- 1Sydney School of Veterinary Science, Faculty of Science, The University of Sydney, Camperdown, NSW, Australia
- 2School of Veterinary Science, Massey University, Palmerston North, New Zealand
- 3Molecular Epidemiology and Public Health Laboratory, Hopkirk Research Institute, Massey University, Palmerston North, New Zealand
Septicaemia due to hypervirulent (HV) Klebsiella pneumoniae is the leading cause of neonatal pup mortality in endangered New Zealand sea lions (Phocarctos hookeri) at Enderby Island, in the New Zealand sub-Antarctic. Accounting for approximately 60% of annual pup mortality at this site following an epizootic event in 2001–02, HV K. pneumoniae is also emerging worldwide as a significant community-acquired human pathogen. To facilitate efficient direct mitigation to reduce pup mortality, a case-control study and prospective cohort study were conducted to identify risk factors amenable to active management. Additionally, to investigate impacts of hookworm (Uncinaria spp.), a nested treatment trial with the anthelmintic ivermectin was undertaken concurrently. During two austral summer field seasons (2016–2018), 698 pups were captured for treatment trial recruitment and the collection of morphometric measurements, biological samples and risk factor data. Gastrointestinal carriage of the virulent phenotype of K. pneumoniae was a consistent risk factor, while ivermectin treatment and higher body condition index consistently reduced risk of HV K. pneumoniae mortality. Significantly fewer ivermectin-treated pups were found dead (24.1% control, 11.1% treatment), with a trend towards a higher proportion of HV K. pneumoniae deaths amongst the control group. This study provides evidence to support ivermectin treatment as a pup mortality mitigation strategy in New Zealand sea lions at Enderby Island. If applied to larger colonies where HV K. pneumoniae and hookworm impact pup survival, this intervention could have population-scale benefits for this endangered species. Further work is required to understand how ivermectin prevents HV K. pneumoniae septicaemia, but removal of hookworms before intestinal mucosal damage occurs could limit systemic spread of virulent bacteria from the gastrointestinal tract.
Introduction
Emerging infectious diseases impact animal and ecosystem health globally (Daszak et al., 2000). Hypervirulent (HV) Klebsiella pneumoniae is the most common cause of death in endangered New Zealand (NZ) sea lion (Phocarctos hookeri) pups at sub-Antarctic Enderby Island, Auckland Islands (Figure 1A; Michael et al., 2019). This emerging pathogen, a virulent form of an otherwise common mucosal commensal of most mammals, also causes human disease and mortality (reviewed by Paczosa and Mecsas, 2016; Russo and Marr, 2019). Infection was first discovered on Enderby Island during an epizootic event in 2001–02, continuing as enzootic disease ever since (Wilkinson et al., 2006; Castinel et al., 2007b; Roe et al., 2015; Michael et al., 2019). However, HV K. pneumoniae-associated pup mortality has since been detected at the northernmost extent of the species’ range on mainland New Zealand (Roe et al., 2015) and at the largest breeding colony – Dundas Island, Auckland Islands (unpublished data). In NZ sea lions, HV K. pneumoniae isolates causing mortality have been identified as a clonal lineage (Pinpimai, 2018), readily identified by their hypermucoviscous (HMV) phenotype, due to a major virulence factor of excessive capsule production. Hypervirulent K. pneumoniae disease and mortality is dependent on pup, pathogen, maternal, spatial and environmental factors influencing susceptibility and disease progression. Understanding risk factors for HV K. pneumoniae infection and mortality in pups is critical for determining where management can aid conservation of this endangered species.
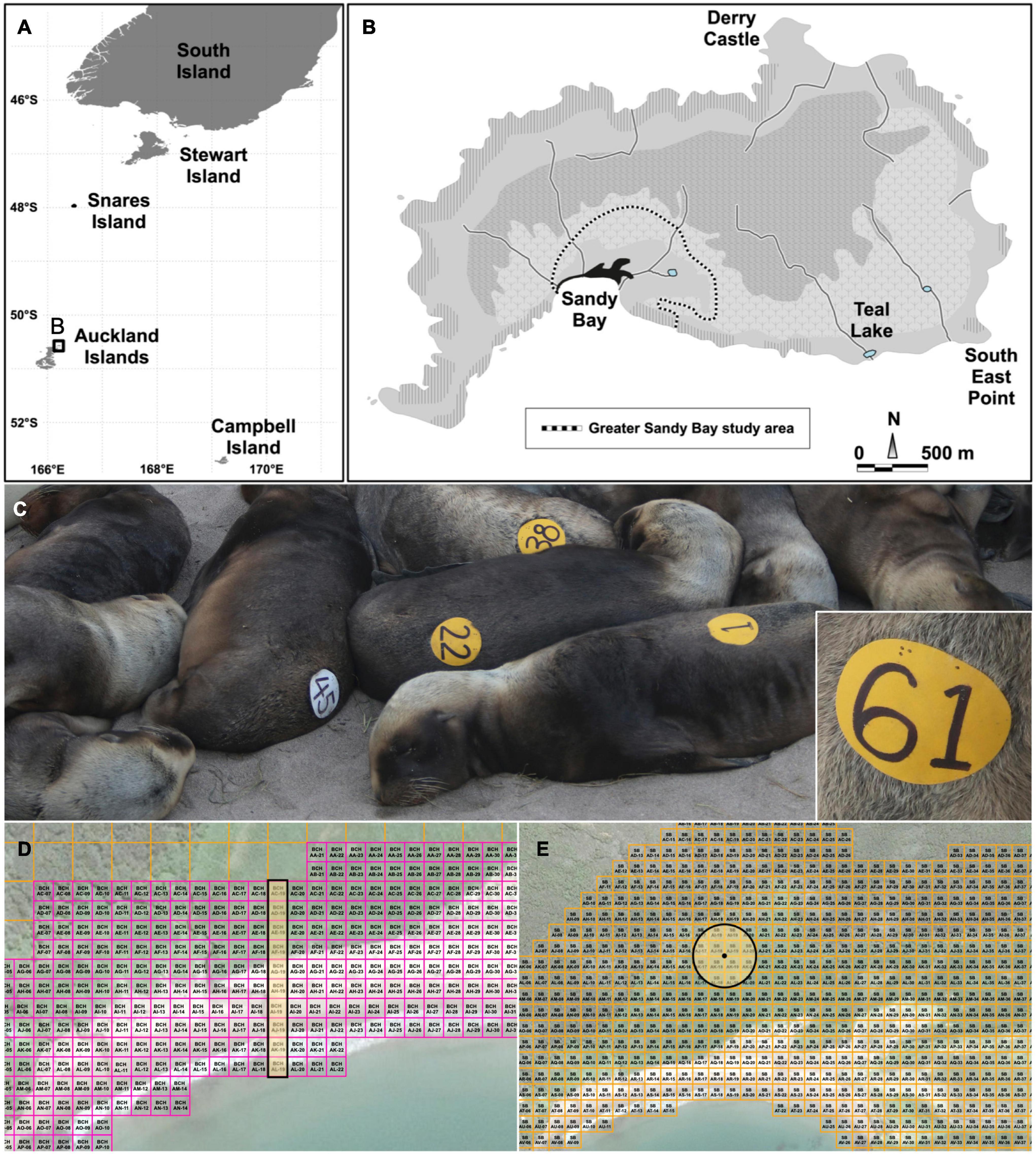
Figure 1. Summary of methodology used in data collection for analysis of risk factors for mortality in New Zealand (NZ) sea lion (Phocarctos hookeri) pups. (A) Map of NZ sea lion breeding sites including the South Island of mainland New Zealand, Stewart Island, Auckland Islands and Campbell Island. (B) Localities of Enderby Island including the study site of greater Sandy Bay area. (C) Uniquely numbered vinyl circular discs glued to NZ sea lion pups. White discs signify cohort study animals, while all other animals were deployed with yellow discs. Inset: Braille numbers were punched into the vinyl before attachment to pups to enable identification after the written number was abraded off by sand. (D) The 10 m2 overlaid GPS grid was used with a randomly selected north-south strip. (E) The 20 m2 overlaid GPS grid was used with a 40 m radius from a randomly selected cell.
Otariid (eared) seals are commonly infected with host-adapted species of hookworm (Uncinaria spp.) resulting in enteric mucosal hemorrhage with anemia, weight loss, weakness and mortality (Lyons et al., 2001; Marcus et al., 2015a; Seguel et al., 2018). Aberrant peritoneal migration of hookworms through the intestinal wall, causing bacteremia and peritonitis has also been reported (Spraker et al., 2004; Lyons et al., 2011; Seguel et al., 2017). A hookworm enteritis-bacteremia (HEB) syndrome has been described in California- (Zalophus californianus) and South American fur seals (Arctocephalus australis) with a proposed link between submucosal enteric hookworm damage and systemic bacterial infection with enteric commensals (Spraker et al., 2007; Seguel et al., 2017). In NZ sea lions, however, although hookworm prevalence can reach 100%, infection is associated with lower burdens and a milder syndrome, with superficial mucosal damage, no significant anemia and no peritoneal migration (Castinel, 2006; Castinel et al., 2007c; Michael et al., 2019). Ivermectin, a macrocyclic lactone anthelmintic drug routinely used for parasite control in domestic animals is effective at reducing or eliminating hookworm burdens in free-ranging otariids (DeLong et al., 2009; Marcus et al., 2015b; Seguel et al., 2018), including NZ sea lions (Castinel, 2006; Chilvers et al., 2009). While the role of hookworm in HV K. pneumoniae disease is unclear, the association requires exploration given the improved survival of a small sample of pups treated with ivermectin during a high K. pneumoniae mortality season at Enderby Island (Chilvers et al., 2009). Given the species’ endangered status and the high proportion of HV K. pneumoniae- associated pup mortality annually (mean 60.2%; Michael et al., 2019), understanding risk and protective factors for pup infection and mortality is integral to active management.
