- 1School of Zoology, George S. Wise Faculty of Life Sciences, Tel Aviv University, Tel Aviv, Israel
- 2The Steinhardt Museum of Natural History, Israel National Center for Biodiversity Studies, Tel Aviv University, Tel Aviv, Israel
Sponges have long been considered as “living hotels” due to the great diversity and abundance of other taxonomic groups often found in association with them. Sponges are the dominant components of benthic communities in the Levant Sea; and especially in the recently discovered mesophotic sponge grounds off the coast of Israel. However, almost no data exist regarding their associated macrofauna. The current study sought to identify the macrofauna associated with massive sponges along the Israeli Mediterranean coast; and to compare the role of sponges, as ecosystem engineers, or “living hotels,” in both the shallow-water and mesophotic habitats. Sixty-four massive sponge specimens, from 10 different species, were collected from shallow and mesophotic habitats by SCUBA diving and Remotely Operated Vehicle, respectively. Sponge volume was estimated, specimens were dissected, and the associated macrofauna were identified. Our results reveal that the sponges supported a diverse assemblage of associated macrofauna. A total of 61 associated taxa were found, including species reported for the first time in Israel. A clear, differentiation existed in the structure of the associated assemblage between the two habitats, which is mainly attributed to four species (two polychaetes, a crustacean, and a brittle star). The trophic composition remained stable across the two habitats. No correlation was found between sponge volume and the associated fauna community parameters. The highest richness of associated fauna was found in the mesophotic habitat, where sponge diversity is also higher. In contrast, a greater endobiont abundance and density were recorded in the shallow habitat, where massive sponges may be a limiting factor due to their lower richness and abundance. Our findings emphasize the importance of sponges as ecosystem engineers, and suggest that sponge diversity may be an important factor that contribute to benthic biodiversity in these regions.
Introduction
Sponges (phylum Porifera) are essential, often dominant components of benthic communities and interact with a wide range of other organisms (Wulff, 2006; Becerro, 2008). They have long been considered as “living hotels” due to the great diversity and abundance of those other taxonomic groups found in association with them, such as polychaetes, crustaceans, echinoderms, and more (Pearse, 1950; Klitgaard, 1995; Ribeiro et al., 2003). The nature of many of these associations remains unclear, but they can represent a variety of ecological interactions (facultative or obligatory) from mutualism to parasitism (Wulff, 2006). The sponges’ bodies, which are composed of a complex network of canals and cavities, provide their associated fauna with a substrate, shelter from predators, food supply, reproduction sites, and nurseries for juveniles (Westinga and Hoetjes, 1981; Çinar et al., 2002; Wulff, 2006; Huang et al., 2008). Various parameters have been thought to affect the composition, diversity, and abundance of sponge-associated fauna. For example, sponge volume is often positively correlated with the diversity of endobionts (Çinar et al., 2002; Özcan and Katağan, 2011), as is the host sponge morphology (Koukouras et al., 1992), geographic location (Klitgaard, 1995), depth (Pearse, 1950), and the surrounding area of the sponges (Westinga and Hoetjes, 1981). In serving as a habitat for a myriad of associated taxa, sponges constitute important reservoirs of marine biodiversity (Cerrano et al., 2006).
Previous studies of sponge-associated fauna have been carried out from the tropics to the poles (Pearse, 1950; Frith, 1976; Peattie and Hoare, 1981; Klitgaard, 1995; Magnino et al., 1999; Abdo, 2007; Schejter et al., 2012; Beepat et al., 2014; Kersken et al., 2014). Although, the sponge-associated fauna of the Mediterranean Sea is considered well-studied (e.g., Rützler, 1976; Koukouras et al., 1985, 1992, 1996; Voultsiadou-Koukoura et al., 1987; Çinar et al., 2002; Gerovasileiou et al., 2016), in the Levantine Sea (Eastern Mediterranean), however, there are only a handful of studies focusing on sponges and their endobiotic community compositions (e.g., Pavloudi et al., 2016; Çinar et al., 2019; Papatheodoulou et al., 2019). Moreover, only one of those studies investigated sponge-associated fauna in the Israeli Mediterranean at a depth of 830 m (Ilan et al., 1994), and examined only three different sponge species (two massive sponges – Sarcotragus foetidus, Ircinia retidermata and a thickly branched sponge – Bubaris sarayi), one specimen of each. The two massive sponges were found to host three species of polychaetes, and one crustacean. No endobionts were recorded inside the branched sponge.
In the last decade, several mesophotic sponge grounds (MSG) were discovered along the Israeli coast of the Mediterranean Sea (Idan et al., 2018). While the Eastern Mediterranean sponge fauna is considered to be impoverished in comparison to the Western Mediterranean (Voultsiadou, 2009), the former are local hotspots for sponge diversity (Idan et al., 2018), and may constitute an oasis of local richness and diversity (Bo et al., 2012). Between the years 2016 and 2020, several expeditions were conducted in order to study the sponge fauna of the Israeli MSGs (Idan et al., 2018; Idan, 2020). These expeditions yielded, among others, dozens of specimens of sponges of several species, which provided us with the opportunity to investigate the macrofauna associated with massive sponges off the Israeli Mediterranean coast. We further compared the role of sponges, as ecosystem engineers, or “living hotels” in both shallow and mesophotic habitats by characterizing the diversity, community composition, and trophic structure of the sponges’ endofauna in both habitats.
Materials and Methods
Sampling
Sponge species (class Demospongiae) with massive growth forms bearing conspicuous cavities and canals that were collected as part of the ongoing sponge-fauna study (Idan et al., 2018; Idan, 2020), were selected for the study of their associated fauna in the surveyed areas.
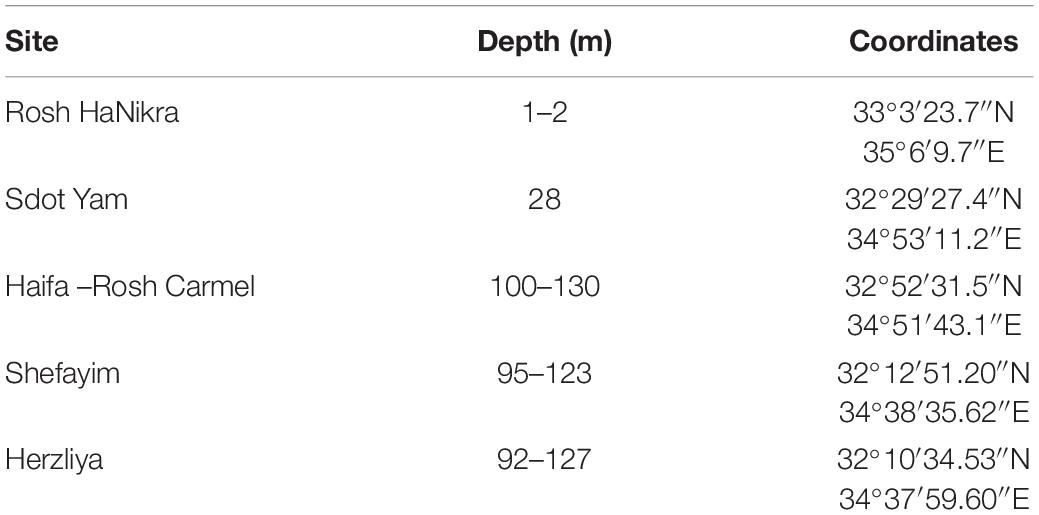
The study sites and their depth range are listed in Table 1. The species included in this study were Ircinia oros, Ircinia variabilis, Sarcotragus foetidus, Sacrotragus spinosulus, Spongia lamella, Spongia nitens, Fasciospongia cavernosa, Thymosiopsis conglomerans, Stryphnus mucronatus, and Agelas oroides. Details of number of specimens are listed in Table 2. The sponges were collected along the Israeli coast of the Mediterranean Sea at five sites (two shallow water, and three from the mesophotic sponge grounds; Table 2). Sampling from shallow water sites (<30 m) was done by SCUBA diving. Sponge specimens were covered with plastic bags to avoid loss of motile macrofauna and then detached using a knife. At the mesophotic sites (90–120 m), samples were collected from the R/V Mediterranean Explorer (EcoOcean) using a Remotely Operated Vehicle (ROV; ECA-Robotics H800), equipped with a five-function-manipulator, a full high-definition camera, and two parallel laser beams for scale. Sponge specimens were brought on board in a collection basket and transferred as quickly as possible to a container with seawater.
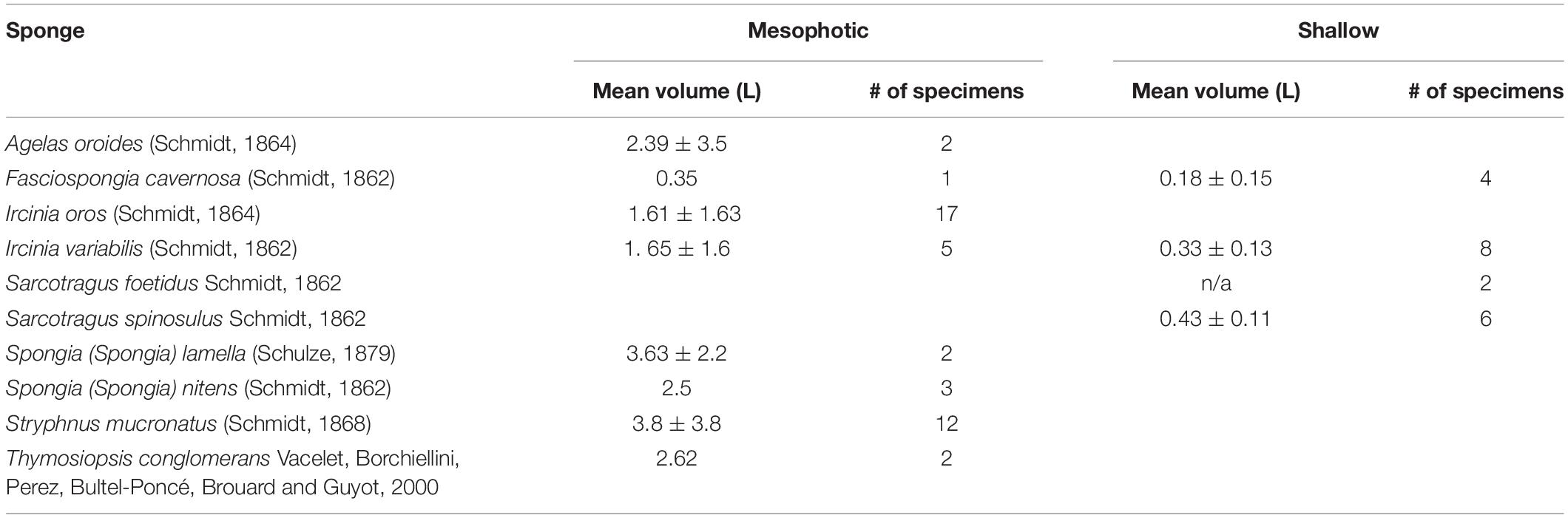
Table 2. Number and mean volume of sponges collected from mesophotic and shallow habitats along the Israeli Mediterranean coast.
