- 1CAS Key Laboratory of Marine Ecology and Environmental Sciences, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 2Laboratory for Marine Ecology and Environmental Science, Qingdao National Laboratory for Marine Science and Technology, Qingdao, China
- 3Center for Ocean Mega-Sciences, Chinese Academy of Sciences, Qingdao, China
- 4CAS Engineering Laboratory for Marine Ranching, Institute of Oceanology, Chinese Academy of Sciences, Qingdao, China
- 5Shandong Province Key Laboratory of Experimental Marine Biology, Qingdao, China
- 6College of Environment and Safety Engineering, Qingdao University of Science and Technology, Qingdao, China
Introduction: Microplastics (MPs) and cadmium (Cd) are persistent pollutants in aquatic environments. Sea cucumbers are susceptible to MPs and Cd due to their feeding behavior.
Methods: This study, based on Illumina sequencing, compared the transcriptomes of A. japonicus before and after Cd and/or MPs exposure. Additionally, we detected the changes of catalase (CAT), and superoxide dismutase (SOD) activity, glutathione (GSH), and malondialdehyde (MDA) content in sea cucumbers.
Results and Discussion: High concentration of MPs caused the increase of SOD activity. High concentration combined treatment resulted in significant up regulation of these four indicators in A. japonicus and had the largest number of differential expression genes (DEGs) reaching 1,618 DEGs, consisting of 789 up regulated along with 829 down regulated DEGs. Transcriptome results showed that Cd induced up regulation of intestinal FAS associated death domain protein (FADD) expression, which may cause apoptosis and inflammation. The increase of intestinal putative heparan sulfate 2-O-sulfotransferase in cadmium treatment groups provided a mechanism for host defense. The imbalance of expression of the NOD-like receptor (NLR) family inflammatory bodies and caspase 6 in the microplastic treatment group also led to the inflammatory reaction in the intestine of sea cucumber. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed that in the process of fatty acid metabolism, MPs and Cd showed antagonistic effects, mainly in the inconsistent expression of Stearoyl CoA Ddesaturase (SCD1) protein. The significant changes of Toll interacting protein (TOLLIP) and E-selectin (SELE) in all Cd and MPs treatment groups may indicate the key immune response genes of sea cucumber to Cd exposure and MPs exposure. These genes were involved in the immune defense of sea cucumber exposed to different levels of Cd and MPs. This study provided insights into the mechanism of dietary MPs and Cd intake in an economically and ecologically important invertebrate species.
1 Introduction
In recent years, there has been widespread concern about environmental pollution caused by the rapid accumulation of plastic debris in the natural environment (Auta et al., 2017; Liao and Yang, 2020). Tiny plastic fragments, fibers, and particles, known as Microplastics (MPs) (1 μm to 5 mm in diameter), are the main form of marine plastic debris (Thompson et al., 2004). MPs are small and easily ingested by predators living in the water column and sediments. The presence of MPs has been demonstrated in zooplankton, fish, crabs, shrimps, echinoderms including sea urchins, sea cucumbers and even marine mammals. (Daniel et al., 2020; Feng et al., 2020; Plee and Pomory, 2020; Taha et al., 2021; Zantis et al., 2021; Zhang et al., 2021). Ingested MPs may accumulate in the digestive tract of aquatic animals and in severe cases even obstruct the digestive tract, resulting in decreased feeding motility and feeding rate (Md Amin et al., 2020). MPs have been shown to cause a variety of negative effects on living organisms, including mechanical damage to digestive organs, inflammatory responses, causing oxidative stress, and alterations in enzyme production and metabolism (Wright et al., 2013; Welden and Cowie, 2016; Lei et al., 2018). Plastics are usually complex mixtures composed of polymers, residual monomers, and chemical additions (Zettler et al., 2013).
Furthermore, the organic matter, bacteria and chemical contaminants adsorbed by MPs increase their complexity (Carbery et al., 2020; Liu et al., 2022; Mohsen et al., 2022). Due to their physicochemical properties, surface characteristics, small particle size, large specific surface area and hydrophobicity, MPs have the potential to adsorb other harmful pollutants such as heavy metals in the surrounding environment (Vedolin et al., 2018; Wang et al., 2020). Heavy metals are classified as harmful to living organisms even at low concentrations due to their high toxicity and carcinogenicity (Akhbarizadeh et al., 2018). MPs and heavy metals are persistent pollutants in aquatic environments due to their bioaccumulation and biomagnification in the food chain (Kim et al., 2017). The accumulation of heavy metals in organisms may destroy enzyme activity, lead to oxidative damage, and affect biological growth and metabolism. In addition, these heavy metals are often concentrated and amplified in organisms at higher levels of the food chain, particularly in the benthos (Boyd and Massaut, 1999; Esmaeilzadeh et al., 2017; Kovacevic et al., 2020). These organisms may become food for humans and then threaten human health. Cd is poorly degradable and highly soluble in lipids, and when ingested by living organisms, it will be enriched in living organisms and not easily decomposed and excreted (Rainbow, 2003). The investigation found that the median concentration of Cd was higher on the microplastics than in the corresponding sediment (Mohsen et al., 2019a). According to another report, MPs can change the bioavailability of heavy metals in environmental media (Zhang et al., 2020). Among marine organisms, considering the combined effects of MPs and heavy metals, the most common organisms studied include phytoplankton algae, invertebrates, and fish (Qiao et al., 2019; Fernandez et al., 2020; Lin et al., 2020). Previous studies have shown that both MPs and heavy metals could show toxic effects on organisms, and their combination may produce three effects, namely synergistic, antagonistic, or potentiating effects (Bhagat et al., 2021).
Sea cucumber (Apostichopus japonicus) has a high market value and medicinal properties. It is an important economic species in the north of China (Fu et al., 2005). Sea cucumbers prefer to inhabit the sea floor where there is a high content of organic matter such as algae (Purcell et al., 2014; Ru et al., 2019). The concentration of metals in sediments is usually much higher than in water. Sediments can be a source of chemicals in the water column and also adversely affect sediment-dwelling organisms through direct toxicity (Roussiez et al., 2006; Dane and Sisman, 2020). The feeding nature of sea cucumbers make them more susceptible to heavy metals and MPs in the sediment (Jiang et al., 2013; Yokoyama, 2013). The environmental pollution problem is especially prominent because coastal industrial effluents and domestic effluents are largely discharged offshore, causing great harm to the sea cucumber cultivation industry along the coast (Khan et al., 2019). In addition, sea cucumbers, as sediment and suspension feeders, can be effective as a bioindicator of environmental pollution (Mohsen et al., 2020; Parra-Luna et al., 2020).
The intestine not only plays a role in absorption of nutrients, but is also the main route for toxic substances to enter the body (Ling et al., 2018; Dane and Sisman, 2020). The ingestion of sediments by sea cucumbers could lead to the unselected entry of MPs and Cd from sediments into the gut. The intestine is the first tissue to be affected by contaminants present in ingested foods, and many studies have been conducted on the effects of heavy metals on its histological changes (Joshy et al., 2022). It is not clear how the intestine of sea cucumber responds to MPs and heavy metal stresses at the molecular level. With high-throughput precision and reproducibility, transcriptome sequencing technology is a useful tool for studying aquatic animal growth, development, the immune system against disease, stress physiology, and other functional processes. To help understand the intrinsic molecular processes of the gut in the face of Cd and MPs stress, we determined the transcriptome changes of its gut by using Illumina HiSeq 2500 sequencing technology. Additionally, we examined the status of oxidative stress enzymes. The findings of the present study are important for further understanding the mechanisms of adaptation of echinoderms to Cd and MPs of the environment, and thus, provide references for the cultivation and food safety of sea cucumber.
2 Materials and methods
2.1 Animal collection
Healthy sea cucumbers weighing 25.54 ± 2.24 g were selected as experimental animals. They were purchased from the Shandong Tonghe Marine Technology Co., Ltd. (Dongying, China). They were randomly divided into seven groups of five in parallel, each with 10 sea cucumbers, and reared in a 120 L (length × width × height: 70 cm × 50 cm × 35 cm) breeding boxes, maintained with ample blast gas and half water changed every 24 h. They were given a period of 2 weeks to acclimate before the start of the experiment. Throughout the rearing process, the breeding box water inlets were filtered with a 74 μm silk sieve. The seawater used in the experiment met the first-class conditions of the seawater quality standard (GB 3097-1997). We maintained a 12 h - 12 h light-dark illumination regime throughout the study. Further information is presented in 2.3 Experimental setup.
2.2 Test diets
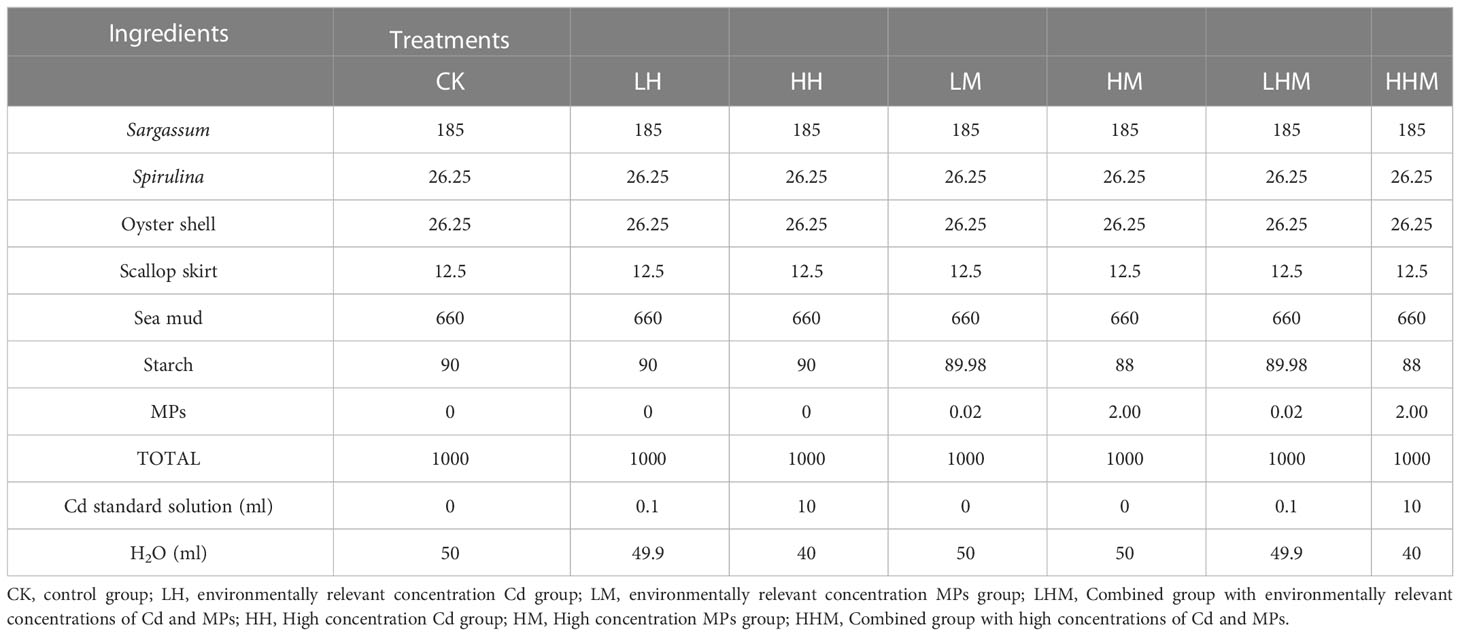
Polyethylene glycol terephthalate (PET) microplastic particles of about 150 μm diameter were purchased from the Hangzhou Hongyuan Polymer Technology Co., Ltd. (Hangzhou, China), and sequentially sieved through stainless steel sieves with mesh diameters of 180 μm and 150 μm. The density of the MPs was about 1.37/(g/cm3). The chemicals used (CdCl2·2.5H2O; analytical grade)) were purchased from the Shanghai Wokai Biotechnology Co., Ltd. (Shanghai, China). CdCl2·2.5H2O was prepared into 5g/L Cd standard solution with ultrapure water. The composition of the experimental diets are shown in Table 1. The actual Cd and MPs concentrations were 0.49mg/kg, 46.2mg/kg, 0.0194g/kg (about 1000 microplastic particles/kg), 1.9358g/kg (about 100000 microplastic particles/kg) dry weight, respectively. They were separately passed through a grinder and pelleted through 3mm diameter extruder. The diets dried at 45 °C for 24 hours and stored in -20°C for further use.
2.3 Experimental setup
Three hundred fifty sea cucumber were randomly divided into seven groups, five parallel in each group (n = 10), and the diet exposure experiment was conducted for 30 days. Seven groups included one control group and six experimental groups. The experimental group included three environmentally relevant treatment groups (approximate natural sediment pollutant concentrations): LH (environmentally relevant concentration Cd group) with 0.5 mg/kg Cd; LM (environmentally relevant concentration MPs group) with 1000 microplastic particles/kg; and LHM (combined group with environmentally relevant concentrations of Cd and MPs) with 0.5 mg/kg Cd + 1,000 microplastic particles/kg (Mohsen et al., 2019; Wu et al., 2022) and three high concentration treatment groups (one hundred times the concentration of both pollutants in natural sediments): HH (high concentration Cd group) with 50 mg/kg Cd; HM (high concentration MPs group) with 100,000 microplastic particles/kg; and HHM (combined group with high concentrations of Cd and MPs) with 50 mg/kg Cd + 100,000 microplastic particles/kg. The ranges of the parameters were as follows: water temperature 14 ± 1°C, pH 8.3 ± 0.2, salinity 30 ± 1, dissolved oxygen 10.2 ± 0.3 mg O2/l. In the experiment periods, sea cucumbers were fed at an amount of 3% of their body weight once a day at 16:00. We set a blank environmental control breeding box to exclude the entry of MPs in the environment. After the test, no MPs similar to the MPs used in this experiment were found in control breeding box.
