- Department of Life and Environmental Sciences, University of Cagliari, Cagliari, Italy
Holothuria tubulosa is one of the most common sea cucumber species inhabiting the Mediterranean Sea. Due to its commercial interest for the international market, it has been harvested without proper management causing the overexploitation of its stocks. Inadequate management is also caused by lack of information on basic biology and ecology not allowing the estimating of the species vulnerability and resilience to growing anthropogenic pressures. In this paper, we have investigated basic life-history traits of H. tubulosa (population structure and reproductive cycle) in a population of Central-Western Mediterranean (Sardinia, Italy). A macroscopic maturity scale for both sexes was defined through an instrumental colorimetric analysis of the gonads and the ramification level of the gonad’s tubules, subsequently confirmed by histological analysis. The seasonal trend of the Gonado-Somatic Index, the changes in color of the gonads and tubules ramification indicated that the spawning period of H. tubulosa was concentrated in summer with a peak in late August, closely related to the increase in water temperature. A synchronous development of the gonads, with a unique and short reproductive event during the year, was also detected. In conclusion, this study provides new evidence on the biological and ecological features of H. tubulosa, essential data for developing a scientifically-based stock assessment as well as conservative management at a local scale. Finally, we provided basic information for the domestication of broodstock in a conservative hatchery.
1 Introduction
Holothurians, known as sea cucumbers, are common benthic marine invertebrates belonging to the Phylum Echinodermata, Class Holothurioidea, represented by more than 1500 species worldwide (Horton et al., 2018). Most sea cucumbers are strong deposit-feeder bioturbators and, as such, are thought to play a key ecological role in benthic biogeochemistry (Roberts et al., 2000; Uthicke, 2001; Mangion et al., 2004; Amaro et al., 2010; Purcell et al., 2016; Neofitou et al., 2019).
Sea cucumbers are part of the culinary culture of Eastern Asian countries and are largely used in traditional Chinese medicine (Yang and Bai, 2015; Yang et al., 2015). They are a valuable source of bioactive compounds used in the pharmaceutical and cosmetic industry and are also considered a gourmet seafood called trepang or bèche de mer (Kinch et al., 2008; Bordbar et al., 2011; Purcell et al., 2012; Janakiram et al., 2015; Yang and Bai, 2015; Hame at al., 2022). The high cost of trepang (generally between 50 to 600 US$ dry kg-1) (Purcell et al., 2012; Ram et al., 2014; Purcell, 2014), and the numerous medical applications of sea cucumbers, led to the overexploitation of most of the Indo-Pacific species (Lovatelli et al., 2004; Conand, 2006; Choo, 2008; Purcell et al., 2011; Conand et al., 2014). In response to the market demand, the fishery of sea cucumbers have shifted to new target species as the Mediterranean ones (González-Wangüemert et al., 2014; González-Wangüemert et al., 2018). In many cases, the sea cucumber harvesting increased without an adequate management, which, coupled with the illegal catches, caused the collapse of several stocks (Toral-Granda et al., 2008; Anderson et al., 2011; Purcell, 2014, González-Wangüemert et al., 2018; Ramírez-González et al., 2020a). One of the main causes of the failure of sea cucumber management can also be a reflection of severe knowledge gaps on basic biological and ecological attributes, and their reproductive biology. Then again, the current lack of information about the sea cucumbers population dynamics depends also upon their peculiar body morphology. Indeed, the lack of a rigid skeleton and the high plasticity of the body wall, that can vary in response to environmental conditions (Bulteel et al., 1992; Kinch et al., 2008; Zang et al., 2012; Prescott et al., 2015; Tolon et al., 2017; Ramírez-González et al., 2020b), limit practically the possibility to estimate appropriately their age/size relationship and growth rates. Moreover, such peculiarity led to different approaches when measuring these organisms with authors reporting the gutted weight, the drained weight or wet weight to build the morphometric relationships producing different reference data (Kazanidis et al., 2010; Aydin, 2020).
Since an accurate knowledge of the reproductive cycle is a crucial step for breeding practices in captivity, more insights about the biology and ecology of sea cucumbers are needed to ultimately produce valuable biomass and reduce the fishing pressure on wild stocks (Morgan, 2000; Agudo, 2006; Purcell et al., 2012; Dominguez-Godino and Gonzàlez-Wangüemert, 2018; Rakaj et al., 2018; Rakaj et al., 2019).
Sea cucumber species are generally gonochoric, only sporadically hermaphrodites, and are mainly broadcast spawners (e.g. Hyman, 1955; Smiley et al., 1988; Smiley, 1990; Smiley et al., 1991; Mohsen and Yang, 2021). The maturation process of gametes seems to be controlled by either exogenous or endogenous factors. Temperature, light intensity, photoperiod, lunar cycle, tidal flux, food quality and its availability are among the most influential exogenous factors (Conand, 1981; Ramofafia et al., 2000; Tan and Zulfigar, 2001; Hamel and Mercier, 2004; Mercier and Hamel, 2009; Lee et al., 2018), but their different role in modulating the reproduction is still far to be fully accomplished.
In the Mediterranean Sea, one of the most common sea cucumber species is Holothuria tubulosa Gmelin 1791, which inhabits organic matter-rich soft bottoms and Posidonia oceanica meadows, where it plays a prominent role in recycling sedimentary organic detritus (Bulteel et al., 1992; Mezali et al., 2006; Mezali and Soualili, 2013; Costa et al., 2014; Boncagni et al., 2019; Pasquini et al., 2021). In the last few years, this species has been one of the most commercially exploited in the whole Mediterranean basin (Gonzàlez-Wangüemert et al., 2014; Gonzàlez-Wangüemert et al., 2015; Gonzàlez-Wangüemert et al., 2018; Dereli and Aydin, 2021) leading the Italian Ministry of Agriculture, Food and Forestry (MIPAAF) to ban sea cucumber fishing along the entire national coastline (Ministerial decree 156/2018), as a precaution for their conservation (Pasquini et al., 2021). Considering its growing economic interest, appropriate biological information is required. Currently, fragmentary data are available about the population structure and reproductive cycle in the Eastern (Bulteel et al., 1992; Despalatović et al., 2004; Dereli et al., 2016; Ocaña and Sanchez Tocino, 2005) and the Western (Tahri et al., 2019) Mediterranean Sea. Nevertheless, no information is reported about the spawning period of H. tubulosa in the Central-Western Mediterranean Sea.
In particular, studies about the reproductive period of H. tubulosa have mainly been focused on the histological observation of the gonads and yet the macroscopic characteristics and their correspondence with the microscopic features have been almost entirely ignored, resulting in a knowledge gap, in contrast to what has been done for other species (e.g., H. fuscogilva; Ramofafia et al., 2000). Furthermore, studies on the reproductive cycle and population dynamics conducted so far on H. tubulosa are related to limited temporal and spatial scales and, as such are not exhaustive.
In order to promote the scientific management of H. tubulosa, it is essential to acquire a baseline knowledge about its ecology, population dynamics, and reproduction. Therefore, the objectives of the present study are: (1) to provide a detailed description of the morphology of reproductive structures in relation to the reproductive cycle; (2) to obtain an insight of the seasonal and inter-population variability of the reproductive cycle.
2 Materials and methods
2.1 Study area and sampling
Specimens of H. tubulosa were collected in three different sites (Gulf of Oristano, Gulf of Teulada, and Tortolì) (Figure 1) of the coastal area of Sardinia Island (Italy, Central-Western Mediterranean Sea).
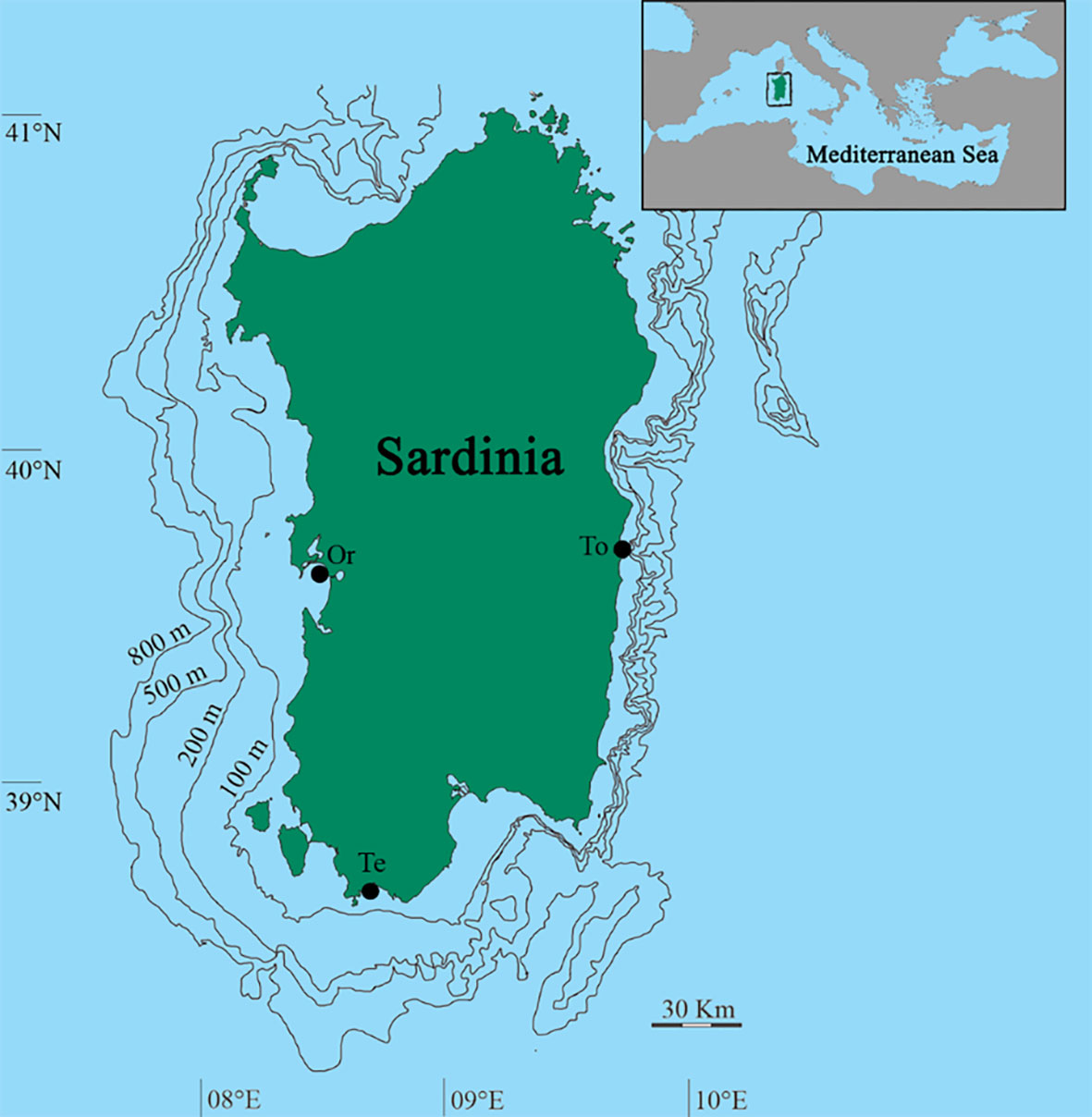
Figure 1 Map of the study area. Sampled sites of Oristano (Or), Teulada (Te), and Tortolì (To) are indicated.
A total of 214 specimens were collected by divers (3 – 10 meters depth) in 21 different sample occasions from 2018 to 2022. At each sampling date, sea surface temperature was recorded using a multiparametric probe (In-Situ SmarTROLL Multiparameter Handheld). After collection, specimens were placed into a 3L plastic bag and transported to the laboratory inside a cooler box (at in situ temperature). In the lab, in order to reduce the intra-specimen variability, sea cucumbers were gently squeezed to remove the excess water from the respiratory tree following the protocol of Costa et al. (2014) and then were weighted (WW, g, ± 0.01 g). The total length (TL, cm, ± 0.1 cm), was recorded using an ichthyometer considering the extended length of the sea cucumber, and the width (TW, cm, ± 0.1 cm) was recorded by caliper. After gutting (the removal of gonads and gut), the cucumbers were also weighted (GW, g, 0.01 g).
2.2 Population structure
Specimens were ordinated according to different size (length) classes and the length-frequency distribution was defined. To analyze relative changes in sea cucumbers morphology, allometric relationships were assessed using the allometric equation established by Keys (1928):
where b is the allometric coefficient and a is the intercept, estimated from least-squares fitting method of the log10 -transformed variables:
This equation was used to assess five different relationships (WW- TL; GW- TL; WW- TW; GW-TW; GW-WW). Since H. tubulosa lacks in sexual dimorphism, specimens were sexed after dissection, observing the gonads’ color, and were divided into four categories: F, females; M, males; I, undetermined, and U, unsexed (that occurred when was not possible to dissect the specimens). The sex-ratio (SR; females:males) was, also, estimated for the entire population.
