- 1Western Australian Fisheries and Marine Research Laboratories, Department of Primary Industries and Regional Development, Government of Western Australia, Hillarys, WA, Australia
- 2Centre for Sustainable Aquatic Ecosystems, Harry Butler Institute, Murdoch University, Murdoch, WA, Australia
- 3Centre for Marine Ecosystems Research, School of Science, Edith Cowan University, Joondalup, WA, Australia
Coastal environments and their associated biota provide numerous environmental, economic and societal services. Cockburn Sound, a temperate embayment on the lower west coast of Western Australia, is immensely important for the State and adjacent capital city of Perth. However, urbanisation and associated terrestrial and marine development has the potential to threaten this important ecosystem. This study collated published and unpublished data to review the current state of the ecological resources of Cockburn Sound and describe how they have changed over the past century. Post-WWII, the embayment began undergoing pronounced anthropogenic changes that limited oceanic water exchange, increased nutrient load, modified benthic habitats and increased fishing pressure. The most visual outcome of these changes was substantial eutrophication and the loss of 77% of seagrass habitats. However, the increased primary productivity from elevated nutrient inputs produced high commercial fishery yields of up to ~1,700 t in the early 1990s before improved wastewater regulation and restricted fishing access steadily reduced commercial catches to ~300 t in recent years. Despite substantial anthropogenic-induced changes, Cockburn Sound has remained a diverse and ecologically important area. For example, the embayment is a key spawning area for large aggregations of Snapper, is a breeding and feeding site for seventeen marine bird species (including Little Penguins) and, is frequented by numerous protected species such as pinnipeds, dolphins, and White and Grey Nurse sharks. In recent decades, numerous projects have been initiated to restore parts of Cockburn Sound with mixed success, including seagrass transplantation, deployment of artificial reefs and stocking of key fish species, mainly Snapper. Nevertheless, while still biodiverse, there are signs of considerable ecological stress from escalating anthropogenic pressures and the cumulative impacts of ongoing and future developments, including climate change, which may severely impact the functioning of this important ecosystem.
Introduction
Coastal environments are among the most productive and biologically valuable ecosystems (Costanza et al., 1997). They have played crucial roles for humans for millennia with many major and mega-cities having developed on coasts worldwide, thereby increasing reliance of these environments for human habitation and infrastructure, commerce and industry, and tourism and recreation (Defeo and Elliott, 2021). However, despite the ecosystem services they provide, coastal environments are deteriorating through intense and increasing anthropogenic usage (Halpern et al., 2008; Barbier et al., 2010). Three major threats were identified in the ‘triple whammy’, i.e. increasing urbanisation and industrialisation, increasing use of resources and decreased resilience to climate change (Defeo and Elliott, 2021).
Many coastal cities have undergone a similar pattern of settlement, followed by industrialisation and the rapid expansion of trade and resource utilisation which has resulted in the severe modification of the environment (e.g. Tinsley, 1998; Cloern and Jassby, 2012; Banks et al., 2016). This has resulted in inputs of nutrients, heavy metals and other contaminants (e.g. microplastics), land reclamation, the clearing of habitats, the extraction of biota and other natural resources and translocation of non-indigenous species (He and Silliman, 2019). Such impacts often result in the degradation or the collapse of coastal ecosystems and the services they provide (Crain et al., 2009; Barbier et al., 2010). For example, globally, 29% of seagrass habitat and 85% of oyster reefs have been lost (Waycott et al., 2009; Beck et al., 2011). Moreover, in the Thames Estuary (UK), sewage inputs caused anoxia in parts of the system resulting in only a single fish species being caught between the 1920s to 1960, before wastewater treatment was improved and fish species returned (Tinsley, 1998; Potter et al., 2015). Increased shipping was a major driver of 232 species being translocated into San Francisco Harbour over 142 years (Costello et al., 2007). Given the value of coastal ecosystems and the extent of degradation in many areas around cities, amelioration and restoration effects are underway (Waltham et al., 2020). The synthesis of data over decades is crucial in understanding how coastal environments have changed and how they may in the future with a growing global population and the increasing effects of climate change (Cloern and Jassby, 2012).
Globally, ~26% of the population live within 50 km of the coastline (Reimann et al., 2023), however, in Australia this proportion is 87% (22 million people), which causes significant and sustained pressures on the coastal environments (Clark et al., 2021). In the most recent State of the Environment Report for Australia for 2021, the condition of i) beaches and shorelines, ii) waterways, iii) ecosystems and habitats, and iv) species were all assessed as “poor” and, in three of the four categories, were “deteriorating” (Clark et al., 2021). Coastal environments located near urban centres were in the worst condition and, among the taxa assessed, invertebrates, fish and shorebirds were undergoing the most pronounced declines. Being an urban coastal nation, population-driven pressures were considered to have a high or very high impact, most notably coastal development and changing land use (i.e. coastal squeeze). Although industrial pressures were, overall, assessed as low impact, nutrient pollution, contamination, and artificial structures were each considered to exert a high impact on coastal environments. In addition, invasive species, which are often associated with shipping, were assessed as a pervasive and significant threat (Clark et al., 2021). Australian coastal environments are also particularly sensitive to the effects of climate change, which can exacerbate existing threats (He and Silliman, 2019; Spencer et al., 2023). Extreme weather events and erosion were both assessed to have a high impact (Clark et al., 2021). The lower west and eastern coasts of Australia are hotspots for marine heat waves and the frequency, intensity and duration of which are increasing (Hobday and Pecl, 2014; Kajtar et al., 2021) and rainfall following wildfires can result in mass mortality events in estuaries (Silva et al., 2020).
Due to the rate of change, the Perth metropolitan region of Western Australia (WA) has been identified as a region of high cumulative human impact, making it at high risk of ecosystem collapse (Halpern et al., 2019). As humans change the natural physical and ecological processes for societal gains, its creating complex social-ecological systems that require transdisciplinary approaches to inform decision-making (Spencer et al., 2023). This study integrates a variety of unpublished and published data to determine the state of the ecological resources of Cockburn Sound (CS), a large temperate marine embayment that is culturally, economically and recreationally important to the people of WA, but which has undergone major ecological changes over the last century due to various anthropogenic influences.
This synthesis was initiated by the proposal to develop a new port in CS capable of handling 4.5 million twenty-foot equivalent units (i.e. standard 6.1 m long shipping containers) annually, which will also require a substantial dredging program to enable ultra-large container vessels to dock (Westport, 2020). This port is in addition to the expansion of other industrial and military facilities around CS and increased urbanisation and human usage from Perth’s expanding population. This article provides a comprehensive overview of the current status of key ecological assets within CS, contextualized against the natural and anthropogenic changes which have occurred since the early 1900s. The subsequent text describes how the anthropogenic footprint on CS has developed over time and, through the lens of ecosystem-based fisheries management, considers changing linkages between taxa, including vulnerable habitats and threatened species. This synthesised information is intended to inform ongoing management of WA aquatic resources and support the development and assessment of potential impacts of marine development projects within CS. Finally, knowledge gaps are identified to guide future research and monitoring programs and support the ongoing management of an increasingly developed CS in a rapidly changing climate.
Site description
Geography and geology
Cockburn Sound is situated at ~32°S on the lower-west coast of WA and, in this study, is considered to include all inshore waters from Fremantle to Rockingham, consistent with the commercial fishery boundary (Figure 1). This region spans 25 km north-south and up to 10 km east-west, with an area of ~200 km2. Along the western boundary is Garden Island (GI; ~10 km long), as well as several smaller islands, shallow limestone reefs, and a 4.2 km long rock-filled causeway (Figure 1). Directly to the north is the entrance to the Swan-Canning Estuary (SCE).
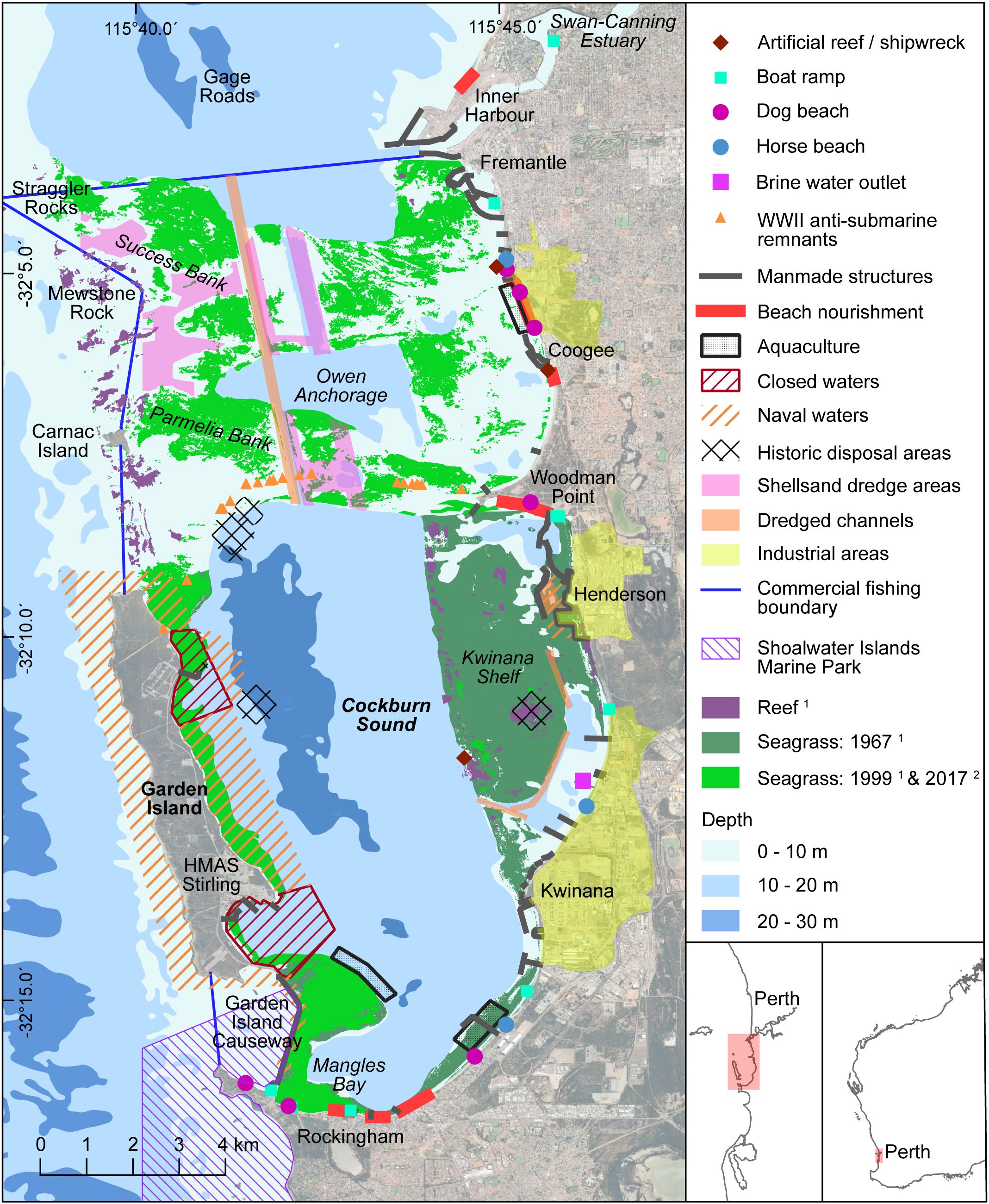
Figure 1. Map of Cockburn Sound detailing the bathymetry and some geographic points mentioned in the text. Key habitats, i.e. 1 (Kendrick et al., 2002) and 2 (Hovey and Fraser, 2018), and the footprint of human uses are overlaid. Inset shows the location of Cockburn Sound within Western Australia. Note there are no available maps of the historic extent of seagrass north of Woodman Point.
