- Crop Research Institute, Liaoning Academy of Agricultural Sciences, Shenyang, China
Introduction: Low temperature is a key environmental factor that threaten sweetpotato growth and development. In-depth studies on the gene functions underlying cold resistance are important for genetic engineering in sweetpotato.
Methods: The IbXTH16 gene was cloned using a homologous cloning approach. Its expression was detected in sweetpotato leaves subjected to low-temperature stress and brassinosteroid treatment. Subsequently, the IbXTH16 gene was introduced into sweetpotato variety Lizixiang to generate IbXTH16-overexpressing plants, thereby enabling the functional validation of the IbXTH16.
Results and discussion: The IbXTH16 gene was cloned from the cold-tolerant variety LHS21. Its 879 bp coding sequence encoded a 292 aa protein with a molecular weight of 32.983 kDa and a pI of 8.47. The 2039 bp genomic sequence of IbXTH16 contained two exons and one intron. The IbXTH16 protein was localized in the cell membrane. IbXTH16 was strongly induced by 4°C and brassinosteroid. IbXTH16 positively regulates cold tolerance of sweetpotato by activating the BR and proline pathways.
1 Introduction
Low temperature is a key environmental factor that threaten crop growth and development worldwide (Peng et al., 2014; Shi et al., 2018). Sweetpotato, Ipomoea batatas (L.) Lam., an important cash crop, serves as both a staple food and a bioenergy resource (Zhang et al., 2019). As sweetpotato is native to tropical America, it exhibits sensitivity to low temperature, highlighting the importance of enhancing cold tolerance to ensure sustained productivity (Jin et al., 2017; Yu et al., 2022). The development and cultivation of low-temperature tolerant sweetpotato varieties hold significant importance for addressing temperature-related challenges and ensuring global food security. Therefore, in-depth studies on the gene functions underlying cold resistance are important for genetic engineering in sweetpotato.
The xyloglucan endotransglycosylase/hydrolase (XTH) superfamily is an important protein widely present in plants, mainly catalyzing the endohydrolysis of the β-1,4 glycosidic bond of xyloglucan and the self-connection of the xyloglucan molecule (Rose et al., 2002; Morales-Quintana et al., 2020). The XTH superfamily has been reported to participate in diverse biological processes of plants such as fruit maturation and drought response (Miedes et al., 2010; Wu et al., 2022; Han et al., 2023). In cold stress, AtXTH21 positively modulated the freezing stress resistance and XTH19 mutant exhibited reduced freezing tolerance in Arabidopsis (Shi et al., 2014; Takahashi et al., 2021). In sweetpotato, under cold treatment, only IbXTH02 and IbXTH12 of the XTH family were downregulated expression (Zhang et al., 2023). However, XTHs’ function on cold stress of sweetpotato remain largely unknow.
Brassinolide (BR) refers to a group of polyhydroxy steroids that include BR and its structural analogs (Kim and Russinova, 2020). As a type of steroid hormone, BR is ubiquitously distributed across various plant tissues (Xu et al., 2020). Previous studies have demonstrated that the exogenous application of BR can enhances plant cold tolerance. Specifically, treatment with 2 mg L-1 BR significantly mitigated leaf surface damage in rice plants, improving their resistance to cold stress (Wang et al., 2020). Under exogenous BR treatment, mangoes exhibited an increased proportion of unsaturated fatty acids in cell membranes, which enhanced membrane fluidity and consequently improved cold tolerance (Li et al., 2012). In addition to exogenous BR treatment, endogenous BR signalling pathways in plants also play a crucial role in regulating cold tolerance. The homologous protein CES of brassinosteroid enhanced expression, which acts as a positive regulatory factor in BR signal transduction, can directly interact with downstream CBF proteins and activate the transcription of CBF1 and CBF3, contributing to enhancing plant cold tolerance (Eremina et al., 2016). Overexpression of the BR receptor BRI1, a key activator of BR signalling, has been shown to improve the cold tolerance of tomato plants (Wang et al., 2022). Conversely, BIN2, a negative regulator of BR signalling in Arabidopsis, negatively regulates plant cold tolerance by modulating the activities of BZR1 and phosphorylated ICE1 (Ye et al., 2019).
Proline serves as a critical osmotic regulatory compound that is ubiquitously present in plants and plays a protective role under low temperature stress conditions (Kidokoro et al., 2022; Kim et al., 2024). Specifically, proline helps maintain the stability of biological membranes and various enzymes while regulating the acid-base and redox balance within the cytoplasm (Takagi, 2008; Hayat et al., 2012). In Arabidopsis, the content of proline increases with prolonged exposure to 4°C low temperature treatment (Kaplan et al., 2007). In cucumber, the ICE1 enhances the cold tolerance of transgenic plants by promoting the accumulation of free proline (Liu et al., 2010). In Rosa multiflora, RmZAT10 specifically binds to and activates the promoter of RmP5CS, thereby regulating proline biosynthesis and positively influencing cold resistance (Luo et al., 2022).
In this study, a XTH superfamily gene IbXTH16 was cloned and characterized from sweetpotato. The IbXTH16 gene was introduced into the sweetpotato variety Lizixiang to verify its function. Functional analysis showed that overexpression of IbXTH16 enhanced cold tolerance of sweetpotato by activating the BR and proline pathways.
2 Materials and methods
2.1 Plant materials
The sweetpotato cold-tolerant variety Liaohanshu21 (LHS21) was employed to clone the IbXTH16 gene. The sweetpotato variety LHS21 and cold-susceptible variety Sushu28 (SS28) were employed to analyze the expression level of IbXTH16. The sweetpotato variety Lizixiang was used to identify the function of IbXTH16.
2.2 Cloning and sequence analysis
Total RNA for cDNA generation (TRIzol reagent, CWBIO, Beijing, China) and genomic DNA (Easy Pure Plant Genomic DNA Kit, Trans Gen, Beijing, China) were isolated from the leaves of LHS21 according to Fan et al. (2024). The coding sequence (CDS), genome sequence, and promoter region of IbXTH16 were obtained based on a homologous cloning approach. Phylogenetic analysis was performed with MEGA 11.0 software. The genomic structure of IbXTH16 was analyzed by GSDS 2.0 (http://gsds.gao-lab.org/). The cis-acting regulatory elements of the IbXTH16 promoter region were analyzed by PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/). All primers in this study were showed in Supplementary Table S1.
2.3 Subcellular localization
The CDS of IbXTH16 (without the stop codon) was integrated into the pCAMBIA1300-GFP vector according to Zhang et al. (2020). The pCAMBIA1300-IbXTH16-GFP was introduced into Agrobacterium tumefaciens strain GV3101 and transiently inoculated into Nicotiana benthamiana leaf hypodermal cells. After 48 h of infection, the GFP signals were observed using a confocal fluorescence microscope (LSM880, Zeiss, Jena, Germany).
