- 1South African Institute for Aquatic Biodiversity, Makhanda, South Africa
- 2Department of Ichthyology and Fisheries Science, Rhodes University, Makhanda, South Africa
Estuaries serve as important nursery habitats for several coastal fishery species. The successful recruitment of larvae and early juveniles into estuaries is paramount for population persistence and maintenance. Several factors have been proposed as stimuli that could elicit a recruitment response in estuary-associated fish species. Larvae and early juveniles may trace land-based cues back to an estuary by following the olfactory concentration gradient or use other visual or acoustic stimuli. Argyrosomus japonicus is an iconic estuarine-associated species. Due to overfishing, reduced freshwater input and habitat degradation in their estuarine nursery habitat, the South African population has suffered severe stock declines. Turbidity associated with high freshwater input is thought to promote recruitment into estuaries. We used choice-chamber laboratory experiments to test the hypothesis that settlement-stage A. japonicus are attracted to turbidity rather than olfactory gradients when recruiting into estuaries. Three choice experiments (with three replicate trials each) were performed over three consecutive days. Each experiment used paired combinations of six estuarine/seawater types with varying turbidity and olfactory characteristics. For each experiment, three trials were repeated in succession with six new fish for each trial. Settlement-stage A. japonicus showed a significant preference for turbid water (with and without olfactory cues) over seawater (no olfactory cues) and clear estuary water (with olfactory cues). No clear choice was made between clear estuary water (with olfactory cues) and clear artificial seawater (without olfactory cues), suggesting that turbidity gradients are most likely the primary factor governing the recruitment of settlement-stage A. japonicus into estuaries.
Introduction
Fish may utilize acoustic, visual, and chemical cues to identify and recruit into nursery habitats (Gouraguine et al., 2017). Studies on early-stage temperate sparids and sciaenids have highlighted the importance of olfaction in locating estuarine nursery habitats (James et al., 2008; Radford et al., 2012; Havel and Fuiman, 2015). Olfactory cues that allow for discrimination between habitat types may come from a variety of compounds including amino acids, lipids as well as mannitol from algae and lignins from seagrasses and terrestrial plants (Dixson et al., 2008; Havel and Fuiman, 2015; Gouraguine et al., 2017). Olfactory cues may be particularly important for species using littoral vegetated habitats in estuaries (such as seagrasses), with larvae of the sparids Rhabosargus holubi and Pagrus auratus and the sciaenid Sciaenops ocellatus preferring water collected from or near seagrass beds over seawater (James et al., 2008; Radford et al., 2012; Havel and Fuiman, 2015). However, it is not clear whether this is also true for demersal species that often utilize the deeper channels of estuaries, which may be responding to visual cues such as turbidity.
The dusky kob, Argyrosomus japonicus, is a widely distributed sciaenid, which occurs in temperate and subtropical waters of the Indian and Pacific Oceans around Africa, Australia, India, Pakistan, China, Korea, and Japan (Silberschneider and Gray, 2008). Spawning occurs in the nearshore marine environment in the vicinity of estuaries, reefs and the surf-zone (Silberschneider and Gray, 2008), with settlement stages in South Africa (10 – 30 mm TL) (Griffiths, 1996; Pattrick and Strydom, 2014; Nodo et al., 2018) and eastern Australia (from ~ 4 weeks post-hatching) (Russell et al., 2021a) recruiting into estuaries soon after spawning. Argyrosomus japonicus in South Africa is most likely estuarine-dependent, with the early juveniles (< 150 mm TL) thought to occur exclusively in estuaries and the larger juveniles found in estuaries and nearshore coastal waters (Griffiths, 1996; Cowley et al., 2008). However, based on acoustic telemetry research on juvenile A. japonicus in South Africa it has been suggested that the A. japonicus stock exists as several subpopulations (within larger metapopulations), each with distinct estuarine and marine contingents (Childs, 2013; Childs et al., 2015). In Australia, although estuaries are critical nursery habitats for A. japonicus and recruitment is likely bolstered by access to turbid estuaries for certain populations there appears to be some plasticity in A. japonicus life history. A range of different research methods provide evidence that the populations are not entirely estuarine associated, with marine centric contingents that do not enter estuaries (Ferguson et al., 2011; Barnes et al., 2016; Barnes et al., 2019; Russell et al., 2022).
It has been suggested that high freshwater flow promotes the recruitment of larval and juvenile A. japonicus into estuaries (reviewed in Stewart et al., 2020). The prevalence of juveniles of the South African population in turbid versus non-turbid estuaries (Marais, 1981; Marais, 1985; Plumstead et al., 1985; Whitfield and Paterson, 2003; Nodo et al., 2017; James et al., 2020), suggests that turbid systems with fairly high freshwater input, such as the Great Fish Estuary on the south-east coast of South Africa, are the preferred nursery habitat for A. japonicus (Griffiths, 1996). Within turbid estuaries throughout their distribution, early juveniles are found predominantly in deeper waters in the upper reaches and are not associated with the shallow littoral vegetated fringes (Silberschneider and Gray, 2008).
In this study, we assessed the behavioural response of settlement-stage larval A. japonicus from the south-east coast of South Africa to several different estuarine and seawater types (with varying turbidity and olfaction characteristics) by using a two-channel choice flume adopted in previous studies (James et al., 2008; Radford et al., 2012; Havel and Fuiman, 2015). We tested the hypothesis that turbidity associated with high freshwater flow is used as a visual cue in the recruitment of A. japonicus into estuaries.
