- 1Department of Entomology, University of Georgia, Athens, GA, United States
- 2Warnell School of Forestry & Natural Resources, University of Georgia, Athens, GA, United States
- 3Applied Statistics, College of Agricultural and Environmental Sciences, University of Georgia, Griffin, GA, United States
- 4SE Fruit and Tree Nut Research Laboratory, USDA-ARS, Byron, GA, United States
- 5Department of Horticulture, University of Georgia, Griffin, GA, United States
- 6Institute of Plant Breeding, Genetics, and Genomics, University of Georgia, Griffin, GA, United States
- 7Plant and Environmental Sciences Department, Clemson University, Clemson, SC, United States
Beauveria bassiana (Balsamo) Vuillemin is a well-known entomopathogenic fungus that occupies diverse ecological niches, including soilborne, epiphytic, and endophytic habitats. Its capacity to function as an endophyte has received growing interest in potential applications for sustainable pest management, particularly in woody perennial systems where delivery and persistence of biological control agents are challenging. This study investigated endophytic colonization of peach (Prunus persica Batsch) seedlings by B. bassiana and quantified production of the insecticidal secondary metabolite beauvericin (BEA) in and on plant tissues. Seedlings were inoculated via foliar spray or soil drench. Fungal recovery was assessed from leaf, stem, and root tissues. Colonization patterns indicated systemic movement, however foliar spray increased recovery from leaf tissues and soil drench increased recovery from roots over time. BEA concentrations varied significantly by tissue type, inoculation method, and surface sterilization status. The highest levels were detected in non-surface-sterilized leaves of foliar-sprayed plants, measured two weeks post-inoculation. Surface sterilization prior to extraction significantly reduced detected concentrations, suggesting that BEA is primarily produced by epiphytic fungal growth. Larval bioassays with Tenebrio molitor L. revealed increased mortality associated with foliar-sprayed tissues, aligning with observed BEA levels and suggesting localized insecticidal activity. These findings demonstrate that the spatial dynamics of fungal colonization and metabolite localization are critical considerations for the effective deployment of B. bassiana in biocontrol strategies. Further research is needed to determine how environmental factors, host physiology, fungal strain, and time influence secondary metabolite production in and on plants treated with B. bassiana.
1 Introduction
Peach [Prunus persica (L.) Batsch] is a culturally and economically important fruit crop in the southeastern United States, with Georgia and South Carolina ranking among the top producers nationally (NASS, 2024). However, the region’s warm, humid climate supports a diverse array of insect pests and pathogens that jeopardize tree health and fruit production. Management of key pests, particularly cryptic borers, relies heavily on calendar-based applications of broad-spectrum insecticides like chlorpyrifos (Blaauw et al., 2025). While effective in the short term, these chemicals pose substantial risks including resistance development, environmental contamination, regulatory restriction, and negative impacts on non-target organisms (Lacey and Shapiro-Ilan, 2008; Foong et al., 2020; Nandi et al., 2022; Wołejko et al., 2022). These challenges underscore the urgent need for sustainable alternatives such as biological control agents that can be incorporated into integrated pest management (IPM) programs.
Beauveria bassiana (Balsamo) Vuillemin (Ascomycota: Hypocreales) is a widely studied, commercially available entomopathogenic fungus (EPF) used for the biological control of many insect pests and plant pathogens (Faria and Wraight, 2007; Ownley et al., 2010; Ferus et al., 2019; Bamisile et al., 2021a; Sharma et al., 2023);. Traditionally applied to plants and substrates as a foliar spray or soil treatment, B. bassiana infects insects through cuticle penetration, and performs its entomopathogenic activity through internal colonization, and ultimately host mortality, mediated in part by the production of insecticidal metabolites (Mascarin and Jaronski, 2016).
In addition to colonizing insects, B. bassiana can colonize plant surfaces as an epiphyte, as well as internal tissues, as an endophyte. In this latter case, B. bassiana lives asymptomatically within different tissues of the plant and has been observed to offer a variety of benefits, including plant growth promotion and pest suppression (Ownley et al., 2010; Vega, 2018; Jaber, 2018; Espinoza et al., 2019; Mantzoukas and Eliopoulos, 2020; Bamisile et al., 2021b; Sui et al., 2023; Vega, 2008). As an insect biocontrol tactic, the endophytic presence of B. bassiana has been associated with reduced insect development, fecundity, and survival, offering the potential to target pests within plant tissues (Cherry et al., 2004; Powell et al., 2009; Garrido-Jurado et al., 2017; Rondot and Reineke, 2018; Russo et al., 2019; Barra-Bucarei et al., 2020; Ramakuwela et al., 2020; Shin et al., 2022; Darsouei et al., 2024; Barta, 2018). Furthermore, the endophytic state may improve fungal persistence under abiotic stressors such as UV radiation, high temperature, and desiccation (Mishra et al., 2015; Acheampong et al., 2020), potentially enhancing its biocontrol efficacy in the field. Despite its promise, however, many aspects of this symbiosis remain poorly understood.
One underexplored dimension is the role of fungal secondary metabolites, such as beauvericin (BEA), in mediating insecticidal effects when B. bassiana resides within or on plant tissues. BEA, a cyclic hexadepsipeptide produced by B. bassiana and other fungi such as Fusarium and Isaria spp., is increasingly recognized as a potent insecticidal secondary metabolite (Wang and Xu, 2012; Caloni et al., 2020). BEA acts as an ionophore that disrupts cellular ion gradients, leading to cytotoxicity and mortality in diverse insect systems (Grove and Pople, 1980; Calò et al., 2003; Fornelli et al., 2004; Bi et al., 2023). Early bioassays demonstrated ingestion-based toxicity, with mortality of Aedes aegypti L. (Diptera: Culicidae) larvae exceeding 80% at 10–20 µg/mL, while adults of Calliphora erythrocephala Meigen (Diptera: Calliphoridae) were less sensitive (Grove and Pople, 1980). Subsequent work has shown that BEA impairs insect fecundity and symbiont function: ingestion of 25 µg/mL reduced reproduction and increased embryonic abortion in the aphid Schizaphis graminum Rondani (Hemiptera: Aphididae), coinciding with DNA disruption in Buchnera bacteriocytes (Ganassi et al., 2002).
In lepidopteran systems, BEA exerts strong cytotoxicity both in vivo and in vitro. In Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) SF-9 cells, cytotoxicity occurred at low micromolar concentrations (IC50 ≈ 2.5 µM), with progressive cell death after prolonged exposure (Calò et al., 2003; Fornelli et al., 2004). In Bombyx mori L. (Lepidoptera: Bombycidae), injection of BEA (lethal concentration 50% (LC50) = 362 µM) caused hemocyte vacuolization, reduced viability, and dysregulated immune encapsulation responses (Bi et al., 2023). Similarly, in Galleria mellonella L. (Lepidoptera: Pyralidae), fungal infection yielded hemolymph BEA concentrations of 136 ng/mL, with higher levels associated with increased larval mortality and paralysis (Safavi, 2013). Field-relevant studies also demonstrate insecticidal activity: purified BEA achieved up to 100% mortality of spider mites, Tetranychus urticae (Acari: Tetranychidae), in laboratory assays and enhanced strawberry yields under greenhouse conditions (Al Khoury et al., 2019). Furthermore, nano-formulated BEA reduced survival of Glyphodes pyloalis Walker (Lepidoptera: Crambidae) larvae with an LC50 of 0.918 µg/mL, while also impairing digestive enzyme activity and inducing oxidative stress (Yousefi-Lardeh and Zibaee, 2024). Collectively, these studies establish BEA as a multifunctional metabolite capable of reducing insect survival, reproduction, and immune competence across taxa. However, its production and spatial localization, within or on plant tissues following B. bassiana inoculation, have not been quantified. This gap limits our understanding of whether and how fungal metabolites contribute to systemic insecticidal effects in plants treated with B. bassiana.
Furthermore, a key limitation to the efficient utilization of B. bassiana in IPM programs is the difficulty in determining and tracking its insecticidal status following field application. Unlike chemical insecticides whose residual activity can be measured through established residue assays, the persistence and biological activity of B. bassiana after application is challenging to measure, limiting growers’ ability to optimize application timing. Developing reliable indicators of fungal activity would greatly enhance the practical use of B. bassiana in IPM programs. Because BEA is an insecticidal secondary metabolite primarily produced by B. bassiana, quantifying its presence in plant tissues colonized by the fungus may provide a biochemical marker of fungal persistence and insecticidal potential. Thus, beyond testing whether B. bassiana produces BEA endophytically, this study explores whether BEA production could serve as a proxy for monitoring the insecticidal status of B. bassiana in applied systems.
Although B. bassiana has been successfully introduced into over 25 plant species via seed treatment, foliar spray, or soil drench (Bamisile et al., 2018a; Yerukala et al., 2022), its ability to colonize peach as an endophyte has not been previously confirmed. Likewise, no study has quantified BEA levels within or on the surface of peach tissues inoculated with B. bassiana.
The objectives of this study were to: (1) determine whether B. bassiana can establish as a fungal endophyte in peach seedlings following foliar spray and soil drench inoculation methods; (2) quantify epiphytic and endophytic BEA concentrations in peach tissues; and (3) evaluate the implications of BEA detection as an indicator of fungal persistence and potential insecticidal activity. This work provides foundational insight into the biochemical interactions between B. bassiana and its host plant and highlights a potential method to assess fungal activity and improve the efficiency of B. bassiana use in IPM programs.