2.4 Detection of indicators related to oxidative stress
After exposure, a total of 35 sea cucumbers (five replicates per group) were collected and dissected on ice. The body coelomic fluid and intestinal tract were quickly frozen with liquid nitrogen, and placed in an Ultra-low temperature freezer for storage until testing. The detection kit was purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China). After thawed, the coelomic fluid was centrifuged at 425 × g for 10 mins at 4°C, and the levels of CAT, SOD and GSH were measured at wavelengths of 405 nm, 450 nm and 405 nm using a microplate reader (Thermo Scientific, Waltham, MA, USA), respectively. After the intestinal sample was thawed, a portion of the sample was weighed and added to normal saline (saline volume: tissue mass, 9:1). It was then homogenized in a centrifuge tube to obtain a 10% tissue homogenate solution, centrifuged at 956 × g for 10 min at 4°C, and the MDA content in the supernatant was determined using a microplate reader at a wavelength of 532 nm. Protein concentrations were measured using the bicinchoninic acid microplate method and a detection kit purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.5 RNA isolation and Illumina sequencing
Total RNA was extracted using TRIzol reagent kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. RNA quality was assessed on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and checked using RNase free agarose gel electrophoresis. After total RNA was extracted, eukaryotic mRNA was enriched by Oligo(dT) beads, while prokaryotic mRNA was enriched by removing rRNA by Ribo-ZeroTM Magnetic Kit (Epicentre, Madison, WI, USA). Then the enriched mRNA was fragmented into short fragments using fragmentation buffer and reverse transcripted into cDNA with random primers. Second-strand cDNA were synthesized by DNA polymerase I, RNase H, dNTP, and buffer. Then the cDNA fragments were purified with QiaQuick PCR extraction kit (Qiagen, Venlo, The Netherlands), end repaired, poly(A) added, and ligated to Illumina sequencing adapters. The ligation products were size selected by agarose gel electrophoresis, PCR amplified, and sequenced using Illumina HiSeq 2500 by Gene Denovo Biotechnology Co. (Guangzhou, China). The raw reads were deposited into the National Center for Biotechnology Information (NCBI) Sequence Read Archive (SRA) database (accession number: PRJNA907491).
2.6 Bioinformatics analysis
To get high quality clean reads, reads were further filtered by fastp (version 0.18.0) (Chen et al., 2018). Short reads alignment tool Bowtie2 (version 2.2.8) was used for mapping reads to ribosome RNA (rRNA) database (Langmead and Salzberg, 2012). The rRNA mapped reads were then removed. The remaining clean reads were further used in assembly and gene abundance calculation. An index of the reference genome was built, and paired-end clean reads were mapped to the reference genome using HISAT2. 2.4. RNAs differential expression analysis was performed by DESeq2 software between two different groups (and by edgeR between two samples) (Robinson et al., 2010; Love et al., 2014; Kim et al., 2015). The Gene Ontology (GO) enrichment analysis along with the Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses for DEGs were performed taking false discovery rate (FDR) ≤ 0.05 as a threshold.
2.7 Real-time PCR validation
To validate the RNA-sequencing results, six genes were randomly selected for real-time PCR. Primers were designed for optimal performance using primer 5 (Supplementary Table 4). Total RNA was extracted from the respiratory tree, the same tissue used for the construction of the RNA-seq profile, by using a MiniBEST Universal RNA Extraction Kit (Takara, Shiga, Japan). A SYBR Green® real-time PCR assay (SYBR PrimeScript™ RT-PCR Kit II, TaKaRa) with an Eppendorf Mastercycler® ep realplex (Eppendorf, Hamburg, Germany) were used for examining the mRNA expression levels, and NADH was used as a reference gene for internal standardization (Zhang et al., 2022). Quantitative real-time PCR was conducted in 20 µL using SYBR Master Mix (TaKaRa, Kusatsu, Japan). The PCR was conducted at 95°C for 90 s, followed by 40 cycles at 95°C for 5 s, 60°C for 15 s, and 72°C for 20 s. Then, a melting curve analysis was performed to assess the specificity of qPCR amplification. Each gene was repeated three technique replications and 2–△△CT method was used for analyzing relative quantification (Schmittgen and Livak, 2008).
2.8 Statistical analysis
The results of the experiments are presented as the means ± SD. CAT, SOD, and MDA were analyzed using the one-way analysis of variance (ANOVA) with multiple comparisons for significant differences between treated and control groups, raw data were diagnosed for their normality of distribution by Shapiro-Wilk test. Meanwhile, two-way ANOVA was used to evaluate the interactive effect of pollutant type and pollutant concentration on the parameters of oxidative stress of sea cucumber. Statistical analyses were performed using GraphPad Prism version 8.0. A value of P < 0.05 was considered significant. The genes with the parameter of P ≤ 0.05 and absolute fold change ≥ 2 were considered differentially expressed genes.
3 Results
3.1 Oxidative stress related indicators
The effects of different treatment methods on catalase (CAT), superoxide dismutase (SOD) activity, glutathione (GSH) content, and malondialdehyde (MDA) content of sea cucumber are shown in the Figure 1. In the environmental concentration exposure experiment, the LH group significantly increased the SOD enzyme activity (P < 0.001) and the LHM group significantly increased the content of MDA (P < 0.05). In the high concentration treatment group, the activity of CAT in the HH and HHM groups was significantly increased, compared with that in the CK group (P < 0.05). The SOD activity was significantly increased in HH; HM and HHM groups (P < 0.001). The content of GSH in HH and HHM groups was significantly increased (P < 0.01), and the content of MDA was significantly increased in HH and HHM groups (P < 0.01). The concentration and type of pollutants showed significant interaction on the three oxidative stress indicators of SOD, GSH and MDA(P < 0.01) (Table 2).
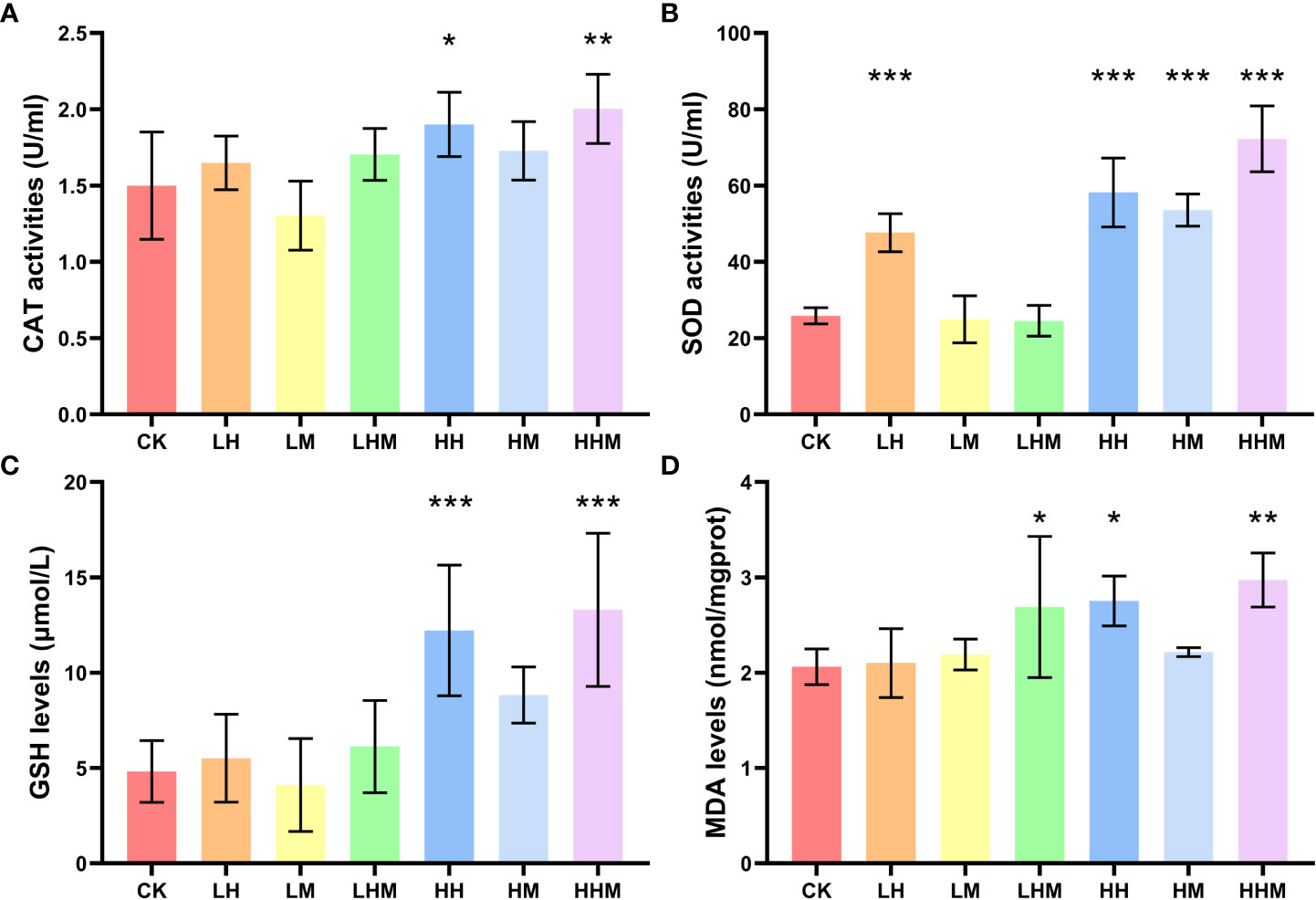
Figure 1 (A) Catalase (CAT) and (B) Superoxide dismutase (SOD) activities, (C) glutathione (GSH) and (D) Malondialdehyde (MDA) contents of A. japonicus at the end of the toxic exposure experiment. Asterisks (*) represent significant differences between treatments and the control group (CK) at the same developmental stage (*P < 0.05, **P < 0.01, ***P< 0.001). CK: control group; LH: environmentally relevant concentration Cd group; LM: environmentally relevant concentration MPs group; LHM: Combined group with environmentally relevant concentrations of Cd and MPs; HH: High concentration Cd group; HM: High concentration MPs group; HHM: Combined group with high concentrations of Cd and MPs.
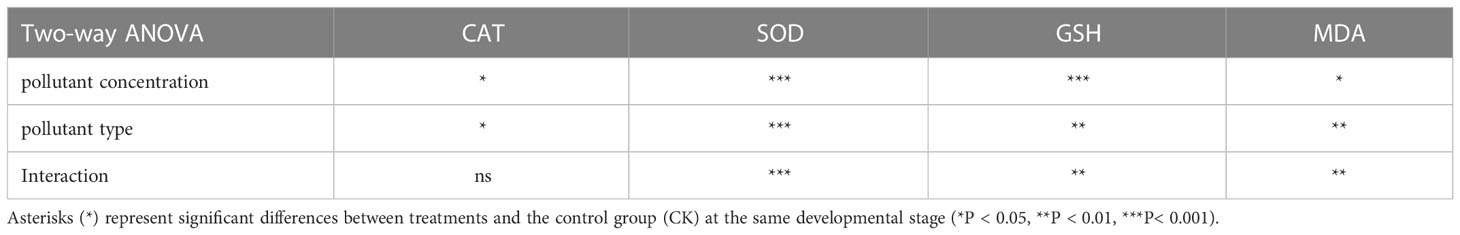
Table 2 The interactive effect of pollutant type and pollutant concentration on the parameters of oxidative stress of sea cucumber.
3.2 Transcriptome assembly and annotation
A total of 35 cDNA libraries were constructed in this study. For data quality assurance, raw data were data filtered before information analysis to reduce analytical interference from invalid data. First, we quality controlled the resulting raw reads using fastp, and filtered low quality data to obtain clean reads (Supplementary Table 1) (Chen et al., 2018). RNA-seq generated 35,986,062−62,000,734 high quality clean reads (Supplementary Table 2). We then aligned clean reads to the ribosomal database of that species using the short reads alignment tool Bowtie2, removed reads mapping to the ribosome on the alignment without allowing mismatches, and retained unmapped reads were used for subsequent transcriptome analysis (Langmead and Salzberg, 2012). Using hisat2 software, we developed reference genome-based alignment analysis. We mapped 25,301,750–44,342,913 reads (67.35%–75.63% of clean reads) to the genome of A. japonicus (Supplementary Table 3).
3.3 Identification of differentially expressed genes
To reveal the mechanism of how A. japonicus responds to MPs and Cd stress, we performed differential gene expression analysis (P < 0.05) in the CK intestine relative to the LH, LM, and LHM intestines of the environmental concentration treated group and the HH, HM, and HHM intestines of the high concentration group. In environmentally relevant concentration groups, there were 1107 DEGs in the LH group, consisting of 452 up regulated along with 655 down regulated DEGs; 1240 DEGs in LM group, consisting of 493 up regulated along with 747 down regulated DEGs; and 1091 DEGs in the LHM group, consisting of 518 up regulated along with 573 down regulated DEGs. In the high concentration group, there were 1,173 DEGs in the HH group, consisting of 505 up regulated along with 668 down regulated DEGs; 1,094 DEGs in the HM group, consisting of 419 up regulated along with 675 down regulated DEGs. The HHM group had the largest number of DEGs reaching 1618, consisting of 789 up regulated along with 829 down regulated DEGs (Figure 2). Figure 3 shows the top 10 DEGs with the highest fold change values under the six group treatments.