2.4 Expression analysis
The four-week-old in vitro-grown LHS21 or SS28 plants was treated with cold (4°C) or 100 mM BR for 0, 1, 3, 6, 12, 24 h and the expression of IbXTH16 was quantified with Real-time quantitative polymerase chain reaction (RT-qPCR) (SYBR Green Master Mix, YEASEN, Shanghai, China). Expression of IbXTH16 in leaf, root, and stem tissues four-week-old in vitro-grown LHS21 was quantified with RT-qPCR. The IbACTIN was used as the internal control.
2.5 Production of transgenic sweetpotato plants
The CDS of IbXTH16 (without the stop codon) was integrated into the pCAMBIA1300 vector according to Fan et al. (2024). The pCAMBIA1300-IbXTH16 was introduced into A. tumefaciens strain EHA105, and then infected Lizixiang embryogenic suspension cultures as described by Yu et al. (2007). The transgenic plants were identified with PCR (LA Taq, TaKaRa, Tokyo, Japan) and RT-qPCR.
2.6 Cold tolerance analysis
4-week-old IbXTH16-overexpressing sweetpotato plants and wide type (WT) with the same status were subjected to cold treatment after a week of acclimatization. The cold treated leaves of the IbXTH16-overexpressing sweetpotato plants and WT were used to determine the superoxide dismutase (SOD) (SOD-1-W, Cominbio, Suzhou, China) and peroxidase (POD) activities (POD-1-Y, Cominbio), proline (PRO-1-Y, Cominbio) and malondialdehyde (MDA) contents (MDA-1-Y, Cominbio) according to manufacturer’s instructions. The BR content and relative electrical conductivity were determined by Norminkoda Biotechnology Co., Ltd (Wuhan, China). The expression of IbDWF4, IbDET2, IbBRI1, IbBES1, IbBEE3, IbBIN2, IbP5CR, IbP5CS, IbP5CDH, and IbPDH were quantified with RT-qPCR.
2.7 Statistical analysis
Data are analyzed using one-way ANOVA followed by post-hoc Tukey’s test or Student’s t-test at P < 0.05 or P < 0.01.
3 Results
3.1 Cloning and sequence analysis of IbXTH16 and its promoter
To identify potential regulators of cold resistance in sweetpotato, we cloned IbXTH16 gene from cold-tolerant variety LHS21. Its 879 bp CDS encoded a 292 aa protein with a molecular weight of 32.983 kDa and a pI of 8.47 (Supplementary Table S2). Phylogenetic analysis showed that IbXTH16 shared the closest relationship with AtXTH16 among Arabidopsis homologs (Figure 1A). The 2039 bp genomic sequence of IbXTH16 contained two exons and one intron, which was different from the three exons and two introns of AtXTH16 (Figure 1B). The 800 bp IbXTH16 promoter region contained a low-temperature response element LTR and an ABA-response element ABRE (Figure 1C). To examine the subcellular location of IbXTH16, the IbXTH16-GFP fusion protein was conducted by transiently expressing in N. benthamiana leaf epidermal cells. The results showed that IbXTH16-GFP was localized in the cell membrane (Figure 1D).
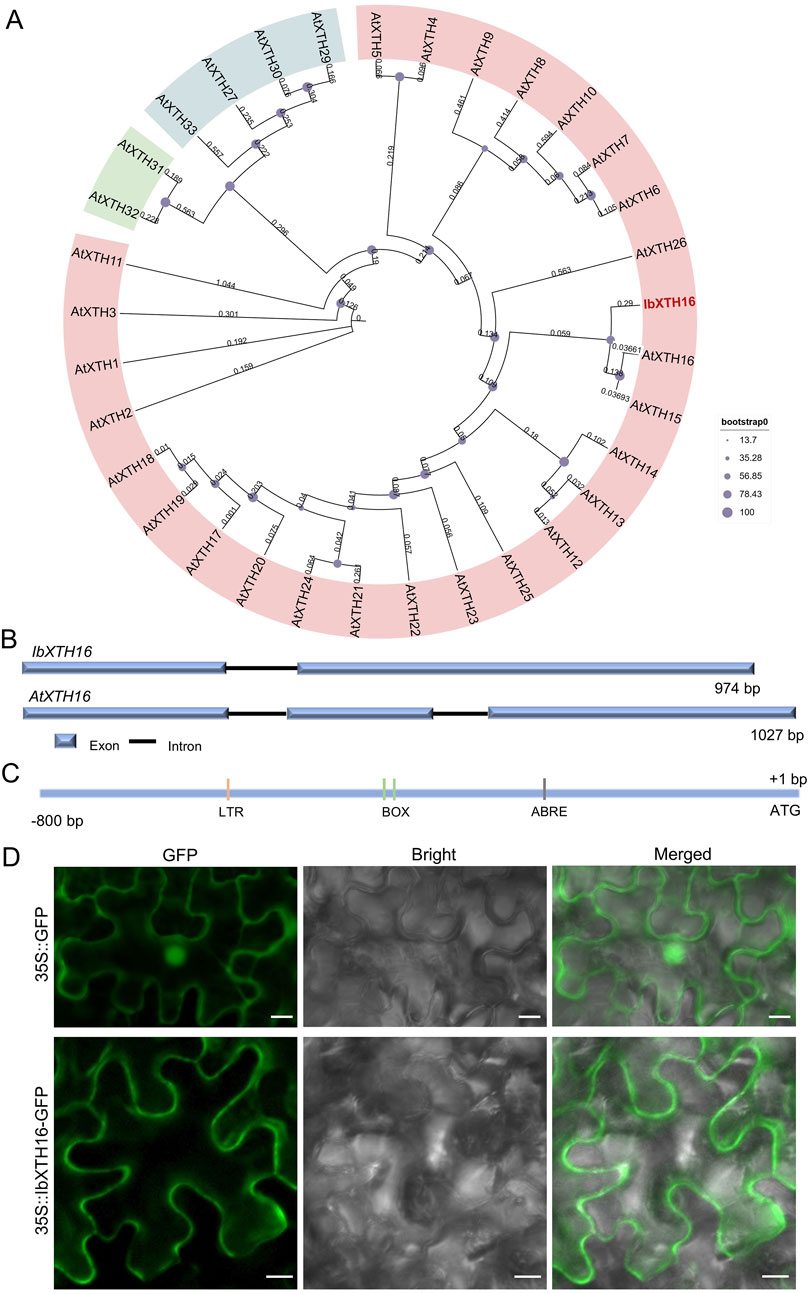
Figure 1. Sequence analysis and subcellular localization of IbXTH16. (A) Phylogenetic tree of IbXTH16 and XTH family of Arabidopsis. The IbXTH16 cloned in this study is marked in red. (B) Comparison of IbXTH16 and AtXTH16 genomic structures. (C) Diagrammatic representation of the IbXTH16 promoter. (D) Subcellular localization of IbXTH16 in N. benthamiana leaf hypodermal cells. Bars = 10 μm.
