- 1Hospital for Skin Disease (Institute of Dermatology), Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing, China
- 2Sino-French Hoffmann Institute, School of Basic Medicine, The Second Affiliated Hospital, Guangzhou Medical University, Guangzhou, China
- 3The State Key Laboratory of Respiratory Disease, The First Affiliated Hospital, Guangzhou Medical University, Guangzhou, China
Psoriasis is a chronic autoimmune inflammatory disease that remains active for a long period, even for life in most patients. The impact of psoriasis on health is not only limited to the skin, but also influences multiple systems of the body, even mental health. With the increasing of literature on the association between psoriasis and extracutaneous systems, a better understanding of psoriasis as an autoimmune disease with systemic inflammation is created. Except for cardiometabolic diseases, gastrointestinal diseases, chronic kidney diseases, malignancy, and infections that have received much attention, the association between psoriasis and more systemic diseases, including the skin system, reproductive system, and oral and ocular systems has also been revealed, and mental health diseases draw more attention not just because of the negative mental and mood influence caused by skin lesions, but a common immune-inflammatory mechanism identified of the two systemic diseases. This review summarizes the epidemiological evidence supporting the association between psoriasis and important and/or newly reported systemic diseases in the past 5 years, and may help to comprehensively recognize the comorbidity burden related to psoriasis, further to improve the management of people with psoriasis.
Introduction
Psoriasis is a common chronic inflammatory disease with prevalence of 0.33%-0.6% in different races (1, 2), and affects around 125 million people worldwide. With the understanding of the biological nature of psoriasis, it has been recognized as an autoimmune disease with significant impacts on health implications extending beyond the skin (3).
The first extracutaneous comorbid disease of psoriasis was diabetes, which was reported in 1897 (4), and research on the association between psoriasis and systemic comorbidities has rapidly grown in past decades, mainly focusing on cardiometabolic diseases, chronic kidney disease (CKD), gastrointestinal diseases, malignancy, mood disorders, infection, and psoriatic arthritis (PsA), which was summarized in a comprehensive review published in 2017 (5). In the past 5 years, increasing epidemiologic evidence supports that more systemic diseases are identified as extracutaneous comorbidities of psoriasis. In this review, we summarized these data to improve comprehensive understanding of the burden of comorbid diseases associated with psoriasis, which may be essential for future medical management for patients with psoriasis.
Materials and Methods
A search was performed on PubMed using the following search strategy: psoriasis AND ((comorbid disease) OR comorbidity) AND (epidemiology OR incidence) between 2016 and 2021. In total, 1874 papers were collected, 1160 of them were removed because of irrelevance and 489 were removed because of non-original article type; finally 216 original articles published were included in the current review.
Cardiovascular Diseases
The incidence of overall atherosclerotic cardiovascular disease is higher in psoriasis compared with controls (2), and the prevalence of atherosclerotic cardiovascular disease varies in different races, as it is 2.4-fold higher in African American than in white patients with psoriasis (1). Psoriasis is independently associated with myocardial infarction along with hypertension, dyslipidemia, and diabetes mellitus (6), in both sexes in adults (2, 6), whereas the risk for ischemic stroke is increased in women with moderate to severe psoriasis (2).
Many studies on the association between cardiovascular diseases and psoriasis have been concluded in at least one meta-analysis (Table 1) (7–13), including coronary artery disease (CAD) (such as stroke, myocardial infarction, and cardiovascular death), atrial fibrillation, and aortic aneurysm. Two meta-analyses specially investigated the risk of cardiovascular diseases according to the disease severity (7, 11). Two systematic reviews checked the associations between psoriasis and cardiovascular comorbidities in pediatric populations, one confirmed the existence of the association (12), however the other one found a null association (13).
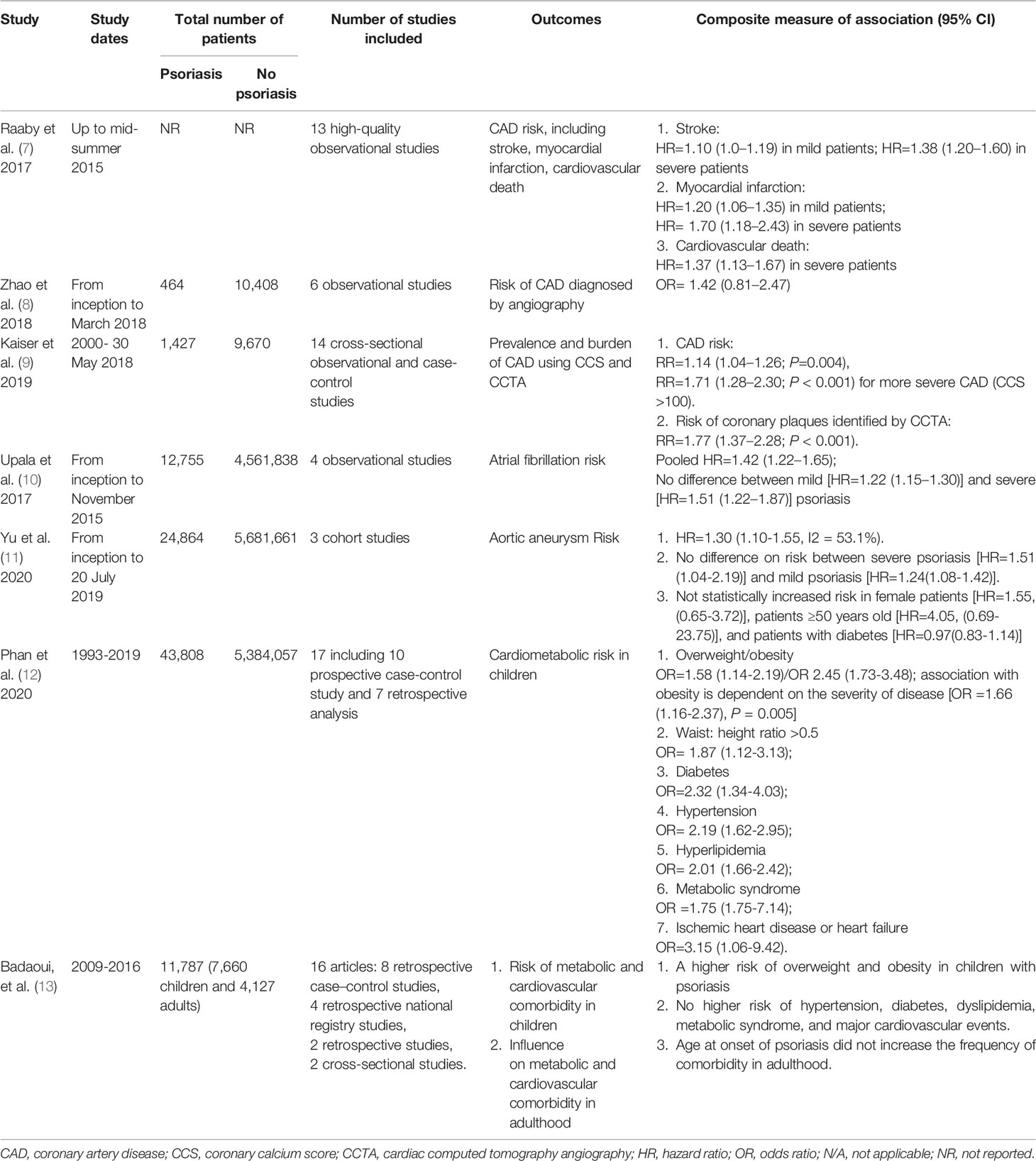
Table 1 Summary of systematic review and meta-analyses assessing the association between psoriasis and cardiovascular diseases.
Psoriasis with chronic inflammation promotes development of pulmonary embolism, and is related to a cardiovascular and venous thromboembolism risk, but lower in-hospital mortality (14). Independent predictors for venous thromboembolism in psoriasis patients included older age, diabetes mellitus, and corticosteroid usage (15). Psoriasis was an independent predictor for gastro-intestinal bleeding (14). A cohort study in Sweden suggested that low cardiorespiratory fitness at an early age was associated with an increased risk of psoriasis and PsA in men (16).
Several clinical and biological variables including anti-HDL antibodies (17), coronary flow reserve (18), lymphocyte ratio (19), total plaque area, and carotid intima-media thickness (20) have been used for evaluation or prediction of CAD in psoriasis. Inflammation (high-sensitivity C-reactive protein) played an important role in the development of visceral adipose tissue and its effect on early atherogenesis (21). Noncalcified coronary burden risk was associated with high-sensitivity C-reactive protein (21) and serum high-sensitivity troponin-T (22). Machine learning methods were successfully applied to identify noncalcified coronary burden predictors in psoriasis patients by coronary computed tomography angiography (23). Inflammasome signaling is correlated with severity of psoriasis disease, proinflammatory endothelial transcripts, and circulating interleukin (IL)-6 (24). A systematic review and meta-analysis including 24 studies found that psoriasis patients had a higher homocysteine level [standardized mean differences (SMD)=0.41, 95% CI:0.21-0.61] and a lower folate level (SMD=-0.94, 95% CI: -1.49 to -0.40) in serum compared with controls (25). These above findings provide a better evaluation on the heightened risk of cardiovascular disease in patients with psoriasis.
Treatment modalities on psoriasis may affect cardiovascular comorbidities of psoriasis. A systematical review including 14 studies confirmed that weight loss can improve the psoriasis area and severity index (PASI) score of patients, and prevent the onset of psoriasis in obese individuals (26). Adalimumab showed anti-inflammatory effects and improved flow-mediated dilation, and fumaric acid esters interacted favorably with the cholesterol metabolism (27). Cyclosporine and mixed conventional systemic treatments were associated with increased major adverse cardiovascular events risk, and the cumulative of major adverse cardiovascular events incidence in the phototherapy and biologic was lower, while methotrexate was not associated with major adverse cardiovascular events (28).
Metabolic Diseases
Metabolic diseases are usually considered as high-risk factors for cardiovascular diseases, and the studies focusing on the association between psoriasis and cardiovascular risk factors are increasing. Most studies have been summarized in at least one meta-analysis (Table 2), and cardiovascular risk factors include obesity measured by body mass index (BMI), waist circumference (WC), waist-to-hip ratio, weight (29), type 2 diabetes (30), and metabolic syndrome (MetS) of adults (31) and children (32).
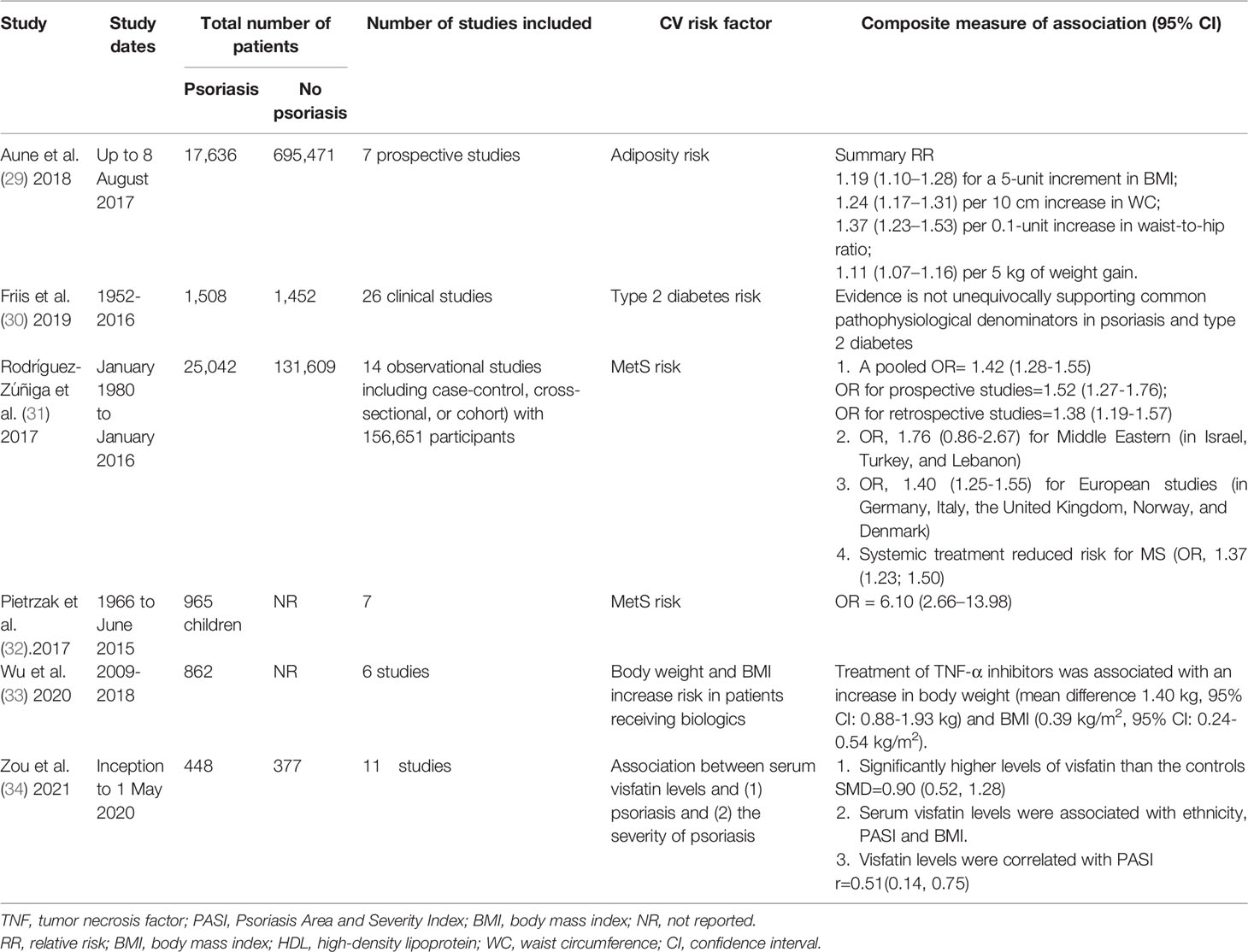
Table 2 Summary of systematic review and meta-analyses assessing the association between psoriasis and metabolic diseases-cardiovascular disease risk factors.
Obesity/Overweight
A nationwide prospective cohort study in Korea found that metabolically unhealthy non-obese subjects and metabolically unhealthy obese subjects had a higher risk of psoriasis compared to metabolically healthy non-obese subjects (35).
A meta-analysis including seven prospective studies concluded that adiposity as measured by BMI, WC, waist-to-hip ratio, and weight gain was associated with increased risk of psoriasis (29). WC measuring central adiposity is a specific factor affecting psoriatic risk (36), after adjustment for confounders and BMI (37). Two Mendelian randomization analyses using genetic variants as instrumental variables confirmed that higher BMI causally increased the risk of psoriasis (38, 39).
A systematic review and network meta-analysis including six studies concluded that compared with conventional systemic treatments, tumor necrosis factor (TNF) α inhibitors were associated with a significant increase in body weight, while anti-IL-12/23 or anti-IL-17 biologics had no effect on an increase of body weight or BMI (33).
Metabolic Syndrome
The prevalence of MetS in patients with psoriasis ranges from 14.3% to 50% (31, 40, 41). The strength of these associations between psoriasis and MetS has been repeatedly confirmed by several observational studies (40, 42–44), and psoriatic patients had at least a double risk of MetS compared with non psoriatic individuals (41). The psoriasis risk tended to increase with the number increase of MetS components, and this trend was evident in obese subjects (44). A systematic review and meta-analysis of 14 observational studies found that a greater risk for MetS was reported in Middle Eastern than European patients (31). However, in Thailand, no significant association was found between MetS and psoriasis severity using PASI (45).