The southern waters of CS (Woodman Point to Rockingham) contain a deep central basin (17–22 m) surrounded by a shallow (<10 m) shelf (Figure 1). To the north are Parmelia and Success Banks, which extend from the mainland to Carnac Island and Stragglers Rocks, respectively. Deeper (~15 m) depressions lie between and to the north of these banks, i.e. Owen Anchorage (OA) and Gage Roads. A shipping channel, maintained to a depth of ~15 m, extends from Gage Roads to the central basin of CS and navigational waters in the Kwinana area and southern GI are regularly dredged to ~12–15 m.
Climate and hydrology
Located in a Mediterranean climate region, CS experiences air temperatures ranging from ~8°C (mean monthly minimum) during July (Austral winter) to ~32°C (mean monthly maximum) in February (BOM, 2024). Average annual rainfall is ~750 mm, of which 80% occurs between May and October (Hallett et al., 2018). From October to April light to moderate offshore easterly winds typically occur in the morning with strong and consistent onshore sea breezes (20–25 knots, south to south-westerly) in the afternoon (Steedman and Craig, 1983; Masselink and Pattiaratchi, 2001). During winter, strong winds are irregular and typically associated with onshore cold fronts. Tidal range is ~0.6 m and coastal waters are characterised by a low mean significant wave height (<2 m), with wave conditions typically driven by sea breezes during summer and storm activity in winter (Lemm et al., 1999).
The protected nature of CS results in a low-energy system with limited hydrodynamic mixing of the water column, particularly in south-eastern waters (Xiao et al., 2022). Water temperatures range from 16°C in late-winter to 24°C in late-summer (Johnston and Yeoh, 2021), and are strongly influenced interannually by the poleward flowing Leeuwin Current (Feng et al., 2003). Bottom salinity remains close to marine (35–37) throughout the year, although freshwater influences from the SCE can reduce surface salinity to ~30, predominately from mid-winter to spring (CSMC, 2018). Stratification is most apparent during autumn when winds are lightest, which can reduce dissolved oxygen concentrations in bottom waters (Xiao et al., 2022).
Physical habitats and manmade features
The substratum in the deeper waters of CS comprises predominately mud with minimal vegetation, hard structure or reef (Skene et al., 2005). Shallower waters (<10 m) contain coarser substrates and seagrass meadows (Kendrick et al., 2002). Small areas of rock and coral reef are present throughout these shallower waters and several shipwrecks and artificial reefs exist at depths ranging from 2–18 m (Figure 1; see Restoration and enhancement).
Sandy beaches extend along most of the mainland shoreline, except for ~1 km of limestone cliffs in Henderson and various manmade structures such as jetties and rock walls (Figure 1). The shoreline from Woodman Point to Kwinana is particularly developed with industrial and port facilities. Marinas for recreational and commercial vessels are in Fremantle, Coogee and Henderson and numerous swing moorings are located in Mangles Bay (Figure 1).
Garden and Carnac Islands are relatively undeveloped apart from naval facilities (HMAS Stirling) on GI, which is also connected to the mainland via a causeway (Figure 1). Sandy beaches extend along the landward sides of both islands while their seaward sides contain predominately limestone cliffs and rocky outcrops.
Human uses
Cockburn Sound is culturally, economically and recreationally important, supporting a vast array of human activities (Table 1). The spatio-temporal extent of these activities, combined with their physical nature (e.g. extractive vs non-extractive), culminates in their level of impact on the marine environment (see Anthropogenic pressures). Moreover, certain activities have little or no reliance on biodiversity (e.g. industry), while others are highly reliant (e.g. recreational activities) and may even aim to increase biodiversity through restoration activities.
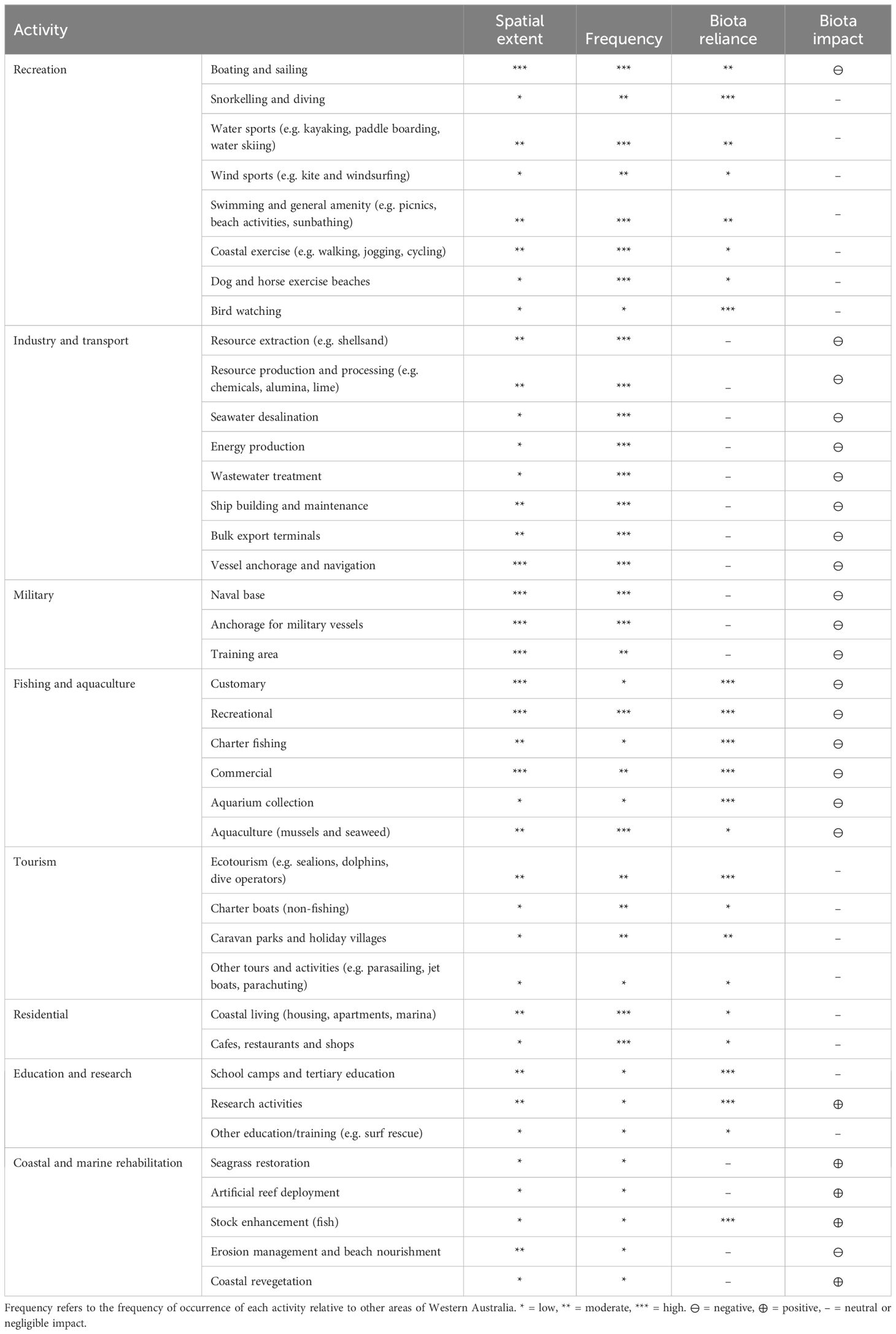
Table 1. Summary of major human activities in Cockburn Sound with their spatial extent, frequency of occurrence, reliance and general impact on marine biota.
Known as Derbal Nara, or “Estuary of the Salmon”, to the indigenous Whadjuk Noongar people, CS has been used for fishing, hunting and gathering resources for thousands of years (Collard and Bracknell, 2012). With the arrival of European settlers in the early-1800s, CS provided safe anchorage and food resources and became an important port and trading hub (Botting et al., 2009). Shipping has remained a major human use of CS over the past two centuries, which has been accompanied by the development of various industries and infrastructure. Fremantle Port opened in 1897, initially comprising an inner harbour at the mouth of the SCE, and expanded in 1955 to include an outer harbour located in CS (Tull, 1985). Today the port handles >99% of WA’s container trade, equating to 1,500 to 1,800 ships carrying 700,000 to 800,000 twenty-foot equivalent units (2012-2023) with a value of ~AU$37.6 billion in 2022 (Fremantle Ports, 2022).
The Kwinana Industrial Area located on the eastern shoreline (Figure 1) contains WA’s largest industrial and shipping complexes, including oil, gas and alumina refineries, chemical manufacturing, shipbuilding and bulk transport (Table 1; MacLachlan, 2013). Production from this large-scale industry generated AU$8.5 billion in revenue in 2007 (Botting et al., 2009; MacLachlan, 2013) and provides substantial local employment. The industrial area also produces a major proportion of WA’s electricity and water through power and seawater desalination plants. Historically, Perth’s electricity was generated by an oil- and coal-fired station in Coogee (Figure 1), which operated from the 1950s to 1985 and used seawater for cooling (Botting et al., 2009). Currently, there are two active gas turbine power stations in Kwinana with their locations of strategic importance due to the proximity of established infrastructure, natural gas supply and seawater for cooling. The seawater desalination plant, which intakes seawater and expels hypersaline warm water back into CS, has an annual capacity output of 50 GL and provides ~15% of Perth’s water supply (Clark et al., 2021).
A wastewater treatment plant at Woodman Point processes wastewater from >500,000 people. It historically discharged secondary treated effluent into CS until a secondary pipe was constructed to transport wastewater further offshore. Historically, the shoreline was also used by other industries such as meatworks and lime kilns. The prevalence of abattoirs peaked in the 1940s, with Robb’s Jetty Abattoir, situated near Fremantle, being the largest (Moredoundt and Kendall, 2012). Cockburn Sound also plays a major role for the Australian Defence Force and is the location for the western naval fleet. The sound provided safe harbour from submarines during WWII, with the southern and northern entrances to the sound protected by anti-submarine scaffolding and nets (Figure 1), which were in place from 1944 to 1964, with some remains still being present (Carter and Anderson, 2010). A causeway from the mainland to GI was constructed during the 1970s to facilitate development of the HMAS Stirling naval base (commissioned in 1978). The base supports domestic and foreign naval surface ships and submarines and contains the only submarine escape training facility in the southern hemisphere.
