- 1School of Biological Sciences and the Oceans Institute, The University of Western Australia, Crawley, WA, Australia
- 2Elizabeth Moore International Center for Coral Reef Research and Restoration, Mote Marine Laboratory, Summerland Key, FL, United States
- 3Western Australian State Department of Biodiversity, Conservation and Attractions, Kensington, WA, Australia
- 4Centre for Marine Ecosystems Research and School of Science, Edith Cowan University, Joondalup, WA, Australia
- 5Centre for Integrative Ecology, School of Life and Environmental Sciences, Deakin University, Warrnambool, VIC, Australia
- 6Save Our Seas Shark Center, Guy Harvey Research Institute, Nova Southeastern University, Fort Lauderdale, FL, United States
- 7Department of Water and Environmental Regulation, Perth, WA, Australia
- 8Oceans Graduate School, The Oceans Institute, The University of Western Australia, Crawley, WA, Australia
- 9Center for Coastal Oceans Research, Department of Biological Sciences, Florida International University, Miami, FL, United States
- 10Virginia Institute of Marine Science, College of William and Mary, Williamsburg, VA, United States
A central question in contemporary ecology is how climate change will alter ecosystem structure and function across scales of space and time. Climate change has been shown to alter ecological patterns from individuals to ecosystems, often with negative implications for ecosystem functions and services. Furthermore, as climate change fuels more frequent and severe extreme climate events (ECEs) like marine heatwaves (MHWs), such acute events become increasingly important drivers of rapid ecosystem change. However, our understanding of ECE impacts is hampered by limited collection of broad scale in situ data where such events occur. In 2011, a MHW known as the Ningaloo Niño bathed the west coast of Australia in waters up to 4°C warmer than normal summer temperatures for almost 2 months over 1000s of kilometers of coastline. We revisit published and unpublished data on the effects of the Ningaloo Niño in the seagrass ecosystem of Shark Bay, Western Australia (24.6–26.6° S), at the transition zone between temperate and tropical seagrasses. Therein we focus on resilience, including resistance to and recovery from disturbance across local, regional and ecosystem-wide spatial scales and over the past 8 years. Thermal effects on temperate seagrass health were severe and exacerbated by simultaneous reduced light conditions associated with sediment inputs from record floods in the south-eastern embayment and from increased detrital loads and sediment destabilization. Initial extensive defoliation of Amphibolis antarctica, the dominant seagrass, was followed by rhizome death that occurred in 60–80% of the bay's meadows, equating to decline of over 1,000 km2 of meadows. This loss, driven by direct abiotic forcing, has persisted, while indirect biotic effects (e.g., dominant seagrass loss) have allowed colonization of some areas by small fast-growing tropical species (e.g., Halodule uninervis). Those biotic effects also impacted multiple consumer populations including turtles and dugongs, with implications for species dynamics, food web structure, and ecosystem recovery. We show multiple stressors can combine to evoke extreme ecological responses by pushing ecosystems beyond their tolerance. Finally, both direct abiotic and indirect biotic effects need to be explicitly considered when attempting to understand and predict how ECEs will alter marine ecosystem dynamics.
Introduction
A key question at the forefront of ecology and evolutionary research in the anthropocene is “how will climate change alter the structure and function of ecological systems?” Evidence suggests widespread, dramatic, climate driven changes to ecosystems with negative consequences including the local extinction of species, major shifts in geographic range and phenology, disruption of fundamental biotic interactions, and a reduction in ecosystem productivity (Poloczanska et al., 2013; Hyndes et al., 2016; Pecl et al., 2017). Recent examples of range shifts and local extinctions that have been documented in marine environments (Johnson et al., 2011; Wernberg et al., 2016) include seagrasses (Kim et al., 2009; Gorman et al., 2016).
Identifying stressors that negatively affect the resilience of ecosystems is fundamental to managing the impacts of climate change (Peterson et al., 1998). Marine ecosystems are being impacted through increasing ocean temperatures, ocean acidification, deglaciation, reduced ocean ice cover, rising sea levels, increasing storm frequency, and intensity (Doney et al., 2012), and strengthening boundary currents (Vergés et al., 2014). These stressors are decreasing ocean productivity, altering food web dynamics, reducing abundance of habitat-forming species, shifting species distributions, and increasing the incidence of diseases (Hoegh-Guldberg and Bruno, 2010; Wernberg et al., 2016). Impacts have been widely documented, despite average global warming of just 1°C (Scheffers et al., 2016). While some of these changes are clearly visible and have received much public attention, such as coral bleaching events, others are much more insidious.
Climate change is predicted to increase the frequency, duration, and intensity of extreme climate events (ECEs), including marine heatwaves (MHWs) (Cai et al., 2014; Pachauri et al., 2014; Oliver et al., 2015; Frölicher and Laufkötter, 2018). ECEs can act as strong and acute agents of change that can generate widespread mortality and collapse of ecosystem services (Smale et al., 2019). ECEs rarely occur in isolation, but generally cause impacts through the combination of multiple abiotic, (e.g., temperature, salinity, pCO2 concentration), and biotic drivers (e.g., changes in food resources, herbivory, predation, competition, disease) acting additively or synergistically through time (Brook et al., 2008). ECEs, including MHWs, can push populations beyond their functional threshold, where range contractions and extinctions are likely (Hyndes et al., 2016; Wernberg et al., 2016). Furthermore, the indirect, biotic effects that ECEs trigger (e.g., biogenic habitat loss) can even affect species that were resilient to the initial abiotic effects of an ECE. Despite the critical need to understand the potential for multiple stressors to affect resilience synergistically, many studies instead treat co-occuring stressors as independent phenomena (Orth et al., 2006; Wernberg et al., 2012). Finally, because studies of ECE impacts are often opportunistic, insights into community scale impacts of these events are relatively rare, at least in marine systems. Understanding how ecosystems respond to stressors is necessary to be able to quantify and ultimately predict the resilience of ecosystems exposed to the increasing stressors of the Anthropocene (Pecl et al., 2017).
Summer temperature extremes associated with MHWs are important drivers for the survival and growth of seagrasses globally and will heavily impact their biogeographical distributions and, therefore, have indirect effects to species that are dependent on seagrass ecosystem services (Orth et al., 2006). For example, during the summers of 2005 and 2010, severe MHWs (Hobday et al., 2018) in Chesapeake Bay resulted in 58% loss of Zostera marina (2005) along with declines in blue crabs, silver perch and bay scallops followed by a further 41% loss of seagrasses (2010) (Lefcheck et al., 2016). Two strong MHWs in 2003 and 2006 in the western Mediterranean caused shoot mortality of Posidonia oceanica to exceed recruitment (Diaz-Almela et al., 2007; Marba and Duarte, 2010). However, with the exception of these studies, monitoring of extreme MHWs in seagrass ecosystems has been rare and generally focussed on individual organisms.
In this paper, we focus on resilience to extreme events in a seagrass-dominated ecosystem, specifically in one of the largest in the world, Shark Bay, Western Australia. We define resilience as “the capacity to undergo disturbance without permanent loss of key ecological structures and functions” (O'Brien et al., 2018: based on Holling, 1973). We focus on the processes of resistance and recovery to assess resilience (sensu Hodgson et al., 2015) in relation to 3 seagrass ecosystem trajectories outlined in O'Brien et al. (2018): reversible degradation where the ecosystem recovers post-disturbance; hysteretic degradation, where feedbacks maintain the disturbed state which requires a lower environmental threshold or another perturbation to start recovery, and; recalcitrant (irreversible) degradation where the damage done by the disturbance is not reversible and the environment is not suitable for recovery of seagrass habitat. We also investigate how the life history strategy of major seagrass species found in Shark Bay, as described by Kilminster et al. (2015), influence resistance and recovery trajectories.
Shark Bay is a large marine embayment (13,500 km2) (Figure 1) on the tropical temperate transition zone on the west coast of Australia that has obtained World Heritage Site (WHS) listing because of its unique environmental values (whc.unesco.org). One of these values is the extensive seagrass meadows that support high marine biodiversity, including significant consumer populations of dugongs, turtles, and their major predator, tiger sharks (Heithaus et al., 2012; Kendrick et al., 2012). Shark Bay is characterized by high seagrass biodiversity as it sits in the transition between the temperate and tropical biomes, with 13 species of temperate and tropical seagrasses, most at the extremes of their respective distributions (Walker et al., 1988). These species also encompass multiple life history strategies including colonizing, opportunistic and persistent (Kilminster et al., 2015). The large temperate seagrasses Amphibolis antarctica and Posidonia australis have historically dominated Shark Bay seagrass cover, creating extensive, persistent meadows measuring 3,676 km2 and 200 km2 of 4,176 km2 total seagrass cover, respectively (Walker et al., 1988). Both species lack a seed bank and exhibit relatively slow rates of rhizome expansion, resulting in slow rates of recovery from disturbance. The remaining 500 km2 of seagrass meadows are dominated by the small tropical colonizing seagrasses Halodule uninervis, Halophila ovalis, Halophila ovata, Halophila spinulosa, and Halophila decipiens and opportunistic tropical species Cymodocea serrulata, Cymodocea angustata, and Syringodium isoetifolium. These tropical species have low initial resistance to disturbances but can recover quickly through seed banks, vegetative fragments, and rapid rhizome elongation rates (Sherman et al., 2018). There is also minor coverage by other persistent temperate species Posidonia angustifolia and Posidonia coriacea.
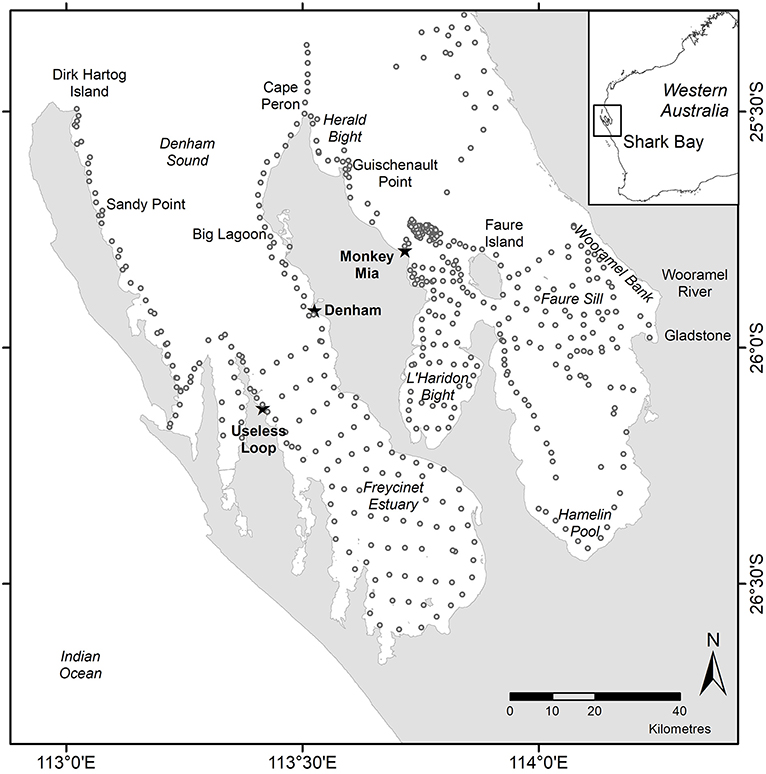
Figure 1. Map of Shark Bay showing marine regions (italics), towns (bold and starred) and specific sampling locations (normal font) used in the text. Open circles show seagrass sampling locations 1982–2018.