Materials and Methods
All procedures were undertaken in the greater Sandy Bay area (50.5°S, 166.28°E; Figure 1B), Enderby Island, during the 2016–17 and 2017–18 austral summers. Field season lengths were 92 and 84 days, respectively, between early December and early March. Three studies were undertaken concurrently: a randomized controlled clinical treatment trial with ivermectin, a case-control study and a prospective cohort study to investigate risk factors for pup mortality. The nested study design resulted in some pups being part of all three studies.
Procedures were permitted by the Department of Conservation, New Zealand (necropsies 39239-MAR, all other procedures permitted as species management). All methods were approved by the Massey University Animal Ethics Committee (approval number 16/89) and Department of Conservation Animal Ethics Committee (approval number 304).
Colony Monitoring and Routine Procedures
All pups at the Sandy Bay colony were flipper tagged (Jumbo Tag; Dalton Continental, Lichtenvoorde, Netherlands) as part of ongoing demographic studies in mid-January (pups approximately 3 weeks of age) in both seasons. Between mid-December and early January, date of pupping was determined for as many permanently identified females as possible, facilitating matching of pupping/birth date from mother-pup associations. The latter was facilitated between early January and mid-March by recording resights of marked pups with their mother and this time was also used to identify pups with abnormal physical or behavioral findings.
Ivermectin Clinical Treatment Trial: Animal Capture and Processing
Pups were first captured by hand or net when unattended by their mother at approximately 1 week of age and manually restrained in a customized canvas bag, typically <10 min, always <15 min. Individuals were allocated to ivermectin treatment and control groups using a random number table, aiming for approximately 50% in each group. Treatment-allocated pups were administered 0.2 mg/kg ivermectin (Ivomec 1% Injection for Cattle, Sheep and Pigs; Boehringer Ingelheim Animal Health, Manukau City, New Zealand) subcutaneously in the interscapular region; control pups were not injected. Pups were assigned permanent individual identification at first capture with a PIT tag inserted subcutaneously in the dorsal lumbar region. Temporary external identification prior to flipper tagging was achieved via a vinyl disc with a unique number attached to the lumbar region using cyanoacrylate adhesive (Loctite 454; Henkel, Kilsyth, VIC, Australia; Figure 1C). Recaptures were undertaken in conjunction with case-control and prospective cohort studies, with all captures involving collection of data as described below.
Morphometric, Physical Examination and Risk Factor Data
Risk factor data were collected (Table 1). Body mass was measured with hanging scales (WS603; Wedderburn, Auckland, New Zealand). Standard length was measured as the straight distance between the tip of the nose and tip of the tail, and girth as the circumference of the pup’s body just caudal to the pectoral flippers, on exhalation. A full physical examination for wounds or abnormalities was undertaken noting discharge or signs of infection from the eyes, nostrils, umbilicus and tag sites.
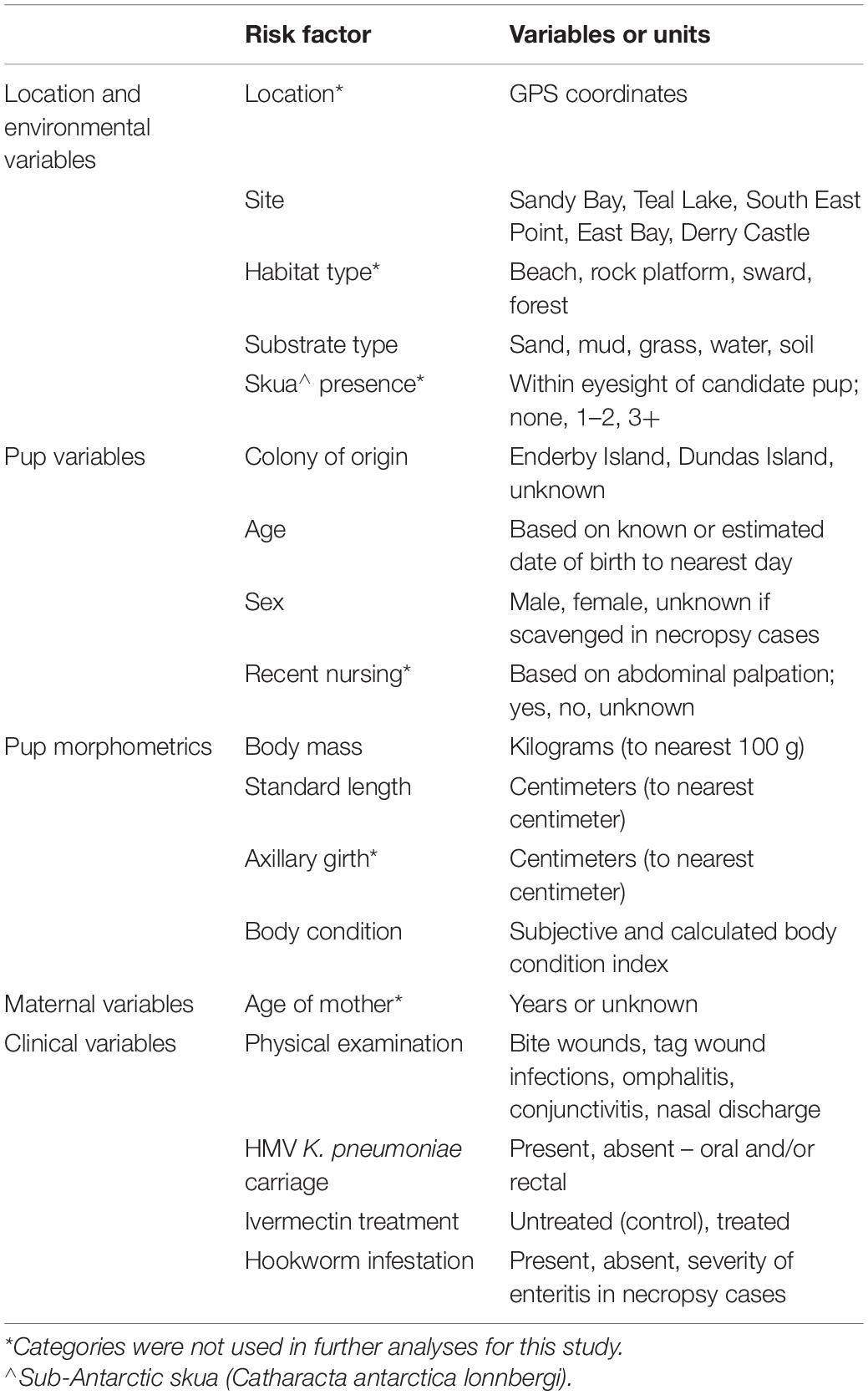
Table 1. Summary of risk factor data collected during and following NZ sea lion (Phocarctos hookeri) pup captures at Sandy Bay, Enderby Island.
Case-Control Study: Design and Animal Selection
The Sandy Bay colony was searched at least daily for dead pups. When found, risk factor data were collected (Table 1). Necropsy examination was undertaken as described by Michael et al. (2019). Within 24 h of finding a dead pup (case), 1–2 control live pups were selected and captured for processing. Randomization of control selection was achieved using a grid system overlaid on a map of Enderby Island, subdivided into zones with 10 m2 cells for Sandy Bay beach and 20 m2 cells for the greater Sandy Bay area (Figures 1D,E). The grid was determined in the field using a handheld GPS (Garmin GPSMAP 64sc; Eastern Creek, NSW, Australia) to locate waypoints at the southwest corner of each cell. During pupping when all pups are on the beach, a random number table was used to select a column of 10 m2 north-south grid cells, allowing marking of the east-west boundaries in the field (Figure 1D). A count of all available pups in the selected area was undertaken and a random number selected to enable a pup to be nominated based on a count of a second researcher blinded to the number chosen. Following dispersal of pups from the beach, the 20 m2 grid was implemented in conjunction with the “distance to destination” function on a handheld GPS allowing visualization of a 40 m radius from the randomly selected point (Figure 1E). If one pup was present in the area, it was chosen as a control. When more than one pup was present, the nearest pup to the waypoint was chosen unless it was part of a group of pups. In this case, a fraction from a random number table was generated and multiplied by the number of pups in the group to select the control. Again, a researcher blinded to the number chosen counted the pups until the designated number was reached and the pup selected. If no pup was present in the area, another point was selected randomly, and the process repeated. Further criteria were exclusion of pups from selection that were actively nursing at the time and pups that had been captured in the preceding 24 h. The selected pup was identified, then samples (oral and rectal swabs, feces) and morphometric and risk factor data were collected (Table 1 and Supplementary Table 1).
