- 1School of Natural Resources and Environment, University of Florida, Gainesville, FL, United States
- 2Laboratorio de Biología de Organismos, Centro de Ecología, Instituto Venezolano de Investigaciones Científicas, San Antonio de los Altos, Venezuela
- 3Fisheries and Aquatic Sciences Program, School of Forest Resources and Conservation, University of Florida, Gainesville, FL, United States
The use of aquatic mammals as bait to enhance the harvest of fisheries species has garnered little attention by the scientific and conservation communities, often receiving only brief mention in reports focused on the human consumption or bycatch of aquatic mammals. A number of studies, however, highlight the negative impact of this practice on affected mammal populations. A systematic review of relevant literature published since 1970 yields new insight into the scope of the issue. Findings indicate that the practice of using aquatic mammals for bait has been and continues to be geographically widespread, has affected at least 42 species, and often involves deliberate killing for the express purpose of securing bait. The nature of the fisheries involved is diverse, encompassing a wide range of target species and gear types; however, shark fisheries that employ longlines appear to be the most widely engaged in using aquatic mammals as bait. This practice appears to be most common in Latin America and Asia. It is evident, based on our review, that there is little information on the impact of the direct take on most targeted mammal populations, commonly small cetaceans, and increased monitoring efforts are needed in many locales. In most instances, the ecology and population dynamics of the targeted fishery species is poorly understood and in some cases the species is classified as threatened, suggesting a fishery sustainability issue that cannot be fully addressed with a substitute for the aquatic mammal bait. It is essential that natural resource managers implement mitigation approaches that consider the socio-economic, cultural, political, and ecological circumstances leading to the use of aquatic mammal bait in each fishery.
Introduction
Direct interactions with fisheries are a major threat to aquatic mammal populations worldwide (Vidal, 1993; Jefferson and Curry, 1994; Perrin et al., 1994a,b; Northridge and Hofman, 1999; Read et al., 2006; Read, 2008; Davidson et al., 2012). While some of these interactions (e.g., such as bycatch and direct take for human consumption) have received considerable attention by the scientific and conservation communities, the use of marine mammals as bait to enhance the harvest of fisheries species has garnered relatively little attention. A number of studies, however, highlight the negative impact of this practice on affected mammal populations (e.g., Cárdenas et al., 1987; Lescrauwaet and Gibbons, 1994; Stensland et al., 2006; Mangel et al., 2010; Mintzer et al., 2013) and efforts should be focused at better understanding this threat. Specifically, examples of small cetacean harvest for crab bait in Tierra del Fuego (Cárdenas et al., 1987; Lescrauwaet and Gibbons, 1994) and more recent studies on Amazon River dolphins utilized as catfish bait (da Silva et al., 2011; Mintzer et al., 2013), reveal that the practice can be unsustainable and become a major threat to a species.
In some fisheries, the practice of using aquatic mammals for bait is directly linked to aquatic mammal bycatch (Read, 2008). Bycatch or non-targeted catch that is incidentally captured in fishing gear is often discarded by fishers. However, the retention and utilization of non-targeted catch as bait is also a widespread activity (Perrin et al., 1994a; Cosentino and Fisher, 2016). This retention of non-targeted catch may develop into a direct take of aquatic mammals (Perrin et al., 1994b; Ofori-Danson et al., 2003, 2012; Read, 2008). Where non-targeted catch has proven to be consumable by humans or effective as bait, it may become a target of the fishery itself. This transition is more likely to occur in locations where poverty and rapid population growth fuel the process (Read, 2008).
The practice of harvesting aquatic mammals for bait raises a suite of ethical issues and ecological considerations. From an ecological perspective, the removal of higher-level consumers can have profound consequences for food web dynamics, energy flow, and material transport (Estes et al., 2011). From an ethical and/or political standpoint, the practice of harvesting aquatic mammals for bait is likely to be viewed negatively. However, the issue might be considered by some to be less contentious than the killing of aquatic mammals for human consumption, particularly if the flesh is a not an essential source of protein and/or the activity itself lacks any important cultural value. Nevertheless, the practice of taking aquatic mammals for bait is poorly understood, and more information is needed to guide conservation efforts and develop effective mitigation measures that consider socio-economic contexts.
Herein, we endeavor to reveal the extent of this practice, and identify knowledge gaps that, at present, hinder our ability to fully assess the implications for conservation and management. Specifically, we aimed to: (1) identify the species utilized as bait, (2) define the location and characteristics of fisheries that use aquatic mammals for bait, (3) synthesize available knowledge on the impacts of the practice on affected mammal populations, and (4) review case-studies to identify possible mitigation measures.
Methods
We accessed 145 source materials (dated between 1970 and 2017) for information on the use of aquatic mammals for bait. Over 40% (n = 61) of the source materials were comprised of peer-reviewed articles and books in the primary scientific literature. Another 42% (n = 61) of the sources reviewed were reports from and to the International Whaling Commission (IWC), the International Union for Nature Conservation (IUCN), the United Nations Environment Programme (UNEP), and other intergovernmental institutions. The remaining sources included government reports (n = 8, 6%), academic theses and other university publications (n = 5, 3%), conference proceedings (n = 2, 1%), and reports and/or newsletters from regional or local non-governmental organizations (n = 8, 6%). Sources in English, Spanish, and Portuguese were reviewed. We contacted source authors or local scientists and/or natural resource managers to obtain additional information and/or seek clarification. Relevant peer-reviewed publications were identified using academic databases including Web of Science and Google Scholar. Key words and phrases (e.g., “dolphins for bait,” “cetacean AND bait”) were used to complete these searches. We then employed a “snowball approach” to identify additional source materials using the literature cited section of each of the sources reviewed and repeated this process for each new source identified. Only sources that explicitly mentioned the utilization of at least one aquatic mammal as bait were included in subsequent analysis.
From the identified source materials, we compiled the following information pertaining to each fishery that exploits aquatic mammals for bait: (1) country where fishery takes place, (2) target fishery/species for which bait is utilized, (3) species utilized as bait, (4) method(s) of bait acquisition, (5) gear utilized for bait deployment, and (6) period of reported use of aquatic mammal bait. We considered countries as locations with two-letter Federal Information Processing Standard codes and categorized them as belonging to one of five aquatic-based regions: North Atlantic, South Atlantic, Indo-Pacific, Eastern Pacific, and large river systems. A few countries, such as Mexico and Brazil, were included in two regions, highlighting the involvement of the country's fisheries in two oceans or in both marine and large river systems. Aquatic mammal species names were adjusted, when necessary, to reflect the most current taxonomy recognized by the Society for Marine Mammalogy (Committee on Taxonomy, 2016).
We recognized three methods of bait acquisition based on the definitions adopted by Hall (1996) and (Robards and Reeves, 2011): (1) Targeted, (2) Non-Targeted-Deliberate, and (3) Non-Targeted-Salvage. “Targeted” refers to those cases where the capture of aquatic mammals for bait was a primary objective. “Non-Targeted-Deliberate” included cases where an animal became accidentally entangled in fishing gear or was stranded alive and subsequently killed for use as bait. Finally, “Non-Targeted-Salvage” refers to the use of animals that accidentally drowned in fishing gear or carcasses found on a beach or elsewhere. When the distinction between “Non-Targeted-Deliberate” and “Non-Target-Salvage” was not clear, we categorized the method of acquisition as “Non-Targeted-?”.
In addition to the information noted above, when available, we retained information on the local magnitude and/or geographical extent of the practice and the impact of deliberate killing of mammals on affected population(s). This included number of animals killed/utilized for bait during a specific period, demographic studies of targeted mammal populations, and observations and/or reports of population trends. Sources that provided such information often included management and conservation implications and historical context/stories that we also amassed.
Results
Extent and Characteristics of the Use of Aquatic Mammals for Bait
A broad suite of fisheries have used aquatic mammals as bait, and accordingly there exists a wide range of gear types and target species associated with the practice. We identified 42 species of aquatic mammals that have been used as bait since 1970 in 33 different countries (Figure 1).
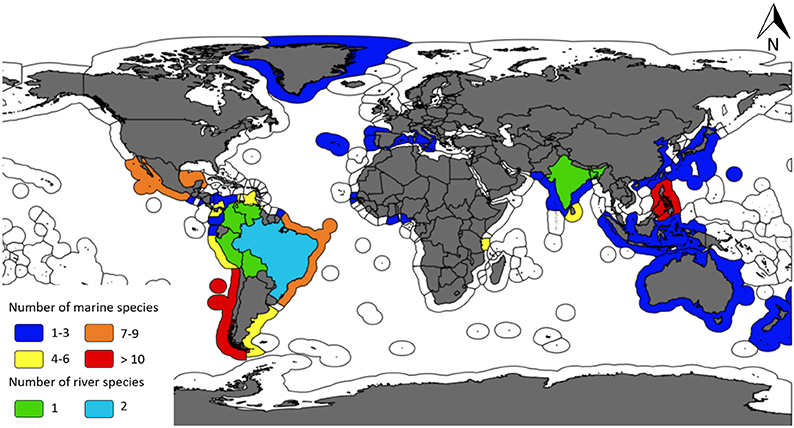
Figure 1. Map highlighting the number of aquatic mammal species by country that have been used as bait in fisheries between 1970 and 2017 (GIS layers: Claus et al., 2018).
Based on the number of sources and number of countries identified within them, the practice is most prevalent in Latin America (sources = n = 96, 60%; countries = n = 13, 39%) and Asia (sources = n = 42, 26%; countries = n = 9, 27%), particularly for those fisheries that target sharks (Tables 1–5). With regard specifically to aquatic mammals utilized for bait, two-thirds of the marine species identified as part of this review (66.67%, n = 26) were reported to be used as shark bait in at least one country. Eighty-three percent (n = 35) of all the identified aquatic mammal bait species were deliberately killed/targeted for use as bait in at least one country. Almost two-thirds of the aforementioned countries (70.37%, n = 19) had at least one fishery that targeted marine mammals for use as bait in the most recent time period assessed: 2000–2017. In large river systems, catfishes are the primary target of fisheries that utilize cetacean bait. Our results also demonstrate that small cetaceans are, by an overwhelming margin, the group of aquatic mammals most vulnerable to this threat (Table 6). Below we provide a summary of our findings at a regional level.
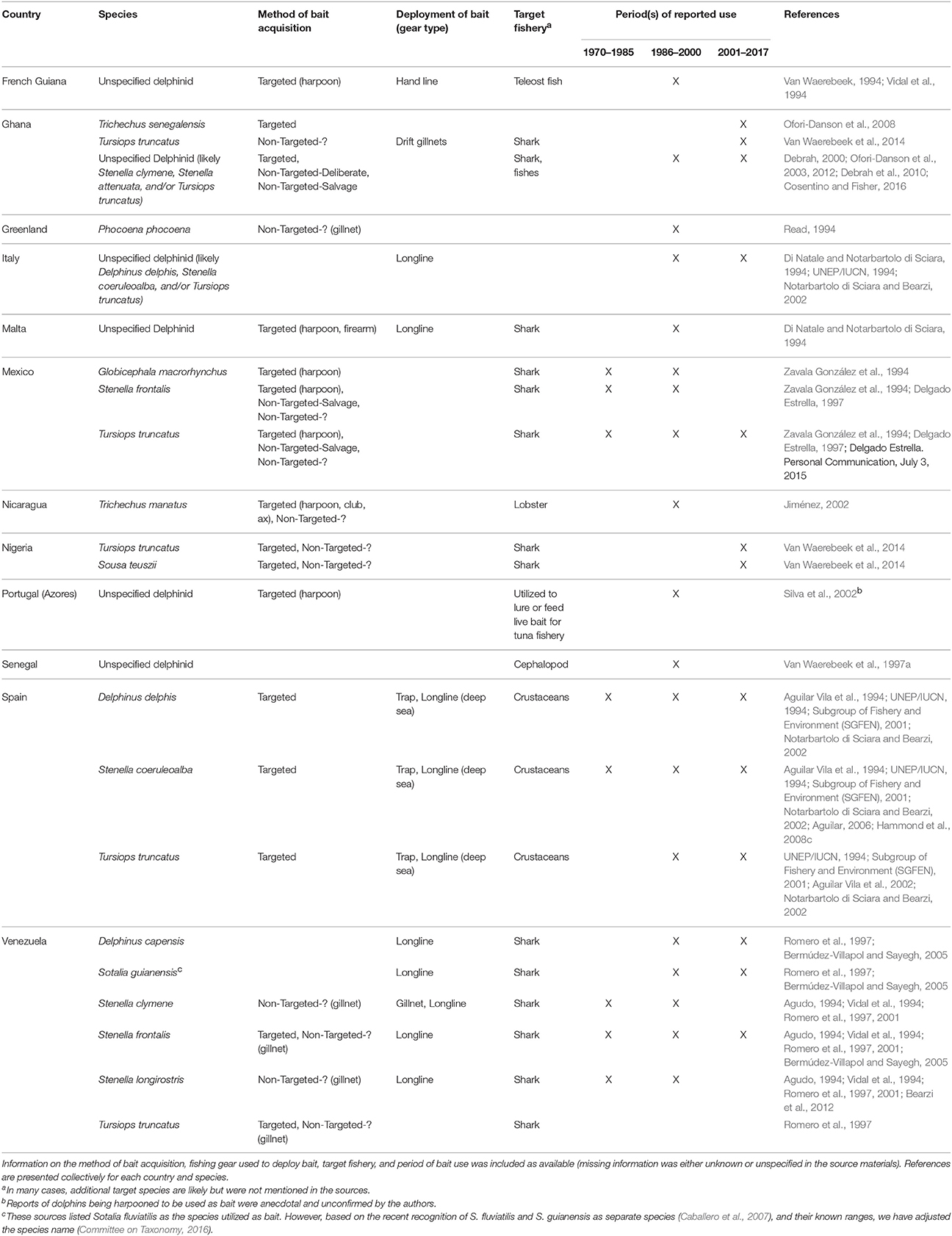
Table 1. Species of aquatic mammals utilized as bait in the North Atlantic region (including the Mediterranean).
