- 1Department of Biological Sciences, Institute of the Environment, Florida International University, North Miami, FL, United States
- 2National Oceanic and Atmospheric Administration (NOAA), National Centers for Coastal Ocean Science, Beaufort, NC, United States
- 3ERT, Inc., in Support of Habitat Conservation Division, Southeast Region, NOAA Fisheries Service, West Palm Beach, FL, United States
The formation of fish spawning aggregations (FSAs) is an essential part of the life history of many economically important fish species; however, their status are often poorly described in the literature either due to their occurrence in remote locations, during seasons with unsafe ocean conditions, or because they move on space and time scales that are difficult to predict and validate. Even in areas that are relatively accessible and heavily fished, such as southeast Florida, regionally relevant information describing FSA dynamics is generally absent from the literature and unaccounted for in existing management plans. We propose that this can be attributed to the fact that information is often held by stakeholders or found in unpublished manuscripts and reports. These sources are not widely disseminated and are therefore difficult to locate and integrate into fisheries management decisions. In this paper, we present a case study demonstrating the value of regional data syntheses as a tool to improve management activities in southeast Florida. Specifically, we engaged with local stakeholders to collect reports of FSA occurrence, and used Web of Science queries to collate information describing the reproductive dynamics of locally occurring snapper and grouper species. Reports were combined with regional FSA literature and provided to managers as a support tool to anticipate FSA occurrence, and to guide policy development and future FSA research. Resource users identified 13 potential aggregations from five species, but Web of Science queries revealed a paucity of information. Echosounder, camera, and fisheries dependent surveys were then used to corroborate reportedly active cubera snapper (Lutjanus cyanopterus), hogfish (Lachnolaimus maximus), and gag grouper (Mycteroperca microlepis) aggregations. Variability in the spatiotemporal aspects of FSA occurrence make them difficult to study, but this may also explain how certain species have avoided detrimental impacts from aggregation fishing. These data represent a first step toward describing FSAs that have historically occurred in the Southeast Florida Coral Reef Ecosystem Conservation Area and can be used by managers to prioritize future research efforts focused on species or hotspots of multispecies activity along the northern extent of the Florida Reef Tract.
Introduction
The formation of Fish Spawning Aggregations (FSAs) is a vital part of the life cycle of many fish species, with each spawning event representing 33–100% of the annual reproductive investment for transient aggregating species (Domeier and Colin, 1997; Sadovy de Mitcheson et al., 2013). This reproductive strategy is shared by over 150 species world-wide (Claydon, 2004), and sites are often used by multiple species, either simultaneously or across multiple seasons (Johannes, 1978; Kobara et al., 2013; Farmer et al., 2017). Despite the documented occurrence of nearly 1,000 aggregations across the globe, the status of approximately 50% of them are unknown due to the difficulty associated with locating FSAs and conducting field research that characterizes their biological and ecological dynamics (Russell et al., 2014).
Location is thought to be primarily dictated by the optimization of larval dispersal into environments where predation risk is minimized and food encounter rate in a heterogeneous landscape is maximized (Johannes, 1978; Karnauskas et al., 2011; Sadovy De Mitcheson and Colin, 2013). However, fluctuations in oceanographic features (e.g., changes in flow direction and speed, temperature, etc.) are known to drive spatiotemporal patterns of occurrence (Heyman and Kjerfve, 2008; Karnauskas et al., 2011). For instance, changes in tidal period or short-term upwelling events may disperse aggregated spawners over a period of a few hours, or shift their focal spawning area, making detection increasingly difficult. Beyond the environmental factors complicating FSA detection, aggregating species exhibit varying degrees of site fidelity and seasonality (Farmer et al., 2017). Certain species within the snapper-grouper complex, such as mutton snapper (Lutjanus analis) and goliath grouper (Epinephelus itajara), are known to maintain localized “home ranges” during discrete spawning periods (Koenig et al., 2017; Feeley et al., 2018), though other species such as gray snapper (L. griseus), yellowtail snapper (Ocyurus chrysurus), and hogfish (Lachnolaimus maximus) aggregate on a range of habitats, have relatively large spawning home-ranges and protracted spawning seasons (Muñoz et al., 2010; Farmer et al., 2017).
While hundreds to thousands of individuals have been documented traveling for weeks, over great distances (10–100 s km) during specific times of the year for the sole purpose of spawning (Sadovy De Mitcheson and Colin, 2013), pinpointing their precise location in space and time is difficult without substantial effort and resources. Even in cases where high-resolution spatial and temporal information on aggregation occurrence have been provided in historical reports from resource users, documentation of FSA formation and spawning can take years, especially where heavy fishing pressure has depressed abundance (Burton et al., 2005; Heyman and Kjerfve, 2008; Feeley et al., 2018). For example, a collaborative multi-agency effort to document the recovery of a mutton snapper aggregation near Dry Tortugas National Park, Florida required approximately 10 years of consistent study before spawning was observed in 2009 (Feeley et al., 2018). Prior to the formation of the Tortugas South Ecological Reserve (TSER) in 2001, commercial fishing on Riley’s Hump (the focal point of the TSER) had consistently occurred for over a decade (Burton et al., 2005). Following sharp declines in mutton snapper landings during the spawning season, concerned fishers approached the Florida Keys National Marine Sanctuary with reports of the decline and began assisting with the implementation of legislation that closed off the region surrounding Riley’s Hump. With endorsement from the commercial fishing community, a comprehensive monitoring program was developed. Over the following 10 years period, mutton snapper and numerous other aggregating species, including ocean triggerfish (Canthidermis sufflamen), cubera snapper (L cyanopterus), permit (Trachinotus falcatus), and horse-eye jacks (Caranx latus) were observed at the aggregation site (Feeley et al., 2018).
Similar to the successes seen in the Dry Tortugas, stakeholder involvement has led to the recovery of FSAs throughout the world (Russell et al., 2014). However, the dynamics of FSA occurrence are still poorly understood in many regions, even those that are easily accessible and widely discussed within the fishing community. The paucity of information can be attributed to biotic and abiotic factors that drive spatial and temporal variability as described, but a significant obstacle to successful FSA management and identification is the lack of peer-reviewed syntheses that combine stakeholder reports, relevant peer-reviewed sources, and gray literature sources to describe regionally specific FSA dynamics. Syntheses such as these may be generated as part of an agency report or technical review, but they are not widely disseminated in peer-reviewed journals due to their scope and are therefore difficult to locate and integrate into current and future FSA management activities. Large spatial-scale reviews are useful and represent a valuable tool to broadly describe the reproductive dynamics of selected species, but regionally specific reviews may provide the level of detail needed to make appropriate management decisions that address local resource needs.
In this paper, we present a case study in support of regional FSA syntheses. Using the FSA research guidelines presented by the Society for the Conservation of Reef fish Aggregations (Collins et al., 2003), we gathered information from peer-reviewed literature, gray literature sources, and stakeholders to inform regional management decisions and develop an FSA validation field survey in the Southeast Florida Coral Reef Ecosystem Conservation Area (ECA). Specifically, we used multiple queries from Web of Science and engaged with local fishers and SCUBA divers to collect historical and current reports of FSA formation in the ECA. Reports from stakeholders were combined with information from regional FSA literature and provided to managers as a geospatial report of the occurrence of FSAs that could be used to guide conservation goals and future FSA research1. Field echosounder surveys, camera surveys, on-water fishing surveys, and trip-interviews were used to validate and assess reportedly active spawning aggregations, for which we had reports with meaningful spatial information. These data represent a first step toward describing FSAs that have historically occurred in the ECA and can be used by managers to prioritize future research and management efforts focused on individual species or hotspots of multispecies activity along the northern extent of the Florida Reef Tract.
The Southeast Florida Coral Reef Ecosystem Conservation Area–A Case Study
The Southeast Florida Coral Reef Initiative (SEFCRI) includes a collaborative advisory team and Technical Advisory Committee tasked with identifying and implementing priority actions needed to reduce key threats to coral reef resources off southeast Florida. The region extends along 150 km of coastline from the northern boundary of Biscayne National Park off Miami-Dade County, to the St. Lucie inlet in Martin County (Figure 1) (SEFCRI 2012). This portion of the Florida reef tract was designated as the Southeast Florida Coral Reef ECA by the Florida Legislature in 2018. The SEFCRI team consists of governmental agencies, non-profit organizations, recreational and commercial fishing and diving stakeholders, and marine industry leaders, focused on providing recommendations to the Florida Department of Environmental Protection (FDEP) Coral Reef Conservation Program (CRCP) resource managers, related to priority projects consistent with their Charter. The SEFCRI Technical Advisory Committee is made up of subject matter experts who advise the SEFCRI Team on technical topics related to coral reef threats. Founded in 2004, the SEFCRI and FDEP CRCP have completed over 140 projects pertaining to awareness and appreciation (i.e., outreach and education), land-based sources of pollution, maritime industry and coastal construction, and fishing, diving and other uses. However, a state-adopted regional management plan has not been developed prior to the designation of the ECA, leaving the northern extent of the Florida Reef Tract largely under managed and under protected.
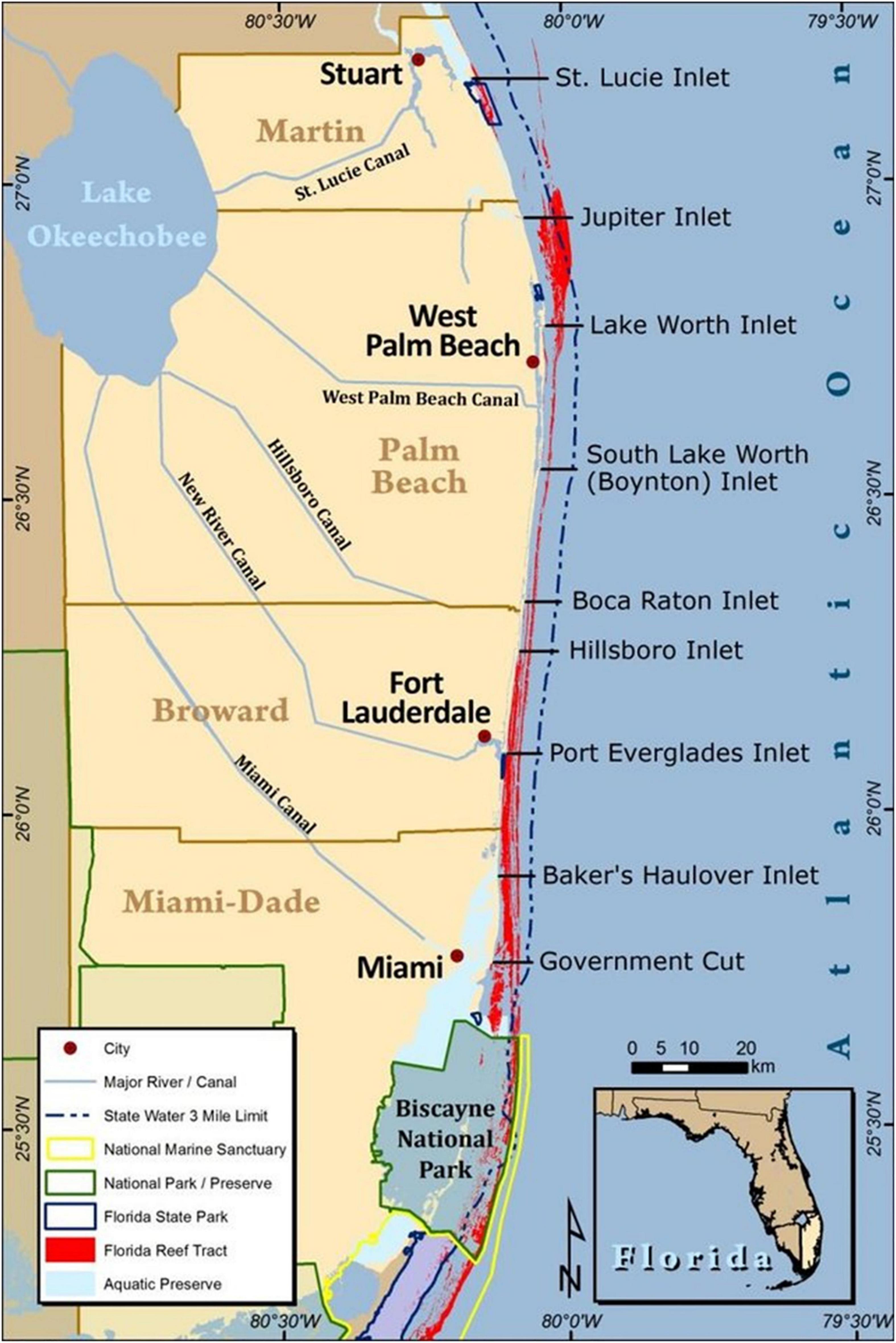
Figure 1. Counties included in the Southeast Florida Coral Reef Ecosystem Conservation Area, including major waterways, cities, and ocean inlets (Credit: Kurtis Gregg 2013).
