- 1Marine Resources Unit, Department of Environment, Grand Cayman, Cayman Islands
- 2Centre for Ecology and Conservation (CEC), University of Exeter, Penryn, United Kingdom
Given differing trajectories of sea turtle populations worldwide, there is a need to assess and report long-term population trends and determine which conservation strategies are effective. In this study, we report on sea turtle nest monitoring in the Cayman Islands over a 22-year period. We found that green (Chelonia mydas) and loggerhead (Caretta caretta) nest numbers increased significantly across the three islands since monitoring began in 1998, but that hawksbill nest numbers remained low with a maximum of 13 nests recorded in a season. Comparing the first 5 years of nest numbers to the most recent 5 years, the greatest percentage increase in green turtle nests was in Grand Cayman from 82 to 1,005 nests (1,126%), whereas the greatest percentage increase for loggerhead turtle nests was in Little Cayman from 10 to 290 nests (3,800%). A captive breeding operation contributed to the increase in the Grand Cayman green turtle population, however, loggerhead turtles were never captive-bred, and these populations began to increase after a legal traditional turtle fishery became inactive in 2008. Although both species have shown significant signs of recovery, populations remain at a fragment of their historical level and are vulnerable to threats. Illegal harvesting occurs to this day, with multiple females taken from nesting beaches each year. For nests and hatchlings, threats include artificial lighting on nesting beaches, causing hatchlings to misorient away from the sea, and inundation of nests by seawater reducing hatch success. The impacts of lighting were found to increase over the monitoring period. Spatial data on nest distribution was used to identify critical nesting habitat for green and loggerhead turtles and is used by the Cayman Islands Department of Environment to facilitate remediation of threats related to beachside development and for targeted future management efforts.
Introduction
For many marine megavertebrate populations, centuries of overharvesting have led to extinction or significant population depletion (e.g., seals: McClenachan and Cooper, 2008, sharks and rays: Dulvy et al., 2014; marine mammals: Springer et al., 2008). The intensity of human impacts in the marine environment is believed to be increasing (McCauley et al., 2015) and population reductions of marine vertebrates are likely to have significant consequences for the health of marine environments (Estes et al., 2011). However, with sufficient reduction in threats, there have been examples of significant population increases. For example, northern elephant seal (Mirounga angustirostris) populations made a remarkable recovery after hunting pressure was removed (Stewart et al., 1992) and many humpback whale (Megaptera novaeangliae) populations have increased after protection from commercial whaling (Zerbini et al., 2019). The most common factors influencing positive population trends are exploitation reduction and habitat protection, although more information on drivers of marine recoveries is needed to facilitate effective management (Lotze et al., 2011).
Like many other large marine vertebrate species, over-exploitation has reduced sea turtle populations to a fraction of their former levels, which may influence the dynamics of reefs and seagrass beds (Jackson, 2001; McClenachan et al., 2006). In addition, sea turtles face other threats such as coastal development of nesting habitats (Casale, 2010). Some previously abundant green turtle (Chelonia mydas) nesting populations have been extirpated, such as in Bermuda (Lagueux, 2001), however, others have shown promising increases. For example, green turtle nester or nest numbers have increased steadily over the past 20–30 years in Japan, Australia, Hawaii, Florida, Costa Rica (Chaloupka et al., 2008), and Ascension Island (Weber et al., 2014). Similarly, there are distinct and differing population trends in other species; some hawksbill (Eretmochelys imbricata) and loggerhead (Caretta caretta) turtle nest numbers are now stable or increasing (Mortimer and Donnelly, 2008; Casale and Tucker, 2017), although worldwide there have been overall declines in abundance.
Globally, much effort has been focused on monitoring sea turtle nesting populations. Nest counts cannot be used as a direct measure of the abundance of nesting female turtles, due to varying reproductive output, nest site fidelity, and internesting and remigration intervals (Casale and Ceriani, 2020). Furthermore, due to the time lag between hatchling production and recruitment to the adult female stage, nest numbers do not indicate absolute abundance for all life-stages or overall trends for entire populations (Mortimer, 1995). However, nest counts are the most common index of sea turtle population monitoring as they can be systematically obtained and compared among locations (Ceriani et al., 2019) and monitoring and protection of nesting assemblages is crucial for successful conservation (McClenachan et al., 2006). Nevertheless, sea turtle nesting abundance time series rarely include long-term data (from more than 20 years of monitoring), which are necessary to detect significant changes in abundance (Mazaris et al., 2017). Comprehensive assessments of local threats and management interventions are also lacking (Rees et al., 2016). Given the divergent trajectories of sea turtle populations worldwide, there is a need to accurately assess and report nesting trends, understand the dynamics of declines or increases, and establish which conservation strategies are effective (Lewison and Crowder, 2007; Rees et al., 2016).
Historically, the Cayman Islands are thought to have been one of the world’s largest green turtle nesting populations (Groombridge and Wright, 1982) and nesting by hawksbill, loggerhead, and leatherback turtles was also previously considered “abundant” (Lewis, 1940). Although the islands were not permanently settled until the 1700s, visiting ships were estimated to harvest over 10,000 green turtles per year from 1688 onward (Lewis, 1940; Jackson, 1997). By the early 1800s, sea turtle nesting populations were exhausted, and these commercial turtle fishing efforts shifted overseas. While green turtle nesting populations were listed as locally extinct in the 1980s (Groombridge and Wright, 1982), it appears that populations persisted, though at very low levels (Wood and Wood, 1994).
Between 1971 and 1991, 78 sea turtle nests (17 green, 43 loggerhead, 1 leatherback, 6 hawksbill, and 11 unidentified) were recorded in ad hoc surveys (Wood and Wood, 1994). The first systematic survey of Cayman Islands sea turtle nesting was undertaken from 1998 to 1999, during which 38 nests were recorded on the three islands (Aiken et al., 2001). Further to this work, nesting activity was reported from 2000 to 2003 by Bell et al. (2007), with a maximum of 75 nests recorded in Grand Cayman and Little Cayman in 1 year (2002: 51 green turtle nests, 13 loggerhead nests, and the remainder unidentified). Despite the small size of the nesting population, a small-scale local traditional turtle fishery continued until 2008 (Bell et al., 2006; Blumenthal et al., 2010). During this time, fishermen were permitted to capture juvenile and adult green and loggerhead turtles over 54.5 kg/120 lb. in an open season between October and April each year. In 2008, the closed season was extended to include May and November and a maximum size limit of 60cm curved carapace length was introduced. While captures by licensed fishermen are still permissible within these restrictions, no legal take of turtles has occurred since 2008 (Nuno et al., 2018).
Changes to turtle fishery regulations to prevent take of adult turtles (Blumenthal et al., 2010) were complemented by the in situ protection efforts by the Cayman Islands Department of Environment (DoE) on nesting beaches and releases by the Cayman Turtle Farm (now the Cayman Turtle Conservation and Education Centre Ltd.). Over 30,000 green turtles were released between 1980 and 2001, of which approximately 50% were hatchlings and 50% yearlings (Bell et al., 2005). Releases have continued at various levels to the present day as one of the world’s most prominent green turtle re-introduction programmes (Wood and Wood, 1994; Bell et al., 2005).
Though understanding threats to the nesting turtles in the Cayman Islands is critical to their conservation, Cayman Islands green and loggerhead turtles are also known to be migratory. Satellite tracking of post-nesting adult females indicated that they utilised foraging grounds in a range of Caribbean jurisdictions, including Mexico, Belize, Guatemala and Nicaragua (Blumenthal et al., 2006). Therefore, these populations are sensitive to threats and environmental changes outside of the Cayman Islands, highlighting the need for international protection on foraging grounds, as well as protection and monitoring on nesting beaches.
Here we present sea turtle nesting numbers for the Cayman Islands over 22 nesting seasons (1998–2019) with reference to trends, spatial patterns, and threats. The study sheds light on the factors which have affected, and which may continue to affect sea turtle nesting population trends.
Methods
The Cayman Islands consist of three small inhabited islands in the Caribbean Sea: Grand Cayman, Little Cayman, and Cayman Brac (Figure 1). To assess nesting numbers, beaches were monitored during the loggerhead (∼May to September) and green (∼June to December) turtle nesting and hatching seasons across all three islands.
Standardised yearly monitoring was carried out in Grand Cayman from 1999 to 2019, in Little Cayman in 1998 (Aiken et al., 2001), 2000–2003 (Bell et al., 2006), and 2014–2019 and in Cayman Brac in 1998 (Aiken et al., 2001) and from 2012 to 2019. Following methods by Bell et al. (2007), all known nesting beaches were surveyed on foot approximately twice weekly throughout the nesting season. All turtle activities were recorded, and GPS locations taken. Only confirmed nests, where clutches were located, were used in nest counts. Clutches were relocated further from the high-water line if deemed at high risk from inundation. After approximately 44 days of incubation, each nest was monitored for signs of hatchling emergence, and nest excavations were carried out after emergence to determine the success of the hatch, according to the methodology of Miller (1999) and Aiken et al. (2001).
