- 1Marine Biology Section, Department of Biology, University of Copenhagen, Helsingør, Denmark
- 2Aix-Marseille Université, Université de Toulon, CNRS, IRD, MIO UM 110, Marseille, France
- 3Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat Gan, Israel
- 4Department of Marine Biology, Charney School of Marine Sciences, University of Haifa, Haifa, Israel
The biological fixation of dinitrogen (N2) by marine prokaryotes called diazotrophs is the major source of nitrogen to the ocean, estimated at ~106–120 Tg N y−1 (Gruber, 2004; Gruber and Galloway, 2008). This process contributes importantly to sustain primary production and maintain the global nitrogen inventory. The nitrogen reservoir is further controlled by fixed nitrogen loss processes including sediment burial, denitrification, and anammox (Falkowski, 1997), which exceed fixed nitrogen gains through N2 fixation, leading to an imbalanced global nitrogen budget (Codispoti et al., 2001; Codispoti, 2007; Eugster and Gruber, 2012). Since the early 1970s, diazotrophic activity has been attributed to autotrophic cyanobacteria constrained to the sunlit and oligotrophic layer of the tropical and subtropical oceans (Zehr, 2011). Yet substantial evidence indicates a high diversity and wide distribution of non-cyanobacterial diazotrophs (bacteria and archaea) in the oceans (Zehr et al., 1998, 2000; Farnelid et al., 2011; Bombar et al., 2016; Moisander et al., 2017). These diazotrophs are potentially not constrained by light as are their cyanobacterial counterparts, and have been detected in wide-ranging environments such as nutrient-rich, cold, and/or dark ecosystems including coastal upwelling regions (Sohm et al., 2011), temperate coastal zones (Bentzon-Tilia et al., 2015), and the deep ocean (Hewson et al., 2007; Hamersley et al., 2011).
Stretching the environmental boundaries, beyond those traditionally thought to constrain N2 fixation, will likely impact current estimates of nitrogen input to the global ocean. Extending the latitudinal limits from the tropics and subtropics to temperate waters would already represent a considerable increase in the potentially active N2 fixation area, but spreading this area vertically to the mesopelagic (200–1,000 m) and bathypelagic (1,000–4,000 m) ocean would be immense. Aphotic N2 fixation rates are usually low when compared to surface activity (<1 nmol N L−1 d−1; see Table 1 in Moisander et al., 2017) but the volume of the deep ocean is enormous. Consequently, studies comprising both photic and aphotic N2 fixation measurements report depth-integrated aphotic rates representing 40–95% of the whole water column activity (Bonnet et al., 2013; Rahav et al., 2013; Benavides et al., 2015). Hence, aphotic fixation can account for a significant or even predominant fraction of water column N2 fixation.
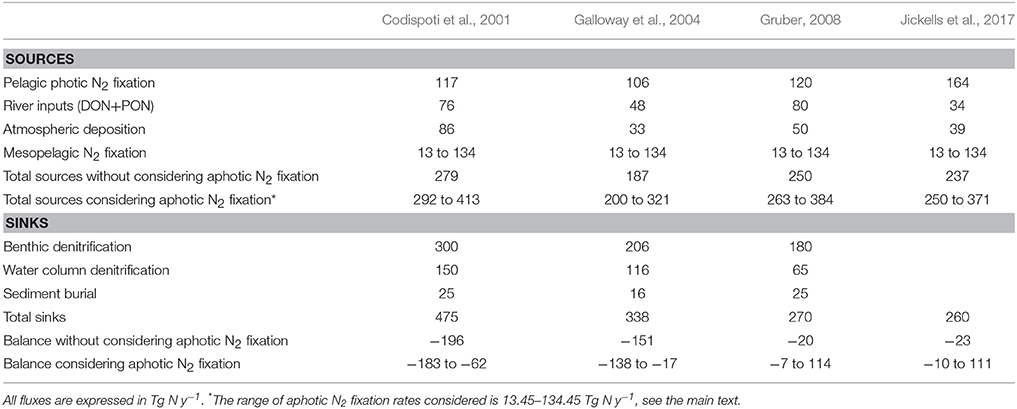
With the mere purpose of illustrating the potential budgetary relevance of the aphotic N2 fixation to the global fixed nitrogen input, a back-of-the-envelope calculation can be carried out. If we consider a scenario for the mesopelagic zone (where the great majority of published aphotic N2 fixation measurements were obtained from): taking the lower-end range of aphotic N2 fixation rates available in the literature (0.01–0.1 nmol N L−1 d−1; Table 1 in Moisander et al., 2017), and the estimated volume of the mesopelagic zone (2.63 × 1017 m3; Arístegui et al., 2005), mesopelagic N2 fixation would range between 13 and 134 Tg N y−1. Fixed nitrogen inputs to the ocean include fluvial inputs, atmospheric deposition and biological N2 fixation, which add up to 187–279 Tg N y−1 (Table 1). Combining denitrification (including sediment burial) and anammox, fixed nitrogen losses add up to 260–475 Tg N y−1 (Table 1). Adding mesopelagic N2 fixation to fixed nitrogen inputs and subtracting losses from gains, we obtain differences ranging from a loss of 183 to a surplus of 114 Tg N y−1 (Table 1). Despite this extrapolation may be questionable given that data on aphotic N2 fixation are so sparse that the spatial distribution of mesopelagic N2 fixation is unknown, it does illustrate that aphotic N2 fixation could be important to global nitrogen budget considerations, and thus deep N2 fixation should be further explored. Considering the stock of fixed nitrogen in the mesopelagic zone (Gruber, 2008) and the range of mesopelagic N2 fixation rates estimated here (i.e., 13–134 Tg N y−1), N2 fixed and eventually remineralized to nitrate in the mesopelagic zone would turn over in 4 to 43 y.
