- 1Tobacco Research Institute of Chinese Academy of Agricultural Sciences, Qingdao, China
- 2Tobacco Research Institute of Nanping, Nanping, China
- 3Plant Protection Station of Shandong Province, Jinan, China
- 4College of Marine Life Sciences, Ocean University of China, Qingdao, China
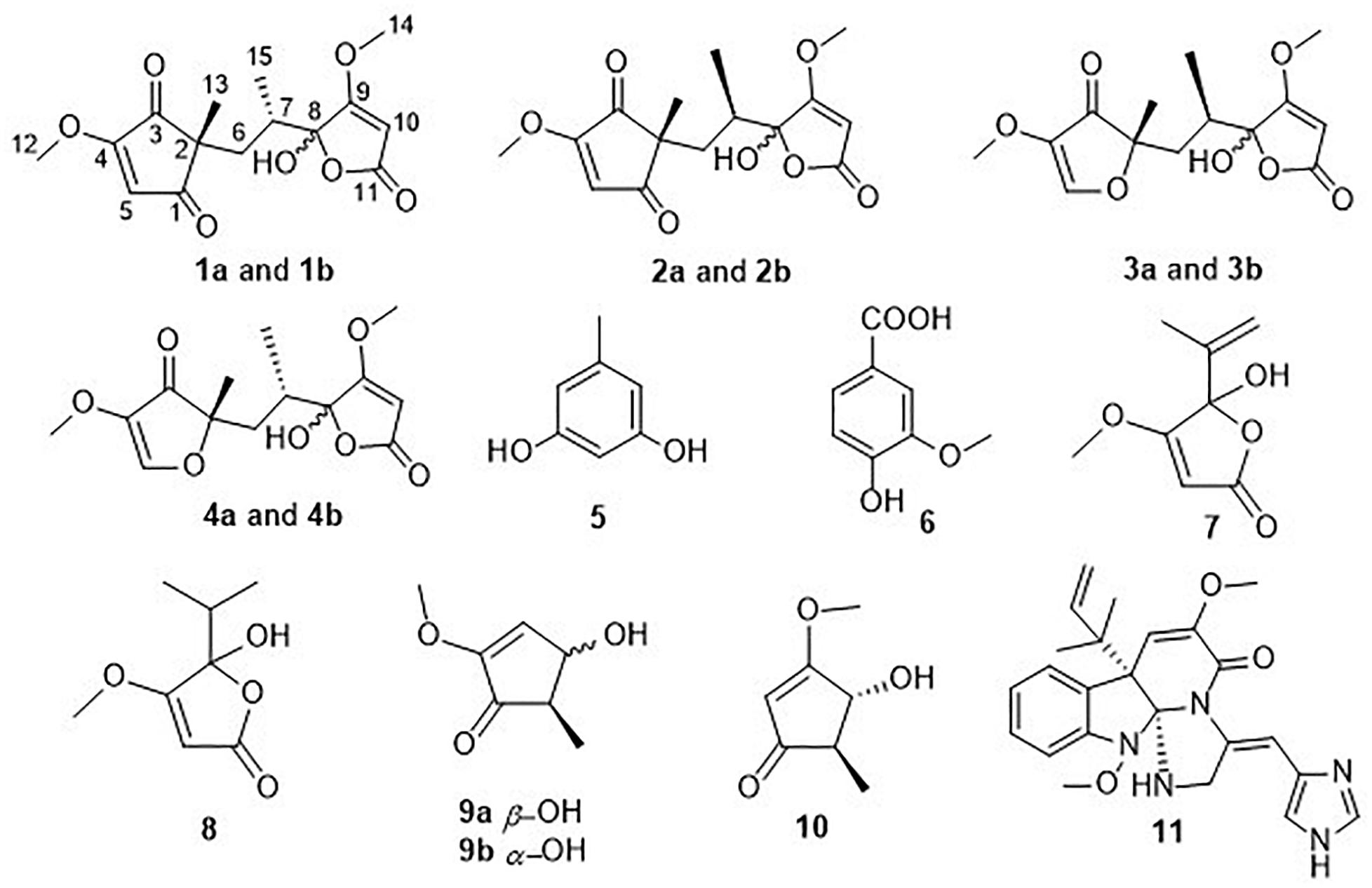
In order to search for new lead compounds with anti-phytopathogenic bacterial activity, three pairs of new furanone derivatives, sclerotiorumins D–F (1–3), and eight known compounds (4–11) were isolated from the seaweed-derived fungus, Aspergillus sp. D40, fermented with potato dextrose seawater (PDW) medium. Their structures were determined using comprehensive spectroscopic analyzes including HRESIMS, 1D and 2D NMR data. Compounds 1–4 and 9 existed as inseparable mixtures of a pair of epimers. Penicillic acid (7) exhibited clear antibacterial activity against Ralstonia solanacearum and several other plant pathogenic bacteria with IC50 values ranging from 11.6 to 58.2 μg/mL.
Introduction
Phytopathogenic bacteria cause many serious diseases in plants and limit the quality and production of crops all over the world, and as a result pose a significant threat to global food safety (Sundin et al., 2016). For instance, Ralstonia solanacearum – the causative agent of bacterial wilt and Moko disease – is ranked second on the top ten bacterial plant pathogen list (Manfield et al., 2012). R. solanacearum affects more than 200 plant species belonging to over 50 different botanical families, including Solanaceae – tomato and potato – and many weeds, crops, shrubs, and trees (dicot as well as monocot). This pathogen invades the xylem conduit from the roots of the plant, and spreads to the aerial parts of the plant through the vascular system. The extensive colonization results in vascular dysfunction, thereby causing the wilting symptoms (Genin and Denny, 2012).