All terrestrial threats to nests and hatchlings were recorded. Wherever possible, nests were left to hatch naturally. If a nest was in a location with artificial lighting, DoE requested that property owners turn off beachside lights for a period surrounding the predicted hatch date, though under Cayman Islands Law, compliance was not a legal requirement. Therefore, if lights were not turned off, interventions to the nest were implemented on a case-by-case basis, including shielding the nest from the light source (e.g., with the use of heavy cloth sheeting or tarpaulin), observing the hatch, inducing the hatch and intervening if hatchlings misoriented, or removing hatchlings prior to, or as they emerged for release elsewhere. The frequency of interventions to nests were recorded, as well as the frequency of hatchling misorientation, defined as the misdirection of a hatchling toward an artificial light source (Sella et al., 2006). Misorientation was determined based on observations of hatchlings or the orientation of hatchling tracks away from the sea.
Threats to mature turtles were determined by recording incidences of legal take (from fishermen reports as a legal requirement) from 1999 to 2008 during the operation of the legal traditional fishery and known incidences of illegal take (from enforcement officer reports) throughout the monitoring period.
Data Analysis
Generalised additive mixed models (GAMMs) were used to model non-linear relationships between nest counts and year for loggerhead and green turtles in Grand Cayman, which incorporated a negative binomial link function and accounted for autocorrelation. Both models were implemented using the “mgcv” package v. 1.8-24 (Wood, 2017) in the statistical programme R v. 3. 5. 1. (R Core Team, 2014). As monitoring data for Little Cayman and Cayman Brac had missing years, GAMMs were not a suitable analysis. Therefore, mean nest counts were compared between two time periods (the first and last 5 years of monitoring for Grand Cayman and Little Cayman), and between the first year and the most recent 5 years for Cayman Brac, according to the method used by the IUCN Marine Turtle Specialist Group (MTSG) to determine population change for Red List Assessments (Casale and Tucker, 2017). Wilcoxon tests were carried out to assess differences in the proportion of hatchling misorientations between loggerhead and green turtles. Hatch success (%) was determined by: number of hatched shells/total clutch size) × 100 (Miller, 1999).
Spatial Analysis
Spatial nesting patterns for green and loggerhead turtles were assessed in Grand Cayman by calculating the proportion of nests by each species in the north, south, east or west sections of the islands and differences were tested using a chi-square test of independence.
To locate critical nesting habitat, green and loggerhead turtle nest GPS points were mapped using (ESRI, 2016). ArcGIS ArcMap v.10.4 to determine beach locations containing >10% of the maximum nesting density value. Critical habitat is defined under the Cayman Islands National Conservation Law (2013) as the specific area or areas of land containing the physical, biological, and ecological features needed for the conservation of a species. For a revised Turtle Conservation Plan, which is under review at the time of writing, it was determined that areas with little (containing <10% of the maximum nesting density value) or no nesting would be excluded, and higher density areas would be designated as critical habitat.
Results
Nesting Numbers
Overall, sea turtle nesting numbers have shown a promising increase across the three islands. In 1998, surveys were carried out in Little Cayman and Cayman Brac only, and a total of 16 nests were recorded. In 1999, surveys were carried out in Grand Cayman only and 23 nests were located. Using the same standardised sampling methodology to these early years, a total of 675 nests were recorded across the three islands in 2019 (69% on Grand Cayman, 23% Little Cayman, and 8% on Cayman Brac). Of total nests recorded (n = 4901), green turtle nests represented 54%, loggerhead turtle nests represented 44%, and hawksbill nests represented only 1%. The remaining proportion (<1%) were unidentified (see Table 1 for total nest numbers per year, by island, and species). Across all three islands, no leatherback turtle nests were recorded during monitoring.
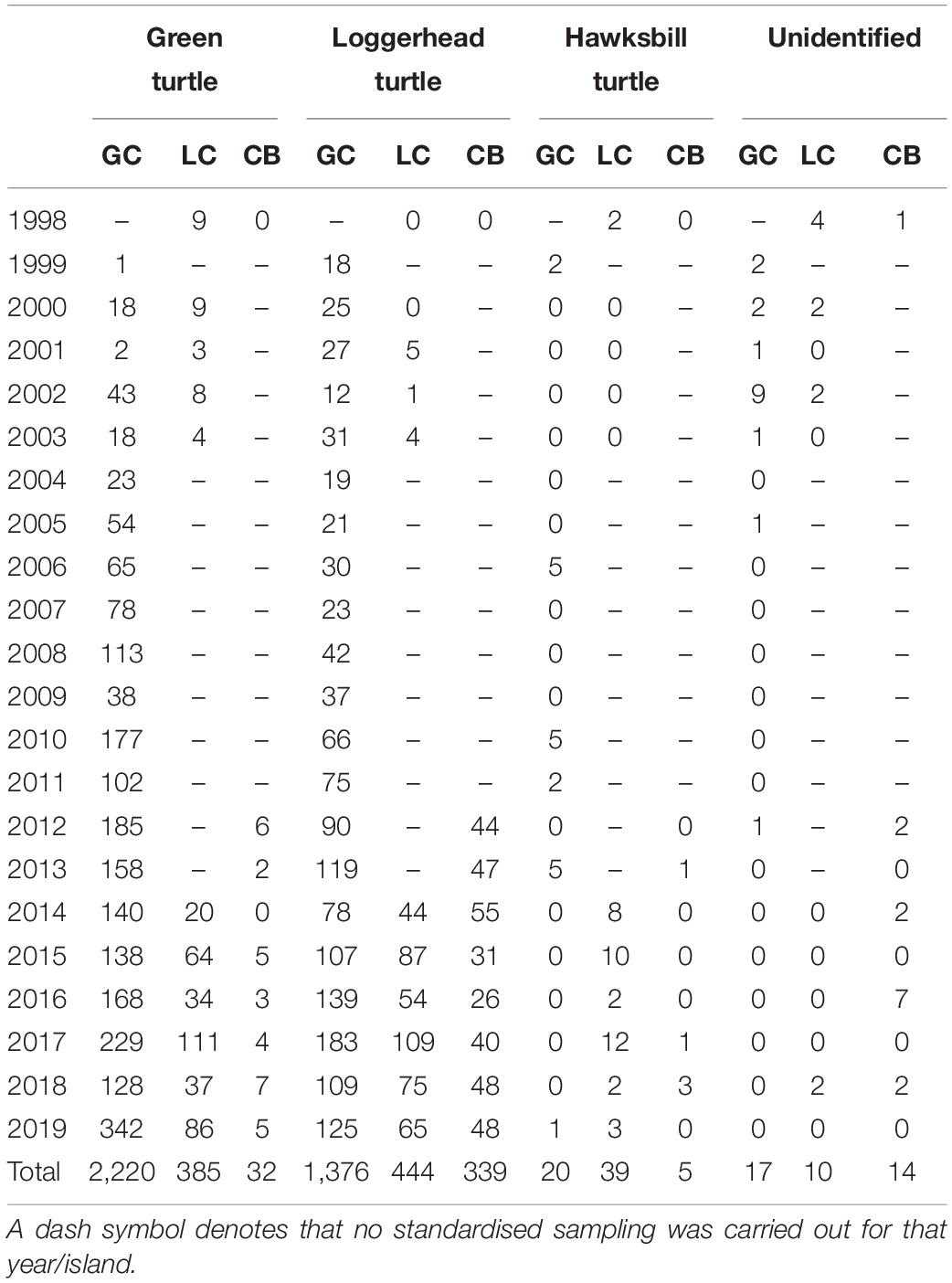
Table 1. Total number of sea turtle nests recorded for each year of standardised monitoring in the Cayman Islands (GC, Grand Cayman; LC, Little Cayman; CB, Cayman Brac).
Grand Cayman Nests
Over the 21-year period of nest monitoring in Grand Cayman, green turtle nest numbers increased by 1,126% from a total of 82 nests recorded in the first 5 years (1999–2003) to a total of 1,005 in the most recent 5 years (2015–2019). Over the same period, loggerhead nests increased by 487% from 113 to 663 nests. Hawksbill nesting numbers have remained low, with only two nests recorded in first years of monitoring and only one in the most recent 5 years.
Despite fluctuations in nesting numbers between years, yearly counts for the two dominant species, green and loggerhead turtles, have shown significant increasing trends (Figure 2 and Supplementary Table 1). Green turtle nest numbers increased throughout the study period (Figure 2A), while loggerhead nest numbers did not start to increase until 2008 (Figure 2B), coinciding with the end of the local traditional turtle fishery (legal catch numbers from 1999 to 2008 are reported in Figure 3 and Supplementary Table 2).