Despite substantial industrialisation, CS remains a popular recreational and tourism destination for boat and shore-based activities. Woodman Point is WA’s most used boat ramp (Afrifa-Yamoah et al., 2021), which along with numerous other public and private boating facilities, support a range of activities, including fishing, sailing, diving, water skiing and nature appreciation (Table 1). Commercial operations in CS also facilitate wildlife encounters, fishing charters and other leisure activities (e.g. parasailing). Shore-based activities include swimming, snorkelling, scuba diving, fishing, kite and windsurfing, walking, animal exercise and nature appreciation. As industrial and commercial development has reduced foreshore accessibility for recreational use, the remaining areas have become increasingly valued by local communities (Afrifa-Yamoah et al., 2021). Although many of the recreational activities in CS utilise the natural environment, some developments have sought to enhance experiences, such as the construction of a dive trail and swimming enclosure net in Coogee.
Anthropogenic pressures
While humans have inhabited the region for thousands of years, CS began to be heavily modified post-WWII as industrial and urban expansion of Perth accelerated. During the 1960s this previously oligotrophic environment became eutrophic, as CS received substantial domestic wastewater and industry effluent from a wastewater treatment plant discharging west of Woodman Point and two factories in Kwinana producing nitrogen and phosphorus fertilisers (Chiffings, 1979). By the 1970s, ~1,820 t of nitrogen and 1,378 t of phosphorus were being discharged into CS annually, increasing nutrient levels 25–30 times higher than previously recorded (Chiffings, 1979). Additional nutrients entered from the SCE (Robson et al., 2008) and other coastal industries (e.g. abattoirs dumping waste products directly into CS; Botting et al., 2009). Nutrient enrichment was compounded by the construction of the GI causeway in the early 1970s, which reduced circulation and mixing with oceanic waters (see below). Subsequent declines in water quality, and increased epiphyte loads on seagrasses (Cambridge et al., 1986) and phytoplankton densities (Chiffings, 1979) ultimately resulted in a 77% decline in seagrass coverage between 1967 and 1999, with the majority occurring before 1972 (Figure 2; Kendrick et al., 2002). The increase in primary productivity did, however, generate a major increase in fisheries production (Marks et al., 2021), particularly of filter-feeding invertebrates and planktivorous fish species. Various small stocks, including mussels (Mytilidae spp.), Blue Swimmer Crab (Portunus armatus), Scaly Mackerel (Sardinella lemuru) and Australian Sardine (Sardinops sagax), expanded significantly during the 1970s–1990s, forming the basis for commercial fisheries and recreational fishing activity (see Fisheries).
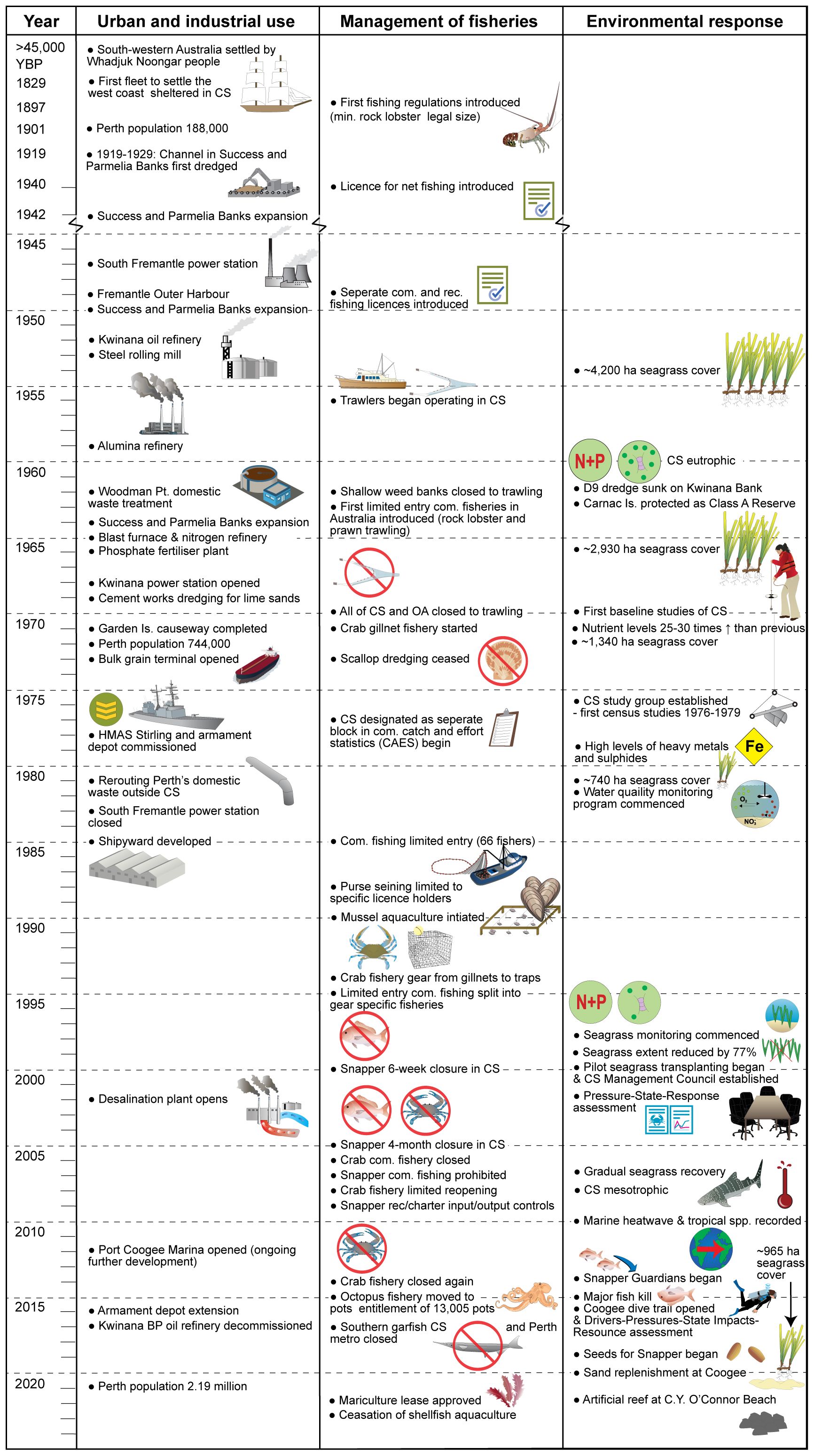
Figure 2. Timeline of key urban and industrial development, fisheries management measures and main environmental responses and human interventions within Cockburn Sound since European settlement. Note that these timelines are non-exhaustive. Additional details provided in the text.
Improved management practices and construction of the Sepia Depression Outlet in 1984, to redirect wastewater outside CS, substantially reduced nutrient loads (Figure 2). By 2000, the annual nitrogen discharge into CS was 57 t, which was reduced to essentially zero by 2015 (BMT, 2018). Despite these significant declines, nitrogen and phosphate levels remained elevated for decades, as stored nutrients in sediments slowly remobilised and contaminated groundwater continued to flow into CS (Greenwood et al., 2016). Nevertheless, CS has returned from a system of nitrogen excess to one of nitrogen deficit (Greenwood et al., 2016), thereby meeting the intent outlined in 1979 of reducing the nitrogen load to the levels that occurred pre-seagrass dieback (DCE, 1979). These nutrient reductions have resulted in substantially decreased primary productivity (CSMC, 2020), which likely contributed to the reduced catches of several fishery species including crabs and mussels.
Historical industrial discharge into CS also resulted in high levels of heavy metals and sulphides accumulating in sediment and biota. Plaskett and Potter (1979) reported appreciable discharge rates of 4–24 kg day-1 for various heavy metals, including cadmium, zinc and lead, and up to 520 kg day-1 for iron. Elevated concentrations of metals were found in a range of invertebrates, seagrasses and the alga Ulva lactuca, whereas levels in fish were within human food health standards (Plaskett and Potter, 1979; Talbot and Chegwidden, 1982). Concerns over heavy metals have focused on the risk to human health, however, along with sulphide pollutants in the sediment, they have been identified as a contributing factor to the decline and lack of recovery of seagrass within CS (Fraser and Kendrick, 2017). In recent decades, discharges into CS were more tightly regulated. For instance, the desalination plant which began operating in 2006 discharges hypersaline, warm water (22-26°C), but ongoing monitoring has determined it has had a negligible influence on dissolved oxygen and salinity levels (Water Corporation, 2019).
The seabed and shoreline of CS have been extensively modified over the last century to meet the needs of an expanding city (Figures 1, 2). The most high-profile modification was the GI solid rock-fill causeway built in the 1970s. After initial public concern and baseline studies of CS (DCE, 1979), the causeway was redesigned with two openings (613 and 304 m wide) to allow seawater flow and vessel traffic to pass beneath. Nevertheless, the causeway severely limited the mixing between CS and oceanic waters resulting in flushing times of up to 47 days (Steedman and Craig, 1983) and reduced water quality, especially around Mangles Bay (Pattiaratchi et al., 1994). Annual water quality monitoring demonstrates a steady improvement since 1977, although the southern sites closest to the causeway have had consistently higher nutrient and chlorophyll-a levels and lower dissolved oxygen and light penetration (CSMC, 2018). Other impacts included physical smothering of seagrass beds by the causeway and deep scouring through seagrass beds from water funneling through the bridge openings (Cambridge and McComb, 1984).
Prior to the causeway, CS had already undergone major physical change due to the repeated deepening and widening of channels through Parmelia and Success Banks and Kwinana Shelf to allow access for larger vessels. Dredging has caused localised but dramatic habitat changes, including the removal of 400 ha of seagrass from Parmelia Bank, and the flattening and/or burial of hard substrates at Sulphur Rocks (Cambridge and McComb, 1984). Dredging continues in designated areas north of Woodman Point to supply shellsand for lime production (Figure 1; BMT, 2018). Shipping infrastructure has resulted in the addition of hard structures (i.e. jetties, groynes, marinas, rock walls and moorings), particularly on the eastern shoreline of CS (Figure 1). These man-made structures also modified coastal processes, leading to increased shoreline erosion and accretion (DCE, 1979; BMT, 2018). Mitigation efforts have included constructing further groynes and ongoing beach nourishment, however, both the initial changes and mitigation efforts modify the dune and intertidal habitats. Further coastal modifications are planned as local government councils prepare for the impacts of climate change and sea level rise (CSCA, 2013).
High levels of commercial and recreational vessel traffic have induced other pressures on the marine environment. The highly-toxic pollutant tributyltin (TBT) historically used in antifouling paints has been detected in elevated levels in the sediment, particularly around ship maintenance sites and jetties (CSMC, 2020), as well as in various marine species including Little Penguins (Eudyptula minor) (Cannell et al., 2016). TBT-induced imposex has been detected in gastropods (BMT, 2018), albeit contaminants in the water were below Environmental Quality Criteria guideline levels (Bourke and Chase, 2009). Commercial and naval vessels also pose a large risk to biosecurity through the spread of non-indigenous species (Hewitt et al., 2000; Montelli and Lewis, 2008; Montelli, 2010). Recreational vessels pose a different set of pressures such as mooring scars in high use areas like Mangles Bay (BMT, 2018); and vessel strikes to dolphins, pinnipeds and penguins (Cannell, 2004).
