- 1Department of Psychiatry, Stellenbosch University, Cape Town, South Africa
- 2Mental Health Project, MRC/UVRI and LSHTM Uganda Research Unit, Entebbe, Uganda
- 3Department of Psychiatry, Makerere University, Kampala, Uganda
- 4Department of Medical Microbiology, Makerere University, Kampala, Uganda
- 5South African Medical Research Council/Stellenbosch University Genomics of Brain Disorders Research Unit, Cape Town, South Africa
- 6School of Biomedical Sciences, College of Health Sciences, Makerere University, Kampala, Uganda
- 7MRC/UVRI and LSHTM Uganda Research Unit, Statistics and Data Science Section, Entebbe, Uganda
Background: Internalizing mental disorders (IMDs) among HIV-positive (HIV+) children and adolescents are associated with poor disease outcomes, such as faster HIV disease progression. Although it has been suggested that the development of IMDs is moderated by interaction of stressful life events and vulnerability factors, the underlying etiology is largely unknown. Serotonin transporter gene [solute carrier family 6 member A4 (SLC6A4)] and human tryptophan hydroxylase 2 gene (TPH2) polymorphisms have been implicated in the development of IMDs. This study investigated the association between acute stress and IMDs, and moderation by chronic stress and genetic variants in SLC6A4 and TPH2.
Hypothesis: Acute stress acts through genetic and environmental vulnerability factors to increase the risk of developing IMDs.
Methods: Polymorphisms in SLC6A4 (5-HTTLPR, rs25531, 5-HTTLPR-rs25531, and STin2 VNTR) and TPH2 (rs1843809, rs1386494, rs4570625, and rs34517220) were genotyped in 368 HIV+ children and adolescents (aged 5–17 years) with any internalizing mental disorder (depression, anxiety disorders, or posttraumatic stress disorder), and 368 age- and sex-matched controls, who were also HIV+. Chronic and acute stress categories were derived by hierarchical cluster analysis. Logistic regression analysis was used to assess the independent moderating effect of chronic stress and each selected polymorphism on the association between acute stress and IMDs.
Results: We observed a statistically significant association between severe acute stress and IMDs (p = 0.001). Children and adolescents who experienced severe acute stress were twice as likely to develop IMDs, compared to children and adolescents who experienced mild acute stress (p = 0.001). Chronic stress interacted with severe acute stress to increase the risk of IMDs (p = 0.033). Acute stress was found to interact with 5-HTTLPR-rs25531 S-A-S-A haplotype to increase the risk for IMDs among Ugandan HIV+ children and adolescents (p = 0.049). We found no evidence for a combined interaction of acute stress, chronic stress, and 5-HTTLPR-rs25531 on IMDs.
Conclusion: The odds of having an internalizing mental disorder (IMD) were higher among HIV+ children and adolescents who experienced severe acute stress compared to HIV+ children and adolescents who experienced mild acute stress. Chronic stress and 5-HTTLPR-rs25531 independently moderated the association between acute stress and IMDs.
Introduction
HIV-positive (HIV+) children and adolescents suffer a considerable burden of internalizing mental disorders (IMDs; Mellins et al., 2012; Nachman et al., 2012; Kinyanda et al., 2019). IMDs are characterized by quiet, internal distress (Tandon et al., 2011) and include depressive and anxiety disorders, as well as posttraumatic stress disorder (PTSD; American Psychiatric Association, 2013). IMDs among people living with HIV/AIDS have generally been associated negative outcomes of more rapid HIV disease progression (Ironson et al., 2005; Chida and Vedhara, 2009), poor adherence to medication (Springer et al., 2012; Kinyanda et al., 2018), risky sexual behavior (Springer et al., 2012; Kinyanda et al., 2018), and poor linkages to care (Bhatia et al., 2011).
Internalizing mental disorders are complex disorders with gene-environment interactions contributing to their etiology (Musci et al., 2019), where a number of genes play a role with each gene contributing a small effect (Plomin and Davis, 2009; Assary et al., 2018). The role of psychosocial factors and their interaction with biological mechanisms in the etiology of IMDs is still poorly understood. HIV+ children and adolescents experience various chronic life stressors such as awareness of their HIV-status, increased levels of stigma and poorer parental mental health (Betancourt et al., 2014). As chronic stressors are reported to be risk factors for IMDs (Adelman et al., 2014; Robles et al., 2014; Revenson et al., 2016), chronic stressors likely contribute to the etiology of IMDs among HIV+ children and adolescents (Boyes and Cluver, 2015; Lwidiko et al., 2018).
Internalizing mental disorders are heritable (Smoller, 2016): twin studies have estimated a genetic heritability of 35% for depression (Otte et al., 2016) and 30-50% for PTSD (Smoller, 2016). In addition, a meta-analysis of family and twin studies estimated a genetic heritability of 31.6% for generalized anxiety disorder (GAD; Hettema et al., 2001). A more recent genome-wide association study (GWAS) of monozygotic and dizygotic female twins has estimated a genetic heritability of 42% for GAD (Davies et al., 2015). Despite being heritable, the underlying etiology of IMDs is largely unknown although dysregulation in serotonergic transmission has been implicated in depression among adults living with HIV (Hammoud et al., 2010).
Serotonergic pathways in the central nervous system is important in regulating mood and anxiety (Olivier, 2015). After an impulse is fired and serotonin (5-HT) is released into the synaptic cleft, 5-HT re-uptake reduces the activity of the serotonergic neurons, preparing the neuron for a new discharge (Artigas, 2013a,b; Olivier, 2015). Encoded by the serotonin transporter gene [solute carrier family 6 member 4 (SLC6A4)], the serotonin transporter (5-HTT) influences serotonergic transmission by regulating the duration of serotonin in the synaptic cleft (Rudnick, 2006; Kristensen et al., 2011). 5-HTT is a target for selective serotonin re-uptake inhibitors (SSRIs) that competitively block substrate binding and thereby prolong neurotransmitter action at the synapse (Kristensen et al., 2011; Cipriani et al., 2018). Tryptophan hydroxylase 2 (TPH2) catalyzes the rate-limiting step in 5-HT biosynthesis (Walther et al., 2003; Carkaci-Salli et al., 2006) and is expressed exclusively in the brainstem (Kennedy et al., 2012), an area which is the major locus of serotonin-producing neurons (Kennedy et al., 2012). Long-term treatment with the SSRI fluoxetine has been found to be associated with concurrent upregulation of TPH2 messenger ribonucleic acid (mRNA) expression and alleviation of depressive symptoms (Shishkina et al., 2007), suggesting that low TPH2 gene expression could represent a dysregulation corrected by fluoxetine.
SLC6A4 is located on chromosome 17 at position 17q11 (National Center for Biotechnology Information, 2021a). The serotonin transporter-linked polymorphic region (5-HTTLPR) within the promoter region of SLC6A4 has been implicated in depression (Clarke et al., 2010; Wang et al., 2016), anxiety-related traits (Munafò et al., 2009b), and PTSD (Xie et al. 2009). 5-HTTLPR variants comprise either 14 (short, S-allele) or 16 (long, L-allele) copies of a 22–23 base pair (bp) imperfect repeat (Heils et al., 1996). In vitro studies show that the L-allele has two to three times higher basal transcriptional activity compared to the S-allele (Lesch et al., 1996; Philibert et al., 2008). In proximity to 5-HTTLPR is an A to G single nucleotide polymorphism (SNP), rs25531 which has been reported to alter expression of the SLC6A4 by creating a functional AP2 transcription-factor binding site (Hu et al., 2006; Ehli et al., 2012). The rs25531 G-allele has been associated with reduced transporter gene expression (Hu et al., 2006; Ehli et al., 2012). This SNP, when analyzed in combination with 5-HTTLPR, results in L-A and L-G haplotypes. The L-G haplotype has been associated with lower SLC6A4 expression levels compared to the L-A haplotype (Hu et al., 2006; Lipsky et al., 2009). However, some studies have found no effect of the L-G haplotype on SLC6A4 expression (Martin et al., 2007; Philibert et al., 2008).
A variable number of tandem repeats (VNTR) polymorphism also occurs within the second intron (STin2) of SLC6A4. The polymorphism consists of multiple repeat copies of a 16–17 bp element (Battersby et al., 1996; Furlong et al., 1998). Three alleles have been reported (National Center for Biotechnology Information, 2021b), containing 9 (STin2.9), 10 (STin2.10), and 12 (STin2.12) copies of the repeat. The alleles have been associated with differential expression of SLC6A4, as STin2.9 has been found to be associated with increased SLC6A4 expression, and an increasing number of repeats associated with reduced reporter gene expression in rat neonate prefrontal cortical cultures (Ali et al., 2010). The STin2 VNTR polymorphism has also been found to interact with 5-HTTLPR to regulate expression of SLC6A4, with the combination of the 5-HTTLPR S-allele and either STin2.10 or STin2.12 associated with increased expression, as compared to STin2.9 whose combination with 5-HTTLPR S-allele directed expression levels comparable to the 5-HTTLPR S-allele alone (Ali et al., 2010; Haddley et al., 2012). The STin2.9 allele has been associated with anxiety in patients with self-harming behaviors (Evans et al., 2013), STin2.10 has been associated with both anxiety and PTSD (Evans, Li and Whipple, 2013; Xiao et al., 2019) and the STin2.12 allele has been associated with depression, neuroticism, and suicide (Lopez de Lara et al., 2007; O’Gara et al., 2008; Kalungi et al., 2017).
The TPH2 gene is located on chromosome 12 at position 12q21.1. Located within intron 5 (rs1843809 and rs1386494), and within the promoter region (rs4570625), rs1843809, rs1386494, and rs4570625 have been linked to depression (Zill et al., 2004; Anttila et al., 2009; Gao et al., 2012). Located within the TPH2 transcription factor binding site, rs34517220 SNP has been reported to modulate TPH2 expression by altering binding sites for foxa1 and foxa2 transcription factors (Pristerà et al., 2015). Also, foxa2 plays a role in establishing progenitor domains for serotonergic neuron precursors in the ventral hindbrain and in activating transcription factors required for the terminal differentiation of serotonergic neurons (Jacob et al., 2007). Therefore, rs34517220-driven variation in foxa2 binding may have important effects on the development of neural serotonergic systems.
The diathesis-stress hypothesis of neuropsychiatric disorders postulates that a lower stress threshold is required for psychiatric disease to occur in individuals who harbor certain vulnerability factors, which may be genetic and/or acquired (Monroe and Simons, 1991; Silberg et al., 2001; Caspi et al., 2003; Figure 1). Stress is a common environmental risk factor for a number of mental disorders, including depression, anxiety, and PTSD (de Kloet et al., 2005; Popoli et al., 2011), and it is currently accepted that gene-environment interactions underlie the etiology of many, if not all, IMDs (Caspi and Moffitt, 2006; Willis and Brock, 2018; Jawahar et al., 2019).