The severity of several MHWs has been characterized (Hobday et al., 2018) and the marine heatwave of austral summer 2011 along the Western Australian coast (Figure 2) was among the most extreme on record (Category IV). An unusual combination of conditions led to this event (Feng et al., 2013). The Western Australian coastline is influenced by the poleward flowing Leeuwin Current (LC) that transports tropical waters from the eastern Indian Ocean southward along the continental slope, particularly during winter. The LC is heavily influenced by the El Niño Southern Oscillation (ENSO) through oceanographic and atmospheric connectivity to the Pacific Ocean. During La Niña years (e.g., 1999–2000, 2011–2012) the LC flows stronger resulting in transport of elevated ocean temperatures down the west coast (Feng et al., 2003). The region is typically dominated by strong southerly wind patterns through the summer months that oppose the LC, contributing to its seasonality, and also acting to moderate heating of coastal ocean temperatures through upwelling (Woo et al., 2006; Rossi et al., 2013) and air-sea heat flux (e.g., evaporative cooling) processes (Feng and Shinoda, 2019). However, relaxation or reversal of the southerly winds can further enhance heating, as occurred during the La Niña event of 2010–2011, when weak, northerly winds combined with an unusually strong summer Leeuwin Current to elevate summer maximum sea temperatures by 2–4°C in the region. This extraordinary build-up of warm Indian Ocean water along the Western Australian coast was coined the “Ningaloo Niño” (Feng et al., 2013; Pearce and Feng, 2013) and has recently been proposed to occur even in the absence of ENSO influences (Kataoka et al., 2018). The shallow, semi-enclosed geography of Shark Bay means it is particularly susceptible to anomalous air-sea heat fluxes such as the conditions observed during the Ningaloo Niño and other climatic events, a factor which has generally been overlooked in broad scale regional studies. Whilst extreme temperatures were experienced over the entire region (Figure 2i), within the bay the local SST response to the extreme conditions varied (Figures 2a–h).
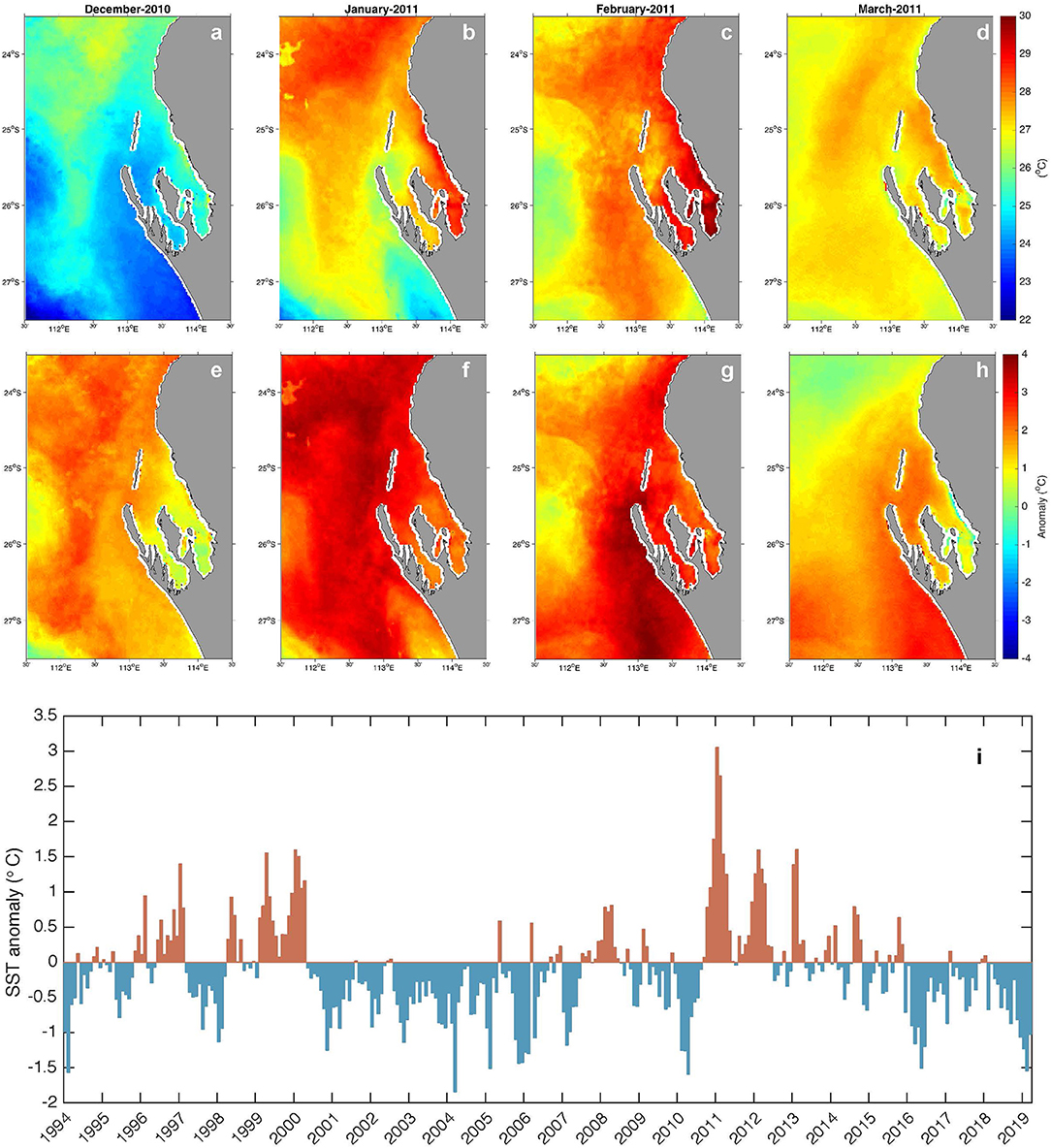
Figure 2. Monthly mean satellite SST (a–d) and anomalies (e–h) calculated from the SSTAARS 1993–2016 climatology (Wijffels et al., 2018) for the 2011 MHW event. Interesting features visible in the images include: intrusion of cooler upwelled water into the bay (a) and subsequent shut down of this cooling mechanism during February (g); spatial variability of MHW signature in shallow areas of the inner bay (higher temperatures, faster cooling (c,h). Time series of the mean anomaly over the map domain is shown in (i), highlighting the elevated temperatures related to La Niña in 1999–2000 and 2010–2012.
Here, we review published literature and unpublished data to characterize the resilience (i.e., resistance and recovery) of a large seagrass-dominated marine ecosystem using a case study focussed on the influence of the 2011 MHW on the seagrasses of Shark Bay. First, we summarize our knowledge of individual species resistance to this environmental “perfect storm” and the trajectory of recovery for seagrasses and seagrass-dependent organisms in Shark Bay across multiple scales from whole ecosystem (>10,000 km2), to regions that cover areas of >100 km2, and local scales of single to multiple sites within a region (<10 km2). Finally, we discuss the future of the system and the management of the WHS values of Shark Bay.
Methods
The size of Shark Bay (13,500 km2), its isolation from major research institutes (>900 km), and the varying taxonomic and regional foci of its researchers has resulted in heterogeneous data on the impact and recovery from the MHW. Furthermore, the data available have been collected during studies not specifically aimed at addressing questions around the MHW. Here we combine the best available science from these studies, both published and unpublished, to address the loss of resistance and the trajectory of recovery in the system.
Satellite Sea Surface Temperature (SST) Data
In order to examine details of the MHW within the bay and avoid biases intrinsic in some coarser SST datasets, high-resolution (2 km) daily nightime AVHRR L3S SST data available through the Integrated Marine Observing System (IMOS) (http://imos.org.au/facilities/srs/sstproducts/sstdata0/, Griffin et al., 2017) were combined with the SST Atlas of Australian Regional Seas (SSTAARS) (1993–2016) climatology (Wijffels et al., 2018) to produce monthly mean SST and anomaly maps from 1993 to 2019. Time series of these variables were extracted by calculating the spatial means over the region shown in Figure 2.
Seagrasses
We collated published and unpublished seagrass data from before, during and after the MHW in Shark Bay to address the magnitude of the disturbance and change in state in relation to the return time of the Shark Bay ecosystem and characterized the scale of impact to one of three scales: ecosystem-wide, regional, and local. Ecosystem-wide data represent the entire 13,500 km2 Shark Bay ecosystem with 4,176 km2 of seagrass-dominated banks and sills (Figure 1). Regional studies represent regions of >100 km2 within the ecosystem, like Faure Sill, Eastern Cape Peron, L'Haridon Bight, Western Cape Peron, Denham Sound, Freycinet Estuary and sills and banks offshore from Monkey Mia. Local studies are those at individual locations <10 km2, like Useless Loop. We only included data that allowed us to address changes across multiple years and that was appropriately collected using comparable methods.
Mapped Changes
Historical mapping of seagrasses between 1983 and 1985 was conducted by Walker et al. (1988). Ecosystem-wide changes in seagrass coverage were determined from a comparison of satellite imagery collected between 2002 and 2014 that mapped 68% of the Shark Bay Marine Park, which were extrapolated to cover the whole Shark Bay region (Arias-Ortiz et al., 2018). We updated these data to include unpublished studies that are currently underway by the Department of Biodiversity Conservation and Attractions (DBCA) in Western Australian to improve both spatial and temporal resolution and coverage of this dataset. The final data set allowed us to illustrate changes in seagrass coverage across multiple years before and after the MHW throughout Shark Bay.
Regional Changes in Shoot Density and % Cover
Regional loss of seagrasses were recorded as changes in presence/absence, percent cover, and shoot density in quadrats (Tables S1, S2). These data were collected before, during and after the 2011 MHW. Shoot densities at 14 locations were collected from six 0.2 × 0.1 m quadrats at each location in the western and eastern Cape Peron and Faure Sill regions in 1982, three decades before the heatwave (Walker, 1985). Similar shoot density data were collected from five 0.04 m−2 cores taken at multiple locations from Useless Loop, Freycinet Estuary (Statton, unpublished data) and from Faure Sill (2011, 2013), and eastern and western Cape Peron (2013–2014, 2017–2018) (Fraser et al., 2014: Fraser and Kendrick, unpublished data). Seagrass density data predominantly from the western embayment and Freycinet Estuary were also included (Seagrass monitoring program for the Shark Bay Marine Park, DBCA, Strydom, unpublished data). Briefly, in the DBCA survey, shoot density was determined at six locations by randomly placing eight 0.2 × 0.2 m quadrats along three 10 m transects at each location and counting shoot densities (Table S2).