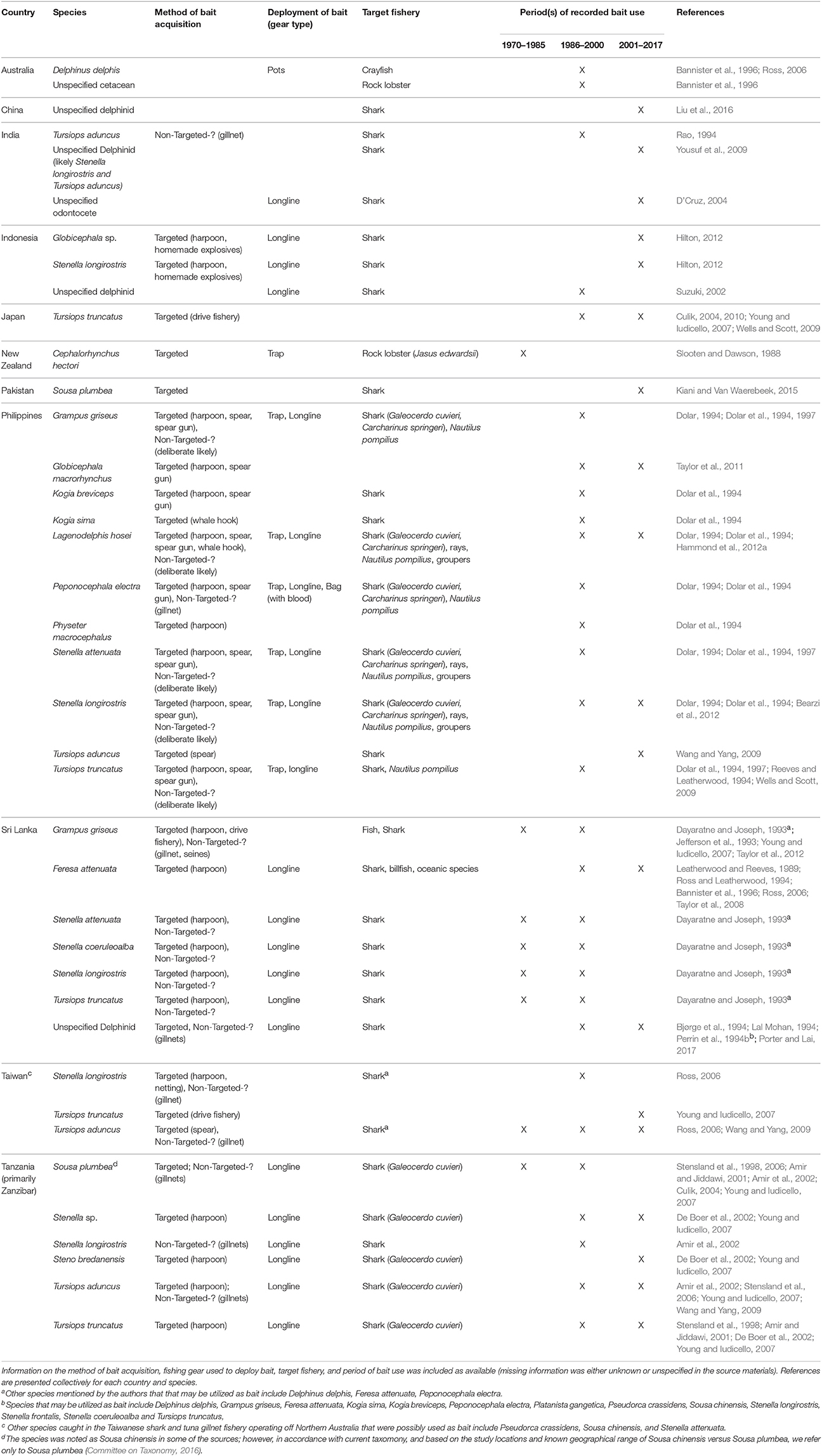
Table 3. Species of aquatic mammals utilized as bait in the Indo-Pacific region (including New Zealand).
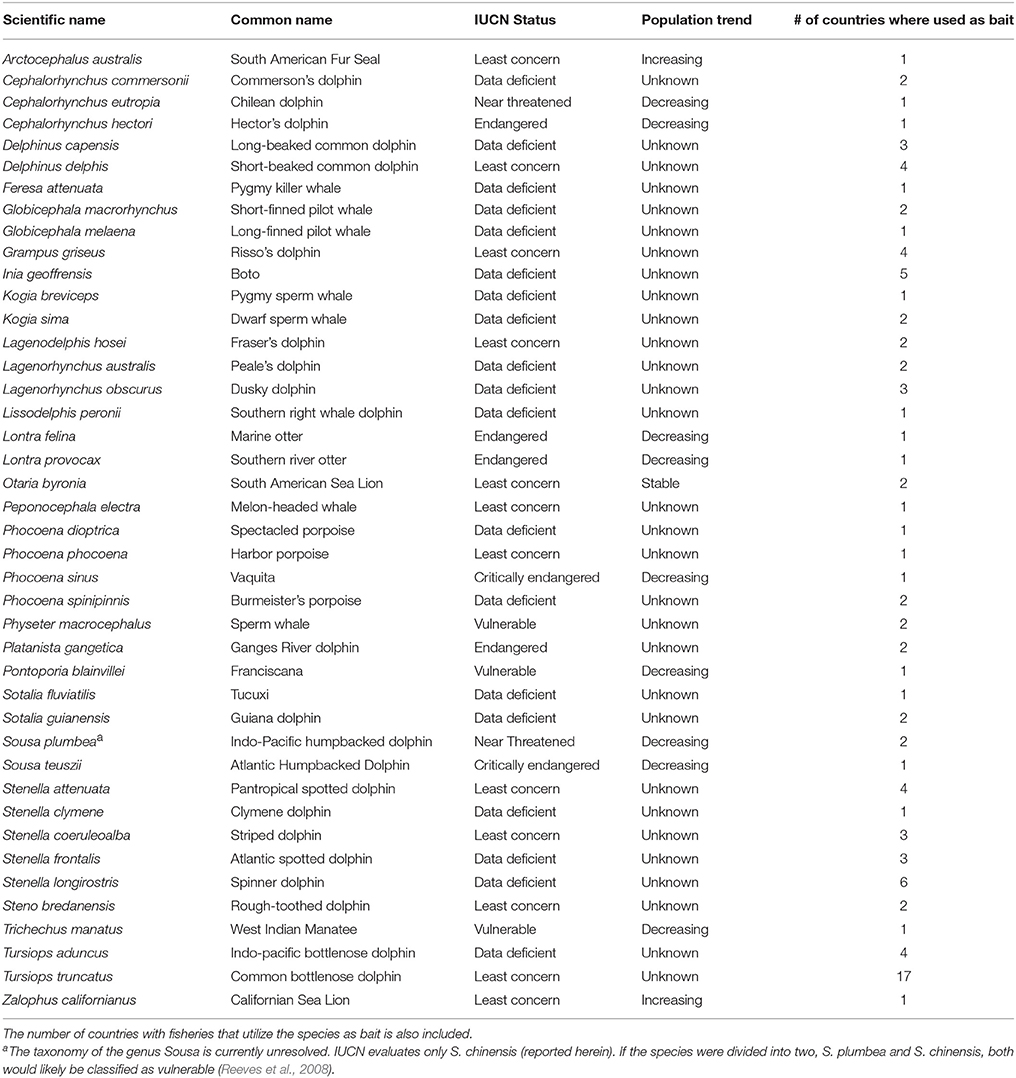
Table 6. Conservation status and population trend of aquatic mammal species utilized as bait as indicated in the International Union for the Conservation of Nature's (IUCN) species Red List (IUCN, 2017).
North Atlantic
Since 1970, 13 species of aquatic mammals have been utilized as bait in 12 countries in the North Atlantic region (Table 1; sources = n = 28, 17%). In the western North Atlantic, primarily off the coasts of Mexico and Venezuela, the fisheries engaged in this practice have been, for the most part, longline fisheries targeting sharks. Small cetaceans are typical bait and killed deliberately with harpoons or collected, often incidentally, with gillnets (Non-Targeted-?) (Table 1). In Venezuela, deliberate killing of small cetaceans for bait was known to occur in the early 2000s (Bermúdez-Villapol and Sayegh, 2005), but more recent information is needed to assess the current status of the activity is lacking. In Mexico, the practice appears to be ongoing in Yucatán and Tabasco as evidenced by the recovery of Tursiops truncatus carcasses with cuts that suggest removal of meat for bait (A. Delgado Estrella, Personal Communication, July 3, 2015).
In the eastern North Atlantic, the Mediterranean was identified by the greatest number of sources as a region where marine mammals have been used as bait (Table 1). In the 1990s and earlier, the use of small cetaceans for bait was recorded numerous times in the Spanish shrimp and longlines fisheries, and in Italian and Maltese longline fisheries (Table 1). Although there is no recent research on the status of these activities in the Mediterranean, the continuation of direct killing of cetaceans for use as bait is unlikely due to changes in public sensitivity toward marine mammals that have occurred in the last couple of decades (G. Notarbartolo di Sciara, Personal Communication, July 3, 2015).
Sources that indicated the hunting or incidental capture of marine mammals in western African nations pointed primarily to the use of this catch for human consumption (see Robards and Reeves, 2011 and sources therein) and not for bait. Exceptions were found in Ghana, Nigeria, and Senegal where sources indicated an existing, but poorly documented practice of using aquatic mammals for shark and cephalopod bait. The bait utilized has been primarily small cetaceans, although manatees (Trichechus senegalensis) have been targeted in Ghana (Table 1).
South Atlantic
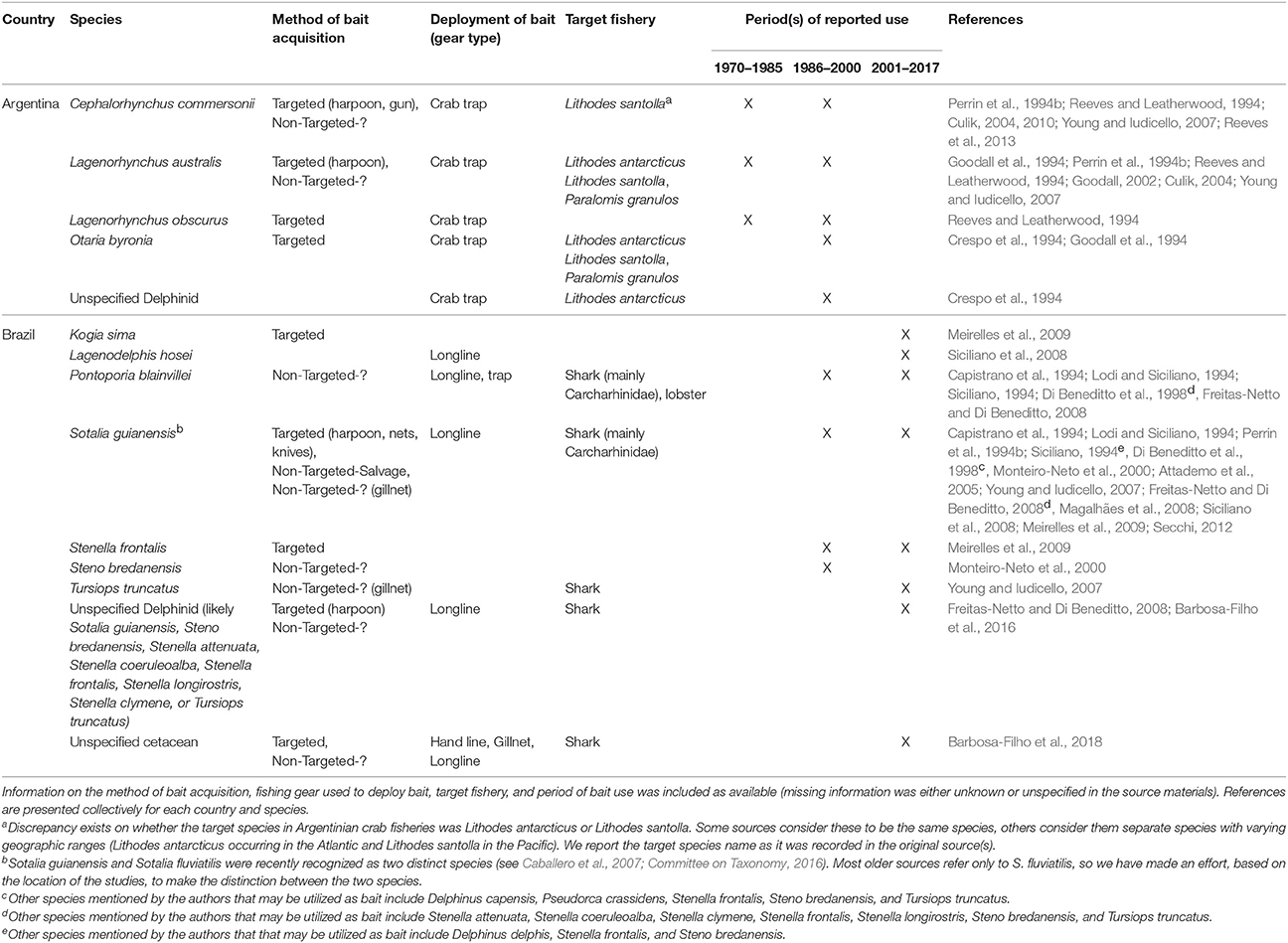
From this region, we only found sources indicating the existence of the practice of using aquatic mammal bait in Argentina and Brazil (sources = n = 22, 14%). Fisheries from these two countries were determined to have utilized at least 10 species of small cetaceans and the South American sea lion for bait, primarily to target crabs and sharks (Table 2).
The crab fishery off the coast of Tierra del Fuego is a relatively well-studied case where aquatic mammals have been utilized for bait (Tables 2, 4). Small cetaceans, fur seals, sea lions, and otters, as well as seabirds and other wildlife have been used in trap fisheries targeting crabs known as centolla (Lithodes antarcticus and/or Lithodes santolla) and centollón (Paralomis granulosa) (Tables 2, 4). The utilization of wildlife bait reached its peak in the 1970s and early 1980s, but by 1990s had decreased substantially. While the practice is thought to continue to a limited extent in neighboring Chile, it has likely ceased completely in Argentina (Goodall, 2002). We provide additional detail in the section on the Eastern Pacific.
In Brazil, the most common oceanic dolphin used for bait is Sotalia guianensis (Table 2). It is captured intentionally and retained also as bycatch in gillnet fisheries where it is, in turn, used as shark bait in longline fisheries. The practice has been reported along the coast, from the states of Pará to São Paulo (Table 2). In recent years, however, Siciliano et al. (2008) pointed to a decrease in harpooning of S. guianensis, for use as bait, in Baía de Marajó and the eastern coast of Pará. The practice in these areas appears to have subsided, in large part, due to enforcement efforts initiated in the 1990s. In the state of Bahia, the use of both dolphins and whales for shark bait is ongoing (Barbosa-Filho et al., 2018). Over 80% of fishers interviewed in 13 communities among the Bahia coast reported that cetaceans were used as shark bait (Barbosa-Filho et al., 2018).
Pontoporia blainvillei has been utilized as bait along the southern coast of Brazil (Siciliano, 1994). Although this species does not appear to be targeted like S. guianensis, the vulnerable conservation status of P. blainvillei (Table 6) warns that the non-targeted acquisition and use of this species as bait should not be ignored. While records are limited, many other small cetacean species (see Table 2 and corresponding footnotes) are likely to be utilized in Brazilian longline shark fisheries.
