Abstract
The armored dinoflagellate Prorocentrum donghaiense distributes globally and has been forming large scale and dense ecosystem disruptive algal blooms (EDABs) in the East China Sea (ECS) almost every year since the 1990s and often in other coastal waters of the world. It has long been a mystery, however, about how these blooms were seeded or where the initiating population came from. In this work, we provide a more feasible and universal seeding mechanism, formation of resting cysts. Using light microscopy, we confirmed sexual reproduction according to the observations of mating cells in pairs, planozygotes having two similar flagella, darkened and thick-walled resting cysts with smooth surface, and germination processes of resting cyst. Using morpho-molecular detection, we confirmed P. donghaiense resting cyst in the field, including the positive detections of polymerase chain reaction (PCR) using species-specific primers and then the fluorescence in situ hybridization (FISH) using species-specific probes, and further confirmation via single-cell sequencing for the individual FISH-detected cysts. Furthermore, the distribution and abundance of P. donghaiense cysts along the coast of China Seas were mapped using an approach combining real-time PCR (qPCR) and FISH, with the qPCR quantification taking into account the doubled copy number of LSU rRNA gene in resting cysts. Resting cysts of this species were found to widely distribute in the Yellow Sea (YS), ECS, and South China Sea (SCS), with a relatively low abundance at most sampling sites, but to be absent in the eight samples from the Bohai Sea (BS). Resting cyst production confirmed with evidences from both laboratory cultures and field sediments and the extensive distribution of cysts in the China Seas, as the first case in planktonic species of Prorocentrum, not only filled up a knowledge gap about the life history of P. donghaiense but also provided a possible mechanistic facility to seed the annual blooms in the ECS and the global distribution of the species.
Introduction
Over the past several decades, harmful algal blooms (HABs) have become more frequent, extensive and severe globally (Anderson et al., 2012, 2021; Fu et al., 2012; Glibert, 2014; Wells et al., 2015; Glibert et al., 2018; Gu et al., 2021; Karlson et al., 2021; Sakamoto et al., 2021). In Chinese coastal waters, the occurrences of HABs have been dramatically increasing since 1980s, and more causative species have been recorded (Lu et al., 2014; Hu et al., 2018, 2021; Yu et al., 2018, 2020; Li et al., 2019; Xu et al., 2019; Zhai et al., 2019; Gu et al., 2021). Among these HABs-causing species, a dinoflagellate, Prorocentrum donghaiense has drawn great attention from the research community, fishery and aquaculture industry, and other stakeholders (Lu and Goebel, 2001; Lu et al., 2014; Yu et al., 2018, 2020). Since 1998, more than 250 P. donghaiense blooming events have been recorded in the East China Sea (ECS) and these blooms covered several km2 to more than 10,000 km2 (Wang and Wu, 2009; Lu et al., 2014; Yu et al., 2018, 2020). These blooms were recorded to have a maximum cell density up to 3.6 × 108 cells⋅L–1, to last several days to 1 month, and to cause severe deleterious impacts on the local marine fisheries, public health, and aquatic ecosystems (Lu and Goebel, 2001; Lin et al., 2014; Yu et al., 2020).
It is noteworthy that although the species investigated in the present work has been recently proposed by Shin et al. (2019a) to be named Prorocentrum obtusidens, we decided to keep the name P. donghaiense as described by Lu and Goebel (2001) because we firmly believe it is absolutely necessary to conduct a more detailed morpho-molecular examination for specimen from the type locality (the Adriatic Sea) of P. obtusidens before it can be accepted to replace P. donghaiense. Prorocentrum donghaiense is a globally distributed dinoflagellate that inhabits in estuarine and coastal waters of Asian countries (Lu and Goebel, 2001; Takano and Matsuoka, 2011; Lee et al., 2013; Lu et al., 2014; Shin et al., 2019a,b), Oceania countries (Chang, 1988; Ajani et al., 2011), European countries (Parke and Dixon, 1976; Viličić et al., 2002; Gómez, 2003; Gómez and Boicenco, 2004; Percopo et al., 2011; Guilloux et al., 2013), North and South American countries (Hernández-Becerril et al., 2000; Scorzetti et al., 2009; Muciño-Márquez et al., 2015; Rezende et al., 2015), and the Arctic areas (Okolodkov, 1998; Chang et al., 2013). However, there has been no study explaining the cosmopolitan distribution of this species. High cell density blooms of P. donghaiense have been reported from many countries (Hernández-Becerril et al., 2000; Ajani et al., 2011; Roselli et al., 2019; Shin et al., 2019a), and particularly in China (Lu et al., 2014). Since 2001, blooms of P. donghaiense have occurred annually in the similar area of the ECS (Lu D. et al., 2005; Lu et al., 2014). Because of the severity of economic and ecological impacts of these blooms, P. donghaiense has been extensively investigated from many facets during the last two decades, including its tolerance to extreme or changing salinity, temperature, and light intensity (Chen et al., 2005; Xu et al., 2010; Boyd et al., 2013; Hu et al., 2014, 2016), nutrient requirements (Huang et al., 2005; Hong et al., 2009; Ou et al., 2010; Hu et al., 2012, 2016; Zhang et al., 2012; Jing et al., 2017), method development for species detection (Wang et al., 2007; Chen et al., 2011, 2013), effects on and interactions with other organisms (Wang et al., 2003, 2020; Yan et al., 2007; Lin et al., 2014; Li et al., 2018; Shi et al., 2018), effects on resource utilization (Chai et al., 2020), and blooming dynamics and oceanographic mechanisms (Cai et al., 2002; Zhu et al., 2009; Li et al., 2010; Dai et al., 2013, 2014; Zhang et al., 2020). All these studies helped us to better understand the biology and ecology of this species, but it is still highly controversial regarding where the initial “seeding population” came from for the annual blooms. Dai et al. (2013) and Zeng et al. (2020) proposed a “Pelagic Seed Bank Hypothesis” based on a claimed “spatio-temporal match” between P. donghaiense abundance and the Taiwan Warm Current (TWC), in which the vegetative cells-containing front of TWC was believed to serve as a ‘seed bank’ and initiated P. donghaiense blooms in the ECS. While we noticed there was no correlation-regression analysis in figure 6 of Dai et al. (2013) and figure 7 of Zeng et al. (2020), both of them were supposed to show the significant correlation (or ‘match’) between P. donghaiense abundance and temperature or salinity, we also noticed that there was no monthly investigation on the abundance of vegetative cells in the water column of the TWC, especially during the winter time. Zeng et al. (2020) deemed that more investigations are needed to ascertain the initial seed source. Therefore, even if TWC in late March (the earliest cruise month in Dai et al., 2013) contained vegetative cells and thus acted as a “seed bank” for the blooms in the ECS later, where did the vegetative cells in the TWC come from? In addition, despite of numerous studies on P. donghaiense, none of them has addressed its overwintering mechanism.
Resting cyst has been proven to play important roles in the biology and ecology of dinoflagellates (Anderson and Wall, 1978; Dale, 2001; Smayda, 2007; Bravo and Figueroa, 2014; Ellegaard et al., 2017; Ellegaard and Ribeiro, 2018; Figueroa et al., 2018; Tang et al., 2021), which highlights the significance of revealing the life history and particularly whether or not it forms resting cysts in the blooming region. Multiple, mostly benthic, Prorocentrum species have been investigated for their sexual reproduction and possible resting cyst formation (Bhaud et al., 1988; Faust, 1990, 1993; Cannon, 1993; Mertens et al., 2017; Berdieva et al., 2020; Matantseva et al., 2020). Unlike other dinoflagellates that were observed a perpendicular orientation for their cell pairs during sexual mating (Figueroa and Bravo, 2005; Tang and Gobler, 2012, 2015; Hu et al., 2020; Liu et al., 2020a,b), the two mating cells of P. micans formed a fertilization tube, through which a donor cell injected its nucleus to the recipient cell (Bhaud et al., 1988). Cannon (1993) observed vegetative cells of P. gracile and P. micans from sediment germination experiments, suggesting these two species could form resting cysts, but the morphologies of their resting cysts have not been observed. Faust (1990) reported sexual reproduction and resting cysts in the benthic P. foraminosum (= P. marinum) from field samples, but she still did not observe the germination process or identify the cyst as P. foraminosum (= P. marinum) using a reliable method, which led Hoppenrath et al. (2013) to comment also that the evidence for cyst production of this species is still fragmental and needs further confirmation. Faust (1993) reported sexuality in the benthic P. lima, e.g., observations of “dancing groups of gametes, gametes forming fertilization bridge, nucleus of the donor cell moving to the recipient cell,” nuclei fusing, and formation of resting cysts. Berdieva et al. (2020) proposed a haplontic life cycle of P. minimum based on the observations of a homothallic but incomplete sexual mating and an absence of resting cyst. More importantly, Mertens et al. (2017) confirmed the first case of resting cyst production in the genus Prorocentrum from the benthic species P. leve via cyst germination from sediment samples and LSU rRNA gene sequencing for both cysts and vegetative cells that were germinated from cysts. Although P. leve belongs to the benthic clade in the phylogenetic tree (Hoppenrath et al., 2013; Mertens et al., 2017), the confirmation of resting cyst production in P. leve and the observations of sexuality in multiple other Prorocentrum species as reviewed above made us to believe in the existence of more resting cyst producers in this ecologically important genus.
However, except for the asexual production of P. donghaiense, there has been no particular examination on the sexual reproduction and possible formation of resting cyst of this organism. Based on the review for the investigations on the ecology of P. donghaiense and life history of other Prorocentrum species, we hypothesized that P. donghaiense is another resting cyst producer. Here, we report our evidences supporting this hypothesis that were obtained via experiments, light microscopy, fluorescence in situ hybridization (FISH) detection, and single-cell polymerase chain reaction (PCR) and sequencing both in laboratory cultures and field sediments. We consider our findings will advance the understanding and further investigations about the ecology of this HABs-forming dinoflagellate.
Materials and Methods
Culture Maintenance
Two strains of P. donghaiense (CCMA206 and CCMA264) were obtained from the Center for Collections of Marine Algae (CCMA), Xiamen University, isolated from the coastal water of Xiamen, Fujian Province, China in 2014 and Changjiang River Estuary, China in 2010, respectively. All cultures were grown in natural seawater with a salinity of ∼32 enriched with f/2-Si medium (Guillard, 1975) and a final concentration of 10–8 M selenium under conditions of 21 ± 1°C, ∼100 μmol quanta m–2⋅s–1, and a photoperiod of 12 h:12 h (light: dark) supplied by white fluorescent light (Ningbo Jiangnan Instrument Factory, Ningbo, China). An antibiotic solution (a mixture of 10,000 I.U. penicillin and 10,000 μg⋅mL–1 streptomycin, Solarbio, Beijing, China) was added into the medium immediately before every time of inoculation (final concentration 2%) to discourage bacterial growth.
Production and Germination of Resting Cysts
Cell pairs in division and in mating, planozygotes, resting cyst formation, morphology of resting cyst, and vegetative cells were observed with an inverted (IX73, Olympus, Tokyo, Japan) and upright microscope (BX53, Olympus, Tokyo, Japan). For epifluorescence microscopy, vegetative cells, cells in mating, and planozygote were stained with SYBR Green (Solarbio, Beijing, China), viewed and photographed for SYBR Green-induced green nuclear fluorescence. Micrographs and videos were obtained with a digital camera (DP80, Olympus, Tokyo, Japan). To observe a single pair of gametes to finish the complete life stage until the cyst formation, single pairs of gametes were micropipetted and transferred into 24-well culture plate wells with one pair of gametes in each well, and observed every day. For cyst germination, individual cysts were isolated by micropipetting from the cultures that had been generally inoculated and cultured for more than 1 month. Resting cysts were washed with fresh medium in a Petri dish at least three times, and placed in individual wells of 24-well culture plate with each well containing 2.5 mL fresh medium and 2% antibiotic solution and incubated at 21 ± 1°C, 12:12 h light: dark cycle, and ∼100 μmol photons m–2⋅s–1. Changes in cyst morphology were observed every or every other day with the inverted microscope and photographed by a DP80 digital camera (Olympus, Tokyo, Japan).
Polymerase Chain Reaction Confirmation of the Presence of Prorocentrum donghaiense in Surface Sediments
Surface (0–2 cm) sediment samples were collected from the coast of Ningde, Fujian Province in April, 2016 using a grab sampler (Table 1 and Figure 1), and stored at 4°C in darkness prior to the cyst detection.
TABLE 1
| Station | Latitude (N)/Longitude (E) | Temperature (°C) | Salinity |
| NE20 | 26.92°/120.19° | 14.0 | 27.5 |
| NE29 | 26.89°/120.18° | 13.9 | 27.5 |
| NE33 | 26.84°/120.19° | 13.9 | 27.6 |
| NE41 | 26.87°/120.19° | 14.5 | 28.5 |
| NE45 | 26.75°/120.19° | 14.4 | 28.7 |
Physicochemical variables of the surface water and locations of the sampling stations.