Regional changes in seagrass cover were also monitored more frequently at five offshore banks north of Monkey Mia, where occurrence and percent cover of A. antarctica, H. uninervis, and macroalgae were determined from 3 × 0.36 m2 across 63 locations between 2007 and 2017 (Nowicki et al., 2017; Nowicki, unpublished data). Seagrass % cover was also taken across Faure Sill and Wooramel Bank regions in March 2011 (28 locations, 5 × 0.25 m2 quadrats location−1) at the height of the MHW, in September 2011 (14 locations, 5 × 0.25 m2 quadrats location−1) and in February 2013 (5 locations, 5 × 0.25 m2 quadrats location−1) (Fraser et al., 2014).
Finally, to attempt an analysis of system resistance and recovery, we took the most complete dataset, shoot density for A. antarctica and P. australis, and plotted it by field program (mean ± SE) for field programs that collected that data between 1982 and 2011−2018. For A. antarctica, there were four field programs between 1982 and 2013, and for P. australis there were 11 field programs between 1982 and 2018. lSampling locations within each field program were treated as replicates to reduce confounding data in space and time. This approach also addressed the effect of differences in both number and placement of locations from each program. Note also that some programs sampled existing seagrass meadows so have a bias over time toward seagrass loss. Statistical differences between each program's data were tested using one way ANOVA, and significance differences determined using Tukeys HSD pairwise tests (aov and Tukeys HSD: R Core Team, 2013).
Local Observations
Local observations of seagrass reproduction and recruitment were used to assess the capacity for recovery. A series of recruitment studies using transplants of both P. australis and A. antarctica were undertaken between 2010 and 2018 at Useless Loop as part of a seagrass restoration program (Poh, Statton, unpublished data). Surveys of flowering and seed production in P. australis were carried out mainly at Useless Loop and Guischenault Point but opportunistic collections were also made at Monkey Mia, Denham, Big Lagoon and Eagle Bluff in 2011, 2012, 2016 and 2017 (Statton and Kendrick, unpublished data).
Effect on Seagrass Associated Fauna
Effects of seagrass loss were assessed on all major species of air-breathing megafauna that occur in Shark Bay via visual transect surveys at the surface (Nowicki et al., 2019). These surveys, running continuously since 1998, have been part of a wider community research project on the seagrass banks immediately north of Monkey Mia (see Heithaus et al., 2012 for descriptions). Briefly, long-term transects 3–4 km in length were established over shallow seagrass banks (~2–4 m depth) or deep sandy channels (~10 m depth). Each transect was run by driving a 5.5 m vessel along the transect at 6–9 km per hour approximately four times per month, with most sampling occurring between Feb-Oct. All air breathing fauna (Indo-Pacific bottlenose dolphins Tursiops aduncus, dugongs Dugong dugon, loggerhead turtles Caretta caretta, green turtles Chelonia mydas, Pied Cormorants Phalacrocorax varius, and sea snakes) that were sighted at the surface within a species-specific sighting band were quantified and recorded.
In addition to air-breathing fauna, Nowicki et al. (2019) also quantified changes to the large shark community via standardized drumline fishing. ~4 days per month (mostly between Feb-Oct), 10 drumlines baited with ~1.5 kg of fish each were set at dawn. All sharks captured were identified, measured, tagged, and released. Catch-per-unit effort (expressed as sharks per 100 hook hours) was compared between 1998–2010 and 2012–2014 to assess whether seagrass loss related to the Ningaloo Niño significantly impacted large shark densities, which are historically dominated by tiger sharks (Galeocerdo cuvier, Heithaus, 2001). We also examined data on bioturbation on establishing seeds (Johnson et al., 2018) at Useless Loop.
Results
Scales of Loss and Recovery in Seagrasses: Ecosystem-Wide
The seagrass-dominated ecosystem in Shark Bay displayed high resistance to change in seagrass cover before the MHW, which varied little between that determined by hand drawn polygons in 1983–85 and computerized mapping from satellite imagery in 2002 (area change of −183 km2 to +124 km2 (Table 1). Differences in aerial coverage between 2002 pre-MHW and 2014 post-MHW resulted in 696–921 km2 lost and 190–261 km2 of dense meadows thinning dramatically becoming sparse meadows of <10% coverage. A brief visual survey of landsat imagery indicated seagrass losses in the landscape occurred 1–2 years after defoliation of A. antarctica (2012–2013), and was most evident in shallow offshore banks and sills and deeper seagrass environments (Figure 3).
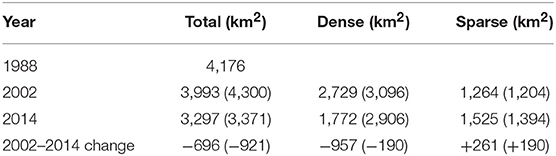
Table 1. Change in total area of seagrasses between 1983–85 (Walker et al., 1988), and dense and sparse seagrasses between 2002 and 2014 (DBCA monitoring program, no brackets) with the larger estimates of Arias-Ortiz et al. (2018) in brackets.
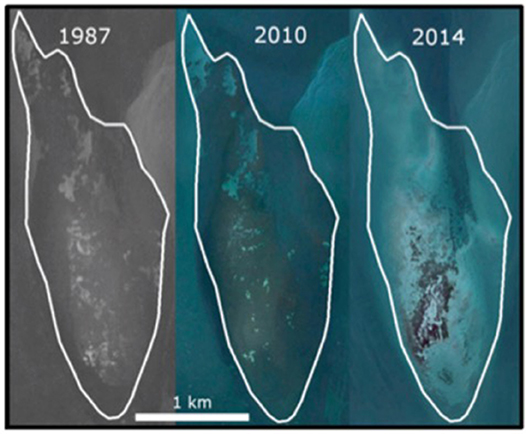
Figure 3. Imagery illustrating changes to seagrass cover (dark areas) within a single seagrass bank (white outline) before and after the 2011 MHW. The decadal stability of small bed features such as sand patches across almost 3 decades illustrates the natural resistance of the system to change, as well as the unusual impact of the MHW on the Shark Bay seagrass landscape (from Nowicki et al., 2017, with permission to reuse from MEPS, Images from Google Earth).
Regional Loss and Recovery of Seagrasses
Using long term and multi-institutional data on shoot density (m−2) we demonstrate there were significant loss in above ground shoot and stem densities in temperate seagrasses during (P. australis) and within a year after (A. antarctica) the 2011 MHW (Figure 4, Table S3). A. antarctica stem densities had not recovered by 2015 [Figure 4A, Table S2: ANOVA F = 9.139, p = 4.28 × 10−6, df = 4, 77; Pairwise Tukey HSD DW1 (1982) = GAK1 (March 2011) ≠ GAK2 (September 2011) ≠ GAK3 (2013) ≠ DPAW1 (2015)]. In contrast, P. australis shoot density collapsed to a third of historical values in 2011 and remained low in 2014, and 2016 [Tukey's HSD DIW (1982) ≠ DBCA1 (2011) ≠ DBCA2 (2014) ≠ DBCA3 (2016)], but by 2016 was showing signs of recovery (DBCA3 = CG1 ≠ JS1). By 2017–2018 shoot densities of P. australis were not significantly different than those recorded in 1982 [Figure 4B, Table S3: ANOVA F = 11.23 p = 2.35 × 10−9, df = 9, 50; Tukey's HSD DIW (1982) = JS2 (2017) = GAK 1, 2, 3 (2018 seasonal sampling)]. Note we show these data with the major caveat that the different sources of data were from field programs that sampled different locations and numbers of locations with different sampling densities (Table S2).
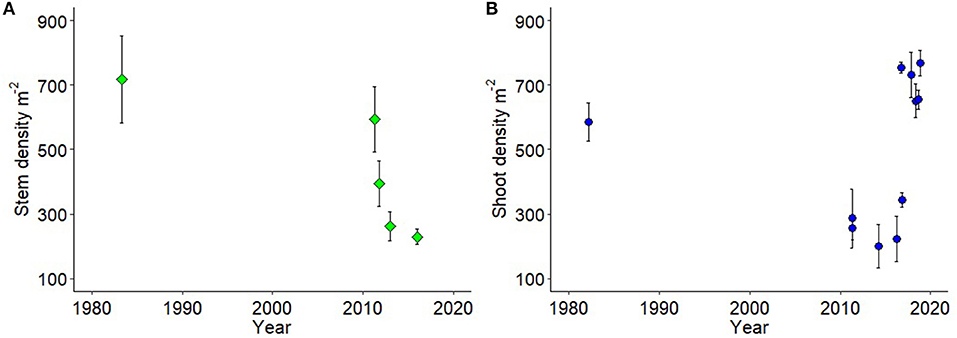
Figure 4. (A) A. antarctica shoot density (m−2; green diamond) from four field programs in the Eastern Embayment from Herald Bight to southern Faure Sill, and 1 from the Western Embayment; and (B) P. australis shoot density (m−2; blue circles) from 11 field programs across the Western Embayment but also including Guischenault Point to Monkey Mia. Each mean is an individual field program (details and data in Table S2).
The state change from A. antarctica meadows to low cover of tropical colonizing and opportunistic seagrasses has persisted to 2017 across five shallow offshore banks near Monkey Mia from 2007–2008, 2011–2014, 2016, 2017 (Figure 5). Statistically significant losses in A. antarctica occurrence and % cover were documented between 2007–2009 and 2012 (% cover of 89.5–4.8% (Friedman test, chi2 = 59.7, df = 2, P < 0.0001), and no recovery between 2012 and 2014 [% cover 3.8 ± 0.9; One-way repeated-measures ANOVAs on ranks, F(1, 125) = 3.16, p = 0.08] was observed. However, the tropical colonizing species, H. uninervis, increased in occurrence and cover by almost 3-fold from 2007–2009 to 2014 (occurrence: 12–29%; logistic regression, t(124) = 6.94, p < 0.0001, and cover: 3.1 ± 0.5 to 8.5 ± 2.6; One-way repeated-measures ANOVAs on ranks, F(1, 125) = 23.64, p < 0.0001), a trend which continued into 2016 (Figure 5). Importantly, this increase is small in comparison to the loss of A. antarctica on these banks from >80 to <5% cover (Figure 5) and does not represent functional ecosystem recovery.
