- 1Higher Institution Centre of Excellence (HICoE), Institute of Tropical Aquaculture and Fisheries, Universiti Malaysia Terengganu, Terengganu, Malaysia
- 2Shantou University-Universiti Malaysia Terengganu (STU-UMT) Joint Shellfish Research Laboratory, Shantou University, Shantou, China
- 3Centre for Chemical Biology, Universiti Sains Malaysia, Minden, Malaysia
- 4Department of Marine Biology, Faculty of Marine Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
- 5School of Biological Sciences, Universiti Sains Malaysia, Minden, Malaysia
Sexual dimorphism is generally obvious in brachyurans, and sexual dimorphism between species is of a higher degree to allow separation of trophic niches compared to the lower intraspecific variations between sexes. Mud crab genus Scylla are distributed along the Indo-West Pacific region, and species within this genus often exhibit overlapping niches and similar external morphologies. This study compared the intra- and interspecific sexual dimorphism patterns of three sympatric Scylla species from four distinct geographical locations along the equatorial region based on 24 morphometric characters. The consistency of sexual dimorphism patterns between locations was higher than between species. However, reproduction-related characters such as cheliped dimensions and abdomen width (AW) exhibited similar sexual dimorphism patterns across species. Discriminant function analysis based on the 23 morphometric ratios revealed the morphometric intraspecific divergence in all three Scylla species from the Asajaya mangrove forest. The cause for this regional intraspecific differentiation of mud crabs from the Asajaya mangrove forest remains unknown.
Introduction
Sexual dimorphism in terms of morphological characters is of functional significance and an outcome of the interaction between natural and sexual selection (Darwin, 1871; Hamasaki et al., 2020). The hard exoskeleton of crustaceans, including brachyurans, allows easy documentation and comparison of external morphological patterns (Barría et al., 2014). The marked difference in morphological characters is pronounced in brachyurans. In general, the larger chela dimensions found in males are associated with agonistic interactions related to mating (Waiho et al., 2015) and male-male competition (Yasuda et al., 2011) whereas females possess larger abdomens due to their reproductive needs, i.e., for brood attachment (Simpson et al., 2016; Parvizi et al., 2017). Although most studies focused on the sexually dimorphic characters linked to reproduction (Parvizi et al., 2017; Hamasaki et al., 2020), other characters might be sexually dimorphic as well, albeit more subtle and require the aid of quantitative analyses to assess the degree of sexual dimorphism (Bertin et al., 2002). Some of these features that are often used in morphometric discrimination of brachyurans include merus and/or propodus length (PL) of periopods (Simpson et al., 2016) and supraorbital dimension (Alencar et al., 2014).
Although sexual dimorphism focuses primarily on highlighting the difference in characters between sexes of a species, interspecific and intraspecific variations exist and could be largely due to sexual selection or niche divergence (Lailvaux and Vincent, 2007; Hirst and Kiørboe, 2014; Selz et al., 2016). Interspecific sexual dimorphism is often used to describe the ancestral relationship and phenotypic conservation between species and has been reported in some anomuran (Barría et al., 2014) and brachyuran species (Miyajima and Wada, 2017). Intraspecific or spatial variation in sexually dimorphic characters between populations are linked to mutation or genetic drift (Ritchie et al., 2007), intraspecific mating system evolution (Baur et al., 2019), predation/resource-mediated ecological selection (Scharnweber et al., 2013), or sexual selection for novel/exaggerated signals such as coloration (Selz et al., 2016). Intraspecific latitudinal variations in the magnitude of sexual dimorphism were also reported in both marine (Lima-Filho et al., 2017) and freshwater fish species (Estlander et al., 2017). Variation in environmental conditions is postulated to cause changes in morphological characters and evolutionary patterns and subsequently influence sexual dimorphism patterns between and within a species (Lima-Filho et al., 2017). However, instead of interspecific comparison, intraspecific comparison of sexual dimorphism patterns could highlight variations between populations due to the reduced phylogenetic effects (Shelomi, 2012; Lima-Filho et al., 2017). Thus, the connection between variation in sexual dimorphism patterns and ecological adaptations could be deduced by studying the differences in external morphological characters between populations (Kalate et al., 2017). However, unlike fish (Gunawickrama, 2008; Laporte et al., 2018), intraspecific character difference is less documented in brachyurans (Kalate et al., 2017). Most studies on sexual dimorphism of brachyurans focused primarily on one species or inter-species due to the obvious morphological differences between sexes and species (Peiró et al., 2012; Alencar et al., 2014; Simpson et al., 2016; Parvizi et al., 2017; Fazhan et al., 2021b).
Mud crabs (genus Scylla) are naturally occurring in the equatorial region and at least two species co-exist sympatrically in most populations (Overton and Macintosh, 2002; Fazhan et al., 2017a). Thus far, the sexual dimorphism of Scylla serrata adults (Presilda et al., 2018) and Scylla paramamosain crablets (Shi et al., 2019) have been described. Recently, we characterized and compared the sexual dimorphism of three sympatric species in the equatorial region, i.e., Scylla olivacea, Scylla tranquebarica, and S. paramamosain based on 10 morphological characters. The overall body size and cheliped dimensions were male-biased whereas abdomen size was female-biased in all Scylla species. However, spatial and temporal factors were excluded in the previous study to allow a general representation of each species (Fazhan et al., 2021b). To understand the interspecific and intraspecific variations in the sexual dimorphism patterns of brachyurans, the sexual dimorphism patterns of 24 morphometric characters for three Scylla species (S. olivacea, S. tranquebarica, and S. paramamosain) from four geographical locations along the equatorial region were determined and compared in this study. These 24 morphologically defining characters of mud crab were used by Keenan et al. (1998), in combination with molecular data, to separate S. serrata into four distinctive Scylla species. Further, spatial differences in morphological characters of each sex were analyzed to determine if morphological variations exist among populations of the same species.