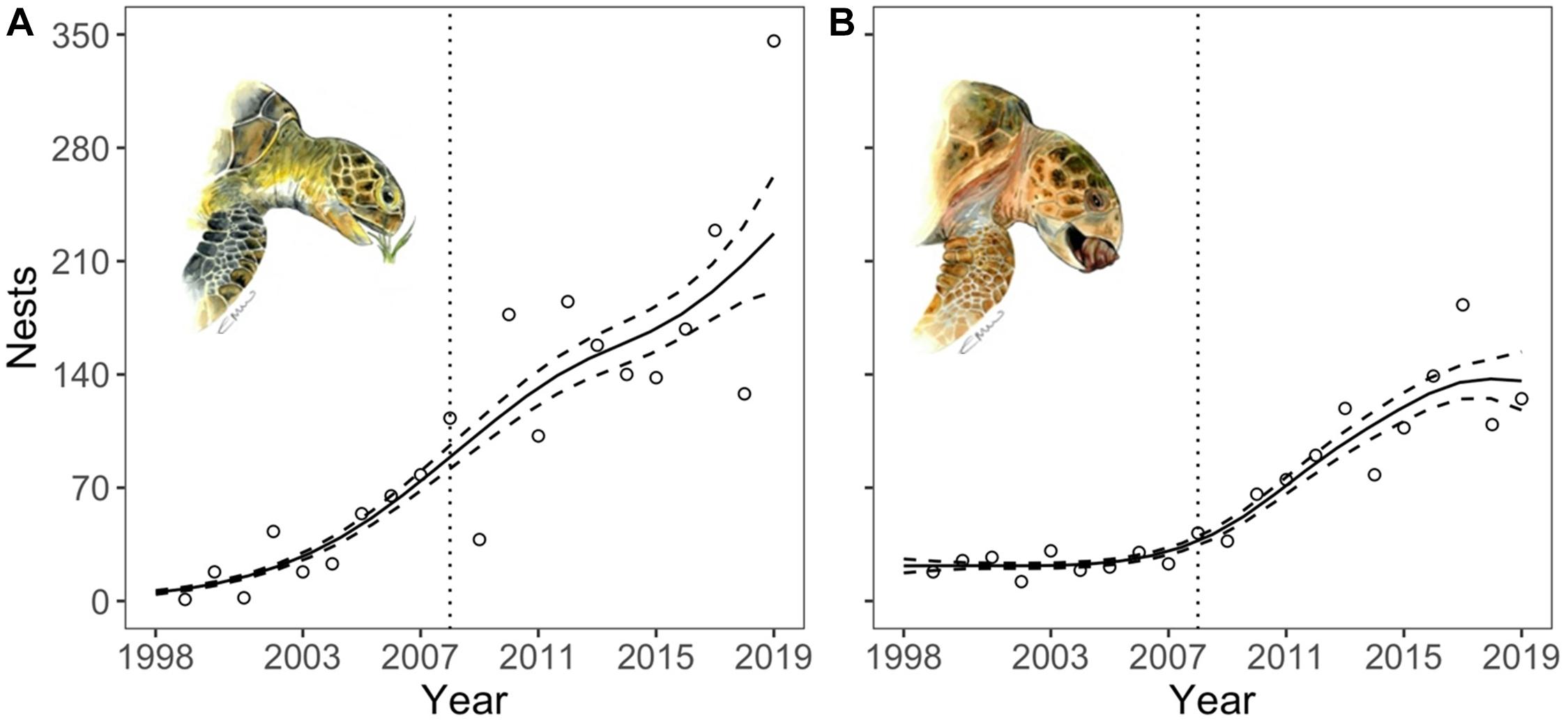
Figure 2. Temporal variation in nest counts for green (A) and loggerhead (B) turtles in Grand Cayman over the study period (1999–2019). Nest counts are show by the dots. Solid lines denote model predictions and dashed lines show standard errors. The dotted vertical lines indicate the end of the Cayman legal turtle fishery for mature green and loggerhead turtles. Artwork: Emma Wood.
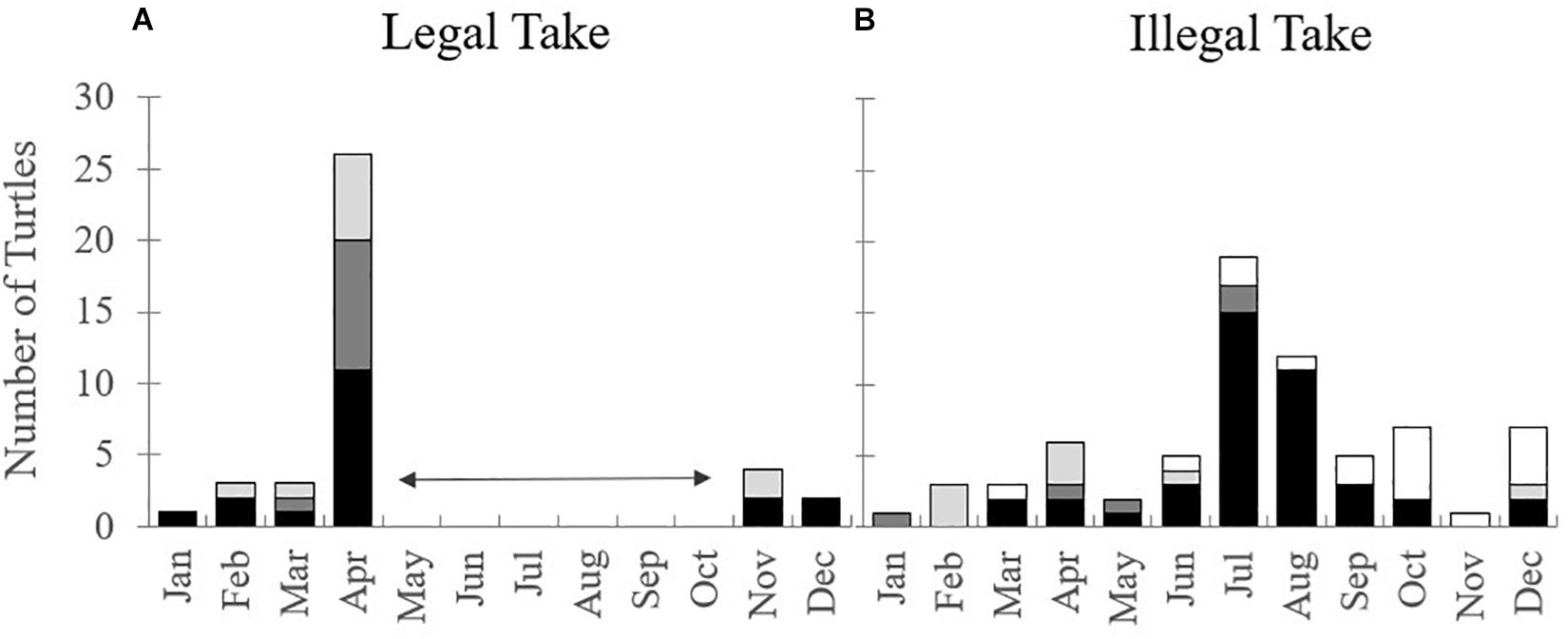
Figure 3. Number of turtles harvested from the wild by month, legally (1999–2008) (A) and illegally (1999–2019) (B). Bar colours represent different species (black: green turtles, dark grey: loggerhead turtles, light grey: hawksbill turtles, and white: unidentified species). Data reported is from all three Cayman Islands. Horizontal arrows represent the government imposed “no-take” season for licensed turtle fishermen.
Little Cayman Nests
In Little Cayman, green turtle nest numbers have increased by 906%, from a total of 33 in the first 5 years of monitoring (1998, 2000–2003) to 332 in the most recent 5 years (2015–2019), while loggerhead nest numbers increased 3,800%, from 10 nests to 390. Little Cayman had the greatest number of hawksbill turtle nests of the three islands, which increased by 190% between the two 5-year time periods, from 10 nests to 29.
Cayman Brac Nests
In 1998 in Cayman Brac, after monitoring for the full season, only one nest was recorded (species unidentified) (Aiken et al., 2001). In the most recent 5 years of monitoring, there have been a total of 24 green turtle nests, 193 loggerhead nests, and four hawksbill turtle nests.
Spatial Nesting Patterns
In Grand Cayman, green and loggerhead turtles had different spatial nesting patterns (X2 = 1231.4, df = 3, p ≤ 0.01). Although proportions of nests on the north side of Grand Cayman were similar for green and loggerhead turtles (27 and 22% of nests, respectively), green turtles preferred to nest on the west side (64% of nests) and loggerheads on the south side (58% of nests) of the island. There was also considerable variation in nesting density, which allowed critical nesting habitat to be identified (Figure 4). Critical nesting habitat in Grand Cayman covered 26.5, 34.7, and 57.3% of potential nesting habitat on Grand Cayman, Little Cayman, and Cayman Brac, respectively, and encompassed at least 89% of total nests on each island. In Grand Cayman, 67% of green turtle nests were located within 3 km of the Cayman Turtle Farm breeding centre which is located on the northwest point of the island (Figure 4), though this only represented 9% of available nesting habitat.