Prospective Cohort Study: Design and Processing
Pups were recruited into the cohort study at first capture, by selecting every sixth pup processed in 2016–17 (n = 50) and every third pup in 2017–18 (n = 100). These pups were recaptured approximately fortnightly to collect serial samples and risk factor data (Table 1 and Supplementary Table 1).
Sample Collection, Handling and Field Processing
Oral and rectal swabs were collected by rubbing a dry rayon swab (155C; Copan, Brescia, Italy) in the oral cavity and oropharynx, and a second into the rectum to later determine the presence of HMV K. pneumoniae. Swabs were frozen in liquid nitrogen within six hours of collection and stored at −80°C prior to culture.
Fecal samples were collected on every capture except the first (Supplementary Table 1), using a modified fine tipped swab (160C; Copan) technique (Marcus et al., 2014). Fecal volume was subjectively scored (scant, moderate, or heavy) and a smear made on a microscope slide with a drop of saline if needed. Presence or absence of hookworm ova under light microscopy at 4× or 10× objectives was recorded (Figure 2A).
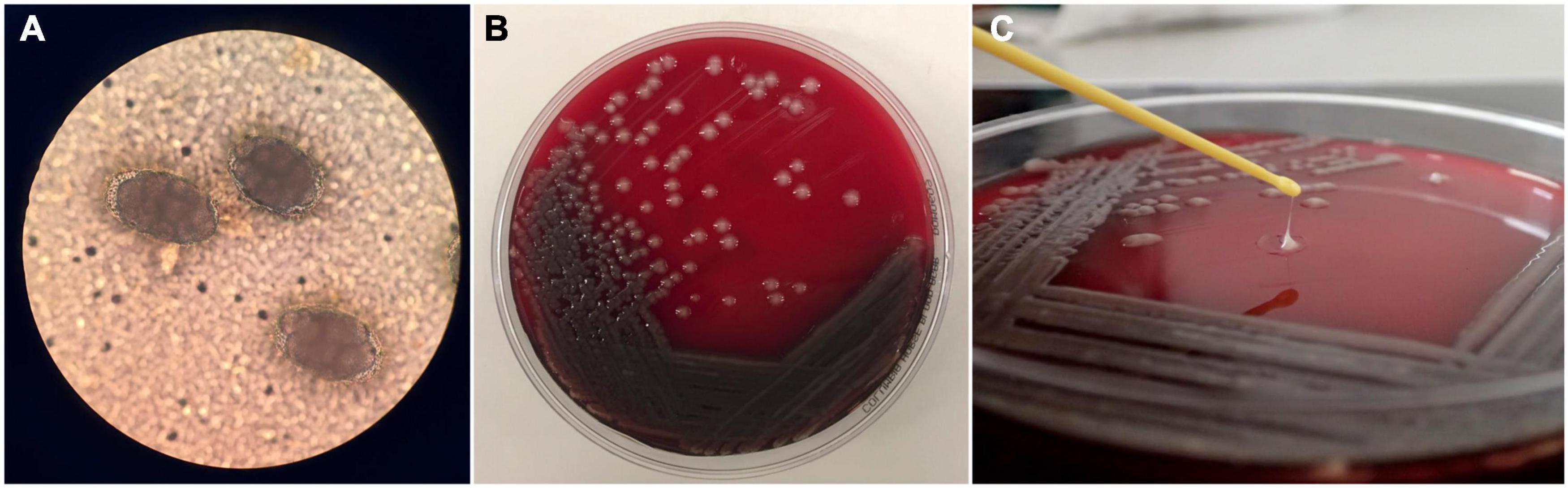
Figure 2. Parasitology and microbiology analysis of samples collected from New Zealand sea lion (Phocarctos hookeri) pups on Enderby Island. (A) Hookworm (Uncinaria spp.) eggs under light microscopy (20× objective) on a direct fecal smear. (B) Pure culture of hypermucoviscous Klebsiella pneumoniae on horse blood agar plate showing large mucoid off-white to translucent colonies. (C) Positive string test on a hypermucoviscous K. pneumoniae isolate, determined by production of a viscous string >5 mm.
Microbiology Laboratory Techniques
Oral and rectal swabs were thawed, subjectively scored on saliva or fecal coverage (scant, moderate, or heavy) and incubated aerobically at 37°C in Luria broth for 18–24 h. The broth was streaked onto differential agar plates (CHROMagarTM Orientation, Fort Richard Laboratories, Auckland, New Zealand) and incubated aerobically at 37°C for 18–24 h. Representative colonies consistent with Klebsiella spp. (mucoid, dark blue, to turquoise) were subcultured onto MacConkeys agar (Fort Richard Laboratories, Auckland, New Zealand) and incubated aerobically at 37°C for a further 18–24 h. Colonies suggestive of Klebsiella spp. (mucoid, positive lactose fermentation) were then subcultured onto horse blood agar (Fort Richard Laboratories) and incubated aerobically at 37°C for another 18–24 h.
Resultant pure cultures (Figure 2B) were then processed as an ethanolic suspension for identification by matrix assisted laser desorption/ionization – time of flight mass spectrometry (MALDI-TOF MS) by homogenizing approximately 2 mm3 of pure bacterial colony in 300 μl sterile water, then mixing with 900 μl high grade 100% ethanol. Targets were prepared according to manufacturer’s recommendations and spectra were measured using the Bruker Biotyper and Bruker isolate database version 7 (7311 RUO). The string test for hypermucoviscosity (Fang et al., 2004) was carried out on representative colonies with a positive recorded if a viscous string >5 mm was produced (Figure 2C).
Statistical Analysis
All data were entered into an Access database (Microsoft Corporation, Auckland, New Zealand), then exported for coding using R (Version 3.6.0) and RStudio (Version 1.2.1335) (R Core Team, 2018). All plots were created using the ggplot2 package (Wickham, 2016). The chi-squared (χ2) test was used to determine associations between categorical variables, with p < 0.05 as the threshold for statistical significance.
For pups with unknown date of birth, “date first seen” was used. This was either the date that an identifiable mother was first seen with a pup if the birth itself was not observed, the date of the pup’s first capture if the mother was not identifiable or the date of tagging if not individually seen before then. Exact ages (calculated from date of birth) and estimated ages (calculated from date first seen) were used in models. Growth rates were calculated by linearly regressing age on mass and standard length for live known-age pups only. Body condition index (BCI) was derived by linearly regressing body mass against standard length, with residuals used as the BCI (Guinet et al., 1998).
Physical examination data was incorporated into wound and discharge scores to indicate extent and severity (Supplementary Table 2). Generalized pair plots and principal component analysis were used to determine correlations between variables so indicative indices representing those parameters could be used in analyses. Due to correlations and the low variability amongst wound variables indices, these were combined into a “wound score” by the addition of all wound categories (head, body, flippers, tag sites, and umbilicus) to indicate degree of skin barrier damage. An overall “wound discharge” score characterized any discharge present (Supplementary Table 2). Models were also run with a “modified wound score” (wound scores of head, body, flippers, and umbilicus only), with interaction terms including tag wound score, wound discharge, and age.
Klebsiella pneumoniae carriage was assigned by whether K. pneumoniae was determined on MALDI-TOF MS speciation of an isolate from an oral or rectal swab. HMV K. pneumoniae carriage was designated for those with a positive K. pneumoniae swab where the isolate had a positive string test. Carriage in models for HMV K. pneumoniae was true when the pup had ever had a swab test positive.
Models were produced using biologically relevant variables for the outcomes of death due to HV K. pneumoniae infection “KlebCase” and death of any cause. Two methods were used to determine the most important factors contributing to risk or protection. Firstly, a series of generalized linear models using the glm function were generated and then stepwise model selection using the step function to assess important contributing factors and interactions (R Core Team, 2018). Regression coefficients included in the candidate models were plotted using the plot_summs function in the jtools package (Long, 2019). The Akaike information criterion (AIC) statistic, which balances goodness of fit and parsimony, was used to compare models (R Core Team, 2018). Generalized mixed effects models were generated using the glmer function in the lme4 package, using individual pup ID as a repeated measure (Bates et al., 2015). Further, cumulative survival rates for HV K. pneumoniae cases and death of any cause were assessed using the NestedCohort package with the Cox proportional hazards regression (coxph) clustered by individual pup ID and Kaplan-Meier survival analysis (nested.km) functions, allowing for missing covariate data (Katki and Mark, 2008) and plotted using the survival (Therneau, 2014) and ggfortify packages (Tang et al., 2016). For both analyses, study time was extrapolated from either date of pup birth or date first seen and date of death or date last seen based on daily colony resighting. Survival after approximately 75 days was censored due to few records following the field season conclusion and expected poor resightability of juvenile sea lions until 4 years of age (Roberts and Doonan, 2016).