Figure 2 Differential expression genes (DEGs) were identified in the intestine of (A) japonicus after exposure. Volcano plots of DEGs in (A) CK vs. LH, (B) CK vs. LM, (C) CK vs. LHM, (D) CK vs. HH, (E) CK vs. HM, and (F) CK vs. HHM. Red dots designate up regulated genes, and orange dots designate down regulated genes. CK: control group; LH: environmentally relevant concentration Cd group; LM: environmentally relevant concentration MPs group; LHM: Combined group with environmentally relevant concentrations of Cd and MPs; HH: High concentration Cd group; HM: High concentration MPs group; HHM: Combined group with high concentrations of Cd and MPs.
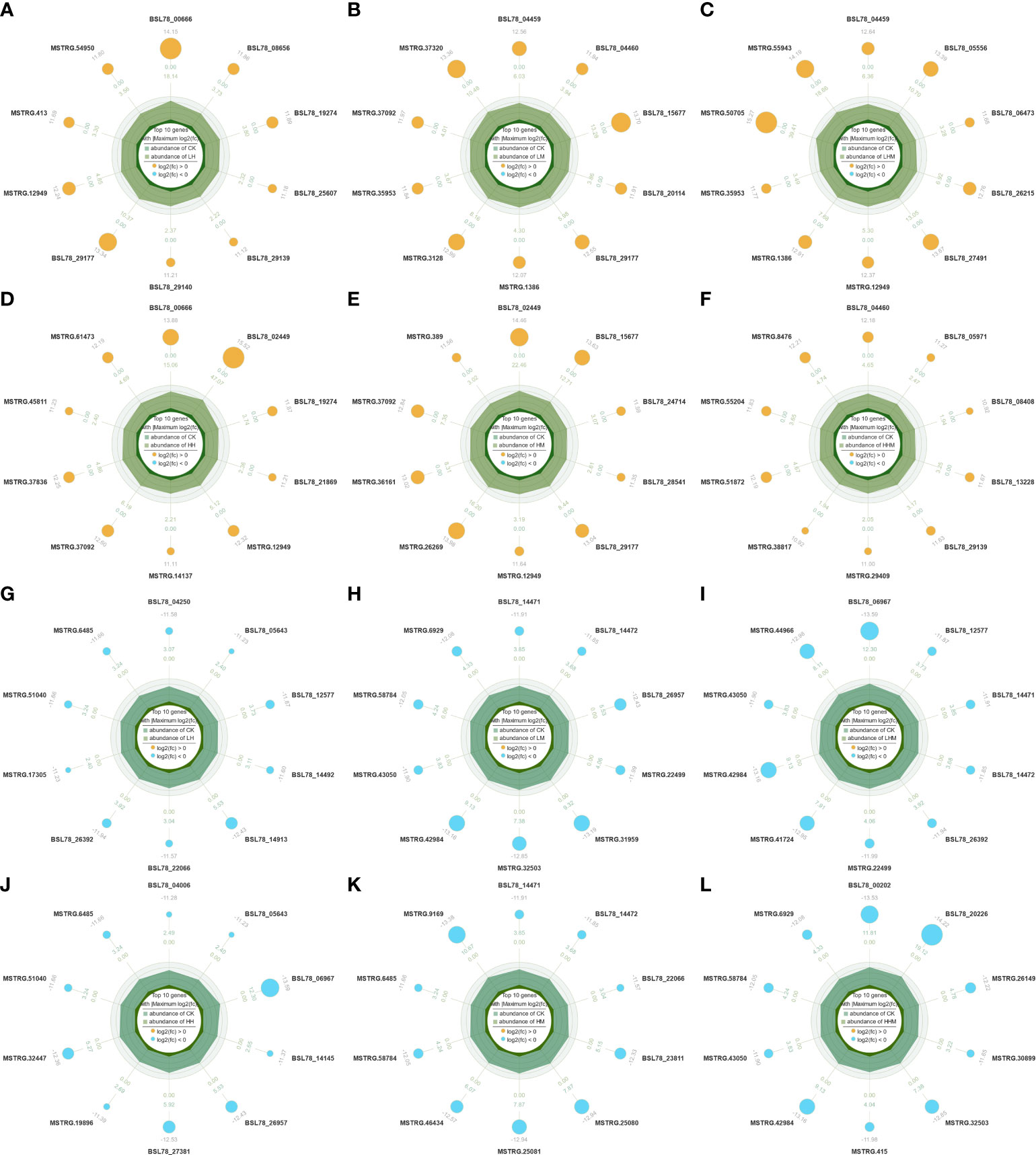
Figure 3 Radar map of the first 10 significantly up regulated differentially expressed genes in (A) CK vs. LH, (B) CK vs. LM, (C) CK vs. LHM, (D) CK vs. HH, (E) CK vs. HM, and (F) CK vs. HHM. Radar map of the first 10 significantly down regulated differentially expressed genes in (G) CK vs. LH, (H) CK vs. LM, (I) CK vs. LHM, (J) CK vs. HH, (K) CK vs. HM, and (L) CK vs. HHM. CK: control group; LH: environmentally relevant concentration Cd group; LM: environmentally relevant concentration MPs group; LHM: Combined group with environmentally relevant concentrations of Cd and MPs; HH: High concentration Cd group; HM: High concentration MPs group; HHM: Combined group with high concentrations of Cd and MPs.
3.4 Gene ontology enrichment analysis of differentially expressed genes
To gain insights into the biological roles of the DEGs, we performed a Gene ontology (GO) categories enrichment analysis. The GO enrichment data denoted that DEGs were classified into Biological Processes (BPs), Cellular Components (CCs), and Molecular Functions (MFs) three major functional categories. In the LH vs CK group, the most significantly enriched terms of DEGs in BPs, MFs and CCs three major functional categories are DNA-dependent DNA replication, magnesium chelatase activity, and replication fork; in the LM vs CK group, the most significantly enriched terms are interneuron axon guidance, extracellular matrix structural constituent, and basolateral plasma membrane; for LHM vs CK group, the most significantly enriched terms of are regulation of atrial cardiac muscle cell action potential, heme binding, and extracellular region; in the HH vs CK group, the most significantly enriched terms are mesonephric tubule formation, ADP binding, and NLS-dependent protein nuclear import complex; in the HM vs CK group, the most significantly enriched terms are Group II intron splicing, aspartic-type endopeptidase activity, and anchored component of membrane; and for HHM vs CK group, the most significantly enriched terms of DEGs in BPs, MFs and CCs three major functional categories are anchored component of membrane, oxidoreductase activity, and microbody.
3.5 Kyoto encyclopedia of genes and genomes enrichment analysis of differentially expressed genes
To further investigate the function of these DEGs, we mapped all these DEGs into the KEGG database. Hypergeometric tests with a P value cutoff of 0.05 was used as the criteria for pathway detection. After mapping to the KEGG database, the results in CK vs. LH, CK vs. LM, CK vs. LHM, CK vs. HH, CK vs. HM, and CK vs HHM groups showed that these DEGs were successfully annotated and assigned to 277, 239, 247, 233, 253, and 294 pathways, respectively. The most significant KEGG pathways in the LH, LM, LHM, HH, HM, HHM groups were DNA replication, pancreatic secretion, biosynthesis of unsaturated fatty acids, other glycan degradation, complement and coagulation cascades, and fatty acid metabolism, respectively. Figures 4, 5 demonstrates the top 20 most significantly altered pathways, with the most differentially gene containing pathways in cancer, metabolic pathways, arachidonic acid metabolism, MAPK signaling pathway, arachidonic acid metabolism, and metabolic pathways in CK vs. LH, CK vs. LM, CK vs. LHM, CK vs. HH, CK vs. HM, and CK vs. HHM, respectively. For better clarity and concise presentation, we analyzed the union of the top 20 GO terms and KEGG pathways in the environmentally relevant concentration and high concentration treatment groups, respectively (Figures 4–7). See Supplementary Figures 2-13 for specific significance and other information of these terms and pathways.
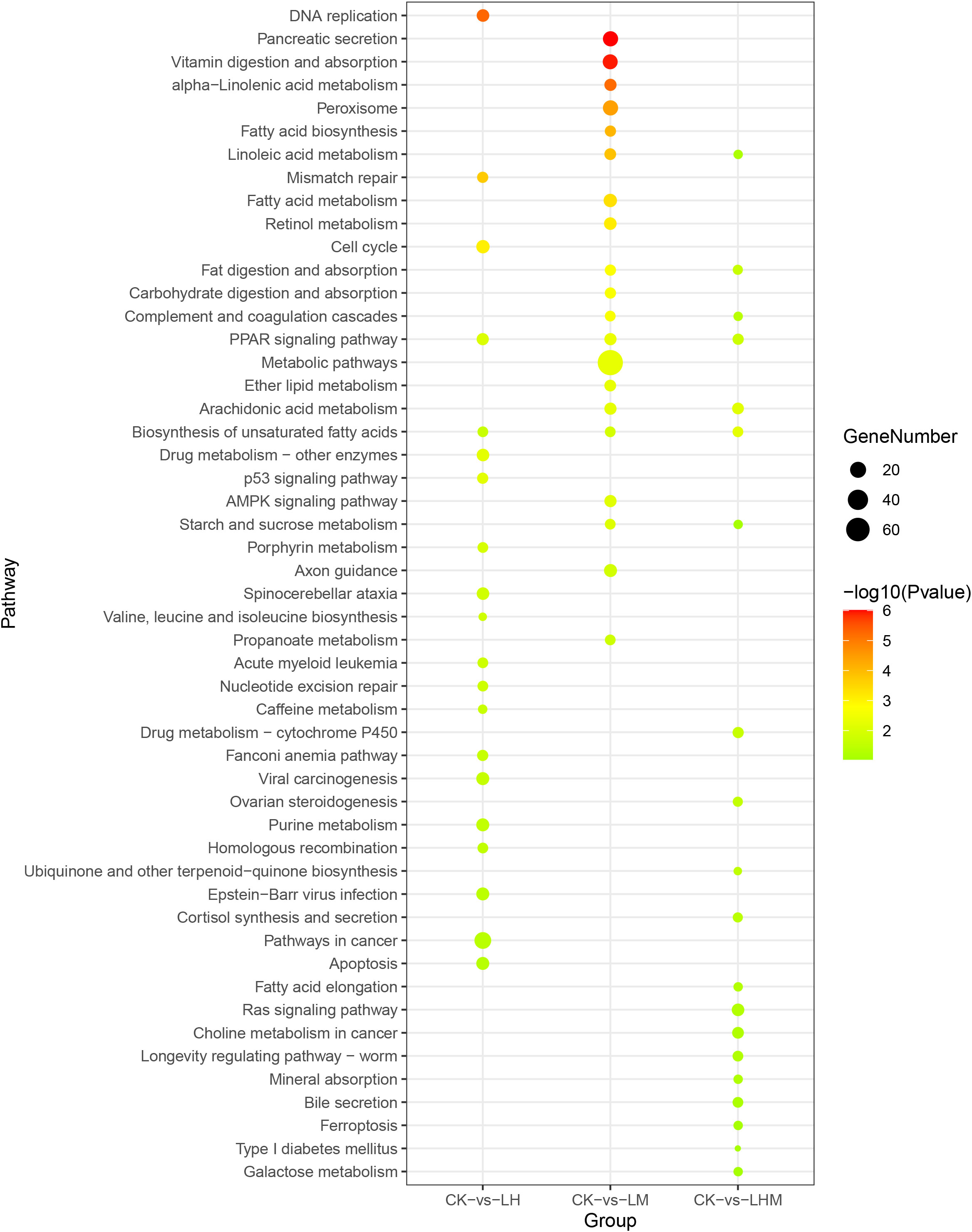
Figure 4 Top 20 KEGG pathways enrichment of DEGs in CK vs. LH, CK vs. LM, and CK vs. LHM. CK: control group; LH: environmentally relevant concentration Cd group; LM: environmentally relevant concentration MPs group; LHM: Combined group with environmentally relevant concentrations of Cd and MPs.