3.2 Expression analyses of IbXTH16 in sweetpotato
To study the potential role of IbXTH16 in cold resistance of sweetpotato, the expression level of IbXTH16 was analyzed. RT-qPCR assay showed that the expression level of IbXTH16 in LHS21 was much higher than that in SS28 (Figure 2A). Tissue-specific expression assay revealed that the expression level of IbXTH16 was relatively high in the roots of in vitro-grown LHS21 plants (Figure 2B). The expression of IbXTH16 was significantly induced by 4°C and 100 mM BR (Figures 2C,D).
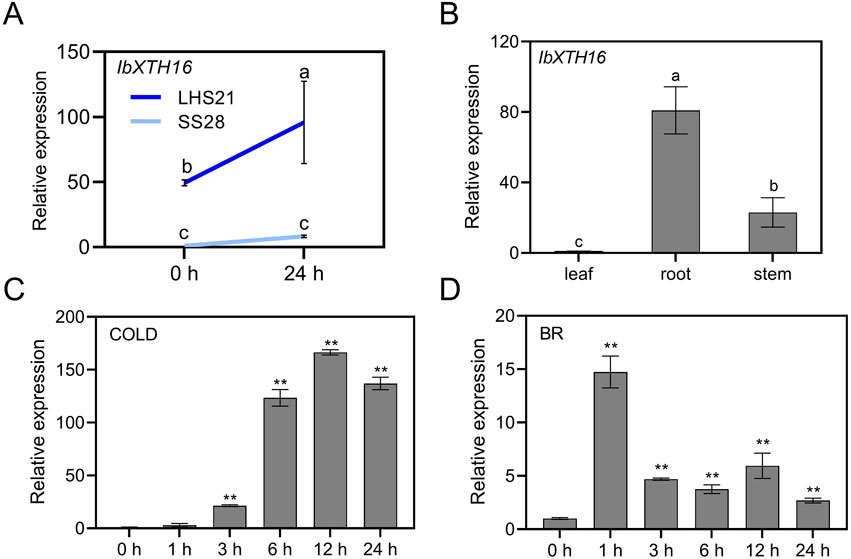
Figure 2. Expression analyses of IbXTH16 in sweetpotato. (A) Expression of IbXTH16 in cold-tolerant variety LHS21 and cold-susceptible variety SS28 under 4°C. (B) Expression of IbXTH16 in 4-week-old in vitro-grown LHS21. (C) Expression of IbXTH16 in cold-tolerant variety LHS21 after different time points (h) under 4°C. (D) Expression of IbXTH16 in cold-tolerant variety LHS21 after different time points (h) in response to 100 mM BR. Different lowercase letters indicate differences at P < 0.05 based on one-way ANOVA followed by post-hoc Tukey’s test. ** indicates a significant difference at P < 0.01 according to Student’s t-test.
3.3 Overexpression of IbXTH16 enhances cold tolerance in sweetpotato
To investigate whether IbXTH16 contributes to cold tolerance in sweetpotato, this gene was transferred into sweetpotato variety Lizixiang via A. tumefaciens-mediated method, and 12 IbXTH16-overexpressing lines (OX-1 to OX-12) were generated (Figures 3A–F). There was no significant difference in the phenotype of sweetpotato storage roots between overexpression lines and WT (Figure 3G). IbXTH16 exhibited increased expression level in the overexpression lines compared with the WT (Figure 3H). Three overexpression lines (OX-2, OX-7, and OX-12) with higher expression levels of IbXTH16 were selected for further study. Furthermore, the overexpression and WT plants were treatment at 4°C and restored at 25°C. The degree of wilting in the overexpression lines at 24 h and 48 h under cold stress was lower compared to that of WT (Figures 4A–C). Additionally, the overexpression lines recovered more rapidly than WT when returned to 25°C (Figure 4D). These results indicated that IbXTH16 functions as a positive regulator of cold tolerance in sweetpotato.
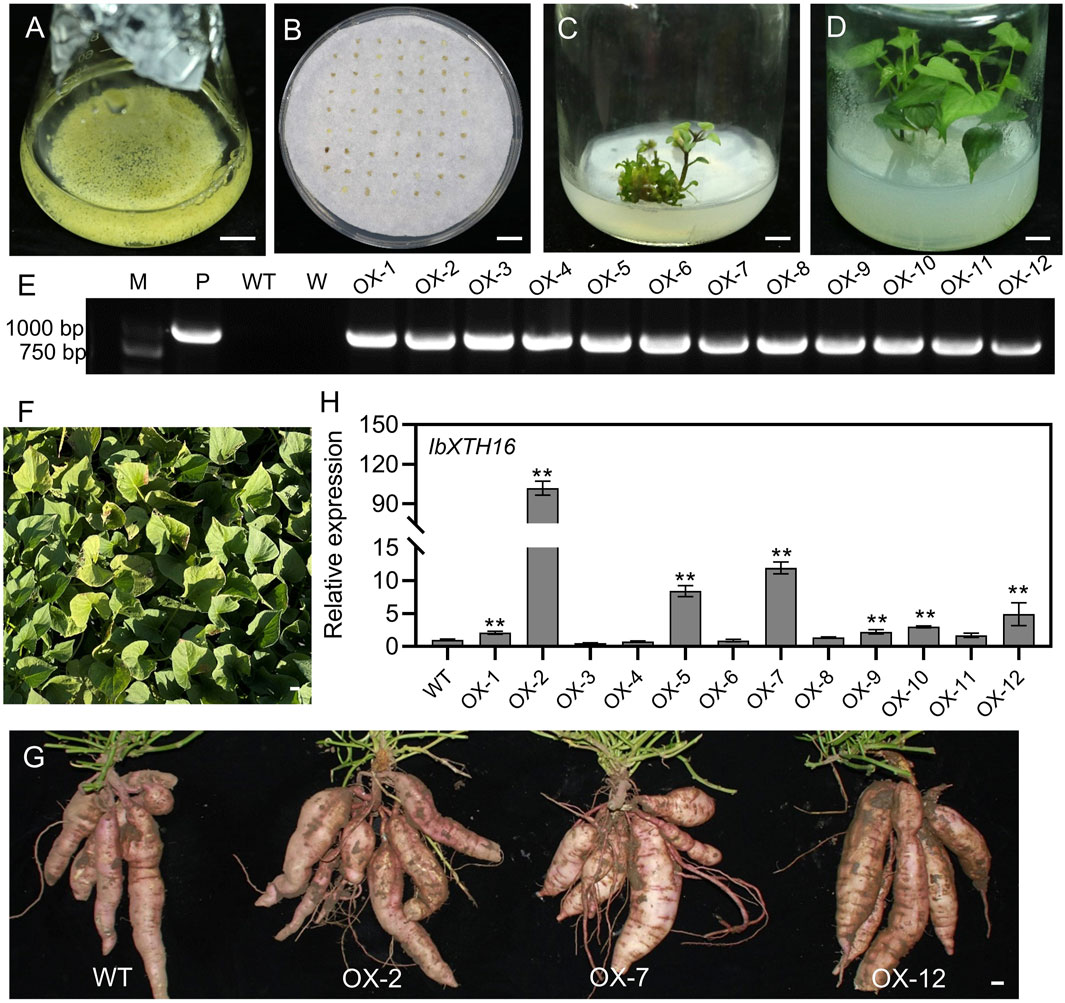
Figure 3. Production of the IbXTH16-overexpressing sweetpotato plants. (A) Lizixiang embryonic suspension cultures. (B) Screening of hygromycin-resistant embryogenic calli. (C) Regeneration of the IbXTH16-overexpressing plantlets. (D) Whole IbXTH16-overexpressing plants. (E) PCR identification of the IbXTH16-overexpressing plants. Lane M, DNA marker; Lane P, plasmid pCAMBIA1300-IbXTH16 (positive control); Lane WT, Lizixiang (negative control); Lane W, water (negative control); OX-1-OX12, IbXTH16-overexpressing plants. (F) IbXTH16-overexpressing plants grown in a field. (G) Storage roots from WT and IbXTH16-overexpressing plants. (H) Expression analysis of IbXTH16 in the overexpression plants by RT-qPCR. ** indicates a significant difference at P < 0.01 according to Student’s t-test. Bars = 1 cm.