The Florida Reef Tract is comprised of nursery, spawning, and foraging habitats for a diverse assemblage of tropical and sub-tropical species (Arena et al., 2007). Characterized by three distinct limestone reefs and nearshore ridge complex habitats that occur at increasing distances from shore, increasing in complexity seaward, the Florida Reef Tract is dominated by micro/macro-algae cover, interspersed with soft-coral colonies, sponges, and stony coral species, and bordered by expanses of sandy unconsolidated soft bottom (Walker and Gilliam, 2013). State waters within the ECA also contain an extensive network of artificial reef complexes, both intentionally and unintentionally sunk (Walker et al., 2009) (Figure 2). These structures vary in spatial extent (i.e., footprint), vertical relief, overall complexity (rugosity) and age, but both natural and artificial reef habitats in the region are “hotspots” of fish aggregation, production and biodiversity in a heterogeneous (patchy) landscape of small and isolated islands (Arena et al., 2007; Walker et al., 2009).
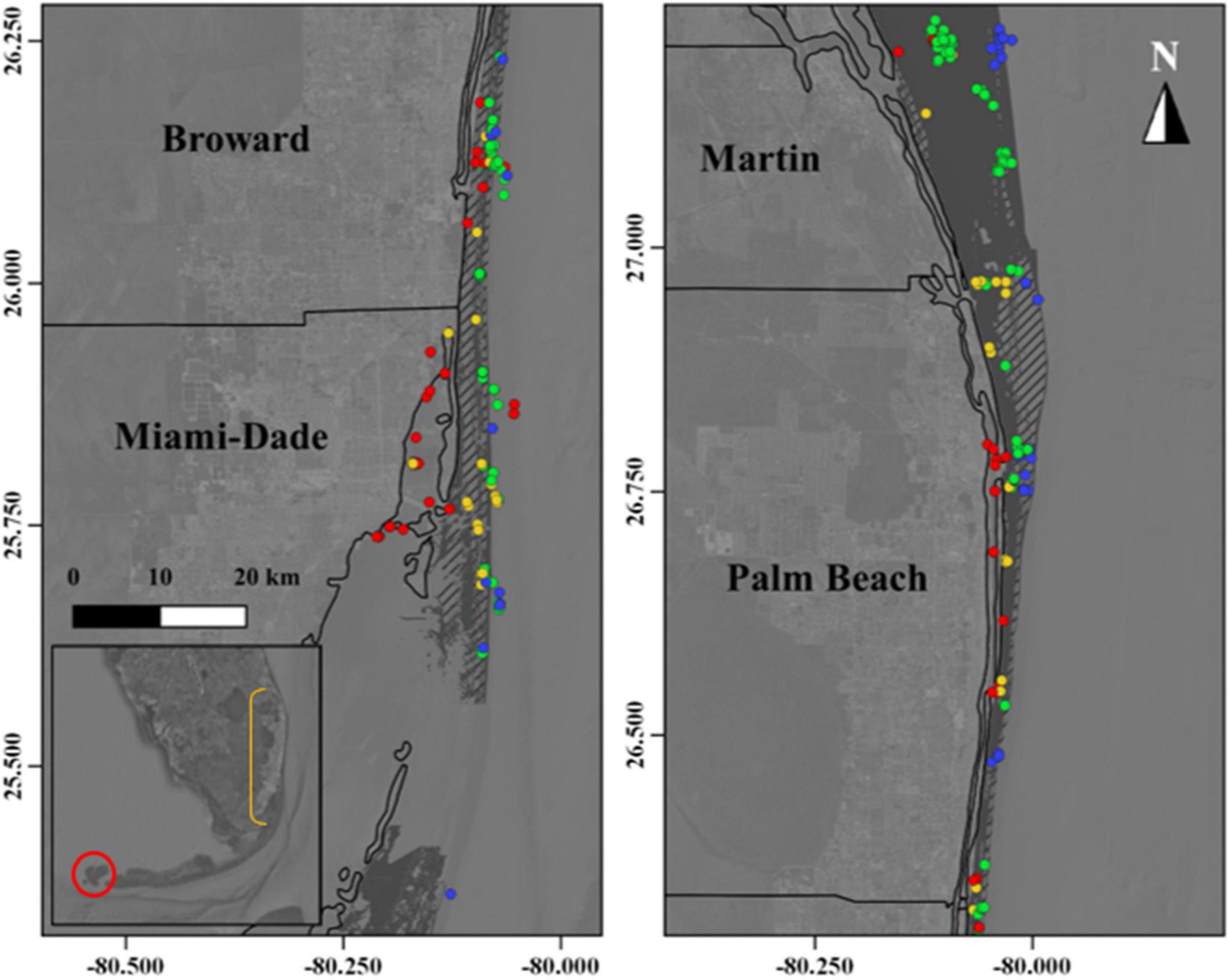
Figure 2. The Florida Reef Tract extends from the Dry Tortugas (Inset–red circle) to the northern extent of Martin County, Florida (north). Comprised of a range of habitats, harbottom limestone reefs (dashed gray polygons) are surrounded by unconsolidated sandy bottom (dark-gray polygons) in the Southeast Florida Coral Reef Ecosystem Conservation Area. Artificial reefs are distributed throughout the region at a range of depths. Colored circles represent reefs at depths between 1 and 25 m (red circles), 26–50 m (yellow circles), 51–75 m (green circles), and 75–100 m (blue circles). Ecosystem Conservation Area counties are labeled, and boundaries denoted by black lines.
Recognizing the importance of protecting sensitive living marine resources, SEFCRI launched the “Our Florida Reefs” campaign in 2013 to engage stakeholders, ocean users and the general public in a collaborative community planning effort that identified knowledge gaps and management priorities for the region (Reisewitz and Harper, 2013). Among the gaps outlined by the team, delineating habitats used by fish (specifically recreationally and commercially important species) during spawning were specifically highlighted as a research priority for integration into the final management plan recommendation. Spawning habitats are already identified as a federal management priority with the provision of the Essential Fish Habitat amendment to the Magnuson Stevens Act in 2002 (Federal Register vol. 67, no. 12, 2002) and subsequent reauthorization in 2006, though information related to the spatial and temporal aspects of FSAs in the ECA are essentially absent from the scientific literature despite their ecological importance.
Materials and Methods
Study Region and Target Species
Data collection and reports used for this synthesis were constrained to coastal state waters and adjacent federal waters (≤75 m depth) between the northern and southern boundaries of the ECA (Figure 1). Focal species were selected based on initial review of reports from users, government reports, theses and peer-reviewed publications from reports of spawning aggregation occurrence in the study region. Select taxa were within the Snapper-Grouper Complex managed by the US South Atlantic Fishery Management Council (Gould and Brawner, 1983): gray snapper, mutton snapper, cubera snapper, gag grouper (M. microlepis), and hogfish. Though hogfish are classified as a wrasse (Family: Labridae), they are a managed species of significant economic value within the snapper-grouper complex. Goliath grouper aggregations were not included in this synthesis, as their aggregations are well described in the literature, and they are currently protected from harvest.
Data Collection
Literature Review and User Reports
A keyword search was performed on Web of Science to compile available FSA literature pertaining to the ECA (Table 1). The search results were considered relevant and retained if they included species of interest occurring in the ECA. Those that pertained to the south Florida region and the species of interest were preserved, and location, time of aggregation occurrence, FSA size (geographic extent and relative abundance), and study dates were all recorded. Scientists in the region known to study snapper and grouper reproduction were also contacted to identify internal government reports and unpublished data sources that may contain relevant information pertaining to FSA spatiotemporal dynamics.

Table 1. A keyword search was performed in Web of Science (Clarivate Analytics, 2018) to identify primary literature relevant to spawning aggregation activity in the Southeast Florida Coral Reef Initiative Ecosystem Conservation Area.
Anecdotal user reports were collected by means of direct interview and through the collection of second-hand reports from resource users in the region. Contacts were initially identified by established scientists working in the region, and additional contacts were generated through resource user interviews. Contacts included retired and active commercial fishers, charter guides, recreational anglers, and SCUBA diving shops. The information collected from users included species, location, time of aggregation occurrence, FSA magnitude, and age of the report.
Spawning Aggregation Validation
Validation efforts were performed using a combination of echosounder transects paired with 360° unbaited remote underwater video (URUV) surveys, drop cameras, fisheries observer surveys, and dockside interviews. All four methods were used to explore and confirm the occurrence of a gag grouper spawning aggregation that was reportedly active near Boynton Beach, Florida between January, and March of 2016. Paired echosounder and URUV/drop camera surveys were conducted near Jupiter, Florida on a reported gray snapper aggregation site, between July and September 2016. Observer surveys paired with dockside interviews were used to confirm the occurrence of cubera and mutton snapper aggregations offshore of Homestead, Florida between May and October of 2014 and 2015 (Figure 3).
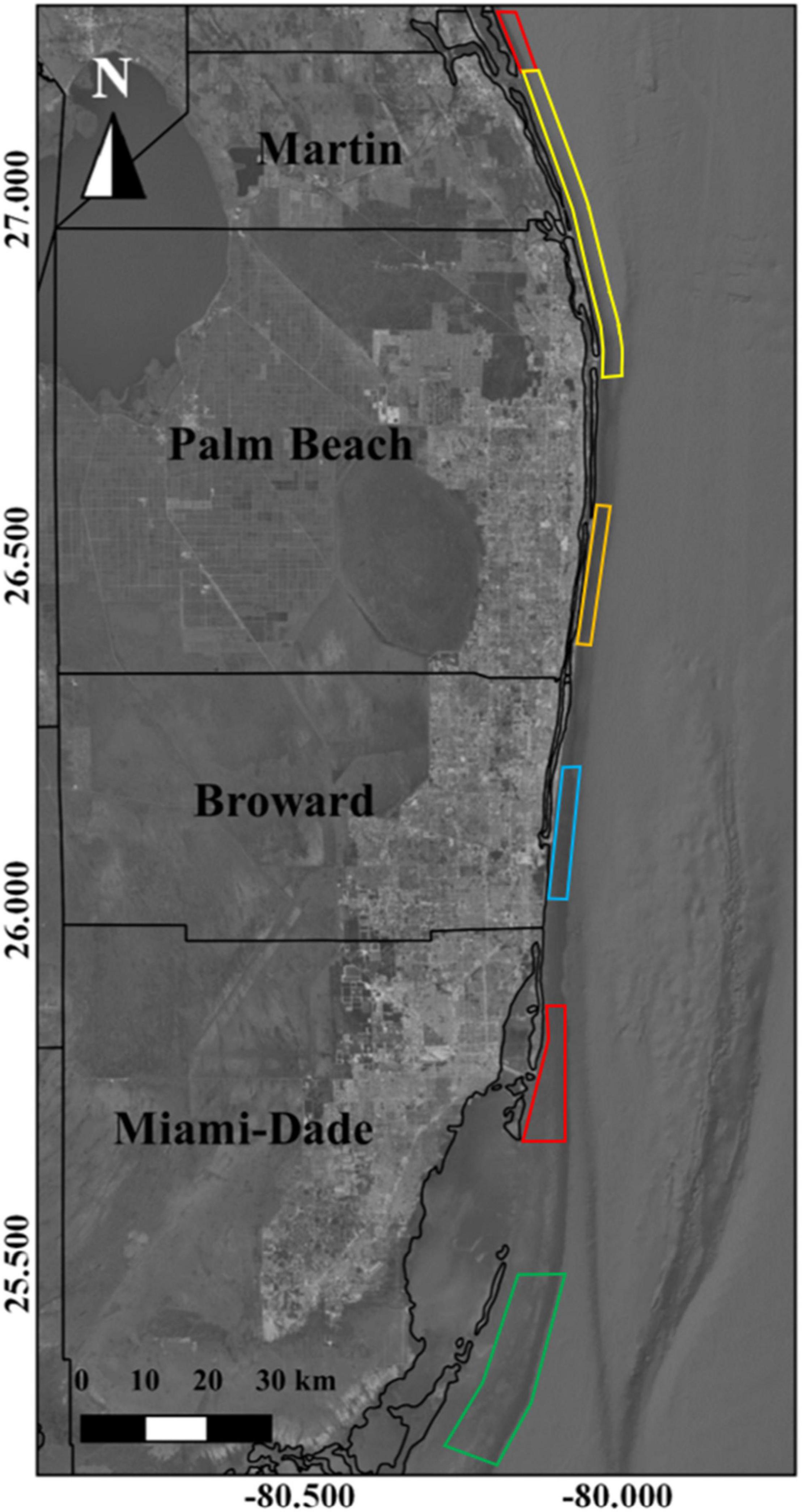
Figure 3. Extent of reports of FSAs for gray snapper (red), goliath grouper (yellow), gag and goliath grouper (orange), hogfish and vermillion snapper (blue polygon), and mutton snapper (green).