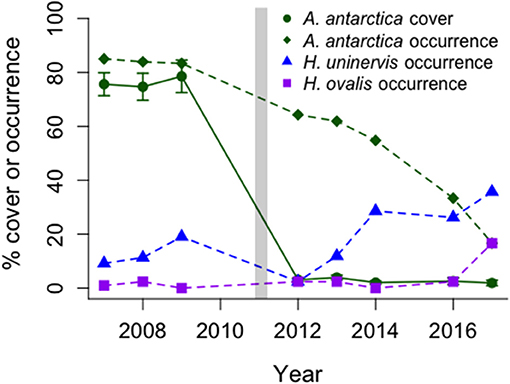
Figure 5. Changes over time in occurrence of two common tropical seagrasses (H. uninervis, H. ovalis) and percent occurrence (as a total) and percent cover (mean ± SE, n = 63) of the dominant temperate seagrass, A. antarctica, before and after the MHW (vertical bar). Data collected from 42 long-term monitoring stations north of Monkey Mia, Shark Bay.
Local Loss and Recovery of Seagrasses
Local studies of shoot mortality and growth at Useless Loop indicated P. australis was not resistant to MHWs although it showed some recovery after 5 years. Higher shoot mortality and slower shoot growth was recorded in P. australis transplants after the 2011 MHW at Useless Loop from restoration studies (Poh, unpublished data 2011). Interestingly, seagrass restoration experiments conducted between 2015 and 2018 at Useless Loop show annual doubling of shoot densities for transplants of both A. antarctica and P. australis suggesting these temperate species do have the ability to recover at the plant scale, but this has not translated into system-wide recovery yet.
Though meadow mortality was low for P. australis, recruitment from seed was heavily impacted. Although P. australis continued to flower, 100% seed abortion was observed in 2011–2012. Subsequent observations of flowering in 2016 and 2017 recorded much higher successful seed production from flowering (Table S4). In 2016, Guischenault Point and Useless Loop produced 350 and 0.65 viable seeds m−2 and in 2017, 350 and 116 viable seeds m−2, respectively. Clearly, reproductive propagules have been missing from Shark Bay until 2016–2017 and have not made a major contribution to recovery for P. australis. Similarly, large numbers of viviparous seedlings of A. antarctica were observed in August 2018 in the Western Gulf (Kendrick and Sinclair, pers obs), though whether this will result in meadow level recovery remains unclear.
Other Published Observations—Wooramel River
Other 2011 MHW observations that are already published include dramatic loss of A. antarctica adjacent to the Wooramel River due to combined high surface sea temperatures and unprecedented flooding. Flooding released over 500 gigaliters of floodwater (Table S5) containing large amounts of fine sediments. The flood was the largest recorded between 1994 and 2015. Reduced light availability over weeks to months associated with resuspended fine sediments, exacerbating the effect of extreme temperatures resulting in a change in state from seagrass meadows to bare sand and patchy meadows in that area. This flood effect was small in area (300 km2) in relation to the total size of Shark Bay and the scale of loss of seagrasses across the whole system where flooding effects were not observed. Though leaf biomass in the area recovered slightly in the 2 years following the event, it was still at 7–20% of historical averages. Belowground biomass decreased by an order of magnitude over the same time period, indicating change in biomass allocation associated with physiological stress and likely reducing the recovery capacity and increasing the return time for extensive seagrass meadows.
Seagrass Associated Biota
The impacts of seagrass loss within Shark Bay on vertebrate consumers varied with species. For example, long term surface transect data from the Eastern Gulf of Shark Bay offshore of Monkey Mia showed significant population declines in Indo-Pacific bottlenose dolphins (39%), dugongs (68%), cormorants (35%), green turtles (39%), and sea snakes (77%) (Figure 6). The mechanisms of decline (i.e., emigration vs. mortality) likely differ by species, and consumers more strongly associated with seagrass for food or habitat were more impacted by seagrass loss. Also, seagrass associated fish populations declined significantly, though density of fish in remaining seagrass habitats actually increased following the MHW (Table S1). The ecological impacts of the MHW and seagrass loss on invertebrate fauna have been less well-studied, with existing studies focusing on impacts to fisheries. The 2011 MHW impacted invertebrate fisheries with closure of scallop and blue swimmer crab fisheries and modification in the size to maturity of prawns in the prawn fishery in Shark Bay (Table S1).
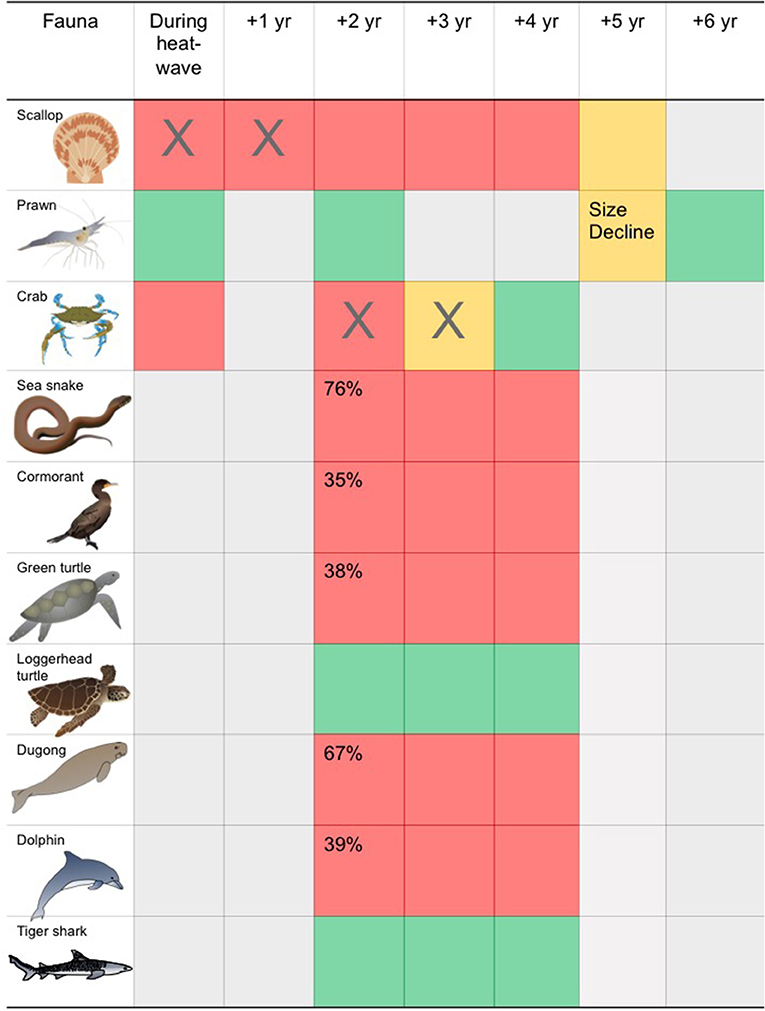
Figure 6. Generalized timeline of change in seagrass associated biota before to after the 2011 heatwave. Red, population decline; Yellow, other change to population; Green, no decline in population; Gray, no data; “X”, fishery closure (see Nowicki et al., 2019 for details).
Discussion
The “Ningaloo Niño” MHW in 2011 pushed the temperate persistent meadow-forming seagrass species A. antarctica and P. australis past their capacity to resist high temperatures in Shark Bay, Western Australia. This drove a change in state where extensive leaf defoliation in A. antarctica (Fraser et al., 2014) and subsequent death of shoots and whole meadows resulted in bed erosion, sediment resuspension and movement (Thomson et al., 2015; Nowicki et al., 2017), and major losses to seagrass-dependant biota (Caputi et al., 2016; D'Anastasi et al., 2016; Nowicki et al., 2019) (Figures 6, 7). The breakdown in resistance is among the largest observed in Australia (Statton et al., 2018) (Figure 7). Several years after the MHW there has been little documented recovery in seagrass extent (Arias-Ortiz et al., 2018). Shark Bay has the largest C stock reported for a seagrass ecosystem globally with up to 1.3% of total C sequestered by seagrasses worldwide stored within the top meter of sediments (Fourqurean et al., 2012). It also experiences a relatively high sediment accumulation rate of 1.6–4.5 mm y−1 (Arias-Ortiz et al., 2018). The 2011 MHW resulted in loss of seagrass stored carbon of between 1.8 and 9 Tg as CO2 over the 3 years between 2011–2013 (Arias-Ortiz et al., 2018). This represents a significant loss to C sequestration.
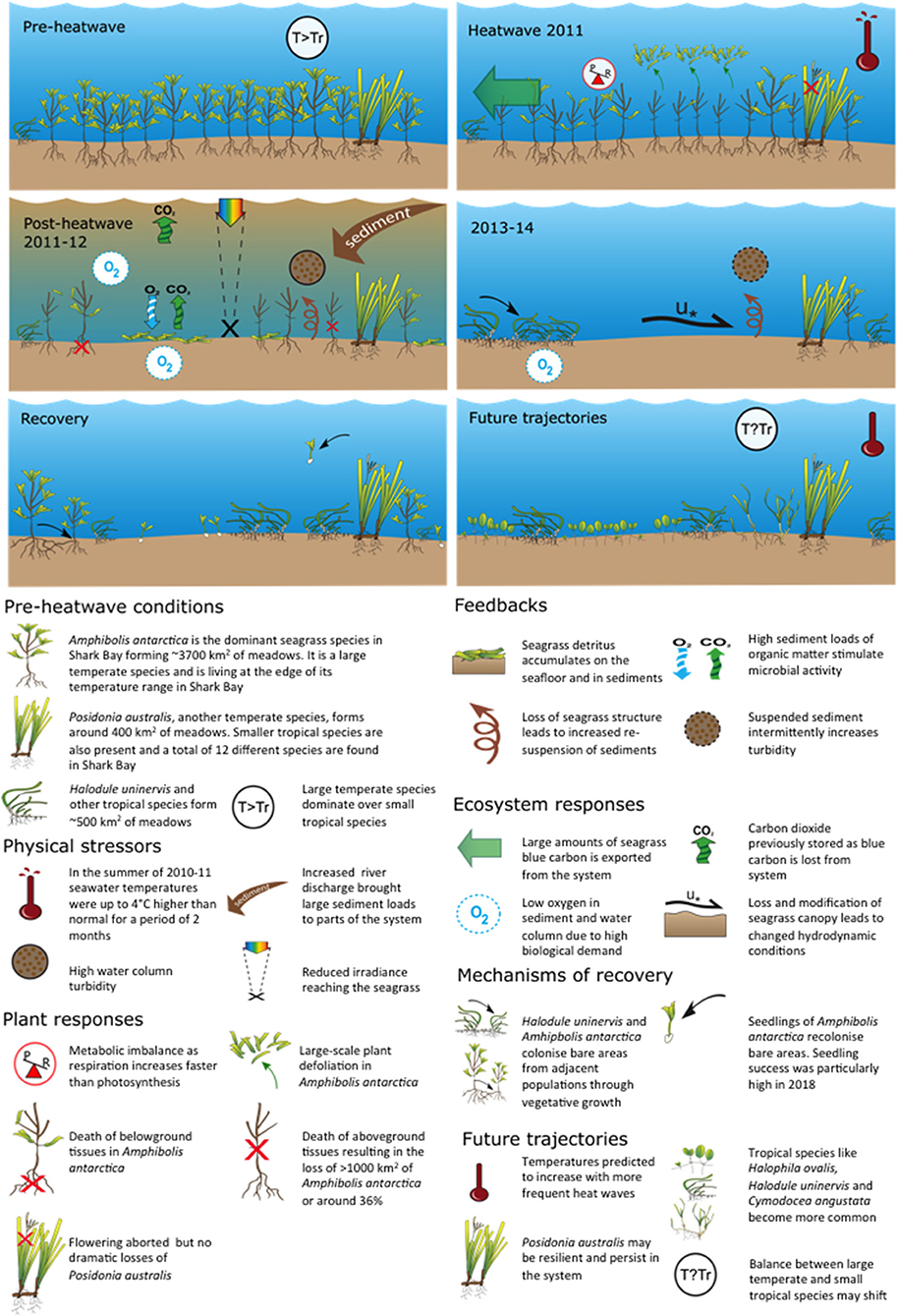
Figure 7. Generalized timeline of change in seagrass structure and composition before during and after the 2011 MHW with potential future shown (Data and publications shown in Table S1).