Materials and Methods
Sampling and Species Identification
Mud crabs of the genus Scylla were sampled from four stations along the equatorial region representing three seas in Malaysia, namely the Malacca Strait, Matang Mangrove Forest Reserve, Perak (4°45'N, 100°37'E); South China Sea, Setiu Wetlands, Terengganu (5°39'N, 102°43'E); South China Sea, Asajaya Mangrove Forest, Sarawak (1°32'N, 110°29'E); and the Sulu Sea, Kota Marudu Mangrove Forest, Sabah (6°44'N, 117°1'E) (Figure 1). Sampling was conducted over 3 years from April 2012 until March 2015. All sampling locations are landing grounds of mud crabs and, hence, no specific license is required for their acquisition. To obtain a targeted number (n = 300) for each sex per species per location, additional crab samples were also measured from fishermen of the same sampling sites. After acquiring the required morphometric measurements, crabs were either returned alive to the fishermen or released to the wild. Crabs were identified to species level according to the morphological characteristics detailed in Keenan et al. (1998) and Fazhan et al. (2020). Only healthy mature specimens with carapace width (CW) above 95.0 mm (Waiho et al., 2016b), with complete appendages and no visible external abnormalities (Waiho et al., 2017; Fazhan et al., 2018) were included in this study to minimize the effect confounding variables including sexual maturation, allometric growth and compromised immunity. Sex was determined based on the abdominal morphology (Waiho et al., 2016a; Fazhan et al., 2017b). Males were identified based on their triangular-shaped abdomens whereas mature females exhibited prominent globular and darkened abdomens (Ikhwanuddin et al., 2011). Three species, i.e., S. olivacea, S. tranquebarica, and S. paramamosain were present in all four geographical locations (Keenan et al., 1998; Fazhan et al., 2017a). A total of 1,800 crabs were obtained from each sampling station within 3 years.
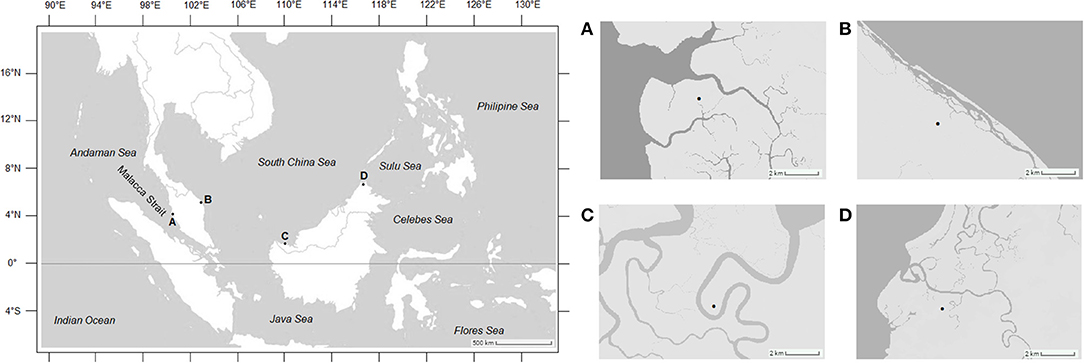
Figure 1. Mud crab sampling locations of this study. (A) Matang Mangrove Forest Reserve, Perak; (B) Setiu Wetlands, Terengganu; (C) Asajaya mangrove forest, Sarawak; (D) Kota Marudu mangrove forest, Sabah.
Morphometric Measurements
Twenty-four (24) morphologically defining characters described by Keenan et al. (1998) were measured for each individual. They are divided into five sections, namely carapace, frontal lobe, sternum, periopods and chelipeds (Supplementary Table 1). Each character was measured to the nearest 0.01 mm using digital Vernier Calipers. CW was used as a body size index (Hartnoll, 1982; McLain and Pratt, 2011).
Data Analysis
All data analyses were performed using IBM SPSS Statistics version 25. As we aimed to characterize sexual dimorphism patterns according to species and locations, possible seasonal variations were not being considered in the analysis. Individuals were grouped according to species, followed by sampled location and sex. All variables were checked for normality using the Kolmogorov-Smirnov test and homogeneity of variance using Levene's test. One-way ANOVA with Welch's correction and subsequent Games-Howell post-hoc test were used to compare CW between sex, location and species. Multivariate analysis of covariance (ANCOVA) was used to test for differences in all morphological characters between sexes and locations (independent variables), after controlling for body size, i.e., CW as the covariate. If the multivariate ANCOVA is statistically significant, ANCOVA was performed on each morphological character between sexes based on each location followed by Bonferroni post-hoc test, after adjusting for the covariate (i.e., CW). To further characterize the intraspecific differences in characters of specific sex across different locations, 23 morphometric ratios (excluding CW) were divided by CW to ensure values were independent of total size and subsequently analyzed using stepwise discriminant function analysis. A minimum F value of 3.0 (0.05 significance level) was used based on previous studies on the same genus (Keenan et al., 1998; Fazhan et al., 2018). Wilks' lambda (U statistic) was used to determine the significance of the discriminant functions and subsequent V transformation of Bartletts (Chi-Square statistic) was performed to validate the significance of the lambda value. The robustness of the function was cross-validated using the split-sample validation method available in the SPSS program.
Results
Sexual size dimorphism in terms of CW was consistently male-biased across locations only in S. olivacea (all P < 0.05; Figure 2). Interestingly, females of S. tranquebarica from Terengganu exhibited larger CW compared to their male counterparts (P = 0.018), whereas CW of S. tranquebarica from Sarawak (P = 0.893) and S. paramamosain from Sabah and Sarawak showed no significant difference between sexes (PSabah = 0.087; PSarawak = 0.793). Males of S. olivacea did not vary greatly in their body size (P = 0.258), while males of S. tranquebarica and S. paramamosain from Terengganu exhibited significantly smaller CW compared to those at different locations (Figure 2). Females of S. tranquebarica and S. paramamosain showed similar patterns, with those from Sabah were of significantly larger body sizes. In contrast, S. olivacea females from Sabah were significantly smaller than those from other locations.