Significant differences were found in relation to the prevalence of cardiovascular risk factors, MetS, and major cardiovascular events in patients with psoriasis compared to non-psoriatic population, however, differences were not seen among psoriasis severity groups (43). After adjusting for all other MetS factors, WC and blood pressure remained significantly associated with noncalcified coronary burden (46).
Women with MetS have a higher chance of being psoriatic (47). Prevalence of MetS (32) and insulin resistance (48) was significantly higher in children with psoriasis. In most studies included in a meta-analysis conducted by Pietrzak et al. (32), a significantly decrease of high-density lipoprotein cholesterol level was found in children with psoriasis.
The increase in expression of surviving and heat shock protein 27, heat shock protein 60, and heat shock protein 90, and the decrease in cyclin D1 expression may be an important molecular mechanism involved in the development of MetS in psoriasis (49). Selenoprotein P was increased in psoriasis and significantly decreased after psoriatic treatment (50).
IL-17A monoclonal antibody treatment cannot only ameliorate psoriatic lesions but also restore dysregulation of lipid metabolism in psoriasis patients (51). MetS negatively affects psoriasis severity and treatment outcomes (45).
Type 2 Diabetes
A systematic review including 26 clinical studies concluded that the available literature does not unequivocally support the association between psoriasis and type 2 diabetes because the low quality of studies included (30). However psoriasis was reported to be associated with type 2 diabetes, independent of visceral fat (52), and psoriasis severity is an independent risk factor of the homeostatic model assessment of insulin resistance (53). Lee et al. (54) firstly confirmed the association between psoriasis and diabetic complications including diabetic retinopathy and end-stage renal disease (ESRD). The effect of age seemed to differ between psoriasis sexes, men from 35 to 49 years old were at a lower risk of type 2 diabetes compared to their peers of 75 years of age and older, whereas women of 50 to 64 years old were at an increased risk of type 2 diabetes (55).
Lipid Metabolism
Psoriasis was significantly associated with hypercholesterolemia, hospital-diagnosed hypertension (56), and hyperlipidemia (57). The accumulating evidence of the nature of psoriasis and the risk of cardiovascular comorbidities is potentially due to an acquired hypercoagulability (58). Psoriasis significantly increased the risk of CKD, and so did hyperlipidemia. Statin treatment for hyperlipidemia reduced the CKD risk in psoriasis patients compared to treatment without statins (57).
A meta-analysis including 11 studies demonstrated that psoriasis patients had higher levels of visfatin (SMD, 0.90; 95% CI, 0.52-1.28), which was associated with ethnicity, PASI, and BMI (34). Visceral fat was associated with psoriasis, hyper-triglyceridemia, low high-density lipoprotein, and type 2 diabetes, and these associations were linked to serum IL-6, adiponectin, tumor necrosis factor, and insulin resistance (52).
Autoimmune Thyroid Disease
A meta-analysis suggests that thyroid peroxidase antibody positivity (pooled OR, 1.71, 95% CI: 1.27-2.31), hypothyroidism, and hyperthyroidism (pooled OR, 1.17, 95% CI: 1.03-1.32) might be associated with prevalent psoriatic disease (59). Patients with thyroid dysfunction have significantly higher PASI scores and elevated serum C-reactive protein levels than those without thyroid dysfunction (60). In Taiwan, the psoriasis group had an increased risk for incident hyperthyroidism, Graves’ disease, hypothyroidism, and Hashimoto thyroiditis compared with controls (61), and a high prevalence of Hashimoto’s thyroiditis is especially observed in women with psoriasis (62).
Gout
A nationwide population-based cross-sectional study in Taiwan found gout was associated with psoriasis (adjusted OR, 1.30, 95% CI:1.20-1.42) (63), and a positive correlation was found between PASI scores and serum uric acid levels in psoriasis patients (64).
Mental Health Diseases
A great deal of literature was produced to assess different aspects of psychology in psoriasis. The hazard ratio (HR) of any mental disorder is 1.75 (95%CI, 1.62-1.89) in psoriasis persons compared with the general population, and the main mental disorders reported were as follows: vascular dementia, schizophrenia, bipolar disorder, unipolar depression, generalized anxiety disorder, and personality disorders (65). In both sexes, psoriatic patients and their partners suffer from psychopathological and sexual consequences related to disease severity (66). A significant increase in psychiatric disorders occurs in pediatric psoriasis, with a 6.65-fold greater risk of depression and a 9.21-fold greater risk of anxiety, compared with the controls (67).
Risk factors of distress include female gender, a younger age of disease onset, those with self-assessment of severe psoriasis (68), type D personality (69), younger patients, and those with lesions on sensitive or visible areas (70). Alcohol disorders, not illicit drug use, are more common in patients with psoriasis (68).
An association was observed between the severity of psoriasis and mood disturbances with an impact on quality of life (71), and men tended to have a shorter time to onset for most mental health disorders than women, except for neurotic disorders and anxiety disorders (72), which may influence the appropriate management of male patients.
The risk of mental disorders increased among psoriasis individuals who had completed short-term education compared with those with medium and long-term education (65), while Zhang et al. (73) concluded there was no significant difference in psychological health between psoriasis patients with different levels of educational attainment. Except for anxiety and depression, psoriasis patients suffer from social distress and social avoidance.
Depression/Anxiety
Psoriasis and depression might have a bidirectional association (74). The prevalence of anxiety/depression among psoriasis patients was 11.52%-27.00% (75–77). Psoriasis patients were at an increased risk for depression (78–81), anxiety disorders (79–81), anxiety and depression co-occurrence (81), and somatoform disorders (79) compared with the referent cohort. Risk factors in psoriasis patients associated with depression were: 20-50 years (77), female sex (77, 82), major comorbid diseases (77, 82), and low income (77, 81). Patients with moderate-to-severe psoriasis had a significant risk of depression and somatoform disorders compared to patients with mild disease (79), and the highest risk was observed among patients with severe psoriasis aged 40-50 years (78). However a HUNT3 study found depressive symptoms do not seem to be a major concern among individuals with psoriasis (83).
Major depressive disorder is a risk factor for the development of psoriasis (84, 85), especially in the male sex (85). Somatic and anxiety symptoms, as well as BMI, are closely linked to dermatology-related quality of life (86).
A unique study conducted in a South East Asian population determined that Indian ethnicity was a predictor of depression (P = 0.024) (87), and it provided invaluable insight into predictive factors of adverse effects of psoriasis on distress in a special population.
A systematic review described that the prevalence of anxiety in patients with psoriasis was significantly higher than healthy controls (OR: 2.91, 95% CI: 2.01-4.21), and an improvement in anxiety symptoms with therapy of psoriasis was demonstrated (Table 3) (88).
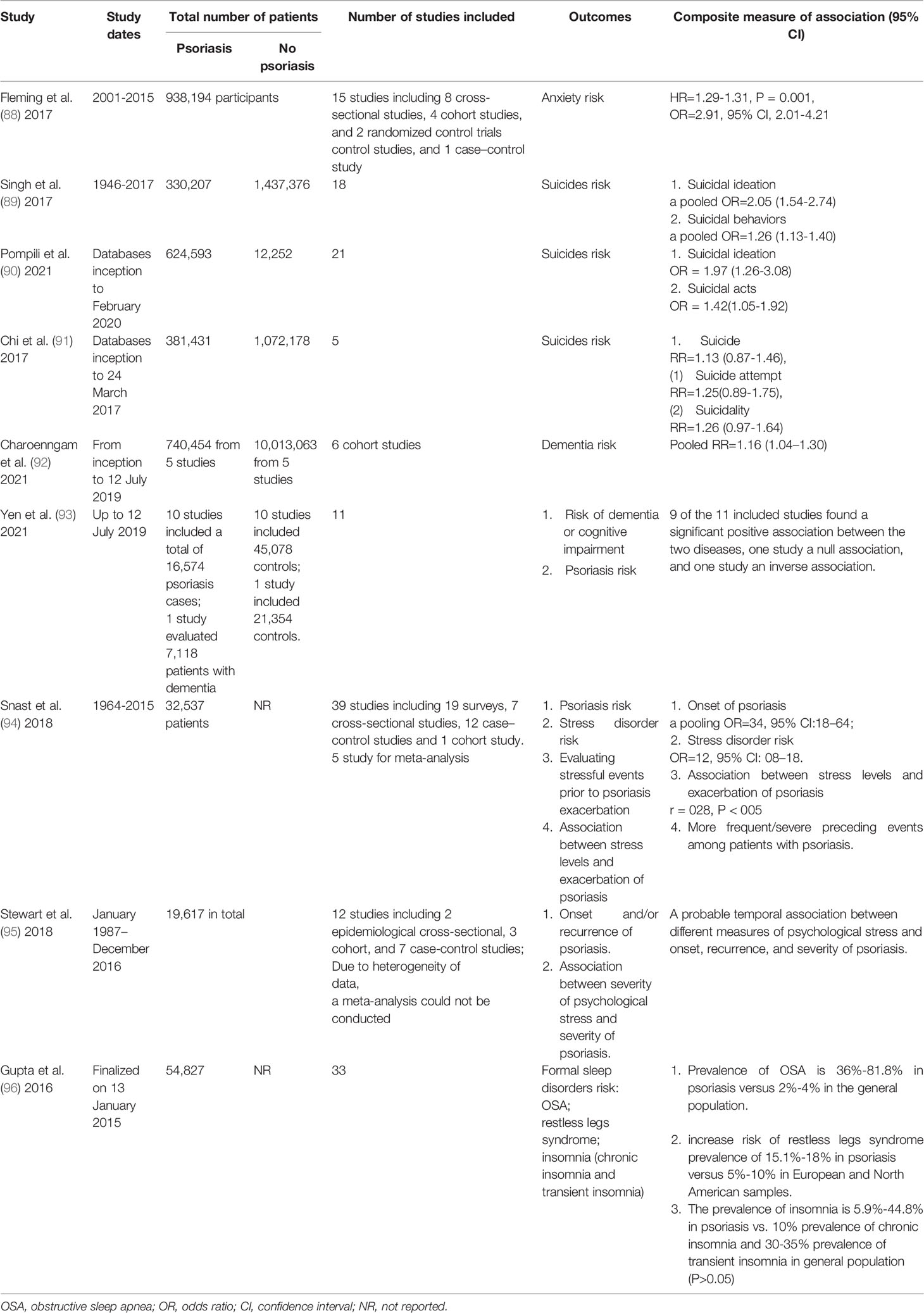
Table 3 Summary of systematic review and meta-analyses assessing the association between psoriasis and mental health diseases.
For moderate-to-severe psoriasis, unadjusted incremental all-cause healthcare costs associated with anxiety/depression were $8,077. Psoriasis treatments that improve psychiatric symptoms may reduce their economic burden (76). Therefore, screening for depressive/anxiety can lead to an increase of utilization of mental health care and improvement of psoriasis.
Suicides
Two systematic reviews and meta-analyses found more suicidal ideation and acts with psoriasis (Table 3) (89, 90), and severe psoriasis and younger age were risk factors (89). However, another systematic review and meta-analysis with five population-based cohort studies found no increase in the risk of suicide, suicide attempt, or suicidality among people with psoriasis (Table 3) (91).
Because the systematic review and meta-analyses from Pompili et al. (90) analyzed the association between suicidal risks with not only psoriasis but also atopic dermatitis, Matterne et al. (97) compared the two systematic reviews and meta-analyses with opposite conclusions (89, 91) focusing on suicidal risks with psoriasis, and found that the differences mainly came from the data source. Chi et al. (91) only included cohort studies while Singh et al. (89) included cross-sectional, case-control, and cohort studies. Chi et al. (91) included five high quality studies according to the Newcastle-Ottawa Scale. Singh et al. (89) included 18 studies that were defined as to be medium quality to high quality.
The results of the association between suicides and psoriasis in later studies are still contradictory: the risk of suicidal behavior in individuals with psoriasis was confirmed in Canada (97), but not in Taiwan (98).
A systematic review and meta-analysis identified no increased risk for depression, anxiety, or suicidality with treatment of secukinumab in a pooled data analysis from 10 studies in patients with moderate-to-severe plaque psoriasis (99).
Dementia
Two systematic review and meta-analyses summarized the association between dementia and psoriatic disease (Table 3) (92, 93), however two population-based studies in Taiwan found contradictory results on the association between psoriasis and dementia (100, 101). Phototherapy and systemic treatment might not have a protective effect against dementia in psoriatic patients (100).
Stress
Psychological stress plays an important role in the development of psoriasis, but the details of this association remain to be clearly defined. A systematic review demonstrates a temporal relationship between stress and psoriasis (Table 3) (95). A tertiary level of education was an independent risk factor while a higher monthly income was a protective factor for psoriasis populations (87). However, another systematic review and meta-analysis including 39 studies concluded there is no convincing evidence that preceding stress is significantly associated with exacerbation/onset of psoriasis because data are primarily based on retrospective studies with nonnegligible limitations (Table 3) (94).
Cohort studies from the UK and Denmark observed no evidence supporting increased long-term risk of psoriasis following bereavement (102).
Sleep Disorders
A systematic review observed an overall 36% to 81.8% prevalence of obstructive sleep apnea in psoriasis (96), and psoriasis is also associated with restless legs syndrome, which is a possible sign of autonomic activation in psoriasis (103).
Fatigue
Psoriasis was associated with an elevated risk of chronic fatigue syndrome, which is differentiated by sex and age (104). Fatigue severity was associated with smoking, pain, and depression, but not with psoriasis severity (105).
Newly Reported Mental Comorbidity of Psoriasis
An increased risk of Parkinson disease (106) and migraine, especially migraine with aura (107) was reported in patients with psoriasis; while a higher risk of psoriasis was found in patients with schizophrenia, and Th17 and pro-inflammatory cytokines may act as a link between these two diseases (108). There is a relationship between psoriasis and alexithymia (109) and dental fear (110), and impulse control proved to be the strongest predictor to psoriasis disability (111).
A meta-analysis showed significant, small-to-medium effects of psychosocial interventions on quality of life (0.28, 0.04-0.51) and anxiety (0.36, 0.15-0.57), but a not significant effect on depression (0.37, -0.05-0.80) (112).
Nervous System Disease
Multiple sclerosis (MS) is an autoimmune-mediated inflammatory disease of the central nervous system, characterized by myelin and various degrees of axonal loss.
Three systematic review and meta-analyses concluded that psoriasis is associated with an increased risk of MS (113), and vice versa (114), especially for family members of those with MS (115) (Table 4).
