- 1Department of Life and Environmental Science, University of Cagliari, Cagliari, Italy
- 2Consorzio Nazionale Interuniversitario per le Scienze del Mar, Rome, Italy
Molecular identifications based on two mitochondrial markers (cytochrome c oxidase subunit I -COI- and 16S ribosomal RNA gene -16S-) have been implemented to confirm the morphological identification of eight specimens collected in the Central western Mediterranean. Molecular data show they belonged to a recently resurrected species of the genus Ommastrephes, i.e., O. caroli, known to be distributed in the Atlantic Ocean and Mediterranean Sea. Despite this, molecular analyses of COI sequences evidenced the presence of potential genetic differentiation between Mediterranean and Atlantic samples, highlighting the need for further studies, with more individuals to investigate the connectivity between individuals living in the two areas. Furthermore, morphological, biometric and reproductive features here reported, could be useful in evaluating possible distinctive biological features between the Mediterranean and Atlantic individuals. Female mature size was larger than the male. The relationships obtained between the beak measurements and body sizes (DML; TW) were better described by a power model. Asynchronous oocytes development with relatively small oocytes (0.05–1.10 mm) and a protracted intermittent spawning with active feeding were observed. This study also reported for the specie O. caroli the first data on the potential fecundity estimated (840061 oocytes), the oviducal load (90000 ripe oocytes) as well as the number of seminal receptacles and the size and morphology of the spermatangia found in the buccal mass of all mated females. Even if on a low sample size, beaks and eye lenses were used for the first time in O. caroli for age estimation. The statistically significant relationship found between increments counted in eye lenses and beaks highlighted the reliability of the lenses to estimate age in O. caroli, even if further studies will be needed for its validation. Assuming a daily increment for both structures, a mean life span of about 12–13 months was estimated for both sexes, which is consistent with the sexual maturity condition observed in all the samples and the semelparity known for cephalopods coleoids.
Introduction
Recently, molecular methods in combination with meristic knowledge have led to re-valuate the long-standing problem of the taxonomy of the genus Ommastrephes (d’Orbigny, 1834; Fernández-Álvarez et al., 2020). Before that, the three species Ommastrephes bartramii (Lesueur, 1821), Ommastrephes caroli (Furtado, 1887) and Ommastrephes pteropus (Steenstrup, 1855) were considered to belong to this genus (Roper et al., 1984). Subsequently, the genus has been considered monotypic with the only species O. bartramii characterized by a cosmopolitan distribution and the species O. pteropus is currently placed within the genus Sthenoteuthis (Verrill, 1880; Roper et al., 2010). Actually. Fernández-Álvarez et al. (2020) revealed the presence of four groups/species of Ommastrephes living in distinct geographical regions. It was designated a neotype for O. bartramii applying this name to the species occurring in the North Pacific, the only one well studied and commercially exploited (Bower and Ichii, 2005; Ichii et al., 2009; Ding et al., 2019). For the remaining groups/species, a revision of the distributional ranges was proposed and attributed the following three, formerly were synonymized names: O. brevimanus, (Gould, 1852) Ommstrephes cylindraceus (d’Orbigny, 1835) and O. caroli. The species O. brevimanus was assigned to South Pacific and, O. cylindraceus to Tropical, South Atlantic and South Indian waters. O. caroli was considered pseudocryptic because of morphological differences from the other species in this genus and assigned to North East Atlantic and Mediterranean specimens (Fernández-Álvarez et al., 2020). For the Mediterranean, only one sequence of O. bartramii, from Croatian waters, is currently available in the public database (accession number KF212462). Franjević et al. (2015) attributed their Adriatic specimen to O. bartramii and not to O. caroli because at that time it was still considered a cosmopolitan species. Overall, Mediterranean findings of Ommastrephes are considered a fortuitous phenomenon, mostly occurring in the Eastern basin, generally due to large senescent post-spawning females beached or accidentally caught by commercial or experimental fisheries (Lefkaditou et al., 2011; Kapiris et al., 2014; Franjević et al., 2015; Tsiamis et al., 2015). Lefkaditou et al. (2011) suggested an increase in the presence of the species in the Mediterranean related to climate change. The low number of specimens recorded in the Mediterranean may be due to the absence of a target fishery; only off the Aeolian Islands (Italy): it is a by-catch species for the jigging fisheries (<3%) (Battaglia et al., 2010; Lefkaditou et al., 2011). However, the scarcity of records contrasts with its frequency of occurrence in the stomach contents of apex predators (Bello, 1991; Romeo et al., 2012) suggesting that the species could be not so rare. Lishchenko et al. (2021), stated that more than 90% of the studies on Ommastrephes specimens have been focused on Pacific populations, the remainder refer to what in the Atlantic and Mediterranean was considered to belong to O. bartramii and now instead has been designated as O. caroli (Fernández-Álvarez et al., 2020). Jereb et al. (2015) noted that general information of the basic biology of O. caroli in the North Atlantic was still needed and that a translation of the published Russian studies into English would help make the available knowledge more accessible (Lishchenko et al., 2021). As regards the Mediterranean findings, biological information addressing reproductive and life cycle data is too fragmentary and lacking. IN this contest, we aim to molecularly identify the specimens collected in the Central western Mediterranean Sea and report their morphological, reproductive features and age estimation. Moreover, the age estimation was based on the beaks and eye lenses, both structures never before used for O. caroli, and eye lenses not even within the genus Ommastrephes.
Materials and Methods
Morphometric and Biological Features
The eight specimens of O. caroli used in this analysis were found accidentally in the Sardinian waters (central western Mediterranean) from 2006 to 2019. Two were stranded, five were caught during commercial fishing (one damaged and without beak) and one within a scientific survey (MEDITS project_2019) (Table 1). Each specimen was weighed (TW, Total Weight, to the nearest g), sexed following Roper and Voss (1983), and measured (DML, dorsal Mantle Length, to the nearest millimeter). The maturity condition was established, following the scale of five stages (immature, maturing, developing, mature/spawning, and spent) currently in use for cephalopods within the MEDITS project (AAVV, 2017). Buccal membranes of all females were analyzed to verify mating signs (spermatangia implanted in the tissue). Spermatangia (sp) were counted and measured taking their total length and width, using the Tps_Dig2 software. With the same program the number of seminal receptacles (SRs) were counted in four females. One mature female was used for the analysis of oocytes size (major axis nearest to 0.01 mm) and to estimate the potential fecundity as the sum of total oocyte (>0.05 mm in diameter) number in the ovary and total egg number in the oviducts (Nigmatullin and Markaida, 2009). All stomachs were removed and assigned to a subjective fullness index (FUI, where 0 = empty; 1 = containing very scarce remains; 2 = from significant remains to full repletion (Rasero et al., 1996). Stomachs with FUI values of 1 and 2 were used for prey identification at category level. The eye lenses of all samples were removed with a scalpel, cleaned with distilled water and fixed in neutralized formalin for age study, according to Baqueiro-Cárdenas et al. (2011). Mandibles (beaks) were removed from seven animals. According to Clarke (1986), standard measurements of the upper (U) and lower (L) jaws were taken using a digital caliper (accurate to a tenth of a millimeter) (Supplementary Figure 1). Linear and power regressions were performed to evaluate the best relationship between beak measurements and the sizes of the bodies (ML and TW) of the animals. After measurements, the beaks were stored in 75% ethanol for further analysis.