Materials and methods
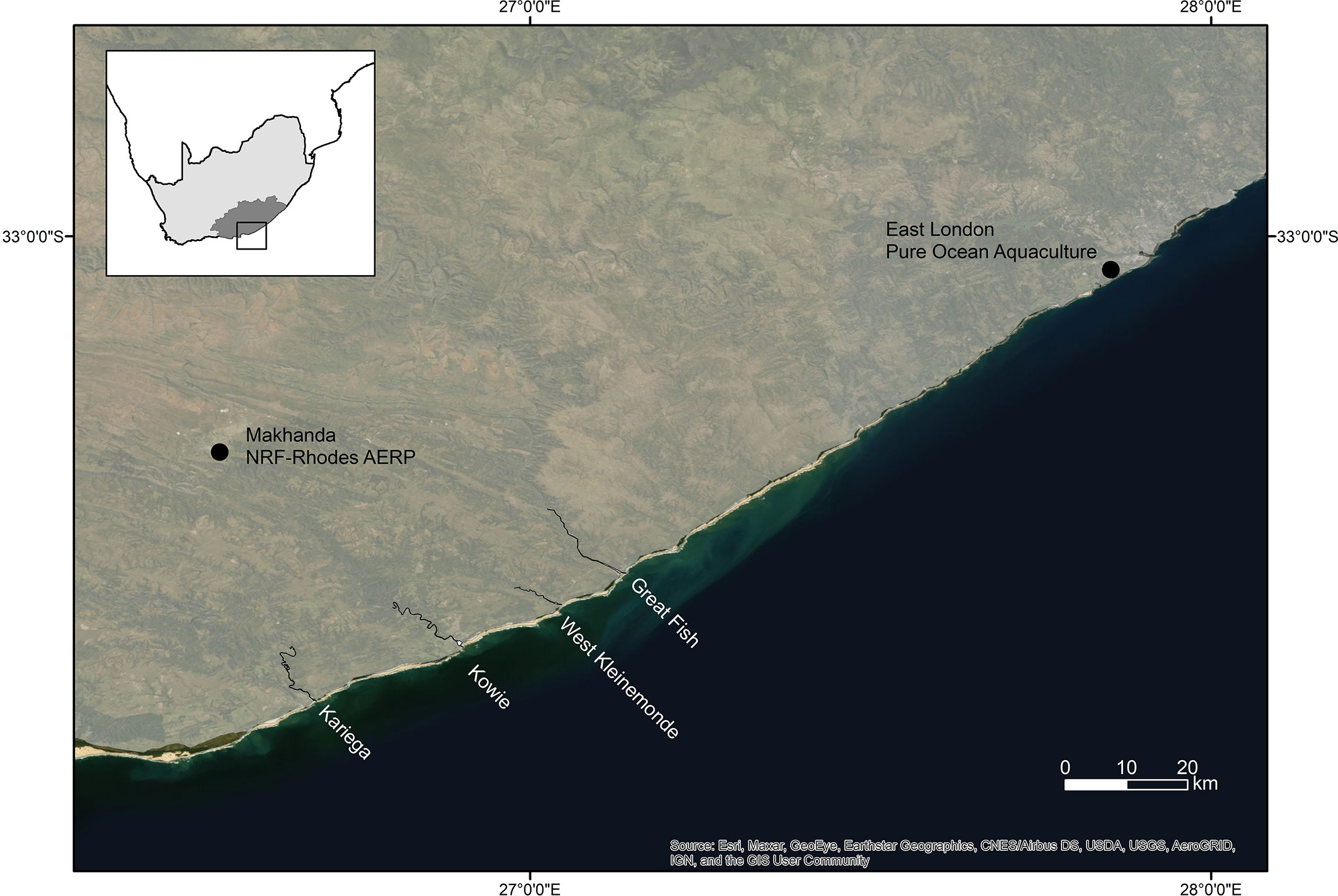
All the choice experiments were conducted in a controlled environment (CE) room at the NRF-SAIAB Aquatic Ecophysiology Research Platform (AERP) laboratory at Rhodes University in Makhanda, South Africa (Figure 1).
Experimental animals
Settlement-stage (size at recruitment, having completed metamorphosis) A. japonicus were sourced from the Pure Ocean Aquaculture facility in East London. The fish were hatched from eggs derived from an induced spawning of wild caught (West Kleinemonde Estuary) broodstock (Figure 1). They were transported from the hatchery to the laboratory in Makhanda and stocked into a single 500 L holding tank although estuaries are critical nursery habitats for A. japonicus a range of different research methods provide evidence that the populations are not entirely estuarine associated, with marine centric contingents that do not enter estuaries containing filtered (1 µm) and ozonated seawater (35 ppt). The seawater in the tanks was continuously re-circulated through a mechanical and biological filter to maintain water quality. The fish were maintained on their hatchery artificial micro-pellet diet and fed three times a day.
Seawater and estuarine water collection sites
Seawater was collected at a site 6 km offshore of the Kowie Estuary (Figure 1) and estuarine water was collected from the freshwater-dominated Great Fish Estuary, and the marine-dominated Kariega Estuary (Figure 1). The Great Fish Estuary has a large catchment area of about 30 000 km2. Although several impoundments have been constructed in the catchment, natural runoff is augmented by water from the Orange River. The high sediment load of the river results in very high turbidity (Nephelometric Turbidity Unit (NTU) >240) (Grange et al., 2000; James and Harrison, 2010; Froneman, 2010; Nodo et al., 2017). In contrast, the Kariega Estuary receives a negligible inflow of freshwater due to relatively low rainfall, a small catchment (686 km2), as well as several impoundments along the river that severely reduce river flow. The estuarine waters are normally clear (NTU <35) as a result of limited freshwater input, with the bed of the system visible in the lower reaches (James and Harrison, 2010; Froneman, 2010).
Choice experiments
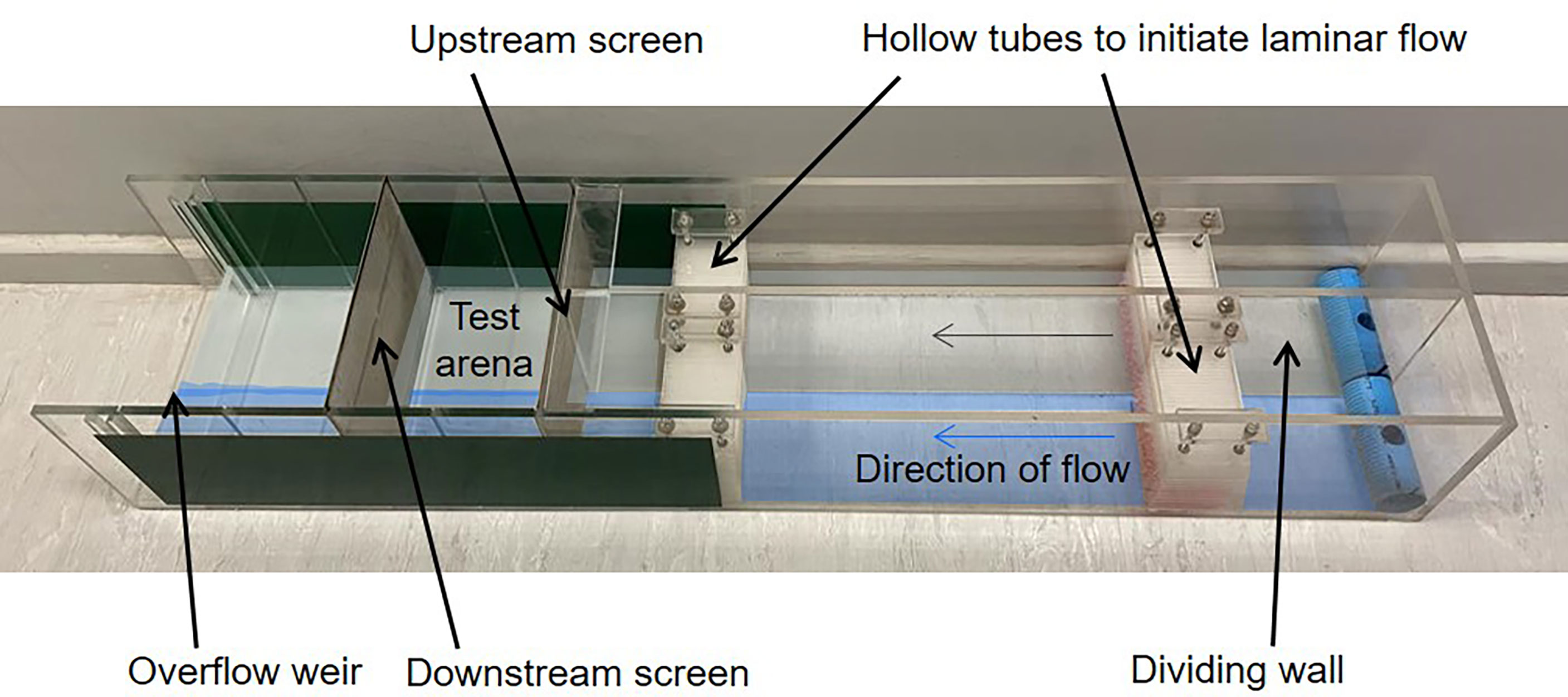
A two-channel choice-flume (Figure 2) constructed from Perspex was used to conduct choice experiments on two water types at a time. The two types of experimental water to be tested were pumped from their respective holding tanks to two small header tanks (20 L) located above the flume, each with an overflow outlet at the top of the tank that returned excess water to the holding tanks. A second outlet at the base of each header tank allowed water to flow by gravity through tubing to the inflow of each channel of the flume. The flow of experimental water was regulated by a double valve and inline flowmeter. The flow rate to each channel of the flume was regulated to 2 cm s-1. Water from each source entered the upstream end of each channel of the flume and immediately passed through a wall constructed of packed, fine-walled PVC tubes to dissipate inflow turbulence and initiate laminar flow. The two water types, still separated by a dividing wall, then flowed down the flume before passing through a second wall of packed tubes. Thereafter the central divider ended and the two water types flowed alongside each other before exiting the system over a beveled overflow weir. Two stainless steel screens (1 mm mesh size) placed across the width of the flume (at the end of the dividing wall and before the overflow weir) were used to define a test