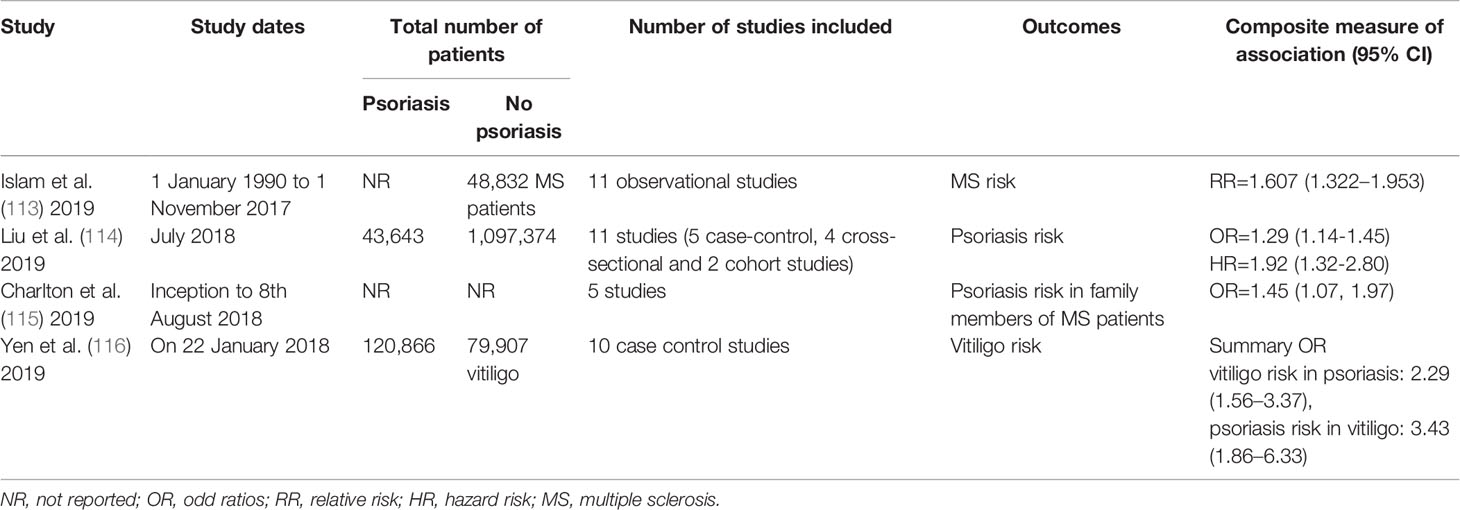
Table 4 Summary of systematic review and meta-analyses assessing the association between psoriasis and MS and vitiligo.
One study evaluated that the RR of MS risk was 0.184 in mild psoriasis (95% CI 1.46–2.30) and 2.61 in severe psoriasis (95% CI 1.44– 4.74) (117). A significant association between psoriasis and MS was detected after adjusting for variables of age, sex, PsA, and exposure of TNF-α agent (118).
Gastrointestinal Disease
Inflammatory Bowel Diseases
IBDs include two chronic idiopathic inflammatory diseases: ulcerative colitis (UC) and Crohn’s disease (CD), which are the main gastrointestinal comorbidities of psoriasis. Two meta-analyses found significant bidirectional associations between psoriasis and IBDs, presence of CD (OR 2.0, 95% CI 1.4-2.9) and UC (OR 1.5, 95% CI 1.2-2.0) was significantly associated with psoriasis (119), and psoriasis had an increased risk of CD (RR= 2.53; 95% CI, 1.65-3.89) and UC (RR=1.71; 95% CI, 1.55-1.89) (120). Psoriasis patients were reported to have a higher prevalence of gastrointestinal signs and symptoms (feeling full/bloated, belly pain, diarrhea, mucus/blood in stool, and unintentional weight loss) (121), and sex and PsA were particular risk factors (122). Psoriasis-UC patients may have a higher BMI and milder skin symptoms than those with psoriasis alone (123), while psoriasis-CD patients have a mild (early-onset) psoriasis but a severe CD phenotype (124). While a cohort study in Denmark found a limited incremental effect of IBDs on risk of comorbidities in patients with psoriasis (125).
Association of psoriasis/PsA with IBD influences management strategy (126), and young patients and those with severe psoriasis may require comprehensive management (127). Time to CD, but not UC, diagnosis was significantly longer for patients with psoriasis compared with the general controls, and patients with systemic treatment had the longest time to CD and UC (122). A systematic review on 132 randomized, controlled, double-blinded studies concluded that infliximab and adalimumab had demonstrated efficacy in psoriasis, PsA, UC, and CD (128).
Non-Alcoholic Fatty Liver Disease
The prevalence of NAFLD in psoriasis patients is high and related to a higher prevalence of MetS, bacterial translocation, and a higher pro-inflammatory state (TNF-α, TGF-β level, and bacterial translocation) (129). Early onset of psoriasis was independently associated with greater odds of NAFLD, hypertriglyceridemia, hyperuricemia, and smaller odds of diabetes compared to late onset (130). The NAFLD fibrosis score proved a high significant correlation (P<0.0001) with fibrosis histological lesions in psoriatic patients (131). Enhanced liver fibrosis score and procollagen-3 N-terminal peptide are elevated in psoriasis and PsA, and enhanced liver fibrosis score may be superior to procollagen-3 N-terminal peptide alone (132).
Other Liver Diseases
A two-way meta-analysis including 18 studies found bidirectional associations between celiac disease and psoriasis; the OR is 2.16 (95% CI, 1.74-2.69) for celiac disease in patients with psoriasis and 1.8 (95% CI, 1.36-2.38) for psoriasis in patients with CD (133). The prevalence of gallstones was higher in the psoriasis patients (adjusted OR is 1.18, 95% CI: 1.14-1.23) (134). Patients with psoriasis may have an elevated risk for diverticulitis, not appendicitis or cholecystitis, compared to the general population (135). Psoriasis is associated with cirrhosis, even among patients without systemic therapy (136).
CKD
Psoriasis and CKD might have a bidirectional association. Psoriasis has a significantly increased risk of CKD (57), and is weakly associated with CKD stages 3-5 (137). The cumulative incidence of psoriasis was higher in CKD patients with anemia than those without, and low hemoglobin levels were significantly related to the risk of psoriasis in CKD patients (138). Statin treatment for hyperlipidemia reduced the CKD risk in patients with psoriasis (57).
In Korea, psoriasis was associated with the risk of ESRD, and the risk of developing ESRD in patients with psoriasis differed according to the type of treatment (with acitretin or not) and the presence of arthritis (139). A retrospective cohort study from Taiwan reported a higher HR for psoriasis in hemodialysis patients than that of the control group, and age <60 years was a risk factor of psoriatic development (140).
Patients with moderate-to-severe psoriasis had a significantly increased risk for development of IgA nephropathy and glomerular disease (141).
Malignancy
A systematic review and meta-analysis including 112 cohort studies found the overall prevalence of cancer in psoriasis patients was 4.78% (95% CI, 4.02%-5.59%), with an RR of 1.21 (95% CI, 1.11-1.33) (142). There was an increased risk of the following cancers in psoriasis patients: keratinocyte cancer, lymphomas, lung cancer, bladder cancer (142), and melanoma or hematologic cancer (143). A prospective longitudinal cohort study showed that a higher standardized incidence ratio of cancer was observed in women compared to men (144). Another systematic review and meta-analysis including 58 cohort and case-control studies found severe psoriasis was associated with a mortality risk of cancer (overall RR, 1.22; 95% CI, 1.08-1.38) (145). However, the included studies had a high level of heterogeneity, which made interpretation challenging.
There is concern that systemic treatment of psoriasis may increase the risk of cancer, however, the effects of systemic treatment including biologics on malignancy are contradictory. Psoriasis patients receiving systemic treatment were reported to have a significantly elevated HR for cancers of bone and cartilage (146), non-Hodgkin lymphoma, and non-melanoma skin cancer (147). However, a systematic review and meta-analysis concluded that no increased risk of cancer was found (RR, 0.97; 95% CI, 0.85-1.10) in psoriasis patients treated with biologics (142), even basal cell carcinoma (BCC) or squamous cell carcinoma (SCC) in patients with a history of BCC or SCC (148), since a history of previous BCC or SCC is by far the strongest predictor of future BCC and SCC. Increased prescribing of acitretin and decreased prescribing of narrowband UVB were clearly evident in psoriasis patients with a history of BCC or SCC (148). The slightly increased risk of cutaneous SCC does not seem to be associated with methotrexate, but rather with disease severity, other anti-psoriatic treatments, and ultraviolet exposure (149). There were no differences in malignancy risk among topical treatments, systemic agents, phototherapy, or biologics, and psoriasis patients with cancer did not have worse survival than patients without psoriasis (143).
Infection
Psoriasis is associated with an unremarkable increase in the risk of serious infection (fully adjusted HR is 1.36, 95% CI 1.31–1.40) (150), and psoriasis severity is a predictor of serious infection risk (151).
COVID‐19
COVID‐19 is the worst epidemic in the last 2 years, and psoriasis patients might discontinue treatment without consulting a dermatologist due to fear of SARS-CoV-2 infections, especially patients who require systemic therapy. However, a Danish study reported that most patients felt to a great extent well treated (67.0%) and safe about their treatment in general (76.4%) (152). A retrospective cohort study in Brazil suggested that systemic therapy did not worsen COVID-19 in psoriasis (150). It is not necessarily concluded that TNFi is safer than biologics targeting IL-17 and IL-23 or not, with respect to risk of respiratory tract and SARS-CoV-2 infections (153). In patients with moderate-to-severe psoriasis, nonbiologic systemic therapies were associated with a higher risk of COVID-19-related hospitalization than with biologic use, and established risk factors included older age, male sex, non-white ethnicity, and having comorbidities (154). A so-called “cytokine storm syndrome” may increase the risk of mortality in COVID-19 patients (155). Although, there are no obvious contraindications to the use of inactivated vaccines, evaluating the risk-benefit ratio of maintaining ongoing immunosuppressive therapy before performing the vaccine is mandatory (156).
Hepatitis B and C
A large US population study reported the prevalence of chronic hepatitis B/C in psoriasis and non-psoriasis patients was 0.5%/0.8% and 1.3/1.6%, respectively, and there was no significant difference between the populations with and without psoriasis in terms of prevalence of hepatitis B or C (157). A higher prevalence of hepatitis C virus was demonstrated in adults with psoriasis and a higher rate of hepatic decompensation in hepatitis C virus+ individuals with moderate-severe psoriasis (158). Long-term use of methotrexate may not be associated with a liver cirrhosis risk among psoriatic patients with chronic viral hepatitis (159). Upregulation of inflammatory cytokines in patients with hepatitis viral infection possibly increases susceptibility to developing psoriasis (160).
HIV
Infection of HIV was an independent risk factor for development of psoriasis (adjusted HR, 1.80; 95%CI: 1.38-2.36) (161), and severe psoriasis was an independent risk factor for steatosis in patients with HIV infection (OR, 12; 95% CI, 1.2-120) (162). Treatment of psoriasis patients with HIV is a challenge as immunosuppressive agents may reactivate or induce infection in such patients, so biologics become a possible effective choice in psoriatic patients with a stable HIV infection, and it is also mandatory to monitor CD4 count and HIV viral load, in order to avoid possible causes of infection (163).
Mycobacterium
Single nucleotide polymorphism of rs1465788 in the zinc finger protein 36 ring finger protein-like 1 gene was identified as an early-onset psoriasis risk gene which demonstrates opposite associations with leprosy and psoriasis in a Chinese Han population (164).
Detection of latent Mycobacterium tuberculosis infection is mandatory before biotherapy in psoriasis as biotherapy may reactivate the infection. The national investigation of latent tuberculosis infection in Italian patients with psoriasis reported a prevalence of 8.5%, and independent risk factors included male sex, age over 55 years, and being entered into conventional treatment (165). The heparin-binding hemagglutinin, a higher sensitivity marker in response to a mycobacterial antigen, may help to prioritize patients who receive prophylactic interventions of Mycobacterium tuberculosis before starting biotherapies (166).
Staphylococcal Colonization
A systematic review and meta-analysis found that psoriatic patients faced an increased risk for colonization of staphylococci compared to healthy controls, and the pooled prevalence was 39.2% (95% CI 33.7-44.8) vs. 35.3% (95% CI 25.0-45.6) (167). A 4.5-fold increase of Staphylococcal aureus colonization was found on the patients’ skin compared to healthy controls, and 60% was in the nares (167).
Herpes Zoster
A Taiwanese cohort study confirmed psoriasis was associated with an increased risk of HZ (adjusted HR of 1.29, 95% CI= 1.07-1.56), which involved differences in sex and age (168). The risk of HZ was significantly increased among the moderate to severe psoriasis group and was associated with immunosuppressive therapy (151). Although systemic therapy may play an important role in the risk of HZ, the intrinsic factors of psoriasis should be considered.
Musculoskeletal System
PsA
Almost 1/3 of patients with psoriasis develop PsA, which showed strong associations with psoriasis (adjusted OR:10.08; 95% CI: 7.97-12.74) (169). A meta-analysis including 266 studies found that the overall pooled proportion of PsA among adult psoriatic patients was 19.7%, and was 3.3% (95% CI, 2.1%-4.9%) in adolescents <18 years. The PsA prevalence was 22.7% (95% CI, 20.6%-25.0%), 21.5% (95% CI, 15.4%-28.2%), 19.5% (95% CI, 17.1%-22.1%), 15.5% (95% CI, 0.009%-51.5%), and 14.0% (95% CI, 11.7%-16.3%) in psoriasis from European, South American, North American, African, and Asian patients respectively (170). However, the high heterogeneity of studies included may have affected the estimates.
Incidence of PsA was 1.48, 3.00, and 5.49 per 100 patient-years in mild, moderate, and severe psoriasis patients, respectively (171). Risk of PsA increases steadily with duration of cutaneous symptoms following psoriasis onset (172, 173). PsA patients have a larger clinical burden, characterized by higher comorbidity rates, than those with psoriasis. Ratios of psoriasis-PsA comorbidity rates relative to psoriasis-only ranged from 1.1 for allergies and infections to 1.7 for fatigue, diabetes, and obesity (174).
Main factors that contribute to the delay in the diagnosis of PsA are lack of awareness among patients of the relationship between skin disease and joint symptoms and the absence of a specific diagnostic marker. Improved PsA screening is suggested in patients with psoriasis because the validated psoriasis epidemiology screening tool identified more than 10% of registry patients who could have had undiagnosed PsA (175). Recent consensus guidelines for managing psoriasis recommend using questionnaires to screen for the presence of PsA, and Salaff et al. (176) developed a self-administered questionnaire, called Simple Psoriatic Arthritis Screening (SiPAS), which screens psoriasis patients for signs and symptoms of PsA with compared high sensibility and specificity. In a systematic review, 259 possible markers associated with the development or presence of PsA in patients with psoriasis were identified in 119 studies (177). Laboratory markers related to inflammation and bone metabolism showed a strong association (not prediction) of PsA in psoriasis, however only C-X-C motif ligand 10 reached a strong level of evidence for a positive predictive value (177), but whether these indicators are clinically useful remains to be further investigated.
Active enthesitis and synovitis could be useful to identify subclinical PsA (178). Fluorescence optical imaging may be a helpful novel tool to analyze microcirculation in psoriasis/PsA patients (179).
Osteoporosis and Fracture
Studies have yielded inconclusive results on the association between psoriatic/PsA and bone loss (180–182), a systematic review and meta-analysis including 12 studies concluded that patients with psoriasis/PsA have an increased risk of fractures (psoriasis: OR = 1.29, 95%CI = 1.02-1.63; PsA: OR = 2.88, 95%CI = 1.51-5.48). However there is little evidence supporting the association between psoriasis and osteoporosis/osteopenia (osteoporosis, psoriasis: OR, 1.28, 95%CI 0.86-1.90; PsA: OR, 1.32, 95%CI: 0.79-2.19; osteopenia, psoriasis: OR, 1.50, 95%CI: 0.75-3.02; PsA: OR, 1.61, 95%CI: 0.67-3.85) (183). Psoriasis increased the risk of osteoporosis in patients aged ≥ 40 years in Korea (182).
