- 1Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Guangzhou, China
- 2Experimental Station for Scientific Observation on Fishery Resources and Environment in the Middle and Lower Reaches of Pearl River, Ministry of Agriculture and Rural Affairs of the People’s Republic of China, Zhaoqing, China
- 3Guangzhou Scientific Observing and Experimental Station of National Fisheries Resources and Environment, Guangzhou, China
- 4Key Laboratory of Aquatic Animal Immune Technology of Guangdong Province, Guangzhou, China
Global warming is influencing the life history traits of fishes globally. However, the impacts of elevated temperature on fish reproduction are diverse in different regions. Previous studies have revealed that the spawning timing of silver carp (Hypophthalmichthys molitrix) in the Pearl River, in China, has changed over the past decade. However, few studies have explored the potential reasons, which are critical for determining fishing-moratorium periods and developing sustainable fisheries. The current study used discharge suitability index (DSI), temperature suitability index (TSI), correlation and time-series analyses to determine (i) the optimal discharge and temperature for silver carp spawning; (ii) relationships among the thermal regime, hydrological parameters, and spawning timing based on an 11-year time-series dataset. Our results indicated that the most suitable discharge and temperature for silver carp spawning were 13,000–15,000 m3/s and 25–26°C, respectively. The start date of spawning fluctuated with a slight tendency to delay, while the spawning peak and end date obviously occurred earlier during the study period. Correlation analyses suggested that the increasing average temperature between January and March likely caused the initial spawning delay. Moreover, elevated temperatures in August and September probably promoted the anticipated end of silver carp spawning. However, increases in discharge did not significantly correlate with the start of spawning but were significantly and positively correlated with the spawning peak. These results indicated that elevated temperatures shorten the spawning period of silver carp in the Pearl River. Moreover, the initial spawning of silver carp seems to be triggered by temperature rather than changes in discharge; flow pulses can probably create more suitable spawning niches for H. molitrix. This study enhances our understanding of the effect of warming on fish reproduction in subtropical regions.
Introduction
Global warming is a critical concern in spawning ecology of fishes, especially in freshwater (Alix et al., 2020; Zhang et al., 2021). By 2017, the Earth’s surface temperature had increased by approximately 1.0°C relative to pre-industrial (1850–1900) levels, with average temperatures rising by 0.2°C per decade over the past three decades (IPBES, 2019). It is predicted that temperatures will rise even faster over the next 25 years than it did over the previous two decades (Smith et al., 2018). The impact of warming is likely to be stronger in freshwater than in terrestrial and marine systems, because discrete ecosystem boundaries in rivers limit fish migrations to follow optimal temperature conditions (Perry et al., 2005; Chen et al., 2011). More specifically, fish reproduction, controlled by the brain-pituitary-gonadal axis, may be more sensitive to warming than fish biodiversity, species interactions, and distributions (Walther et al., 2002; Miranda et al., 2013).
Determining the impact of global warming on fish spawning (e.g., spawning time) is challenging, since spawning characteristics are also regulated by other factors such as hydrographic parameters, spawning population stocks (Gillet and Dubois, 2007; Paumier et al., 2020). Generally, warm temperature and increases in river discharge can trigger spawning initiation (Yu et al., 2018), while spawning stock abundance mainly determines the interannual variability in larvae production (Glover et al., 2020). However, in some cases (e.g., a cold winter), temperature and river discharge affect larval abundance of some species (e.g., Clupea harengus membras) more significantly than spawning stock size (Ojaveer et al., 2011). Temperature and natural river floods can also affect oviposition amount and hatchability (O’Brien et al., 2012; Garcia et al., 2013; Wakefield et al., 2015). Therefore, understanding the influences of changes in temperature and hydrological conditions on fish reproduction is essential to preserving fish resources and developing sustainable fisheries practices.
For freshwater fish species that form spawning aggregations, understanding how global warming and other factors influence their spawning time is of great interest, since such information can be vital for determining management policies such as closed fishing seasons (Tuz-Sulub and Brulé, 2015; van Overzee and Rijnsdorp, 2015). Mature fishes that form transient aggregates to spawn at specific times and places are productivity hubs and vulnerable to overfishing (Erisman et al., 2017). Generally, the timing of spawning is influenced by the age of the spawning fish, water temperature, and hydrological conditions (Wang et al., 2014; Li et al., 2020). The age of mature fish slightly influences the spawning time, in that large individuals tend to produce eggs later than small ones (Gillet and Dubois, 2007; Gordoa and Carreras, 2014). Low-temperature can delay fish spawning by inhibiting vitellogenesis or reducing the mature fish size (Kjesbu, 1994; Carscadden et al., 1997; Wang et al., 2014), although low temperature may contribute to earlier spawning migration in temperate fish species (Sims et al., 2004). Extreme high temperatures seem to be more readily impair or even quit some fish (e.g., Odontesthes bonariensis) spawning by inhibiting gene expression throughout the brain-pituitary-gonad axis (Miranda et al., 2013). Hydrological conditions such as flood peaks during spawning seasons can promote fish spawning by favoring gonad development (Bailly et al., 2008), stimulating spawning migration (Thorstad et al., 2008), generating eddies that promote fertilization (Yi et al., 1988), and assisting the eggs and larvae in drifting downstream (Zhang et al., 2018).
Silver carp (Hypophthalmichthys molitrix) is a common freshwater fish that forms large spawning aggregations and is widely distributed in Asia, especially in China’s rivers. As one of the four major Chinese carp, it is a commercially important fish species in the Pearl River in southern China, accounting for about 13.6% of the total weight of the catches (Zhang et al., 2020). Mature silver carp form transient aggregations in spawning grounds in flood seasons and produce semi-buoyant eggs that drift with the flow for hundreds of kilometers in Asia (Yi and Liang, 1964; Zhang et al., 2000). Spawning characteristics of silver carp populations around the world demonstrated high local adaptation. Their spawning timings vary slightly in different regions: mid-May to the beginning of July in the Caspian Basin (Abdusamadov, 1987); from mid-May to mid-June in Arkansas (Freeze and Crawford, 1983); from June to early August in the Amur River (Gorbach and Krykhtin, 1989); from May to August in the Yangtze River (Zhang et al., 2000); and early April to early October in the Pearl River in China (Shuai et al., 2018). In China, some researchers consider the occurrences of silver carp eggs and larvae to be stimulated by high water discharge (Shuai et al., 2018; Yu et al., 2018). However, this fish can reproduce in the Wabash River without changes in discharges (Coulter et al., 2016). Notably, the spawning of silver carp in China is impacted by changes in thermal and hydrological regimes caused by the construction of dams (Wang et al., 2014; Li et al., 2016). In general, thermally stratified reservoirs releasing hypolimnetic water could reduce the temperature below large dams (Preece and Jones, 2002; Wang et al., 2014), while small surface-release dams could increase downstream temperatures by releasing warm epilimnetic water (Lessard and Hayes, 2003).
Currently, we lack a clear understanding of the impacts of global warming and other factors upon the spawning characteristics of silver carp in one of its largest habitats, the Pearl River in China (Shuai et al., 2018). First, there is a debate on the change of spawning timing of silver carp (delayed vs. advanced) in the Pearl River. Based on fish larvae data from 2006 to 2008, Tan et al. (2010) reported silver carp spawning delays in the Pearl River. In contrast, Shuai et al. (2018) reported that the spawning peak of this fish occurred at an earlier date from 2006 to 2013 in the same region. The spawning processes of fish have natural fluctuations, and long-term data are required to reveal the dynamics. Second, few studies have examined the possible reasons that drive the changes in spawning characteristics of silver carp in this river (Shuai et al., 2016).
The present study uses a long time-series dataset (2006–2013 and 2017–2019) to reveal the changing spawning characteristics of silver carp in the Pear River and the underlying drivers. We focus on answering the following three questions: (1) what are the temperature and hydrological requirements for spawning of silver carp in the river; (2) how has their spawning timing varied (delayed or advanced) over the past decade; (3) which factors (e.g., global warming, river discharge) were mainly correlated with such variation?