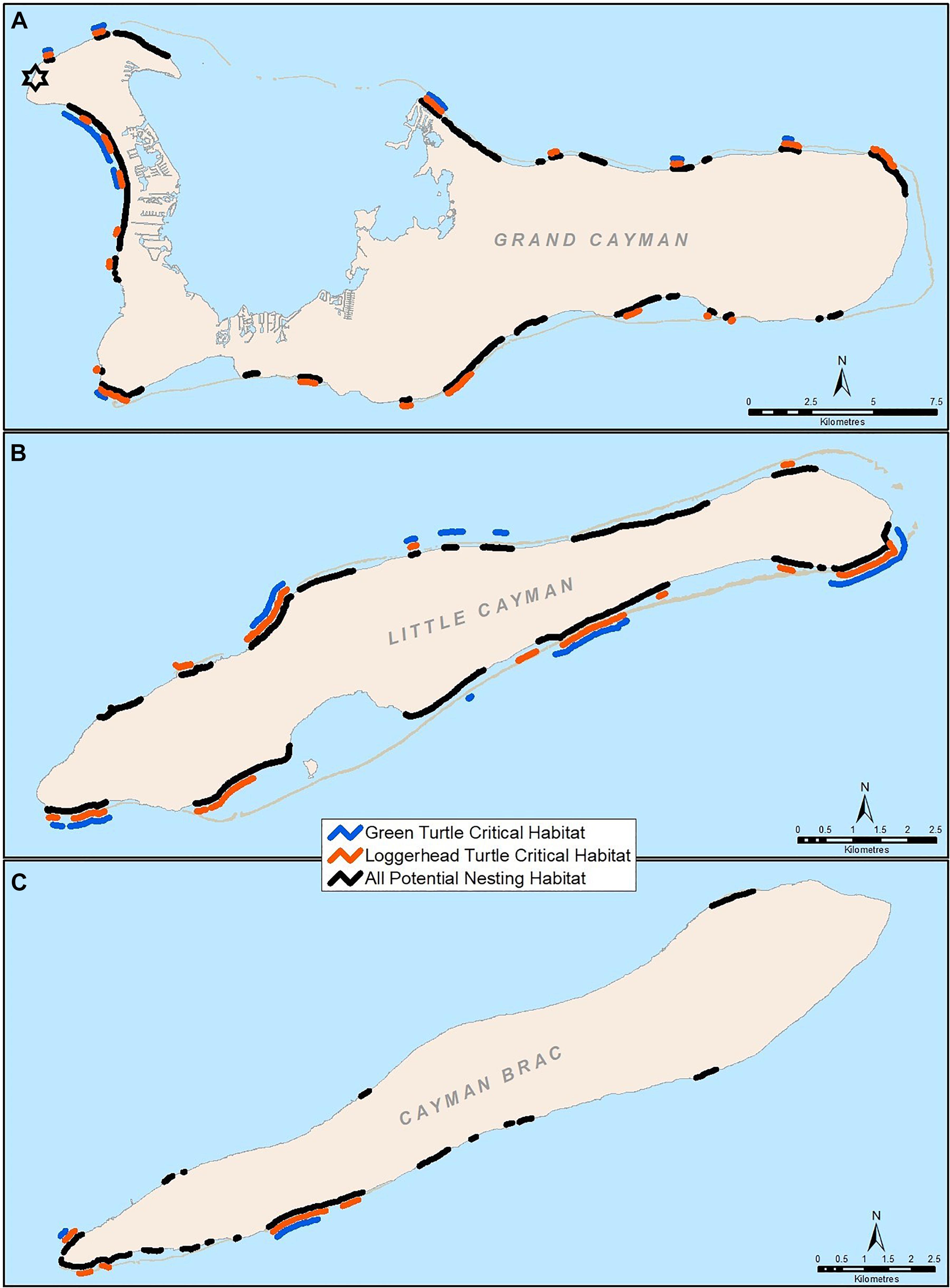
Figure 4. Critical habitat for green and loggerhead turtles in Grand Cayman (A), and Little Cayman (B) and Cayman Brac (C). The location of the Cayman Turtle Farm in Grand Cayman is noted by the star.
Threats to Mature Turtles
Legal Take From the Cayman Traditional Turtle Fishery
A total of 39 turtles were taken from the wild legally by licensed fishermen between 1999 and 2008 (Figure 3 and Supplementary Table 2). This included 19 green, 10 loggerhead, and 10 hawksbill turtles. More than 65% were captured in April (Figure 3) which is the beginning of the loggerhead breeding/nesting season, and most were large juveniles or adults (Bell et al., 2006). The sex of the turtles was often not reported, but it is known that at least eight were females.
Illegal Take From the Wild
From 1999 to 2019, 71 turtles are known to have been illegally harvested from the wild around the Cayman Islands (Figure 3 and Supplementary Table 3). Of these cases, at least 21 were known to be mature females and 72% of cases occurred in Grand Cayman. Green turtles were the most targeted species (58% of all illegal take) and the peak months of nesting (July and August), were also the peak months for illegal take of this species (Figure 3). Furthermore, law enforcement officials from DoE and the Royal Cayman Islands Police intercepted the illegal take of 37 turtles, 57% of which were mature females (3 loggerhead and 18 green turtles); these animals were released alive (Supplementary Table 3).
Threats to Nests and Hatchlings
Artificial Lighting
Artificial lighting along nesting beaches was one of the most frequently occurring threats to nests in Grand Cayman. From 1999 to 2019, for 3,242 nests that were known to have successfully hatched, hatchling misorientation was confirmed from 369 nests (11%). Additionally, interventions were applied to 288 nests (9%) to prevent hatchling misorientation, but in 74 cases misorientation occurred despite having an intervention in place. It should be noted that the true misorientation rate is likely to be significantly underestimated, as signs of hatchling misorientation are not always visible during bi-weekly monitoring. There was no difference in the proportion of misorientations by green or loggerhead turtles over time (W = 228, df = 40, p = 0.43), however, in recent years, the proportion of misorientations has been greater than in earlier years (mean of 5% from 1999 to 2003 vs. 14% from 2015 to 2019). In contrast, artificial lighting affected fewer nests in the less developed islands of Little Cayman and Cayman Brac. In Little Cayman, from 899 nests, hatchling misorientation was confirmed for less than 1% of total nests. In Cayman Brac, of 307 nests, hatchling misorientation was confirmed for 4% of total nests.
Inundation
Another relatively frequent threat to nests in the Cayman Islands was that of inundation from high tides or storm events. In Grand Cayman, from 3,590 confirmed turtle nests, 193 nests (5%) were inundated or washed away during incubation. Additionally, over the 21-year period, 208 nests (6%) were relocated, 26 of which were still inundated after relocation. The proportion of nests where inundation occurred varied by year, with highest rates linked to extreme weather events. The greatest weather impacts were in 2001 (Tropical storm Chantal and Hurricane Michelle-17% of nests inundated), 2004 (Hurricane Ivan inundation to 52% of nests), 2017 (Hurricane Irma-10%) and 2018 (Hurricane Michael-16%).
Inundation was also a threat to nests in Little Cayman and Cayman Brac. Between 2014 and 2019, 5 and 3% of turtle nests were inundated on Little Cayman (n = 803) and Cayman Brac (n = 287), respectively. Over the same period, 0.6 and 18% of nests were relocated in Little Cayman and Cayman Brac, respectively.
Other Threats
Records of predation on sea turtle nests/hatchlings on land in the Cayman Islands are very rare. For example in Grand Cayman, predation only impacted 46 out of 3,284 (1.4%) nests that were excavated. These incidents involved fire ants, crabs, dogs, worms and two accounts of snake predation. Other threats to nests included human disturbances/interference, roots growing into the nests, debris in or on the nests, and illegal take of eggs. Each of these were low in Grand Cayman and Little Cayman, affecting <3% of nests. In Cayman Brac, however, where green turtle nests were scarce (34 nests recorded in total), illegal take of eggs had occurred to 7% of these nests (but less than 2% of total nests).
Discussion
We present results of 22 years of sea turtle nest monitoring in the Cayman Islands and show that both green and loggerhead nesting populations have made a significant recovery since being historically over-exploited. A total of 78 turtle nests were reported between the 1970s and 1990s (Wood and Wood, 1994) and almost 700 turtle nests were recorded in the 2019 green and loggerhead turtle nesting season. While nesting numbers have increased dramatically for these two species, it is important to consider this modern-day population increase in the context of historical levels of abundance in the 1600s, when the nesting turtle population in the Cayman Islands was estimated to be more than 2.5 million (Jackson, 1997). Today, the green turtle nesting population has been estimated at around 100–150 females (Barbanti et al., 2019) and nesting data suggests there are even fewer loggerheads turtles. Additionally, these populations are still experiencing several anthropogenic threats including illegal take of adult turtles and the impact of artificial lighting causing hatchling mortality. This research provides a case study on the recovery of a depleted sea turtle population and facilitates successful national management efforts.
Green Turtle Population Trend
In the Caribbean, the abundance of green turtles has been reported as less than 1% of pre-exploitation levels (McClenachan et al., 2006). We found that green turtle nest numbers in the Cayman Islands were initially critically reduced but increased significantly throughout our monitoring period. Increases in green turtle nesting numbers have been observed in other populations within the region in recent years. For example, in Florida, from 1989 to 2019, nest numbers increased eightyfold [Florida Wildlife Fish and Wildlife Conservation Commission (FWC), 2019] and the trends in the Cayman Islands mirror these trends to a certain degree, on a smaller scale. In Hawaii, green turtles have been exploited over various phases as a means of subsistence and for commercial trade, and by the mid-1900s nesting was essentially extirpated everywhere except a single remote atoll. Subsequently, green turtles were protected in US waters under the Endangered Species Act in 1978 (Witzell, 1994; van Houtan and Kittinger, 2014). Since this time there have been no major anthropogenic threats and the nesting population has increased dramatically (Balazs and Chaloupka, 2004). By 2012, this population was listed as “Least Concern” by the IUCN (Pilcher et al., 2012).