2 Materials and methods
2.1 Source of Beauveria bassiana inoculum
The B. bassiana strain GHA (BotaniGard 22WP) was purchased from Arbico Organics (Oro Valley, AZ USA) and subcultured on potato dextrose agar (PDA; Fisher Scientific, Waltham, MA USA). The fungus was incubated for approximately two weeks at 25°C in the dark, and conidia were harvested by flooding plates in 1000 μL of sterile water, scraping the agar surface with a sterile spatula and filtering the collection liquid through a sterile cheesecloth. Conidial concentrations were determined using a Bright-Line™ Hemacytometer (Hausser Scientific, Horsham, PA USA) and the suspensions adjusted to 1 × 108 conidia mL− 1 in sterile distilled water containing 0.05% Silwet L-77 (Fisher Scientific) according to Parsa et al. (2013). For all experiments, conidial viability was evaluated by taking a 100 μL sample of each inoculum, plating it on PDA, and incubating it at 25°C for 24 h in the dark. Germination was then assessed under light microscopy by counting germinated spores from a total of 100 randomly selected conidia. Conidia were deemed to have germinated if the germ tube was at least twice the conidia’s length. Only inoculum with a germination of ≥ 90% was used for experiments. The remaining inoculum was stored in the dark at 4°C until viability was confirmed.
2.2 Peach seed preparation
Adapting methods from Ramakuwela et al. (2020), under a flow hood, Guardian® rootstock peach pits (sourced from Clemson University, Clemson, SC USA) were surface sterilized by immersion for two minutes in 0.5% sodium hypochlorite (The Clorox Company, Oakland, CA USA) and two minutes in 70% ethanol (Fisher Scientific) then rinsed three times in sterile distilled water. The success of the sterilization process was evaluated by plating 100 µL of the last rinsing water on PDA media, incubating the plate for 10 days at 25 °C and checking for contaminant growth. If growth was seen, the corresponding pits were removed from the experiment and replaced. Pits were cracked using double-bladed pruning shears, which were flame sterilized between each use. Seeds were then submerged in sterile distilled water to hydrate for five days, changing water daily. Under the flow hood, hydrated seeds were placed in stratifying bags containing sterile perlite moistened with sterile distilled water and left for about three months in the dark at °C to germinate.
2.3 Inoculation of peach seedlings with B. bassiana
Seventy-five germinated seeds were planted in 15.2 cm X 15.2 cm plastic pots containing steam sterilized propagation media (SunGro® Sunshine Mix #5 Propagation Mix, BFG, Burton, OH USA) and Osmocote® 18-6–12 slow-release fertilizer (ICL Specialty Fertilizers, Dublin, OH USA). Plants were then placed in the greenhouse at 25°C under natural light (RH ~55%) where they were watered via drip irrigation for two minutes every other day until they reached their first true leaf stage (approximately three weeks). Forty-eight hours prior to inoculation plants were no longer watered to enhance the uptake of the fungal inoculum. Two treatments, soil drench with a B. bassiana inoculum, foliar spray with a B. bassiana inoculum, and a control were tested (25 plants each). For soil drench inoculation, a graduated cylinder was used to apply 150 mL suspension of B. bassiana (1 x 108 conidia mL-1 containing 0.05% Silwet L-77) to the surface of the soil at the base of each of twenty-five plants. For foliar inoculation, a conventional CO2 pressurized backpack sprayer was used (BellSpray LLC – R&D Sprayers, Opelousas, LA USA; TeeJet 8002VD yellow flat spray tip, TeeJet Technologies, Glendale Heights, IL USA) to apply approximately 10 mL of B. bassiana inoculum (1 x 108 conidia mL-1 containing 0.05% Silwet L-77d) to the surface of leaves to each of twenty-five seedlings. With foliar spray treatments the soil base was left uncovered and fungal inoculum allowed to drip off leaves onto soil to mimic field application. The control consisted of both a 10 mL treatment of 0.05% Silwet L-77 sprayed on the surfaces of leaves as well as a 150 mL of 0.05% Silwet L-77 treatment applied to the surface of the soil, applied to each of the remaining twenty-five seedlings. After inoculation, plants were arranged in a randomized block design and allowed to continue to grow in the greenhouse until subsequent tissue sampling.
2.4 Re-isolation of endophytic B. bassiana in peach tissues by surface sterilization and tissue culturing
Five peach seedlings per treatment (i.e. treated with B. bassiana inoculum via foliar spray, treated with B. bassiana inoculum through soil drench) and the control were examined two, four, six, eight-, and twelve-weeks post inoculation (WPI), to confirm endophytic colonization of the fungus. Plants were carefully uprooted and two leaflets, two pieces of the main stem, and two pieces of root were sampled from each plant. Leaves were randomly selected from the middle canopy of the seedling. No leaves were selected from the apical or basal area of the plant. Of the two parts of the main stem, one was sampled towards the middle of the plant and the second closer to the soil surface. The two root sections were obtained by dividing the root system into two parts. Working under a laminar flow hood, all tissue samples were separately surface sterilized to remove epiphytic fungi and surface contaminants as follows: Immersion in 0.5% sodium hypochlorite for thirty seconds, followed by immersion in 70% ethanol for thirty seconds, and rinsing in sterile distilled water three times and success of sterilization was evaluated as previously described. Tissue samples were then allowed to dry on sterile cheesecloths. The outer edges of each tissue sample were trimmed and further cut into six ~ 4 mm X 4mm pieces. Tissue pieces were then plated on selective Doberski and Tribe medium (DBT; Doberski and Tribe, 1980). Cultures were incubated at room temperature in the dark for ~ 14 days. Tissue cultures exhibiting typical mycelial growth of B. bassiana (Figure 1) were re-isolated onto fresh DBT and allowed to grow for 21 days at room temperature in the dark for subsequent molecular identification.

Figure 1. Leaf tissue cultures from Beauveria bassiana inoculum soil-drenched (right) and control treated (left) plants. Leaves were sampled two weeks post inoculation (WPI), surface sterilized, plated on DBT media and incubated for ~14 days. Fungi from all plates with tissue samples exhibiting morphological signs of B. bassiana (white mycelial growth, left) were re-isolated to fresh media and subjected to subsequent molecular confirmation of B. bassiana.
2.5 Molecular validation of fungal re-isolates as B. bassiana
The identity of the re-isolated fungal from tissue sample cultures grown on DBT was confirmed via DNA barcoding, including a pure culture of the commercial B. bassiana strain GHA as a positive control. DNA was extracted from mycelia collected from each colony using a ZR Fungal/Bacterial DNA MiniPrep Kit (Zymo Research, Irvine, CA USA) following the manufacturer’s protocol. Sterile distilled water was used as a negative control to check for contamination and DNA samples were stored at –20°C until they were processed for PCR.
Following methods similar to Posada and Vega, 2006, the ITS region of the nuclear ribosomal DNA repeat was sequenced for each re-isolate utilizing the primers ITS1-F (fungal-specific) (Gardes & Bruns, 1993) and ITS4 (White et al., 1990). PCRs were performed in 25 μL reaction volumes with 12.5 μL GoTaq Green Master Mix (Promega, Madison, WI USA), 1 μL each of 10 mM primers, and 1 μL of 10 ng/μL DNA or 1 μL of a sterile water control. Amplification was achieved with an initial denaturation step of 5 min at 94°C; 35 cycles of 30 s at 94°C, 45 s at 50°C, and 45 s at 72°C; and a final extension of 7 min at 72°C. PCR products were run on an agarose gel, stained with SYBR Safe (Fisher Scientific), and viewed under UV light. An All-Purpose Hi-Lo DNA Marker (Bio-nexus, Oakland CA USA) was used to visually estimate amplicon size. Samples with amplicons of the correct size (~464 bp) were sent to Eurofins Genomics for Sanger sequencing. Sequencing results were trimmed as needed and contiguous sequences for each isolate were assembled in Geneious Prime version 2024.0.3 (www.geneious.com). Sequences were compared to those of the reference B. bassiana strain using the BLAST tool on GenBank (National Center for Biotechnology Information, National Institute for Health, Bethesda, MD USA) to confirm identity. Based on this molecular validation, tissue sample plates (leaf, stem, or root) resulting in positive B. bassiana fungal re-isolates were recorded as 1 and those not containing B. bassiana fungal re-isolates were recorded as 0.
2.6 Quantification of epiphytic and endophytic BEA
2.6.1 Plant material and sample preparation
A total of thirty seedlings were germinated, planted, and grown in the greenhouse as previously described. Ten plants were treated with the fungal inoculum via foliar spray and ten plants were treated with the fungal inoculum via soil drench. The ten remaining plants were prepared as controls. At two WPI, six leaves, six pieces of stem, and six roots were collected from each plant. The first set of two leaves, two pieces of stem, and two roots from each plant were immediately frozen in liquid nitrogen. The second set of two leaves, two pieces of stem, and two root samples from each plant were surface sterilized separately by immersion in 0.5% sodium hypochlorite for thirty seconds, followed by immersion in 70% ethanol for thirty seconds, and rinsing in sterile distilled water three times, prior to freezing in liquid nitrogen. This surface sterilization was performed to remove epiphytic fungi, enabling comparison of BEA concentrations associated with external (epiphytic) versus internal (endophytic) fungal presence. The success of sterilization was evaluated by plating 100 µL of the last rinsing water on PDA media, incubating the plate for 10 days at 25 °C and checking for contaminant growth. If growth was seen these samples were removed from the experiment and replaced with a new collection from additionally treated plants. The remaining tissue samples were also subjected to surface sterilization, re-isolation and molecular confirmation of B. bassiana following the same protocol as previously described to confirm successful endophytic colonization of the fungus. The entire experiment was conducted twice.