Skin
Onychomycosis was the most frequent skin comorbidity of psoriasis followed by rosacea and telangiectasia (184). Psoriatic patients with skin diseases had a worse quality of life than those without, as measured by Skindex 29, Dermatology Life Quality Index, and psoriasis disability index scores (185).
Vitiligo
A meta-analysis including 10 case-control and cross-sectional studies found psoriasis and vitiligo are bidirectionally associated with each other: increased odds for psoriasis in vitiligo patients (summary OR: 3.43, 95% CI: 1.86-6.33) as well as elevated odds for vitiligo in psoriatic patients (summary OR: 2.29, 95% CI: 1.56-3.37) (116) (Table 4).
Bullous Disease
A systematic review and meta-analysis of 12 observational studies found that the overall pooled prevalence of psoriasis was 2.4% (95% CI, 1.0-4.4) among pemphigus patients (186). Pemphigus patients face an increased risk of psoriasis (187).
Psoriasis was independently associated with an increased risk of bullous pemphigoid (188, 189), with an important risk factor of younger age, and over one-third of bullous pemphigoid cases were diagnosed in the first year after incident psoriasis (188).
Chronic Itch
Higher itch intensity was associated with women, lower educational level, pustular psoriasis, lesions on visible or sensitive areas, palmoplantar areas, severe disease, disease duration <15 years, and no or few prior systemic treatments (190). Reszke et al. (191) firstly reported the possible associations between psoriatic pruritus and drugs administrated in various systemic conditions, including antacids, beta-blockers, angiotensin enzyme converting inhibitors, and xerosis intensity angiotensin receptor blockers.
Palmoplantar Pustulosis
Three large population-based cohorts reported that the prevalence of psoriasis was between 14.2% and 61.3% in patients with palmoplantar pustulosis, and patients both with palmoplantar pustulosis and psoriasis had a higher PsA prevalence and antipsoriatic drug use (192).
Allergy
A positive association was reported between psoriasis and contact allergy (193), while an inverse association was reported in psoriasis and atopic diseases (194). The polarization of the activated immune response by allergens may affect the occurrence and significance of allergies in underlying immune-mediated diseases, even beyond the skin (193).
Reproductive System
Male
Two comprehensive meta-analyses summarized that psoriasis was associated with an increased risk of erectile dysfunction (OR, 1.22; 95% CI 1.08-1.37; P = 0.002 (195); OR, 1.62, 95%CI: 1.37-1.91, P < 0.001 (196)), especially in men with mild psoriasis (1.13; 1.09-1.20) and severe psoriasis (1.17; 1.04-1.32) (197). A pilot study supported a correlation between hypogonadism and obesity in male psoriasis patients, while no psoriasis-specific reduction of testosterone can be assumed (198).
A comprehensive literature review and meta-analysis including 28 studies found that the prevalence of sexual dysfunction (SD) ranged from 40.0% to 55.6% in psoriasis patients (199). Physical and psychological comorbidities are risk factors for SD, and the strongest association with SD is anxiety and depression, PsA, and genital psoriasis. Biologic drugs have benefits for the improvement of SD (199).
Female
A meta-analysis including 16 studies found that pregnant women with psoriasis had a significantly higher risk of adverse maternal outcomes [preterm birth: 1.32 (1.15, 1.52); caesarean delivery: 1.33 (1.17, 1.52); (pre)eclampsia: 1.28 (1.14, 1.43); gestational hypertension: 1.30 (1.18, 1.44); gestational diabetes: 1.19 (1.10, 1.30)], but not adverse neonatal events (200).
Respiratory System
Psoriasis and asthma might have a bidirectional association. A meta-analysis including six studies indicated that the patients with psoriasis had a higher risk of asthma susceptibility (OR 1.32 [95% CI, 1.20-1.46]), especially among the older patients (≥50 years) (201). A cohort study in Korean children found asthma was associated with the elevated risk of psoriasis (adjusted HR = 1.19; 95% CI, 1.07-1.33) (202). Patients with psoriasis who do not smoke may not have an increased risk of developing chronic obstructive pulmonary disease in US adults (203), so counselling psoriasis patients on the benefits of smoking cessation remains a valuable approach for preventing chronic obstructive pulmonary disease risk.
Oral Comorbidities
Geographic Tongue
GT has been described as a predictor of psoriasis, however, reports are inconclusive. A systematic review and meta-analysis including 11 case-control studies found that the frequency of GT was statistically associated with psoriasis (pooled OR was 3.53, 95% CI: 2.56-4.86) (204), however prevalence rates vary from 0.51% to 11.43% and data for Europe are sparse (205). A hospital-based cross-sectional study in Saudi Arabia found a positive association of both GT and fissured tongue in adult patients with psoriasis compared with those without (206). The PASI score was statistically higher in patients affected by GT, and exhibited less improvement after treatment (204). However a prospective case-control study in an Austrian cohort found that psoriasis was associated with fissured tongue but not with GT (207), and the severity of the disease evaluated by the PASI scale did not influence mucosal involvement (208).
Periodontitis
A meta-analysis including two cohort studies and three case-control studies found that patients with periodontitis had a significantly elevated risk of psoriasis (pooled RR is 1.55, 95% CI, 1.35-1.77) (209). Vice versa, studies confirmed a significant psoriasis-associated increased risk of periodontitis (210–212), which was highest in patients with severe psoriasis and PsA (210). Severity of psoriasis also presented a strong relationship with periodontal clinical parameters (211). A link was identify between the inverse type of psoriasis and periodontitis (212).
Ocular Comorbidities
Studies on ocular comorbidities of psoriasis are mainly from Taiwan. A retrospective cohort study concluded that psoriasis was associated with an increased risk of keratopathy in patients without preexisting prominent corneal disease; age older than 60 years and dry eye disease were risk factors of developing keratopathy (213). The risk of retinal diseases was significantly higher in psoriasis, including retinal detachment, retinal vascular occlusion, and retinopathy (214), and an increased risk of uveitis (215). Ocular morbidity was increased with increasing duration and PASI score (216), so it is important to screen psoriasis patients to prevent sight-threatening complications.
Rare Comorbidities
An association was also confirmed between psoriasis and hearing loss (217), Behçet’s disease (218), scabies infection (219), and pediatric infection (220).
Conclusion
With the increase of epidemiologic and basic scientific evidence, the nature of psoriasis is recognized as a systemic inflammatory disease. The pathogenesis of systemic inflammation of psoriasis is the development basis of extracutaneous comorbid diseases, and can explain why therapies for psoriasis including traditional systematic agents and biologics benefit the extracutaneous comorbidities of psoriasis.
In addition to CAD, IBDs, and CKD, which are recognized major comorbidities of psoriasis, more systematic diseases, such as bullous disease, asthma, and periodontitis, are identified as new comorbidities of psoriasis, and it helps to complete the understanding of psoriasis and comorbid diseases. Mental health becomes a new hot area of psoriasis study, as psoriasis not only causes a negative impact on psychological health in patients, which leads to a range of psycho-emotional consequences, but also causes substantial pathological changes of the nervous system by immune dysregulation-mediated inflammation. Bidirectional associations are proved between psoriasis and extracutaneous comorbidities, such as depression, CKD, celiac disease, and vitiligo, and it provides epidemiological evidence that psoriasis treatment benefits comorbidities.
Based on the association and pathogenesis research, biochemical indicators and technical means including artificial intelligence technology that can be used as disease evaluation and prediction indicators have entered a stage of rapid development, which may facilitate the management of diseases. However, the clinical application of these assessments and predictors requires the strong support of studies with large sample sizes.
Although the association between increasing extracutaneous comorbidities and psoriasis has been identified, the correlation between certain diseases and psoriasis is still contradictory, even when using the conclusions of systematic review and meta-analyses, which needs to be confirmed by more high-quality studies included.
Malignancy risk for biologics treatment of psoriasis draws much attention, especially in the era of the COVID-19 pandemic. however the conclusions of related studies remain conflicting. Therefore, patients receiving biologics treatment should be closely monitored.
With the in-depth study of psoriasis and its comorbidities, including epidemiological and pathological basic research and genetic molecular research, the traditional system for disease patterns is likely to be broken, therefore it is critical for both clinicians and patients to recognize the potentially risk of important comorbidities associated with psoriasis, which leads to a great disease burden for people and society. Apart from the disease itself, socio-economic and cultural factors, such as sex, age, income, and education level, all possibly act as risk factors for comorbidities, so, increased awareness of psoriasis comorbidities is critical to the management of psoriasis.
Author Contributions
JB draft the manuscript, RD made the literature searching, LZ and XC performed the selection of articles, ES managed the program and reviewed the manuscript. All of the authors approved the submission of the current manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Mr. Qi Li for his help with literature searching and access.
References
1. Arnold KA, Treister AD, Lio PA, Alenghat FJ. Association of Atherosclerosis Prevalence With Age, Race, and Traditional Risk Factors in Patients With Psoriasis. JAMA Dermatol (2019) 155(5):622–3. doi: 10.1001/jamadermatol.2018.5462
2. Jung KJ, Kim TG, Lee JW, Lee M, Oh J, Lee SE, et al. Increased Risk of Atherosclerotic Cardiovascular Disease Among Patients With Psoriasis in Korea: A 15-Year Nationwide Population-Based Cohort Study. J Dermatol (2019) 46(10):859–66. doi: 10.1111/1346-8138.15052
3. Committee of Psoriasis, Dermatology Branch, Chinese Medical Association. Guidelines for the Diagnosis and Treatment of Psoriasis in China: 2019 Concise Edition. Int J Dermatol Venereol (2020) 3(1):14–26. doi: 10.1097/JD9.0000000000000074
4. Kawazoe S, Tokuyama N, Mizukami H, Ohashi H. Zur Lehre Von Der Neurogenen Und Der Thyreogenen Glykosurie (Schluss Aus No. 18.). Dtsch Med Wochenschr (1897) 23(20):309–12.
5. Takeshita J, Grewal S, Langan SM, Mehta NN, Ogdie A, Van Voorhees AS, et al. Psoriasis and Comorbid Diseases: Epidemiology. J Am Acad Dermatol (2017) 76(3):377–90. doi: 10.1016/j.jaad.2016.07.064
6. Shiba M, Kato T, Izumi T, Miyamoto S, Nakane E, Haruna T, et al. Risk of Myocardial Infarction in Patients With Psoriasis: A Cross-Sectional Patient-Population Study in a Japanese Hospital. J Cardiol (2019) 73(4):276–9. doi: 10.1016/j.jjcc.2018.10.008
7. Raaby L, Ahlehoff O, de Thurah A. Psoriasis and Cardiovascular Events: Updating the Evidence. Arch Dermatol Res (2017) 309(3):225–8. doi: 10.1007/s00403-016-1712-1
8. Zhao J, Zhang X, Sun H, An R. Is Psoriasis a Risk Factor of Angiography-Proven Coronary Artery Disease? Br J Dermatol (2018) 179(5):1218–9. doi: 10.1111/bjd.16925
9. Kaiser H, Abdulla J, Henningsen KMA, Skov L, Hansen PR. Coronary Artery Disease Assessed by Computed Tomography in Patients With Psoriasis: A Systematic Review and Meta-Analysis. Dermatology (2019) 235(6):478–87. doi: 10.1159/000502138
10. Upala S, Shahnawaz A, Sanguankeo A. Psoriasis Increases Risk of New-Onset Atrial Fibrillation: A Systematic Review and Meta-Analysis of Prospective Observational Studies. J Dermatolog Treat Q2 (2017) 28(5):406–10. doi: 10.1080/09546634.2016.1255703
11. Yu X, Feng X, Xia L, Cao S, Wei X. Risk of Aortic Aneurysm in Patients With Psoriasis: A Systematic Review and Meta-Analysis of Cohort Studies. Clin Cardiol (2020) 43(11):1266–72. doi: 10.1002/clc.23438
12. Phan K, Lee G, Fischer G. Pediatric Psoriasis and Association With Cardiovascular and Metabolic Comorbidities: Systematic Review and Meta-Analysis. Pediatr Dermatol Q3-4 (2020) 37(4):661–9. doi: 10.1111/pde.14208
13. Badaoui A, Tounian P, Mahé E. Psoriasis and Metabolic and Cardiovascular Comorbidities in Children: A Systematic Review. Arch Pediatr Q4 (2019) 26(2):86–94. doi: 10.1016/j.arcped.2018.12.005