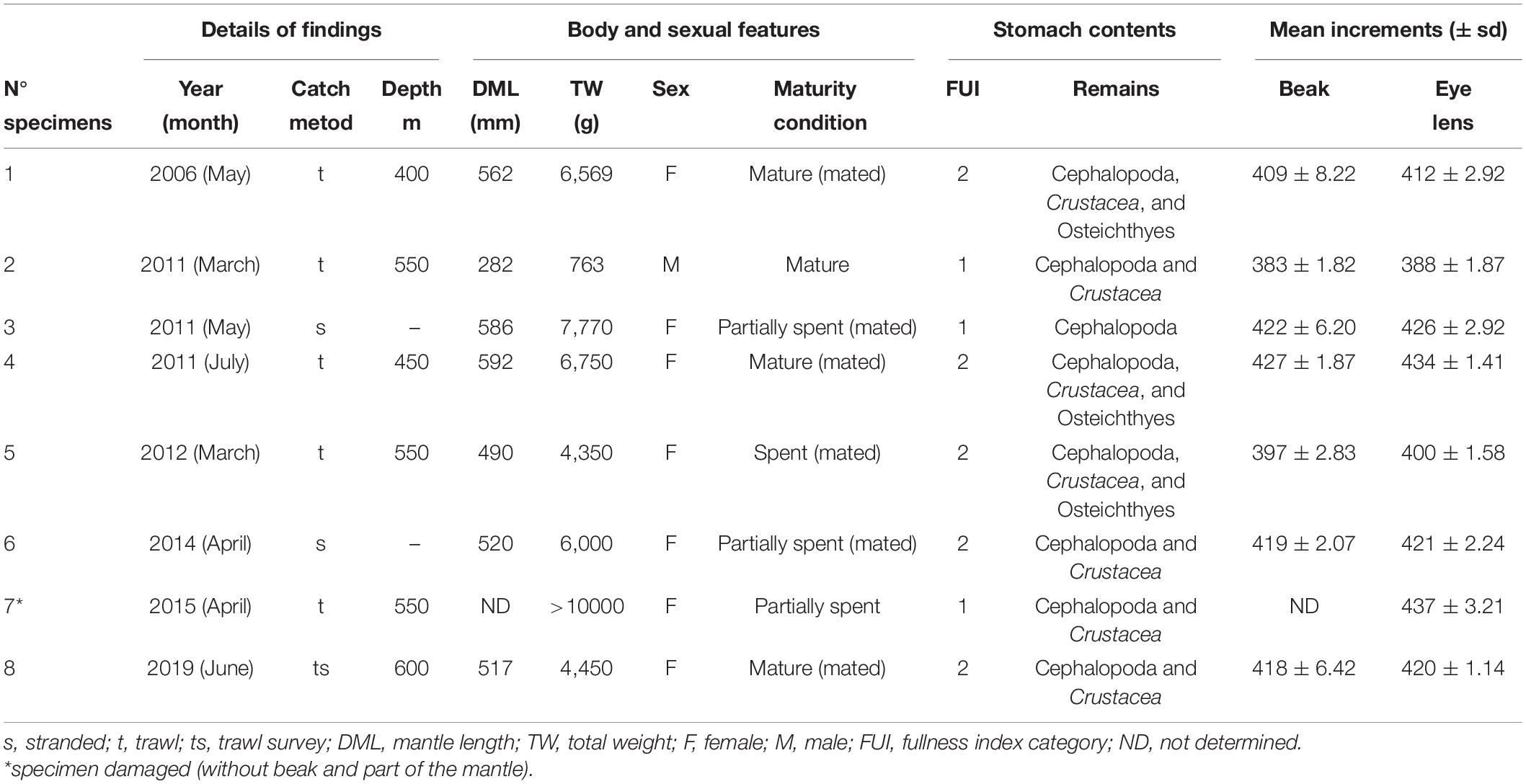
Table 1. Details on body size, sex, maturity condition, stomach contents, and mean increments counted in beaks and eye lenses of eight specimens of Ommastrephes caroli recorded from 2006 to 2019 in Sardinian waters.
Molecular Analysis
Total genomic DNA was extracted from ethanol fixed tissue of three individuals (hereafter referred to as: Ommastrephes sp. 2006, 2012, and 2014) using the PureLinkTM Genomic DNA kit (Invitrogen), following the manufacturer’s protocol, and resuspended in a final volume of 50 μL. A negative control that contained no tissue was included in every round of DNA extraction to check for contamination. Sequences from the partial mitochondrial cytochrome c oxidase subunit I (COI) gene were amplified using the primer pair LCO1490 and HCO2198 (Folmer et al., 1994). The partial mitochondrial 16S ribosomal RNA (16S) fragment was amplified using the primer pair 16Sbr and 16Sar (Palumbi et al., 1991). Standard polymerase chain reactions (PCRs) were performed using the Dream Taq DNA polymerase (Thermofisher), following manufacturer’s protocol, in a total volume of 25 μL. The PCRs consisted of an initial denaturation at 95°C for 2 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 50°C for COI and 45°C for 16S for 30 s and extension at 72°C for 45 s, with a final extension of 5 min at 72°C. The PCR products were sequenced by Macrogen Europe (Amsterdam, Netherlands). The sequences were deposited in the public repository GenBank (Accession Numbers MW752849-W752851 and MW732699-MW732701 for COI and 16S sequences, respectively). Two approaches were employed for the genetic identification of the three individuals. The first step involved similarity-based methods: identification engine of BOLD-IDS (only for COI sequences) and BLASTn search routine implemented in GenBank (for both COI and 16S sequences). Both methods provided a list of similar sequences with the associated taxon name and the percent sequence similarity. In the second step, our sequences were included in a phylogenetic analysis with those available in public databases belonging to O. bartramii and the other three recently resurrected species (i.e., O. brevimanus, O. caroli, and O. cylindraceus; see Fernández-Álvarez et al., 2020; Supplementary Tables 1, 2). The final alignment included 28 and 72 sequences for COI and 16S, respectively. The Clustal-Wallis algorithm (Thompson et al., 1994) implemented in MEGA v10 (Kumar et al., 2018) was used to align the sequences, resulting in 589 and 379 bp final alignments for COI and 16S, respectively. In order to assess the relationship between our query sequences and its neighboring reference sequences, all the COI and 16S sequences were separately analyzed using tree-based approaches: Neighbor-Joining (NJ, implemented in MEGA v10; Kumar et al., 2018), Bayesian inference (BA, using MrBayes v3.2.7; Ronquist et al., 2012), and Maximum Likelihood method (ML, with PhyML; Guindon et al., 2010). Furthermore, to visualize genealogical relationships at the intraspecific level, as well as to make inference about biogeography, haplotype networks have been inferred for both genes using the TCS method (Clement et al., 2000) implemented in POPART (Leigh and Bryant, 2015). Uncorrected p-distances within and among Atlantic and Mediterranean O. caroli specimens were calculated with MEGA for both molecular markers.
Age Estimation
Beaks and eye lenses, were analyzed for age estimation, assuming daily increments (Sakai et al., 2007; Arkhipkin et al., 2018). According to Hernández-López et al. (2001), upper beaks were sectioned sagittally in two symmetrical pieces along the posterior edge of the hood and crest, to the rostral tip. Following Perales-Raya et al. (2010), one section was embedded in epoxy resin for 24 h for hardening and then glued on to microscope slide. After the section, it was gradually grounded parallel to the cutting plane to approach the sagittal section surface with waterproof sandpaper at different grit. Following Agus et al. (2018), eye lenses, were kept under flowing water for 24 h and dried for a few hours in a laminar flow hood, in order to eliminate any residual of water. Then, they were incorporated into epoxy resin and the blocks were grounded using a lapping machine with different grit waterproof sandpapers, a section of 1 mm thickness was obtained. The sections were mounted on a microscope slide and stained using Gill’s Ematoxilin and Eosin before being examined using an optical microscope equipped with CANON EOS 1100 D camera to take digital images (magnifications: 100× and 400×). Increments were counted starting from the centre (nucleus) to the outer end of the lenses. To avoid duplicating counts, each increment was numbered using the Tps_Dig2 software. The counting of the increments in beaks and eye lenses was performed five times by the same operator at different times, using the average value of the number of increments for both structures. The overall countings precision and accuracy for both structures, were evaluated by the Coefficient of Variation (%CV) (Chang, 1982) and the Average Percent Error (APE) (Beamish and Fournier, 1981) calculated as
where Xij is the ith age determination of the jth fish, Xj is the mean age estimate of the jth fish, and R is the number of times each fish is aged. For each specimen the average of increments obtained by beaks and lenses, were correlated and compared by the ANOVA test and hypothesis Z-test, using the software Statgraphics Centurion XVI. For the z-hypothesis test it has been assumed that the difference between means is zero (null hypothesis) or different (Alternative hypothesis).