Current integrated management strategies for R. solanacearum and other phytopathogenic bacteria include the use of resistant cultivars, pathogen-free transplants, and crop rotation with non-host cover crops, all of which have had limited effects (Pradhanang et al., 2005). A few – mainly chemical – pesticides are used to control phytopathogenic bacteria, however, this leads to environmental pollution, pesticide residues, food safety issues, and pathogen resistance (Fujiwara et al., 2011). It is well known that marine fungi can produce secondary metabolites with novel structures and potential antibacterial activities, as part of their repertoire of survival strategies and metabolic mechanisms endowed by the unique marine environment. Thus, they have been a hotspot for study of new antibacterial agents (Carroll et al., 2020). However, there have been few reports focused on their potential for applications in agriculture, making this a promising new field for identification and study of antibacterial biopesticides.
In our ongoing search for new bioactive secondary metabolites from marine-derived fungi (Huang et al., 2018; Zhao et al., 2018; Zhao et al., 2019), our attention was drawn to Aspergillus sp. D40, isolated from a red seaweed Grateloupia filicina, because an ethyl acetate (EtOAc) extract of a culture grown in potato dextrose seawater (PDW) medium exhibited obvious antibacterial activity against R. solanacearum (5 mg/mL). Further chemical examination of the EtOAc extract resulted in the discovery of three new furanone derivatives, sclerotiorumins D–F (1–3), and eight known compounds, including penicillic acid (4–11) (Figure 1). Herein, we report the isolation, identification, and antibacterial activity of these compounds.
Materials and Methods
General Experimental Procedures
Optical rotations were measured on a JASCO P-1020 digital display with a 1 dm cell polarimeter (Jasco, Inc., Tokyo, Japan). UV spectra were acquired using a Techcomp on the UV2310II spectrophotometer (Techcomp, Ltd., Shanghai, China). NMR spectra were recorded on an Agilent DD2 NMR spectrometer (500 MHz for 1H, 125 MHz for 13C; Agilent Technologies, Santa Clara, CA, United States) with tetramethylsilane (TMS) as internal standard. High-resolution ESIMS (HRESIMS) and electrospray ionization mass spectrometry (ESIMS) data were measured using a Thermo Scientific LTQ Orbitrap XL spectrometer (Thermo Fisher Scientific, Waltham, MA, United States) and a Micromass Q-TOF spectrometer (Waters, Milford, MA, United States). Semi-preparative HPLC was performed on a C18 (Waters, 5 μm, 10 × 250 mm) column using a Waters e2695 separation module equipped with a 2998 detector (Waters). Silica gel (200–300 mesh; Qingdao Ocean Chemistry Group Co., Ltd., Qingdao, China), octadecylsilyl silica gel (ODS) (RP18, 40–63 mm; Merck, Billerica, MA, United States), and Sephadex LH-20 (GE Healthcare, Pittsburgh, PA, United States) were used for column chromatography. Precoated silica gel plates (Yan Tai Zi Fu Chemical Group Co.; G60, F-254) were used for thin-layer chromatography.
Fungal Material
The fungal strain Aspergillus sp. D40 was isolated from a red seaweed Grateloupia filicina, which was collected from coastal habitats in Qingdao, China (120°20′18.18′′E, 36°03′15.90′′N), in July 2016. The fungus was identified based on its morphological characteristics and by a molecular protocol based on the amplification of the ITS region of the rDNA gene followed by sequence determination (Zhao et al., 2018). The strain was deposited in the Marine Agriculture Research Center, Tobacco Research Institute of Chinese Academy of Agricultural Sciences, Qingdao, China, with the GenBank (NCBI) accession number MK968521.