FIGURE 1
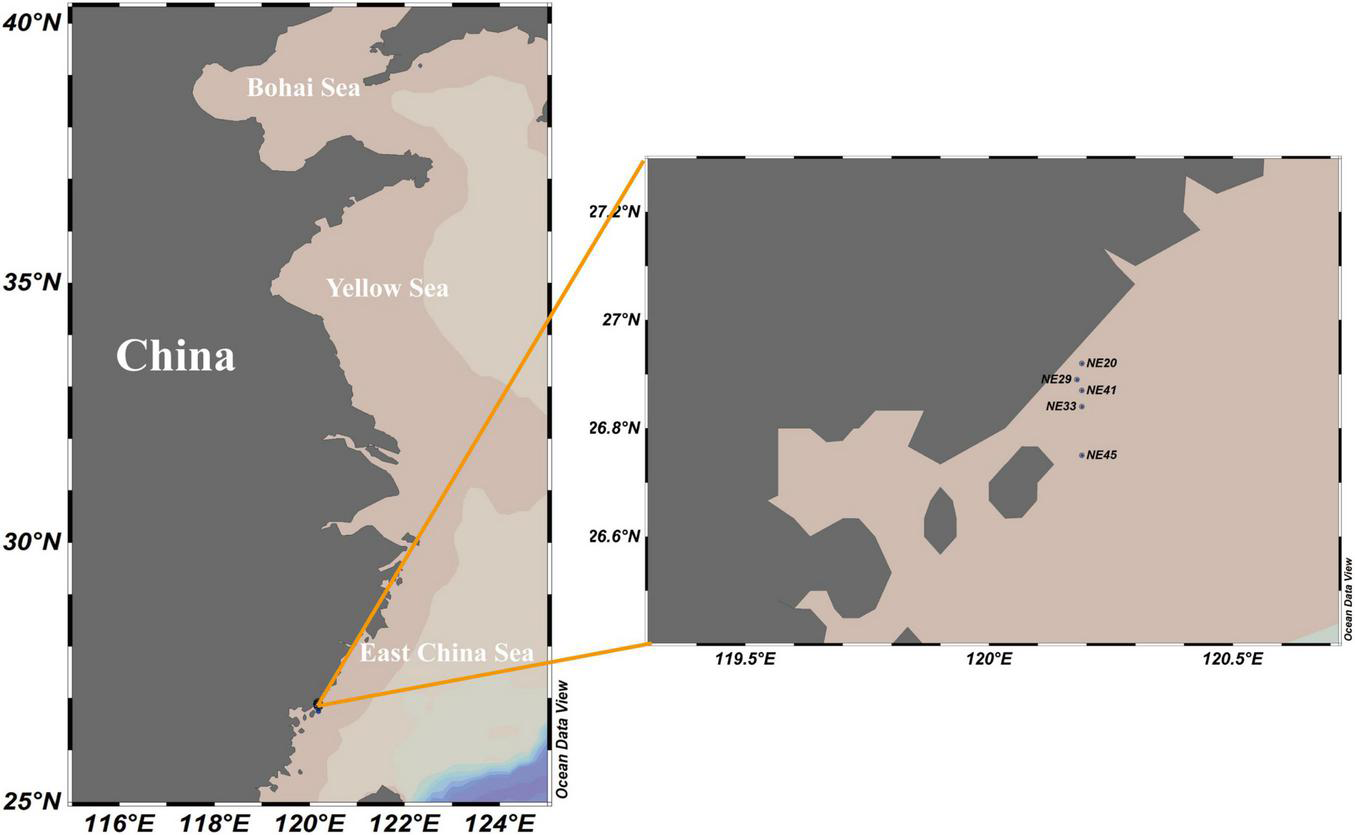
Sediment sampling stations in Ningde coast, China.
Total genomic DNA in sediment sample was extracted using FastDNA Spin Kit for Soil (MP Biomedicals, Solon, OH, United States) according to the manufacturer’s protocol. The concentration and quality of DNA were measured using a ND-2000 Nanodrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, United States). All reference 28S rRNA gene sequences (D1--D2) of P. donghaiense were retrieved from NCBI database1, assembled into a contig using the DNAstar software (version 7.1.02), and then aligned with other related dinoflagellates (Table 2) using Clustal X (version 2.0; Thompson et al., 1994). The species-specific primers targeting a 201-bp LSU fragment for P. donghaiense were designed based on the alignment: the forward primer PD201F (5′-CAGGATTGTCGGGTGCATGA), and the reverse primer PD201R (5′-TGGCAGGCCAAGCCGAGAT). The specificity of primers was tested using BLASTn (Basic Local Alignment Search Tool3). Primers were synthesized at Tsingke Biotechnology (Qingdao, China). The PCR reactions, amplification, PCR products purification, cloning and sequencing followed that of Liu et al. (2020a). All sequences obtained were blasted in GenBank using BLASTn (see footnote 3) for their identity.
TABLE 2
| Reference sequence | GenBank accession number |
| Prorocentrum donghaiense | KX656893, KF032444, AY833513, AY822610, AY863007, AY863006, KF998562 |
| P. minimum | EU532479, DQ054539 |
| P. micans | EF613361, EU780638 |
| Karenia mikimotoi | AF200681, AY355460, EF469238, HM807325, HM807328, HM807331, KJ508363, KT733618 |
| K. brevis | AY355459, AY335458, AY355457, AY355456, AY355455 |
| K. umbella | KY287670 |
| Karlodinium gentienii | KJ508379, KJ508378 |
| K. veneficum | KJ508384, KJ508383, KP790220 |
| Takayama acrotrocha | FJ024703, FJ024702 |
| T. helix | AY464691, AY284951, AY284950 |
| T. tasmanic | KC485077, AY284948, KP790230, AY284949 |
| Akashiwo sanguinea | KF533111 |
| Gymnodinium aureolum | DQ917486 |
| Levanderina fissa | EF613354 |
Representative species and accession numbers of their LSU rRNA gene sequences included in the alignment.
Fluorescence in situ Hybridization Detection of Resting Cysts in Marine Sediment and Microscopic Observation
Eight species-specific oligonucleotide probes of P. donghaiense targeted the 28S rRNA gene (D1–D2 domains) were designed according to the abovementioned alignment: PDprobe-1 (5′-GAGATCTCCAAGGTGCCAAAG), PDprobe-2 (5′-CTTTGCGTCAGGGAAATGCCCA), PDprobe-3 (5′-ACTGTGGCAGGCCAAGCCGAGAT), PDprobe-4 (5′-AGGC AGGAGACGATCAGGA), PDprobe-5 (5′-CACCCGA CAATCCTGCCAAGCAAC), PDprobe-6 (5′-CCAAGCCGAGA TCTCCAAGG), PDprobe-7 (5′-AGCGTCATGCACCCGAC AATCC), and PDprobe-8 (5′-GCTATTCACTCACCCGAAG ACAA). All probes were monolabeled with FITC at the 5′ end, and tested their fluorescence signal intensity and specificity using vegetative cells of P. donghaiense, P. minimum, Karenia mikimotoi, and Karlodinium veneficum. The best probe was then selected and monolabeled with either Cy3 or FITC for further FISH detection.
Before FISH detection, resting cysts in sediment samples were firstly concentrated using the sodium polytungstate (SPT) protocol (Bolch, 1997). The SPT-concentrated cysts were fixed with 400 μL 4% paraformaldehyde overnight (∼14 h) at 4°C, centrifuged at 4,000 rpm for 3 min, dehydrated twice in 85% PBS-ethanol on ice for 15 min, and then dried at room temperature. Samples were added 200 μL Triton X-100 solution (final concentration 0.5%) prior to an incubation in water bath at 37°C for 15 min, centrifuged at 4,000 rpm for 3 min, and supernatant removed. To permeabilize the cyst wall, 200 μL proteinase K solution (1 μg⋅mL–1 in 1 × PBS) was added into each cyst pellet and then incubated at 37°C for 1 h. After permeabilization, the samples were centrifuged at 4,000 rpm for 3 min and further washed with 1 × PBS twice before hybridization in water bath as described above. The FISH protocol was basically the same as that of Liu et al. (2020a).
Further Confirmation of the Identity of Resting Cyst Detected With Fluorescence in situ Hybridization via Single-Cell Polymerase Chain Reaction Sequencing and Phylogenetic Analyses
To further confirm the identity of resting cysts as P. donghaiense that were positively detected with FISH, single-cell nested-PCR and subsequent sequencing were conducted. The first pair of primers for nested-PCR was adopted from Litaker et al. (2003) with minor modification: LSUF1, 5′-CAAGTACCATGAGGGAAA; LSUR1, 5′-ACGAACGATTTGCACGTCAGTA, targeting a fragment of ∼500 bases in D1–D2 domains. The second pair of primers, PD201F and PD201R, were designed inside the targeted sequence of the first pair of primers. The details of breaking FISH probe-labeled resting cyst, PCR amplification, cloning, and sequencing followed that used in Liu et al. (2020a). As a routine procedure, a sterile water sample was always used as the negative control, which was subjected to the same extraction and PCR protocol. New sequences were shown in Supplementary Appendix 1.
Newly obtained LSU rRNA gene sequences were incorporated into those of closely related species available in the GenBank (their accession numbers are shown after each taxon in the phylogenetic tree) and that of outgroup taxa were aligned using the MAFFT v7.473 with the default settings (Katoh et al., 2002)4 and manually checked with BioEdit (v7.2.5; Hall, 1999). The program jModelTest (Darriba et al., 2012) was used to select the most appropriate model of molecular evolution with Akaike information criterion. This test chose general time-reversible (GTR) model with gamma-distributed rate variation across sites and a proportion of invariable sites was selected as the best-fit model (GTR + G + I). The Bayesian inference (BI) analysis was conducted with MrBayes 3.2.6 with the best-fitting substitution model (GTR + I + G) (Ronquist and Huelsenbeck, 2003). Four independent Markov Chain Monte Carlo simulations were run simultaneously for 10,000,000 generations and trees were sampled every 1,000 generations. The first 25% trees were discarded as burn-in. The convergence was judged based on the average standard deviation of split frequencies (all less than 0.01). The remaining trees were used to generate a consensus tree and calculate the posterior probabilities of all branches using a majority-rule consensus approach. FigTree (v1.4.4) was used to view and edit trees for publication.
Determination of the rRNA Gene Copy Numbers in Single Cell or Cyst of Prorocentrum donghaiense
For a more accurate PCR-based quantification of resting cysts abundance in sediments, the copy numbers of LSU rRNA gene in a single vegetative cell or resting cyst of P. donghaiense were determined using Taqman qPCR. The specific primers were the above-described PD201F and PD201R, and a P. donghaiense-specific probe (PDprobe, 5′-AGGCAGGAGACGATCAGGA) was designed to target the middle segment of the LSU rRNA gene fragment targeted by PD201F and PD201R. The probe was inspected for its specificity using BLASTn and labeled with FAM at 5′ end and BHQ1 at 3′ end (Tsingke, Beijing, China). The average LSU rRNA gene copy number in a single vegetative cell was estimated by single-cell qPCR assay for 56 vegetative cells (n = 56). The single-cell samples were pretreated with a set of previously optimized conditions to pursue a complete release of cellular DNA: a single cell was micropipetted with an inverted microscope, washed three times with 2 μL of 1 × PBS on a sterile slide, and then transferred into qPCR tube. The tube was incubated at 94°C for 10 min and immediately frozen at –80°C for 10 min, repeated this frozen-thawed process once more, and then subjected to the qPCR. The qPCR had a 20 μL mixture system including 10 μL of premix Ex Taq II (2×), 0.8 μL of each primer (10 μM), 0.8 μL probe (10 μM), and 2 μL DNA template, and was conducted with the following parameters: 94°C for 10 min, 45 cycles at 94°C for 20 s, and then 56°C for 45 s. Triplicate reactions, as well as negative controls, were performed for each DNA template. Calibration curves were constructed using 10-fold serial dilutions of plasmid DNA (approximately 102–107 copies of recombinant plasmid). The amplification efficiency of real-time PCR was 94.5% (R2 = 0.9995), a sound linear relationship between the gene copy number and Ct value.
Quantification of Prorocentrum donghaiense Resting Cysts in Sediment Samples
One hundred and twenty-five surface sediment samples were collected from China Seas covering the Bohai Sea (BS), the Yellow Sea (YS), the East China Sea (ECS), and the South China Sea (SCS) (Supplementary Table 1), with the longitude ranging from 108.58°E to 123.37°E, and the latitude from 18.29°N to 39.85°N. The abundance of P. donghaiense resting cysts in sediment was quantified with two approaches: qPCR and FISH, as applied in Liu et al. (2020b). (1) For the Taqman qPCR assay based on LSU rRNA gene copy number in P. donghaiense cyst, the specific primers and probe were PD201F, PD201R, and PDprobe described above. In order to remove possibly presented dead vegetative cells and fragmental DNA in sediment samples, all cysts in each sample (32 g wet weight) were concentrated using the SPT protocol (Bolch, 1997) and incubated at 30°C for 1 h (Park et al., 2016) before being pelleted in a 2 mL tube at 4°C. A volume of 800 μL buffer GP1 (Tiangen, Beijing, China) and 0.25 g quartz sands were added to cysts pellet, and the sample was then lysed using FastPerp-24 classic grinder (MP Biomedicals, Solon, OH, United States). Thereafter, the genomic DNA from lysate was extracted following the protocol of plant genomic DNA kit (Tiangen, Beijing, China), with the quality and quantity being measured using an ND-2000 Nanodrop spectrophotometer (Thermo Fisher Scientific, Wilmington, DE, United States), and then stored at –80°C prior to qPCR. The qPCR had a 20 μL mixture system including 10 μL of premix Ex Taq II (2×), 0.8 μL of each primer (10 μM), 0.8 μL probe (10 μM), and 2 μL DNA template, and was conducted with the following parameters: 94°C for 10 min, 45 cycles at 94°C for 20 s, and then 60°C or 56°C for 45 s. Triplicate reactions, as well as negative controls, were performed for each DNA template. Calibration curves were constructed using 10-fold serial dilutions of plasmid DNA (approximately 102–107 copies of recombinant plasmid). The amplification efficiency of real-time PCR was 99.5% (R2 = 0.9999; for 60°C of annealing), a sound linear relationship between the gene copy number and Ct value. One third of the qPCR products amplified from all sediment samples was cloned and sequenced to confirm the specificity of assays. The P. donghaiense cyst abundance in marine sediments was quantified on the basis that the rRNA gene copy number in a resting cyst (diploid) is twice that in a vegetative cell (haploid). The quantification results for P. donghaiense cyst in sediment samples were corrected as follows: The extraction efficiency of genomic DNA was estimated through comparing the number of P. donghaiense vegetative cell by two methods including microscopic count and qPCR, and then the LSU rRNA gene copies in each sediment sample via qPCR were multiplied by the coefficient of extraction efficiency. (2) The FISH quantification of cysts followed the protocol described above and was applied to 40 sediment samples (32 g each) covering BS, YS, ECS, and SCS (Supplementary Table 1).