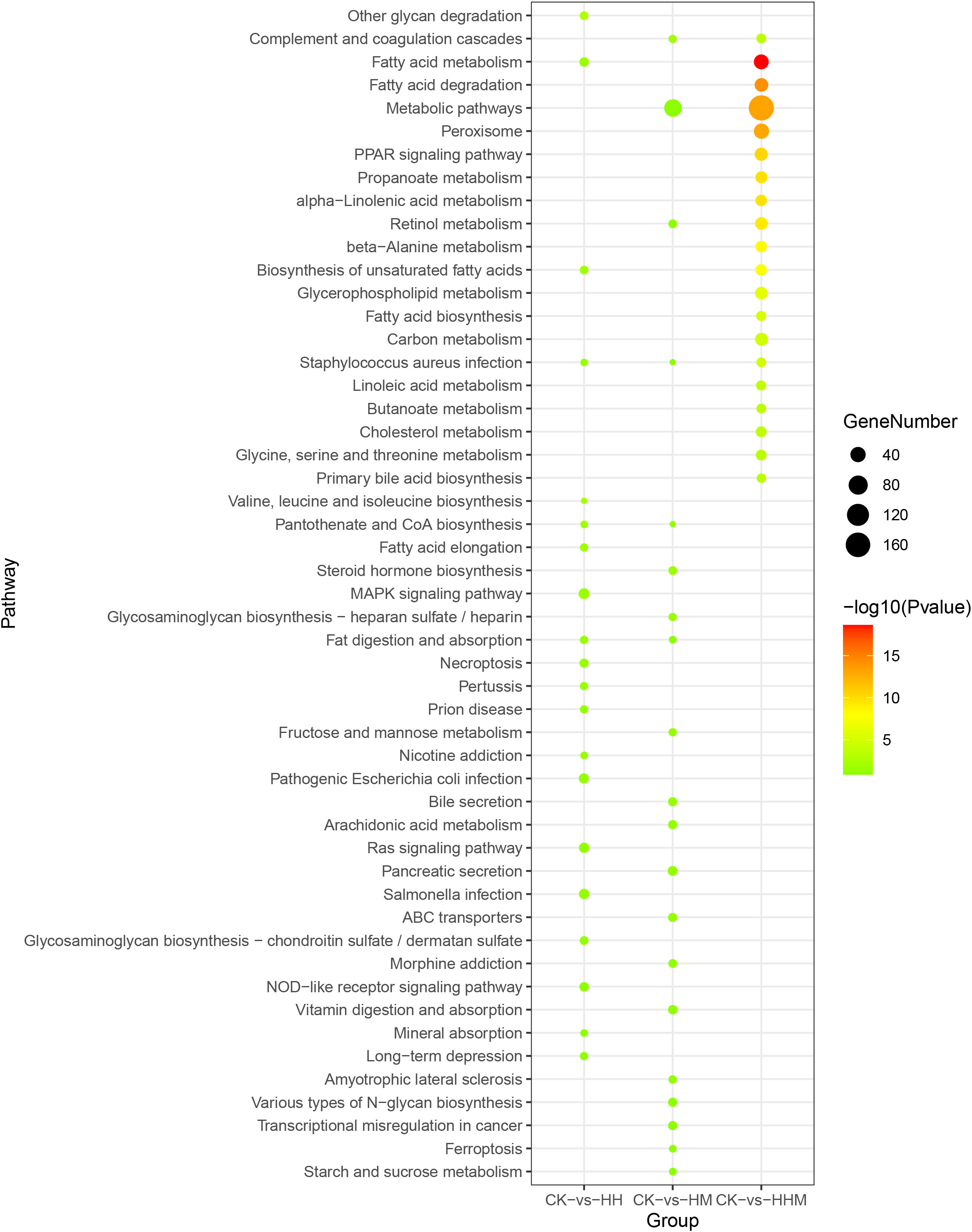
Figure 5 Top 20 KEGG pathways enrichment of DEGs in CK vs. HH, CK vs. HM, and CK vs. HHM. CK: control group; HH: High concentration Cd group; HM: High concentration MPs group; HHM: Combined group with high concentrations of Cd and MPs.
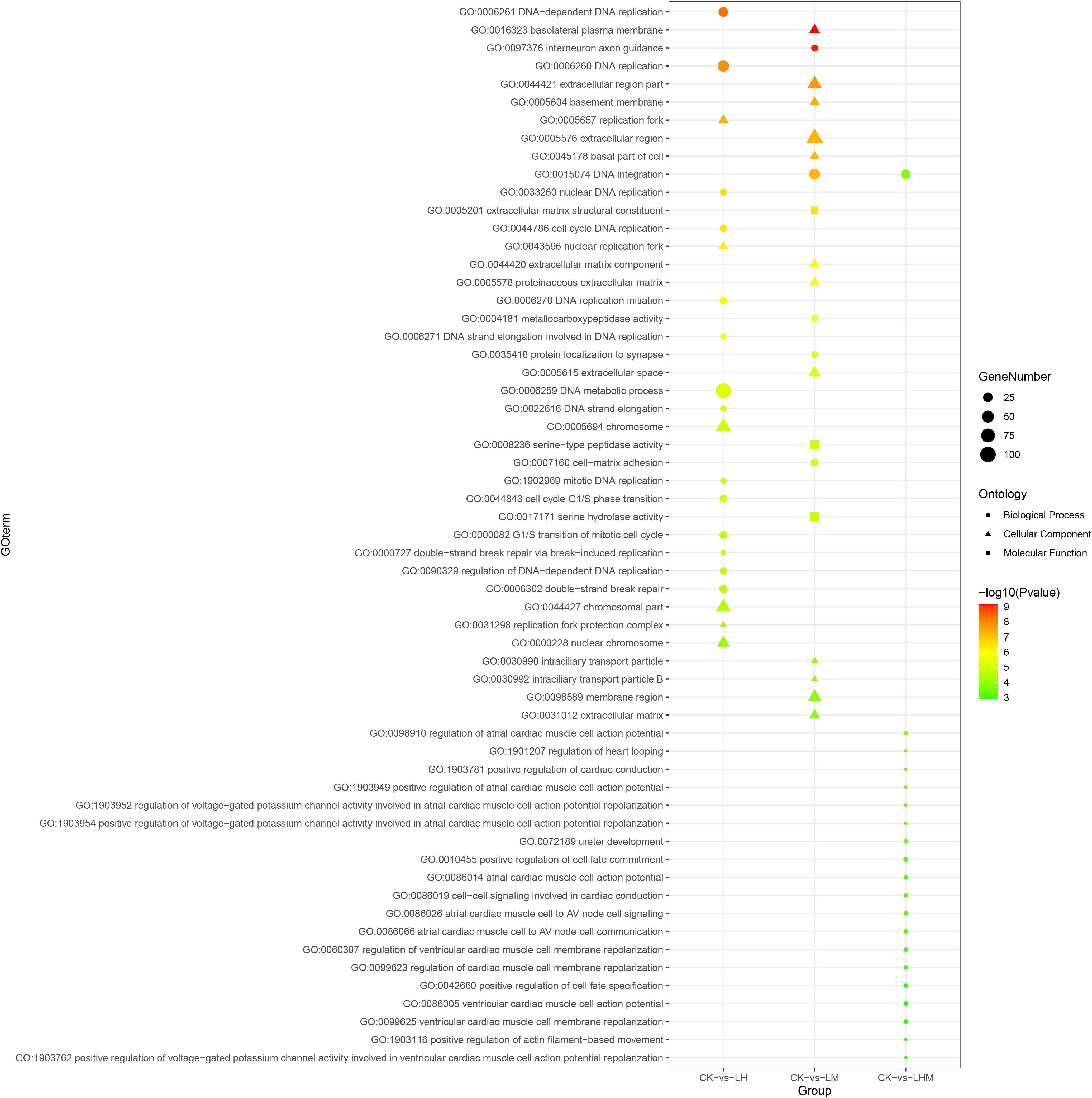
Figure 6 Top 20 Gene Ontology (GO) terms enrichment of DEGs in CK vs. LH, CK vs. LM, and CK vs. LHM. CK: control group; LH: environmentally relevant concentration Cd group; LM: environmentally relevant concentration MPs group; LHM: Combined group with environmentally relevant concentrations of Cd and MPs.
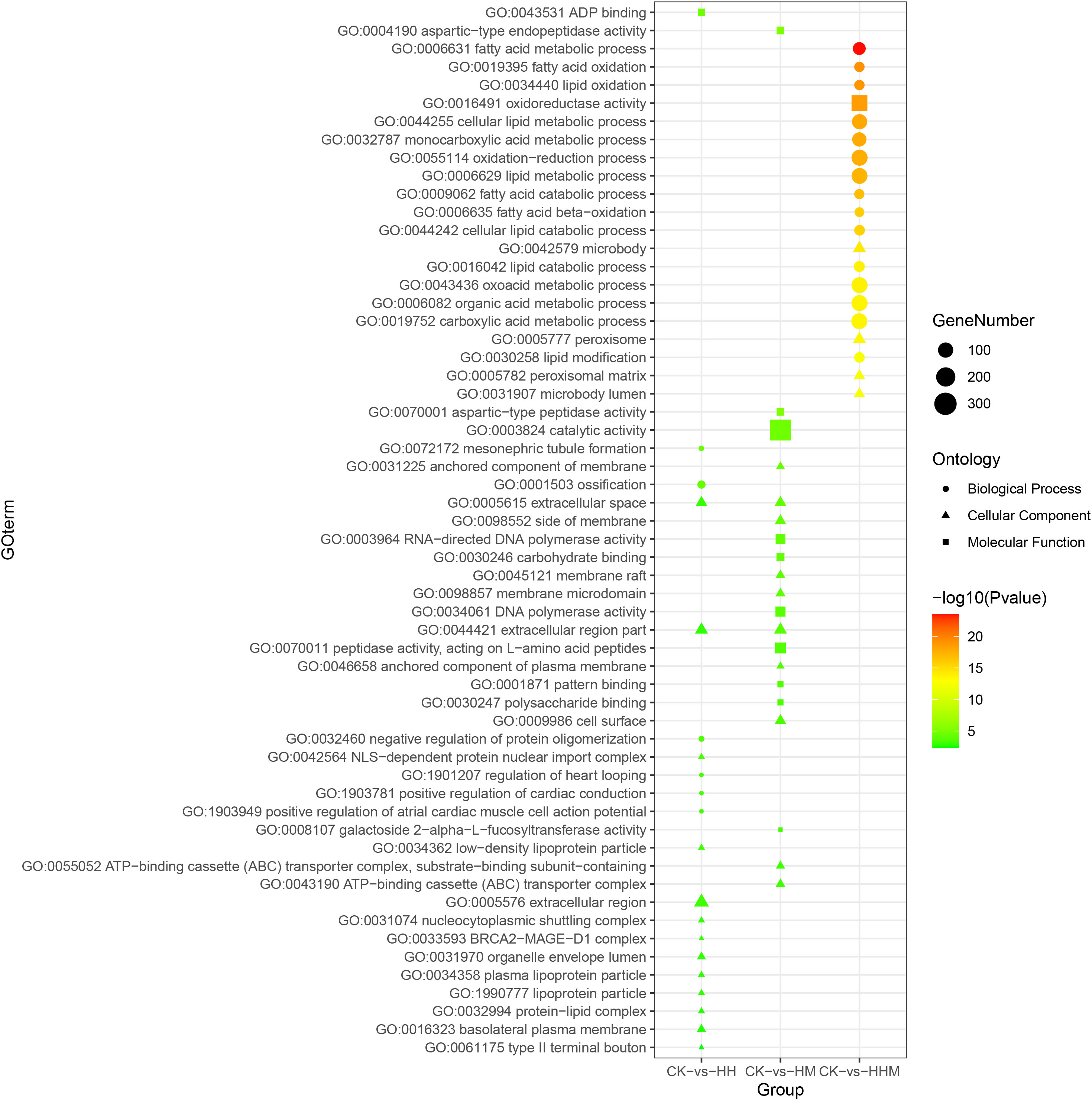
Figure 7 Top 20 Gene Ontology (GO) terms enrichment of DEGs in CK vs. HH, CK vs. HM, and CK vs. HHM. CK: control group; HH: High concentration Cd group; HM: High concentration MPs group; HHM: Combined group with high concentrations of Cd and MPs.
3.6 Targeted gene analysis
By comparing the genes of all treatment groups containing Cd treatments, we found 30 DEGs in common. To clarify the function of these 30 DEGs, they were subjected to KEGG enrichment and found to be significantly enriched in a total of three pathways in lipid metabolism, glycan biosynthesis and metabolism and immune system. Similarly, in the DEGs Venn plots of all the MPs treated groups, 27 DEGs were found (Figure 8). We performed KEGG enrichment analysis and found that it was significantly enriched in a total of seven pathways including endocrine system, immune system, digestive system, glycan biosynthesis and metabolism, lipid metabolism, endocrine and metabolic disease, and infectious disease: bacterial. MPs and Cd treatment had significant effects on lipid metabolism and immune system. In the Cd treatment group, three lipid metabolism pathways of fatty acid elongation, biosynthesis of unsaturated fatty acids, and steroid household biosynthesis changed significantly. In the MPs treatment group, the steroid hormone biosynthesis pathway of lipid metabolism changed. Toll-like receptor signaling pathway and complex and coordination cascades pathways were significantly enriched in the immune system of Cd and microplastic treatment groups, respectively. In addition, MPs also caused changes in cortisol synthesis and secret, prolactin signaling pathway, and ovarian steroidogenesis endocrine system pathways.