Similar large-scale seagrass declines have been recorded from other seagrass-dominated ecosystems. A downward trend in coverage of eelgrass (Zostera marina) in Chesapeake Bay has been observed since 1984 and was driven by multiple disturbance events including heating, cooling, turbidity and freshwater inputs from flooding ECEs (Lefcheck et al., 2016). More locally in Western Australia, system-wide loss of seagrasses between 1968 and 1972 produced a recalcitrant state change to bare sediments in over 700 ha of previous seagrass habitat in Cockburn Sound, Western Australia that has lasted for 47 years (Kendrick et al., 2002). High inputs of nutrients and other pollutants were determined to be the cause of the initial rapid loss of seagrasses but subsequent reduction in nutrient inputs and a shift in the system toward oligotrophic conditions have not resulted in recovery of the seagrasses in either system.
Time and Space Scales of Loss and Recovery
Fast local scale (individual to population, weeks to months) responses of temperate seagrass to the MHW included defoliation of large areas of A. antarctica and higher shoot mortalities and seed abortion in P. australis (Figure 7). Loss of both species resulted in landscapes changing from seagrass-dominated to sand dominated but this slower, larger scale process took 1–2 years to develop after the 2011 MHW. These sand- and silt-dominated areas have persisted years after the MHW ended (Nowicki et al., 2017). Since 2016, both A. antarctica and P. australis (2016–2017) have been reproductive and in some locations have produced numbers of seedlings and seeds, respectively. However, limitations remain to recruitment and re-establishment of temperate seagrasses from seeds or seedlings. For example, seed predation has been observed near Useless Loop as well as seedling disturbance by bioturbators in the sediments (especially the heart urchin Breynia desori: Johnson et al., 2018).
The slower 1–2 year system-wide seagrass loss after the 2011 MHW demonstrate how indirect, biotic legacies of MHWs can be significant, even for organisms that are resistant to the direct abiotic effects (Figure 7) (Nowicki et al., 2019). This multi-faceted nature of resistance needs to be considered in the context of ECEs, including MHWs. Indeed, changes to seagrass-associated fauna have continued for 4 years after the influence of the initial stressor (temperature). Nowicki et al. (2017) also reported increased turbidity and sediment resuspension and movement after A. antarctica was lost from offshore banks near Monkey Mia, affecting both stability of sediments and incident light reaching seagrasses. There were also local observations of phytoplankton blooms across many locations suggesting continuing microbial remineralization of organic matter associated with the high input of organic detritus into the system since defoliation in 2011 (Thomson et al., 2015; Nowicki et al., 2017).
Impacts to Seagrass
Life history traits of temperate and tropical seagrass species (Kilminster et al., 2015) have influenced both the scale of loss and extremely slow rate of recovery of the Shark Bay ecosystem (Figure 7) (Kilminster et al., 2015). Energy budgets of the persistent temperate species A. antarctica, indicate a strong dependency on high photosynthesis rates to compensate for respiratory load associated with the complex aerial canopy of multiple leaf clusters, some in full sunlight and others significantly shaded by the canopy above, and little ability to store carbohydrates in rhizomes. Without sufficient light, respiration will exceed production in plants of A. antarctica (Carruthers and Walker, 1997). Also, experiments on the temperature tolerance of A. antarctica indicate increased mortality above water temperatures of 28°C (Walker and Cambridge, 1995). Therefore, A. antarctica is at the limits of its physiological tolerance in Shark Bay and based purely on physiology, would expect to become locally extinct under climate change scenarios (Hyndes et al., 2016) unless thermally resistant genets exist among surviving beds.
The persistent temperate species P. australis appeared more resistant to the 2011 MHW, however it still underwent loss in shoot density (Figure 4B) and showed a multi-annual reproductive collapse despite widespread flowering (Figure 7). This is important to note because some Posidonia species (like P. oceanica in the Mediterranean) increase flowering intensity during warm events (Ruiz et al., 2018), suggesting some persistent seagrasses may demonstrate resilience to warming through reproduction. However, the total seed abortion of P. australis documented in Shark Bay in 2011–2012 (Sinclair et al., 2016) suggests flowering alone may be a poor proxy for resilience. More than 7 years after the MHW there is no evidence that recruitment from seed has occurred in Shark Bay.
The tropical colonizing seagrass, H. uninervis appear to be less impacted by the 2011 MHW and increased in cover during post-MHW recovery (Nowicki et al., 2017) (Figure 7). H. uninervis is a colonizing and sediment stabilizing species common in the Indo-Pacific (Ooi et al., 2011a) and has a low level of clonal integration making it resistant to physiological stress and sediment burial (Ooi et al., 2011b). However, it is one of the preferred seagrasses in fish, turtle and dugong diets and top down control has been shown to limit its abundance and distribution (Anderson, 1986; Burkholder et al., 2012, 2013; Thomson et al., 2015; Bessey et al., 2016). As such, certain biotic legacy effects of MHWs may be more important to these species than they are for persistent species.
Community to Ecosystem Response
Little research has focussed on community to ecosystem responses to ECEs (Langtimm and Beck, 2003; Cahill Abigail et al., 2013), particularly in marine ecosystems. Most consumer species in Shark Bay were negatively impacted by the seagrass loss, although some remained less affected (Figure 6). In general, the level of population decline was roughly correlated to the direct reliance of the species on seagrasses. For example, sea snakes, which use seagrass meadows as both foraging grounds and refuge, suffered the largest declines from seagrass losses, while dugongs, obligate seagrass herbivores, suffered the second highest loss (Nowicki et al., 2019). Resource loss can influence the capacity of consumers to engage in anti-predator behavior because they must balance anti-predator behavior with other needs (such as obtaining food) (Clark, 1994; Werner and Peacor, 2006). Indeed, green turtles in poor body condition in Shark Bay spend more time in the middle of shallow seagrass habitats, which offers higher quality food resources but also reduces the potential for escape from tiger shark encounters (Heithaus et al., 2007). Long-term demographic data on Shark Bay's resident Indo-Pacific bottlenose dolphin (Tursiops aduncus) population revealed a significant decline in female reproductive rates following the MHW, with capture–recapture analyses indicated 5.9 and 12.2% post-MHW declines in the survival of dolphins that use tools to forage relative to those that do not (Wild et al., 2019). Lower survival has persisted, suggesting that habitat loss following extreme weather events may have prolonged, negative impacts on even behaviourally flexible, higher-trophic level predators, but that the tool-using dolphins may be somewhat buffered against the cascading effects of habitat loss following the MHW (Wild et al., 2019). The Indo-Pacific bottlenose dolphins altered their habitat use patterns similarly following seagrass loss, increasing their use of profitable but dangerous shallow banks during periods of high tiger shark abundance, suggesting a need to increase foraging in these habitats despite predation risk (Nowicki et al., 2019). Finally, surface surveys and shark fishing data indicated that loggerhead turtles and tiger sharks, which are both generalist and opportunistic consumers (Matich et al., 2011; Thomson et al., 2012), showed no short-term population declines following seagrass loss (Nowicki et al., 2019). This aligns with the theory that generalist consumers are likely to be more resilience to habitat loss than specialists (Ryall and Fahrig, 2006). Indeed, seagrass loss may increase foraging success of these species, which often hunt species that can be obscured by dense seagrass meadows. However, even these generalists may be impacted if seagrass recovery does not occur.
The mechanism of decline likely differs by species and can be inferred with knowledge of the species' biology. For example, sea snakes are known to have extremely small home ranges (Burns and Heatwole, 1998; Lukoschek et al., 2008; Lukoschek and Shine, 2012) and to be highly reliant on seagrass for both foraging ground and refuge in Shark Bay (e.g., Kerford et al., 2008; Wirsing and Heithaus, 2009), suggesting that population declines are likely mostly driven by starvation and predation mortality (D'Anastasi et al., 2016; Nowicki et al., 2019). In contrast, dugong population declines are almost certainly driven by emigration; indeed, dugongs often migrate between foraging regions in response to resource loss, including between Shark Bay and Ningaloo reef (Preen and Marsh, 1995; Holley et al., 2006). This, combined with a lack of strandings that would be expected if mass mortality had occurred (Marsh, 1989; Preen and Marsh, 1995), suggest that dugongs left the interior of Shark Bay in response to seagrass loss.
These different mechanisms of population decline have important ecological implications for the recovery of Shark Bay's seagrasses. A rapid functional return of dugongs is more likely than for sea snakes, and will likely alter the relative functional role of the seagrass consumer community (Preen et al., 1995). For example, dugongs structure seagrass ecosystems via herbivory, that targets tropical species, but their grazing can damage climax species when they co-occur. Because dugong foraging decisions and modes are risk sensitive (Wirsing et al., 2007a,b,c), their overall impact on systems and climax species may be greater with the loss of top predators or reductions in predation risk sensitivity that are predicted under conditions of resource scarcity (Heithaus et al., 2008). Dugongs can actively choose habitat based on the location of preferred seagrass forage, and they maintain a spatial memory of these locations (Holley et al., 2006; Sheppard et al., 2010). Similarly, the species-specific changes in risk-sensitive foraging (Nowicki et al., 2019) suggest that the possible magnitude and nature of top-down control by tiger sharks (i.e., predation risk vs. direct predation) has likely changed for some prey species within Shark Bay. Understanding how consumer populations, habitat use patterns, and species interactions change in response to the direct impacts (i.e., physical forcing) and indirect impacts (i.e., resource loss) of MHWs will remain critically important to accurately predicting the recovery trajectories of primary producer communities to these disturbances (Nowicki et al., 2019). This is particularly important because overfishing continues to be a major problem for true apex predators, like tiger sharks, in most areas of the world and overfishing likely is a multiplier of ECE effects.