In Grand Cayman, it is likely that the Cayman Turtle Farm “re-seeded” the nearly extirpated green turtle population. The number of hatchlings and yearlings released each year by the farm has varied but was highest in the early years of the programme (1980–1989), when more than 10,000 yearling and 15,000 hatchling turtles were released (Bell et al., 2005). Through documentation of permanent marks known as “living tags,” the first farm-released green turtles were recorded nesting on Grand Cayman beaches in 2002, at an age of 15 years (1 individual) and 17 years (2 individuals) (Bell et al., 2005). A genetic study determined that 90% of wild nesting green turtles (n = 57) in Grand Cayman in 2013/2014 were related as either offspring or full or half-siblings to the turtle farm individuals (Barbanti et al., 2019) and our study showed that the majority of green turtle nesting occurs in close proximity to the Cayman Turtle Farm breeding location. Therefore, the increase in nest numbers detected in the early years of nest monitoring may have been driven by the turtles released from the Cayman Turtle Farm in the 1980s reaching maturity after more than two decades in the wild.
In the context of effective sea turtle conservation strategies, turtle farming is a controversial technique, with debate over its efficacy (e.g., Frazer, 1992; Campbell, 2002; D’Cruze et al., 2015; Bennett et al., 2017; Walker et al., 2019). Criticisms of “headstarting” (captive rearing prior to release to reduce mortality in the younger life stages) as a conservation measure have focused on several points, which due to a lack of robust data, have seldom been evaluated (Bennett et al., 2017). Demographic models hypothesise that population growth rate is most influenced by subadult and adult survivorship, suggesting that headstarting hatchlings is ineffective compared to protection of mature turtles in the wild (Heppell et al., 1996; Heppell, 1998). It has also been speculated that headstarting could alter natural behaviours, including predator avoidance and foraging, and therefore decrease fitness and survival (Bennett et al., 2017). Concerns have also been raised relating to animal welfare standards at Cayman Turtle Centre, such as low hatchling survival rates and high levels of turtle morbidity and mortality (D’Cruze et al., 2015) and signs of physical injury, disease, and stress (Arena et al., 2014) and the resultant potential for disease transfer through releases of animals from an intensive-rearing facility into the wild (Seigel and Dodd, 2000; Bell et al., 2005; Arena et al., 2014; Bennett et al., 2017).
Assessing the success of headstarting in terms of population-level recovery requires several decades of data (Heppell, 1998). Here, we report on Grand Cayman wild turtle population trends in the decades after one of the world’s largest green turtle re-introduction programmes to contribute to this assessment. It is essential that re-introduction programmes have pre-determined goals and careful consideration of benefits and risks and undertake robust monitoring and reporting (Bennett et al., 2017). Evaluation of the effectiveness of an ex-situ breeding programme as a conservation tool must take into account the genetic diversity of the founder stock and the re-established population (Barbanti et al., 2019), and the effect on wild populations must also be evaluated to determine whether these efforts are worth the associated costs (D’Cruze et al., 2015). In Grand Cayman, from more than 25,000 yearling and hatchling turtles released between 1980 and 1989 (Bell et al., 2005), the green turtle nesting population is now estimated at 100–150 adult females (Barbanti et al., 2019).
Loggerhead Population Trend
Cayman Islands loggerhead turtle nest numbers have also increased, though this species was never captive bred. For loggerhead turtles, the nest number increase may be partially attributable to changes in Cayman Islands sea turtle fishery legislation which occurred at the end of 2007. Prior to that change, licensed turtle fishermen were permitted to capture adult loggerhead and green turtles, provided this occurred outside of the fishery closed season of May to October. As April falls within the Cayman Islands loggerhead breeding season, the prescribed size limit and closed season still allowed mature loggerhead turtles to be captured (Bell et al., 2007). Since 2008, the legal turtle fishery has been inactive (Nuno et al., 2018). While only a relatively low number of legal captures were recorded overall, due to the small nesting population at this time the fishery likely had an impact on the population growth rates. The legal fishery may also have masked additional illegal take and it appears that when this source of mortality was removed, loggerhead nesting numbers began to increase. Data on illegal take from recent years also shows that green turtles are harvested more than loggerheads, with no loggerhead captures recorded since 2014, further allowing these populations to increase.
The increase in loggerhead turtle nesting numbers suggests that if threats to adult turtles are ameliorated, small populations can recover—and indeed, an absence of Allee effects has been noted in the Cayman Islands and in other nesting populations (Bell et al., 2010; Mazaris et al., 2017). A reduction of wild sea turtle harvesting is also thought to have played an important role in the increase in loggerhead turtle nest numbers in Cape Verde (Marco et al., 2012), which showed a 15-fold increase over a 10-year period (2008–2017) (Laloë et al., 2020). However, wider environmental factors also likely influence these trends; between 1999 and 2007, the same period where there was no increase in loggerhead nests in the Cayman Islands, growth rates of Northwest Atlantic loggerhead turtles declined significantly (Bjorndal et al., 2013). During this period, nest counts in Florida showed a decline, before rebounding (Witherington et al., 2009; Ceriani et al., 2019).
Both loggerhead and green turtles are migratory species and therefore whole population level trends are challenging to assess. Because these species are likely impacted by threats and conservation efforts elsewhere within their range (Blumenthal et al., 2006), factors affecting turtles on foraging grounds may be working in concert with the increase in protection of nesting turtles within the Cayman Islands to recover Cayman Islands green and loggerhead nesting populations.
Threats and Management
Threats on Foraging Grounds
Adult foraging grounds of some post-nesting Cayman Islands green and loggerhead turtles have been identified through satellite tracking (Blumenthal et al., 2006) and findings revealed highly dispersed foraging areas spanning a >2,000 km stretch of Caribbean coastline and the Florida Keys. However, juvenile foraging grounds have not been identified for green and loggerhead turtles nesting in the Cayman Islands. Therefore, it is not possible to comprehensively assess threats to Cayman Islands turtles when not on nesting beaches.
Globally, juvenile and adult turtles on foraging grounds are threatened by legal and illegal take, pollution, disease, and other threats (Rees et al., 2016). However, resources from the turtle research community are often biased toward addressing terrestrial threats (Donlan et al., 2010). In order to protect sea turtles throughout their life-cycle, a coordinated approach to management is needed through multiple life stages and jurisdictions.
Threats Within the Cayman Islands
Illegal Take
While sea turtle consumption has occurred in many parts of the world over the last 7,000 years and holds both cultural and economic values (Frazier, 2003), unsustainable practices have been a key factor in the decline of sea turtle population numbers worldwide (McClenachan et al., 2006), including in the Cayman Islands (Aiken et al., 2001; Bell et al., 2006). While captures of wild turtles in the legal fishery have ceased, illegal harvesting continues in the Cayman Islands and the number of turtles harvested each year are likely much higher than those known and reported here. Similarly, there are many reports of the ongoing illegal turtle meat consumption and trade in other countries (e.g., Mancini and Koch, 2009; de Vasconcellos Pegas and Stronza, 2010; Nada and Casale, 2011; Hancock et al., 2017; Veríssimo et al., 2020). In 2014, interviews conducted in the Cayman Islands reported that 30% of respondents had consumed turtle products in the past 12 months and 0.3–3.5% had consumed wild rather than farmed meat (Nuno et al., 2018). Despite the Cayman Turtle farm having produced farmed green turtle meat for consumption for approximately 50 years, 13.5% of consumers strongly preferred wild turtle products, mainly due to the taste, indicating that farmed products do not entirely substitute for wild products (Nuno et al., 2018). This represents an important threat due to the reduced size of wild populations.
According to Tensen (2016), a number of criteria must be met to ensure that wildlife farming is beneficial to wild populations; among these, there must be no laundering of wild products into commercial trade. Our data reports a minimum of 24 turtles were harvested illegally between 2015 and 2019, indicating that this threat is ongoing and requires further management. Crucial initiatives include the marking of farmed turtle products to ensure that they are distinguishable from wild products and managing their pricing and distribution to avoid incentivising illegal take, a process which is still ongoing, as well as continued monitoring and robust evaluation of legal and illegal trade and consumption of turtle products (Nuno et al., 2018). As an additional management technique, the use of a beachside camera with a live-feed (designed by Security Centre International1 (funded by community donations) was trialled in 2019 to protect nesting females on a secluded nesting beach which experienced high levels of illegal take. This proved successful for this location, however, the use of cameras is not a viable option for all nesting locations due to the high cost and limited range. Expansion of awareness campaigns (Hancock et al., 2017) and introduction of conservation marketing and demand reduction initiatives (Veríssimo et al., 2020) also hold promise for the future.
Threats to Nests and Hatchlings
Sea turtles have a long generation time and spend the majority of their lifecycles at sea, so the impact of threats on nesting beaches that cause either low hatch success or hatchling survival will not be apparent for decades (Seminoff and Shanker, 2008). This means that threats must be recognised and addressed in the present to prevent irreversible future nesting population declines.