At least 38 non-indigenous marine species have been detected in CS and Fremantle Port waters (Supplementary Material Table 1). The most common introduction pathway is through shipping, via hull fouling and/or ballast water, albeit aquaculture equipment and stock movements are also viable pathways for introduction and translocation. Many of the species listed as non-indigenous in baseline surveys were likely introduced decades ago, became established and have not displayed pest-like characteristics. However, several species have exhibited negative influences, most notably the European Fan Worm Sabella spallanzanii, which dominated benthic substrates in the 1990s, before populations reportedly subsided (McDonald and Wells, 2009). The Asian Date Mussel Arcuatula senhousia forms extensive beds in the SCE, although its presence in CS has been sporadic (Wellington pers. obs. 2024). Didemnum perlucidum (a colonial tunicate), which was first reported in CS in 2011, displayed fouling behaviour and was subsequently found to be ubiquitous in many regions of WA (Smale and Childs, 2012; Dias et al., 2021). Finally, Didemnum vexillum was recently detected at two port locations in CS and one location in NSW, resulting in a nationally coordinated response for the control and management of this species (DPIRD, 2023).
Compounding this diverse array of localised pressures are impacts from regional climate change that have been particularly evident in south-western WA during recent decades (Hobday and Pecl, 2014). Impacts on CS include warmer water, reduced rainfall and riverine inputs, and increased frequency and severity of extreme weather events such as marine heat waves and storms (Andrys et al., 2017; BMT, 2018). Most notably, the 2010/11 marine heat wave increased water temperatures 2-4°C above normal to their highest on record (Rose et al., 2012). This produced dramatic changes to habitats and fisheries stocks within CS, including decreased seagrass shoot density and severely inhibited spawning and subsequent population declines for Southern Garfish (Hyporhamphus melanochir) and Sandy Sprat (Hyperlophus vittatus) (Caputi et al., 2014; Smith and Grounds, 2020). The heatwave event was also linked to range extensions for several tropical species, some of which established self-recruiting populations that survived successive winters (see Tropicalisation).
Fisheries
Commercial fishing history and overview
Cockburn Sound has historically been a fishing ground for a range of species including scallops, mussels, crabs, teleosts, elasmobranchs and mammals. Records from the 1800s describe how whales (Southern Right (Eubalaena australis) and Humpback (Megaptera novaeangliae)) and “seals” (likely Australian Sea Lion (Neophoca cinerea)) were hunted and processed for their meat, oil and bone (McIlroy, 1986; Gibbs, 2012). Populations of these taxa declined over the next century and by the early-1900s harvesting ceased. Commercial fishing operations targeting finfish and invertebrates (e.g. squid) for the Perth market began in the early-1900s, including beach seining and horse-towed trawl nets, followed by small-scale otter trawling over the macrophyte beds from 1955 to 1962 (Botting et al., 2009). Due to concerns about trawl fishing damaging the seagrass beds, all areas <10 m depth in CS were closed to trawling in the mid-1960s. A further extension to the shallow seagrass trawl closures was implemented in 1970 and encompassed all remaining areas of CS (Penn, 1977). This closure was implemented to prevent trawling for a newly discovered small stock of Western King Prawn (Penaeus latisulcatus) in the shipping lanes, as well as to protect sensitive marine habitats. The closure also aimed to increase protection for fish stocks and allowed the area to be used for fisheries research. A large stock of Commercial Scallop (Pecten fumatus) was discovered by divers in the deeper southern waters of CS (14–22 m depth) in the late 1960s. This unusually large population of scallops was the subject of a short and intense 12-month period of dredge fishing during 1970–71, with ~2,000 t of catch taken. Dredge fishing continued for a short period before the method was outlawed in WA waters in 1973 (Figure 2).
Mandatory reporting of commercial catch and effort for CS began in 1977, at which time there were 34 licensed commercial operators (Figure 3). Fishing activities expanded during the 1970s and early 1980s as an open access fishery, meaning any WA commercial fishing license holder could operate in CS (except for trawling and dredging). As part of a state-wide commercial fishery management initiative in the early 1980s, entry to CS was restricted in 1985 to only 66 fishers who had a significant history of fishing CS (Figure 3). Although vessel and fisher numbers were limited, total catch steadily increased until the early 1990s, peaking at ~1,700 t in 1992 (Figure 3). Targeted species in this catch included the clupeids Australian Sardine (~1,274 t) and Scaly Mackerel (56 t), together with Blue Swimmer Crab (92 t), mussels (54 t) and other fish and invertebrates (177 t). Economic value (based on total landings) of the commercial fishery also peaked throughout the 1990s at ~AU$5 mil (1997 value adjusted to 2021/22 financial year inflated value; Figure 3). Beginning in 1985, managed fisheries for crabs, line and pot fishing, fish netting, and purse seining were introduced (Figure 2), which limited the number of licenses (and gear used) in each fishery and the species they were able to retain. These controls, adjusted to maintain sustainability, all remain in use today. Commercial fishing effort and total fishery production (biomass and economic value) decreased markedly during the late 2000s and has remained relatively low since (harvested biomass <300 t and economic value typically <AU$1 million; Figure 3), potentially driven by decreasing primary productivity following reduced nutrient inputs, but changing fisheries management policies, climate variability, habitat degradation and market demands also potential contributing factors. Current commercial fishing activities focus on octopus and small pelagic finfish species.
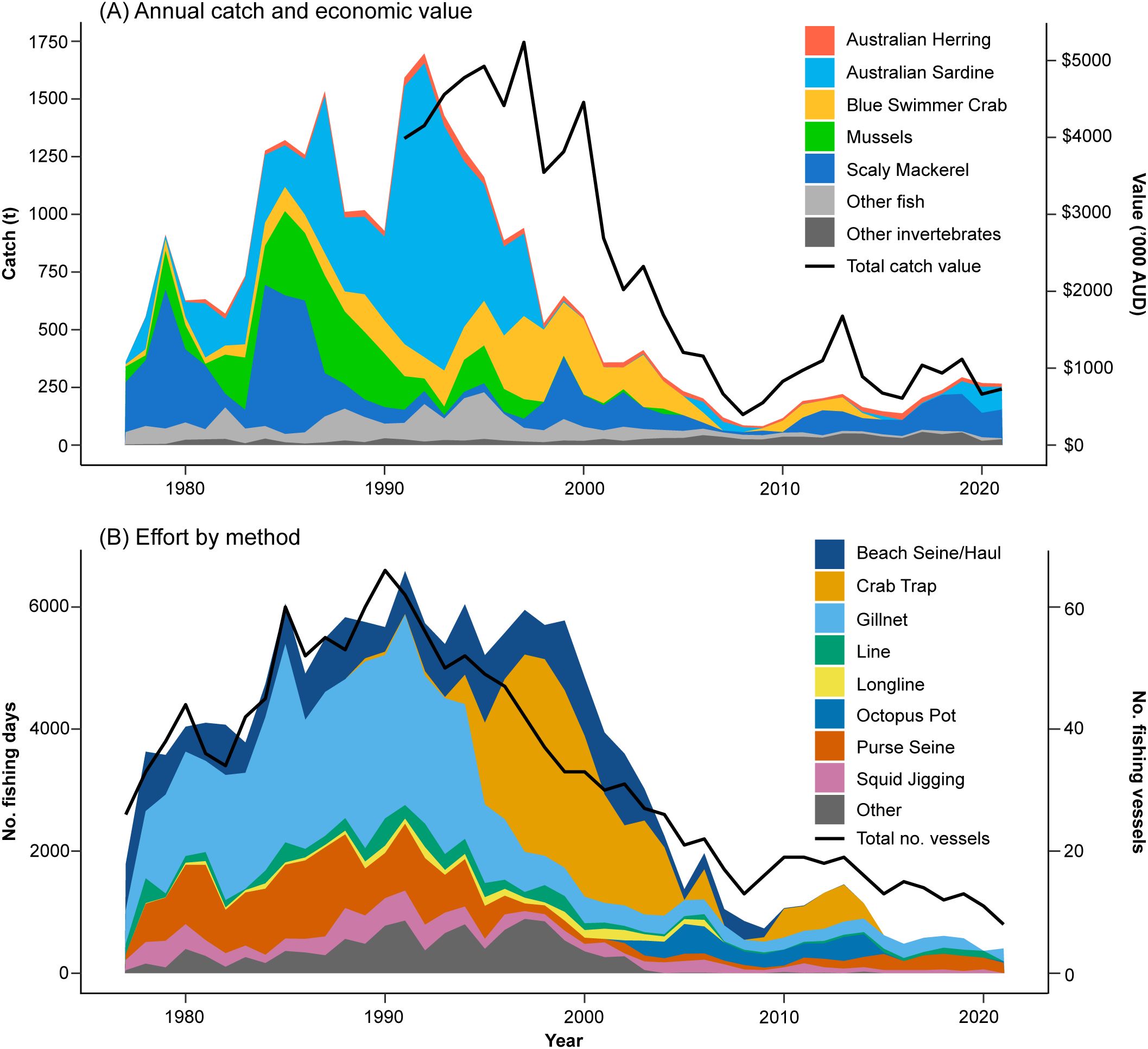
Figure 3. Commercial fishing (A) catch and (B) effort for Cockburn Sound from 1977 to 2021. All data obtained from the Department of Primary Industries and Regional Development Catch and Effort Statistics Database. Catch data are presented at a broader spatial scale in Newman et al. (2023). Note, robust catch per unit effort data are not available for the majority of species targeted in CS with the exception of Blue Swimmer Crab (Johnston et al., 2020) and Southern Calamari (Yeoh et al., 2021).
Commercial invertebrate fisheries
Eleven invertebrate taxa have been recorded from commercial catches in CS since mandatory reporting began in 1977. Five taxa were regularly caught for several decades or more, i.e. Blue Swimmer Crab, mussels, Western Rock Octopus (Octopus djinda, formerly O. aff. tetricus), Southern Calamari (Sepioteuthis australis) and cuttlefish (Sepiidae spp.), with Blue Swimmer Crabs and mussels comprising the vast majority (~87%) of total invertebrate landings over that time (Figure 3). Roe’s Abalone (Haliotis roei) were also regularly caught within the CS commercial boundary (Figure 1) but are taken from exposed reef habitats surrounding the area, rather than in CS proper. Mussel stocks in CS, primarily Mediterranean Mussel (Mytilus galloprovincialis), developed in the early 1970s when CS was highly eutrophic and grew on dead seagrass root masses. These bivalves were the main commercially harvested invertebrate throughout the 1980s, with a peak annual harvest of 423 t in 1987, almost exclusively taken by diving (Figure 3). During the early 1990s mussel aquaculture began, and wild harvest ceased in 2004 (see Aquaculture).
The commercial fishery for Blue Swimmer Crab in CS was once the largest for this portunid in Australia, with annual landings steadily increasing from ~50 t in the late 1970s to a peak of 360 t in the late-1990s (Figure 3). Initially a gill net fishery, it transitioned to baited traps in the mid-1990s to increase efficiency and minimise bycatch. However, additional effort due to the extension of fishing into winter inadvertently increased pressure on mated pre-spawning females. Combined with several consecutive years of cooler temperatures during spawning months and an overall reduction in productivity of CS, stocks declined significantly in the early-2000s and the fishery was closed in 2006 (de Lestang et al., 2010; Johnston et al., 2011). Briefly re-opening in 2009 under strict management conditions, the fishery closed again in 2014 due to further stock declines (Johnston et al., 2020). The stock is currently considered to be environmentally limited, due to reduced primary productivity (Marks et al., 2021).