Specimens from both the shallow and the mesophotic zone were either processed on board or in the laboratory (following preservation in 85% ethanol). To reduce the possibility that the difference in methods of collection between the two habitats might affect our results, six of the shallow habitat sponges (three I. variabilis and three S. spinosulus) were collected without being covered with a bag and borne along in the water throughout the entire dive before being taken to the surface and preserved. No significant differences were found in richness and abundance of the non-covered specimens of each species, when compared to their covered counterparts (Kruskal Wallis test: χ2 = 0.29, p = 0.58, df = 1). Sponge volume was measured by water displacement. Sponges were then cut into small pieces along their canals and cavities (to minimize endofauna damage), and the associated fauna was removed. The water and the associated macrofauna, which remained in the bags and containers, were washed through a 0.5-mm mesh sieve. Sponge-associated fauna was sorted, counted, and identified to the lowest taxonomic level. Polychaetes were identified mainly according to Fauchald (1977); Fitzhugh (1989), Knight-Jones et al. (1991); Giangrande (1994), Barnich and Fiege (2003); San Martin (2003), Carrera-Parra (2006); Tovar-Hernández and Harris (2010), and Nogueira et al. (2015). The remaining endobionts were identified with the help of experts in the Steinhardt Museum of Natural History or at the Hellenic Centre for Marine Research in Crete, Greece.
Statistical and Faunal Analysis
To compare the endobionts’ communities between the mesophotic and shallow sponges, richness per sponge specimen, species density (richness per L sponge), individuals’ density (abundance per L sponge), and Shannon-Wiener diversity (H’) were calculated. Because statistical analysis was constrained by the un-even availability of sponge specimens of each species, the above-noted parameters were tested only for the six sponge species for which we had more than three replicates: three from the mesophotic habitat (Ircinia oros, Ircinia variabilis, and Stryphnus mucronatus) and three from the shallow habitat (Fasciospongia cavernosa, Ircinia variabilis, and S. spinosulus). The comparison was made using Kruskal Wallis test, first between the sponge species of each habitat, and then when no differences were found, the data were pooled and compared between the two habitats. Spearman’s rank correlation coefficient was used to examine the relationship between sponge volume, number of taxa, and diversity (H’) of the endobionts.
Resemblance analysis of the endobionts communities in the six selected sponge species was performed with nMDS based on Bray-Curtis dissimilarity index. ANOSIM was used in order to compare the difference between the composition of endobionts communities in the selected sponges. SIMPER analysis was used to estimate the contribution of each endobiont species to the dissimilarity. For the trophic characterization of the endobionts fauna, all species were assigned to feeding groups based on Fauchald and Jumars (1979); Dauer (1984), Gambi et al. (1995); Chintiroglou (1996), Damianidis and Chintiroglou (1998), Martin et al. (2000); Antoniadou and Chintiroglou (2005), Leclerc et al. (2013a,b), Antoniadou (2014); Faulwetter et al. (2014), Guerra-García et al. (2014); Manousis and Galinou-Mitsoudi (2014), and Gerovasileiou et al. (2016).
Statistical analyses were performed with R version 3.6.1 (R Core Team, 2019), RStudio (RStudio Team, 2020), and the Vegan package (Oksanen et al., 2013).
Results
Host Sponges
A total of 64 massive sponge specimens belonging to 10 sponge species were analyzed (Table 2 and Figure 1). As the shallow coast of Israel contains a lower richness of massive sponges (Idan et al., 2018), only four of these sponge species, Ircinia variabilis, S. spinosulus, S. foetidus, and Fasciospongia cavernosa, were present and collected from these sites (Achziv, Sdot Yam); while eight species were collected from the mesophotic sites: Ircinia oros, Ircinia variabilis, Spongia lamella, S. nitens, Fasciospongia cavernosa, Thymosiopsis conglomerans, Stryphnus mucronatus, and Agelas oroides. The size of the specimens varied among species, but, specimens from the shallow habitat were smaller (0.08–0.65 L; Table 2) than those from the mesophotic habitat (0.32–11 L; Table 2).
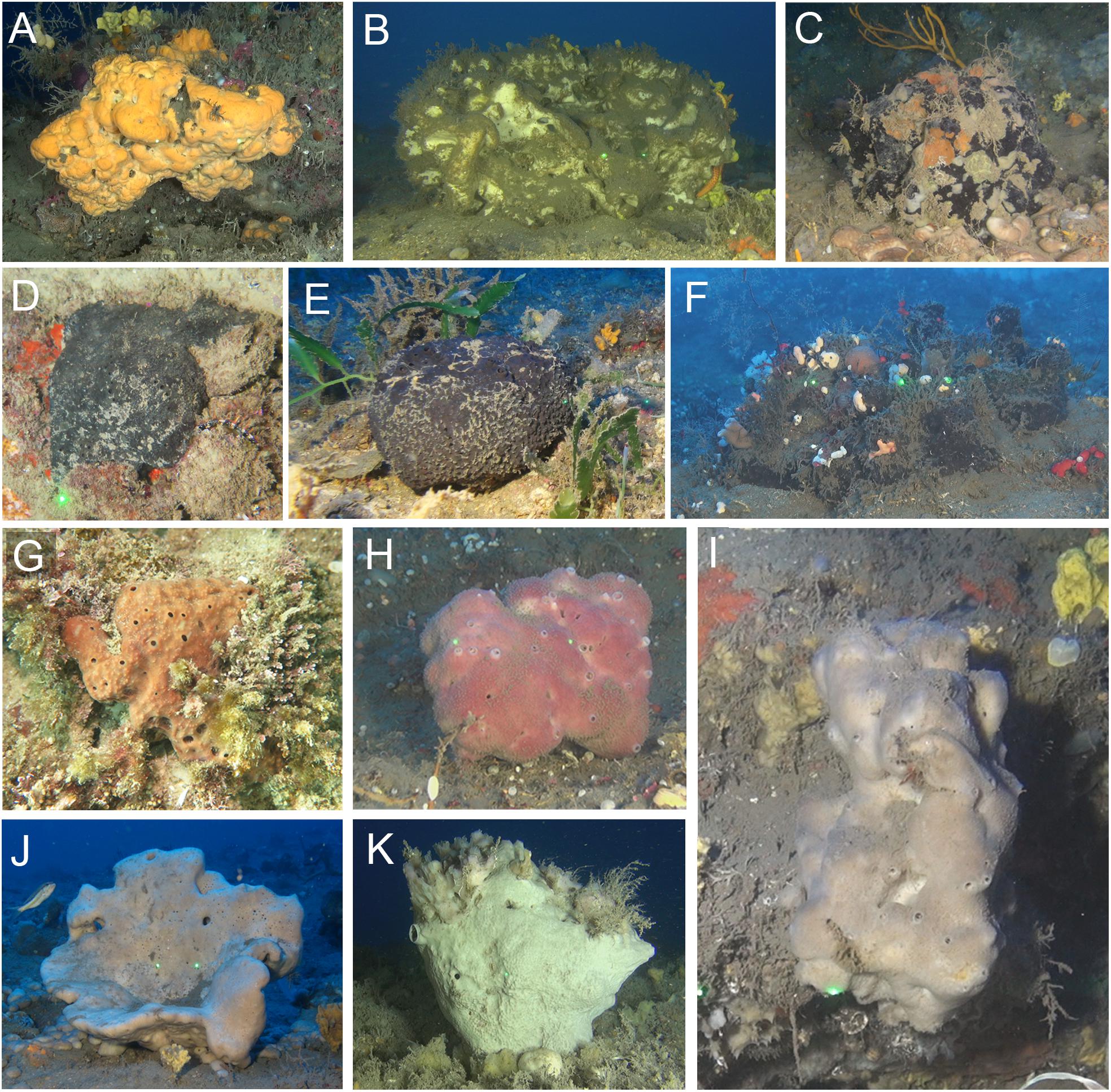
Figure 1. Massive sponge species, collected from the mesophotic sponge grounds and shallow-water habitat along the Mediterranean coast of Israel. (A) Agelas oroides; (B) Thymosiopsis conglomerans; (C) Stryphnus mucronatus; (D) Sarcotragus spinosulus; (E) Sarcotragus foetidus; (F) Fasciospongia cavernosa; (G) Ircinia variabilis from the shallow zone; (H) Ircinia variabilis from the MSG; (I) Spongia nitens; (J) Spongia lamella; (K) Ircinia oros.
Associated Macrofauna
A total of 1099 individuals were identified living within the canals of the 10 sponge species, representing 60 taxa and six major taxonomic groups: Polychaeta, Sipuncula, Crustacea, Insecta, Ophiuroidea, and Mollusca (Table 3). The richest group was Polychaeta, accounting for 57% and 79% of the species in the MSG and shallow zone, respectively, followed by Crustacea (19%) in the MSG and Ophiuroidea (7%) in the shallow zone (Figures 2A,B). Seven taxa of Isopods and Amphipods were not included in this analysis, as it was uncertain as to whether they were endobionts or epibionts. Of particular interest was the presence of chironomid larvae (Insecta) in the canals of S. spinosulus from the shallow zone. To the best of our knowledge, this is the first time an insect has been recorded inhabiting a marine sponge.