The currently available dataset (Table 1 from Moisander et al., 2017, this issue) lacks robustness because (i) the number of measurements is limited and geographically sparse, and (ii) methodological difficulties are entailed in the detection of low N2 fixation rates. While aphotic N2 fixation has been consistently reported in several tropical and temperate waters (Table 1; Moisander et al., 2017, this issue), it is unknown whether it occurs homogeneously throughout the dark water column or only in micro-niches where suitable conditions are found. Such hospitable niches may comprise aggregates, or organic matter accumulation zones like ecotones, fronts or water mass boundaries (Benavides et al., 2015; Bombar et al., 2016). Only a few studies have documented nifH gene expression in aphotic waters (e.g., Jayakumar et al., 2012), and it is debated whether reported abundances of non-cyanobacterial diazotrophs can account for measured rates of N2 fixation when considering published cell specific rates of cultivated strains (Turk-Kubo et al., 2014; Bentzon-Tilia et al., 2015). This introduces uncertainty to the reliability of measuring especially low N2 fixation rates (Gradoville et al., 2017), and emphasizes the need for continued refinement of the 15N2 incorporation method (Moisander et al., 2017).
In this context, it is pertinent to consider the methodological difficulties encompassed in the detection of low N2 fixation rates using 15N2 as a tracer. The precision of N2 fixation rates may be affected by (i) a slower than theoretically assumed dissolution of the 15N2 bubble in seawater (Mohr et al., 2010; Großkopf et al., 2012), (ii) the contamination of 15N2 gas stocks with nitrogenous species other than N2 (Dabundo et al., 2014), and (iii) failure to measure time zero δ15N values of the particulate nitrogen pool. As any other tracer method, 15N2-based N2 fixation rates are subject to a number of other sources of error, including variability in incubation and/or filtration time among replicates, sample particle size and its retention in filters varying with filter pore size (Bombar et al., 2018), as well as heterogeneous distribution of particles in Niskin bottles (Suter et al., 2017). Moreover, the vast majority of 15N2-based published N2 fixation measurements report net rates, whereas the leakage of 15N-labeled dissolved organic nitrogen and/or ammonium can be significant in certain cases (e.g., Berthelot et al., 2017).
Most of the compiled aphotic rates (Moisander et al., 2017, this issue) were measured using the bubble method (Montoya et al., 1996), and should be considered as minimum estimates, despite the fact that they were performed in cold waters (typically ~10°C), which enhances gas dissolution and hence optimizes isotopic equilibrium in seawater samples enriched with 15N2 gas. Moreover, the majority of the studies i) used an isotope brand that provides high purity 15N2 gas, affecting aphotic N2 fixation rates by <1% when 15N-labeled nitrogen molecules other than N2 are taken up (Benavides et al., 2015) and/or ii) provided time zero δ15N values of the particulate nitrogen pool at each sampling depth, making their results robust (Bonnet et al., 2013; Rahav et al., 2013; Benavides et al., 2015, 2016). Finally, the variability between replicates in all terms included in the N2 fixation calculation equation (as outlined in Montoya et al., 1996) may throw back minimum quantifiable rates values below estimated aphotic N2 fixation rates (Gradoville et al., 2017). Propagating errors of the data (Birge, 1940), in five out of the nine aphotic N2 fixation studies currently available, results in minimum quantifiable rates ranging from 0.01 to 2.7 nmol N L−1 d−1 (Table S1), suggesting that most of the aphotic N2 fixation rates published are significant.