Extraction and Isolation
The fungal strain Aspergillus sp. D40 was fermented in 80 L of potato dextrose seawater (PDW) medium at 28°C for 30 days. The mycelia were mechanically broken and then ultrasonically disrupted for 10 min, and were then extracted twice with CH2Cl2:MeOH (1:1, v/v). The solutions were concentrated under reduced pressure to yield a residue, which was extracted three times with EtOAc. The culture media was also extracted three times with EtOAc, and the combined EtOAc extracts from both the mycelia and culture media were concentrated under reduced pressure to yield the total EtOAc extract (22.3 g). The extract was subjected to silica gel vacuum liquid chromatography (VLC), eluted using a linear gradient of petroleum ether (PE)–EtOAc (0–100%) and subsequently EtOAc–MeOH (0–100%) to obtain seven fractions (Fr.1–Fr.7). Fraction 2 was initially fractionated using reverse silica gel column chromatography (CC) with a step gradient elution of MeOH–H2O (50–90%), followed by separation on Sephadex LH-20 CC (CH2Cl2/MeOH, v/v, 1/1) to afford Fr. 2-1 and Fr. 2-2. Fraction 2-1 was then purified by reversed phase (RP)-HPLC and eluted with 30% MeOH–H2O to obtain 5 (168.0 mg) and 6 (30.6 mg). Fraction 3 was run on a reverse silica gel column and separated on silica gel CC (CH2Cl2/MeOH, v/v, 200/1–0/100) to obtain Fr. 3-1–Fr. 3-6. Fraction 3–5 was further separated by HPLC using 15% MeCN–(H2O + 0.1% TFA) to yield 7 (271.1 mg) and 8 (5.8 mg). Fraction 4 was subjected to octadecylsilyl silica gel (ODS) CC separation using a gradient elution of 30–90% MeOH–H2O to afford subfractions Fr.4-1–Fr.4-2. Fraction 4-1 and Fraction 4-2 were separated by silica gel CC (CH2Cl2/MeOH, v/v, 500/1–0/100) to obtain Fr.4-1-1–Fr.4-1-3 and Fr.4-2-1–Fr.4-2-2, respectively. Fraction 4-1-3 was purified by HPLC using 5% MeOH–H2O to obtain 9 (72.0 mg) and 10 (5.5 mg). Fraction 4-2-2 was purified by HPLC utilizing 30% MeOH–(H2O + 0.1% TFA) to obtain 1 (16.0 mg), 2 (12.0 mg), 3 (42.5 mg), and 4 (80.7 mg). Fraction 6 was run on a reverse silica gel CC, eluted with 30–90% MeOH in H2O, and further separated on silica gel CC (CH2Cl2/MeOH, v/v, 200/1–0/100) to obtain Fr. 6-1–Fr. 6-4. Fraction 6-4 was finally separated by HPLC using 30% MeCN–(H2O + 0.1% TFA) to yield 11 (25.0 mg). The purities of all the isolated compounds were >95% based on the peak area normalization method.