Results
A total of 698 pups were captured a total of 1,397 times including 657 first captures, 211 control captures, 383 cohort captures, 126 cases and 20 combined captures (first capture/control or control/cohort). Mean number of captures was four for pups in the cohort study and two for all other pups (range 1–7 captures). All pups were born at Sandy Bay except for two Dundas Island tagged pups and 23 untagged pups presumed to have originated from Dundas Island. Actual dates of birth were determined for 31.4% of pups (211/673) born on Enderby Island (n = 65, 2016–17; n = 146, 2017–18); the remainder estimated based on date first seen.
Ivermectin Clinical Treatment Trial
In 2016–17, 341 Enderby-born pups were randomly recruited into the treatment trial at first capture between 16 December and 15 January. Of these, 163 (47.8%; 83 females, 80 males) were treated and 178 were controls (80 females, 98 males). In 2017–18, 325 pups were recruited between 21 December and 22 January, where 180 (55.4%; 96 females, 84 males) were treated and 145 were controls (66 females, 79 males). Median age of treatment for known age pups was 7 days (n = 107; range 2–19 days).
Overall, 301 pups had at least one fecal sample tested for hookworm ova (n = 295, 2016–17; n = 410, 2017–18) as part of case-control and prospective cohort study recaptures. Of untreated retested Enderby-born pups, 45.5% (50/110) were hookworm positive on at least one test. Of treated retested pups (n = 181), all were negative, except one pup in 2016–17. Detection of hookworm ova in fecal smears was significantly influenced by fecal volume extracted (χ2 = 38.18, p < 0.0001). Moderate or heavy volume fecal samples were more than three times more likely to be positive than when scant feces were collected (37%, 30.1–44.3% 95% CI moderate to heavy; 9.4%, 6.7–12.8% 95% CI scant).
Growth Rate
Calculated daily mass gain including both sexes and treatment groups was 195 g (178–221 g 95% CI). There was no significant difference between sexes, but consistent with sexual dimorphism, males gained more weight per day (male 207 g, 182–233 g 95% CI; female 173 g, 150–198 g 95% CI; Supplementary Figure 1). Within each sex cohort, there was a non-significant trend of increased mean daily growth rate with treatment (male treatment 216 g, 191–243 g 95% CI; male control 197 g, 173–223 g 95% CI; female treatment 181 g, 158–206 g 95% CI; female control 161 g, 139–185 g 95% CI).
There was no significant difference between sex or ivermectin treatment group on growth rate in length, with an overall mean of 3.4 mm per day (male treatment 3.5 mm, 3.2–3.9 mm 95% CI; male control 3.2 mm, 2.9–3.5 mm 95% CI; female treatment 3.3 mm, 3.0–3.6 mm 95% CI; female control 3.4 mm, 3.1–3.7 mm 95% CI; Supplementary Figure 1).
Survival
Of all ivermectin-treated pups in both seasons, 11.1% (38/343) were found dead, significantly fewer than 24.1% (78/323) of untreated controls (χ2 = 18.86, p < 0.001). There was a higher proportion of HV K. pneumoniae deaths in the untreated group (44/78; 56%) than the treated group (16/38; 42%), but this was not significant (χ2 = 1.56, p = 0.21). These analyses exclude untagged untreated pups presumed to originate from Dundas Island that also died at Enderby Island (n = 10), of which 60% died due to HV K. pneumoniae.
Risk Factor Analysis
Standard length correlated well with age (Pearson’s r = 0.86, 0.84–0.88 95% CI), and was included in further analyses as a surrogate index. Of six initial models including biologically relevant predictor variables for HV K. pneumoniae deaths (“KlebCase”; Table 2), model 2 accounted for 78.3% of weight in AIC analysis. In model 2, significant protective factors (or factors reducing risk) included ivermectin treatment, higher BCI, the 2017–18 season, sand substrate and an interaction between wound score and length (Table 3 and Figure 3A). Significant risk factors for death due to HV K. pneumoniae included gastrointestinal HMV K. pneumoniae carriage, greater length (and therefore age), higher wound score, water substrate and higher nasal discharge scores (Table 3 and Figure 3A). Model 3 accounted for 15% weight in AIC analysis (the second highest) and contained identical variables excluding the interaction term between wound score and length.
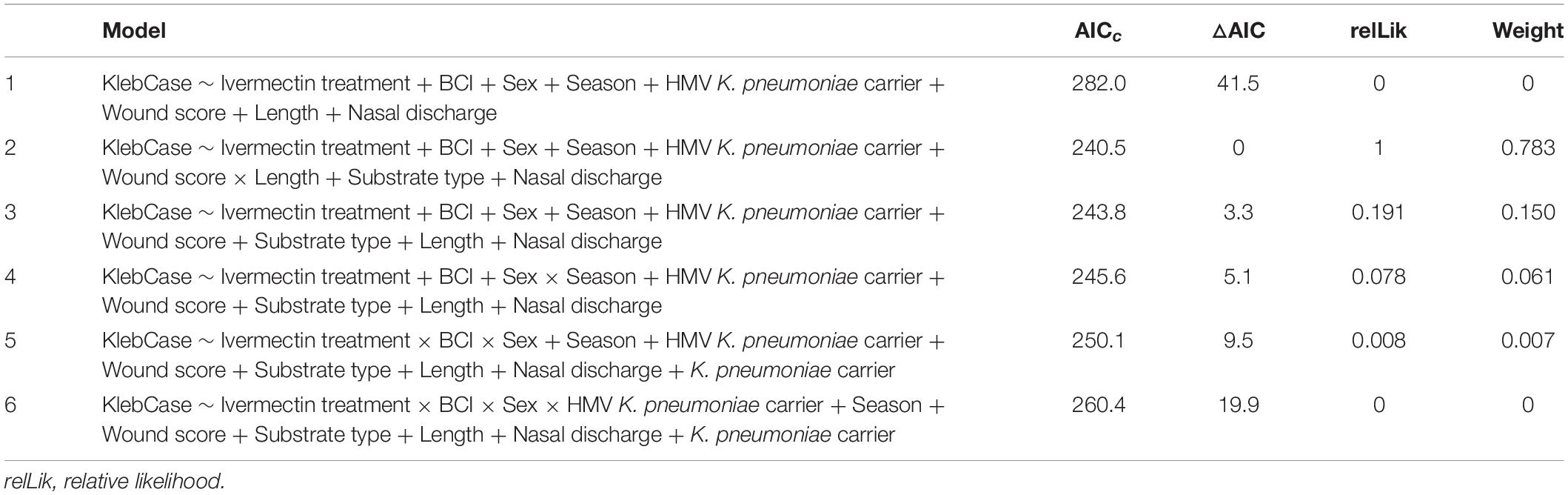
Table 2. Summary of predictor variables used in six candidate models for generalized linear model analysis and Akaike information criterion (AIC) statistics for hypervirulent Klebsiella pneumoniae mortality in New Zealand sea lion (Phocarctos hookeri) pups at Sandy Bay, Enderby Island.
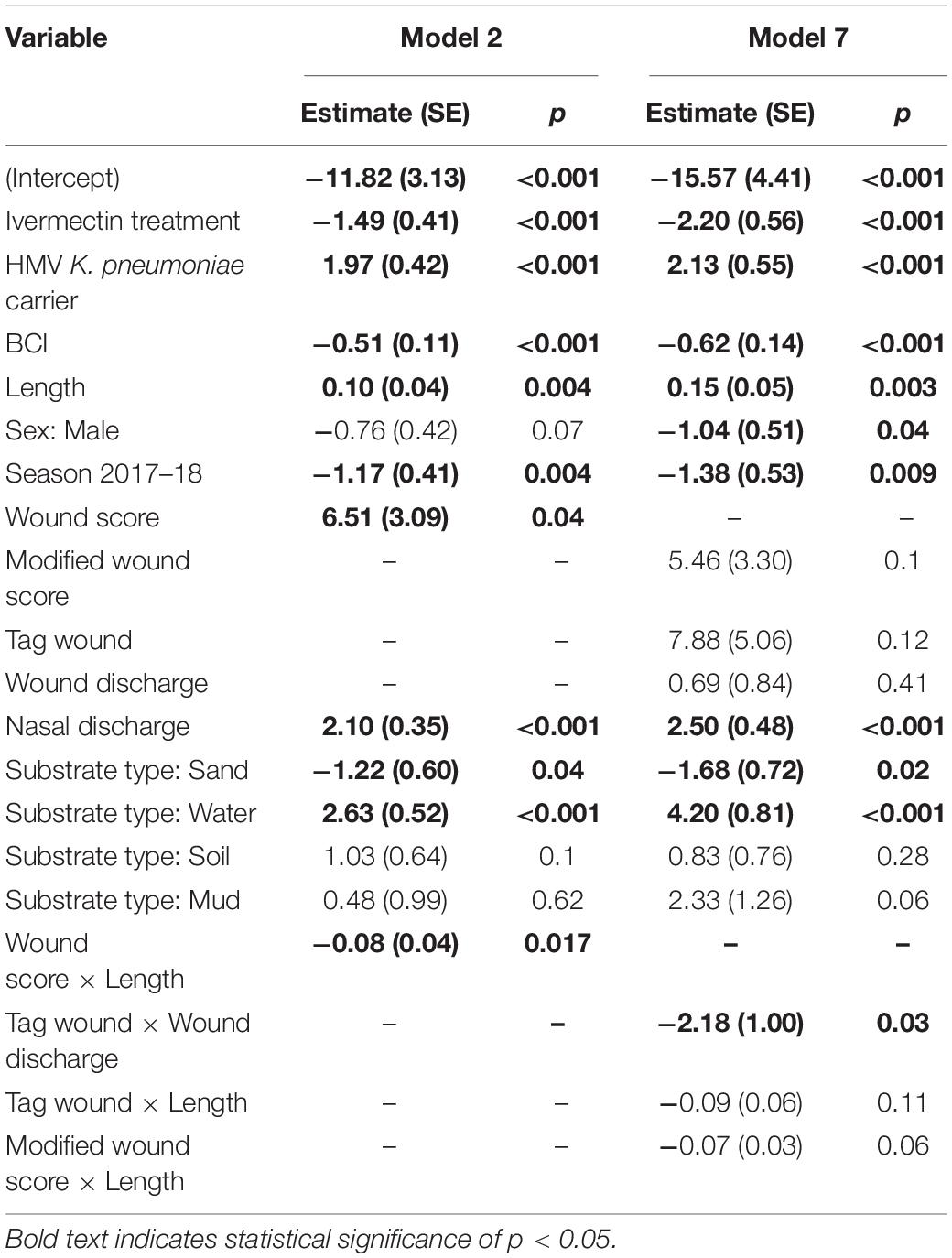
Table 3. Summary results of generalized linear models 2 and 7, analyzing risk factors for hypervirulent Klebsiella pneumoniae mortality in New Zealand sea lion (Phocarctos hookeri) pups at Sandy Bay, Enderby Island.