Results
Molecular Analysis
Using BLASTn search routine implemented in GenBank, COI sequences of the three specimens matched at 99% identity with a sequence (GenBank Accession MK995127) of O. caroli. Likewise, 16S sequences of our individuals returned a match with a 16S sequence (GenBank Accession MK991814) of O. caroli at 97–99% identity. The BOLD identification engine (using only COI sequences) reported that the queried specimens were likely to be O. bartramii or O. caroli; the best ID (99% similarity) was found with the only available Mediterranean sequence of O. bartramii, from Croatia (GenBank Accession KF212462). In the NJ, BA and ML trees (performed separately on both COI and 16S datasets), COI sequences clustered in four groups corresponding to four distinct species: O. bartramii and the other three recently resurrected species (i.e., O. brevimanus, O. caroli, and O. cylindraceus; Figure 1). The sequences of our specimens clustered together in a single well supported clade with public sequences from the Atlantic assigned by Fernández-Álvarez et al. (2020) to O. caroli and one Mediterranean sequence (GenBank Accession KF212462; Figure 1), deposited as O. bartramii by Franjević et al. (2015). Within this clade, the analyses indicated the possible occurrence of a distinct subcluster, composed by all the Atlantic sequences, which appear to be separated from the Mediterranean ones, even if they differ by a few mutations (p-distance 0.29%). The 16S trees showed only two supported clades (Supplementary Figure 2). O. caroli sequences clustered together, separated from the remaining sequences. Our 16S sequences clustered with sequences of Ommastrephes group 1 sensu Fernández-Álvarez et al. (2020) in the O. caroli clade. No further clades were formed, and hence all other Ommastrephes, apart from O. caroli, were not differentiated. This result confirms that 16S might be very conserved to resolve the taxonomy of this genus. Similarly, COI haplotype network (Supplementary Figure 3A) confirmed the clear separation of Mediterranean and North Atlantic O. caroli specimens from all other sequences from different areas, while the O. caroli haplotypes in the 16S network were far less differentiated from the other areas (p-distance between North Atlantic and Mediterranean 0.31%; Supplementary Figure 3B).
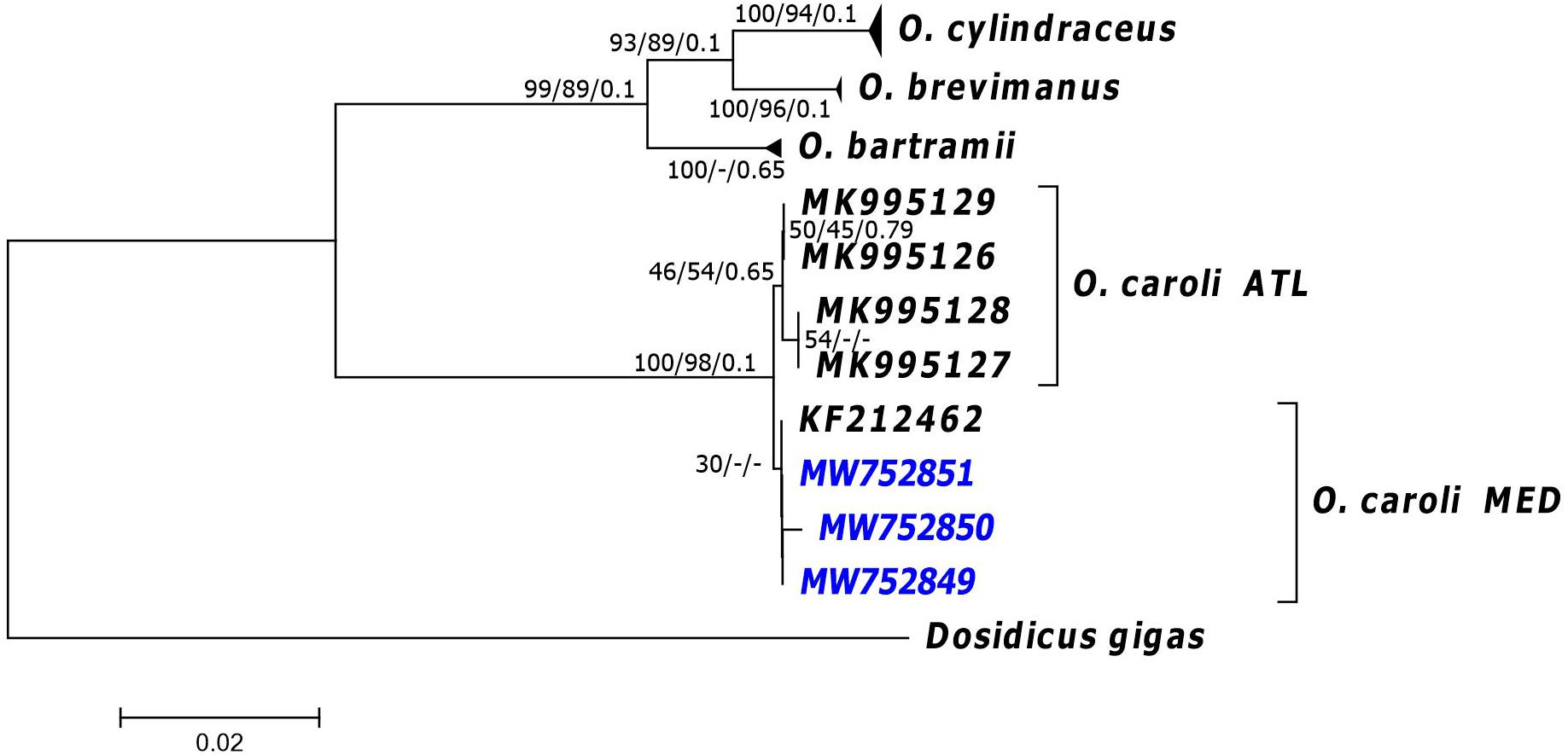
Figure 1. Neighbour joining (NJ) phylogenetic tree of the COI sequences. Near the nodes are the support values >30 obtained for NJ, Maximum Likelihood (ML) and Bayesian (BA) trees, respectively. NJ and ML values refer to bootstrap while BA support are posterior probabilities. Codes in the tips of branches correspond to the GenBank Accession number of sequences (see main text for details). ATL, samples from the Atlantic Ocean, MED, samples caught in the Mediterranean Sea. In blue, the sequences from individuals obtained in the present study.
Morphometric and Biological Features
The sample (Table 1) was composed of seven females (three mature and four partially spent) (Figures 2A,B) ranging from 490 to 592 mm in mantle length, and from 4350 to about 10,000 g in weight (TW) and one mature male (282 mm ML and 763 g TW) (Figure 2C). Buccal membranes of the females displayed from 53 to 178 seminal receptacles (SRs) of which most appeared white. Moreover, in all females, around SRs there were also spermatangia implanted in the tissue (Figure 2D). Their number varied from 12 to 92 (45 ± 31.28) and the size ranged from 1.82 to 6.29 mm (3.87 ± 0.80 mm) in length, and from 0.30 to 1.02 mm (0.60 ± 0.15 mm) in width (Figure 2E). In the mature female analyzed (ML: 517 mm; TW: 4450 g) the ovary was composed of oocytes of different size (0.05–1.10 mm) and each oviduct contained, respectively, 45796 and 44689 brownish ripe oocytes ranging from 0.98 to 1.32 mm (1.11 ± 0.10 mm). Potential fecundity was estimated in 840061 oocytes. The seven beaks analyzed had the hood and rostrum completely pigmented as showed in Figure 2F. The relationships obtained between the beak measurements and body sizes of the specimens (ML; TW) was better described by a power model (Supplementary Table 3). All stomachs, even if at different fullness levels, showed remains of preys belonging to Cephalopoda, Crustacea, and Osteichthyes categories (Table 1).