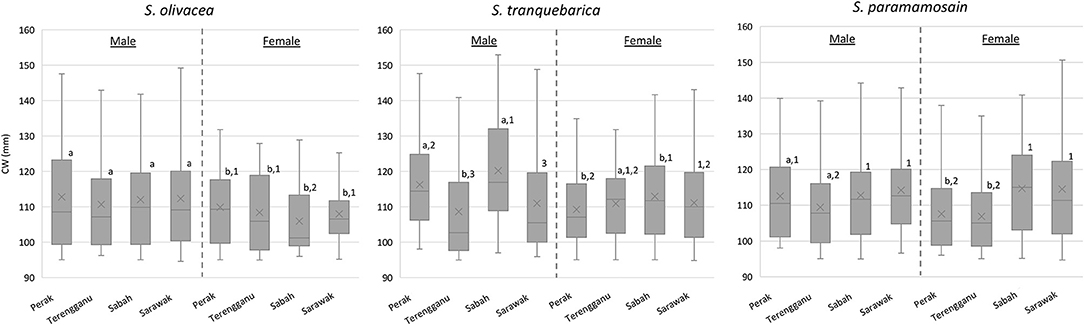
Figure 2. The boxplots of carapace width (CW) of Scylla species are based on sex and location. Superscript alphabets represent significant differences among male-female comparison within the same species and location (P < 0.05); Superscript Arabic numerals represent significant differences among locations within the same species and sex (P < 0.05); No superscript alphabet/Arabic numeral was used for groups with no significant difference.
The mean and SE of the morphometric measurements for both sexes of S. olivacea, S. paramamosain and S. tranquebarica from four locations are shown in Supplementary Table 2. After corrected for body size (CW), there was a statistically significant difference between sex and location on the combined morphological characters in all three Scylla species [S. olivacea: Fsex(22, 2,370) = 3710.47, P < 0.001, Wilks' Λ = 0.028, Flocation(66, 7,078) = 114.88, P < 0.001, Wilks' Λ = 0.114; S. paramamosain: Fsex(22, 2,370) = 5141.84, P < 0.001, Wilks' Λ = 0.021, Flocation(66, 7,116) = 122.90, P < 0.001, Wilks' Λ = 0.102; S. tranquebarica: Fsex(23, 2,369) = 3730.79, P < 0.001, Wilks' Λ = 0.027, Flocation(69, 7,078) = 67.13, P < 0.001, Wilks' Λ = 0.222]. The interaction effect between sex and location on the morphological characters was also significant for all three species [S. olivacea: F(66, 7,078) = 106.44, P < 0.001, Wilks' Λ = 0.128; S. paramamosain: F(66, 7,078) = 117.65, P < 0.001, Wilks' Λ = 0.110; S. tranquebarica: F(69, 7,078) = 54.90, P < 0.001, Wilks' Λ = 0.278].
Among the carapace characters, the eighth CW (8CW) and carapace length (CL) were almost exclusively male-biased, except for the 8CW and CL of S. paramamosain from Kota Marudu mangrove forest and Asajaya mangrove forest, respectively, were female-biased, and the 8CW of S. paramamosain from Asajaya mangrove forest showed no significant difference between sexes. Although the sexual dimorphism pattern of internal CW (ICW) was inconsistent across species and location, that of posterior width of the carapace (PWC) were consistently based on species, i.e., the PWC of S. paramamosain were male-biased whereas the PWC of S. tranquebarica and S. olivacea were female-biased regardless of location, except for males and females of S. olivacea from Asajaya mangrove forest with no apparent dimorphism in PWC character (Figure 3; Supplementary Table 3).
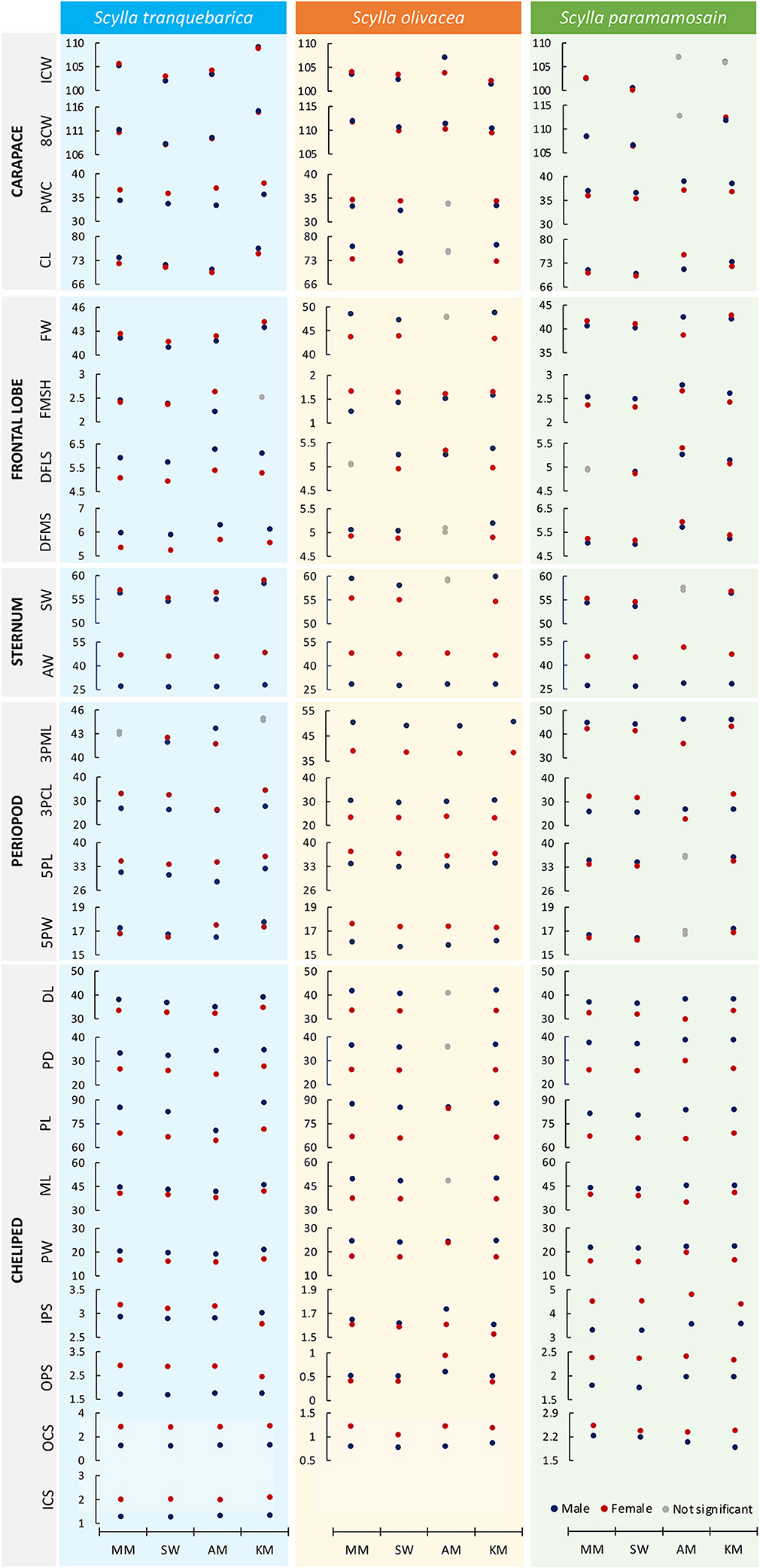
Figure 3. Comparison of sexual dimorphism patterns across geographical locations for three Scylla species based on the estimated marginal means after controlling for the covariate (carapace width, CW) using ANCOVA. For detailed statistical values, refer to Supplementary Tables 6–8. MM, Matang Mangrove Forest Reserve, Perak; SW, Setiu Wetlands, Terengganu; AM, Asajaya mangrove forest, Sarawak; KM, Kota Marudu mangrove forest, Sabah.
The sexual dimorphism patterns of frontal lobe characters were consistent within but not across Scylla species (Figure 3; Supplementary Table 3), except for Scylla species from Asajaya mangrove forest. For example, the frontal width (FW) of S. paramamosain from all locations were female-biased but those from the Asajaya mangrove forest exhibited larger frontal width in males. The two sternum characters, i.e., sternum width (SW) and abdomen width (AW), were almost exclusively female-biased in all three Scylla species from four locations, except for the male-biased SW of S. olivacea.