Indo-Pacific
In the Indo-Pacific, small cetaceans, represented by 17 different species, have been utilized as bait in support of regional fisheries (Table 3; sources = n = 37, 23%). All but one of these species has been killed for the express purpose of procuring bait. Of these species, 14 (82.35%) have been used to attract sharks, primarily through the deployment of longlines. According to fishers from this region, dolphin meat and blubber are durable longline baits that remain on the hooks and do not disintegrate easily, and dolphin blood is an efficient shark attractant (Dayaratne and Joseph, 1993). Dolphins have been preferred over other commonly available baits such as bony fishes and dog (Dolar, 1994). The use of small cetaceans as bait in this region appears to be most prevalent in Indonesia, the Philippines, Taiwan, and Tanzania.
Markets for dolphin carcasses have developed in some locales including Zanzibar and Indonesia (Amir et al., 2002; Hilton, 2012). In a large market in Lombok, Indonesia, dolphins were observed to be landed during 7 out of 10 visits (Hilton, 2012). However, dolphin meat is consumed in some of these locales, so it is difficult to ascertain the proportion of carcasses that are sold for human consumption versus bait. Nevertheless, with the high price of shark fins and continuing demand (Clarke et al., 2007), efficient shark fishing may outweigh the market value or desirability of consuming cetacean meat in some areas.
The Philippines is one of only two countries in this review where fisheries have utilized more than 10 different species of aquatic mammals (Figure 1). Dolar et al. (1994) described the use of dolphins as bait, in detail, in several Philippine islands in the early 1990s. In Brooke's Point, Palawan, 125–150 dolphins were estimated to have been killed per season for use as bait in traps for chambered nautilis. Demand for dolphins was such that fishers adapted fishing gear and methods to increase their effectiveness in hunting cetaceans. Dolar (1994) observed a shift in the types of hunting gear used from harpoon, to spear, to spear gun. On Selinog Island, it was also estimated that about 100 dolphins were killed annually for use as bait (primarily for longlines targeting sharks, rays, and groupers) (Dolar et al., 1994). Hunting of dolphins decreased in some villages after the implementation of the Fisheries Administrative Order 185 in 1992 that prohibited the catching of dolphins. However, hunting still occurred in villages visited by Dolar et al. (1997), where fishers were unware of the law. More current information on the use of cetacean bait in the Philippines is not available.
Although only one source confirmed the utilization of cetacean bait in Taiwan, it is likely that this practice is much more widespread within Taiwanese fisheries than what is presently recorded. From 1974 to 1986, the Taiwanese shark and tuna gillnet fishery exploited the waters off northern Australia, including the Arafua and Timor Seas (Young and Iudicello, 2007). This fishery was responsible for the incidental catch of a variety of small cetaceans, with Tursiops aduncus comprising 60% (8,400) of the total cetacean catch. This species, along with Stenella longirostris, was retained and utilized as bait (Table 3). The fishery was banned by the Australian government in 1986 after it was determined that cetacean take was severely affecting local populations (Young and Iudicello, 2007). Nevertheless, illegal dolphin hunting, through the use of harpoons, was thought to continue for some time in Australian waters (Ross, 2006). Eventually, however, the Taiwanese moved north and continued the driftnet fishery in Indonesian waters, and there is no indication that the practice of using cetaceans as bait ceased with the relocation.
In spite of the severity of the situation in some Indo-Pacific nations, the region showcases three examples where the use of cetacean bait has decreased. In the Maldives, dolphins were used as shark bait in a traditional fishery called Maa keyolhu kan. The demand for dolphins decreased, for unknown reasons, with the introduction of longlines in the 1960's (Anderson and Ahmed, 1993). More recently, the Maldives, recognizing the important role of sharks in their tourism industry, banned the fishing of sharks, as well as the trade and exportation of shark products (Ward-Paige, 2017). Thus, there is currently no need to take dolphins for use as bait.
New Zealand serves as another example in this region where the use of dolphins for bait diminished markedly. Historically, the endangered Hector's dolphin (Cephalorhynchus hectori) was hunted for use as bait in rock lobster (Jasus edwardsii) traps (Slooten and Dawson, 1988). The practice appears to have stopped in the 1970's due to (1) changes in public perception of dolphins because of their popularization in television and (2) the implementation of New Zealand's Marine Mammal Protection Act in 1978 (Slooten and Dawson, 1988).
In parts of Zanzibar, the direct harvest of dolphins has given way to the tourism industry (Stensland et al., 2006). Although Stenella sp., Steno bredonensis, T. aduncus, and T. truncatus, are likely still utilized as tiger shark bait in longline fisheries off the coast of Tanzania (De Boer et al., 2002; Young and Iudicello, 2007; Wang and Yang, 2009), the practice has decreased off the south western coast of Zanzibar. In Menai Bay, Sousa plumbea and T. truncatus were hunted and utilized as shark bait well into the 1990's (Amir and Jiddawi, 2001). In 1996, for example, 23 dolphins were caught and utilized as bait by fishers in the village of Kizimkazi-Dimbani. Dolphin meat was reported to be preferred by fishers because it attracted sharks from long distances due to its strong odor (Amir and Jiddawi, 2001). Although the total number of dolphins taken was unknown, Stensland et al. (2006) estimated an annual mortality of 12% of the combined population of the two dolphin species, a rate that far exceeded the 2% recommended level of take for small cetacean populations. Consequently, the practice likely decreased the abundance of both populations (Young and Iudicello, 2007).
Dolphin watching and swimming tours began in Menai Bay in 1992, and fishers soon realized the value of utilizing dolphins as an ecotourism attraction (Amir and Jiddawi, 2001). The number of villagers involved in the new industry grew rapidly and motors boats were introduced as means to increase profits. Villagers created informal agreements to fix the price and routine of dolphin-watching boats, and to prohibit the killing of dolphins. However, formal legislation and enforcement is lacking, and concern exists regarding the lack of rules placed on the tour boats, as well as the distribution of wealth arising from the industry (Amir and Jiddawi, 2001; Stensland et al., 2006). Nevertheless, the hunting of dolphins has ceased in these villages (Stensland et al., 2006).
Eastern Pacific
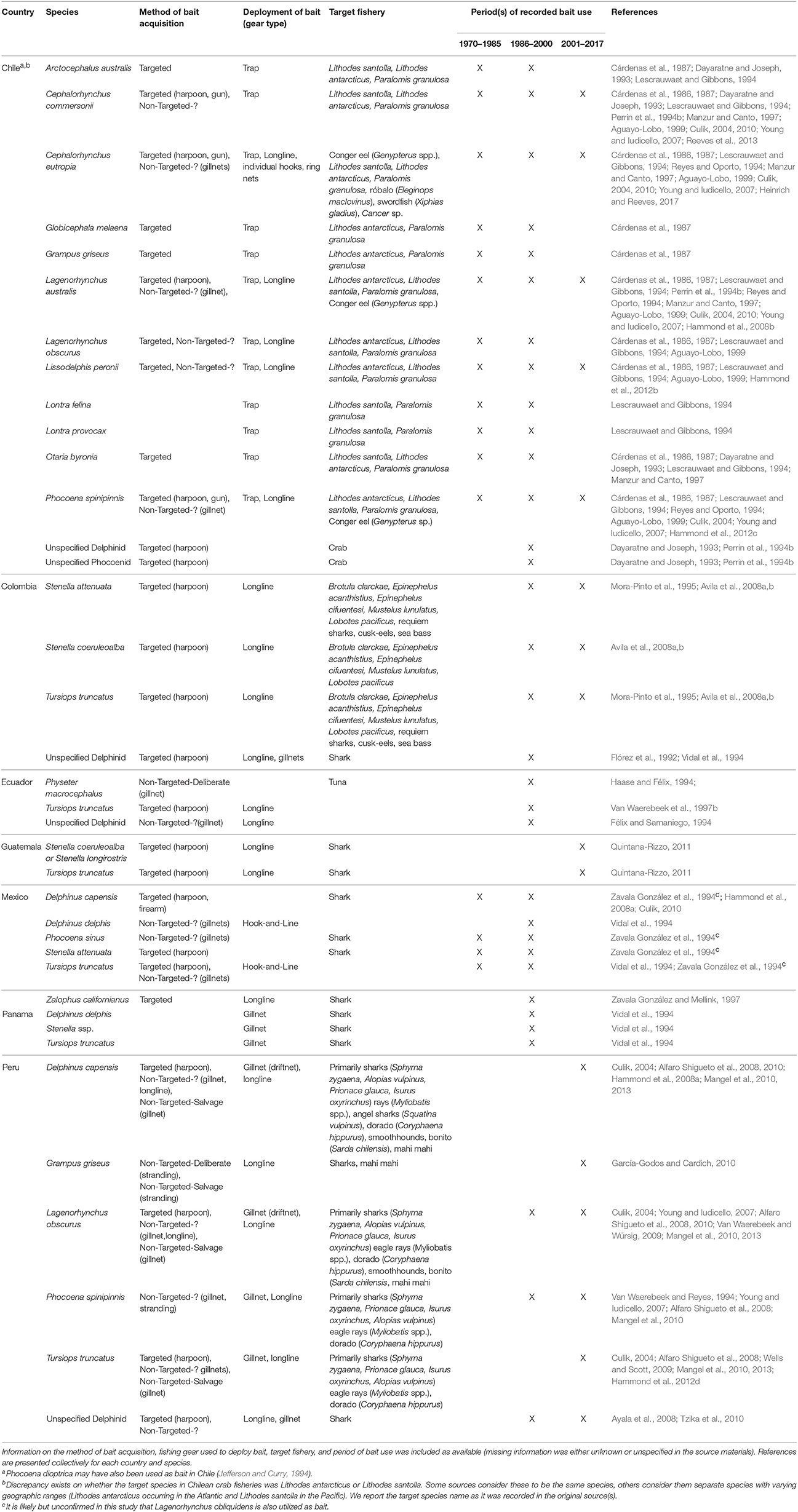
Twenty species of marine mammals, including small cetaceans, fur seals, sea lions, and otters, have been utilized as bait in seven countries in the Eastern Pacific since 1970 (Table 4; sources = n = 40, 25%). All but five of these species are known to have been directly targeted for the purpose of acquiring bait in support of fishing activity. The practice in this region has, in fact, been well documented with substantial harvest of marine mammals in Chile reported in the 1970s and 80s. In the two most recent decades, the killing of cetaceans appears to be more common in the northern South American Pacific coast, where small cetaceans are being utilized for shark bait in Peru, Colombia, Guatemala, and likely other nations (Table 4).
The use of marine mammals in the Chilean crab fishery, as alluded to above, is perhaps the most well-studied case included in this review (as indicated by the number of sources and information within, see Table 4). Over a dozen species of marine mammals were targeted until the late 1980s to provide bait for traps targeting centolla (L. antarcticus and/or L. santolla) and centollón (P. granulosa) (Table 4). Recorded estimates of the number of dolphins killed for this purpose was highest in the 1978–1979 season when 4,120 dolphins were killed for use as bait, primarily Cephalorhynchus commersonii and Lagenorhynchus australis (Torres et al., 1979; Cárdenas et al., 1987). The abundance of C. commersonii (Cárdenas et al., 1987; Reeves et al., 2013) and L. australis (Lescrauwaet and Gibbons, 1994) is presumed to have been drastically reduced, but rates of decline are unavailable. Differences in survey methodologies utilized during the different time periods limits sound statistical analyses pertaining to these abundance trends (Lescrauwaet et al., 2000). Moreover, take estimates for later years are unavailable because fishers stopped reporting cetacean take after the establishment of new legislation protecting marine mammals (Cárdenas et al., 1987).
Cetacean mortality in the region is thought to have decreased since 1990 due to a variety of factors (Goodall, 2002). First, a depletion of the crab stocks reduced fishing effort that, in turn, decreased the need for bait (Lescrauwaet and Gibbons, 1994; Manzur and Canto, 1997). The capture of centolla decreased in the Magallanes region from 2,688 tons in 1983 to one ton in 1996 (Manzur and Canto, 1997). Second, although most of the bait between 1983 and 1988 was wildlife, by the early 1990s a considerable amount of waste from slaughter houses and industrial fisheries was provisioned as bait (Lescrauwaet and Gibbons, 1994; Manzur and Canto, 1997). Moreover, small cetaceans appear to have become less abundant, decreasing the ease of their capture and use as bait (Lescrauwaet and Gibbons, 1994). There was also a reorientation of fisheries in the Magallanes and the focus was switched from crabs to sea urchins (Loxechinus albas; Manzur and Canto, 1997).
In 1993, various organizations in the United States petitioned the federal government to place an embargo on the importation of centolla and centollón into the United States. As a result, the Chilean government, under an agreement between the United States National Marine Fisheries Service and the Fishery Subsecretary of Chile implemented various measures to address the remaining issues surrounding the use of marine mammal bait (Young and Iudicello, 2007). The measures included increases in enforcement efforts, additional legislation, education campaigns, and better management of and provisioning of alternative baits (Manzur and Canto, 1997; Young and Iudicello, 2007). As a result of these measures, no marine mammals were reported to be captured in either 1994 or 1995. However, in 1996, there was a shortage of alternative baits, which allegedly led to the reoccurrence of the use of marine mammals for this purpose (Manzur and Canto, 1997). Regardless, these measures taken by the Chilean government were deemed successful enough to forego the embargo. The killing of small cetaceans for bait is presumed to continue to some degree, but there are no recent studies that inform of the magnitude or extent of the take (Young and Iudicello, 2007; Heinrich and Reeves, 2017).
In recent years, the use of cetaceans as bait has become more common along the northern coast of South America, particularly Peru, where the use of cetacean bait to attract sharks has garnered the interest of scientists as well as the international press (e.g., Manning, 2013; Rodriguez and Romo, 2013). Following the collapse of the anchoveta fishery in 1972, a directed fishery for dolphins and porpoises began in Peru (Culik, 2004). Small cetaceans were targeted intensely using nets and harpoons and sold readily for food (Culik, 2004). In the early 1990s, small cetacean catches were reported to be as high as 15,000–20,000 animals per year (Van Waerebeek and Reyes, 1994). Dolphin hunting was banned by law in 1996, and it is believed that the legislation, together with the depleted status of small cetacean populations, led to the decrease in direct exploitation for human food consumption (Young and Iudicello, 2007). However, while the legislation appears to have reduced landings and limited the market for cetacean meat, it has not reduced the level of cetacean bycatch (Mangel et al., 2010).