arena (280 mm wide x 200 mm long) and prevented the fish from moving upstream of the end of the central divider or towards the overflow. A GoPro camera (GoPro Hero 3) was mounted directly above the test arena to record the activity of fish during each trial. The walls of the flume were covered with opaque vinyl film to eliminate external influences that may have resulted in side-bias. A light source was located centrally above the chamber to ensure equal lighting to each chamber. Tracer dye tests were performed after each experiment to ensure the two water flows were laminar throughout the flume.
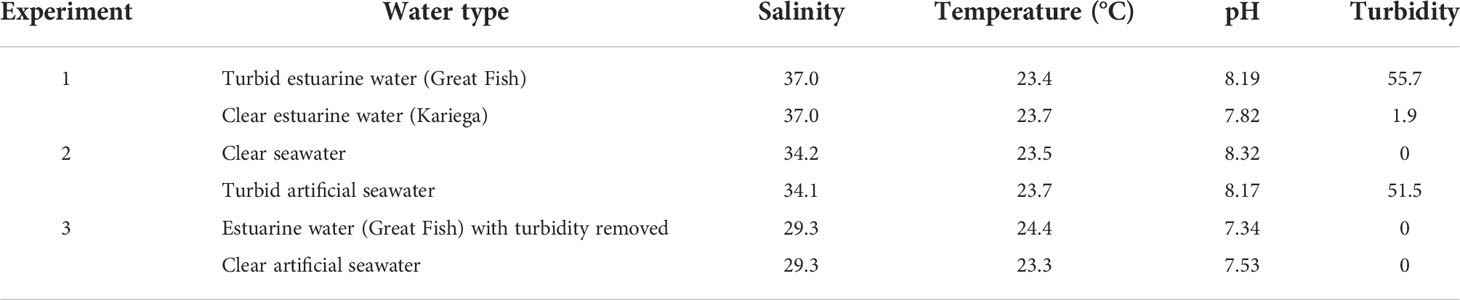
Three choice experiments (with three replicate trials each) were performed over three consecutive days just prior to the midday feed. Each experiment used paired combinations of six seawater/estuary water types (Table 1), with varying turbidity and olfactory characteristics, namely:
Experiment 1) Preference of A. japonicus for Great Fish estuarine water over Kariega estuarine water
Turbid estuarine water (Great Fish: + turbidity, + odor) over clear estuarine water (Kariega: - turbidity, + odor)
Experiment 2) Preference of A. japonicus for turbidity cues
Clear oceanic seawater (- turbidity, - odor) with turbid artificial seawater (+ turbidity, - odor)
Experiment 3) Preference of A. japonicus for odor in Great Fish estuarine water over turbidity
Estuarine water (Great Fish) with turbidity removed (- turbidity, + odor) with clear artificial seawater (- turbidity, - odor)
Oceanic seawater (1000 L) (- turbidity, - odor) was collected at high tide using a submersible pump. The turbid estuarine water (2000 L) (+ turbidity, + odor) and clear estuarine water (1000 L) (- turbidity, + odor) were pumped during low tide from the lower reaches of the freshwater-dominated Great Fish Estuary and the marine-dominated Kariega Estuary, respectively. The collected oceanic seawater and estuarine water were transported to the AERP laboratory and stored for 1 – 4 days at 24°C in separate 500 L containers containing circulation pumps to maintain water movement.
In order to determine if A. japonicus were responding to odor rather than turbidity in Great Fish Estuary water, a portion (1000 L) of the water sample collected from the Great Fish Estuary was treated to remove turbidity by settlement and filtration (1 µm and 0.2 µm filter pore). This resulted in estuarine water with turbidity removed (- turbidity, + odor). Artificial seawater (- turbidity, - odor) was also used in addition to oceanic seawater (- turbidity, - odor) due to sampling constraints. Artificial seawater (- turbidity, - odor) was made by adding artificial sea salt (Tetra Marine Sea Salt, Tetra® - Spectrum Brands Pet LLC, USA) to rainwater to achieve a salinity of 35 ppt. Turbid artificial seawater (+ turbidity, - odor) was created by adding sediment collected from the lower reaches of the Great Fish Estuary to artificial seawater. The sediment was first dried in a circulating oven (8 h at 100°C) and then placed in a muffle oven (12 h at 400°C) to burn off organic matter (Carrasco et al., 2013) and associated olfactory cues. The resulting product was crushed and sieved (2 µm mesh) before mixing with artificial seawater. The turbidity was matched to untreated Great Fish water by assessing a sample (1 L) of Great Fish water that was vacuum filtered (0.2 µm) in ten 100 ml batches onto pre-weighed filter papers. These were then oven dried (four hours at 100°C) and weighed to estimate the turbidity (grams of sediment L-1). The required portion of treated sediment was then added to the artificial seawater and adjusted using turbidity meter (HANNA 98703-02) readings.