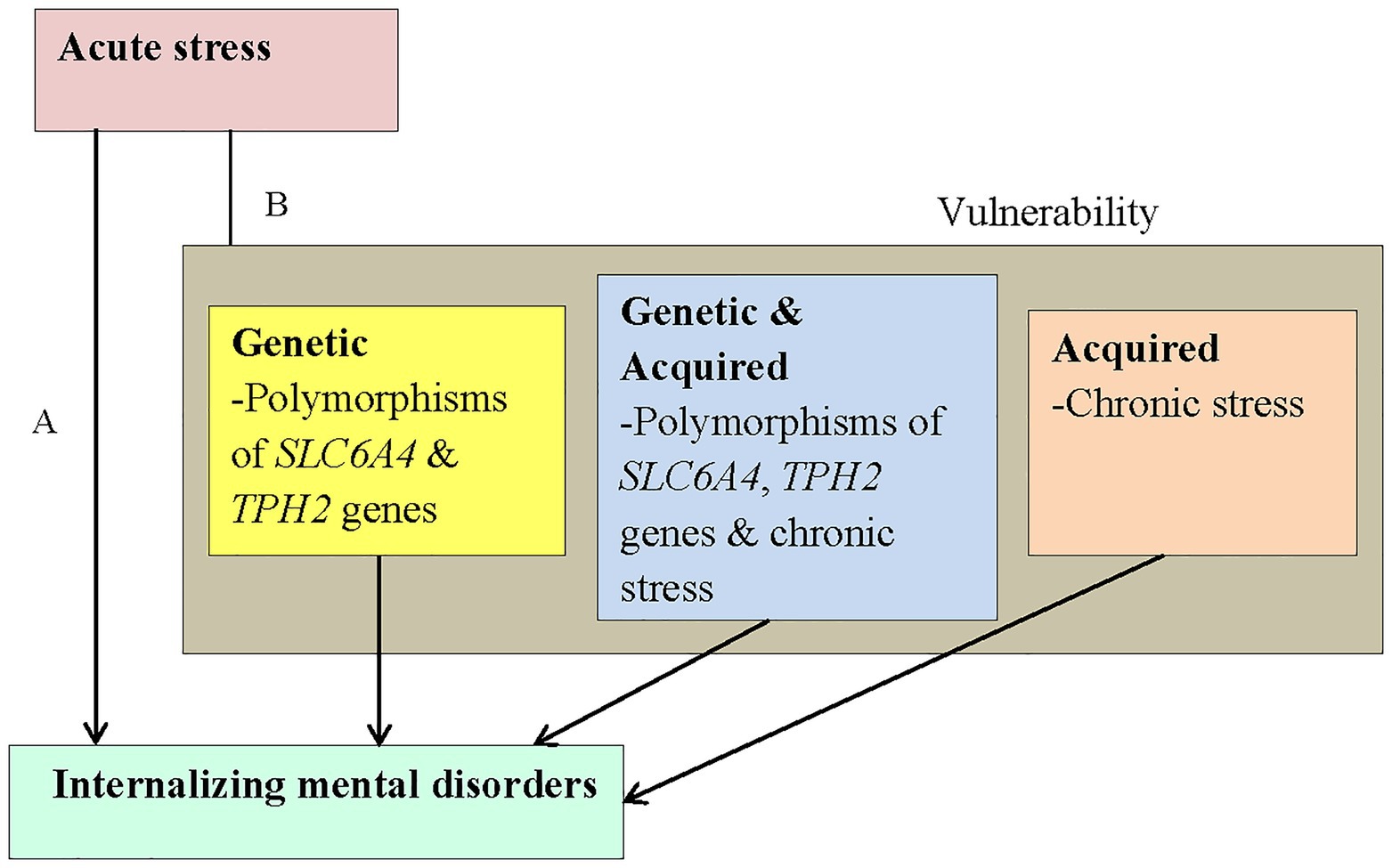
Figure 1. The conceptual framework is adapted from the diathesis-stress model for depression (Monroe and Simons, 1991). The aim of the present study was to investigate the association between acute stress and IMDs (A). We postulated that acute stress would interact with vulnerability factors [B; genetic (yellow block), acquired (pink block), or both (blue block)] to increase the risk for IMDs. SLC6A4, serotonin transporter gene; TPH2, tryptophan hydroxylase 2 gene.
In the context of the diathesis-stress hypothesis, with acute stress representing the exposure variable in a sample of HIV+ children and adolescents, we investigated polymorphisms in SLC6A4 (5-HTTLPR, rs25531, and STin2 VNTR) and TPH2 (rs1843809, rs1386494, rs4570625, and rs34517220) as genetic vulnerability factors and chronic stress as an acquired vulnerability factor for IMDs. Our a priori selection of these polymorphisms was based on their associations with IMDs (5-HTTLPR, STin2 VNTR, TPH2 rs1843809, rs1386494, rs4570625, and rs34517220) and regulation of SLC6A4 (STin2 VNTR, rs25531).
Materials and Methods
Study Design
This case-control study was nested within a study which investigated mental health among HIV infected children and adolescents in Kampala and Masaka, Uganda (CHAKA study). Characteristics of the study participants and the study design for the CHAKA study are detailed elsewhere (Mpango et al., 2017; Kinyanda et al., 2019).
All study participants were of Bagandan ethnicity, the largest ethnic group in Uganda, and were thus expected to be genetically similar since relatively modest genetic differentiation has been observed among populations representing the major sub-populations in sub-Saharan Africa (Gurdasani et al., 2015). All participants in the present study were HIV+ children and adolescents (aged 5–17 years) of black African (Ugandan) ancestry (Kinyanda et al., 2019). Cases (n = 368) were defined as participants who had any depressive disorder [depression or dysthymia (persistent depressive disorder)] or any anxiety disorder (GAD, separation anxiety disorder, social anxiety disorder, panic disorder, and agoraphobia) or PTSD. Controls (n = 368) were age-, site-, socio-economic status (SES)-, and sex-matched without any psychiatric disorder. Genomic DNA was extracted from an archived cell pellet sample for each participant.
Clinical Assessments
Children and assented adolescents, as well as their caregivers, were interviewed using a structured questionnaire. The questionnaire included, among others, socio-demographic characteristics (sex, study site, age, caregiver level of education, and SES) and modules on different psychiatric disorders from the DSM-5 referenced Children and Adolescent Symptom Inventory 5 (CASI-5; caregiver reported; Gadow and Sprafkin, 2013) and the Youth Inventory-4R (YI-4R; youth reported; Gadow and Sprafkin, 1999). The CASI-5 and YI-4R list the symptoms of a wide range of psychiatric disorders including major depressive disorder, GAD, PTSD, and attention-deficit/hyperactivity disorder, among others. Individual CASI-5 items are rated on a 4-point frequency of occurrence scale ranging from never (0) to very often (3). Though there are several CASI-5 scoring algorithms, in the present study, we used symptom count cut-off scores that reflect the prerequisite number of symptoms for a clinical diagnosis. About 4 ml of blood was withdrawn from each study participant by venipuncture into an EDTA vacutainer and was stored at −80°C pending DNA extraction for the genetics analyses.
Inclusion and Exclusion Criteria
Inclusion criteria were: (i) HIV-infected outpatients, registered with an HIV clinic at any of the study sites; (ii) aged between 5 and 17 years at the time of enrolment; (iii) conversant in English or Luganda, the language into which research assessment tools were translated; and (iv) able to provide written informed consent (caregiver) and assent (adolescents). Exclusion criteria were: (i) seriously ill and (ii) being unable to understand study procedures.
Ethical Considerations
The study was conducted in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). The CHAKA study obtained ethical and scientific clearance from the Uganda Virus Research Institute’s Science and Ethical Committee (# GC/127/15/06/459) and the Uganda National Council of Science and Technology (# HS 1601). The study reported here obtained approval from the Higher Degrees Research and Ethics Committee of the School of Biomedical Sciences, Makerere University (# SBS 421) and the Health Research Ethics Committee of Stellenbosch University (# S17/09/179). All caregivers provided informed consent for their children/adolescents to participate in the study and for a blood specimen to be drawn from them (child/adolescent) for genetics analyses. Adolescents further provided informed assent to participate in the study. Study participants who were diagnosed with significant psychiatric problems were referred to mental health units at Entebbe and Masaka government hospitals.
Selection of Cases and Controls
The procedure for selection of cases and controls has been previously described (Kalungi et al., 2021). Briefly, all cases were stratified by site, sex, age category, and SES, resulting in a total of 36 strata. In each stratum, the number of cases was ascertained (e.g., for males in site 1 in the youngest age category and the lowest SES group there were nine cases). An equal number of controls were then randomly sampled from the stratum concerned, thus the controls were matched to the cases by site, sex, age, and SES.
DNA Extraction
DNA was extracted from whole blood using the QiAmp Mini DNA Extraction Kit according to manufacturer’s instructions (Qiagen GmbH, Germany).
Selection of Variants Within the Serotonin Transporter and Tryptophan Hydroxylase 2 Genes
The selection of variants was based on their role in regulating gene expression or association with any of the IMDs. Also, because the Bantu group of people in East Africa have been reported to genetically cluster together as per principal components analysis (unpublished results from NeuroGAP data), a minor allele frequency cut-off of at least 0.1 was set for each selected SNP based on the Luhya, a population that belongs to same Bantu group as the population of the present study. The minor allele frequency data were obtained from an online genomic project (Clarke et al., 2017). Supplementary Table S1 summarizes the biological data based on for selection for each of the SLC6A4 and TPH2 variants.
SLC6A4 Genotyping
All polymerase chain reactions (PCR) for SLC6A4 polymorphisms were performed in a GeneAmp PCR System 9700 (Perkin Elmer Biosystems, Foster City, CA, United States). Amplification reactions were carried out in 25 μl reaction volumes containing: DNA template, 200 μM dNTP (Kapa Biosystems, Cape Town, South Africa), 5 μl of 10X Taq DNA polymerase buffer (Kapa Biosystems), 1.0 mM magnesium chloride (Kapa Biosystems), 0.625 units (U) Taq DNA polymerase (Kapa Biosystems), and 0.5 μM of each primer (Integrated DNA Technologies, Coralville, IA, United States), with bi-distilled water.