Although small cetaceans are still consumed by humans in the region, there has been a partial shift from the utilization of small cetacean meat for human food to shark bait (Young and Iudicello, 2007). It should be noted that dolphins (both harpooned and non-targeted) can be processed and transferred to shark-fishing boats at sea, and fishers can avoid bringing carcasses to shore where enforcement presence is greater (Dolar et al., 1994; Van Waerebeek et al., 2003; Young and Iudicello, 2007). The increasing demand and price of shark fins, which corresponded in time with the government-imposed ban (Clarke et al., 2007), likely helped to encourage this shift. Mangel et al. (2013) recorded the use of small cetacean blubber in approximately 27% of gillnet sets that they observed while conducting a study on the use of acoustic pingers in the Peruvian small-scale driftnet fishery. During these sets, larger bycatch such as bottlenose dolphins and pilot whales were usually discarded, while dusky dolphins (L. obscurus) were used for bait and Burmeister's porpoise for human food. Additionally, the observers recorded the harpooning of 23 common dolphins (Delphinus spp.) and two dusky dolphins (L. obscurus) for use as bait (to target blue and mako sharks). This direct killing was documented during 10 fishing trips (11%), when 1–4 individuals were killed at a time (Mangel et al., 2013). The use of small cetacean bait in Peruvian small-scale fisheries continues, and based on fleet sizes and known bycatch rates, it is reasonable to estimate that many hundreds or thousands of dolphins may be used for bait annually (J.C. Mangel, Personal Communication, September 1, 2017).
Given the high demand for sharks, it is not surprising that fishers in other nearby nations are also utilizing small cetaceans for shark bait. Small-scale fishers throughout Latin America have reported on the effectiveness of cetacean meat as shark bait. Its high blood and fat content make it an efficient attractant, while its hardy nature allows it to remain attached to hooks after extended periods of soaking (unlike other baits, fishes in particular) (Mangel et al., 2010). However, it is important to note that many fishers also report that they have shifted to utilizing cetacean bait only after traditional bait has become unattainable. For example, in Peru, fishers have indicated that they utilize small cetacean bait due to the high cost of traditional bait like mackerel (Scomber japonics; Mangel et al., 2010). In Ecuador, dolphins were reported to be used as bait when whitebait became scarce (Félix and Samaniego, 1994). Quintana-Rizzo (2011) and Van Waerebeek et al. (1997b) also reported that fishers utilized cetacean bait when traditional baits were unavailable in Guatemala and Colombia, respectively.
Large River Systems
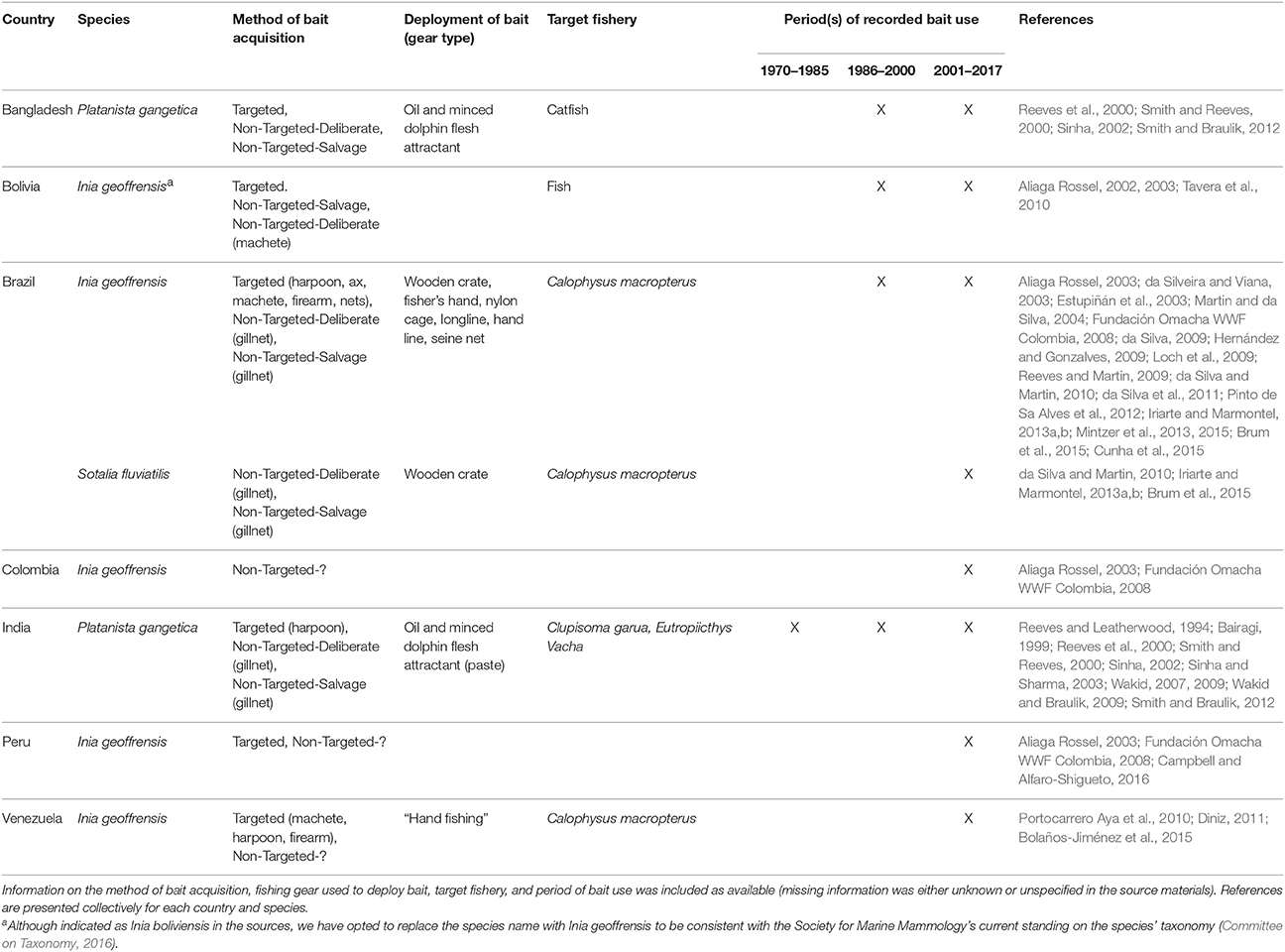
All extant river dolphin species are reported to have been used as bait in at least some part of their range since 1970 (Table 5; sources = n = 34, 21%). Given the close proximity of these species to impoverished communities where fishing is the main source of livelihood, this finding is not surprising. Dolphin bait has been deemed to be highly effective catfish bait by both fishers in South American and Asian rivers. Consequently, the practice of killing river dolphins for use as bait has reached unsustainable levels in some locales (da Silva et al., 2011; Smith and Braulik, 2012; Mintzer et al., 2013). Of the seven countries where freshwater fisheries have utilized cetacean bait, all were identified in source materials published since 2010 suggesting the recent and likely ongoing occurrence of this practice.
In the Amazon and Orinoco basins of South America, the two endemic river dolphin species, boto (Inia geoffrensis) and tucuxi (Sotalia fluviatilis) are used as bait to attract the catfish known as piracatinga or mota (Calophysus macropterus) (Table 5). Although the practice has been documented in Venezuela, Colombia, Bolivia, and Peru, it is most popular in the Brazilian Amazon where botos are commonly harpooned for this purpose (Table 5). In one floodplain system in the Central /Brazilian Amazon, take levels (estimated at 1650 dolphins per year, da Silva et al., 2011) likely exceed sustainable limits, as indicated by decreased survival rates (Mintzer et al., 2013) and a marked population decline (i.e., a per annum mean reduction of 10% since 2000; da Silva et al., 2011). Although tucuxi are rarely targeted, if they become entangled in fishing gear, the carcasses are utilized as bait (Iriarte and Marmontel, 2013a,b; Brum et al., 2015).
The use of boto bait has increased and expanded throughout the Amazon basin during the last decade. Accordingly, the issue has received considerable attention from scientists, conservationists, and the international press. Although botos have protected status in all countries where they occur, such designation provides little protection due to the difficulty in enforcing natural resource laws in Amazonian towns and villages where staffing and financial resources are limited (De Oliveira, 2002). Moreover, the international nature of the market for C. macropterus has complicated things further, as the consumers (in large Colombian and Brazilian cities) are far away and disconnected from the suppliers (in small Amazonian villages and towns) and have little knowledge of where their fish originates, let alone that it is being caught using what is culturally perceived as a charismatic and important animal. In both Colombia and Brazil, C. macropterus is sold under false names, confounding the issue even further (Gómez et al., 2008; Cunha et al., 2015). In response to pressure from conservation groups, the Ministry of the Environment in Brazil announced a 5-year moratorium on the selling and trade of piracatinga effective January 2015 (Brum et al., 2015). Because piracatinga is transported to and handled in large processing facilities, monitoring for compliance and enforcement are expected to be relatively straightforward endeavors. However, it is possible that the moratorium will lead to a black market for piracatinga (Brum et al., 2015). Nevertheless, an expected decrease in killing should afford an opportunity to develop alternative baits or livelihoods. Continued monitoring of boto population dynamics will be essential to assess, with any confidence, the effectiveness of the ban.
Direct killing for use as bait is one of the principal threats affecting survival of the endangered Ganges river dolphin (Platanista gangetica) (Sinha and Sharma, 2003; Smith and Braulik, 2012). This age-long practice has been well-documented by several authors that describe the extraction and use of dolphin blubber oil to attract the catfishes Eutropiichthys vacha and Clupisoma garua (e.g., Bairagi, 1999; Sinha, 2002). Dolphins are used as bait in both India and Bangladesh, in the Ganges and Brahmaputra rivers, although the practice appears to be most common in the central Ganges. Killing with harpoons is thought to have declined since the enactment of the Indian Wildlife (Protection) Act in 1972 (Sinha, 2002). However, this law has been deemed largely ineffective, because instead of harpooning, fishers place nets in areas where they are likely to catch dolphins, a process referred to as “assisted incidental capture” (Sinha, 2002). Considerable effort has been directed at finding alternative baits and fish scrap oil is reported to be an effective attractant (Sinha, 2002). Long-term and widespread extension and outreach programs are needed to educate fishers on this alternative (Sinha and Sharma, 2003).
Conservation Status of the Species Involved
Of the 42 species identified as having been utilized as bait, 11 (26.91%) are listed as near threatened or threatened (vulnerable to critically endangered) by the IUCN (Table 6). Direct killing for use as bait has been identified in redlist assessments as a major threat for two of these species, i.e., the Ganges River dolphin (P. gangetica) (Smith and Braulik, 2012) and the Chilean dolphin (Cephalorhynchus eutropia) (Heinrich and Reeves, 2017). In addition to the threatened status of almost a quarter of the species utilized as bait, the classification of 19 (45.24%) of these species as data deficient is disconcerting (Table 6). Hunting for use as bait is an important threat affecting at least one of these species, i.e., I. geoffrensis (Reeves et al., 2011). Finally, it is worth noting that the common bottlenose dolphin (T. truncatus), although classified as least concern, has been utilized as bait in over half of the countries (n = 17) with fisheries that have employed aquatic mammal bait (Table 6), a result that can likely be explained by the species' widespread distribution and occurrence in nearshore and coastal waters.
Of the 24 species identified as the target of a fishery utilizing aquatic mammals as bait, seven (29.20%) are classified by the IUCN as near threatened or vulnerable. These include one grouper species (Epinephelus cifuentesi) and six sharks: blue shark (Prionace glauca), common thresher shark (Alopias vulpinus), tiger shark (Galeocerdo cuvier), shortfin mako (Isurus oxyrinchus), Caribbean reef shark (Carcharinus springeri), and the smooth hammerhead (Sphyrna zygaena). Eight species (33.33%) that are targeted by the fisheries are either unassessed or listed as data deficient. The remaining target fishery species (n = 9, 37.5%) are classified to be of least concern (IUCN, 2017).
Discussion
Geography of the Use of Aquatic Mammal Bait
The synthesis herein revealed that the practice of utilizing marine mammals for bait has been and continues to be most common in Latin America, followed by Asian countries. Latin America had the most sources reporting the practice and the most countries with fisheries that utilize aquatic mammal bait. While the greater number of sources acquired from this region might be explained, in part, by the authors' languages and regional expertise, it also reflects a large number of sources (n = 41) pertaining to two cases in the region: Chilean crab fishery and Amazon catfish fishery. The occurrence of these cases and the number of countries with fisheries engaging in the practice, strongly suggest that Latin America is a region that needs careful monitoring. The socio-economic climate in Latin America may be a driver of the practice and growth of harvesting aquatic mammals for use as bait.
The majority of the countries in Latin America and Asia with fisheries that use aquatic mammal bait are categorized as having a “medium” to “high” Human Development Index (HDI; United Nations Development Programme (UNDP), 2016). As of this century, with the exception of Chile and Japan, no nations categorized as having a “very high” HDI have fisheries that utilize aquatic mammals for bait. This is likely due to a combination of factors, including: (1) strict legislation and enforcement pertaining to marine mammal protection (e.g., the Marine Mammal Protection Acts of the United States and New Zealand), (2) public perceptions and attitudes toward marine mammals (e.g., positive attitudes in the Mediterranean and New Zealand), and (3) the relatively low presence or importance of artisanal or subsistence fisheries in “very high” HDI countries (Berkes, 2001). While many Latin American and Asian countries listed in this review have legislation protecting aquatic mammals, enforcement is close to non-existent in most areas due primarily to limited financial and staffing resources (De Oliveira, 2002; Salas et al., 2007). Moreover, with artisanal fisheries as an important source of livelihood along much of the Latin American and Asian coasts (Berkes, 2001; Salas et al., 2007), there are ample opportunities for aquatic mammal-human interactions.
The lack of sources indicating the occurrence of this activity in Africa, where most countries have a “low” HDI index and artisanal fisheries have a strong presence, warrants further investigation. An absence of general cetacean research in many countries in the region may, in part, explain the scarcity of relevant source material in this review [(Elwen et al., 2011); only 13 sources (8%) provided information on bait use in African countries]. It is more likely, however, that widespread human consumption of marine mammal meat in Africa supersedes its importance as a bait in regional fisheries (Van Waerebeek et al., 2000; Andrianarivelo, 2001; Debrah et al., 2010; Robards and Reeves, 2011; Weir and Pierce, 2013; Cerchio et al., 2015). With the scarcity and high cost of protein in many locations, marine mammal meat is consumed readily and using it for bait would be difficult to justify from either a health or economic perspective.
In Ghana, for example, although dolphins are used occasionally for bait, the price of cetacean meat for human consumption has equaled that of billfishes such as marlin and sailfish, so there has been little financial incentive to use the meat as bait (Debrah et al., 2010). Nevertheless, as the demand for shark fins in China continues with the growth of its middle class (Clarke et al., 2007), the financial incentives for using cetaceans as bait may increase (Debrah, 2000; Debrah et al., 2010). In fact, Ofori-Danson et al. (2012) specifically describe that the shift from incidental catches to targeted fishing of small cetaceans, for use as shark bait, has already started to occur.