14. Keller K, Hobohm L, Ostad MA, Karbach S, Espinola-Klein C, Münzel T, et al. Psoriasis and Its Impact on the Clinical Outcome of Patients With Pulmonary Embolism. Int J Cardiol (2021) 343:114–21. doi: 10.1016/j.ijcard.2021.08.042
15. Damian AC, Colaco K, Rohekar S, Boyd T, Chandran V, Gladman DD, et al. The Incidence and Risk Factors for Venous Thromboembolic Events in Patients With Psoriasis and Psoriatic Arthritis. Semin Arthritis Rheum Q1 (2021) 51(3):547–52. doi: 10.1016/j.semarthrit.2021.04.008
16. Laskowski M, Schiöler L, Gustafsson H, Wennberg AM, Åberg M, Torén K. Cardiorespiratory Fitness in Late Adolescence and Long-Term Risk of Psoriasis and Psoriatic Arthritis Among Swedish Men. PloS One (2021) 16(1):e0243348. doi: 10.1371/journal.pone.0243348
17. Paiva-Lopes MJ, Batuca JR, Gouveia S, Alves M, Papoila AL, Alves JD. Antibodies Towards High-Density Lipoprotein Components in Patients With Psoriasis. Arch Dermatol Res (2020) 312(2):93–102. doi: 10.1007/s00403-019-01986-x
18. Piaserico S, Osto E, Famoso G, Montisci R, De Michieli L, Zanetti I, et al. Long-Term Prognostic Value of Coronary Flow Reserve in Psoriasis Patients. Atherosclerosis (2019) 289:57–63. doi: 10.1016/j.atherosclerosis.2019.08.009
19. Kvist-Hansen A, Kaiser H, Wang X, Krakauer M, Gørtz PM, McCauley BD, et al. Neutrophil Pathways of Inflammation Characterize the Blood Transcriptomic Signature of Patients With Psoriasis and Cardiovascular Disease. Int J Mol Sci (2021) 22(19):10818. doi: 10.3390/ijms221910818
20. Sobchak C, Akhtari S, Harvey P, Gladman D, Chandran V, Cook R, et al. Value of Carotid Ultrasound in Cardiovascular Risk Stratification in Patients With Psoriatic Disease. Arthritis Rheumatol Q1 (2019) 71(10):1651–9. doi: 10.1002/art.40925
21. Sajja A, Abdelrahman KM, Reddy AS, Dey AK, Uceda DE, Lateef SS, et al. Chronic Inflammation in Psoriasis Promotes Visceral Adiposity Associated With Noncalcified Coronary Burden Over Time. JCI Insight (2020) 5(22):e142534. doi: 10.1172/jci.insight.142534
22. Zhou W, Abdelrahman KM, Dey AK, Reddy A, Uceda DE, Lateef SS, et al. Association Among Noncalcified Coronary Burden, Fractional Flow Reserve, and Myocardial Injury in Psoriasis. J Am Heart Assoc (2020) 9(22):e017417. doi: 10.1161/jaha.119.017417
23. Munger E, Choi H, Dey AK, Elnabawi YA, Groenendyk JW, Rodante J, et al. Application of Machine Learning to Determine Top Predictors of Noncalcified Coronary Burden in Psoriasis: An Observational Cohort Study. J Am Acad Dermatol (2020) 83(6):1647–53. doi: 10.1016/j.jaad.2019.10.060
24. Garshick MS, Barrett TJ, Wechter T, Azarchi S, Scher JU, Neimann A, et al. Inflammasome Signaling and Impaired Vascular Health in Psoriasis. Arterioscler Thromb Vasc Biol (2019) 39(4):787–98. doi: 10.1161/atvbaha.118.312246
25. Tsai TY, Yen H, Huang YC. Serum Homocysteine, Folate and Vitamin B(12) Levels in Patients With Psoriasis: A Systematic Review and Meta-Analysis. Br J Dermatol (2019) 180(2):382–9. doi: 10.1111/bjd.17034
26. Mahil SK, McSweeney SM, Kloczko E, McGowan B, Barker JN, Smith CH. Does Weight Loss Reduce the Severity and Incidence of Psoriasis or Psoriatic Arthritis? A Critically Appraised Topic. Br J Dermatol (2019) 181(5):946–53. doi: 10.1111/bjd.17741
27. Holzer G, Hoke M, Sabeti-Sandor S, Perkmann T, Rauscher A, Strassegger B, et al. Disparate Effects of Adalimumab and Fumaric Acid Esters on Cardiovascular Risk Factors in Psoriasis Patients: Results From a Prospective, Randomized, Observer-Blinded Head-To-Head Trial. J Eur Acad Dermatol Venereol (2021) 35(2):441–9. doi: 10.1111/jdv.16635
28. Hong JR, Jeong H, Kim H, Yang HS, Hong JY, Kim SM, et al. The Potential Impact of Systemic Anti-Inflammatory Therapies in Psoriasis on Major Adverse Cardiovascular Events: A Korean Nationwide Cohort Study. Sci Rep (2021) 11(1):8588. doi: 10.1038/s41598-021-87766-y
29. Aune D, Snekvik I, Schlesinger S, Norat T, Riboli E, Vatten LJ. Body Mass Index, Abdominal Fatness, Weight Gain and the Risk of Psoriasis: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Eur J Epidemiol Q1 (2018) 33(12):1163–78. doi: 10.1007/s10654-018-0366-z
30. Friis NU, Hoffmann N, Gyldenløve M, Skov L, Vilsbøll T, Knop FK, et al. Glucose Metabolism in Patients With Psoriasis. Br J Dermatol (2019) 180(2):264–71. doi: 10.1111/bjd.17349
31. Rodríguez-Zúñiga MJM, García-Perdomo HA. Systematic Review and Meta-Analysis of the Association Between Psoriasis and Metabolic Syndrome. J Am Acad Dermatol (2017) 77(4):657–66.e8. doi: 10.1016/j.jaad.2017.04.1133
32. Pietrzak A, Grywalska E, Walankiewicz M, Lotti T, Roliński J, Myśliński W, et al. Psoriasis and Metabolic Syndrome in Children: Current Data. Clin Exp Dermatol (2017) 42(2):131–6. doi: 10.1111/ced.13014
33. Wu MY, Yu CL, Yang SJ, Chi CC. Change in Body Weight and Body Mass Index in Psoriasis Patients Receiving Biologics: A Systematic Review and Network Meta-Analysis. J Am Acad Dermatol (2020) 82(1):101–9. doi: 10.1016/j.jaad.2019.07.103
34. Zou Q, Si J, Guo Y, Yu J, Shi H. Association Between Serum Visfatin Levels and Psoriasis and Their Correlation With Disease Severity: A Meta-Analysis. J Int Med Res (2021) 49(3):3000605211002381. doi: 10.1177/03000605211002381
35. Kim ES, Han K, Kim MK, Park YM, Baek KH, Moon SD, et al. Impact of Metabolic Status on the Incidence of Psoriasis: A Korean Nationwide Cohort Study. Sci Rep (2017) 7(1):1989. doi: 10.1038/s41598-017-01983-y
36. Han JH, Lee JH, Han KD, Kim HN, Bang CH, Park YM, et al. Increased Risk of Psoriasis in Subjects With Abdominal Obesity: A Nationwide Population-Based Study. J Dermatol (2019) 46(8):695–701. doi: 10.1111/1346-8138.14939
37. Ferguson LD, Brown R, Celis-Morales C, Welsh P, Lyall DM, Pell JP, et al. Association of Central Adiposity With Psoriasis, Psoriatic Arthritis and Rheumatoid Arthritis: A Cross-Sectional Study of the Uk Biobank. Rheumatology (Oxford) (2019) 58(12):2137–42. doi: 10.1093/rheumatology/kez192
38. Ogawa K, Stuart PE, Tsoi LC, Suzuki K, Nair RP, Mochizuki H, et al. A Transethnic Mendelian Randomization Study Identifies Causality of Obesity on Risk of Psoriasis. J Invest Dermatol (2019) 139(6):1397–400. doi: 10.1016/j.jid.2018.11.023
39. Budu-Aggrey A, Brumpton B, Tyrrell J, Watkins S, Modalsli EH, Celis-Morales C, et al. Evidence of a Causal Relationship Between Body Mass Index and Psoriasis: A Mendelian Randomization Study. PloS Med (2019) 16(1):e1002739. doi: 10.1371/journal.pmed.1002739
40. Gui XY, Yu XL, Jin HZ, Zuo YG, Wu C. Prevalence of Metabolic Syndrome in Chinese Psoriasis Patients: A Hospital-Based Cross-Sectional Study. J Diabetes Investig (2018) 9(1):39–43. doi: 10.1111/jdi.12663
41. Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the Metabolic Syndrome. Clin Dermatol (2018) 36(1):21–8. doi: 10.1016/j.clindermatol.2017.09.005
42. Snekvik I, Nilsen TIL, Romundstad PR, Saunes M. Metabolic Syndrome and Risk of Incident Psoriasis: Prospective Data From the Hunt Study, Norway. Br J Dermatol (2019) 180(1):94–9. doi: 10.1111/bjd.16885
43. Fernández-Armenteros JM, Gómez-Arbonés X, Buti-Soler M, Betriu-Bars A, Sanmartin-Novell V, Ortega-Bravo M, et al. Psoriasis, Metabolic Syndrome and Cardiovascular Risk Factors. A Population-Based Study. J Eur Acad Dermatol Venereol (2019) 33(1):128–35. doi: 10.1111/jdv.15159
44. Kim HN, Han K, Park YG, Lee JH. Metabolic Syndrome Is Associated With an Increased Risk of Psoriasis: A Nationwide Population-Based Study. Metabolism (2019) 99:19–24. doi: 10.1016/j.metabol.2019.07.001
45. Chularojanamontri L, Wongpraparut C, Silpa-Archa N, Chaweekulrat P. Metabolic Syndrome and Psoriasis Severity in South-East Asian Patients: An Investigation of Potential Association Using Current and Chronological Assessments. J Dermatol (2016) 43(12):1424–8. doi: 10.1111/1346-8138.13540
46. Teklu M, Zhou W, Kapoor P, Patel N, Dey AK, Sorokin AV, et al. Metabolic Syndrome and Its Factors Are Associated With Noncalcified Coronary Burden in Psoriasis: An Observational Cohort Study. J Am Acad Dermatol (2021) 84(5):1329–38. doi: 10.1016/j.jaad.2020.12.044
47. Sondermann W, Djeudeu Deudjui DA, Körber A, Slomiany U, Brinker TJ, Erbel R, et al. Psoriasis, Cardiovascular Risk Factors and Metabolic Disorders: Sex-Specific Findings of a Population-Based Study. J Eur Acad Dermatol Venereol (2020) 34(4):779–86. doi: 10.1111/jdv.16029
48. Caroppo F, Galderisi A, Ventura L, Belloni Fortina A. Metabolic Syndrome and Insulin Resistance in Pre-Pubertal Children With Psoriasis. Eur J Pediatr (2021) 180(6):1739–45. doi: 10.1007/s00431-020-03924-w
49. Korkmaz S, Korkmaz H. Effect of Alterations in Apoptotic Pathway on Development of Metabolic Syndrome in Patients With Psoriasis Vulgaris. Br J Dermatol (2017) 176(6):1549–57. doi: 10.1111/bjd.15185
50. Baran A, Nowowiejska J, Krahel JA, Kaminski TW, Maciaszek M, Flisiak I. Higher Serum Selenoprotein P Level as a Novel Inductor of Metabolic Complications in Psoriasis. Int J Mol Sci (2020) 21(13):4594. doi: 10.3390/ijms21134594
51. Cao H, Su S, Yang Q, Le Y, Chen L, Hu M, et al. Metabolic Profiling Reveals Interleukin-17a Monoclonal Antibody Treatment Ameliorate Lipids Metabolism With the Potentiality to Reduce Cardiovascular Risk in Psoriasis Patients. Lipids Health Dis (2021) 20(1):16. doi: 10.1186/s12944-021-01441-9
52. Goolam Mahyoodeen N, Crowther NJ, Pillay L, Snyman T, Toman M, Daya S, et al. Relationship of Visceral Fat and Adipokines With Cardiometabolic Diseases in Psoriasis. Acta Derm Venereol (2019) 99(13):1218–23. doi: 10.2340/00015555-3327
53. Polic MV, Miskulin M, Smolic M, Kralik K, Miskulin I, Berkovic MC, et al. Psoriasis Severity-A Risk Factor of Insulin Resistance Independent of Metabolic Syndrome. Int J Environ Res Public Health (2018) 15(7):1486. doi: 10.3390/ijerph15071486
54. Lee JH, Han JH, Han KD, Park YM, Lee JY, Park YG, et al. Psoriasis Risk in Patients With Diabetic Retinopathy: A Nationwide Population-Based Study. Sci Rep (2018) 8(1):9086. doi: 10.1038/s41598-018-27147-0
55. Milan R, LeLorier J, Litvinov IV, Dasgupta K, Rahme E. Sex Differences in the Risk of Diabetes Mellitus Among Individuals With Psoriasis: A Retrospective Cohort Study in Québec, Canada. J Am Acad Dermatol (2021) 85(1):213–5. doi: 10.1016/j.jaad.2020.07.082
56. Blegvad C, Nybo Andersen AM, Adam A, Zachariae C, Skov L. Psoriasis as a Predictor of Cardiometabolic Comorbidity in Women: A Study Based on the Danish National Birth Cohort. Acta Derm Venereol (2019) 99(3):274–8. doi: 10.2340/00015555-3090
57. Liu KL, Tsai WC, Tu HP, Lee CH. Statin Use and the Risk of Chronic Kidney Disease in Patients With Psoriasis: A Nationwide Cohort Study in Taiwan. PloS One (2020) 15(8):e0237816. doi: 10.1371/journal.pone.0237816
58. Visser MJE, Venter C, Roberts TJ, Tarr G, Pretorius E. Psoriatic Disease Is Associated With Systemic Inflammation, Endothelial Activation, and Altered Haemostatic Function. Sci Rep (2021) 11(1):13043. doi: 10.1038/s41598-021-90684-8
59. Khan SR, Bano A, Wakkee M, Korevaar TIM, Franco OH, Nijsten TEC, et al. The Association of Autoimmune Thyroid Disease (Aitd) With Psoriatic Disease: A Prospective Cohort Study, Systematic Review and Meta-Analysis. Eur J Endocrinol (2017) 177(4):347–59. doi: 10.1530/eje-17-0397
60. Namiki K, Kamata M, Shimizu T, Chijiwa C, Uchida H, Okinaga S, et al. Thyroid Dysfunction in Patients With Psoriasis: Higher Prevalence of Thyroid Dysfunction in Patients With Generalized Pustular Psoriasis. J Dermatol (2020) 47(2):133–9. doi: 10.1111/1346-8138.15178
61. Wang SH, Wang J, Lin YS, Tung TH, Chi CC. Increased Risk for Incident Thyroid Diseases in People With Psoriatic Disease: A Cohort Study. J Am Acad Dermatol (2019) 80(4):1006–12. doi: 10.1016/j.jaad.2018.11.049
62. Valduga JAG, Rebeiko LB, Skare TL. Prevalence of Hashimoto's Thyroiditis in Psoriasis Patients. Rev Assoc Med Bras (1992) (2021) 67(1):52–7. doi: 10.1590/1806-9282.67.01.20200274
63. Hu SC, Lin CL, Tu HP. Association Between Psoriasis, Psoriatic Arthritis and Gout: A Nationwide Population-Based Study. J Eur Acad Dermatol Venereol 2区 (2019) 33(3):560–7. doi: 10.1111/jdv.15290
64. Yilmaz E, Tamer E, Artüz F, Külcü Çakmak S, Köktürk F. Evaluation of Serum Uric Acid Levels in Psoriasis Vulgaris. Turk J Med Sci (2017) 47(2):531–4. doi: 10.3906/sag-1512-5
65. Leisner MZ, Riis JL, Schwartz S, Iversen L, Østergaard SD, Olsen MS. Psoriasis and Risk of Mental Disorders in Denmark. JAMA Dermatol (2019) 155(6):745–7. doi: 10.1001/jamadermatol.2019.0039
66. Alariny AF, Farid CI, Elweshahi HM, Abbood SS. Psychological and Sexual Consequences of Psoriasis Vulgaris on Patients and Their Partners. J Sex Med (2019) 16(12):1900–11. doi: 10.1016/j.jsxm.2019.08.017
67. Kara T, Topkarcı Z, Yılmaz S, Akaltun İ, Erdoğan B. Pediatric Patients With Psoriasis and Psychiatric Disorders: Premorbidity and Comorbidity in a Case-Control Study. J Dermatolog Treat (2019) 30(2):129–34. doi: 10.1080/09546634.2018.1476653
68. Lim DS, Bewley A, Oon HH. Psychological Profile of Patients With Psoriasis. Ann Acad Med Singap (2018) 47(12):516–22.