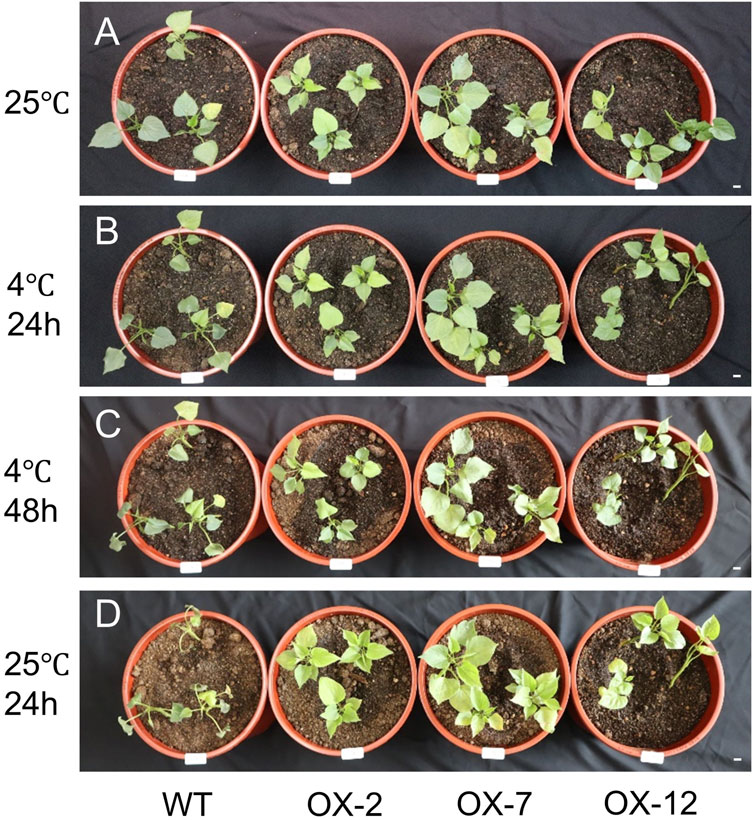
Figure 4. 4°C treatment assays of the WT and IbXTH16-overexpressing sweetpotato plants. (A) Phenotype before 4°C treatments. (B) Phenotype after treatment at 4°C for 24 h. (C) Phenotype after treatment at 4°C for 48 h. (D) Phenotype after recovery at 4°C for 24 h. Bars = 1 cm.
3.4 IbXTH16 alters the contents of components and expression of genes related to stress response
To further explore how IbXTH16 mediate the cold tolerance in sweetpotato, the contents of stress response-related components were measured. Under 4°C treatments for 24 h and 48 h, higher SOD and POD activities, higher proline and BR contents, and lower relative electrical conductivity and MDA content were found in the overexpression lines relative to WT (Figures 5A–F). In BR biosynthesis and signalling pathway, key enzyme genes IbDWF4 and IbDET2 and positive regulatory factors IbBRI1, IbBES1, and IbBEE3 in the transgenic plants were upregulated under 4°C treatment (Figures 6A–E), while negative regulatory factor IbBIN2 was downregulated (Figure 6F). In proline biosynthesis and signalling pathway, key enzyme genes IbP5CR and IbP5CS in the transgenic plants were upregulated under 4°C treatment (Figures 6G,H), while degradation pathway IbP5CDH and IbPDH were downregulated (Figures 6I,J). These results indicated that IbXTH16 positively regulates cold tolerance of sweetpotato by activating the BR and proline pathways.
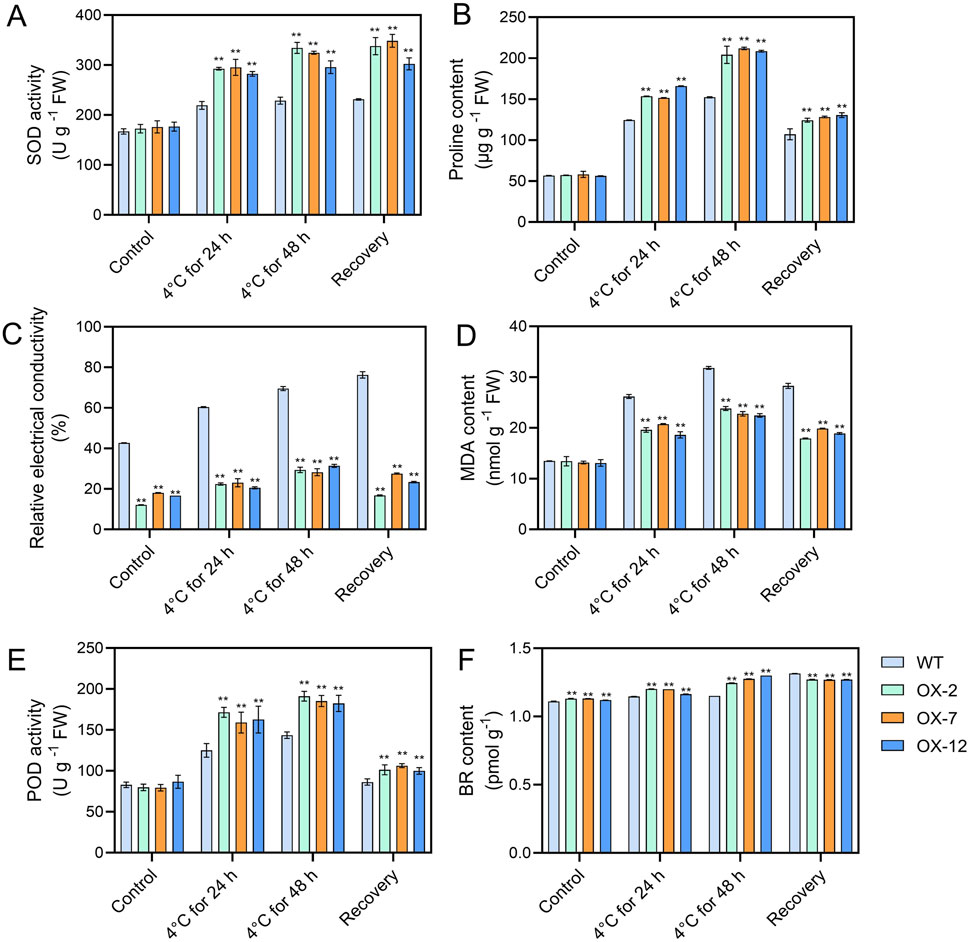
Figure 5. Analyses of components in the WT and IbXTH16-overexpressing sweetpotato plants. (A) SOD activity. (B) Proline content. (C) Relative electrical conductivity. (D) MDA content. (E) POD activity. (F) BR content. ** indicates a significant difference at P < 0.01 according to Student’s t-test.