Materials and Methods
Study Area
The Pearl River is the largest river in southern China, with a total length of 2,214 km and a mean annual discharge of 3.3 × 1011 m3. The sampling location, i.e., Zhaoqing section, is in the lower reach of the Pearl River, about 180 km upstream from the estuary (Figure 1). The annual average air temperature, annual rainfall, and annual discharge of this section are about 22.0°C, 1,644.5 mm, and 6,810 m3/s, respectively (Lu, 1990). There are 76 fish species recorded in the lower reach of the Pearl River, excluding the estuary area (Zhang et al., 2020). Over the past century, 18 spawning grounds of silver carp have been reported in the middle and upper reaches of the Pearl River (Committee of Pearl River Fishery Resources Investigation, 1985), and approximately 26 dams had been built near the spawning grounds by 2007. Our long-term monitoring results (unpublished data), along with a recent study (Gao et al., 2020), indicate that only the spawning grounds in the lower reaches of the tributary Liujiang River and the Laibin–Pingnan section of the mainstream still exist after 2007.
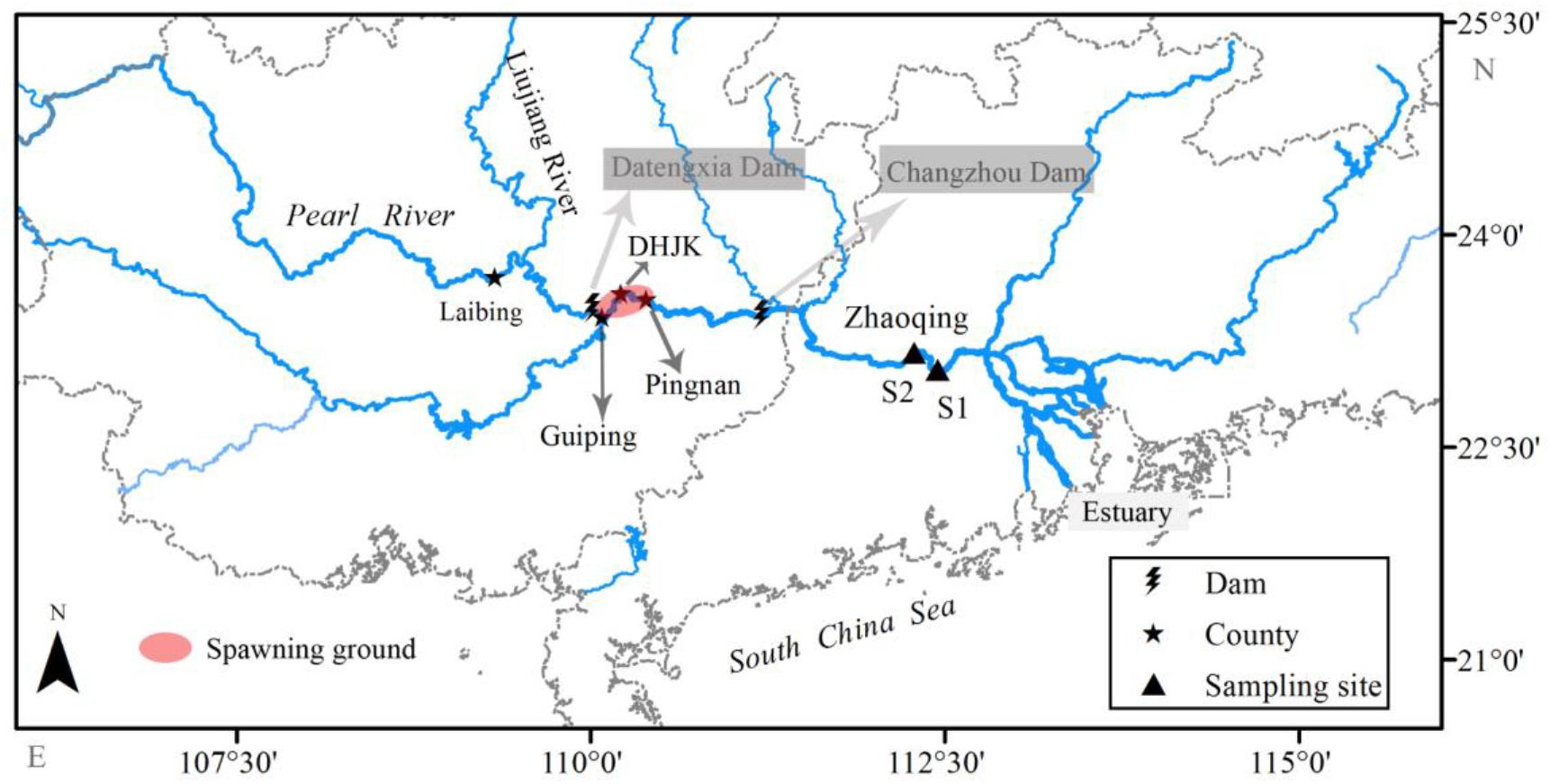
Figure 1. Locations of dams near the spawning ground, sampling sites, and spawning ground of the collected larvae in the Pearl River (China). Sampling of larvae was conducted during 2006–2013 and 2017–2019. The daily temperatures and discharges of the spawning ground were collected from the DHJK gauge station from 2006 to 2019.
For our samples, the spawning grounds are located in the Guiping–Pingnan section, estimated by the development periods of captured larvae and water velocity (Pearl River Water Resources Commission, unpublished data). Eleven dams have been constructed in the mainstream of the Pearl River. All these dams were completed before 2005 and are located at the upper reaches, except for two downstream dams: Changzhou Dam and Datengxia Dam (Chen et al., 2018). The Changzhou Dam is a low head dam (height < 30 m, highest water level = 16 m) built in 2007 with only a daily discharge regulation. The Datengxia Dam began to impound in 2020, with a maximum water level of 61 m expected in 2023. Taken together, all large dams were located in the headwater and upper reaches of the Pearl River before 2019, far away from the current silver carp spawning grounds. Therefore, the impoundment would not influence the water temperature in the current spawning grounds during the study period (2006–2019).
Data Collection
We sampled three times a day (06:00–08:00, 13:00–15:00, and 19:00–21:00) every other day throughout the year. Larval fish were collected using a pyramid trap net (mesh size = 0.5 mm, length = 6 m). The net had a rectangular mouth (width × height: 1.5 m × 1.0 m), and the end opened to a larval collection cage (0.8 m × 0.4 m × 0.4 m) (Shuai et al., 2018). A cup-type flowmeter (LS45-2) was fixed at the net mouth center to measure the water volume flowing through the net. The net was submerged in the water with mouth open and facing the river flow. The net position would change slightly with the rise and fall of the water level, but it was kept 10–15 m away from the bank, where the flow velocity was approximately 0.3 m/s. Sampling was initially conducted at S1 (23°2′40″ N, 112°27′5″ E) from 2006 to 2013. But the location was changed to S2 (23°9′54″ N, 112°16′58″ E, about 28 km upstream of S1) from 2017 to 2019, because cargo ships anchored near S1 (Figure 1). No samples were collected from 2014 to 2016.
Larvae samples were kept in a 5% formalin solution immediately after collection. The larvae of silver carp were identified (based on morphological characteristics) and counted by an experienced researcher in the laboratory (Yi et al., 1988). The abundance of larvae was calculated as the number of larvae per 24 h. The collected silver carp larvae were mainly at two developmental stages: the emergence of air bladder and one-chamber air bladder. Spawning dates of larvae were estimated using back-calculation methods from the captured dates (Yi et al., 1988). The spawning date of larvae was defined as the day of spawning occurrence.
We collected daily water temperatures and discharges at the Dahuangjiangkou (DHJK) gauge station to represent the environmental conditions of the spawning ground from 2006 to 2019 (Figure 1). Daily water temperature was collected by an automatic water temperature monitor, which recorded the temperature at least four times a day. Daily river discharge (Dis) was obtained from the Pearl River Water Resources Commission.