69. Aguayo-Carreras P, Ruiz-Carrascosa JC, Molina-Leyva A. Type D Personality Is Associated With Poor Quality of Life, Social Performance, and Psychological Impairment in Patients With Moderate to Severe Psoriasis: A Cross-Sectional Study of 130 Patients. Indian J Dermatol Venereol Leprol (2020) 86(4):375–81. doi: 10.4103/ijdvl.IJDVL_114_19
70. Kwan Z, Bong YB, Tan LL, Lim SX, Yong ASW, Ch'ng CC, et al. Determinants of Quality of Life and Psychological Status in Adults With Psoriasis. Arch Dermatol Res (2018) 310(5):443–51. doi: 10.1007/s00403-018-1832-x
71. Tribó MJ, Turroja M, Castaño-Vinyals G, Bulbena A, Ros E, García-Martínez P, et al. Patients With Moderate to Severe Psoriasis Associate With Higher Risk of Depression and Anxiety Symptoms: Results of a Multivariate Study of 300 Spanish Individuals With Psoriasis. Acta Derm Venereol (2019) 99(4):417–22. doi: 10.2340/00015555-3114
72. Bang CH, Yoon JW, Chun JH, Han JH, Park YM, Lee SJ, et al. Association of Psoriasis With Mental Health Disorders in South Korea. JAMA Dermatol (2019) 155(6):747–9. doi: 10.1001/jamadermatol.2019.0315
73. Zhang Q, Han J, Zhang Y, Li C, Chen P, Zhang J, et al. Study on the Psychological Health and Related Risk Factors in 245 Patients With Psoriasis in Inner Mongolia. Psychol Health Med (2019) 24(7):769–80. doi: 10.1080/13548506.2019.1574352
74. Min C, Kim M, Oh DJ, Choi HG. Bidirectional Association Between Psoriasis and Depression: Two Longitudinal Follow-Up Studies Using a National Sample Cohort. J Affect Disord (2020) 262:126–32. doi: 10.1016/j.jad.2019.10.043
75. Read C, Armstrong AW. Association Between the Mental Health of Patients With Psoriasis and Their Satisfaction With Physicians. JAMA Dermatol (2020) 156(7):754–62. doi: 10.1001/jamadermatol.2020.1054
76. Cai Q, Teeple A, Wu B, Muser E. Prevalence and Economic Burden of Comorbid Anxiety and Depression Among Patients With Moderate-To-Severe Psoriasis. J Med Econ (2019) 22(12):1290–7. doi: 10.1080/13696998.2019.1638788
77. Hu SC, Chen GS, Tu HP. Epidemiology of Depression in Patients With Psoriasis: A Nationwide Population-Based Cross-Sectional Study. Acta Derm Venereol (2019) 99(6):530–8. doi: 10.2340/00015555-3145
78. Egeberg A, Thyssen JP, Wu JJ, Skov L. Risk of First-Time and Recurrent Depression in Patients With Psoriasis: A Population-Based Cohort Study. Br J Dermatol (2019) 180(1):116–21. doi: 10.1111/bjd.17208
79. Oh J, Jung KJ, Kim TG, Kim HW, Jee SH, Lee MG. Risk of Psychiatric Diseases Among Patients With Psoriasis in Korea: A 12-Year Nationwide Population-Based Cohort Study. J Dermatol (2021) 48(11):1763–71. doi: 10.1111/1346-8138.16115
80. Tian Z, Huang Y, Yue T, Zhou J, Tao L, Han L, et al. A Chinese Cross-Sectional Study on Depression and Anxiety Symptoms in Patients With Psoriasis Vulgaris. Psychol Health Med (2019) 24(3):269–80. doi: 10.1080/13548506.2018.1529323
81. Tzur Bitan D, Krieger I, Comaneshter D, Cohen AD, Feingold D. The Association Between the Socioeconomic Status and Anxiety-Depression Comorbidity in Patients With Psoriasis: A Nationwide Population-Based Study. J Eur Acad Dermatol Venereol (2019) 33(8):1555–61. doi: 10.1111/jdv.15651
82. Lamb RC, Matcham F, Turner MA, Rayner L, Simpson A, Hotopf M, et al. Screening for Anxiety and Depression in People With Psoriasis: A Cross-Sectional Study in a Tertiary Referral Setting. Br J Dermatol (2017) 176(4):1028–34. doi: 10.1111/bjd.14833
83. Modalsli EH, Åsvold BO, Snekvik I, Romundstad PR, Naldi L, Saunes M. The Association Between the Clinical Diversity of Psoriasis and Depressive Symptoms: The Hunt Study, Norway. J Eur Acad Dermatol Venereol (2017) 31(12):2062–8. doi: 10.1111/jdv.14449
84. Vallerand IA, Lewinson RT, Parsons LM, Lowerison MW, Patten SB, Barnabe C. Depression as a Risk Factor for the Development of Psoriasis: A Retrospective Cohort Study in the Uk. Br J Dermatol (2020) 183(4):776–8. doi: 10.1111/bjd.19160
85. Chen YH, Wang WM, Li IH, Kao HH, Yeh CB, Kao LT. Major Depressive Disorder Increased Risk of Psoriasis: A Propensity Score Matched Cohort Study. J Affect Disord (2021) 278:407–12. doi: 10.1016/j.jad.2020.09.108
86. Sondermann W, Schreiber A, Körber A, Fiege O, Scherbaum N, Benson S, et al. Psychosocial Burden and Body Mass Index Are Associated With Dermatology-Related Quality of Life in Psoriasis Patients. Eur J Dermatol (2020) 30(2):140–7. doi: 10.1684/ejd.2020.3755
87. Kwan Z, Bong YB, Tan LL, Lim SX, Yong AS, Ch'ng CC, et al. Socioeconomic and Sociocultural Determinants of Psychological Distress and Quality of Life Among Patients With Psoriasis in a Selected Multi-Ethnic Malaysian Population. Psychol Health Med (2017) 22(2):184–95. doi: 10.1080/13548506.2016.1220603
88. Fleming P, Bai JW, Pratt M, Sibbald C, Lynde C, Gulliver WP. The Prevalence of Anxiety in Patients With Psoriasis: A Systematic Review of Observational Studies and Clinical Trials. J Eur Acad Dermatol Venereol (2017) 31(5):798–807. doi: 10.1111/jdv.13891
89. Singh S, Taylor C, Kornmehl H, Armstrong AW. Psoriasis and Suicidality: A Systematic Review and Meta-Analysis. J Am Acad Dermatol (2017) 77(3):425–40.e2. doi: 10.1016/j.jaad.2017.05.019
90. Pompili M, Bonanni L, Gualtieri F, Trovini G, Persechino S, Baldessarini RJ. Suicidal Risks With Psoriasis and Atopic Dermatitis: Systematic Review and Meta-Analysis. J Psychosom Res (2021) 141:110347. doi: 10.1016/j.jpsychores.2020.110347
91. Chi CC, Chen TH, Wang SH, Tung TH. Risk of Suicidality in People With Psoriasis: A Systematic Review and Meta-Analysis of Cohort Studies. Am J Clin Dermatol (2017) 18(5):621–7. doi: 10.1007/s40257-017-0281-1
92. Charoenngam N, Rittiphairoj T, Ponvilawan B, Ungprasert P. Patients With Psoriasis Have a Higher Risk of Dementia: A Systematic Review and Meta-Analysis. Indian J Dermatol Venereol Leprol (2021) 87(3):364–70. doi: 10.25259/ijdvl_732_19
93. Yen H, Yen H, Chi CC. Is Psoriasis Associated With Dementia or Cognitive Impairment? A Critically Appraised Topic. Br J Dermatol (2021) 184(1):34–42. doi: 10.1111/bjd.19025
94. Snast I, Reiter O, Atzmony L, Leshem YA, Hodak E, Mimouni D, et al. Psychological Stress and Psoriasis: A Systematic Review and Meta-Analysis. Br J Dermatol (2018) 178(5):1044–55. doi: 10.1111/bjd.16116
95. Stewart TJ, Tong W, Whitfeld MJ. The Associations Between Psychological Stress and Psoriasis: A Systematic Review. Int J Dermatol (2018) 57(11):1275–82. doi: 10.1111/ijd.13956
96. Gupta MA, Simpson FC, Gupta AK. Psoriasis and Sleep Disorders: A Systematic Review. Sleep Med Rev (2016) 29:63–75. doi: 10.1016/j.smrv.2015.09.003
97. Laverde-Saad A, Milan R, Mohand-Saïd S, LeLorier J, Litvinov IV, Rahme E. The Risk of Suicidal Behaviour in Individuals With Psoriasis: A Retrospective Cohort Study in Quebec, Canada. J Eur Acad Dermatol Venereol (2020) 34(12):e800–e2. doi: 10.1111/jdv.16647
98. Wang SH, Wang J, Chi CC, Lin YS, Liao SC, Chen PE, et al. Risk for Suicidal Behavior Among Psoriasis Patients: A Nationwide Cohort Study. Am J Clin Dermatol (2020) 21(3):431–9. doi: 10.1007/s40257-019-00489-9
99. Strober BE, Langley RGB, Menter A, Magid M, Porter B, Fox T, et al. No Elevated Risk for Depression, Anxiety or Suicidality With Secukinumab in a Pooled Analysis of Data From 10 Clinical Studies in Moderate-To-Severe Plaque Psoriasis. Br J Dermatol (2018) 178(2):e105–e7. doi: 10.1111/bjd.16051
100. Wu CY, Hu HY, Chou YJ, Li CP, Chang YT. Psoriasis Is Not a Risk Factor for Dementia: A 12-Year Nationwide Population-Based Cohort Study. Arch Dermatol Res (2020) 312(9):657–64. doi: 10.1007/s00403-020-02057-2
101. Lin CC, Lin HC, Chiu HW. Association Between Psoriasis and Dementia: A Population-Based Case-Control Study. Am J Clin Dermatol (2019) 20(3):457–63. doi: 10.1007/s40257-018-00420-8
102. Wong AYS, Frøslev T, Forbes HJ, Kjaersgaard A, Mulick A, Mansfield K, et al. Partner Bereavement and Risk of Psoriasis and Atopic Eczema: Cohort Studies in the U.K. And Denmark. Br J Dermatol (2020) 183(2):321–31. doi: 10.1111/bjd.18740
103. Gupta MA, Gupta AK. Psoriasis Is Associated With a Higher Prevalence of Obstructive Sleep Apnea and Restless Legs Syndrome: A Possible Indication of Autonomic Activation in Psoriasis. J Clin Sleep Med (2018) 14(6):1085. doi: 10.5664/jcsm.7194
104. Tsai SY, Chen HJ, Chen C, Lio CF, Kuo CF, Leong KH, et al. Increased Risk of Chronic Fatigue Syndrome Following Psoriasis: A Nationwide Population-Based Cohort Study. J Transl Med (2019) 17(1):154. doi: 10.1186/s12967-019-1888-1
105. Skoie IM, Dalen I, Ternowitz T, Jonsson G, Kvivik I, Norheim K, et al. Fatigue in Psoriasis: A Controlled Study. Br J Dermatol (2017) 177(2):505–12. doi: 10.1111/bjd.15375
106. Lee JH, Han K, Gee HY. The Incidence Rates and Risk Factors of Parkinson Disease in Patients With Psoriasis: A Nationwide Population-Based Cohort Study. J Am Acad Dermatol (2020) 83(6):1688–95. doi: 10.1016/j.jaad.2019.07.012
107. Capo A, Affaitati G, Giamberardino MA, Amerio P. Psoriasis and Migraine. J Eur Acad Dermatol Venereol (2018) 32(1):57–61. doi: 10.1111/jdv.14472
108. Yu S, Yu CL, Huang YC, Tu HP, Lan CE. Risk of Developing Psoriasis in Patients With Schizophrenia: A Nationwide Retrospective Cohort Study. J Eur Acad Dermatol Venereol (2017) 31(9):1497–504. doi: 10.1111/jdv.14303
109. Dehghani F, Dehghani F, Kafaie P, Taghizadeh MR. Alexithymia in Different Dermatologic Patients. Asian J Psychiatr (2017) 25:42–5. doi: 10.1016/j.ajp.2016.10.011
110. Graetz C, Woeste S, Mrowietz U, Ehrenthal JC. The Impact of Psychological Attachment on the Relationship Between Periodontal Health and Dental Fear in Patients With Versus Without Psoriasis: A Questionnaire-Based, Cross-Sectional Study. BMC Oral Health (2021) 21(1):95. doi: 10.1186/s12903-021-01457-8
111. Almeida V, Taveira S, Teixeira M, Almeida I, Rocha J, Teixeira A. Emotion Regulation in Patients With Psoriasis: Correlates of Disability, Clinical Dimensions, and Psychopathology Symptoms. Int J Behav Med (2017) 24(4):563–70. doi: 10.1007/s12529-016-9617-0
112. Zill JM, Christalle E, Tillenburg N, Mrowietz U, Augustin M, Härter M, et al. Effects of Psychosocial Interventions on Patient-Reported Outcomes in Patients With Psoriasis: A Systematic Review and Meta-Analysis. Br J Dermatol (2019) 181(5):939–45. doi: 10.1111/bjd.17272
113. Islam MM, Poly TN, Yang HC, Wu CC, Li YC. Increase Risk of Multiple Sclerosis in Patients With Psoriasis Disease: An Evidence of Observational Studies. Neuroepidemiology (2019) 52(3-4):152–60. doi: 10.1159/000495112
114. Liu CY, Tung TH, Lee CY, Chang KH, Wang SH, Chi CC. Association of Multiple Sclerosis With Psoriasis: A Systematic Review and Meta-Analysis of Observational Studies. Am J Clin Dermatol (2019) 20(2):201–8. doi: 10.1007/s40257-018-0399-9
115. Charlton O, Phan K, Smith SD, Parratt J. Psoriasis in Family Members of Patients With Multiple Sclerosis. Mult Scler Relat Disord (2019) 36:101421. doi: 10.1016/j.msard.2019.101421
116. Yen H, Chi CC. Association Between Psoriasis and Vitiligo: A Systematic Review and Meta-Analysis. Am J Clin Dermatol (2019) 20(1):31–40. doi: 10.1007/s40257-018-0394-1
117. Egeberg A, Mallbris L, Gislason GH, Skov L, Hansen PR. Risk of Multiple Sclerosis in Patients With Psoriasis: A Danish Nationwide Cohort Study. J Invest Dermatol (2016) 136(1):93–8. doi: 10.1038/jid.2015.350
118. Guido N, Cices A, Ibler E, Huynh T, Majewski S, Sable K, et al. Multiple Sclerosis Association With Psoriasis: A Large U.S. Population, Single Centre, Retrospective Cross-Sectional Study. J Eur Acad Dermatol Venereol (2017) 31(9):e397–e8. doi: 10.1111/jdv.14205
119. Alinaghi F, Tekin HG, Burisch J, Wu JJ, Thyssen JP, Egeberg A. Global Prevalence and Bidirectional Association Between Psoriasis and Inflammatory Bowel Disease-A Systematic Review and Meta-Analysis. J Crohns Colitis (2020) 14(3):351–60. doi: 10.1093/ecco-jcc/jjz152
120. Fu Y, Lee CH, Chi CC. Association of Psoriasis With Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis. JAMA Dermatol (2018) 154(12):1417–23. doi: 10.1001/jamadermatol.2018.3631
121. Feldman SR, Srivastava B, Abell J, Hoops T, Fakharzadeh S, Chakravarty S, et al. Gastrointestinal Signs and Symptoms Related to Inflammatory Bowel Disease in Patients With Moderate-To-Severe Psoriasis. J Drugs Dermatol (2018) 17(12):1298–308.