All tissue samples frozen in liquid nitrogen were then freeze-dried (FreeZone 2.5 Liter Benchtop Freeze Dry System, Cat no. 7670520; Labconco, Kansas City, MO USA) and processed immediately. The tissue was ground into powder using a tissue homogenizer (SPEX SamplePrep 1600 MiniG; Cole-Parmer SamplePrep, Metuchen,NJ USA) and 10 mg were weighed into 1.7 mL microcentrifuge tubes. The extraction procedure was conducted following the protocol by Rämö et al., 2021. Briefly, 250 uL of extraction solvent [3:1 methanol: water, v/v; (LC-MS grade; Sigma-Aldrich, Burlington, MA, USA)] were added to the tissue with 100 ng of isotopically labeled cinnamic acid-13C3 (Cat. No. 513962; Sigma-Aldrich) in each sample as the internal standard. Samples were then sonicated and vortexed for 15 min in ice-cold water and centrifuged (5 min at 4°C, at 15,000 rpm), the supernatant was transferred to a new microcentrifuge tube, and the pellet re-extracted with 250 uL of extraction solvent. All supernatants were pooled, filtered through 0.22um PTFE filters (Agilent Technologies, Santa Clara, CA USA, Cat no. 5191-5912), concentrated using a nitrogen stream, re-suspended in the extraction buffer, and stored at -80 °C until further analysis.
2.6.2 UHPLC-ESI-MS analysis
BEA was quantified using reverse-phase ultra-high performance liquid chromatography-electrospray ionization-mass spectrometry (UHPLC-ESI-MS), as described in detail by Rämö et al., 2021. Analyses were performed on an Agilent 1290 Infinity II UHPLC system with autosampler and high-speed pump (Agilent Technologies, Santa Clara, CA, USA) coupled to an Agilent 6546 LC/QTOF mass spectrometer (Agilent Technologies). Chromatographic separation was achieved on a Zorbax Eclipse Plus C18 column (2.1 x 50 mm, 1.8 μm; Agilent Technologies, Cat. No 959757-902) maintained at 40 °C. Mobile phase A was water with 0.1% formic acid (v/v; Fisher Scientific, Waltham, MA, USA) and mobile phase B was acetonitrile with 0.1% formic acid (v/v; Sigma-Aldrich). The flow rate was 0.50 mL min-1, and the injection volume was 1 μL. The gradient was: 3% B from 0.0-2.5 min, increased to 15% B at 4.0 min, to 30% B at 6.0 min, to 50% B at 8.0 min, and to 100% B at 10.0 min, held at 100% B until 12.0 min, followed by return to initial conditions and re-equilibrium. Mass spectrometric detection was in positive ESI mode with nitrogen gas temperature 250 °C, nebulizer pressure 40 psi, and capillary voltage 3,000 V. Data were acquired in MS mode and processed using Agilent MassHunter Workstation (Qualitative Analysis, v10.0). Quality control procedures included solvent blanks, calibration check standards, and system suitability standards at the start of the sequence and after each analytical batch to monitor retention time stability, sensitivity, and carryover, as described in detail by Rämö et al., 2021.
2.6.3 Standard curve analysis
Quantification was performed against a calibration curve prepared from an authentic BEA standard (≥97% HPLC purity, Cat. No. B7510; Sigma-Aldrich) in the extraction solvent mixture as described by Rämö et al., 2021. The standard curve consisted of 10 points (1 μL working solutions ranging from 0.1–10 ng μL-1) prepared in methanol (LC-MS grade; Sigma-Aldrich) containing the same concentration of isotopically labeled internal standard, cinnamic acid-13C3 (Cat. No. 513962; Sigma-Aldrich), as used for samples. Each standard and sample were analyzed in triplicate injections to determine concentration. The calibration curve showed an R2 of 0.981, generated using Agilent MassHunter Workstation (Quantitative Analysis v11.0).
2.6.4 Limit of BEA detection and quantification
The limit of detection (LOD) and limit of quantification (LOQ) were used to evaluate method sensitivity. The criteria for a detectable signal included correct retention time, a sufficient signal-to-noise ratio (S/N), and presence of confirmation ions for BEA. LOD was calculated as the average peak area plus three times the standard deviation (SD), and LOQ as the average peak area plus six times the SD (Rämö et al., 2021). In addition, as an alternative definition, LOD and LOQ may be expressed as the lowest concentrations at which the analyte produced a peak signal three- and tenfold higher than the background noise, respectively (Yogendrarajah et al., 2013). Using this approach, the method LOD and LOQ for BEA were determined to be 1.950 ng g-1 and 5.910 ng g-1, respectively. We considered samples with resulting BEA concentrations that fell between LOD and LOQ to contain trace amounts of the mycotoxin and samples containing values below the LOD to contain none of the mycotoxin.
2.7 Insect leaf feeding assay
To evaluate the functional significance of BEA detection as an indicator of fungal persistence and potential insecticidal activity, a total of thirty seedlings were germinated, planted, and grown in the greenhouse as previously described. Ten plants were treated with the fungal inoculum via foliar spray and ten plants were treated with the fungal inoculum via soil drench. The ten remaining plants were prepared as controls. Two WPI, six leaves of around the same size were collected from each plant to be utilized for feeding assays and confirmation of endophytic presence of B. bassiana. Of the collected leaves, two leaves from each plant were surface sterilized separately by immersion in 0.5% sodium hypochlorite for thirty seconds, followed by immersion in 70% ethanol for thirty seconds, and rinsing in sterile distilled water three times to remove epiphytic fungi, enabling comparison of feeding assay outcomes and BEA exposure between epiphytic and endophytic fungal associations, while the other two leaves were not surface sterilized prior to use in feeding assays. The success of the sterilization procedure was evaluated by plating 100 µL of the last rinsing water on PDA media, incubating the plate for 10 days at 25°C, and checking for contaminant growth. If growth was seen, these samples were removed from the experiment and replaced with new samples from additionally treated plants. The remaining two leaf samples per plant were subjected to re-isolation and molecular confirmation of B. bassiana as previously described, to confirm the endophytic presence of the fungus.
Leaves collected and prepared for feeding assays were placed in an individual feeding chamber, which consisted of a 100 mm Petri dish (Fisher Scientific), lined with 9.0 cm filter paper (Fisher Scientific) moistened with 1000 µL of sterile distilled water. One third instar yellow mealworm, Tenebrio molitor L. (Coleoptera: Tenebrionidae) larva, (Fluker Farms, Port Allen, LA, USA) was added to each chamber and the dish sealed with parafilm. Although T. molitor is not an insect pest of peach this species was chosen as a model in our study due to its generalist feeding behavior, ease of rearing, and potential to replicate these experiments in other plant systems. The feeding chambers were then incubated at 26°C and mortality was assessed and recorded daily for 21 days. Larvae were considered dead if they failed to respond to gentle prodding. Twenty larval feeding chambers were created per foliar spray, soil drench, and control treatment for the experiment and the entire experiment was conducted twice.
2.8 Statistical analysis
All statistical analyses and data visualization were performed in R version [4.3.2] (R Core Team, 2023) using base functions and relevant packages.
2.8.1 Assessment of B. bassiana as an endophyte in peach
To test the hypothesis that the probability of observing the presence of B. bassiana as an endophyte would depend on the continuous variable of Time (WPI), three levels of Treatment (foliar spray, soil drench, or control), and three levels of Tissue (leaf, root, stem), as well as the interaction between Treatment and Tissue, we fit a generalized linear mixed model with a binomial distribution and a logit link function using the glmer function in the lme4 package (Bates et al., 2014). To account for repeated measurements from the same individual plant, we included plant identity as a random intercept. Following the fitting of the model, the simulateResiduals function from the DHARMa package (Hartig, 2024) was used to assess the distribution of residuals and confirm that the model assumptions (e.g., normally distributed residuals, and homogeneity of variance, etc.) were met. Model significance was assessed with the Anova () function in the car package (Fox and Weisberg, 2019) and post hoc comparisons were conducted via the emmeans package (Lenth, 2025) using Sidak’s test (α = 0.05). Lastly, sample predictions were fit using the predict function in the stats package (R Core Team, 2024) and plotted with the raw data for visualization with the ggplot function in the ggplot2 package (Wickham, 2016).