2.3 Macroscopic and colorimetric analyses of the gonads
2.3.1 Macroscopic observation of the gonads
The macroscopic gonadal stage was assigned considering the following parameters: Gonado-Somatic index (GSI), color and the number of ramifications of the gonadal tubules. The gonad was separated from the gut and the respiratory tree and weighted (GoW, g, ± 0.01 g) and the GSI was calculated as follows:
whenever possible, the sex of the specimens was assigned by observing the gonads’ color according to Despalatović et al. (2004): white for males and pink for females. The number of the gonad tubules ramifications (from 1 to 5, used as a descriptor of the gonad maturation stage; the high the number of ramifications, the more developed the gonad; Ramofafia et al., 2000) was considered as reported in Figure S1, and the highest ramification level recorded.
2.3.2 Colorimetric analysis of the gonads
The gonad color is the most common macroscopic clues to identifying sea cucumbers sex and maturity (Ramofafia et al., 2000; Ramofafia et al., 2003; Despalatović et al., 2004). The color was defined using a reference palette with 10 main colors and the respective RGB scores and Pantones® codes (Pantone Inc. USA) in natural daylight (Table S1) (Prato et al., 2018). However, the capacity to assign colors may vary between observers, hence, an analytical method to assign the color to the gonads was used (Addis et al., 2014). The gonads’ colorimetric analyses were conducted using a digital colorimeter (Chroma meter CR-400, Konica Minolta, Tokyo, Japan), which specified the color according to the Commission Internationale de l’Eclairage (CIE, Commission Internationale de l’Eclairage (2008) Vienna, Austria) as lightness (L*), redness (a*), yellowness (b*) color space (CIELAB).
2.4 Histological analysis
Gonads were processed for histological analysis. For each sample, a piece of gonad tissue (0.5 to 1 cm long) was immediately fixed in a buffered 5% formalin solution (0.1 mol L-1, pH 7.4) for 48 h. The tissues were then dehydrated and embedded in a synthetic resin (GMA, Technovit 7100, Bio-Optica, Milan, Italy) following routine protocols and sectioned at 3.5 µm with a rotating microtome (ARM3750, Histo-Line Laboratories, Pantigliate, Italy). Slides were stained with Gill hematoxylin followed by eosin counterstain (H&E) for standard histology and with periodic acid–Schiff (PAS) and Alcian blue (AB) in combination to assess the production of neutral and sulfated acid mucins (Cerri and Sasso-Cerri, 2003). Subsequently, sections were dehydrated in graded ethanol (96–100%), cleared in Histolemon (Carlo Erba Reagents, Cornaredo, Italy) and mounted in resin (Eukitt, Bio-Optica).
Female histological samples were examined to determine the developmental stage of oocytes. The microscopic maturity stages of females were determined based on the ovarian wall thickness, the position of the oocytes in the gonad section and the oocyte size (following the description used by Ramofafia et al., 2000 and modified ad hoc for H. tubulosa.). The size composition of oocytes was obtained measuring only oocytes where the nucleus was clear, and given that these oocytes are rarely perfectly spherical in shape, in order to reduce the variance, the diameter of each oocyte was taken using Tps.dig v. 2.12 software (Rohlf, 2009) and calculated as average of the major and minor axis.
As in females, to assess the maturity stage of males’ gonad, the following clues were considered: the thickness of the testis, the extension of the germinal layers and its folds and the abundance (by rank) of spermatozoa filling the gonad lumen. For each individual, the average thickness was determined by measuring the thickness at five different points.
2.5 Reproductive period and maturity
The seasonality of spawning of H. tubulosa was estimated through an analysis of the monthly distribution of the percentage of maturity stages of females and males and the evolution of the mean GSI (see above). A range of mature individuals TL (males and females separately, and total population) was also reported.
2.6 Statistical analyses
To test the adequacy of the model used to assess the TL-WW, TW-WW, TL-GW, WW-GW relationships, an ANOVA test, using the P value for Lack-of-Fit, was performed on previously transformed (ln) data.
The significance of deviation of sex-ratio from the 1:1 null hypothesis was tested using the Chi-Square test (χ2) (Zar, 1999), while to assess differences among length-frequency distributions, the Kolmogorov-Smirnov test was used.
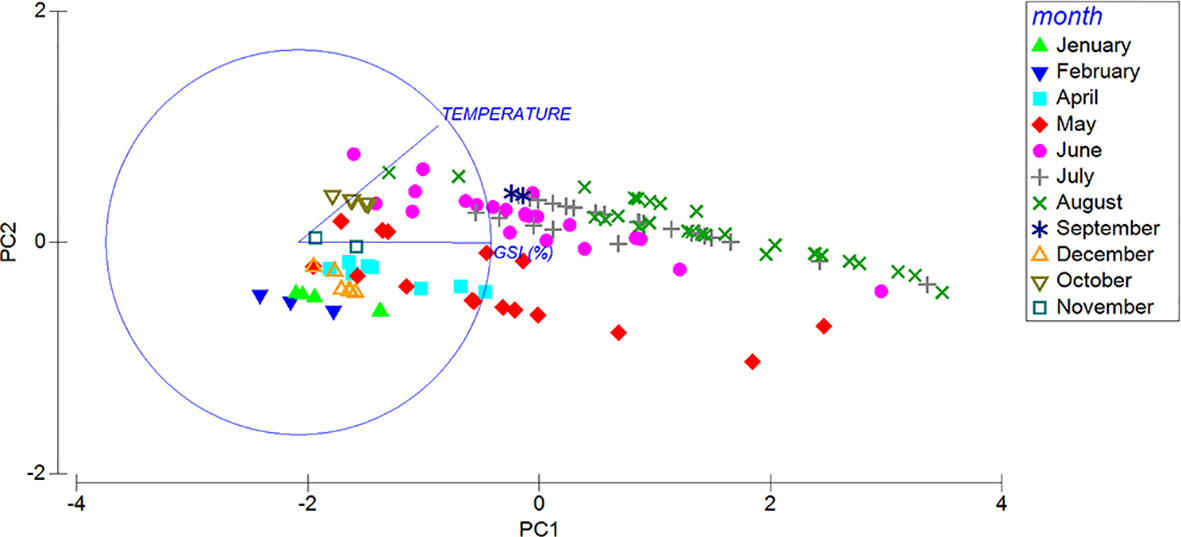
To evaluate differences between the GSI of the specimens and season, a univariate permutational analysis of variance (PERMANOVA) was applied using maturity stages, season and month as fixed factor. Prior to the statistical analysis, the GSI data were arcsin transformed (x’ = arcsin√x). The PERMANOVA was also performed to test differences in the gonad colors spaces L*, a*, b* (using sex and maturity stages as fixed and orthogonal factors) and in the oocytes’ diameters (using season as fixed factor). Principal-components analysis (PCA) was carried out using the PRIMER 7 software (Clarke and Gorley, 2015), to visualize the relationship between GSI and temperature with months considering the whole population and by sex, showing the importance of the contribution of each variable to the PC axes. All PERMANOVA tests were based on Euclidean distances of previously normalized data, using 999 random permutations of the appropriate units. When significant differences were observed, pairwise tests were also performed. P values in the PERMANOVA and pairwise tests were obtained from Monte Carlo asymptotic distributions (Anderson and Robinson, 2003) using the routines included in the PRIMER 7 software (Clarke and Gorley, 2006).
3 Results
3.1 Population structure
Among 214 sea cucumber collected, 170 specimens of H. tubulosa were dissected to define the sex. Males (n=47) and females (n=57) are equally distributed (SR= 0.82) showing no significant differences among them (χ2 = 0.4825; p =0.4876).
Considering the whole population, the WW of sea cucumbers ranges between 52.78 and 989.10 g (278.76g ± 160.85 s.d.), the GW ranges between 33.54 and 296.43 g (133.17g± 97.99 s.d.), the TW ranges between 2.5 and 9.7cm (5.3cm ± 1.2 s.d.), the TL ranges between 9 to 37 cm (20.5cm ± 5.7 s.d.). In detail, in females, the TL ranges from 14 to 37 cm (22.66cm ± 5.56 s.d.), while in males the TL ranges from 11 to 35.5 cm (22.38cm ± 6.03 s.d.) (Figure 2) with both females and males attaining substantially the same size. The results from Kolmogorov–Smirnov two-sample test do not indicate a statistically significant difference in length-frequency distribution among sexes (K–S test = 0.62; p = 0.832).
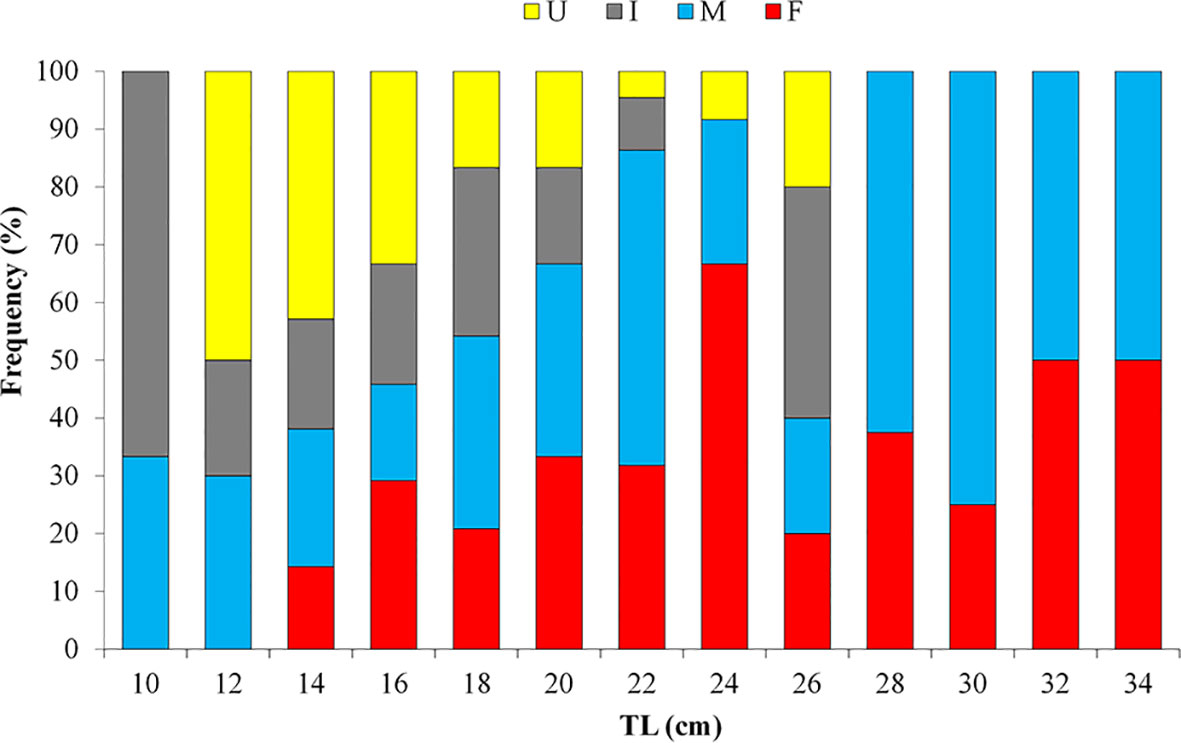
Figure 2 Length – frequency distribution in different size classes of female (F), male (M), undetermined sexes (I) and unsexed (U) specimens of Holothuria tubulosa.
The allometric equations (y = axb) between all taken parameters (TL, TW, WW and GW) and estimates are present in Figure 3. The relationships between weights and lengths (Figures 3A–D) show a b value always lower than 3, highlighting a negative allometric growth, with significant power curves. Among them, the best relationship is WW-TL with a slope of 1.725 and R2 of 0.739. The results of the Analysis of Variance with Lack-of-Fit of the four relationships (p>0.05) indicate that the models used to describe the relationships fit with the observed data. A negative allometric growth (b<1) is observed also between WW and GW (Figure 3E).