Flow on Effects to Human Activities
Impacts of the MHW to human activities in the Shark Bay WHS can be measured in terms of changes to commercial and recreational fisheries and tourism. The response to recruitment and catch declines in the Blue Swimmer crab and scallop fishery was 1–3 year closures and catch has returned to pre-MHW levels subsequent to the fisheries being opened (Caputi et al., 2016). The economic and social impact to fishermen was severe and points to a need to build in climate adaptation strategies for fisheries management. These include early identification of temperature hot spots, early detection of abundance changes (preferably using pre-recruit surveys), and flexible harvest strategies that allow a quick response to minimize the effect of heavy fishing on poor recruitment to enable protection of the spawning stock (Caputi et al., 2016). Major declines in the tourism experience also occurred. Sightings recorded in daily operator logs declined for dugongs, turtles, sharks, dolphins, and fish that forced operators to move their activities spatially to compensate although total loss of tourism revenue was not determined.
Concluding Remarks
To be able to predict future impacts from climate change and increased frequency of MHWs, we need a detailed ecosystem level understanding of how and when such events exceed the ecological resistance of foundation species. Also we need to understand how the ensuing habitat loss can impact fisheries, species of conservation concern, or other species that may be resistant to the direct abiotic forcing of MHWs, but not to the ensuing biotic effects of habitat loss. Furthermore, we need an understanding of the role of species interactions in generating feedbacks. This requires us to be able to identify which interactions are likely to be dominant drivers of patterns (including competition between seagrasses, predation, etc.). Understanding mechanisms that drive dominant interactions will better allow us to predict whether those interactions will remain strong or not after a system changes.
We also need to understand the potential for surviving seagrass to persist through future extreme events. Genomic tools offer new insights into local adaptation (Savolainen et al., 2013) to increase our understanding of species' response to climate change (Stillman and Armstrong, 2015), although there are challenges for translating into conservation practice (Shafer et al., 2015). More importantly, the factors that allow us to “future-proof” seagrasses warrant substantial consideration to ensure contemporary restoration efforts are not compromised by future conditions. In Shark Bay and the west coast of Western Australia, genomic studies designed to understand the interaction between plasticity, adaptation and range shifts will contribute to better translation for adaptive management and conservation responses to ECEs for the dominant habitat-forming temperate seagrasses. This is needed for both temperate and tropical seagrasses that are at both (trailing and leading edge, respectively) extremes of their geographical distributions. Recent research on terrestrial plants has shown such edge populations show similar or less resilience than core populations and are typically characterized by lower levels of genetic diversity, increasing genetic differentiation due to reduced gene flow, lower effective population sizes, and reduction in sexual reproduction although it is unknown whether trailing edge populations have a lower or higher capacity for plasticity (Donelson et al., 2019). A decline in genetic diversity was not observed in tropical colonizing species H. ovalis and H. uninervis along a Western Australian tropical to subtropical gradient with Shark Bay as the most southerly location. Instead the biggest trend was that areas of high dugong grazing show higher genetic diversity in both H. ovalis and H. uninervis, so for these species loss of dugongs may lead to lower genetic diversity over time (McMahon et al., 2017).
Finally, we stress that long term and broad spatial monitoring of iconic flora and fauna, and the initiation of continuous recording of in-situ environmental data linked to oceanographic models is required to better understand resilience of seagrass-dominated ecosystems to MHWs into the future, as well as a commitment to continue funding existing long term biological research. Individual researchers and government scientists volunteered their research effort to the understanding of the 2011 MHW, but this is not the best model for future events. A more interdisciplinary approach is required to facilitate greater understanding of the complex interactions among seagrasses and their environment, seagrass-dependent communities and trophic webs, and seagrass ecosystems. Several such models already exist and could be adapted to an Australian context, including the U.S. National Science Foundation Long Term Ecological Research Network (LTER) (https://lternet.edu/), the Zostera Experimental Network (Zenscience.org), or the U.S. NSF National Ecological Observatory Network (NEON) (Neonscience.org). Such initiatives are critical to increasing our ability to understand and predict ecosystem resilience to change in the Anthropocene.
Data Availability
All datasets generated for this study are included in the manuscript and/or the Supplementary Files.
Author Contributions
GK conceived the study. GK, RN, YO, SS, MF, ES, JS, RH, JT, DB, KM, KK, JF, MH, and RO designed the study. GK, RN, SS, MF, ES, JS, JT, DB, JF, and MH supplied published and unpublished data. GK, RN, YO, SS, RH, YH, JT, KM, and KK analyzed data. GK, RN, YO, SS, MF, ES, JS, RH, JT, DB, KM, YH, KK, JF, MH, and RO wrote and edited the manuscript.
Funding
The research into recovery of temperate seagrasses in Shark Bay was funded through successive ARC Linkage and Discovery grants (LP130100918, LP130100155, LP160101011, DP180100668), with industry partners Shark Bay Resources and the Botanic Gardens and Parks Authority. All collections were made under valid WA Department of Parks and Wildlife permits (now Department of Biodiversity, Conservation and Attractions). This is contribution #141 from the Center for Coastal Oceans Research in the Institute of Water and Environment at Florida International University, and contribution #3835 from the Virginia Institute of Marine Science.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2019.00455/full#supplementary-material
Table S1. Impacts to marine organisms and data sources by year before, during and after the 2011 MHW in Shark Bay. All cited papers are in the reference list of the main paper.
Table S2. Stem and shoot density (Mean, SE, and n) for Amphibolis antarctica and Posidonia australis, respectively, for field programs in Shark Bay held between 1982 and 2018.
Table S3. Description of results of a one way ANOVA and post-hoc Tukeys HSD statistics with field trip as the factor and shoot density of Posidonia australis and stem density of Amphibolis antarctica as dependent variables.
Table S4. Posidonia australis inflorescence densities and seed production between 2011 and 2018.
Table S5. Wooramel River Discharge by month (megaL) and sea temperatures between 1994 and 2015 (courtesy of WA Dept. of Water and Environmental Regulation).
References
Anderson, P. K. (1986). Dugongs of Shark Bay, Australia-Seasonal migration, water temperature, and forage. Natl. Geogr. Res. 2, 473–490.
Arias-Ortiz, A., Serrano, O., Masqué, P., Lavery, P. S., Mueller, U., Kendrick, G. A., et al. (2018). A marine heatwave drives massive losses from the world's largest seagrass carbon stocks. Nat. Clim. Chang. 8, 338–344. doi: 10.1038/s41558-018-0096-y
Bessey, C., Heithaus, M. R., Fourqurean, J. W., Gastrich, K. R., and Burkholder, D. A. (2016). Importance of teleost macrograzers to seagrass composition in a subtropical ecosystem with abundant populations of megagrazers and predators. Mar. Ecol. Prog. Ser. 553, 81–92. doi: 10.3354/meps11790
Brook, B. W., Sodhi, N. S., and Bradshaw, C. J. A. (2008). Synergies among extinction drivers under global change. Trends Ecol. Evol. 23, 453–460. doi: 10.1016/j.tree.2008.03.011
Burkholder, D. A., Heithaus, M. R., and Fourqurean, J. W. (2012). Feeding preferences of herbivores in a relatively pristine subtropical seagrass ecosystem. Mar. Freshwater Res. 63, 1051–1058. doi: 10.1071/MF12029
Burkholder, D. A., Heithaus, M. R., Fourqurean, J. W., Wirsing, A., and Dill, L. M. (2013). Patterns of top-down control in a seagrass ecosystem: could a roving apex predator induce a behaviour-mediated trophic cascade? J. Anim. Ecol. 82, 1192–1202. doi: 10.1111/1365-2656.12097
Burns, G., and Heatwole, H. (1998). Home range and habitat use of the olive sea snake, Aipysurus laevis, on the great barrier reef, Australia. J. Herpetol. 32, 350–358. doi: 10.2307/1565449
Cahill Abigail, E., Aiello-Lammens Matthew, E., Fisher-Reid, M., Caitlin Hua, X., Karanewsky Caitlin, J., Yeong Ryu, H., et al. (2013). How does climate change cause extinction? Proc. R. Soc. B Biol. Sci. 280:20121890. doi: 10.1098/rspb.2012.1890
Cai, W., Borlace, S., Lengaigne, M., van Rensch, P., Collins, M., Vecchi, G., et al. (2014). Increasing frequency of extreme El Niño events due to greenhouse warming. Nat. Clim. Change 4, 111–116. doi: 10.1038/nclimate2100
Caputi, N., Kangas, M., Denham, A., Feng, M., Pearce, A., Hetzel, Y., et al. (2016). Management adaptation of invertebrate fisheries to an extreme marine heat wave event at a global warming hot spot. Ecol. Evol. 6, 3583–3593. doi: 10.1002/ece3.2137
Carruthers, T. J. B., and Walker, D. I. (1997). Light climate and energy flow in the seagrass canopy of Amphibolis griffithii (J. M. Black) den Hartog. Oecologia 109, 335–341. doi: 10.1007/s004420050091
Clark, C. W. (1994). Antipredator behavior and the asset-protection principle. Behav. Ecol. 5, 159–170. doi: 10.1093/beheco/5.2.159
D'Anastasi, B. R., van Herwerden, L., Hobbs, J. A., Simpfendorfer, C. A., and Lukoschek, V. (2016). New range and habitat records for threatened Australian sea snakes raise challenges for conservation. Biol. Conserv. 194, 66–70. doi: 10.1016/j.biocon.2015.11.032
Diaz-Almela, E., Marbà, N., and Duarte, C. M. (2007). Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Glob. Chang. Biol. 13, 224–235. doi: 10.1111/j.1365-2486.2006.01260.x
Donelson, J. M., Sunday, J. M., Figueira, W. F., Gaitán-Espitia, J. D., Hobday, A. J., Johnson, C. R., et al. (2019). Understanding interactions between plasticity, adaptation and range shifts in response to marine environmental change. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374:20180186. doi: 10.1098/rstb.2018.0186
Doney, S. C., Ruckelshaus, M., Duffy, J. E., Barry, J. P., Chan, F., English, C. A., et al. (2012). Climate change impacts on marine ecosystems. Ann. Rev. Mar. Sci. 4, 11–37. doi: 10.1146/annurev-marine-041911-111611
Feng, M., McPhaden, M. J., Xie, S.-P., and Hafner, J. (2013). La Nina forces unprecedented Leeuwin Current warming in 2011. Sci. Rep. 3:1277. doi: 10.1038/srep01277
Feng, M., Meyers, G., Pearce, A., and Wijffels, S. (2003). Annual and interannual variations of the Leeuwin Current at 32 degrees S. J. Geophys. Research-Oceans, 108(C11) doi: 10.1029/2002JC001763
Feng, X., and Shinoda, T. (2019). Air-sea heat flux variability in the southeast indian ocean and its relation with Ningaloo Niño. Front. Mar. Sci. 6:266. doi: 10.3389/fmars.2019.00266
Fourqurean, J. W., Duarte, C. M., Kennedy, H., Marba, N., Holmer, M., Mateo, M. A., et al. (2012). Global carbon stocks in seagrass ecosystems. Nat. Geosci. 5, 505–509. doi: 10.1038/ngeo1477
Fraser, M. W., Kendrick, G. A., Statton, J., Hovey, R. K., Zavala-Perez, A., and Walker, D. I. (2014). Extreme climate events lower resilience of foundation seagrass at edge of biogeographical range. J. Ecol. 102, 1528–1536. doi: 10.1111/1365-2745.12300
Frölicher, T. L., and Laufkötter, C. (2018). Emerging risks from marine heat waves. Nat. Commun. 9:650. doi: 10.1038/s41467-018-03163-6
Gorman, D., Turra, A., Bergstrom, E. R., and Horta, P. A. (2016). Population expansion of a tropical seagrass (Halophila decipiens) in the southwest Atlantic (Brazil). Aquat. Bot. 132, 30–36. doi: 10.1016/j.aquabot.2016.04.002
Griffin, C., Beggs, H., and Majewski, L. (2017). GHRSST compliant AVHRR SST products over the Australian region – Version 1, Technical Report. 151 p. Bureau of Meteorology, Melbourne, VIC: Australia.