2.8.2 Quantification of BEA
We recorded samples containing BEA values below the analytical limit of detection (LOD = 1.950 µg/g) as zero. Surface sterilization was excluded from analyses because all sterilized samples were below the LOD and contained no detectable BEA. To test the hypothesis that both the probability of detecting BEA above the LOD and the conditional concentration of BEA would depend on the interaction between the three levels of Treatment (foliar spray, soil drench, or control) and the three levels of Tissue (leaf, root, or stem), we initially fit a linear model with Treatment, Tissue, and their interaction as predictors. However, diagnostic tests indicated strong violations of assumptions: residuals were significantly non-normal (Shapiro–Wilk p < 0.001) and heteroscedastic (Breusch–Pagan test p < 0.001). A Poisson generalized linear model was also evaluated but proved unsuitable due to extreme overdispersion (dispersion ratio ≈ 201, p < 0.001) and significant zero inflation (p < 0.001; DHARMa residual diagnostics via the DHARMa package, Hartig, 2024).
Given these results, the data were more appropriately modeled with a two-part hurdle approach, which accommodates both excess zeros and skewed positive values (Zuur et al., 2009; Brooks et al., 2017). The first component was a binary logistic regression modeling the probability of detecting BEA above the LOD (BEA > 1.950 µg/g). The second component was a Gamma generalized linear model with a log link, fit only to samples with positive concentrations, to estimate conditional mean levels of BEA. Both models included Treatment, Tissue, and their interaction as fixed effects and were fit utilizing the glmmTMB package in R (Brooks et al., 2017). Residual diagnostics from the DHARMa package (Hartig, 2024) confirmed that the hurdle models exhibited no overdispersion, residual bias, or outliers. Model significance was assessed with the Anova () function via the car package (Fox and Weisberg, 2019) and post hoc comparisons were conducted via the emmeans package (Lenth, 2025) using Sidak’s test (α = 0.05). To facilitate interpretation, we combined the predicted detection probabilities from the logistic component (p) with the conditional mean concentrations from the Gamma component (μ+). Overall expected concentrations were calculated as:
In plain terms, this value is the probability of detecting BEA multiplied by the average concentration among those samples where it was detected, yielding an overall expected mean that represents the average concentration across all samples, including both non-detects and positives.
2.8.3 Assessment of T. molitor mortality
To test the hypothesis of a two-way interaction between the three levels of B. bassiana Treatment (foliar spray, soil drench, control) and the two levels of Surface Sterilization (yes or no) on the probability of yellow mealworm mortality, we used a generalized linear mixed model with a binomial distribution and logit link function using the glmer() function in the lme4 package (Bates et al., 2014). The probability of yellow mealworm mortality was the dependent variable and the two-way interaction of treatment and surface sterilization, and main effects were the independent variables. The experimental trial was designated as a random effect. Following the fitting of the model, the simulateResiduals function from the DHARMa package (Hartig, 2024) was used to assess the distribution of residuals to determine if the model assumptions (e.g. normally distributed residuals, and homogeneity of variance, etc.) were met. Model significance was assessed using the Anova () function in the car package (Fox and Weisberg, 2019). Post hoc comparisons were conducted via the emmeans package (Lenth, 2025) using Sidak’s test (α = 0.05) nesting treatment within surface sterilization status and plotted for visualization with the ggplot function in the ggplot2 package (Wickham, 2016).
3 Results
3.1 Systemic endophytic colonization of B. bassiana in peach
Results from fungal re-isolation via tissue culturing indicate that the entomopathogenic fungus B. bassiana can be successfully established as an endophyte in peach seedlings using both foliar spray and soil drench inoculation methods (Figure 2). The PCR amplification of fungal re-isolates was consistent in generating an amplicon of the appropriate size (~464 bp), specific to B. bassiana. Sequencing confirmed the identity of amplified fragments as B. bassiana and pairwise alignment against previously characterized sequences of B. bassiana on GenBank (Accession number: PP318546) was recorded for all positive samples. The main effects of Treatment (χ² = 23.72, df = 1, p < 0.001), Tissue type (χ² = 6.17, df = 2, p = 0.046), and Time (WPI; χ² = 33.17, df = 1, p < 0.001) significantly affected the probability of recovery of B. bassiana as an endophyte from peach seedlings. In addition, a significant Treatment × Tissue interaction was detected (χ² = 27.59, df = 2, p < 0.001), indicating that the effect of treatment varied among plant tissues.
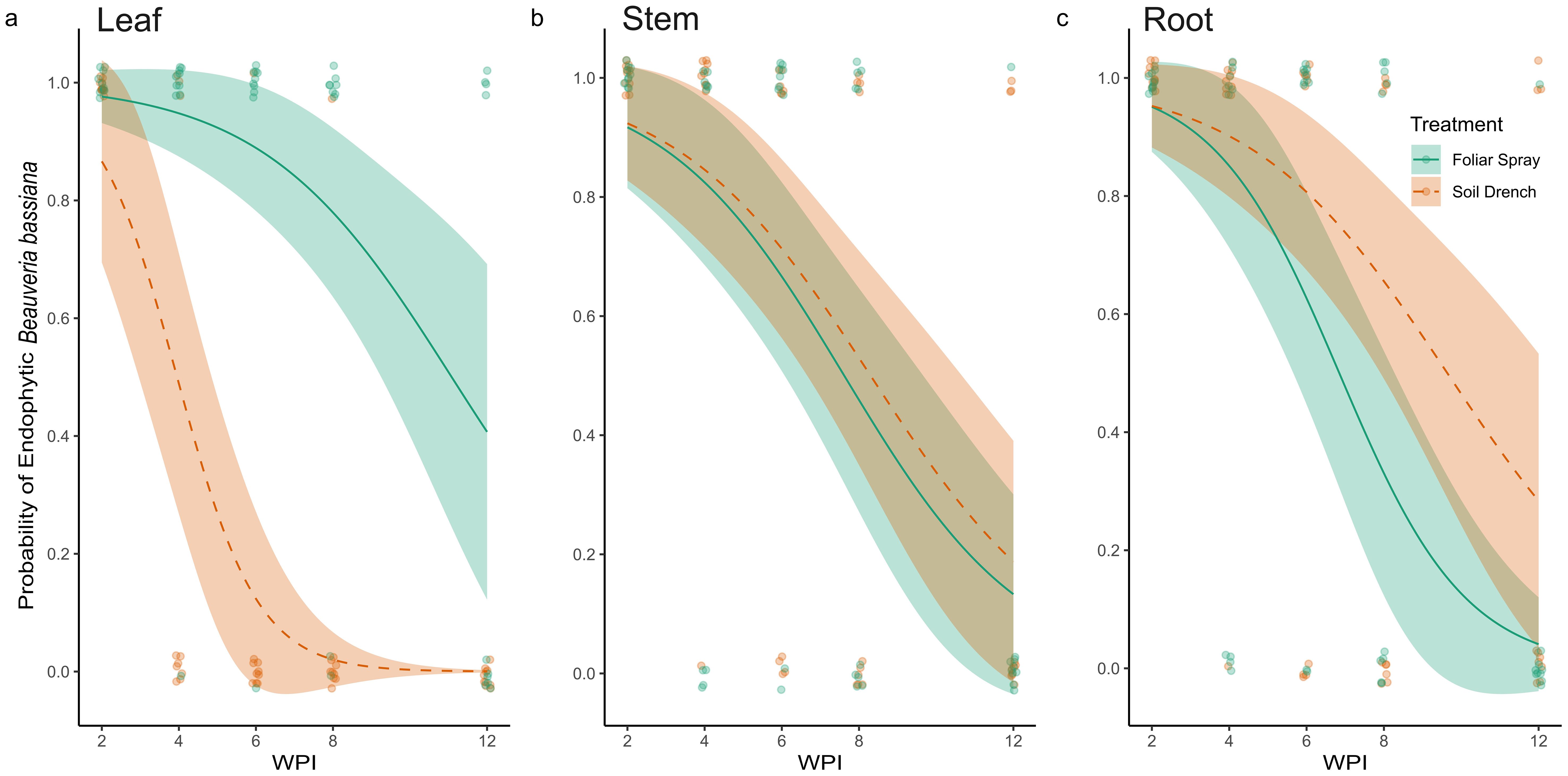
Figure 2. Probability of the presence of endophytic Beauveria bassiana in leaves (A), stem (B), and root (C) tissues of plants foliar sprayed (green) and soil drenched (orange) with fungal inoculum two to twelve weeks post inoculation (WPI). No control samples tested positive for the presence of B. bassiana, therefore are not represented in the figure.
At two WPI, all sampled tissues (leaves, stems, and roots) from both foliar-sprayed and soil-drenched plants tested positive for endophytic colonization by B. bassiana (Figures 2, 3). We recognized this as an opportunity to investigate what occurs metabolically with BEA when B. bassiana can be detected systemically, therefore all other experiments in our study were conducted 2 WPI. Over time, the probability of recovering B. bassiana from leaf tissues cultures was higher in foliar-sprayed plants compared to soil-drenched plants (Figure 2A). In soil-drenched plants, recovery from leaves declined sharply around six WPI and ceased entirely by twelve weeks (Figure 2A). In contrast, recovery from stem tissues was similar between foliar spray and soil drench treatments throughout the study period (Figure 2B). By eight WPI, the likelihood of recovery from stems was approximately 50%, regardless of treatment method (Figure 2B). For root tissues, the probability of endophytic recovery was slightly higher in soil-drenched plants compared to those treated via foliar spray (Figure 2C). Overall, the probability of recovering B. bassiana from all tissue types declined over time. However, in foliar-sprayed plants, the fungus was still detectable in representative samples of all tissue types at twelve WPI (Figure 2). There was no recovery of B. bassiana in any of the control plants.