Cultural beliefs may play a role in limiting the use of dolphin as bait in some locations in Africa and elsewhere, as taboos associated with killing dolphins exist throughout the world. In Madagascar, for example, the Sakalava people of the northwest discard dolphin carcasses from incidental captures in observance of local taboos, whereas the Vezo people of the southwest, who have no such taboo, hunt and readily consume marine mammals (Andrianarivelo, 2001; Cerchio et al., 2015). Beliefs surrounding the harming of dolphins may refrain them from using dolphins for human consumption or bait in the northwest, whereas value of the meat in the southwest is too great to use it as bait (N. Andrianarivelo, Personal Communication, August 29, 2017).
Two of the most well-studied cases presented in this review: the killing of cetaceans in the Magellan region of Chile in support of the crab fishery and the hunting of botos in the Amazon for catfish bait have geographical similarities that should be highlighted. First, both fisheries are fueled by external markets, with the products (i.e., crab and catfish) being exported to foreign markets or large, metropolitan cities (Cárdenas et al., 1987; Gómez et al., 2008; Cunha et al., 2015). The killing and use of cetaceans as bait for fisheries, in general, occurs far from the ultimate consumption point of the harvest species. As a consequence, consumers are largely ignorant with regard to these issues. Second, the killing of cetaceans takes place in remote areas where enforcement is difficult and costly; there was a lack of government intervention to prevent overexploitation in both cases (Lescrauwaet and Gibbons, 1994; Mintzer et al., 2013).
Knowledge Gaps
Findings summarized herein very likely underrepresent the full extent of the issues surrounding the utilization of small cetaceans as bait in global fisheries. Although marine mammals are legally protected in many countries, artisanal fisheries generally occur in remote areas where management and enforcement are limited. Fishers can easily discard evidence of mammal harvest, utilize the carcass quickly for bait on their vessels, and avoid reprehension (Van Waerebeek et al., 2003; Young and Iudicello, 2007). The illegal nature of the practice makes it difficult to study and certainly leads to underreporting and poor coverage in the literature.
Two large knowledge gaps stand out as being particularly disconcerting: (1) the data deficient or unassessed status of over a third of the species involved (bait or target) (see the following section Provisioning of Alternative Baits for further discussion) and, (2) the lack of studies presenting estimates of the number of marine mammals killed for bait and/or impacts to these exploited populations. Of all the cases included in this review, only five (the crab fishery in South America, catfish fishery in the Amazon, small-scale driftnet fishery in Peru, Palawan and Selinog Island fisheries in the Philippines, and shark fishery along the southern coast of Zanzibar) have corresponding source materials that provide some estimates of the number of cetaceans taken and/or show declines in population parameters (Tables 4, 5). Without more of these estimates, conservation actions will be hindered and may simply not take place because the need is not evident.
We recognize that the take of marine mammals for use as bait may be negligible for some affected populations. However, because of the clandestine nature of the practice, there is a high degree of uncertainty surrounding even the best estimates of take for cetacean bait. The line between Targeted, Non-Targeted-Deliberate, and Non-Targeted-Salvage take is often blurred, as indicated by several authors (e.g., Sinha, 2002; Young and Iudicello, 2007; Read, 2008; Robards and Reeves, 2011) and also by the amount of times we reported “Non-Target-?” herein (see Tables 1–5). We encourage marine mammal scientists, particularly those studying small cetaceans, to implement research methods (fisher interviews, examination of carcasses, stomach analyses of fishery catch, etc.) to better gage the extent of the activity (i.e., take specifically for use as bait).
Provisioning of Alternative Baits
Developing and providing alternative baits was the most common solution identified in our review of the literature to encourage a shift away from the utilization of cetacean baits (e.g., Goodall et al., 1994; Lal Mohan, 1994; Perrin et al., 1994b; Sinha, 2002; Culik, 2004; Alfaro Shigueto et al., 2008; Beltrão et al., 2017). The use of alternative baits was, in part, responsible for the decrease in the use of cetaceans as bait in Chilean crab fisheries (Lescrauwaet and Gibbons, 1994; Manzur and Canto, 1997; Young and Iudicello, 2007). Considering that so many fishers, particularly in South America, mentioned that they utilize cetacean bait only because preferred or traditional bait is expensive or unavailable, we can presume that, in at least some cases, fishers would be willing to adopt a change without resistance (Alfaro Shigueto et al., 2008). However, it is clear, based on our findings, that the procurement and distribution of an alternative bait to replace a cetacean bait would not address the heart of the issue.
Two-thirds of aquatic mammal species used as bait are utilized in shark fisheries. An increase in the demand for shark products, particularly for fins, has led to an increase in shark fishing effort and landings worldwide (Clarke et al., 2007; Debrah et al., 2010). Consequently, many shark populations have experienced a drastic and widespread decline in the past decades (Momigliano and Harcourt, 2014). In fact, sharks, generally speaking, are now considered to be among the most threatened marine animals (Lucifora et al., 2011). It is imperative that marine resource managers address the issue of cetacean bait and/or its alternatives in this light.
Although an argument could be made for focusing on the procurement of alternative baits in cases were the target fishery is not threatened, only three targeted fishery species identified in this review are categorized as “Least Concern” with populations known to be “Stable”. Most other species have been unassessed by the IUCN or are classified as “Data Deficient”. As noted above, this lack of knowledge surrounding the issue of cetacean bait is cause for legitimate concern and scientific effort should be directed at alleviating these information deficiencies.
Mitigation Measures
In addition to developing and using alternative baits, it was noted by several authors that education campaigns, dolphin-centered tourism, and increases in enforcement might serve as effective measures to mitigate the use of aquatic mammal bait. With regard to education campaigns, with the exception of Chile, where fishers were informed about the ecological importance of cetaceans and utilization of alternative baits (Manzur and Canto, 1997; Young and Iudicello, 2007), we found no records demonstrating the application or success of this measure. There was some indication that a shift in public perception toward dolphins caused a decrease in the use of cetacean bait in New Zealand and the Mediterranean, but no particular education efforts were described in the source materials that might have precipitated a change in awareness or perception. Nevertheless, authors argue for the need for local programs that will educate fishers on marine mammal protection laws and their importance (e.g., Culik, 2004). Although education should be a component of programs aimed at decreasing the use of aquatic mammal bait, we stress the need for implementing this measure concurrently with other actions, as exemplified in Chile. Given the socio-economic status of most fishers involved in this practice, education programs should present fishers with alternative ways to secure their livelihoods.
Dolphin-centered tourism might be a viable alternative in cases where the dollar value of keeping a dolphin alive outweighs the value of the fishery catch a dead dolphin would help procure. Evidence in support of this idea comes from the southwest coast of Zanzibar where a dolphin hunt was replaced by profitable dolphin watching tours (see section on the Indo-Pacific). Researchers from Brazil and Indonesia have recognized similar potential within their study regions (Hilton, 2012; Pinto de Sa Alves et al., 2012). However, several sources in this review call attention to the negative side of dolphin-based tourism and how lack of regulations placed on boats can lead to detrimental effects on dolphin behavior and health (Amir and Jiddawi, 2001; Stensland et al., 2006; Pinto de Sa Alves et al., 2012).
Lack of enforcement of existing laws that protect aquatic mammals from being killed is widely recognized as a serious impediment to resolving the issues discussed in this review (e.g., Jiménez, 2002; Young and Iudicello, 2007). As noted previously, many locations where the direct take of mammals occurs lack the staffing and financial resources to implement the necessary enforcement effort. Moreover, the very nature of using mammals for bait (i.e., essentially disposing of the carcass) makes it relatively easy for fishers to conceal the illicit activity (Van Waerebeek et al., 2003; Young and Iudicello, 2007). Although an increase in enforcement could very well be the solution to halting the use of aquatic mammal bait, the economic and political reality at many locales hinders this possibility. Nevertheless, authorities in some locales may be in a position to develop and implement successful enforcement efforts. For example, Siciliano et al. (2008) reported on the decrease of hunting of S. guianensis in the waters off the coast of Brazil. The practice appears to have subsided, since the 1990s, due largely to increased enforcement efforts. Accessibility to the hunting locations and markets in Baía de Marajó and the eastern coast of Para likely facilitated enforcement in these coastal areas.
In another example described in this review (see section on Large River Systems), Brazil established a 5-year moratorium on the fishing and selling of C. macropterus that began in January 2016 (Brum et al., 2015; Beltrão et al., 2017). This ban was put in place to decrease the killing of botos for use as bait and to allow time for the development of alternatives to present to fishers (Brum et al., 2015). Regardless of the fact that the killing of botos takes place in secluded, hard-to-enforce, areas of the Amazon, substantial catches of C. macropterus are processed in large, centralized freezing/distribution plants that should be relatively easy to monitor (Brum et al., 2015). As of mid-2017, the piracatinga fishery and hunt for botos continues (da Silva and Martin, 2017). However, the overall catch of piracatinga appears to have decreased and efforts continue to train enforcement agents (V. da Silva, Personal Communication, August 29, 2017). Although it will be years before the outcomes of the moratorium are known, initiatives surrounding the implementation of the moratorium will provide a series of lessons learned regarding the enforcement of natural resource laws in remote areas.
Enforcement improvements need not be federal, but may be implemented through local means such as protected areas and/or fishing communities. The Mamirauá Sustainable Development Reserve, in the Brazilian Amazon, where Amazon River dolphin are hunted, provides a model for developing local efforts. Although hunting occurs in some areas in the reserve, it appears to be limited in locations where there is strong protected area enforcement and researcher presence (Mintzer et al., 2015). Moreover, community-based ecotourism and education initiatives have also played a role in developing positive behaviors toward dolphins within reserve communities (Mintzer et al., 2015). Albeit the current scale of influence of these initiatives has been insufficient to prevent boto population decline (da Silva et al., 2011; Mintzer et al., 2013).
As the Chilean crab fishery case study shows, several measures will likely need to be implemented concurrently to decrease cetacean harvest for bait. Where possible, natural resource managers are encouraged to consider implementing a holistic approach. Presenting fishers with ecotourism or other economic incentives, educating them on marine mammal laws and cetacean ecology, and enforcing rules related to sound dolphin-watching, could lead to a system that benefits both the fishing communities and dolphin populations.
Conclusion
The use of small cetaceans as bait is a widespread activity and is documented in this review to have occurred in at least 33 countries, across six continents, since 1970. At least one fishery in 21 of these countries has utilized aquatic mammals as bait in the most recent period assessed in this study (2000–2017). This practice appears to be most common and ongoing in Latin America and Asia, where socio-economic conditions fuel the need for effective and free or cheap bait to enhance artisanal fishery harvest. Although there is very limited information on the impact of the direct take on most targeted small cetacean populations, a couple of case-studies, explained herein, indicate significant population-level impacts.
Resource managers and conservation scientists are advised to consider the results of this review within the context of their home regions. We encourage those studying or working with fisheries and/or species listed in Tables 1–6 to make an effort to gage the level of activity directed at procuring and using aquatic mammals for bait in their areas of study and be vigilant of increases in this elusive practice. With the continuing demand for shark fins, the practice of using small cetaceans for bait will likely increase in locations already engaged in this practice, and possibly spread to new ones.
The successful reduction of this threat will, in general, require a combination of measures that include, for example, education, ecotourism, and enforcement initiatives. The use of aquatic mammals occurs in many artisanal fisheries, where the practice is tied to the economic viability of human populations, and needs to be addressed within its intricate ecological, social and political system. Combining conservation knowledge and sound economic reasoning could help alleviate stresses imposed on both dolphins and sharks in many locations around the world.
Author Contributions
VM led and executed the systematic review, synthesis, and writing of the manuscript. KD contributed the initial idea for the review and conducted a large segment of the systematic review. TF synthesized source materials and organized and developed the manuscript.
Funding
This work was supported by the School of Natural Resources and Environment at the University of Florida and the Instituto Venezolano de Investigaciones Científicas. The views expressed are those of the authors and do not necessarily reflect the views of these organizations.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful to the many authors of the sources that informed this review. We also thank the researchers who took the time to provide us with additional details and insights into their reported findings.
References
Aguayo-Lobo, A. (1999). The cetaceans and their conservation perspectives. Estud. Oceanol. 18, 35–39.
Agudo, A. I. (1994). “Preliminary report on death of cetaceans in gillnets in northeastern Venezuelan waters,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 623.
Aguilar Vila, A., Borrell Thió, A., Gazo Pérez, M., del Mar Fernández Contreras, M., Cantos Font, G., Arderiu i Bofill, A., et al. (2002). Actuaciones Para la Conservación del Delfín Mular. Grup D'Estudi I Conservacio de Mamífers Marins, Universitat de Barcelona.
Aguilar Vila, A., Forcada, J., Borrell, A., Silvani, L., Grau, G., Gazo, M., et al. (1994). Inventario de Cetáceos Mediterráneos Ibéricos: Status y Problemas de Conservación. Departamento de Biología Animal, Universitat de Barcelona.
Aguilar, A. (2006). “Striped dolphin (Stenella coeruleoalba), Mediterranean subpopulation,” in The Status and Distribution of Cetaceans in the Black Sea and Mediterranean Sea, eds R. R. Reeves and G. Notarbartolo di Sciara (Malaga: IUCN Centre for Mediterranean Cooperation), 57–63.
Alfaro Shigueto, J., Mangel, J. C., and Van Waerebeek, K. (2008). Small cetacean captures and CPUE estimates in artisanal fisheries operating from a port in northern Peru, 2005-2007. Document SC/60/SM19 Presented to the International Whaling Commission (Santiago).
Alfaro Shigueto, J., Mangel, J. C., Pajuelo, M., Dutton, P. H., Seminoff, J. A., and Godley, B. J. (2010). Where small can have a large impact: structure and characterization of small-scale fisheries in Peru. Fish. Res. 106, 8–17. doi: 10.1016/j.fishres.2010.06.004
Aliaga Rossel, E. (2002). Distribution and abundance of the river dolphin (Inia geoffrensis) in the Tijamuchi River, Beni, Bolivia. Aquat. Mammals 28, 312–323.
Aliaga Rossel, E. (2003). Situación actual del delfín de río (Inia geoffrensis) en Bolivia. Ecol. Bolivia 38, 167–178.
Amir, O. A., and Jiddawi, N. S. (2001). “Dolphin tourism and community participation in Kizmkazi village, Zanzibar,” in Proceedings of the 20th Anniversary Conference on Advances in Marine Science in Tanzania, eds M. D. Richmond and J. Francis, Marine Science development in Tanzania and East Africa (Zanzibar: IMS/WIOMSA), 551–560.
Amir, O. A., Berggren, P., and Jiddawi, N. (2002). The incidental catch of dolphins in gillnet fisheries in Zanzibar, Tanzania. Western Indian Ocean J. Mar. Sci. 1, 155–162.