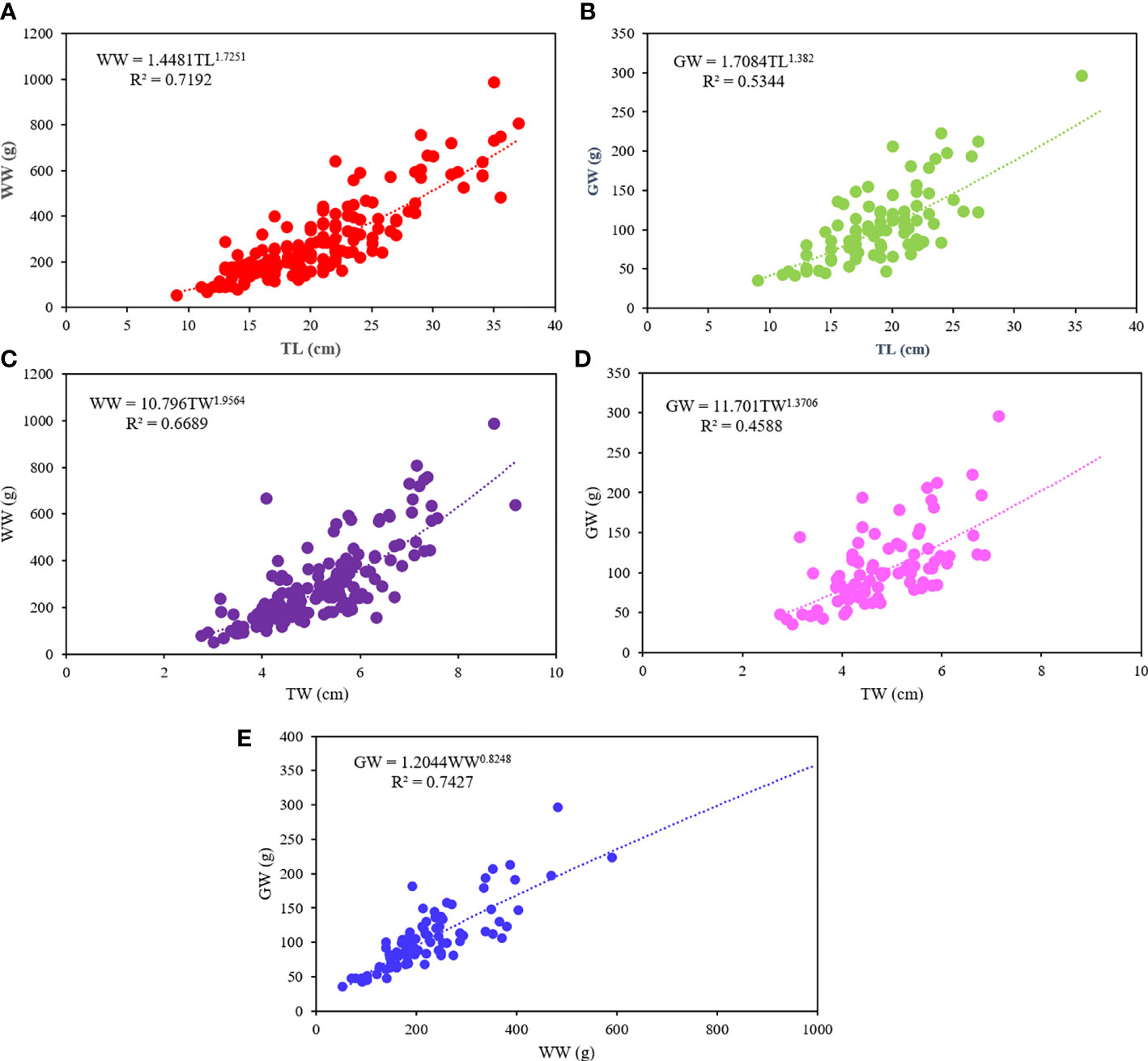
Figure 3 Allometric relationships for Holothuria tubulosa. (A) WW-TL relationship; (B) GW-TL relationship; (C) WW-TW relationship; (D) GW-TW relationship; (E) GW-WW relationship. TL, total length; WW, wet weight; GW, gutted weight; TW, total width.
3.2 Macroscopic maturity stages
The reproductive apparatus of H. tubulosa consists in a single gonad ramified in several tubules and a gonoduct that lead to the gonopore situated in the anterior part of the organism. All sea cucumbers collected in the present study had a visible and recognizable gonad, leading us to identify these sea cucumbers as adults. Juveniles (i.e. immature specimens) and recruits were not recorded in the present study. Based on the GSI, the number of gonad ramifications and their color, five macroscopic gonadal stages can be identified for H. tubulosa:
3.2.1 Recovery
The gonad is very small, and its weight (GoW) is generally<2 g. The color is pale and translucent (Table S1, ID 454; 461) and is not possible to distinguish between females and males. In this stage, the number of ramifications cannot be assigned macroscopically (Figures 4A, M). The GSI varies between 0 and 1 (Figure 5). The TL of sea cucumbers in this stage is 15.92cm ± 3.4 s.d.
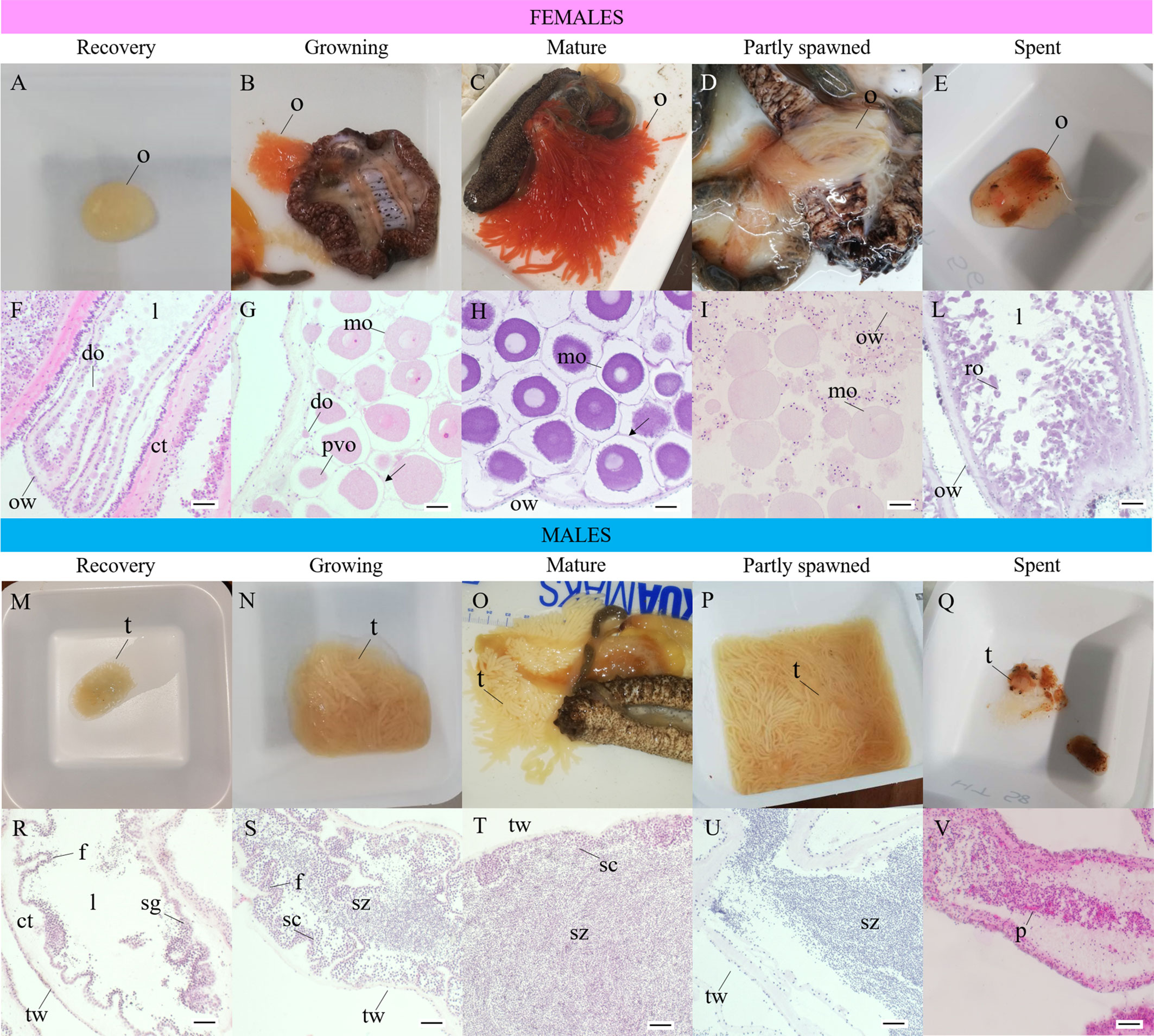
Figure 4 Macroscopic and histological maturity stages of Holothuria tubulosa females (A–L) and males (M–V). (A), ovary at recovery stage (GSI<2, observed in autumn and winter); (B), female in growing stage (TL: 21.3 ± 1.2 s.d.; GSI: 4.0 ± 1.4 s.d.; observed in spring and summer); (C), female in mature stage (TL: 22.8 ± 5.2 s.d.; GSI: 15.8 ± 8.2 s.d.; observed mainly in summer); (D), female in partly spawned stage (TL: 20.1 ± 1.2 s.d.; GSI: 2.29 ± 2.1 s.d.; observed in summer and autumn); (E), ovary in spent stage (GSI<1; observed in autumn and winter); (F), longitudinal section of ovary in recovery stage with small developing oocytes arranged in a single layer, thick ovarian wall (E–H); (G), ovary in growing stage with developing and mature oocytes arranged in multiple layers (E–H); (H) ovary in mature stage characterized by fully-grown mature oocytes densely packed; thin ovarian wall (AB/PAS); (I), ovary in partly spawned stage with mature oocytes less abundant and empty spaces in the lumen (E–H); (L) ovary in spent stage with thick ovarian wall and unspawned relict oocytes (E–H); (M), testis at recovery stage (GSI< 2, observed in winter); (N), testis in growing stage (TL 20.6 ± 5.3 s.d., GSI 2.3 ± 2.1 s.d., observed in spring and summer); (O), male in mature stage (TL 25.1 ± 5.5 s.d., month of sample GSI 8.5 ± 4.0 s.d. observed in spring and summer); (P), testis in partly spawned stage (TL 17.3 ± 3.1 s.d.; GSI 7.4 ± 4.9 s.d., observed in summer); (Q), testis in spent stage (GSI<1, observed in winter and autumn); (R), testis in recovery stage with spermatogonia on the germinal layer, thick testicular wall (E-H); (S), testis in growing stage with spermatocytes extending towards the lumen and spermatozoa start to fill the lumen (E–H); (T), testis in mature stage with densely packed spermatozoa in the lumen (E–H); (U), testis in partly spawned stage with spermatozoa less densely packed (E–H); (V), testis in spent stage with unspawned spermatozoa and phagocytes visible (E–H). ct, connective tissue; do, developing oocyte; l, lumen; mo, mature oocyte; o, ovary; ow, ovarian wall; p, phagocyte; ro, relicted oocyte; sc, spermatocyte; sg, spermatogonium; sz, spermatozoa; t, testis; tw, testicular wall. (H-E, Hematoxylin and eosin, AB/PAS, Alcian Blu/Periodic Acid Schiff.
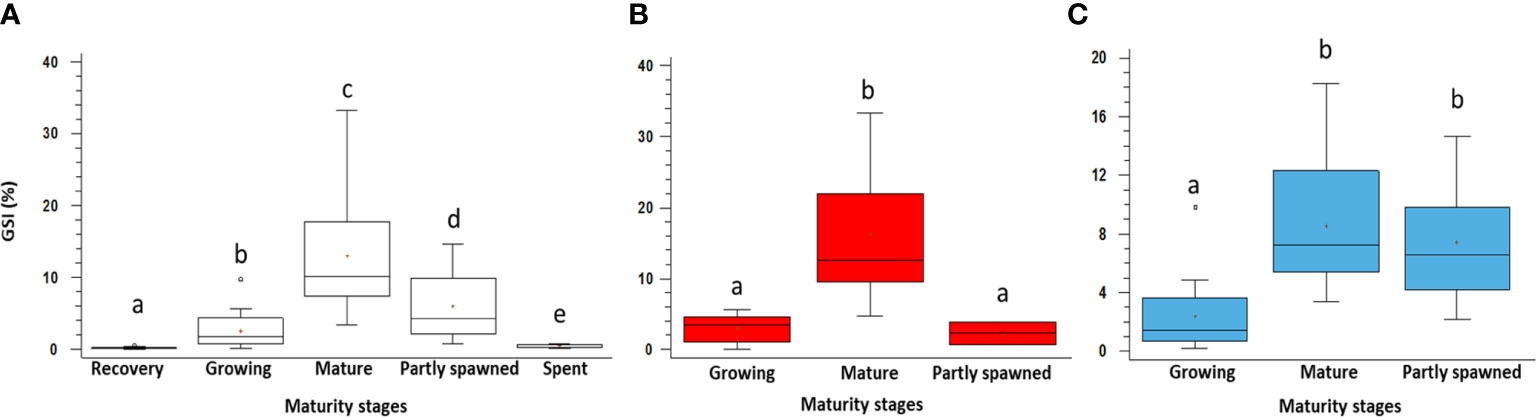
Figure 5 GSI variation among different maturity stages of the gonads in Holothuria tubulosa specimens. In (A) whole sample showing all the maturity stages; (B) females; (C) males. Lowercase letters indicate the results of the post-hoc tests. The boxes show the interquartile range, with the median value indicated by the horizontal line and the ‘+’ sign indicating the mean; whiskers show the range. Individual symbols show outliers.
3.2.2 Growing
The weight of the gonad is greatly variable, ranging between 2 and 25g (8.39g ± 9.35 s.d.), with female gonads (9.84g ± 8.90 s.d.) bigger than male ones (8.52g ± 10.02 s.d.). In both sexes, the gonad occupies up to one third of the coelomic cavity (Figures 4B, M). Males have a matt pale yellow gonad (Figure 4M; Table S1, ID 461; 454), whereas females have a translucent red–coral gonad (Figure 4B, Table S1, ID 151; 157). The number of ramifications varies between 2 and 3. The GSI increases and varies between 1 and 10, in detail in females 2.97 ± 1.86 s.d. and males 2.38 ± 2.16 s.d. (Figure 5). The TL of sea cucumbers in this stage is 20.63cm ± 5.949 s.d.