In recent years, octopus has been the main invertebrate landed, with catches from 2012 to 2021 ranging 16–51 t. The fishery was the most economically valuable of all commercial species during that period, representing ~40% of the total fishery value (Table 2). Octopus fishers exclusively use shelter pots, i.e. unbaited open-ended tubes that are highly selective (Hart et al., 2018). Southern Calamari has been regularly caught since the 1970s, with annual catches of 0.6–8 t and peaking in the late-1980s to early-1990s (Yeoh et al., 2021). All licenced fishers operating are permitted to retain Southern Calamari (and other squid or cuttlefish). Up to 22 different vessels have landed this species annually and almost exclusively using squid jigging, a highly efficient and species-selective fishing method (Yeoh et al., 2021).
Commercial finfish fisheries
Since catch records began in 1977, 71 teleost and nine elasmobranch taxa have been landed in CS (Table 2). However, except for several small pelagic species, catches of finfish have been relatively small by tonnage and economic value compared to those of invertebrates (Figure 3). Scaly Mackerel and Australian Sardine have comprised 83% of total finfish landings by weight since 1977. Catches of Scaly Mackerel were highest during the 1970s and 1980s with a peak catch of ~612 t in 1984, and have been relatively consistent at ~105 t (range 57–162 t) over the last decade. The Australian Sardines were by far the most abundant of all commercial species in CS (invertebrates and finfish) during the early- to mid-1990s and peaked at 1,274 t in 1992, before decreasing to practically 0 t in 1998 which coincided with a mass mortality event caused by pilchard herpesvirus across the southern and western WA stocks (Gaughan et al., 2008). While stocks showed a strong recovery, sardine catches in CS over the last decade (2012-2021) have ranged from 0 to 113.2 t (mean 29.7). Before 2000, several other small pelagic species were also regularly targeted by purse seine, with annual catches of Australian Anchovy (Engraulis australis), Yellowtail Scad (Trachurus novaezelandiae), Perth Herring (Nematalosa vlaminghi) and Maray (Etrumeus jacksoniensis) exceeding 10 t in certain years (Table 2).
Two other small pelagic species, Sandy Sprat and Blue Sprat (Spratelloides robustus), are also regularly caught by beach seines. When initiated, this fishery was focused in CS but now encompasses the wider Perth region (Millington, 1990). Annual catches of Sandy Sprat peaked in 1982 at 33 t (Table 2), coinciding with concerns about the potential for increasing fishing effort (Millington, 1990). Management changes were implemented in 1995 to prevent new fishers and fishing methods from entering the fishery. However, in the following decade, most fishers exited the fishery as annual catches steadily declined to <9 t, and no catch has been landed in CS since 2013. It is unclear what triggered the decline, although Sandy Sprat populations are thought to be strongly driven by recruitment variability (Newman et al., 2023), with the strength of the Leeuwin Current thought to positively influence recruitment (Caputi et al., 1996; Lenanton et al., 2009). However, in the last decade, there have been several years of strong Leeuwin Current, particularly during the 2010–2011 marine heat wave, yet this appeared to negatively impact Sandy Sprat recruitment and catches have continued to decline (Smith and Grounds, 2020). Based on observations from South Australia, increased rainfall has also been linked to better recruitment years for Sandy Sprat (Bice et al., 2015; Greenwell et al., 2021).
Australian Herring (Arripis georgianus) and Southern Garfish have primarily been captured using gillnets, beach hauls and garfish nets (Table 2). In 1995, the CS Fish Net fishery was introduced to manage these two species that accounted for 90% of the license holder’s catch, although a further 29 species had also been landed. During the 1990s, CS supported the largest commercial and recreational fishery for Southern Garfish in WA (Smith et al., 2017). However, catches declined from a peak of ~37 t in 1999 to just 2 t in 2016, which was attributed to the combined effects of fishing mortality and unfavourable environmental conditions, i.e. the 2010–2011 marine heat wave (Smith et al., 2017). The stock was closed to recreational and commercial fishing in 2017, however was reopened to recreational fishing and limited commercial fishing in 2024. Although in lower quantities, several higher-value species have been regularly landed in CS, including Snapper (Chrysophrys auratus) and King George Whiting (Sillaginodes punctatus) (Table 2). These species were primarily caught by line (including longlines) and gillnet, respectively. Two mugilids, i.e. Sea Mullet (Mugil cephalus) and Yelloweye Mullet (Aldrichetta forsteri), contributed substantially to catches before the 2000s, but have been essentially absent from catches since.
Before 2006, several fisheries operating in CS were permitted to retain elasmobranchs and nine taxa including several species of rays, Dusky Whaler (Carcharhinus obscurus), Whiskery Shark (Furgaleus macki) and Spinner Shark (Carcharhinus brevipinna) were landed, albeit in low quantities (average annual catch <0.5 to 2.1 t; Table 2), using mainly longlines and gillnets. Management changes in 2006 prohibited the landing of elasmobranchs from CS and the broader Perth metropolitan area and no landings have occurred since.
Recreational fishing
Recreational fishing is immensely popular in WA, with an estimated 753,000 individuals fishing at least once annually, and generates ~AU$1.1 billion to the economy (Ryan et al., 2022; Moore et al., 2023; DPIRD, 2024). As the only sheltered embayment in the Perth metropolitan region, CS is heavily utilised by boat- and shore-based fishers. Between 2011 and 2016 Woodman Point had 27,000-30,000 vessel retrievals annually and Point Peron had 12,000-19,000, making them two of the busiest boat ramps in WA (Afrifa-Yamoah et al., 2021). Most fishers in CS (~99%) use hook and line, with a small proportion also operating lobster pots, crab drop nets and diving (Sumner and Lai, 2012).
The most recent recreational boat-based catch survey specific to CS was in 2005/06, reporting that ~16 t of Australian Herring and 9 t of whiting (mainly King George Whiting and School Whiting Sillago spp.) were retained (Sumner and Lai, 2012). Roving creel surveys conducted along the CS coastline in 2023 revealed shore-based fishing effort to be ~81,696 (64,302-99,090 95%CI) fisher hours. The total kept number of all species retained was ~148,334 (46,740-249,928 95%CI) which included 38 species groupings (Tate et al., 2024). Among teleosts, Australian Herring were the most commonly retained (~30% of total catch by numbers), with Scaly Mackerel, Yellowtail Scad, Western Striped Grunter (Helotes octolineatus), and Western Butterfish (Pentapodus vitta) also frequently retained (Table 3).
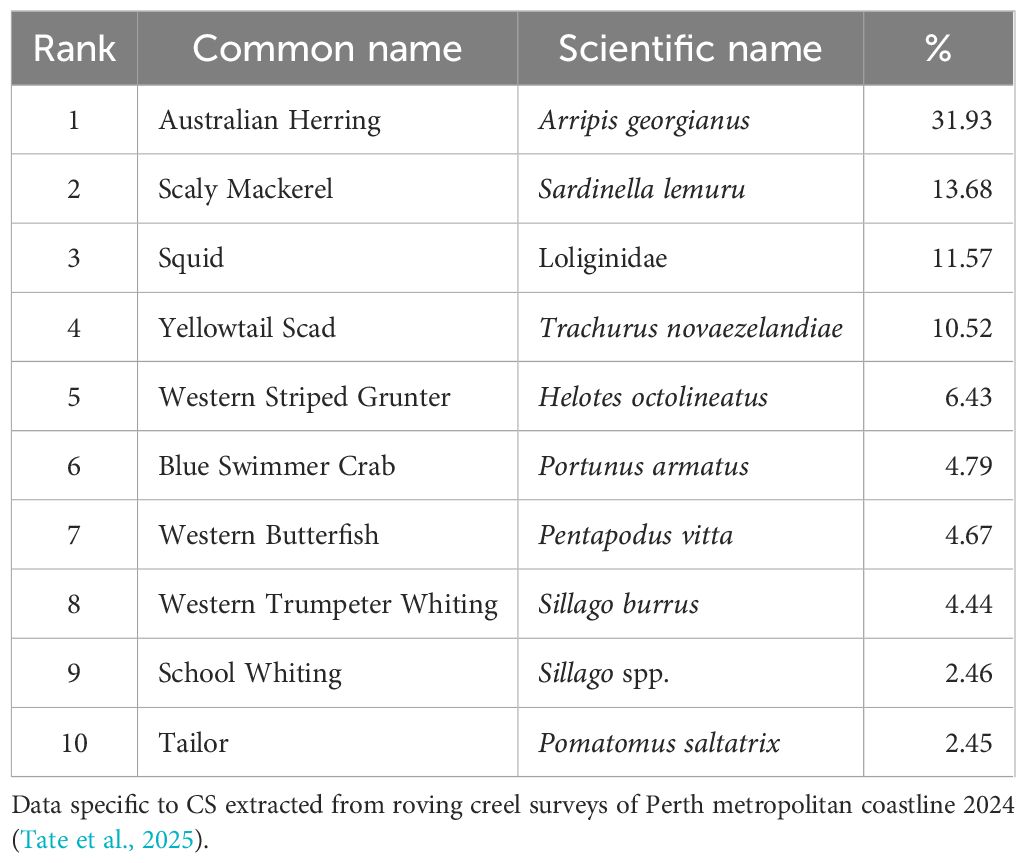
Table 3. Top 10 species retained by shore-based recreational fishers in CS, and proportion as the percentage of total catch.
Blue Swimmer Crab and loliginid squid are the main invertebrates that have been caught recreationally in CS over time. The portunid was historically very abundant and recreational catches of ~25 t annually were recorded during surveys in 1996/97 and 2002-04 (south of Woodman Point only; Bellchambers et al., 2006). However, the recreational catch for 2005/06 was estimated to be only 4 t (Sumner and Lai, 2012), coinciding with a significant stock decline (Figure 3). As a result, recreational crabbing was prohibited in CS south of Woodman Pt until December 2024, when the fishery reopened to the recreational sector only under precautionary arrangements (bag limit of five). More recent surveys of boat-based fishers suggest that 6–13 t of crabs are caught annually from the northern waters of CS (Johnston et al., 2020). The sheltered seagrass meadows of CS are popular areas for targeting squid (mainly Southern Calamari), particularly the Parmelia and Success banks and along the eastern margin of GI (Coulson et al., 2016; Yeoh et al., 2021). Squid are consistently among the most retained of all species caught by boat fishers in the Perth metropolitan area (Ryan et al., 2022). Squid are also the third most retained species by shore-based fishers in CS (Table 3). Other invertebrates recreationally caught from CS in smaller numbers include octopus, Western Rock Lobster (Panulirus cygnus), cuttlefish, mussels and prawns, which are mainly targeted through potting and/or diving (Ryan et al., 2022).