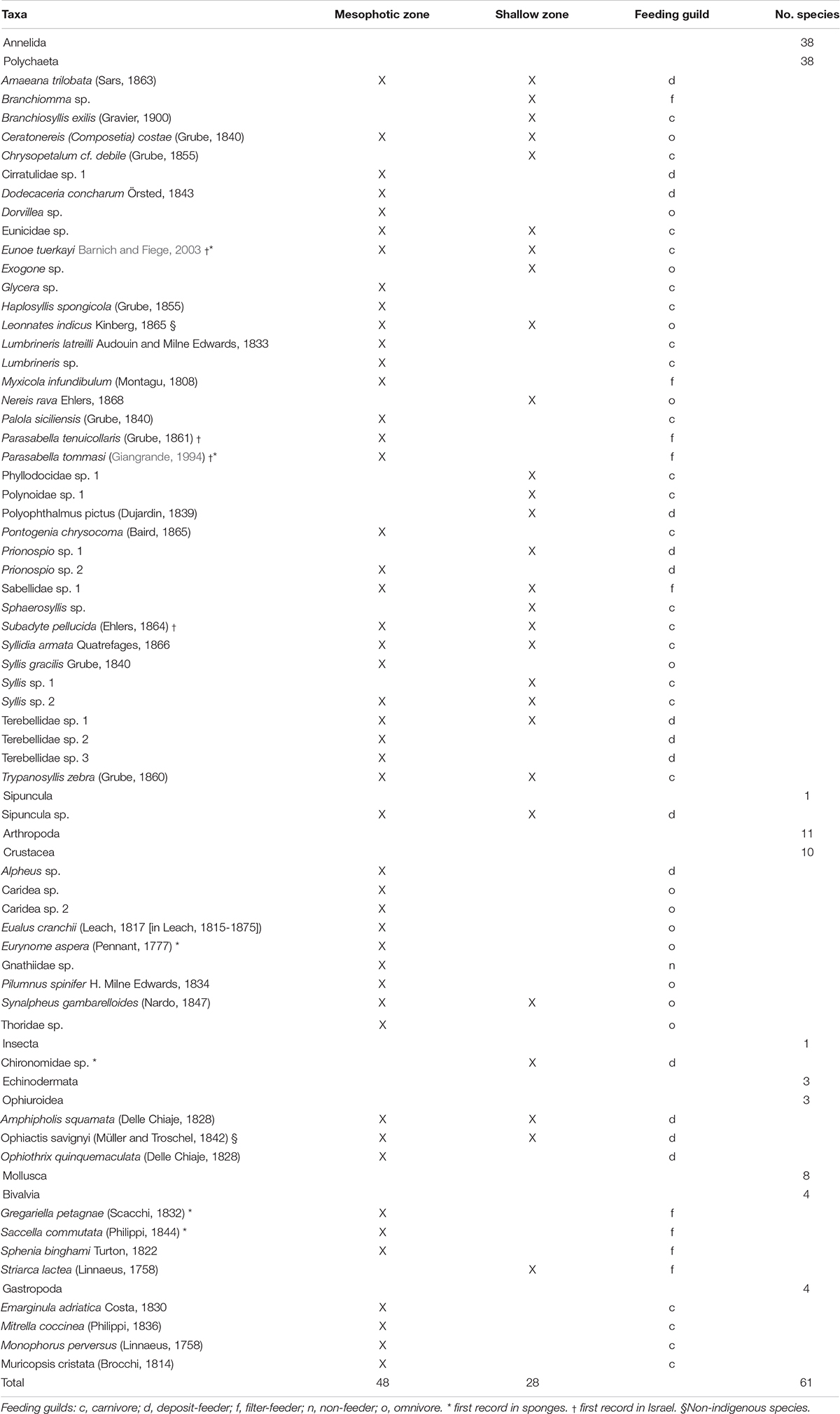
Table 3. Macrofaunal endobionts found in the canals of massive sponges in the shallow and mesophotic habitats of the Israeli Mediterranean coast.
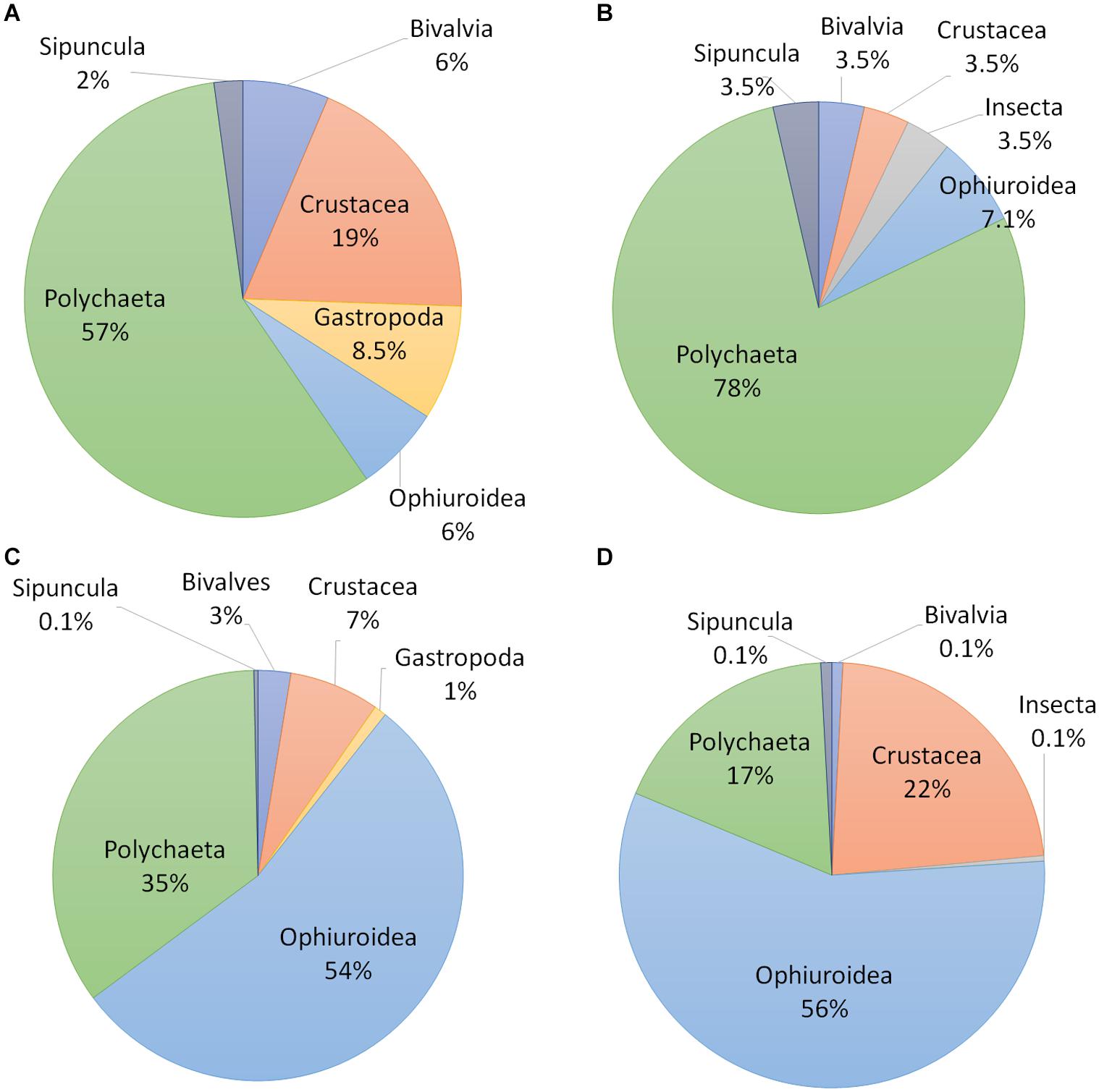
Figure 2. Taxonomic groups inhabiting sponges along the Mediterranean coast of Israel: (A) by the number of taxa in the mesophotic habitat; (B) by the number of taxa in the shallow habitat; (C) by the number of individuals in the mesophotic habitat; (D) by the number of individuals in the shallow habitat.
The sponge S. mucronatus demonstrated the richest cumulative endobiont fauna (29 species), followed by I. variabilis (21 species), while S. foetidus and A. oroides hosted the poorest fauna (four species; Figure 3). The total richness of endobiont species was higher in the MSG (48 species) than in the shallow water (28 species).
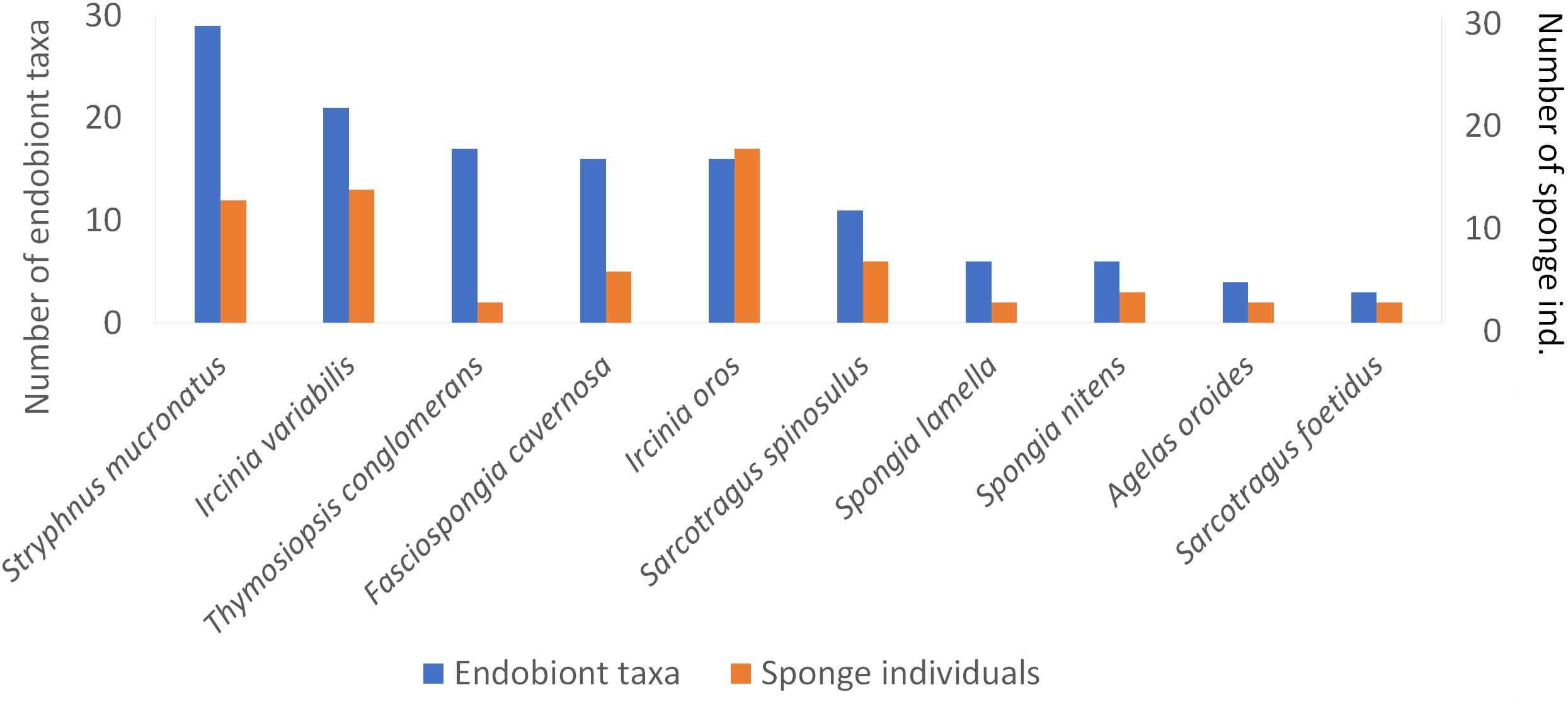
Figure 3. Total richness of sponge endobiont taxa (in orange) found in 10 sponge species (in blue) in the shallow and mesophotic habitats along the Mediterranean coast of Israel.
