.01.026
111. Ganos C, Edwards MJ, Bhatia KP. The phenomenology of functional (psychogenic) dystonia. Mov Disord Clin Pract. (2014) 1(1):36–44. doi: 10.1002/mdc3.12013
112. Gray J, Welck M, Cullen NP, Singh D. Functional dystonia in the foot and ankle. Bone Joint J. (2021) 103(6):1127–32. doi: 10.1302/0301-620X.103B6.BJJ-2020-2187.R2
113. Monday K, Jankovic J. Psychogenic myoclonus. Neurology. (1993) 43(2):349–349. doi: 10.1212/WNL.43.2.349
114. Rubino FA. Gait disorders. Neurologist. (2002) 8(4):254–62. doi: 10.1097/00127893-200207000-00005
115. Adang LA. How Do You Tell a Pathologic Gait From a Functional Gait? Curbside Consultation in Pediatric Neurology. Oxfordshire: CRC Press (2024). p. 231–4.
116. Fasano A, Bloem BR. Gait disorders. Continuum. (2013) 19(5):1344–82. doi: 10.1212/01.CON.0000436159.33447.69
117. Issak S, Kanaan R, Nielsen G, Fini NA, Williams G. Functional gait disorders: clinical presentations, phenotypes and implications for treatment. Brain Inj. (2023) 37(5):437–45. doi: 10.1080/02699052.2023.2165158
118. Erro R, Sheerin UM, Bhatia KP. Paroxysmal dyskinesias revisited: a review of 500 genetically proven cases and a new classification. Mov Disord. (2014) 29(9):1108–16. doi: 10.1002/mds.25933
121. Stone J, Aybek S. Functional limb weakness and paralysis. Handb Clin Neurol. (2016) 139:213–28. doi: 10.1016/B978-0-12-801772-2.00018-7
122. Tinazzi M, Simonetto S, Franco L, Bhatia KP, Moretto G, Fiaschi A, et al. Abduction finger sign: a new sign to detect unilateral functional paralysis of the upper limb. Mov Disord. (2008) 23(16):2415–9. doi: 10.1002/mds.22268
123. Tremolizzo L, Susani E, Riva MA, Cesana G, Ferrarese C, Appollonio I. Positive signs of functional weakness. J Neurol Sci. (2014) 340(1–2):13–8. doi: 10.1016/j.jns.2014.03.003
124. Yoshida K. Movement disorders of the stomatognathic system: a blind spot between dentistry and medicine. Dent Med Problems. (2024) 61(4):613–25. doi: 10.17219/dmp/185249
125. Yi M, Li J, Liu G, Ou Z, Liu Y, Li J, et al. Mental health and quality of life in patients with craniofacial movement disorders: a cross-sectional study. Front Neurol. (2022) 13:938632. doi: 10.3389/fneur.2022.938632
126. Mishra A, Pandey S. Functional neurological disorders: clinical spectrum, diagnosis, and treatment. Neurologist. (2022) 27(5):276–89. doi: 10.1097/NRL.0000000000000453
127. Di Giuda D, Camardese G, Bentivoglio AR, Cocciolillo F, Guidubaldi A, Pucci L, et al. Dopaminergic dysfunction and psychiatric symptoms in movement disorders: a 123 I-FP-CIT SPECT study. Eur J Nucl Med Mol Imaging. (2012) 39:1937–48. doi: 10.1007/s00259-012-2232-7
128. Keynejad RC, Frodl T, Kanaan R, Pariante C, Reuber M, Nicholson TR. Stress and functional neurological disorders: mechanistic insights. Journal of neurology. Neurosurg Psychiatry. (2019) 90(7):813–21. doi: 10.1136/jnnp-2018-318297
129. Ludwig L, Pasman JA, Nicholson T, Aybek S, David AS, Tuck S, et al. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry. (2018) 5(4):307–20. doi: 10.1016/S2215-0366(18)30051-8
130. LaFaver K, Maurer CW, Nicholson TR, Perez DL. Functional Movement Disorder. Berlin: An Interdisciplinary Case-Based Approach (2022). p. 320.