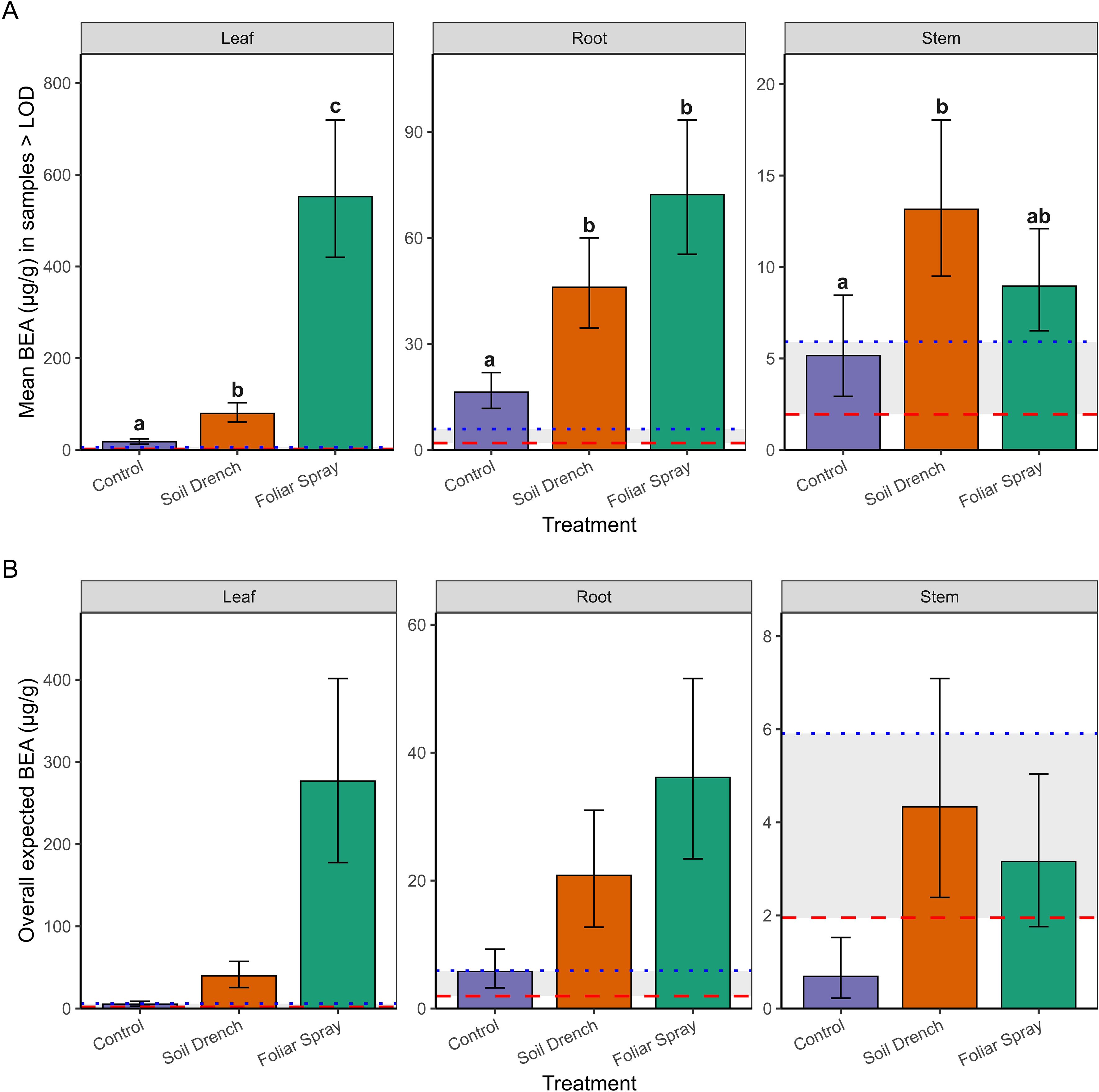
Figure 3. Concentrations of beauvericin (BEA; µg/g tissue) detected in peach seedlings following inoculation with Beauveria bassiana strain GHA. (A) Mean concentrations (± SE) among samples with detectable BEA (>LOD = 1.95 µg/g; >LOQ = 5.91 µg/g) across treatments (Control, Soil Drench, Foliar Spray) and tissues (Leaf, Root, Stem). (B) Overall expected concentrations (± SE), which include zero values for samples without detectable BEA, thereby representing treatment and tissue-specific means on a per-sample basis. Red dashed line indicates the LOD threshold and blue dashed line indicates the LOQ threshold. The same letters indicate no significant between mean beauvericin concentrations (Sidak’s test, a = 0.05).
3.2 Quantification of BEA
Surface sterilized tissues consistently contained BEA concentrations below the limit of detection (LOD = 1.95 µg/g), indicating that in this study measurable BEA production was only present epiphytically. Therefore, analyses were restricted to non-sterilized tissues to evaluate treatment effects on epiphytic BEA production.
To do so we used a two-part hurdle modeling framework. The first component modeled the probability of detecting BEA above the limit of detection (LOD = 1.95 µg/g) in non-surface sterilized tissues. Detection probability did not differ significantly among Treatments (foliar spray, soil drench, control), Tissue (leaf, root, stem), or their interaction (Anova, χ² = 4.25, df = 2, p = 0.12 for Treatment; χ² = 5.41, df = 2, p = 0.07 for Tissue; χ² = 1.39, df = 4, p = 0.85 for Treatment × Tissue; Supplementary Figure S1). Because detectability did not differ among treatments or tissues, subsequent differences reflect true variation in concentrations rather than artifacts of threshold sensitivity.
The second component of the analysis estimated mean concentrations conditionally among samples with detectable BEA (BEA >LOD; Figure 3A). Treatment and tissue type as well as the interaction between treatment and tissue type significantly influenced BEA concentrations in non-surface sterilized peach tissue samples. Foliar spray inoculation consistently yielded the highest concentrations, especially in leaves (mean: 549 µg/g; 95% CI: 422–714 µg/g, range: 127 – 1517 µg/g), which were significantly greater than both soil drench and control treatments (p < 0.0001). Roots also exhibited elevated concentrations under foliar spray (71 µg/g; 95% CI: 55–93 µg/g, range: 27.7-119 µg/g) compared to controls (p < 0.0001). Stems contained lower concentrations overall (≤18 µg/g), with only the soil drench treatment producing levels significantly higher than the control (p = 0.0068).
Finally, by combining detection probability with conditional means, we derived overall expected BEA concentrations (i.e., the average amount expected across all samples, including non-detects; Figure 3B). This metric revealed the strongest treatment effects in leaves, where foliar spray application produced mean expected concentrations nearly an order of magnitude greater than soil drench and two orders of magnitude greater than controls. Roots showed intermediate effects, while stems exhibited only trace to low expected concentrations. Notably, concentrations between the LOD (1.95 µg/g) and LOQ (5.91 µg/g) were considered trace, and such levels were observed primarily in control and stem tissues. Together, these results demonstrate that foliar spray inoculation is the most effective method for achieving high BEA concentrations in peach seedlings, with the strongest accumulation on leaves. Soil drench application yielded intermediate concentrations, whereas stems rarely accumulated levels above trace amounts.
3.3 T. molitor mortality coincides with BEA Production
Tenebrio molitor feeding assays revealed significant main effects of treatment (x2 = 18.33, df = 2, P = < 0.001) and surface sterilization status (x2 = 19.40, df = 1, P = < 0.0001) on yellow mealworm mortality. However, the interaction between treatment and sterilization was not statistically significant (x2 = 3.04, df = 2, P = 0.219), suggesting that treatment and surface sterilization independently influenced mortality. Post hoc comparisons within each sterilization group clarified these effects. In the non-surface sterilized group, yellow mealworm mortality was significantly higher when exposed to leaf tissue foliar sprayed with the fungus (P < 0.05; prob = 0.578 ± 0.106 SE) compared to both control (prob = 0.118 ± 0.059 SE) and soil drench (prob = 0.167 ± 0.072 SE) treatments (Figure 4A). No significant difference in mortality was observed between control and soil drench treatments in this group. In contrast, when leaves were surface sterilized yellow mealworm mortality was uniformly low across all treatments (prob range: 0.023 – 0.047), with no significant differences detected among control, foliar spray, or soil drench treatments (Figure 4B).

Figure 4. Probability (mean ± SE) of yellow mealworm (Tenebrio molitor) mortality exposed to non-surface sterilized (A) and surface sterilized (B) leaf tissues sampled from plants treated with a control, soil-drenched, or foliar-sprayed with a Beauveria bassiana inoculum. The same letters indicate no difference in the probability of mealworm mortality between treatments under each surface sterilization status (Sidak’s test, a = 0.05).