Ophiuroidea was the most abundant group in both zones (54% in the MSG and 57% in the shallow zone), with more than half of all 609 specimens belonging to three species. Of these three species, Ophiactis savignyi was by far the most abundant in both habitats, with more than 96% and 99% of the ophiurid individuals in the mesophotic habitat and the shallow habitat, respectively. Polychaetes were the second most dominant group, in terms of richness and abundance, in the mesophotic habitat (35% of endobionts), while crustaceans were the second-most dominant group in the shallow habitat (23% of endobionts; Figures 2C,D).
The number of individuals in the sponges ranged from 1 to 210, and from 1 to 135 in the mesophotic and shallow habitats, respectively.
In order to compare the endobiont community parameters between the MSG and the shallow habitat, a statistical analysis was conducted on the six selected sponge species for which we had a sufficient number of replicates (more than three replicates each).
No significant differences were found among the endobionts of each habitat in mean richness, species density individuals’ density, and Shannon-Wiener diversity (Table 4). When data for the three sponge species in each habitat were pooled together, no significant differences were found between the shallow and MSG in mean endobionts richness and Shannon-Wiener diversity index (Table 4 and Figure 4A). This was further supported in the sample-based rarefaction curves (Sanders, 1968; Hurlbert, 1971; Figure 5) which did not differ between the two habitats. However, species density, and individual density were significantly higher in the shallow habitat than in the MSG (richness per L sponge: Kruskal Wallis, χ2 = 22.4, p < 0.001, df = 1; density: Kruskal Wallis, χ2 = 26.4, p < 0.001, df = 1; Table 4 and Figures 4B,C).

Table 4. Results of Kruskal–Wallis tests between community parameters of sponges’ endobionts in the shallow water and mesophotic habitats.
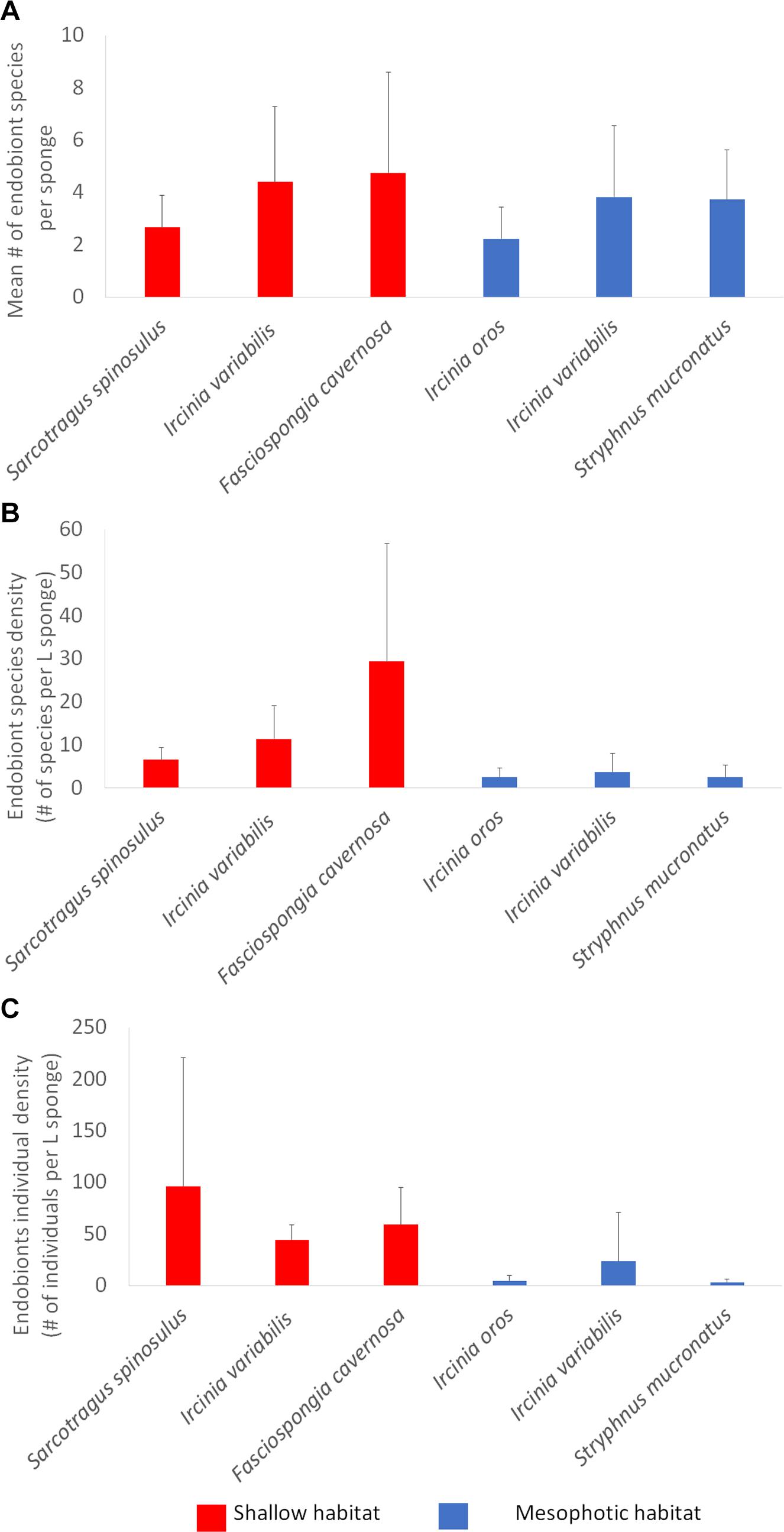
Figure 4. Community parameters of endobionts found in the six sponge species with a sufficient number of specimens (n ≥ 3). (A) Mean number of endobiont taxa per sponge specimen; (B) Species density (mean number of endobiont species per L sponge); (C) Endobiont density (number of individuals per L sponge). Error bars represent standard deviation.
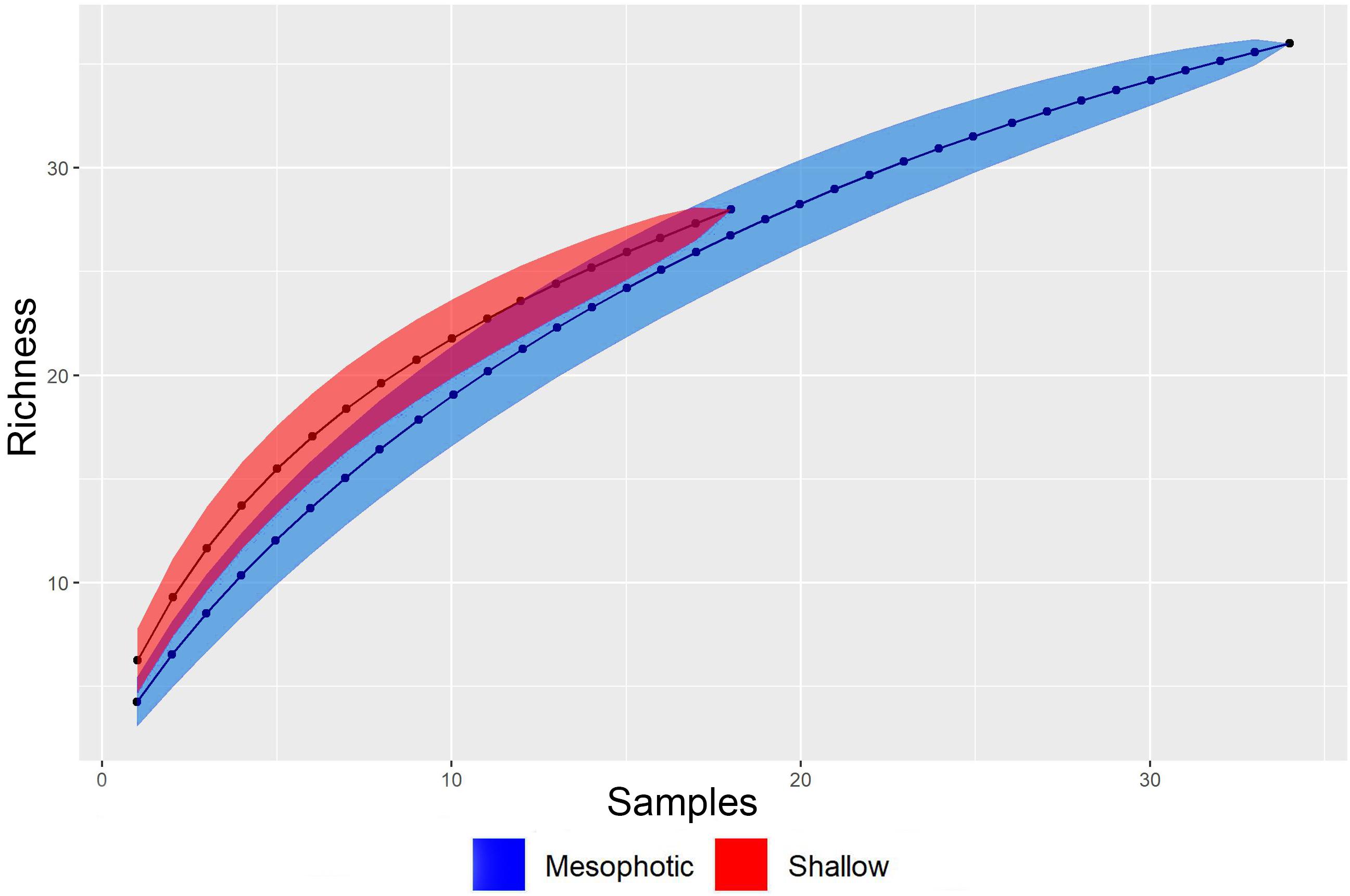
Figure 5. Rarefaction curves from pooled data of sponge-associated taxa found in the six sponge species with more than three replicates in both shallow and mesophotic habitats along the Mediterranean coast of Israel.