Aquaculture
The aquaculture industry in CS has focused on the non-indigenous Mediterranean Mussel. A ~50 ha lease was established on the Southern Flats in the 1980s and 47 ha at the Kwinana Grain Jetty during the 1990s (Figure 1). Although harvests increased from ~400 t in the early-1990s to 700 t in the early-2000s, annual harvests over the last decade have declined and remained low at <200 t. Although there is limited information indicating the causes of the marked reductions in harvests, several factors have been suggested. The most likely is the decline in nutrient levels from improved environmental management and the reduction of flow from the SCE, which caused a ~50% reduction in chlorophyll-a concentrations between the 1990s and 2000s (CSMC, 2016). Predation from Snapper during their spawning aggregations has also been proposed as an issue (CSMC, 2022), with anecdotal reports suggesting that, in some years, >120 t of mussels were consumed. Other pressures include warming temperatures (including marine heatwaves) that push the mussels to their upper thermal temperature tolerance of 26°C (Cottingham et al., 2023) and non-indigenous species that can smother mussels and reduce phytoplankton quality. One producer pivoted their production to Akoya Pearl Oysters (Pinctada imbricata) to overcome the declines in mussel production (CSMC, 2016) and all production ceased in 2023.
A 32-ha lease was approved in 2022 for the mariculture of 15 species of macroalgae including Asparagopis taxiformis and Asparagopis armata. These two species are of particular interest as a supplement for livestock feed to reduce methane production (Roque et al., 2019, 2021).
Aquatic vegetation
Seagrasses, along with their epiphytic algae, dominate the aquatic vegetation of CS (Cambridge et al., 1986), reflecting the large expanses of shallow (<10 m depth), soft-sediment areas around the periphery and limited reef habitat available for macroalgae (Figure 1). Seagrass meadows extended across 4,200 ha in the 1950s, but reduced dramatically to 900 ha by the 1970s (Cambridge et al., 1986). Posidonia sinuosa and Posidonia australis are the most dominant seagrasses in CS, with Posidonia coriacea and Amphibolis griffithii also forming extensive meadows on Parmelia and Success Banks. In addition, the smaller Halophila ovalis, Halophila decipiens, Heterozostera nigricaulis and Syringodium isoetifolium can occur as understory species or in disturbed areas (Cambridge et al., 1986).
Posidonia species form meadows with higher total biomass than many other seagrass genera, with the average above-ground biomass for P. sinuosa and P. australis reaching nearly 200 g DW m-2 (Cambridge and Hocking, 1997). Although highly seasonal, above-ground Net Primary Production (NPP) for these two species ranges from 600–900 and 900–1,100 g m-2 yr-1, respectively, which is similar to many other seagrasses (Cambridge and Hocking, 1997; Strydom et al., 2023). The persistent life history characteristics of Posidonia species lead to higher loads of epiphytic algae than many other seagrasses (Walker et al., 1999). The two dominant seagrass species in CS provide substrate for a highly variable but diverse epiphytic flora (Cambridge et al., 2007) with high biomass and NPP (11–78 g DW m-2 and 50 to 400 g m-2 yr-1, respectively) (Cambridge and Hocking, 1997). However, with leaf turnover rates of 1.1-1.8% day-1, considerable biomass of both seagrass leaves and their epiphytes are dislodged from the meadows and enter the detrital cycle within the meadows and in other habitats.
Despite this productivity, it appears the role of seagrasses in the food web is primarily as a substrate for epiphytic growth and as a contributor to the pool of particulate organic matter in sediments (Smit et al., 2006). Direct grazing by invertebrates and fishes on temperate Australian seagrasses is limited (York et al., 2018), with both seagrass-associated fish and invertebrates in CS appearing to consume epiphytes, based on dietary (Smit et al., 1998) and stable isotope studies (Smit et al., 2005, 2006). Fish assemblages within temperate Australian seagrass meadows are typically dominated by meso-carnivores, which derive their nutrients indirectly from invertebrates that feed on epiphytes rather than seagrasses (York et al., 2018). The thick wracks of seagrass that accumulate along the sandy beaches and deeper regions of CS also appear to play a minimal role in the food web. Instead, stable isotope studies suggest the detrital macroalgae dislodged from nearby reefs or imported into CS appear to be the main contributors to invertebrate production in the shallow waters of sandy beaches with wrack accumulates rather than seagrass and seagrass detritus (Hyndes and Lavery, 2005). This finding is supported by other studies in the region (Crawley et al., 2006; Crawley and Hyndes, 2007).
Benthic invertebrates
The benthic environment in CS is classified as an ecological protection zone of ‘moderate’ to ‘high’ importance for maintaining ecosystem integrity (EPA, 2015), with monitoring advised for nearshore, ‘moderate’ protection areas (EPA, 2017). To date, no systematic biotic monitoring occurs and knowledge stems from sporadic unrelated surveys of benthic habitats and their associated invertebrates. Spatially extensive, stratified surveys in the 1950s-70s documented 379 invertebrate species from select groups of Cnidaria, Echinodermata, and Mollusca (Devaney, 1978; Marsh, 1978a, b; Wilson et al., 1978). A later trawl survey of deep areas in 2007/08 recorded 141 relatively large (>45 mm) invertebrate taxa from six phyla (Arthropoda, Chordata, Cnidaria, Echinodermata, Mollusca and Porifera). Coupled with 75 fish species, diversity increased northward from Mangles Bay to OA (Sampey et al., 2011). Six unique communities were identified based on the abundance of these taxa, with the portunids Blue Swimmer Crab and Trionectes rugosus, Western King Prawn, the sea cucumbers Colochirus quadrangularis and Cercodemas anceps, and Southern Calamari partly distinguishing these communities (Sampey et al., 2011).
Infaunal and epifaunal species have been collected using sediment cores/grabs (<1.7 mm mesh) from vegetated and bare sediments. Venerid and tellinid bivalves, deposit-feeding polychaetes (Aricidea sp. and Capitella spp.), and errant polychaete families (Lumbrineridae, Onuphidae and Syllidae) are particularly abundant in Posidonia and Amphibolis meadows, with seagrass beds supporting a greater species richness, abundance, and biomass of invertebrates than neighbouring bare sands (Wildsmith et al., 2005; Dapson, 2011). This is also true for seagrass wrack on beaches of GI that can temporarily host more terrestrial invertebrates than macroalgae wrack or sand in the same location, likely due to its enhanced structural complexity (Mellbrand et al., 2011). In sparsely vegetated areas, the bivalves Donax columbella and Donacilla sp., the amphipods Phoxocephalopsidae sp. and Exoediceroides sp., and the spionid polychaete Scolelepis carunculata occur (Wildsmith et al., 2005). The infauna of soft sediments in the deep basin are dominated by molluscs (e.g. Arcuatula glaberrima, Bedeva paivae, and Dosinia incisa), polychaetes (e.g. Capitellidae sp.) and echinoderms (e.g. Temnopleurus michaelseni), while epifauna include sea pens and sea cucumbers (Wilson et al., 1978; Wells and Threlfall, 1980; BMT Oceanica, 2018). Differences among these communities were related to sediment type and the quantity of microphytobenthos present (BMT Oceanica, 2018).
Invertebrates, including several non-indigenous species, have colonised natural hard substrates including low-relief limestone reefs and artificial structures such as wharves, breakwaters, channel markers and artificial reefs (Sutton and Shaw, 2019). Twelve species of reef-building corals attached to dead coral mounds or groynes provide habitat for bivalves and echinoderms (Marsh, 1978b; Wilson et al., 1978). Common hard substrate fauna include solitary ascidians (Herdmania sp., Polycarpa viridis), stony corals (Turbinaria peltata, Turbinaria reniformis) and encrusting coral (Plesiastrea versipora) (Hammond et al., 2020). The limestone reefs and associated flora may provide limited spawning grounds for Southern Calamari, which deposit eggs near the base of macrophytes (Coulson et al., 2016). The invertebrate fauna of CS enhance its biodiversity and provide a key food source for crabs, fishes, birds, and mammals (Dunlop, 1997; Platell and Potter, 2001; Campbell et al., 2021).
Fish
Coastal waters are crucial environments for many fish species due to their high productivity and sheltered nature (Beck et al., 2001). A total of 571 fish species from 142 families have been recorded along the Perth Coast, of which 83 (15%) are endemic to WA (Whisson and Hoschke, 2021).
Benthic/demersal fish communities
Beach seining in seagrass habitats in and near CS yielded 67 fish species (Valesini et al., 2004). Catches mainly comprised small pelagic atherinids such as Silverfish (Leptatherina presbyteroides) and Common Hardyhead (Atherinomorus vaigiensis), together with juveniles of larger-bodied fishes such as Western Striped Grunter and Western Trumpeter Whiting (Sillago burrus; Table 4). The size composition of these and other species, including King George Whiting and Sea Mullet, demonstrates the nursery function these seagrass habitats provide. In contrast, sandy habitats were less speciose (Valesini et al., 2004), comprising schooling clupeids, i.e. Sandy Sprat and Blue Sprat, the Flathead Sandfish (Lesueurina platycephala), a dorsoventrally-flattened and camouflaged ambush predator, and juveniles of various whiting species (Table 4). Cockburn Sound and Warnbro Sound, a smaller marine embayment directly south of CS, are well-known nursery areas for Sandy Sprat and Blue Sprat (Valesini and Tweedley, 2015), two important commercial species that are also a food source see Marine birds.