3.2.3 Mature
The gonad reaches the maximum weight (43.13g ± 28.03 s.d.), with female gonad (51.84g ± 33.40 s.d.) bigger than males one (31.27g ± 10.61 s.d.), up to occupy the whole coelomic cavity. Males have a matt pale yellow gonad (Figure 4O; Table S1, ID 157; 461), whereas females have a translucent red-coral and red gonad (Figure 4C; Table S1, ID 172, 485). The number of ramifications varies between 3 and 5 and the GSI is comprised between 3 and 35 (12.93 ± 7.66 s.d.) (Figure 5). In particular, in females, GSI shows values 2 times higher than males, 16.18 ± 8.12 s.d. and 8.52 ± 4.02 s.d. respectively. The mean TL of sea cucumbers in this stage is 23.80cm ± 5.48 s.d.
3.2.4 Partly spawned
The gonad is reduced in size, and its weight is 11.42g ± 8.35 s.d., with males’ gonad (13.74g ± 8.55 s.d.) bigger than females one (5.61g ± 5.59 s.d.). Males have a matt pale yellow gonad (Figure 4P; Table S1, ID 461), whereas female gonad loses its translucency and appears dull coral-red (Figure 4D; Table S1, ID 157). The gonads are emptied, the tubules are elongated and thin, and the number of ramifications varies between 3 and 4. The GSI is between 1 and 15 (Figure 5), in particular in females is 2.29 ± 2.16 s.d. and in males is 7.47 ± 4.92 s.d. The mean TL of sea cucumbers in this stage is 17.90cm ± 3.00 s.d.
3.2.5 Spent
The gonad is much reduced and the weight is <2g. It shows segments with different colors and is not possible to distinguish between females and males (Figures 4Q, E). Branches ramifications are not visible or recognizable. The GSI is between 0 and 1 (Figure 5). The mean TL of sea cucumbers in this stage is 19.16cm ± 4.65 s.d.
The statistical analyses reveal significant differences in the GSI among all the maturity stages described above (Table S2, S3; Figure 5). Females and males show a similar trend (Figures 5A, B) despite females have a higher GSI value in Mature (2 times) and males in Partly spawned (3 times), whereas the lowest value is observed in recovery and spent specimens when they could not be sexed with the macroscopic approach.
The color of the gonads differs significantly between Growing and Mature stages in both females and males. The pairwise comparison shows that in Mature females, when compared to the Growing ones, the L* (lightness) component decreases, the a* (redness) one increases, and the b* (yellowness) one does not change (Figures 6A–C). Males’ gonad color is much less variable and significant differences occur between Growing and Mature males only for the a* (redness) component (Figures 6D–F).
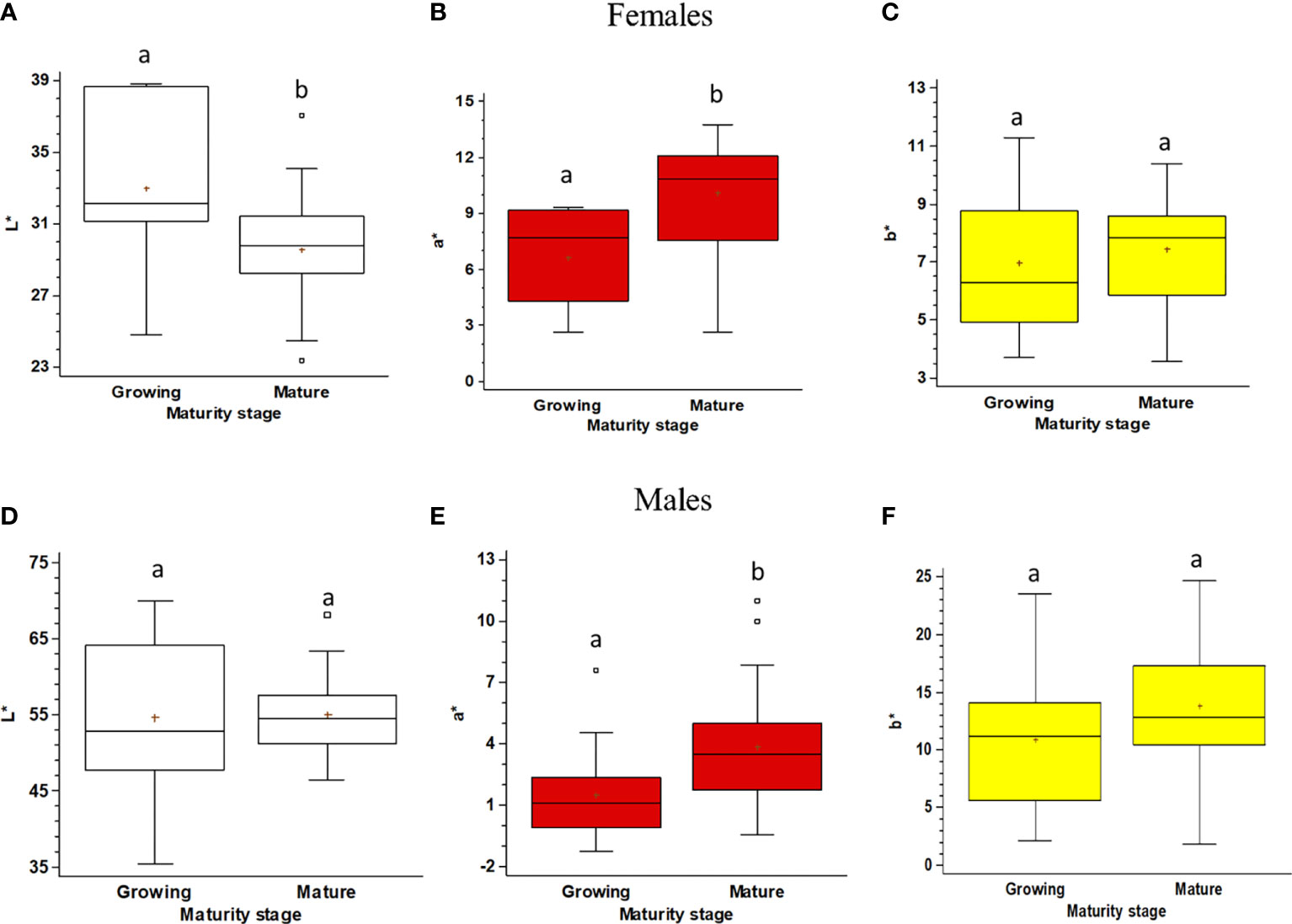
Figure 6 Boxplot of the color space (lightness, L*; redness, a*; yellowness, b*) of the gonads of Holothuria tubulosa, for females (A–C) and males (D–F) at Growing and Mature maturity stages. The boxes show the interquartile range, with the median value indicated by the horizontal line and the ‘+’ sign indicating the mean; whiskers show the range. Individual symbols show outliers. Lowercase letters indicate the results of the pairwise comparison of the L*a*b* value between Maturity stages.
3.3 Microscopic maturity stages
Based on the histology of the gonads of males and females (Figure 4) and the diameter distribution of oocytes (Figure 7A) in females, five female gonadal stages are recognized (Figures 4F–M, R–V) and described in Table 1. The oocyte size varies significantly among all the identified maturity stages (Tables S4, S5), except for Mature and Partly spawned, where oocytes show similar diameter (Figure 7A). Moreover, in Mature stage, the oocyte population shows a unimodal distribution with a peak at 110 µm, indicating that H. tubulosa females have a synchronized development of the ovaries (Figure 7B).
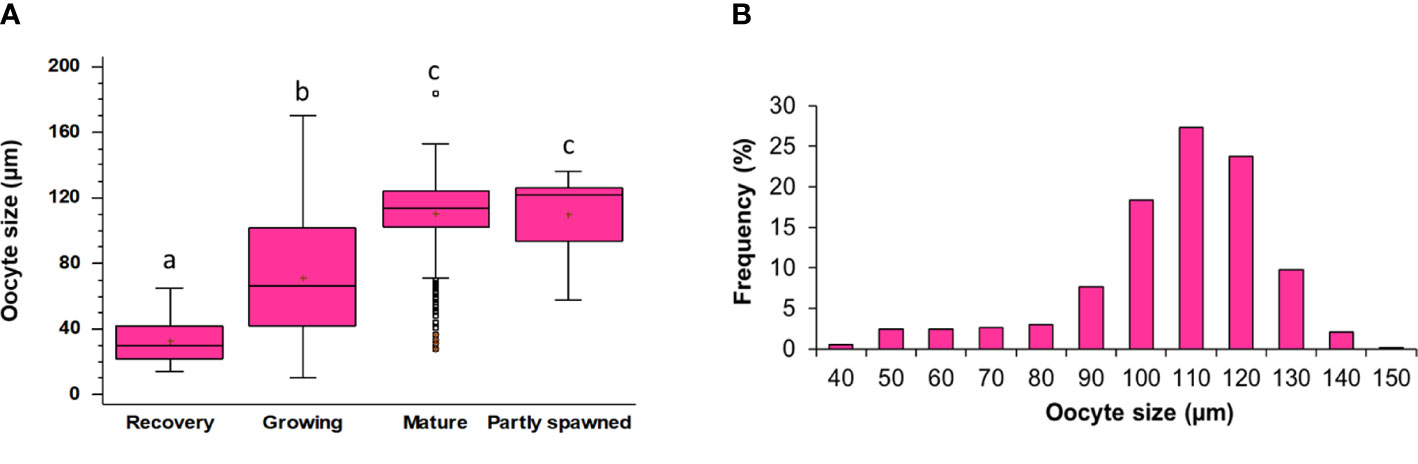
Figure 7 (A) Oocytes size in different maturity stages. Lowercase letters indicate the results of the pairwise comparison between Maturity stages. The boxes show the interquartile range, with the median value indicated by the horizontal line and the ‘+’ sign indicating the mean; whiskers show the range. Individual symbols show outliers. Lowercase letters indicate the results of the pairwise comparison of the maturity stages. (B) Detail of the size-frequency distribution of oocytes in Mature ovaries.
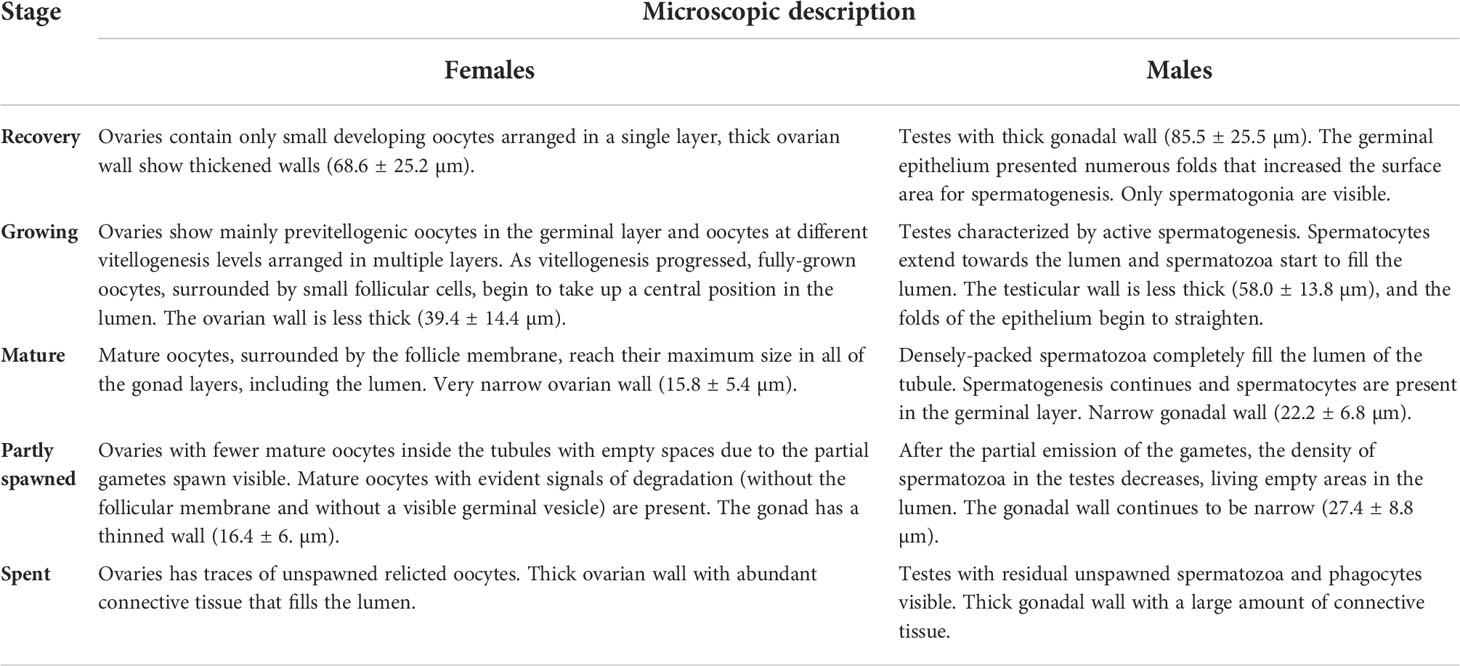
Table 1 Description of the microscopic gonadal stages in female and male of Holothuria tubulosa specimens.