The potentially high budgetary significance of aphotic N2 fixation to the global nitrogen budget calls for further studies that will establish the geographical and temporal distribution of aphotic N2 fixation and consolidate the volumetric rates published thus far. In future studies, we encourage researchers in the field of marine nitrogen cycling to place emphasis on documenting N2 fixation in the aphotic ocean and identifying environmental drivers of aphotic N2 fixation: including oxygen, dissolved organic matter availability and particle colonization (Riemann et al., 2010; Benavides et al., 2015; Bombar et al., 2016). The availability of more data is essential to facilitate modeling and assessment of the distribution and magnitude of aphotic N2 fixation in the global ocean (i.e., association with water masses, ecotones or density fronts). Eventually, a more comprehensive understanding of the ecophysiology of aphotic N2 fixers and their contribution to global nitrogen input, will reveal their ecological importance and may help answer such question as what are the evolutionary advantages of the energetically-expensive process of N2 fixation in an environment rich in dissolved inorganic nitrogen, and how does it affect oceanic carbon sequestration.
Author Contributions
MB gathered N2 fixation rates and made the calculations shown in the tables. MB, IB-F, SB, and LR wrote the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
MB and LR were supported by grant 6108-00013 from the Danish Council for independent research and by the BONUS BLUEPRINT project receiving funding from BONUS (Art 185) funded jointly from the European Union's Seventh Programme for research, technological development and demonstration and from The Danish Council for Strategic Research (LR).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2018.00108/full#supplementary-material
Table S1. Error propagation analysis of aphotic N2 fixation rates from various published and unpublished studies.
References
Arístegui, J., Agustí, S., Middelburg, J. J., and Duarte, C. M. (2005). “Respiration in the mesopelagic and bathypelagic zones of the oceans,” in Respiration in Aquatic Ecosystems, eds P. A. Del Giorgio and P. Williams (Oxford: Oxford University Press), 182–206.
Benavides, M. H., Moisander, P., Berthelot, H., Dittmar, T., Grosso, O., and Bonnet, S. (2015). Mesopelagic N2 fixation related to organic matter composition in the Solomon and Bismarck Seas (Southwest Pacific). PLoS ONE 10:e0143775. doi: 10.1371/journal.pone.0143775
Benavides, M., Bonnet, S., Hernández, N., Martínez-Pérez, A. M., Nieto-Cid, M., Álvarez-Salgado, X. A., et al. (2016). Basin-wide N2 fixation in the deep waters of the Mediterranean Sea. Glob. Biogeochem. Cycles 30, 952–961. doi: 10.1002/2015GB005326
Bentzon-Tilia, M., Traving, S. J., Mantikci, M., Knudsen-Leerbeck, H., Hansen, J. L. S., Markager, S., et al. (2015). Significant N2 fixation by heterotrophs, photoheterotrophs and heterocystous cyanobacteria in two temperate estuaries. ISME J. 9, 273–285. doi: 10.1038/ismej.2014.119
Berthelot, H., Benavides, M., Moisander, P. H., Grosso, O., and Bonnet, S. (2017). High-nitrogen fixation rates in the particulate and dissolved pools in the Western Tropical Pacific (Solomon and Bismarck Seas). Geophys. Res. Lett. 44, 8414–8423. doi: 10.1002/2017GL073856