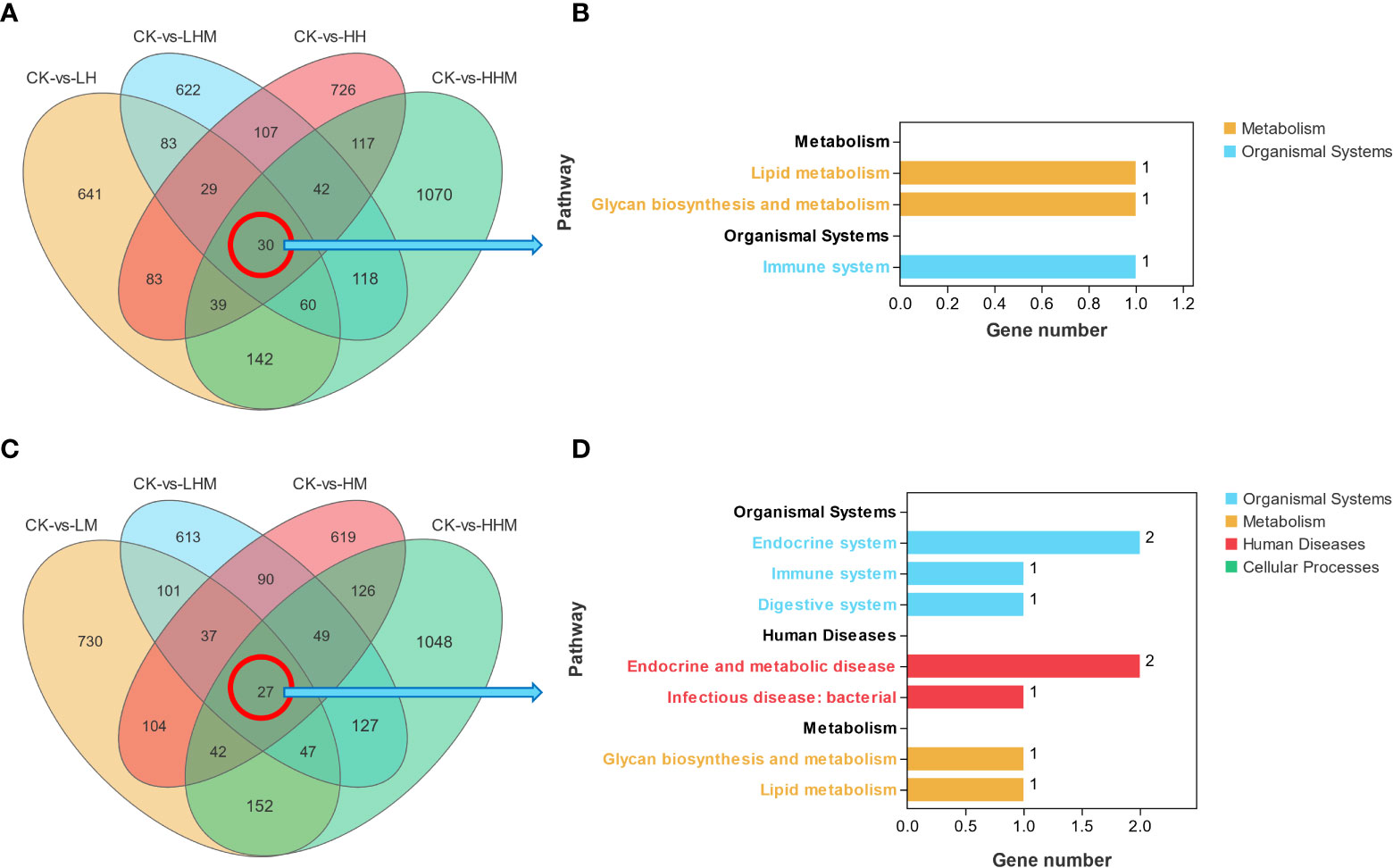
Figure 8 Wayne map of common differentially expressed genes (DEGs) in (A) Cd and (C) microplastic treatment groups. Enrichment of KEGG pathway by (B) Cd and (D) microplastic treatment groups target DEGs. CK: control group; LH: environmentally relevant concentration Cd group; LM: environmentally relevant concentration MPs group; LHM: Combined group with environmentally relevant concentrations of Cd and MPs; HH: High concentration Cd group; HM: High concentration MPs group; HHM: Combined group with high concentrations of Cd and MPs.
3.7 Real-time PCR validation
In order to verify the gene expression profile identified by RNA Seq, six DEGs were selected to verify the correctness of transcriptome analysis (BSL78_01257, BSL78_04100, BSL78_07802, BSL78_08543, BSL78_12019, BSL78_20141). The relative expression level of mRNA was further detected by qRT-PCR. These data were congruent with RNA-seq data in terms of the variation trend (Figure 9), demonstrating the accuracy and reliability of the RNA-seq investigation.
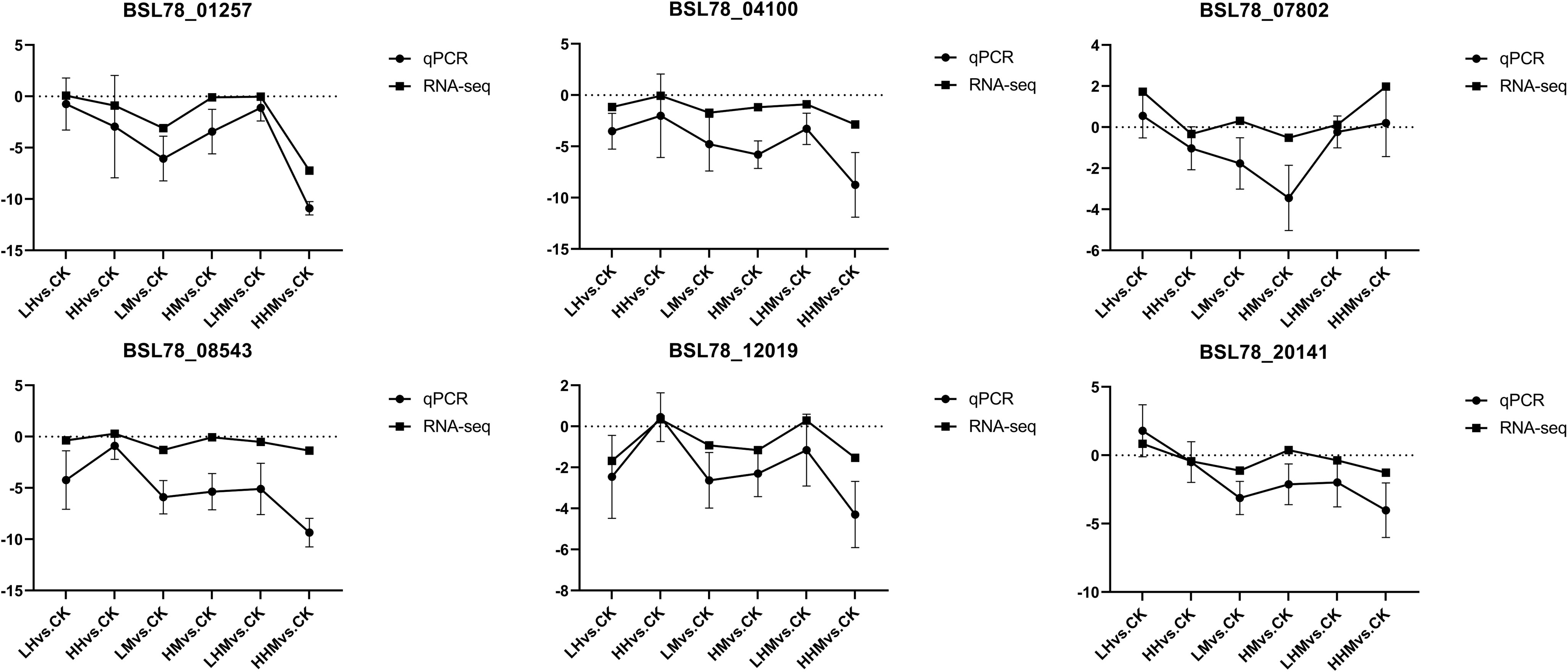
Figure 9 Relative expression of 6 selected genes differentially expressed after treatment. CK: control group; LH: environmentally relevant concentration Cd group; LM: environmentally relevant concentration MPs group; LHM: Combined group with environmentally relevant concentrations of Cd and MPs; HH: High concentration Cd group; HM: High concentration MPs group; HHM: Combined group with high concentrations of Cd and MPs.
4 Discussion
The pollution of MPs in the water column often has a great visual impact on people, but the transfer of MPs from the water column to the sediment environment has a huge impact on benthos. Sediment environment is considered as a long-term sink of MPs (Cozar et al., 2014; Avio et al., 2017). Heavy metal pollution in sediment environment is also an important environmental pollution problem (Hanebuth et al., 2018). The feeding characteristics of sea cucumber as deposit feeders or suspension feeders will cause it to be in the complex pollution environment of MPs and heavy metals (Roberts et al., 2000; Taylor et al., 2016). In our study, sea cucumbers were exposed to MPs and Cd under simulated environmental concentrations and severe pollution. We detected their indicators related to oxidative stress, and used RNA-Seq technology to build the gene expression profile of the intestine most vulnerable to these two pollutants. The changes of catalase (CAT) and superoxide dismutase (SOD) enzyme activities and the contents of glutathione (GSH) and malondialdehyde (MDA) mainly occurred in the high concentration treatment group. The transcriptome sequencing results showed that the MPs and Cd caused the intestinal inflammatory reaction of sea cucumber and affected the immune system and endocrine system.
4.1 Changes of indexes related to oxidative stress
In the process of evolution, organisms have formed an effective antioxidant defense system including antioxidant enzymes and non-enzymatic antioxidants. In the present study these parameters were not severely affected by the treatment of pollutants at environmentally relevant concentrations, and the absence of oxidative stress at this concentration may be due to the protective effect of the gut, which sequestered Cd more than liver and muscle (Hani et al., 2018).
SOD, CAT enzyme activities and GSH, MDA contents were significantly increased in the high concentration Cd treatment group. Heavy metals can damage organisms through various pathways including ROS generation, weakening of the antioxidant defense, enzyme inactivation, oxidative stress and some of them have selective binding to specific macromolecules (Balali-Mood et al., 2021). When aquatic invertebrates are exposed to heavy metals, animals respond by activating detoxification mechanisms (Wenli et al., 2008). But with increasing concentration or time of exposure, Cd may exceed the detoxification limit of the organism. Cd will bind to macromolecular proteins (such as various enzymes) in the body, resulting in toxic effects.
In this study, high concentrations of MPs caused an increase in SOD activity. Current research has found that the toxic effects of MPs on marine organisms are affected by various factors. The size, concentration, and type will affect the accumulation and distribution of MPs in organisms to a certain extent, and then have different toxic effects on organisms (Kim et al., 2021). The MPs used in this study are large in size (Supplementary Figure 1), the defecation cycle of A. japonicus is short, and the large-sized MPs stay in the intestine for a short time, so they have less impact. Overall, micron-sized MPs can be efficiently excreted in the form of fecal pellets. However, it is important to note that smaller MPs, such as nano-sized MPs, may have retention times longer than 24 hours or even days (Jeong et al., 2018).
Combined exposure to high concentrations of experimental groups did not cause more severe oxidative stress in this study, however, some studies have shown that the interaction between MPs and heavy metals may greatly affect the bioaccumulation and toxicity of heavy metals (Barboza et al., 2018). MPs can aggravate copper toxicity in zebrafish liver and gut by inhibiting copper ion transport while enhancing oxidative stress, resulting in elevated levels of malondialdehyde (MDA) and metallothionein (MT), and decreased levels of superoxide dismutase (SOD) (Qiao et al., 2019). When MPs and heavy metals coexist, the toxicity to organisms changes, and even the toxicity trend of the same substance may vary due to the functional groups and other characteristics of MPs (Kim et al., 2017).
4.2 Function classification of DEGs
Among the top 10 genes with significant difference between each experimental group and the control group (Figure 2), gene BSL78_00666 was significantly up regulated in LH and HH treatment groups, which may be related to the synthesis of FAS-associated death domain protein (FADD). FADD is a central mediator of death receptor-initiated apoptosis that directly activates the caspase-8 protease (Chinnaiyan et al., 1995; Kim et al., 2003). In addition, overexpression of FADD complex may cause apoptosis and inflammation (Salaun et al., 2007). Abnormal inflammatory reaction is an important link of cell and tissue damage caused by heavy metals (Fagerberg et al., 2017). Cd has always been considered as a kind of xenobiotics related to inflammation, because it can induce complex inflammatory reactions in a variety of cell types (Anka et al., 2022). After Cd exposure for 9 weeks, the expression of two enzymes associated to inflammation, cyclooxygenase type 2 (COX-2), and iNOS in mice increased significantly. Chronic Cd nephrotoxicity is associated with overexpression of COX-2 and iNOS (Morales et al., 2006).
Gene BSL78_19274 was significantly up regulated in LH and HH treatment groups, which was related to the synthesis of heparan sulfate 2-O-sulfotransferase. Heparin sulfate is a glycosaminoglycan sulfate, which can facilitate neutrophil recruitment based on the reduction of neutrophil infiltration in mice (Axelsson et al., 2012). Cytokines (TNF-α, IL-1, and others), L- and P-selectins, and chemokines bind to therapeutic heparin and purified heparan sulfate, suggesting the importance of heparan sulfate in inflammation (Ley et al., 1991; Wang et al., 2002). Neutrophil recruitment and exosmosis at the inflammatory site provide a mechanism for host defense. The up-regulation of this gene indicated the response mechanism of sea cucumber in response to the inflammation caused by Cd exposure.
Gene BSL78_29177 was significantly up regulated in LH, LM and HM treatment groups, which may be related to the activation of NLRC4 inflammatory bodies. The NLR family inflammatory bodies are activated by various exogenous and endogenous drugs, and then promote and enhance the inflammatory response. NLRC4 inflammatory corpuscles are considered to be the main promoter of Hemophagocytic lymphohistiocytosis (Schulert and Cron, 2020; Tomasik and Basak, 2022). Hemophagocytic lymphohistiocytosis is a disease characterized by immune system damage associated with excessive inflammation. Excessive release of proinflammatory cytokines triggers pathological systemic inflammation, which ultimately leads to multiple organ failure (Hayden et al., 2016). A total of 1% to 4% of polystyrene Pparticles ingested in the intestine are believed to migrate into the blood. Transferring nano plastics into the blood may cause local inflammation or cause allergic reaction in the tissues (Sass et al., 1990; Hwang et al., 2020).