The cross-inspection of the gonadal stages as determined using separately macroscopical and histological cues provides evidence of a validation of the two different approaches, summarized in Table S6.
3.4 Reproductive period and maturity
The GSI, in both sexes, varies significantly among seasons and months (Figures 8A, B; Table S3), increasing progressively from late autmn (December) to summer, when it reaches the highest value in August, then dropping in September. In general, in spring (April, May and June) and summer (July and August), the GSI in female specimens reaches higher values than that observed in males (Figures 8A, B). Based on the maturity scale of female and male gonads reported above, monthly variations in the frequency of the different gonadal stages are also investigated (Figure 8). As far as the females are concerned, in winter, in correspondence of the lowest surface temperature (14.52°C ± 0.75 s.d.) only the Recovery (December and February) and the Growing stages (January 50% each) are observed (Figure 8A). In spring (April), when the sea surface temperature begins to rise (17.99°C ± 1.90 s.d.), we observe a dominance of the Growing stage (ca. 66%) that progressively gives way to the Mature stage in May and June. During the summer season, the frequency distribution of mature females increases up to 93% when maximum sea surface temperature are recorded (25.45°C ± 1.29 s.d.). Moreover, in August the first Partly spawned females are observed (ca. 6%). Mature and Growing stages disappear in September, when only the Partly spawned stage is observed. No females are observed in October and November. A similar trend is observed in males, characterized by a peak of mature individuals in July and August (Figure 8B). Unassigned specimens (I) in the Spent stage appear only from October to February (Figure 8C). The relationship between temperature and GSI with months and sexes are clearly visualized, Figure 9, S2 respectively, showed by the PCA ordination.
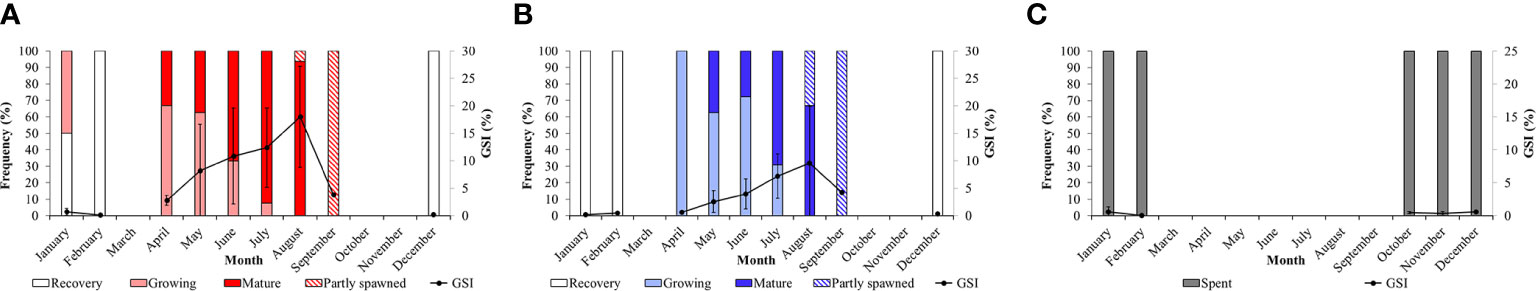
Figure 8 Monthly distribution of Holothuria tubulosa females (A), males (B) and unassigned specimens (C) at each gonadal stages during the sampling months. Gonado-Somatic Index (± s.d.) evolution along months is also reported.
Observing the female sea cucumbers length at different maturity stages, the results reveal that the range of matures is 15-37 cm (22.75 ± 5.15 cm TL), while the TL range of males is 14-35.5 cm TL (23.34 ± 7.15 cm TL). Since H. tubulosa does not show any sexual dimorphism, the interval of mature population is 14-37 cm TL (23.01 ± 5.15 cm TL).
The ovary is characterized by different oocyte diameters along the different gonadal stages (Figure 7A). Such variation is also reflected in their size when considering different seasons. Oocyte’s diameter varies significantly among seasons, with minimum values in winter, and much higher values in all other seasons. Values increase progressively in spring and summer, while in autumn, the oocyte diameter is greatly variable due to the presence of both female in Recovery and in Growing stages (Tables S5; Figure 10).
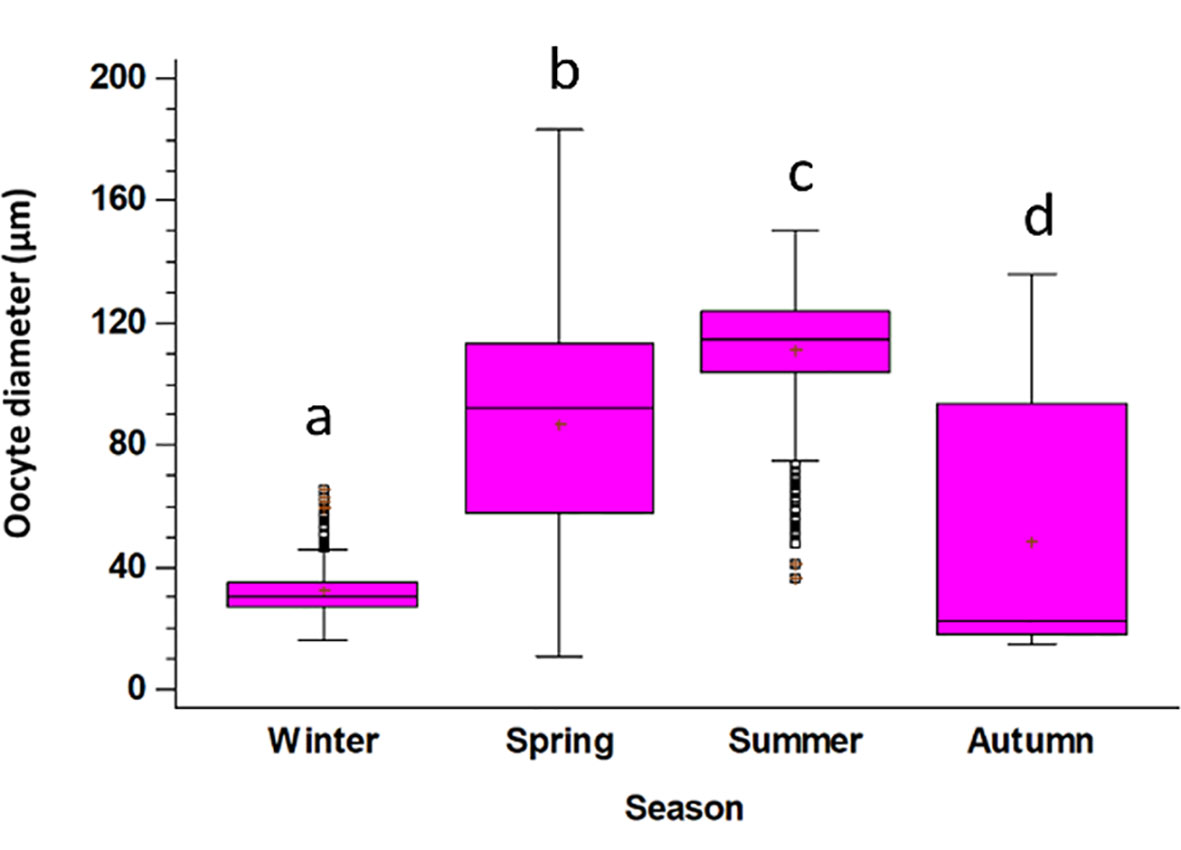
Figure 10 Oocyte diameters in the different seasons (Winter, January - March; Spring: April - June; Summer: July - September; Autumn: October - December). The boxes show the interquartile range, with the median value indicated by the horizontal line and the ‘+’ sign indicating the mean; whiskers show the range. Individual symbols show outliers. Lowercase letters indicate the results of the pairwise comparison between the oocytes size in different season.
4 Discussion
Several field and modelling studies have been conducted so far on the population structure of historically exploited sea cucumbers such as Apostichopus japonicus, Cucumaria frondosa, and Isostichopus fuscus (e.g., Herrero-Pérezrul et al., 1999; Reyes-Bonilla and Herrero-Pérezrul, 2003; Hamel and Mercier, 2008; Purcell et al., 2011; Glockner-Fagetti et al., 2016; Ramı́rez–González et al., 2020b). However, Gonzalez-Wangüemert et al. (2016) put the baseline for the study of population dynamics of the new target species including Holothuria polii, H. mammata, H. arguinensis and H. tubulosa. The analysis of sea cucumbers population structure and monitoring, based on common tagging methods, is typically biased by the high morphological plasticity of the body wall (Prescott et al., 2015), which makes poorly effective the use of tags (Kinch et al., 2008). At the same time, body size of holothurians can vary in response to environmental conditions, food availability and stress level of the organism (including the evisceration of the internal organs) (Bulteel et al., 1992; Wilkie, 2001; Zang et al., 2012; Tolon et al., 2017). Consequently, different authors considered the gutted weight and the gutted length to reduce the intraspecific variability (Kazanidis et al., 2010; González-Wangüemert et al., 2014; Prescott et al., 2015; Dereli et al., 2016; Marquet et al., 2017; Aydin, 2020). This approach can be used in fish-dependent data (González-Wangüemert et al., 2014). On the other hand, for restocking and sea ranching activities, where it is necessary to follow the growth of sea cucumbers, this option is not applicable. Another way to reduce the intraspecific variability, avoiding the death of the sea cucumbers, is to gently squeeze the specimens to remove the excess water from the respiratory tree (Sewell, 1990; Costa et al., 2014), the same approach used in the present study to record data of alive specimens. We reported here that, whatever the morphometrical relationship considered, sea cucumber H. tubulosa shows a negative allometric growth, indicating that it would preferably invest its resources in increasing the length rather than its wall thickness. This result is corroborated by previous studies on this species (Kazanidis et al., 2010; Aydin, 2020), and for other species belonging to the same genus (e.g. González-Wangüemert at al., 2014; Marquet et al., 2017; Azevedo et al., 2021). Moreover, our data indicate that among the relationships considered here, the Wet Weight (WW)-Total length (TL) relationship represents the most reliable and can be used as proxy for investigating the size structure of H. tubulosa and comparing different populations. The use of the WW and the TL parameters, which can be easily taken on alive specimens, has the advantage, not negligible, to avoid the sacrifice of sea cucumbers, representing a useful tool for their monitoring and management (e.g., restocking and/or sea ranching actions).
The sea cucumbers collected in the present study show bigger sizes (max 35 cm TL) and weights (max 989.10 g WW) than those collected in the Dardanelles Strait (max TL 30.3; Dereli et al., 2016), the south Aegean Sea (max 19.7 cm TL and 350g WW, Antoniadou and Vafidis, 2011) and Turkish coasts (max TL 23 cm, max GW 130 g; González-Wangüemert et al., 2014). Such differences could be related to the local different trophic settings of the environment occupied by sea cucumbers combined also with the effect of the local fishery, which can lead to lose the larger individuals in the exploited populations (Vafeiadou et al., 2010, González-Wangüemert et al., 2014).
The sex-ratio of H. tubulosa reveals that males and females are equally represented in the studied population as also reported by Kazanidis et al. (2014) and Despalatović et al. (2004) in the Adriatic Sea, and Dereli et al. (2016) in the Dardanelles Strait on the same species, and by Mezali et al. (2014) on H. sanctori in Algerian coasts. However, several studies on the reproductive biology of sea cucumbers such as H. mammata in the Atlantic Ocean (Santos et al., 2017; Venâncio et al., 2022), H. whitmaei in the Pacific Ocean (Shiell and Uthike, 2006), and H. leucospilota in the Western Indian Ocean (Gaudron et al., 2008) highlighted an imbalanced sex ratio with females outnumbered males. Our study was carried out in areas where the population is unexploited, suggesting that this ratio is a natural trait for these non-fissiparous sea cucumbers, while unbalanced sex-ratio described above could be due to evisceration events, association in same-sex groups, unsexed reproduction (i.e. fission), as well as fishing pressure as a possible causes (Shiell and Uthike, 2006; Muthiga et al., 2009; Santos et al., 2017).
Population structure is characterized mainly by adult individuals (TL > 9 cm), which reach the same range in sizes in both sexes, as also reported for the same species in the Aegean (Kazanidis et al., 2010) and Adriatic Sea (Despalotović et al., 2004). However, the failure to detect a separate mode for juveniles and immature holothurians, a common pattern observed also in other species (e.g. Venâncio et al., 2022), may be due to the sampling method used in this study, which is size-selective because the small specimens show cryptic behavior, or even could occupy different environment (Mercier et al., 2000; Hamel et al., 2001; Kazanidis et al., 2010; Aydin, 2019). On the other hand, a very fast growth during the first age of the species (Massin and Jangoux, 1976), a different habitat preference or, most likely, a cryptic or diel behaviour, as observed for other sea cucumber species (e.g., Sloan, 1979; Shiell, 2004; Purcell, 2010; Soliman et al., 2019; Félix et al., 2021) could also explain the few (or absent) juveniles and recruits in Sardinian populations.