122. Egeberg A, Thyssen JP, Burisch J, Colombel JF. Incidence and Risk of Inflammatory Bowel Disease in Patients With Psoriasis-A Nationwide 20-Year Cohort Study. J Invest Dermatol (2019) 139(2):316–23. doi: 10.1016/j.jid.2018.07.029
123. Masaki S, Bayaraa B, Imafuku S. Prevalence of Inflammatory Bowel Disease in Japanese Psoriatic Patients. J Dermatol (2019) 46(7):590–4. doi: 10.1111/1346-8138.14900
124. Eppinga H, Poortinga S, Thio HB, Nijsten TEC, Nuij V, van der Woude CJ, et al. Prevalence and Phenotype of Concurrent Psoriasis and Inflammatory Bowel Disease. Inflamm Bowel Dis (2017) 23(10):1783–9. doi: 10.1097/mib.0000000000001169
125. Egeberg A, Mallbris L, Gislason G, Skov L. Limited Incremental Effect of Inflammatory Bowel Disease on Risk of Comorbidities in Patients With Psoriasis. J Dermatol (2017) 44(10):1176–7. doi: 10.1111/1346-8138.13658
126. Shaw CA, Kole LCS, Elewski BE. Association of Psoriasis/Psoriatic Arthritis With Inflammatory Bowel Disease Influences Management Strategy. J Eur Acad Dermatol Venereol (2019) 33(11):e431–e2. doi: 10.1111/jdv.15752
127. Lee JY, Kang S, Bae JM, Jo SJ, Koh SJ, Park HS. Psoriasis Increases the Risk of Concurrent Inflammatory Bowel Disease: A Population-Based Nationwide Study in Korea. Indian J Dermatol Venereol Leprol (2019) 85(2):145–52. doi: 10.4103/ijdvl.IJDVL_875_17
128. Whitlock SM, Enos CW, Armstrong AW, Gottlieb A, Langley RG, Lebwohl M, et al. Management of Psoriasis in Patients With Inflammatory Bowel Disease: From the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol (2018) 78(2):383–94. doi: 10.1016/j.jaad.2017.06.043
129. Belinchón-Romero I, Bellot P, Romero-Pérez D, Herraiz-Romero I, Marco F, Frances R, et al. Non-Alcoholic Fatty Liver Disease Is Associated With Bacterial Translocation and a Higher Inflammation Response in Psoriatic Patients. Sci Rep (2021) 11(1):8593. doi: 10.1038/s41598-021-88043-8
130. Xu X, Su L, Gao Y, Ding Y. The Prevalence of Nonalcoholic Fatty Liver Disease and Related Metabolic Comorbidities Was Associated With Age at Onset of Moderate to Severe Plaque Psoriasis: A Cross-Sectional Study. PloS One (2017) 12(1):e0169952. doi: 10.1371/journal.pone.0169952
131. Neagoe CD, Farmazon AS, Amzolini AM, Singer CE, Ianoşi SL, Tutunaru CV, et al. The Role of Non-Invasive Scores in Determining the Liver Fibrosis in Nafld and Psoriatic Patients. Rom J Morphol Embryol (2020) 61(2):503–11. doi: 10.47162/rjme.61.2.20
132. van der Voort EAM, Wakkee M, Veldt-Kok P, Darwish Murad S, Nijsten T. Enhanced Liver Fibrosis Test in Patients With Psoriasis, Psoriatic Arthritis and Rheumatoid Arthritis: A Cross-Sectional Comparison With Procollagen-3 N-Terminal Peptide (P3np). Br J Dermatol (2017) 176(6):1599–606. doi: 10.1111/bjd.15220
133. Acharya P, Mathur M. Association Between Psoriasis and Celiac Disease: A Systematic Review and Meta-Analysis. J Am Acad Dermatol (2020) 82(6):1376–85. doi: 10.1016/j.jaad.2019.11.039
134. Egeberg A, Andersen YMF, Gislason GH, Skov L, Thyssen JP. Gallstone Risk in Adult Patients With Atopic Dermatitis and Psoriasis: Possible Effect of Overweight and Obesity. Acta Derm Venereol (2017) 97(5):627–31. doi: 10.2340/00015555-2622
135. Lee EB, Amin M, Duan L, Egeberg A, Wu JJ. Risk for Appendicitis, Cholecystitis, or Diverticulitis in Patients With Psoriasis. Cutis (2019) 103(3):175–9;e1;e2.
136. Ogdie A, Grewal SK, Noe MH, Shin DB, Takeshita J, Chiesa Fuxench ZC, et al. Risk of Incident Liver Disease in Patients With Psoriasis, Psoriatic Arthritis, and Rheumatoid Arthritis: A Population-Based Study. J Invest Dermatol (2018) 138(4):760–7. doi: 10.1016/j.jid.2017.10.024
137. Schonmann Y, Mansfield KE, Mulick A, Roberts A, Smeeth L, Langan SM, et al. Inflammatory Skin Diseases and the Risk of Chronic Kidney Disease: Population-Based Case-Control and Cohort Analyses. Br J Dermatol (2021) 185(4):772–80. doi: 10.1111/bjd.20067
138. Lee SH, Kim M, Han KD, Lee JH. Low Hemoglobin Levels and an Increased Risk of Psoriasis in Patients With Chronic Kidney Disease. Sci Rep (2021) 11(1):14741. doi: 10.1038/s41598-021-94165-w
139. Lee E, Han JH, Bang CH, Yoo SA, Han KD, Kim HN, et al. Risk of End-Stage Renal Disease in Psoriatic Patients: Real-World Data From a Nationwide Population-Based Cohort Study. Sci Rep (2019) 9(1):16581. doi: 10.1038/s41598-019-53017-4
140. Wang CC, Tang CH, Huang KC, Huang SY, Sue YM. Increased Risk of Incident Psoriasis in End-Stage Renal Disease Patients on Chronic Hemodialysis: A Nationwide Population-Based Cohort Study. J Dermatol (2018) 45(9):1063–70. doi: 10.1111/1346-8138.14531
141. Grewal SK, Wan J, Denburg MR, Shin DB, Takeshita J, Gelfand JM. The Risk of Iga Nephropathy and Glomerular Disease in Patients With Psoriasis: A Population-Based Cohort Study. Br J Dermatol (2017) 176(5):1366–9. doi: 10.1111/bjd.14961
142. Vaengebjerg S, Skov L, Egeberg A, Loft ND. Prevalence, Incidence, and Risk of Cancer in Patients With Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-Analysis. JAMA Dermatol (2020) 156(4):421–9. doi: 10.1001/jamadermatol.2020.0024
143. Reddy SP, Martires K, Wu JJ. The Risk of Melanoma and Hematologic Cancers in Patients With Psoriasis. J Am Acad Dermatol 1区 (2017) 76(4):639–47.e2. doi: 10.1016/j.jaad.2016.09.047
144. Polachek A, Muntyanu A, Lee KA, Ye JY, Chandran V, Cook RJ, et al. Malignancy in Psoriatic Disease: Results From Prospective Longitudinal Cohorts. Semin Arthritis Rheum 2区 (2021) 51(1):144–9. doi: 10.1016/j.semarthrit.2020.12.008
145. Trafford AM, Parisi R, Kontopantelis E, Griffiths CEM, Ashcroft DM. Association of Psoriasis With the Risk of Developing or Dying of Cancer: A Systematic Review and Meta-Analysis. JAMA Dermatol (2019) 155(12):1390–403. doi: 10.1001/jamadermatol.2019.3056
146. Jensen P, Egeberg A, Gislason G, Thyssen JP, Skov L. Risk of Uncommon Cancers in Patients With Psoriasis: A Danish Nationwide Cohort Study. J Eur Acad Dermatol Venereol (2018) 32(4):601–5. doi: 10.1111/jdv.14610
147. Lee JW, Jung KJ, Kim TG, Lee M, Oh J, Jee SH, et al. Risk of Malignancy in Patients With Psoriasis: A 15-Year Nationwide Population-Based Prospective Cohort Study in Korea. J Eur Acad Dermatol Venereol (2019) 33(12):2296–304. doi: 10.1111/jdv.15783
148. Mason KJ, Burden AD, Barker J, Lunt M, Ali H, Kleyn CE, et al. Characteristics and Skin Cancer Risk of Psoriasis Patients With a History of Skin Cancer in Badbir. J Eur Acad Dermatol Venereol (2021) 35(8):e498–501. doi: 10.1111/jdv.17230
149. Giannopoulos F, Gillstedt M, Laskowski M, Bruun Kristensen K, Polesie S. Methotrexate Use for Patients With Psoriasis and Risk of Cutaneous Squamous Cell Carcinoma: A Nested Case-Control Study. Acta Derm Venereol (2021) 101(1):adv00365. doi: 10.2340/00015555-3725
150. Lima XT, Cueva MA, Lopes EM, Alora MB. Severe Covid-19 Outcomes in Patients With Psoriasis. J Eur Acad Dermatol Venereol (2020) 34(12):e776–e8. doi: 10.1111/jdv.16867
151. Takeshita J, Shin DB, Ogdie A, Gelfand JM. Risk of Serious Infection, Opportunistic Infection, and Herpes Zoster Among Patients With Psoriasis in the United Kingdom. J Invest Dermatol (2018) 138(8):1726–35. doi: 10.1016/j.jid.2018.01.039
152. Loft ND, Halling AS, Iversen L, Vestergaard C, Deleuran M, Rasmussen MK, et al. Concerns Related to the Coronavirus Disease 2019 Pandemic in Adult Patients With Atopic Dermatitis and Psoriasis Treated With Systemic Immunomodulatory Therapy: A Danish Questionnaire Survey. J Eur Acad Dermatol Venereol (2020) 34(12):e773–e6. doi: 10.1111/jdv.16863
153. Syed MN, Shah M, Shin DB, Wan MT, Winthrop KL, Gelfand JM. Effect of Anti-Tumor Necrosis Factor Therapy on the Risk of Respiratory Tract Infections and Related Symptoms in Patients With Psoriasis-A Meta-Estimate of Pivotal Phase 3 Trials Relevant to Decision Making During the Covid-19 Pandemic. J Am Acad Dermatol (2021) 84(1):161–3. doi: 10.1016/j.jaad.2020.08.095
154. Mahil SK, Dand N, Mason KJ, Yiu ZZN, Tsakok T, Meynell F, et al. Factors Associated With Adverse Covid-19 Outcomes in Patients With Psoriasis-Insights From a Global Registry-Based Study. J Allergy Clin Immunol (2021) 147(1):60–71. doi: 10.1016/j.jaci.2020.10.007
155. Yiu ZZN, Parisi R, Lunt M, Warren RB, Griffiths CEM, Langan SM, et al. Risk of Hospitalization and Death Due to Infection in People With Psoriasis: A Population-Based Cohort Study Using the Clinical Practice Research Datalink. Br J Dermatol (2021) 184(1):78–86. doi: 10.1111/bjd.19052
156. Diotallevi F, Campanati A, Radi G, Martina E, Rizzetto G, Barbadoro P, et al. Vaccination Against Sars-Cov-2 and Psoriasis: The Three Things Every Dermatologist Should Know. J Eur Acad Dermatol Venereol (2021) 35(7):e428–e30. doi: 10.1111/jdv.17256
157. Orrell KA, Vakharia PP, Hagstrom EL, Brieva J, West DP, Nardone B. Prevalence of Chronic Hepatitis B and C in Psoriasis Patients: A Cross-Sectional Study in a Large Us Population. J Am Acad Dermatol (2017) 77(3):572–3. doi: 10.1016/j.jaad.2017.05.020
158. Noe MH, Grewal SK, Shin DB, Ogdie A, Takeshita J, Gelfand JM. Increased Prevalence of Hcv and Hepatic Decompensation in Adults With Psoriasis: A Population-Based Study in the United Kingdom. J Eur Acad Dermatol Venereol (2017) 31(10):1674–80. doi: 10.1111/jdv.14310
159. Tang KT, Chen YM, Chang SN, Lin CH, Chen DY. Psoriatic Patients With Chronic Viral Hepatitis Do Not Have an Increased Risk of Liver Cirrhosis Despite Long-Term Methotrexate Use: Real-World Data From a Nationwide Cohort Study in Taiwan. J Am Acad Dermatol (2018) 79(4):652–8. doi: 10.1016/j.jaad.2018.05.004
160. Chun K, Afshar M, Audish D, Kabigting F, Paik A, Gallo R, et al. Hepatitis C May Enhance Key Amplifiers of Psoriasis. J Eur Acad Dermatol Venereol (2017) 31(4):672–8. doi: 10.1111/jdv.13578
161. Yen YF, Jen IA, Chen M, Lan YC, Lee CY, Chuang PH, et al. Hiv Infection Increases the Risk of Incident Psoriasis: A Nationwide Population-Based Cohort Study in Taiwan. J Acquir Immune Defic Syndr (2017) 75(5):493–9. doi: 10.1097/qai.0000000000001431
162. Busca Arenzana C, Quintana Castanedo L, Chiloeches Fernández C, Nieto Rodríguez D, Herranz Pinto P, Delgado Hierro AB, et al. Psoriasis and Liver Damage in Hiv-Infected Patients. Cells (2021) 10(5):1099. doi: 10.3390/cells10051099
163. Bardazzi F, Magnano M, Campanati A, Loconsole F, Carpentieri A, Potenza C, et al. Biologic Therapies in Hiv-Infected Patients With Psoriasis: An Italian Experience. Acta Derm Venereol (2017) 97(8):989–90. doi: 10.2340/00015555-2698
164. Wang H, Wang C, Wang Z, Fu X, Yu G, Sun L, et al. Identification of Zfp36l1 as an Early-Onset Psoriasis Risk Gene Demonstrates Opposite Associations With Leprosy and Psoriasis in the Chinese Population. J Eur Acad Dermatol Venereol (2020) 34(9):e520–e3. doi: 10.1111/jdv.16437
165. Gisondi P, Cazzaniga S, Chimenti S, Maccarone M, Picardo M, Girolomoni G, et al. Latent Tuberculosis Infection in Patients With Chronic Plaque Psoriasis: Evidence From the Italian Psocare Registry. Br J Dermatol (2015) 172(6):1613–20. doi: 10.1111/bjd.13539
166. Benhadou F, Dirix V, Domont F, Willaert F, Van Praet A, Locht C, et al. Tuberculosis Risk Stratification of Psoriatic Patients Before Anti-Tnf-A Treatment. Front Immunol (2021) 12:672894. doi: 10.3389/fimmu.2021.672894
167. Ng CY, Huang YH, Chu CF, Wu TC, Liu SH. Risks for Staphylococcus Aureus Colonization in Patients With Psoriasis: A Systematic Review and Meta-Analysis. Br J Dermatol (2017) 177(4):967–77. doi: 10.1111/bjd.15366
168. Tsai SY, Chen HJ, Lio CF, Ho HP, Kuo CF, Jia X, et al. Increased Risk of Herpes Zoster in Patients With Psoriasis: A Population-Based Retrospective Cohort Study. PloS One (2017) 12(8):e0179447. doi: 10.1371/journal.pone.0179447
169. Blegvad C, Egeberg A, Tind Nielsen TE, Gislason GH, Zachariae C, Nybo Andersen AM, et al. Autoimmune Disease in Children and Adolescents With Psoriasis: A Cross-Sectional Study in Denmark. Acta Derm Venereol (2017) 97(10):1225–9. doi: 10.2340/00015555-2743
170. Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of Psoriatic Arthritis in Patients With Psoriasis: A Systematic Review and Meta-Analysis of Observational and Clinical Studies. J Am Acad Dermatol (2019) 80(1):251–65.e19. doi: 10.1016/j.jaad.2018.06.027
171. Lindberg I, Lilja M, Geale K, Tian H, Richardson C, Scott A, et al. Incidence of Psoriatic Arthritis in Patients With Skin Psoriasis and Associated Risk Factors: A Retrospective Population-Based Cohort Study in Swedish Routine Clinical Care. Acta Derm Venereol (2020) 100(18):adv00324. doi: 10.2340/00015555-3682
172. Belman S, Walsh JA, Carroll C, Milliken M, Haaland B, Duffin KC, et al. Psoriasis Characteristics for the Early Detection of Psoriatic Arthritis. J Rheumatol (2021) 48(10):1559–65. doi: 10.3899/jrheum.201123
173. Egeberg A, Skov L, Zachariae C, Gislason GH, Thyssen JP, Mallbris L. Duration of Psoriatic Skin Disease as Risk Factor for Subsequent Onset of Psoriatic Arthritis. Acta Derm Venereol (2018) 98(6):546–50. doi: 10.2340/00015555-2912
174. Skornicki M, Prince P, Suruki R, Lee E, Louder A. Clinical Burden of Concomitant Joint Disease in Psoriasis: A Us-Linked Claims and Electronic Health Records Database Analysis. Adv Ther (2021) 38(5):2458–71. doi: 10.1007/s12325-021-01698-7
175. Mease PJ, Palmer JB, Hur P, Strober BE, Lebwohl M, Karki C, et al. Utilization of the Validated Psoriasis Epidemiology Screening Tool to Identify Signs and Symptoms of Psoriatic Arthritis Among Those With Psoriasis: A Cross-Sectional Analysis From the Us-Based Corrona Psoriasis Registry. J Eur Acad Dermatol Venereol (2019) 33(5):886–92. doi: 10.1111/jdv.15443
176. Salaffi F, Di Carlo M, Luchetti MM, Di Donato E, Campanati A, Benfaremo D, et al. A Validation Study of the Simple Psoriatic Arthritis Screening (Sipas) Questionnaire to Screen Psoriasis Patients for Psoriatic Arthritis. Clin Exp Rheumatol (2018) 36(1):127–35.