Results
Observations of Cell Pairs in Sexual Mating, Planozygotes and Resting Cysts
The normal vegetative cells of P. donghaiense were slightly asymmetric, elongated, and narrowing toward the posterior end (Figures 2A,B), with the anterior end slightly indented and posterior end generally rounded (Figures 2A,B). Solitary cells and colonies in chain of 2 to 64 cells, usually in even number, were observed in cultures (Figures 2A–D). Gametes in mating, planozygotes (judged from two similar long flagella), and thick-walled cysts were observed in the clonal culture of P. donghaiense strain CCMA264 (Figures 2E–K). Mating gametes usually occurred in late exponential or stationary cultures, with the two gametes connecting to each other via anterior ends and tangling for several hours before fusion (Figure 2E). Regarding to the changes of single pair of gametes in each well, unfortunately, all the fusing gametes separated within 1–2 days after the manipulation. On the contrast, the two daughter cells during asexual division usually formed a chain and significantly smaller than normal vegetative cells (Figure 2D). Then the two gametes fused from the apical end (Figures 2F,G), and four flagella were observed (Supplementary Video 1). After a while, the fused cell became a planozygote with two similar flagella (the other two might lost; Figure 2H and Supplementary Video 2). The motile planozygote lost all flagella, and formed a non-motile planozygote, with an oval and larger nucleus than the vegetative cell (Figures 2I,J). After a while, the non-motile planozygote became a mature resting cyst with a round shape, thick wall, darkened color, smooth surface, accumulation body, and shrunken chloroplasts (Figures 1K,M). The new germling releasing from the archeopyle was occasionally observed (Figure 1L). Resting cysts from the culture had a diameter ranging from 13.1 to 27.3 μm (mean = 19.8 ± 3.5 μm, n = 50), while that from the field sediment ranging from 10.8 to 20.0 μm (mean = 14.9 ± 3.1 μm, n = 15).
FIGURE 2
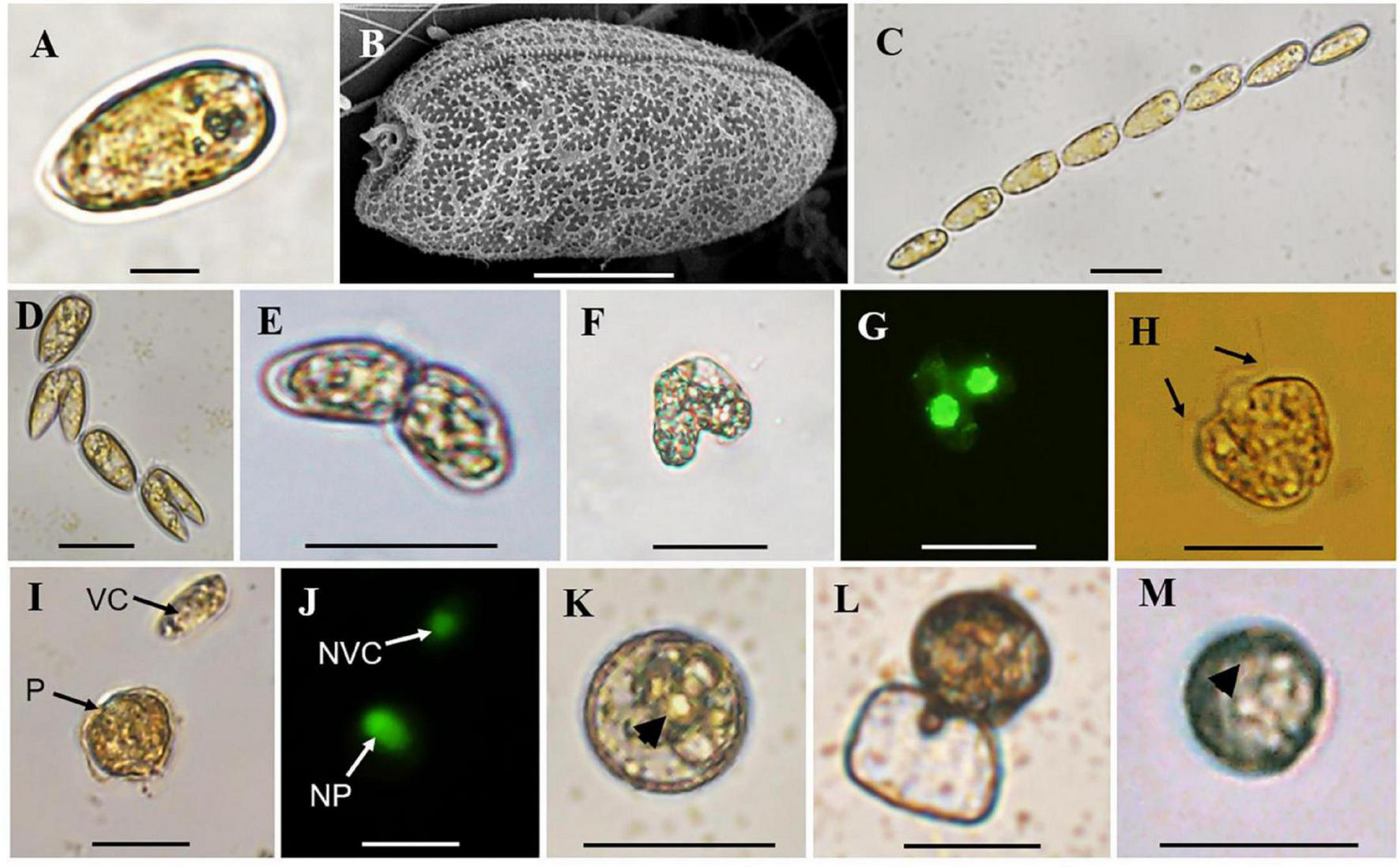
Observations of vegetative cells of Prorocentrum donghaiense, cell chain, cells in division, cell pairs in mating, planozygote, resting cysts from culture and sediment. (A,B) Vegetative cell observed by light (LM) and scanning electron microscope (SEM); (C) a cell chain with eight vegetative cells observed by LM; (D) cells in division; (E) cell pairs in mating with two apical ends connected together observed by LM; (F,G) two mating cells in fusion observed by bright field LM and green epifluorescent LM for SYBR Green-induced green nuclear fluorescence; (H) planozygote with two similar flagella (black arrows) observed by LM; (I,J) a vegetative cell (VC) and planozygote (P) and their nuclei [nucleus of vegetative cell (NVC) and nucleus of planozygote (NP)] observed by bright field LM and green epifluorescent LM for SYBR Green-induced green nuclear fluorescence; (K) round and brown resting cyst from culture with thick wall and accumulation body (black arrowhead) observed by LM; (L) a new germling releasing from the archeopyle; (M) round and brown resting cyst detected from sediment with an accumulation body (black arrowhead) with LM. Scale bars for (A) = 10 μm, (B) = 5 μm, (C–M) = 20 μm.
Resting Cyst Germination
The germination experiment was conducted with the resting cysts isolated from cultures about 1-month after inoculation. During 2 weeks’ incubation, 5 of 24 resting cysts (20.8%) successfully germinated, while the others were broken during incubation. After 1 day of incubation under normal culturing conditions used to maintain cultures, the pigmentation changed and started to distribute through the cell (Figures 3A,B). On day 3, two cells connecting to each other with irregular morphology and an empty, subspherical cyst were observed, indicating a fast meiotic division for the new germling (Figures 3C,D). The morphology of archeopyle was not clearly observable due to many fine particles around the empty cyst and the small sizes of cysts. All new germling swam very fast, which made us difficult to determine whether they had one or two similar flagella.
FIGURE 3
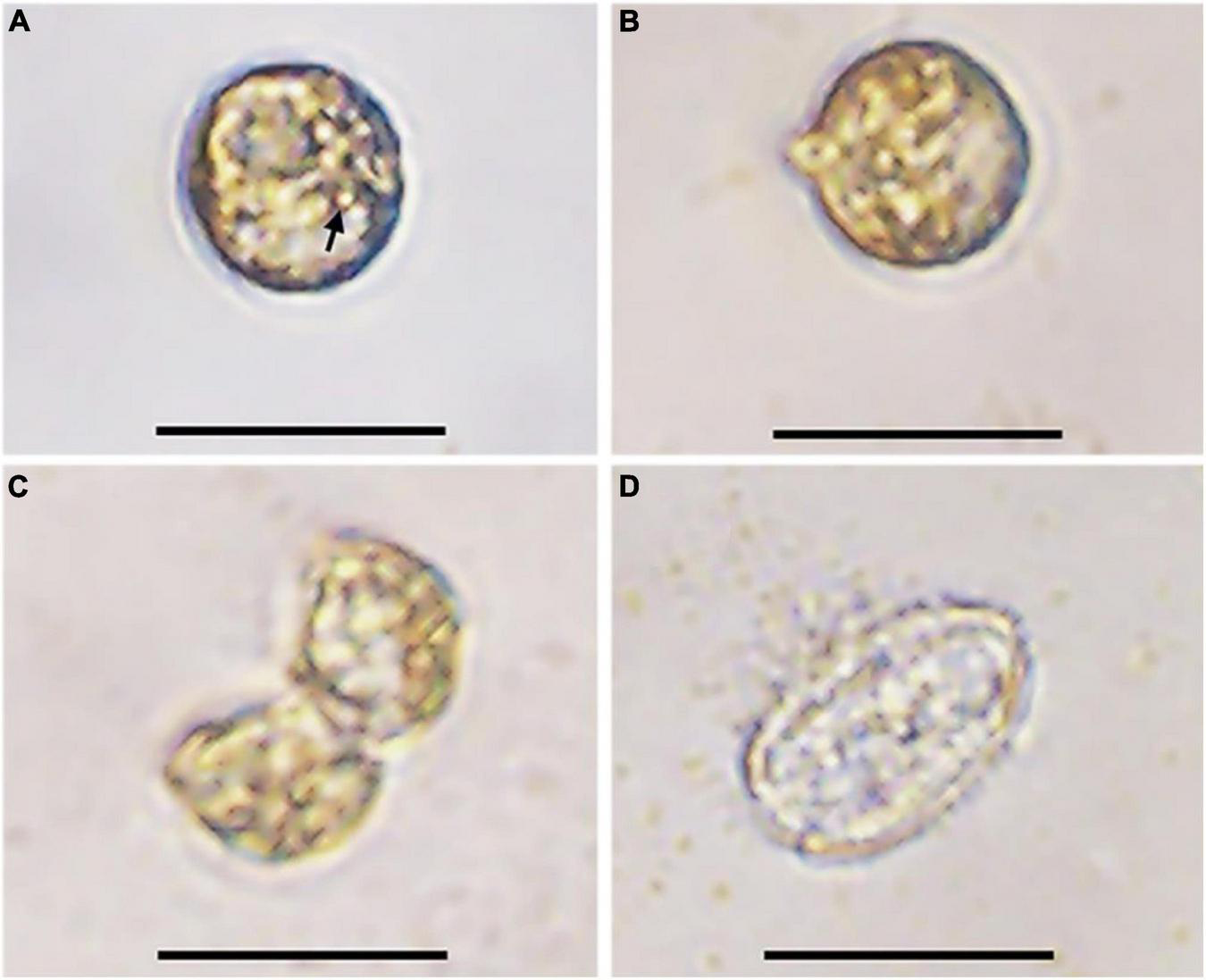
Germination process of a resting cyst of Prorocentrum donghaiense (strain CCMA264) at 21°C. (A) The resting cyst at day 1 was newly isolated and the accumulation body was clear (arrow); (B) the resting cyst at day 2 was observed with morphological changes; (C) the planomeiocyte division at day 3; (D) the empty cyst with a thick wall observable. Scale bars = 20 μm.
Polymerase Chain Reaction Detection of Prorocentrum donghaiense in Surface Sediments
Using P. donghaiense-specific primers (PD201F and PD201R), two of five surface sediment samples obtained from Ningde, Fujian, China were detected positive in the presence of P. donghaiense. The two sequences of PCR products (Supplementary Appendix 1) exhibited 100% identity to a reference sequence of P. donghaiense deposited in NCBI database (Genbank accession No. KX656893), which was from Shin et al. (2019a), demonstrating the presence of P. donghaiense in surface sediments most possibly as resting cyst, or, less likely, as other forms (e.g., vegetative cells or DNA residues).
Validation for the Specificity of the Oligonucleotide Probes Used in Fluorescence in situ Hybridization Detection
For the fluorescence intensity, probes 1, 2, 3, 4, and 6 exhibited weak green fluorescence (Supplementary Figure 1), then probes 5, 7, and 8 were used for specificity test. Prorocentrum donghaiense-specific probes PDprobe-5 (data not shown), PDprobe-7 (data not shown), and PDprobe-8 (Figure 4) exhibited the brightest fluorescence intensity in labeling vegetative cells of P. donghaiense strain CCMA264 among the eight candidate probes. Specificity test with the mixed samples in which Karenia mikimotoi, Karlodinium veneficum, and Prorocentrum minimum was respectively mixed with P. donghaiense showed that all probes were not hybridizing with K. mikimotoi and K. veneficum, while, however, PDprobe-5 and PDprobe-7 hybridized with P. minimum judged from fluorescence intensity (Supplementary Figure 2), but PDprobe-8 did not (Figure 4I). Both the FITC- and Cy3-labeled PDprobe-8 exhibited bright fluorescence in hybridizing P. donghaiense cells (Figures 4B,C) and thus was applied to the following FISH detection of P. donghaiense resting cysts in marine sediment samples.