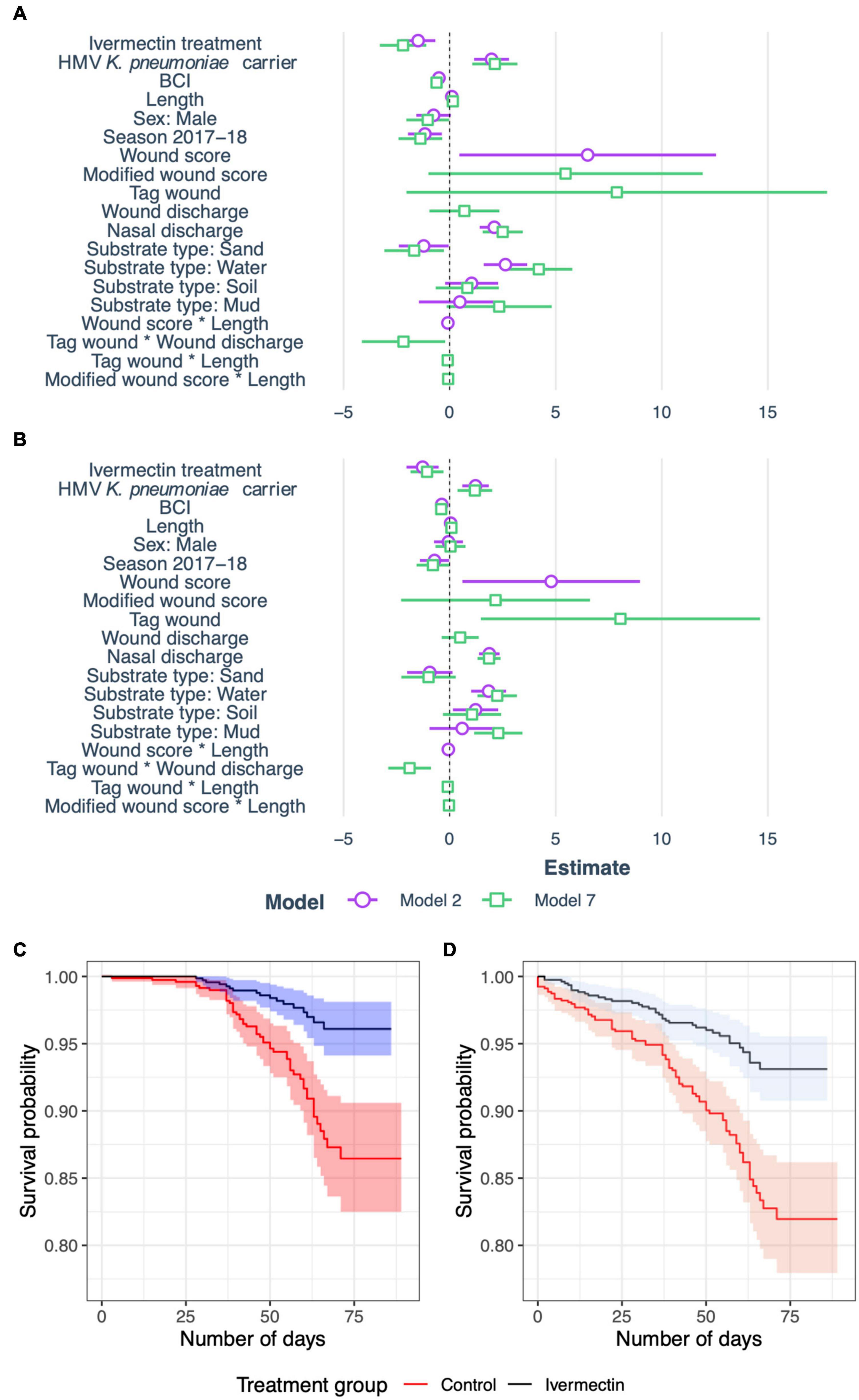
Figure 3. Summary plots of risk factors for mortality in New Zealand sea lion (Phocarctos hookeri) pups at Sandy Bay, Enderby Island. (A) Coefficients from the best generalized linear model selected by AIC for pup mortality due to hypervirulent Klebsiella pneumoniae (Model 2) and adjusted model to investigate wound score uncertainty (Model 7). 95% confidence intervals are shown. (B) Coefficients from the Cox proportional hazards model for pup mortality due to hypervirulent K. pneumoniae, clustered by individual pup for Model 2 and Model 7. 95% confidence intervals are shown. (C) Proportion of surviving New Zealand sea lion pups in ivermectin treatment and control groups with shaded 95% confidence intervals. Outcome has been censored to include only mortality due to hypervirulent K. pneumoniae. (D) Proportion of surviving New Zealand sea lion pups in ivermectin treatment and control groups with shaded 95% confidence intervals. Outcome includes all causes of mortality.
Due to the uncertainty surrounding the large confidence interval on wound score, components were analyzed in groups to investigate potential intrinsic interactions. Modified wound score included all wounds not inflicted through tagging, while tag wound and wound discharge scores were examined separately. Stepwise model selection using AIC determined the best model to be:
Model 7 results mirrored Model 2, however, male sex became protective in terms of reducing risk and there was a significant protective association between tag wound and wound discharge, such that all wound parameters (modified wound score, tag wound and wound discharge) became non-significant (Table 3 and Figure 3A). Generalized linear mixed effects models incorporating repeated measures were attempted but failed to converge.
When the same covariates were used in the same six generalized linear models for all causes of death (Supplementary Table 3), Model 2 was again the best model according to the AIC analysis, accounting for 92.7% of weight with similar significant factors determined (Supplementary Table 4).
Cox Proportional Hazards Analysis
The Cox proportional hazards analysis, clustered for individual pups, with the response variable of HV K. pneumoniae mortality, mirrored those found in models 2 and 7 described above. Figure 3B and Table 4 show the Cox analyses for these models with consistent findings in both analyses of ivermectin as protective along with BCI and the 2017–18 season. Wound coefficients again produced variable results, with overall wound score a significant risk factor in model 2 (4.79, 0.60–8.97 95% CI), but when separated in model 7, tag wounds were the only component with statistical significance, albeit with a broad confidence interval (8.05, 1.47–14.63 95% CI). Sand substrate was no longer significant in either analysis. The same trend is present with HMV K. pneumoniae carriage, nasal discharge and water substrate being a risk factor for both analyses. Length and mud substrate were significant risk factors only in model 7.