Artificial lighting presented one of the most frequent threats to nests in Grand Cayman and has been recognised as a substantial anthropogenic threat in many coastal environments (Depledge et al., 2010). Firstly, it can deter turtles from nesting (Weishampel et al., 2016; Silva et al., 2017). In Brazil, artificial lighting in coastal areas, driven by tourism has increased over time and light levels were found to impact nest densities of northern loggerhead and hawksbill turtles (Colman et al., 2020). Artificial lighting also attracts emerging hatchlings away from the sea or can lure them back to shore where they may experience mortality through entrapment, predation or exhaustion (Thums et al., 2016; Truscott et al., 2017; Erb and Wyneken, 2019). Our results showed that 18% of nests were negatively impacted by lighting, either due to misorientation or because of required interventions due to the location of the nest. Direct interventions to turtle nests are labour intensive and prone to failure or have unknown, but likely deleterious, impacts on hatchling survival or nest to surf orientation, for example, when hatchlings are removed early for later release (Lorne and Salmon, 2007).
While population-level impacts of lighting-related hatchling mortality have not been determined in the Cayman Islands, the threat is increasing and may have serious potential to limit hatchling production if not mitigated. Challenges in managing these impacts are particularly concerning given increasing nesting numbers and coastal development in the Cayman Islands, as well as other coastal locations where humans and turtles overlap worldwide. If managed well, turtles and humans can co-exist with the establishment of targeted dark protected areas during the nesting season (Colman et al., 2020). While more research is needed on the determinates of nest site selection (including proximity to the Cayman Turtle Farm for green sea turtles, beach topography, sand characteristics, and levels of development), our spatial analysis of nesting density has allowed recognition of critical nesting habitat in the Cayman Islands. This analysis also highlights where important nesting beaches are in developed areas or on high-value land, presenting an acute conflict between economic considerations and sea turtle survival. As a potential management solution, the Cayman Islands Government is currently funding a “Turtle Friendly Lighting Initiative” to remediate existing beachfront lighting, targeting areas of critical habitat in highly developed locations.
An additional threat to sea turtle nests in the Cayman Islands was inundation caused by storms or changing tides. This threat was most prominent in years where hurricanes had hit, or come close to, the Islands. Attention must be given to this as an emerging threat that could change over time. It is predicted that sea levels may rise 0.14–0.91 cm per year in the Cayman Islands [Caribbean Community Climate Change Centre (CCCCC), 2011], and that storms and hurricanes will intensify (Climate Studies Group, 2014). These emerging threats are widely acknowledged for small tropical islands (Nurse et al., 2014) and can increase nest inundation, egg loss and loss of nesting habitat (Ehrhart et al., 2014).
Efforts should be made to mitigate climate change impacts through sustainable development policies including sufficient setback of development from the beach (Fish et al., 2008). In the Cayman Islands, designation of critical habitat could also allow these measures to be targeted into the areas where they are needed the most.
Conclusion
Overall, this study shows that despite a massive reduction from early population levels, the Cayman Islands nesting population has indeed increased for both loggerhead and green sea turtles. However, continuing threats, such as artificial lighting, and emerging threats, such as climate change, indicate that strategically targeted management efforts are needed to secure the future survival of these populations. This study highlights the importance of assessing long-term sea turtle nesting abundance data, where trends are more easily detectable than in shorter time series and protection efforts may take decades to prove successful (Mazaris et al., 2017). These data can be used on a larger scale to increase the understanding of changes to populations throughout the region and to compare to other populations worldwide.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the animal study because no animals were handled, harmed or sacrificed for the purpose of the study.
Author Contributions
JMB, BJG, ACB, TJA, and GEP conceived of the project. JMB oversaw and collected the data collection. JLH and JMB drafted and edited the manuscript. LG and PC managed fieldwork. LC, LDL, JLH, and APV were involved in data collection and data management. LDL developed an app for data management. JO carried out the GIS analysis. LCMO carried out GAMM analysis and commented/edited the manuscript. BJG and ACB and also assisted with manuscript comments. All authors contributed to the article and approved the submitted version.
Funding
Funding was provided by the Cayman Islands Department of Environment (DoE) between 1998 and current day for the nest monitoring program, with assistance from the Foreign and Commonwealth Office, the Turtles in the Overseas Territories (TCOT project), the Turtles in the UK Overseas Territories Project (TUKOT), the Cayman Islands Governor’s Fund, and the Darwin Initiative.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank the Cayman Islands Department of Environment for the ongoing funding of the program and the large number of DoE staff members, volunteers and interns who were integral to the collection of data, in particular Jonathan Aiken, Joni Kirkconnell, Catherine Bell, Joseph Roche-Chaloner, Jennifer Swiggs, Jack Boyle, Bonnie Scott, Gene Edwards, Caroline and Dwayne Fredericks, Debra Vascik, Helen Leroy, Sharon Baker, Bill Woods, Jennifer Gough (Mills), Elizabeth Chafin, Liz Holmes, Maisy Fuller, Dexter Frago, and many others. We extend our thanks to the Conservation Enforcement Officers, in particular Mark Orr and Chadd Bush, who have helped protect the sea turtles around the three islands. We would also like to thank Michael Kader who created and donated the “Turtle Tracker” app, and Mike Ridley for arranging the donation of the beach security camera in 2019. We are also grateful for feedback from two reviewers which improved the manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.663856/full#supplementary-material
Footnotes
References
Aiken, J. J., Godley, B. J., Broderick, A. C., Austin, T., Ebanks-Petrie, G., and Hays, G. C. (2001). Two hundred years after a commercial marine turtle fishery: the current status of marine turtles nesting in the Cayman Islands. Oryx 35, 145–151. doi: 10.1046/j.1365-3008.2001.00168.x
Arena, P. C., Warwick, C., and Steedman, C. (2014). Welfare and environmental implications of farmed sea turtles. J. Agric. Environ. Ethics 27, 309–330. doi: 10.1007/s10806-013-9465-8
Balazs, G. H., and Chaloupka, M. (2004). Thirty-year recovery trend in the once depleted Hawaiian green sea turtle stock. Biol. Conserv. 117, 491–498. doi: 10.1016/j.biocon.2003.08.008
Barbanti, A., Martin, C., Blumenthal, J. M., Boyle, J., Broderick, A. C., Collyer, L., et al. (2019). How many came home? Evaluating ex situ conservation of green turtles in the Cayman Islands. Mol. Ecol. 28, 1637–1651. doi: 10.1111/mec.15017
Bell, C., Parsons, J., Austin, T., Broderick, A., Ebanks-Petrie, G., and Godley, B. (2005). Some of them came home: the Cayman Turtle Farm headstarting project for the green turtle Chelonia mydas. Oryx 39, 137–148. doi: 10.1017/s0030605305000372
Bell, C. D., Blumenthal, J. M., Austin, T. J., Solomon, J. L., Ebanks-Petrie, G., Broderick, A. C., et al. (2006). Traditional Caymanian fishery may impede local marine turtle population recovery. Endanger. Species Res. 2, 63–69. doi: 10.3354/esr002063
Bell, C. D., Blumenthal, J. M., Broderick, A. C., and Godley, B. J. (2010). Investigating potential for depensation in marine turtles: how low can you go? Conserv. Biol. 24, 226–235. doi: 10.1111/j.1523-1739.2009.01313.x
Bell, C. D., Solomon, J. L., Blumenthal, J. M., Austin, T. J., Ebanks-Petrie, G., Broderick, A. C., et al. (2007). Monitoring and conservation of critically reduced marine turtle nesting populations: lessons from the Cayman Islands. Anim. Conserv. 10, 39–47. doi: 10.1111/j.1469-1795.2006.00068.x
Bennett, A. M., Steiner, J., Carstairs, S., Gielens, A., and Davy, C. M. (2017). A question of scale: replication and the effective evaluation of conservation interventions. Facets 2, 892–909. doi: 10.1139/facets-2017-0010
Bjorndal, K. A., Schroeder, B. A., Foley, A. M., Witherington, B. E., Bresette, M., Clark, D., et al. (2013). Temporal, spatial, and body size effects on growth rates of loggerhead sea turtles (Caretta caretta) in the Northwest Atlantic. Mar. Biol. 160, 2711–2721. doi: 10.1007/s00227-013-2264-y
Blumenthal, J. M., Austin, T. J., Bothwell, J. B., Broderick, A. C., Ebanks-Petrie, G., Olynik, J. R., et al. (2010). Life in (and out of) the lagoon: fine-scale movements of green turtles tracked using time-depth recorders. Aquat. Biol. 9, 113–121. doi: 10.3354/ab00222
Blumenthal, J. M., Solomon, J. L., Bell, C. D., Austin, T. J., Ebanks-Petrie, G., Coyne, M. S., et al. (2006). Satellite tracking highlights the need for international cooperation in marine turtle management. Endanger. Species Res. 2, 51–61. doi: 10.3354/esr002051
Campbell, L. M. (2002). Science and sustainable use: views of marine turtle conservation experts. Ecol. Appl. 12, 1229–1246. doi: 10.1890/1051-0761(2002)012[1229:sasuvo]2.0.co;2
Caribbean Community Climate Change Centre (CCCCC) (2011). Climate and Weather Assessment for the Cayman Islands. Appendix 2: National Climate Assessment. Available online at: http://doe.ky/wp-content/uploads/2015/01/Part-4-VCA-Report_-June-2011.pdf (accessed March 18, 2021).