The 5-HTTLPR, 5-HTTLPR-rs25531, and STin2 VNTR polymorphisms were genotyped following a procedure described by Kalungi et al. (2017). Fragment sizes revealed by PCR were confirmed by restriction fragment length analysis on the ABI prism. Expected fragment sizes of the alleles at the 5-HTTLPR-rs25531 locus were as follows: S-A = 281 bp, L-A = 325 bp and S-G-L-G = 151 bp, resulting into the following genotypes: S-A-S-A = 281 bp; L-A-L-A = 325 bp; S-G-S-G, L-G-L-G, L-G-S-G = 151 bp; L-G-S-A = 151 + 281 bp and L-A-S-G, L-A-L-G = 325 + 151 bp. Expected fragment sizes of the alleles at the STin2 VNTR locus were as follows: 9-repeat (STin2.9) = 250 bp, 10-repeat (STin2.10) = 265 bp and 12-repeat (STin2.12) = 300 bp, resulting in the following genotypes: 9/9 = 250 bp, 9/10 = 250 + 265 bp, 9/12 = 250 + 300 bp, 10/10 = 265 bp, 10/12 = 265 + 300 bp, and 12/12 = 300 bp.
Determining Genotypes for Selected Single Nucleotide Polymorphims in Tryptophan Hydroxylase 2 Gene
DNA samples were quantified and sent to LGC laboratory (LGC, Middlesex, United Kingdom) for automated SNP genotyping using the kompetitive allele-specific PCR (KASP) assay. Genotypes were determined for TPH2 rs1843809, rs1386494, rs4570625, and rs34517220. However, none of these genotypes were validated using an alternate method.
Haplotype Analyses
We used Haploview version 4.2 (Barrett, 2009) to analyze linkage disequillibrium (LD) in SLC6A4 and TPH2. SLC6A4 analyses included 5-HTTLPR, rs25531, and STin2 VNTR polymorphisms. 5-HTTLPR and STin2 VNTR alleles were coded as dummy variables, i.e., S = 1, L = 2, and STin2.10 = 1 and STin2.12 = 2. As very few participants carried the STin2.9 allele (n = 6), they were excluded from the analysis.
TPH2 analyses included the three single nucleotide polymorphisms (SNPs) genotyped. Participants with more than 50% missing genotypes were excluded from the analysis, yielding data from 692 participants for each of the SLC6A4 and TPH2 analyses. None of the investigated TPH2 polymorphisms were in LD (D’ < 0.8), while SLC6A4 5-HTTLPR and rs25531 were in LD (D’ > 0.8) (Supplementary Figures S1, S2).
Generation of Acute and Chronic Stress Class Categories
Social disadvantage variables were grouped into an index of acute and chronic stress. Caregiver mental state (assessed as psychological distress using the Self-report Questionnaire-20; Beusenberg et al., 1994), child-caregiver relationship [assessed as child-caregiver interactions, using data on how often the caregiver (i) beats, (ii) insults, (iii) spanks, or (iv) yells at the child/adolescent] and HIV symptoms were grouped together to constitute “acute stress” and orphanhood, study site (urban vs. rural), and caregiver level of education as variables constituting “chronic stress.” Variables were scored on a disadvantage scale where, for example, double orphanhood carried a higher chronic stress score vs. single orphanhood or not orphaned; food availability: not enough food carried a higher chronic stress score vs. enough food; study site: urban carried a higher chronic stress score than rural; and caregiver level of education: no formal education carried a higher chronic stress scores than primary and primary a higher stress score than secondary, etc. Hierarchical cluster analysis using Statistica 13.5 software (TIBCO, CA, United States), Euclidian distance, as distance measure and Ward’s method for clustering (Ward, 1963), was used to generate the different cut-off points for each acute and chronic stress class, respectively.
The acute stress index ranged from 0 to 2.46, with a normal distribution, while the chronic stress index ranged from 0 to 3.75, with a normal distribution as well. A total of three classes were generated for each type of stress by the hierarchical cluster analysis, i.e., mild, moderate, and severe. For acute stress, the mild class had an acute stress score of less than 0.362, the moderate class a score of 0.362–0.622, while the severe class a score of greater than 0.622. For chronic stress, the mild class had a chronic stress score of less than 1.375, the moderate class a score of 1.375–2.375, while the severe class a score of greater than 2.375.
Power of the Study
Using Stata 15 (StataCorp, TX, United States) software, we did a post hoc power calculation. Given a case-control ratio of 1:1, and assuming a zero correlation of exposure between cases and controls, a probability of 0.5 of exposure among controls at a 0.05 significance level, and an expected odds ratio of at least 1.8 for IMDs under severe acute stress, we needed 188 cases to achieve a power of 80% (Lachin, 1992). Our study comprising 368 cases was, therefore, adequately powered to address the main aim. Assuming similar assumptions, our interaction analyses achieved a power of 87.5%.
Statistical Methods
Statistical analyses were conducted using Stata 15 (StataCorp, TX, United States). Socio-demographic characteristics were compared between cases and controls. SES was generated from a scale of nine household items owned (car, motorcycle, refrigerator, electricity, bicycle, radio, telephone, cupboard, and flask) as previously described (Kalungi et al., 2019). A t-test was used to compare the distribution of cluster of differentiation 4 (CD4) counts between cases and controls. We computed 95% confidence intervals and statistical significance was set at a value of p less than or equal to 0.05.
Genotype distributions were compared between cases and controls. Likelihood-ratio tests were used to test genotypes for the Hardy-Weinberg equilibrium (HWE) in both cases and controls. Logistic regression was used to assess the relationship between TPH2 rs1843809 and IMDs (equation for the model was Y = β0 + β1X1, where Y = IMDs, β0 = constant, X1 = rs1843809, and β1 = coefficient for rs1843809). Logistic regression models were used to assess the relationship between acute stress and IMDs (equation for the model was Y = β0 + β1X1, where Y = IMDs, β0 = constant, X1 = acute stress, and β1 = coefficient for acute stress).
The moderating effect of chronic stress on the association between acute stress and IMDs was assessed by comparing logistic regression models of the association between acute stress and IMDs with and without chronic stress. Interactions between acute stress and chronic stress were tested using a likelihood ratio test (equation for the model was Y = β0 + β1X1 + β2X2 + β3X1X2, where Y = IMDs, β0 = constant, X1 = acute stress, X2 = chronic stress and β1–β3 are the coefficients for acute stress, chronic stress, and the interaction term, respectively).
The moderating effect of each genotype on the association between acute stress and IMDs was assessed by comparing logistic regression models of the association between acute stress and IMDs with and without the polymorphism controlling for chronic stress. Interactions between acute stress and each of the polymorphism were tested using a likelihood ratio test, controlling for chronic stress (equation for the model was Y = β0 + β1X1 + β2X2 + β3X3 + β4X1X2, where Y = IMDs, β0 = constant, X1 = acute stress, X2 = polymorphism/SNP, X3 = chronic stress and β1–β4 are the coefficients for acute stress, the polymorphism/SNP, chronic stress, and the interaction, respectively). These interaction models were performed on all the explanatory variables even without observing significant main effects, in order to rule out the possibility of cross-over interaction, where significant interactions may be observed for non-significant main effects (Widaman et al., 2012). Three-way interactions of acute stress, chronic stress, and any selected polymorphism on IMDs were not assessed as the two-way models were better than the three-way models, based on goodness of fit test, Arkaike informatics criteria, and the Bayesian information criterion. The 95% confidence intervals were calculated for all analyses.
Results
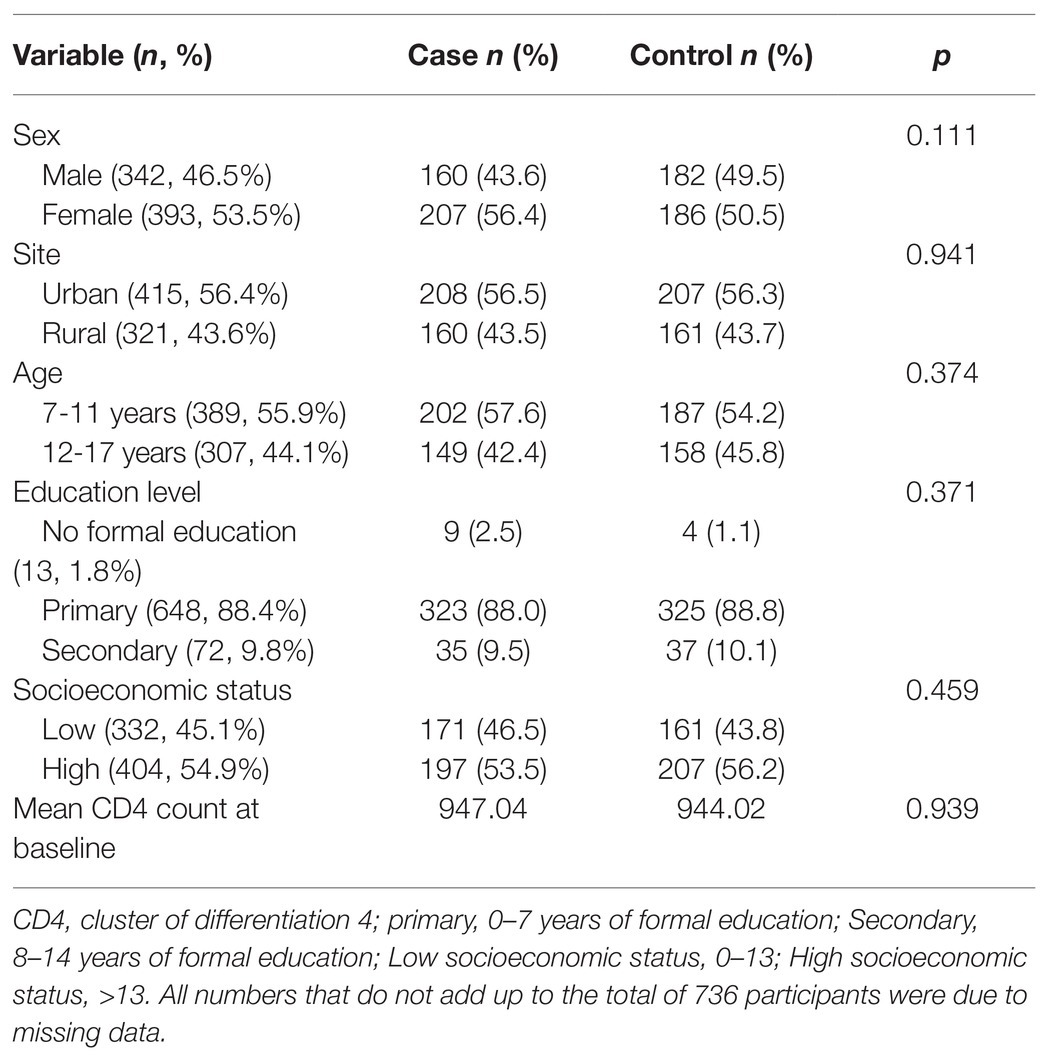
Socio-demographic factors were similarly distributed between cases and controls as shown in Table 1.