Inter- but not intraspecific variation in sexual dimorphism of periopod characters was obvious (Figure 3; Supplementary Table 3). When comparing between sexes of each species, the third right periopod merus length (3PML) was longer in males of S. olivacea and S. paramamosain, but shorter in males of S. tranquebarica. The third right periopod carpus length (3PCL), however, was male-biased only in S. olivacea. The fifth right periopod dactyl length (5PL) was female-biased in S. olivacea and S. tranquebarica, but male-biased in S. paramamosain. The fifth right periopod dactyl width (5PW) was male-biased in S. paramamosain and S. tranquebarica, but female-biased in S. olivacea. The 3PML and 5PW of S. tranquebarica and 3PCL of S. paramamosain from Asajaya mangrove forest, however, showed opposite sexual dimorphism patterns compared to the same species from other locations.
The main cheliped characters such as the dactyl length (DL), propodus depth (PD), PL, merus length (ML), and propodus width (PW) were exclusively larger in males regardless of species and locations, except for DL, PD and ML of S. olivacea from Asajaya mangrove forest with no significant difference among sexes (Figure 3; Supplementary Table 3). The inner and outer propodus spines (IPS, OPS) and carpus spines (ICS, OCS) of S. tranquebarica were almost exclusively longer in females, except the IPS of S. tranquebarica from Kota Marudu mangrove forest that showed a male-biased pattern. Similarly, the IPS, OPS, and OCS of S. paramamosain were longer in females. However, except for OCS, the IPS and OPS of S. olivacea were almost male-biased.
Stepwise discriminant function analysis was used to discern if spatial differences in terms of morphometric characters exist in males and females of Scylla species. Three significant canonical discriminant functions were obtained for females of S. olivacea and S. tranquebarica and males of S. olivacea, whereas two significant functions were obtained for males and females of S. paramamosain and males of S. tranquebarica (all P < 0.005; Table 1; Supplementary Tables 4, 5). When each species was analyzed according to sex, we noticed intraspecific differences in characters that significantly discern females or males across the four sampling locations. For example, ICW and one of the frontal lobe characters, i.e., the distance between median spines (DFMS), were useful in separating either males or females of all three species across four locations (Table 1). Interestingly, the spinations on cheliped and cheliped dimensions were important discerning criteria of the intraspecific differences of the three Scylla species (Table 1; Supplementary Table 4). In all three Scylla species, the PD and inner propodus spine (IPS) of the right cheliped of females were important criteria to discriminate the corresponding species among locations; in males, spination patterns, especially outer carpus spine (OCS) of the right cheliped of S. olivacea and S. paramamosain and most cheliped dimensions of S. tranquebarica helped discriminate intraspecific dimorphism (Table 1; Supplementary Table 4). Based on the group centroids (Supplementary Table 4), the first function of males and females of all species was specifically useful to discern specimens from the Asajaya mangrove forest from the other three locations. The remaining functions allowed more general discrimination among the four locations. When the functions were integrated (Supplementary Figure 1), the differences in morphometric characters of mud crabs (all Scylla species) in both sexes, especially prominent in females, between Asajaya mangrove forest and the other three geographical locations were highlighted. All combination of functions has a correct classification rate of more than 60% and a cross-validation rate of more than 58% (Supplementary Figure 1).