Echosounder surveys consisted of parallel linear transects, spaced approximately 25–30 m apart, that bisected the reefs and surrounding habitat centered on the geographic position where aggregations were reported to occur (Figure 4). Survey extent and transect line lengths varied by site and were determined by precision of report. Echosounder data were collected with calibrated 38 and 120 kHz split-beam echosounders (SIMRAD EK60/EK80), operating at 0.256 μs pulse duration with a 10° and 7° beam-angle, respectively. The transducers were deployed from a pole mount, approximately 1 m below the surface. Echosounder surveys were primarily used as a tool to identify areas of increased fish biomass for camera (URUV and drop camera) surveys. Specifically, an adaptive sampling approach was implemented, where echosounder data were monitored for the presence of backscatter indicating fish aggregations, and cameras were immediately deployed when elevated backscatter was observed. The URUV system consisted of a weighted (10 kg of lead weights) aluminum tripod, with three GoPro Hero 3 action cameras (170° horizontal field of view). The cameras were mounted on a platform attached to the top of the tripod, that allowed for 360° viewing of the surrounding habitat. The overall height of the URUV was approximately 1 m, to allow for unobstructed viewing over low lying visual obstructions (Supplementary Material). GoPro Hero 3 action cameras were also used for drop camera surveys. The three cameras were arranged on a weighted pipe to create a 360° video and deployed over the side with polypropylene rope. The pipe was deployed to the bottom, then recovered to suspend approximately 1–2 m over the substrate while the survey vessel held position over the site. Data collected during camera deployments were processed by a trained analyst proficient in reef fish identification, and the presence of aggregating target species was recorded along with additional reef fish species relative abundance (based on the maximum number of conspecifics seen in a single frame) (Ellis and DeMartini, 1995).
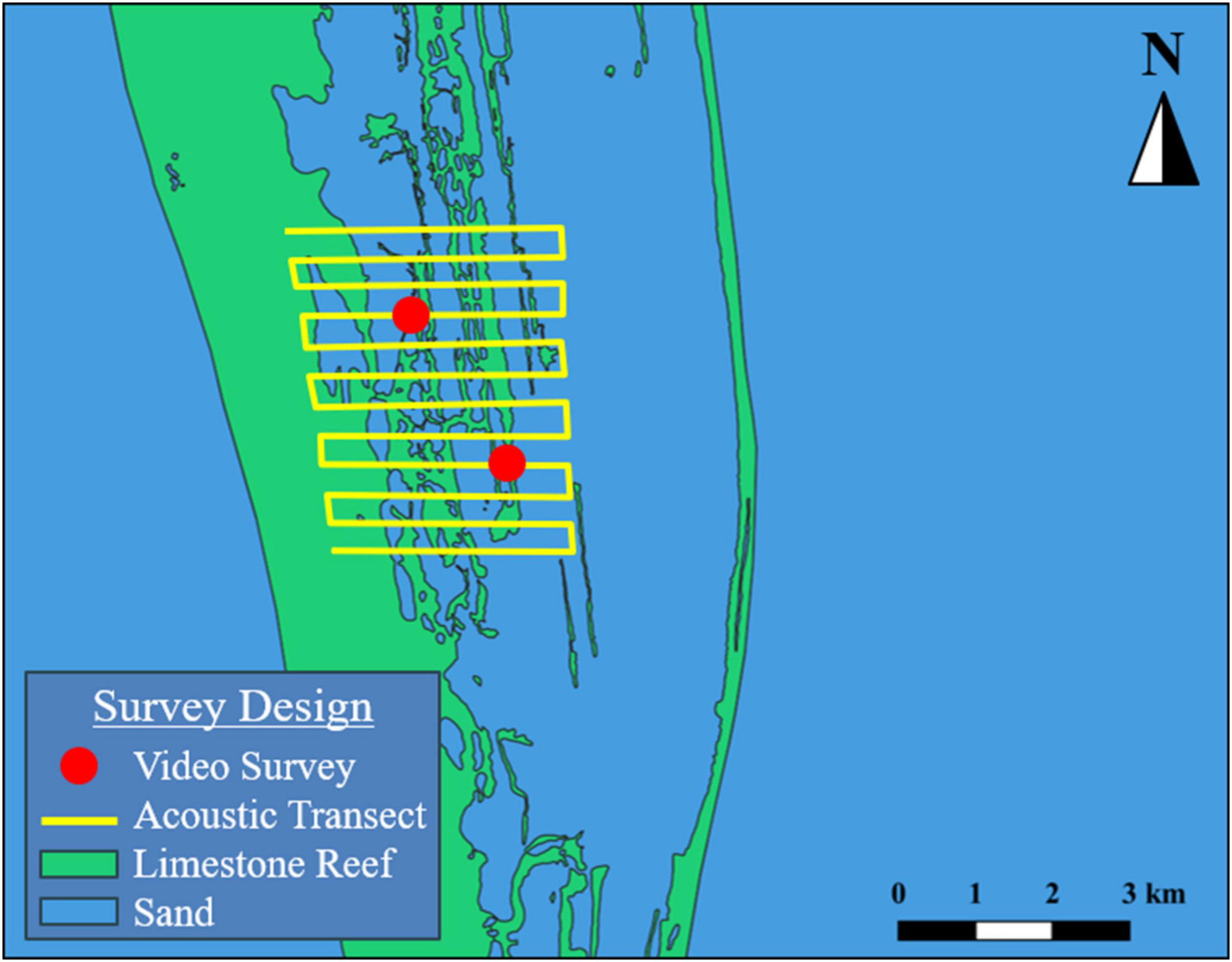
Figure 4. Example echosounder surveys (parallel line) paired with stationary video deployments (dots) along reef sand interface near promontories used to validate user reports.
Fisheries observer surveys consisted of on-water surveys aboard a charter fishing vessel. The observer recorded fishing pressure and landings at the reported aggregation, and reproductive state of fish that were harvested using standard gonad assessment protocols consistent with Lowerre-Barbieri et al. (2011). In addition to on-water surveys, participating charter fishers were interviewed upon returning from fishing activities and the reproductive state of harvested fish was assessed. Biweekly interviews were made during the aggregation period to confirm the occurrence of aggregation activity and consisted of general questions related to: (1) the targeted species, (2) locations and timing of any aggregations observed, (3) depth where aggregation fishing took place, (4) observations of milt or eggs flowing from captured fish, (5) size of aggregation(s), (6) age of report (i.e., when did they see an aggregation relative to when they were interviewed), (7) a general description of habitat where aggregations were observed (e.g., artificial reef or natural reef), (8) and observations of notable behaviors exhibited by aggregating fish (Supplementary Material).
Results
Literature Review
Between the three Web of Science queries conducted, 178 articles were identified. Several of these articles were represented in multiple queries, reducing the total unique sources to 116 peer-reviewed journal articles (Table 2). Only 27 of these studies related to the target species, 24 of which were conducted outside of our current study region. The three remaining articles focused on mutton snapper age, growth, and mortality (Burton, 2002); and the life history, movement and management of gray snapper (Faunce and Serafy, 2007; Luo et al., 2009). No articles pertaining to the target species spawning in the ECA were identified, but eight articles related to spawning were identified from other regions in the coastal United States (i.e., Gulf of Mexico and South Atlantic). Nine additional Florida-centric references offer insight into the life history, management, movement, spawning, and general ecology of the study species and those grouper and snapper found in the ECA that are taxonomically similar (Table 3). Goliath grouper are not among the target species in this review, but they are known to spawn in the ECA. Seven studies characterizing aspects of goliath grouper life history, management, movement, spawning, and ecology were identified by our queries, four of which were conducted in the ECA. Black and red grouper were also excluded from our synthesis due to an absence of aggregation reports in the study region, but 11 studies characterizing their life history, management, movement, spawning, and ecology were identified. Those studies were conducted near the West Florida Shelf, Dry Tortugas, Florida Keys, Puerto Rico, and US Virgin Islands.
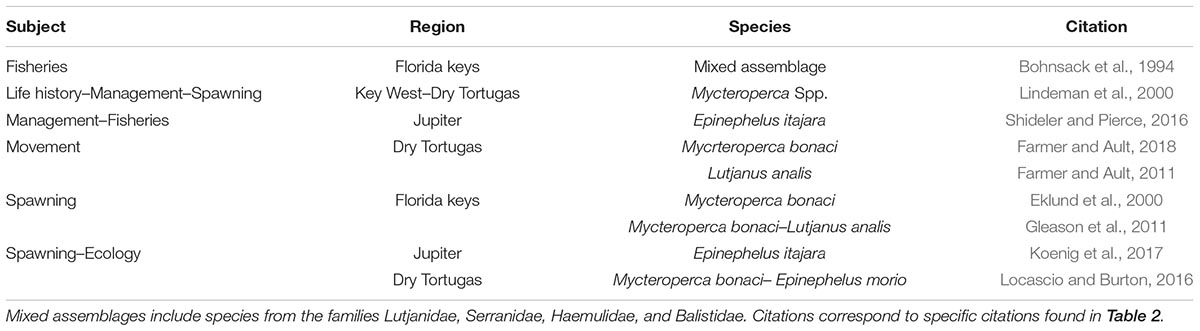
Table 3. Literature related to target and non-target (taxonomically similar) species found in Florida.
Five articles describing hogfish life history, movement and habitat use in the eastern Gulf of Mexico, Eastern United States, and Florida Keys were identified outside of the Web of Science query, but information pertaining to spawning in the ECA is absent from the literature.
Unpublished Theses
Potential spawning locations for various snapper species along the east coast of Florida were identified by Tishler-Meadows (2012) who presented a survey that capitalized on fishers’ ecological knowledge and identified 51 potential spawning locations for red snapper (L. campechanus) (27), gray snapper (19), mutton snapper (8), cubera snapper (6), and vermilion snapper (3). Nine of the reported aggregations were considered multi-species aggregations, four of which occurred just beyond the northern extent of the ECA. Species included in these aggregations were gray and mutton snapper (3), and gray, mutton and cubera snapper (1). Eight gray snapper aggregations, nineteen mutton snapper, and three cubera snapper aggregations were reported to occur within the northern extent of the ECA. Due to confidentiality agreements with fishers, the precise location of reported aggregations were not presented, thus it is uncertain whether all the reported aggregations lie within the ECA. Direct evidence of spawning (gametes released in water column) was only observed at two of the reported spawning sites (gray snapper), but advanced stage gonadal development was observed at 49 of the reported sites (all species). Reproductive seasonality varied for all species when compared to conspecific spawning periods in other regions throughout the United States and Greater Caribbean, but reports peaked between June and July, and ranged from April to September (Table 4).
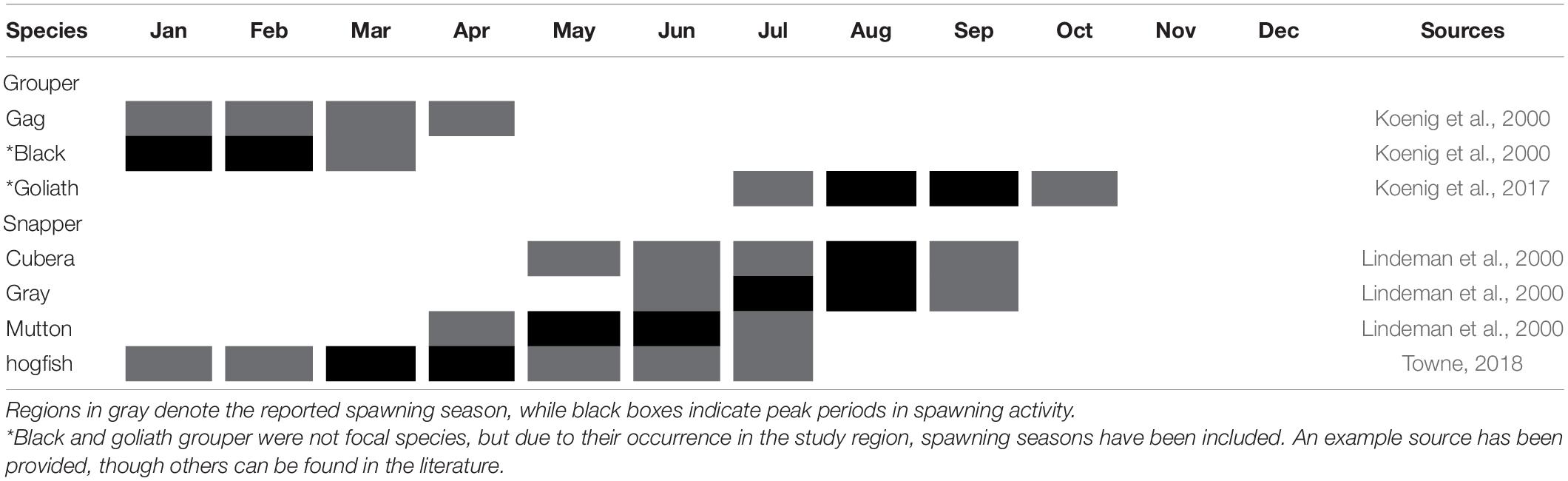
Table 4. Spawning seasonality for study species found in the Southeast Florida Coral Reef Ecosystem Conservation Area.