Each choice experiment was conducted according to the following protocol. Prior to the experiments to eliminate salinity as a confounding factor and to prevent water types of different salinities from mixing in the test arena the salinity of the experimental water was standardized by the addition of artificial sea salt (Tetra Marine Sea Salt, Tetra® - Spectrum Brands Pet LLC, USA). Six fish were placed in the test arena containing water from their holding tank and allowed five minutes to acclimate without flow. Water flow from the two header tanks (containing the experimental water as per the paired combinations listed above) to each channel was then initiated for three minutes after which the inflows to the flume channels were switched around and run for a further three minutes to control for potential side bias. For each experiment, three trials were repeated in succession with six new fish for each trial. Water quality parameters were measured at the start of each experiment (Table 1).
Video image analyses
VLC media player was used to crop the last six minutes from each of the 11 minutes of video footage for analysis in the automated video tracking software id Tracker (Romero-Ferrero et al., 2019). See https://www.youtube.com/watch?v=fk6QzEd-iZ8&list=PLbDylBDlLLT2wYjfdTsbOrlB2P8MeXp10 for the cropped video footage from Trial 1 of each experiment. Id Tracker extracts trajectory data for each of the six individuals in the test arena. The location of each individual was noted every second and allocated either a 1 (for presence in the cue) or 0 (absence in the cue) to create a binomial dataset for each individual.
Statistical analyses
The binomial data for each individual were used to calculate the time (seconds) that the individual fish spent in the turbid (Experiment 1 and 2) or odor (Experiment 3) cue for each trial. We fitted a generalized linear model (GLM) with a gamma distribution (Zuur et al., 2009), estimated with a maximum likelihood estimator, to test the significance of time spent in each cue. Gamma distribution was selected due to the response variable having a maximum time (Jutfelt et al., 2017). Separate models were run for each experiment using the glm function in the ‘lme4’ package (Bates et al., 2015), where time spent (seconds) of each fish was the response variable and presence in the cue (or not) and side preference (side 1 vs side 2) were added as fixed effects. Model diagnostics were conducted to ensure assumptions were not violated (Zuur et al., 2010). We initially ran a generalized mixed effects model to include the random effect structure of individual fish nested within each trial (Harrison et al., 2018). However, since the effect was negligible (variance of the random effects was zero), they were dropped from the model and the generalized linear models were then fitted for each experiment. All plots and analyses were conducted in R (version 4.0.2) and RStudio (version 1.4.1717).
Results
Settlement stage (21.9 ± 5.3 mm TL) A. japonicus spent more time in the turbid cues in both experiments 1 and 2 (turbid estuarine water (Great Fish water) and turbid artificial seawater, respectively) occurring in the turbid cues on average 79.54 (± 5.06 SE, range 43.33 – 100, n = 18) and 69.72 (± 3.02 SE, range 50 – 94.16, n = 18) percent of the time during each experiment, respectively (Figure 3). The average time spent in the clear estuarine odor cue (Experiment 3), however, was only 45.97 (± 4.01 SE, range 20 – 93.33, n = 18) percent (Figure 3).
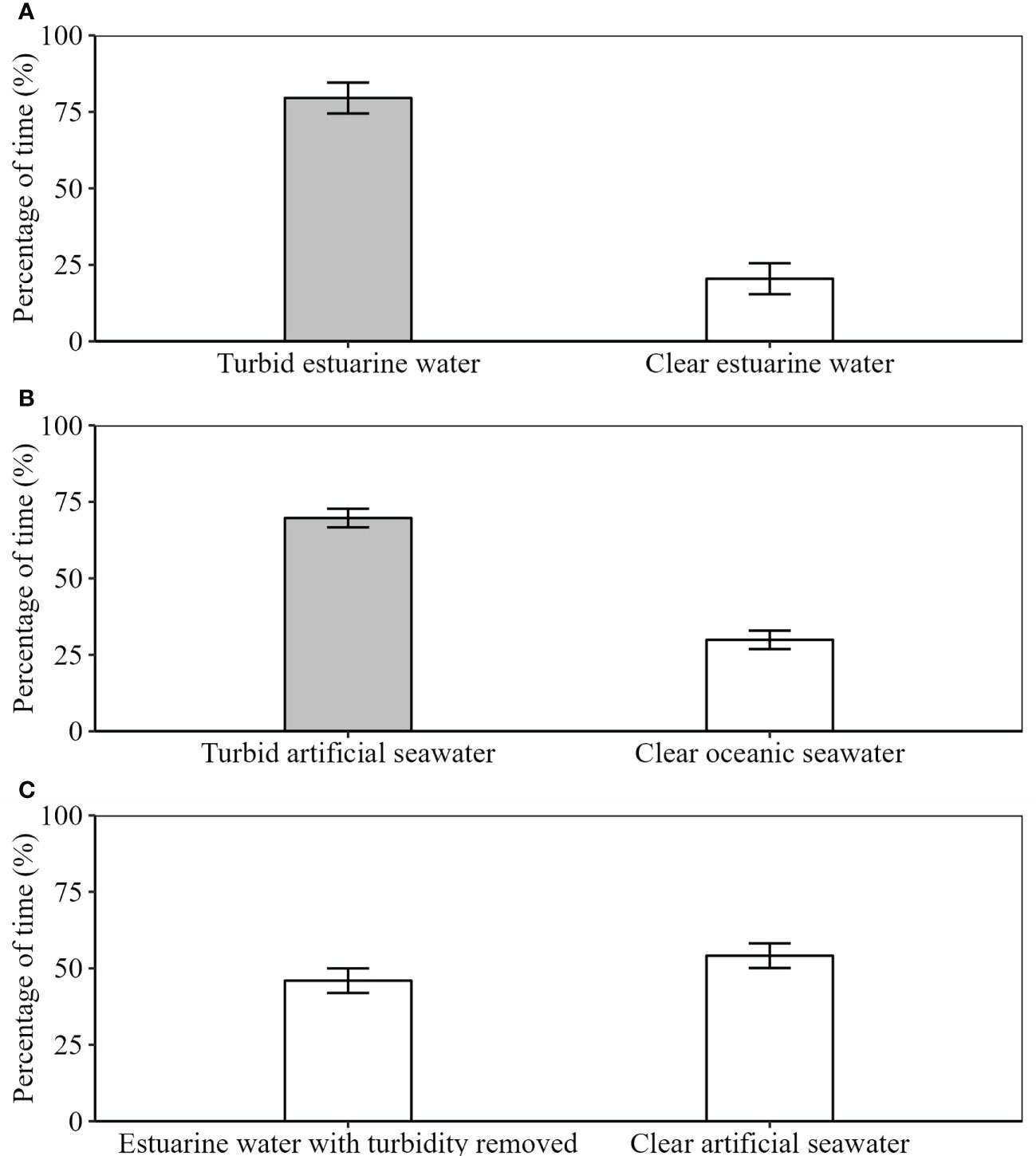
Figure 3 Mean percent preference ( ± SE) of settlement-stage Argyrosomus japonicus (n = 54) when given the choice of (A) turbid estuarine water (sourced from the Great Fish Estuary) or clear estuarine water (sourced from the Kariega Estuary), (B) clear seawater or turbid artificial seawater and (C) estuarine water with turbidity removed (sourced from the Great Fish Estuary) or clear artificial seawater. Grey bars indicate the turbid water and white bars indicate clear water.