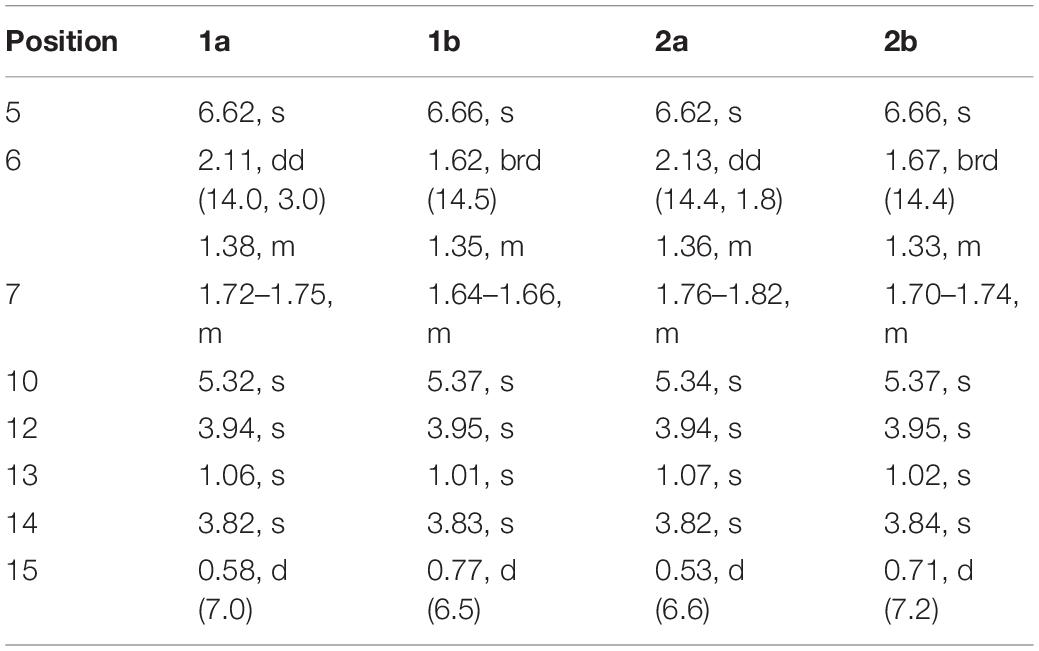
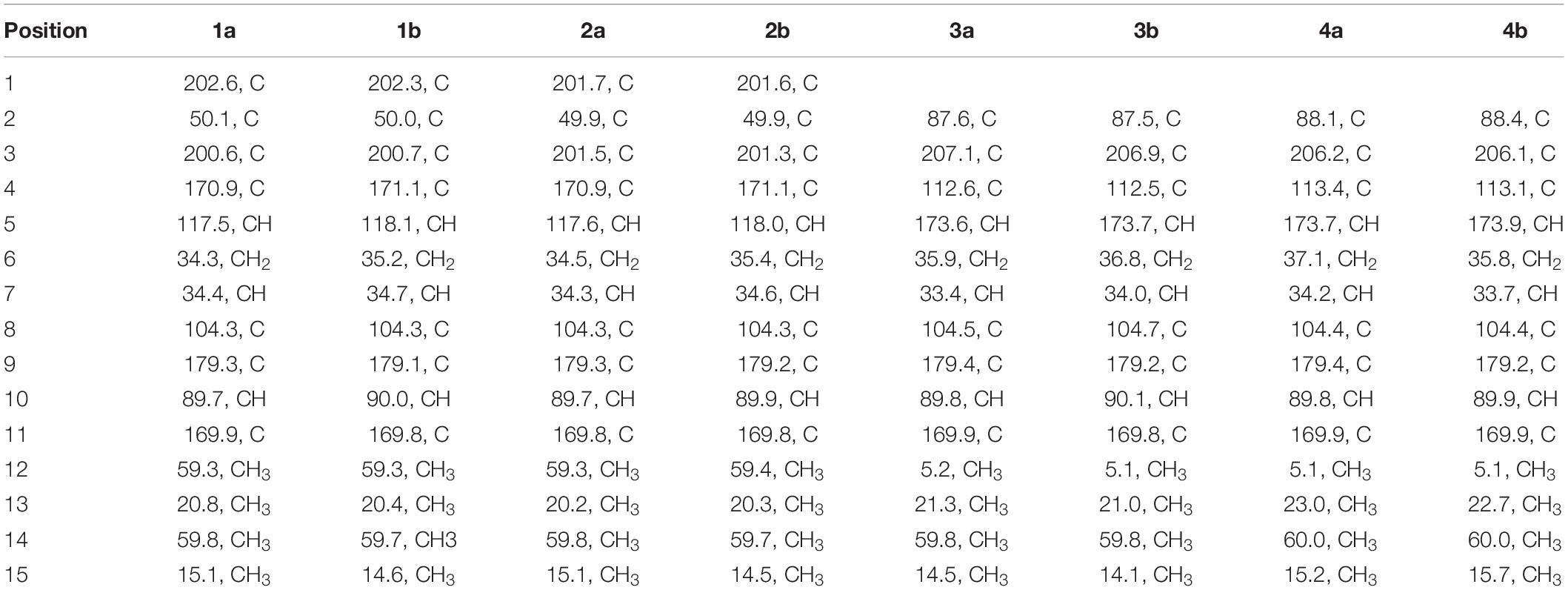
Sclerotiorumin D (1): pale yellow powder; [α]20D -1.9 (c 0.34, MeOH); UV (MeOH) λmax (log ε) 228 (3.51), 260 (3.47) nm; 1H and 13C NMR data, Tables 1, 2; HRESIMS m/z 333.0940 [M + Na]+ (calcd for C15H18O7Na, 333.0945).
Sclerotiorumin E (2): pale yellow powder; [α]20D -0.82 (c 0.38, MeOH); UV (MeOH) λmax (log ε) 225 (3.84), 260 (3.71) nm; 1H and 13C NMR data, Tables 1, 2; HRESIMS m/z 328.1392 [M + NH4]+ (calcd for C15H22O7N, 328.1391).
Sclerotiorumin F (3): pale yellow oil; [α]20D -0.37 (c 0.48, MeOH); UV (MeOH) λmax (log ε) 223 (3.80), 269 (3.67) nm; 1H and 13C NMR data, Tables 2, 3; HRESIMS m/z 283.1181 [M +H]+ (calcd for C14H19O6, 283.1176).
Sclerotiorumin B (4): pale yellow oil; [α]20D -0.41 (c 0.42, MeOH); UV (MeOH) λmax (log ε) 226 (3.71), 269 (3.57) nm; 1H and 13C NMR data, Tables 2, 3; HRESIMS m/z 283.1176 [M +H]+ (calcd for C14H19O6, 283.1176).
Antibacterial Assay for the Isolated Compounds
The antibacterial activity of the isolated compounds was determined using a conventional broth dilution assay (Oppong-Danquah et al., 2020). Apart from R. solanacearum (bacterial wilt of tobacoo), another five phytopathogenic bacterial strains, including Acidovorax avenae (bacterial fruit blotch), Clavibacter michiganensis (bacterial wilt and canker of tomato), Erutima carafavora (tobacco hollow stalk), Xanthomonas campestris (cotton angular leaf spot), and Xanthomonas citri (bacterial canker of citrus) were used. Streptomycin sulfate was used as the positive control. Dimethyl sulfoxide was used as the solvent and the concentration was 1%. The MIC was determined as the lowest concentration at which no growth was observed. The inhibition rate was calculated according to the following formula, and the IC50 was calculated from the regression equation.
bs, bacterial suspension; t, tested compounds; ck, blank control.