Towne (2018) examined age and growth of hogfish in southeast Florida. The field effort was limited in scope, and focused on the expected peak of the spawning season between March and May (McBride and Richardson, 2007). Evidence of spawning in the ECA was presented, based on observations of courtship behavior by divers. Personal communication with the author (i.e., Towne) confirms that both male and female hogfish had fully developed gonads during the spring season, based on a macroscopic assessment of reproductive stage from harvested specimens. This interpretation is consistent with the observed spawning period identified in the Florida Keys, Puerto Rico, and eastern Gulf of Mexico (Colin, 1982; McBride and Richardson, 2007; Muñoz et al., 2010; Collins and Mcbride, 2015). Four additional reports related to hogfish were identified, including the most current Southeast Data, Assessment, and Review (SEDAR) hogfish stock assessment (SEDAR 37) (Cooper et al., 2012). An addendum to SEDAR 37 was released in 2018, though this pertained to the West Florida Shelf hogfish stock (Addis et al., 2018). Information related to spawning in the study area was absent from the identified reports.
User Reports
From 2014 through 2016, 13 potential aggregations were identified for the five different study species, between the southern extent of Miami-Dade County and the northern extent of Martin County (Table 5 and Figure 3). Reports were collected from long-time professional fishers (>10 years of experience) and members of the South Atlantic Fisheries Management Council with connections to the fishing community. Reports were also provided by state and federal fisheries biologist that work primarily with the focal species. Goliath grouper spawning aggregations in Palm Beach County were identified by resource users as economically and ecologically important, and have been reported here, but were not a priority study species identified by the SEFCRI due to the harvest moratorium currently in place. One vermillion snapper (Rhomboplites aurorubens) aggregation was also reported to occur during the summer months, but precise information pertaining to timing and location could not be verified, and it was not prioritized as a study species.
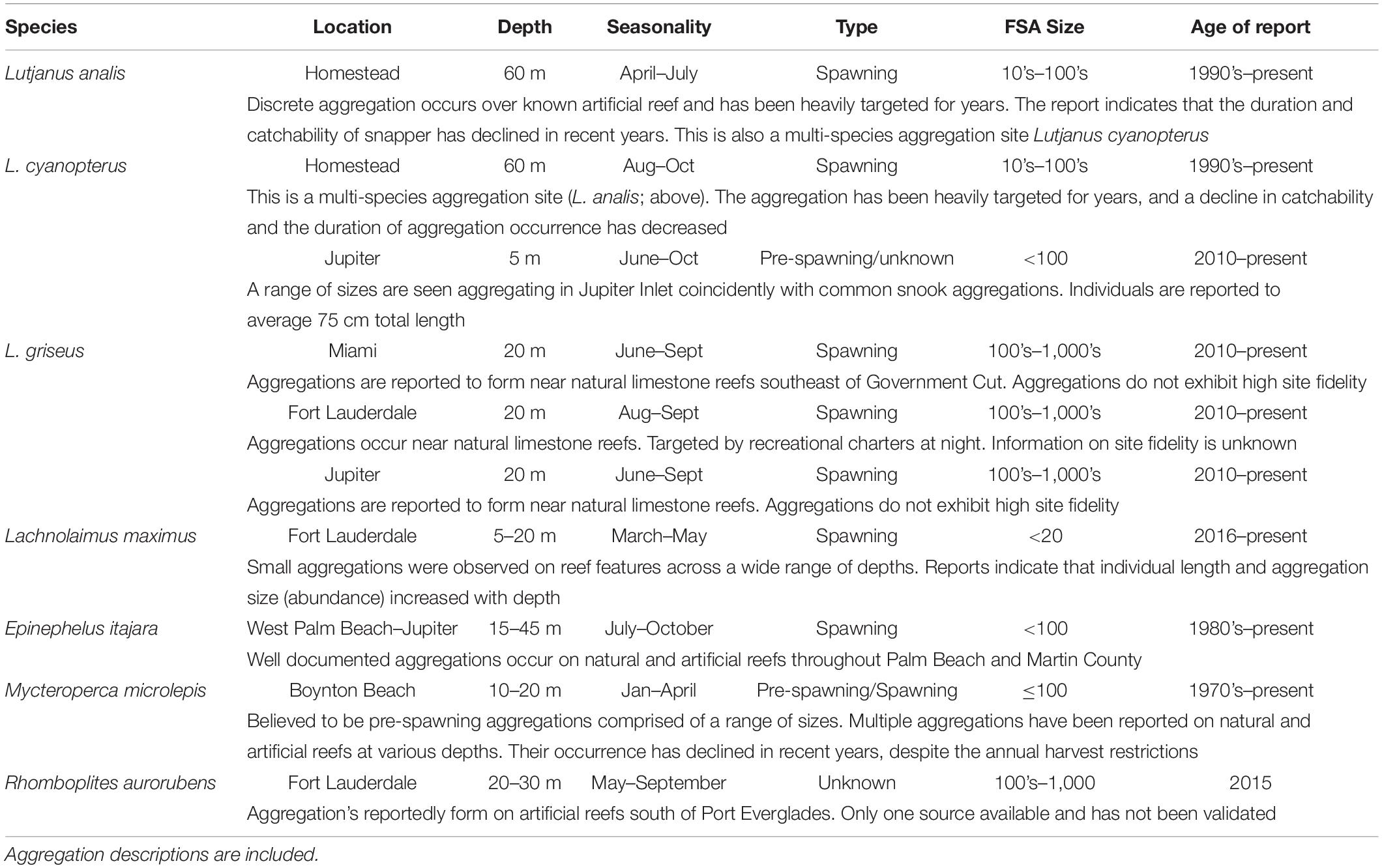
Table 5. Anecdotal reports of spawning aggregations in the Southeast Florida Coral Reef Ecosystem Conservation Area collected between 2014 and 2016.
Spawning Aggregation Validation
Only three aggregations out of the 13 identified were reported to be active with precise spatial information and selected for field validation. A gag grouper aggregation reported to occur near Boynton Beach, Florida was not observed despite multiple attempts to confirm their presence during the expected reproductive season in 2016. Echosounder surveys (n = 8) were conducted over an approximately 60 km2 region between January and March during full moon periods. URUV surveys (n = 19) were conducted at high relief reef locations where small schools were detected, though gag grouper were not observed in URUV data. Video data consistently revealed a mixed reef fish assemblage and high-density schools of tomtates (Haemulon aurolineatum) (Figure 5). Bi-weekly interviews with a collaborating SCUBA diving shop during the 2016 and 2017 season (n = 10) also indicated that gag grouper were never seen aggregated at the suspected aggregation site.
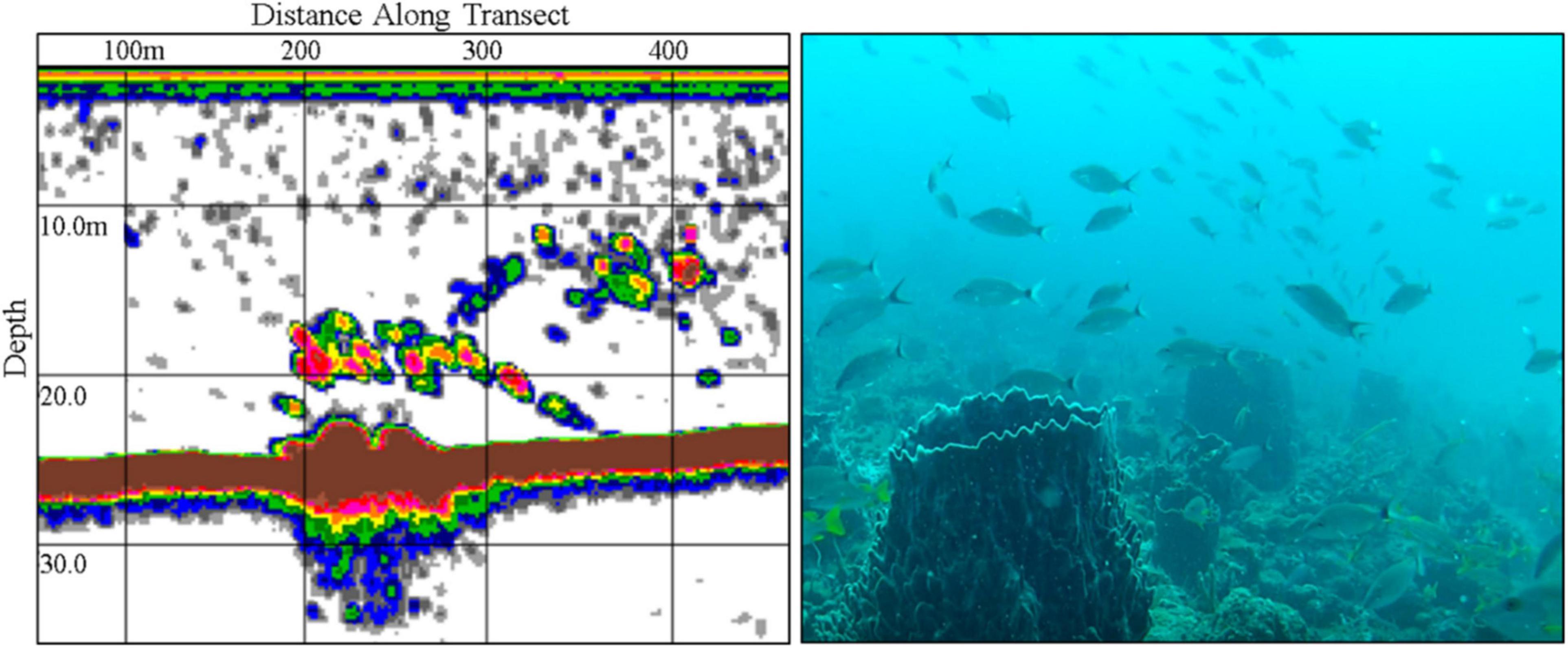
Figure 5. Echosounder surveys were conducted on a historically recognized gag grouper aggregation site east of Boynton Beach, Florida (left). Unbaited remote underwater video (URUV) tripods were deployed (right) where elevated backscatter was observed in echosounder surveys, to determine species identity and abundance, though no evidence of aggregating gag grouper was observed. Schools consisted of a mixed assemblage of reef fish, dominated by dense schools of tomtates (Haemulon aurolineatum).
Paired echosounder and URUV surveys near Jupiter, Florida were also used to validate a reported gray snapper aggregation occurring over a discrete natural reef area (approx. 1.5 km2), between July and September 2016 (n = 6). No areas of concentrated backscatter were identified by echosounders, but URUV (n = 5) and drop camera surveys (n = 5) were performed near high-relief reef-sand interfaces (i.e., promontories), where gray snapper were expected to aggregate. Gray snapper were not observed in videos, and subsequent resource user interviews indicated that gray snapper in the study area are not often isolated to discrete regions as described by the original source.
A cubera and mutton snapper aggregation was reported to occur offshore of Homestead, Florida. The report indicated that the aggregations occurred annually from April to July (mutton snapper), and August–September (cubera snapper) during full moons. However, the mutton snapper aggregation had not been reported as active for several years, and both FSAs were heavily fished since the early 2000’s (no specific date could be provided). Researchers joined a recreational fishing charter on two occasions, in August 2014 and 2015, during full moon overnight fishing charters to confirm the occurrence of the cubera snapper FSA. Biweekly interviews were also conducted to assess the status of the cubera snapper aggregation. During field surveys, eight mature cubera snapper were captured (four each year), and four were harvested (two from each year). All eight fish were >80.0 cm total length, and the four harvested cubera snapper were spawning capable males with fully ripe gonads, qualified using the classification system developed by Domeier and Colin (1997). Surveys were not conducted in September of 2014 and 2015, or in the 2016 season, based on reports from the charter captain that the aggregations had not formed.