Anderson, R., and Ahmed, H. (1993). The Shark Fisheries of the Maldives. Ministry of Fisheries and Agriculture and Food and Agriculture Organization of the United Nations, Male.
Andrianarivelo, N. (2001). Essai D'évaluation de l'importance de la Pêche aux Dauphins Dans la Région d'Anakao (sud-ouest de Madagascar). DEA thesis. Institut Halieutique et des Sciences Marines, Université de Toliara, Madagascar.
Attademo, F. L. N., de Lima Silva, F. J., de Moura, J. A. F., Firmino, A. S. L., Gomes, P. T., and de Oliveira, A. F. (2005). Uso de Cetáceos Como ISCA no Rio Grande do Norte. VII Congresso de Ecologia do Brasil, Sociedade de Ecologia do Brasil, Caxambu, 2.
Avila, I. C., García, C., and Bastidas, J. C. (2008a). A note on the use of dolphins as bait in the artisanal fisheries off Bahía Solano, Chocó, Colombia. J. Cetacean Res. Manage 10, 179–182.
Avila, I. C., García, C., and Bastidas, J. C. (2008b). Use of dolphins for bait in the artisanal fisheries of Bahía Solano, Chocó, Colombia. Document SC/60/SM6 presented to the International Whaling Commission (Santiago).
Ayala, L., Amorós, S., and Céspedes, C. (2008). By-catch of Albatross and Petrel in Artisan Longline and gillnet Fisheries in Northern Peru. APECO final report to the Rufford Small Grants for Nature Conservation. Available online at: https://www.rufford.org/files/31.06.06%20Detailed%20Final%20Report.pdf (Accessed May 31, 2016).
Bairagi, S. P. (1999). Oil bait fishery of catfishes in Brahmaputra River affecting river dolphin populations in Assam, India. J. Bombay Natural History Soc. 96, 424–426.
Bannister, J., Kemper, C. M., and Warneke, R. M. (1996). The Action Plan for Australian Cetaceans. Australian Nature Conservation Agency (Canberra, ACT), 272.
Barbosa-Filho, M. L. V., Barreto, R. M. F., Siciliano, S., Seminara, C. I., and Costa-Neto, E. M. (2018). Use of Cetaceans as Bait in Southern Bahia, Brazil, by expert fishermen that market shark fins: a lucrative trade and two threatened zoological groups. Ethnobiol. Lett. 9, 12–18. doi: 10.14237/ebl.9.2.2018.953
Barbosa-Filho, M., Costa-Neto, E., and Danilewicz, D. (2016). Dolphin harpooning off the coast of Bahia, Brazil. Mar. Biodivers. Rec. 9:42. doi: 10.1186/s41200-016-0046-1
Bearzi, G., Bjørge, A., Forney, K. A., Hammond, P. S., Karkzmarski, L., Kasuya, T., et al. (2012). Stenella Longirostris. The IUCN Red List of Threatened Species. Version 2015.2. Available online at: www.iucnredlist.org
Beltrão, H., M. Porto-Braga, T., and Schwartz-Benzaken, Z. (2017). Alternative bait usage during the piracatinga (Calophysus macropterus) fishery in the Manacapuru region, located at the lower Solimões-Amazonas River, Amazon basin, Brazil. Panam J. Aquat. Sci. 12, 194–205.
Berkes, F. (2001). Managing Small-Scale Fisheries: Alternative Directions and Methods. International Development Research Centre, Ottawa, ON.
Bermúdez-Villapol, L. A., and Sayegh, A. J. (2005). Informe Técnico de Varamientos de Cetáceos en el edo. Nueva Esparta, Venezuela, periodo 2000-2004. Informe Técnico Depositado en la Dirección Estadal Ambiental del Estado Nueva Esparta y en la Dirección Nacional de Diversidad Biológica MARN. Centro de Investigación de Cetáceos, Los Robles.
Bjørge, A., Brownell, R. L. Jr., Donovan, G. P., and Perrin, W. F. (1994). “Significant direct and incidental catches of small cetaceans,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 73–130.
Bolaños-Jiménez, J., Boede, E. O., Ferrer-Pérez, A., Herrera-Trujillo, O., Linares, O., Portocarrero-Aya, M., et al. (2015). “Tonina del Orinoco, Inia geoffrensis,” in Libro Rojo de la Fauna Venezolana. Cuarta Edición, eds J. P. Rodríguez, A. García-Rawlins, and F. Rojas-Suárez (Caracas: Provita y Fundación Empresas Polar), 113.
Brum, S., Silva, V., Rossoni, F., and Castello, L. (2015). Use of dolphins and caimans as bait for Calophysus macropterus (Lichtenstein, 1819) (Siluriforme: Pimelodidae) in the Amazon. J. Appl. Ichthyol. 31, 675–680.
Caballero, S., Trujillo, F., Vianna, J., Barrios-Garrido, H., Montiel, M., Beltrán-Pedreros, S., et al. (2007). Taxonomic status of the genus Sotalia: species level ranking for “tucuxi”(Sotalia fluviatilis) and “costero”(Sotalia guianensis) dolphins. Mar. Mammal Sci. 23, 358–386. doi: 10.1111/jai.12772
Campbell, E., and Alfaro-Shigueto, J. (2016). “Diagnóstico sobre el estado de conservación de delfines de río y manatíes amazónicos,” in Diversidad Biológica del Sudeste de la Amazonía Peruana: Avances en la Investigación, eds J. L. Mena and C. Germaná (Lima: Consorcio Purús Manu: WWF, CARE Perú, ProNaturaleza, ProPurús, Sociedad Zoológica de Fráncfort, ORAU), 194–210.
Capistrano, C., Ramos, R., and Beneditto, A. (1994). “Incidental capture of small cetaceans on the coasts of Rio de Janeiro, Espititu Santo and Bahia States, Brazil,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 623.
Cárdenas, J. C., Gibbons, J., Oporto, J., and Stutzin, M. (1987). Impacto de la pesquería de centolla y centollón sobre las poblaciones de mamíferos marinos de Magallanes, Chile. Ambiente y Desarrollo 3, 111–119.
Cárdenas, J., Oporto, J., and Stutzin, M. (1986). Problemas de manejo que afectan a las poblaciones de cetáceos en Chile: Proposiciones para una política de conservación y manejo. Ambiente y Desarrollo 2, 107–116.
Cerchio, S., Andrianarivelo, N., and Andrianantenaina, B. (2015). “Ecology and conservation status of Indian Ocean Humpback Dolphins (Sousa plumbea) in Madagascar,” in Advances in Marine Biology, Vol. 72, eds T. A. Jefferson and B. E. Curry (Oxford: Academic Press), 163–199.
Clarke, S., Milner-Gulland, E. J., and Bjørndal, T. (2007). Social, economic, and regulatory drivers of the shark fin trade. Mar. Resour. Econ. 22, 305–327. doi: 10.1086/mre.22.3.42629561
Claus, S., De Hauwere, N., Vanhoorne, B., Souza Dias, F., Oset García, P., Schepers, L., et al. (2018). MarineRegions.org. Available online at: http://www.marineregions.org
Committee on Taxonomy (2016). List of Marine Mammal Species and Subspecies. Society for Marine Mammalogy. Available online at: https://www.marinemammalscience.org/species-information/list-marine-mammal-species-subspecies/
Cosentino, A. M., and Fisher, S. (2016). The utilization of aquatic bushmeat from small cetaceans and manatees in South America and West Africa. Front. Mar. Sci. 3:163. doi: 10.3389/fmars.2016.00163
Crespo, E., Corcuera, J., and López Cazorla, A. (1994). “Interactions between marine mammals and fisheries in some coastal fishing areas of Argentina,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 269–281.
Culik, B. (2004). Review of Small Cetaceans: Distribution, Behaviour, Migration and Threats. UNEP/CMS Secretaria (Bonn).
Culik, B. (2010). Odontocetes-The Toothed Whales: Distribution, Behaviour, Migration and Threats. UNEP/CMS Secretaria (Bonn).
Cunha, H. A., da Silva, V. M., Santos, T. E., Moreira, S. M., do Carmo, N. A., and Solé-Cava, A. M. (2015). When you get what you haven't paid for: Molecular identification of “Douradinha” fish fillets can help end the illegal use of river dolphins as bait in Brazil. J. Hered. 106, 565–572. doi: 10.1093/jhered/esv040
D'Cruz, T. (2004). Artisanal deep-sea fishing in Kerala: prospects and problems. Discussion Paper No. 74. Kerala Research Programme on Local Level Development, Centre for Development Studies, Thiruvananthapuram.
da Silva, V. M. F. (2009). “Amazon River dolphin,” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (London: Academic Press), 26–28.
da Silva, V. M. F., and Martin, A. R. (2010). “Status, threats, conservation initiatives and possible solutions for Inia geoffrensis and Sotalia fluviatilis in Brazil,” in The Action Plan for South American River Dolphins 2010–2020, eds F. Trujillo, E. Crespo, P. A. Van Damme, and J. S. Usma (Bogotá: WWF, Fundación Omacha, W. D. S., WDCS, and SOLAMAC), 123–143.
da Silva, V. M. F., and Martin, A. R. (2017). A note on the continuing hunt for botos (Inia geoffrensis) in the Brazilian Amazon and the continuing rapid decline of this dolphin. Document SC/67A/SM/13 presented to the International Whaling Commission (Bled).
da Silva, V. M. F., Martin, A. R., and do Carmo, N. A. S. (2011). Boto bait - Amazonian fisheries pose threat to elusive dolphin species. IUCN Magazine of the Species Survival Commission, 10–11.
da Silveira, R., and Viana, J. P. (2003). Amazonian crocodilians: a keystone species for ecology and management… Or simply bait? Crocodile Specialist Group Newsletter, 16–17.
Davidson, A. D., Boyer, A. G., Kim, H., Pompa-Mansilla, S., Hamilton, M. J., Costa, D. P., et al. (2012). Drivers and hotspots of extinction risk in marine mammals. Proc. Natl Acad. Sci. U.S.A. 109, 3395–3400. doi: 10.1073/pnas.1121469109
Dayaratne, P., and Joseph, L. (1993). A Study on Dolphin Catches in Shri Lanka. Bay of Bengal Programme, Madras, 47.
De Boer, M., Baldwin, R., Burton, C., Eyre, E., Jenner, K., Jenner, M., et al. (2002). Cetaceans in the Indian Ocean Sanctuary: A Review. Whale and Dolphin Conservation Society, Wiltshire.
De Oliveira, J. A. P. (2002). Implementing environmental policies in developing countries through decentralization: the case of protected areas in Bahia, Brazil. World Dev. 30, 1713–1736. doi: 10.1016/S0305-750X(02)00067-0
Debrah, J. S. (2000). Taxonomy, Exploitation and Conservation of Dolphins in the Marine Waters of Ghana. Masters thesis, Department of Oceanography and Fisheries, University of Ghana, 86.
Debrah, J. S., Ofori-Danson, P. K., and Van Waerebeek, K. (2010). An update on the catch composition and other aspects of cetacean exploitation in Ghana. Document SC/62/SM10 Presented to the International Whaling Commission, Agadir.
Delgado Estrella, A. (1997). Relación de las toninas, Tursiops truncatus, y las toninas moteadas, Stenella frontalis, con la actividad camaronera en la Sonda de Campeche, México. Anal. Instit. Biol. Ser. Zool. 68, 317–338.
Di Beneditto, A. P. M., Ramos, R. M. A., and Lima, N. R. W. (1998). Fishing activity in northern Rio de Janeiro State (Brazil) and its relation with small cetaceans. Braz. Arch. Biol. Technol. 41, 296–302. doi: 10.1590/S1516-89131998000300004
Di Natale, A., and Notarbartolo di Sciara, G. (1994). “A review of passive fishing nets and trap fisheries in the Mediterranean Sea and of the cetacean bycatch,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 189–202.
Diniz, K. S. (2011). La pesca del Bagre Zamurito (Calophysus macropterus, Siluformes: Pimelodidade) y su Efecto Potencial Sobre la Extracción de Toninas (Inia geoffrensis, Cetacea: Iniidae) y Babas (Caiman Crocodilus, Crocodilia: Aligatoridae) en Venezuela. Master's thesis, Instituto Venezolano de Investigaciones Científicas, Caracas.
Dolar, M. L. L. (1994). “Incidental takes of small cetaceans in fisheries in Palawan, central Visayas and northern Mindanao in the Philippines,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 355–363.
Dolar, M., Leatherwood, S., Wood, C., Alava, M., Hill, C., and Aragones, L. (1994). Directed Fisheries for Cetaceans in the Philippines. Report - International Whaling Commission, Cambridge, 439–449.
Dolar, M., Perrin, W., Yaptinchay, A., Jaaman, S. A. B. H., Santos, M. D., Alava, M. N., et al. (1997). Preliminary investigation of marine mammal distribution, abundance, and interactions with humans in the southern Sulu Sea. Asian Mar. Biol. 14, 61–81.
Elwen, S. H., Findlay, K. P., Kiszka, J., and Weir, C. (2011). Cetacean research in the southern African subregion: a review of previous studies and current knowledge. Afr. J. Mar. Sci. 33, 469–493. doi: 10.2989/1814232X.2011.637614
Estes, J. A., Terborgh, J., Brashares, J. S., Power, M. E., Berger, J., Bond, W. J., et al. (2011). Trophic downgrading of planet Earth. Science 333, 301–306. doi: 10.1126/science.1205106
Estupiñán, G. M. B., Marmontel, M., de Queiroz, H. L., e Souza, P. R., Valsecchi, J., da Silva Batista, G., et al. (2003). A Pesca da Piracatinga (Calophysus macropterus) na Reserva de Desenvolvimento Sustentável Mamirauá. Relatório Técnico. Ministério da Ciência e Tecnologia, Instituto de Desenvolvimento Sustentável Mamirauá, Tefé. Available online at: http://www.socioambiental.org/website/noticias/agenda/fks/rel_piracatinga.htm (Accessed May 31, 2012).
Félix, F., and Samaniego, J. (1994). “Incidental catches of small cetaceans in the artisanal fisheries of Ecuador,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 475–480.
Flórez, L., Prieto, M., and Bohórquez, O. (1992). Informe Nacional Sobre la Situación de los Mamíferos Marinos en Colombia. Regional Seas Programme, United Nations Environmental Programme.
Freitas-Netto, R., and Di Beneditto, A. P. M. (2008). Interactions between fisheries and cetaceans in Espírito Santo State coast, southeastern Brazil. Rev. Bras. Zoociências 10, 55–63.
Fundación Omacha WWF Colombia (2008). First Evaluation of Abundance of the Three River Dolphin Species (Inia geoffrensis, I. boliviensis, and Sotalia fluviatilis) in the Orinoco and Amazon River Basins, South America. Fundación Omacha and WWF Colombia, 21. Available online at: https://www.wwf.de/fileadmin/fm-wwf/Publikationen-PDF/WWF_Report_River_Dolphins.pdf (Accessed February 09, 2017).