4 Discussion
This study demonstrates that endophytic colonization of peach seedlings by B. bassiana strain GHA is feasible, thereby expanding the range of woody perennials – currently including pecan (Carya illinoinensis Koch), pine (Pinus radiata D. Don), date palm (Phoenix dactylifera L.), cacao (Theobroma cacao L.), horse chestnut (Aesculus hippocastanum L.), cassava (Manihot esculenta Crantz), and coffee (Coffea arabica L.) - known to support artificial endophytic establishment of this fungus (Posada and Vega, 2005, 2006; Brownbridge et al., 2012; Barta, 2008; Greenfield et al., 2016; Ramakuwela et al., 2020; Husain et al., 2024). Our findings indicate that peach seedlings are highly receptive to colonization, with both soil drench and foliar spray inoculation methods resulting in successful recovery of B. bassiana from all sampled leaf, main stem, and root tissues two WPI. Re-isolation of the fungus from tissues distal to the inoculation sites, such as leaf tissues from soil-drenched plants, suggests systemic movement and vertical transmission within the host. As in other plant hosts, endophytic colonization of peach by B. bassiana was transient, with a general decline in fungal recovery over time and patterns of persistence varied by inoculation method and tissue type (Garrido-Jurado et al., 2017; Bamisile et al., 2018b; Vega, 2018; Ramakuwela et al., 2020). By 12 WPI, the probability of fungal recovery had dropped below 50% across all treatments. Notably, the foliar spray treatment was associated with a higher probability of re-isolation from leaf tissues, while the soil drench treatment showed slightly greater persistence in root tissues over time. These differences suggest that the method of inoculation may influence the longevity and distribution of B. bassiana colonization within specific peach tissues. Together, these results highlight the potential of B. bassiana to establish systemic transient endophytic associations in peach seedlings and underscore the importance of considering inoculation method, timing, and repeated application when aiming to establish this fungus as an endophyte in specific tissue types and time periods.
A central novel finding of this study is the quantification of beauvericin (BEA), a cyclic hexadepsipeptide with insecticidal, antimicrobial, and cytotoxic properties (Caloni et al., 2020), in peach tissues following B. bassiana inoculation. Our results demonstrate that measurable BEA accumulation occurred primarily in non-surface-sterilized tissues, suggesting that epiphytic colonization and surface-associated fungal growth contribute most strongly to its production under greenhouse conditions. The highest concentrations were detected in leaves from foliar-sprayed plants, averaging 549 µg/g tissue (95% CI: 422–714 µg/g), with maximum levels reaching 1,517 µg/g. Roots also accumulated moderate concentrations (mean 71 µg/g), while stems contained comparatively low amounts (≤18 µg/g).
These values are biologically meaningful. The mean concentrations on foliar-sprayed leaves (549 µg/g; range 127–1517 µg/g) overlap with or exceed thresholds previously shown to negatively affect insects. For example, ingestion of 1–5 µg/g impaired aphid reproduction (Ganassi et al., 2002), while 10–20 µg/mL caused >80% mosquito larval mortality (Grove and Pople, 1980). Thus, the concentrations observed here fall within biologically active ranges known to reduce insect survival and fecundity. Importantly, BEA levels measured here also exceeded concentrations reported from broth culture or conidial suspensions (often <10 ng/mL; Leland et al., 2005; Safavi, 2013), suggesting that phyllosphere conditions such as humidity, nutrient exudates, and microbial interactions may enhance secondary metabolite biosynthesis.
Environmental and host-associated factors are likely to play key roles in regulating BEA production. Temperature, humidity, and time since inoculation have all been shown to affect mycotoxin biosynthesis in entomopathogenic and phytopathogenic fungi (James et al., 1998; Gutierrez-Pozo et al., 2024; Oluwakayode et al., 2024). For instance, Fusarium spp. exhibit peak BEA synthesis under specific temperature and humidity regimes (Gutierrez-Pozo et al., 2024), while B. bassiana has been shown to adjust metabolite expression in response to host or environmental conditions (James et al., 1998). In addition, plant factors such as exudates, surface microflora, and tissue physiology may influence fungal metabolic activity. The complete absence of detectable BEA in sterilized tissues highlights the importance of spatial context. Epiphytic associations may stimulate metabolite production more strongly than internal colonization under some conditions. Future studies should therefore quantify temporal patterns of BEA and other B. bassiana mycotoxins in both epiphytic and endophytic niches, across different environmental conditions and hosts.
Interestingly, low but detectable levels of BEA were also observed in non-surface-sterilized control tissues, especially in roots. Although not directly tested in this study, one possible explanation is the presence of naturally occurring fungi such as Fusarium spp., which are known BEA producers and exist as endophytes in peach (Wang and Xu, 2012; Mannai et al., 2018; Gautier et al., 2020). This complicates interpretation and highlights a broader challenge for field-level monitoring: distinguishing BEA produced by inoculated Beauveria from background Fusarium contamination is agronomically important. Because Fusarium-derived BEA can accumulate in agricultural products (Santini et al., 2012; Pietruszka et al., 2023), its occurrence in untreated peach tissues underscores the need for robust metabolite monitoring in systems where B. bassiana is utilized as a biological control tactic potentially increasing BEA in the environment where it is applied.
The functional significance of BEA accumulation was supported by our insect bioassays. Non-surface sterilized leaves from foliar-sprayed plants, which contained the highest BEA concentrations, caused significantly higher mortality in T. molitor larvae compared to controls or soil-drenched treatments. While this correlation suggests a role for BEA in insecticidal activity, synergistic effects with other fungal metabolites or enzymes cannot be excluded (Mascarin and Jaronski, 2016). However, these results support BEA detection as an indicator of B. bassiana persistence and potential insecticidal activity in general. It is important to note that T. molitor serves as a generalist model and does not represent the insect pest complex associated with peach. Feeding guilds differ substantially among peach herbivores: phloem-feeding aphids (Myzus persicae Sulzer), cell-piercing thrips (Frankliniella spp.), and fruit-, xylem- or cambium-feeding borers may encounter BEA at different concentrations and tissue locations than T. molitor (Blaauw et al., 2025). Therefore, while our results demonstrate toxicity, future bioassays with peach-relevant pests are needed to determine the significance of B. bassiana and BEA-mediated suppression in peach specific pests.
From an applied perspective, foliar inoculation produced higher BEA accumulation than soil drench, especially on leaves, suggesting this method may yield stronger short-term pest suppression. However, given the transient nature of colonization, repeated applications are likely needed to sustain efficacy. In agricultural systems, understanding and managing the variables associated with BEA production is essential to harness the benefits of B. bassiana as a biocontrol agent while minimizing unintended risks. The increased addition of a biologically active mycotoxin to agricultural environments necessitates careful consideration in terms of application timing, harvest intervals, post-harvest processes, and regulatory thresholds. While BEA is not currently regulated in most agricultural contexts, its detection and known bioactivities emphasize the need for monitoring protocols, particularly as mycoinsecticides gain traction in sustainable agriculture. BEA has been reported not only for its insecticidal properties but also for its potential pharmaceutical applications, raising concerns about impacts on non-target organisms, soil microbiomes, food safety, and the potential development of antimicrobial resistance (Sood et al., 2017; Gautier et al., 2020; Křížová et al., 2021; Pietruszka et al., 2023; Al Khoury et al., 2024). Given its antibiotic-like activity, there is a pressing need for monitoring BEA and similar fungal metabolites in treated crops, especially as the use of endophytic and epiphytic fungi and mycotoxins expands in IPM and organic farming practices.
The ability of B. bassiana to occupy both endophytic and epiphytic niches and produce metabolites such as BEA underscores the ecological flexibility of this fungus. This dual capacity may facilitate persistence in variable environments and enhance interactions with both plant hosts and herbivores. However, it also complicates predictions of biocontrol outcomes, as metabolite production may be shaped by inoculation method, tissue colonization patterns, host physiology, and environmental stressors. Integrating metabolite monitoring with functional outcomes such as pest suppression, plant growth promotion, and shifts in phytochemistry will be essential to fully understand the roles of endophytic B. bassiana and other endophytic entomopathogenic fungi in crop systems as well as proper integration into IPM programs. These results provide the first evidence in peach that endophytic colonization can occur without concomitant metabolite accumulation or insecticidal effect. By distinguishing epiphytic from endophytic pathways, this study clarifies why field outcomes with B. bassiana are variable and underscores the importance of timing, formulation, and monitoring in deploying entomopathogenic fungi for integrated pest management. Future studies should evaluate colonization and metabolite production in mature orchard trees under field conditions. Assessing persistence, spatial distribution, and ecological impacts at the orchard scale will be critical to determine the long-term feasibility of deploying B. bassiana as a biocontrol tool in peach production.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
SE: Funding acquisition, Methodology, Writing – original draft, Formal analysis, Visualization, Conceptualization, Investigation, Data curation, Project administration. CV: Supervision, Conceptualization, Writing – review & editing, Methodology, Resources, Validation. CF: Writing – review & editing, Visualization, Validation. DS-I: Supervision, Writing – review & editing, Conceptualization, Funding acquisition, Validation, Resources, Methodology. DC: Writing – review & editing, Supervision, Resources, Validation, Conceptualization, Methodology. BB: Validation, Resources, Writing – review & editing, Funding acquisition, Conceptualization, Project administration, Methodology, Supervision.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This research was supported by the Sustainable Agriculture Research and Education (SARE) Projects Graduate Student Grant GS23–286 as well as the USDA-NIFA Crop Protection and Pest Management (CPPM) Program (grant number: 2022-70006-37992).