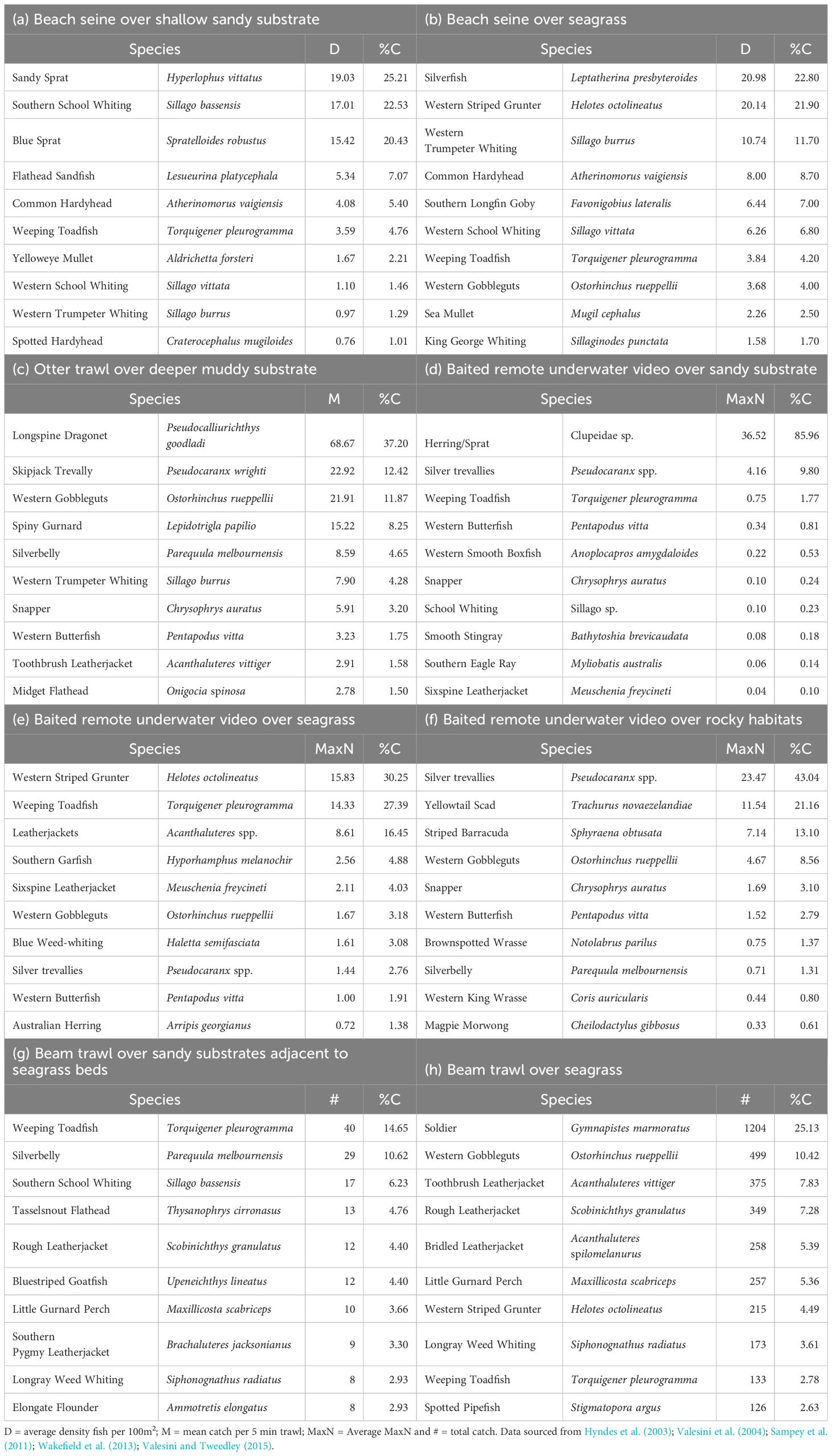
Table 4. Percentage contribution (%C) of the ten most abundant fish species caught in broad habitat types in Cockburn Sound using a range of methods.
Trawling in the deeper waters of CS yielded 75 fish species (Sampey et al., 2011). Among these, the Longspine Dragonet (Pseudocalliurichthys goodladi), Skipjack Trevally (Pseudocaranx wrighti) and Gobbleguts (Ostorhinchus rueppellii) each represented >10% of the catch (Table 4). A spatial gradient was evident, with greater numbers of species recorded in OA and along GI, with the latter region also harbouring the most fish. Community composition also shifted on a north-south axis through CS. Sampey et al. (2011) suggested the Western Butterfish, Longspine Dragonet and Silverbelly (Parequula melbournensis) as indicator species, that were easily identified in the field and good surrogates for monitoring changes in faunal composition.
A wider range of habitats, including different sandy areas, seagrass, limestone outcrops and man-made rock walls (both subsequently referred to as reef), have been sampled using Baited Remote Underwater Video (BRUV; Wakefield et al., 2013). Sandy areas were dominated by the pelagic Clupeidae spp. and silver trevallies Pseudocaranx spp. (Table 4). More species and individuals were recorded over seagrass and reef habitats than sandy areas. The predominantly herbivorous Western Striped Grunter and the benthic invertivores Weeping Toadfish (Torquigener pleurogramma) and leatherjackets (Acanthaluteres spp.) characterised seagrass habitats (Table 4; Poh et al., 2018). These species are camouflaged and directly or indirectly utilise this habitat for foraging. The reef fish community was largely dominated by silver trevallies and Yellowtail Scad, which aggregate around hard structures (Florisson et al., 2018). Despite the limited spatial extent of seagrass and reef habitats in CS (Figure 1), given their rich and abundant fish fauna, they likely play an important role in the fish ecology of this embayment (Wakefield et al., 2013).
Hyndes et al. (2003) investigated the fish fauna over three types of seagrass beds (A. griffithii, P. sinuosa and Posidonia coriacea) and adjacent unvegetated habitats and found a greater number of species, individuals and biomass in vegetated vs unvegetated habitats and a different faunal composition among seagrass species (Table 4). This suggested that fish species prefer a particular seagrass architecture. The number of species and density of fish were greatest in P. sinuosa beds with their uniformly dense blade-like leaves, albeit these fish were smaller (Hyndes et al., 2003). Larger fish were able to penetrate and occupy the A. griffithii beds, as below the leaf canopy there are relatively open spaces between the woody stems. The patchy habitat provided by P. coriacea showed a similar number of species and density of fish as unvegetated habitats.
The most notable temporal shift in the demersal fish fauna occurs between September and January, when Snapper from up to 700 km away aggregate in CS to spawn (Crisafulli et al., 2019). The geomorphology and prevailing south-westerly winds result in a counterclockwise gyre facilitating the retention of eggs and larvae (Wakefield, 2010). This unique environment makes CS an important source of recruitment for nearby adult stocks along the lower west coast of Australia (Crisafulli et al., 2019). These seasonal increases in adult Snapper abundance have also been linked with increases in higher trophic-level predators such as dolphins and sharks. A survey targeting larval Snapper in and around CS recorded larvae of 53 fish taxa from 30 families (Breheny et al., 2012). Larval concentrations were greater in CS than in nearby areas including OA and Warnbro Sound, due to large concentrations of anchovies and to a lesser extent, dragonets and leatherjackets.
Syngnathids
Twenty-three syngnathid species, i.e. 17 pipefish, three sea dragons, two seahorses and one pipehorse, occur in waters around Perth, with five endemic to WA (Whisson and Hoschke, 2021). This family is listed in the Environment Protection and Biodiversity Conservation (EPBC) Act 1999 and, therefore, protected in Commonwealth waters; however, currently, only the Leafy Seadragon (Phycodurus eques) and Common Seadragon (Phyllopteryx taeniolatus) are protected in WA state waters (Table 5).
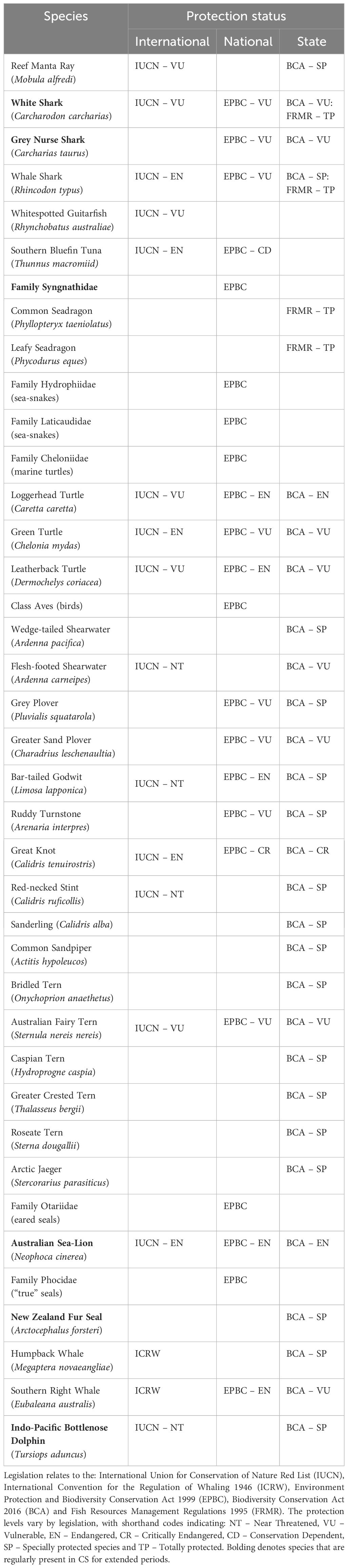
Table 5. List of species that have been frequently observed within Cockburn Sound and have state, national or international protected status.
Syngnathids are weak swimmers, relying on camouflage to avoid predation and thus often exhibit strong preferences for structurally complex habitats. The most abundant pipefishes, i.e. Spotted Pipefish (Stigmatopora argus) and Wide-bodied Pipefish (Stigmatopora nigra), were recorded mainly in P. sinuosa and/or P. coriacea beds where their green and brown colourations mimic seagrass leaves that they grasp with their prehensile tails (Kendrick and Hyndes, 2003). Among the sea dragons, the Leafy Seadragon resides mainly in Posidonia beds, whereas the Common Seadragon preferred floating above Ecklonia-dominated reefs (Connolly et al., 2002; Allan et al., 2022). Both seahorse species present in CS, i.e. Short-snouted Seahorse (Hippocampus breviceps) and West Australian Seahorse (Hippocampus subelongatus), are associated with hard substrates, including man-made structures (Whisson and Hoschke, 2021). Swimming enclosure nets, such as that at Coogee Beach, can have a positive effect on seahorse populations elsewhere (Simpson et al., 2021).
Dietary information for 12 synganthid species demonstrated they predominantly consume small crustaceans but with a high degree of trophic specialisation influenced by their snout morphologies, body form and mobility (Kendrick and Hyndes, 2005). Based on their strong habitat associations and trophic specialisation towards taxa that are associated with seagrass (Kendrick and Hyndes, 2005), synganthid populations (especially pipefish) have been linked to the health and extent of these crucial habitats in CS. Declines in several pipefish species have been correlated with the loss of seagrasses elsewhere (Adams et al., 2022; Cowley et al., 2022) and have been a suggested flagship group for conservation planning (Shokri et al., 2009).
Pelagic fishes
Although there has been no targeted survey of the pelagic fish fauna of CS, information can be derived from commercial fishing. Small to medium-bodied pelagic species recorded in commercial catches include Scaly Mackerel, Australian Sardine, Australian Anchovy, Maray, Blue Sprat, Sandy Sprat and Yellowtail Scad (Table 2). In recent decades, the most abundant species by weight is the Scaly Mackerel, a tropical species at the southern limit of its distribution (Whitehead, 1985). Otolith chemistry suggests fish in this stock are highly mobile and regularly enter and leave CS with no discernible seasonality or habitat preference (Gaughan and Mitchell, 2000). The year-round presence of larvae in the SCE suggests protracted local spawning (Gaughan et al., 1990; Neira et al., 1992).
Catches of Australian Sardine are greatest during autumn, possibly due to this temperate species avoiding warming waters on the outer continental shelf when the Leeuwin Current typically strengthens (Feng et al., 2003). Adult Australian Anchovy are relatively abundant in commercial catches (Table 2) and also occur as larvae and juveniles (Breheny et al., 2012), suggesting CS is important throughout multiple life stages of this species. Samson Fish (Seriola hippos), a large piscivorous pelagic species targeted by recreational fishers and of minor commercial importance, visit CS as adults but is also known to use it as a nursery area. Demersal trawls in the 1970s recorded 0+ juveniles (caudal fork length 8–25 cm) mainly in autumn (Penn, 1977).
Elasmobranchs
Several shark and ray species use CS for at least part of their life cycle, from mesopredators such as Southern Eagle Ray (Myliobatis tenuicaudatus), and Whiskery (Furgaleus macki) and Dusky sharks to apex predators including White Shark (Carcharodon carcharias). The degree of residency within CS varies among species, with smaller elasmobranchs such as the Southern Eagle Ray spending more time in CS than larger more oceanic species such as White Shark (Wakefield et al., 2009; McAuley et al., 2017), therefore exerting different ecological pressures.