CASP6 (BSL78_15677) gene was significantly up regulated in LM and HM groups. Caspases are a family of intracellular cysteine proteases that play an important role in tissue homeostasis by regulating inflammation and apoptosis. The disorder of these proteases can lead to inflammatory diseases, neurodegenerative diseases, and cancer (Creagh et al., 2003). Intracellular protease caspase-6 (CASP6) regulates neuronal apoptosis and axonal degeneration. Higher levels of Casp6 activity are associated with lower cognitive ability (Albrecht et al., 2007; Berta et al., 2014). MSTRG.12949 and MSTRG.6485 showed significant changes in expression in multiple treatment groups, which may be an important gene for intestinal response to pollutants. However, their annotation is unknown.
4.3 Gene ontology and Kyoto encyclopedia of genes and genomes enrichment analysis of differentially expressed genes
GO enrichment and KEGG enrichment analysis provided biological variations and molecular mechanisms possibly participate in detoxification of MPs and Cd in sea cucumber. Among the GO enrichment items, there were few items shared by the combined treatment group and the single MPs and Cd treatment group. Only DNA integration (GO: 0015074) was significantly enriched in LM and LHM treatment groups. In the high concentration treatment, the two entries of extracellular space (GO: 0005615) and extracellular region part (GO: 0044421) were significantly enriched in the HH and HM treatment groups. These results indicated that the toxic effects of MPs and Cd on sea cucumber were complex, and the effects of mixed exposure of the two pollutants were not simple addition effects.
In the environmental related concentration treatment group, the differentially expressed genes in LM, LH, and LHM treatment groups were significantly enriched in Peroxisome proliferator activated receptor (PPAR) signaling pathway and biosynthesis of unsaturated fat acids pathway. PPAR can regulate the metabolism of many cells, regulate the transcription of target genes, and play a very important role in various metabolic processes (Ma et al., 2017). PPAR signaling pathway is closely related to lipid metabolism. Stearoyl CoA Desaturase (SCD1) and acyl CoA oxidase (ACO) are important proteins in PPAR signaling pathway, which play a regulatory role in fatty acid composition. SCD1 is the rate-limiting enzyme of biosynthesis of monounsaturated fatty acids. It plays an important role in de novo synthesis of fatty acids (Dumas and Ntambi, 2017; Tracz-Gaszewska and Dobrzyn, 2019). LM treatment may have a negative effect on fatty acid metabolism of sea cucumber. Interestingly, after LH treatment, SCD1 and long chain acyl CoA synthase (ACS) were up regulated, and LH treatment promoted the metabolism of fatty acids to some extent. It may be related to the detoxification of pollutants by organisms themselves. SCD1 protein is located on the endoplasmic reticulum membrane and mainly controls the endoplasmic reticulum stress response. The increase of saturated phospholipid components leads to the destruction of the endoplasmic reticulum, which activates the endoplasmic reticulum stress response. The stress reaction can be repaired by supplementing unsaturated fatty acids (Borradaile et al., 2006; Peng et al., 2011).
MPs and Cd play an antagonistic role in the metabolism of fatty acids at low concentrations. Similarly, in biosynthesis of unsaturated fatty acids pathway, there were more up regulated genes after LH treatment and more down regulated genes after LM treatment. Furthermore, the arachidonic acid metabolism pathway was significantly enriched in the LM and LHM treatment groups. In addition, the linoleic acid metabolism pathway and the starch and sucrose metabolism pathway were both enriched in the LM and LHM groups. Further analysis of DEGs enriched in the pathway showed that after LM treatment, DEGs in the linoleic acid metabolism and arachidonic acid metabolism pathways were mainly down regulated, while DEGs in the starch and sucrose metabolism pathways were up regulated. After LHM treatment, DEGs in the linoleic acid metabolism and radionic acid metabolism pathways were up regulated, and those in the starch and sucrose metabolism were down regulated. In our study, MPs inhibited lipid metabolism, promoted carbohydrate metabolism, and antagonized Cd. It is speculated that MPs may have a significant impact on intestinal metabolism related pathways. Metabolic pathways are also significantly enriched in HM and HHM groups. Similarly, ingesting MPs can affect the metabolism of fish by changing the proportion of triglycerides and cholesterol in the blood and the distribution of cholesterol in muscle and liver tissues (Jovanovic, 2017). It will also affect the induction or inhibition of enzymes related to lipid metabolism, as well as the changes of hormone levels related to lipid metabolism, leading to changes in triglyceride and cholesterol levels (Banaee et al., 2019).
In addition, the complex and coagulation cascades pathway was significantly enriched in LM and LHM groups. In LM, the completion component (3b/4b) receiver 1 (CR1) was up regulated. In LHM, the completion factor H (CFH) and completion component (3b/4b) receiver 1 (CR1) were up regulated. Similarly, this pathway was significantly enriched in HM and HHM treatment groups, and most of the genes were up regulated. Complement factor H (CFH), a multifunctional soluble complement regulatory protein, can bind to a variety of pathogens and play a crucial role in host innate immune defense. CFH is also an important regulator of immune cells. It can regulate the phagocytosis of monocytes/macrophages and the production of inflammatory factors (Abdul-Aziz et al., 2016). CFH plays an important role in protecting host cells against damage mediated by disturbance of completion system and normal function of immune cells (Roy et al., 2016). CFH also aids non-inflammatory clearance of damaged cells and cell debris. These results may be related to the physical damage of MPs to the intestine (Jozsi et al., 2022). Simple chemical forces can produce nanoscale particles from PS particles and cause direct cell damage. Absorbed MPs and nanomaterials with a diameter less than 1.5 µm can directly damage cells (Mattsson et al., 2017; Sharma and Chatterjee, 2017).
4.4 Analysis of target functional genes related to immune response
Analysis of common differential genes in all Cd-containing treatment groups showed that gene BSL78_29595 is enriched to Toll-like receiver signaling pathway. The Toll interacting protein (TOLLIP) is an inhibitor of TLR4 signal pathway. On one hand, it can interact with interleukin 1 receptor associated kinase 4 (IRAK4) in TLR4-MYD88 dependent signal pathway, and inhibit the phosphorylation of IRAK4, thereby inhibiting the activation of the downstream of TLR4 signal pathway (Burns et al., 2000). On the other hand, TOLLIP interacts with TLR2, TLR4, and IL-1R receptor to inhibit the occurrence of natural immune response activated by LPS (Bulut et al., 2001). In Toll-like signaling pathway, TOLLIP plays a negative regulatory role and inhibits the occurrence of Toll signaling pathway. Recent studies have shown that TOLLIP negative regulation of TLR4 signaling pathway may help to limit the production of excessive cytokines during inflammation and various pathogenic infections (Zhang and Ghosh, 2002; Shibolet and Podolsky, 2007). Therefore, TOLLIP seems to be a key regulatory factor involved in the initiation and maintenance of inflammatory process in TLR4 mediated inflammatory process (Didierlaurent et al., 2006; Kowalski and Li, 2017). Kim et al. (2020) knockdown of TOLLIP expression by RNA interference, RM treated activated macrophages showed augmented expression of inflammatory mediators (pro-inflammatory cytokines, NO, inducible nitric oxidase, and cyclooxygenase-2, and surface molecules) and restored the expression of MAPK and NF-κB signals inhibited by RM treatment (Kim et al., 2020). In this study, TOLLIP plays an important role in the negative regulation of the intestinal tract of sea cucumber on Cd-induced inflammation.
High concentrations of MPs or toxic substances adsorbed by MPs may cause acute damage to the human intestinal tract, inflammation of the inner wall of the intestinal tract, and a sharp decline in the amount of intestinal mucus (Wright and Kelly, 2017). Intestinal immune system disorder can recruit inflammatory cells to release inflammatory mediators, causing tissue damage and dysfunction. Among them, cell adhesion molecules play an important role in intestinal immune response and inflammation process.
The SELE gene encodes E-selectin, which is found in cytokine-stimulated endothelial cells and is thought to be responsible for the accumulation of leukocytes at sites of inflammation by mediating the adhesion of cells to the vascular wall (Li et al., 2021). Inflammatory factor interleukin-1 (IL-1), tumor necrosis factor-α (TNF-α), bacterial lipopolysaccharide (LPS) stimulated endothelial cells, the expression of E-selectin increased significantly, which can increase the permeability of blood vessel surface, promote the activation of inflammatory cells, and mediate the occurrence of inflammation (McEver, 2015). Similarly, high concentrations of polystyrene MPs/NPs may induce intestinal inflammation due to the disorder of zebrafish intestinal microbiota. The proinflammatory cytokines were significantly up regulated in the 1 mg/L NPS treatment group. The correlation between intestinal microbiota composition and immune related genes showed that the change of intestinal microbiota comparison was positively correlated with the expression of immune cytokines. Different amounts of MPs can increase the secretion of pro-inflammatory cytokine IL-1α in serum (Li et al., 2020). Different amounts of MPs can also increase the secretion of proinflammatory cytokines IL-1α in mice serum, but the amount of each MPs will affect the secretion of specific cytokines. High concentrations of MPs tend to induce intestinal inflammation by activating TLR4 signal transduction (Li et al., 2020). MPs are considered as foreign substances, which can stimulate the immune response of fish or inhibit the immune function by inducing immunotoxicity, i.e., MPs can affect the immunity of fish through a variety of mechanisms. However, a detailed study of the immune response of fish after MP exposure is needed (Kim et al., 2021).
5 Conclusions
In conclusion, the oxidative stress of sea cucumber was mainly influenced by Cd. Although the high concentration of MPs also caused a certain increase in SOD activity, this effect may not cause substantial damage to the sea cucumber. This study is the first to report the transcriptome information of A. japonicus intestine after combined exposure to Cd and MPs. In the environmental concentration group, 1,107, 1,240, and 1,091 DEGs were identified respectively in LH, LM, and LHM groups, and 1,173, 1,094 and 1,618 DEGs were identified, respectively, in HH, HM and HHM groups in the high concentration group. Cd-induced up-regulation of intestinal FAS associated death domain protein (FADD) expression may cause apoptosis and inflammation. At the same time, the increase of intestinal putative heparan sulfate 2-O-sulfotransferase in LH and HH treatment groups provides a mechanism for host defense. The imbalance of expression of NLR family inflammatory bodies and CASP6 in the MPs treatment group also led to the inflammatory reaction in the intestine of sea cucumber. GO and KEGG pathway analysis showed that the mixed toxicity of MPs and Cd was not a simple additive effect. In the process of fatty acid metabolism, MPs and Cd showed antagonistic effects, mainly in the inconsistent expression of SCD1 protein. MPs and Cd also showed antagonistic effects in lipid metabolism and carbohydrate metabolism pathways. The significant up-regulation of completion factor H after MPs treatment may be related to the physical damage caused by microplastics. The significant changes of TOLLIP and SELE in all Cd and MPs treatment groups may indicate the key immune response genes of sea cucumber to Cd exposure and MPs exposure. These genes are involved in the immune defense of sea cucumber exposed to different levels of Cd and MPs. These findings indicated that the exposure of MPs and Cd caused oxidative stress in sea cucumber, inflammatory reaction in the intestine, and activated the intestinal immune defense system. The results of this study will provide clues for the molecular process of the combined toxicity of MPs and Cd.
Data availability statement
The data presented in the study are deposited in the NCBI Sequence Read Archive repository, accession number PRJNA907491.