Data Analysis
We back-calculated the earliest birth date of the captured larvae, date of the spawning peak, and the end date of spawning by subtracting their development periods from the capturing dates of the larvae. These variables were used to describe the spawning period across our study. A previous study reported that the minimum discharge required to observe the occurrence of silver carp larvae was 10,000 m3/s in the Pearl River (Shuai et al., 2018). Thus, we used the first date of the discharge increased to 10,000 and 13,000 m3/s to index the possible flow-related reasons for changes in spawning timing. The minimum water temperature for silver carp spawning was 18°C (Yi et al., 1988). We counted the number of days with a temperature not lower than 18°C (hereafter, N_18) prior to the spawning season (from January to March). The threshold temperature for initial gonad development of adult silver carp was 15°C. We thus regarded the date when the water temperature reached 15°C during spring as the start date of stage IV (Wang et al., 2014). The end date of stage V denoted the date when larval abundance reached 5% of the total observed annual abundance. The cumulative temperature from stages IV to V was calculated by the sum of daily water temperature from stages IV to V in the year. The above variables and the mean monthly water temperature of the spawning ground were used to describe the changes in water temperature of spawning and cumulative temperature. Using the day of year calendar, the date is a sequential day number starting with day 1 on January 1.
The discharge suitability index (DSI) and the temperature suitability index (TSI) were applied to define the discharge and temperature suitability for silver carp’ spawning (Yu et al., 2018). The DSI was calculated as , where DSIi is the discharge suitability index of the discharge bin i, Di is the value of larvae abundance weighted by the number of spawning occurrences of the discharge bin i, Dmax is the maximum value of larvae abundance weighted by the number of spawning occurrences across all discharge bins. The TSI was calculated as , where TSIj is the temperature suitability index of the temperature bin j, Tj is the value of larvae abundance weighted by the number of spawning occurrences of the temperature bin j, Tmax is the maximum value of larvae abundance weighted by the number of spawning occurrences across all temperature bins.
All datasets of discharge, temperature, and abundance of larvae have normal distributions. The analysis were performed using Shapiro–Wilk tests in the “stats” package in R 3.6.1 (R Core Team, 2019). Pearson correlation analysis was used to determine the statistical significance of correlations between the spawning timing, discharge increase, and monthly temperature variables. The correlation analysis was performed using SPSS 13.0. Data for daily temperature (from January to March, August to September) was converted to weekly time-series for trend analysis using the “stats” package in R. The structural change in a time-series was detected by the chow test/F statistic using the “strucchange” package in R to plot the corresponding p-values (Zeileis et al., 2002). Differences were considered statistically significant at p < 0.05.
Results
Discharge and Temperature Suitability for Silver Carp Spawning
The silver carp started to spawn when the river discharge reached 2,310 m3/s (Figure 2A). The occurrence of spawning gradually increased with increased discharge before 7,000–9,000 m3/s, and then decreased (Figure 2A). However, the highest discharge suitability index (DSI) was observed at a discharge of 13,000–15,000 m3/s (Figure 2A). Moreover, there was a relatively lower peak of DSI at a discharge of 19,000–21,000 m3/s (Figure 2A).
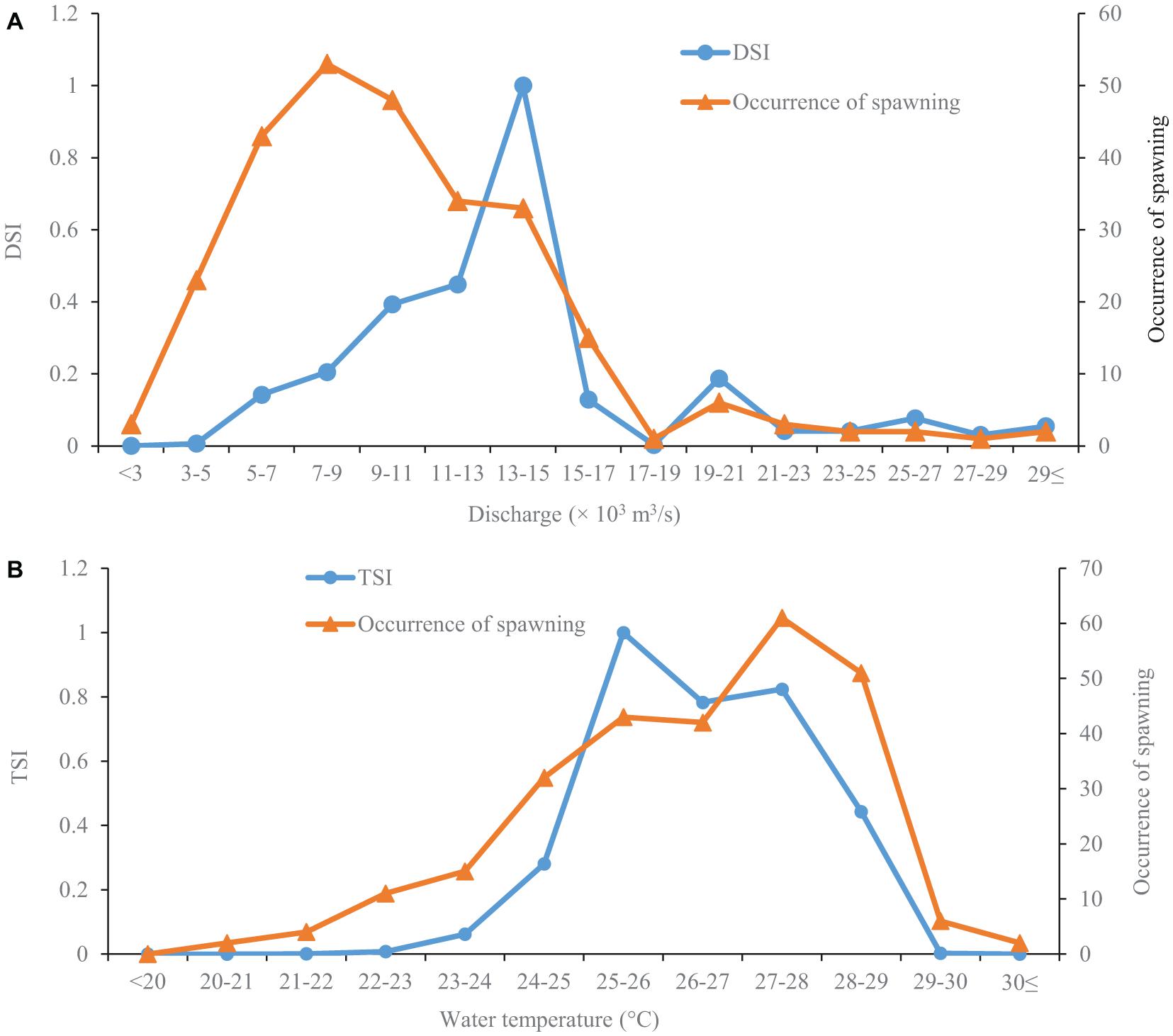
Figure 2. Discharge (A) and temperature (B) suitability curves for the spawning of silver carps. The data are collected in the Pearl River (China) during 2006–2013 and 2017–2019. The occurrence of spawning represents the number of spawning days at a given discharge or temperature. The discharge suitability index (DSI) and temperature suitability index (TSI) range from 0–1.
The lowest temperature observed for the occurrence of silver carp spawning was 20.7°C (Figure 2B). The occurrence of spawning gradually increased with increasing temperature before 27–28°C, slowly declined after that, and then plummeted (Figure 2B). The temperature suitability index (TSI) stayed at a low level when the temperature was lower than 22°C, then sharply increased to the peak at 25–26°C. Then TSI rapidly declined and remained at a relatively stable level at temperatures of 26–28°C after that; finally, it sharply decreased at temperatures above 28°C (Figure 2B).
Increased Temperature Shortening Spawning Period of Silver Carp
Variation of Spawning Timing and Temperature Variables
Silver carp’ spawning began with the onset of floods at the end of April, peaking by June/July (Figure 3). The onset of spawning (spawning start; Figure 3) fluctuated from 2006 to 2017, with a delay of 55 days in 2017 relative to 2009 (the trough). By 2019, the onset of spawning was 47 days earlier than in 2017. In contrast, there were relatively stronger temporal trends in peak and end of spawning dates in that they were both earlier (Figure 3). For instance, peak spawning occurred earlier stepwise from 2006 to 2012, with the earliest peak occurring in 2012. Specifically, peak spawning was 80-days earlier in 2012 than 2006; in 2019, peak spawning was delayed by 18 compared to 2012. A similar temporal trend was also observed for the end of the spawning season, which tended to occur earlier stepwise from 2006 to 2011, with the earliest end date in 2011. This 2011 end date was 61-days earlier than 2006 and only 3 days earlier than in 2019. Consequentially, the interval between the onset and the end of spawning decreased from 130 days in 2006 to 81 days in 2019. In contrast, the number of days temperatures were greater than or equal to 18°C from January to March (N_18) generally increased. The N_18 first gradually increased from 0 in 2006 to 71 in 2017 (the peak), although there were two zeros in 2011 and 2012 and the peak value halved by 2019.