García-Godos, I., and Cardich, C. (2010). First mass stranding of Risso's dolphins (Grampus griseus) in Peru and its destiny as food and bait. Mar. Biodivers. Rec. 3:e3. doi: 10.1017/S1755267209991084
Gómez, C., Trujillo, F., Diazgranados, M. C., and Alonso, J. (2008). “Capturas dirigidas de delfines de río en la Amazonia para la pesca de mota (Calophysus macropterus): una problemática regional de gran impacto,” in Fauna acuática amenazada en la Amazonia Colombiana – Análisis y Propuestas Para su Conservación, eds F. Trujillo, J. C. Alonso, M. C. Diazgranados, and C. Gómez (Bogotá: Fundación Omacha, Fundación Natura, Instituto Sinchi, Corpoamazonia), 39–57.
Goodall, R. N. P. (2002). “Peale's Dolphin - Lagenorhynchus australis,” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (London: Academic Press), 890–894.
Goodall, R., Schiavini, A., and Fermani, C. (1994). “Net fisheries and net mortality of small cetaceans off Tierra del Fuego, Argentina,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 295–304.
Haase, B., and Félix, F. (1994). “A note on the incidental mortality of sperm whales (Physeter macrocephalus) in Ecuador,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 481–483.
Hammond, P. S., Bearzi, G., Bjørge, A., Forney, K. A., Karkzmarski, L., Kasuya, T., et al. (2012a). Lagenodelphis hosei. The IUCN Red List of Threatened Species 2012:e.T11140A17807828. doi: 10.2305/IUCN.UK.2012.RLTS.T11140A17807828.en
Hammond, P. S., Bearzi, G., Bjørge, A., Forney, K. A., Karkzmarski, L., Kasuya, T., et al. (2012b). Lissodelphis peronii. The IUCN Red List of Threatened Species 2012:e.T12126A17877993. doi: 10.2305/IUCN.UK.2012.RLTS.T12126A17877993.en
Hammond, P. S., Bearzi, G., Bjørge, A., Forney, K. A., Karkzmarski, L., Kasuya, T., et al. (2012c). Phocoena spinipinnis. The IUCN Red List of Threatened Species 2012:e.T17029A17117957. doi: 10.2305/IUCN.UK.2012.RLTS.T17029A17117957.en
Hammond, P. S., Bearzi, G., Bjørge, A., Forney, K. A., Karkzmarski, L., Kasuya, T., et al. (2012d). Tursiops truncatus. The IUCN Red List of Threatened Species 2012:e.T22563A17347397. doi: 10.2305/IUCN.UK.2012.RLTS.T22563A17347397.en
Hammond, P. S., Bearzi, G., Bjørge, A., Forney, K., Karczmarski, L., Kasuya, T., et al. (2008a). Delphinus capensis. The IUCN Red List of Threatened Species 2008:e.T6337A12663800. doi: 10.2305/IUCN.UK.2008.RLTS.T6337A12663800.en
Hammond, P. S., Bearzi, G., Bjørge, A., Forney, K., Karczmarski, L., Kasuya, T., et al. (2008b). Lagenorhynchus australis. The IUCN Red List of Threatened Species 2008:e.T11143A3256488. doi: 10.2305/IUCN.UK.2008.RLTS.T11143A3256488.en
Hammond, P. S., Bearzi, G., Bjørge, A., Forney, K., Karczmarski, L., Kasuya, T., et al. (2008c). Stenella coeruleoalba. The IUCN Red List of Threatened Species 2008:e.T20731A9223182. doi: 10.2305/IUCN.UK.2008.RLTS.T20731A9223182.en
Heinrich, S., and Reeves, R. (2017). Cephalorhynchus eutropia. The IUCN Red List of Threatened Species 2017:e.T4160A50351955. doi: 10.2305/IUCN.UK.2017-3.RLTS.T4160A50351955.en
Hernández, S., and Gonzalves, J. (2009). Evaluation of deliberate killing of Amazon River dolphins used as bait for Mota fishery in the Javari River, Brazil. Instituto de Desenvolvimento Socioambiental Vale do Javari, Brazil. Available online at: http://idsavj.org/pages/mota_project.html (Accessed September 02, 2017).
Iriarte, V., and Marmontel, M. (2013a). Insights on the use of dolphins (boto, Inia geoffrensis and tucuxi, Sotalia fluviatilis) for bait in the piracatinga (Calophysus macropterus) fishery in the Western Brazilian Amazon. J. Cetacean Res. Manage 13, 163–173.
Iriarte, V., and Marmontel, M. (2013b). River dolphin (Inia geoffrensis, Sotalia fluviatilis) mortality events attributed to artisanal fisheries in the western Brazilian Amazon. Aquat. Mammals 39, 116–124. doi: 10.1578/AM.39.2.2013.116
IUCN (2017). The IUCN Red List of Threatened Species. Version 2017-1. Available online at: http://www.iucnredlist.org
Jefferson, T. A., and Curry, B. E. (1994). A global review of porpoise (Cetacea: Phocoenidae) mortality in gillnets. Biol. Conserv. 67, 167–183. doi: 10.1016/0006-3207(94)90363-8
Jefferson, T. A., Leatherwood, S., and Webber, M. A. (1993). Marine Mammals of the World (FAO Species Identification Guide). United Nations Environment Programme and Food and Agriculture Organization of the United Nations, Rome.
Jiménez, I. (2002). Heavy poaching in prime habitat: the conservation status of the West Indian manatee in Nicaragua. Oryx 36, 272–278. doi: 10.1017/S0030605302000492
Kiani, M. S., and Van Waerebeek, K. (2015). Chapter eight-a review of the status of the Indian ocean Humpback Dolphin (Sousa plumbea) in Pakistan. Adv. Mar. Bio. 72, 201–228. doi: 10.1016/bs.amb.2015.09.002
Lal Mohan, R. (1994). “Review of gillnet fisheries and cetacean bycatches in the northeastern Indian Ocean,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 329–343.
Leatherwood, S., and Reeves, R. R. (1989). Marine Mammal Research and Conservation in Sri Lanka, 1985-1986. United Nations Environmental Programme, Nairobi.
Lescrauwaet, A. C., and Gibbons, J. (1994). “Mortality of Small Cetaceans and the Crab Bait Fishery in the Magallanes Area of Chile Since 1980,” in Gillnets and Cetaceans, W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 485–494.
Lescrauwaet, A.-C., Gibbons, J., Guzman, L., and Schiavini, A. (2000). Abundance estimation of Commerson's dolphin in the eastern area of the Strait of Magellan-Chile. Revista Chilena de Historia Natural 73, 473–478.
Liu, M., Lin, M., Turvey, S. T., and Li, S. (2016). Fishers' knowledge as an information source to investigate bycatch of marine mammals in the South China Sea. Anim. Conserv. 20, 182–192. doi: 10.1111/acv.12304
Loch, C., Marmontel, M., and Simões-Lopes, P. C. (2009). Conflicts with fisheries and intentional killing of freshwater dolphins (Cetacea: Odontoceti) in the Western Brazilian Amazon. Biodivers. Conserv. 18, 3979–3988. doi: 10.1007/s10531-009-9693-4
Lodi, L., and Siciliano, S. (1994). “Survey of incidental net catches on marine Sotalia fluviatilis, Pontoporia blainvillei, and other small cetaceans in Brazil,” in Gillnet and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int.Whaling Comm.), 625.
Lucifora, L. O., García, V. B., and Worm, B. (2011). Global diversity hotspots and conservation priorities for sharks. PLoS ONE 6:e19356. doi: 10.1371/journal.pone.0019356
Magalhães, F., Tosi, C., Garri, R., Chellappa, S., and Silva, F. (2008). Cetacean diversity on the Parnaiba Delta, Maranhão state, northeastern Brazil. Braz. J. Biol. 68, 545–551. doi: 10.1590/S1519-69842008000300012
Mangel, J. C., Alfaro-Shigueto, J., Van Waerebeek, K., Cáceres, C., Bearhop, S., Witt, M. J., et al. (2010). Small cetacean captures in Peruvian artisanal fisheries: high despite protective legislation. Biol. Conserv. 143, 136–143. doi: 10.1016/j.biocon.2009.09.017
Mangel, J. C., Alfaro-Shigueto, J., Witt, M. J., Hodgson, D. J., and Godley, B. J. (2013). Using pingers to reduce bycatch of small cetaceans in Peru's small-scale driftnet fishery. Oryx 47, 595–606. doi: 10.1017/S0030605312000658
Manning, A. (2013). Dolphin Slaughter Fueled by Illegal Shark Trade. National Geographic, Ocean Views. Available online at: https://voices.nationalgeographic.org/2013/10/24/dolphin-slaughter-fueled-by-illegal-shark-trade/ (Accessed September 02, 2017).
Manzur, M., and Canto, J. (1997). Las pesquerías de centolla y centollón y su interferencia con mamíferos marinos. Ambiente y Desarrollo 13, 64–69.
Martin, A. R., and da Silva, V. M. F. (2004). Number, seasonal movements, and residency characteristics of river dolphins in an Amazonian floodplain lake system. Can. J. Zool. 82, 1307–1315. doi: 10.1139/Z04-109
Meirelles, A. C. O., Monteiro-Neto, C., Martins, A. M. A., Costa, A. F., Barros, H., and Alves, M. D. O. (2009). Cetacean strandings on the coast of Ceará, north-eastern Brazil (1992-2005). J. Mar. Biol. Assoc. 89, 1083–1090. doi: 10.1017/S0025315409002215
Mintzer, V. J., Da Silva, V. M. F., Martin, A. R., Barbour, A. B., Frazer, T. K., and Lorenzen, K. (2013). Effect of illegal harvest on apparent survival of Amazon River dolphins (Inia geoffrensis). Biol. Conserv. 158, 280–286. doi: 10.1016/j.biocon.2012.10.006
Mintzer, V. J., Schmink, M., Lorenzen, K., Frazer, T. K., Martin, A. R., and da Silva, V. M. F. (2015). Attitudes and behaviors toward Amazon River dolphins (Inia geoffrensis) in a sustainable use protected area. Biodivers. Conserv. 24, 247–269. doi: 10.1007/s10531-014-0805-4
Momigliano, P., and Harcourt, R. (2014). “Shark conservation, governance and management. Sharks: conservation, governance and management: the science-law disconnect,” in Sharks: Conservation, Governance and Management, eds N. Klein and E. Techera (Abingdon: Earthscan Series, Routledge), 89–106.
Monteiro-Neto, C., Alves-Júnior, T. T., Avila, F. C., Campos, A. A., Costa, A. F., Silva, C. N., et al. (2000). Impact of fisheries on the tucuxi (Sotalia fluviatilis) and rough-toothed dolphin (Steno bredanensis) populations off Ceará state, northeastern Brazil. Aquat. Mammals 26, 49–56.
Mora-Pinto, D. M., Muñoz-Hincapi,é, M. F., Mignucci-Giannoni, A. A., and Acero-Pizarro, A. (1995). Marine mammal mortality and strandings along the Pacific coast of Colombia. Rep. Int. Whaling Comm. 45, 427–429.
Northridge, P. S., and Hofman, R. J. (1999). “Marine mammal interactions with fisheries,” in Conservation and Management of Marine Mammals, eds J. R. Twiss, and R. R. Reeves (Washington, DC: Smithsonian Institution Press), 99–119.
Notarbartolo di Sciara, G., and Bearzi, G. (2002). “Cetacean direct killing and live capture in the Mediterranean Sea,” in Cetaceans of the Mediterranean and Black Seas: State of Knowledge and Conservation Strategies. A Report to the ACCOBAMS Secretariat, ed G. Notarbartolo di Sciara (Monaco), 5.1–5.2.
Ofori-Danson, P., Debrah, J., and Van Waerebeek, K. (2012). Survey for the Conservation Status of Small Cetaceans in Ghanaian coastal Waters in Conserving cetaceans and manatees in the western African region. University of Ghana, Centre for Migration Studies Technical Series No. 26, Bonn.
Ofori-Danson, P., Self-Sullivan, C., Mombu, V. M., and Yelibora, M. A. (2008). Enhancing conservation of the West African Manatee in Ghana. Annual Report 2007, Nature Conservation Research Centre (Accra).
Ofori-Danson, P., Van Waerebeek, K., and Debrah, S. (2003). A survey for the conservation of dolphins in Ghanaian coastal waters. J. Ghana Sci. Assoc. 5, 45–54.
Perrin, W. F., Donovan, G. P., and Barlow, J. (1994a) (eds.). Gillnets Cetaceans. Cambridge: Rep. Int. Whaling Comm.
Perrin, W. F., Donovan, G. P., and Barlow, J. (1994b). “Report of the workshop on mortality of cetaceans in passive fishing nets and traps,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 1–72.
Pinto de Sa Alves, L. C., Zappes, C. A., and Andriolo, A. (2012). Conflicts between river dolphins (Cetacea: Odontoceti) and fisheries in the Central Amazon: a path toward tragedy? Zoologia 29, 420–429. doi: 10.1590/S1984-46702012000500005
Porter, L., and Lai, H. Y. (2017). Marine mammals in asian societies; trends in consumption, bait, and traditional use. Front. Mar. Sci. 4:47. doi: 10.3389/fmars.2017.00047
Portocarrero Aya, M., Ferrer, A., Lasso, C. A., Ruiz-Garcia, M., Bolaños-Jiménez, J., and Caballero, S. (2010). “Status, distribution and conservation of the river dolphins Inia geoffrensis and Sotalia spp. in Venezuela,” in The Action Plan for South American River Dolphins 2010–2020, eds F. Trujillo, E. Crespo, P. A. Van Damme, and J. S. Usma (Bogotá: WWF, Fundación Omacha, WDS, WDCS, and SOLAMAC), 17–28.
Quintana-Rizzo, E. (2011). Harpooning and entanglement of wild dolphins in the Pacific coast of Guatemala. Latin Am. J. Aquat. Mamm. 9, 179–182. doi: 10.5597/lajam00187
Rao, R. J. (1994). “Status and conservation of sea dolphins along the east coast of Andhra Pradesh, India,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int.Whaling Comm.), 626–627.
Read, A. J. (1994).” Interactions between cetaceans and gillnet and trap fisheries in the northwest Atlantic,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 133–147.
Read, A. J. (2008). The looming crisis: interactions between marine mammals and fisheries. J. Mammal. 89, 541–548. doi: 10.1644/07-MAMM-S-315R1.1
Read, A. J., Drinker, P., and Northridge, S. (2006). Bycatch of marine mammals in US and global fisheries. Conserv. Biol. 20, 163–169. doi: 10.1111/j.1523-1739.2006.00338.x
Reeves, R. R., and Leatherwood, S. (1994). Dolphins, Porpoises, and Whales: 1994-1998 Action Plan for the Conservation of Cetaceans. IUCN, Gland.