The HWE p-values among cases and controls for each polymorphism are shown in Table 2. None of the genotype frequencies deviated significantly from HWE among controls except for rs1386494, which was thus omitted from further analysis. For SLC6A4 polymorphisms, 5-HTTLPR was in LD with rs25531 (D’ > 0.8) (Supplementary Figure S1), while for TPH2 SNPs, none of the selected polymorphisms were in LD (D’ < 0.8) (Supplementary Figure S2).
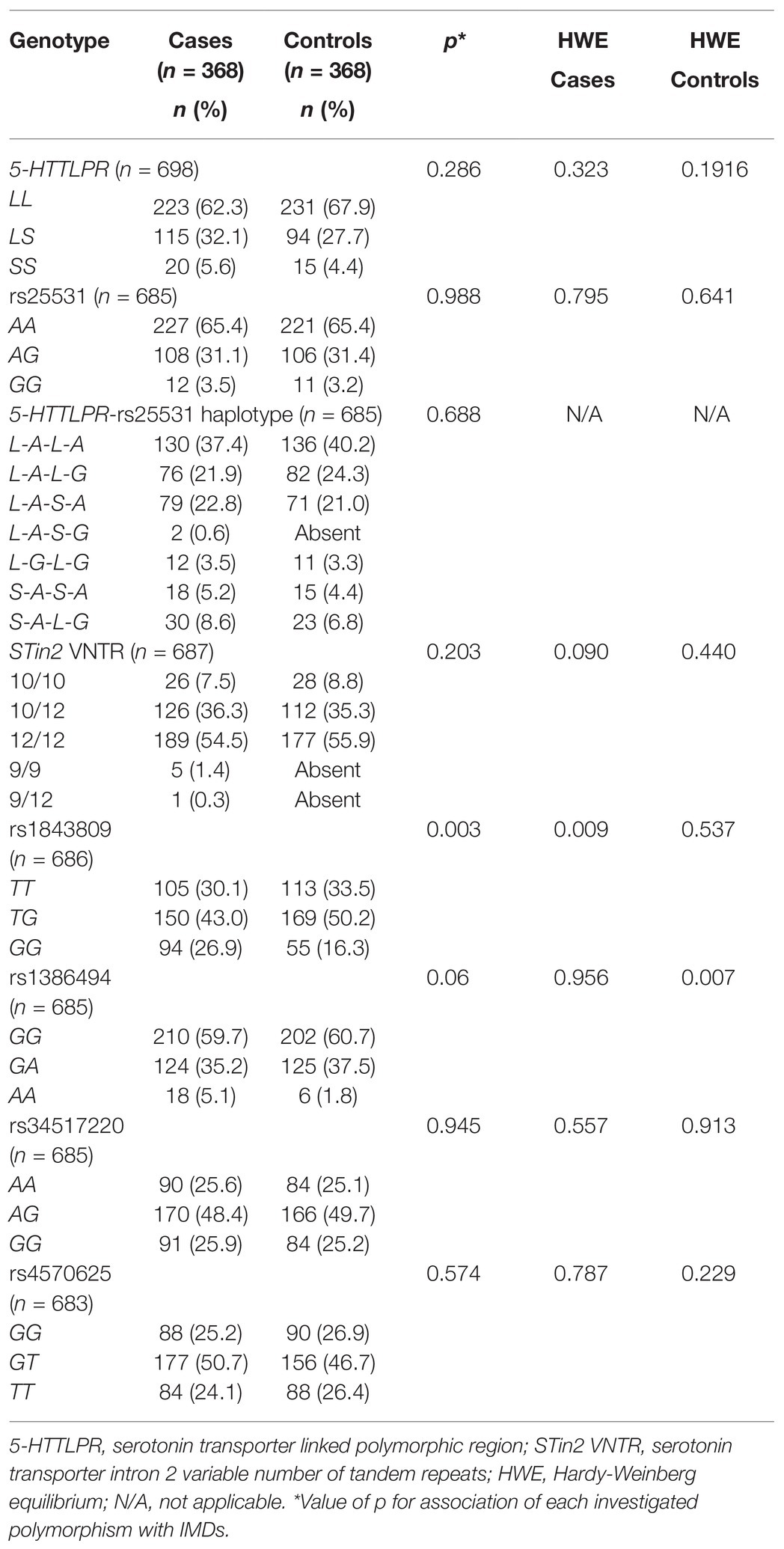
Table 2. Distribution of genotypes and Hardy-Weinberg equilibrium analysis between cases and controls for SLC6A4 [5-HTTLPR, rs25531, 5-HTTLPR-rs25531, and STin2 VNTR] and TPH2 [rs1843809, rs1386494, rs34517220, and rs4570625] polymorphisms.
The association between each investigated polymorphism and IMDs is shown in Table 2. There was a protective effect against IMDs in participants with TPH2 rs1843809 TG and TT genotypes compared with participants with the GG genotype (Table 3).
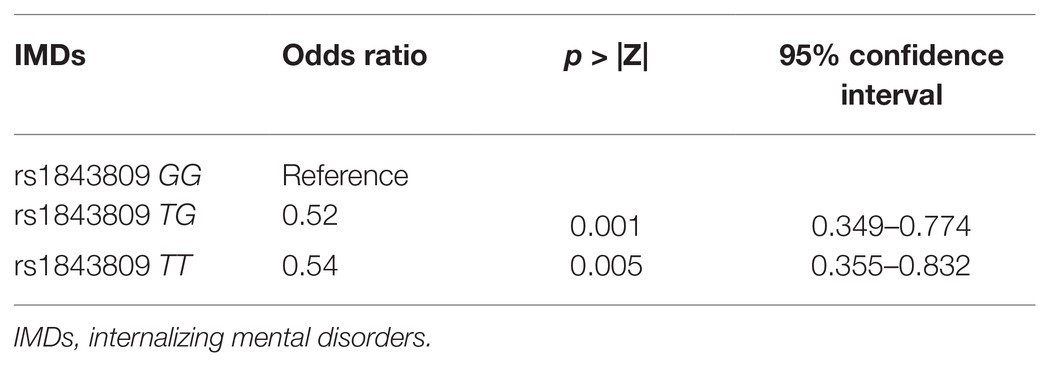
Table 3. Association between tryptophan hydroxylase 2 gene rs1843809 and internalizing mental disorders (IMDs).
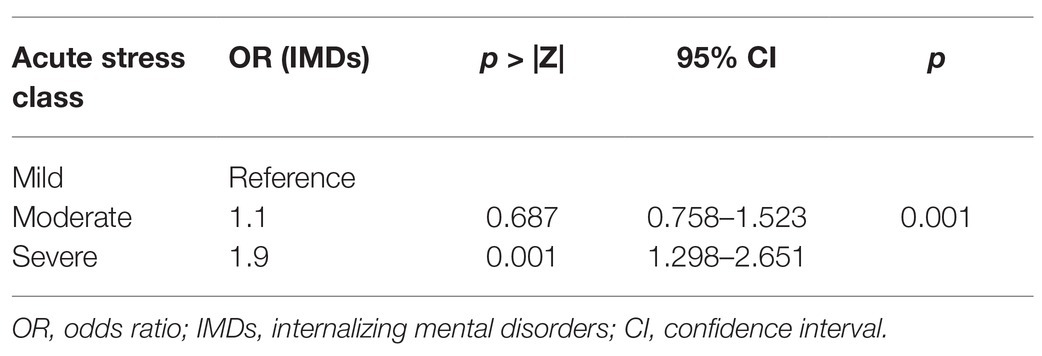
Table 4 shows the association between acute stress and IMDs. A significant association was observed between acute stress and being an IMD case. Table 5 shows the independent effect of chronic stress and SLC6A4 5-HTTLPR-rs25531 haplotype on the association seen in Table 4 (between acute stress and IMDs). The independent effect of all other polymorphisms on the association between acute stress and IMDs are shown in Supplementary Table S2.
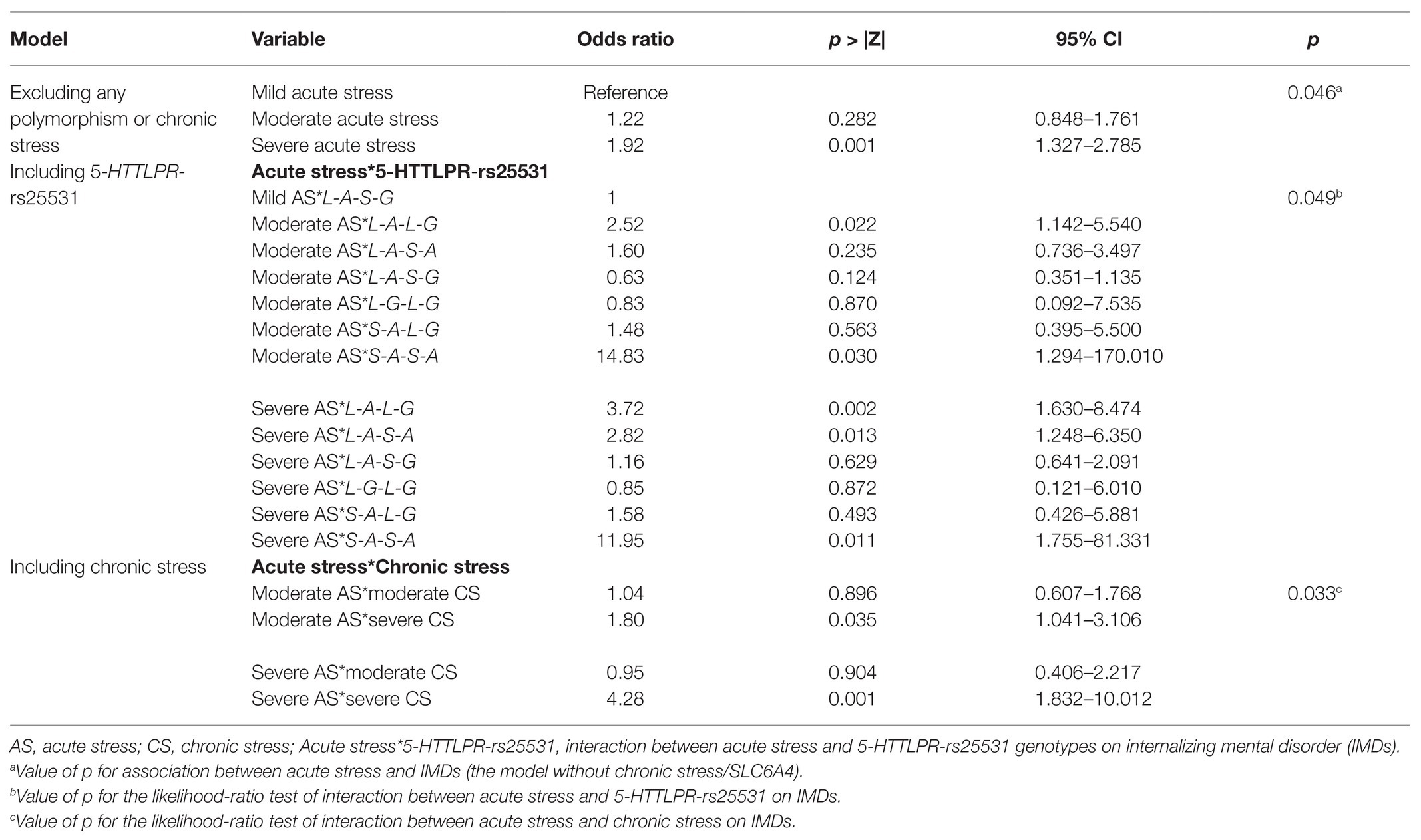
Table 5. Logistic regression analyses for the interaction of acute stress with 5-HTTLPR-rs25531 and chronic stress, respectively, on IMDs.