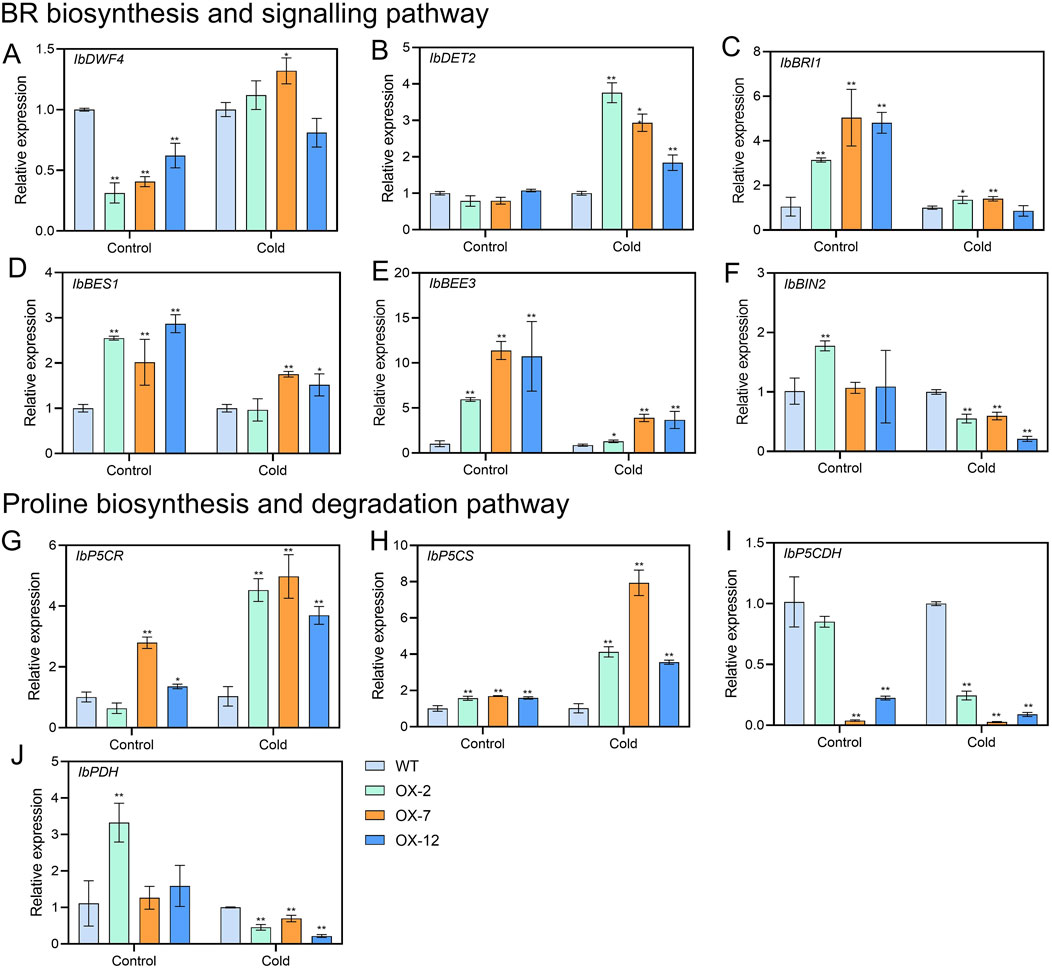
Figure 6. Expression analyses of BR and proline-related genes in the WT and IbXTH16-overexpressing sweetpotato plants under 4°C. (A) IbDWF4. (B) IbDET2. (C) IbBRI1. (D) IbBES1. (E) IbBEE3. (F) IbBIN2. (G) IbP5CR. (H) IbP5CS. (I) IbP5CDH. (J) IbPDH. * and ** indicate significant differences at P < 0.05 and P < 0.01 according to Student’s t-test.
4 Discussion
4.1 IbXTH16 positively regulates cold tolerance of sweetpotato
Many crops are well-suited for growth in tropical or subtropical regions (Chinnusamy et al., 2007). However, the average minimum temperature of most land areas on Earth is <0°C (Rihan et al., 2017). Low temperatures adversely affect crop growth and development, limiting their geographical distribution (Pearce, 2001; Ding et al., 2019). Sweetpotato is an important crop for ensuring national food security, but it is vulnerable to yield reductions caused by low-temperature damage (Jin et al., 2017; Yu et al., 2022). Genetic engineering has emerged as an effective strategy for enhancing sweetpotato’s tolerance to cold stress (Jin et al., 2017; 2021; 2022; Yu et al., 2022). Nevertheless, the function of XTH in cold stress of sweetpotato remains to be further studied. In this study, a novel IbXTH16 gene was cloned from the cold-tolerant variety LHS21 (Figure 1). The plant cell membrane serves as a crucial barrier for maintaining stable cellular metabolism and also plays a key role in sensing low temperatures (Zhang et al., 2019). After plants are exposed to low temperatures, the permeability and fluidity of their cell membranes decline, activating cold-response genes (Muratan, 1997). The localization of IbXTH16 in the cell membrane and the induction of IbXTH16 by cold suggest that IbXTH16 might serve as a signalling molecule in cold tolerance of sweetpotato (Figures 1D, 2C). The expression of IbXTH16 was induced by the BR (Figure 2D), and its overexpression enhanced cold tolerance in sweetpotato (Figure 4). Therefore, IbXTH16 is believed to be involved in the cold tolerance of sweetpotato.