Discussion
Information related to commercially and recreationally important snapper and grouper reproduction (specifically spawning aggregations) in the ECA is essentially absent from the peer-reviewed literature based on our Web of Science queries and a thorough review of additional primary and gray literature sources. No research specifically characterizing the spatial aspects of spawning aggregation formation (for the focal species), and their seasonality in the ECA was identified. However, numerous literature sources describing spawning seasonality in other regions were available and used to develop a calendar to forecast FSA seasonality, which showed a high degree of overlap with the reports of FSA occurrence provided by fishers. The information available in the literature was primarily limited to research describing life-history, movement, and seasonality of reproductive development. Thus, the paucity of data identified is likely an accurate reflection of the state of FSA science in the ECA.
When compared to the number of current and historical aggregations reported throughout the region by users, it is clear that a focused effort is required to confirm the presence of and characterize the state of regional FSAs that may still occur. Considering the high degree of spatiotemporal variability associated with FSA formation, it is not unreasonable to presume that aggregations were missed by our field and interview approach, which were dependent on up-to-date user reports distributed over a large geographical area for many species. While user reports do offer the highest spatial and temporal resolution, a lack of reports is not necessarily sufficient evidence to conclude that aggregations are not occurring in the reported region. This is exceptionally true in cases where users are not actively targeting the species of interest. For example, the gag grouper fishery is closed from January through April to protect their populations from exploitation during the reproductive season2. This precluded any targeted fishing by commercial and recreational anglers during the study period, which may have produced spawning reports useful to our field efforts had the fishery been open. Thus, in the case of the reported gag grouper aggregation near Boynton Beach, Florida, we were solely dependent on SCUBA diving charter reports and our own exploratory field surveys across a wide expanse of continuous reef. Had the commercial and recreational fishing community been targeting the inshore gag grouper fishery at the time, it is possible that field survey efforts may have been more successful.
Fish Spawning Aggregations have also been historically reported to occur near natural and artificial promontories, which function as recurrent spawning sites for various species. Indeed, spawning aggregations have been found near promontories in the Florida Keys (Feeley et al., 2018), northeast Florida (Koenig et al., 2000), and west Florida (Coleman et al., 1996), while in contrast, there are fewer promontories along the northern extent of the Florida Reef Tract, and fewer confirmed reports of spawning aggregation occurrence. This may explain why aggregations reported to occur in the study region are difficult to locate and exhibit lower site fidelity (i.e., they are not concentrated on discrete features). Furthermore, while high relief features do occur along the northern extent of the Florida Reef Tract, some features that may be ordinarily attractive to aggregating species occur beyond their typical spawning depths. For example, Tishler-Meadows (2012) reported gray snapper aggregations at depths between 15 and 60 m, but this is deeper than reported spawning depths in Florida Keys, Dry Tortugas, and Cuba (9–37 m) (Domeier and Colin, 1997; Lindeman et al., 2000; Claro and Lindeman, 2003).
In addition to abiotic considerations, species-specific reproductive behaviors and regional differences may further hinder our ability to detect aggregations. For instance, gray snapper aggregations are known to be less predictable in time and space, as they spawn repeatedly over protracted time periods and exhibit lower fidelity to discrete locations (Domeier and Colin, 1997; Sadovy De Mitcheson et al., 2008; Farmer et al., 2017). They may form aggregations on large swaths of reef for short periods of time and inadvertently avoid exploitation because their occurrence is unpredictable, brief, and their movements are frequent. Even with respect to species that typically form predictable aggregations in discrete areas (i.e., mutton and cubera snapper), detection along continuous reefs has been historically difficult. For example, a black grouper aggregation was observed on one occasion by researchers near Key Largo, Florida (Eklund et al., 2000), but a subsequent study between 2008 and 2012 was only able to re-locate the aggregation on one occasion, despite repeated diver surveys paired with echosounder surveys over the 4 years period (Taylor et al. Unpubl. data).
Anthropogenic factors may also explain why FSAs have remained undetected and thus understudied in the ECA. Specifically, FSA identification (initial detection by managers and scientists), and subsequent investigation, has typically been tied to reports from resource users participating in targeted aggregation fishing activities that have occurred over extended periods. Drawing from examples found in the literature, aggregation fishing had occurred for extended periods, and only after decreases in catches became noticeable to resource users, did reports reach fisheries managers and scientists. At that point, most of the aggregations reported in the literature were overfished, extirpated, or were suffering substantial losses due to on-going fishing activities (Luckhurst, 1998, 2010; Burton et al., 2005; Nemeth, 2005). In the context of reports gathered during this study, only the cubera and mutton snapper aggregations identified near Homestead, Florida were reported by fishers as heavily fished, and had been for over a decade (Binder personal comm.). The user indicated that both cubera and mutton snapper abundance at the aggregation site had decreased steadily over a 10-years period and indicated that management intervention was needed to protect the two resources.
No users indicated that aggregating species in the ECA were consistently fished beyond the two reported cases. Indeed, despite the generally high pressure exerted on fishery resources in south Florida, specifically the snapper and grouper fisheries, very little evidence of on-going aggregation fishing was documented. A paucity of information in the media (i.e., newspapers and social media) also suggests that aggregating species are not exposed to aggregation fishing activity within the ECA. Conversely, media sources (newspaper, radio broadcast, and social media) and charter fishing services widely publicize and offer permit and mutton snapper aggregation fishing opportunities in the Florida Keys, which have resulted in heavy fishing pressure during spring and summer full moon periods at discrete FSA sites. Thus, it is plausible that the variability of aggregation occurrence, which is driven by ephemeral hydrodynamic events (i.e., current changes, upwelling, etc.) and the heterogeneous landscape (i.e., limited promontories and expanses of continuous reef interspersed with sandy substrate), mitigates aggregation fishing activity in the ECA.
Potentially the largest obstacle hindering effective FSA assessments and effective conservation has been the lack of real-time data streams in regions of concern (Kobara et al., 2013). Reports from resource users, that make their livelihoods using coastal resources, offer a wealth of real-time information collected over expansive geographical areas (Gerhardinger et al., 2006). Additionally, individuals from the local fishing community are capable of tracking fine-scale changes in environmental factors, using decades of experience (in some cases), to interpret environmental conditions that dictate where target fish schools may be on a given day. Indeed, numerous studies have successfully utilized local and traditional knowledge from fishers to achieve a baseline understanding of the spatial and temporal dynamics of aggregations (Johannes, 1978; Lindeman et al., 2000; Sadovy De Mitcheson et al., 2008; Freitas et al., 2011), and invested resource users have contributed directly to the recovery of FSAs throughout the United States and Greater Caribbean (Lindeman et al., 2000; Burton et al., 2005; Nemeth et al., 2006; Feeley et al., 2018).
Fish Spawning Aggregations represent “hotspots” of fish production during ephemeral periods in time and space that often support multiple aggregating spawning species, and play a role in promoting overall ecosystem health through the stimulation of fish biomass and biodiversity (Schärer et al., 2010; Archer et al., 2015; Grüss et al., 2018). Unfortunately, FSAs also represent attractive targets to fishers, and there are many cases of decline and extirpation after extended periods of excessive fishing (Sadovy De Mitcheson et al., 2008). An erosion of trust between resource users and managers has resulted in challenges assimilating their knowledge into assessments and management process (Boonstra and Nhung, 2012; Jagers et al., 2012). The SEFCRI was specifically created to bridge that gap between resource users and managers and develop effective long-term solutions to coastal and fisheries management issues. Cooperation between users and managers that result in actionable reports from users for field investigations are essential to the future of integrated fisheries management, especially with respect to protecting FSAs. The approach presented here is an important first step toward understanding the spatiotemporal dynamics of regional FSA occurrence, and represents a thorough synthesis of information describing the state of knowledge for recreational and commercially important aggregating species found in the ECA. These data can be used to inform future management plan development, and we hope that these data will be used as a framework for future studies focused on improving our understanding of FSA dynamics in south Florida.
Data Availability Statement
The identity of resource users and precise locations of reported aggregations are not provided due to the sensitivity of this material. The data layers described herein can be found at: https://www.arcgis.com/apps/webappviewer/index.html?id=0825dda753674dabbd184ea5cae8a8c8&extent=-9381939.8924%2C2833900.6103%2C-8442681.6888%2C3250941.0367%2C102100.
Author Contributions
BB gathered reports, carried out fieldwork and prepared the manuscript. JT NOAA partner assisted with the manuscript preparation. KG SEFCRI partner assisted with the manuscript preparation. KB project principal investigator advised BB (Ph.D. student) and assisted with manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
Funding for this project was provided by the National Oceanic Atmospheric Administration Coral Reef Conservation Program (FY 2014-18). The Florida Department of Environmental Protection Southeast Coral Reef Initiative (SEFCRI) advisory council awarded this project to Florida International University, and the award was administered by the Cooperative Institute for Marine and Atmospheric Studies.
Conflict of Interest
KG was employed by ERT Inc. during the time of field data collection and manuscript preparation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to acknowledge the Southeast Florida Coral Reef Initiative Team and Technical Advisory Committee, the Florida Department of Environmental Protection Coral Reef Conservation Program, the National Oceanic and Atmospheric Administration Coral Reef Conservation Program, and the Cooperative Institute for Marine and Atmospheric Studies for administering the funds to complete this effort. We would also like to thank Kenyon Lindeman for his advice while preparing this manuscript, as well as Michelle Tishler-Meadows and Ian Towne for providing us with their theses. Note that the scientific results and conclusions, as well as any views or opinions expressed herein, are those of the authors and do not necessarily reflect those of NOAA or the Department of Commerce. This is contribution 267 from the Coastlines and Oceans Division of the Institute of Environment at Florida international University.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.671477/full#supplementary-material
Footnotes
- ^ https://ourfloridareefs.org/tool/
- ^ https://safmc.net/regulations/regulations-by-species/gag-grouper/
References
Adams, A., Wolfe, R. K., Barkowski, N., and Overcash, D. (2009). Fidelity to spawning grounds by a catadromous fish, Centropomus undecimalis. Mar. Eco. Prog. Ser. 389, 213–222. doi: 10.3354/meps08198
Addis, D. T., Smith, E. H., and Swanson, C. E. (2018). “Stock assessment of hogfish in the west Florida shelf stock 1986-2016,” in SEDAR 37U, (Florida, FL: SEDAR).
Aguilar-Perera, A. (2004). Detection of fishing effects on a nassau grouper spawning aggregation from southern Quintana Roo, Mexico. Proc. 55th GCFI 2004, 543–556.
Allee, R. J., David, A. W., and Naar, D. F. (2012). Two shelf-edge marine protected areas in the eastern Gulf of Mexico. Seafloor Geomorphol. Benthic Habit. 2012, 435–448. doi: 10.1016/b978-0-12-385140-6.00030-x
Allman, R. J., and Grimes, C. B. (2002). Temporal and spatial dynamics of spawning, settlement, and growth of gray snapper (Lutjanus griseus) from the West Florida Shelf as determined from otolith microstructures. Fish. Bul. 3, 391–403.
Amezcua, F., Soto-Avila, C., and Green-Ruiz, Y. (2006). Age, growth, and mortality of the spotted rose snapper Lutjanus guttatus from the Southeastern Gulf of California. Fish Res. 3, 293–300. doi: 10.1016/j.fishres.2005.10.012
Archer, S. K., Allgeier, J. E., Semmens, B. X., Heppell, S. A., Pattengill-Semmens, C. V., Rosemond, A. D., et al. (2015). Hot moments in spawning aggregations: implications for ecosystem-scale nutrient cycling. Coral Reefs 34, 19–23. doi: 10.1007/s00338-014-1208-4
Arena, P. T., Jordan, L. K. B., and Spieler, R. E. (2007). Fish assemblages on sunken vessels and natural reefs in southeast Florida, USA. Hydrobiologia 580, 157–171. doi: 10.1007/s10750-006-0456-x
Ault, J. S., Bohnsack, J. A., and Meester, G. A. (1998). A retrospective (1979-1996) multispecies assessment of coral reef fish stocks in the Florida Keys. Fish Bul. 3, 395–414.
Barbour, A. B., and Adams, A. J. (2012). Biologging to examine multiple life stages of an estuarine-dependent fish. Mar. Eco. Prog. Ser. 457, 241–250. doi: 10.3354/meps09669
Baumberger, R. E., Brown-Peterson, N. J., Reed, J. K., and Gilmore, R. G. (2010). Spawning aggregation of beardfish, Polymixia lowei, in a deep-water sinkhole off the Florida Keys. Copeia 1, 41–46. doi: 10.1643/ce-09-004
Bohnsack, J. A., Harper, D. E., and McClellan, D. B. (1994). Fisheries trends from Monroe County, Florida. Bul. Mar. Sci. 3, 982–1018.