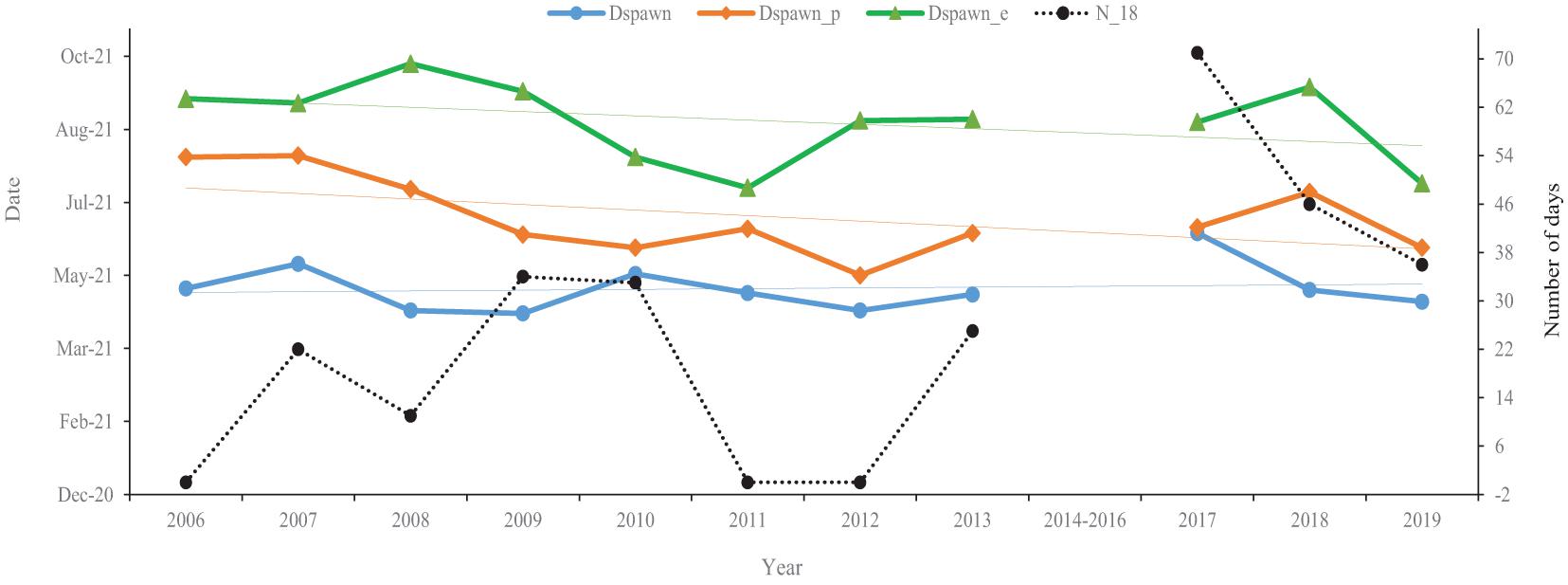
Figure 3. Variations in the timing of silver carps’ spawning characteristics and the number of days with a temperature greater than or equal to 18°C from January to March (N_18). The data are collected in the Pearl River (China) during 2006–2013 and 2017–2019. The earliest date of spawning (blue; Dspawn), the peak spawning date (orange; Dspawn_p), the end of spawning (green; Dspawn_e). Faint lines of similar colors denote the trend lines.
Correlation Between Flow and Temperature Variables and Spawning Timing
Pearson correlation coefficients between timings of silver carp spawning, thermal regime, and discharge variables are shown in Table 1. The number of days with a temperature greater than or equal to 18°C from January to March (N_18) and the mean temperature in January (T1) were significantly and positively correlated with the initial spawning date (Dspawn) (p < 0.05), but significantly and negatively correlated with the days of spawning occurrence (Occu) (p < 0.05). Spawning peak (Dspawn_p) was significantly and positively correlated with (i) the end of spawning (Dspawn_e), (ii) the onset of the discharge increased to 10,000 m3/s (Ddis_1), and (iii) the cumulative temperature from stages IV to V (DdegdayIV-V) (p < 0.05). The Dspawn_e was positively correlated with the mean temperature in April (T4, p < 0.05), but negatively correlated with mean temperatures in August (T8) and September (T9) (both p < 0.05). The onset of the discharge increased to 13,000 m3/s (Ddis_13) was positively correlated with Ddis_1, DdegdayIV-V, and mean temperatures in January (T1), April (T4), May (T5), and December (T12) (all p < 0.05). The Ddis_1 was positively correlated with Dspawn_p, Ddis_13, DdegdayIV-V, and mean temperature in May (T5) (all p < 0.05). The Occu was negatively correlated with Dspawn, N_18, and mean temperatures in January (T1) and February (T2) (all p < 0.05).
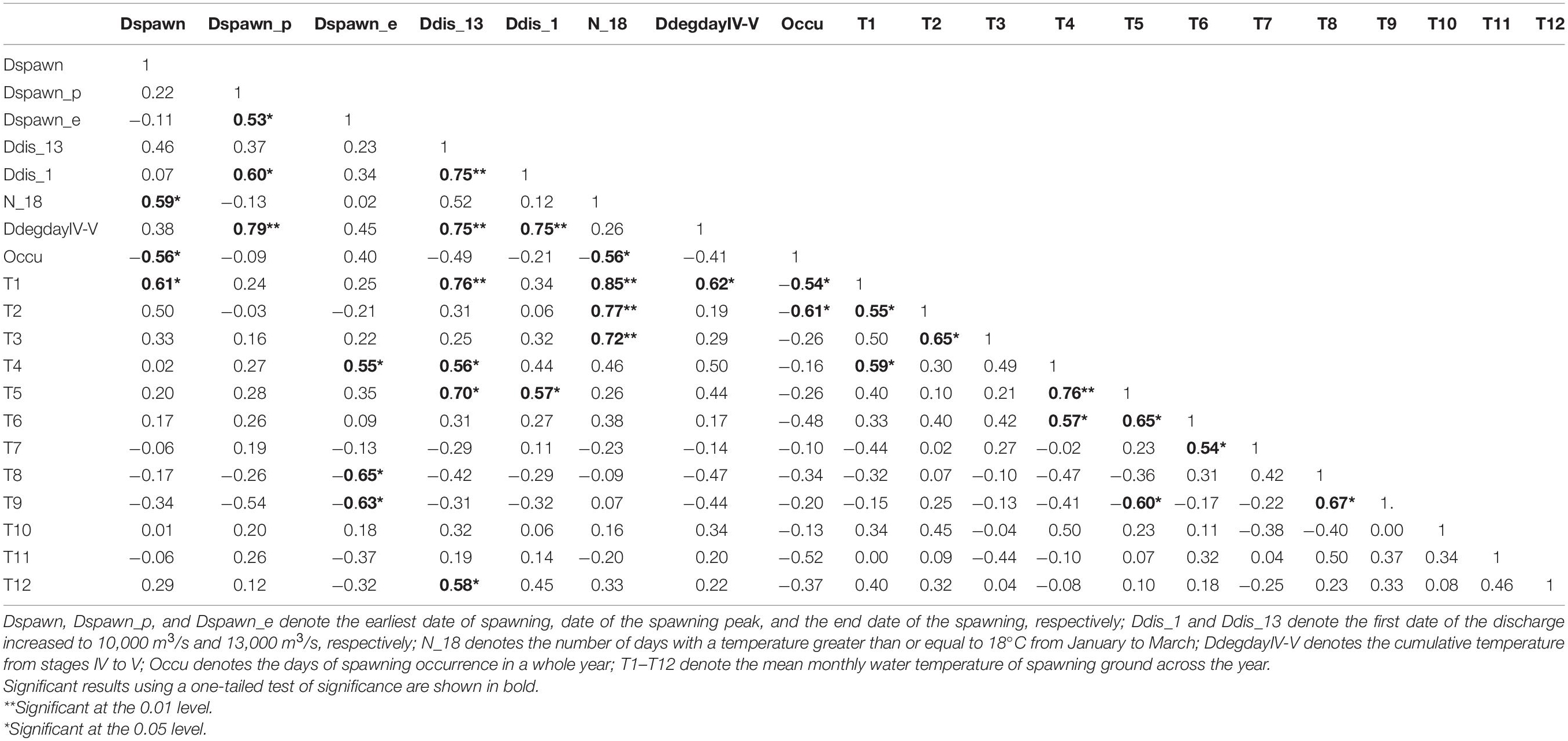
Table 1. Pearson correlation coefficients among thermal, discharge, and spawning timing variables in the Pearl River (China) during 2006–2013 and 2017–2019.