Results and Discussion
Structural Elucidation of Compounds 1–11
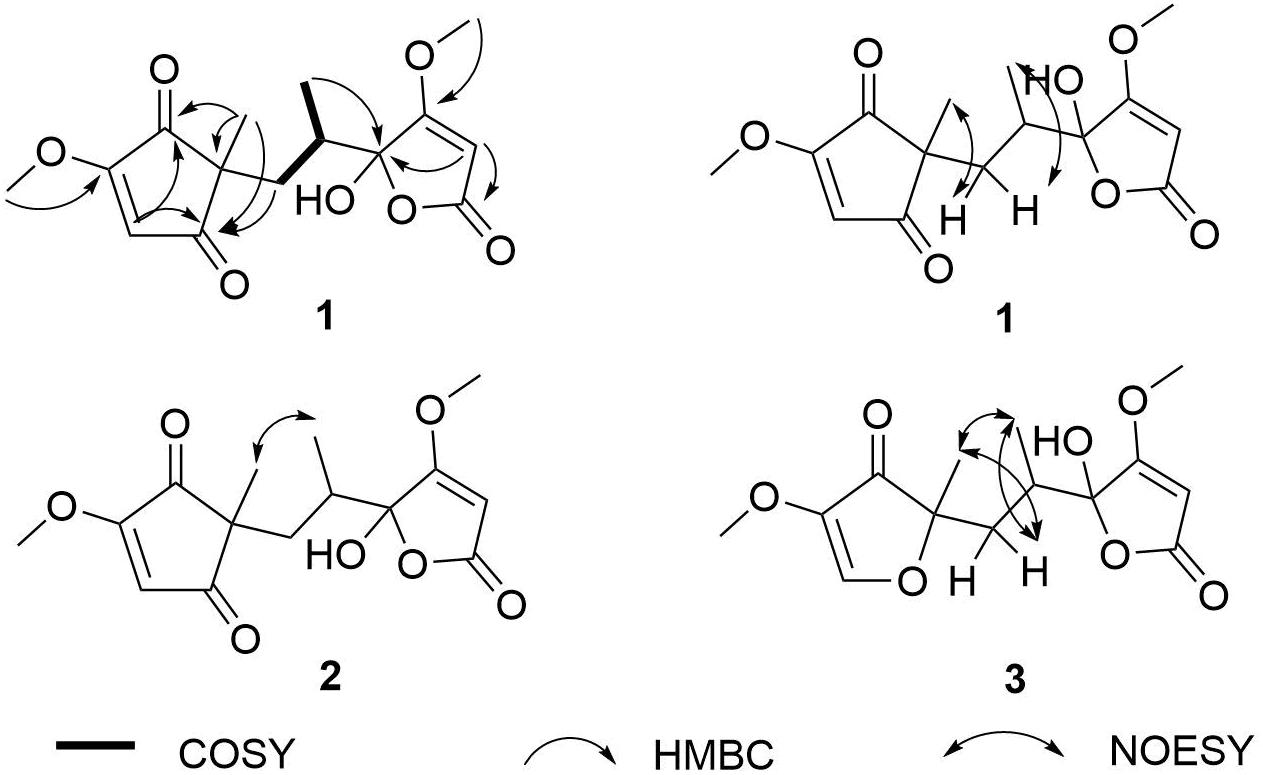
Sclerotiorumin D (1) was obtained as a pale-yellow powder, and had a molecular formula of C15H18O7 based on HRESIMS data (m/z 333.0942 [M + Na]+) (Supplementary Figure S7), accounting for seven degrees of unsaturation. Although it was isolated as a pure compound by HPLC, its 1H- and 13C-NMR signals (Supplementary Figures S1–S5) appeared as a mixture of two geometric isomers (1a and 1b) with a ratio of 1:1.3 (Supplementary Figures S1,S2). Further attempts to separate the two isomers using a chiral column failed due to spontaneous and immediate isomerization between the two forms.
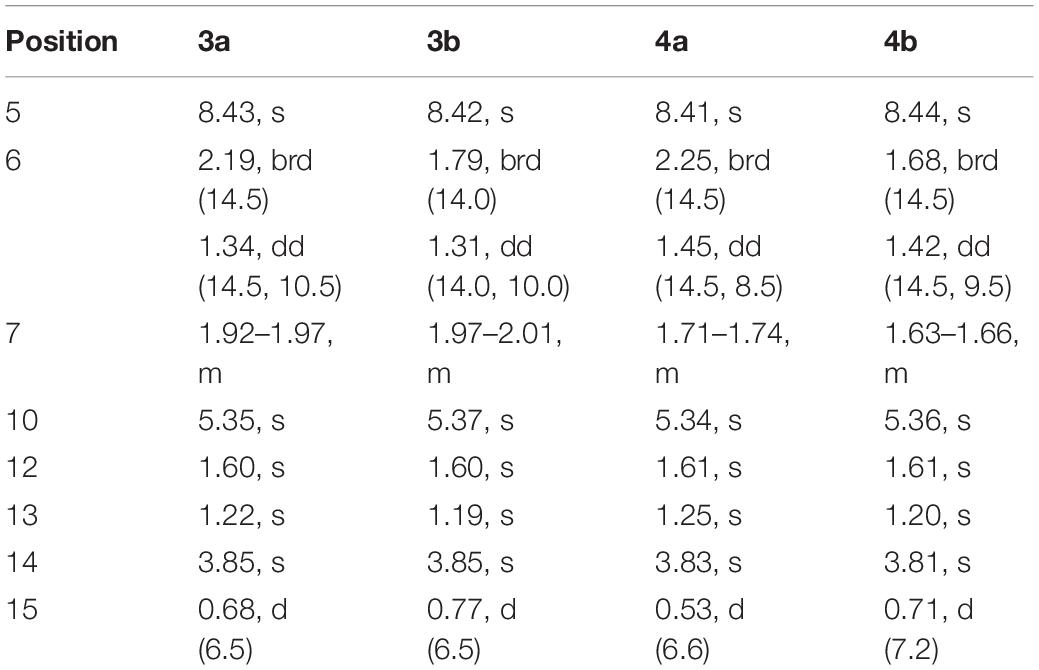
The 1H NMR spectrum of 1a (Table 1) displayed signals for two olefinic protons at δH 6.62 (s) and 5.32 (s), two methoxy groups at δH 3.94 (s) and 3.82 (s), one set of non-equivalent methylene protons at δH 2.11 (dd, J = 14.0, 3.0 Hz) and δH 1.38 (m), one methine proton at δH 1.72–1.75 (m), and two methyl groups at δH 1.06 (s) and 0.58 (d, J = 7.0 Hz). The 13C NMR and DEPT spectra of 1a (Table 2) showed resonances for 15 carbon signals which could be classified as one methylene, three methines (one aliphatic and two olefinic), seven quaternary carbons including two α, β-unsaturated ketones (δC 202.6, 200.7), two methoxy groups (δC 59.8, 59.3), and two methyl groups (δC 20.8, 15.1). These spectroscopic features suggested that 1a belongs to the family of furanones and that ring B in 1a is very similar to sclerotiorumin B (4), isolated from a co-culture of Aspergillus sclerotiorum and Penicillium citrinum (Bao et al., 2017). Analysis of their 1H and 13C NMR spectra indicated that the main differences were present in the ring A. The additional methoxy group (δH 3.94, δC 59.3) in 1a, and the disappearance of one methyl group (δH 1.61, δC 5.1) compared to 4a, suggested that there was a methoxy rather than a methyl group anchored at C-4 in 1a. The additional α,β-unsaturated ketone (δC 202.6) and the upfield shifts of C-2 (δC 49.9) and C-5 (δC 117.5) in 1a, revealed that C-1 in 4a was replaced by a carbonyl. The observed HMBC correlations (Figure 2 and Supplementary Figure S5) from H-5 to C-1, C-2, and C-3, from H-6 to C-1, from H-12 to C-4, and from H-13 to C-1, C-2, and C-3 confirmed the above deduction. Thus, the planar structure of 1a was determined.