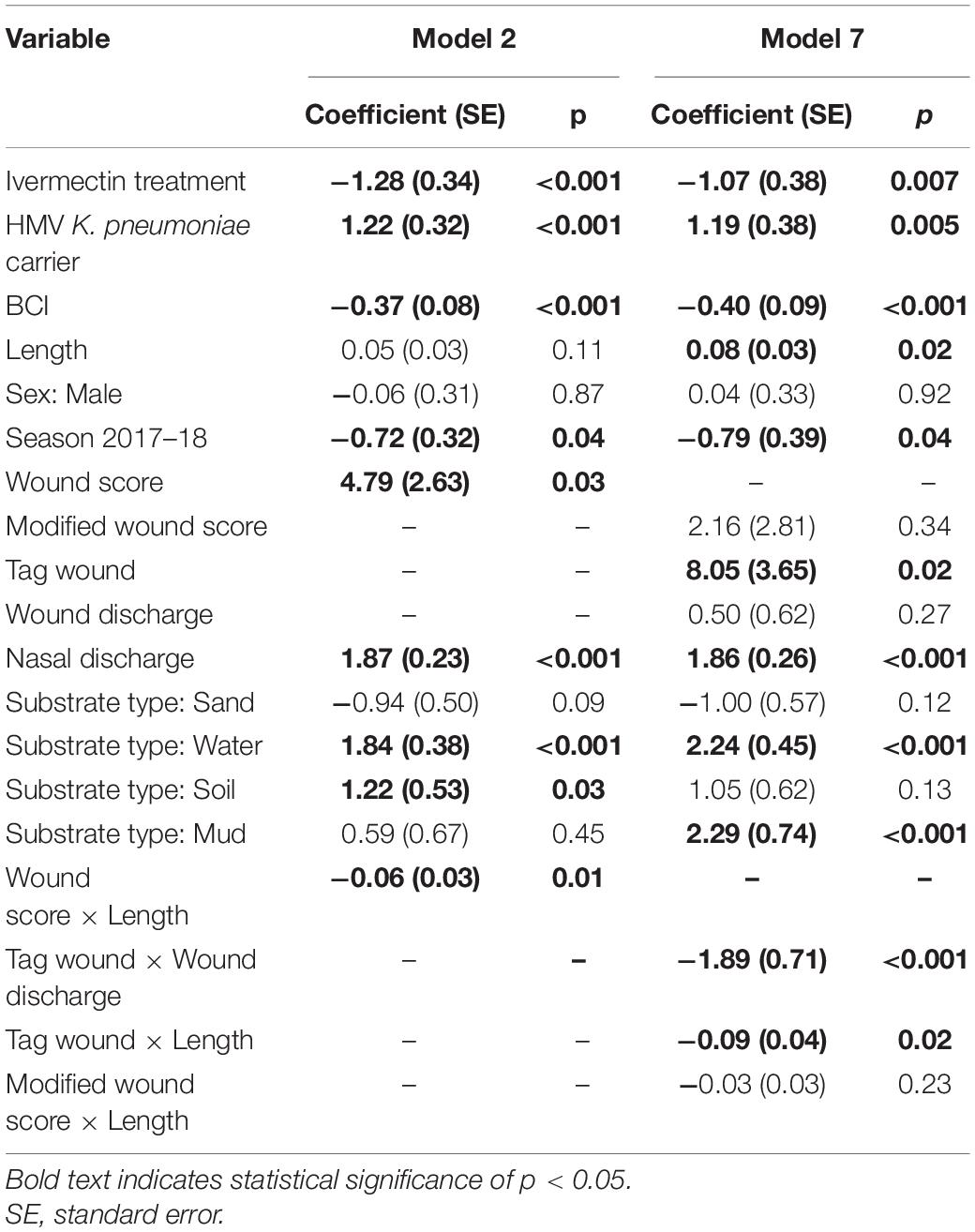
Table 4. Summary results of Cox proportional hazards models 2 and 7, analyzing risk factors for hypervirulent Klebsiella pneumoniae mortality in New Zealand sea lion (Phocarctos hookeri) pups at Sandy Bay, Enderby Island.
Survival Analysis
The risk for a pup dying due to HV K. pneumoniae as determined by the Kaplan Meier survival analysis was 9.6% (5.1–14.0% 95% CI) higher for controls compared to those pups treated with ivermectin, during the first 3 months of life (Figure 3C). The risk of dying due to any cause was 10.8% (4.5–17.0 95% CI) higher for controls, compared to ivermectin-treated pups (Figure 3D) during the same time period.
Discussion
Ivermectin treatment significantly improved NZ sea lion pup survival in the first 3 months of life, in the largest reported controlled trial of anthelmintic treatment in free-ranging pinnipeds, consistent with previous small sample size experiments in the species (Chilvers et al., 2009; Michael et al., 2015). Further, gastrointestinal carriage of HMV K. pneumoniae was a consistently significant risk factor for systemic infection and mortality. Consequently, we hypothesize that the enhancement of pup survival by ivermectin treatment is not solely due to removal of direct hookworm effects, rather the disruption of a synergistic relationship between hookworms and HV K. pneumoniae. This relationship resembles the HEB syndrome previously reported (Spraker et al., 2007; Seguel et al., 2017), however, there are distinct contrasts. Unlike in the American otariids, hookworms have relatively mild systemic effects in NZ sea lions with no evidence of enteric submucosal penetration or peritoneal migration (Castinel et al., 2007a, b): the proposed entry route for HEB infections (Spraker et al., 2007). In other otariid species diagnosed with HEB, a range of enteric microbiological isolates cause bacteremia, including E. coli, non-HMV K. pneumoniae and Salmonella spp. (Spraker et al., 2007; Seguel et al., 2017). In NZ sea lion pups at Enderby Island, however, the vast majority of bacterial infections are due to HV K. pneumoniae and most of those that are not, occur during the hookworm pre-patent period (Michael et al., 2019), making hookworms an unlikely mediator in these cases. Although the link between hookworm and HV K. pneumoniae accounts for a major proportion of infection and mortality, ivermectin-treated pups still died from HV K. pneumoniae bacteremia, suggesting pups were infected via alternative routes such as the respiratory tract or cutaneous wounds, where hookworm removal would have no effect.
Infection of pups with HV K. pneumoniae via alternative routes, and initial gastrointestinal tract colonization requires an environmental reservoir. Several studies investigating reservoirs at Enderby Island including adult NZ sea lions (Gonzalez Argandoña, 2017), substrate (Pinpimai, 2018) and sympatric species [sub-Antarctic skua (Catharacta antarctica lonnbergi) and yellow-eyed penguins (Megadyptes antipodes) (unpublished data; Pinpimai et al., 2018b)] have confirmed presence of HV K. pneumoniae in all to varying degrees, with a temporal pattern reflecting the onset of HV K. pneumoniae deaths in pups on the cusp of January and February.
Gastrointestinal carriage of HMV K. pneumoniae was a significant risk factor for pup mortality, particularly for HV K. pneumoniae-associated mortality. This is consistent with human cases, where colonized individuals are at greater risk of developing infection (Martin et al., 2016; Gorrie et al., 2017). The mechanisms behind this, including contributing host factors, relative virulence of K. pneumoniae strains and threshold burden of colonizing organisms are poorly understood. In the present study, the earliest positive swabs from live pups occurred on 31 January in 2016–17, the day before the first HV K. pneumoniae death and interestingly in 2017–18 on 22 December near the peak of pupping, with detection in an 8-day old pup. While this may represent colonization from the mother in the perinatal period, in 2017–18, two HV K. pneumoniae deaths occurred in early January, well before the expected onset of cases, such that environmental contamination in 2017–18 may have occurred earlier than usual. Substrate samples were not collected in that season, so this is unable to be confirmed. These three positive pups survived, two were later captured and still culture-positive, so whilst carriage of HMV K. pneumoniae is a risk factor for mortality, colonized pups also survive and likely contribute to environmental contamination, potentially serving as a source of contamination for localities preferentially inhabited by pups, including freshwater ponds and streams.
The voluminous viscous capsule of HMV K. pneumoniae aids resistance to the host’s immune system, and a wide range of other virulence factors could allow this pathogen to take advantage of a hookworm alliance. Pinpimai (2018) showed that NZ sea lion HMV K. pneumoniae isolates were virulent in vitro, being more resistant to phagocytosis, serum-mediated and oxidative-mediate killing than non-HMV K. pneumoniae and E. coli human isolates. Whilst the HMV phenotype was initially used as the hallmark of K. pneumoniae hypervirulence, further work has shown this to be less sensitive and specific than first thought; rather a suite of biomarkers on a large virulence plasmid are more reliable indicators (Lin et al., 2011; Catalan-Najera et al., 2017; Russo et al., 2018). K. pneumoniae isolates from pup mortality cases at Enderby Island were HMV phenotype, K2 capsule serotype and sequence type 86, with likely clonality (Pinpimai, 2018; Pinpimai et al., 2018a), so the string test for hypermucoviscosity (Fang et al., 2004) was conducted to indicate these in this study. Further work would involve analyzing isolates from this study for virulence biomarkers including rmpA, which could detect virulent K. pneumoniae isolates without a HMV phenotype.