FIGURE 4
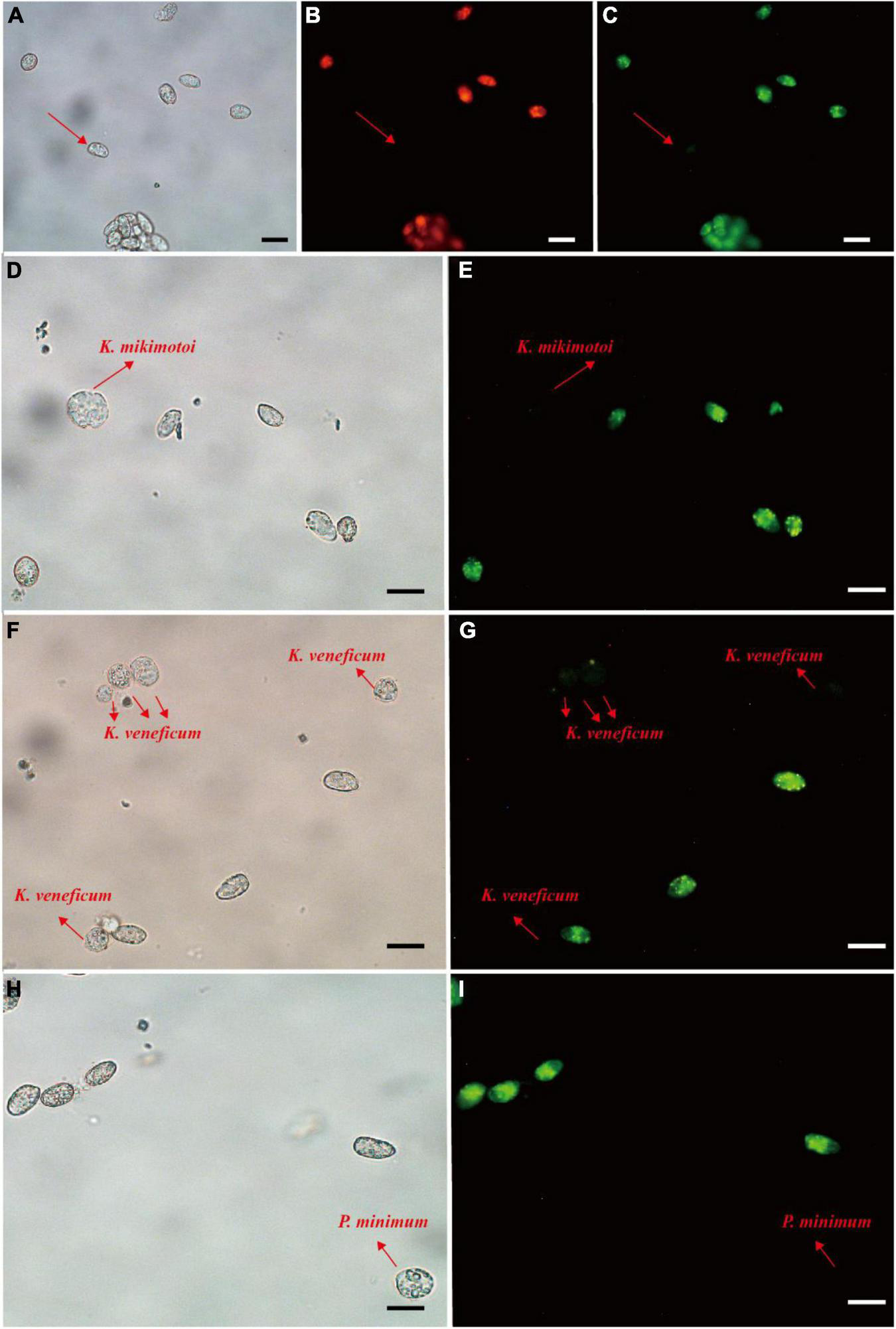
Prorocentrum donghaiense vegetative cell labeled with two fluorescence signal probes (Cy3 and FITC). Cells were observed at bright field LM, orange epifluorescent LM for Cy3-labeled cells and green epifluorescent LM for FITC-labeled cells (A–C). Specificity tests for the PDprobe-8 using mixed samples (D–I), containing P. donghaiense and K. mikimotoi, P. donghaiense and K. veneficum, P. donghaiense and P. minimum, respectively. The arrows indicated the cells were not labeled with probe. Scale bars = 20 μm.
Fluorescence in situ Hybridization Detection of Resting Cysts Presence in Marine Surface Sediments
Seven P. donghaiense resting cysts were detected by FISH using PDprobe-8 labeled with either FITC or Cy3 from the samples collected from Sansha Bay, Ningde, China, which were observed at bright field LM, orange epifluorescent LM for Cy3-labeled cysts and green epifluorescent LM for FITC-labeled cyst, respectively (Figure 5). The cysts hybridized with both the FITC-labeled and Cy3-labeled probes were ellipsoidal to spherical, with a diameter of 10.8–20.0 μm, a relatively thick cyst wall, an accumulation body, some granular inclusions, and absence of chloroplasts (Figure 5), which were almost the same as those cysts observed from clonal cultures described above.
FIGURE 5
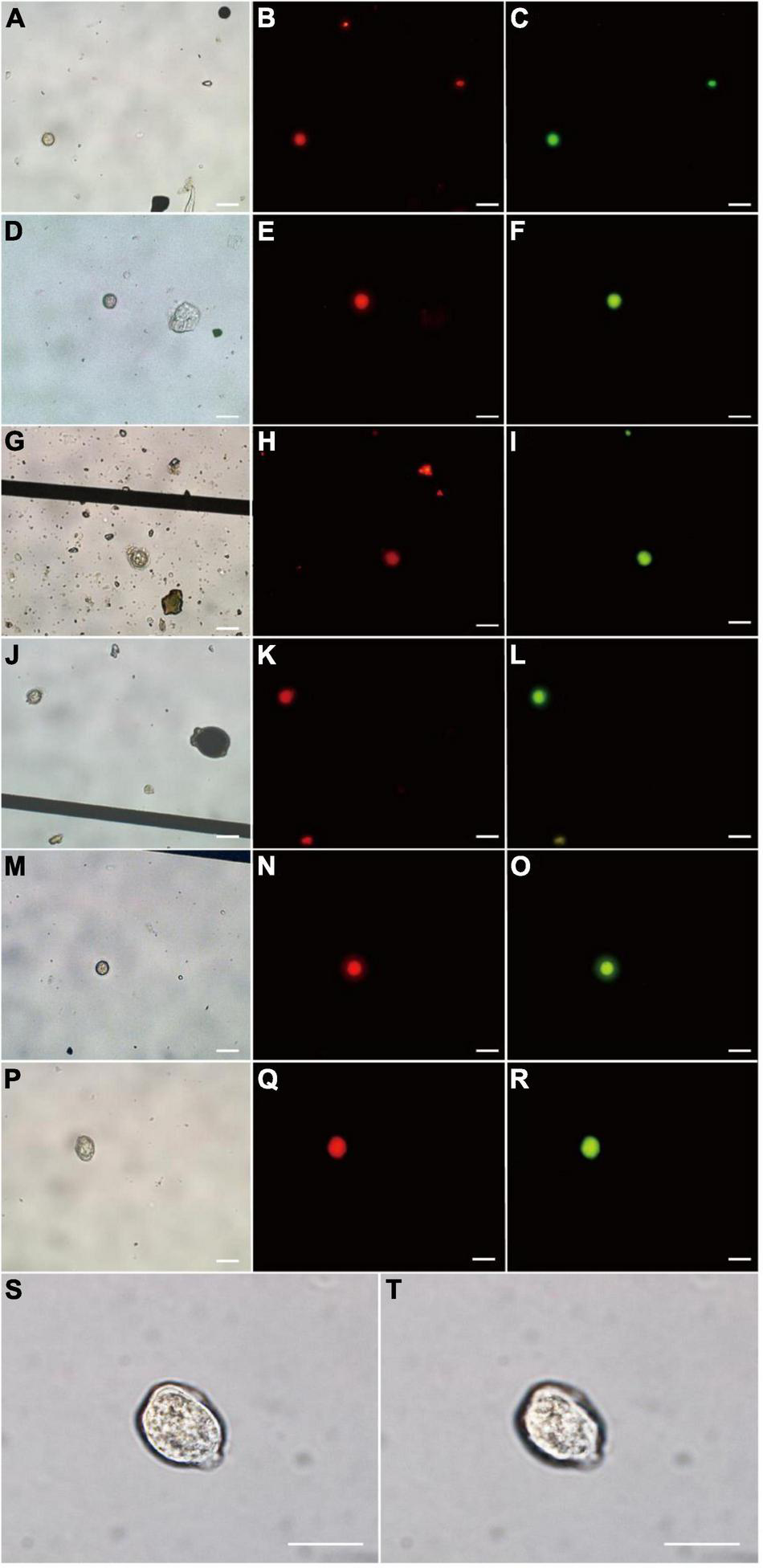
Microscopic observations of Prorocentrum donghaiense resting cysts labeled with two fluorescence signal probes simultaneously (Cy3 and FITC) in sediment. Each cyst was observed at bright field LM (A,D,G,J,M,P), orange epifluorescent LM for Cy3-labeled cysts (B,E,H,K,N,Q) and green epifluorescent LM for FITC-labeled cyst (C,F,I,L,O,R), respectively. (S,T) The cyst in (P) observed at 100× objective lens with different focusing distances. Scale bars = 20 μm.
Confirmation of the Identity of Resting Cysts Using Single-Cell Polymerase Chain Reaction Sequencing
In order to avoid possible pseudo-positive FISH results (e.g., autofluorescence), seven FISH-detected cysts were successfully isolated from the FISH test slide and then identified through single-cell PCR sequencing. Six sequences with 201 bases from the D2 domain of 28S rRNA gene obtained from two of the seven cysts (see Supplementary Appendix 1) were 98–99% identical to the reference sequence of P. donghaiense (accession number KX656893), the identity of which was confirmed by Shin et al. (2019a). In addition, the phylogenetic analysis (Figure 6) also confirmed the identity of those FISH-detected cysts shown in Figure 5 as P. donghaiense (Prorocentrum donghaiense PD2-1, 2-3, 3-3, 3-4, 2-5, 3-1). Note that the other four cysts were simply not successfully sequenced but not identified to be other non-target species.
FIGURE 6
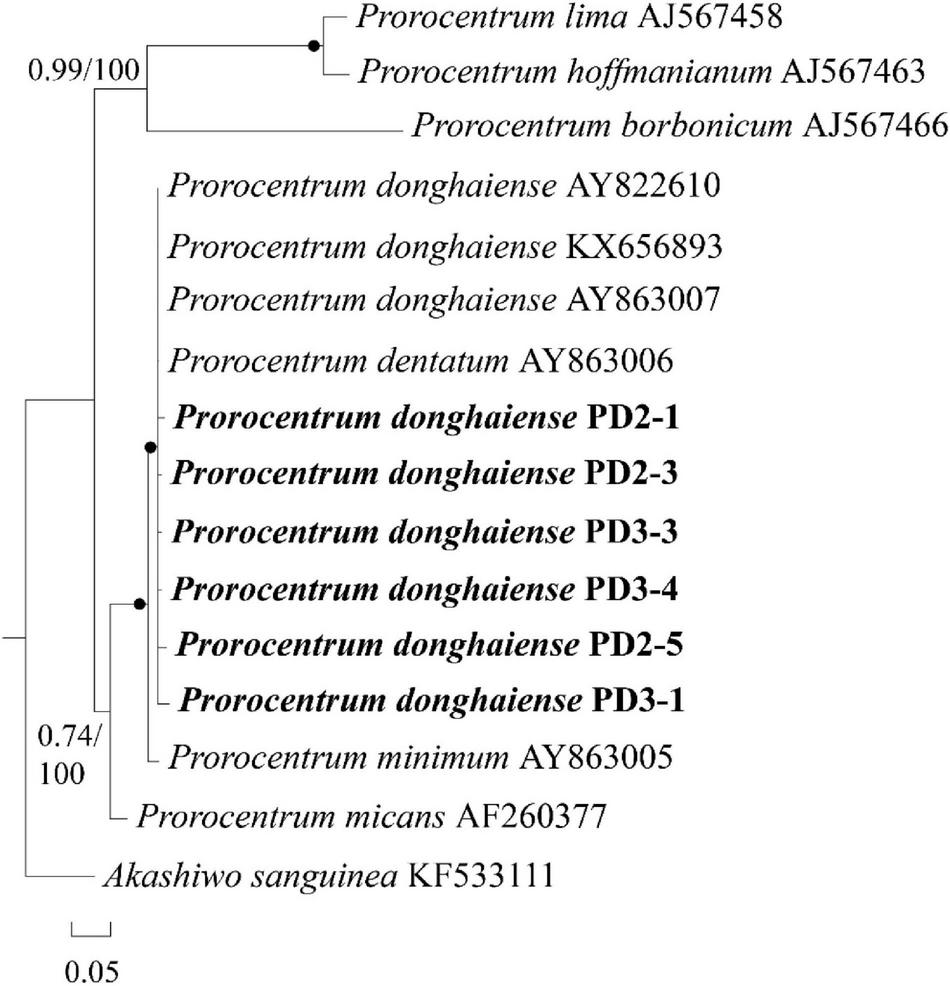
A phylogenetic tree of Prorocentrum donghaiense inferred from partial LSU rRNA gene sequences using Bayesian inference. The two sequences PD-2 and PD-3 were obtained via sing-cell PCR sequencing for the resting cysts that had been detected with FISH from sediment. Numbers on branches are statistical support values [left, Bayesian posterior probabilities; right, maximum likelihood (ML) bootstrap support values]. Bootstrap values > 50% and posterior probabilities (pp) above 0.5 are shown. Black circles (•) indicate maximal support (pp = 1.00 in BI and bootstrap support = 100% in ML, respectively). All branches are drawn to scale.