Supporting our hypothesis and overall trends observed in the data, the results of the GLMs showed that A. japonicus exhibited a significant preference for turbid estuarine water when compared to clear estuarine water (Experiment 1) (β = -0.03, SE = 0.004, t(69) = -7.101, P < 0.001) and turbid artificial seawater when compared to clear seawater (Experiment 2) (β = -0.03, SE = 0.004, t(69) = -7.157, P < 0.001). Conversely, when given the choice of clear estuarine water and clear artificial seawater, fish showed no significant preference for the clear estuarine water (β = 0.0005, SE = 0.003, t(69) = 1.382, P = 0.172). For all experiments, side was not a significant predictor of time spent in the cue (Experiment 1 (β = 0.00005, SE = 0.003, t(69) = 0.000, P = 0.999), Experiment 2 (β = 0.00005, SE = 0.003, t(69) = 0.017, P = 0.987) and Experiment 3 (β = 0.005, SE = 0.003, t(69) = 0.008, P = 0.994).
Discussion
Results from this study clearly indicate that settlement-stage A. japonicus show a preference for turbid water (with and without odor) over clear water. This suggests that A. japonicus use turbidity as a cue to navigate towards estuarine nursery habitats. These findings also confirm the importance of turbid estuaries, particularly the Great Fish Estuary, as settlement and nursery areas for the South African population of this species. Unlike other estuarine-associated species, which are associated with shallow littoral vegetation, turbidity may be more important as a recruitment cue than olfaction for this demersal species.
The habitat and associated behavioural transitions associated with maturing larvae of estuary-associated marine species are dependent on the presence of external stimuli that induce a migratory response (Boehlert and Munday, 1988). When the behavioural or biological significance of a stimuli is unknown, choice chamber experiments using a group (or an individual) of test animals provide an opportunity to understand behavioural responses to the controlled release of physico-chemical stimuli (Scarfe et al., 1985). It is also highly likely that the importance of certain environmental stimuli are species-specific (Cyrus and Blaber, 1987).
Although studies have highlighted the importance of both olfaction (James et al., 2008; Radford et al., 2012; Havel and Fuiman, 2015) and turbidity (this study) in recruitment of estuarine-associated species into estuaries, identifying the mechanism is challenging, and in many instances, a myriad of environmental and sensory system stimuli may also be used to locate estuarine nurseries. For example, the use of sound (not tested in the present study) as a cue for settlement-stage larval reef fishes to locate nursery areas for settlement is well documented (e.g. Simpson et al., 2004; Radford et al., 2011). Juvenile reef fishes use a variety of sensory cues and the utilization of different types of cues may vary according to different spatial scales (Kingsford et al., 2002; Leis et al., 2011).
Estuaries with large amounts of riverine input have higher turbidity in comparison to smaller estuaries and freshwater deprived estuaries in the same region (Marais, 1988) and discharge turbid, nutrient-rich plumes into adjacent nearshore waters (shown graphically in Figure 4). These turbid, nutrient-rich plumes have the greatest potential to affect fish recruitment (Grimes and Kingsford, 1996). Vorwerk (2006) found that outflow of estuarine water from the Great Fish Estuary results in a plume of turbid water, with increased particulate organic matter (POM) as well as phytoplankton and zooplankton concentrations adjacent to and downstream of the estuary mouth. In contrast, no evidence of freshwater outflow (reduced salinity and increased POM) is evident adjacent to the Kariega Estuary. The absence of a marked turbidity gradient in the marine environment adjacent to several estuaries on the south-eastern coastline of South Africa, led Whitfield (1994) to suggest that olfaction rather than turbidity is likely the driving force behind the recruitment of most marine fishes into South African estuaries. Although this hypothesis is supported by the results of James et al. (2008) for the estuarine-dependent Rhabdosargus holubi, Whitfield (1994) and Cyrus and Blaber (1987) suggested that certain species may follow turbidity gradients into estuaries.

Figure 4 Turbidity plumes adjacent to the two study estuaries (A) Google Earth Pro V 7.3.3.7786. (6 December 2003). The lower reaches and adjacent nearshore of the Kariega Estuary -33.683882; 26.684485, Eye alt 4.49 km. Maxar Technologies 2022. http://www.earth.google.com [14 May 2022] and (B) Google Earth Pro V 7.3.3.7786. (3 August 2004) the lower reaches and adjacent nearshore of the Great Fish Estuary -33.492921, 27.126729, Eye alt 12.41 km. Landsat/Copernicus 2022, Maxar Technologies 2022. http://www.earth.google.com [14 May 2022].
Settlement stage A. japonicus showed a significant preference for turbid water (with and without olfactory cues) over seawater (no olfactory cues) and clear estuary water from the Kariega Estuary (with olfactory cues). No clear choice was made between clear estuary water (with olfactory cues) and clear oceanic seawater (without olfactory cues). These findings suggest that turbidity gradients are likely the primary factor governing the recruitment of settlement stage A. japonicus into estuaries. At settlement, larval A. japonicus are capable of active swimming (12 BL s-1) in response to environmental cues from estuaries (Clark et al., 2005). It is likely that these settlement stage fish use vision (along with other senses) to follow estuarine turbidity gradients into freshwater-dominated estuaries. Early-stage A. japonicus have well developed visual senses from 5 mm TL, with vision being the main sensory system used for feeding (Ballagh, 2011).
In South African estuaries, A. japonicus early juveniles (< 150 - 400 mm TL) are common in turbid systems and are relatively scarce in clearer estuaries (Ter Morshuizen et al., 1996; Whitfield and Paterson, 2003). Preliminary otolith microchemistry classification analyses on young-of-the-year A. japonicus captured from six estuaries with contrasting environmental attributes (including the Kariega and Great Fish estuaries sampled in the present study) showed high levels of classification (> 90%) when only turbid freshwater-influenced estuaries were grouped compared to other adjacent less turbid estuaries, suggesting that larvae and early juveniles may recruit primarily into turbid estuaries, and then either remain in these important estuaries or later move to less turbid estuaries (Childs, unpublished data). The results of the present study, which show that settlement stage A. japonicus have a preference for turbid cues support this hypothesis and highlight the importance of turbidity and freshwater inflow for the successful recruitment of settlement-stage fish. Additionally, geographic variation in growth rates of A. japonicus suggest that the increased productivity in the freshwater-influenced estuaries (including Great Fish) support higher growth rates of juveniles and hence increased nursery values when compared to other less productive estuaries (Childs, 2013). Indeed, the availability of nutrients in freshwater-dominated estuaries supports elevated primary, secondary and tertiary productivity providing an abundant food supply to meet diverse food requirements of estuarine fishes (e.g. Froneman, 2010) including the newly recruited A. japonicus larvae and early juveniles. The spring/summer rainfall pattern and highest river discharge along the south-east coast of South Africa coincides with peak spawning of A. japonicus and give rises to higher mysid and copepod biomasses, important prey items for early juvenile A. japonicus (< 50 mm TL) migrating into South African estuaries (Griffiths, 1997). An increase in detritus accumulations (organic material) also cause a significant increase in detritivorous teleosts, such as Mugilidae, which are preyed upon by larger A. japonicus juveniles (> 150 mm TL) (Griffiths, 1997).