Several risk factor variables returned inconsistent results, most likely due to intrinsic biological, spatial and temporal correlations. At Enderby Island, pupping is tightly synchronized, with 69% of pups born in a 2 week period on Sandy Bay beach, centered approximately around 26 December (Chilvers et al., 2007). Pups are tagged in mid-January corresponding with the beginning of dispersal from the beach (Augé et al., 2009). Consequently, substrate is linked with pup age and since HV K. pneumoniae mortality occurs late in the season once pups have dispersed (Michael et al., 2019), sand substrate is a protective factor. Water was a significant risk factor, likely due to pups dispersing to this area later in the season and the relationship between drowning deaths and underlying HV K. pneumoniae infection, with sick pups hypothesized to have difficulty exiting pools and streams due to neurological and musculoskeletal lesions (Michael et al., 2019). Similarly, the association between wound scores and risk of death was difficult to interpret due to age-linked events. Newborn pups had higher wound scores due to their fresh umbilicus, then all pups were tagged over a 2-day interval, followed by a variable period of tag-site discharge, granulation, and healing (pers. obs). When modified wound score was considered, removing effects of tag wounds and discharge, wound scores became statistically insignificant. Likewise, higher nasal discharge score as a risk factor for HV K. pneumoniae death may be confounded as purulent discharge in the nasal turbinates was a common finding at necropsy in HV K. pneumoniae cases but was difficult to detect in live pups. Whilst wounds and nasal discharge were included in the analysis as semi-quantitative measures to investigate potential entry points for environmental HV K. pneumoniae, it is difficult to ascertain their contribution to pathogenesis.
Field season had a significant effect in all models with higher pup mortality in 2016–17, partly due to a stochastic event where an adult male crushed seven pups over a 2 day period, however, this did not fully explain the 6% difference in mortality rate, especially given the proportion of HV K. pneumoniae cases was lower in 2016–17 (Michael et al., 2019). The contribution of inter-annual climatic variation is unclear, with similar temperature and wind speed extremes between years but higher rainfall in 2016–17 (Enderby Island automatic weather station, K92900; National Institute of Water and Atmospheric Research, 2019). For future mitigation, this annual variation in pup mortality demonstrates the importance of monitoring over multiple seasons to determine the effectiveness of ivermectin for improving pup survival.
Calculated growth rates were slightly but not significantly higher than previously reported (195 g/day versus 178 g; Chilvers et al., 2009). Unexpectedly, higher BCI was protective against HV K. pneumoniae death, a counterintuitive finding since pups that die from HV K. pneumoniae are generally in excellent body condition as measured by axillary blubber depth (Michael et al., 2019).
The evidence for the significantly protective effect of ivermectin against NZ sea lion pup mortality at Enderby Island is promising, as treatment can be practically implemented. As a management tool, ivermectin treatment should be continually reassessed for intended outcomes in improving pup survival, which can be balanced to prevent the development of anthelmintic resistance. Near 100% effectiveness of ivermectin against hookworm as shown in the current study, accurate subcutaneous dosing following precise body mass measurement and several life history traits of NZ sea lions enabling natural refugia all contribute to delayed selection of anthelmintic resistance. Refugia, a population of parasites that have not experienced selective pressure by anthelmintic treatment would be likely at Sandy Bay, even if all Sandy Bay-born pups were treated, due to dispersal of untreated pups during the hookworm patent period from nearby Dundas Island (unpublished data). In addition, females are unlikely to return to colonies every year, maintaining anthelmintic-susceptible dormant larvae in their blubber.
In conclusion, this study provides further evidence for interactions between hookworms and HV K. pneumoniae in NZ sea lion pups at Enderby Island. By manipulating this relationship, pup mortality can be significantly decreased. These results provide a practical mitigation option for NZ sea lion conservation, and address a key knowledge gap, improving our understanding of a complex system of risk and protective factors for NZ sea lion pup mortality overall, and specifically for HV K. pneumoniae-associated pup mortality.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Massey University Animal Ethics Committee and New Zealand Department of Conservation Animal Ethics Committee.
Author Contributions
SM, DH, and WR conceived the ideas. SM and DH designed methodology and analyzed the data. SM collected the data, performed the laboratory analyses, and led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Funding
This work was supported by Massey University and the New Zealand Department of Conservation as part of the New Zealand Sea Lion Threat Management Plan. SM was supported by a Research Training Program Stipend (SC1999) and a University of Sydney Vice-Chancellor’s Research Scholarship (SC0912). DH was supported by the Royal Society Te Apārangi Rutherford Discovery Fellowship (RDF-MAU1701). Open access publication fees were contributed by SSVS, The University of Sydney; Massey University and the Wildlife Disease Association Student Scholarship Award received by SM. The funders had no role in collection, analysis or interpretation of data, in the writing of the report or the decision to submit the article for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the New Zealand sea lion field teams on Enderby Island for their hard work, with particular mentions to Thomas Burns, Shannon Taylor, and Aditi Sriram. The Department of Conservation National Office, Murihiku/Southland Office, Blue Planet Marine, and Simon Childerhouse provided logistical support for field work. A huge number of people provided assistance in making this study possible, but particular contributions were made by Drew V. Lee for creating maps, Andrea Sward of the Department of Conservation Geospatial Services team for creating GPS waypoint-linked grid overlays of Enderby Island, Lynn Rogers and Anne Midwinter for microbiological assistance, Ahmed Fayaz for database design and Bryce Ilton, Kirsty Anderson, Tony Russell, Kristene Gedye, and Verne Ward for logistical support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.680678/full#supplementary-material
References
Augé, A. A., Chilvers, B. L., Moore, A., Mathieu, R., and Robertson, B. C. (2009). Aggregation and dispersion of female New Zealand sea lions at the Sandy Bay breeding colony, Auckland Islands: how unusual is their spatial behaviour? Behaviour 146, 1287–1311. doi: 10.1163/15683909X427687
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Castinel, A. (2006). Causes of Neonatal Mortality in the New Zealand Sea Lion (Phocarctos hookeri). Ph.D. thesis. Palmerston North: Massey University.
Castinel, A., Duignan, P. J., Lyons, E. T., Pomroy, W. E., Gibbs, N., Lopez-Villalobos, N., et al. (2007a). Epidemiology of hookworm (Uncinaria spp.) infection in New Zealand (Hooker’s) sea lion (Phocarctos hookeri) pups on Enderby Island, Auckland Islands (New Zealand) during the breeding seasons from 1999/2000 to 2004/2005. Parasitol. Res. 101, 53–62. doi: 10.1007/s00436-006-0453-z
Castinel, A., Duignan, P. J., Pomroy, W. E., Lopez-Villalobos, N., Gibbs, N. J., Chilvers, B. L., et al. (2007b). Neonatal mortality in New Zealand sea lions (Phocarctos hookeri) at Sandy Bay, Enderby Island, Auckland Islands from 1998 to 2005. J. Wildl. Dis. 43, 461–474. doi: 10.7589/0090-3558-43.3.461
Castinel, A., Pomroy, B., and Grinberg, A. (2007c). Hookworm infection and Klebsiella pneumoniae epidemics in New Zealand sea lion pups. Vet. Microbiol. 125, 388–389. doi: 10.1016/j.vetmic.2007.08.009
Catalan-Najera, J. C., Garza-Ramos, U., and Barrios-Camacho, H. (2017). Hypervirulence and hypermucoviscosity: two different but complementary Klebsiella spp. phenotypes? Virulence 8, 1111–1123. doi: 10.1080/21505594.2017.1317412
Chilvers, B. L., Duignan, P. J., Robertson, B. C., Castinel, A., and Wilkinson, I. S. (2009). Effects of hookworms (Uncinaria sp.) on the early growth and survival of New Zealand sea lion (Phocarctos hookeri) pups. Polar Biol. 32, 295–302. doi: 10.1007/s00300-008-0559-0
Chilvers, B. L., Robertson, B. C., Wilkinson, I. S., and Duignan, P. J. (2007). Growth and survival of New Zealand sea lions, Phocarctos hookeri: birth to 3 months. Polar Biol. 30, 459–469. doi: 10.1007/s00300-006-0203-9
Daszak, P., Cunningham, A. A., and Hyatt, A. D. (2000). Emerging infectious diseases of wildlife- threats to biodiversity and human health. Science 287, 443–449. doi: 10.1126/science.287.5452.443
DeLong, R. L., Orr, A. J., Jenkinson, R. S., and Lyons, E. T. (2009). Treatment of northern fur seal (Callorhinus ursinus) pups with ivermectin reduces hookworm-induced mortality. Mar. Mamm. Sci. 25, 944–948. doi: 10.1111/j.1748-7692.2008.00274.x
Fang, C.-T., Chuang, Y.-P., Shun, C.-T., Chang, S.-C., and Wang, J.-T. (2004). A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J. Exp. Med. 199, 697–705. doi: 10.1084/jem.20030857
Gonzalez Argandoña, A.-K. (2017). Carriage of Pathogens in New Zealand Sea Lions (Phocarctos hookeri) Frozen Faecal Samples. Masters thesis. Palmerston North: Massey University.
Gorrie, C. L., Mirceta, M., Wick, R. R., Edwards, D. J., Thomson, N. R., Strugnell, R. A., et al. (2017). Gastrointestinal carriage is a major reservoir of Klebsiella pneumoniae infection in intensive care patients. Clin. Infect. Dis. 65, 208–215. doi: 10.1093/cid/cix270
Guinet, C., Roux, J. P., Bonnet, M., and Mison, V. (1998). Effect of body size, body mass, and body condition on reproduction of female South African fur seals (Arctocephalus pusillus) in Namibia. Can. J. Zool. 76, 1418–1424. doi: 10.1139/z98-082
Katki, H. A., and Mark, S. D. (2008). Survival analysis for cohorts with missing covariate information. R J. 8, 14–19.