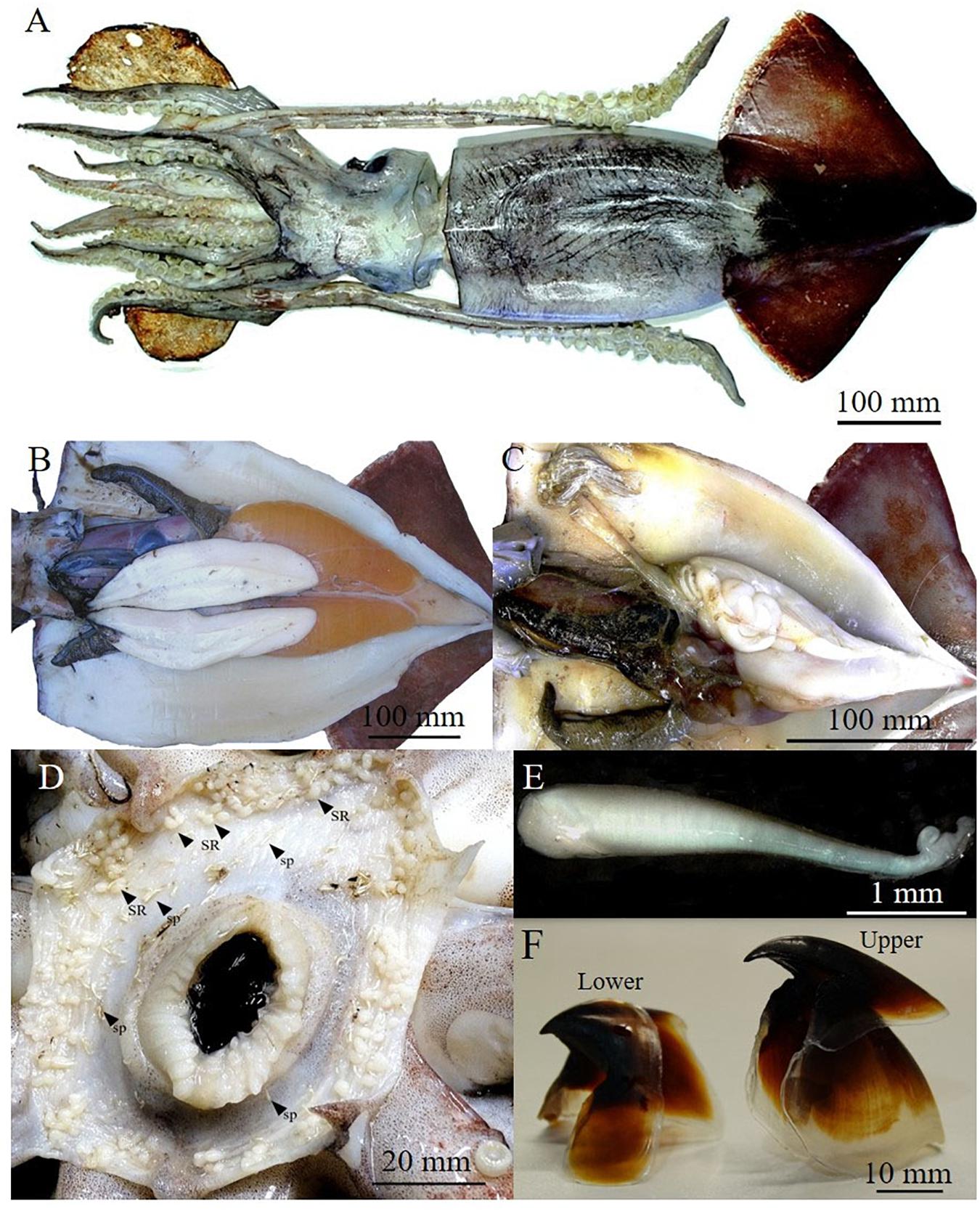
Figure 2. Dorsal vision of a mature female of Ommastrephes caroli of 520 mm of mantle length (A), mature reproductive apparatus of a female (B) and male (C). Buccal membrane of mated female with seminal receptacles (SR) and spermatangia (sp) (D), a spermatangium (E) and a lower and upper beak isolated (F).
Age Estimation
After their processing, the upper beaks showed a distinct band pattern (increments) from the rostrum tip to the posterior region of the lateral wall. The increments in the tip region were narrower than those in the medial part (Figure 3). The mean number of increments counted in the male was 383 and within the females, it ranged from 396 to 427 (Tables 1, 2). All the eye lenses analyzed showed a well-defined nucleus surrounded by subsequent layers (increments), varying in number from 388 to 437 (Figure 4 and Tables 1, 2). For each specimen low values of APE and CV for both structures were obtained showing a high level of precision and accuracy in the readings (Table 2). A significant statistically linear relationship was observed between the number of increments counted in the eye lenses and in the beaks, with R2 and slope close to 1 (Figure 5). ANOVA test confirmed a strong relationship (P < 0.05; 95% confidence level) between the average of increments obtained by beaks and lenses for each specimen. The Z-test showed a computed Z statistic = 0.0 and a P-value = 1.0 (P > 0.05) therefore we can accept that the variance between means is equal, with 95% confidence level.
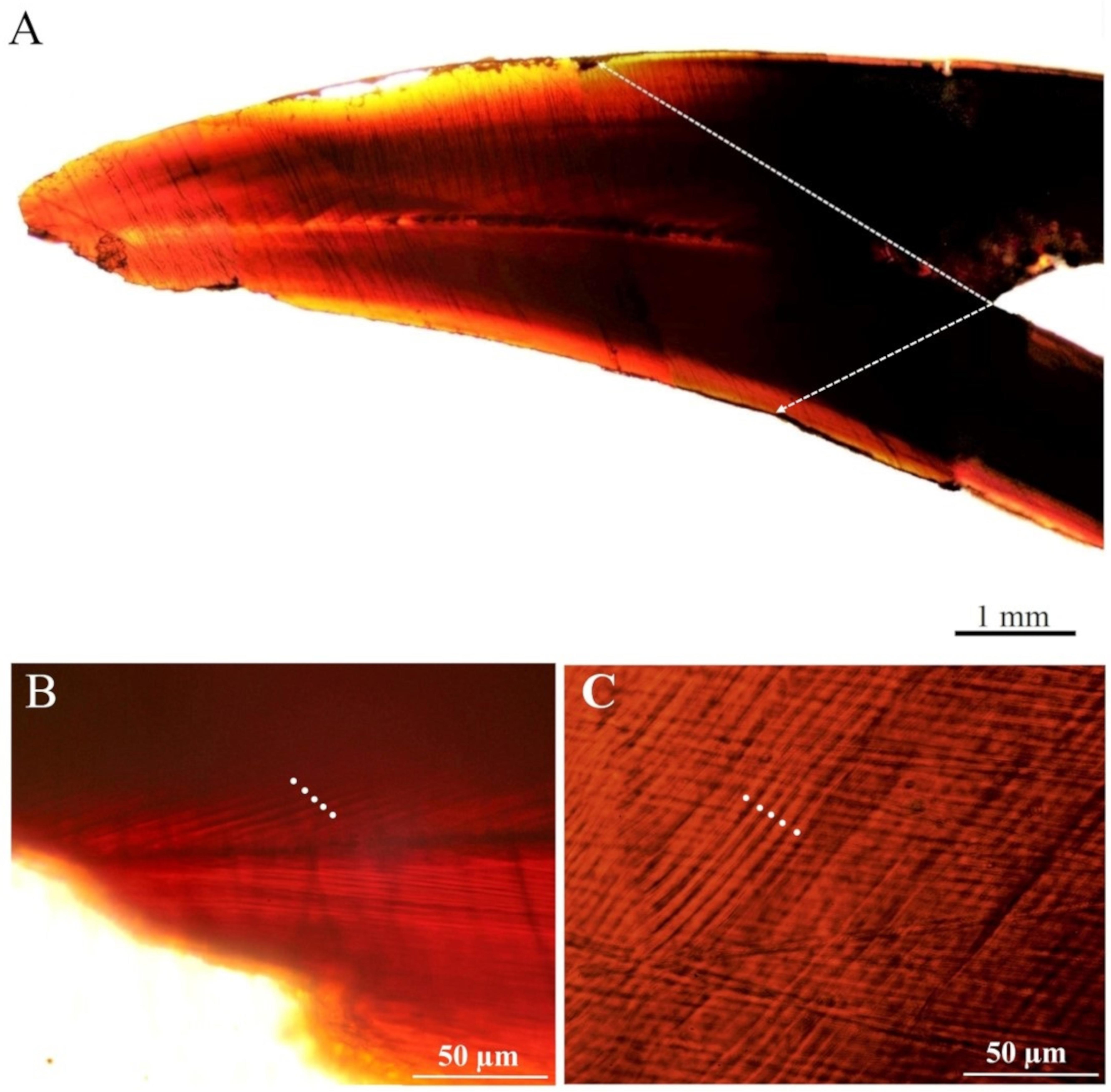
Figure 3. Upper beak section of Ommastrephes caroli (A). Increments in the tip (B) and in medial (C) regions.