131. Berg L, Pringsheim TM, Martino D. Sudden onset tic and tic-like presentations in older adolescents and adults. Curr Dev Disord Rep. (2022) 9(4):146–55. doi: 10.1007/s40474-022-00263-3
132. Tinazzi M, Morgante F, Marcuzzo E, Erro R, Barone P, Ceravolo R, et al. Clinical correlates of functional motor disorders: an Italian multicenter study. Mov Disord Clin Pract. (2020) 7(8):920–9. doi: 10.1002/mdc3.13077
133. Kletenik I, Sillau SH, Isfahani SA, LaFaver K, Hallett M, Berman BD. Gender as a risk factor for functional movement disorders: the role of sexual abuse. Mov Disord Clin Pract. (2020) 7(2):177–81. doi: 10.1002/mdc3.12863
134. Schwingenschuh P, Pont-Sunyer C, Surtees R, Edwards MJ, Bhatia KP. Psychogenic movement disorders in children: a report of 15 cases and a review of the literature. Mov Disord. (2008) 23(13):1882–8. doi: 10.1002/mds.22280
135. Batla A, Stamelou M, Edwards MJ, Edwards MJ, Bhatia KP. Functional movement disorders are not uncommon in the elderly. Mov Disord. (2013) 28(4):540–3. doi: 10.1002/mds.25350
136. Ganos C, Edwards M, Bhatia K. Posttraumatic functional movement disorders. Handb Clin Neurol. (2016) 139:499–507. doi: 10.1016/B978-0-12-801772-2.00041-2
137. Conte A, Falla M, Diana MC, Bologna M, Suppa A, Fabbrini A, et al. Spread of muscle spasms in hemifacial spasm. Mov Disord Clin Pract. (2015) 2(1):53–5. doi: 10.1002/mdc3.12106
138. Johnson LN, Lapour RW, Johnson GM, Johnson PJ, Madsen RW, Hackley SA. Closely spaced stressful life events precede the onset of benign essential blepharospasm and hemifacial spasm. J Neuroophthalmol. (2007) 27(4):275–80. doi: 10.1097/WNO.0b013e31815c4233
139. Lefaucheur J-P, Daamer NB, Sangla S, Le Guerinel C. Diagnosis of primary hemifacial spasm. Neurochirurgie. (2018) 64(2):82–6. doi: 10.1016/j.neuchi.2017.12.003
140. Kaski D, Pradhan V, Bronstein AM. Clinical features of functional (psychogenic) eye movement disorders. Journal of neurology. Neurosurg Psychiatry. (2016) 87(12):1389–92. doi: 10.1136/jnnp-2016-313608
141. Szczęśniak M, Sikorska E, Rajca M, Koper M, Kopacz W, Sikorski P, et al. The etiology, diagnostics, and treatment of the spasm of the near reflex-a narrative review. Eur J Ophthalmol. (2024) 34(6):1655–66. doi: 10.1177/11206721241237309
142. Scoppetta C, Di Gennaro G. Psychogenic convergence spasm mimicking ocular myasthenia. Eur Rev Med Pharmacol Sci. (2017) 21(5):1088–90.28338183
143. Fasano A, Valadas A, Bhatia KP, Prashanth LK, Lang AE, Munhoz RP, et al. Psychogenic facial movement disorders: clinical features and associated conditions. Mov Disord. (2012) 27(12):1544–51. doi: 10.1002/mds.25190
144. Baizabal-Carvallo JF, Jankovic J. Functional (psychogenic) stereotypies. J Neurol. (2017) 264:1482–7. doi: 10.1007/s00415-017-8551-7
145. Hiiemae KM, Palmer JB. Tongue movements in feeding and speech. Crit Rev Oral Biol Med. (2003) 14(6):413–29. doi: 10.1177/154411130301400604
146. Clavé P, Terré R, De Kraa M, Serra M. Approaching oropharyngeal dysphagia. Rev Esp Enferm Dig. (2004) 96(2):119–31. doi: 10.4321/S1130-01082004000200005
147. Panebianco M, Marchese-Ragona R, Masiero S, Restivo D. Dysphagia in neurological diseases: a literature review. Neurol Sci. (2020) 41:3067–73. doi: 10.1007/s10072-020-04495-2