Turbid water may also provide juveniles with protection from predation, including by conspecifics (Cyrus and Blaber, 1987; Stewart et al., 2020). Stewart et al. (2020) found that the recruitment success of A. japonicus in eastern Australia is linked to freshwater inflow into estuaries and subsequent salinity and turbidity gradients. Freshwater inflow also increased the availability of key prey (Metapenaeus macleayi) for juvenile A. japonicus in eastern Australia and may be linked to the enhanced growth and survival of cohorts (Stewart et al., 2020). Similarly, Russell et al. (2021a) and Russell et al. (2021b), using otolith microchemical analysis, also identified important estuarine nursery areas, characterized by an abundance of prey items and reasonable freshwater inflow, for the successful recruitment of A. japonicus. In Australia, the populations are not always estuary-associated, with some individuals and populations being marine resident throughout their life-cycle (Ferguson et al., 2011; Barnes et al., 2016; Barnes et al., 2019). The variation in time spent in the turbid and odor cues by individual early life stage settlement-stage A. japonicus in the present study provides further evidence that the South African A. japonicus populations could also exist as separate estuarine and marine contingents, as proposed by Childs et al. (2015). The existence of such a strategy would improve the species resilience to major anthropogenic impacts such as estuarine degradation (i.e. freshwater abstraction) and overfishing (Childs et al., 2015), and ultimately confer survival benefits (Russell et al., 2022).
High levels of growth (overexploitation of juveniles) and recruitment (overexploitation of mature individuals) overfishing have led to the collapse of the South African A. japonicus stock (Griffiths, 1996; Childs et al., 2015; Mirimin et al., 2015). The dependence on turbid estuaries, such as the Great Fish Estuary, as a nursery area for A. japonicus may have contributed to the decline and collapse of this species in South Africa owing to the high fishing effort these estuaries experience. Exploitation pressure for juveniles of this species in estuaries such as the Great Fish Estuary is significant, which results in growth overfishing. Cowley et al. (2008), in a tagging study of juveniles (150 – 400 mm TL) in the Great Fish Estuary, found that 41% of tagged juveniles were caught in the fishery. The importance of freshwater inflow and turbidity in recruitment, when viewed in the context of continued freshwater abstraction from estuaries, may also limit the nursery habitat available for the estuary-associated early juveniles (<150 mm TL). Based on the concentration of early juveniles in the upper reaches of turbid, freshwater-rich estuaries, such as the Great Fish, and the absence of early juveniles in the clear, freshwater-deprived Kariega Estuary, Whitfield and Paterson (2003) suggested that freshwater abstraction reduces the nursery habitat available to A. japonicus in this region. Confirming the importance of freshwater input and increased turbidity to the recruitment of A. japonicus, Nodo et al. (2018) also found that settlement stage A. japonicus (30 – 100 mm TL) were only recorded in the freshwater deprived Kariega Estuary following major river flooding and increases in turbidity and food resources in the middle and upper reaches of the estuary.
The results of this study provide valuable information for conservation and resource management initiatives for estuaries and overexploited estuarine-associated fishery species. Our results highlight the importance of turbid estuaries as nursery areas for early juvenile A. japonicus and the important role of turbidity gradients in the nearshore marine environment in the successful recruitment of settlement-stage A. japonicus. Estuaries with large amounts of riverine input have higher turbidity gradients within and outside of the estuary in comparison to smaller estuaries in the same region. Climate change is already altering rainfall patterns, with changes in rainfall affecting the amount and timing of freshwater entering estuaries. These changes are exacerbated in estuaries where humans have modified freshwater delivery through freshwater abstraction (James et al., 2013). The south-east coast of South Africa is predicted to be drier (with an increase in dry days and rainfall variability) by the end of the century (Engelbrecht et al., 2015). Our results also highlight the importance of adequate and effective catchment and flow management in our changing climate.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was reviewed and approved by Rhodes University Animal Ethics Committee. All animals were treated in accordance with the guidelines established by the South African Institute for Aquatic Biodiversity and Rhodes University ethics committees (DIFS 11 2015).
Author contributions
NJ, the primary author, was responsible for the conceptualization of the research, funding acquisition, project administration, development of the methodology, student supervision, collection of data, primary data curation and analysis and development of the manuscript draft. A-RC was responsible for conceptualization of the research, project administration, development of the methodology, student supervision, collection of data, primary data curation and analysis and editing of the original manuscript draft. JK was responsible for the development of the methodology, design of the experimental setup and choice chamber, collection of data and development of the manuscript draft. SW was the student conducting the research and was responsible for conceptualization of the research, project administration, development of the methodology, collection of data, primary data curation and analysis and development of the manuscript draft. CE was responsible for collection of data, primary data curation and analysis and editing the manuscript draft. All authors contributed to the article and approved the submitted version.
Funding
Research funding for this work was provided by the South African National Research Foundation (NRF) Research Development Grants for y-rated researchers (UID 93382) and Rhodes University – JRC funds.
Acknowledgments
We hereby acknowledge use of infrastructure, and equipment provided by the NRF-SAIAB Ecophysiology Research Platform channeled through the SAIAB-NRF Institutional Support System and Rhodes University. We also thank the Pure Ocean Aquaculture facility (East London) and Andre Bok for their assistance in providing the experimental animals. Finally, we are thankful to the anonymous reviewers for their valuable comments on an earlier draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ballagh D. A. (2011). Intensive rearing techniques and feeding behaviour of larval and juvenile mulloway, Argyrosomus japonicus (James Cook University) 145. pp
Barnes T. C., Junge C., Myers S. A., Taylor M. D., Rogers P. J., Ferguson G. J., et al. (2016). Population structure in a wide-ranging coastal teleost (Argyrosomus japonicus, sciaenidae) reflects marine biogeography across southern Australia. Mar. Freshw. Res. 67, 1103–1113. doi: 10.1071/MF15044
Barnes T. C., Rogers P. J., Wolf Y., Madonna A., Holman D., Ferguson G. J., et al. (2019). Dispersal of an exploited demersal fish species (Argyrosomus japonicus, sciaenidae) inferred from satellite telemetry. Mar. Biol. 166, 1–12. doi: 10.1007/s00227-019-3575-4
Bates D., Mächler M., Walker S. (2015). Fitting linear mixed effects models using lme4. J. Stat. Software 67, 1–48. doi: 10.18637/jss.v067.i01
Boehlert G. W., Munday B. C. (1988). Roles of behavioural and physical factors in larval and juvenile fish recruitment to estuarine nursery areas. Am. Fish. Soc Symp. 3, 51–67.