Casale, P. (2010). Sea Turtles in the Mediterranean: Distribution, Threats and Conservation Priorities. Gland: IUCN.
Casale, P., and Ceriani, S. A. (2020). Sea turtle populations are overestimated worldwide from remigration intervals: correction for bias. Endanger. Species Res. 41, 141–151. doi: 10.3354/esr01019
Casale, P., and Tucker, A. D. (2017). Caretta caretta (Amended Version of 2015 Assessment). The IUCN Red List of Threatened Species 2017: e.T3897A119333622. doi: 10.2305/IUCN.UK.2017-2.RLTS.T3897A119333622.en
Cayman Islands National Conservation Law (2013). Section 17 (7). Available online at: https://conservation.ky/wp-content/uploads/2020/11/Interim-Directive-for-Sea-Turtle-Critical-Habitat.pdf (accessed March, 2020).
Ceriani, S. A., Casale, P., Brost, M., Leone, E. H., and Witherington, B. E. (2019). Conservation implications of sea turtle nesting trends: elusive recovery of a globally important loggerhead population. Ecosphere 10:e02936.
Chaloupka, M., Bjorndal, K. A., Balazs, G. H., Bolten, A. B., Ehrhart, L. M., Limpus, C. J., et al. (2008). Encouraging outlook for recovery of a once severely exploited marine megaherbivore. Glob. Ecol. Biogeogr. 17, 297–304. doi: 10.1111/j.1466-8238.2007.00367.x
Climate Studies Group (2014). Climate Profile for the Cayman Islands. Variability, Trends and Projections. The University of the West Indies. Available online at: http://doe.ky/wp-content/uploads/2016/12/App_B-2_ClimateChangeRiskAssessment_FinalDraft.pdf (accessed March 18, 2021).
Colman, L. P., Lara, P. H., Bennie, J., Broderick, A. C., de Freitas, J. R., Marcondes, A., et al. (2020). Assessing coastal artificial light and potential exposure of wildlife at a national scale: the case of marine turtles in Brazil. Biodivers. Conserv. 29, 1135–1152. doi: 10.1007/s10531-019-01928-z
D’Cruze, N., Alcock, R., and Donnelly, M. (2015). The Cayman Turtle Farm: why we can’t have our green turtle and eat it too. J. Agric. Environ. Ethics 28, 57–66. doi: 10.1007/s10806-014-9519-6
de Vasconcellos Pegas, F., and Stronza, A. (2010). Ecotourism and sea turtle harvesting in a fishing village of Bahia, Brazil. Conserv. Soc. 8, 15–25. doi: 10.4103/0972-4923.62676
Depledge, M. H., Godard-Codding, C. A., and Bowen, R. E. (2010). Light pollution in the sea. Mar. Pollut. Bull. 60, 1383–1385. doi: 10.1016/j.marpolbul.2010.08.002
Donlan, C. J., Wingfield, D. K., Crowder, L. B., and Wilcox, C. (2010). Using expert opinion surveys to rank threats to endangered species: a case study with sea turtles. Conserv. Biol. 24, 1586–1595. doi: 10.1111/j.1523-1739.2010.01541.x
Dulvy, N. K., Fowler, S. L., Musick, J. A., Cavanagh, R. D., Kyne, P. M., Harrison, L. R., et al. (2014). Extinction risk and conservation of the world’s sharks and rays. eLife 3:e00590.
Ehrhart, L., Redfoot, W., Bagley, D., and Mansfield, K. (2014). Long-term trends in loggerhead (Caretta caretta) nesting and reproductive success at an important western Atlantic rookery. Chelonian Conserv. Biol. 13, 173–181. doi: 10.2744/ccb-1100.1
Erb, V., and Wyneken, J. (2019). Nest-to-surf mortality of loggerhead sea turtle (Caretta caretta) hatchlings on Florida’s east coast. Front. Mar. Sci. 6:271. doi: 10.3389/fmars.2019.00271
Estes, J. A., Terborgh, J., Brashares, J. S., Power, M. E., Berger, J., Bond, W. J., et al. (2011). Trophic downgrading of planet. Earth Sci. 333, 301–306.
Fish, M. R., Cote, I. M., Horrocks, J. A., Mulligan, B., Watkinson, A. R., and Jones, A. P. (2008). Construction setback regulations and sea-level rise: mitigating sea turtle nesting beach loss. Ocean Coast. Manag. 51, 330–341. doi: 10.1016/j.ocecoaman.2007.09.002
Florida Wildlife Fish and Wildlife Conservation Commission (FWC) (2019). Index Nesting Beach Survey Totals (1989-2019). Available online at: https://myfwc.com/research/wildlife/sea-turtles/nesting/beach-survey-totals/ (accessed January 2020).
Frazer, N. B. (1992). Sea turtle conservation and halfway technology. Conserv. Biol. 6, 179–184. doi: 10.1046/j.1523-1739.1992.620179.x
Frazier, J. (2003). “Prehistoric and ancient historic interactions between humans and marine turtles,” in The Biology of Sea Turtles, Vol. II, eds P. L. Lutz, J. A. Musick, and J. Wyneken (Boca Raton, FL: CRC Press), 1–38. doi: 10.1201/9781420040807.ch1
Groombridge, B., and Wright, L. (1982). The IUCN Amphibia-Reptilia Red Data Book, Part 1. Gland: IUCN.
Hancock, J. M., Furtado, S., Merino, S., Godley, B. J., and Nuno, A. (2017). Exploring drivers and deterrents of the illegal consumption and trade of marine turtle products in Cape Verde, and implications for conservation planning. Oryx 51, 428–436. doi: 10.1017/s0030605316000107
Heppell, S. S. (1998). Application of life-history theory and population model analysis to turtle conservation. Copeia 2, 367–375. doi: 10.2307/1447430
Heppell, S. S., Crowder, L. B., and Crouse, D. T. (1996). Models to evaluate headstarting as a management tool for long-lived turtles. Ecol. Appl. 6, 556–565. doi: 10.2307/2269391
Jackson, J. B. (2001). What was natural in the coastal oceans? Proc. Natl. Acad. Sci. U.S.A. 98, 5411–5418. doi: 10.1073/pnas.091092898
Lagueux, C. (2001). “Status and distribution of the green turtle, Chelonia mydas, in the Wider Caribbean Region,” in Proceedings of the Marine Turtle Conservation in the Wider Caribbean region: A Dialogue for Effective Regional Management, Santo Domingo, 32–35.
Laloë, J. O., Cozens, J., Renom, B., Taxonera, A., and Hays, G. C. (2020). Conservation importance of previously undescribed abundance trends: increase in loggerhead turtle numbers nesting on an Atlantic island. Oryx 54, 315–322. doi: 10.1017/s0030605318001497
Lewis, C. B. (1940). “The Cayman Islands and marine turtle,” in The Herpetology of the Cayman Islands. Bulletin of the Institute of Jamaica Science Series 2, ed. C. Grant (Kingston: Institute of Jamaica), 56–65.
Lewison, R. L., and Crowder, L. B. (2007). Putting longline bycatch of sea turtles into perspective. Conserv. Biol. 21, 79–86. doi: 10.1111/j.1523-1739.2006.00592.x
Lorne, J. K., and Salmon, M. (2007). Effects of exposure to artificialre to artificial lighting on orientation of hatchling sea turtles on the beach and in the ocean. Endanger. Species Res. 3, 23–30. doi: 10.3354/esr003023
Lotze, H. K., Coll, M., Magera, A. M., Ward-Paige, C., and Airoldi, L. (2011). Recovery of marine animal populations and ecosystems. Trends Ecol. Evol. 26, 595–605. doi: 10.1016/j.tree.2011.07.008
Mancini, A., and Koch, V. (2009). Sea turtle consumption and black market trade in Baja California Sur, Mexico. Endanger. Species Res. 7, 1–10. doi: 10.3354/esr00165
Marco, A., Abella, E., Liria-Loza, A., Martins, S., López, O., Jiménez-Bordón, S., et al. (2012). Abundance and exploitation of loggerhead turtles nesting in Boa Vista island, Cape Verde: the only substantial rookery in the eastern Atlantic. Anim. Conserv. 15, 351–360. doi: 10.1111/j.1469-1795.2012.00547.x
Mazaris, A. D., Schofield, G., Gkazinou, C., Almpanidou, V., and Hays, G. C. (2017). Global sea turtle conservation successes. Sci. Adv. 3:e1600730. doi: 10.1126/sciadv.1600730
McCauley, D. J., Pinsky, M. L., Palumbi, S. R., Estes, J. A., Joyce, F. H., and Warner, R. R. (2015). Marine defaunation: animal loss in the global ocean. Science 347:1255641. doi: 10.1126/science.1255641
McClenachan, L., and Cooper, A. B. (2008). Extinction rate, historical population structure and ecological role of the Caribbean monk seal. Proc. R. Soc. B Biol. Sci. 275, 1351–1358. doi: 10.1098/rspb.2007.1757
McClenachan, L., Jackson, J. B., and Newman, M. J. (2006). Conservation implications of historic sea turtle nesting beach loss. Front. Ecol. Environ. 4, 290–296. doi: 10.1890/1540-929520064[290:CIOHST]2.0.CO;2
Miller, J. D. (1999). “Determining clutch size and hatching success,” in Research and Management Techniques for the Conservation of Sea Turtles, eds K. L. Eckert, K. A. Bjorndal, A. Abreu-Grobois, and M. Donnelly (Washington, DC: IUCN/SSC Marine Turtle Specialist Group Publication No. 4), 124–139.