Discussion
Consistent male-based sexual size dimorphism patterns among Scylla species have been reported in previous studies (Fazhan et al., 2021a,b) when geographical and seasonal factors were excluded. However, based on CW, 8CW and CL, this study further shows that although the general pattern was male-biased, intraspecific variation in sexual size dimorphism within Scylla species exist as well, at least for S. tranquebarica and S. paramamosain. The differences in growth-promoting environmental parameters and sex-specific phenotypic plasticity could be a plausible explanation (Cox and Calsbeek, 2010). Lengkeek et al. (2008) also postulated that the intraspecific variation in sexual size dimorphism of Mediterranean blennies might be due to phenotypic plasticity based on the demonstration that intraspecific variation exceeded interspecific variation. For example, if the intensity of combat is related to resource availability (e.g., food, territory or mate) and the degree of competition directly influences energy investment in body size, then the difference in resource availability across population could result in intraspecific body size differences in Scylla species. In addition, high predation pressure could also cause variation in sexual size dimorphism patterns between populations, as observed in the lower degree of sexual size dimorphism of freshwater fish Salaria fluviatilis from lake populations compared to the river counterparts (Laporte et al., 2018). Another possible explanation of the variation of sexual dimorphism pattern in carapace dimensions of Scylla species across locations could be due to the different age structures in different populations (Cox and Calsbeek, 2010). However, this could not be verified due to the lack of data on the mortality and age of each Scylla species in every sampling location.
Sexual dimorphism patterns of reproduction-related characters such as sternum and cheliped characters were almost exclusively female- and male-biased, respectively, across geographical locations of all three Scylla species (Figure 3). This is expected as both cheliped dimensions and AW were obvious sexually dimorphic characters in almost all brachyuran species (Simpson et al., 2016; Parvizi et al., 2017), including in Scylla spp. (Waiho et al., 2016b; Fazhan et al., 2021b). The conserved patterns in reproduction-related morphological characters between Scylla spp. could be explained by the genetic variation that exists between them, as high interspecific but low intraspecific genetic distances were reported for the three Scylla species along the equatorial region (inclusive of the sampling sites in this study) by Naim et al. (2020).
Other subtle characters, such as the dimensions of third and fifth right periopods and frontal lobe dimensions, however, were more similar within than between species (Figure 3). The longer ML of the third periopod in males of Japanese mitten crab Eriocheir japonica was linked to active mate searching and female-cradling and -guarding during the mating process (Kobayashi, 2002). This could hold true for S. olivacea and S. paramamosain as similar male-biased MLs of their third periopods were observed in this study. The mating behavior of S. olivacea whereby males were involved in mate selection and guarding further supports this postulate (Waiho et al., 2015). Comparatively, S. tranquebarica females exhibited longer ML of their third periopods. In sand fiddler crab Uca pugilator, the long rear legs of females were believed to be involved in egg mass support and digging out of breeding burrows (McLain and Pratt, 2008). However, future research on the mating and brooding behaviors of S. tranquebarica is warranted to verify the involvement of female-biased 3PML in the breeding or spawning processes. In addition, future works on the genetic differentiation among geographically close populations of Scylla and the differences in environmental factors could reveal the potential involvement of adaptive phenotypic plasticity in the inter- and intraspecific variation of sexual dimorphism patterns in Scylla species along the equatorial region.
Cheliped spinations were female-biased across locations for S. paramamosain and S. tranquebarica, whereas those of S. olivacea showed male-biased patterns in some locations. Cheliped spinations serve as important defense accessories and we postulate that the female-biased sharp spination patterns (inner and outer propodus and carpus spines on the cheliped) protect females from predators, especially when they are carrying eggs externally during their migration toward offshore to spawn (Meynecke and Richards, 2014), in compensating with the lesser cheliped size compared to their male counterparts (Fazhan et al., 2021b). The variation in dimorphism patterns of cheliped spinations observed in S. olivacea, however, might be ecologically related. S. olivacea is known to prefer habitats of lower salinity, such as upstream of river mouths and mangrove forests, whereas S. tranquebarica and S. paramamosain are often found in estuaries and intertidal and subtidal zones of higher salinities with less salinity fluctuation (Fazhan et al., 2017a, 2021b). A less protruding propodus spines in females of S. olivacea might facilitate easier movement in between pneumatophores and enable convenient burrowing to escape from predators. This hypothesis, however, requires further verification via observing their behavior and movement in the natural environment.
When comparing each species according to sex using discriminant function analysis, Scylla spp. from Asajaya mangrove forest, especially females, were distinct from the other three remaining locations. Various interplaying factors, including evolutionary selection pressure (Kim et al., 2017), diet (Papacostas and Freestone, 2016) and predation pressure (Laporte et al., 2018) might be involved in causing this phenomenon. The morphological divergence between populations (intraspecific variation) was also reported in eight species of Uca along the coast of Brazil and ecological modulation of phenotype was proposed as a possible inducing factor (Hampton et al., 2014). However, it is beyond the scope of this study to identify potential causative agents to the intraspecific morphological variations of Scylla spp. due to the lack of environmental data. Another possible explanation is that the distinct variation in morphological characters of Scylla spp. from the Asajaya mangrove forest could be linked with the latitudinal factor. Among the four locations, Asajaya mangrove forest is the closest to the equator (latitude: 1°32'N) whereas other locations are at 4°45'N, 5°39'N and 6°44'N, respectively. Instead of reduced intraspecific sexual dimorphism with increasing latitude as reported in other aquatic species (Estlander et al., 2017; Lima-Filho et al., 2017), this study shows that increasing latitude resulted in reversal of some morphometric characters. Latitudinal variation influences temperature and other biotic and abiotic factors, which in turn, could impact trophic interactions between species (Estlander et al., 2017). Future studies on the life-history traits and their correlation with biotic and abiotic factors are needed to validate this pattern.