Regarding the macroscopic observation of the gonads, it allowed us to identify five different gonadal stages, similarly as observed by Ramofafia et al. (2000, 2003) for the tropical H. fuscogilva and H. scabra. In detail, the RGB color palette enabled us to distinguish clearly the sex of the developed individuals, but it was almost ineffective in the determination of the sex of undeveloped specimens (Recovery and Spent maturity stages) with gonads characterized by a pale-white color or a heterogeneous color with rust blotches (Marquet et al., 2017). In addition, the discrimination between the Growing and Mature stages within the sexes is not always clear because of the numerous nuances of the gonads color and the observer’s sensitivity, which can lead to different color assignments. Thus, the innovative colorimetric method using the CIELAB color space, here used for the first time in holothurians, allowed us to overpass such subjective assignment of the gonad color avoiding misinterpretations of a certain reproductive stage, analysing the different coordinates, lightness, redness and yellowness.
A good correspondence between the maturity stages based on tubule appearance (e.g. color, dimension, ramification degree), and those based on histology is observed. Thus, the tubule morphology in alive specimens, which is possible to observe after a dorsal incision in the body wall near the gonopore, can be used to detect the release of gametes in the breeding programs when the sea cucumbers cannot be sacrificed, as also reported for H. fuscogilva (Ramofafia et al., 2000). However, this is an invasive practice which can cause pathogen infection and stress in the sea cucumbers (Becker et al., 2004).
H. tubulosa shows a synchronic gonad development at level of tubules and oocyte size distribution in females, revealed by the histological observation of gonads. This reproductive strategy is widespread among holothurians such as Holothuria atra, H. mexicana, H. nobilis, Actinopyga echinites, Stichopus variegatus and Thelenota ananas (Sewell et al., 1997). The results indicate that H. tubulosa does not fit the tubule recruitment model which involves several stages of gonad development in the same gonad, observed in other holothurians as Isostichopus badionotus (Foglietta et al., 2004), H. scabra (Ramofafía et al., 2003) and H. floridiana (Ramos-Miranda et al., 2017).
Both macroscopic and histological approaches have proven to be reliable in identifying the reproductive period of H. tubulosa in the study sites. Monthly changes in GSI and the frequency distribution of the maturity stages clearly depict the restricted reproductive season, which is concentrated, in the Sardinian population, at the end of the summer season. Our result is in good agreement with those reported earlier for the same species by Despalatović et al. (2004) in the Adriatic Sea, Kazanidis et al. (2014) in the Aegean Sea, Dereli et al. (2016) in the Dardanelles Strait, and Tahri et al. (2019) in Oran coast (Algeria).
The clear association between the peak of H. tubulosa spawning and the maximum values of sea surface temperature recorded in the sampled sites is a common pattern of sea cucumber species (e.g. Bulteel et al., 1992; Despalatović et al., 2004; Kazanidis et al., 2010; Antoniadou and Vafidis, 2011; Acosta et al., 2021; Venâncio et al., 2022). Moreover, the seasonal variation of temperature is accompanied by the increase of the day-length and the availability of phytoplankton in the water-column (Venâncio et al., 2022). The increasing of temperature can also modify the metabolism of sea cucumbers (Kühnhold et al., 2019) that use their energy to develop the gonads as observed in other holothurian species such as A. japonicas (Yang et al., 2006) and Australostichopus mollis (Slater et al., 2011). Furthermore, the seasonality may have an indirect effect on reproduction by increasing food availability for broodstock through increased benthic productivity (Muthiga, 2006), and fostering the phytoplankton abundance for larval development (Boidron-Metairon, 1995; Cameron and Frankboner, 1989; Rakaj et al., 2018). Another environmental factor that can influence the gametogenesis and spawning in holothurians is the rainfall (e.g. Asha and Muthiah, 2008; Benítez-Villalobos et al., 2013; Leite-Castro et al., 2016; Acosta et al., 2021). Indeed, during the rainy season, there is a significant increase in phytoplankton, related to the contribution of nutrients from runoff and rivers (Acosta et al., 2021). However, in our investigated areas, it does not seem to be a determinant factor, because the pluvial precipitations do not follow a clear seasonal pattern as observed in the tropical regions to determine and characterize the spawning cycle of this species.
Among the life history traits of new target species, the size at maturity represents one of the most common and useful information for the estimation of the minimum fishing size (Navarro et al., 2012) ensuring spawning of mature sea cucumbers and protecting their natural stocks (Abdel-Razek et al., 2005). The absence of immature sea cucumbers did not allow us to define the size at maturity, but here we report that the smallest mature female is observed at 15 cm TL and the smallest mature male measured 14 cm TL. Few information about the size at maturity of the Holothuria genus are available in literature. Indeed, the only information about the maturity of H. tubulosa comes from the Adriatic Sea (Kazanidis et al., 2014), where it was estimated only in terms of the drained weight of the individuals (approximately 220 g). Beside this, our range of maturity is in agreement with that reported for the congeneric species H. mammata showing the size at first maturity in males TL=14.2 cm and females TL=16.7 cm (Venâncio et al., 2022), with which H. tubulosa shares a similar geographical distribution (Tortonese, 1965; Borrero-Pérez et al., 2009; González-Wangüemert et al., 2014; González-Wangüemert et al., 2018; Dereli and Aydin, 2021). Hence, our data should be considered as a preliminary evaluation of the adult size-structure, requiring the investigation of lower size classes, not included in this study, to have an exhaustive description of the whole population.
Conclusion
The results of this study provided evidence that the sea cucumber H. tubulosa has a synchronous development of the gonads, with an apparently unique reproductive event during the year, corresponding to the end of the summer season. Our results provides a first insight on size-range of matures of this valuable species, giving useful information for the management policy.
This study also shows that either the macroscopic or microscopic approach used to identify the different gonadal stages provide overlapping information. Nevertheless, the macroscopic proxy is faster than, and similarly reliable to, the microscopic one. Additionally, the macroscopic approach can be used in the field following the maturity scale here provided, allowing even not-expert observers such as fishermen to expeditiously determine sex and maturity stage of natural populations of sea cucumbers.
Given our results, this study is essential to increase the biological and ecological knowledge of the populations of H. tubulosa, create conditions for the domestication of broodstock in captivity and precise measures for the conservation of biodiversity.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VP and PA conceived and planned the experiments. VP and AG carried out the sampling. VP, CP and MM laboratory analyses. VP, CP, AG carried out the statistical analyses. All authors contributed to the interpretation of the results. VP and CP took the lead in writing the manuscript followed by extensive revision by PA and MF to achieve the final version. All authors provided critical feedback and helped shape the research, analysis and manuscript. VP and CP contributed equally to the study. All authors contributed to the article and approved the submitted version.
Funding
This study has been carried out in the framework of the projects “Innovative species of commercial interest for Sardinian aquaculture: development of experimental protocols for the breeding of sea cucumbers, (project n.1/INA/2.47/2017; cUP:F26C19000000006)” funded by the EuropeanMaritime and Fisheries Fund (EMFF) Programme 2014/2020, Measure:2.47 – Innovation; and co-founded by the “lnEVal: lncreasing Echinoderm Value Chains” (grant n. ID 101 InEVal; CUP: F24I20000160006) funded by ERA-NET BlueBio programme.
Acknowledgments
The authors wish to express their gratitude to the Marine Protected Area Penisola del Sinis – Isola di Mal di Ventre in the people of the director Ing. Massimo Marras and Dr. Roberto Brundu; Dr. Gaspare Barbera technical manager of the L.P.A. Group fish farm and the Marina di Teulada in the person of Mrs. Barbara Lai, for the logistic support in the field sampling. We are thankful to Dr. Marco Secci and Mr. Marco Maxia (Agris Sardinia Agency) for their help in the field and sampling activities. The collection of sea cucumbers was carried out under the authorization released by Regione Autonoma della Sardegna (Prot. N. 1845, 06/02/2019; Prot. N. 20735, 28/11/2019; Prot. N. 810, 13/01/2021; Prot. N. 0023738, 03/02/2022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.1029147/full#supplementary-material
References
Abdel-Razek F. A., Abdel-Rahmen S. H., El-Shumy N. A., Omar H. A. (2005). Reproductive biology of the tropical sea cucumber Holothuria atra (Echinodermata: Holothuroidea) in the red Sea coast of Egypt. Egypt. J. Aquat. Res. 31, 383–402.
Acosta E. J., Rodríguez-Forero A., Werding B., Kunzmann A. (2021). Ecological and reproductive characteristics of holothuroids Isostichopus badionotus and Isostichopus sp. in Colombia. PloS One 16 (2), e0247158. doi: 10.1371/journal.pone.0247158
Addis P., Moccia D., Secci M. (2014). Effect of two different habitats on spine and gonad colour in the purple sea urchin Paracentrotus lividus. Mar. Ecol. 36 (2), 178–184. doi: 10.1111/maec.12133
Agudo N. (2006). “Sandfish hatchery techniques,” in Australian Centre for international agricultural research (Noumea, New Caledonia: Secretariat of the Pacific Community and WorldFish Center), 45.
Amaro T., Bianchelli S., Billett D. S. M., Cunha M. R., Pusceddu A., Danovaro R. (2010). The trophic biology of the holothurian Molpadia musculus: Implications for organic matter cycling and ecosystem functioning in a deep submarine canyon. Biogeosciences 7, 2419–2432. doi: 10.5194/bg-7-2419-2010
Anderson S. C., Flemming J. M., Watson R., Lotze H. K. (2011). Serial exploitation of global sea cucumber fisheries. Fish. Fish. 12, 317–339. doi: 10.1111/1467-842X.00285
Anderson M. J., Robinson J. (2003). Generalised discriminant analysis based on distances. Aust. N. Z. J. Stat. 45, 301–318. doi: 10.1111/1467-842X.00285
Antoniadou C., Vafidis D. (2011). Population structure of the traditionally exploited holothurian Holothuria tubulosa in the south Aegean Sea. Cah. Biol. Mar. 52, 171–175.
Asha P. S., Muthiah P. (2008). Reproductive biology of the commercial sea cucumber Holothuria spinifera (Echinodermata: Holothuroidea) from tuticorin, Tamil nadu, India. Aquacult. Int. 16, 231–242. doi: 10.1007/s10499-007-9140-z
Aydin M. (2020). Length – weight relationships and condition factor of four different sea cucumber species in the Aegean Sea. J. Anatol. Environ. Anim. Sci. 1, 80–85. doi: 10.35229/jaes.677940
Aydin M. (2019). Density and biomass of commercial sea Cucumber species relative to Depth in the Northern Aegean Sea. Thalassas 35, 541–550. doi: 10.1007/s41208-019-00144-4
Azevedo e Silva F., Brito A. C., Simões T., Pombo A., Marques T. A., Rocha C., et al. (2021). Allometric relationships to assess ontogenetic adaptative changes in three NE Atlantic commercial sea cucumbers (Echinodermata, Holothuroidea). Aquat Ecol. 55, 711–720. doi: 10.1007/s10452-021-09856-3
Becker P., Gillan D., Lanterbecq D., Jangoux M., Rasolofonirina R., Rakotovao J., et al. (2004). The skin ulceration disease in cultivated juveniles of Holothuria scabra (Holothuroidea, Echinodermata). Aquaculture 242 (1 – 4), 13–30. doi: 10.1016/j.aquaculture.2003.11.018
Benítez-Villalobos F., Avila-Poveda O. H., Gutiérrez-Méndez I. S. (2013). Reproductive biology of Holothuria fuscocinerea (Echinodermata: Holothuroidea) from Oaxaca, Mexico. Sex Early Dev. Aquat. Org. 1, 13–24. doi: 10.3354/sedao00003
Boidron-Métairon I. F. (1995). “Larval nutrition,” in Ecology of marine invertebrate larvae. Ed. McEdward L. (Boca Raton, FL, USA: CRC Press), 223–248.
Boncagni P., Rakaj A., Fianchini A., Vizzini S. (2019). Preferential assimilation of seagrass detritus by two coexisting Mediterranean sea cucumbers: Holothuria polii and holothuria tubulosa. Estuar. Coast. 231, 106464. doi: 10.1016/j.ecss.2019.106464
Bordbar S., Anwar F., Saari N. (2011). High – value components and bioactives from sea cucumbers for functional food – a review. Mar. Drugs 9 (10), 1761–1805. doi: 10.3390/md9101761
Borrero-Pérez G., Pérez-Ruzafa A., Marcos C., González-Wangüemert M. (2009). The taxonomic status of some Atlanto-Mediterranean species in the subgenus Holothuria (Echinodermata: Holothuroidea: Holothuriidae) based on molecular evidence. Zool. J. Lin. Soc 157, 51–69. doi: 10.1111/j.1096-3642.2009.00529.x
Bulteel P., Jangoux M., Coulon P. (1992). Biometry, bathymetric distribution, and reproductive cycle of the holothuroid Holothuria tubulosa (Echinodermata) from Mediterranean Sea grass beds. Mar. Ecol. 13, 53–62. doi: 10.1111/j.1439-0485.1992.tb00339.x
Cameron J. L., Fankboner P. V. (1989). Reproductive biology of the commercial sea cucumber Parastichopus californicus (Stimpson) (Echinodermata: Holothuroidea). II. Observations on the ecology of development, recruitment, and the juvenile life stage. J. Exp. Mar. Biol. Ecol. 127, 43–67. doi: 10.1016/0022-0981(89)90208-6
Cerri P. S., Sasso-Cerri E. (2003). Staining methods applied to glycol methacrylate embedded tissue sections. Micron 34, 365–372. doi: 10.1016/S0968-4328(03)00098-2
Choo P. S. (2008). “Population status, fisheries and trade of sea cucumbers in Asia,” In: Sea Cucumbers: a Global Review on Fisheries and Trade. FAO Fisheries and Aquaculture Technical Paper.No.516. Eds. Toral – Granda V., Lovatelli A., Vasconcellos M. (Rome: FAO), 81–118. Available at: http://www.fao.org/docrep/011/i0375e/i0375e00.htm.