Estimation of rRNA Gene Copy Numbers in Resting Cysts of Prorocentrum donghaiense
The measured mean value of LSU rRNA gene copies in a single vegetative cell was 1,519 ± 1,249 (Figure 7). Since the variation of LSU rRNA gene copy numbers may reflect that the vegetative cells were at different stages of cell cycle, the data in the inner box plot (between the upper and lower quartile) were thus considered to represent the vegetative cells at the haploid stage, which generated a copy number of 1,279 ± 483 copies per cell. Considering the diploidy of resting cysts, the LSU rRNA gene copy number in P. donghaiense resting cysts was thus estimated to be ∼2,560 copies per cyst.
FIGURE 7
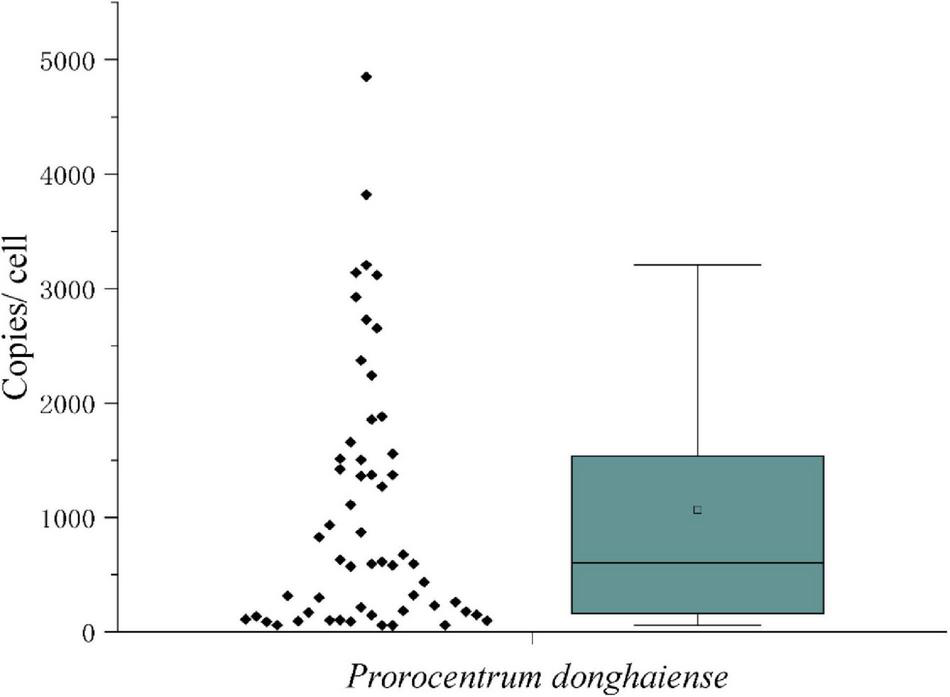
The LSU rRNA gene copy numbers in Prorocentrum donghaiense vegetative cells.
Distribution and Abundance of Prorocentrum donghaiense Resting Cysts in the Sediments of China Seas
Among the 125 sediment samples from China Seas (SCS, ECS, YS, and BS), 36 were detected positive in the presence of P. donghaiense cyst via either qPCR or FISH (Supplementary Table 1). Based on qPCR detection, the calculated abundance of P. donghaiense cysts ranged from 0 to 62 cysts/32 g wet sediment (Figure 8). Via FISH detection, the measured abundance ranged from 0 to 8 cysts/32 g wet sediment in 40 samples (Figure 8). Comparatively, the abundance of P. donghaiense cysts in the ECS was the highest among all four seas (p < 0.05). The percent of samples that were detected positive was 42.8% (N = 70) in the ECS, followed by 20% (N = 25) in the SCS, 4.54% (N = 22) in the YS, and 0 (N = 8) in the BS. Although a regression analysis showed a significant positive correlation between the cyst abundance measurements using qPCR and FISH (R2 = 0.3533, n = 40, p < 0.05; Figure 9), the slope of the regression line (0.495) indicated that FISH method generally generated a lower cyst abundance than qPCR did. In spite of this discrepancy, both FISH and qPCR detected extremely low abundance but wide distribution of P. donghaiense cyst in the sediments collected from the ECS, the SCS, and the YS. The negative detection from the BS deserves a further attention.
FIGURE 8
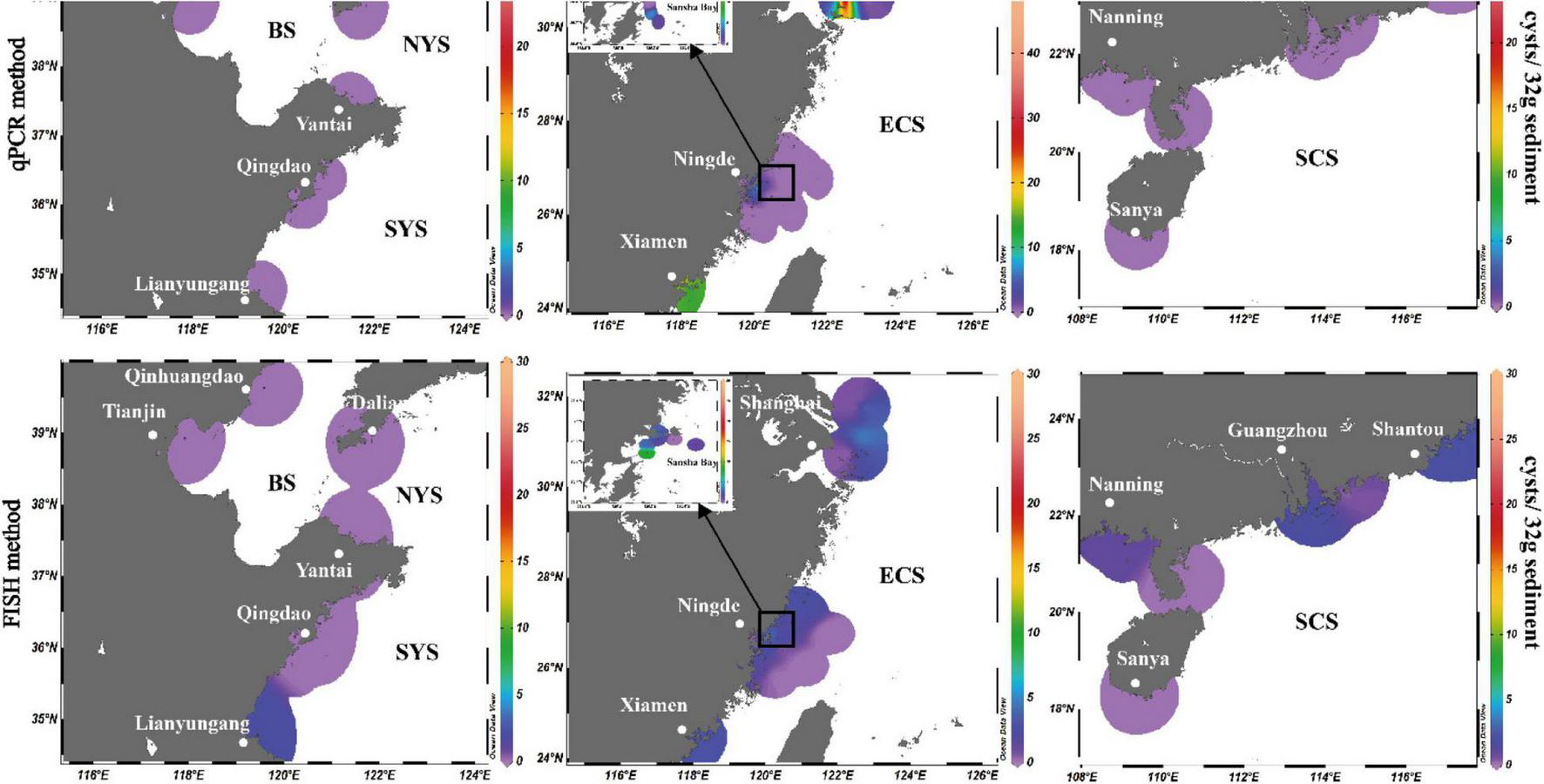
Distribution and abundance of Prorocentrum donghaiense cysts in the sediments along the China coasts as quantified using real-time PCR in combination with FISH method.
FIGURE 9
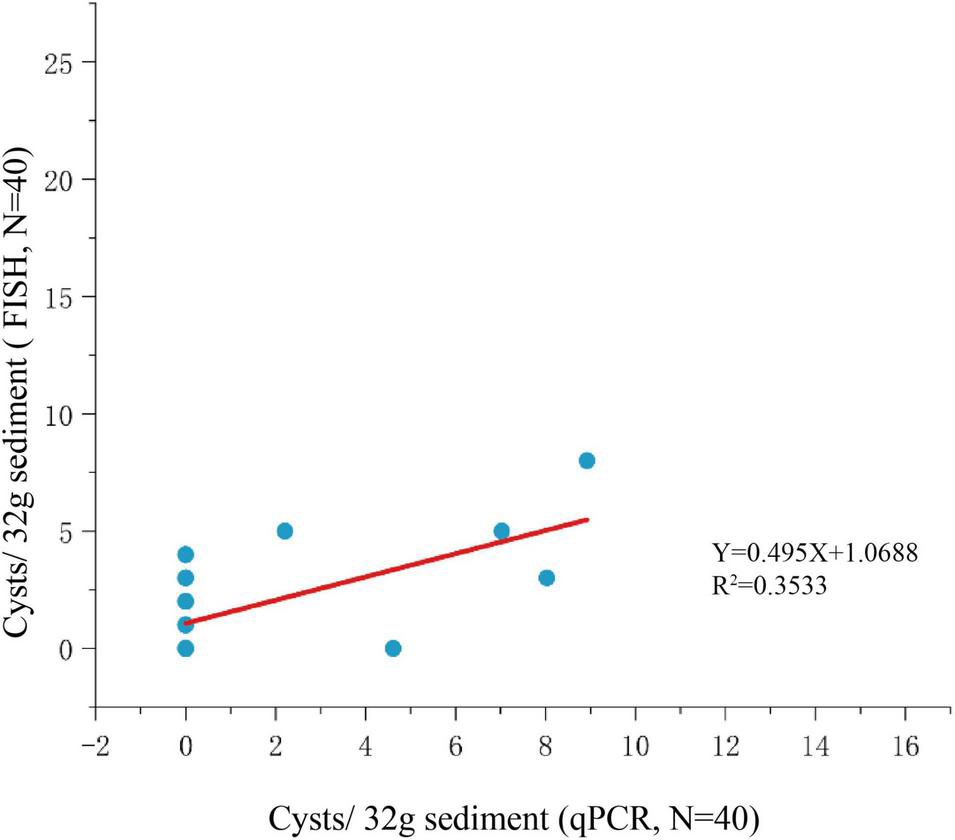
Regression analysis for comparison of qPCR and FISH methods for cyst enumeration.
Discussion
Prorocentrum donghaiense Produces Resting Cysts
Although sexual reproduction has been observed in multiple Prorocentrum species prior to the present work (Bhaud et al., 1988; Faust, 1990, 1993; Cannon, 1993; Berdieva et al., 2020), only three benthic species have been reported to form resting cysts, namely P. lima (Faust, 1993), P. foraminosum (as P. marinum) (Faust, 1990, 1993), and P. leve (Mertens et al., 2017), with the former two cases being questionable in their reliability or completeness of evidences (Hoppenrath et al., 2013). Prorocentrum leve can be considered the first confirmed resting cyst-producing species of the genus (Mertens et al., 2017), as the direct cyst germination from sediment samples together with the subsequent molecular confirmation of species identity are convincing evidences. However, phylogenetic analyses have shown that Prorocentrum is a polyphyletic genus that were composed of at least three groups, with benthic (e.g., P. leve) and planktonic (e.g., P. donghaiense) species consistently belonging to separate clades (Hoppenrath et al., 2013; Mertens et al., 2017). Therefore, P. donghaiense should be viewed as the first planktonic species of Prorocentrum proven to form resting cyst in the life history, as presented here. This encourages us to believe that many more Prorocentrum species (note that Prorocentrum is a specious genus) may be resting cyst producers as well, as suggested in some previous works. For instance, P. cordatum, P. koreanum, and P. micans have been detected in marine sediments via molecular sequencing (Dzhembekova et al., 2018; Jung et al., 2018), suggesting they all may form resting cysts, although the detected molecular signal might be also from fragmental DNA residues of vegetative cells. We provided both microscopic and molecular evidences from laboratory cultures and field sediment samples to document the production of sexual resting cysts by P. donghaiense. Our laboratory culture experiments generated microscopic evidences including cell pairs in mating (cell pair twined in the anterior end at the early stage and fusing at the central area at a later stage), planozygotes with two similar flagella (compared to the vegetative cells having two dissimilar flagella), and thick-walled resting cysts. The resting cysts of P. donghaiense were also sieved out from field sediments that were stored in ∼4°C in darkness for at least 1 year (some longer than 3 years) using species-specific oligonucleotide probes by FISH method and further single cell PCR and sequencing for the cysts detected with FISH. The germination of cyst from cultures together with the cyst from sediment maintaining intactness under a long storage at 4°C justify that the cysts are “resting cysts” rather than “temporary cysts.” The P. donghaiense resting cyst was subspherical to spherical, had a smooth surface, golden brown accumulation body, and thick wall, which is different from the resting cyst of P. leve having two layers of wall and being urn-shaped with plug (Mertens et al., 2017). The resting cyst of P. donghaiense was similar to the cysts of Akashiwo sanguinea (Tang and Gobler, 2015), Alexandrium andersonii (Matsuoka and Fukuyo, 2003), Cochlodinium polykrikoides Malaysia-American ribotype (= Margalefidinium polykrikoides) (Tang and Gobler, 2012), Karenia mikimotoi (Liu et al., 2020a), Karlodinium veneficum (Liu et al., 2020b), and Wangodinium sinense (Luo et al., 2018) in the sense of having a subspherical to spherical shape and a smooth surface (no spine or regular ornament), but different from the cysts of C. polykrikoides East Asian ribotype (= Margalefidinium polykrikoides) (Li et al., 2015), Gymnodinium catenatum (Anderson et al., 1988; Matsuoka and Fukuyo, 2003), Lingulodinium polyedrum (Matsuoka and Fukuyo, 2003), Pheopolykrikos hartmannii (Matsuoka and Fukuyo, 2003), Polykrikos spp. (Matsuoka and Fukuyo, 2003), Pseudocochlodinium profundisulcus (Hu et al., 2021), Protoperidinium spp. (Matsuoka and Fukuyo, 2003), Scrippsiella spp. (Luo et al., 2016), and Tovellia spp. (Lindberg et al., 2005; Pandeirada et al., 2019) in terms of that the resting cysts of all these species have characteristic ornaments or spines on their surface.