Conclusions
The interspecific variation in sexual dimorphism patterns was stronger than intraspecific among the three Scylla species. However, the results also highlight a divergence in phenotype in all Scylla species of Asajaya mangrove forest compared to the other three geographical locations. Future characterization of the ecological niche and molecular characteristics could aid in identifying the mechanisms behind the phenotypic divergence and further the understanding of the potential causative factors of morphological variation between and among brachyurans.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
KW: methodology, investigation, formal analysis, writing the original draft, and writing the review and editing. MI: methodology, validation, and writing the review and editing. MA and AS-C: formal analysis, validation, and writing the review and editing. SI: visualization and formal analysis. MJ and GA: investigation and formal analysis. HF: conceptualization, investigation, writing the original draft, writing the review, and editing and supervision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Universiti Malaysia Terengganu under the Talent and Publication Enhancement-Research Grant (TAPE-RG) (Vot. No. 55288) and the Ministry of Higher Education, Malaysia under the Higher Institution Centre of Excellence (HICoE) program (Vot. No. 63933 and Vot. No. 56042).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Yong Sui Sien, Khor Yip Mau, and Noraidah Omar for providing transportation and accommodation during mud crab sampling. An adjunct Academic Fellow position from Universiti Sains Malaysia (USM) to KW is also acknowledged.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2021.690836/full#supplementary-material
References
Alencar, C. E. R. D., Lima-Filho, P. A., Molina, W. F., and Freire, F. A. M. (2014). Sexual shape dimorphism of the mangrove crab Ucides cordatus (Linnaeus, 1763) (Decapoda, Ucididae) accessed through geometric morphometric. Sci. World J. 2014:206168. doi: 10.1155/2014/206168
Barría, E. M., Santos, S., Jara, C. G., and Butler, C. J. (2014). Sexual dimorphism in the cephalothorax of freshwater crabs of genus Aegla Leach from Chile (Decapoda, Anomura, Aeglidae): an interspecific approach based on distance variables. Zoomorphology 133, 379–389. doi: 10.1007/s00435-014-0231-x
Baur, J., Roy, J., Schäfer, M. A., Puniamoorthy, N., Blanckenhorn, W. U., and Rohner, P. T. (2019). Intraspecific mating system evolution and its effect on complex male secondary sexual traits: does male–male competition increase selection on size or shape? J. Evol. Biol. 33, 297–308. doi: 10.1111/jeb.13565
Bertin, A., David, B., Cézilly, F., and Alibert, P. (2002). Quantification of sexual dimorphism in Asellus aquaticus (Crustacea: Isopoda) using outline approaches. Biol. J. Lin. Soc. 77, 523–533. doi: 10.1046/j.1095-8312.2002.00125.x
Cox, R. M., and Calsbeek, R. (2010). Sex-specific selection and intraspecific variation in sexual size dimorphism. Evolution 64, 798–809. doi: 10.1111/j.1558-5646.2009.00851.x
Darwin, C. R. (1871). La Descendencia del Holbre y la Selección en Relación al Sexo. Biblioteca de la Revista de Medicina y Cirugía Práctica, Madrid.
Estlander, S., Kahilainen, K. K., Horppila, J., Olin, M., Rask, M., Kubečka, J., et al. (2017). Latitudinal variation in sexual dimorphism in life-history traits of a freshwater fish. Ecol. Evol. 7, 665–673. doi: 10.1002/ece3.2658
Fazhan, H., Waiho, K., Al-Hafiz, I., Kasan, N. A., Ishak, S. D., Afiqah-Aleng, N., et al. (2021a). Composition, size distribution, length-weight relationship of sympatric mud crab species (Scylla) and the case of presumed hybrids. Estuar. Coast. Shelf Sci. 250:107154. doi: 10.1016/j.ecss.2020.107154
Fazhan, H., Waiho, K., Darin Azri, M. F., Al-Hafiz, I., Wan Norfaizza, W. I., Megat, F. H., et al. (2017a). Sympatric occurrence and population dynamics of Scylla spp. in equatorial climate: effects of rainfall, temperature and lunar phase. Estuar Coast Shelf Sci. 198, 299–310. doi: 10.1016/j.ecss.2017.09.022
Fazhan, H., Waiho, K., Fujaya, Y., Rukminasari, N., Ma, H., and Ikhwanuddin, M. (2021b). Sexual dimorphism in mud crabs: a tale of three sympatric Scylla species. PeerJ 9:e10936. doi: 10.7717/peerj.10936
Fazhan, H., Waiho, K., Quinitio, E., Baylon, J. C., Fujaya, Y., Rukminasari, N., et al. (2020). Morphological descriptions and morphometric discriminant function analysis reveal an additional four groups of Scylla spp. PeerJ 8:e8066. doi: 10.7717/peerj.8066
Fazhan, H., Waiho, K., Wan Norfaizza, W. I., Megat, F. H., and Ikhwanuddin, M. (2017b). Assortative mating by size in three species of mud crabs, genus Scylla De Haan, 1833 (Brachyura: Portunidae). J. Crust Biol. 37, 654–660. doi: 10.1093/jcbiol/rux063
Fazhan, H., Waiho, K., Wee, H. B., Surzanne, M. A., Ma, H., and Ikhwanuddin, M. (2018). Predicting the sacculinid Sacculina beauforti infection status of the orange mud crab Scylla olivacea by discriminant analysis. Aquaculture 491, 128–134. doi: 10.1016/j.aquaculture.2018.03.009
Gunawickrama, K. B. S. (2008). Intraspecific variation in morphology and sexual dimorphism in Puntius singhala (Teleostei: Cyprinidae). Ceylon J. Sci. 37, 167–175. doi: 10.4038/cjsbs.v37i2.504
Hamasaki, K., Osabe, N., Nishimoto, S., Dan, S., and Kitada, S. (2020). Sexual dimorphism and reproductive status of the red swamp crayfish Procambarus clarkii. Zool. Stud. 59:e7. doi: 10.6620/ZS.2020.59-07
Hampton, K. R., Hopkins, M. H., McNamara, J. C., and Thurman, C. L. (2014). Intraspecific variation in carapace morphology among fiddler crabs (Genus Uca) from the Atlantic coast of Brazil. Aquat. Biol. 20, 53–67. doi: 10.3354/ab00545
Hartnoll, R. G. (1982). “Growth,” in The Biology of Crustacea: Embryology, Morphology and Genetics, Vol. 3, ed D. E. Bliss (New York, NY: Academic Press), 111–196.