4.2 IbXTH16 activates the biosynthesis of SOD and POD
Under low-temperature stress, plants accumulate excessive reactive oxygen species (ROS), which can be detrimental to plant cells (Guo et al., 2022; Mittler et al., 2022). The ROS scavenging system can detoxify ROS by enhancing the activity of ROS-scavenging enzymes, such as SOD and POD, preventing oxidative damage to plant cells (Gill and Tuteja, 2010; Bose et al., 2014; Choudhury et al., 2017). In Zoysia japonica, overexpression of ZjICE1 conferred cold tolerance in transgenic plants by increasing SOD, POD, and proline contents, as well as decreasing MDA content (Zuo et al., 2019). In Betula platyphylla, overexpression of BpERF13 improved the cold tolerance of transgenic plants by binding to cis-elements of SOD and POD and increasing SOD and POD contents (Lv et al., 2020). In this study, the SOD and POD contents were significantly increased in the transgenic plants under 4°C (Figures 5A,E). It is suggested that overexpression of IbXTH16 enhances cold tolerance by activating the biosynthesis of SOD and POD in transgenic sweetpotato. Previous reports have indicated that the plant hormone ABA is also involved in the cold tolerance of plants (Huang et al., 2017;Ma et al., 2018; Li et al., 2021). The IbXTH16 promoter region contained an ABA-response element ABRE (Figure 1C). However, whether the XTH16 gene regulates cold tolerance in sweetpotato via the ABA pathway requires further investigation.
4.3 IbXTH16 positively regulates BR signalling pathway and proline accumulation
BR signalling not only participates in plant growth and development, but also has been reported in plant resistance to low temperature (Kinoshita et al., 2005; Vriet et al., 2012). In the initial phase when plants are exposed to cold stress, the activity of BIN2 kinase is suppressed, while OST1 kinase is activated, this synergistic regulation stabilizes ICE1, thereby enhancing the expression of CBF and improving the cold tolerance of plants (Barrero-Gil and Salinas, 2018; Ye et al., 2019). In Arabidopsis, compared with the WT, the cold resistance of overexpressing BRI1 plants was enhanced, while that of mutant BRI1 plants was decreased (Eremina et al., 2016). In this study, the transgenic plants showed a significant increase in BR content, which might be due to the overexpression of IbXTH16 increasing the BR biosynthesis of transgenic plants, thus conferring cold tolerance (Figure 5F). Interestingly, the BR content in the transgenic plants was significantly decreased after restoring the room temperature (Figure 5F). The BR biosynthetic pathway involves the participation of a series of genes (Zhao and Li, 2012). In this study, the expression levels of BR biosynthesis and signalling pathway-related positive regulatory factors were significantly upregulated, while negative regulatory factor was significantly downregulated (Figures 6A–F). More proline accumulation can protect plants from low-temperature stress and ROS damage (Ghosh et al., 2022; Kidokoro et al., 2022). In this study, the proline biosynthesis-related genes were significantly upregulated, while degradation pathway-related genes were significantly downregulated (Figures 6G–J). Collectively, these findings suggest that IbXTH16 positively regulates cold tolerance of sweetpotato by activiting BR signalling pathway and proline accumulation (Figure 7).
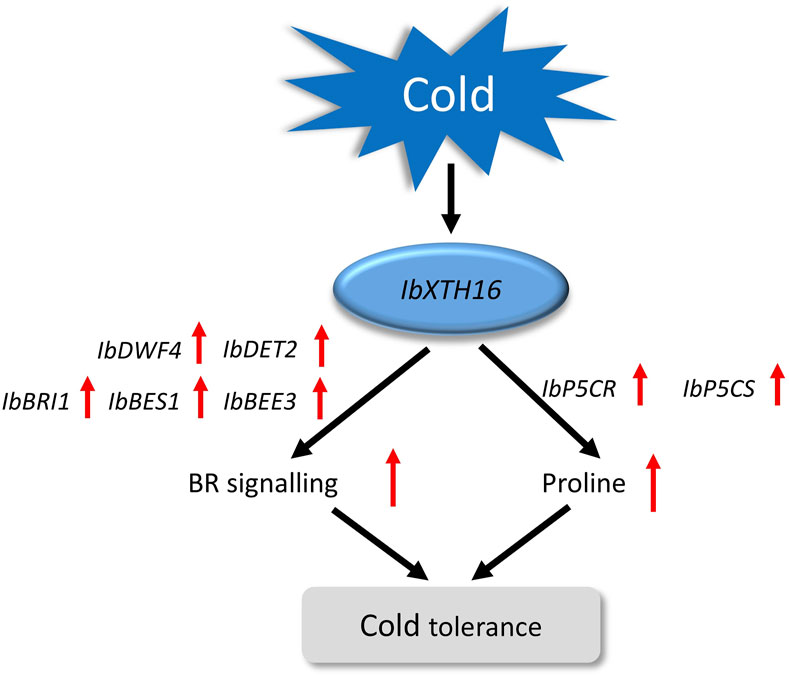
Figure 7. Proposed regulatory model of IbXTH16 in the transgenic sweetpotato plants response to cold stress.