Interannual Fluctuations of Temperature
The temperature before the spawning season (from January to March) fluctuated and can be generally viewed from two periods: before and after 2012 (Figure 4). Prior to 2012, the temperature fluctuated without significant trends. After 2012, there was a warming trend by 2019 (all p values < 0.05, except those in 2019). During 2006–2019, the average minimum and maximum temperatures from January to March increased by approximately 0.95 and 1.76°C, respectively.
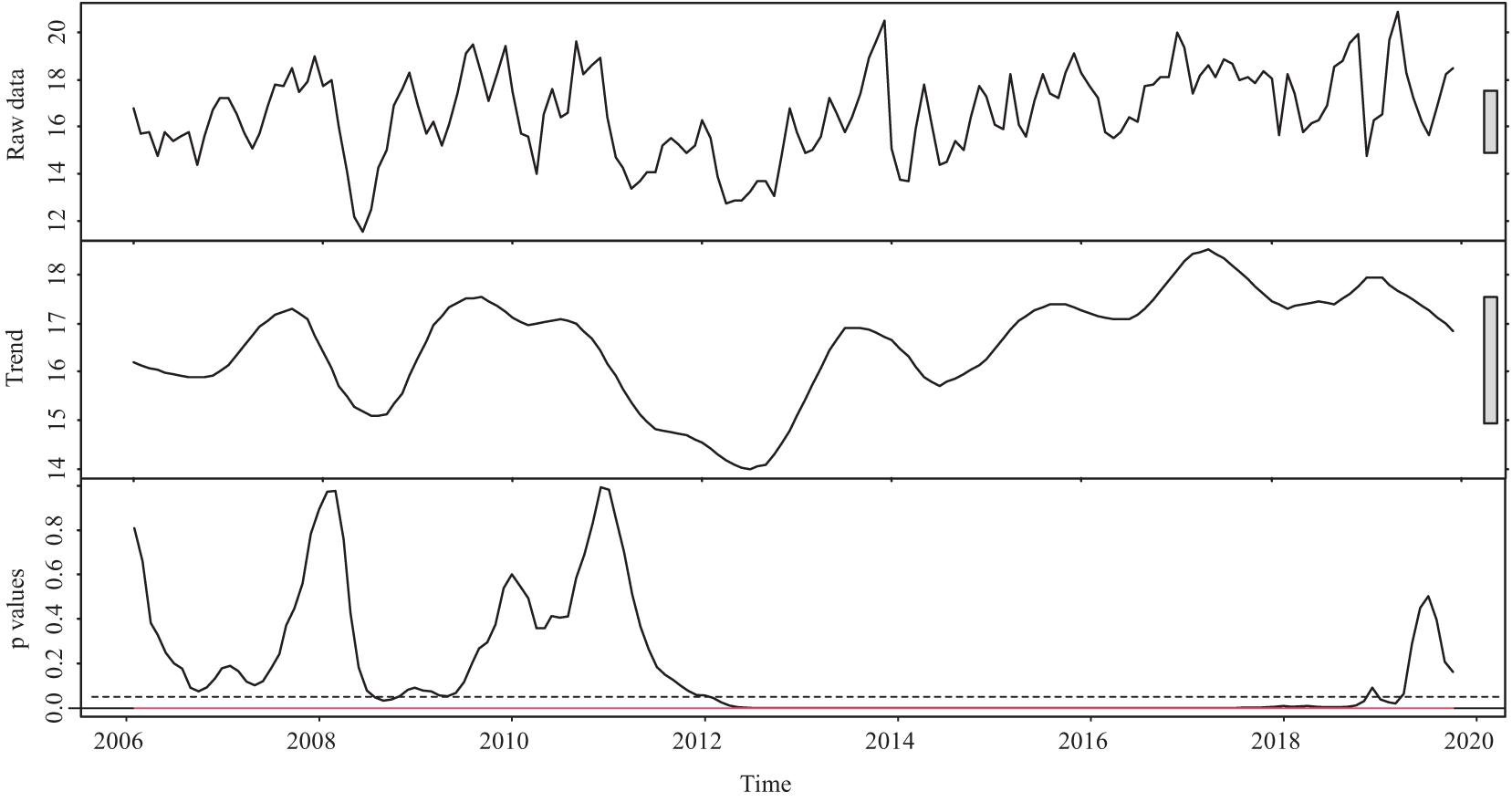
Figure 4. The temperature (two components: raw data and trend) and corresponding p-value before the spawning season (from January to March) during 2006–2019 in the Pearl River (China). The gray boxes represent the relative magnitude of variation in each component. The trend component is derived by locally weighted scatterplot smoothing. The red line represents the corresponding p-value of the boundary of the F statistics at the confidence level of 99%. The dotted line shows where the p-value equals 0.05.
The temperature from August to September (during the late period of the spawning season) can be viewed from three periods: 2006–2011, 2011–2015, and 2015–2019 (Figure 5). The temperature during the late period of the spawning season showed a generally significant increase trend between 2006 and 2011, when the minimum and maximum temperatures increased by approximately 1.61 and 2.39°C, respectively (Figure 5). In contrast, the temperature fluctuated without a significant trend from 2011 to 2015, when the minimum and maximum temperatures decreased by approximately 1.91 and 2.44°C, respectively. From 2015 to 2019, however, there existed another warming trend, and the minimum and maximum temperatures increased by approximately 1.73 and 2.49°C, respectively.

Figure 5. The temperature (two components: raw data and trend) and corresponding p-value from August to September during 2006–2019 in the Pearl River (China). The gray boxes represent the relative magnitude of variation in each component. The trend component is derived by locally weighted scatterplot smoothing. The red line represents the corresponding p-value of the boundary of the F statistics at the confidence level of 99%. The dotted line shows where the p-value equals 0.05.
Discussion
Discharge and Temperature Suitability for Silver Carp Spawning
Spawning environment suitability of fish in rivers is determined by hydrodynamic, geomorphological, and biological interactions that generate complex and localized linkages (Alvarez-Mieles et al., 2019). The same species distributed over a variety of environmental conditions could probably demonstrate different ecological and hydrological requirements for spawning. The silver carp is distributed all over the world and has high local adaptation in reproduction characteristics. The initiation of silver carp spawning without changes in discharge was noted in the Wabash River, but the occurrences of eggs and larvae of this fish seemed to be triggered by flood pulses in the Yangtze River and Pearl River (Coulter et al., 2016; Shuai et al., 2018; Yu et al., 2018). The most suitable discharge and temperature of silver carp’ spawning habitats in the middle and lower reaches of the Yangtze River were 15,000–21,300 m3/s and 21–24°C, respectively (Yi et al., 1988; Yu et al., 2018). In contrast, the current study demonstrated that spawning in the Pearl River was initiated when discharge was much lower, approximately 2,310 m3/s, indicating that the fish can start to spawn without significant increases in discharge. The most suitable discharge for silver carp’ spawning in this study (13,000–15,000 m3/s) was also lower than that in the Yangtze River. The distance between the middle and lower reaches of the Yangtze River and those of the Pearl River is approximately 800 km, and there is a relatively high genetic distance between these two silver carp populations (Ji et al., 2009). Therefore, different hydrological requirements for silver carp spawning in those two large rivers in China could probably attribute to local adaptation.
Our study provides some new insights about the mechanisms of how river discharge affects the spawning characteristics of silver carp. First, our findings suggest that the spawning peak of silver carp, rather than the initial spawning, is probably triggered by flow increase in the Pearl River. This was evidenced by the fact that the start date of spawning was not significantly correlated with high discharge (Ddis_1 and Ddis_13), while the spawning peak was significantly and positively correlated with high discharge (Ddis_1). Second, we showed that spawning occurrence of silver carp occurred at a relatively low discharge level, but with relatively lower daily larvae abundance. This finding is likely because only part of the mainstream is suitable for fish spawning when the discharge is at a low level. Then as the discharge gradually increases, some hydro-fluctuation zones become suitable for spawning, which explains why the highest average daily larvae appeared at high discharge. Flow pulses can probably create more suitable spawning niches for fish.