Hirst, A. G., and Kiørboe, T. (2014). Macroevolutionary patterns of sexual size dimorphisms in copepods. Proc. R. Soc. 281:20140739. doi: 10.1098/rspb.2014.0739
Ikhwanuddin, M., Azmie, G., Juariah, H. M., Zakaria, M. Z., and Ambak, M. A. (2011). Biological information and population features of mud crab, genus Scylla from mangrove areas of Sarawak, Malaysia. Fish. Res. 108, 299–306. doi: 10.1016/j.fishres.2011.01.001
Kalate, A., Keikhosravi, A., Naderloo, R., Hajjar, T., and Schubart, C. D. (2017). Morphometric characterization of the freshwater crab Potamon elbursi Pretzmann, 1962 in the Caspian Sea and Namak Lake hydrographic systems. J. Crust Biol. 38, 91–100. doi: 10.1093/jcbiol/rux090
Keenan, C. P., Davie, P., and Mann, D. (1998). A revision of the genus Scylla De Haan, 1833 (Crustacea: Decapoda: Brachyura: Portunidae). Raffles Bull. Zool. 46, 217–245.
Kim, T. W., Lee, J. H., and Choe, J. C. (2017). Not all crabs are created equal: diverse evolutionary paths of female preferences for courtship structures in fiddler crabs (genus Uca). Behav. Ecol. Sociobiol. 71:33. doi: 10.1007/s00265-016-2235-7
Kobayashi, S. (2002). Relative growth pattern of walking legs of the Japanese mitten crab Eriocheir japonica. J. Crust Biol. 22, 601–606. doi: 10.1163/20021975-99990272
Lailvaux, S. P., and Vincent, S. E. (2007). Ecological dimorphisms: an introduction to the symposium. Integr. Comp. Biol. 47, 169–171. doi: 10.1093/icb/icm003
Laporte, M., Berrebi, P., Claude, J., Vinyoles, D., Pou-Rovira, Q., Raymond, J.-C., and Magnan (2018). The ecology of sexual dimorphism in size and shape of the freshwater blenny Salaria fluviatilis. Curr. Zool. 64, 183–191. doi: 10.1093/cz/zox043
Lengkeek, W., Didderen, K., Côté, I. M, van der Zee, E. M., Snoek, R. C., and Reynolds, J. D. (2008). Plasticity in sexual size dimorphism and Rensch's rule in Mediterranean blennies (Blenniidae). Can. J. Zool. 86, 1173–1178. doi: 10.1139/Z08-103
Lima-Filho, P. A., Bidau, C. J., Alencar, C. E. R. D., and Molina, W. F. (2017). Latitudinal influence on the sexual dimorphism of the marine fish Bathgobius soporator (Gobiidae: Teleostei). Evol. Biol. 44, 374–385. doi: 10.1007/s11692-017-9416-9
McLain, D. K., and Pratt, A. E. (2008). Asymmetry of leg size and differential leg usage in the sand fiddler crab, Uca pugilator. J. Crust Biol. 28, 601–606. doi: 10.1651/07-2932.1
McLain, D. K., and Pratt, A. E. (2011). Body and claw size at autotomy affect the morphology of regenerated claws of the sand fiddler crab, Uca pugilator. J. Crust Biol. 31, 1–8. doi: 10.1651/10-3298.1
Meynecke, J.-O., and Richards, R. G. (2014). A full life cycle and spatially explicit individual-based model for the giant mud crab (Scylla serrata): a case study from a marine protected area. ICES J. Mar. Sci. 71, 484–498. doi: 10.1093/icesjms/fst181
Miyajima, A., and Wada, K. (2017). Relationships between life history traits and sexual dimorphisms in two varunid crabs, Hemigrapsus takanoi Asakura and Watanabe, 2005 and H. sinensis Rathbun, 1931 (Brachyura: Varunidae). J. Crust Biol. 37, 21–28. doi: 10.1093/jcbiol/ruw011
Naim, D. M., Nor, S. A. M., and Mahboob, S. (2020). Reassessment of species distribution and occurrence of mud crab (Scylla spp., Portunidae) in Malaysia through morphological and molecular identification. Saudi J. Biol. Sci. 27, 643–652. doi: 10.1016/j.sjbs.2019.11.030
Overton, J. L., and Macintosh, D. J. (2002). Estimated size at sexual maturity for female mud crabs (genus Scylla) from two sympatric species within Ban Don Bay, Thailand. J. Crust Biol. 22, 790–797. doi: 10.1163/20021975-99990293
Papacostas, K. J., and Freestone, A. L. (2016). Latitudinal gradient in niche breadth of brachyuran crabs. Global Ecol. Biogeogr. 25, 207–217. doi: 10.1111/geb.12400
Parvizi, E., Naderloo, R., Keikhorsravi, A., and Schubart, C. D. (2017). Life history traits and patterns of sexual dimorphism in the freshwater crab Potamon ibericum (Bieberstein, 1809) (Decapoda: Brachyura: Potamidae) from the western Alborz Mountains, Iran. J. Crust Biol. 37, 323–331. doi: 10.1093/jcbiol/rux029
Peiró, D. F, Baeza, J. A., and Mantelatto, F. L. (2012). Host-use pattern and sexual dimorphism reveals the mating system of the symbiotic pea crab Austinixa aidae (Crustacea: Brachyura: Pinnotheridae). J. Mar. Biolog. Assoc. 93, 715–723. doi: 10.1017/S0025315412000720
Presilda, C. J., Salcedo, M. A., Moreno, M. J., Cogenera, J., Japitana, R. A., Jumawan, J. H., et al. (2018). Sexual dimorphism in the carapace of mud crab (Scylla serrata, Forsskål, 1775) in Magallanes, Agusandel Norte using geometric morphometric analysis. Comput. Ecol. Softw. 8, 88–97.