Comparison of the 1D and 2D NMR data for 1a and 1b indicated that they had the same planar structure. There were also no differences between the two compounds in the NOESY spectrum of 1a and 1b (Figure 2 and Supplementary Figure S6). The correlations of H-6a with H-13, H-6b with H-15, and the lack of signals between H-13 and H-15 indicated a trans-relationship between these two groups. Although the single bonds in the open chain could rotate freely at room temperature, and the NOESY correlation signals of the open chain could not be used to determine the stereoscopic relationship; these data indicated that 1a and 1b are a pair of epimers at C-8 (Bao et al., 2017). Detailed analysis of their structures revealed that the hemiacetal group was the reason why 1a and 1b were unstable, and resulted in the R/S configurations of C-8.
Sclerotiorumin E (2) was also obtained as a pale-yellow powder with the molecular formula of C15H18O7, the same as 1 (Supplementary Figure S14). Similar to 1, compound 2 also existed as geometric isomers in the same ratio of 1:1.3. Comparison of the 1D (Tables 1, 2 and Supplementary Figures S8, S9) and 2D NMR data of 2 with those of 1 revealed that these compounds have the same planar structures (Supplementary Figures S10–S12). The correlation of H-13 with H-15 in the NOESY spectrum (Figure 2 and Supplementary Figure S13), which was different from that of 1, indicated that these two groups have a syn-relationship. These data indicated that 2a and 2b were also epimers at C-8.
Sclerotiorumin F (3) was isolated as pale-yellow oil and also presented as an inseparable mixture of two geometric isomers (3a and 3b). Its HRESIMS data (m/z 283.1176 [M + H]+) revealed a molecular formula of C14H18O6, requiring six degrees of unsaturation (Supplementary Figure S21). Detailed analysis of the 1D (Table 3 and Supplementary Figures S15, S16) and 2D NMR data showed that 3a and 3b have the same planar structure as 4, indicating that they were stereoisomers of 4 (Supplementary Figures S17–S19). In the NOESY spectrum (Figure 2 and Supplementary Figure S20), the correlation signals were the same between 3a and 3b, but different from those of 4. The correlations of H-6b with H-13 and H-15, and of H-15 with H-13 indicated that the two groups are positioned in the same plane.
It may be impossible to determine the absolute configurations of 1–4 due to the unstable hemiacetal group. Methylation was performed to fix the hydroxyl group at C-8, but failed, possibly because the lactone was disrupted under alkaline conditions.
The known compounds 4–11 were identified on the basis of their spectroscopic data by comparison with those in the literature. These compounds were identified as sclerotiorumin B (4) (Supplementary Figures S22–S28) (Bao et al., 2017), orcinol (5) (Witiak et al., 1967), vanillic acid (6) (Lee et al., 1992), penicillic acid (7) (Suzuki et al., 1971), dihydropenicillic acid (8) (Phainuphong et al., 2017), 4-hydroxy-2-methoxy-5-methylcyclopent-2-enone (9) (Wang et al., 2015), 4-hydroxy-3-methoxy-5-methylcyclopent-2-enone (10) (Wang et al., 2015), and oxaline (11) (Konda et al., 1980). Compound 9 was previously reported as a pure compound (Wang et al., 2015), however, in the present study, 9 existed as a pair of epimers with a trans relationship of H-4/H-5 (9a, J4,5 = 1.0 Hz), while H-4/H-5 of 9b had a cis relationship (J4,5 = 5.5 Hz). Therefore, 9b was identified as a new compound with a relative configuration different from that of 9a.