148. Goh K-H, Acharyya S, Ng SY-E, Boo JP, Kooi AH, Ng HL, et al. Risk and prognostic factors for pneumonia and choking amongst Parkinson’s disease patients with dysphagia. Parkinsonism Relat Disord. (2016) 29:30–4. doi: 10.1016/j.parkreldis.2016.05.034
149. Yoshida K. Peripherally induced movement disorders in the stomatognathic system after oral surgical or dental procedures. Oral Maxillofac Surg. (2024) 28(4):1579–86. doi: 10.1007/s10006-024-01285-4
150. Britton D, Alty J, Mannion C. Oromandibular dystonia: a diagnosis not to miss. Br J Oral Maxillofac Surg. (2020) 58(5):520–4. doi: 10.1016/j.bjoms.2020.02.018
151. Crofford LJ. Use of NSAIDs in treating patients with arthritis. Arthritis Res Ther. (2013) 15:1–10. doi: 10.1186/ar4174
152. Shekelle PG, Newberry SJ, FitzGerald JD, Motala A, O'Hanlon CE, Tariq A, et al. Management of gout: a systematic review in support of an American college of physicians clinical practice guideline. Ann Intern Med. (2017) 166(1):37–51. doi: 10.7326/M16-0461
153. Pardutz A, Schoenen J. NSAIDs in the acute treatment of migraine: a review of clinical and experimental data. Pharmaceuticals. (2010) 3(6):1966–87. doi: 10.3390/ph3061966
154. Ravindran A, Bialik R, Lapierre Y. Therapeutic efficacy of specific serotonin reuptake inhibitors (SSRIs) in dysthymia. Canad J Psychiatry. (1994) 39(1):21–6. doi: 10.1177/070674379403900106
155. Bakker A, Van Balkom A, Spinhoven P. SSRIs vs. TCAs in the treatment of panic disorder: a meta-analysis. Acta Psychiatr Scand. (2002) 106(3):163–7. doi: 10.1034/j.1600-0447.2002.02255.x
156. Hollander E. Treatment of obsessive-compulsive spectrum disorders with SSRIs. Br J Psychiatry. (1998) 173(S35):7–12. doi: 10.1192/S0007125000297845
157. Chiu SY, Burns MR, Malaty IA. An update on botulinum toxin in neurology. Neurol Clin. (2021) 39(1):209–29. doi: 10.1016/j.ncl.2020.09.014
158. Yeung W, Richards AL, Novakovic D. Botulinum neurotoxin therapy in the clinical management of laryngeal dystonia. Toxins (Basel). (2022) 14(12):844. doi: 10.3390/toxins14120844
159. Kaplan EH, Vecchio M, Simpson DM. Botulinum toxin for treatment of focal limb dystonia. Toxins (Basel). (2025) 17(3):122. doi: 10.3390/toxins17030122
Keywords: functional movement disorder, craniofacial region, orthodontics, spasm, tongue movement
Citation: Al-Jewair T, Zylalaj A and Poursattar Bejehmir A (2025) Orthodontic considerations for managing patients with functional movement disorders: a narrative review and clinical guide. Front. Dent. Med. 6:1628802. doi: 10.3389/fdmed.2025.1628802
Received: 10 June 2025; Accepted: 21 July 2025;
Published: 7 August 2025.
Edited by:
Francisco Nociti, American Dental Association, United StatesReviewed by:
Michele Tepedino, University of L'Aquila, ItalyEduardo César Almada Santos, State University of Campinas, Brazil
Copyright: © 2025 Al-Jewair, Zylalaj and Poursattar Bejehmir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thikriat Al-Jewair, dGhpa3JpYXRAYnVmZmFsby5lZHU=
 Thikriat Al-Jewair
Thikriat Al-Jewair Ajola Zylalaj
Ajola Zylalaj Arash Poursattar Bejehmir
Arash Poursattar Bejehmir