Ritchie, M. G., Hamill, R. M., Graves, J. A., Magurran, A. E., Webb, S. A., and Macías Garcia, C. (2007). Sex and differentiation: population genetic divergence and sexual dimorphism in Mexican goodeid fish. J. Evol. Biol. 20, 2048–2055. doi: 10.1111/j.1420-9101.2007.01357.x
Scharnweber, K., Watababe, K., Syväranta, J., Wanke, T., Monaghan, M. T., and Mehner, T. (2013). Effects of predation pressure and resource use on morphological divergence in omnivorous prey fish. BMC Evol. Biol. 13:132. doi: 10.1186/1471-2148-13-132
Selz, O. M., Thommen, R., Pierotti, M. E. R., Anaya-Rojas, J. M., and Seehausen, O. (2016). Differences in male coloration are predicted by divergent sexual selection between populations of a cichlid fish. Proc. R. Soc. 283:20160172. doi: 10.1098/rspb.2016.0172
Shelomi, M. (2012). Where are we now? Bergmann's rule sensu lato in insects. Am. Nat. 180, 511–519. doi: 10.1086/667595
Shi, X., Lu, J., Wu, Q., Waiho, K., Aweya, J. J., Fazhan, H., et al. (2019). Comparative analysis of growth performance between female and male mud crab Scylla paramamosain crablets: evidences from a four-month successive growth experiment. Aquaculture 505, 351–362. doi: 10.1016/j.aquaculture.2019.02.062
Simpson, L. A., Ambrosio, L. J., and Baeza, J. A. (2016). Sexual dimorphism and allometric growth in the enigmatic pygmy crab Petramithrax pygmaeus (Bell, 1836) (Decapoda: Brachyura: Mithracidae), with a formal test of Rensch's rule in spider crabs (Superfamily Majoidea). J. Crust Biol. 36, 792–803. doi: 10.1163/1937240X-00002486
Waiho, K., Fazhan, H., Baylon, J. C., Wan Norfaizza, W. I., and Ikhwanuddin, M. (2016a). Use of abdomen looseness as an indicator of sexual maturity in male mud crab Scylla spp. J. Shellfish Res. 35, 1027–1035. doi: 10.2983/035.035.0425
Waiho, K., Fazhan, H., Glenner, H., and Ikhwanuddin, M. (2017). Infestation of parasitic rhizocephalan barnacles Sacculina beauforti (Cirripedia, Rhizocephala) in edible mud crab, Scylla olivacea. PeerJ 5:e3419. doi: 10.7717/peerj.3419
Waiho, K., Fazhan, H., and Ikhwanuddin, M. (2016b). Size distribution, length-weight relationship and size at the onset of sexual maturity of the orange mud crab, Scylla olivacea, in Malaysian waters. Mar. Biol. Res. 12, 726–738. doi: 10.1080/17451000.2016.1200726
Waiho, K., Mustaqim, M., Fazhan, H., Wan Norfaizza, W. I., Megat, F. H., and Ikhwanuddin, M. (2015). Mating behaviour of the orange mud crab, Scylla olivacea: the effect of sex ratio and stocking density on mating success. Aquacult. Rep. 2, 50–57. doi: 10.1016/j.aqrep.2015.08.004
Keywords: sexual dimorphism, Scylla, discriminant function analysis, morphometric ratios, brachyuran
Citation: Waiho K, Ikhwanuddin M, Abualreesh MH, Shu-Chien AC, Ishak SD, Jalilah M, Azmie G and Fazhan H (2021) Intra- and Interspecific Variation in Sexual Dimorphism Patterns of Mud Crab Genus Scylla Along the Equatorial Region. Front. Mar. Sci. 8:690836. doi: 10.3389/fmars.2021.690836
Received: 06 April 2021; Accepted: 22 June 2021;
Published: 04 August 2021.
Edited by:
Gustavo Fonseca, Federal University of São Paulo, BrazilReviewed by:
Antonio Castilho, São Paulo State University, BrazilNasreen Peer, Stellenbosch University, South Africa
Copyright © 2021 Waiho, Ikhwanuddin, Abualreesh, Shu-Chien, Ishak, Jalilah, Azmie and Fazhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hanafiah Fazhan, ZmF6aGFuQHVtdC5lZHUubXk=
†Present address: Mhd Ikhwanuddin, Faculty of Fisheries and Marine, Airlangga University, Surabaya, Indonesia
 Khor Waiho
Khor Waiho Mhd Ikhwanuddin
Mhd Ikhwanuddin Muyassar H. Abualreesh
Muyassar H. Abualreesh Alexander Chong Shu-Chien
Alexander Chong Shu-Chien Sairatul Dahlianis Ishak
Sairatul Dahlianis Ishak Mohamad Jalilah
Mohamad Jalilah Ghazali Azmie
Ghazali Azmie Hanafiah Fazhan
Hanafiah Fazhan