Antibacterial Activity of 1–11 From D40 in PDW Medium
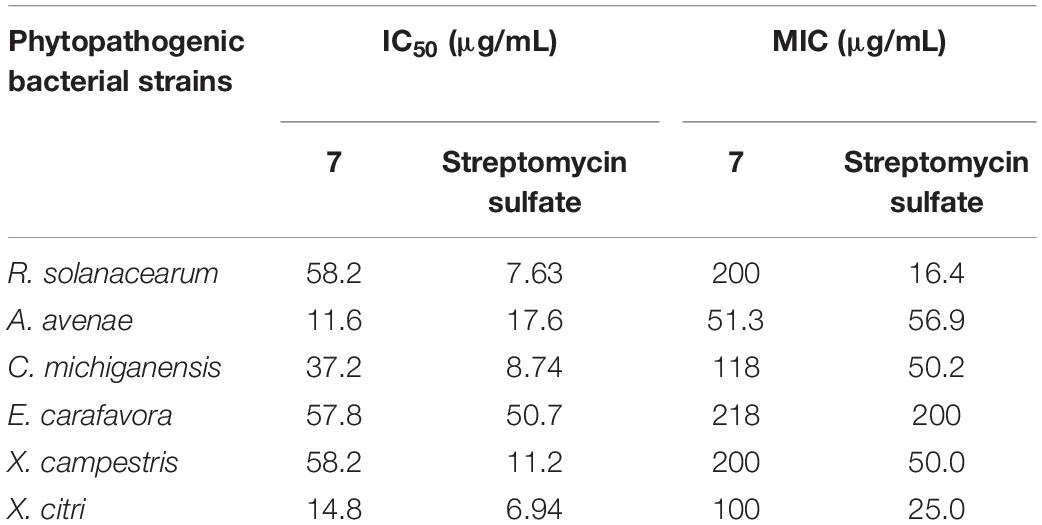
All the isolated compounds were evaluated for their antibacterial activity against R. solanacearum (bacterial wilt of tobacoo), and another five phytopathogenic bacterial strains – Acidovorax avenae (bacterial fruit blotch), Clavibacter michiganensis (bacterial wilt and canker of tomato), Erutima carafavora (tobacco hollow stalk), Xanthomonas campestris (cotton a ngular leaf spot), and Xanthomonas citri (bacterial canker of citrus) – and only penicillic acid (7) showed potent antibacterial activity. Penicillic acid has been reported to have anti-plant pathogenic bacterial activity (Nguyen et al., 2016). In the present study, its antibacterial activity toward other phytopathgens or those from different plants were studied. The IC50 values of 7 against six plant pathogens are shown in Table 4 and Supplementary Figure S29. As indicated, 7 exhibited obvious antibacterial effects against all 6 tested strains. Although the positive control streptomycin sulfate showed a stronger effect than 7 (Supplementary Figure S30), its application has been prohibited in agriculture in China since 2016. Penicillic acid was first reported in an examination of fungal growth in maize and the potential for fungal involvement in pellagra, and was found to be more common on stored cereals, as the best producers are mostly cereal-borne (Frisvad, 2018). Its toxicity to poultry, mice, rats, and rabbits, as well as human beings was soon discovered (Barkai-Golan, 2008). However, hormesis, the phenomena of low-dose stimulation/signaling and high-dose toxicity by the same molecule, is very common for microbial natural products (Schmidt et al., 2019).
Conclusion
In summary, we successfully isolated and identified 11 compounds from the seaweed-derived Aspergillus sp. D40 cultured with PDW medium, including three pairs of new furanone derivatives, sclerotiorumins D–F (1–3), which existed as inseparable mixtures of epimers. Among these compounds, penicillic acid exhibited potent anti-bacterial activity toward different plant pathogens that showing the potential to develop into an anti-bacterial biopesticide.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
C-SZ and D-LZ conceived and designed the experiments. R-HH, WLin, J-YL, and DW performed the experiments. PZ, Y-QL, and X-QW analyzed the data. D-LZ wrote the manuscript. WLi provided the fungal material and performed fungal strain screening. D-LZ and WLi revised the manuscript. All authors reviewed the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (41806194), the Major Agricultural Application Technology Innovation Projects of Shandong Province (SD2019ZZ002), and the Foundation of Key Laboratory of Tropical Medicinal Resource Chemistry of Ministry of Education (RDZH2019001).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.00313/full#supplementary-material
References
Bao, J., Wang, J., Zhang, X. Y., Nong, X. H., and Qi, S. H. (2017). New furanone derivatives and alkaloids from the co-culture of marine-derived fungi Aspergillus sclerotiorum and Penicillium citrinum. Chem. Biodivers. 14:e1600327. doi: 10.1002/cbdv.201600327
Barkai-Golan, R. (2008). “Aspergillus mycotoxins,” in Mycotoxins in Fruits and Vegetables, eds R. Barkai-Golan and N. Paster (Amsterdam: Elsevier), 115–151.
Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R. (2020). Marine natural products. Nat. Prod. Rep. 35, 8–53. doi: 10.1039/c9np00069k
Frisvad, J. C. (2018). A critical review of producers of small lactone mycotoxins: patulin, penicillic acid and moniliformin. World Mycotoxin J. 11, 73–100. doi: 10.3920/WMJ2017.2294
Fujiwara, A., Fujisawa, M., Hamasaki, R., Kawasaki, T., Fujie, M., and Yamada, T. (2011). Biocontrol of Ralstonia solanacearum by treatment with lytic bacteriophages. Appl. Environ. Microbiol. 77, 4155–4162. doi: 10.1128/AEM.02847-10
Genin, S., and Denny, T. P. (2012). Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 50, 67–89. doi: 10.1146/annurev-phyto-081211-173000
Huang, R. H., Gou, J. Y., Zhao, D. L., Wang, D., Liu, J., Ma, G. Y., et al. (2018). Phytotoxicity and anti-phytopathogenic activities of marine-derived fungi and their secondary metabolites. RSC Adv. 8, 37573–37580. doi: 10.1039/c8ra08047j
Konda, Y., Onda, M., Hirano, A., and Ômura, S. (1980). Oxaline and neoxaline. Chem. Pharm. Bull. 28, 2987–2993. doi: 10.1248/cpb.28.2987
Lee, M. W., Morimoto, S., Nonaka, G. I., and Nishioka, I. (1992). Flavan-3-ol gallates and proanthocyanidins from Pithecellobium lobatum. Phytochemistry 31, 2117–2120. doi: 10.1016/0031-9422(92)80375-O
Manfield, J., Genin, S., Magori, S., Citovsky, V., Sriariyanum, M., Ronald, P., et al. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13, 614–629. doi: 10.1111/J.1364-3703.2012.00804.X
Nguyen, H. T., Yu, N. H., Jeon, S. J., Lee, H. W., Bae, C. H., Yeo, J. H., et al. (2016). Antibacterial activities of penicillic acid isolated from Aspergillus persii against various plant pathogenic bacteria. Lett. Appl. Microbiol. 62, 488–493. doi: 10.1111/lam.12578
Oppong-Danquah, E., Budnicka, P., Blümel, M., and Tasdemir, D. (2020). Design of fungal co-cultivation based on comparative metabolomics and bioactivity for discovery of marine fungal agrochemicals. Mar. Drugs 18, 73. doi: 10.3390/md18020073
Phainuphong, P., Rukachaisirikul, V., Tadpetch, K., Sukpondma, Y., Saithong, S., Phongpaichit, S., et al. (2017). γ-Butenolide and furanone derivatives from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. Phytochemistry 137, 165–173. doi: 10.1016/j.phytochem.2017.02.008
Pradhanang, P. M., Ji, P., Momol, M. T., Olson, S. M., Mayfield, J. L., and Jones, J. B. (2005). Application of acibenzolar-S methyl enhances host resistance in tomato against Ralstonia solanacearum. Plant Dis. 89, 989–993. doi: 10.1094/PD-89-0989
Schmidt, R., Ulanova, D., Wick, L. Y., Bode, H. B., and Garbeva, P. (2019). Microbe-driven chemical ecology: past, present and future. ISME J. 13, 2656–2663. doi: 10.1038/s41396-019-0469-x
Sundin, G. W., Castiblanco, L. F., Yuan, X. C., Zeng, Q., and Yang, C. H. (2016). Bacterial disease management: challenges, experience, innovation and future prospects. Mol. Plant Pathol. 17, 1506–1518. doi: 10.1111/mpp.12436
Suzuki, S., Kimura, T., Saito, F., and Ando, K. (1971). Antitumor and antiviral properties of penicillic acid. Agric. Biol. Chem. 35, 287–290. doi: 10.1080/00021369.1971.10859915
Wang, Z., Ma, Z., Wang, L., Tang, C., Hu, Z., Chou, G. X., et al. (2015). Active anti-acetylcholinesterase component of secondary metabolites produced by the endophytic fungi of Huperzia serrata. Electron. J. Biotechnol. 18, 399–405. doi: 10.1016/j.ejbt.2015.08.005
Witiak, D. T., Patel, D. B., and Lin, Y. (1967). Nuclear magnetic resonance. Influence of substituents on the long-range spin-spin coupling constant between benzylic and ring protons in the orcinol series. J. Am. Chem. Soc. 89, 1908–1911. doi: 10.1021/ja00984a027
Zhao, D. L., Han, X. B., Wang, D., Liu, M. H., Gou, J. Y., Peng, Y. L., et al. (2019). Bioactive 3-decalinoyltetramic acids derivatives from a marine-derived strain of the fungus Fusarium equiseti D39. Front. Microbiol. 10:1285. doi: 10.3389/fmicb.2019.01285
Keywords: furanones, penicillic acid, Ralstonia solanacearum, phytopathogenic bacteria, antibacterial activity
Citation: Huang R-H, Lin W, Zhang P, Liu J-Y, Wang D, Li Y-Q, Wang X-Q, Zhang C-S, Li W and Zhao D-L (2020) Anti-phytopathogenic Bacterial Metabolites From the Seaweed-Derived Fungus Aspergillus sp. D40. Front. Mar. Sci. 7:313. doi: 10.3389/fmars.2020.00313
Received: 21 February 2020; Accepted: 16 April 2020;
Published: 15 May 2020.
Edited by:
Susana P. Gaudêncio, New University of Lisbon, PortugalReviewed by:
Nelson Gonçalo Mortágua Gomes, University of Porto, PortugalRamasamy Ramasubburayan, Manonmaniam Sundaranar University, India
Copyright © 2020 Huang, Lin, Zhang, Liu, Wang, Li, Wang, Zhang, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Li, bGl3ZWkwMUBvdWMuZWR1LmNu; Dong-Lin Zhao, emhhb2RvbmdsaW5AY2Fhcy5jbg==
†These authors have contributed equally to this work
 Rui-Huan Huang1†
Rui-Huan Huang1† Peng Zhang
Peng Zhang Cheng-Sheng Zhang
Cheng-Sheng Zhang Dong-Lin Zhao
Dong-Lin Zhao