Relationships Between Sponge Volume and Endobionts’ Community Parameters
Five sponge species from which more than three specimens were collected (Ircinia variabilis, Ircinia oros, Fasciospongia cavernosa, Stryphnus mucronatus, and Sarcotragus spinosulus) were tested for the relationship between their volume and the endobionts’ community parameters mentioned above (richness, abundance, diversity H’, Supplementary Table 1).
In general, no significant correlation was found between the volume of sponges and community parameters in both habitats. A positive correlation between the sponge volume and the abundance of endobionts was found only in one species (I. variabilis, ρ = 1, p < 0.003).
Similarities Among Sponge-Associated Communities
Only 25% of the total endobiont taxa were found in both habitats. A two-dimensional nMDS ordination revealed a clear differentiation between the community composition of the two habitats (stress 0.18; Figure 6), which was found to be significant in an ANOSIM test (R = 0.43, P = 0.001, permutations = 1000). SIMPER analysis indicated that the associated endobiotic species that contributed most to the dissimilarity between the two habitats were the crustacean Synalpheus gambarelloides and the nereidid polychaete Leonnates indicus which were more common in the shallow habitat; and the brittle star Ophiactis savignyi and the nereidid polychaete Ceratonereis (Composetia) costae which were more common in the mesophotic habitat. These four species contributed about 67% of the dissimilarity between the two habitats. All four could be found across the entire depth range of the study, but were more common in their respective preferred habitats.
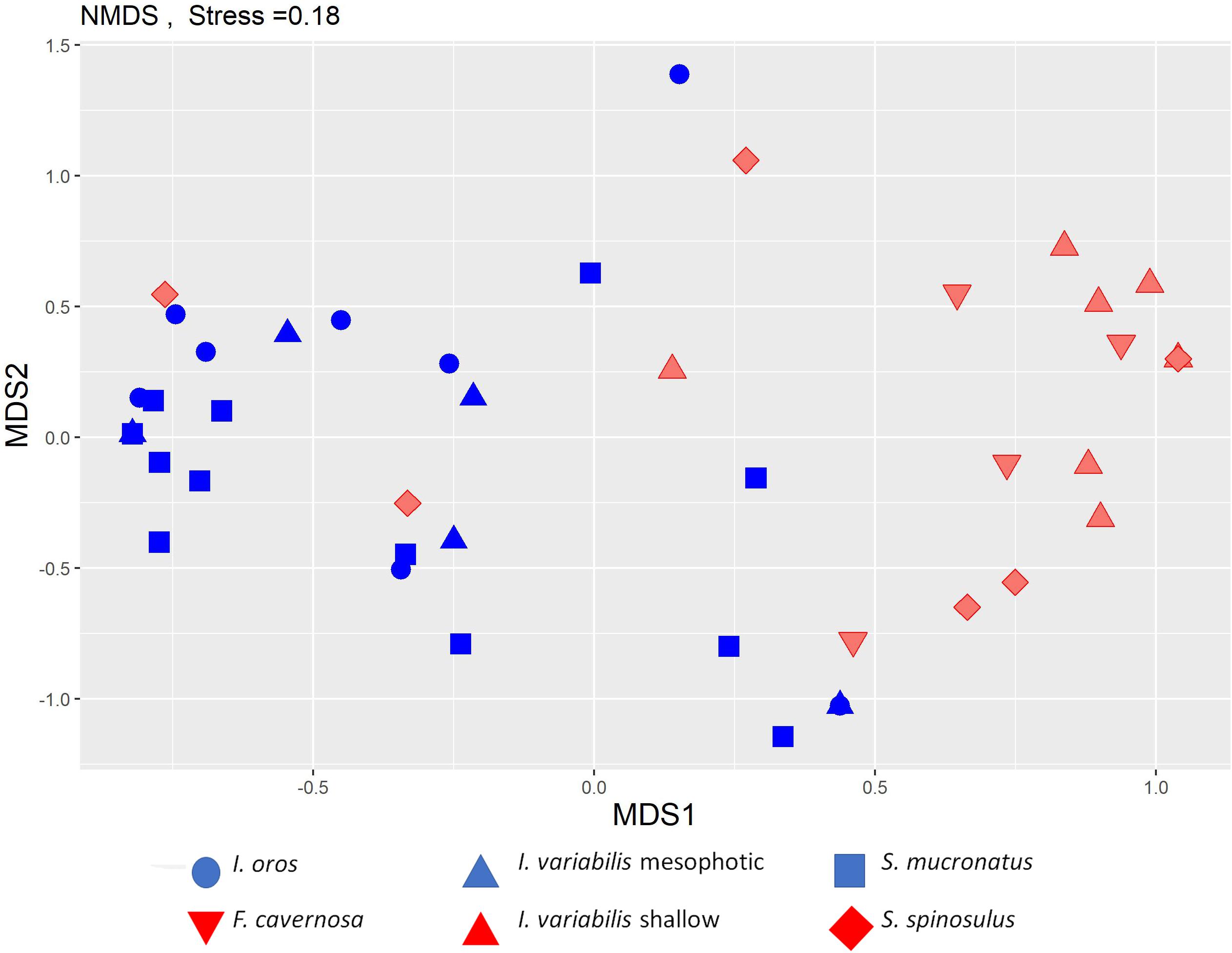
Figure 6. NMDS ordination of the endobiont communities found in the sponges along the Mediterranean coast of Israel. Each symbol represents a community within a sponge. 2D stress: 0.18. Analysis is shown for the six sponge species with sufficient replicates (n > 3).
The trophic composition, of the sponge-associated fauna, in terms of richness, did not change significantly across the two habitats (χ2 = 0.95, df = 3, p > 0.05). Carnivores dominated both habitats, followed by deposit feeders, omnivores, and filter feeders (Figure 7A). No significant difference was found between the two habitats in term of number of individuals as well (χ2 = 7.42, df = 3, p > 0.05). However, in this case deposit feeders dominated the community, followed by omnivores, and both carnivores and filter feeders comprised only a small portion of the community (about 10%; Figure 7B)
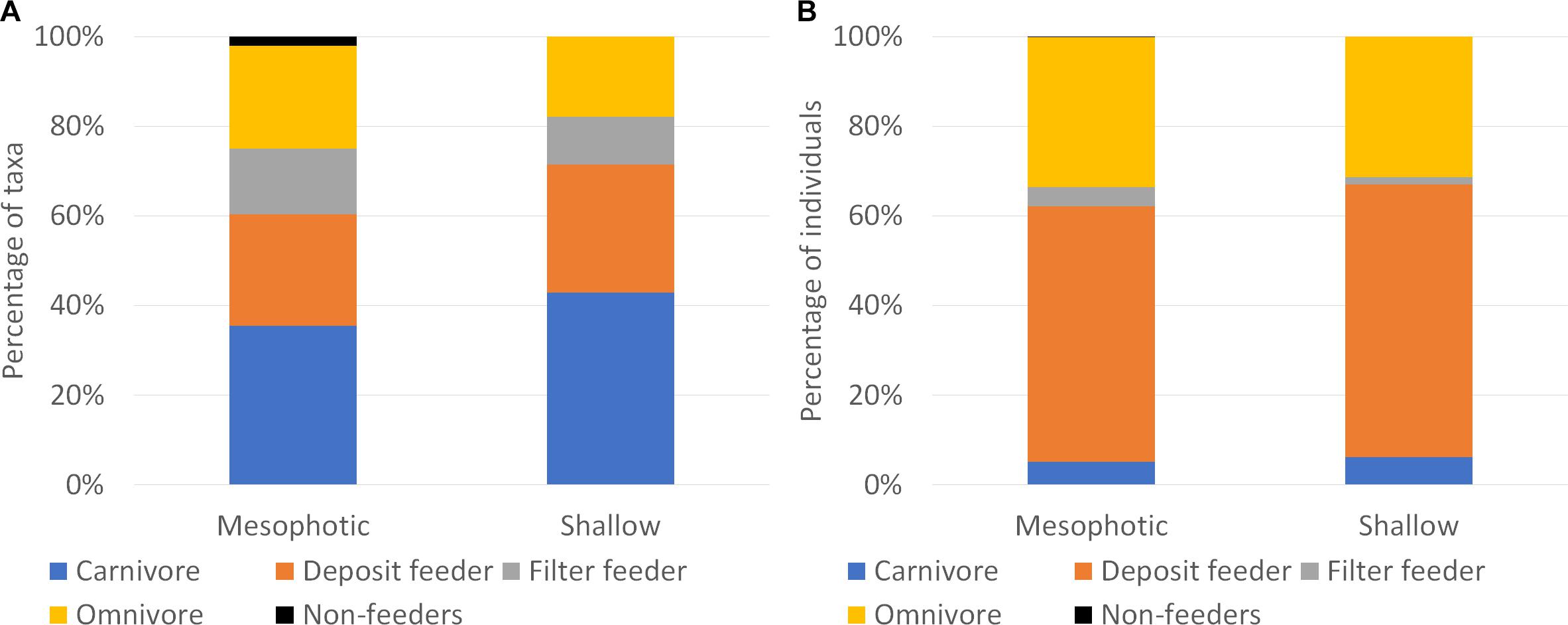
Figure 7. Trophic structure of endobionts found in massive sponges along the Mediterranean coast of Israel, in terms of (A) number of taxa (S) and (B) abundance (N) of sponge-associated fauna across the surveyed habitats.