Apart from the SLC6A4 5-HTTLPR-rs25531 haplotype (Table 5), we found no significant interactions between acute stress and any polymorphism on IMDs (Supplementary Table S2). A significant interaction between acute and chronic stress (p = 0.033) and acute stress and 5-HTTLPR-rs25531 S-A-S-A haplotype (p = 0.049) was observed on IMDs (Table 5). The odds of having an IMD were 4.3 times higher among participants under both severe acute stress and severe chronic stress, compared to those under mild acute stress and mild chronic stress (Table 5). The odds for being a case of an IMD were 14.8 times higher in participants with the S-A-S-A haplotype who experienced acute stress as compared to participants with the L-A-L-A haplotype who experienced mild acute stress. Similarly, the odds of having an IMD were 11.95 times higher in S-A-S-A participants with severe acute stress compared to L-A-L-A participants with mild stress (Table 5).
Discussion
This study investigated the association between acute stress and IMDs, and whether this association was moderated by chronic stress or selected genetic variants in SLC6A4 and TPH2. To our knowledge, this is the first sub-Saharan African study to investigate these interactions among HIV+ children and adolescents. Results revealed a statistically significant association between acute stress and IMDs. This association was found to be moderated by 5-HTTLPR-rs25531 in a haplotype-dependent manner. Specifically, in comparison to L-A-L-A haplotype carriers with experience of mild acute stress, individuals carrying the S-A-S-A haplotypes were more likely to have an IMD under conditions of moderate and severe acute stress. We found no evidence for a significant combined interaction of acute stress, chronic stress, and 5-HTTLPR-rs25531 on IMDs.
Stress represents a prevalent environmental risk factor for many mental disorders, including depression and anxiety (de Kloet et al., 2005; Popoli et al., 2011). Acute stress has been associated with the likelihood of developing IMDs among samples of adolescent school children and adult disaster workers (Fullerton et al., 2004; O’Connor et al., 2010; Brown et al., 2016). In line with previous studies, where acute stress has been associated with IMDs such as depression, PTSD (Fullerton et al., 2004), and anxiety (Grillon et al., 2007), we found that risk for IMDs increased with increasing acute stress and was highest for severe acute stress. Chronic stress was found to significantly moderate the association between acute stress and IMDs. Previous studies have reported on chronic stress as a risk factor for IMDs (Charney and Manji, 2004; de Kloet et al., 2005; Evans et al., 2013; Adelman et al., 2014; Robles et al., 2014; Revenson et al., 2016). Vulnerability to IMDs by chronic stress (through an interaction with acute stress) could be due to alterations in the functioning of the hypothalamic-pituitary-adrenal axis that follow exposure to chronic stress (de Kloet et al., 2005; Fuchs and Flïugge, 2006). In addition, chronic stress has been reported to affect serotonergic signaling in the brain (van den Buuse and Hale, 2019). As serotonergic systems have been implicated in threat appraisal, psychophysiological measures of stress response (skin conductance and startle reactions), and attentional bias to negative stimuli, it is possible that alterations in 5-HT signaling due to chronic stress may at least partially contribute to some of the behavioral features of IMDs (Pergamin-Hight et al., 2012; Klumpp et al., 2014; Klumpers et al., 2015).
In contrast to previous findings of associations between 5-HTTLPR and STin2 VNTR and IMDs (Battersby et al., 1996; Evans et al., 2013; Gressier et al., 2013; Zhao et al., 2013; Smoller, 2016; Wang et al., 2016; Xiao et al., 2019), we found no direct association between any of the investigated SLC6A4 polymorphisms and IMDs. This may be due to the complex nature of IMDs, where many genetic variants contribute to disease risk, each contributing a small effect (Plomin and Davis, 2009) and thus the effect of a few variants may not be powerful enough to show significant association. Indeed, studies examining the contributions of 5-HTTLPR and STin2 variants have yielded inconsistent results, with other published work similarly failing to find a significant association (Munafò et al., 2009a,b; Culverhouse et al., 2018; Xiao et al., 2019). In order to resolve these contradictory results, studies looking at epistatic interactions are required.
Based on the diathesis-stress hypothesis of neuropsychiatric disorders, we postulated that the association between acute stress and IMDs would be moderated by genetic polymorphisms, chronic stress, or a combination of genetic polymorphisms and chronic stress. Results revealed chronic stress and the 5-HTTLPR-rs25531 S-A-S-A haplotype to each significantly moderate the association between acute stress and IMDs.
The interaction between the 5-HTTLPR and stress has previously been investigated in gene-environment studies of IMDs (Karg et al., 2011; Sharpley et al., 2014). In contrast to previous studies that have reported the moderating role of 5-HTTLPR on the association between stress and IMDs (Caspi et al., 2003; Karg et al., 2011; Conway et al., 2014; Sharpley et al., 2014), we found no significant moderating effect of 5-HTTLPR on the association between acute stress and IMDs. However, our results revealed that the combined 5-HTTLPR-rs25531 haplotype significantly moderates the association between acute stress and IMDs. These results revealed the S-A-S-A genotype as a vulnerability factor for IMDs. HIV+ children who experienced moderate and severe acute stress and possessed the S-A-S-A haplotype were more likely to have an IMD, compared to HIV+ children who experienced mild acute stress and possessed the L-A-L-A haplotype.
Previous studies have reported the SS genotype to be a risk for different IMD psychopathologies including depression (Zalsman et al., 2006; Cervilla et al., 2007; Karg et al., 2011; Sharpley et al., 2014; Haberstick et al., 2016), anxiety (Armbruster et al., 2009), and IMDs in general (Conway et al., 2014). However, we found no association between the SS genotype and IMDs in the present study. This observation could be due to insufficient power to detect the effect of 5-HTTLPR on the association between acute stress and IMDs among our study participants. Our study sample size had a post hoc power of greater than 80% (87.5% for the interaction analyses) under assumption that there was zero correlation of exposure between cases and controls and that the probability of exposure among controls was 0.5. There is, however, a possibility that the probability of exposure could have been less than 0.5 among controls, thus reducing the power in the present study. The association between the SS genotype and IMDs has been suggested to be due to its lower transcriptional activity (Baca-García et al., 2002; Hu et al., 2006). Future functional studies that examine the effects of the S-A-S-A compared to the L-A-L-A haplotype are required for us to determine whether lower 5-HTT levels could be driving our results.
A significant association was observed between TPH2 rs1843809 and IMDs. The functional role of TPH2 rs1843809 is currently not known. Being an intronic variant, it is less likely that rs1843809 would have an effect on gene expression or protein structure, as introns are spliced during mRNA processing, although potential effects of intronic variants have been suggested (Kleinjan and van Heyningen, 2005). It is also possible that rs1843809 is in LD with a causal variant. For example, rs1843809 is in LD with a missense rs142055199 SNP among the Luhya, a population that speaks the same Niger-Congo language as the population of the present study (Baganda; Countries and their Cultures, 2021). East African populations that speak this class of language have been found to be genetically similar by principal components analysis on GWAS data from NeuroGAP pilot study (Unpublished results). The SNP rs142055199 is located in the zinc finger C3H1-type containing gene, which modulates interleukin-8 (IL-8) transcription (National Center for Biotechnology Information, 2021c). Modulation of IL-8 would be of interest since IMDs have been associated with inflammatory processes (Miller et al., 2009; Musinguzi et al., 2018; Hori and Kim, 2019; Wang et al., 2019). Further studies will be needed to determine the mechanisms underlying the influence of this SNP in IMDs.
None of the investigated polymorphisms in TPH2 moderated the association between acute stress and IMDs. This suggests that although TPH2 is critical in 5-HT biosynthesis (Walther et al., 2003; Carkaci-Salli et al., 2006), genetic variants investigated in this study neither moderate an individual’s response to stress nor are they causal polymorphisms that moderate an individual’s response to stress.
Limitations and Recommendations
The following limitations should be noted. We defined IMDs as having any depressive disorder, anxiety disorder, or PTSD. As much as anxiety disorders are commonly comorbid with depression, the inclusion of PTSD (n = 60) is contentious as it has been excluded from the anxiety disorder category in the Diagnostic and Statistical Manual of Mental Disorders – 5th edition (DSM-5) and is not widely accepted as an IMD (American Psychiatric Association, 2013). The inclusion of PTSD samples allowed us to achieve the desired statistical power. However, the low correlation between genetic risk factors for PTSD and those of depression and anxiety would have reduced the power to detect true significant associations and interactions. Future studies should endeavor to stratify analyses by specific IMDs although this will require larger samples. In addition, both acute and chronic stress indices were measured using a number of context-specific indicators. This approach was adopted because there is no locally adapted tool for assessing these indicators in this setting and the variables used to generate these indices are known stressors in this population. However, this tool remains to be validated. Moreover, we did not control for population stratification at analysis. The study participants belong to the Bagandan population group, for which principal components analysis on a GWAS sample of over 4,700 individuals has shown less genetic variation among the Bagandan (unpublished data). There is, however, a possibility that some participants were not Bagandan, even though they could speak Luganda (the main language of Baganda), indicating that we may have had an admixed sampled. Future studies should, therefore, control for population stratification, in order to circumvent confounding of results due to possible ancestry associated differences in genetic architecture. Also, due to the exploratory nature of the study, multiple testing corrections were not done. Correcting for multiple testing would have rendered the interaction analysis non-significant. We, therefore, acknowledge the risk of false positive findings in this study which have potentially important clinical implications and need to be verified in future research.
Conclusion
Acute stress was found to be associated with an increased risk of IMDs, and this association was found to be moderated by the 5-HTTLPR-rs25531 S-A-S-A haplotype and chronic stress among Ugandan HIV+ children and adolescents. These results support the diathesis-stress model, though the mechanisms through which acute stress interacts with 5-HTTLPR-rs25531 S-A-S-A haplotype to moderate the risk of IMDs need to be elucidated. This will allow for interventions to be targeted to at-risk individuals, an important consideration in resource constrained settings.