Acknowledgments
The authors thank the University of Georgia Plant Metabolomics Laboratory for assistance with BEA quantification. We are also grateful to Hayley Daniels and Zahra Khan for their support with sample processing and data collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffunb.2025.1714008/full#supplementary-material
References
Acheampong M. A., Hill M. P., Moore S. D., and Coombes C. A. (2020). UV sensitivity of Beauveria bassiana and Metarhizium anisopliae isolates under investigation as potential biological control agents in South African citrus orchards. Fungal Biol. 124, 304–310. doi: 10.1016/j.funbio.2019.11.006
Al Khoury C., Nemer G., Nemer N., Baroudi E., and Tokajian S. (2019). Insecticidal activity of beauvericin against the two-spotted spider mite Tetranychus urticae (Acari: Tetranychidae) and its impact on strawberry yield. Exp. Appl. Acarol 78, 101–115. doi: 10.1007/s10493-019-00379-4
Al Khoury C., Tokajian S., Nemer N., Nemer G., Rahy K., Thoumi S., et al. (2024). Computational applications: beauvericin from a mycotoxin into a humanized drug. Metabolites 14, 232. doi: 10.3390/metabo14040232
Bamisile B. S., Akutse K. S., Siddiqui J. A., and Xu Y. (2021a). Model application of entomopathogenic fungi as alternatives to chemical pesticides: prospects, challenges, and insights for next-generation sustainable agriculture. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.741804
Bamisile B. S., Dash C. K., Akutse K. S., Keppanan R., Afolabi O. G., Hussain M., et al. (2018b). Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: an insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 217, 34–50. doi: 10.1016/j.micres.2018.09.001
Bamisile B. S., Dash C. K., Akutse K. S., Keppanan R., and Wang L. (2018a). Fungal endophytes: beyond herbivore management. Front. Microbiol. 9. doi: 10.3389/fmicb.2018.00544
Bamisile B. S., Siddiqui J. A., Akutse K. S., Ramos Aguila L. C., and Xu Y. (2021b). General limitations to endophytic entomopathogenic fungi use as plant growth promoters, pests and pathogens biocontrol agents. Plants 10, 10102119. doi: 10.3390/plants10102119
Barra-Bucarei L., González M. G., Iglesias A. F., Aguayo G. S., Peñalosa M. G., and Vera P. V. (2020). Beauveria bassiana Multifunction as an Endophyte: Growth Promotion and Biologic Control of Trialeurodes vaporariorum, (Westwood) (Hemiptera: Aleyrodidae) in Tomato. Insects 11, 591. doi: 10.3390/insects11090591
Barta M. (2018). In planta bioassay on the effects of endophytic Beauveria strains against larvae of horse-chestnut leaf miner (Cameraria ohridella). Biol. Control 121, 88–98. doi: 10.1016/j.biocontrol.2018.02.009
Bi Y., Wu L., Li B., Hao Y., Li Z., Zhang J., et al. (2023). Effects of beauvericin on the blood cells of Bombyx mori. J. Invertebr Pathol. 201, 108003. doi: 10.1016/j.jip.2023.108003
Blaauw B. R., Brannen P., Lockwood D., Schnabel G., and Ritchie D. (2025). “Southeastern peach, nectarine, and plum pest management and culture guide,” in UGA Coop. Ext. Bull (University of Georgia, Athens, GA), 1171.
Brooks M. E., Kristensen K., van Benthem K. J., Magnusson A., Berg C. W., Nielsen A., et al. (2017). glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400. doi: 10.32614/RJ-2017-066
Brownbridge M., Reay S. D., Nelson T. L., and Glare T. R. (2012). Persistence of Beauveria bassiana as an endophyte following inoculation of radiata pine seed and seedlings. Biol. Control 61, 194–200. doi: 10.1016/j.biocontrol.2012.01.007
Calò L., Fornelli F., Nenna S., Tursi A., Caiaffa M., and Macchia L. (2003). Beauvericin cytotoxicity to the invertebrate cell line SF-9. J. Appl. Genet. 44, 515–520.
Caloni F., Fossati P., Anadón A., and Bertero A. (2020). Beauvericin: The beauty and the beast. Environ. Toxicol. Pharmacol. 75, 103349. doi: 10.1016/j.etap.2020.103349
Cherry A. J., Banito A., Djegui D., and Lomer C. (2004). Suppression of the stem-borer Sesamia calamistis (Lepidoptera; Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. Int. J. Pest Manage. 50, 67–73. doi: 10.1080/09670870310001637426
Darsouei R., Karimi J., and Stelinski L. L. (2024). Endophytic colonization of sugar beet by Beauveria varroae and Beauveria bassiana reduces performance and host preference in army worm, Spodoptera littoralis. Crop Prot. (Guildford Surrey) 175, 106441. doi: 10.1016/j.cropro.2023.106441
Espinoza F., Vidal S., Rautenbach F., Lewu F., and Nchu F. (2019). Effects of Beauveria bassiana (Hypocreales) on plant growth and secondary metabolites of extracts of hydroponically cultivated chive (Allium schoenoprasum L. [Amaryllidaceae]). Heliyon 5, e03038. doi: 10.1016/j.heliyon.2019.e03038
Faria M. R. d. and Wraight S. P. (2007). Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 43, 237–256. doi: 10.1016/j.biocontrol.2007.08.001
Ferus P., Barta M., and Konôpková J. (2019). Endophytic fungus Beauveria bassiana can enhance drought tolerance in red oak seedlings. Trees (Berlin Germany: West) 33, 1179–1186. doi: 10.1007/s00468-019-01854-1
Foong S. Y., Ma N. L., Lam S. S., Peng W., Low F., Lee B. H. K., et al. (2020). A recent global review of hazardous chlorpyrifos pesticide in fruit and vegetables: prevalence, remediation and actions needed. J. Hazard Mater. 400, 123006. doi: 10.1016/j.jhazmat.2020.123006
Fornelli F., Minervini F., and Logrieco A. (2004). Cytotoxicity of the mycotoxins beauvericin and enniatins on human cells. J. Appl. Genet. 45, 389–393.
Ganassi S., Moretti A., Bonvicini Pagliai A. M., Logrieco A., and Sabatini M. A. (2002). Effects of beauvericin on Schizaphis graminum (Aphididae). J. Invertebr Pathol. 80, 90–96. doi: 10.1016/S0022-2011(02)00013-4
Garrido-Jurado I., Resquín-Romero G., Amarilla S. P., Ríos-Moreno A., Carrasco L., and Quesada-Moraga E. (2017). Transient endophytic colonization of melon plants by entomopathogenic fungi after foliar application for the control of Bemisia tabaci. J. Pest Sci. 90, 319–330. doi: 10.1007/s10340-016-0770-2
Gautier C., Pinson-Gadais L., and Richard-Forget F. (2020). Fusarium mycotoxins enniatins: an updated review of their occurrence, the producing Fusarium species, and the abiotic determinants of their accumulation in crop harvests. J. Agric. Food Chem. 68, 4788–4798. doi: 10.1021/acs.jafc.9b07817
Greenfield M., Gómez-Jiménez M. I., Ortiz V., Vega F. E., Kramer M., and Parsa S. (2016). Beauveria bassiana and Metarhizium anisopliae endophytically colonize cassava roots following soil drench inoculation. Biol. Control 95, 40–48. doi: 10.1016/j.biocontrol.2015.12.002
Grove J. F. and Pople M. (1980). The insecticidal activity of beauvericin and the enniatins. Mycopathologia 70, 103–105. doi: 10.1007/BF00443665
Gutierrez-Pozo A., Cano J., and Calvo A. M. (2024). Environmental regulation of beauvericin biosynthesis by Fusarium species. Int. J. Food Microbiol. 410, 110321. doi: 10.1016/j.ijfoodmicro.2023.110321
Hartig F. (2024). DHARMa: residual diagnostics for hierarchical (multi-level / mixed) regression models. R package version 0.4.7. Available online at: https://github.com/florianhartig/DHARMa (Accessed August 19, 2025).
Husain M., Sutanto K. D., Rasool K. G., Qureshi J. A., and Aldawood A. S. (2024). Translocation and survival of trunk injected Beauveria bassiana in healthy date palm trees. J. King Saud Univ. Sci. 36, 103077. doi: 10.1016/j.jksus.2023.103077
Jaber L. R. (2018). Seed inoculation with endophytic fungal entomopathogens promotes plant growth and reduces crown and root rot (CRR) caused by Fusarium culmorum in wheat. Planta 248, 1525–1535. doi: 10.1007/s00425-018-2991-x
James R. R., Croft B. A., and Shaffer B. T. (1998). Environmental effects on the persistence of Beauveria bassiana in soil: the role of temperature and soil type. Biol. Control 12, 1–10. doi: 10.1006/bcon.1998.0614
Křížová L., Dadáková K., Dvořáčková M., and Kašparovský T. (2021). Feedborne mycotoxins beauvericin and enniatins and livestock animals. Toxins 13, 32. doi: 10.3390/toxins13010032
Lacey L. A. and Shapiro-Ilan D. I. (2008). Microbial control of insect pests in temperate orchard systems: potential for incorporation into IPM. Annu. Rev. Entomol. 53, 121–146. doi: 10.1146/annurev.ento.53.103106.093419
Leland J. E., McGuire M. R., Grace J. A., Jaronski S. T., Ulloa M., Park Y.-H., et al. (2005). Strain selection of a fungal entomopathogen, Beauveria bassiana, for control of plant bugs (Lygus spp.). Biol. Control 35, 104–114. doi: 10.1016/j.biocontrol.2005.06.002
Lenth R. (2025). emmeans: estimated marginal means, aka least-squares means. R package version 1.11.2-8. Available online at: https://rvlenth.github.io/emmeans/ (Accessed August 19, 2025).