Carrasco N. K., Perissinotto R., Jones S. (2013). Turbidity effects on feeding and mortality of the copepod acartiella natalensis (Connell and grindle) in the St Lucia estuary, south Africa. J. Exp. Mar. Biol. Ecol. 446, 45–61. doi: 10.1016/j.jembe.2013.04.016
Childs A.-R. (2013). Estuarine-dependency and multiple habitatuse by dusky kob argyrosomus japonicus (Pisces: Sciaenidae) (Makhanda, South Africa: Rhodes University).
Childs A.-R., Cowley P. D., Naesje T. F., Bennett R. H. (2015). Habitat connectivity and intra-population structure of an estuary-dependent fishery species. Mar. Ecol. Prog. Ser. 537, 233–245. doi: 10.3354/meps11456
Clark D. L., Leis J. M., Hay A. C., Trnski T. (2005). Swimming ontogeny of larvae of four temperate marine fishes. Mar. Ecol. Prog. Ser. 292, 287–300. doi: 10.3354/meps292287
Cowley P. D., Kerwath S. E., Childs A.-R., Thorstad E. B., Okland F., Naesje T. F. (2008). Estuarine habitat use by juvenile dusky kob Argyrosomus japonicus (Sciaenidae), with implications for management. Afr. J. Mar. Sci. 30, 247–253. doi: 10.2989/AJMS.2008.30.2.5.555
Cyrus D. P., Blaber S. J. M. (1987). The influence of turbidity on juvenile marine fishes in estuaries. part 2. laboratory studies, comparisons with field data and conclusions. J. Exp. Mar. Biol. Ecol. 109, 71–91. doi: 10.1016/0022-0981(87)90186-9
Dixson D. L., Jones G. P., Munday P. L., Planes S., Pratchett M. S., Srinivasan M., et al. (2008). Coral reef fish smell leaves to find island homes. Proc. Royal. Soc Biol. 275, 2831–2839. doi: 10.1098/rspb.2008.0876
Engelbrecht F., Adegoke J., Bopape M.-J., Naidoo M., Garland R., Thatcher M., et al. (2015). Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ. Res. Lett. 10. doi: 10.1088/1748-9326/10/8/085004
Ferguson G. J., Ward T. M., Gillanders B. M. (2011). Otolith shape and elemental composition: Complementary tools for stock discrimination of mulloway (Argyrosomus japonicus) in southern Australia. Fish. Res. 110, 75–83. doi: 10.1016/j.fishres.2011.03.014
Froneman P. W. (2010). Preliminary study on the food web structure of two contrasting estuaries along the Eastern cape coast, south Africa. Afr. J. Aqua. Sci. 25, 13–22. doi: 10.2989/160859100780177622
Gouraguine A., Diaz-Gil C., Reñones O., Otegui D. S., Palmer M., Hinz H., et al. (2017). Behavioral response to detection, feeding and schooling in a temperate juvenile fish. J. Exp. Mar. Biol. Ecol. 486, 140–147. doi: 10.1016/j.jembe.2016.10.003
Grange N., Whitfield A. K., de Villiers C. J., Allanson B. R. (2000). The response of two south African east coast estuaries to altered river flow regimes. Aqua. Cons. Mar. Fresh. Eco. 10, 155–177. doi: 10.1002/1099-0755(200005/06)10:3<155::AID-AQC406>3.0.CO;2-Z
Griffiths M. H. (1996). Life history of the dusky kob argyrosomus japonicus (scianidae) off the east coast of south Africa. S. Afr. J. Mar. Sci. 17, 135–154. doi: 10.2989/025776196784158653
Griffiths M. H. (1997). Influence of prey availability on the distribution of dusky kob argyrosomus japonicus (Sciaenidae) in the great fish river estuary, with notes on the diet of early juveniles from three other estuarine systems. S. Afr. J. Mar. Sci. 18, 137–145. doi: 10.2989/025776197784161153
Grimes C. B., Kingsford M. J. (1996). How do riverine plumes of different sizes influence fish larvae: do they enhance recruitment? Mar. Fresh. Res. 47, 191–208. doi: 10.1071/MF9960191
Harrison X. A., Donaldson L., Correa-Cano M. E., Evans J., Fisher D. N., Goodwin C. E. D., et al. (2018). A brief introduction to mixed effects modelling and multi-model inference in ecology. Peer J. 6, e4794. doi: 10.7717/peerj.4794
Havel L. N., Fuiman L. A. (2015). Settlement-size larval red drum (Sciaenops ocellatus) respond to estuarine chemical cues. Estuaries Coasts 39, 560–570. doi: 10.1007/s12237-015-0008-6
James N. C., Adams J. B., Connell A. D., Lamberth S. J., MacKay C. F., Snow G. C., et al. (2020). High flow variability and storm events shape the ecology of the mbhashe estuary, south Africa. Afr. J. Aqua. Sci. 45, 131–151. doi: 10.2989/16085914.2020.1733472
James N. C., Cowley P. D., Whitfield A. K., Kaiser H. (2008). Choice chamber experiments to test the attraction of postflexion rhabdosargus holubi larvaie to water of estuarine and riverine origin. Estuar. Coast. Shelf Sci. 77, 143–149. doi: 10.1016/j.ecss.2007.09.010
James N. C., Harrison T. D. (2010). A preliminary survey of the estuaries on the southeast coast of south Africa, cape padrone - great fish river, with particular reference to the fish fauna. Trans. R. Soc South Afr. 65, 149–164. doi: 10.1080/00359191003652165
James N. C., van Niekerk L., Whitfield A. K., Potts W. M., Götz A., Paterson A. (2013). A review of the possible effects of climate change on south African estuaries and associated fish species. Clim. Res. 57, 233–248. doi: 10.3354/cr01178
Jutfelt F., Sundin J., Raby G. D., Krång A.-S., Clark T. D. (2017). Two-current choice flumes for testing avoidance and preference in aquatic animals. Methods Ecol. Evol. 8, 379–390. doi: 10.1111/2041-210X.12668
Kingsford M. J., Leis J. M., Shanks A., Lindeman K. C., Morgan S. G., Pineda J. (2002). Sensory environments, larval abilities and local self-recruitment. Bull. Mar. Sci. 70, 309–340.