Recently, we proved that two Kareniaceae species, K. mikimotoi and K. veneficum, can form either thin- or thick-walled resting cysts both in laboratory cultures and the field, especially identified the cysts from marine sediments using a combination of FISH and single cell PCR sequencing (Liu et al., 2020a,b). This combined approach provided an effective and reliable means to discover resting cysts with small sizes but without surface ornaments or spines, which was also applied in the present work, as the resting cyst of P. donghaiense is small (10.8–27.3 μm), of no discernible surface features, and of low abundance in marine sediments. Therefore, our practices have tested a feasible approach that may be applicable to the detection of novel cyst species or types of dinoflagellates.
Distribution and Abundance of Prorocentrum donghaiense Cyst in the Sediments of Four Seas of China and Possible Implications
As introduced above, P. donghaiense is widely distributed in estuaries and coasts around the world across Asia, Oceania, America, and Europe (Chang, 1988; Lu and Goebel, 2001; Gómez, 2003; Gómez and Boicenco, 2004; Scorzetti et al., 2009; Ajani et al., 2011; Percopo et al., 2011; Takano and Matsuoka, 2011; Muciño-Márquez et al., 2015; Rezende et al., 2015; Shin et al., 2019b). In China, P. donghaiense has been reported in the Bohai Sea since 1970s (recorded as P. dentatum or P. donghaiense) (Song et al., 2016), the Yellow Sea (recorded as P. dentatum) (Liu et al., 2015; Zhang et al., 2016), the South China Sea since 1980s (Hong Kong, also recorded as P. dentatum) (Law, 2018), and the East China Sea since 1990s (recorded as P. dentatum or P. donghaiense) (Lu and Goebel, 2001; Lu et al., 2014). Especially in the East China Sea, the species formed blooms with different scales almost every year during the last three decades, which caused serious impacts on fisheries, public health, and aquatic ecosystems (Lin et al., 2014; Lu et al., 2014). Therefore, mapping the spatial distribution of the resting cysts of this species in the sediments of China Seas will strengthen our understanding of its ecology and provide valuable information for assessing the risk associated with P. donghaiense booms.
Presence or abundance of vegetative cells of P. donghaiense in water body may be affected by seasonality, vertical variation, and environmental factors, but the sediment provides the best place to obtain an inventory of dinoflagellates accumulated for a long period as resting cysts (Ribeiro et al., 2011, 2012). Our detections showed that P. donghaiense cysts are widely distributed in the sediments of the South China Sea, the East China Sea, and the Yellow Sea, which is generally in accordance with the reported presence range of this organism via morphological identification of vegetative cells in water samples (Lu and Goebel, 2001; Lu et al., 2014; Liu et al., 2015; Song et al., 2016; Zhang et al., 2016; Law, 2018). Resting cysts of P. donghaiense exhibited a high frequent presence in the sediment samples taken from the East China Sea, concomitant to the frequent outbreaks of P. donghaiense blooms in this region since 1990s. We also found the distribution of P. donghaiense cysts in the southern Yellow Sea but not northern Yellow Sea, which is also in accordance with previous investigations (Liu et al., 2015; Zhang et al., 2016). In the South China Sea, P. donghaiense has been reported in Hong Kong waters (Law, 2018), we now detected its resting cysts in the entire region (from Beibu Gulf to Shantou, Guangdong province, covering a latitude and longitude range of 1.8° and 8.5°, respectively). Interestingly but also surprisingly, we did not find the resting cysts of this organism in the eight sediment samples of the Bohai Sea, although vegetative cells were reported from this sea area before (Song et al., 2016). Three possibilities may explain this absence: misidentification of the species based solely on LM for such a small-sized species in the previous work (Song et al., 2016), the fewer number of sediment samples we detected, and/or the extremely low abundance of cysts in those samples. This issue deserves further clarification as it pertains to the question whether or not this species has a northmost edge of distribution in the northwest Pacific.
Although resting cyst has been proven to be a vital factor regulating HABs initiation and geographical expansion of dinoflagellates (Anderson et al., 1988, 2014; Bolch and de Salas, 2007; Smayda, 2007; Garrett et al., 2014; Figueroa et al., 2018; Shaw et al., 2019; Kim et al., 2020; Tang et al., 2021) and almost all other well-known HABs-forming dinoflagellates in China have been confirmed for resting cyst production in the field (as reviewed in Tang et al., 2021), it has long been an intriguing and also controversial issue regarding whether or not P. donghaiense produces resting cysts and where the initial “seeding population” came from for the annual blooms of P. donghaiense in the East China Sea. Dai et al. (2013) proposed a “Pelagic Seed Bank Hypothesis” in which the front of the Taiwan Warm Current (TWC) is believed to contain vegetative cells and thus serve as a ‘seed bank’ for the initiation of P. donghaiense blooms in the East China Sea. While we cannot exclude this possibility with data currently available, we also think that the germination of resting cysts may provide the initial population during the early spring and possibly more so in those temperate regions of higher latitudes. By assuming an abundance of P. donghaiense cysts in the sediment as 4 cysts/32 g wet sediment (for the surface sediment of a depth of 2 cm; the abundance of P. donghaiense cysts ranged from 0 to 62 cysts/32 g wet sediment for qPCR assay), a germination rate of 100%, a specific growth rate of 0.4 d–1 (specific growth rates in most treatments above 0.4 d–1 in Hu et al. (2012); a maximum specific growth rate of 1.50 d–1 reported in Hu et al. (2014), a specific density of the wet sediment as 2 g⋅cm–3 (Lu B. et al., 2005), a zero mortality, and a water depth of 2 m, we could estimate that P. donghaiense needs only 23 days to reach a cell density of 107 cells⋅L–1 in the water column, and the time will be shortened if assuming the growth rate above 0.4 d–1. This time requirement is a realistic scenario in the field if cysts germinate in April when the temperature is appropriate for germination as blooms are frequently observed at late April or early May in the East China Sea. The highest abundance (62 cysts/32 g wet sediment) and frequency were detected in Changjiang River Estuary, which is in accordance to the fact that P. donghaiense has been forming HABs annually in this area and at least indicates that the area with frequent and dense blooms is more likely to accumulate more abundant cysts.
In addition, production of resting cysts may have been a mechanistic facility enabled the cosmopolitan distribution of the species. Considering the data from the blooming field are highly limited, these arguments, admittedly, are speculative and the possible roles played by resting cysts of P. donghaiense in initiating blooms and in expanding population distribution need more intensive, particularly onsite, investigations.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
YT: conceptualization, supervision, and funding acquisition. ZH, YL, YD, and YT: methodology, validation, investigation, and writing—review and editing. ZH and YT: resources. ZH and YL: data curation and writing—original draft preparation. ZH: project administration. All authors read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Science Foundation of China, grant number 41476142, the Science & Technology Basic Resources Investigation Program of China, grant number 2018FY100200, the Marine S&T Fund of Shandong Province for Pilot National Laboratory for Marine Science and Technology (Qingdao), grant number 2018SDKJ0504-2, the National Natural Science Foundation of China, grant numbers 41976134 and 41776125, and the Youth Talent Support Program of the Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology (Qingdao), grant number LMEES-YTSP-2018-01-04.
Acknowledgments
We thank Yuanyuan Sun from CAS Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences for her assistance in SEM sample preparation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2022.826736/full#supplementary-material
References
1
Ajani P. Ingleton T. Pritchard T. Armand L. (2011). Microalgal blooms in the coastal waters of New South Wales, Australia.Proc. Linn. Soc. N.S.W.13315–31. 10.3316/informit.820628615111869
2
Anderson D. M. Cembella A. D. Hallegraeff G. M. (2012). Progress in understanding harmful algal blooms: paradigm shifts and new technologies for research, monitoring, and management.Annu. Rev. Mar Sci.4143–176. 10.1146/annurev-marine-120308-081121
3
Anderson D. M. Fensin E. Gobler C. J. Hoeglund A. E. Hubbard K. A. Kulis D. M. et al (2021). Marine harmful algal blooms (HABs) in the United States: history, current status and future trends.Harmful Algae102:101975. 10.1016/j.hal.2021.101975
4
Anderson D. M. Jacobson D. M. Bravo I. Wrenn J. H. (1988). The unique, microreticulate cyst of the naked dinoflagellate Gymnodinium catenatum.J. Phycol.24255–262. 10.1111/j.1529-8817.1988.tb04241.x
5
Anderson D. M. Keafer B. A. Kleindinst J. L. McGillicuddy D. J. Martin J. L. Norton K. et al (2014). Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms.Deep-Sea Res. PT II1036–26. 10.1016/j.dsr2.2013.10.002
6
Anderson D. M. Wall D. (1978). Potential importance of benthic cysts of Gonyaulax tamarensis and G. excavata in initiating toxic dinoflagellate blooms.J. Phycol.14224–234. 10.1111/j.1529-8817.1978.tb02452.x
7
Berdieva M. Kalinina V. Lomert E. Knyazev N. Skarlato S. (2020). Life cycle stages and evidence of sexual reproduction in the marine dinoflagellate Prorocentrum minimum (Dinophyceae, Prorocentrales).J. Phycol.56941–952. 10.1111/jpy.12989
8
Bhaud Y. Soyer-Gobillard M.-O. Salmon J. M. (1988). Transmission of gametic nuclei through a fertilization tube during mating in a primitive dinoflagellate, Prorocentrum micans Ehr.J. Cell Sci.89197–206. 10.1242/jcs.89.2.197
9
Bolch C. J. S. (1997). The use of sodium polytungstate for the separation and concentration of living dinoflagellate cysts from marine sediments.Phycologia36472–478. 10.2216/i0031-8884-36-6-472.1
10
Bolch C. J. S. de Salas M. F. (2007). A review of the molecular evidence for ballast water introduction of the toxic dinoflagellates Gymnodinium catenatum and the Alexandrium “tamarensis complex” to Australasia.Harmful Algae6465–485. 10.1016/j.hal.2006.12.008
11
Boyd P. W. Rynearson T. A. Armstrong E. A. Fu F. Hayashi K. Hu Z. et al (2013). Marine phytoplankton temperature versus growth responses from polar to tropical waters-outcome of a scientific community-wide study.PLoS One8:e63091. 10.1371/journal.pone.0063091
12
Bravo I. Figueroa R. I. (2014). Towards an ecological understanding of dinoflagellate cyst functions.Microorganisms211–32. 10.3390/microorganisms2010011
13
Cai Y. Jiang X. Huang X. (2002). Studies on the red tide of Prorocentrum dentatum in Zhoushan archipelago sea area.Mar. Environ. Sci.2142–45. 10.1007/s11769-002-0041-9
14
Cannon J. A. (1993). “Toxic phytoplankton blooms in the sea,” in Germination of the Toxic Dinoflagellate Alexandrium Minutum, from Sediments in the Port River, South Australia, edsSmaydaT. J.ShimizuY. (Amsterdam: Elsevier Science Publishers B.V.), 103–107.
15
Chai Z. Y. Wang H. Deng Y. Hu Z. Zhong Tang Y. (2020). Harmful algal blooms significantly reduce the resource use efficiency in a coastal plankton community.Sci. Total Environ.704:135381. 10.1016/j.scitotenv.2019.135381
16
Chang F. H. (1988). Distribution, abundance, and size composition of phytoplankton off Westland, New Zealand, February 1982.N. Z. J. Mar. Freshwat. Res.22345–367. 10.1080/00288330.1988.9516307
17
Chang H. F. Williams M. J. M. Schwarz J. N. Hall J. A. Maas E. W. Stewart R. (2013). Spatial variation of phytoplankton assemblages and biomass in the New Zealand sector of the Southern Ocean during the late austral summer 2008.Polar Biol.36391–408. 10.1007/s00300-012-1270-8
18
Chen B. Wang Z. Zhu M. Li R. (2005). Effects of temperature and salinity on growth of Prorocentrum dentatum and comparisons between growths of Prorocentrum dentatum and Skeletonema costatum.Adv. Mar. Sci.2360–64. 10.1111/j.1744-7909.2005.00184.x
19
Chen G. Ma C. Zhang C. Zhou J. Wang Y. Wang G. et al (2013). A rapid and sensitive method for field detection of Prorocentrum donghaiense using reverse transcription-coupled loop-mediated isothermal amplification.Harmful Algae2931–39. 10.1016/j.hal.2013.08.001
20
Chen G. Zhang C. Zhang B. Wang G. Lu D. Xu Z. et al (2011). Development of a PNA probe for fluorescence in situ hybridization detection of Prorocentrum donghaiense.PLoS One6:e25527. 10.1371/journal.pone.0025527
21
Dai X. Lu D. Guan W. Xia P. Wang H. He P. et al (2013). The correlation between Prorocentrum donghaiense blooms and the Taiwan Warm Current in the East China Sea-evidence for the “Pelagic Seed Bank” hypothesis.PLoS One8:e64188. 10.1371/journal.pone.0064188
22
Dai X. Lu D. Xia P. Wang H. He P. Li D. (2014). Impact of water stratification on Prorocentrum donghaiense Lu blooms in high-frequency HAB occurrence area of the East China Sea in 2010-2011.Oceanol. Limnol. Sin.45218–224.