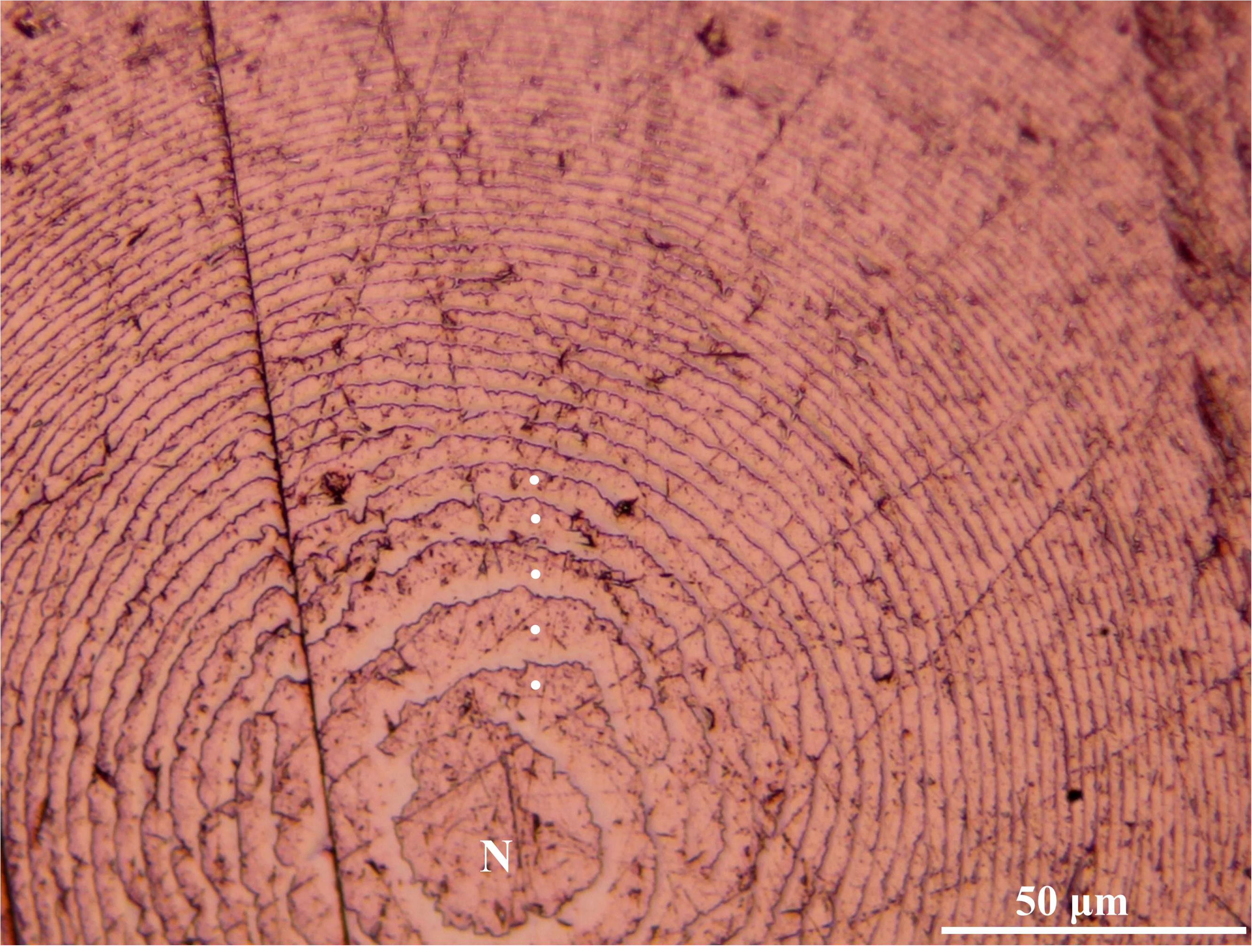
Figure 4. Microscopic vision (400×) of growth increments in an eye lens of Ommastrephes caroli specimen. Nucleus (N).

Figure 5. The relationship between the number of increments counted in the beaks and in the eye lenses of seven specimens of Ommastrephes caroli.
Discussion
Molecular Analysis
Both the similarity-based identifications and tree-based methods showed that the three specimens caught in Sardinian waters belonged to the species O. caroli, a recently resurrected species of the genus Ommastrephes distributed in the Atlantic Ocean and Mediterranean Sea (Fernández-Álvarez et al., 2020). It is noteworthy that the only Mediterranean sequence available in public database and assigned to O. bartramii (Franjević et al., 2015) clustered with our sequences, as well as the others attributed to O. caroli by Fernández-Álvarez et al. (2020). Our study confirms that the specimens collected and analyzed so far in the Mediterranean Sea belonged to the same species O. caroli. It further highlights how the presence of erroneous records in international repositories are particularly abundant for cephalopods as previously reported (Groenenberg et al., 2009; Fernández-Álvarez et al., 2021) and reaffirms the urgent need to check and update the information reported therein, especially when the taxonomy of species has been recently revised, as in the case of Ommastrephes (Fernández-Álvarez et al., 2020). The analyses of COI sequences suggested a potential genetic differentiation of Atlantic O. caroli from the Mediterranean ones, even if with a very low support by only two (ML and BA) out of the three methods used to obtain our phylogenetic trees (Figure 1). Therefore, this result should be interpreted with caution for at least three main reasons: the bootstrap values supporting the Atlantic clade were low-to-moderate, the low number of sequences available from both areas could produce biased results, and the differentiation was not as clear as for COI with the other marker used (16S sequences). Further studies, with more individuals and markers would shed light on connectivity and potential genetic differentiation among individuals living in the Atlantic and the Mediterranean Sea.