Mortimer, J. A. (1995). Teaching critical concepts for the conservation of sea turtles. Mar. Turtl. Newslett. 71, 1–4. doi: 10.1515/9780824860196-003
Mortimer, J. A., and Donnelly, M. (2008). Eretmochelys imbricata. The IUCN Red List of Threatened Species 2008: e.T8005A12881238. doi: 10.2305/IUCN.UK.2008.RLTS.T8005A12881238.en
Nada, M., and Casale, P. (2011). Sea turtle bycatch and consumption in Egypt threatens Mediterranean turtle populations. Oryx 45, 143–149. doi: 10.1017/s0030605310001286
Nuno, A., Blumenthal, J. M., Austin, T. J., Bothwell, J., Ebanks-Petrie, G., Godley, B. J., et al. (2018). Understanding implications of consumer behavior for wildlife farming and sustainable wildlife trade. Conserv. Biol. 32, 390–400. doi: 10.1111/cobi.12998
Nurse, L. A., McLean, R. F., Agard, J., Briguglio, L., Duvat-Magnan, V., Pelesikoti, N., et al. (2014). “Small islands,” in Climate Change 2014 : Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the 5th Assessment Report of the Intergovernmental Panel on Climate Change, eds V. R. Barros, C. B. Field, D. J. Dokken, M. D. Mastrandrea, K. J. Mach, T. E. Bilir, et al. (Cambridge: Cambridge University Press), 1613–1654.
Pilcher, N. J., Chaloupka, M. Y., and Woods, E. (2012). Chelonia mydas (Hawaiian subpopulation). The IUCN Red List of Threatened Species 2012:e.T16285718A16285879. doi: 10.2305/IUCN.UK.2012-1.RLTS.T16285718A16285879
R Core Team (2014). R: A Language and Environment for Statistical Computing. Vienna: R Found Stat Comput.
Rees, A. F., Alfaro-Shigueto, J., Barata, P. C. R., Bjorndal, K. A., Bolten, A. B., Bourjea, J., et al. (2016). Are we working towards global research priorities for management and conservation of sea turtles? Endanger. Species Res. 31, 337–382.
Seigel, R. A., and Dodd, C. K. (2000). “Manipulation of turtle population for conservation: halfway technologies or viable options?,” in Turtle Conservation, ed. M. W. Klemmens (Washington, DC: Smithsonian Institution Press), 218–238.
Sella, K. N., Salmon, M., and Witherington, B. E. (2006). Filtered streetlights attract hatchling marine turtles. Chelonian Conserv. Biol. 5, 255–261. doi: 10.2744/1071-8443(2006)5[255:fsahmt]2.0.co;2
Seminoff, J. A., and Shanker, K. (2008). Marine turtles and IUCN Red Listing: a review of the process, the pitfalls, and novel assessment approaches. J. Exp. Mar. Biol. Ecol. 356, 52–68. doi: 10.1016/j.jembe.2007.12.007
Silva, E., Marco, A., da Graça, J., Pérez, H., Abella, E., Patino-Martinez, J., et al. (2017). Light pollution affects nesting behavior of loggerhead turtles and predation risk of nests and hatchlings. J. Photochem. Photobiol. B Biol. 173, 240–249. doi: 10.1016/j.jphotobiol.2017.06.006
Springer, A. M., Estes, J. A., van Vliet, G. B., Williams, T. M., Doak, D. F., Danner, E. M., et al. (2008). Mammal-eating killer whales, industrial whaling, and the sequential megafaunal collapse in the North Pacific Ocean: a reply to critics of Springer et al. 2003. Mar. Mamm. Sci. 24, 414–442. doi: 10.1111/j.1748-7692.2008.00185.x
Stewart, B. S., Yochem, P. K., Huber, H. R., Delong, R. L., Sydeman, W., and Allen, S. G. (1992). “History and present status of the northern elephant seal population,” in Elephant Seals: Population Ecology, Behaviour, and Physiology, eds B. J. Le Boeuf and R. M. Laws (Berkeley, CA: University of California Press), 30–48.
Tensen, L. (2016). Under what circumstances can wildlife farming benefit species conservation? Glob. Ecol. Conserv. 6, 286–298. doi: 10.1016/j.gecco.2016.03.007
Thums, M., Whiting, S. D., Reisser, J., Pendoley, K. L., Pattiaratchi, C. B., Proietti, M., et al. (2016). Artificial light on water attracts turtle hatchlings during their near shore transit. R. Soc. Open Sci. 3:160142. doi: 10.1098/rsos.160142
Truscott, Z., Booth, D. T., and Limpus, C. J. (2017). The effect of on-shore light pollution on sea-turtle hatchlings commencing their off-shore swim. Wildl. Res. 44, 127–134. doi: 10.1071/wr16143
van Houtan, K. S., and Kittinger, J. N. (2014). Historical commercial exploitation and the current status of Hawaiian green turtles. Biol. Conserv. 170, 20–27. doi: 10.1016/j.biocon.2013.11.011
Veríssimo, D., Vieira, S., Monteiro, D., Hancock, J., and Nuno, A. (2020). Audience research as a cornerstone of demand management interventions for illegal wildlife products: demarketing sea turtle meat and eggs. Conserv. Sci. Pract. 2:e164.
Walker, J. M., Godley, B. J., and Nuno, A. (2019). Media framing of the Cayman Turtle Farm: implications for conservation conflicts. J. Nat. Conserv. 48, 61–70. doi: 10.1016/j.jnc.2019.01.001
Weber, S. B., Weber, N., Ellick, J., Avery, A., Frauenstein, R., Godley, B. J., et al. (2014). Recovery of the South Atlantic’s largest green turtle nesting population. Biodivers. Conserv. 23, 3005–3018. doi: 10.1007/s10531-014-0759-6
Weishampel, Z. A., Cheng, W. H., and Weishampel, J. F. (2016). Sea turtle nesting patterns in Florida vis-à-vis satellite-derived measures of artificial lighting. Remote Sens. Ecol. Conserv. 2, 59–72. doi: 10.1002/rse2.12
Witherington, B., Kubilis, P., Brost, B., and Meylan, A. (2009). Decreasing annual nest counts in a globally important loggerhead sea turtle population. Ecol. Appl. 19, 30–54. doi: 10.1890/08-0434.1
Witzell, W. N. (1994). The origin, evolution, and demise of the US sea turtle fisheries. Mar. Fish. Rev. 56, 8–23.
Wood, F. E., and Wood, J. R. (1994). “Sea turtles of the Cayman Islands,” in The Cayman Islands: Natural History and Biogeography: Monographiae Biologicae, Vol. 71, eds M. A. Brunt and J. E. Davies (Dordrecht: Kluwer Academic Publishers), 229–236. doi: 10.1007/978-94-011-0904-8_12
Wood, S. N. (2017). Generalized Additive Models: An Introduction With R. Boca Raton, FL: Chapman and Hall/CRC.
Keywords: artificial beachside lighting, Caribbean, Caretta caretta, Chelonia mydas, Eretmochelys imbricata, illegal take, threats, turtle re-introduction
Citation: Blumenthal JM, Hardwick JL, Austin TJ, Broderick AC, Chin P, Collyer L, Ebanks-Petrie G, Grant L, Lamb LD, Olynik J, Omeyer LCM, Prat-Varela A and Godley BJ (2021) Cayman Islands Sea Turtle Nesting Population Increases Over 22 Years of Monitoring. Front. Mar. Sci. 8:663856. doi: 10.3389/fmars.2021.663856
Received: 03 February 2021; Accepted: 07 April 2021;
Published: 03 May 2021.
Edited by:
Wen-Cheng Wang, National Taiwan Normal University, TaiwanReviewed by:
Paul Richards, Southeast Fisheries Science Center (NOAA), United StatesCeline Alexia Godard-Codding, Texas Tech University, United States
Copyright © 2021 Blumenthal, Hardwick, Austin, Broderick, Chin, Collyer, Ebanks-Petrie, Grant, Lamb, Olynik, Omeyer, Prat-Varela and Godley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Janice M. Blumenthal, SmFuaWNlQEJsdW1lbnRoYWwua3k=; Jane L. Hardwick, SmFuZS5IYXJkd2lja0Bnb3Yua3k=
†These authors share first authorship
 Janice M. Blumenthal
Janice M. Blumenthal Jane L. Hardwick
Jane L. Hardwick Timothy J. Austin1
Timothy J. Austin1 Annette C. Broderick
Annette C. Broderick Lucy C. M. Omeyer
Lucy C. M. Omeyer Alejandro Prat-Varela
Alejandro Prat-Varela Brendan J. Godley
Brendan J. Godley