Boonstra, W. J., and Nhung, P. T. H. (2012). The Ghosts of Fisheries Management. J. Nat. Resour. Policy Res. 4, 1–25. doi: 10.1080/19390459.2012.642634
Bryan, D. R., Luo, J., Ault, J. S., Mcclellan, D. B., Smith, S. G., Snodgrass, D., et al. (2015). Transport and connectivity modeling of larval permit from an observed spawning aggregation in the dry tortugas, Florida. Env. Bio. Fishes 98, 2263–2276. doi: 10.1007/s10641-015-0445-x
Bueno, L. S., Bertoncini, A. A., Koenig, C. C., Coleman, F. C., Freitas, M. O., Leite, J. R., et al. (2016). Evidence for spawning aggregations of the endangered atlantic goliath grouper Epinephelus itajara in southern Brazil. J. Fish Bio. 89, 876–889. doi: 10.1111/jfb.13028
Buitrago, J., Capelo, J., Gutierrez, J., Rada, M., Hernandez, R., and Grune, S. (2006). Living macromolluscs from a paleo-reef region on the northeastern Venezuelan continental shelf. Estuar. Coastal Shelf Sci. 66, 634–642. doi: 10.1016/j.ecss.2005.11.006
Bullock, L. H., and Murphy, M. D. (1994). Aspects of the life-history of the yellowmouth grouper, Mycteroperca-interstitialis, in the eastern Gulf of Mexico. Bul. Mar. Sci. 55, 30–45.
Burton, M. l., Brennan, K. J., Munoz, R. C., and Parker, R. O. (2005). Preliminary evidence of increased spawning aggregations of mutton snapper (Lutjanus analis) at Riley’s Hump two years after establishment of the Tortugas South Ecological Reserve. Fisher. Bul. 103, 404–410.
Burton, M. L. (2002). Age, growth and mortality of mutton snapper, Lutjanus analis, from the east coast of Florida, with a brief discussion of management implications. Fish. Res. 59, 31–41. doi: 10.1016/S0165-7836(02)00007-3
Carson, E. W., Saillant, E., Renshaw, M. A., Cummings, N. J., and Gold, J. R. (2011). Population structure, long-term connectivity, and effective size of mutton snapper (Lutjanus analis) in the Caribbean Sea and Florida Keys. Fisher. Bul. 109, 416–428.
Chiappone, M., Sluka, R., and Sealey, K. S. (2000). Groupers (pisces : Serranidae) in fished and protected areas of the Florida Keys, Bahamas and northern Caribbean. Mar. Eco. Prog. Ser. 198, 261–272. doi: 10.3354/meps198261
Claro, R., and Lindeman, K. C. (2003). Practical approaches to achieve economic and conservation goals. Gulf Caribb. Res. 14, 139–154.
Claydon, J. (2004). Spawning aggregations of coral reef fishes: Characteristics, hypotheses, threats and management. Oceanogr. Mar. Biol. Annu. Rev. 42, 265–302. doi: 10.1201/9780203507810
Coleman, F. C., Koenig, C. C., and Collins, L. A. (1996). Reproductive styles of shallow-water groupers (Pisces: Serranidae) in the eastern Gulf of Mexico and the consequences of fishing spawning aggregations. Environ. Biol. Fishes 47, 129–141. doi: 10.1007/BF00005035
Coleman, F. C., Scanlon, K. M., and Koenig, C. C. (2011). Groupers on the edge: shelf edge spawning habitat in and around marine reserves of the northeastern Gulf of Mexico. Pro. Geo. 63, 456–474. doi: 10.1080/00330124.2011.585076
Colin, P. L. (1982). Spawning and larval development of the hogfish, Lachnolaimus maximus (Pisces: Labriadae). Fish. Bull. 80, 853–862.
Collins, A. B., and Mcbride, R. S. (2015). Variations in reproductive potential between nearshore and offshore spawning contingents of hogfish in the eastern Gulf of Mexico. Fish. Manag. Ecol. 22, 113–124. doi: 10.1111/fme.12102
Collins, L. A., Walling, W. T., Brusher, J. H., Remy, M. G., Mikulas, J. J., Chandler, G. M., et al. (2003). Preliminary results from a study of reproduction in the vermilion snapper (Lutjanidae : Rhomboplites aurorubens) from the eastern US Gulf of Mexico, 1991-2001. Proc. 54th GCFI 2003, 580–591.
Cooper, W., O’Hop, J., Murphy, M., and Chagaris, D. (2012). The 2013 Stock Assessment Report for Hogfish in the South Atlantic and Gulf of Mexico. Florida, FL: Florida Fish and Wildlife Conservation Commission.
Crabtree, R. E., and Bullock, L. H. (1998). Age, growth, and reproduction of black grouper, Mycteroperca bonaci, in Florida waters. Fish Bul. 96, 735–753.
D’Alessandro, E. K., Sponaugle, S., and Serafy, J. E. (2010). Larval ecology of a suite of snappers (Family: Lutjanidae) in the straits of Florida, western Atlantic Ocean. Mar. Eco. Prog. Ser. 410, 159–175. doi: 10.3354/meps08632
Danylchuk, A. J., Cooke, S. J., Goldberg, T. L., Suski, C. D., Murchie, K. J., Danylchuk, S. E., et al. (2011). Aggregations and offshore movements as indicators of spawning activity of bonefish (Albula vulpes) in the Bahamas. Mar. Bio. 158, 1981–1999. doi: 10.1007/s00227-011-1707-6
Degidio, J. L. A., Yanong, R. P. E., Watson, C. A., Ohs, C. L., Cassiano, E. J., and Barden, K. (2017). Spawning, embryology, and larval development of the milletseed butterflyfish Chaetodon miliaris in the laboratory. N. Amer. J. Aquacult. 79, 205–215. doi: 10.1080/15222055.2017.1302025
Denit, K., and Sponaugle, S. (2004). Growth variation, settlement, and spawning of gray snapper across a latitudinal gradient. Trans. Amer. Fish Soc. 133, 1339–1355. doi: 10.1577/t03-156.1
Domeier, M. l (2004). A potential larval recruitment pathway originating from a Florida marine protected area. Fish Oceanogr. 13, 287–294. doi: 10.1111/j.1365-2419.2004.00287.x
Domeier, M. L., and Colin, P. L. (1997). Tropical reef fish spawning aggregations: defined and reviewed. Bull. Mar. Sci. 60, 698–726.
Donahue, M. J., Karnauskas, M., Toews, C., and Paris, C. B. (2015). Location isn’t everything: timing of spawning aggregations optimizes larval replenishment. PLoS One 10:0130694. doi: 10.1371/journal.pone.0130694
Eklund, A., Mcclellan, D. B., and Harper, D. E. (2000). Black grouper aggregations in relation to protected areas within the Florida Keys national marine sanctuary. Bul. Mar. Sci. 66, 721–728.
Ellis, D. M., and DeMartini, E. E. (1995). Evaluation of a video camera technique for indexing abundances of juvenile pink snapper, Pristipomoides filamentosus, and other Hawaiian insular shelf fishes. Fishery Bull. 93, 67–77.
Farmer, N. A., and Ault, J. S. (2011). Grouper and snapper movements and habitat use in Dry Tortugas, Florida. Mar. Eco. Prog. Ser. 433, 169–184. doi: 10.3354/meps09198
Farmer, N. A., and Ault, J. S. (2018). Accounting for detection gaps when evaluating reef fish habitat use in an acoustic array. Canad. J. Fish Aquat. Sci. 75, 375–388. doi: 10.1139/cjfas-2016-0494
Farmer, N. A., Heyman, W. D., Karnauskas, M., Kobara, S., Smart, T. I., Ballenger, J. C., et al. (2017). Timing and locations of reef fish spawning off the southeastern united states. PLoS One 12:0172968. doi: 10.1371/journal.pone.0172968
Faunce, C. H., and Serafy, J. E. (2007). Nearshore habitat use by gray snapper (Lutjanus griseus) and bluestriped grunt (Haemulon sciurus): environmental gradients and ontogenetic shifts. Bull. Mar. Sci. 80, 473–495.
Faunce, C. H., and Serafy, J. E. (2008). Growth and secondary production of an eventual reef fish during mangrove residency. Estuar. Coast. Shelf Sci. 79, 93–100. doi: 10.1016/j.ecss.2008.03.006
Feeley, M. W., Luiz, O. J. Jr., and Zurcher, N. (2009). Colour morph of a probable queen angelfish Holacanthus ciliaris from Dry Tortugas, Florida. J. Fish Bio. 74, 2415–2421. doi: 10.1111/j.1095-8649.2009.02259.x
Feeley, M. W., Morley, D., Acosta, A., Barbera, P., Hunt, J., Switzer, T., et al. (2018). Spawning migration movements of Mutton Snapper in Tortugas, Florida: Spatial dynamics within a marine reserve network. Fish. Res. 204, 209–223. doi: 10.1016/j.fishres.2018.02.020
Fitzhugh, G., Koenig, C. C., Coleman, F. C., Grimes, C. B., and Sturges, W. (2005). Spatial and temporal patterns in fertilization and settlement of young gag (Mycteroperca microlepis) along the West Florida Shelf. Bul. Mar. Sci. 77, 377–396.
Flaherty, K. E., Switzer, T. S., Winner, B. L., and Keenan, S. F. (2014). Regional correspondence in habitat occupancy by gray snapper (Lutjanus griseus) in estuaries of the southeastern United States. Estuar. Coasts 37, 206–228. doi: 10.1007/s12237-013-9652-x
Freitas, M. O., de Moura, R. L., Francini-Filho, R. B., and Minte-Vera, C. V. (2011). Spawning patterns of commercially important reef fish (Lutjanidae and Serranidae) in the tropical western South Atlantic. Sci. Mar. 75, 135–146. doi: 10.3989/scimar.2011.75n1135
Frias-Torres, S. (2013). Should the critically endangered goliath grouper Epinephelus itajara be culled in Florida? Oryx 47, 88–95. doi: 10.1017/s0030605312000361
Garlock, T. M., Camp, E. V., and Lorenzen, K. (2017). Using fisheries modeling to assess candidate species for marine fisheries enhancement. Fish Res. 186, 460–467. doi: 10.1016/j.fishres.2016.08.024
Gerhardinger, L., Marenzi, R., Andrade, A., Medeiros, R., and Hostim-Silva, M. (2006). Local Ecological Knowledge on the Goliath Grouper Epinephelus itajara (Teleostei: Serranidae) in Southern Brazil. Neotrop. Ichthyol. 4, 441–450. doi: 10.1590/s1679-62252006000400008
Gilmore, R. G., and Jones, R. S. (1992). Color variation and associated behavior in the Epinepheline groupers, Mycteroperca-microlepis and M. phenax. Bul. Mar. Sci. 51, 83–103.
Gleason, A. C. R., Kellison, G. T., and Reid, R. P. (2011). Geomorphic characterization of reef fish aggregation sites in the upper Florida Keys, USA, using single-beam acoustics. Pro. Geo. 63, 443–455. doi: 10.1080/00330124.2011.585075
Gledhill, C., and David, A. (2004). Survey of fish assemblages and habitat within two marine protected areas on the West Florida Shelf. Proc. 55th GCFI 2004, 614–625.
Grüss, A., Biggs, C., Heyman, W. D., and Erisman, B. (2018). Prioritizing monitoring and conservation efforts for fish spawning aggregations in the U.S. Gulf of Mexico. Sci. Rep. 8, 1–10. doi: 10.1038/s41598-018-26898-0
Gruss, A., Thorson, J. T., Sagarese, S. R., Babcock, E. A., Karnauskas, M., Walter, J. F., et al. (2017). Ontogenetic spatial distributions of red grouper (Epinephelus morio) and gag grouper (Mycteroperca microlepis) in the US Gulf of Mexico. Fish Res. 193, 129–142. doi: 10.1016/j.fishres.2017.04.006
Hare, J. A., and Walsh, H. J. (2007). Planktonic linkages among marine protected areas on the South Florida and southeast United States continental shelves. Canad. J. Fish Aquat. Sci. 64, 1234–1247. doi: 10.1139/f07-089
Hernandez, K. M., Risch, D., Cholewiak, D. M., Dean, M. J., Hatch, L. T., Hoffman, W. S., et al. (2013). Acoustic monitoring of atlantic cod (Gadus morhua) in Massachusetts Bay: implications for management and conservation. ICES J. Mar. Sci. 70, 628–635. doi: 10.1093/icesjms/fst003
Heyman, W. D., and Kjerfve, B. (2008). Characterization of transient multi-species reef fish spawning aggregations at Gladden Spit, Belize. Bull. Mar. Sci. 83, 531–551.