We indicate that the minimum and most suitable temperatures for silver carp’ spawning in the Pearl River were higher than those in the Yangtze River (Yi et al., 1988). Temperature can both affect the development of gonad and trigger spawning of fish (Morgan et al., 2013). In the present study, silver carp spawned at 20.7–30.1°C in the Pearl River, while they spawned at 18–28°C in the Yangtze River (Chen et al., 2009). Fish are very capable of finding habitat patches with suitable spawning temperature (Górski et al., 2010), which might explain why silver carp can spawn at a wide range of temperatures. The lowest temperature for silver carp spawning is 18°C indicating that water temperature is not a limiting factor in the Pearl River where average annual water temperature in spawning grounds is approximately 23°C (Yi et al., 1988). Although there is a wide range of temperatures for silver carp spawning in the present study, the most suitable temperature was closely related to the flood season.
Increased Temperature Shortening Spawning Period of Silver Carp
We showed that the spawning period of silver carp has been gradually shortened, given the start date of spawning fluctuated with a slight tendency to delay, while the spawning peak and end date obviously occurred earlier over the years. The start date of spawning of silver carp in the Pear River was largely regulated by water temperature, and warmer temperature prior to the spawning season can delay the spawning (Warren et al., 2012). The temperature-related changes in spawning timing are evident in a large number of freshwater fish species. For example, elevated water temperatures caused earlier spawning of rainbow smelt (Osmerus mordax) in the Great Lakes (O’Brien et al., 2012). Warmer temperatures prompted earlier phenology that led to 17 species of larval fishes occurring earlier in the year in southern California (Asch, 2015). In the Yangtze River, a water temperature decline of 2–4°C between March and May resulting from the Three Gorges Dam impoundment led to a delay in silver carp spawning (Wang et al., 2014). However, the rising temperature can also delay fish spawning. It has been reported that the elevated summer temperature caused delayed spawning of brook trout (Salvelinus fontinalis) and reduce spawning activities in Rock Lake (Warren et al., 2012). Likewise, in our study, the results suggest that rising temperature from January to March (prior to the spawning season) may delay the spawning start date and decreases the occurrence of spawning. Given the uncertainty between 2014 and 2016 and the limited time-series points, our analyses and conclusions on the trend of Dspawn should be viewed with caution.
We indicated that water temperature increases before the spawning season may be the principal reason for the initial spawning delay in the present study. However, an observed delay in silver carp spawning in the Yangtze River was caused by water temperature declines before spawning (Wang et al., 2014). This could attribute to the reasons that when the mean temperature before the spawning season is lower than 15°C (threshold temperature for gonad development of silver carp), the increase of temperature may favor body growth and promoted gonad development (Yi et al., 1988). However, when the mean temperature substantially exceeds 18°C (threshold temperature for silver carp spawning), we speculated that the elevated temperature would increase metabolism, diverting energy from the fat accumulation, which is needed to complete gonad development, ultimately causing spawning delay (Luksiene and Svedang, 1997; Pankhurst and Munday, 2011). The mean water temperature before and during the spawning season in the spawning ground (DHJK) in the Pearl River were approximately 18 and 26°C, respectively. However, in the spawning grounds of silver carp in the Yangtze River, the mean water temperatures before and during the spawning period were approximately 13 and 21°C, respectively (Wang, 2016). These differences in ambient temperatures might be the principal reason for the divergence in warming effects on the onset of spawning. Although temperature-dependent adaptation allows parents to adjust the spawning time so that the larvae match the timing of the food source (Neuheimer et al., 2018), most studies consider that temperature-related spawning time variations can be attributed to changes in effective accumulated temperature (Robinson et al., 2010; Chezik et al., 2014; Hansen et al., 2017). The initiation of spawning in the present study did not significantly correlate with cumulative temperature. Although cumulative degree-day is an important factor affecting spawning initiation, it is not the main limiting factor in a subtropical river with high temperatures (Wang et al., 2014; Hansen et al., 2017).
The average temperature in August and September was significantly and negatively correlated with the end of spawning, indicating that high temperature in August and September promotes the earlier termination of silver carp spawning. The mean water temperature in the Pearl River between August and September is approximately 28.6°C, which is higher than mean temperatures in other months. The optimum temperature for embryo development of silver carp is 22–28°C (Chen et al., 2009). Therefore, it is likely that silver carp in the Pearl River complete spawning early to avoid the adverse effects of rising temperature on embryo development in a freshwater system with limited thermal refugia (McCullough et al., 2009; Warren et al., 2012).
We revealed that the temperature before the spawning period (from January to March) and near the end of spawning (August and September) were increased during the study period. According to the relationships between the timing of spawning and thermal regime, this warming trend was likely the principal reason for the decreasing trend of the spawning time of silver carp in the present study. Given there was no large dam near the spawning ground during the sampling period, the temperature variation could not be attributed to dam impoundment. It has been reported that the Pearl River basin has been undergoing air temperature increases since the 1950s caused by climate change (Zhang et al., 2012; Tian and Yang, 2017). Therefore, the upward trend in the water temperature in the present study is most likely due to global warming.
Conclusion
The discharge suitability index for spawning of silver carp in the Pearl River showed a peak at the discharge of 13,000–15,000 m3/s. While the temperature suitability index peak was observed at temperatures of 25–26°C, the most suitable temperature for spawning was between 25 and 28°C. In general, the start date of spawning fluctuated with a slight tendency to delay, while the spawning peak and end date obviously occurred earlier during the study period. The initial spawning delay mainly attributed to the increasing temperatures between January and March. In addition, the end date of the spawning became earlier, likely due to elevated temperatures in August and September. Increases in discharge did not significantly correlate with the start of spawning but significantly positively correlated with the spawning peak. These results indicated that (i) the initial spawning of silver carp seems to be triggered by temperature rather than changes in discharge; (ii) flow pulses can probably create more suitable spawning niches for fish; and (iii) elevated temperature has shortened the spawning period of silver carp in the Pearl River.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
All experiments were performed under the approval of the Ethics Committee of Pearl River Fisheries Research Institute, Chinese Academy of Fishery Sciences. These policies align with the Chinese Association for the Laboratory Animal Sciences and the Institutional Animal Care and Use Committee (IACUC) protocols.
Author Contributions
YX: data analysis, writing—original draft, writing—review and editing, and visualization. XL: conceptualization, methodology, and funding acquisition. JY, SZ, ZW, and JL: sampling collection and data curation. YL: conceptualization, methodology, investigation, and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (2018YFD0900903), the Guangzhou Science Technology Project (202102020270), and the Pearl River Fishery Resources Survey and Evaluation Innovation Team Project (2020TD10, 2020ZJTD-04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to technicians Shaofang Su and Zhirong Zhi for their assistance with fieldwork and larvae collection.
References
Abdusamadov, A. S. (1987). Biology of white Amur (Ctenopharyngodon idella), silver carp (Hypophthalmichthys molitrix), and bighead (Aristichthys nobilis), acclimatized in the Terek Region of the Caspian Basin. J. Ichthyol. 26, 41–49.
Alix, M., Kjesbu, O. S., and Anderson, K. C. (2020). From gametogenesis to spawning: how climate-driven warming affects teleost reproductive biology. J. Fish Biol. 97, 607–632. doi: 10.1111/jfb.14439
Alvarez-Mieles, G., Corzo, G., and Mynett, A. E. (2019). “Spatial and temporal variations’ of habitat suitability for fish: a case study in Abras de Mantequilla Wetland, Ecuador,” in Spatiotemporal Analysis of Extreme Hydrological Events, eds G. Corzo and E. A. Varouchakis (Amsterdam: Elsevier), 113–141.