Discussion
Endobiont Diversity
The results of this study reveal that massive sponges along the coast of Israel support diverse assemblages of invertebrates (61 species), including six species that are reported for the first time as sponge endobionts, and four species of polychaetes that are new records for Israel. However, a comparison between the endobiont communities of the shallow waters and the mesophotic depth revealed only partial overlap, with merely 15 species being found in both habitats. Moreover, the species richness in the shallow-water habitat was much lower than that in the mesophotic habitat (28 vs. 48 spp., respectively). Other studies in the Eastern Mediterranean have documented much higher richness of associated invertebrates within shallow-water sponges, ranging from 86 species around the island of Lesvos to more than 200 along the continental coasts of the northern Aegean Sea (Koukouras et al., 1985, 1996; Voultsiadou-Koukoura et al., 1987; Çinar et al., 2002, 2019; Gerovasileiou et al., 2016). Further east, in Cyprus, the number of species shallow water is lower (44 spp., Pavloudi et al., 2016) but still higher than our findings in the shallow water. The low richness may be in accordance with other studies that documented an NNW-SSE gradual decrease in biodiversity (Voultsiadou, 2009). This decrease is considered to be related to temperature and nutrient concentration differences, which affect both pelagic and benthic communities, with higher temperatures and lower nutrient concentrations associated with lower diversity (Arvanitidis et al., 2002; Coll et al., 2010). The shallow water along the Israeli coast reaches the most extreme summer-temperatures in the Mediterranean (>30°C; Idan et al., 2018; Idan, 2020), thus possibly explaining the lower endobiont richness. In accordance, in the mesophotic habitat, where the temperature is lower and more stable (Idan et al., 2018, 2020), one would expect a higher richness and indeed, the numbers of species found there is higher (Table 3), and similar to that found at the same depth in Cyprus (Papatheodoulou et al., 2019).
While the cumulative endobiont richness was higher in the MSG, our quantitative analysis of endobiont community parameters, conducted for the six sponge species with a sufficient number of replicates (n > 3), showed contrasting results. In this case mean endobiont richness and Shannon-Wiener diversity per sponge specimen did not differ between the two habitats. Furthermore, the mean endobionts’ individual and species densities (# per L sponge) were significantly higher in the shallow habitat than in the MSG. To understand these discrepancies, we should also consider other differences between the two habitats that relate to sponge community parameters:
1. Massive sponge richness in the shallow habitat is lower than in the MSG (4 vs. 16 species, respectively; Idan et al., 2018).
2. Massive sponges in the shallow habitat are scarcer and much smaller than in the MSG (mean 0.7 vs. 2.7 L, respectively). This is probably due to the harsher abiotic conditions in the shallow waters (Burton, 1936; Lévi, 1957; Voultsiadou, 2009; Coll et al., 2010; Idan, 2020; Idan et al., 2020).
We suggest that massive sponge richness, being higher in the MSG, has a positive effect on endobiont richness. Species-specific characteristics of sponges, such as diameter or volume of canals, or chemical composition of the sponge, which were beyond the scope of this study, are known to affect endobiont diversity and richness (Fiore and Jutte, 2010; Ávila and Ortega-Bastida, 2015). Thus, increasing sponge richness may in turn increase endobionts’ richness. This is supported by the difference in the rarefaction curves constructed for the six sponge species with more than three replicates (Figure 3), and for all 10 species collected in the study (Supplementary Figure 1). In the former, no significant difference was found between the habitats, while in the latter the MSG curve rises higher and more quickly. Furthermore, massive sponges, being less common in the shallow habitat, in terms of volume and density (Idan et al., 2018; Idan, 2020), may act as a limiting factor, reducing the total endobiont richness, but also forcing the macroinvertebrates to aggregate within them. This may explain their much higher density along the shallow waters of the Israeli coast, and why the well-documented link between sponge volume and their endobiont richness and abundance (Koukouras et al., 1992, 1996; Cinar and Ergen, 1998; Gherardi et al., 2001) is not expressed there (Supplementary Table 1).
Nonetheless, the true richness in both habitats is probably higher, as evidenced by the rarefaction curves, which did not approach an asymptotic value, and by the fact that sampling constrains resulted in an un-even number of sponge species collected.
Furthermore, the richness in the mesophotic sponge grounds may be still higher, as some of the endobionts may have been lost during the ascent of the ROV to sea level. However, we believe that this loss was minimal, as no significant difference was found in endobiont richness between the sponges covered at the time of collection and those that were borne along uncovered during the SCUBA dive (see “Materials and Methods”).
Endobiont Community Composition
The dominance of Polychaeta in terms of number of taxa is in accordance with other studies (Klitgaard, 1995; Gerovasileiou et al., 2016; Çinar et al., 2019). The numeric dominance of Ophiuroidea in the sponges, however, is uncharacteristic, since in the majority of previous studies, the most important group in terms of abundance, was Crustacea (Koukouras et al., 1996; Gerovasileiou et al., 2016). The result of the community composition analysis in this study showed a clear dissimilarity between the two habitats, which was mainly attributed to four species: Ophiactis savignyi (54%), Ceratonereis (Composetia) costae (14%), Synalpheus gambarelloides (12%), and Leonnates indicus (3%), which together comprised 83% of total number of individuals found in the sponges.
Ophiactis savignyi was found to be the most abundant endobiont in both the shallow and mesophotic habitats. It comprised more than 50% of individuals found in the sponges, and it is also the only endobiont that was found in high and equal abundance in both habitats. It is considered to be a Lessepsian migrant, which in the Mediterranean Sea dwells almost exclusively inside sponges (Çinar et al., 2019), unlike in its native geographic area where it also dwells in other environments such as algae and coral rubble (Mladenov and Emson, 1988).
Ceratonereis costae, the second most abundant organism, and the dominant polychaete in the mesophotic zone, was often reported as an abundant and even dominant species in Mediterranean sponges (Alos et al., 1981; Koukouras et al., 1992; Ilan et al., 1994; Gherardi et al., 2001; Çinar et al., 2002, 2019). In the shallow water C. costae is replaced by L. indicus as the dominant species. Leonnates indicus is another non-indigenous species and a Lessepsian, migrant previously reported from Levantine Sea sponges (Ilan et al., 1994; Çinar et al., 2019). Its dominance in the shallow water suggests that it may competitively exclude C. costae. The lower numbers of L. indicus in the MSG might reflect this tropical and sub-tropical species preference to shallow (usually warmer) habitats (Ben-Eliahu, 1991; Qiu and Qian, 2000; Çinar, 2009; Çinar et al., 2019).
Synalpheus gambarelloides, is a well-known gregarious crustacean whose adults and juveniles inhabit sponges, and feed off their host (Erdman and Blake, 1987; Ilan et al., 1994). In this study, juveniles and females with eggs were often found.
Other notable organisms that resided in the sponges in both the mesophotic and shallow habitats, were four polychaete species, previously unreported from Israel: Parasabella tenuicollaris, Parasabella tommasi, Subadyte pellucida, and Eunoe tuerkayi. All of these are known from other parts of the Mediterranean sea (Giangrande, 1994; Barnich and Fiege, 2003; Tovar-Hernández and Harris, 2010; Gerovasileiou et al., 2016). P. tenuicollaris and S. pellucida are also recognized as sponge inhabitants (Cinar and Ergen, 1998; Gerovasileiou et al., 2016). Three individuals of Chironomidae were found in two sponge specimens from the shallow water. This is the first record of insects inhabiting sponges in the Mediterranean Sea. While, chironomids are known to inhabit demosponges in freshwater habitats (Gugel, 2001; Roque et al., 2004) and are also known from algae and submerged wood panels in marine environments (Olander and Palmén, 1968; Santhakumaran et al., 1984), to the best of our knowledge this is the first record of an insect inhabiting sponges in a marine environment. Of the Mollusca, Sphenia binghami was the most common. It is known to inhabit crevices as well as habitats previously inhabited by the bivalve Hiatella arctica. Both S. binghami, and H. arctica are outwardly similar, and can be easily misidentified. The latter is often reported from sponges in the Levant (Pavloudi et al., 2016; Çinar et al., 2019). Other molluscs found in the sponges included bivalves such as Saccella commutata and Gregariella petagnae, which have never previously been recorded from sponges, and gastropods (Muricopsis cristata, Mitrella coccinea, Emarginula adriatica, and Monophorus perversus). The last three are known, or thought to, feed off sponges (Taylor, 1987; Kantor and Medinsakaya, 1991; Manousis and Galinou-Mitsoudi, 2014).
Trophic Structure of the Shallow and Mesophotic Habitats
The trophic structure of the endobiont assemblages was similar between the two habitats. with carnivores being the most prevalent group in both, followed by deposit feeders. Gerovasileiou et al. (2016) also found carnivores and deposit-feeders to be the most speciose groups in cave-dwelling A. oroides and Aplysina aerophoba. In regards to abundance, the dominance of deposit feeders in both habitats, is attributed to the extremely high numbers of O. savignyi. The second most prevalent group was the omnivores, mainly represented by S. gambarelloides, which was the most dominant species in the shallow water and C. costae in the MSGs. Other studies have usually found carnivorous endobionts to be the most prevalent group, mainly due to the abundance of amphipods (Gerovasileiou et al., 2016; Navarro-Barranco et al., 2016). In the present study, however, amphipods were not prevalent and nor were they considered in the analysis, as they were usually found on only the external parts of the sponge.
Summary
The present study has shown that sponges along the Israeli coast provide a habitat for a diverse community of endobionts. The highest richness of endobionts was found in the MSGs, where sponge diversity is higher; while, in contrast, the highest endobiont density and mean abundance was found in the shallow zone, where massive sponges may be a limiting factor. This emphasizes the importance of sponges as ecosystem engineers and indicating that their role as habitat builders is an important factor affecting benthic biodiversity in this region. This role of sponges may also promote the establishment of non-indigenous species that use them as (primary) habitat, with the sponges essentially acting as stepping stones for their dispersion. While many studies have explained endobiont diversity according to factors such as volume, morphology and surroundings habitat and biota of the sponges, the results of the present study suggest that sponge diversity and density too, constitute variables that should be considered in relation to endobiont diversity.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
LG, SS, and MI contributed to conception and design of the study. LG, SS, and TI performed the field and laboratory work. LG organized the database and wrote the first draft of the manuscript. LG and TI performed the statistical analysis. TI wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Funding
This research was partially funded by grants from the Israel Ministry of Energy and Infrastructures (#217-17-020 to MI) and the Nature and Parks Authority (#01023182 to MI). LG was supported by a VATAT fellowship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge and thank the members of Ilan’s group, who participated and assisted in sponge and endobionts collections during the many dives and expeditions on board the research vessel. We thank the environmental NGO EcoOcean personnel and the many volunteers on board the R/V Mediterranean Explorer and Oded Ezra, Itay Katzman and Asi Gil’adi for the operation of the Remotely Operated Vehicle. The many species that were collected were identified with the help of experts from The Steinhardt Museum of Natural History at Tel Aviv University: Dr. Henk Mienis (Mollusca), Dr. Omri Bronstein (Echinodermata), Dr. Bella Galil, and Ya’arit Levitt-Barmats (Crustacea). Some of the polychaetes were identified with the help of Dr. Christos Arvanitidis from the Hellenic Centre for Marine Research in Crete, Greece.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2020.612779/full#supplementary-material
References
Abdo, D. A. (2007). Endofauna differences between two temperate marine sponges (Demospongiae; Haplosclerida; Chalinidae) from southwest Australia. Mar. Biol. 152, 845–854. doi: 10.1007/s00227-007-0736-7
Alos, C., Campoy, A., and Pereira, F. (1981). Contribución al estudio de los anélidos poliquetos endobiontes de esponjas. Actas do II Simposio Iberico de Estudos do Benthos Marino. 3, 139–157.