Author contributions
CZ: Conceptualization, Investigation, Formal analysis, Writing – original draft, Writing – review & editing. LZ: Funding acquisition, Writing – review & editing. LL: Writing – review & editing. MM: Writing – review & editing. FS: Methodology. XW: Formal analysis. CL: Writing – review & editing, Resources, Funding acquisition, Supervision. All authors contributed to the article and approved the submitted version
Funding
This work was supported by the National Natural Science Foundation of China (42176106; 31960225; 32150410376) and the Shandong Provincial Key R&D Program, China (2022RZB07051).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2023.1109691/full#supplementary-material
References
Abdul-Aziz M., Tsolaki A. G., Kouser L., Carroll M. V., Al-Ahdal M. N., Sim R. B., et al. (2016). Complement factor h interferes with mycobacterium bovis BCG entry into macrophages and modulates the pro-inflammatory cytokine response. Immunobiology 221 (9), 944–952. doi: 10.1016/j.imbio.2016.05.011
Akhbarizadeh R., Moore F., Keshavarzi B. (2018). Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian gulf. Environ. pollut. 232, 154–163. doi: 10.1016/j.envpol.2017.09.028
Albrecht S., Bourdeau M., Bennett D., Mufson E. J., Bhattacharjee M., LeBlanc A. C. (2007). Activation of caspase-6 in aging and mild cognitive impairment. Am. J. Pathol. 170 (4), 1200–1209. doi: 10.2353/ajpath.2007.060974
Anka A. U., Usman A. B., Kaoje A. N., Kabir R. M., Bala A., Arki M. K., et al. (2022). Potential mechanisms of some selected heavy metals in the induction of inflammation and autoimmunity. Eur. J. Inflammation 20. doi: 10.1177/1721727X221122719
Auta H. S., Emenike C. U., Fauziah S. H. (2017). Distribution and importance of microplastics in the marine environment: A review of the sources, fate, effects, and potential solutions. Environ. Int. 102, 165–176. doi: 10.1016/j.envint.2017.02.013
Avio C. G., Gorbi S., Regoli F. (2017). Plastics and microplastics in the oceans: From emerging pollutants to emerged threat. Mar. Environ. Res. 128, 2–11. doi: 10.1016/j.marenvres.2016.05.012
Axelsson J., Xu D., Kang B. N., Nussbacher J. K., Handel T. M., Ley K., et al. (2012). Inactivation of heparan sulfate 2-o-sulfotransferase accentuates neutrophil infiltration during acute inflammation in mice. Blood 120 (8), 1742–1751. doi: 10.1182/blood-2012-03-417139
Balali-Mood M., Naseri K., Tahergorabi Z., Khazdair M. R., Sadeghi M. (2021). Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 12. doi: 10.3389/fphar.2021.643972
Banaee M., Soltanian S., Sureda A., Gholamhosseini A., Haghi B. N., Akhlaghi M., et al. (2019). Evaluation of single and combined effects of cadmium and micro-plastic particles on biochemical and immunological parameters of common carp (Cyprinus carpio). Chemosphere 236, 124335. doi: 10.1016/j.chemosphere.2019.07.066
Barboza L. G. A., Vieira L. R., Branco V., Carvalho C., Guilhermino L. (2018). Microplastics increase mercury bioconcentration in gills and bioaccumulation in the liver, and cause oxidative stress and damage in dicentrarchus labrax juveniles. Sci. Rep. 8 (1), 15655. doi: 10.1038/s41598-018-34125-z
Berta T., Park C. K., Xu Z. Z., Xie R. G., Liu T., Lu N., et al. (2014). Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-alpha secretion. J. Clin. Invest. 124 (3), 1173–1186. doi: 10.1172/JCI72230
Bhagat J., Nishimura N., Shimada Y. (2021). Toxicological interactions of microplastics/nanoplastics and environmental contaminants: Current knowledge and future perspectives. J. Hazard. Mater. 405, 123913. doi: 10.1016/j.jhazmat.2020.123913
Borradaile N. M., Han X. L., Harp J. D., Gale S. E., Ory D. S., Schaffer J. E. (2006). Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J. Lipid Res. 47 (12), 2726–2737. doi: 10.1194/jlr.M600299-JLR200
Boyd C. E., Massaut L. (1999). Risks associated with the use of chemicals in pond aquaculture. Aquacult. Eng. 20 (2), 113–132. doi: 10.1016/S0144-8609(99)00010-2
Bulut Y., Faure E., Thomas L., Equils O., Arditi M. (2001). Cooperation of toll-like receptor 2 and 6 for cellular activation by soluble tuberculosis factor and borrelia burgdorferi outer surface protein a lipoprotein: Role of toll-interacting protein and IL-1 receptor signaling molecules in toll-like receptor 2 signaling. J. Immunol. 167 (2), 987–994. doi: 10.4049/jimmunol.167.2.987
Burns K., Clatworthy J., Martin L., Martinon F., Plumpton C., Maschera B., et al. (2000). Tollip, a new component of the IL-1RI pathway, links IRAK to the IL-1 receptor. Nat. Cell Biol. 2 (6), 346–351. doi: 10.1038/35014038
Carbery M., MacFarlane G. R., O’Connor W., Afrose S., Taylor H., Palanisami T. (2020). Baseline analysis of metal(loid)s on microplastics collected from the Australian shoreline using citizen science. Mar. Pollut. Bull. 152, 110914. doi: 10.1016/j.marpolbul.2020.110914
Chen S. F., Zhou Y. Q., Chen Y. R., Gu J. (2018). Fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinf. 34 (17), 884–890. doi: 10.1093/bioinformatics/bty560
Chinnaiyan A. M., Orourke K., Tewari M., Dixit V. M. (1995). FADD, a novel death domain-containing protein, interacts with the death domain of FAS and initiates apoptosis. Cell 81 (4), 505–512. doi: 10.1016/0092-8674(95)90071-3
Cozar A., Echevarria F., Gonzalez-Gordillo J. I., Irigoien X., Ubeda B., Hernandez-Leon S., et al. (2014). Plastic debris in the open ocean. Proc. Natl. Acad. Sci. U. S. A. 111 (28), 10239–10244. doi: 10.1073/pnas.1314705111
Creagh E. M., Conroy H., Martin S. J. (2003). Caspase-activation pathways in apoptosis and immunity. Immunol. Rev. 193 (1), 10–21. doi: 10.1034/j.1600-065X.2003.00048.x
Dane H., Sisman T. (2020). A morpho-histopathological study in the digestive tract of three fish species influenced with heavy metal pollution. Chemosphere 242, 125212. doi: 10.1016/j.chemosphere.2019.125212
Daniel D. B., Ashraf P. M., Thomas S. N. (2020). Abundance, characteristics and seasonal variation of microplastics in Indian white shrimps (Fenneropenaeus indicus) from coastal waters off Cochin, kerala, India. Sci. Total. Environ. 737, 139839. doi: 10.1016/j.scitotenv.2020.139839
Didierlaurent A., Brissoni B., Velin D., Aebi N., Tardivel A., Kaslin E., et al. (2006). Tollip regulates proinflammatory responses to interleukin-1 and lipopolysaccharide. Mol. Cell. Biol. 26 (3), 735–742. doi: 10.1128/MCB.26.3.735-742.2006
Dumas S., Ntambi J. M. (2017). Co-Conspirators in a new mechanism for the degradation of 9-desaturase. J. Biol. Chem. 292 (49), 19987–19988. doi: 10.1074/jbc.H117.801936
Esmaeilzadeh M., Karbassi A. R., Bastami K. D. (2017). Antioxidant response to metal pollution in phragmites australis from anzali wetland. Mar. pollut. Bull. 119 (1), 376–380. doi: 10.1016/j.marpolbul.2017.03.030
Fagerberg B., Borne Y., Barregard L., Sallsten G., Forsgard N., Hedblad B., et al. (2017). Cadmium exposure is associated with soluble urokinase plasminogen activator receptor, a circulating marker of inflammation and future cardiovascular disease. Environ. Res. 152, 185–191. doi: 10.1016/j.envres.2016.10.019
Feng Z., Wang R., Zhang T., Wang J., Huang W., Li J., et al. (2020). Microplastics in specific tissues of wild sea urchins along the coastal areas of northern China. Sci. Total. Environ. 728, 138660. doi: 10.1016/j.scitotenv.2020.138660
Fernandez B., Santos-Echeandia J., Rivera-Hernandez J. R., Garrido S., Albentosa M. (2020). Mercury interactions with algal and plastic microparticles: Comparative role as vectors of metals for the mussel, mytilus galloprovincialis. J. Hazard. Mater. 396, 122739. doi: 10.1016/j.jhazmat.2020.122739
Fu X. Y., Xue C. H., Miao B. C., Li Z. J., Gao X., Yang W. G. (2005). Characterization of proteases from the digestive tract of sea cucumber (Stichopus japonicus): High alkaline protease activity. Aquaculture 246 (1-4), 321–329. doi: 10.1016/j.aquaculture.2005.01.012
Hanebuth T. J. J., King M. L., Mendes I., Lebreiro S., Lobo F. J., Oberle F. K., et al. (2018). Hazard potential of widespread but hidden historic offshore heavymetal (Pb, zn) contamination (Gulf of cadiz, Spain). Sci. Total Environ. 637, 561–576. doi: 10.1016/j.scitotenv.2018.04.352
Hani Y. M. I., Turies C., Palluel O., Delahaut L., Gaillet V., Bado-Nilles A., et al. (2018). Effects of chronic exposure to cadmium and temperature, alone or combined, on the threespine stickleback (Gasterosteus aculeatus): Interest of digestive enzymes as biomarkers. Aquat. Toxicol. 199, 252–262. doi: 10.1016/j.aquatox.2018.04.006
Hayden A., Park S., Giustini D., Lee A. Y. Y., Chen L. Y. C. (2016). Hemophagocytic syndromes (HPSs) including hemophagocytic lymphohistiocytosis (HLH) in adults: A systematic scoping review. Blood Rev. 30 (6), 411–420. doi: 10.1016/j.blre.2016.05.001
Hwang J., Choi D., Han S., Jung S., Choi J., Hong J. (2020). Potential toxicity of polystyrene microplastic particles. Sci. Rep. 10 (1), 7391. doi: 10.1038/s41598-020-64464-9
Jeong C.-B., Kang H.-M., Lee Y. H., Kim M.-S., Lee J.-S., Seo J. S., et al. (2018). Nanoplastic ingestion enhances toxicity of persistent organic pollutants (POPs) in the monogonont rotifer brachionus koreanus via multixenobiotic resistance (MXR) disruption. Environ. Sci. Technol. 52 (19), 11411–11418. doi: 10.1021/acs.est.8b03211
Jiang S. H., Dong S. L., Gao Q. F., Wang F., Tian X. L. (2013). Comparative study on nutrient composition and growth of green and red sea cucumber, apostichopus japonicus (Selenka 1867), under the same culture conditions. Aquacult. Res. 44 (2), 317–320. doi: 10.1111/j.1365-2109.2011.03033.x
Joshy A., Sharma S. R. K., Mini K. G., Gangadharan S., Pranav P. (2022). Histopathological evaluation of bivalves from the southwest coast of India as an indicator of environmental quality. Aquat. Toxicol. 243, 106076. doi: 10.1016/j.aquatox.2022.106076
Jovanovic B. (2017). Ingestion of microplastics by fish and its potential consequences from a physical perspective. Integr. Environ. Assess. Manage. 13 (3), 510–515. doi: 10.1002/ieam.1913
Jozsi M., Barlow P. N., Meri S. (2022). Editorial: Function and dysfunction of complement factor h. Front. Immunol. 12. doi: 10.3389/fimmu.2021.831044
Khan M. I., Zahoor M., Khan A., Gulfam N., Khisroon M. (2019). Bioaccumulation of heavy metals and their genotoxic effect on freshwater mussel. Bull. Environ. Contam. Toxicol. 102 (1), 52–58. doi: 10.1007/s00128-018-2492-4
Kim D., Chae Y., An Y. J. (2017). Mixture toxicity of nickel and microplastics with different functional groups on daphnia magna. Environ. Sci. Technol. 51 (21), 12852–12858. doi: 10.1021/acs.est.7b03732
Kim W. S., Kim K., Byun E. B., Song H. Y., Han J. M., Park W. Y., et al. (2020). RM, a novel resveratrol derivative, attenuates inflammatory responses induced by lipopolysaccharide via selectively increasing the tollip protein in macrophages: A partial mechanism with therapeutic potential in an inflammatory setting. Int. Immunopharmacol. 78, 106072. doi: 10.1016/j.intimp.2019.106072
Kim D., Landmead B., Salzberg S. L. (2015). HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 12 (4), 357–U121. doi: 10.1038/NMETH.3317
Kim P. K. M., Park S. Y., Koty P. P., Hua Y., Luketich J. D., Billiar T. R. (2003). Fas-associating death domain protein overexpression induces apoptosis in lung cancer cells. J. Thorac. Cardiovasc. Surg. 125 (6), 1336–1342. doi: 10.1016/S0022-5223(02)73227-3
Kim J. H., Yu Y. B., Choi J. H. (2021). Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mater. 413, 125423. doi: 10.1016/j.jhazmat.2021.125423
Kovacevic M., Jovanovic Z., Andrejic G., Dzeletovic Z., Rakic T. (2020). Effects of high metal concentrations on antioxidative system in phragmites australisgrown in mine and flotation tailings ponds. Plant Soil 453 (1-2), 297–312. doi: 10.1007/s11104-020-04598-x
Kowalski E. J. A., Li L. W. (2017). Toll-interacting protein in resolving and non-resolving inflammation. Front. Immunol. 8. doi: 10.3389/fimmu.2017.00511
Langmead B., Salzberg S. L. (2012). Fast gapped-read alignment with bowtie 2. Nat. Methods 9 (4), 357–U354. doi: 10.1038/NMETH.1923