Asch, R. G. (2015). Climate change and decadal shifts in the phenology of larval fishes in the California Current ecosystem. Proc. Natl. Acad. Sci. U.S.A. 112, E4065–E4074. doi: 10.1073/pnas.1421946112
Bailly, D., Agostinho, A. A., and Suzuki, H. I. (2008). Influence of the flood regime on the reproduction of fish species with different reproductive strategies in the Cuiaba River, upper Pantanal, Brazil. River Res. Appl. 24, 1218–1229. doi: 10.1002/rra.1147
Carscadden, J., Nakashima, B. S., and Frank, K. T. (1997). Effects of fish length and temperature on the timing of peak spawning in capelin (Mallotus villosus). Can. J. Fish. Aquat. Sci. 54, 781–787. doi: 10.1139/f96-331
Chen, F., Lei, H., Zheng, H., Wang, W., Fang, Y., Yang, Z., et al. (2018). Impacts of cascade reservoirs on fishes in the mainstream of Pearl River and mitigation measures. J. Lake Sci. 30, 1097–1108. doi: 10.18307/2018.0422
Chen, I. C., Hill, J. K., Ohlemüller, R., Roy, D. B., and Thomas, C. D. (2011). Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. doi: 10.1126/science.1206432
Chen, Y. B., Liao, W. G., Peng, Q. D., Chen, D. Q., and Gao, Y. (2009). A summary of hydrology and hydrodynamics conditions of Four Chinese Carps’ spawning. J. Hydroecol. 2, 130–133. doi: 10.15928/j.1674-3075.2009.02.023
Chezik, K. A., Lester, N. P., Venturelli, P. A., and Tierney, K. (2014). Fish growth and degree-days I: selecting a base temperature for a within-population study. Can. J. Fish. Aquat. Sci. 71, 47–55. doi: 10.1139/cjfas-2013-0295
Committee of Pearl River Fishery Resources Investigation (1985). Investigation Report on Fishery Resources in the Pearl River, Vol. 6. 33–54. (Guangzhou: Unpublished report).
Coulter, A. A., Bailey, E. J., Keller, D., and Goforth, R. R. (2016). Invasive Silver Carp movement patterns in the predominantly free-flowing Wabash River (Indiana, USA). Biol. Invasions 18, 471–485. doi: 10.1007/s10530-015-1020-2
Erisman, B., Heyman, W., Kobara, S., Ezer, T., Pittman, S., Aburto-Oropeza, O., et al. (2017). Fish spawning aggregations: where well-placed management actions can yield big benefits for fisheries and conservation. Fish Fish. 18, 128–144. doi: 10.1111/faf.12132
Gao, M., Wu, Z., Tan, X., Huang, L., Huang, H., and Liu, H. (2020). Composition and abundance of drifting fish eggs on the upper reaches of Xijiang River, China, after the formation of the cascade reservoirs. BioRxiv [preprint] doi: 10.1101/2020.01.13.904110
Garcia, T., Jackson, P. R., Murphy, E. A., Valocchi, A. J., and Garcia, M. H. (2013). Development of a fluvial egg drift simulator to evaluate the transport and dispersion of Asian carp eggs in rivers. Ecol. Modell. 263, 211–222. doi: 10.1016/j.ecolmodel.2013.05.005
Gillet, C., and Dubois, J. P. (2007). Effect of water temperature and size of females on the timing of spawning of perch Perca fluviatilis L. in Lake Geneva from 1984 to 2003. J. Fish Biol. 70, 1001–1014. doi: 10.1111/j.1095-8649.2007.01359.x
Glover, R. S., Soulsby, C., Fryer, R. J., Birkel, C., and Malcolm, I. A. (2020). Quantifying the relative importance of stock level, river temperature and discharge on the abundance of juvenile Atlantic salmon (Salmo salar). Ecohydrology 13:e2231. doi: 10.1002/eco.2231
Gorbach, E. I., and Krykhtin, M. L. (1989). Migration of the white Amur, Ctenopharyngodon idella, and silver carp, Hypophthalmichthys molitrix, in the Amur River Basin. J. Ichthyol. 28, 47–53.
Gordoa, A., and Carreras, G. (2014). Determination of temporal spawning patterns and hatching time in response to temperature of Atlantic bluefin tuna (Thunnus thynnus) in the western Mediterranean. PLoS One 9:e90691. doi: 10.1371/journal.pone.0090691
Górski, K., Winter, H. V., De Leeuw, J. J., Minin, A. E., and Nagelkerke, L. A. J. (2010). Fish spawning in a large temperate floodplain: the role of flooding and temperature. Freshw. Biol. 55, 1509–1519. doi: 10.1111/j.1365-2427.2009.02362.x
Hansen, G. J., Read, J. S., Hansen, J. F., and Winslow, L. A. (2017). Projected shifts in fish species dominance in Wisconsin lakes under climate change. Glob. Change Biol. 23, 1463–1476. doi: 10.1111/gcb.13462
IPBES. (2019). “Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services,” in IPBES Secretariat, eds S. Díaz, J. Settele, E. S. Brondizio, H. T. Ngo, M. Guèze, J. Agard, et al. (Bonn: IPBES), 13.
Ji, C., Gu, J., Mao, R., Zhu, X., and Sun, X. (2009). Analysis of genetic diversity among wild silver carp (Hypophthalmichthys molitrix) populations in the Yangtze River, Helongjiang and Pearl River using microsatellite markers. J. Fish. China 33, 364–371. doi: 10.3321/j.issn:1000-0615.2009.03.002
Kjesbu, O. S. (1994). Time of start of spawning in Atlantic cod (Gadus morhua) females in relation to vitellogenic oocyte diameter, temperature, fish length and condition. J. Fish Biol. 45, 719–735. doi: 10.1111/j.1095-8649.1994.tb00939.x
Lessard, J. A. L., and Hayes, D. B. (2003). Effects of elevated water temperature on fish and macroinvertebrate communities below small dams. River Res. Appl. 19, 721–732. doi: 10.1002/rra.713
Li, F., Wei, J., Qiu, J., and Jiang, H. (2020). Determining the most effective flow rising process to stimulate fish spawning via reservoir operation. J. Hydrol. 582:124490. doi: 10.1016/j.jhydrol.2019.124490
Li, M., Duan, Z., Gao, X., Cao, W., and Liu, H. (2016). Impact of the three Gorges Dam on reproduction of four major Chinese carps species in the middle reaches of the Changjiang River. Chin. J. Oceanol. Limn. 34, 885–893. doi: 10.1007/s00343-016-4303-2
Lu, K. (1990). Fisheries Resources in Pearl River. Guangzhou: Guangdong science and Technology Press, 1–5.
Luksiene, D., and Svedang, H. (1997). A Review on Fish Reproduction With Special Reference to Temperature Anomalies. Öregrund: Fiskeriverket, Kustlaboratoriet, 35.