Birge, R. T. (1940). The propagation of errors. Am. Phys. Teacher 7, 351–357. doi: 10.1119/1.1991484
Bombar, D., Paerl, R. W., Anderson, R., and Riemann, L. (2018). Filtration via conventional glass fiber filters in 15N2 tracer assays fails to capture all nitrogen-fixing Prokaryotes. Front. Mar. Sci. 5:6. doi: 10.3389/fmars.2018.00006
Bombar, D., Paerl, R. W., and Riemann, L. (2016). Marine non-cyanobacterial diazotrophs: moving beyond molecular detection. Trends Microbiol. 24, 916–927. doi: 10.1016/j.tim.2016.07.002
Bonnet, S., Dekaezemacker, J., Turk-Kubo, K. A., Moutin, T., Hamersley, R. M., Grosso, O., et al. (2013). Aphotic N2 fixation in the eastern tropical South Pacific Ocean. PLoS ONE 8:e81265. doi: 10.1371/journal.pone.0081265
Codispoti, L. A. (2007). An oceanic fixed nitrogen sink exceeding 400 Tg N y−1 vs the concept of homeostasis in the fixed-nitrogen inventory. Biogeosciences 4, 233–253. doi: 10.5194/bg-4-233-2007
Codispoti, L. A., Brandes, J. A., Christensen, J. P., Devol, A. H., Naqvi, S. W. A., Paerl, H. W., et al. (2001). The oceanic fixed nitrogen and nitrous oxide budgets: moving targets as we enter the anthropocene? Sci. Mar. 65, 85–105. doi: 10.3989/scimar.2001.65s285
Dabundo, R., Lehmann, M. F., Treibergs, L., Tobias, C. R., Altabet, M. A., Moisander, P. H., et al. (2014). The contamination of commercial 15N2 gas stocks with 15N-labeled nitrate and ammonium and consequences for nitrogen fixation measurements. PLoS ONE 9:e110335. doi: 10.1371/journal.pone.0110335
Eugster, O., and Gruber, N. (2012). A probabilistic estimate of global marine N-fixation and denitrification. Global Biogeochem. Cycles 26:4013. doi: 10.1029/2012GB004300
Falkowski, P. G. (1997). Evolution of the nitrogen cycle and its influence on the biological sequestration of CO2 in the ocean. Nature 387, 272–275. doi: 10.1038/387272a0
Farnelid, H., Andersson, A. F., Bertilsson, S., Al-Soud, W. A., Hansen, L. H., Sørensen, S., et al. (2011). Nitrogenase gene amplicons from global marine surface waters are dominated by genes of non-cyanobacteria. PLoS ONE 6:e19223. doi: 10.1371/journal.pone.0019223
Galloway, J. N., Dentener, F. J., Capone, D. G., Boyer, E. W., Howarth, R. W., Seitzinger, S. P., et al. (2004). Nitrogen cycles: past, present, and future. Biogeochemistry 70, 153–226. doi: 10.1007/s10533-004-0370-0
Gradoville, M. R., Bombar, D., Crump, B. C., Letelier, R. M., Zehr, J. P., and White, A. E. (2017). Diversity and activity of nitrogen-fixing communities across ocean basins. Limnol Oceanogr. 62, 1895–1909. doi: 10.1002/lno.10542
Großkopf, T., Mohr, W., Baustian, T., Schunck, H., Gill, D., Kuypers, M. M. M., et al. (2012). Doubling of marine dinitrogen-fixation rates based on direct measurements. Nature 488, 361–364. doi: 10.1038/nature11338
Gruber, N. (2004). The dynamics of the marine nitrogen cycle and its influence on atmospheric CO2 variations. Ocean Carbon Cycle Clim. 40, 97–148. doi: 10.1007/978-1-4020-2087-2_4
Gruber, N. (2008). “The Marine Nitrogen Cycle: overview and challenges,” in Nitrogen in the Marine Environment, eds D. G. Capone, D. A. Bronk, M. R. Mulholland, and E. J. Carpenter (Academic Press), 1–50.