Antoniadou, C. (2014). Succession patterns of polychaetes on algal-dominated rocky cliffs (Aegean Sea, Eastern Mediterranean). Mar. Ecol. 35, 281–291. doi: 10.1111/maec.12079
Antoniadou, C., and Chintiroglou, C. (2005). Response of Polychaete populations to distrubance: an evaluation of methods in hard substratum. Fresenius Environ. Bull. 14, 1066–1073.
Arvanitidis, C., Bellan, G., Drakopoulos, P., Valavanis, V., Dounas, C., Koukouras, A., et al. (2002). Seascape biodiversity patterns along the Mediterranean and the Black Sea: lessons from the biogeography of benthic polychaetes. Mar. Ecol. Prog. Ser. 244, 139–152. doi: 10.3354/meps244139
Ávila, E., and Ortega-Bastida, A. L. (2015). Influence of habitat and host morphology on macrofaunal assemblages associated with the sponge Halichondria melanadocia in an estuarine system of the southern Gulf of Mexico. Mar. Ecol. 36, 1345–1353. doi: 10.1111/maec.12233
Barnich, R., and Fiege, D. (2003). The Aphroditoidea (Annelida: Polychaeta) of the Mediterranean Sea. Abhandlungen der Senckenbergischen Naturforschenden Gesellschaft 559, 1–167.
Becerro, M. A. (2008). Quantitative trends in sponge ecology research. Mar. Ecol. 29, 167–177. doi: 10.1111/j.1439-0485.2008.00234.x
Beepat, S. S., Appadoo, C., Marie, D. E. P., and Paula, J. P. M. (2014). Macrofauna associated with the sponge Neopetrosia exigua (Kirkpatrick, 1900) from Mauritius. West. Indian Ocean J. Mar. Sci. 13, 133–142.
Ben-Eliahu, M. N. (1991). Nereididae of the Suez Canal—potential lessepsian migrants? Bull. Mar. Sci. 48, 318–329.
Bo, M., Bertolino, M., Bavestrello, G., Canese, S., Giusti, M., Angiolillo, M., et al. (2012). Role of deep sponge grounds in the Mediterranean Sea: a case study in southern Italy. Hydrobiologia 687, 163–177. doi: 10.1007/s10750-011-0964-1
Burton, M. (1936). The fishery grounds near Alexandria. IX. Sponges. Notes Mem. Fish Res. Cairo 17, 1–28.
Carrera-Parra, L. F. (2006). Revision of Lumbrineris de Blainville, 1828 (Polychaeta: Lumbrineridae). Zootaxa 1336, 1–64.
Cerrano, C., Calcinai, B., Pinca, S., and Bavestrello, G. (2006). “Reef sponges as hosts of biodiversity: cases from North Sulawesi,” in Proceedings of the 10th International. Coral Reef Symposym. Okinawa 2006, Okinawa.
Chintiroglou, C. C. (1996). Feeding guilds of polychaetes associated with Cladocora Caespitosa (L.) (anthozoa, cnidaria) in the North Aegean Sea. Isr. J. Ecol. Evol. 42, 261–274. doi: 10.1080/00212210.1996.10688847
Çinar, M. E. (2009). Alien polychaete species (Annelida: Polychaeta) on the southern coast of Turkey (Levantine Sea, eastern Mediterranean), with 13 new records for the Mediterranean Sea. J. Nat. Hist. 43, 2283–2328. doi: 10.1080/00222930903094654
Çinar, M. E., Bakir, K., Doğan, A., Açik, S., Kurt, G., Katağan, T., et al. (2019). Macro-benthic invertebrates associated with the black sponge Sarcotragus foetidus (Porifera) in the Levantine and Aegean Seas, with special emphasis on alien species. Estuar. Coast. Shelf Sci. 227:106306. doi: 10.1016/j.ecss.2019.106306
Cinar, M. E., and Ergen, Z. (1998). Polychaetes associated with the sponge sarcotragus muscarum schmidt, 1864 from the turkish aegean coast. Ophelia 48, 167–183. doi: 10.1080/00785236.1998.10426964
Çinar, M. E., Katağan, T., Ergen, Z., and Sezgin, M. (2002). Zoobenthos-inhabiting Sarcotragus muscarum (Porifera: Demospongiae) from the Aegean Sea. Hydrobiologia 482, 107–117. doi: 10.1023/A:1021260314414
Coll, M., Piroddi, C., Steenbeek, J., Kaschner, K., Lasram, F. B. R., Aguzzi, J., et al. (2010). The biodiversity of the Mediterranean Sea: estimates, patterns, and threats. PLoS One 5:e11842. doi: 10.1371/journal.pone.0011842
Damianidis, P., and Chintiroglou, C. C. (1998). Feeding guilds of polychaetes associated with Mytilus Galloprovincialis (Lam.) Assemblage in the North Aegean Sea. Rapp. Comm. Int. Mer. Médit. 35, 416–417.
Dauer, D. M. (1984). The use of polychaete feeding guilds as biological variables. Mar. Pollut. Bull. 15, 301–305. doi: 10.1016/0025-326X(84)90199-1
Erdman, R. B., and Blake, N. J. (1987). Population dynamics of the sponge-Dwelling Alpheid Synalpheus longicarpus, with Observations on S. brooksi and S. pectiniger, in Shallow-Water assemblages of the Eastern Gulf of Mexico. J. Crustac. Biol. 7, 328–337. doi: 10.2307/1548613
Fauchald, K. (1977). The Polychaete Worms. Definitions and keys to the Orders, Families and Genera. Nat. Hist. Museum Los Angeles County. Sci. Ser. 28:188. doi: 10.3354/dao063107
Fauchald, K., and Jumars, P. A. (1979). The diet of worms: a study of polychaete feeding guilds. Oceanogr. Mar. Biol. Annu. Rev. 17, 193–284. doi: 10.12691/marine-1-1-6
Faulwetter, S., Markantonatou, V., Pavloudi, C., Papageorgiou, N., Keklikoglou, K., Chatzinikolaou, E., et al. (2014). Polytraits: a database on biological traits of marine polychaetes. Biodivers. Data J. 2:e1024. doi: 10.3897/BDJ.2.e1024
Fiore, C. L., and Jutte, P. C. (2010). Characterization of macrofaunal assemblages associated with sponges and tunicates collected off the southeastern United States. Invertebr. Biol. 129, 105–120. doi: 10.1111/j.1744-7410.2010.00184.x
Fitzhugh, K. (1989). A systematic revision of the sabellidae-caobangiidae- sabellongidae complex (Annelida: Polychaeta). Bull. Am. Museum Nat. Hist. 192, 1–104.
Frith, D. W. (1976). Animals associated with sponges at North Hayling, Hampshire. Zool. J. Linn. Soc. 58, 353–362. doi: 10.1111/j.1096-3642.1976.tb01005.x
Gambi, M., Giangrande, A., Martinelli, M., and Chessa, L. (1995). Polychaetes of a Posidonia oceanica bed off Sardinia (Italy): spatio-temporal distribution and feeding guild analysis. Sci. Mar. 59, 129–141.
Gerovasileiou, V., Chintiroglou, C. C., Konstantinou, D., and Voultsiadou, E. (2016). Sponges as “living hotels” in Mediterranean marine caves. Sci. Mar. 80, 279–289. doi: 10.3989/scimar.04403.14B
Gherardi, M., Giangrande, A., and Corriero, G. (2001). Epibiontic and endobiontic polychaetes of Geodia cydonium (Porifera, Demospongiae) from the Mediterranean Sea. Hydrobiologia 443, 87–101. doi: 10.1023/A:1017500321330
Giangrande, A. (1994). The genus demonax (Polychaeta, sabellidae) in the mediterranean sea, with description of d. tommasi n.sp. Bolletino di Zool. 61, 229–233. doi: 10.1080/11250009409355890
Guerra-García, J. M., Tierno de Figueroa, J. M., Navarro-Barranco, C., Ros, M., Sánchez-Moyano, J. E., and Moreira, J. (2014). Dietary analysis of the marine Amphipoda (Crustacea: Peracarida) from the Iberian Peninsula. J. Sea Res. 85, 508–517. doi: 10.1016/j.seares.2013.08.006
Gugel, J. (2001). Life cycles and ecological interactions of freshwater sponges (Porifera, Spongillidae) in the River Rhine in Germany. Limnologica 31, 185–198. doi: 10.1016/S0075-9511(01)80020-7
Huang, J. P., McClintock, J. B., Amsler, C. D., and Huang, Y. M. (2008). Mesofauna associated with the marine sponge Amphimedon viridis. Do its physical or chemical attributes provide a prospective refuge from fish predation? J. Exp. Mar. Bio. Ecol. 362, 95–100. doi: 10.1016/j.jembe.2008.06.007
Hurlbert, S. H. (1971). The nonconcept of species diversity: a critique and alternative parameters. Ecology 52, 577–586.
Idan, T. (2020). Mesophotic Sponge Grounds in the Levant basin: Community Composition and Dynamics. Ph.D. thesis, Tel Aviv University, Tel Aviv.
Idan, T., Goren, L., Shefer, S., and Ilan, M. (2020). Sponges in a changing climate: survival of Agelas oroides in a warming Mediterranean Sea. Front. Mar. Sci. 7:1064.
Idan, T., Shefer, S., Feldstein, T., Huchon, D., and Ilan, M. (2018). Shedding light on an East-Mediterranean mesophotic sponge ground community and the regional sponge fauna. Mediterr. Mar. Sci. 19, 84–106. doi: 10.12681/mms.13853
Ilan, M., Ben-Eliahu, M. N., and Galil, B. S. (1994). Three deep water sponges from the eastern mediterranean and their associated fauna. Ophelia 39, 45–54. doi: 10.1080/00785326.1994.10429901
Kantor, Y. I., and Medinsakaya, A. I. (1991). Morphology and feeding of Mitrella burchardi (Gastropoda: Columbellidae). Asian Mar. Biol. 8, 25–33.