Leis J. M., Siebeck U., Dixson D. L. (2011). How nemo finds home: the neuroecology of dispersal and of population connectivity in larvae of marine fishes. Int. Compar. Biol. 51, 826–843. doi: 10.1093/icb/icr004
Marais J. F. K. (1981). Seasonal abundance, distribution and catch per unit effort by gillnets of fishes in the sundays estuary. S. Afri. J. Zool. 16, 144–150. doi: 10.1080/02541858.1981.11447749
Marais J. F. K. (1985). Some factors influencing the size of fishes caught in gillnets in Eastern cape estuaries. Fish. Res. 3, 251–261. doi: 10.1016/0165-7836(85)90026-8
Marais J. F. K. (1988). Some factors that influence fish abundance in south African estuaries. S. Afri. J. Mar. Sci. 6, 67–77. doi: 10.2989/025776188784480609
Mirimin L., Macey B., Kerwath S., Lamberth S., Bester-van der Merwe A., Cowley P., et al. (2015). Genetic analyses reveal declining trends and low effective population size in an overfished south African sciaenid species, the dusky kob (Argyrosomus japonicus). Mar. Fresh. Res. 66, 1–11. doi: 10.1071/MF14345
Nodo P., James N. C., Childs A. R., Nakin M. D. V. (2017). The impact of river flooding and high flow on the demersal fish assemblages of the freshwater-dominated great fish estuary, south Africa. Afr. J. Mar. Sci. 39, 491–502. doi: 10.2989/1814232X.2017.1404494
Nodo P., James N. C., Childs A.-R., Nakin M. D. V. (2018). Response of demersal fish assemblages to an extreme flood event in a freshwater-deprived estuary in south Africa. Mar. Fresh. Res. 69, 253–266. doi: 10.1071/MF17096
Pattrick P., Strydom N. A. (2014). Recruitment of fish larvae and juveniles into two estuarine nursery areas with evidence of ebb tide use. Estuar. Coast. Shelf Sci. 149, 120–132. doi: 10.1016/j.ecss.2014.08.003
Plumstead E. E., Prinsloo J. F., Schoonbee H. J. (1985). A survey of the fish fauna of transkei estuaries. part 1. Kei River estuary. S. Afri. J. Zool. 20, 213–220. doi: 10.1080/02541858.1985.11447938
Radford C. A., Sim-Smith C. J., Jeffs A. G. (2012). Can larval snapper, pagrus auratus, smell their new home? Mar. Fresh. Res. 63, 898–904. doi: 10.1071/MF12118
Radford C. A., Stanley J. A., Simpson S. D., Jeffs A. G. (2011). Juvenile coral reef fishes use sound to locate habitats. Coral Reefs 30, 295–305. doi: 10.1007/s00338-010-0710-6
Romero-Ferrero F., Bergomi M. G., Hinz R. C., Heras F. J. H., de Polavieja G. G. (2019). Idtracker.ai: tracking all individuals in small or large collectives of unmarked animals. Nat. Methods 16, 179–182.
Russell A., Gillanders B. M., Barnes T. C., Johnson D. D., Taylor M. D. (2021a). Inter-estuarine variation in otolith chemistry in a large coastal predator: a viable tool for identifying coastal nurseries. Estuar. Coast. 44, 1132–1146. doi: 10.1007/s12237-020-00825-x
Russell A. L., Taylor M. D., Barnes T. C., Johnson D. D., Gillanders B. M. (2021b). Potential linkages between juvenile nurseries and exploited populations of mulloway (Argyrosomus japonicus), explored using otolith chemistry. Fish. Res 11. doi: 10.1016/j.fishres.2021.106063
Russell A., Taylor M. D., Barnes T. C., Johnson D. D., Gillanders B. M. (2022). Habitat transitions by a large coastal sciaenid across life history stages, resolved using otolith chemistry. Mar. Enviro. Res. 176, 105614. doi: 10.1016/j.marenvres.2022.105614
Scarfe A. D., Steele C. W., Rieke G. K. (1985). Quantitative chemobehavior of fish: an improved methodology. Environ. Biol. Fish. 13, 183–194. doi: 10.1007/BF00000930
Silberschneider V., Gray C. A. (2008). Synopsis of biological, fisheries and aquaculture-related information on argyrosomus japonicus (Pisces: Sciaenidae), with particular reference to Australia. J. App. Ichthy. 24, 7–17. doi: 10.1111/j.1439-0426.2007.00913.x
Simpson S. D., Meekan M. G., McCauley R. D., Jeffs A. (2004). Attraction of settlement-stage coral reef fishes to reef noise. Mar. Ecol. Prog. Ser. 276, 263–268. doi: 10.3354/meps276263
Stewart J., Hughes J. M., Stanley C., Fowler A. M. (2020). The influence of rainfall on recruitment success and commercial catch for the large sciaenid, argyrosomus japonicus, in eastern Australia. Mar. Environ. Res. 157, 1–8. doi: 10.1016/j.marenvres.2020.104924
Ter Morshuizen L. D., Whitfield A. K., Paterson A. W. (1996). Influence of freshwater flow regime on fish assemblages in the great fish river and estuary. S. Afri. J. Aqua. Sci. 22, 52–61. doi: 10.1080/10183469.1996.9631372
Vorwerk P. D. (2006). A preliminary examination of selected biological links between four Eastern cape estuaries and the inshore marine environment (Rhodes University) 245 pp.
Whitfield A. K. (1994). Abundance of larval and 0+ juvenile marine fishes in the lower reaches of three southern African estuaries with differing freshwater inputs. Mar. Ecol. Prog. Ser. 105, 257–267. doi: 10.3354/meps105257
Whitfield A. K., Paterson A. W. (2003). Distribution patterns of fishes in a freshwater deprived Eastern cape estuary, with particular emphasis on the geographical head water region. Water SA. 29, 61–68.
Zuur A. F., Ieno E. N., Elphick C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 1, 3–14. doi: 10.1111/j.2041-210X.2009.00001.x
Keywords: Argyrosomus japonicus, turbidity, recruitment, nursery habitats, estuaries
Citation: James NC, Childs A-R, Kemp J, Wilsnagh S and Edworthy C (2022) Turbidity influences the recruitment of Argyrosomus japonicus to estuarine nurseries. Front. Mar. Sci. 9:953607. doi: 10.3389/fmars.2022.953607
Received: 26 May 2022; Accepted: 01 September 2022;
Published: 26 September 2022.
Edited by:
Susanne Eva Tanner, University of Lisbon, PortugalReviewed by:
Tom Barnes, NSW Government, AustraliaFilipe Martinho, University of Coimbra, Portugal
Patrick Astruch, GIS Posidonie, France
Copyright © 2022 James, Childs, Kemp, Wilsnagh and Edworthy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicola Caroline James, bmMuamFtZXNAc2FpYWIubnJmLmFjLnph
 Nicola Caroline James
Nicola Caroline James Amber-Robyn Childs
Amber-Robyn Childs Justin Kemp2
Justin Kemp2 Shannon Wilsnagh
Shannon Wilsnagh Carla Edworthy
Carla Edworthy