Gruber, N., and Galloway, J. N. (2008). An Earth-system perspective of the global nitrogen cycle. Nature 451, 293–296. doi: 10.1038/nature06592
Hamersley, M. R., Turk, K. A., Leinweber, A., Gruber, N., Zehr, J. P., Gunderson, T., et al. (2011). Nitrogen fixation within the water column associated with two hypoxic basins in the Southern California Bight. Aquat. Microb. Ecol. 63, 193–205. doi: 10.3354/ame01494
Hewson, I., Moisander, P. H., Achilles, K. M., Carlson, C. A., Jenkins, B. D., Mondragon, E. A., et al. (2007). Characteristics of diazotrophs in surface to abyssopelagic waters of the Sargasso Sea. Aquat. Microb. Ecol. 46, 15–30. doi: 10.3354/ame046015
Jayakumar, A., Al-Rshaidat, M. M. D., Ward, B. B., and Mulholland, M. R. (2012). Diversity, distribution, and expression of diazotroph nifH genes in oxygen-deficient waters of the Arabian Sea. FEMS Microbiol. Ecol. 82, 597–606. doi: 10.1111/j.1574-6941.2012.01430.x
Jickells, T. D., Buitenhuis, E., Altieri, K., Baker, A. R., Capone, D., Duce, R. A., et al. (2017). A reevaluation of the magnitude and impacts of anthropogenic atmospheric nitrogen inputs on the ocean. Glob. Biogeochem. Cycles 31, 289–305. doi: 10.1002/2016GB005586
Moisander, P. H., Benavides, M., Bonnet, S., Berman-Frank, I., White, A. E., and Riemann, L. (2017). Chasing after non-cyanobacterial nitrogen fixation in marine pelagic environments. Front. Microbiol. 8:1736. doi: 10.3389/fmicb.2017.01736
Mohr, W., Großkopf, T., Wallace, D. W. R., and LaRoche, J. (2010). Methodological underestimation of oceanic nitrogen fixation rates. PLoS ONE 5:e12583. doi: 10.1371/journal.pone.0012583.t001
Montoya, J. P., Voss, M., Kahler, P., and Capone, D. G. (1996). A simple, high-precision, high-sensitivity tracer assay for N2 fixation. Appl. Environ. Microbiol. 62, 986–993.
Rahav, E., Bar-Zeev, E., Ohayon, S., Elifantz, H., Belkin, N., Herut, B., et al. (2013). Dinitrogen fixation in aphotic oxygenated marine environments. Front. Microbiol. 4:227. doi: 10.3389/fmicb.2013.00227
Riemann, L., Farnelid, H., and Steward, G. F. (2010). Nitrogenase genes in non-cyanobacterial plankton: prevalence, diversity and regulation in marine waters. Aquat. Microb. Ecol. 61, 235–247. doi: 10.3354/ame01431
Sohm, J. A., Hilton, J. A., Noble, A. E., Zehr, J. P., Saito, M. A., and Webb, E. A. (2011). Nitrogen fixation in the South Atlantic Gyre and the Benguela Upwelling System. Geophys. Res. Lett. 38, 1–6. doi: 10.1029/2011GL048315
Suter, E. A., Scranton, M. I., Chow, S., Stinton, D., Medina Faull, L., and Taylor, G. T. (2017), Niskin bottle sample collection aliases microbial community composition biogeochemical interpretation. Limnol. Oceanogr. 62, 606–617. doi: 10.1002/lno.10447
Turk-Kubo, K. A., Karamchandani, M., Capone, D. G., and Zehr, J. P. (2014). The paradox of marine heterotrophic nitrogen fixation: abundances of heterotrophic diazotrophs do not account for nitrogen fixation rates in the Eastern Tropical South Pacific. Environ. Microbiol. 16, 3095–3114. doi: 10.1111/1462-2920.12346
Zehr, J. P. (2011). Nitrogen fixation by marine cyanobacteria. Trends Microbiol. 19, 162–173. doi: 10.1016/j.tim.2010.12.004
Zehr, J. P., Carpenter, E., and Villareal, T. A. (2000). New perspectives on nitrogen-fixing microrganisms in tropical and subtropical oceans. Trends Microbiol. 8, 68–73. doi: 10.1016/S0966-842X(99)01670-4
Keywords: nitrogen budget, mesopelagic, non-cyanobacterial diazotrophs, nifH gene, aphotic layer
Citation: Benavides M, Bonnet S, Berman-Frank I and Riemann L (2018) Deep Into Oceanic N2 Fixation. Front. Mar. Sci. 5:108. doi: 10.3389/fmars.2018.00108
Received: 06 November 2017; Accepted: 14 March 2018;
Published: 06 April 2018.
Edited by:
Angela Landolfi, GEOMAR Helmholtz Centre for Ocean Research Kiel, GermanyReviewed by:
Luisa I. Falcon, Universidad Nacional Autónoma de México, MexicoArvind Singh, Physical Research Laboratory, India
Copyright © 2018 Benavides, Bonnet, Berman-Frank and Riemann. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mar Benavides, bWFyLmJlbmF2aWRlc0BiaW8ua3UuZGs=; bWFyLmJlbmF2aWRlc0BtaW8ub3N1cHl0aGVhcy5mcg==
 Mar Benavides
Mar Benavides Sophie Bonnet
Sophie Bonnet Ilana Berman-Frank
Ilana Berman-Frank Lasse Riemann
Lasse Riemann