Reeves, R. R., Crespo, E. A., Dans, S., Jefferson, T. A., Karczmarski, L., Laidre, K., et al. (2013). Cephalorhynchus commersonii. The IUCN Red List of Threatened Species 2013:e.T4159A44204030. doi: 10.2305/IUCN.UK.2013-1.RLTS.T4159A44204030.en
Reeves, R. R., Dalebout, M. L., Jefferson, T. A., Karczmarski, L., Laidre, K., O'Corry-Crowe, G., et al. (2008). Sousa chinensis. The IUCN Red List of Threatened Species 2008:e.T20424A9197694. doi: 10.2305/IUCN.UK.2008.RLTS.T20424A9197694.en
Reeves, R. R., Jefferson, T. A., Karczmarski, L., Laidre, K., O'Corry-Crowe, G., Rojas-Bracho, L., et al. (2011). Inia geoffrensis. The IUCN Red List of Threatened Species 2011:e.T10831A3220342. doi: 10.2305/IUCN.UK.2011-1.RLTS.T10831A3220342.en
Reeves, R. R., Smith, B. D., and Kasuya, T. (2000). Biology and Freshwater Cetaceans in Asia – Occasional Papers of the IUCN Species Survival Commission No. 23. IUCN, Gland; Cambridge, UK, 164.
Reeves, R., and Martin, T. (2009). “River dolphins,” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (London: Academic Press), 976–979.
Reyes, J., and Oporto, J. (1994). “Gillnet fisheries and cetaceans in the southeast Pacific,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 467–474.
Robards, M., and Reeves, R. R. (2011). The global extent and character of marine mammal consumption by humans. Biol. Conserv. 144, 2770–2786. doi: 10.1016/j.biocon.2011.07.034
Rodriguez, C. Y., and Romo, R. (2013). Dolphins Killed for Shark Bait in Peru. CNN. Available online at: http://www.cnn.com/2013/10/22/world/americas/dolphins-killed-peru/index.html (Accessed September 02, 2017).
Romero, A., Agudo, A. I., and Green, S. M. (1997). Exploitation of cetaceans in Venezuela. Rep. Int. Whaling Comm. 47, 735–746.
Romero, A., Agudo, A. I., Green, S. M., and di Sciara, G. N. (2001). Cetaceans of Venezuela: Their Distribution and Conservation Status. NOAA Technical Report NMFS 151. U.S. Department of Commerce (Seattle, WA), 59.
Ross, G. J. (2006). Review of the Conservation Status of Australia's Smaller Whales and Dolphins. Department of the Environment and Energy, Australian Government. Available online at: http://www.environment.gov.au/resource/review-conservation-status-australias-smaller-whales-and-dolphins (Accessed September 02, 2017).
Ross, G. J., and Leatherwood, S. (1994). “Pygmy killer whale - Feresa attenuata (Gray, 1874),” in Handbook of Marine Mammals: The Dolphins, eds S. H. Ridgway and R. J. Harrison (London: Academic Press), 387–404.
Salas, S., Chuenpagdee, R., Seijo, J. C., and Charles, A. (2007). Challenges in the assessment and management of small-scale fisheries in Latin America and the Caribbean. Fish. Res. 87, 5–16. doi: 10.1016/j.fishres.2007.06.015
Secchi, E. (2012). Sotalia guianensis. The IUCN Red List of Threatened Species 2012:e.T181359A17583662. doi: 10.2305/IUCN.UK.2012.RLTS.T181359A17583662.en
Siciliano, S. (1994). “Review of small cetaceans and fishery interactions in coastal waters of Brazil,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 329–343.
Siciliano, S., Emin-Lima, N. R., Costa, A. F., Rodrigues, A. L., de Magalhães, F. A., Tosi, C. H., et al. (2008). Revisão do conhecimento sobre os mamíferos aquáticos da costa norte do Brasil. Arquivos do Museo Nacional 66, 381–401.
Silva, M. A., Feio, R., Prieto, R., Gonçalves, J., and Santos, R. (2002). Interactions between cetaceans and the tuna fishery in the Azores. Mar. Mammal Sci. 18, 893–901. doi: 10.1111/j.1748-7692.2002.tb01080.x
Sinha, R. (2002). An alternative to dolphin oil as a fish attractant in the Ganges River system: conservation of the Ganges River dolphin. Biol. Conserv. 107, 253–257. doi: 10.1016/S0006-3207(02)00058-7
Sinha, R., and Sharma, G. (2003). Current status of the Ganges River dolphin, Platanista gangetica in the rivers Kosi and Son, Bihar, India. J. Bombay Nat. Hist. Soc. 100, 27–37.
Slooten, E., and Dawson, S. (1988). “Studies on Hector's dolphin, Cephalorhynchus hectori: a progress report,” in Biology of the Genus Cepharlorhynchus, eds R. L. Brownell and G. P. Donovan (Cambridge: Rep. Int. Whaling Comm.), 325–338.
Smith, B. D., and Braulik, G. T. (2012). Platanista gangetica. The IUCN Red List of Threatened Species 2012:e.T41758A17355810. doi: 10.2305/IUCN.UK.2012.RLTS.T41758A17355810.en
Smith, B. D., and Reeves, R. R. (2000). “Survey methods for population assessment of Asian River Dolphins,” in Biology and Conservation of Freshwater Cetaceans in Asia, eds R. Reeves, B. Smith, T. Kasuya (Gland; Cambridge, UK: IUCN), 97–115.
Stensland, E., Berggren, P., Johnstone, R., and Jiddawi, N. (1998). Marine mammals in Tanzanian waters: urgent need for status assessment. Ambio 27, 771–774.
Stensland, E., Carlen, I., Särnblad, A., Bignert, A., and Berggren, P. (2006). Population size, distribution, and behavior of indo-pacific bottlenose (Tursiops aduncus) and humpback (Sousa chinensis) dolphins off the south coast of Zanzibar. Mar. Mammal Sci. 22, 667–682. doi: 10.1111/j.1748-7692.2006.00051.x
Subgroup of Fishery and Environment (SGFEN) (2001). Incidental catches of small cetaceans. Commission Staff Working Paper (Brussels), 86.
Suzuki, T. (2002). “Development of shark fisheries and shark fin export in Indonesia: Case study of Karangson Village, Indramayu, West Java,” in Elasmobranch Biodiversity, Conservation and Management – Occasional paper of the IUCN Species Survival Commission No. 25, eds S. L. Fowler, T. M. Reed, F. A. Dipper (Gland; Cambridge, UK: IUCN), 149–157.
Tavera, G., Aliaga-Rossel, E., Van Damme, P. A., and Crespo, A. (2010). “Distribution and conservation status of the Bolivian river dolphin Inia boliviensis (d'Orbigny 1832),” in The Action Plan for South American River Dolphins 2010–2020, F. Trujillo, E. Crespo, P. A. Van Damme, and J. S. Usma (Bogotá: WWF, Fundación Omacha, W. D. S., WDCS, and SOLAMAC), 99–1120.
Taylor, B. L., Baird, R., Barlow, J., Dawson, S. M., Ford, J. K. B., Mead, J. G., et al. (2012). Grampus griseus. The IUCN Red List of Threatened Species 2012:e.T9461A17386190. doi: 10.2305/IUCN.UK.2012.RLTS.T9461A17386190.en
Taylor, B. L., Baird, R., Barlow, J., Dawson, S. M., Ford, J., Mead, J. G., et al. (2008). Feresa attenuata. The IUCN Red List of Threatened Species 2008:e.T8551A12921135. doi: 10.2305/IUCN.UK.2008.RLTS.T8551A12921135.en
Taylor, B. L., Baird, R., Barlow, J., Dawson, S. M., Ford, J., Mead, J. G., et al. (2011). Globicephala macrorhynchus. The IUCN Red List of Threatened Species 2011:e.T9249A12972356. doi: 10.2305/IUCN.UK.2011-2.RLTS.T9249A12972356.en
Torres, D., Yañez, J., and Cattan, P. (1979). Mamíferos marinos de Chile: antecedentes y situación actual. Biol. Pesq. Chile 11, 49–81.
Tzika, A. C., D'Amico, E., Alfaro-Shigueto, J., Mangel, J. C., Van Waerebeek, K., and Milinkovitch, M. C. (2010). Molecular identification of small cetacean samples from Peruvian fish markets. Conser. Genet. 11, 2207–2218. doi: 10.1007/s10592-010-0106-8
United Nations Development Programme (UNDP) (2016). Human Development Report 2016: Human Development for Everyone. Lowe-Martin Group.
UNEP/IUCN (1994). United Nations Environment Programme/International Union for Conservation of Nature (UNEP/IUCN): Technical report on the state of cetaceans in the Mediterranean. MAP Technical Reports Sertes No. 82, UNEP, Regional Activity Centre for Specially Protected Areas, Tunis.
Van Waerebeek, K. (1994). Preliminary notes on the existence of a dolphin by-catch off French Guiana. Aquat. Mamm. 16, 71–72.
Van Waerebeek, K. V., and Würsig, B. (2009). “Dusky dolphin - Lagenorhynchus obscurus,” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (London: Academic Press), 26–28.
Van Waerebeek, K., and Reyes, J. C. (1994). “Post-ban small cetacean takes off Peru: a review,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 503–520.
Van Waerebeek, K., Barnett, L., Camara, A., Cham, A., Diallo, M., Djiba, A., et al. (2003). Conservation of Cetaceans in the Gambia and Senegal, 1999-2001, and Status of the Atlantic Humpback Dolphin. UNEP/CMS Secretariat, Bonn.
Van Waerebeek, K., Diallo, M., Djiba, A., Ndiaye, P., and Ndiaye, E. (1997a). Cetacean research in Senegal 1995-97, an overview. Document SC/49/SM10 presented to the International Whaling Commission (Bournemouth), 8.
Van Waerebeek, K., Ndiaye, E., Djiba, A., Diallo, M., Murphy, P., Jallow, A., et al. (2000). A Survey of the Conservation Status of Cetaceans in Senegal, the Gambia and Guinea-Bissau. UNEP/CMS Secretariat, Bonn, 80.
Van Waerebeek, K., Ofori-Danson, P. K., Debrah, J., Collins, T., Djiba, A., and Samba Ould Bilal, A. (2014). On the status of the common bottlenose dolphin Tursiops truncatus in western Africa, with emphasis on fisheries interactions, 1947-2015. Document SC/66b/SM19 Presented to the International Whaling Commission, Bled, 19.
Van Waerebeek, K., Van Bressem, M.-F., Félix, F., Alfaro-Shigueto, J., García-Godos, A., Chávez-Lisambart, L., et al. (1997b). Mortality of dolphins and porpoises in coastal fisheries off Peru and southern Ecuador in 1994. Biol. Conserv. 81, 43–49.
Vidal, O. (1993). Aquatic mammal conservation in Latin America: problems and perspectives. Conserv. Biol. 7, 788–795. doi: 10.1046/j.1523-1739.1993.740788.x
Vidal, O., Van Waerebeek, K., and Findley, L. T. (1994). “Cetaceans and gillnet fisheries in Mexico, Central America and the wider Caribbean: a preliminary review,” in Gillnets and Cetaceans, eds W. F. Perrin, G. P. Donovan, and J. Barlow (Cambridge: Rep. Int. Whaling Comm.), 221–234.
Wakid, A. (2007). Report on the Initiatives to Involve the Major Stakeholders of Assam in the Conservation of Gangetic Dolphin. Gangetic Dolphin Research and Conservation Programme, Aaranyak, Guwahati, Assam, 65.
Wakid, A. (2009). Status and distribution of the endangered Gangetic dolphin (Platanista gangetica gangetica) in the Brahmaputra River within India in 2005. Curr. Sci. 97, 1143–1151.
Wakid, A., and Braulik, G. (2009). Protection of Endangered Ganges River Dolphin in Brahmaputra River, Assam, India. Final Technical Report to Sir Peter Scott Fund, IUCN, 44.
Wang, J. Y., and Yang, S. H. (2009). “Indo-Pacific Bottlenose Dolphin - Tursiops aduncus,” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (London: Academic Press), 602–609.
Ward-Paige, A. (2017). A global overview of shark sanctuary regulations and their impact on shark fisheries. Mar. Policy 82, 87–97. doi: 10.1016/j.marpol.2017.05.004
Weir, C. R., and Pierce, G. J. (2013). A review of the human activities impacting cetaceans in the eastern tropical Atlantic. Mammal Rev. 43, 258–274. doi: 10.1111/j.1365-2907.2012.00222.x
Wells, R. S., and Scott, M. D. (2009). “Common Bottlenose Dolphin- Tursiops truncatus,” in Encyclopedia of Marine Mammals, eds W. F. Perrin, B. Würsig, and J. G. M. Thewissen (London: Academic Press), 249–255.
Young, N., and Iudicello, S. (2007). Worldwide Bycatch of Cetaceans: An Evaluation of the Most Significant Threats to Cetaceans, the Affected Species and the Geographic Areas of High Risk, and the Recommended Actions from Various Independent Institutions. U.S. Dep. Commerce, NOAA Tech. Memo. NMFS-OPR-36, 276.
Yousuf, K., Anoop, A., Anoop, B., Afsal, V., Vivekanandan, E., Kumarran, R., et al. (2009). Observations on incidental catch of cetaceans in three landing centres along the Indian coast. Mar. Biodivers. Rec. 2:e64. doi: 10.1017/S175526720900075X
Zavala González, A., and Mellink, E. (1997). Entanglement of California sea lions, Zalophus californianus californianus, in fishing gear in the central-northern part of the Gulf of California, Mexico. Fish. Bull. 95, 180–184.
Keywords: marine mammal, dolphin, cetacean, fishery-dolphin interactions, bycatch, sharks, conservation, artisanal fisheries
Citation: Mintzer VJ, Diniz K and Frazer TK (2018) The Use of Aquatic Mammals for Bait in Global Fisheries. Front. Mar. Sci. 5:191. doi: 10.3389/fmars.2018.00191
Received: 27 February 2018; Accepted: 11 May 2018;
Published: 07 June 2018.
Edited by:
Wen-Cheng Wang, National Taiwan Normal University, TaiwanReviewed by:
Danielle Kreb, Scientific Program Manager at the Conservation Foundation for Rare Aquatic Species of Indonesia, IndonesiaKevin Alexander Hovel, San Diego State University, United States
Copyright © 2018 Mintzer, Diniz and Frazer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vanessa J. Mintzer, dmpzQHVmbC5lZHU=
 Vanessa J. Mintzer
Vanessa J. Mintzer Karen Diniz
Karen Diniz Thomas K. Frazer
Thomas K. Frazer