McCullough, D. A., Bartholow, J. M., Jager, H. I., Beschta, R. L., Cheslak, E. F., Deas, M. L., et al. (2009). Research in thermal biology: burning questions for coldwater stream fishes. Rev. Fish. Sci. 17, 90–115. doi: 10.1080/10641260802590152
Miranda, L. A., Chalde, T., Elisio, M., and Strüssmann, C. A. (2013). Effects of global warming on fish reproductive endocrine axis, with special emphasis in pejerrey Odontesthes bonariensis. Gen. Comp. Endocr. 192, 45–54. doi: 10.1016/j.ygcen.2013.02.034
Morgan, M. J., Wright, P. J., and Rideout, R. M. (2013). Effect of age and temperature on spawning time in two gadoid species. Fish. Res. 138, 42–51. doi: 10.1016/j.fishres.2012.02.019
Neuheimer, A. B., MacKenzie, B. R., and Payne, M. R. (2018). Temperature-dependent adaptation allows fish to meet their food across their species’ range. Sci. Adv. 4:eaar4349. doi: 10.1126/sciadv.aar4349
O’Brien, T. P., Taylor, W. W., Briggs, A. S., and Roseman, E. F. (2012). Influence of water temperature on rainbow smelt spawning and early life history dynamics in St. Martin Bay, Lake Huron. J. Great Lakes Res. 38, 776–785. doi: 10.1016/j.jglr.2012.09.017
Ojaveer, E., Arula, T., Lankov, A., and Shpilev, H. (2011). Impact of environmental deviations on the larval and year-class abundances in the spring spawning herring (Clupea harengus membras L.) of the Gulf of Riga (Baltic Sea) in 1947–2004. Fish. Res. 107, 159–168. doi: 10.1016/j.fishres.2010.11.001
Pankhurst, N. W., and Munday, P. L. (2011). Effects of climate change on fish reproduction and early life history stages. Mar. Freshw. Res. 62, 1015–1026. doi: 10.1071/MF10269
Paumier, A., Drouineau, H., Boutry, S., Sillero, N., and Lambert, P. (2020). Assessing the relative importance of temperature, discharge, and day length on the reproduction of an anadromous fish (Alosa alosa). Freshw. Biol. 65, 253–263. doi: 10.1111/fwb.13418
Perry, A. L., Low, P. J., Ellis, J. R., and Reynolds, J. D. (2005). Climate change and distribution shifts in marine fishes. Science 308, 1912–1915. doi: 10.1126/science.1111322
Preece, R. M., and Jones, H. A. (2002). The effect of Keepit Dam on the temperature regime of the Namoi River, Australia. River Res. Appl. 18, 397–414. doi: 10.1002/rra.686
R Core Team (2019). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Robinson, J. M., Josephson, D. C., Weidel, B. C., and Kraft, C. E. (2010). Influence of variable interannual summer water temperatures on brook trout growth, consumption, reproduction, and mortality in an unstratified Adirondack Lake. Trans. Am. Fish. Soc. 139, 685–699. doi: 10.1577/T08-185.1
Shuai, F., Lek, S., Baehr, C., Park, Y. S., Li, Y., and Li, X. (2018). Silver carp larva abundance in response to river flow rate revealed by cross-wavelet modelling. Ecol. Modell. 383, 98–105. doi: 10.1016/j.ecolmodel.2018.05.020
Shuai, F., Li, X., Li, Y., Li, J., Yang, J., and Lek, S. (2016). Temporal patterns of larval fish occurrence in a large subtropical river. PLoS One 11:e0146441. doi: 10.1371/journal.pone.0146441
Sims, D. W., Wearmouth, V. J., Genner, M. J., Southward, A. J., and Hawkins, S. J. (2004). Low-temperature-driven early spawning migration of a temperate marine fish. J. Anim. Ecol. 73, 333–341. doi: 10.1111/j.0021-8790.2004.00810.x
Smith, D. M., Scaife, A. A., Hawkins, E., Bilbao, R., Boer, G. J., Caian, M., et al. (2018). Predicted chance that global warming will temporarily exceed 1.5°C. Geophys. Res. Lett. 45, 895–811,903. doi: 10.1029/2018GL079362
Tan, X., Li, X., Lek, S., Li, Y., Wang, C., Li, J., et al. (2010). Annual dynamics of the abundance of fish larvae and its relationship with hydrological variation in the Pearl River. Environ. Biol. Fish. 88, 217–225. doi: 10.1007/s10641-010-9632-y
Thorstad, E. B., Økland, F., Aarestrup, K., and Heggberget, T. G. (2008). Factors affecting the within-river spawning migration of Atlantic salmon, with emphasis on human impacts. Rev. Fish Biol. Fish. 18, 345–371. doi: 10.1007/s11160-007-9076-4
Tian, Q., and Yang, S. (2017). Regional climatic response to global warming: trends in temperature and precipitation in the Yellow, Yangtze and Pearl River basins since the 1950s. Quatern. Int. 440, 1–11. doi: 10.1016/j.quaint.2016.02.066
Tuz-Sulub, A., and Brulé, T. (2015). Spawning aggregations of three protogynous groupers in the southern Gulf of Mexico. J. Fish Biol. 86, 162–185. doi: 10.1111/jfb.12555
van Overzee, H. M. J., and Rijnsdorp, A. D. (2015). Effects of fishing during the spawning period: implications for sustainable management. Rev. Fish Biol. Fisher. 25, 65–83. doi: 10.1007/s11160-014-9370-x
Wakefield, C. B., Potter, I. C., Hall, N. G., Lenanton, R. C. J., and Hesp, S. A. (2015). Marked variations in reproductive characteristics of snapper (Chrysophrys auratus, Sparidae) and their relationship with temperature over a wide latitudinal range. ICES J. Mar. Sci. 72, 2341–2349. doi: 10.1093/icesjms/fsv108
Walther, G. R., Post, E., Convey, P., Menzel, A., Parmesan, C., Beebee, T. J. C., et al. (2002). Ecological responses to recent climate change. Nature 416, 389–395.
Wang, J. N., Li, C., Duan, X. B., Luo, H. H., Feng, S. X., Peng, Q. D., et al. (2014). The relationship between thermal regime alteration and spawning delay of the four major Chinese carps in the Yangtze River below the three Gorges Dam. River Res. Appl. 30, 987–1001. doi: 10.1002/rra.2691
Wang, Y. F. (2016). The Three Gorges Project on Downstream River Eco-Hydrological Impact Assessment Study. Master Dissertation. Zhengzhou: North China University of Water Resources and Electric Power.
Warren, D. R., Robinson, J. M., Josephson, D. C., Sheldon, D. R., and Kraft, C. E. (2012). Elevated summer temperatures delay spawning and reduce redd construction for resident brook trout (Salvelinus fontinalis). Glob. Change Biol. 18, 1804–1811. doi: 10.1111/j.1365-2486.2012.02670.x
Yi, B. L., and Liang, Z. S. (1964). Natural conditions of the spawning grounds of the “domestic fishes” in Yangtze River and essential external factor for spawning. Acta Hydrobiol. Sin. 5, 1–15.
Yi, B. L., Liang, Z. S., Yu, Z. T., Lin, R. D., and He, M. J. (1988). “A comparative study on the early development of grass carp, black carp, silver carp and big head of the Yangtze River,” in Gezhouba Water Control Project and Four Famous Fishes in Yangtze River, eds B. L. Yi, Z. T. Yu, and Z. S. Liang (Wuhan: Hubei Science and Technology Press), 69–116.
Yu, L., Lin, J., Chen, D., Duan, X., Peng, Q., and Liu, S. (2018). Ecological flow assessment to improve the spawning habitat for the four major species of carp of the Yangtze River: a study on habitat suitability based on ultrasonic telemetry. Water 10:600. doi: 10.3390/w10050600
Zeileis, A., Leisch, F., Hornik, K., and Kleiber, C. (2002). Strucchange: an R package for testing for structural change in linear regression models. J. Stat. Softw. 7, 1–38.
Zhang, F., Lei, X., Jiang, Y., and Bai, J. (2012). Analysis on character of temperature variation in upstream of Pearl River basin during 55 years. J. Water Res. Water Eng. 23, 20–25.
Zhang, G. H., Chang, J. B., and Shu, G. F. (2000). Applications of factor-criteria system reconstruction analysis in the reproduction research on grass carp, black carp, silver carp and bighead in the Yangtze River. Int. J. Gen. Syst. 29, 419–428.
Zhang, P., Li, K., Wu, Y., Liu, Q., Zhao, P., and Li, Y. (2018). Analysis and restoration of an ecological flow regime during the Coreius guichenoti spawning period. Ecol. Eng. 123, 74–85. doi: 10.1016/j.ecoleng.2018.08.009
Zhang, P., Qiao, Y., Grenouillet, G., Lek, S., Cai, L., and Chang, J. (2021). Responses of spawning thermal suitability to climate change and hydropower operation for typical fishes below the Three Gorges Dam. Ecol. Indic. 121:107186. doi: 10.1016/j.ecolind.2020.107186
Keywords: fish reproduction, thermal regime, fish larvae, Pearl River (China), hydrological regime
Citation: Xia Y, Li X, Yang J, Zhu S, Wu Z, Li J and Li Y (2021) Elevated Temperatures Shorten the Spawning Period of Silver Carp (Hypophthalmichthys molitrix) in a Large Subtropical River in China. Front. Mar. Sci. 8:708109. doi: 10.3389/fmars.2021.708109
Received: 11 May 2021; Accepted: 27 August 2021;
Published: 16 September 2021.
Edited by:
Jennifer Marie Donelson, James Cook University, AustraliaReviewed by:
Heather Diana Veilleux, University of Alberta, CanadaVânia Baptista, University of Algarve, Portugal
Copyright © 2021 Xia, Li, Yang, Zhu, Wu, Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuefei Li, bGl5dWVmZWk4MTVAMTYzLmNvbQ==
 Yuguo Xia1,2
Yuguo Xia1,2 Yuefei Li
Yuefei Li