Holt, S. A. (2008). Distribution of red drum spawning sites identified by a towed hydrophone array. Trans. Amer. Fish Soc. 137, 551–561. doi: 10.1577/t03-209.1
Hostetter, E. B., and Munroe, T. A. (1993). Age, growth, and reproduction of tautog Tautoga onitis (Labridae, Perciformes) from coastal waters of Virginia. Fishery Bul. 91, 45–64.
Jagers, S. C., Berlin, D., and Jentoft, S. (2012). Why comply? Attitudes towards harvest regulations among Swedish fishers. Mar. Policy 36, 969–976. doi: 10.1016/j.marpol.2012.02.004
Johannes, R. E. (1978). Reproductive strategies of coastal marine fishes in the tropics. Environ. Biol. Fishes 3, 65–84. doi: 10.1007/BF00006309
Johnson, D. R., Perry, H. M., and Lyczkowski-Shultz, J. (2013). Connections between Campeche Bank and red snapper populations in the Gulf of Mexico via modeled larval transport. Trans. Amer. Fish Soc. 142, 50–58. doi: 10.1080/00028487.2012.720630
Jue, N. K., Coleman, F. C., and Koenig, C. C. (2014). Wide-spread genetic variability and the paradox of effective population size in the gag, Mycteroperca microlepis, along the west Florida shelf. Mar. Bio. 161, 1905–1918. doi: 10.1007/s00227-014-2473-z
Kadison, E., Brandt, M., Nemeth, R., Martens, J., Blondeau, J., and Smith, T. (2017). Abundance of commercially important reef fish indicates different levels of over-exploitation across shelves of the US Virgin Islands. PLoS One 12:0180063. doi: 10.1371/journal.pone.0180063
Karnauskas, M., Chérubin, L. M., and Paris, C. B. (2011). Adaptive significance of the formation of multi-species fish spawning aggregations near submerged capes. PLoS One 6:0022067. doi: 10.1371/journal.pone.0022067
Karnauskas, M., Walter, J. F., Campbell, M. D., Pollack, A. G., Drymon, J. M., and Powers, S. (2017). Red snapper distribution on natural habitats and artificial structures in the northern Gulf of Mexico. Mar. Coastal Fish 9, 50–67. doi: 10.1080/19425120.2016.1255684
Kobara, S., Heyman, W. D., Pittman, S. J., and Nemeth, R. S. (2013). Biogeography of transient reeffish spawning aggregations in the Caribbean?: a synthesis for future research and management. Oceanogr. Mar. Biol. Annu. Rev. 51, 281–326.
Koenig, C. C., Bueno, L. S., Coleman, F. C., Cusick, J. A., Ellis, R. D., Kingon, K., et al. (2017). Diel, lunar, and seasonal spawning patterns of the Atlantic goliath grouper, Epinephelus itajara, off Florida, United States. Bull. Mar. Sci. 93, 391–406. doi: 10.5343/bms.2016.1013
Koenig, C. C., Coleman, F. C., and Kingon, K. (2011). Pattern of recovery of the goliath grouper Epinephelus itajara population in the southeastern US. Bul. Mar. Sci. 87, 891–911. doi: 10.5343/bms.2010.1056
Koenig, C. C., Coleman, F. C., Grimes, C. B., Fitzhugh, G. R., Scanlon, K. M., Gledhill, C. T., et al. (2000). Protection of fish spawning habitat for the conservation of warm-temperate reef-fish fisheries of shelf-edge reefs of Florida. Bull. Mar. Sci. 66, 593–616.
Le Port, A., Montgomery, J. C., and Croucher, A. E. (2014). Biophysical modelling of snapper Pagrus auratus larval dispersal from a temperate MPA. Mar. Eco. Prog. Ser. 515, 203–215. doi: 10.3354/meps10973
Lee, T. N., Clarke, M. E., Williams, E., Szmant, A. F., and Berger, T. (1994). Evolution of the Tortugas Gyre and its influence on recruitment in the Florida Keys. Bul. Mar. Sci. 54, 621–646.
Leichter, J. J., Stokes, M. D., and Genovese, S. J. (2008). Deep water macroalgal communities adjacent to the Florida Keys Reef Tract. Mar. Eco. Prog. Ser. 356, 123–138. doi: 10.3354/meps07230
Lindeman, K. C., Pugliese, R., Waugh, G. T., and Ault, J. S. (2000). Developmental patterns within a multispecies reef fishery: management applications for essential fish habitats and protected areas. Bul. Mar. Sci. 66, 929–956.
Locascio, J. V., and Burton, M. I. (2016). A passive acoustic survey of fish sound production at Riley’s Hump within Tortugas South Ecological Reserve: implications regarding spawning and habitat use. Fishery Bul. 114, 103–116. doi: 10.7755/fb.114.1.9
Locascio, J. V., and Mann, D. A. (2011). Diel and seasonal timing of sound production by black drum (Pogonias cromis). Fishery Bul. 109, 327–338.
Lowerre-Barbieri, S. K., Brown-Peterson, N. J., Murua, H., Tomkiewicz, J., Wyanski, D. M., and Saborido-Rey, F. (2011). Emerging issues and methodological advances in fisheries reproductive biology. Mar. Coast. Fish. 3, 32–51. doi: 10.1080/19425120.2011.555725
Lowerre-Barbieri, S. K., Vose, F. E., and Whittington, J. A. (2003). Catch-and-release fishing on a spawning aggregation of common snook: does it affect reproductive output? Trans. Amer. Fish Soc. 132, 940–952. doi: 10.1577/t02-001
Lowerre-Barbieri, S. K., Walters-Burnsed, S. L., and Bickford, J. W. (2016). Assessing reproductive behavior important to fisheries management: a case study with red drum, Sciaenops ocellatus. Eco. App. 26, 979–995. doi: 10.1890/15-0497
Luckhurst, B. (1998). Site fidelity and return migration of tagged red hinds to a spawning aggregation site in Bermuda. Proc. 50th Gulf Caribb. Fish. Inst. 1998, 750–763.
Luckhurst, B. E. (2010). Observations of a black grouper (Mycteroperca bonaci) spawning aggregation in Bermuda. Gulf Caribb. Res. 22, 43–49. doi: 10.18785/gcr.2201.05
Luo, J., Serafy, J. E., Sponaugle, S., Teare, P. B., and Kieckbusch, D. (2009). Movement of gray snapper Lutjanus griseus among subtropical seagrass, mangrove, and coral reef habitats. Mar. Ecol. Prog. Ser. 380, 255–269. doi: 10.3354/meps07911
Mann, D. (2016). Acoustic communication in fishes and potential effects of noise. Effects Noise Aquat. Life 875, 673–678. doi: 10.1007/978-1-4939-2981-8_81
Mann, D., Locascio, J., and Wall, C. (2016). Listening in the ocean: new discoveries and insights on marine life from autonomous passive acoustic recorders. Listening Ocean 2016, 309–324. doi: 10.1007/978-1-4939-3176-7_12
Manooch, C. S., Potts, J. C., Vaughan, D. S., and Burton, M. l (1998). Population assessment of the red snapper from the Southeastern United States. Fish Res. 38, 19–32. doi: 10.1016/s0165-7836(98)00112-x
Manuel Castro-Perez, J., Ernesto Arias-Gonzalez, J., Acosta-Gonzalez, G., and Defeo, O. (2018). Comparison of catch, cpue and length distribution of spawning aggregations of mutton snapper (Lutjanus analis) and grey triggerfish (Balistes capriscus) on a Mesoamerican coral reef. Latin Amer. J. Aquat. Res. 46, 717–726. doi: 10.3856/vol46-issue4-fulltext-9
Marancik, K. E., Richardson, D. E., Lyczkowski-Shultz, J., Cowen, R. K., and Konieczna, M. (2012). Spatial and temporal distribution of grouper larvae (Serranidae: Epinephelinae: epinephelini) in the Gulf of Mexico and Straits of Florida. Fish Bul. 110, 1–20. doi: 10.1007/s10228-006-0367-x
McBride, R. S., and Richardson, A. K. (2007). Evidence of size-selective fishing mortality from an age and growth study of hogfish (Labridae: Lachnolaimus maximus), a hermaphroditic reef fish. Bull. Mar. Sci. 80, 401–417.
McGovern, J. C., Collins, M. R., Pashuk, O., and Meister, H. S. (2002). Temporal and spatial differences in life history parameters of black sea bass in the Southeastern United States. N. Amer. J. Fish Mgmt. 22, 1151–1163. doi: 10.1577/1548-8675(2002)022<1151:tasdil>2.0.co;2
McGovern, J. C., Sedberry, G. R., Meister, H. S., Westendorff, T. M., Wyanski, D. M., and Harris, P. J. (2005). A tag and recapture study of gag, Mycteroperca microlepis, off the southeastern US. Bul. Mar. Sci. 76, 47–59.
McGovern, J. C., Wyanski, D. M., Pashuk, O., Manooch, C. S., and Sedberry, G. R. (1998). Changes in the sex ratio and size at maturity of gag, Mycteroperca microlepis, from the Atlantic coast of the Southeastern United States during 1976-1995. Fishery Bul. 96, 797–807.
Molloy, P. P., Reynolds, J. D., Gage, M. J. G., and Cote, I. M. (2009). Effects of an artisanal fishery on non-spawning grouper populations. Mar. Eco. Prog. Series 392, 253–262. doi: 10.3354/meps08236
Muñoz, R. C., Burton, M. L., Brennan, K. J., and Parker, R. O. Jr. (2010). Reproduction, habitat utilization, and movements of hogfish (Lachnolaimus maximus) in the Florida Keys, USA: comparisons from fished versus unfished habitats. Bul. Mar. Sci. 86, 93–116.
Murchie, K. J., Danylchuk, A. J., Cooke, S. J., O’Toole, A. C., Shultz, A., Haak, C., et al. (2012). Considerations for tagging and tracking fish in tropical coastal habitats: lessons from bonefish, barracuda, and sharks tagged with acoustic transmitters. Telemetry Techniq. 2012, 389–412.
Nadon, M. O., Ault, J. S., Williams, I. D., Smith, S. G., and Dinardo, G. T. (2015). Length-based assessment of coral reef fish populations in the main and Northwestern Hawaiian Islands. PLoS One 10:g003. doi: 10.1371/journal.pone.0133960.g003
Nelson, J., Wilson, R., Coleman, F., Koenig, C., Devries, D., Gardner, C., et al. (2012). Flux by fin: fish-mediated carbon and nutrient flux in the Northeastern Gulf of Mexico. Mar. Bio. 159, 365–372. doi: 10.1007/s00227-011-1814-4
Nelson, M. D., Koenig, C. C., Coleman, F. C., and Mann, D. A. (2011). Sound production of red grouper Epinephelus morio on the West Florida Shelf. Aquat. Bio. 12, 97–108. doi: 10.3354/ab00325
Nemeth, R. S. (2005). Population characteristics of a recovering US Virgin Islands red hind spawning aggregation following protection. Mar. Ecol. Prog. Ser. 286, 81–97. doi: 10.3354/meps286081
Nemeth, R. S., Blondeau, J., Herzlieb, S., and Kadison, E. (2006). Spatial and temporal patterns of movement and migration at spawning aggregations of red hind, Epinephelus guttatus, in the U.S. Virgin Islands. Environ. Biol. Fishes 78, 365–381. doi: 10.1007/s10641-006-9161-x
Paris, C. B., Cowen, R. K., Claro, R., and Lindeman, K. C. (2005). Larval transport pathways from cuban snapper (Lutjanidae) spawning aggregations based on biophysical modeling. Mar. Eco. Prog. Series 296, 93–106. doi: 10.3354/meps296093
Peebles, E. B., Hall, J. R., and Tolley, S. G. (1996). Egg production by the bay anchovy Anchoa mitchilli in relation to adult and larval prey fields. Mar. Eco. Prog. Series 131, 61–73. doi: 10.3354/meps131061
Pichorim, S. F., and Suzuki, D. F. (2015). Proposal of a biological database to research for the fish Epinephelus itajara. New Jersey, NJ: IEEE.
Pinkard, D. R., and Shenker, J. M. (2001). Seasonal variation in density, size, and habitat distribution of juvenile yellowtail snapper (Ocyurus chrysurus) in relation to spawning patterns in the Florida Keys. Amer. Zool. 41, 1556–1557.
Porch, C. E., Eklund, A. M., and Scott, G. P. (2006). A catch-free stock assessment model with application to goliath grouper (Epinephelus itajara) off southern Florida. Fish Bul. 104, 89–101.