Data Availability Statement
All information gathered about study subjects and their samples is confidential, with access limited to the research team. However, upon request, data from the MRC/UVRI and LSHTM Uganda Research Unit is currently accessed under a data sharing policy via: http://www.mrcuganda.org/sites/default/files/publications/MRC_UVRI_Data_sharing_policy_December2015.pdf.
Ethics Statement
This study was reviewed and approved by the Health Research Committee of Stellenbosch University (# S17/09/179) and the higher Degrees Research and Ethics Committee, School of Biomedical Sciences, College of Health Sciences, Makerere University (# SBS 421). The parent study (CHAKA) obtained ethics approval from the Uganda Virus Research Institute (UVRI) Science and Ethical Committee (# GC/127/15/06/459) and the Uganda National Council of Science and Technology (# HS 1601). All parents/caregivers provided written informed consent for their children/adolescents to participate in the study and for a blood specimen to be withdrawn from them for the genetics analyses. Adolescents further provided written informed assent to participate in the study. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
AK, SH, EK, and SS: concept. AK, EK, SH, SS, and JW: data collection. WS, AK, RN, SH, JW, SS, and EK: data analysis. AK, SH, SS, EK, JW, MJ, WS, and RN: First draft and final revision. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the Alliance for Global Health and Science of the Center for Emerging and Neglected Diseases, grant number: 50288/N7145, the Medical Research Council/Department for International Development – African Leadership Award (MRC/DfID/ARL) to EK, grant number: MR/L004623/1, the Africa Center of Excellence in Materials, Product Development and Nanotechnology (MAPRONANO ACE), Project ID/Number: P151847, IDA Number: 5797-UG, the South African Research Chairs Initiative in Post-traumatic Stress Disorder, funded by the Department of Science and Technology and the National Research Foundation of South Africa, and the South African Medical Research Council. AK was also supported by both the South African Research Chairs Initiative in Post-traumatic Stress Disorder and the mental health section of MRC/UVRI and LSHTM Uganda Research unit.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with the author RN.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2021.649055/full#supplementary-material
Acknowledgments
The assistance of the following are gratefully acknowledged: study subjects, research assistants of the mental health section of MRC/UVRI and LSHTM Uganda Research Unit, Joint Clinical Research Centre, Nsambya Home Care, TASO – Masaka, Kitovu Mobile Clinic, Uganda Cares – Masaka, members of the Neuropsychiatric Genetics Laboratory at Stellenbosch University, Data and Statistics Section of the MRC/UVRI and LSHTM Uganda Research Unit, and the National Research Foundation of South Africa.
References
Adelman, R. D., Tmanova, L. L., Delgado, D., Dion, S., and Lachs, M. S. (2014). Caregiver burden: a clinical review. JAMA 311, 1052–1060. doi: 10.1001/jama.2014.304
Ali, F. R., Vasiliou, S. A., Haddley, K., Paredes, U. M., Roberts, J. C., Miyajima, F., et al. (2010). Combinatorial interaction between two human serotonin transporter gene variable number tandem repeats and their regulation by CTCF. J. Neurochem. 112, 296–306. doi: 10.1111/j.1471-4159.2009.06453.x
American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders. 5th Edn.
Anttila, S., Viikki, M., Huuhka, K., Huuhka, M., Huhtala, H., Rontu, R., et al. (2009). TPH2 polymorphisms may modify clinical picture in treatment-resistant depression. Neurosci. Lett. 464, 43–46. doi: 10.1016/j.neulet.2009.08.018
Armbruster, D., Moser, D. A., Strobel, A., Hensch, T., Kirschbaum, C., Lesch, K. P., et al. (2009). Serotonin transporter gene variation and stressful life events impact processing of fear and anxiety. Int. J. Neuropsychopharmacol. 12, 393–401. doi: 10.1017/S1461145708009565
Artigas, F. (2013a). Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 137, 119–131. doi: 10.1016/j.pharmthera.2012.09.006
Artigas, F. (2013b). Future directions for serotonin and antidepressants. ACS Chem. Neurosci. 4, 5–8. doi: 10.1021/cn3001125
Assary, E., Vincent, J. P., Keers, R., and Pluess, M. (2018). Gene-environment interaction and psychiatric disorders: review and future directions. Semin. Cell Dev. Biol. 77, 133–143. doi: 10.1016/j.semcdb.2017.10.016
Baca-García, E., Vaquero, C., Diaz-Sastre, C., Saiz-Ruiz, J., Fernández-Piqueras, J., and de Leon, J. (2002). A gender-specific association between the serotonin transporter gene and suicide attempts. Neuropsychopharmacology 26, 692–695. doi: 10.1016/S0893-133X(01)00394-3
Barrett, J. C. (2009). Haploview: visualization and analysis of SNP genotype data. Cold Spring Harb. Protoc. 2009:pdb.ip71. doi: 10.1101/pdb.ip71
Battersby, S., Ogilvie, A. D., Smith, C. A., Blackwood, D. H., Muir, W. J., Quinn, J. P., et al. (1996). Structure of a variable number tandem repeat of the serotonin transporter gene and association with affective disorder. Psychiatr. Genet. 6, 177–181. doi: 10.1097/00041444-199624000-00001
Betancourt, T., Scorza, P., Kanyanganzi, F., Fawzi, M. C., Sezibera, V., Cyamatare, F., et al. (2014). HIV and child mental health: a case-control study in Rwanda. Pediatrics 134, e464–e472. doi: 10.1542/peds.2013-2734
Beusenberg, M., and Orley, J. H. (1994). A User's Guide to The Self Reporting Questionnaire (SRQ). Geneva, Switzerland: World Health Organization.
Bhatia, R., Hartman, C., Kallen, M. A., Graham, J., and Giordano, T. P. (2011). Persons newly diagnosed with HIV infection are at high risk for depression and poor linkage to care: results from the steps study. AIDS Behav. 15, 1161–1170. doi: 10.1007/s10461-010-9778-9
Boyes, M. E., and Cluver, L. D. (2015). Relationships between familial HIV/AIDS and symptoms of anxiety and depression: the mediating effect of bullying victimization in a prospective sample of south African children and adolescents. J. Youth Adolesc. 44, 847–859. doi: 10.1007/s10964-014-0146-3
Brown, R. C., Nugent, N. R., Hawn, S. E., Koenen, K. C., Miller, A., Amstadter, A. B., et al. (2016). Predicting the transition from acute stress disorder to posttraumatic stress disorder in children with severe injuries. J. Pediatr. Health Care 30, 558–568. doi: 10.1016/j.pedhc.2015.11.015
Carkaci-Salli, N., Flanagan, J. M., Martz, M. K., Salli, U., Walther, D. J., Bader, M., et al. (2006). Functional domains of human tryptophan hydroxylase 2 (hTPH2). J. Biol. Chem. 281, 28105–28112. doi: 10.1074/jbc.M602817200
Caspi, A., and Moffitt, T. E. (2006). Gene-environment interactions in psychiatry: joining forces with neuroscience. Nat. Rev. Neurosci. 7, 583–590. doi: 10.1038/nrn1925
Caspi, A., Sugden, K., Moffitt, T. E., Taylor, A., Craig, I. W., Harrington, H., et al. (2003). Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389. doi: 10.1126/science.1083968
Cervilla, J. A., Molina, E., Rivera, M., Torres-González, F., Bellón, J. A., Moreno, B., et al. (2007). The risk for depression conferred by stressful life events is modified by variation at the serotonin transporter 5HTTLPR genotype: evidence from the Spanish PREDICT-gene cohort. Mol. Psychiatry 12, 748–755. doi: 10.1038/sj.mp.4001981
Charney, D. S., and Manji, H. K. (2004). Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci. STKE 2004, re5. doi: 10.1126/stke.2252004re5
Chida, Y., and Vedhara, K. (2009). Adverse psychosocial factors predict poorer prognosis in HIV disease: a meta-analytic review of prospective investigations. Brain Behav. Immun. 23, 434–445. doi: 10.1016/j.bbi.2009.01.013
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366. doi: 10.1016/S0140-6736(17)32802-7
Clarke, H., Flint, J., Attwood, A. S., and Munafò, M. R. (2010). Association of the 5- HTTLPR genotype and unipolar depression: a meta-analysis. Psychol Med. 40, 1767–1778. doi: 10.1017/S0033291710000516
Clarke, L., Fairley, S., Zheng-Bradley, X., Streeter, I., Perry, E., Lowy, E., et al. (2017). The international genome sample resource (IGSR): a worldwide collection of genome variation incorporating the 1000 genomes project data. Nucleic Acids Res. 45, D854–D859. doi: 10.1093/nar/gkw829
Conway, C. C., Slavich, G. M., and Hammen, C. (2014). Daily stress reactivity and serotonin transporter gene (5-HTTLPR) variation: internalizing responses to everyday stress as a possible transdiagnostic phenotype. Biol. Mood Anxiety Disord. 4:2. doi: 10.1186/2045-5380-4-2
Countries and their Cultures (2021). Baganda. [Place unknown]. [Publisher unknown]. [Date unknown, cited 2021 Jan 02]. Available at: https://www.everyculture.com/wc/Tajikistan-to-Zimbabwe/Baganda.html (Accessed January 02, 2020).
Culverhouse, R. C., Saccone, N. L., Horton, A. C., Ma, Y., Anstey, K. J., Banaschewski, T., et al. (2018). Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol. Psychiatry 23, 133–142. doi: 10.1038/mp.2017.44
Davies, M. N., Verdi, S., Burri, A., Trzaskowski, M., Lee, M., Hettema, J. M., et al. (2015). Generalised anxiety disorder--a twin study of genetic architecture, genome-wide association and differential gene expression. PLoS One 10:e0134865. doi: 10.1371/journal.pone.0134865
de Kloet, E. R., Joëls, M., and Holsboer, F. (2005). Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 6, 463–475. doi: 10.1038/nrn1683
Ehli, E. A., Hu, Y., Lengyel-Nelson, T., Hudziak, J. J., and Davies, G. E. (2012). Identification and functional characterization of three novel alleles for the serotonin transporter-linked polymorphic region. Mol. Psychiatry 17, 185–192. doi: 10.1038/mp.2010.130
Evans, G. W., Li, D., and Whipple, S. S. (2013). Cumulative risk and child development. Psychol. Bull. 139, 1342–1396. doi: 10.1037/a0031808
Fuchs, E., and Flïugge, G. (2006). Experimental animal models for the simulation of depression and anxiety. Dialogues Clin. Neurosci. 8, 323–333.