5 Conclusion
Overexpression of the cloned IbXTH16 gene increased cold tolerance of sweetpotato by activating the BR and proline pathways. This study for the first time sheds light on the important role of IbXTH16 in cold tolerance. IbXTH16 has the potential to increase cold tolerance in sweetpotato and other plants.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
TY: Formal Analysis, Funding acquisition, Investigation, Writing – original draft, Writing – review and editing. JP: Data curation, Investigation, Writing – review and editing. SL: Investigation, Writing – review and editing. ZY: Investigation, Writing – review and editing. ZL: Conceptualization, Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by grants from Potato International Joint Laboratory (2025LHSYS05) and Independent Research Project of Liaoning Academy of Agricultural Sciences (2025XKJBS8507).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgene.2025.1629260/full#supplementary-material
References
Barrero-Gil, J., and Salinas, J. (2018). Gene regulatory networks mediating cold acclimation: the CBF pathway. Adv. Exp. Med. Biol. 1081, 3–22. doi:10.1007/978-981-13-1244-1_1
Bose, J., Rodrigo-Moreno, A., and Shabala, S. (2014). ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 65, 1241–1257. doi:10.1093/jxb/ert430
Chinnusamy, V., Zhu, J., and Zhu, J. K. (2007). Cold stress regulation of gene expression in plants. Trends Plant Sci. 12, 444–451. doi:10.1016/j.tplants.2007.07.002
Choudhury, F. K., Rivero, R. M., Blumwald, E., and Mittler, R. (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J. 90, 856–867. doi:10.1111/tpj.13299
Ding, Y. L., Shi, Y. T., and Yang, S. H. (2019). Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 222, 1690–1704. doi:10.1111/nph.15696
Eremina, M., Unterholzner, S. J., Rathnayake, A. I., Castellanos, M., Khan, M., Kugler, K. G., et al. (2016). Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc. Natl. Acad. Sci. U. S. A. 113, E5982–E5991. doi:10.1073/pnas.1611477113
Fan, Y., Chen, T., Xue, L., Zhang, H., Gao, S., Zhao, N., et al. (2024). A glutathione S-transferase IbGSTL2 interacts with IbcPGM to increase starch content and improve starch quality in sweetpotato. Crop J. 12, 1666–1676. doi:10.1016/j.cj.2024.08.007
Ghosh, U. K., Islam, M. N., Siddiqui, M. N., Cao, X., and Khan, M. A. R. (2022). Proline, a multifaceted signalling molecule in plant responses to abiotic stress: understanding the physiological mechanisms. Plant Biol. 24, 227–239. doi:10.1111/plb.13363
Gill, S. S., and Tuteja, N. (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. biochem. 48, 909–930. doi:10.1016/j.plaphy.2010.08.016
Guo, Z., Cai, L., Liu, C., Chen, Z., Guan, S., Ma, W., et al. (2022). Low-temperature stress affects reactive oxygen species, osmotic adjustment substances, and antioxidants in rice (Oryza sativa L.) at the reproductive stage. Sci. Rep. 12, 6224. doi:10.1038/s41598-022-10420-8
Han, J., Liu, Y., Shen, Y., and Li, W. (2023). A surprising diversity of xyloglucan endotransglucosylase/hydrolase in wheat: new in sight to the roles in drought tolerance. Int. J. Mol. Sci. 24, 9886. doi:10.3390/ijms24129886
Hayat, S., Hayat, Q., Alyemeni, M. N., Wani, A. S., Pichtel, J., and Ahmad, A. (2012). Role of proline under changing environments: a review. Plant Signal. Behav. 7, 1456–1466. doi:10.4161/psb.21949
Huang, X., Shi, H., Hu, Z., Liu, A., Amombo, E., Chen, L., et al. (2017). ABA is involved in regulation of cold stress response in Bermudagrass. Front. Plant Sci. 8, 1613. doi:10.3389/fpls.2017.01613
Jin, R., Kim, B. H., Ji, C. Y., Kim, H. S., Li, H. M., Ma, D. F., et al. (2017). Overexpressing IbCBF3 increases low temperature and drought stress tolerance in transgenic sweetpotato. Plant Physiol. biochem. 118, 45–54. doi:10.1016/j.plaphy.2017.06.002
Jin, R., Kim, H. S., Yu, T., Liu, M., Yu, W., Zhao, P., et al. (2022). Overexpression of IbMPK3 increases low-temperature tolerance in transgenic sweetpotato. Plant Biotechnol. Rep. 16, 91–100. doi:10.1007/s11816-021-00730-0
Jin, R., Kim, H. S., Yu, T., Zhang, A., Yang, Y., Liu, M., et al. (2021). Identification and function analysis of bHLH genes in response to cold stress in sweetpotato. Plant Physiol. biochem. 169, 224–235. doi:10.1016/j.plaphy.2021.11.027
Kaplan, F., Kopka, J., Sung, D. Y., Zhao, W., Popp, M., Porat, R., et al. (2007). Transcript and metabolite profiling during cold acclimation of Arabidopsis reveals an intricate relationship of cold-regulated gene expression with modifications in metabolite content. Plant J. 50, 967–981. doi:10.1111/j.1365-313X.2007.03100.x
Kidokoro, S., Shinozaki, K., and Yamaguchi-Shinozaki, K. (2022). Transcriptional regulatory network of plant cold-stress responses. Trends Plant Sci. 27, 922–935. doi:10.1016/j.tplants.2022.01.008
Kim, E., and Russinova, E. (2020). Brassinosteroid signalling. Curr. Biol. 30, 294–298. doi:10.1016/j.cub.2020.02.011
Kim, J. S., Kidokoro, S., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2024). Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 195, 170–189. doi:10.1093/plphys/kiae105
Kinoshita, T., Caño-Delgado, A., Seto, H., Hiranuma, S., Fujioka, S., Yoshida, S., et al. (2005). Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433, 167–171. doi:10.1038/nature03227
Li, B., Zhang, C., Cao, B., Qin, G., Wang, W., and Tian, S. (2012). Brassinolide enhances cold stress tolerance of fruit by regulating plasma membrane proteins and lipids. Amino Acids 43, 2469–2480. doi:10.1007/s00726-012-1327-6
Li, M., Wang, C., Shi, J., Zhang, Y., Liu, T., and Qi, H. (2021). Abscisic acid and putrescine synergistically regulate the cold tolerance of melon seedlings. Plant Physiol. biochem. 166, 1054–1064. doi:10.1016/j.plaphy.2021.07.011
Liu, L., Duan, L., Zhang, J., Zhang, Z., Mi, G., and Ren, H. (2010). Cucumber (Cucumis sativus L.) over-expressing cold-induced transcriptome regulator ICE1 exhibits changed morphological characters and enhances chilling tolerance. Sci. Hortic. 124, 29–33. doi:10.1016/j.scienta.2009.11.018
Luo, P., Chen, L. M., Chen, Y. N., Shen, Y. X., and Cui, Y. Y. (2022). RmZAT10, a novel Cys2/His2 zinc finger transcription factor of Rosa multiflora, functions in cold tolerance through modulation of proline biosynthesis and ROS homeostasis. Environ. Exp. Bot. 198, 104845. doi:10.1016/j.envexpbot.2022.104845