Potts, J. C., and Burton, M. L. (2017). Preliminary observations on the age and growth of dog snapper (Lutjanus jocu) and mahogany snapper (Lutjanus mahogoni) from the Southeastern US. Peerj 5:e3167. doi: 10.7717/peerj.3167
Powell, A. B. (2003). Larval abundance, distribution, and spawning habits of spotted seatrout (Cynoscion nebulosus) in Florida Bay, Everglades National Park, Florida. Fish Bul. 101, 704–711.
Powell, A. B., Cheshire, R. T., Laban, E. H., Colvocoresses, J., O’Donnell, P., and Davidian, M. (2004). Growth, mortality, and hatch date distributions of larval and juvenile spotted seatrout (Cynoscion nebulosus) in Florida Bay, Everglades National Park. Fishery Bul. 102, 142–155.
Provancha, M. J., and Hall, C. R. (1991). Ecology and life-history of the clown goby inhabiting the upper Banana River, Cape-Canaveral, Florida. Env. Bio. Fishes 31, 41–54. doi: 10.1007/bf00002158
Reed, J. K., Koenig, C. C., and Shepard, A. N. (2007). Impacts of bottom trawling on a deep-water oculina coral ecosystem off Florida. Bul. Mar. Sci. 81, 481–496.
Reed, J. K., Shepard, A. N., Koenig, C. C., Scanlon, K. M., and Gilmore, R. G. (2005). Mapping, habitat characterization, and fish surveys of the deep-water oculina coral reef marine protected area: a review of historical and current research. Cold-Water Corals Ecosyst. 2005, 443–465. doi: 10.1007/3-540-27673-4_22
Reisewitz, A., and Harper, J. (2013). Our Florida Reefs Community Working Group Communications Plan. Miami, FL: FDEP CRCP.
Renan, X., Montero-Munoz, J., Garza-Perez, J. R., and Brule, T. (2016). Age and stock analysis using otolith shape in gags from the southern Gulf of Mexico. Trans. Amer. Fish Soc. 145, 1252–1265. doi: 10.1080/00028487.2016.1217928
Rotman, F. J., Matzie, W., Benetti, D. D., Feeley, M. W., Alarcon, J. F., Zimmerman, S., et al. (2003). Advances in aquaculture technology of mutton snapper (Lutjanus analis) and greater amberjack (Seriola dumerili), two candidate species for offshore grow-out. Open Ocean Aquacult. Res. Commercial Reality 2003, 215–221.
Rowell, T. J., Schaerer, M. T., Appeldoorn, R. S., Nemeth, M. I., Mann, D. A., and Rivera, J. A. (2012). Sound production as an indicator of red hind density at a spawning aggregation. Mar. Eco. Prog. Series 462, 241–250. doi: 10.3354/meps09839
Russell, M., Sadovy, de Mitcheson, Y., Erisman, B., Hamilton, R., Luckhurst, B., et al. (2014). Status Report World’s Fish Aggregations 2014 Report by Science and Conservation of Fish Aggregations (SCRFA) in collaboration with the ICRI Ad Hoc Committee for Reef Associated Fisheries. Berlin: ResearchGate.
Sadovy De Mitcheson, Y., and Colin, P. L. (2013). Reef Fish Spawning Aggregations: Biology, Research and Management. Berlin: Springer, doi: 10.1007/978-94-007-1980-4
Sadovy De Mitcheson, Y., Cornish, A., Domeier, M., Colin, P. L., Russell, M., et al. (2008). A global baseline for spawning aggregations of reef fishes. Conserv. Biol. 22, 1233–1244. doi: 10.1111/j.1523-1739.2008.01020.x
Sadovy de Mitcheson, Y., Craig, M. T., Bertoncini, A. A., Carpenter, K. E., Cheung, W. W. L., et al. (2013). Fishing groupers towards extinction: a global assessment of threats and extinction risks in a billion dollar fishery. Fish Fish. 14, 119–136. doi: 10.1111/j.1467-2979.2011.00455.x
Saillant, E., Bradfield, S. C., and Gold, J. R. (2010). Genetic variation and spatial autocorrelation among young-of-the-year red snapper (Lutjanus campechanus) in the Northern Gulf of Mexico. ICES J. Mar. Sci. 67, 1240–1250. doi: 10.1093/icesjms/fsq011
Sanchez, P. J., Appeldoorn, R. S., Schaerer-Umpierre, M. T., and Locascio, J. V. (2017). Patterns of courtship acoustics and geophysical features at spawning sites of black grouper (Mycteroperca bonaci). Fishery Bul. 115, 186–195. doi: 10.7755/fb.115.2.5
Saucier, M. H., and Baltz, D. M. (1993). Spawning site selection by spotted sea-trout, Cynoscion nebulosus, and black drum, Pogonias cromis, in Louisiana. Env. Bio. Fishes 36, 257–272. doi: 10.1007/bf00001722
Saul, S. E., Walter, J. F. III, Die, D. J., Naarc, D. F., and Donahue, B. T. (2013). Modeling the spatial distribution of commercially important reef fishes on the West Florida Shelf. Fish Res. 143, 12–20. doi: 10.1016/j.fishres.2013.01.002
Schärer, M. T., Nemeth, M. I., and Appeldoorn, R. S. (2010). Protecting a multi-species spawning aggregation at Mona Island, Puerto Rico. Proc. 62nd Gulf Caribb. Fisher. Instit. 2010, 252–259.
Sedberry, G. R., Mcgovern, J. C., and Pashuk, C. (2001). The Charleston Bump: an island of essential fish habitat in the Gulf stream. Island in the Stream. Oceanogr. Fish Charleston Bump 25, 3–23.
Shideler, G. S., and Pierce, B. (2016). Recreational diver willingness to pay for goliath grouper encounters during the months of their spawning aggregation off eastern Florida, USA. Ocean Coastal Mgmt. 129, 36–43. doi: 10.1016/j.ocecoaman.2016.05.002
Shulzitski, K., McCartney, M. A., and Burton, M. L. (2009). Population connectivity among Dry Tortugas, Florida, and Caribbean populations of mutton snapper (Lutjanus analis), inferred from multiple microsatellite loci. Fishery Bul. 107, 501–509.
Strelcheck, A. J., Fitzhugh, G. R., Coleman, F. C., and Koenig, C. C. (2003). Otolith-fish size relationship in juvenile gag (Mycteroperca microlepis) of the eastern Gulf of Mexico: a comparison of growth rates between laboratory and field populations. Fish Res. 60, 255–265. doi: 10.1016/s0165-7836(02)00171-6
Switzer, T. S., Keenan, S. F., Stevens, P. W., McMichael, R. H. Jr., and Macdonald, T. C. (2015). Incorporating ecology into survey design: monitoring the recruitment of age-0 gags in the eastern Gulf of Mexico. N. Amer. J. Fish Mgmt. 35, 1132–1143. doi: 10.1080/02755947.2015.1082517
Taylor, R. G., Whittington, J. A., and Haymans, D. E. (2001). Catch-and-release mortality rates of common snook in Florida. N. Amer. J. Fish Mgmt. 21, 70–75. doi: 10.1577/1548-8675(2001)021<0070:carmro>2.0.co;2
Tishler-Meadows, M. S. (2012). Spawning indicators of snappers (Lutjanidae) on the east coast of Florida determined from commercial and recreational fisher surveys. Ph. D. thesis. Florida, FL: Florida Institute of Technology.
Todd, A. C., Morey, S. L., and Chassignet, E. P. (2014). Circulation and cross-shelf transport in the Florida big bend. J. Mar. Res. 72, 445–475. doi: 10.1357/002224014815540660
Towne, I. (2018). Age and Growth of Hogfish (Lachnolaimus maximus) in Southeast Florida. MS thesis, Fort Lauderdale, FL: NOVA Southeastern University.
Tupper, M. (2002). Essential fish habitat and marine reserves for groupers in the Turks & Caicos Islands. Proc. 53rd GCFI 2002, 606–622.
Turano, M. J., Davis, D. A., and Arnold, C. R. (2000). Observations and techniques for maturation, spawning, and larval rearing of the yellowtail snapper Ocyurus chrysurus. J. World Aquacult. Soc. 31, 59–68. doi: 10.1111/j.1749-7345.2000.tb00698.x
Tzadik, O. E., Jones, D. L., Peebles, E. B., Koenig, C. C., and Stallings, C. D. (2017). The effects of spatial scale on assigning nursery habitats in atlantic goliath groupers (Epinephelus itajara) using non-lethal analyses of fin rays. Estuar. Coasts 40, 1785–1794. doi: 10.1007/s12237-017-0244-z
Vaughan, D. S., Zhao, B. X., Collins, M. R., Mcgovern, J. C., and Meister, H. S. (1998). Evaluation of multiple survey indices in assessment of black sea bass from the US south Atlantic coast. Fishery Stock Assess. Models 15, 121–136. doi: 10.4027/fsam.1998.06
Walker, B. K., and Gilliam, D. S. (2013). Determining the extent and characterizing coral reef habitats of the northern latitudes of the Florida Reef Tract (Martin County). PLoS One 8:0080439. doi: 10.1371/journal.pone.0080439
Walker, B. K., Jordan, L. K. B., and Spieler, R. E. (2009). Relationship of Reef Fish Assemblages and Topographic Complexity on Southeastern Florida Coral Reef Habitats. J. Coast. Res. 10053, 39–48. doi: 10.2112/SI53-005.1
Wall, C. C., Donahue, B. T., Naar, D. F., and Mann, D. (2011). Spatial and temporal variability of red grouper holes within Steamboat Lumps Marine Reserve, Gulf of Mexico. Mar. Eco. Prog. Series 431, 243–254. doi: 10.3354/meps09167
Wall, C. C., Simard, P., Lindemuth, M., Lembke, C., Naar, D. F., Hu, C., et al. (2014). Temporal and spatial mapping of red grouper Epinephelus morio sound production. J. Fish Bio. 85, 1470–1488. doi: 10.1111/jfb.12500
Walters, S., Lowerre-Barbieri, S., Bickford, J., and Mann, D. (2009). Using a passive acoustic survey to identify spotted seatrout spawning sites and associated habitat in Tampa bay, Florida. Trans. Amer. Fish Soc. 138, 88–98. doi: 10.1577/t07-106.1
Walters, S., Lowerre-Barbieri, S., Bickford, J., Tustison, J., and Landsberg, J. H. (2013). Effects of Karenia brevis red tide on the spatial distribution of spawning aggregations of sand seatrout Cynoscion arenarius in Tampa bay, Florida. Mar. Eco. Prog. Series 479, 191–202. doi: 10.3354/meps10219
Weisberg, R. H., Zheng, L., and Peebles, E. (2014). Gag grouper larvae pathways on the West Florida Shelf. Continental Shelf Res. 88, 11–23. doi: 10.1016/j.csr.2014.06.003
White, D. B., and Palmer, S. M. (2004). Age, growth, and reproduction of the red snapper, Lutjanus campechanus, from the Atlantic waters of the Southeastern US. Bul. Mar. Sci. 75, 335–360.
Woodson, C. B. (2018). The fate and impact of internal waves in nearshore ecosystems. Annu. Rev. Mar. Sci. 10, 421–441. doi: 10.1146/annurev-marine-121916-063619
Young, J. M., Yeiser, B. G., and Whittington, J. A. (2014). Spatiotemporal dynamics of spawning aggregations of common snook on the east coast of Florida. Mar. Eco. Prog. Series 505, 227–240. doi: 10.3354/meps10774
Young, J. M., Yeiser, B. G., Ault, E. R., Whittington, J. A., and Dutka-Gianelli, J. (2016). Spawning site fidelity, catchment, and dispersal of common snook along the east coast of Florida. Trans. Amer. Fish Soc. 145, 400–415. doi: 10.1080/00028487.2015.1131741
Keywords: fish spawning aggregations, South Florida, fisheries management, local knowledge, snapper grouper complex, fishing
Citation: Binder BM, Taylor JC, Gregg K and Boswell KM (2021) Fish Spawning Aggregations in the Southeast Florida Coral Reef Ecosystem Conservation Area: A Case Study Synthesis of User Reports, Literature, and Field Validation Efforts. Front. Mar. Sci. 8:671477. doi: 10.3389/fmars.2021.671477
Received: 23 February 2021; Accepted: 12 May 2021;
Published: 02 July 2021.
Edited by:
Simone Libralato, National Institute of Oceanography and Applied Geophysics, ItalyReviewed by:
Kim de Mutsert, University of Southern Mississippi, United StatesClaudio Vasapollo, Independent Researcher, Ancona, Italy
Copyright © 2021 Binder, Taylor, Gregg and Boswell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Benjamin M. Binder, YmJpbmQwMDJAZml1LmVkdQ==
 Benjamin M. Binder
Benjamin M. Binder J. Christopher Taylor
J. Christopher Taylor Kurtis Gregg
Kurtis Gregg Kevin M. Boswell
Kevin M. Boswell