Morphometric and Reproductive Features
Morphological and reproductive information obtained from the analyzed samples will contribute to define the biological features of O. caroli species, confirming and bridging the fragmentary available knowledge on the Mediterranean specimens. The finding of mature/spawning specimens, even if in a low number and accidentally caught, occurred in spring – summer like for all the other large and mature specimens previously recorded in the Mediterranean Sea, both in the Western and Eastern basins (Lefkaditou et al., 2011). As observed by Lefkaditou et al. (2011), the relationships between the beak measurements and body sizes were best fitted by the power model regression; however, we aware of the limitation of the our sample size. Feeding behavior confirms the same most common prey categories (Cephalopoda, Crustacea, and Osteichthyes) observed in specimens from the Mediterranean (Lefkaditou et al., 2011) and also in other ommastrephid squids (e.g., Ivanovic and Brunetti, 2004; Bruno et al., 2021). According to the semelparous life cycle of these organisms, our samples were close to the end of their life span. Considering our samples and also previous findings (Lefkaditou et al., 2011; Kapiris et al., 2014; Franjević et al., 2015), Mediterranean specimens seem to reach a less maximum size respect to the Atlantic ones. Indeed, a female of 90 cm and 25 kg, and a male of 42 cm and 2.2 kg have been reported in the North Atlantic Ocean (Nigmatullin, 1989). This possible discrepancy in sizes among the Atlantic and the Mediterranean specimens could be linked to ecological reasons such as temperature and food supply. Typically, elevated temperatures and limited nutrition result in a smaller size at maturity for squids (e.g., Hoving et al., 2013; Arkhipkin et al., 2014). Similarity to O. bartramii (Roper et al., 2010), our specimens confirm that asynchronous oocytes development, a relatively small oocytes (0.05–1.10 mm) and a protracted intermittent spawning with active feeding, are features of the species O. caroli. Considering that the biological knowledge on O. caroli is limited (e.g., Pinchukov, 1975; Nigmatullin, 1989; Jereb et al., 2015; Lishchenko et al., 2021), the value on potential fecundity here estimated (840061 oocytes), as well as the oviductal load (about 90000 ripe oocytes), at the moment represent the only information available for this species. Potential fecundity is decidedly lower when compared to O. bartramii for which between 3 to 8 million of oocytes were found in mature females of a size comparable with our sample (Roper et al., 2010; Vijai et al., 2014). The number and the size of ripe oocytes in the oviducts are instead comparable with the species O. bartramii (Roper et al., 2010; Vijai et al., 2014). All the studied females showed signs of mating, indeed most of the seminal receptacles in the buccal membrane appeared white, probably because they contain sperm, and around them there were also spermatangia implanted in the tissue. Sperm storage modality in the tissue of the buccal membrane and the number of seminal receptacles are analogous to what was observed in O. bartramii (Roper et al., 2010) but also within the Ommastrephidae family considering the species Sthenoteuthis oualaniensis (Lesson, 1830) and S. pteropus (Steenstrup, 1855; Zuyev et al., 2002). In many species of squid and cuttlefish (Nigmatullin et al., 1995; Sato et al., 2010, 2016), including flying squids of the family Ommastrephidae (Fernández-Álvarez et al., 2018), sperm from spermatangia migrates to the seminal receptacles until used for egg fertilization. In our samples, due to the presence of spermatangia, we are led to assume recent mating activity. However, we do not know how long spermatangia could remain implanted in the tissue and how long sperm takes to migrate from spermatangium to the receptacles. In Idiosepius paradoxus (Ortmann, 1888) spermatangia remain implanted in the female for only 24–48 h (Sato et al., 2014). Data on spermatangia morphology and size here reported, are the first available for O. caroli and, in the future which will be useful to evaluate possible distinctive reproductive features of the Mediterranean specimens.
Age Estimation
To the best knowledge of the authors, age estimation within the genus Ommastrephes had been performed only using statoliths (Yatsu et al., 1997; Yatsu, 2000; Yatsu and Mori, 2000), and more recently through the analysis of Rostral Sagittal Section (RSS) of the beaks of Pacific O. bartramii (Liu et al., 2015; Fang et al., 2016). In our study, similarly to what was observed by Liu et al. (2015) in O. bartamii, growth increments observed in the RSS of O. caroli specimens were easily visible and counted. As regards the use of eye lenses for age estimation in cephalopods, it has been tried in Sepia officinalis (Linnaeus, 1758), Enteroctopus megalocyatus (Gould, 1852), Octopus maya (Voss and Solís, 1966), and more recently in Loligo vulgaris (Lamark, 1798) and Loligo forbesii (Steenstrup, 1856) (Clarke, 1993; Baqueiro-Cárdenas et al., 2011; Rodríguez-Domínguez et al., 2013; Agus et al., 2018). Even if this structure is considered attractive as a potential aging tool, its use has not been satisfactory in all species, suggesting the need of further studies for its validation (Rodríguez-Domínguez et al., 2013). However, in our case, as well as observed for the beaks, the eye lenses are easier to extract and manipulate than statoliths and positive results in reading and counting increments have been confirmed by the high level of reading precision obtained (APE and CV). The statistically significant relationship found between the number of increments counted in eye lenses and beaks (ANOVA, P < 0.05), highlighted the reliability of the lens to estimate age in O. caroli, even if further studies are needed for its validation. Taking into account that a daily deposition of increments in the upper beaks was confirmed and validated in several Ommastrephidae squids such as O. bartramii, Dosidicus gigas (d’Orbigny, 1835) and S. oualaniensis (Sakai et al., 2007), we assumed the same for the beaks of O. caroli and also for the eye lenses. On this basis, in this study, for each specimen emerged an age consistent with their maturity stage, both in the male and females, which were close to the end of life. Consequently for the species O. caroli, even if it greatly exceeds the average size of the coleoids we can hypothesize a short mean life span of 12–13 months.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Ethics Statement
Ethical review and approval was not required for the animal study because the animal’s analyzed were found dead.
Author Contributions
BA, LC, and DC conceived the study. BA, JC, SR, AB, and AC conducted the data acquisition. DC, BA, JC, SR, and AB performed the morphological analysis and interpretation. LC, RC, RM, and EC performed the molecular analysis and interpretation. BA, JC, SR, and DC performed the age analysis and interpretation. All authors contributed to the writing of the manuscript, its critical review, and approve the final version submitted.
Funding
Some data for the purposes of the analyses were obtained from the International Mediterranean Trawl survey MEDITS within the Data Collection Framework (EU Reg. 199/2008).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor declared a past co-authorship with several of the authors, AB, AC, DC, RC, and RM.
Acknowledgments
We are grateful to the editor and the reviewers for their constructive comments and suggestions, which greatly helped to improve the manuscript quality. LC acknowledges the support from PON AIM 1854833 – PON Ricerca e Innovazione 2014-2020 – Azione I.2 – D.D. n. 407, 27/02/2018 – Attraction and International Mobility.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.683856/full#supplementary-material
References
AAVV (2017). MEDITS-Handbook. Version n_9. MEDITS Working Group. Available online at: https://www.sibm.it/MEDITS%202011/principaledownload.htm (accessed November, 2020).
Agus, B., Mereu, M., Cannas, R., Cau, A., Coluccia, E., Follesa, M. C., et al. (2018). Age determination of Loligo vulgaris and Loligo forbesii using eye lens analysis. Zoomorphology 137, 63–70. doi: 10.1007/s00435-017-0381-8
Arkhipkin, A. I., Argüelles, J., Shcherbich, Z., and Yamashiro, C. (2014). Ambient temperature influences adult size and life span in jumbo squid (Dosidicus gigas). Can. J. Fish. Aquat. Sci. 72, 400–409. doi: 10.1139/cjfas-2014-0386
Arkhipkin, A. I., Bizikov, V. A., Doubleday, Z. A., Laptikhovsky, V. V., Lishchenko, F. V., Perales-Raya, C., et al. (2018). Techniques for estimating the age and growth of Molluscs: Cephalopoda. J. Shellfish Res. 37, 783–792. doi: 10.2983/035.037.0409
Baqueiro-Cárdenas, E. R., Medrano Correa, S., Contreras Guzman, R., Barahona, N., Briceño, F., Villegas, M. J., et al. (2011). Eye lens structure of the octopus Enteroctopus megalocyathus: evidence of growth. J. Shell. Res. 30, 199–204. doi: 10.2983/035.030.0201
Battaglia, P., Romeo, T., Consoli, P., Scotti, G., and Andaloro, F. (2010). Characterization of the artisanal fishery and its socio-economic aspects in the central Mediterranean Sea (Aeolian Islands, Italy). Fish. Res. 102, 87–97. doi: 10.1016/j.fishres.2009.10.013
Beamish, R. J., and Fournier, D. A. (1981). A method for comparing the precision of a set of age determinations. Can. J. Fish. Aquat. Sci. 38, 982–983. doi: 10.1139/f81-132
Bello, G. (1991). Role of Cephalopods in the diet of the Swordfish, Xiphias gladius, from the Eastern Mediterranean Sea. Bull. Mar. Sci. 49, 312–324.