CIE, Commission Internationale de l’Eclairage (2008). Colorimetry – part 4: CIE 1976 l*a*b* color spaces (Vienna: Publication CIE), 12.
Conand C. (1981). Sexual cycle of three commercially important holothurian species (Echinodermata) from the lagoon of new Caledonia. Bull. Mar. Sci. 31 (3), 523–543.
Conand C. (2006). Sea Cucumber Biology, Taxonomy, Distribution and Conservation Status. In: Proceedings of the CITES workshop on the conservation of sea cucumbers in the families Holothuriidae and Stichopodidae. Bruckner A.W. (editor). NOAA Technical Memorandum NMFS- OPR 34 , Silver Spring, MD, 244 pp.
Conand C., Polidoro B., Mercier A., Gamboa R., Hamel J. F., Purcell S. (2014). The IUCN red list assessment of aspidochirotid sea cucumbers and its implications. SPC Beche-de-mer Inf. Bull. 34, 3–7.
Costa V., Mazzola A., Vizzini S. (2014). Holothuria tubulosa Gmelin 1791 (Holothuroidea, Echinodermata) enhances organic matter recycling in Posidonia oceanica meadows. J. Exp. Mar. Bio. Ecol. 461, 226–232. doi: 10.1016/j.jembe.2014.08.008
Dereli H., Aydin M. (2021). Sea Cucumber fishery in Turkey: Management regulations and their efficiency. Reg. Stud. Mar. Sci. 41, 101551. doi: 10.1016/j.rsma.2020.101551
Dereli H., Culha S. T., Culha M., Ozalp B. H., Tekinay A. A. (2016). Reproduction and population structure of the sea cucumber Holothuria tubulosa in the dardanelles strait, Turkey. Med. Mar. Sci. 17, 47–55. doi: 10.12681/mms.1360
Despalatović M., Grubelić I., Šimunović A., Antolić B., Žuljević A. (2004). Reproductive biology of the holothurian Holothuria tubulosa (Echinodermata) in the Adriatic Sea. J. Mar. Biol. Assoc. UK. 84, 409–414. doi: 10.1017/S0025315404009361h
Domínguez-Godino J., Gonzàlez-Wangüemert M. (2018). Breeding and larval development of Holothuria mammata, a new target species for aquaculture. Aqua. Res. 49, 1430–1440. doi: 10.1111/are.13597
Félix P. M., Pombo A., Azevedo Silva F., Simões T., Marques T. A., Melo R., et al (2021). Modelling the Distribution of a Commercial NE-Atlantic Sea Cucumber, Holothuria mammata: Demographic and Abundance Spatio-Temporal Patterns. Front. Mar. Sci. 8, 675330. doi: 10.3389/fmars.2021.675330
Foglietta L. M., Camejo M. I., Gallardo L., Herrera F. C. (2004). A maturity index for holothurians exhibiting asynchronous development ofgonad tubules. J. Exp. Mar. Biol. Ecol. 303, 19–30. doi: 10.1016/j.jembe.2003.10.019
Gaudron S. M., Kohler S. A., Conand C. (2008). Reproduction of the sea cucumber Holothuria leucospilota in the Western Indian Ocean: biological and ecological aspects. Invertebr. Reprod. Dev. 51, 19–31.
Glockner-Fagetti A., Calderon – Aguilera L. E., Herrero – Pérezrul M. D. (2016). Density decrease in an exploited population of brown sea cucumber Isostichopus fuscus in a biosphere reserve from the Baja California peninsula, Mexico. Ocean Coast. Manage. 121, 49–59. doi: 10.1016/j.ocecoaman.2015.12.009
González-Wangüemert M., Aydin M., Conand C. (2014). Assessment of sea cucumber populations from the Aegean Sea (Turkey): first insights to sustainable management of new fisheries. Ocean Coast. Manage. 92, 87–94. doi: 10.1016/j.ocecoaman.2014.02.014
González-Wangüemert M., Domínguez – Godino J. A., Cánovas F. (2018). The fast development of sea cucumber fisheries in the Mediterranean and NE Atlantic waters: from a new marine resource to its over – exploitation. Ocean Coast. Manage. 151, 165–177. doi: 10.1016/J.OCECOAMAN.2017.10.002
González – Wangüemert M., Valente S., Aydin M. (2015). Effects of fishery protection on biometry and genetic structure of two target sea cucumber species from the Mediterranean Sea. Hydrobiologia 743, 65–74. doi: 10.1007/s10750-014-2006-2
González-Wangüemert M., Valente S., Henriques F., Domínguez – Godino J. A., Serrão E. A. (2016). Setting preliminary biometric baselines for new target sea cucumbers species of the NE Atlantic and Mediterranean fisherie. Fish. Res. 17957, 66. doi: 10.1016/j.fishres.2016.02.008
Hamel J.-F., Conand C., Pawson D. L., Mercier A. (2001). Biology of the sea cucumber Holothuria scabra (Holothuroidea: Echinodermata) and its exploitation as beche-de-mer. Adv. Mar. Biol. 41, 129–223.
Hamel J., Eeckhaut I., Conand C., Sun J., Caulier G., Mercier A. (2022). Global knowledge on the commercial sea cucumber Holothuria scabra. Adv. Mar. Biol. 91, 1–286. doi: 10.1016/bs.amb.2022.04.001
Hamel J.–F., Mercier A. (2004). “Synchronous gamete maturation and reliable spawning induction method in holothurians,” in Advances in Sea cucumber aquaculture and management. Eds. Lovatelli A., Conand C., Purcell S., Uthicke S., Hamel J.–F., Mercier A. (Rome: FAO), 359–371. Fisheries Technical Paper. No. 463.
Hamel J., Mercier A. (2008). “Population status, fisheries and trade of sea cucumbers in temperate areas of the northern hemisphere,” in Sea Cucumbers. a global review of fisheries and trade. Eds. Toral – Granda V., Lovatelli A., Vasconcellos M. (Rome: FAO), 257–291. FAO Fisheries and Aquaculture Technical Paper. No. 516.
Herrero-Pérezrul D., Reyes-Bonilla H., Garcia-Dominguez F., Cintra-Buenrostro C. E. (1999). Reproduction and growth of Isostichopus fuscus in the southern gulf of California, Mexico. Mar. Biol. 135, 521–532. doi: 10.1007/s002270050653
Horton T., Kroh A., Ahyong S., Bailly N., Boyko C. B., Brandão S. N., et al. (2018). World register of marine species (WoRMS). Available at: https://www.marinespecies.org/imis.php?module=ref&refid=291592.
Janakiram N. B., Mohammed A., Rao C. V. (2015). Sea Cucumbers metabolites as potent anti – cancer agents. Mar. Drugs 13 (5), 2909–2923. doi: 10.3390/md13052909
Kazanidis G., Lolas A., Vafidis D. (2014). Reproductive cycle of the traditionally exploited sea cucumber Holothuria tubulosa (Holothuroidea: Aspidochirotida) in Pagasitikos Gulf, western Aegean Sea. Greece. Turk. J. Zool. 38, 306–315. doi: 10.3906/zoo-1302-31
Kazanidis G., Antoniadou C., Lolas A. P., Neofitou N., Vafidis D., Chintiroglou C., et al. (2010). Population dynamics and reproduction of holothuria tubulosa (Holothuroidea: Echinodermata) in the Aegean Sea. J. Mar. Biol. Assoc. UK 90 (5), 895–901. doi: 10.1017/S0025315410000251
Keys A. B. (1928). The weight-length relationship in fishes. in Proceedings of the National Academy of Science, Vol. XIV. 12, 922–925(Washington, DC).
Kinch J., Purcell S., Uthicke S., Friederich K. (2008). “Population status, fisheries and trade of sea cucumbers in the Western central pacific,” in Sea Cucumbers: A global review of fisheries and trade. Eds. Toral – Granda M. V., Lovatelli A., Vasconcellos M. (Rome: FAO), 7–55. FAO Fisheries and Aquaculture Technical Paper 516.
Kühnhold H., Novais S. C., Alves L. M. F., Kamyab E., Lemos M. F. L., Slater M. J., et al. (2019). Acclimation capability inferred by metabolic performance in two sea cucumber species from different latitudes. J. Therm. Biol. 84, 407–413. doi: 10.1016/j.jtherbio.2019.07.019
Lee S., Ford A. K., Mangubhai S., Wild C., Ferse S. C. A. (2018). Length – weight relationship, movement rates, and in situ spawning observations of Holothuria scabra (sandfish) in Fiji. SPC Beche – de – mer Inf. Bull. 38, 11–14.
Leite-Castro L. V., Souza J., Salmito-Vanderley C. S., Nunes J. F., Hamel J. F., Mercier A. (2016). Reproductive biology of the sea cucumber Holothuria grisea in Brazil: importance of social and environmental factors in breeding coordination. Mar. Biol. 163, 67. doi: 10.1007/s00227-016-2842-x
Lovatelli A., Conand C., Purcell S., Uthicke S., Hamel J., Mercier A. (eds). (2004). Advances in sea cucumber aquaculture and management.
Mangion P., Taddei D., Conand C., Frouin P. (2004). “Feeding rate and impact of sediment reworking by two deposit feeders Holothuria leucospilota and Holothuria atra on fringing reef (Reunion island, Indian ocean),” in Echinoderms: München. Eds. Heinzeller T., Nebelsick J. H. (London: Taylor and Francis), 311–317.
Marquet M., Conand C., Power D. M., Canàrio A. V. M., González – Wangüemert M. (2017). Sea Cucumbers, Holothuria arguinensis and H. mammata, from the southern Iberian peninsula: Variation in reproductive activity between populations from different habitats. Fish. Res. 191, 120–130. doi: 10.1016/S0065-2881(09)55001-8
Massin C., Jangoux M. (1976). Observations ecologiques sur Holothuria tubulosa, H. poli et H. forskali (Echinodermata- Holothuroidea) et comportement alimentaire de H. tubulosa. Cah. Biol. Mar. 17, 45–59.
Mercier A., Hamel J.-F. (2009). Endogenous and exogenous control of gametogenesis and spawning in echinoderms. Adv. Mar. Biol. 55, 1–302.
Mercier A., Battaglene S. C., Hamel J. (2000). Settlement preferences and early migration of the tropical sea cucumber Holothuria scabra. J. Exp. Mar. Biol. Ecol. 249, 89–110. doi: 10.1016/S0022-0981(00)00187-8
Mezali K., Soualili D. L. (2013). The ability of holothurians to select sediment particles and organic matter. SPC Beche-de-mer Inf. Bull. 33, 38–43.
Mezali K., Zupo V., Francour P. (2006). Population dynamics of Holothuria (holothuria) tubulosa and Holothuria (lessonothuria) polii of an Algerian Posidonia oceanica meadow. Biol. Mar. Medit. 13 (4), 158–161.
Mezali K., Soualili D. L., Neghli L., Conand C. (2014). Reproductive cycle of the sea cucumber Holothuria (Platyperona) sanctori (Holothuroidea: Echinodermata) in the southwestern Mediterranean Sea: interpopulation variability. Invertebr. Reprod. Dev. 58, 179–189. doi: 10.1080/07924259.2014.883337
Mohsen M., Yang H. (2021). “Sea Cucumbers research in the Mediterranean and the red seas,” in Sea Cucumbers aquaculture, biology and ecology. Eds. Mohsen M., Yang H. (Academic Press), 61–101. doi: 10.1016/B978-0-12-824377-0.00002-5
Morgan A. D. (2000). Induction of spawning in the sea cucumber Holothuria scabra (Echinodermata: Holothuroidea). J. World Aqua. Soci. 31, 186–194. doi: 10.1111/j.1749-7345.2000.tb00352.x
Muthiga N. A. (2006). The reproductive biology of a new species of sea cucumber, Holothuria (Mertensiothuria) arenacava in a Kenyan marine protected area: The possible role of light and temperature on gametogenesis and spawning. Mar. Biol. 149, 585–593. doi: 10.1007/s00227-005-0224-x
Muthiga N., Kawaka J., Ndirangu S. (2009). The timing and reproductive output of the commercial sea cucumber Holothuria scabra on the Kenyan coast. Estuar. Coast. Shelf Sci. 84, 353–360. doi: 10.1016/j.ecss.2009.04.011
Navarro P. G., García-Sanz S., Tuya F. (2012). Reproductive biology of the sea cucumber Holothuria sanctori (Echinodermata: Holothuroidea). Sci. Mar. 76, 741–752. doi: 10.3989/scimar.03543.15
Neofitou N., Lolas A., Ballios I., Skordas K., Tziantziou L., Vafidis D. (2019). Contribution of sea cucumber Holothuria tubulosa on organic load reduction from fish farming operation. Aquaculture 501, 97–103. doi: 10.1016/j.aquaculture.2018.10.071
Ocaña A., Sanchez Tocino L. (2005). Spawning of Holothuria tubulosa (Holothurioidea, Echinodermata) in the Alboran Sea (Mediterranean Sea). Zool. Baetica 16, 147–150.