Fullerton, C. S., Ursano, R. J., and Wang, L. (2004). Acute stress disorder, posttraumatic stress disorder, and depression in disaster or rescue workers. Am. J. Psychiatry 161, 1370–1376. doi: 10.1176/appi.ajp.161.8.1370
Furlong, R. A., Ho, L., Walsh, C., Rubinsztein, J. S., Jain, S., Paykel, E. S., et al. (1998). Analysis and meta-analysis of two serotonin transporter gene polymorphisms in bipolar and unipolar affective disorders. Am. J. Med. Genet. 81, 58–63. doi: 10.1002/(SICI)1096-8628(19980207)81:1<58::AID-AJMG11>3.0.CO;2-V
Gadow, K. D., and Sprafkin, J. (1999). Youth’s Inventory-4. Stony Brook, New York: Checkmate plus; [Revised 2016 Jan 23]. Available at: https://www.checkmateplus.com/product/yi-4.htm (Accessed April 07, 2021).
Gadow, K. D., and Sprafkin, J. (2013). Child and Adolescent Symptom Inventory-5. Stony Brook, New York: Checkmate plus; [Revised 2016 Jan 23]. Available at: https://www.checkmateplus.com/product/casi5.htm (Accessed April 07, 2021).
Gao, J., Pan, Z., Jiao, Z., Li, F., Zhao, G., Wei, Q., et al. (2012). TPH2 gene polymorphisms and major depression--a meta-analysis. PLoS One 7:e36721. doi: 10.1371/journal.pone.0036721
Gressier, F., Calati, R., Balestri, M., Marsano, A., Alberti, S., Antypa, N., et al. (2013). The 5-HTTLPR polymorphism and posttraumatic stress disorder: a meta-analysis. J. Trauma. Stress. 26, 645–653. doi: 10.1002/jts.21855
Grillon, C., Duncko, R., Covington, M. F., Kopperman, L., and Kling, M. A. (2007). Acute stress potentiates anxiety in humans. Biol. Psychiatry 62, 1183–1186. doi: 10.1016/j.biopsych.2007.06.007
Gurdasani, D., Carstensen, T., Tekola-Ayele, F., Pagani, L., Tachmazidou, I., Hatzikotoulas, K., et al. (2015). The African genome variation project shapes medical genetics in Africa. Nature 517, 327–332. doi: 10.1038/nature13997
Haberstick, B. C., Boardman, J. D., Wagner, B., Smolen, A., Hewitt, J. K., Killeya-Jones, L. A., et al. (2016). Depression, stressful life events, and the impact of variation in the serotonin transporter: findings from the National Longitudinal Study of adolescent to adult health (add health). PLoS One 11:e0148373. doi: 10.1371/journal.pone.0148373
Haddley, K., Bubb, V. J., Breen, G., Parades-Esquivel, U. M., and Quinn, J. P. (2012). Behavioural genetics of the serotonin transporter. Curr. Top. Behav. Neurosci. 12, 503–535. doi: 10.1007/7854_2011_186
Hammoud, D. A., Endres, C. J., Hammond, E., Uzuner, O., Brown, A., Nath, A., et al. (2010). Imaging serotonergic transmission with [11C]DASB-PET in depressed and non-depressed patients infected with HIV. NeuroImage 49, 2588–2595. doi: 10.1016/j.neuroimage.2009.10.037
Heils, A., Teufel, A., Petri, S., Stöber, G., Riederer, P., Bengel, D., et al. (1996). Allelic variation of human serotonin transporter gene expression. J. Neurochem. 66, 2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x
Hettema, J. M., Neale, M. C., and Kendler, K. S. (2001). A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am. J. Psychiatry 158, 1568–1578. doi: 10.1176/appi.ajp.158.10.1568
Hori, H., and Kim, Y. (2019). Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 73, 143–153. doi: 10.1111/pcn.12820
Hu, X. Z., Lipsky, R. H., Zhu, G., Akhtar, L. A., Taubman, J., Greenberg, B. D., et al. (2006). Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am. J. Hum. Genet. 78, 815–826. doi: 10.1086/503850
Ironson, G., O'Cleirigh, C., Fletcher, M. A., Laurenceau, J. P., Balbin, E., Klimas, N., et al. (2005). Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom. Med. 67, 1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8
Jacob, J., Ferri, A. L., Milton, C., Prin, F., Pla, P., Lin, W., et al. (2007). Transcriptional repression coordinates the temporal switch from motor to serotonergic neurogenesis. Nat. Neurosci. 10, 1433–1439. doi: 10.1038/nn1985
Jawahar, M. C., Toben, C. G., and Baune, B. T. (2019). “Gene-environment interactions and epigenetic mechanisms in depression” in Neurobiology of Depression. eds. J. Quevedo, A. F. Carvalho, and C. A. Zarate (Academic Press), 17–25.
Kalungi, A., Kinyanda, E., Womersley, J. S., Joloba, M. L., Ssembajjwe, W., Nsubuga, R. N., et al. (2021). TERT rs2736100 and TERC rs16847897 genotypes moderate the association between internalizing mental disorders and accelerated telomere length attrition among HIV+ children and adolescents in Uganda. BMC Med. Genet. 14:15. doi: 10.1186/s12920-020-00857-z
Kalungi, A., Seedat, S., Hemmings, S. M. J., van der Merwe, L., Joloba, M. L., Nanteza, A., et al. (2017). Association between serotonin transporter gene polymorphisms and increased suicidal risk among HIV positive patients in Uganda. BMC Genet. 18:71. doi: 10.1186/s12863-017-0538-y
Kalungi, A., Womersley, J. S., Kinyanda, E., Joloba, M. L., Ssembajjwe, W., Nsubuga, R. N., et al. (2019). Internalizing mental disorders and accelerated cellular aging among perinatally HIV-infected youth in Uganda. Front. Genet. 10:705. doi: 10.3389/fgene.2019.00705
Karg, K., Burmeister, M., Shedden, K., and Sen, S. (2011). The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry 68, 444–454. doi: 10.1001/archgenpsychiatry.2010.189
Kennedy, A. P., Binder, E. B., Bowman, D., Harenski, K., Ely, T., Cisler, J. M., et al. (2012). A common TPH2 haplotype regulates the neural processing of a cognitive control demand. Am. J. Med. Genet. B Neuropsychiatr. Genet. 159B, 829–840. doi: 10.1002/ajmg.b.32090
Kinyanda, E., Levin, J., Nakasujja, N., Birabwa, H., Nakku, J., Mpango, R., et al. (2018). Major depressive disorder: longitudinal analysis of impact on clinical and behavioral outcomes in Uganda. J. Acquir. Immune Defic. Syndr. 78, 136–143. doi: 10.1097/QAI.0000000000001647
Kinyanda, E., Salisbury, T. T., Levin, J., Nakasujja, N., Mpango, R. S., Abbo, C., et al. (2019). Rates, types and co-occurrence of emotional and behavioural disorders among perinatally HIV-infected youth in Uganda: the CHAKA study. Soc. Psychiatry Psychiatr. Epidemiol. 54, 415–425. doi: 10.1007/s00127-019-01675-0
Kleinjan, D. A., and van Heyningen, V. (2005). Long-range control of gene expression: emerging mechanisms and disruption in disease. Am. J. Hum. Genet. 76, 8–32. doi: 10.1086/426833
Klumpers, F., Kroes, M. C., Heitland, I., Everaerd, D., Akkermans, S. E., Oosting, R. S., et al. (2015). Dorsomedial prefrontal cortex mediates the impact of serotonin transporter linked polymorphic region genotype on anticipatory threat reactions. Biol. Psychiatry 78, 582–589. doi: 10.1016/j.biopsych.2014.07.034
Klumpp, H., Fitzgerald, D. A., Cook, E., Shankman, S. A., Angstadt, M., and Phan, K. L. (2014). Serotonin transporter gene alters insula activity to threat in social anxiety disorder. Neuroreport 25, 926–931. doi: 10.1097/WNR.0000000000000210
Kristensen, A. S., Andersen, J., Jørgensen, T. N., Sørensen, L., Eriksen, J., Loland, C. J., et al. (2011). SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol. Rev. 63, 585–640. doi: 10.1124/pr.108.000869
Lachin, J. M. (1992). Power and sample size evaluation for the McNemar test with application to matched case-control studies. Stat. Med. 11, 1239–1251. doi: 10.1002/sim.4780110909
Lesch, K. P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., et al. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531. doi: 10.1126/science.274.5292.1527
Lipsky, R. H., Hu, X. Z., and Goldman, D. (2009). Additional functional variation at the SLC6A4 gene. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B:153. doi: 10.1002/ajmg.b.30766
Lopez de Lara, C., Brezo, J., Rouleau, G., Lesage, A., Dumont, M., Alda, M., et al. (2007). Effect of tryptophan hydroxylase-2 gene variants on suicide risk in major depression. Biol. Psychiatry 62, 72–80. doi: 10.1016/j.biopsych.2006.09.008
Lwidiko, A., Kibusi, S. M., Nyundo, A., and Mpondo, B. C. T. (2018). Association between HIV status and depressive symptoms among children and adolescents in the southern highlands zone, Tanzania: a case-control study. PLoS One 13:e0193145. doi: 10.1371/journal.pone.0193145
Martin, J., Cleak, J., Willis-Owen, S. A., Flint, J., and Shifman, S. (2007). Mapping regulatory variants for the serotonin transporter gene based on allelic expression imbalance. Mol. Psychiatry 12, 421–422. doi: 10.1038/sj.mp.4001952
Mellins, C. A., Elkington, K. S., Leu, C. S., Santamaria, E. K., Dolezal, C., Wiznia, A., et al. (2012). Prevalence and change in psychiatric disorders among perinatally HIV-infected and HIV-exposed youth. AIDS Care 24, 953–962. doi: 10.1080/09540121.2012.668174
Miller, A. H., Maletic, V., and Raison, C. L. (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. doi: 10.1016/j.biopsych.2008.11.029
Monroe, S. M., and Simons, A. D. (1991). Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol. Bull. 110, 406–425. doi: 10.1037/0033-2909.110.3.406
Mpango, R. S., Kinyanda, E., Rukundo, G. Z., Gadow, K. D., and Patel, V. (2017). Cross-cultural adaptation of the child and adolescent symptom Inventory-5 (CASI-5) for use in central and South-Western Uganda: the CHAKA project. Trop. Dr. 47, 347–354. doi: 10.1177/0049475517724688
Munafò, M. R., Durrant, C., Lewis, G., and Flint, J. (2009a). Gene X environment interactions at the serotonin transporter locus. Biol. Psychiatry 65, 211–219. doi: 10.1016/j.biopsych.2008.06.009
Munafò, M. R., Freimer, N. B., Ng, W., Ophoff, R., Veijola, J., Miettunen, J., et al. (2009b). 5-HTTLPR genotype and anxiety-related personality traits: a meta-analysis and new data. Am. J. Med. Genet. B Neuropsychiatr. Genet. 150B, 271–281. doi: 10.1002/ajmg.b.30808
Musci, R. J., Augustinavicius, J. L., and Volk, H. (2019). Gene-environment interactions in psychiatry: recent evidence and clinical implications. Curr. Psychiatry Rep. 21:81. doi: 10.1007/s11920-019-1065-5
Musinguzi, K., Obuku, A., Nakasujja, N., Birabwa, H., Nakku, J., Levin, J., et al. (2018). Association between major depressive disorder and pro-inflammatory cytokines and acute phase proteins among HIV-1 positive patients in Uganda. BMC Immunol. 19:1. doi: 10.1186/s12865-017-0239-3
Nachman, S., Chernoff, M., Williams, P., Hodge, J., Heston, J., and Gadow, K. D. (2012). Human immunodeficiency virus disease severity, psychiatric symptoms, and functional outcomes in perinatally infected youth. Arch. Pediatr. Adolesc. Med. 166, 528–535. doi: 10.1001/archpediatrics.2011.1785
National Center for Biotechnology Information (2021a). SLC6A4 solute carrier family 6 member 4 [Homo sapiens (human)]. Updated 2020 Dec 27. Available at: https://www.ncbi.nlm.nih.gov/gene/6532#location (Accessed December 29, 2020).