Bower, J. R., and Ichii, T. (2005). The red flying squid (Ommastrephes bartramii): a review of recent research and the fishery in Japan. Fish. Res. 76, 39–55. doi: 10.1016/j.fishres.2005.05.009
Bruno, C., Cornejo, C. F., Riera, R., and Ibáñez, C. M. (2021). What is on the menu? Feeding, consumption and cannibalism in exploited stocks of the jumbo squid Dosidicus gigas in south-central Chile. Fish. Res. 233:105722. doi: 10.1016/j.fishres.2020.105722
Chang, W. Y. B. (1982). A statistical method for evaluating the reproducibility of age determination. Can. J. Fish. Aquat. Sci. 39, 1208–1210. doi: 10.1139/f82-158
Clarke, M. R. (1986). A Handbook for the Identification of Cephalopod Beaks. Marine Biology Association U.K. Plymouth: Clarendon Press Oxford.
Clarke, M. R. (1993). “Age determination and common sense - a free discussion on difficulties encountered by the author,” in Recent Advances in Cephalopod Fisheries Biology, eds T. Okutani, R. K. O’Dor, and T. Kubodera (Tokyo: Tokai University Press), 670–678.
Clement, M., Posada, D., and Crandall, K. (2000). TCS: a computer program to estimate gene genealogies. Mol. Ecol. 9, 1657–1659. doi: 10.1046/j.1365-294x.2000.01020.x
Ding, Q., Cao, J., and Chen, X. (2019). Stock assessment of the western winter-spring cohort of Ommastrephes bartramii in the Northwest Pacific Ocean using a Bayesian hierarchical DeLury model based on daily natural mortality during 2005-2015. Sci. Mar 83, 155–166. doi: 10.3989/scimar.04783.10A
Fang, Z., Li, J., Thompson, K., Hu, F., Chen, X., Liu, B. L., et al. (2016). Age growth, and population structure of the red flying squid (Ommastrephes bartramii) in the North Pacific Ocean, determined from beak structure. Fish. Bull. 114, 34–44. doi: 10.7755/FB.114.1.3
Fernández-Álvarez, F. Á, Braid, H. E., Nigmatullin, C. M., Bolstad, K. S. R., Haimovici, M., Sánchez, P., et al. (2020). Global biodiversity of the genus Ommastrephes (Ommastrephidae: Cephalopoda): an allopatric cryptic species complex. Zool. J. Linn. Soc. 190, 460–482. doi: 10.1093/zoolinnean/zlaa014
Fernández-Álvarez, F. Á, Sánchez, P., and Villanueva, R. (2021). Morphological and molecular assessments of bobtail squids (Cephalopoda: Sepiolidae) reveal a hidden history of biodiversity. Front. Mar. Sci. 7:632261. doi: 10.3389/fmars.2020.632261
Fernández-Álvarez, F. Á, Villanueva, R., Hoving, H. J. T., and Gilly, W. F. (2018). The journey of squid sperm. Rev. Fish. Biol. Fish 28, 191–199. doi: 10.1007/s11160-017-9498-6
Folmer, O., Black, M., Hoeh, W., Lutz, R., and Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299.
Franjević, D., Skaramuca, D., Katavić, V., Rajević, N., and Skaramuca, B. (2015). Genetic identification of a rare record of Ommastrephes bartramii (Cephalopoda: Ommastrephidae) from the Eastern Adriatic Sea. Folia Biol. Biol. (Kraków) 63, 19–23. doi: 10.3409/fb63_1.19
Groenenberg, D. S. J., Goud, J., De Heij, A., and Gittenberger, E. (2009). Molecular phylogeny of North Sea Sepiolinae (Cephalopoda: Sepiolidae) reveals an overlooked Sepiola species. J. Molluscan Stud. 75, 361–369. doi: 10.1093/mollus/eyp032
Guindon, S., Dufayard, J. F., Lefort, V., Anisimova, M., Hordijk, W., and Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 59, 307–321. doi: 10.1093/sysbio/syq010
Hernández-López, J. L., Castro-Hernández, J. J., and Hernández-García, V. (2001). Age determined from the daily deposition of concentric rings on common octopus (Octopus vulgaris) beaks. Fish. Bull. 99, 679–684.
Hoving, H. J., Gilly, W. F., Markaida, U., Benoit-Bird, K. J., Brown, Z. W., Daniel, P., et al. (2013). Extreme plasticity in life-history strategy allows a migratory predator (jumbo squid) to cope with a changing climate. Glob. Change Biol. 19, 2089–2103. doi: 10.1111/gcb.12198
Ichii, T., Mahapatra, K., Sakai, M., and Okada, Y. (2009). Life history of the neon flying squid: effect of the oceanographic regime in the North Pacific Ocean. Mar. Ecol. Prog. Ser. 378, 1–11. doi: 10.3354/meps07873
Ivanovic, M. L., and Brunetti, N. E. (2004). Diet of red squid (Ommastrephes bartramii) in the Southwest Atlantic. Rev. Invest. Desarr. Pesq. 16, 67–75.
Jereb, P., Allcock, A. L., Lefkaditou, E. G., Piatkowski, U., Hastie, L. C., and Pierce, G. J. (2015). “Ommastrephes bartramii,” in Cephalopod biology and Fisheries in Europe: II. Species Accounts. ICES Cooperative Research Report, eds P. Jereb, A. L. Allcock, E. Lefkaditou, U. Piatkowski, L. C. Hastie, and G. J. Pierce (Copenhagen: ICES), 219–228. doi: 10.13140/RG.2.1.1081.6164
Kapiris, K., Apostolidis, C., Baldacconi, R., Başusta, N., Bilecenoglu, M., Bitar, G., et al. (2014). New Mediterranean marine biodiversity records (April, 2014). Mediterr. Mar. Sci. 15, 198–212. doi: 10.12681/mms.737
Kumar, S., Stecher, G., Li, M., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Lefkaditou, E., Peristeraki, P., Chartosia, N., and Salman, A. (2011). Recent findings of Ommastrephes bartramii (Cephalopoda: Ommastrephidae) in the eastern Mediterranean and the implication on its range expansion. Mediterr. Mar. Sci 12, 413–428. doi: 10.12681/mms.41
Leigh, J. W., and Bryant, D. (2015). POPART: full-feature software for haplotype network construction. Methods Ecol. Evol. 6, 1110–1116. doi: 10.1111/2041-210X.12410
Lishchenko, F., Perales-Raya, C., Barrett, C., Oesterwind, D., Power, A. M., Larivain, A., et al. (2021). A review of recent studies on the life history and ecology of European cephalopods with emphasis on species with the greatest commercial fishery and culture potential. Fish. Res 236:105847. doi: 10.1016/j.fishres.2020.105847
Liu, B. L., Chen, X. J., Chen, Y., and Hu, G. Y. (2015). Determination of squid age using upper beak rostrum sections: technique improvement and comparison with the statolith. Mar. Biol. 162, 1685–1693. doi: 10.1007/s00227-015-2702-0
Nigmatullin, C. M. (1989). “Biomass, production, role in the world ocean ecosystem, and fishery potential of squids family Ommastrephidae,” in Proceedings of the 6th All-Russian Conference on Commercial Invertebrates, Kaliningrad (Lesnoye), 3 – 6 September 2002, Abstracts of Reports, eds G. I. Ivanov and C. M. Nigmatullin (Moscow: VNIRO Publishing), 155–157. [in Russian, with English title].