Lei L. L., Wu S. Y., Lu S. B., Liu M. T., Song Y., Fu Z. H., et al. (2018). Microplastic particles cause intestinal damage and other adverse effects in zebrafish danio rerio and nematode caenorhabditis elegans. Sci. Total Environ. 619, 1–8. doi: 10.1016/j.scitotenv.2017.11.103
Ley K., Cerrito M., Arfors K. E. (1991). SULFATED POLYSACCHARIDES INHIBIT LEUKOCYTE ROLLING IN RABBIT MESENTERY VENULES. Am. J. Physiol. 260 (5), 1667–1673. doi: 10.1152/ajpheart.1991.260.5.H1667
Liao Y. L., Yang J. Y. (2020). Microplastic serves as a potential vector for cr in an in-vitro human digestive model. Sci. Total Environ. 703, 134805. doi: 10.1016/j.scitotenv.2019.134805
Li B. Q., Ding Y. F., Cheng X., Sheng D. D., Xu Z., Rong Q. Y., et al. (2020). Polyethylene microplastics affect the distribution of gut microbiota and inflammation development in mice. Chemosphere 244, 125492. doi: 10.1016/j.chemosphere.2019.125492
Ling S. C., Luo Z., Chen G. H., Zhang D. G., Liu X. (2018). Waterborne zn influenced zn uptake and lipid metabolism in two intestinal regions of juvenile goby synechogobius hasta. Ecotoxicol. Environ. Saf. 148, 578–584. doi: 10.1016/j.ecoenv.2017.10.064
Lin W., Su F., Lin M. Z., Jin M. F., Li Y. H., Ding K. W., et al. (2020). Effect of microplastics PAN polymer and/or Cu2+ pollution on the growth of chlorella pyrenoidosa. Environ. pollut. 265, 114985. doi: 10.1016/j.envpol.2020.114985
Liu S., Chen N., Yang X. (2022). Research progress on adsorption-desorption characteristics of organic pollutants by microplastics and their combined toxic effects. Ecol. Environ. Sci. 31 (3), 610–620. doi: 10.16258/j.cnki.1674-5906.2022.03.020
Li N., Xiao H. H., Shen J. L., Qiao X. M., Zhang F. J., Zhang W. B., et al. (2021). SELE gene as a characteristic prognostic biomarker of colorectal cancer. J. Int. Med. Res. 49 (4). doi: 10.1177/03000605211004386
Love M. I., Huber W., Anders S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15 (12), 550. doi: 10.1186/s13059-014-0550-8
Mattsson K., Johnson E. V., Malmendal A., Linse S., Hansson L. A., Cedervall T. (2017). Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 7, 11452. doi: 10.1038/s41598-017-10813-0
Ma Z. G., Yuan Y. P., Zhang X., Xu S. C., Wang S. S., Tang Q. Z. (2017). Piperine attenuates pathological cardiac fibrosis Via PPAR-gamma/AKT pathways. EBioMedicine 18, 179–187. doi: 10.1016/j.ebiom.2017.03.021
McEver R. P. (2015). Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 107 (3), 331–339. doi: 10.1093/cvr/cvv154
Md Amin R., Sohaimi E. S., Anuar S. T., Bachok Z. (2020). Microplastic ingestion by zooplankton in terengganu coastal waters, southern south China Sea. Mar. pollut. Bull. 150, 110616. doi: 10.1016/j.marpolbul.2019.110616
Mohsen M., Lin C. G., Hamouda H. I., Al-Zayat A. M., Yang H. S. (2022). Plastic-associated microbial communities in aquaculture areas. Front. Mar. Sci. 9. doi: 10.3389/fmars.2022.895611
Mohsen M., Wang Q., Zhang L., Sun L., Lin C., Yang H. (2019). Microplastic ingestion by the farmed sea cucumber apostichopus japonicus in China. Environ. pollut. 245, 1071–1078. doi: 10.1016/j.envpol.2018.11.083
Mohsen M., Wang Q., Zhang L., Sun L., Lin C., Yang H. (2019a). Heavy metals in sediment, microplastic and sea cucumber apostichopus japonicus from farms in China. Mar. pollut. Bull. 143, 42–49. doi: 10.1016/j.marpolbul.2019.04.025
Mohsen M., Zhang L. B., Sun L. N., Lin C. G., Wang Q., Yang H. S. (2020). Microplastic fibers transfer from the water to the internal fluid of the sea cucumber Apostichopus japonicus. Environ. pollut. 257, 113606. doi: 10.1016/j.envpol.2019.113606
Morales A. I., Vicente-Sanchez C., Jerkic M., Santiago J. M., Sanchez-Gonzalez P. D., Perez-Barriocanal F., et al. (2006). Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol. Appl. Pharmacol. 210 (1-2), 128–135. doi: 10.1016/j.taap.2005.09.006
Parra-Luna M., Martin-Pozo L., Hidalgo F., Zafra-Gomez A. (2020). Common sea urchin (Paracentrotus lividus) and sea cucumber of the genus holothuria as bioindicators of pollution in the study of chemical contaminants in aquatic media. a revision. Ecol. Indic. 113, 106185. doi: 10.1016/j.ecolind.2020.106185
Peng G., Li L. H., Liu Y. B., Pu J., Zhang S. Y., Yu J. H., et al. (2011). Oleate blocks palmitate-induced abnormal lipid distribution, endoplasmic reticulum expansion and stress, and insulin resistance in skeletal muscle. Endocrinol. 152 (6), 2206–2218. doi: 10.1210/en.2010-1369
Plee T. A., Pomory C. M. (2020). Microplastics in sandy environments in the Florida keys and the panhandle of Florida, and the ingestion by sea cucumbers (Echinodermata: Holothuroidea) and sand dollars (Echinodermata: Echinoidea). Mar. pollut. Bull. 158, 111437. doi: 10.1016/j.marpolbul.2020.111437
Purcell S. W., Lovatelli A., Pakoa K. (2014). Constraints and solutions for managing pacific island sea cucumber fisheries with an ecosystem approach. Mar. Policy 45, 240–250. doi: 10.1016/j.marpol.2013.11.005
Qiao R., Lu K., Deng Y., Ren H., Zhang Y. (2019). Combined effects of polystyrene microplastics and natural organic matter on the accumulation and toxicity of copper in zebrafish. Sci. Total. Environ. 682, 128–137. doi: 10.1016/j.scitotenv.2019.05.163
Rainbow P. S. (2003). Trace metal concentrations in aquatic invertebrates: why and so what? Environ. pollut. 121 (3), 489–489. doi: 10.1016/S0269-7491(02)00445-1
Roberts D., Gebruk A., Levin V., Manship B. A. D. (2000). Feeding and digestive strategies in deposit-feeding holothurians. Oceanogr. Mar. Biol. 38, 257–310.
Robinson M. D., McCarthy D. J., Smyth G. K. (2010). edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26 (1), 139–140. doi: 10.1093/bioinformatics/btp616
Roussiez V., Ludwig W., Monaco A., Probst J. L., Bouloubassi I., Buscail R., et al. (2006). Sources and sinks of sediment-bound contaminants in the gulf of lions (NW Mediterranean sea): A multi-tracer approach. Cont. Shelf Res. 26 (16), 1843–1857. doi: 10.1016/j.csr.2006.04.010
Roy D., Grenier D., Segura M., Mathieu-Denoncourt A., Gottschalk M. (2016). Recruitment of factor h to the streptococcus suis cell surface is multifactorial. Pathog. 5 (3), 47. doi: 10.3390/pathogens5030047
Ru X. S., Zhang L. B., Li X. N., Liu S. L., Yang H. S. (2019). Development strategies for the sea cucumber industry in China. J. Oceanol. Limnol. 37 (1), 300–312. doi: 10.1007/s00343-019-7344-5
Salaun B., Rorner P., Lebecque S. (2007). Toll-like receptors’ two-edged sword: when immunity meets apoptosis. Eur. J. Immunol. 37 (12), 3311–3318. doi: 10.1002/eji.200737744
Sass W., Dreyer H. P., Seifert J. (1990). RAPID INSORPTION OF SMALL PARTICLES IN THE GUT. Am. J. Gastroenterol. 85 (3), 255–260.
Schmittgen T. D., Livak K. J. (2008). Analyzing real-time PCR data by the comparative C(T) method. J. Nat. Protoc. 3 (6), 1101–1108. doi: 10.1038/nprot.2008.73
Schulert G. S., Cron R. Q. (2020). The genetics of macrophage activation syndrome. Genes Immun. 21 (3), 169–181. doi: 10.1038/s41435-020-0098-4
Sharma S., Chatterjee S. (2017). Microplastic pollution, a threat to marine ecosystem and human health: a short review. Environ. Sci. pollut. Res. 24 (27), 21530–21547. doi: 10.1007/s11356-017-9910-8
Shibolet O., Podolsky D. K. (2007). TLRs in the gut. IV. negative regulation of toll-like receptors and intestinal homeostasis: addition by subtraction. Am. J. Physiol-Gastr. L. 292 (6), G1469–G1473. doi: 10.1152/ajpgi.00531.2006
Taha Z. D., Md Amin R., Anuar S. T., Nasser A. A. A., Sohaimi E. S. (2021). Microplastics in seawater and zooplankton: A case study from terengganu estuary and offshore waters, Malaysia. Sci. Total. Environ. 786, 147466. doi: 10.1016/j.scitotenv.2021.147466
Taylor M. L., Gwinnett C., Robinson L. F., Woodall L. C. (2016). Plastic microfibre ingestion by deep-sea organisms. Sci. Rep. 6, 33997. doi: 10.1038/srep33997
Thompson R. C., Olsen Y., Mitchell R. P., Davis A., Rowland S. J., John A. W., et al. (2004). Lost at sea: where is all the plastic? Science 304 (5672), 838. doi: 10.1126/science.1094559
Tomasik J., Basak G. W. (2022). Inflammasomes-new contributors to blood diseases. Int. J. Mol. Sci. 23 (15), 8129. doi: 10.3390/ijms23158129
Tracz-Gaszewska Z., Dobrzyn P. (2019). Stearoyl-CoA desaturase 1 as a therapeutic target for the treatment of cancer. Cancers 11 (7), 948. doi: 10.3390/cancers11070948
Vedolin M. C., Teophilo C. Y. S., Turra A., Figueira R. C. L. (2018). Spatial variability in the concentrations of metals in beached microplastics. Mar. pollut. Bull. 129 (2), 487–493. doi: 10.1016/j.marpolbul.2017.10.019
Wang L. C., Brown J. R., Varki A., Esko J. D. (2002). Heparin’s anti-inflammatory effects require glucosamine 6-o-sulfation and are mediated by blockade of l- and p-selectins. J. Clin. Invest. 110 (1), 127–136. doi: 10.1172/JCI200214996
Wang Q. J., Zhang Y., Wangjin X. X., Wang Y. L., Meng G. H., Chen Y. H. (2020). The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 87, 272–280. doi: 10.1016/j.jes.2019.07.006
Welden N. A. C., Cowie P. R. (2016). Long-term microplastic retention causes reduced body condition in the langoustine, nephrops norvegicus. Environ. pollut. 218, 895–900. doi: 10.1016/j.envpol.2016.08.020
Wenli M., Lan W., Yongji H., Yao Y. (2008). Tissue-specific cadmium and metallothionein levels in freshwater crab sinopotamon henanense during acute exposure to waterborne cadmium. Environ. Toxicol. 23 (3), 393–400. doi: 10.1002/tox.20339
Wright S. L., Kelly F. J. (2017). Plastic and human health: A micro issue? Environ. Sci. Technol. 51 (12), 6634–6647. doi: 10.1021/acs.est.7b00423
Wright S. L., Thompson R. C., Galloway T. S. (2013). The physical impacts of microplastics on marine organisms: A review. Environ. pollut. 178, 483–492. doi: 10.1016/j.envpol.2013.02.031
Wu Z., Dong Y., Liu R., Liu L., Gao J., Song W., et al. (2022). Assessment of heavy metal contamination in surface sediments off the dongying coast, bohai Sea. Mar. pollut. Bull. 180, 113826. doi: 10.1016/j.marpolbul.2022.113826
Yokoyama H. (2013). Growth and food source of the sea cucumber apostichopus japonicus cultured below fish cages - potential for integrated multi-trophic aquaculture. Aquaculture 372, 28–38. doi: 10.1016/j.aquaculture.2012.10.022
Zantis L. J., Carroll E. L., Nelms S. E., Bosker T. (2021). Marine mammals and microplastics: A systematic review and call for standardisation. Environ. pollut. 269, 116142. doi: 10.1016/j.envpol.2020.116142
Zettler E. R., Mincer T. J., Amaral-Zettler L. A. (2013). Life in the “Plastisphere”: Microbial communities on plastic marine debris. Environ. Sci. Technol. 47 (13), 7137–7146. doi: 10.1021/es401288x
Zhang G. L., Ghosh S. (2002). Negative regulation of toll-like receptor-mediated signaling by tollip. J. Biol. Chem. 277 (9), 7059–7065. doi: 10.1074/jbc.M109537200
Zhang S. W., Han B., Sun Y. H., Wang F. Y. (2020). Microplastics influence the adsorption and desorption characteristics of cd in an agricultural soil. J. Hazard. Mater. 388, 121775. doi: 10.1016/j.jhazmat.2019.121775
Zhang S. Y., Ru X. S., Su F., Liang W. K., Zhang L. B., Yang H. S. (2022). Use of quantitative real-time PCR to select reference genes in the testis and ovary of apostichopus japonicus during the breeding period. Aquacult. Rep. 22, 101010. doi: 10.1016/j.aqrep.2022.101010
Keywords: microplastics, cadmium, sea cucumber, combined toxicity, RNA sequencing
Citation: Zhang C, Zhang L, Li L, Mohsen M, Su F, Wang X and Lin C (2023) RNA sequencing provides insights into the effect of dietary ingestion of microplastics and cadmium in the sea cucumber Apostichopus japonicus. Front. Mar. Sci. 10:1109691. doi: 10.3389/fmars.2023.1109691
Received: 28 November 2022; Accepted: 24 January 2023;
Published: 03 February 2023.
Edited by:
Juan D. Gaitan-Espitia, The University of Hong Kong, Hong Kong SAR, ChinaReviewed by:
James Scott Maki, Marquette University, United StatesMing Cong, Yantai University, China
Copyright © 2023 Zhang, Zhang, Li, Mohsen, Su, Wang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chenggang Lin, bGluY2hlbmdnYW5nQHFkaW8uYWMuY24=
 Chenxi Zhang
Chenxi Zhang Libin Zhang
Libin Zhang Lingling Li6
Lingling Li6 Mohamed Mohsen
Mohamed Mohsen Fang Su
Fang Su Chenggang Lin
Chenggang Lin