Kersken, D., Göcke, C., Brandt, A., Lejzerowicz, F., Schwabe, E., Anna Seefeldt, M., et al. (2014). The infauna of three widely distributed sponge species (Hexactinellida and Demospongiae) from the deep Ekström Shelf in the Weddell Sea, Antarctica. Deep. Res. Part II Top. Stud. Oceanogr. 108, 101–112. doi: 10.1016/j.dsr2.2014.06.005
Klitgaard, A. B. (1995). The fauna associated with outer shelf and upper slope sponges (porifera, demospongiae) at the faroe islands, northeastern Atlantic. Sarsia 80, 1–22. doi: 10.1080/00364827.1995.10413574
Knight-Jones, P., Knight-Jones, W., and Ergen, Z. (1991). Sabelliform polychaetes, mostly from turkey’s aegean coast. J. Nat. Hist. 25, 837–858. doi: 10.1080/00222939100770561
Koukouras, A., Russo, A., Voultsiadou-Koukoura, E., Arvanitidis, C., and Stefanidou, D. (1996). Macrofauna associated with sponge species of different morphology. Mar. Ecol. 17, 569–582. doi: 10.1111/j.1439-0485.1996.tb00418.x
Koukouras, A., Russo, A., Voultsiadou-Koukoura, E., Dounas, C., and Chintiroglou, C. (1992). Relationship of sponge macrofauna with the morphology of their hosts in the North Aegean Sea. Int. Rev. der gesamten Hydrobiol. Hydrogr. 77, 609–619. doi: 10.1002/iroh.19920770406
Koukouras, A., Voultsiadou-Koukoura, E., Chintiroglou, H., and Dounas, C. (1985). Benthic bionomy of the North Aegean sea. III: a comparison of the macrobenthic animal assemblages associated with seven sponge species. Cah. Biol. Mar. 26, 300–319.
Leclerc, J. C., Riera, P., Leroux, C., Lévêque, L., and Davoult, D. (2013a). Temporal variation in organic matter supply in kelp forests: linking structure to trophic functioning. Mar. Ecol. Prog. Ser. 494, 87–105. doi: 10.3354/meps10564
Leclerc, J. C., Riera, P., Leroux, C., Lévêque, L., Laurans, M., Schaal, G., et al. (2013b). Trophic significance of kelps in kelp communities in Brittany (France) inferred from isotopic comparisons. Mar. Biol. 160, 3249–3258. doi: 10.1007/s00227-013-2306-5
Magnino, G., Sara, A., Lancioni, T., and Gaino, E. (1999). Endobionts of the Coral Reef Sponge Theonella swinhoei (Porifera, Demospongiae). Invertebr. Biol. 118:213. doi: 10.2307/3226993
Manousis, T., and Galinou-Mitsoudi, S. (2014). New gastropod records for the Eastern mediterranean sea and one new alien (Emarginula decorata deshayes, 1863) for the mediterranean sea from NW Aegean sea, Greece. J. Biol. Res. 21:20. doi: 10.1186/2241-5793-21-20
Martin, D., Pinedo, S., and Sardá, R. (2000). Distribution patterns and trophic structure of soft-bottom polychaete assemblages in a North-Western Mediterranean shallow-water bay. Ophelia 53, 1–17. doi: 10.1080/00785326.2000.10409431
Mladenov, P., and Emson, R. (1988). Density, size structure and reproductive characteristics of fissiparous brittle stars in algae and sponges: evidence for interpopulational variation in levels of sexual and asexual reproduction. Mar. Ecol. Prog. Ser. 42, 181–194. doi: 10.3354/meps042181
Navarro-Barranco, C., Guerra-García, J. M., Sánchez-Tocino, L., Florido, M., and García-Gómez, J. C. (2016). Amphipod community associated with invertebrate hosts in a Mediterranean marine cave. Mar. Biodivers. 46, 105–112. doi: 10.1007/s12526-015-0328-6
Nogueira, J. M. D. M., Carrerette, O., and Hutchings, P. (2015). Review of Amaeana Hartman, 1959 (Annelida, Terebelliformia, Polycirridae), with descriptions of seven new species. Zootaxa 3994, 1–52. doi: 10.11646/zootaxa.3994.1.1
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., and O’Hara, R. B. (2013). Package Vegan. R Package Version 2.5–7. Available online at: https://CRAN.R-project.org/package=vegan
Olander, R., and Palmén, E. (1968). Taxonomy, ecology and behaviour of the northern Baltic Clunio marinus Halid. (Dipt., Chironomidae). Ann. Zool. Fennici 5, 97–110.
Özcan, T., and Katağan, T. (2011). Decapod Crustaceans associated with the sponge Sarcotragus muscarum Schmidt, 1864 (Porifera: Demospongiae) from the Levantine coasts of Turkey. Iran. J. Fish. Sci. 10, 286–293.
Papatheodoulou, M., Jimenez, C., Petrou, A., and Thasitis, I. (2019). Endobiotic communities of Marine Sponges in Cyprus (Levantine Sea). Heliyon 5:e01392. doi: 10.1016/j.heliyon.2019.e01392
Pavloudi, C., Christodoulou, M., and Mavidis, M. (2016). Macrofaunal assemblages associated with the sponge Sarcotragus foetidus Schmidt, 1862 (Porifera: Demospongiae) at the coasts of Cyprus and Greece. Biodivers. Data J. 30:e8210. doi: 10.3897/BDJ.4.e8210
Pearse, A. A. S. (1950). Notes on the Inhabitants of Certain Sponges at Bimini. Hoboken, NJ: Wiley, 149–151.
Peattie, M. E., and Hoare, R. (1981). The sublittoral ecology of the Menai Strait. II. The sponge Halichondria panicea (Pallas) and its associated fauna. Estuar. Coast. Shelf Sci. 13, 621–635. doi: 10.1016/S0302-3524(81)80044-8
Qiu, J., and Qian, P. (2000). Revision of the genus Leonnates Kinberg, 1866 (Polychaeta: Nereididae), with descriptions and comments on other species described in Leonnates. Proc. Biol. Soc. Washingt. 113, 1111–1146.
R Core Team (2019). R: A Language and Environment for Statistical Computing. Industrial and Commercial Training. Vienna: R Core Team. doi: 10.1108/eb003648
Ribeiro, S. M., Omena, E. P., and Muricy, G. (2003). Macrofauna associated to Mycale microsigmatosa (Porifera, Demospongiae) in Rio de Janeiro State, SE Brazil. Estuar. Coast. Shelf Sci. 57, 951–959. doi: 10.1016/S0272-7714(02)00425-0
Roque, F., de, O., Trivinho-Strixino, S., Couceiro, S. R. M., Hamada, N., Volkmer-Ribeiro, C., et al. (2004). Species of Oukuriella Epler (Diptera, Chironomidae) inside freshwater sponges in Brazil. Rev. Bras. Entomol. 48, 291–292. doi: 10.1590/s0085-56262004000200020
RStudio Team (2020). RStudio: Integrated Development for R. Boston, MA: Rstudio Team. doi: 10.1145/3132847.3132886
San Martin, G. (2003). Fauna Iberica Annelida Polychaeta II: Syllidae, Vol. 21. Madrid: Museo National de Ciencias Natureles.
Sanders, H. L. (1968). Marine benthic diversity: a comparative study. Am. Nat. 102, 243–282. doi: 10.1086/282541
Santhakumaran, L. N., Sneli, J. A., and Sundnes, G. (1984). The larvae of halocladius (halocladius) variabilis (diptera: Chironomidae) from the fouling assemblages on wooden test panels submerged in Trondheims-Fjorden, Norway. Sarsia 69, 155–158. doi: 10.1080/00364827.1984.10420601
Schejter, L., Chiesa, I. L., Doti, B. L., and Bremec, C. (2012). Mycale (aegogropila) Magellanica (Porifera: Demospongiae) en el Atlántico suroeste: Fauna endobiótica y nuevos datos de su distribución. Sci. Mar. 76, 753–761. doi: 10.3989/scimar.03490.21A
Taylor, J. D. (1987). Feeding ecology of some common intertidal neogastropods at Djerba, Tunisia. Vie Milieu 37, 13–20.
Tovar-Hernández, M. A., and Harris, L. H. (2010). Parasabella Bush, 1905, replacement name for the polychaete genus Demonax Kinberg, 1867 (Annelida, Polychaeta, Sabellidae). Zookeys 60, 13–19. doi: 10.3897/zookeys.60.547
Voultsiadou, E. (2009). Reevaluating sponge diversity and distribution in the Mediterranean Sea. Hydrobiologia 628, 1–12. doi: 10.1007/s10750-009-9725-9
Voultsiadou-Koukoura, H. E., Koukouras, A., and Eleftheriou, A. (1987). Macrofauna associated with the sponge Verongia aerophoba in the north Aegean Sea. Estuar. Coast. Shelf Sci. 24, 265–278. doi: 10.1016/0272-7714(87)90069-2
Westinga, E., and Hoetjes, P. C. (1981). The intrasponge fauna of Spheciospongia vesparia (Porifera, Demospongiae) at Curaçao and bonaire. Mar. Biol. 62, 139–150. doi: 10.1007/BF00388176
Keywords: sponge grounds, Polychaeta, Ophiuroidea, biodiversity, Crustacea, community ecology, feeding guild
Citation: Goren L, Idan T, Shefer S and Ilan M (2021) Macrofauna Inhabiting Massive Demosponges From Shallow and Mesophotic Habitats Along the Israeli Mediterranean Coast. Front. Mar. Sci. 7:612779. doi: 10.3389/fmars.2020.612779
Received: 30 September 2020; Accepted: 24 December 2020;
Published: 18 January 2021.
Edited by:
Joana R. Xavier, University of Porto, PortugalReviewed by:
Carlo Cerrano, Marche Polytechnic University, ItalyVasilis Gerovasileiou, Hellenic Centre for Marine Research (HCMR), Greece
Copyright © 2021 Goren, Idan, Shefer and Ilan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liron Goren, Z29yZW4ubGlyb25AZ21haWwuY29t; Sigal Shefer, c2hlZkB0YXVleC50YXUuYWMuaWw=
 Liron Goren
Liron Goren Tal Idan
Tal Idan Sigal Shefer
Sigal Shefer Micha Ilan
Micha Ilan