23
Dale B. (2001). The sedimentary record of dinoflagellate cysts: looking back into the future of phytoplankton blooms.Sci. Mar.65257–272. 10.3989/scimar.2001.65s2257
24
Darriba D. Taboada G. L. Doallo R. Posada D. (2012). jModelTest 2: more models, new heuristics and parallel computing.Nat. Methods9:772. 10.1038/nmeth.2109
25
Dzhembekova N. Moncheva S. Ivanova P. Slabakova N. Nagai S. (2018). Biodiversity of phytoplankton cyst assemblages in surface sediments of the Black Sea based on metabarcoding.Biotechnol. Biotec. Eq.321507–1513. 10.1080/13102818.2018.1532816
26
Ellegaard M. Dale B. Mertens K. N. Pospelova V. Ribeiro S. (2017). “Applications of paleoenvironmental techniques in estuarine studies,” in Dinoflagellate Cysts as Proxies for Holocene Environmental Change in Estuaries: Diversity, Abundance and Morphology, edsWeckströmK.SaundersK. M.GellP. A.SkilbeckC. G. (Dordrecht: Springer Netherlands), 295–312.
27
Ellegaard M. Ribeiro S. (2018). The long-term persistence of phytoplankton resting stages in aquatic ‘seed banks’.Biol. Rev.93166–183. 10.1111/brv.12338
28
Faust M. A. (1990). “Toxic marine phytoplankton,” in Cysts of Prorocentrum Marinum (Dinophyceae) in Floating Detritus at Twin Cays, Belize Mangrove Habitats, ed.GranéliE. (New York, NY: Elsevier Science Publishing Co., Inc.), 138–143.
29
Faust M. A. (1993). “Toxic phytoplankton blooms in the sea,” in Sexuality in a Toxic Dinoflagellate, Prorocentrum lima, edsSmaydaT. J.ShimizuY. (New York, NY: Elsevier Science Publishing Co., Inc.), 121–126.
30
Figueroa R. I. Bravo I. (2005). A study of the sexual reproduction and determination of mating type of Gymnodinium nolleri (Dinophyceae) in culture.J. Phycol.4174–83. 10.1111/j.1529-8817.2005.04045.x
31
Figueroa R. I. Estrada M. Garcés E. (2018). Life histories of microalgal species causing harmful blooms: haploids, diploids and the relevance of benthic stages.Harmful Algae7344–57. 10.1016/j.hal.2018.01.006
32
Fu F. Tatters A. O. Hutchins D. A. (2012). Global change and the future of harmful algal blooms in the ocean.Mar. Ecol. Prog. Ser.470207–233. 10.3354/meps10047
33
Garrett M. J. Puchulutegui C. Selwood A. I. Wolny J. L. (2014). Identification of the harmful dinoflagellate Vulcanodinium rugosum recovered from a ballast tank of a globally traveled ship in Port Tampa Bay. Florida, USA.Harmful Algae39202–209. 10.1016/j.hal.2014.07.014
34
Glibert P. M. (2014). Harmful Algal Blooms in Asia: an insidious and escalating water pollution phenomenon with effects on ecological and human health.ASIA Netw. Exchange2152–68. 10.16995/ane.46
35
Glibert P. M. Berdalet E. Burford M. A. Pitcher G. C. Zhou M. (2018). “Global ecology and oceanography of harmful algal blooms,” in Harmful Algal Blooms and the Importance of Understanding Their Ecology and Oceanography, edsGlibertP. M.BerdaletE.BurfordM. A.PitcherG. C.ZhouM. (Cham: Springer International Publishing), 9–25.
36
Gómez F. (2003). Checklist of mediterranean free-living dinoflagellates.Bot. Mar.46215–242. 10.1515/BOT.2003.021
37
Gómez F. Boicenco L. (2004). An annotated checklist of dinoflagellates in the Black Sea.Hydrobiologia51743–59. 10.1023/B:HYDR.0000027336.05452.07
38
Gu H. Wu Y. Lü S. Lu D. Tang Y. Z. Qi Y. (2021). Emerging harmful algal bloom species over the last four decades in China.Harmful Algae111:102059. 10.1016/j.hal.2021.102059
39
Guillard R. R. L. (1975). “Culture of marine invertebrate animals,” in Culture of Phytoplankton for Feeding Marine Invertebrates, edsSmithW. L.ChanleyM. H. (New York, NY: Plenum Press), 26–60.
40
Guilloux L. Rigaut-Jalabert F. Jouenne F. Ristori S. Viprey M. Not F. et al (2013). An annotated checklist of Marine Phytoplankton taxa at the SOMLIT-Astan time series off Roscoff (Western English Channel, France): data collected from 2000 to 2010.Cah. Biol. Mar.54247–256.
41
Hall T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT.Nucleic Acids Symp. Ser.4195–98. 10.1021/bk-1999-0734.ch008
42
Hernández-Becerril D. U. Cortés Altamirano R. Alonso R. R. (2000). The dinoflagellate genus Prorocentrum along the coasts of the Mexican Pacific.Hydrobiologia418111–121. 10.1023/A:1003806719515
43
Hong H. Wang M. Huang X. Wang D. (2009). Effects of macronutrient additions on nickel uptake and distribution in the dinoflagellate Prorocentrum donghaiense Lu.Environ. Pollut.1571933–1938. 10.1016/j.envpol.2009.01.009
44
Hoppenrath M. Chomérat N. Horiguchi T. Schweikert M. Nagahama Y. Murray S. (2013). Taxonomy and phylogeny of the benthic Prorocentrum species (Dinophyceae)—A proposal and review.Harmful Algae271–28. 10.1016/j.hal.2013.03.006
45
Hu Z. Deng Y. Li Y. Tang Y. Z. (2018). The morphological and phylogenetic characterization for the dinoflagellate Margalefidinium fulvescens (=Cochlodinium fulvescens) isolated from the Jiaozhou Bay, China.Acta Oceanol. Sin.3711–17. 10.1007/s13131-018-1295-0
46
Hu Z. Deng Y. Luo Z. Shang L. Kong F. Gu H. et al (2020). Characterization of the unarmored dinoflagellate Pseliodinium pirum (Ceratoperidiniaceae) from Jiaozhou Bay, China.Phycol. Res.683–13. 10.1111/pre.12385
47
Hu Z. Duan S. Xu N. Mulholland M. R. (2014). Growth and nitrogen uptake kinetics in cultured Prorocentrum donghaiense.PLoS One9:e94030. 10.1371/journal.pone.0094030
48
Hu Z. Mulholland M. R. Duan S. Xu N. (2012). Effects of nitrogen supply and its composition on the growth of Prorocentrum donghaiense.Harmful Algae1372–82. 10.1016/j.hal.2011.10.004
49
Hu Z. Mulholland M. R. Xu N. Duan S. (2016). Effects of temperature, irradiance and pCO2 on the growth and nitrogen utilization of Prorocentrum donghaiense.Aquat. Microb. Ecol.77155–166. 10.3354/ame01793
50
Hu Z. Xu N. Gu H. Chai Z. Takahashi K. Li Z. et al (2021). Morpho-molecular description of a new HAB species, Pseudocochlodinium profundisulcus gen. et sp. nov., and its LSU rRNA gene based genetic diversity and geographical distribution.Harmful Algae108:102098. 10.1016/j.hal.2021.102098
51
Huang B. Ou L. Hong H. Luo H. Wang D. (2005). Bioavailability of dissolved organic phosphorus compounds to typical harmful dinoflagellate Prorocentrum donghaiense Lu.Mar. Pollut. Bull.51838–844. 10.1016/j.marpolbul.2005.02.035
52
Jing X. Lin S. Zhang H. Koerting C. Yu Z. (2017). Utilization of urea and expression profiles of related genes in the dinoflagellate Prorocentrum donghaiense.PLoS One12:e0187837. 10.1371/journal.pone.0187837
53
Jung S. W. Kang D. Kim H.-J. Shin H. H. Park J. S. Park S. Y. et al (2018). Mapping distribution of cysts of recent dinoflagellate and Cochlodinium polykrikoides using next-generation sequencing and morphological approaches in South Sea, Korea.Sci. Rep.8:7011. 10.1038/s41598-018-25345-4
54
Karlson B. Andersen P. Arneborg L. Cembella A. Eikrem W. John U. et al (2021). Harmful algal blooms and their effects in coastal seas of Northern Europe.Harmful Algae102:101989. 10.1016/j.hal.2021.101989
55
Katoh K. Misawa K. Kuma K. I. Miyata T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform.Nucleic Acids Res.303059–3066. 10.1093/nar/gkf436
56
Kim Y. O. Choi J. Baek S. H. Lee M. Oh H.-M. (2020). Tracking Alexandrium catenella from seed-bed to bloom on the southern coast of Korea.Harmful Algae99:101922. 10.1016/j.hal.2020.101922
57
Law S. P. C. (2018). Red Tide Species in Hong Kong.Hong Kong: Agriculture, fisheries and conservation department the government of the Hong Kong special administrative region.
58
Lee C. K. Park T. G. Park Y. T. Lim W. A. (2013). Monitoring and trends in harmful algal blooms and red tides in Korean coastal waters, with emphasis on Cochlodinium polykrikoides.Harmful Algae30S3–S14. 10.1016/j.hal.2013.10.002
59
Li D. Zhang H. Chen X. Xie Z. Zhang Y. Zhang S. et al (2018). Metaproteomics reveals major microbial players and their metabolic activities during the blooming period of a marine dinoflagellate Prorocentrum donghaiense.Environ. Microbiol.20632–644. 10.1111/1462-2920.13986
60
Li J. Glibert P. M. Zhou M. (2010). Temporal and spatial variability in nitrogen uptake kinetics during harmful dinoflagellate blooms in the East China Sea.Harmful Algae9531–539. 10.1016/j.hal.2010.03.007
61
Li L. Lü S. Cen J. (2019). Spatio-temporal variations of Harmful algal blooms along the coast of Guangdong, Southern China during 1980–2016.J. Oceanol. Limnol.37535–551. 10.1007/s00343-019-8088-y
62
Li Z. Han M. S. Matsuoka K. Kim S. Y. Shin H. H. (2015). Identification of the resting cyst of Cochlodinium polykrikoides Margalef (Dinophyceae, Gymnodiniales) in Korean coastal sediments.J. Phycol.51204–210. 10.1111/jpy.12252
63
Lin J. Yan T. Zhang Q. Wang Y. Liu Q. Zhou M. (2014). In situ detrimental impacts of Prorocentrum donghaiense blooms on zooplankton in the East China Sea.Mar. Pollut. Bull.88302–310. 10.1016/j.marpolbul.2014.08.026
64
Lindberg K. Moestrup O. Daugbjerg N. (2005). Studies on woloszynskioid dinoflagellates I: Woloszynskia coronata re-examined using light and electron microscopy and partial LSU rDNA sequences, with description of Tovellia gen. nov. and Jadwigia gen. nov. (Tovelliaceae fam. nov.).Phycologia44416–440.
65
Litaker R. W. Vandersea M. W. Kibler S. R. Reece K. S. Stokes N. A. Steidinger K. A. et al (2003). Identification of Pfiesteria piscicida (Dinophyceae) and pfiesteria-like organisms using internal transcribed spacer-specific PCR assays.J. Phycol.39754–761. 10.1046/j.1529-8817.2003.02112.x
66
Liu H. Huang Y. Zhai W. Guo S. Jin H. Sun J. (2015). Phytoplankton communities and its controlling factors in summer and autumn in the southern Yellow Sea, China.Acta Oceanol. Sin.34114–123. 10.1007/s13131-015-0620-0
67
Liu Y. Hu Z. Deng Y. Tang Y. Z. (2020a). Evidence for production of sexual resting cysts by the toxic dinoflagellate Karenia mikimotoi in clonal cultures and marine sediments.J. Phycol.56121–134. 10.1111/jpy.12925
68
Liu Y. Hu Z. Deng Y. Tang Y. Z. (2020b). Evidence for resting cyst production in the cosmopolitan toxic dinoflagellate Karlodinium veneficum and the cyst distribution in the China seas.Harmful Algae93:101788. 10.1016/j.hal.2020.101788
69
Lu B. Li G. X. Huang S. J. Zhang F. S. (2005). The comparing of seabed sediment acoustic-physical properties in the Yellow Sea, the East China Sea and northern the South China Sea.Ocean Technol.2428–32. 10.3969/j.issn.1003-2029.2005.02.008
70
Lu D. Goebel J. (2001). Five red tide species in genus Prorocentrum including the description of Prorocentrum donghaiense Lu SP. nov. from the East China Sea.J. Oceanol. Limnol.19337–344. 10.1007/BF02850738
71
Lu D. Goebel J. Qi Y. Zou J. Han X. Gao Y. et al (2005). Morphological and genetic study of Prorocentrum donghaiense Lu from the East China Sea, and comparison with some related Prorocentrum species.Harmful Algae4493–505. 10.1016/j.hal.2004.08.015
72
Lu D. Qi Y. Gu H. Dai X. Wang H. Gao Y. et al (2014). Causative species of harmful algal blooms in Chinese coastal waters.Algol. Stud.145145–168. 10.1127/1864-1318/2014/0161
73
Luo Z. Hu Z. Tang Y. Mertens K. N. Leaw C. P. Lim P. T. et al (2018). Morphology, ultrastructure, and molecular phylogeny of Wangodinium sinense gen. et sp. nov. (Gymnodiniales, Dinophyceae) and revisiting of Gymnodinium dorsalisulcum and Gymnodinium impudicum.J. Phycol.54744–761. 10.1111/jpy.12780
74
Luo Z. Mertens K. N. Bagheri S. Aydin H. Takano Y. Matsuoka K. et al (2016). Cyst-theca relationship and phylogenetic positions of Scrippsiella plana sp. nov. and S. spinifera (Peridiniales, Dinophyceae).Eur. J. Phycol.51188–202. 10.1080/09670262.2015.1120348
75
Matantseva O. Berdieva M. Kalinina V. Pozdnyakov I. Pechkovskaya S. Skarlato S. (2020). Stressor-induced ecdysis and thecate cyst formation in the armoured dinoflagellates Prorocentrum cordatum.Sci. Rep.10:18322. 10.1038/s41598-020-75194-3
76
Matsuoka K. Fukuyo Y. (2003). “Manual on harmful marine microalgae,” in Taxonomy of Cysts, edsHallegraeffG. M.AndersonD. M.CembellaA. D. (Paris: United Nations Educational, Scientific and Cultural Organization), 563–592.