Heithaus, M. R. (2001). The biology of tiger sharks, Galeocerdo cuvier, in Shark Bay, Western Australia: sex ratio, size distribution, diet, and seasonal changes in catch rates. Environ. Biol. Fishes 61, 25–36. doi: 10.1023/A:1011021210685
Heithaus, M. R., Frid, A., Wirsing, A. J., Dill, L. M., Fourqurean, J. W., Burkholder, D., et al. (2007). State-dependent risk-taking by green sea turtles mediates top-down effects of tiger shark intimidation in a marine ecosystem. J. Anim. Ecol. 76, 837–844. doi: 10.1111/j.1365-2656.2007.01260.x
Heithaus, M. R., Frid, A., Wirsing, A. J., and Worm, B. (2008). Predicting ecological consequences of marine top predator declines. Trends Ecol. Evol. 23, 202–210. doi: 10.1016/j.tree.2008.01.003
Heithaus, M. R., Wirsing, A. J., and Dill, L. M. (2012). The ecological importance of intact top-predator populations: a synthesis of 15 years of research in a seagrass ecosystem. Mar. Freshwater Res. 63, 1039–1050. doi: 10.1071/MF12024
Hobday, A. J., Oliver, E. C. J., Gupta, A. S., and Benthuysen, J. A. (2018). Categorizing and naming marine heatwaves. Oceanography 31, 162–173 doi: 10.5670/oceanog.2018.205
Hodgson, D., McDonald, J. L., and Hosken, D. J. (2015). What do you mean, “resilient”? Trends Ecol. Evol. 30, 503–506. doi: 10.1016/j.tree.2015.06.010
Hoegh-Guldberg, O., and Bruno, J. F. (2010). The impact of climate change on the world's marine ecosystems. Science 328, 1523–1528. doi: 10.1126/science.1189930
Holley, D. K., Lawler, I. R., and Gales, N. J. (2006). Summer survey of dugong distribution and abundance in Shark Bay reveals additional key habitat area. Wildl. Res. 33, 243–250. doi: 10.1071/WR05031
Holling, C. S. (1973). Resilience and stability of ecological systems. Annu. Rev. Ecol. Syst. 4, 1–23. doi: 10.1146/annurev.es.04.110173.000245
Hyndes, G. A., Heck, K. L. Jr., Vergés, A., Harvey, E. S., Kendrick, G. A., Lavery, P. S., et al. (2016). Accelerating tropicalization and the transformation of temperate seagrass meadows. Bioscience 66, 938–948. doi: 10.1093/biosci/biw111
Johnson, A. J., Statton, J., Orth, R. J., and Kendrick, G. A. (2018). A sediment bioturbator bottleneck to seedling recruitment for the seagrass Posidonia australis. Mar. Ecol. Prog. Ser. 595, 89–103. doi: 10.3354/meps12550
Johnson, C. R., Banks, S. C., Barrett, N. S., Cazassus, F., Dunstan, P. K., Edgar, G. J., et al. (2011). Climate change cascades: shifts in oceanography, species' ranges and subtidal marine community dynamics in eastern Tasmania. J. Exp. Mar. Bio. Ecol. 400, 17–32. doi: 10.1016/j.jembe.2011.02.032
Kataoka, T., Masson, S., Izumo, T., Tozuka, T., and Yamagata, T. (2018). Can ningaloo nino/nina develop without El Nino-Southern oscillation? Geophys. Res. Lett. 45, 7040–7048. doi: 10.1029/2018GL078188
Kendrick, G. A., Aylward, M. J., Hegge, B. J., and Cambridge, M. L. (2002). Changes in seagrass coverage in Cockburn Sound, Western Australia between 1967 and 1999. Aquat. Bot. 73, 75–87. doi: 10.1016/S0304-3770(02)00005-0
Kendrick, G. A., Fourqurean, J. W., Fraser, M. W., Heithaus, M. R., Jackson, G., Friedman, K., et al. (2012). Science behind management of Shark Bay and Florida Bay, two P-limited subtropical systems with different climatology and human pressures Introduction. Mar. Freshwater Res. 63, 941–951. doi: 10.1071/MF12280
Kerford, M., Wirsing, A. J., Heithaus, M. R., and Dill, L. M. (2008). Danger on the rise: habitat use by bar-bellied sea snakes in Shark Bay, Western Australia. Mar. Ecol. Prog. Ser. 358, 289–294. doi: 10.3354/meps07346
Kilminster, K., McMahon, K., Waycott, M., Kendrick, G. A., Scanes, P., McKenzie, L., et al. (2015). Unravelling complexity in seagrass systems for management: Australia as a microcosm. Sci. Total Environ. 534, 97–109. doi: 10.1016/j.scitotenv.2015.04.061
Kim, J. B., Park, J.-I., Jung, C.-S., Lee, P.-Y., and Lee, K.-S. (2009). Distributional range extension of the seagrass Halophila nipponica into coastal waters off the Korean peninsula. Aquat. Bot. 90, 269–272. doi: 10.1016/j.aquabot.2008.10.007
Langtimm, C. A., and Beck, C. A. (2003). Lower survival probabilities for adult Florida manatees in years with intense coastal storms. Ecol. Appl. 13, 257–268. doi: 10.1890/1051-0761(2003)013[0257:LSPFAF]2.0.CO;2
Lefcheck, J. S., Wilcox, D. J., Murphy, R. R., Marion, S. R., and Orth, R. J. (2016). Multiple stressors threaten an important coastal foundation species. doi: 10.7287/peerj.preprints.2544v1
Lukoschek, V., and Shine, R. (2012). Sea snakes rarely venture far from home. Ecol. Evol. 2, 1113–1121. doi: 10.1002/ece3.256
Lukoschek, V., Waycott, M., and Keogh, J. S. (2008). Relative information content of polymorphic microsatellites and mitochondrial DNA for inferring dispersal and population genetic structure in the olive sea snake, Aipysurus laevis. Mol. Ecol. 17, 3062–3077. doi: 10.1111/j.1365-294X.2008.03815.x
Marba, N., and Duarte, C. M. (2010). Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob. Change Biol. 16, 2366–2375. doi: 10.1111/j.1365-2486.2009.02130.x
Marsh, H. E. (1989). Mass strandings of dugongs by a tropical cyclone in northern Australia. Mar. Mamm. Sci. 5, 78–84. doi: 10.1111/j.1748-7692.1989.tb00215.x
Matich, P., Heithaus, M. R., and Layman, C. A. (2011). Contrasting patterns of individual specialization and trophic coupling in two marine apex predators. J. Anim. Ecol. 80, 294–305. doi: 10.1111/j.1365-2656.2010.01753.x
McMahon, K. M., Evans, R. D., van Dijk, K.-J., Hernawan, U., Kendrick, G. A., Lavery, P. S., et al. (2017). Disturbance is an important driver of clonal richness in tropical seagrasses. Front. Plant Sci. 8:2026. doi: 10.3389/fpls.2017.02026
Nowicki, R., Heithaus, M., Thomson, J., Burkholder, D., Gastrich, K., and Wirsing, A. (2019). Indirect legacy effects of an extreme climactic event on a marine megafaunal community. Ecol. Monogr. e01365. doi: 10.1002/ecm.1365
Nowicki, R. J., Thomson, J. A., Burkholder, D. A., Fourqurean, J. W., and Heithaus, M. R. (2017). Predicting seagrass recovery times and their implications following an extreme climate event. Mar. Ecol. Prog. Ser. 567, 79–93. doi: 10.3354/meps12029
O'Brien, K. R., Waycott, M., Maxwell, P., Kendrick, G. A., Udy, J. W., Ferguson, A. J. P., et al. (2018). Seagrass ecosystem trajectory depends on the relative timescales of resistance, recovery and disturbance. Mar. Pollut. Bull. 134, 166–176. doi: 10.1016/j.marpolbul.2017.09.006
Oliver, T. H., Marshall, H. H., Morecroft, M. D., Brereton, T., Prudhomme, C., and Huntingford, C. (2015). Interacting effects of climate change and habitat fragmentation on drought-sensitive butterflies. Nat. Clim. Change 5, 941–945. doi: 10.1038/nclimate2746
Ooi, J., Kendrick, G. A., Van Niel, K. P., and Affendi, Y. A. (2011a). Knowledge gaps in tropical Southeast Asian seagrass systems. Estuar. Coast. Shelf Sci. 92, 118–131. doi: 10.1016/j.ecss.2010.12.021
Ooi, J. L. S., Kendrick, G. A., and Van Niel, K. P. (2011b). Effects of sediment burial on tropical ruderal seagrasses are moderated by clonal integration. Cont. Shelf Res. 31, 1945–1954. doi: 10.1016/j.csr.2011.09.005
Orth, R. J., Carruthers, T., Dennison, W. C., Duarte, C. M., Fourqurean, J. W., Heck, K. L., et al. (2006). A global crisis for seagrass ecosystems. Bioscience 56, 987–996. doi: 10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Pachauri, R. K., Allen, M. R., Barros, V. R., Broome, J., Cramer, W., Christ, R., et al. (2014). “Climate change 2014: synthesis report,” in Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, eds R. K. Pachauri and L. Meyer (Geneva: IPCC), 1–112.