National Center for Biotechnology Information (2021b). STIN2-VNTR serotonin transporter intronic VNTR enhancer [Homo sapiens (human)]. Updated 2020 Nov 24. Available at: https://www.ncbi.nlm.nih.gov/gene/?term=serotonin+transporter+intronic+VNTR+enhancer+%5BHomo+sapiens+(human)%5D (Accessed December 29, 2020).
National Center for Biotechnology Information (2021c). ZFC3H1 zinc finger C3H1-type containing [Homo sapiens (human)]. Updated 2020 Dec 06. Available at: https://www.ncbi.nlm.nih.gov/gene/196441 (Accessed December 29, 2020).
O’Connor, R. C., Rasmussen, S., and Hawton, K. (2010). Predicting depression, anxiety and self-harm in adolescents: the role of perfectionism and acute life stress. Behav. Res. Ther. 48, 52–59. doi: 10.1016/j.brat.2009.09.008
O’Gara, C., Knight, J., Stapleton, J., Luty, J., Neale, B., Nash, M., et al. (2008). Association of the serotonin transporter gene, neuroticism and smoking behaviours. J. Hum. Genet. 53, 239–246. doi: 10.1007/s10038-007-0243-1
Olivier, B. (2015). Serotonin: a never-ending story. Eur. J. Pharmacol. 753, 2–18. doi: 10.1016/j.ejphar.2014.10.031
Otte, C., Gold, S. M., Penninx, B. W., Pariante, C. M., Etkin, A., Fava, M., et al. (2016). Major depressive disorder. Nat. Rev. Dis. Primers. 2:16065. doi: 10.1038/nrdp.2016.65
Pergamin-Hight, L., Bakermans-Kranenburg, M. J., van Ijzendoorn, M. H., and Bar-Haim, Y. (2012). Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: a meta-analysis. Biol. Psychiatry 71, 373–379. doi: 10.1016/j.biopsych.2011.10.030
Philibert, R. A., Sandhu, H., Hollenbeck, N., Gunter, T., Adams, W., and Madan, A. (2008). The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa adoption studies. Am. J. Med. Genet. B Neuropsychiatr. Genet. 147B, 543–549. doi: 10.1002/ajmg.b.30657
Plomin, R., and Davis, O. S. (2009). The future of genetics in psychology and psychiatry: microarrays, genome-wide association, and non-coding RNA. J. Child Psychol. Psychiatry 50, 63–71. doi: 10.1111/j.1469-7610.2008.01978.x
Popoli, M., Yan, Z., McEwen, B. S., and Sanacora, G. (2011). The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 13, 22–37. doi: 10.1038/nrn3138
Pristerà, A., Lin, W., Kaufmann, A. K., Brimblecombe, K. R., Threlfell, S., Dodson, P. D., et al. (2015). Transcription factors FOXA1 and FOXA2 maintain dopaminergic neuronal properties and control feeding behavior in adult mice. Proc. Natl. Acad. Sci. U. S. A. 112, E4929–E4938. doi: 10.1073/pnas.1503911112
Revenson, T., Griva, K., Luszczynska, A., Morrison, V., Panagopoulou, E., Vilchinsky, N., et al. (2016). Caregiving in the Illness Context. London: Palgrave Macmillan.
Robles, T. F., Slatcher, R. B., Trombello, J. M., and McGinn, M. M. (2014). Marital quality and health: a meta-analytic review. Psychol. Bull. 140, 140–187. doi: 10.1037/a0031859
Rudnick, G. (2006). Serotonin transporters--structure and function. J. Membr. Biol. 213, 101–110. doi: 10.1007/s00232-006-0878-4
Sharpley, C. F., Palanisamy, S. K., Glyde, N. S., Dillingham, P. W., and Agnew, L. L. (2014). An update on the interaction between the serotonin transporter promoter variant (5-HTTLPR), stress and depression, plus an exploration of non-confirming findings. Behav. Brain Res. 273, 89–105. doi: 10.1016/j.bbr.2014.07.030
Shishkina, G. T., Kalinina, T. S., and Dygalo, N. N. (2007). Up-regulation of tryptophan hydroxylase-2 mRNA in the rat brain by chronic fluoxetine treatment correlates with its antidepressant effect. Neuroscience 150, 404–412. doi: 10.1016/j.neuroscience.2007.09.017
Silberg, J. L., Rutter, M., and Eaves, L. (2001). Genetic and environmental influences on the temporal association between earlier anxiety and later depression in girls. Biol. Psychiatry 49, 1040–1049. doi: 10.1016/S0006-3223(01)01161-1
Smoller, J. W. (2016). The genetics of stress-related disorders: PTSD, depression, and anxiety disorders. Neuropsychopharmacology 41, 297–319. doi: 10.1038/npp.2015.266
Springer, S. A., Dushaj, A., and Azar, M. M. (2012). The impact of DSM-IV mental disorders on adherence to combination antiretroviral therapy among adult persons living with HIV/AIDS: a systematic review. AIDS Behav. 16, 2119–2143. doi: 10.1007/s10461-012-0212-3
Tandon, M., Si, X., and Luby, J. (2011). Preschool onset attention-deficit/hyperactivity disorder: course and predictors of stability over 24 months. J. Child Adolesc. Psychopharmacol. 21, 321–330. doi: 10.1089/cap.2010.0045
van den Buuse, M., and Hale, M. W. (2019). “Serotonin in stress” in Stress: Physiology, Biochemistry, and Pathology. ed. G. Fink (Academic Press), 115–123.
Walther, D. J., Peter, J. U., Bashammakh, S., Hörtnagl, H., Voits, M., Fink, H., et al. (2003). Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science 299:76. doi: 10.1126/science.1078197
Wang, Y., Sun, N., Liu, Z., Li, X., Yang, C., and Zhang, K. (2016). Psychosocial mechanisms of serotonin transporter's genetic polymorphism in susceptibility to major depressive disorder: mediated by trait coping styles and interacted with life events. Am. J. Transl. Res. 8, 1281–1292.
Wang, W., Wang, L., Xu, H., Cao, C., Liu, P., Luo, S., et al. (2019). Characteristics of pro- and anti-inflammatory cytokines alteration in PTSD patients exposed to a deadly earthquake. J. Affect. Disord. 248, 52–58. doi: 10.1016/j.jad.2019.01.029
Ward, J. J. H. (1963). Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 58, 236–244.
Widaman, K. F., Helm, J. L., Castro-Schilo, L., Pluess, M., Stallings, M. C., and Belsky, J. (2012). Distinguishing ordinal and disordinal interactions. Psychol. Methods 17, 615–622. doi: 10.1037/a0030003
Willis, A. M., and Brock, M. S. (2018) Adverse Childhood Experience and Serotonin Transporters: A Gene Environmental Study of the Risk of PTSD in Soldiers (ACES). 59 MDW San Antonio United States.
Xiao, Y., Liu, D., Liu, K., Wu, C., Zhang, H., Niu, Y., et al. (2019). Association of DRD2, 5-HTTLPR, and 5-HTTVNTR gene polymorphisms with posttraumatic stress disorder in Tibetan adolescents: a case-control study. Biol. Res. Nurs. 21, 286–295. doi: 10.1177/1099800419838325
Xie, P., Kranzler, H. R., Poling, J., Stein, M. B., Anton, R. F., Brady, K., et al. (2009). Interactive effect of stressful life events and the serotonin transporter 5-HTTLPR genotype on posttraumatic stress disorder diagnosis in 2 independent populations. Arch. Gen. Psychiatry 66, 1201–1209. doi: 10.1001/archgenpsychiatry.2009.153
Zalsman, G., Huang, Y. Y., Oquendo, M. A., Burke, A. K., Hu, X. Z., Brent, D. A., et al. (2006). Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression. Am. J. Psychiatry 163, 1588–1593. doi: 10.1176/ajp.2006.163.9.1588
Zhao, J., Miao, K., Wang, H., Ding, H., and Wang, D. W. (2013). Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PLoS One 8:e79993. doi: 10.1371/journal.pone.0079993
Keywords: internalizing mental disorders, acute stress, serotonin transporter gene, 5-HTTLPR-rs25531, chronic stress, HIV+ children and adolescents, Uganda
Citation: Kalungi A, Womersley JS, Kinyanda E, Joloba ML, Ssembajjwe W, Nsubuga RN, Seedat S and Hemmings SMJ (2021) The 5-HTTLPR-rs25531 S-A-S-A Haplotype and Chronic Stress Moderate the Association Between Acute Stress and Internalizing Mental Disorders Among HIV+ Children and Adolescents in Uganda. Front. Genet. 12:649055. doi: 10.3389/fgene.2021.649055
Edited by:
Tinashe Chikowore, University of the Witwatersrand, South AfricaReviewed by:
Aniefiok John Udoakang, University of Ghana, GhanaQingsen Ming, First Affiliated Hospital of Soochow University, China
Copyright © 2021 Kalungi, Womersley, Kinyanda, Joloba, Ssembajjwe, Nsubuga, Seedat and Hemmings. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Allan Kalungi, YWxsYW5rYWx1bmdpMUBnbWFpbC5jb20=
 Allan Kalungi
Allan Kalungi Jacqueline S. Womersley
Jacqueline S. Womersley Eugene Kinyanda2,3
Eugene Kinyanda2,3 Soraya Seedat
Soraya Seedat Sian M. J. Hemmings
Sian M. J. Hemmings