77
Mertens K. N. Gu H. Pospelova V. Chomérat N. Nézan E. Gurdebeke P. R. et al (2017). First record of resting cysts of the benthic dinoflagellate Prorocentrum leve in a natural reservoir in Gujan-Mestras, Gironde, France.J. Phycol.531193–1205. 10.1111/jpy.12582
78
Muciño-Márquez R. E. Gárate-Lizárraga I. López-Cortés D. J. (2015). Variación estacional del género Prorocentrum (Dinophyceae) en dos granjas atuneras en la bahía de la paz, México.Acta Biol. Colomb.20195–206. 10.15446/abc.v20n1.42442
79
Okolodkov Y. B. (1998). A checklist of dinoflagellates recorded from the Russian Arctic seas.Sarsia83267–292. 10.1080/00364827.1998.10413687
80
Ou L. Huang B. Hong H. Qi Y. Lu S. (2010). Comparative alkaline phosphatase characteristics of the algal bloom dinoflagellates Prorocentrum donghaiense and Alexandrium catenella, and the diatom Skeletonema costatum.J. Phycol.46260–265. 10.1111/j.1529-8817.2009.00800.x
81
Pandeirada M. S. Craveiro S. C. Daugbjerg N. Moestrup O. Domingues P. Calado A. J. (2019). Studies on Woloszynskioid dinoflagellates X: ultrastructure, phylogeny and colour variation in Tovellia rubescens n. sp. (Dinophyceae).J. Eukaryot. Microbiol.66937–953. 10.1111/jeu.12745
82
Park T. G. Kim J. J. Kim W. J. Won K. M. (2016). Development of real-time RT-PCR for detecting viable Cochlodinium polykrikoides (Dinophyceae) cysts in sediment.Harmful Algae6036–44. 10.1016/j.hal.2016.10.005
83
Parke M. Dixon P. S. (1976). Check-list of British marine algae-third revision.J. Mar. Biol. Assoc. U.K.56527–594. 10.1017/S002531540002066X
84
Percopo I. Siano R. Cerino F. Sarno D. Zingone A. (2011). Phytoplankton diversity during the spring bloom in the northwestern Mediterranean Sea.Bot. Mar.54243–267. 10.1515/bot.2011.033
85
Rezende K. R. V. D. Hatherly M. M. F. Pimenta C. M. M. Eduardo J. Vianna S. D. C. Mangiavacchi N. (2015). Phytoplankton community structure in one sector of Guanabara Bay (RJ, Brazil) during 2011 and 2012.Braz. J. Oceanogr.63239–254. 10.1590/S1679-87592015086506303
86
Ribeiro S. Amorim A. Andersen T. J. Abrantes F. Ellegaard M. (2012). Reconstructing the history of an invasion: the toxic phytoplankton species Gymnodinium catenatum in the Northeast Atlantic.Biol. Invasions14969–985. 10.1007/s10530-011-0132-6
87
Ribeiro S. Berge T. Lundholm N. Andersen T. J. Abrantes F. Ellegaard M. (2011). Phytoplankton growth after a century of dormancy illuminates past resilience to catastrophic darkness.Nat. Commun.2:311. 10.1038/ncomms1314
88
Ronquist F. Huelsenbeck J. P. (2003). MrBayes 3: bayesian phylogenetic inference under mixed models.Bioinformatics191572–1574. 10.1093/bioinformatics/btg180
89
Roselli L. Vadrucci M. R. Fanelli F. Ungaro N. Caroppo C. (2019). First bloom event of the small dinoflagellate Prorocentrum shikokuense in the Mediterranean Sea: cryptogenic or introduced?Mar. Pollut. Bull.139197–204. 10.1016/j.marpolbul.2018.12.034
90
Sakamoto S. Lim W. A. Lu D. Dai X. Orlova T. Iwataki M. (2021). Harmful algal blooms and associated fisheries damage in East Asia: current status and trends in China, Japan, Korea and Russia.Harmful Algae102:101787. 10.1016/j.hal.2020.101787
91
Scorzetti G. Brand L. E. Hitchcock G. L. Rein K. S. Sinigalliano C. D. Fell J. W. (2009). Multiple simultaneous detection of Harmful Algal Blooms (HABs) through a high throughput bead array technology, with potential use in phytoplankton community analysis.Harmful Algae8196–211. 10.1016/j.hal.2008.05.003
92
Shaw J. L. A. Weyrich L. S. Hallegraeff G. Cooper A. (2019). Retrospective eDNA assessment of potentially harmful algae in historical ship ballast tank and marine port sediments.Mol. Ecol.282476–2485. 10.1111/mec.15055
93
Shi X. Liu L. Li Y. Xiao Y. Ding G. Lin S. et al (2018). Isolation of an algicidal bacterium and its effects against the harmful-algal- bloom dinoflagellate Prorocentrum donghaiense (Dinophyceae).Harmful Algae8072–79. 10.1016/j.hal.2018.09.003
94
Shin H. H. Li Z. Mertens K. N. Seo M. H. Gu H. Lim W. A. et al (2019a). Prorocentrum shikokuense Hada and P. donghaiense Lu are junior synonyms of P. obtusidens Schiller, but not of P. dentatum Stein (Prorocentrales, Dinophyceae).Harmful Algae89:101686. 10.1016/j.hal.2019.101686
95
Shin H. H. Li Z. Seo M. H. Soh H. Y. Lim W. A. Park J. W. (2019b). Harmful dinoflagellate Prorocentrum donghaiense Lu is widely distributed along the East China Sea and Korean coastal area.Ocean Sci. J.54685–691. 10.1007/s12601-019-0028-4
96
Smayda T. J. (2007). Reflections on the ballast water dispersal—harmful algal bloom paradigm.Harmful Algae6601–622. 10.1016/j.hal.2007.02.003
97
Song N. Wang N. Lu Y. Zhang J. (2016). Temporal and spatial characteristics of harmful algal blooms in the Bohai Sea during 1952–2014.Cont. Shelf Res.12277–84. 10.1016/j.csr.2016.04.006
98
Takano Y. Matsuoka K. (2011). A comparative study between Prorocentrum shikokuense and P. donghaiense (Prorocentrales, Dinophyceae) based on morphology and DNA sequences.Plankton Bethos Res.6179–186. 10.3800/pbr.6.179
99
Tang Y. Z. Gobler C. J. (2012). The toxic dinoflagellate Cochlodinium polykrikoides (Dinophyceae) produces resting cysts.Harmful Algae2071–80. 10.1016/j.hal.2012.08.001
100
Tang Y. Z. Gobler C. J. (2015). Sexual resting cyst production by the dinoflagellate Akashiwo sanguinea: a potential mechanism contributing to the ubiquitous distribution of a harmful alga.J. Phycol.51298–309. 10.1111/jpy.12274
101
Tang Y. Z. Gu H. Wang Z. Liu D. Wang Y. Lu D. et al (2021). Exploration of resting cysts (stages) and their relevance for possibly HABs-causing species in China.Harmful Algae107:102050. 10.1016/j.hal.2021.102050
102
Thompson J. D. Higgins D. G. Gibson T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice.Nucleic Acids Res.224673–4680. 10.1093/nar/22.22.4673
103
Viličić D. Marasović I. Mioković D. (2002). Checklist of phytoplankton in the eastern Adriatic Sea.Acta Bot. Croat.6157–91.
104
Wang D. Huang X. Chan L. L. Hong H. (2007). Development of an immunofluorescence technique for detecting Prorocentrum donghaiense Lu.J. Appl. Phycol.19325–332. 10.1007/s10811-006-9139-2
105
Wang H. Hu Z. Chai Z. Deng Y. Zhan Z. Tang Y. Z. (2020). Blooms of Prorocentrum donghaiense reduced the species diversity of dinoflagellate community.Acta Oceanol. Sin.39110–119. 10.1007/s13131-020-1585-1
106
Wang J. Wu J. (2009). Occurrence and potential risks of harmful algal blooms in the East China Sea.Sci. Total Environ.4074012–4021. 10.1016/j.scitotenv.2009.02.040
107
Wang L. Yan T. Tan Z. Zhou M. (2003). Effects of Alexandrium tamarense and Prorocentrum donghaiense on rotifer Brachionus plicatilis population.Chin. J. Appl. Ecol.141151–1155.
108
Wells M. L. Trainer V. L. Smayda T. J. Karlson B. S. O. Trick C. G. Kudela R. M. et al (2015). Harmful algal blooms and climate change: learning from the past and present to forecast the future.Harmful Algae4968–93. 10.1016/j.hal.2015.07.009
109
Xu N. Duan S. Li A. Zhang C. Cai Z. Hu Z. (2010). Effects of temperature, salinity and irradiance on the growth of the harmful dinoflagellate Prorocentrum donghaiense Lu.Harmful Algae913–17. 10.1016/j.hal.2009.06.002
110
Xu Y. Zhang T. Zhou J. (2019). Historical occurrence of algal blooms in the Northern Beibu Gulf of China and implications for future trends.Front. Microbiol.10:451. 10.3389/fmicb.2019.00451
111
Yan T. Zhang Y. Han G. Chen Y. Zhou M. (2007). Experimental study on the harmful effects of large-scale HABs in the East China Sea-the toxicity to Neomysis awatschensis and Artemia salina.Stud. Mar. Sin.48166–175.
112
Yu R. Lü S. H. Qi Y. Zhou M. J. (2020). Progress and perspectives of harmful algal bloom studies in China.Oceanol. Limnol. Sin.51768–788. 10.11693/hyhz20200400127
113
Yu R. C. Lü S. H. Liang Y. B. (2018). “Global ecology and oceanography of harmful algal blooms,” in Harmful Algal Blooms in the Coastal Waters of China, edsGlibertP. M.BerdaletE.BurfordM. A.PitcherG. C.ZhouM. (Cham: Springer International Publishing), 309–316.
114
Zeng Y. Lu D. Wang P. Guo R. Guan W. Dai X. (2020). Advanced researches on the relationship between Prorocentrum donghaiense blooms and the Taiwan Warm Current.J. Mar. Sci.3838–48. 10.3969/j.issn.1001-909X.2020.02.005
115
Zhai X. Deng Y. Sun Y. Leaw C. P. Lim P. T. Zhao Z. et al (2019). Morphological and ultrastructural comparison and phylogenetic analyses for the East Asian and American/Malaysian ribotypes of Margalefidinium polykrikoides.Oceanol. Limnol. Sin.501252–1262.
116
Zhang S. Leng X. Feng Y. Ding C. Yang Y. Wang J. et al (2016). Ecological provinces of spring phytoplankton in the Yellow Sea: species composition.Acta Oceanol. Sin.35114–125. 10.1007/s13131-016-0872-3
117
Zhang S. Zhang Y. Ou L. Qi Y. Lu S. (2012). Study on phagotrophic behavior of six harmful dinoflagellates on fluorescent labeled algae.Oceanol. Limnol. Sin.43602–608.
118
Zhang Y. Lin X. Li T. Li H. Lin L. Luo H. et al (2020). High throughput sequencing of 18S rRNA and its gene to characterize a Prorocentrum shikokuense (Dinophyceae) bloom.Harmful Algae94:101809. 10.1016/j.hal.2020.101809
119
Zhu D. Lu D. Wang Y. Su J. (2009). The low temperature characteristics in Zhejiang coastal region in the early spring of 2005 and its influence on harmful algae bloom occurrence of Prorocentrum donghaiense.Acta Oceanol. Sin.3131–39. 10.3321/j.issn:0253-4193.2009.06.004
Summary
Keywords
resting cyst mapping, fluorescence in situ hybridization (FISH), harmful algal blooms (HABs), life cycle (history), Prorocentrum donghaiense
Citation
Hu Z, Liu Y, Deng Y and Tang YZ (2022) The Notorious Harmful Algal Blooms-Forming Dinoflagellate Prorocentrum donghaiense Produces Sexual Resting Cysts, Which Widely Distribute Along the Coastal Marine Sediment of China. Front. Mar. Sci. 9:826736. doi: 10.3389/fmars.2022.826736
Received
01 December 2021
Accepted
24 January 2022
Published
28 February 2022
Volume
9 - 2022
Edited by
Gleyci A. O. Moser, Rio de Janeiro State University, Brazil
Reviewed by
Haifeng Gu, Third Institute of Oceanography, State Oceanic Administration, China; Chih-Ching Chung, National Taiwan Ocean University, Taiwan
Updates
Copyright
© 2022 Hu, Liu, Deng and Tang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Zhong Tang, yingzhong.tang@qdio.ac.cn
†These authors have contributed equally to this work
This article was submitted to Marine Ecosystem Ecology, a section of the journal Frontiers in Marine Science
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.