Lin, Y.-C., Lu, M.-C., Tang, H.-L., Liu, H.-C., Chen, C.-H., Liu, K.-S., et al. (2011). Assessment of hypermucoviscosity as a virulence factor for experimental Klebsiella pneumoniae infections: comparative virulence analysis with hypermucoviscosity-negative strain. BMC Microbiol. 11:50. doi: 10.1186/1471-2180-11-50
Long, J. A. (2019). jtools: Analysis and Presentation of Social Scientific Data. R package version 2.0.1 [Online]. Available online at: https://cran.r-project.org/package=jtools (accessed March 12, 2021).
Lyons, E., Melin, S., Delong, R., Orr, A., Gulland, F., and Tolliver, S. (2001). Current prevalence of adult Uncinaria spp. in northern fur seal (Callorhinus ursinus) and California sea lion (Zalophus californianus) pups on San Miguel Island, California, with notes on the biology of these hookworms. Vet. Parasitol. 97, 309–318. doi: 10.1016/s0304-4017(01)00418-6
Lyons, E. T., Delong, R. L., Nadler, S. A., Laake, J. L., Orr, A. J., Delong, B. L., et al. (2011). Investigations of peritoneal and intestinal infections of adult hookworms (Uncinaria spp.) in northern fur seal (Callorhinus ursinus) and California sea lion (Zalophus californianus) pups on San Miguel Island, California (2003). Parasitol. Res. 109, 581–589. doi: 10.1007/s00436-011-2289-4
Marcus, A. D., Higgins, D. P., and Gray, R. (2014). Epidemiology of hookworm (Uncinaria sanguinis) infection in free-ranging Australian sea lion (Neophoca cinerea) pups. Parasitol. Res. 113, 3341–3353. doi: 10.1007/s00436-014-3997-3
Marcus, A. D., Higgins, D. P., and Gray, R. (2015a). Health assessment of free-ranging endangered Australian sea lion (Neophoca cinerea) pups: effect of haematophagous parasites on haematological parameters. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 184, 132–143. doi: 10.1016/j.cbpa.2015.02.017
Marcus, A. D., Higgins, D. P., and Gray, R. (2015b). Ivermectin treatment of free-ranging endangered Australian sea lion (Neophoca cinerea) pups: effect on hookworm and lice infection status, haematological parameters, growth, and survival. Parasitol. Res. 114, 2743–2755. doi: 10.1007/s00436-015-4481-4
Martin, R. M., Cao, J., Brisse, S., Passet, V., Wu, W., Zhao, L., et al. (2016). Molecular epidemiology of colonizing and infecting isolates of Klebsiella pneumoniae. mSphere 1, e00261-16.
Michael, S. A., Chilvers, B. L., Roe, W. D., and Gartrell, B. D. (2015). Long-term survival and reproductive success of New Zealand sea lions (Phocarctos hookeri) treated with ivermectin as pups. Wildl. Res. 42, 660–667. doi: 10.1071/wr15120
Michael, S. A., Hayman, D. T. S., Gray, R., Zhang, J., Rogers, L., and Roe, W. D. (2019). Pup mortality in New Zealand sea lions (Phocarctos hookeri) at Enderby Island, Auckland Islands, 2013-18. PLoS One 14:e0225461. doi: 10.1371/journal.pone.0225461
National Institute of Water and Atmospheric Research. (2019). The National Climate Database [Online]. Available online at: https://cliflo.niwa.co.nz/ (accessed August 12, 2019).
Paczosa, M. K., and Mecsas, J. (2016). Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol. Mol. Biol. Rev. 80, 629–661. doi: 10.1128/MMBR.00078-15
Pinpimai, K. (2018). Klebsiella pneumoniae in New Zealand Sea Lions. Ph.D Thesis. Palmerston North: Massey University.
Pinpimai, K., Roe, W. D., Biggs, P. J., and Dittmer, K. E. (2018a). Draft whole-genomesequences of seven isolates of Klebsiella pneumoniae from New Zealand sea lions. Microbiol. Resour. Announc. 7:e1270-18. doi: 10.1128/mra.01270-18
Pinpimai, K., Roe, W. D., Biggs, P. J., Dittmer, K. E., and Michael, S. A. (2018b). Draft whole-genome sequences of five Klebsiella pneumoniae isolates from the subantarctic islands of New Zealand. Microbiol. Resour. Announc. 7:e1328-18. doi: 10.1128/mra.01328-18
R Core Team. (2018). R: a Language and Environment for Statistical Computing. [Online]. Vienna: R Foundation for Statistical Computing.
Roberts, J., and Doonan, I. (2016). Quantitative Risk Assessment of Threats to New Zealand Sea Lions. New Zealand Aquatic Environment and Biodiversity Report No. 166. Wellington: Ministry for Primary Industries.
Roe, W. D., Rogers, L., Pinpimai, K., Dittmer, K., Marshall, J., and Chilvers, B. L. (2015). Septicaemia and meningitis caused by infection of New Zealand sea lion pups with a hypermucoviscous strain of Klebsiella pneumoniae. Vet. Microbiol. 176, 301–308. doi: 10.1016/j.vetmic.2015.01.019
Russo, T. A., and Marr, C. M. (2019). Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 32, e00001–e00019. doi: 10.1128/CMR.00001-19
Russo, T. A., Olson, R., Fang, C.-T., Stoesser, N., Miller, M., Macdonald, U., et al. (2018). Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae. J. Clin. Microbiol. 56:e00776-18. doi: 10.1128/jcm.00776-18
Seguel, M., Munoz, F., Navarrete, M. J., Paredes, E., Howerth, E., and Gottdenker, N. (2017). Hookworm infection in South American fur seal (Arctocephalus australis) pups: pathology and factors associated with host tissue damage and mortality. Vet. Pathol. 54, 288–297. doi: 10.1177/0300985816677151
Seguel, M., Muñoz, F., Perez-Venegas, D., Müller, A., Paves, H., Howerth, E., et al. (2018). The life history strategy of a fur seal hookworm in relation to pathogenicity and host health status. Int. J. Parasitol. Parasites Wildl. 7, 251–260. doi: 10.1016/j.ijppaw.2018.07.003
Spraker, T. R., Delong, R. L., Lyons, E. T., and Melin, S. R. (2007). Hookworm enteritis with bacteremia in California sea lion pups on San Miguel Island. J. Wildl. Dis. 43, 179–188. doi: 10.7589/0090-3558-43.2.179
Spraker, T. R., Lyons, E. T., Delong, R. L., and Zink, R. R. (2004). Penetration of the small intestine of a California sea lion (Zalophus californianus) pup by adult hookworms (Uncinaria spp). Parasitol. Res. 92, 436–438. doi: 10.1007/s00436-003-1050-z
Tang, Y., Horikoshi, M., and Li, W. (2016). ggfortify: unified interface to visualize statistical result of popular R packages. R J. 8, 478–489.
Therneau, T. (2014). A Package for Survival Analysis in S. R package version 2.44-1.1 [Online]. Available online at: http://CRAN.R-project.org/package=survival (accessed April 24, 2021)
Wilkinson, I. S., Duignan, P. J., Castinel, A., Grinberg, A., Chilvers, B. L., and Robertson, B. C. (2006). “Klebsiella pneumoniae epidemics: possible impact on New Zealand sea lion recruitment,” in Sea Lions of the World, eds A. W. Trites, S. K. Atkinson, D. P. DeMaster, L. W. Fritz, T. S. Gelatt, L. D. Rea, et al. (Fairbanks, AK: Alaska Sea Grant College Program), 385–403. doi: 10.4027/slw.2006.26
Keywords: case-control study, ivermectin, Klebsiella pneumoniae, pinniped, risk factor, hypervirulent
Citation: Michael SA, Hayman DTS, Gray R and Roe WD (2021) Risk Factors for New Zealand Sea Lion (Phocarctos hookeri) Pup Mortality: Ivermectin Improves Survival for Conservation Management. Front. Mar. Sci. 8:680678. doi: 10.3389/fmars.2021.680678
Received: 15 March 2021; Accepted: 17 June 2021;
Published: 09 July 2021.
Edited by:
Randall William Davis, Texas A&M University at Galveston, United StatesReviewed by:
Pamela Tuomi, Alaska SeaLife Center, United StatesMikaylie Wilson, Auckland Zoo, New Zealand
Copyright © 2021 Michael, Hayman, Gray and Roe. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sarah A. Michael, c2FyYWguYS5taWNoYWVsQGdtYWlsLmNvbQ==
†ORCID: Sarah A. Michael, orcid.org/0000-0002-7176-0774; David Hayman, orcid.org/0000-0003-0087-3015
‡Present address: Sarah A. Michael, Department of Primary Industries, Parks, Water and Environment, Hobart, TAS, Australia
 Sarah A. Michael
Sarah A. Michael David T. S. Hayman
David T. S. Hayman Rachael Gray
Rachael Gray Wendi D. Roe
Wendi D. Roe