Nigmatullin, C. M., Arkhipkin, A. I., and Sabirov, R. M. (1995). Age, growth and reproductive biology of diamond-shaped squid Thysanoteuthis rhombus (Oegopsida: Thysanoteuthidae). Mar. Ecol. Prog. Ser 124, 73–87. doi: 10.3354/meps124073
Nigmatullin, C. M., and Markaida, U. (2009). Oocyte development, fecundity and spawning strategy of large sized jumbo squid Dosidicus gigas. J. Mar. Biol. Assoc. UK 89, 789–801. doi: 10.1017/S0025315408002853
Palumbi, S. R., Martin, A., Romano, S., Mcmillan, W. O., Stice, L., and Grabowski, G. (1991). The Simple Fool’s Guide to PCR. A Collection of PCR Protocols, Version 2. Honolulu: University of Hawaii.
Perales-Raya, C., Bartolomé, A., García-Santamaría, M. T., Pascual-Alayón, P., and Almansa, E. (2010). Age estimation obtained from analysis of octopus (Octopus vulgaris Cuvier, 1797) beaks: improvements and comparisons. Fish. Res. 106, 171–176. doi: 10.1016/j.fishres.2010.05.003
Pinchukov, M. A. (1975). Distribution, Biology and Intraspecies Structure of the Atlantic flying squid Ommastrephes bartramii Lesueur, 1821. Master’s thesis. Kazan: Kazan University. [in Russian].
Rasero, M., González, ÁF., Castro, B. G., and Guerra, Á (1996). Predatory relationships of two sympatric squid, Todaropsis eblanae and Illex coindetii (Cephalopoda: Ommastrephidae) in Galician waters. J. Mar. Biol. Assoc. UK 76, 73–87. doi: 10.1017/S0025315400029027
Rodríguez-Domínguez, A., Rosas, C., Méndez-Loeza, I., and Markaida, U. (2013). Validation of growth increments in stylet, beaks and lenses as ageing tools in Octopus maya. J. Exp. Mar. Biol. Ecol. 449, 194–199. doi: 10.1016/j.jembe.2013.10.001
Romeo, T., Battaglia, P., Pedà, C., Perzia, P., Consoli, P., Esposito, V., et al. (2012). Pelagic cephalopods of the central Mediterranean Sea determined by the analysis of the stomach content of large fish predators. Helgol. Mar. Res. 66, 295–306. doi: 10.1007/s10152-011-0270-3
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Roper, C. F. E., Nigmatullin, C. M., and Jereb, P. (2010). “Family Ommastrephidae,” in Cephalopods of the World. An Annotated and Illustrated Catalogue of Species Known to Date. Myopsid and Oegopsid Squids, Vol. 2, eds P. Jereb and C. F. E. Roper (Rome: FAO Species Catalogue for Fishery Purposes), 269–347.
Roper, C. F. E., Sweeney, M. J., and Nauen, C. E. (1984). FAO Species Catalogue. Cephalopods of the World: An Annotated And Illustrated Catalogue of Species of Interest to Fisheries. FAO Fisheries Synopsis, Vol. 3. Rome: FAO Species Catalogue for Fishery Purposes.
Roper, C. F. E., and Voss, G. L. (1983). Guidelines for taxonomic descriptions of Cephalopod species. Mem. Natl. Museum Victoria. 44, 48–63. doi: 10.24199/j.mmv.1983.44.03
Sakai, M., Brunetti, N., Bower, J., Elena, B., Ichii, T., Ivanovic, M., et al. (2007). Daily growth increments in upper beak of five ommastrephid paralarvae, Illex argentinus, Ommastrephes bartramii, Dosidicus gigas, Sthenoteuthis oualaniensis, Todarodes pacificus. Squids Resour. Res. Conf. 9, 1–7. doi: 10.1007/978-3-319-23534-9_1
Sato, N., Kasugai, T., Ikeda, Y., and Munehara, H. (2010). Structure of the seminal receptacle and sperm storage in the Japanese pygmy squid. J. Zool. 282, 151–156. doi: 10.1111/j.1469-7998.2010.00733.x
Sato, N., Kasugai, T., and Munehara, H. (2014). Spermatangium formation and sperm discharge in the Japanese pygmy squid Idiosepius paradoxus. Zoology 117, 192–199. doi: 10.1016/j.zool.2014.02.001
Sato, N., Yoshida, M., and Kasugai, T. (2016). Impact of cryptic female choice on insemination success: larger sized and longer copulating male squid ejaculate more, but females influence insemination success by removing spermatangia. Evolution 71, 111–120. doi: 10.1111/evo.13108
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Tsiamis, K., Aydogan, Ö, Bailly, N., Balistreri, P., Bariche, M., Carden-Noad, S., et al. (2015). New Mediterranean biodiversity records (July 2015). Mediterr. Mar. Sci 16, 472–488. doi: 10.12681/mms.1440
Vijai, D., Sakai, M., Kamei, Y., and Sakurai, Y. (2014). Spawning pattern of the neon flying squid Ommastrephes bartramii (Cephalopoda: Oegopsida) around the Hawaiian Islands. Sci. Mar. 78, 511–519. doi: 10.3989/scimar.04112.27B
Yatsu, A. (2000). Age Estimation of Four Oceanic Squids, Ommastrephes bartramii, Dosidicus gigas, Sthenoteuthis oualaniensis, and Illex argentinus (Cephalopoda, Ommastrephidae) Based on Statolith Microstructure. Jpn. Agric. Res. Q. 34, 75–80.
Yatsu, A., Midorikawa, S., Shimada, T., and Uozumi, Y. (1997). Age and growth of the neon flying squid, Ommastrephes bartramii, in the North Pacific Ocean. Fish. Res. 29, 257–270. doi: 10.1016/S0165-7836(96)00541-3
Yatsu, A., and Mori, J. (2000). Early growth of the autumn cohort of neon flying squid, Ommastrephes bartramii, in the North Pacific Ocean. Fish. Res. 45, 189–194. doi: 10.1016/S0165-7836(99)00112-5
Keywords: Ommastrephes caroli, molecular analysis, reproduction, age and growth, Mediterranean Sea
Citation: Agus B, Carugati L, Bellodi A, Cannas R, Cau A, Cera J, Coluccia E, Melis R, Ruiu S and Cuccu D (2021) Molecular and Biological Analysis on Ommastrephes caroli Findings in the Central Western Mediterranean Sea (Sardinian Waters) Including First Age Investigation Using Eye Lenses and Beaks. Front. Mar. Sci. 8:683856. doi: 10.3389/fmars.2021.683856
Received: 22 March 2021; Accepted: 28 May 2021;
Published: 18 June 2021.
Edited by:
Pierluigi Carbonara, COISPA Tecnologia & Ricerca, ItalyReviewed by:
Fernando Ángel Fernández-Álvarez, National University of Ireland Galway, IrelandAlba Jurado-Ruzafa, Oceanographic Center of the Canary Islands – Spanish Institute of Oceanography (IEO), Spain
Dharmamony Vijai, National Centre for Biological Sciences, India
Copyright © 2021 Agus, Carugati, Bellodi, Cannas, Cau, Cera, Coluccia, Melis, Ruiu and Cuccu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danila Cuccu, Y3VjY3VAdW5pY2EuaXQ=
†These authors have contributed equally to this work and share first authorship
 Blondine Agus
Blondine Agus Laura Carugati
Laura Carugati Andrea Bellodi
Andrea Bellodi Rita Cannas
Rita Cannas Alessandro Cau
Alessandro Cau Jacopo Cera
Jacopo Cera Elisabetta Coluccia
Elisabetta Coluccia Riccardo Melis
Riccardo Melis Stefano Ruiu
Stefano Ruiu Danila Cuccu
Danila Cuccu