Pasquini V., Giglioli A. A., Pusceddu A., Addisp P.. (2021). Biology, ecology and management perspectives of overexploited deposit-feeders sea cucumbers, with focus on Holothuria tubulosa (Gmelin, 1788). Adv. Oceanogr. Limnol. 12, 9995. doi: 10.4081/aiol.2021.9995
Prato E., Fanelli G., Biandolino F., Chiantore M., Secci M., Angioni A., et al. (2018). Influence of a prepared diet and a macroalga (Ulva sp.) on the growth, nutritional and sensory qualities of gonads of the sea urchin Paracentrotus lividus. Aquaculture 493, 240–250. doi: 10.1016/j.aquaculture.2018.05.010
Prescott J., Zhou S., Prasetyo A. P. (2015). Soft bodies make estimation hard: correlations among body dimensions and weights of multiple species of sea cucumbers. Mar. Freshw. Res. 66, 857–865. doi: 10.1071/MF14146
Purcell S. W. (2014). Value, market preferences and trade of beche – de – mer from pacific island Sea cucumbers. PloS One 9 (4), 95075. doi: 10.1371/journal.pone.0095075
Purcell S. W., Conand C., Uthicke S., Byrne M. (2016). Ecological roles of exploited sea cucumbers. Oceanography and Marine Biology. An Annual Review 54, 367–386. doi: 10.1201/9781315368597-8
Purcell S. W., Hair C. A., Mills D. J. (2012). Sea Cucumber culture, farming and sea ranching in the tropics: Progress, problems and opportunities. Aquaculture 368 – 369, 68–81. doi: 10.1016/j.aquaculture.2012.08.053
Purcell S. W., Mercier A., Conand S., Hamel J. F., Toral – Granda M. V., Lovatelli A., et al. (2011). Sea Cucumber fisheries: global analysis of stocks, management measures and drivers of overfishing. Fish. Fish. 14, 34–59. doi: 10.1111/j.1467-2979.2011.00443.x
Purcell S. W. (2010). Managing sea cucumber fisheries with an ecosystem approach. Eds. Lovatelli A., Vasconcellos M., Yimin Y. (Rome: FAO Fisheries and Aquaculture Technical Paper), 520, 157.
Rakaj A., Fianchini A., Boncagni P., Lovatelli A., Scardi M., Cataudella S. (2018). Spawning and rearing of Holothuria tubulosa: a new candidate for aquaculture in the Mediterranean region. Aquacult. Res. 49, 557–568. doi: 10.1111/are.13487
Rakaj A., Fianchini A., Boncagni P., Scardi M., Cataudella S. (2019). Artificial reproduction of Holothuria polii: a new candidate for aquaculture. Aquaculture 498, 444–453. doi: 10.1016/j.aquaculture.2018.08.060
Ram R., Chand R. V., Southgate P. (2014). Effects of processing methods on the value of bêche – de – mer from the Fiji islands. J. Mar. Sci. Res. Dev. 4 (3), 1–7. doi: 10.4172/2155-9910.1000152
Ramírez-González J., Moity N., Andrade – Vera S., Mackliff H. R. (2020b). Estimation of age and growth and mortality parameters of the sea cucumber Isostichopus fuscus (Ludwig 1875) and implications for the management of its fishery in the Galapagos marine reserve. Aquac. Fish. 5 (5), 245–252. doi: 10.1016/j.aaf.2020.01.002
Ramírez-González J., Moity N., Andrade – Vera S., Reyes H. (2020a). Overexploitation and more than a decade of failed management leads to no recovery of the galápagos sea cucumber fishery. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.554314
Ramofafia C., Battaglene S. C., Bell J. D., Byrne M. (2000). Reproductive biology of the commercial sea cucumber Holothuria fuscogilva in the Solomon islands. Mar. Biol. 136, 1045–1056. doi: 10.1007/s002270000310
Ramofafia C., Byrne M., Battaglene C. (2003). Reproduction of the commercial sea cucumber Holothuria scabra (Echinodermata: Holothuroidea) in the Solomon islands. Mar. Biol. 142, 281–288. doi: 10.1007/s00227-002-0947-x
Ramos-Miranda J., del Río-Rodríguez R., Flores-Hernández D., Rojas‑González R. I., Gómez‑Solano M., Cu‑Escamilla A.d., et al (2017). Reproductive cycle of the sea cucumber Holothuria floridana in the littorals of Campeche, Mexico. Fish. Sci. 83, 699–714. doi: 10.1007/s12562-017-1100-6
Reyes-Bonilla H., Herrero – Pérezrul M. D. (2003). Population parameters of an exploited population of Isostichopus fuscus (Holothuroidea) in the southern gulf of California, Mexico. Fish. Res. 59 (3), 423–430. doi: 10.1016/S0165-7836(02)00023-1
Roberts D., Gebruk A. V., Levin V., Manship B. A. D. (2000). Feeding and digestive strategies in deposit – feeding holothurians. Oceanogr. Mar. Biol. Ann. Rev. 38, 257–310.
Santos R., Dias S., Tecelão C., Pedrosa R., Pombo A. (2017). Reproductive biological characteristics and fatty acid profile of Holothuria mammata (Grube, 1840). SPC Beche-de-mer Inf. Bull. 37, 57–64.
Sewell M. A. (1990). Aspects of the ecology of Stichopus mollis (Echinodermata: Holothuroidea) in north-eastern New Zealand. New Zeal. J. Mar. Freshw. Res. 24, 97–103.
Sewell M. A., Tyler P. A., Young C. M., Conand C. (1997). Ovarian development in the class Holothuroidea: a reassessment of the ‘‘tubule recruitment model’’. Biological Bulletin. Mar. Biol. Laboratory,Woods Hole 192, 17–26.
Shiell G. (2004). “Density of Holothuria nobilis and distribution patterns of common holothurians on coral reefs of Northwestern Australia,” in Advances in Sea Cucumber Aquaculture and Management. Eds. Lovateli A., Conand C., Purcell S., Uthicke S., Hamel J.-F., Mercier A. (Rome: FAO), 231–237.
Shiell G. R., Uticke S. (2006). Reproduction of the commercial sea cu- cumber Holothuria whitmaei (Holothuroidea: Aspidochirotida) in the Indian and Pacific Ocean regions of Australia. Mar. Biol. 148, 973–986.
Slater M. J., Lassudrie M., Jeffs A. G. (2011). Method for determining apparent digestibility of carbohydrate and protein sources for artificial diets for juvenile sea cucumber, Australostichopus mollis. J. World Aquacult. Soc. 42 (5), 714–725. doi: 10.1111/j.1749-7345.2011.00510.x
Sloan N. A. (1979). Microhabitat and resource utilization in cryptic rocky intertidal echinoderms at Aldabra Atoll, Seychelles. Mar. Biol. 54, 269–279. doi: 10.1007/BF00395789
Smiley S. (1990). A review of echinoderm oogenesis. J. Electron Microsc. 16, 96–114. doi: 10.1002/jemt.1060160203
Smiley S. (1988). The dynamics of oogenesis and the annual ovarian cycle of Stichopus californicus (Echinodermata: Holothuroidea). Biological Bulletin. Marine Biological Laboratory, Woods Hole 175, 79–93.
Smiley S., McEuen F. S., Chaffee C., Krishnan S. (1991). “Echinodermata: Holothuroidea,” in Reproduction of marine invertebrates, echinoderms and iophophorates, vol. VI. Eds. Giese A. C., Pearse J. S., Pearse V. B. (Pacific Grove, California, USA: Boxwood Press), 663–750.
Soliman T., Reimer J. D., Kawamura I., van der Meij S. E. T., Reijnen B. T., Paulay G. (2019). Description of the juvenile form of the sea cucumber Thelenota anax H. L. Clar. Mar. Biodivers. 49, 547–554. doi: 10.1007/s12526-017-0820-2
Tahri Y., Dermeche S., Chahrour F., Bouderbala M. (2019). The reproduction cycle of the sea cucumber Holothuria (Holothuria) tubulosa Gmelin 1791 (Echinodermata Hlothuroidea Holothuriidae) in Oran coast, Algeria. Biodivers. J. 10, 159–172. doi: 10.31396/Biodiv.Jour.2019.10.2.159.172
Tan S. H., Zulfigar Y. (2001). “Reproductive cycle of stichopus chloronotus (Brand) in the straits of malacca,” in Echinoderms (Lisse: Swets & Zeitlinger)2000.
Tolon T., Emiroglu D., Guinai D., Hanci B. (2017). Effect of stocking density on growth performance of juvenile sea cucumber Holothuria tubulosa (Gmelin 1788). Aquac. Res. 48 (8), 4124–4131. doi: 10.1111/are.13232
Toral-Granda V., Lovatelli A., Vasconcellos M. (2008). Sea Cucumbers. A global review of fisheries and trade (Rome: FAO), 231–253. FAO Fisheries and Aquaculture Technical Paper. No. 516.
Uthicke S. (2001). Nutrient regeneration by abundant coral reef holothurians. J. Exp. Mar. Bio. Ecol. 265 (2), 153–170. doi: 10.1016/S0022-0981(01)00329-X
Vafeiadou A. M., Antoniadou C., Vafidis D., Fryganiotis K., Chintiroglou C. (2010). Density and biometry of the exploited holothurian Holothuria tubulosa at the Dodecanese, south Aegean Sea. Rapp. Commun. Int. Mer. Médit 9, 661.
Venâncio E., Félix P. M., Brito A. C., Azevedo e Silva F., Simões T., Sousa J., et al. (2022). Reproductive biology of the Sea cucumber Holothuria mammata (Echinodermata: Holothuroidea). Biology 11, 622. doi: 10.3390/biology11050622
Wilkie I. C. (2001). Autotomy as a prelude to regeneration in echinoderms. Microsc. Res. Tech. 55 (6), 369–396. doi: 10.1002/jemt.1185
Yang H., Bai Y. (2015). “Apostichopus japonicus in the life of Chinese people”. in The Sea cucumber Apostichopus japonicus. history, biology, and aquaculture. Eds. Yang H., Hamel J. F., Mercier A. (Academic Press), 1–24.
Yang H., Hamel J., Mercier A. (2015). The Sea cucumber Apostichopus japonicus: History, biology and aquaculture (London, UK: Elsevier), 454.
Yang H., Zhou Y., Zhang T., Yuan X., Li X., Liu Y., et al. (2006). Metabolic characteristics of sea cucumber, Apostichopus japonicus (Selenka) during aestivation. J. Exp. Mar. Biol. Ecol. 330, 505–510. doi: 10.1016/j.jembe.2005.09.010
Zang Y., Tian X., Dong S., Dong Y. (2012). Growth, metabolism and immune responses to evisceration and the regeneration of viscera in sea cucumber, Apostichopus japonicus. Aquaculture 358 – 359, 50–60. doi: 10.1016/j.aquaculture.2012.06.007
Keywords: sea cucumber, Holothuria tubulosa, maturity scale, histology, spawning cycle, Mediterranean Sea
Citation: Pasquini V, Porcu C, Marongiu MF, Follesa MC, Giglioli AA and Addis P (2022) New insights upon the reproductive biology of the sea cucumber Holothuria tubulosa (Echinodermata, Holothuroidea) in the Mediterranean: Implications for management and domestication. Front. Mar. Sci. 9:1029147. doi: 10.3389/fmars.2022.1029147
Received: 26 August 2022; Accepted: 06 October 2022;
Published: 26 October 2022.
Edited by:
Chenghua Li, Ningbo University, ChinaReviewed by:
Qiang Xu, Hainan University, ChinaCarlos Rosas, National Autonomous University of Mexico, Mexico
Copyright © 2022 Pasquini, Porcu, Marongiu, Follesa, Giglioli and Addis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Viviana Pasquini, dml2aWFuYS5wYXNxdWluaUB1bmljYS5pdA==
†These authors have contributed equally to this work
 Viviana Pasquini
Viviana Pasquini Cristina Porcu
Cristina Porcu Martina Francesca Marongiu
Martina Francesca Marongiu Maria Cristina Follesa
Maria Cristina Follesa Ambra Angelica Giglioli
Ambra Angelica Giglioli Pierantonio Addis
Pierantonio Addis