Pearce, A. F., and Feng, M. (2013). The rise and fall of the “marine heat wave” off Western Australia during the summer of 2010/2011. J. Mar. Syst. 111, 139–156. doi: 10.1016/j.jmarsys.2012.10.009
Pecl, G. T., Araújo, M. B., Bell, J. D., Blanchard, J., Bonebrake, T. C., Chen, I.-C., et al. (2017). Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 355:eaai9214. doi: 10.1126/science.aai9214
Peterson, G., Allen, C. R., and Holling, C. S. (1998). Ecological Resilience, Biodiversity, and Scale. Ecosystems 1, 6–18. doi: 10.1007/s100219900002
Poloczanska, E. S., Brown, C. J., and Sydeman, W. J. (2013). Global imprint of climate change on marine life. Nat. Clim. Change 3, 919–925. doi: 10.1038/nclimate1958
Preen, A., and Marsh, H. (1995). Response of dugongs to large-scale loss of seagrass from Hervey Bay, Queensland Australia. Wildl. Res. 22, 507–519. doi: 10.1071/WR9950507
Preen, A. R., Long, W., and Coles, R. G. (1995). Flood and flood and cyclone related loss, and partial recovery, of more than 1000 km2 of seagrass in Hervey Bay, Queensland, Australia. Aquat. Bot. 52, 3–17. doi: 10.1016/0304-3770(95)00491-H
R Core Team (2013). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: http://www.R-project.org/ (accessed July 5, 2019).
Rossi, V., Feng, M., Pattiaratchi, C., Roughan, M., and Waite, A. M. (2013). On the factors influencing the development of sporadic upwelling in the Leeuwin Current system. J. Geophys. Res. Oceans 118, 3608–3621. doi: 10.1002/jgrc.20242
Ruiz, J. M., Marín-Guirao, L., García-Muñoz, R., Ramos-Segura, A., Bernardeau-Esteller, J., Pérez, M., et al. (2018). Experimental evidence of warming-induced flowering in the Mediterranean seagrass Posidonia oceanica. Mar. Pollut. Bull. 134, 49–54. doi: 10.1016/j.marpolbul.2017.10.037
Ryall, K. L., and Fahrig, L. (2006). Response of predators to loss and fragmentation of prey habitat: a review of theory. Ecology 87, 1086–1093. doi: 10.1890/0012-9658(2006)87[1086:ROPTLA]2.0.CO;2
Savolainen, O., Lascoux, M., and Merilä, J. (2013). Ecological genomics of local adaptation. Nat. Rev. Genet. 14, 807–820. doi: 10.1038/nrg3522
Scheffers, B. R., De Meester, L., Bridge, T. C. L., Hoffmann, A. A., Pandolfi, J. M., Corlett, R. T., et al. (2016). The broad footprint of climate change from genes to biomes to people. Science 354:aaf7671. doi: 10.1126/science.aaf7671
Shafer, A. B. A., Wolf, J. B. W., Alves, P. C., Bergström, L., Bruford, M. W., Brännström, I., et al. (2015). Genomics and the challenging translation into conservation practice. Trends Ecol. Evol. 30, 78–87. doi: 10.1016/j.tree.2014.11.009
Sheppard, J. K., Marsh, H., Jones, R. E., and Lawler, I. R. (2010). Dugong habitat use in relation to seagrass nutrients, tides, and diel cycles. Mar. Mamm. Sci. 26, 855–879. doi: 10.1111/j.1748-7692.2010.00374.x
Sherman, C. D. H., Smith, T. M., York, P. H., Jarvis, J. C., Ruiz-Montoya, L., and Kendrick, G. A. (2018). “Reproductive, dispersal and recruitment strategies in australian seagrasses,” in Seagrasses of Australia: Structure, Ecology and Conservation, eds A. W. D. Larkum, G. A. Kendrick, and P. J. Ralph (Cham: Springer International Publishing), 213–256. doi: 10.1007/978-3-319-71354-0_8
Sinclair, E. A., Statton, J., Hovey, R., Anthony, J. M., Dixon, K. W., and Kendrick, G. A. (2016). Reproduction at the extremes: pseudovivipary, hybridization and genetic mosaicism in Posidonia australis (Posidoniaceae). Ann. Bot. 117, 237–247. doi: 10.1093/aob/mcv162
Smale, D. A., Wernberg, T., Oliver, E. C. J., and Thomsen, M. (2019). Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Chang. 9, 306–312. doi: 10.1038/s41558-019-0412-1
Statton, J., Dixon, K. W., Irving, A. D., Jackson, E. L., Kendrick, G. A., Orth, R. J., et al. (2018). “Decline and restoration ecology of australian seagrasses,” in Seagrasses of Australia: Structure, Ecology and Conservation, eds A. W. D. Larkum, G. A. Kendrick, and P. J. Ralph (Cham: Springer International Publishing), 665–704. doi: 10.1007/978-3-319-71354-0_20
Stillman, J. H., and Armstrong, E. (2015). Genomics are transforming our understanding of responses to climate change. Bio Sci. 65, 237–246. doi: 10.1093/biosci/biu219
Thomson, J. A., Burkholder, D. A., Heithaus, M. R., Fourqurean, J. W., Fraser, M. W., Statton, J., et al. (2015). Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Glob. Chang. Biol. 21, 1463–1474. doi: 10.1111/gcb.12694
Thomson, J. A., Heithaus, M. R., and Burkholder, D. A. (2012). Site specialists, diet generalists? Isotopic variation, site fidelity, and foraging by loggerhead turtles in Shark Bay, Western Australia. Mar. Ecol. 453, 213–226. doi: 10.3354/meps09637
Vergés, A., Steinberg, P. D., Hay, M. E., Poore, A. G. B., Campbell, A. H., Ballesteros, E., et al. (2014). The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. Biol. Sci. 281:20140846. doi: 10.1098/rspb.2014.0846
Walker, D. I. (1985). Correlations between salinity and growth of the seagrass Amphibolis antarctica (labill.) Sonder and Aschers., In Shark Bay, Western Australia, using a new method for measuring production rate. Aquat. Bot. 23, 13–26. doi: 10.1016/0304-3770(85)90017-8
Walker, D. I., and Cambridge, M. L. (1995). An experimental assessment of the temperature responses of two sympatric seagrasses, Amphibolis antarctica and Amphibolis griffithii, in relation to their biogeography. Hydrobiologia 302, 63–70. doi: 10.1007/BF00006399
Walker, D. I., Kendrick, G. A., and Mccomb, A. J. (1988). the distribution of seagrass species in Shark Bay, Western Australia, with notes on their ecology. Aquat. Bot. 30, 305–317. doi: 10.1016/0304-3770(88)90063-0
Wernberg, T., Bennett, S., Babcock, R. C., de Bettignies, T., Cure, K., Depczynski, M., et al. (2016). Climate-driven regime shift of a temperate marine ecosystem. Science 353, 169–172. doi: 10.1126/science.aad8745
Wernberg, T., Smale, D. A., and Thomsen, M. S. (2012). A decade of climate change experiments on marine organisms: procedures, patterns and problems. Glob. Change Biol. 18, 1491–1498. doi: 10.1111/j.1365-2486.2012.02656.x
Werner, E. E., and Peacor, S. D. (2006). Lethal and nonlethal predator effects on an herbivore guild mediated by system productivity. Ecology 87, 347–361. doi: 10.1890/05-0091
Wijffels, S. E., Beggs, H., Griffin, C., Middleton, J. F., Cahill, M., King, E., et al. (2018). A fine spatial-scale sea surface temperature atlas of the Australian regional seas (SSTAARS): seasonal variability and trends around Australasia and New Zealand revisited. J. Mar. Systems 187, 156–196. doi: 10.1016/j.jmarsys.2018.07.005
Wild, S., Krützen, M., Rankin, R. W., Hoppitt, W. J. E., Gerber, L., and Allen, S. J. (2019). Long-term decline in survival and reproduction of dolphins following a marine heatwave. Curr. Biol. 29, R239–R240. doi: 10.1016/j.cub.2019.02.047
Wirsing, A. J., and Heithaus, M. R. (2009). Olive-headed sea snakes Disteria major shift seagrass microhabitats to avoid shark predation. Mar. Ecol. Prog. Ser. 387, 287–293. doi: 10.3354/meps08127
Wirsing, A. J., Heithaus, M. R., and Dill, L. M. (2007a). Can measures of prey availability improve our ability to predict the abundance of large marine predators? Oecologia 153, 563–568. doi: 10.1007/s00442-007-0769-0
Wirsing, A. J., Heithaus, M. R., and Dill, L. M. (2007b). Fear factor: do dugongs (Dugong dugon) trade food for safety from tiger sharks (Galeocerdo cuvier)? Oecologia 153, 1031–1040. doi: 10.1007/s00442-007-0802-3
Wirsing, A. J., Heithaus, M. R., and Dill, L. M. (2007c). Living on the edge: dugongs prefer to forage in microhabitats that allow escape from rather than avoidance of predators. Anim. Behav. 74, 93–101. doi: 10.1016/j.anbehav.2006.11.016
Keywords: extreme climate events, marine heatwaves, seagrass, resilience, multiple stressors, resistance, recovery
Citation: Kendrick GA, Nowicki RJ, Olsen YS, Strydom S, Fraser MW, Sinclair EA, Statton J, Hovey RK, Thomson JA, Burkholder DA, McMahon KM, Kilminster K, Hetzel Y, Fourqurean JW, Heithaus MR and Orth RJ (2019) A Systematic Review of How Multiple Stressors From an Extreme Event Drove Ecosystem-Wide Loss of Resilience in an Iconic Seagrass Community. Front. Mar. Sci. 6:455. doi: 10.3389/fmars.2019.00455
Received: 22 April 2019; Accepted: 08 July 2019;
Published: 29 July 2019.
Edited by:
Peng Jin, University of Guangzhou, ChinaReviewed by:
Dan Alexander Smale, Marine Biological Association of the United Kingdom, United KingdomMads Solgaard Thomsen, University of Canterbury, New Zealand
Copyright © 2019 Kendrick, Nowicki, Olsen, Strydom, Fraser, Sinclair, Statton, Hovey, Thomson, Burkholder, McMahon, Kilminster, Hetzel, Fourqurean, Heithaus and Orth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gary A. Kendrick, Z2FyeS5rZW5kcmlja0B1d2EuZWR1LmF1
 Gary A. Kendrick
Gary A. Kendrick Robert J. Nowicki
Robert J. Nowicki Ylva S. Olsen
Ylva S. Olsen Simone Strydom
Simone Strydom Matthew W. Fraser
Matthew W. Fraser Elizabeth A. Sinclair
Elizabeth A. Sinclair John Statton
John Statton Renae K. Hovey
Renae K. Hovey Jordan A. Thomson
Jordan A. Thomson Derek A. Burkholder
Derek A. Burkholder Kathryn M. McMahon
Kathryn M. McMahon Kieryn Kilminster
Kieryn Kilminster Yasha Hetzel
Yasha Hetzel James W. Fourqurean
James W. Fourqurean Michael R. Heithaus
Michael R. Heithaus Robert J. Orth
Robert J. Orth