Mannai S., Benfradj N., Horrigue-Raouani N., and Boughalleb-M’Hamdi N. (2018). Prevalence of Fusarium species associated with peach decline in Tunisian nurseries. Microbiol. Res. J. Int. 23, 1–16. doi: 10.9734/mrji/2018/40746
Mantzoukas S. and Eliopoulos P. A. (2020). Endophytic entomopathogenic fungi: a valuable biological control tool against plant pests. Appl. Sci. 10, 360. doi: 10.3390/app10010360
Mascarin G. M. and Jaronski S. T. (2016). The production and uses of Beauveria bassiana as a microbial insecticide. World J. Microbiol. Biotechnol. 32, 177. doi: 10.1007/s11274-016-2131-3
Mishra S., Kumar P., and Malik A. (2015). Effect of temperature and humidity on pathogenicity of native Beauveria bassiana isolate against Musca domestica. J. Parasitol. Dis. 39, 697–704. doi: 10.1007/s12639-013-0398-3
Nandi N. K., Vyas A., Akhtar M. J., and Kumar B. (2022). The growing concern of chlorpyrifos exposures on human and environmental health. Pestic. Biochem. Physiol. 185, 105138. doi: 10.1016/j.pestbp.2022.105138
Oluwakayode O. O., Chen L., and Zhang Y. (2024). Regulation of secondary metabolite production in entomopathogenic fungi: influence of temperature, humidity, and host-derived factors. Fungal Biol. Rev. 44, 100–112. doi: 10.1016/j.fbr.2024.100112
Ownley B. H., Gwinn K. D., and Vega F. E. (2010). Endophytic fungal entomopathogens with activity against plant pathogens: ecology and evolution. Biocontrol 55, 113–128. doi: 10.1007/s10526-009-9241-x
Pietruszka K., Panasiuk Ł., and Jedziniak P. (2023). Survey of the enniatins and beauvericin in raw and UHT cow’s milk in Poland. J. Vet. Res. 67, 259–266. doi: 10.2478/jvetres-2023-0032
Posada F. and Vega F. E. (2005). Establishment of the fungal entomopathogen Beauveria bassiana as an endophyte in cocoa seedlings. Mycologia 97, 1195–1200. doi: 10.1080/15572536.2006.11832729
Posada F. and Vega F. E. (2006). Inoculation and colonization of coffee seedlings with the fungal entomopathogen Beauveria bassiana. Mycoscience 47, 284–289. doi: 10.1007/s10267-006-0306-6
Powell W. A., Klingeman W. E., Ownley B. H., and Gwinn K. D. (2009). Evidence of endophytic Beauveria bassiana in seed-treated tomato plants acting as a systemic entomopathogen to larval Helicoverpa zea (Lepidoptera: Noctuidae). J. Entomol Sci. 44, 391–396. doi: 10.18474/0749-8004-44.4.391
Ramakuwela T., Hatting J., Bock C., Vega F. E., Wells L., Mbata G. N., et al. (2020). Establishment of Beauveria bassiana as a fungal endophyte in pecan seedlings and its virulence against pecan insect pests. Biol. Control 140, 104102. doi: 10.1016/j.biocontrol.2019.104102
Rämö S., Haapalainen M., and Latvala S. (2021). Development and validation of a UHPLC-MS/MS method for the analysis of Fusarium mycotoxins in onion. Food Anal Methods 14, 1524–1536. doi: 10.1007/s12161-021-01992-8
R Core Team (2023). R: a language and environment for statistical computing (Vienna, Austria: R Foundation for Statistical Computing). Available online at: https://www.R-project.org/ (Accessed August 19, 2025).
Rondot Y. and Reineke A. (2018). Endophytic Beauveria bassiana in grapevine Vitis vinifera (L.) reduces infestation with piercing-sucking insects. Biol. Control: Theory Appl. Pest Manage. 116, 82–89. doi: 10.1016/j.biocontrol.2016.10.006
Russo M. L., Scorsetti A. C., Vianna M. F., Cabello M., Ferreri N., and Pelizza S. (2019). Endophytic Effects of Beauveria bassiana on Corn (Zea mays) and Its Herbivore, Rachiplusia nu (Lepidoptera: Noctuidae). Insects 10, 110. doi: 10.3390/insects10040110
Safavi S. (2013). In vitro and in vivo induction, and characterization of beauvericin isolated from Beauveria bassiana and its bioassay on Galleria mellonella larvae. J. Agric. Sci. Technol. 15, 1–10. doi: 10.5555/20133038048
Santini A., Meca G., Uhlig S., and Ritieni A. (2012). Fusaproliferin, beauvericin and enniatins: occurrence in food – a review. World Mycotoxin J. 5, 71–81. doi: 10.3920/wmj2011.1331
Sharma A., Sharma S., and Yadav P. K. (2023). Entomopathogenic fungi and their relevance in sustainable agriculture: a review. Cogent Food Agric. 9, 2180857. doi: 10.1080/23311932.2023.2180857
Shin T. Y., Lee M. R., Kim J.-C., Nai Y.-S., and Kim J. S. (2022). A new strategy using entomopathogenic fungi for the control of tree borer insects. Entomol Res. 52, 327–333. doi: 10.1111/1748-5967.12605
Sood S., Sandhu S. S., and Mukherjee T. K. (2017). Pharmacological and therapeutic potential of beauvericin: a short review. J. Proteomics Bioinform. 10, 42–46. doi: 10.4172/jpb.1000421
Sui L., Lu Y., Zhou L., Li N., Li Q., and Zhang Z. (2023). Endophytic Beauveria bassiana promotes plant biomass growth and suppresses pathogen damage by directional recruitment. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1227269
Vega F. E. (2008). Insect pathology and fungal endophytes. J. Invertebr Pathol. 98, 277–279. doi: 10.1016/j.jip.2008.01.008
Vega F. E. (2018). The use of fungal entomopathogens as endophytes in biological control: a review. Mycologia 110, 4–30. doi: 10.1080/00275514.2017.1418578
Wang Q. and Xu L. (2012). Beauvericin, a bioactive compound produced by fungi: a short review. Molecules 17, 2367–2377. doi: 10.3390/molecules17032367
Wickham H. (2016). ggplot2: Elegant Graphics for Data Analysis (New York: Springer-Verlag). doi: 10.1007/978-3-319-24277-4
Wołejko E., Łozowicka B., Jabłońska-Trypuć A., Pietruszyńska M., and Wydro U. (2022). Chlorpyrifos occurrence and toxicological risk assessment: a review. Int. J. Environ. Res. Public Health 19, 12209. doi: 10.3390/ijerph191912209
Yerukala S., Butler D. M., Bernard E. C., Gwinn K. D., Grewal P. S., and Ownley B. H. (2022). Colonization efficacy of the endophytic insect-pathogenic fungus Beauveria bassiana across the plant kingdom: a meta-analysis. Crit. Rev. Plant Sci. 41, 241–270. doi: 10.1080/07352689.2022.2109474
Yogendrarajah P., Van Poucke C., De Meulenaer B., and De Saeger S. (2013). Development and validation of a QuEChERS based LC–MS/MS method for the determination of multiple mycotoxins in spices. J. Chromatogr. A 1297, 1–11. doi: 10.1016/j.chroma.2013.04.075
Yousefi-Lardeh L. and Zibaee A. (2024). Nano-formulation of beauvericin shows insecticidal properties against Glyphodes pyloalis. Biocatal Agric. Biotechnol. 59, 103264. doi: 10.1016/j.bcab.2024.103264
Keywords: bioinsecticide, biological control, antibiotic, integrated pest management, yellow mealworm, insecticide, mycotoxin
Citation: Elgar SA, Villari C, Fair CG, Shapiro-Ilan DI, Chavez D and Blaauw BR (2025) Beauvericin production by endophytic and epiphytic Beauveria bassiana in peach (Prunus persica) and implications for insect biocontrol. Front. Fungal Biol. 6:1714008. doi: 10.3389/ffunb.2025.1714008
Received: 26 September 2025; Accepted: 05 November 2025; Revised: 03 November 2025;
Published: 27 November 2025.
Edited by:
Soumyadev Sarkar, Arizona State University, United StatesReviewed by:
Lukasz Lech Stelinski, University of Florida, United StatesSoumya Moonjely, Michigan State University, United States
Copyright © 2025 Elgar, Villari, Fair, Shapiro-Ilan, Chavez and Blaauw. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Brett R. Blaauw, YmJsYWF1d0B1Z2EuZWR1
 Sabrina A. Elgar
Sabrina A. Elgar Caterina Villari
Caterina Villari Conor G. Fair
Conor G. Fair David I. Shapiro-Ilan
David I. Shapiro-Ilan Dario Chavez
Dario Chavez Brett R. Blaauw
Brett R. Blaauw