Lv, K., Li, J., Zhao, K., Chen, S., Nie, J., Zhang, W., et al. (2020). Overexpression of an AP2/ERF family gene, BpERF13, in birch enhances cold tolerance through upregulating CBF genes and mitigating reactive oxygen species. Plant Sci. 292, 110375. doi:10.1016/j.plantsci.2019.110375
Ma, H., Liu, C., Li, Z., Ran, Q., Xie, G., Wang, B., et al. (2018). ZmbZIP4 contributes to stress resistance in maize by regulating ABA synthesis and root development. Plant Physiol. 178, 753–770. doi:10.1104/pp.18.00436
Miedes, E., Herbers, K., Sonnewald, U., and Lorences, E. P. (2010). Overexpression of a cell wall enzyme reduces xyloglucan depolymerization and softening of transgenic tomato fruits. J. Agric. Food Chem. 12, 5708–5713. doi:10.1021/jf100242z
Mittler, R., Zandalinas, S. I., Fichman, Y., and Van Breusegem, F. (2022). Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 23, 663–679. doi:10.1038/s41580-022-00499-2
Morales-Quintana, L., Beltrán, D., Mendez-Yañez, Á., Valenzuela-Riffo, F., Herrera, R., and Moya-León, M. A. (2020). Characterization of FcXTH2, a novel xyloglucan endotransglycosylase/hydrolase enzyme of chilean strawberry with hydrolase activity. Int. J. Mol. Sci. 21, 3380. doi:10.3390/ijms21093380
Muratan, L., and Los, D. A. (1997). Membrane fluidity and temperature perception. Plant Physiol. 115, 875–879. doi:10.1104/pp.115.3.875
Peng, T., Zhu, X., Duan, N., and Liu, J. H. (2014). PtrBAM1, a β-amylase-coding gene of Poncirus trifoliata, is a CBF regulon member with function in cold tolerance by modulating soluble sugar levels. Plant Cell Environ. 37, 2754–2767. doi:10.1111/pce.12384
Rihan, H. Z., Al-Issawi, M., and Fuller, M. P. (2017). Advances in physiological and molecular aspects of plant cold tolerance. J. Plant Interact. 12, 143–157. doi:10.1080/17429145.2017.1308568
Rose, J., Braam, J., Fry, S., and Nishitani, K. (2002). The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: current perspectives and a new unifying nomenclature. Plant Cell Physiol. 43, 1421–1435. doi:10.1093/pcp/pcf171
Shi, H., Ye, T., Zhong, B., Liu, X., Jin, R., and Chan, Z. (2014). AtHAP5A modulates freezing stress resistance in Arabidopsis through binding to CCAAT motif of AtXTH21. New Phytol. 203, 554–567. doi:10.1111/nph.12812
Shi, Y., Ding, Y., and Yang, S. (2018). Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 23, 623–637. doi:10.1016/j.tplants.2018.04.002
Takagi, H. (2008). Proline as a stress protectant in yeast: physiological functions, metabolic regulations, and biotechnological applications. Appl. Microbiol. Biotechnol. 81, 211–223. doi:10.1007/s00253-008-1698-5
Takahashi, D., Johnson, K., Hao, P., Tuong, T., Erban, A., Sampathkumar, A., et al. (2021). Cell wall modification by the xyloglucan endotransglucosylase/hydrolase XTH19 influences freezing tolerance after cold and sub-zero acclimation. Plant Cell Environ. 44, 915–930. doi:10.1111/pce.13953
Vriet, C., Russinova, E., and Reuzeau, C. (2012). Boosting crop yields with plant steroids. Plant Cell 24, 842–857. doi:10.1105/tpc.111.094912
Wang, D., Yang, Z., Wu, M., Wang, W., Wang, Y., and Nie, S. (2022). Enhanced brassinosteroid signaling via the overexpression of SlBRI1 positively regulates the chilling stress tolerance of tomato. Plant Sci. 320, 111281. doi:10.1016/j.plantsci.2022.111281
Wang, S., Zhao, H., Zhao, L., Gu, C., Na, Y., Xie, B., et al. (2020). Application of brassinolide alleviates cold stress at the booting stage of rice. J. Integr. Agric. 19, 975–987. doi:10.1016/S2095-3119(19)62639-0
Wu, J., Zong, Y., Tu, Z., Yang, L., Li, W., Cui, Z., et al. (2022). Genome-wide identification of XTH genes in Liriodendron chinense and functional characterization of LcXTH21. Front. Plant Sci. 13, 1014339. doi:10.3389/fpls.2022.1014339
Xu, P., Fang, S., Chen, H., and Cai, W. (2020). The brassinosteroid-responsive xyloglucan endotransglucosylase/hydrolase 19 (XTH19) and XTH23 genes are involved in lateral root development under salt stress in Arabidopsis. Plant J. 104, 59–75. doi:10.1111/tpj.14905
Ye, K., Li, H., Ding, Y., Shi, Y., Song, C., Gong, Z., et al. (2019). BRASSINOSTEROID-INSENSITIVE2 negatively regulates the stability of transcription factor ICE1 in response to cold stress in Arabidopsis. Plant Cell 31, 2682–2696. doi:10.1105/tpc.19.00058
Yu, B., Zhai, H., Wang, Y. P., Zang, N., He, S. Z., and Liu, Q. C. (2007). Efficient Agrobacterium tumefaciens-mediated transformation using embryogenic suspension cultures in sweetpotato, Ipomoea batatas (L.) Lam. Plant Cell Tiss. Org. 90, 265–273. doi:10.1007/s11240-007-9265-9
Yu, T., Zhou, H. A., Liu, Z. L., Zhai, H., and Liu, Q. C. (2022). The sweet potato transcription factor IbbHLH33 enhances chilling tolerance in transgenic tobacco. Czech J. Genet. Plant Breed. 58, 210–222. doi:10.17221/115/2021-CJGPB
Zhang, H., Dong, J., Zhao, X., Zhang, Y., Ren, J., Xing, L., et al. (2019). Research progress in membrane lipid metabolism and molecular mechanism in Peanut cold tolerance. Front. Plant Sci. 10, 838. doi:10.3389/fpls.2019.00838
Zhang, H., Zhang, Q., Zhai, H., Gao, S., Yang, L., Wang, Z., et al. (2020). IbBBX24 promotes the jasmonic acid pathway and enhances fusarium wilt resistance in sweet potato. Plant Cell 32, 1102–1123. doi:10.1105/tpc.19.00641
Zhang, J. Z., He, P. W., Xu, X. M., Lü, Z. F., Cui, P., George, M. S., et al. (2023). Genome-wide identification and expression analysis of the xyloglucan endotransglucosylase/hydrolase gene family in sweet potato [Ipomoea batatas (L.) Lam]. Int. J. Mol. Sci. 24, 775. doi:10.3390/ijms24010775
Zhao, B., and Li, J. (2012). Regulation of brassinosteroid biosynthesis and inactivation. J. Integr. Plant Biol. 54, 746–759. doi:10.1111/j.1744-7909.2012.01168.x
Keywords: sweetpotato, cold tolerance, IbXTH16, BR, proline
Citation: Yu T, Pan J, Liu S, Yang Z and Liu Z (2025) A xyloglucan endotransglucosylase/hydrolase gene, IbXTH16, increases cold tolerance in transgenic sweetpotato. Front. Genet. 16:1629260. doi: 10.3389/fgene.2025.1629260
Received: 15 May 2025; Accepted: 09 June 2025;
Published: 18 June 2025.
Edited by:
Lianjun Wang, Hubei Academy of Agricultural Sciences, ChinaReviewed by:
Rong Jin, Chinese Academy of Agricultural Sciences, ChinaSun Houjun, Xuzhou Institute of Architectural Technology, China
Copyright © 2025 Yu, Pan, Liu, Yang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenlei Liu, bGl1emhlbmxlaUBjYXUuZWR1LmNu
 Tao Yu
Tao Yu Jiaquan Pan
Jiaquan Pan Zitong Yang
Zitong Yang