177. Mulder MLM, van Hal TW, Wenink MH, Koenen H, van den Hoogen FHJ, de Jong E, et al. Clinical, Laboratory, and Genetic Markers for the Development or Presence of Psoriatic Arthritis in Psoriasis Patients: A Systematic Review. Arthritis Res Ther (2021) 23(1):168. doi: 10.1186/s13075-021-02545-4
178. Zuliani F, Zabotti A, Errichetti E, Tinazzi I, Zanetti A, Carrara G, et al. Ultrasonographic Detection of Subclinical Enthesitis and Synovitis: A Possible Stratification of Psoriatic Patients Without Clinical Musculoskeletal Involvement. Clin Exp Rheumatol (2019) 37(4):593–9.
179. Schmidt A, Glimm AM, Haugen IK, Hoff P, Schmittat G, Burmester GR, et al. Detection of Subclinical Skin Manifestation in Patients With Psoriasis and Psoriatic Arthritis by Fluorescence Optical Imaging. Arthritis Res Ther (2020) 22(1):192. doi: 10.1186/s13075-020-02277-x
180. Kathuria P, Gordon KB, Silverberg JI. Association of Psoriasis and Psoriatic Arthritis With Osteoporosis and Pathological Fractures. J Am Acad Dermatol (2017) 76(6):1045–53.e3. doi: 10.1016/j.jaad.2016.11.046
181. Modalsli EH, Åsvold BO, Romundstad PR, Langhammer A, Hoff M, Forsmo S, et al. Psoriasis, Fracture Risk and Bone Mineral Density: The Hunt Study, Norway. Br J Dermatol (2017) 176(5):1162–9. doi: 10.1111/bjd.15123
182. Lee JW, Min C, Bang CH, Kwon BC, Choi HG. Psoriasis Is Associated With an Increased Risk of Osteoporosis: Follow-Up and Nested Case-Control Studies Using a National Sample Cohort. Osteoporos Int (2021) 32(3):529–38. doi: 10.1007/s00198-020-05724-2
183. Sepehri NZ, Raeisi T, Razi B, Janmohammadi P, Darand M, Alizadeh S. The Association Between Psoriasis and Psoriatic Arthritis With the Risk of Osteoporosis, Osteopenia and Bone Fractures: A Systematic Review and Meta-Analysis. Int J Clin Pract (2021) 75(11):e14630. doi: 10.1111/ijcp.14630
184. Zander N, Schäfer I, Radtke M, Jacobi A, Heigel H, Augustin M. Dermatological Comorbidity in Psoriasis: Results From a Large-Scale Cohort of Employees. Arch Dermatol Res (2017) 309(5):349–56. doi: 10.1007/s00403-017-1741-4
185. Caldarola G, De Simone C, Talamonti M, Moretta G, Fossati B, Bianchi L, et al. Prevalence of Cutaneous Comorbidities in Psoriatic Patients and Their Impact on Quality of Life. Eur J Dermatol (2019) 29(2):192–6. doi: 10.1684/ejd.2019.3529
186. Phan K, Ramachandran V, Smith SD. Association Between Pemphigus and Psoriasis: A Systematic Review and Meta-Analysis. Dermatol Online J (2020) 26(8):13030/qt5g78q4f4. doi: 10.5070/D3268049899
187. Chiu YW, Chen YD, Hua TC, Wu CH, Liu HN, Chang YT. Comorbid Autoimmune Diseases in Patients With Pemphigus: A Nationwide Case-Control Study in Taiwan. Eur J Dermatol (2017) 27(4):375–81. doi: 10.1684/ejd.2017.3060
188. Ho YH, Hu HY, Chang YT, Li CP, Wu CY. Psoriasis Is Associated With Increased Risk of Bullous Pemphigoid: A Nationwide Population-Based Cohort Study in Taiwan. J Dermatol (2019) 46(7):604–9. doi: 10.1111/1346-8138.14902
189. Kridin K, Bergman R. Association Between Bullous Pemphigoid and Psoriasis: A Case-Control Study. J Am Acad Dermatol (2017) 77(2):370–2. doi: 10.1016/j.jaad.2017.02.057
190. Damiani G, Cazzaniga S, Conic RR, Naldi L. Pruritus Characteristics in a Large Italian Cohort of Psoriatic Patients. J Eur Acad Dermatol Venereol (2019) 33(7):1316–24. doi: 10.1111/jdv.15539
191. Reszke R, Białynicki-Birula R, Szepietowski JC. Itch in Psoriasis: A New Look at Well-Known Subject. Acta Derm Venereol (2019) 99(4):429–34. doi: 10.2340/00015555-3147
192. Andersen YMF, Augustin M, Petersen J, Hagenström K, Mallbris L, Burge R, et al. Characteristics and Prevalence of Plaque Psoriasis in Patients With Palmoplantar Pustulosis. Br J Dermatol (2019) 181(5):976–82. doi: 10.1111/bjd.17832
193. Claßen A, Buhl T, Schubert S, Worm M, Bauer A, Geier J, et al. The Frequency of Specific Contact Allergies Is Reduced in Patients With Psoriasis. Br J Dermatol (2019) 180(2):315–20. doi: 10.1111/bjd.17080
194. Kirsten N, Mohr N, Maul JT, Augustin M. Incidence of Atopic Conditions in People With Psoriasis: A Population-Based Analysis. Eur J Dermatol (2021) 31(1):60–4. doi: 10.1684/ejd.2021.3963
195. Wu T, Duan X, Chen S, Chen X, Yu R, Yu X. Association Between Psoriasis and Erectile Dysfunction: A Meta-Analysis. J Sex Med (2018) 15(6):839–47. doi: 10.1016/j.jsxm.2018.04.630
196. Zhao S, Wang J, Xie Q, Liu Y, Luo L, Zhu Z, et al. High Prevalence of Erectile Dysfunction in Men With Psoriasis: Evidence From a Systematic Review and Meta-Analysis. Int J Impot Res (2019) 31(2):74–84. doi: 10.1038/s41443-018-0093-8
197. Egeberg A, Hansen PR, Gislason GH, Skov L, Thyssen JP. Erectile Dysfunction in Male Adults With Atopic Dermatitis and Psoriasis. J Sex Med (2017) 14(3):380–6. doi: 10.1016/j.jsxm.2016.12.233
198. Hillary T, Gutermuth J. Should Psoriasis Be Considered a Risk Factor for Hypogonadism in Male Patients? A Monocentric, Prospective, Observational Pilot Study. J Eur Acad Dermatol Venereol (2017) 31(4):e197–e8. doi: 10.1111/jdv.13944
199. Molina-Leyva A, Salvador-Rodriguez L, Martinez-Lopez A, Ruiz-Carrascosa JC, Arias-Santiago S. Association Between Psoriasis and Sexual and Erectile Dysfunction in Epidemiologic Studies: A Systematic Review. JAMA Dermatol (2019) 155(1):98–106. doi: 10.1001/jamadermatol.2018.3442
200. Xie W, Huang H, Ji L, Zhang Z. Maternal and Neonatal Outcomes in Pregnant Women With Psoriasis and Psoriatic Arthritis: A Systematic Review and Meta-Analysis. Rheumatology (Oxford) (2021) 60(9):4018–28. doi: 10.1093/rheumatology/keab357
201. Wang J, Ke R, Shi W, Yan X, Wang Q, Zhang Q, et al. Association Between Psoriasis and Asthma Risk: A Meta-Analysis. Allergy Asthma Proc (2018) 39(2):103–9. doi: 10.2500/aap.2018.39.4109
202. Kim SY, Min C, Oh DJ, Choi HG. Increased Risk of Psoriasis in Children and Elderly Patients With Asthma: A Longitudinal Follow-Up Study Using a National Sample Cohort. Int Forum Allergy Rhinol (2019) 9(11):1304–10. doi: 10.1002/alr.22429
203. Liu J, Martin A, Thatiparthi A, Wu JJ. Effect Modification by Smoking Status on the Association Between Psoriasis and Chronic Obstructive Pulmonary Disease Among Adults in the USA. Acta Derm Venereol (2021) 101(8):adv00518. doi: 10.2340/00015555-3864
204. González-Álvarez L, García-Martín JM, García-Pola MJ. Association Between Geographic Tongue and Psoriasis: A Systematic Review and Meta-Analyses. J Oral Pathol Med (2019) 48(5):365–72. doi: 10.1111/jop.12840
205. Michalek IM, Loring B, John SM. A Systematic Review of Worldwide Epidemiology of Psoriasis. J Eur Acad Dermatol Venereol (2017) 31(2):205–12. doi: 10.1111/jdv.13854
206. Al Qahtani NA, Deepthi A, Alhussain NM, Al Shahrani BAM, Alshehri H, Alhefzi A, et al. Association of Geographic Tongue and Fissured Tongue With Abo Blood Group Among Adult Psoriasis Patients: A Novel Study From a Tertiary Care Hospital in Saudi Arabia. Oral Surg Oral Med Oral Pathol Oral Radiol (2019) 127(6):490–7. doi: 10.1016/j.oooo.2019.01.080
207. Monshi B, Grabovac S, Gulz L, Ellersdorfer C, Vujic M, Richter L, et al. Psoriasis Is Associated With Fissured Tongue But Not Geographic Tongue: A Prospective, Cross-Sectional, Case-Control Study. J Dtsch Dermatol Ges (2021) 19(8):1170–6. doi: 10.1111/ddg.14451
208. Olejnik M, Osmola-Mańkowska A, Ślebioda Z, Adamski Z, Dorocka-Bobkowska B. Oral Mucosal Lesions in Psoriatic Patients Based on Disease Severity and Treatment Approach. J Oral Pathol Med (2020) 49(8):822–8. doi: 10.1111/jop.13095
209. Ungprasert P, Wijarnpreecha K, Wetter DA. Periodontitis and Risk of Psoriasis: A Systematic Review and Meta-Analysis. J Eur Acad Dermatol Venereol (2017) 31(5):857–62. doi: 10.1111/jdv.14051
210. Egeberg A, Mallbris L, Gislason G, Hansen PR, Mrowietz U. Risk of Periodontitis in Patients With Psoriasis and Psoriatic Arthritis. J Eur Acad Dermatol Venereol (2017) 31(2):288–93. doi: 10.1111/jdv.13814
211. Mendes VS, Cota LOM, Costa AA, Oliveira A, Costa FO. Periodontitis as Another Comorbidity Associated With Psoriasis: A Case-Control Study. J Periodontol (2019) 90(4):358–66. doi: 10.1002/jper.18-0394
212. Painsi C, Hirtenfelder A, Lange-Asschenfeldt B, Quehenberger F, Wolf P. The Prevalence of Periodontitis Is Increased in Psoriasis and Linked to Its Inverse Subtype. Skin Pharmacol Physiol (2017) 30(6):324–8. doi: 10.1159/000481544
213. Lee CY, Chen HC, Lin HW, Huang JY, Lin TL, Yang CH, et al. Increased Risk of Keratopathy After Psoriasis: A Nationwide Population-Based Study. PloS One (2018) 13(7):e0201285. doi: 10.1371/journal.pone.0201285
214. Dai YX, Tai YH, Lee DD, Chang YT, Chen TJ, Chen MH. Risk of Retinal Diseases in Patients With Psoriasis: A Population-Based Cohort Study in Taiwan. J Dermatol (2021) 48(10):1550–6. doi: 10.1111/1346-8138.16062
215. Chi CC, Tung TH, Wang J, Lin YS, Chen YF, Hsu TK, et al. Risk of Uveitis Among People With Psoriasis: A Nationwide Cohort Study. JAMA Ophthalmol (2017) 135(5):415–22. doi: 10.1001/jamaophthalmol.2017.0569
216. Abbagani S, Kamath YS, Nayak S. A Study on Ocular Morbidity Among Patients With Psoriasis Visiting a Tertiary Care Hospital in Karnataka, Southern India. Ocul Immunol Inflamm (2019) 27(4):531–4. doi: 10.1080/09273948.2017.1414271
217. Borgia F, Ciodaro F, Guarneri F, Bartolotta A, Papaianni V, Guarneri C, et al. Auditory System Involvement in Psoriasis. Acta Derm Venereol (2018) 98(7):655–9. doi: 10.2340/00015555-2937
218. Hahn HJ, Kwak SG, Kim DK, Kim JY. Association of Behçet Disease With Psoriasis and Psoriatic Arthritis. Sci Rep (2021) 11(1):2531. doi: 10.1038/s41598-021-81972-4
219. Liu JM, Lin CY, Chang FW, Liu YP, Liang CP, Hsu RJ. Increased Risk of Psoriasis Following Scabies Infection: A Nationwide Population-Based Matched-Cohort Study. J Dermatol (2018) 45(3):302–8. doi: 10.1111/1346-8138.14221
Keywords: psoriasis disease, comorbid disease, epidemiology, autoimmune chronic diseases, prevalence
Citation: Bu J, Ding R, Zhou L, Chen X and Shen E (2022) Epidemiology of Psoriasis and Comorbid Diseases: A Narrative Review. Front. Immunol. 13:880201. doi: 10.3389/fimmu.2022.880201
Received: 21 February 2022; Accepted: 28 April 2022;
Published: 10 June 2022.
Edited by:
Michele Maria Luchetti Gentiloni, Marche Polytechnic University, ItalyReviewed by:
Federico Diotallevi, Marche Polytechnic University, ItalyAnna Campanati, Marche Polytechnic University, Italy
Copyright © 2022 Bu, Ding, Zhou, Chen and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Erxia Shen, erxia_shen@gzhmu.edu.cn
 Jin Bu
Jin Bu Ruilian Ding
Ruilian Ding Liangjia Zhou1
Liangjia Zhou1