Large predatory sharks (>3 m total length) such as the EPBC-listed White, Grey Nurse (Carcharias taurus; Table 5) and Copper (Carcharias brachyurus) sharks have mostly been detected in CS during October-December, attracted by the seasonal aggregations of Snapper (Jakobs and Braccini, 2019). In turn, some of these species can increase predation rates on marine mammals and seabirds (King, 2014). These sharks leave CS to then return the following spawning aggregation season. The smaller elasmobranch species, which tend to spend more time in CS, prey on small demersal teleosts and benthic invertebrates (Simpfendorfer et al., 2001; Sommerville et al., 2011). Finally, in addition to being an important source of prey for a range of shark and ray species, CS may be a nursing ground for Spinner Shark (Carcharias brevipinna), with an aggregation of neonate and small juveniles identified (M. Braccini, unpublished data).
Tropicalisation
The west coast of Australia represents a transition zone between tropical and temperate environments (Saville-Kent, 1897; Fairclough, 2021). The southward-flowing Leeuwin Current is strongest during autumn and winter and disperses the early life history stages of tropical fishes into normally temperate regions, including CS. South-western WA is a global hotspot for marine warming and, in recent decades, has experienced periodic extreme events, such as marine heat waves (Pearce and Feng, 2007; Hobday et al., 2018). As a result, 89 of the 571 fish species recorded in marine waters off Perth are considered possible range extensions of tropical species (Whisson and Hoschke, 2021). While many of the larvae transported on the Leeuwin Current do not survive to maturity, either due to their temperature tolerance or a lack of specific resource needs (e.g. habitat and food), the combination of gradual warming and heatwaves has resulted in the survival of some tropical species in temperate regions of this coast, including CS (Pearce et al., 2016; Lenanton et al., 2017).
An extreme marine heatwave in the summer of 2010/11, followed by two years of above-average water temperatures, allowed survival to sexual maturity, reproduction and recruitment of the tropical Black Rabbitfish (Siganus fuscescens) in seagrass habitats throughout CS (Zarco-Perello et al., 2022). This species was subsequently recorded for several years in commercial catches and faunal surveys (Daviot, 2017; Red Map Australia, 2023). Various other tropical species have also been recorded during warmer years, such as Whale Shark (Rhincodon typus), Reef Manta Ray (Mobula alfredi) and tropical carangids (Whisson and Hoschke, 2021). While a wide range of tropical species have periodically been observed only a limited number have become abundant (e.g., Western Butterfish; (Daviot, 2017), demonstrating that this embayment still primarily comprises a temperate fish fauna. This is influenced by the fact that in between warming events, CS also experiences cool anomalies, limiting the long-term survival of warm water-adapted species (Fairclough, 2021). Albeit, several cooler-water species have undergone population declines, e.g. Southern Garfish and Sandy Sprat.
Fish kills
An average of 25 mass mortality events of fish and invertebrates have been recorded annually in WA since 2000, with most occurring in estuaries and marine embayments adjacent to developed areas in south-western WA (DPIRD, unpublished data). These events are typically the result of natural ‘biological’ (e.g. life-cycle related) or ‘environmental’ (e.g. weather-related) events with, based on the limited records available, 30% attributed to algal blooms and hypoxia (often related to elevated riverine flows following storm events), while 30% were unknown and 10% were related to chemical spills or contaminated water.
Twenty-seven fish kills were recorded in CS and Warnbro Sound between 2001-2023. Although the location of the carcasses is not necessarily where the mortalities occurred, most have been reported on the eastern shoreline of CS including Coogee Beach, Woodman Point, Challenger Beach, and around the Kwinana Grain Jetty. The fish kill that attracted the most public attention occurred in November 2015 and involved Snapper, Southern Calamari, Cobbler and the Weeping Toadfish and others such as Parma spp (reef-associated species). This event was linked with a phytoplankton bloom of the diatom Chaetoceros spp. possibly caused by increased nutrients, higher-than-normal water temperatures, and reduced flushing conditions. However, most events (71%) have involved invertebrates such as sea stars, sea hares and Blue Swimmer Crabs.
Reptiles
Juvenile turtles, typically Loggerhead (Caretta caretta) and Green (Chelonia mydas) and occasionally Leatherback (Dermochelys coriacea), become translocated and stranded in CS due to the Leeuwin Current and/or midwinter westerly storm systems (Prince, 2004; Young, 2022). Larger adult turtles have also been observed (D. Yeoh, pers. obs.). All three turtle species are listed as either vulnerable or endangered under the EPBC Act and by the IUCN (Table 5). Stranded turtles are often rehabilitated and released near Exmouth, Karratha and Broome (Robson et al., 2017).
Sea snakes are not normally present in WA south of Shark Bay, however, six species have been observed in CS, usually washed-up on beaches (Storr and Johnstone, 1983). The most common are the Yellow-bellied (Hydrophis platurus) and Olive-headed (Hydrophis major), which are likely waif populations. Strandings of the former species, which is not a strong swimmer, typically occur between May and October, with 70% in July/August (Hecht et al., 1974) coinciding with the strongest southward flow of the Leeuwin Current (Cresswell, 1991).
Terrestrial Tiger Snakes (Notechis scutatus) are found naturally on GI although there is some debate whether the Carnac Island population are a remnant or the result of a translocation in the 1930s (Ladyman et al., 2020; Lettoof et al., 2024). Carnac supports ~20 adult snakes ha-1, with the high density attributed to their diet of nesting seabird chicks (Bonnet et al., 2002). Remarkably, these snakes can survive, grow and reproduce successfully even after being blinded by birds defending their nests (Aubret et al., 2005) and such is their reliance on avian prey that in less than 90 years individuals have developed modified jaws capable of swallowing chicks whole (Ammresh et al., 2023). GI also supports a population of the threatened Carpet Python (Morelia imbricata) (Pearson et al., 2005).
Marine birds
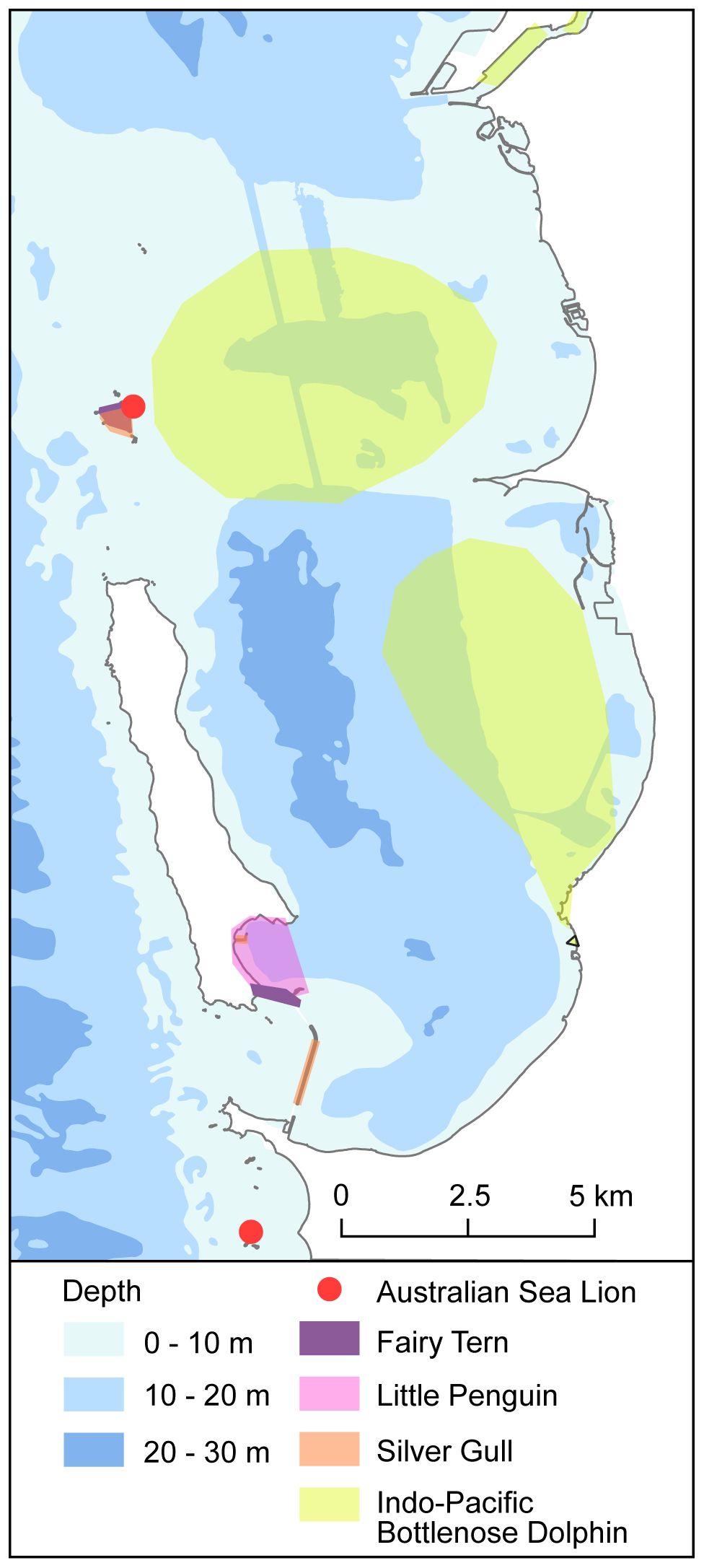
As a sheltered environment with diverse habitats, CS is an attractive point of convergence for seabirds, waterbirds and shorebirds. At least 17 bird species breed regularly on the islands, rocks and/or mainland adjoining CS, while many more non-breeding visitors utilise its waters or shorelines for foraging (Table 6). Resident species, including the Little Penguin and Australian Pied Oystercatcher (Haemotopus longstriosis), are present year-round and breed on GI. During spring and summer, species such as the Bridled Tern (Onychoprion anaethetus), White-faced Storm-petrel (Pelagodroma marina) and Wedge-tailed Shearwater (Ardenna pacificus) return to the region to breed but are rarely seen outside of these seasons (Table 6). Careening Bay is a key area for Little Penguins and provides access to their nesting areas on the island. The sand spit on Parkin Point (south-eastern GI; Figure 4) is particularly important for migratory shorebirds, terns, and Red-capped Plovers (Charadrius ruficapillus) (BirdLife Australia, 2023). Leading up to the breeding season (spring), the spit is a major night roost for the Australian Fairy Tern (Sternula nereis nereis) (Dunlop and Greenwell, 2021). Carnac Island and nearby rocks and islets (Figure 4) remain an important breeding and roosting site for various gulls, terns and cormorants. The area also represents the southernmost breeding extent of the Wedge-tailed Shearwater and northernmost breeding extent of the Little Penguin. However, Little Penguins have not been recorded breeding on the island since 1980 and recent surveys indicate a significant decline in the breeding population of Silver Gull (Chroicocephalus novaehollandiae) (Dunlop and Storr, 1981; E. Clitheroe, unpublished data).
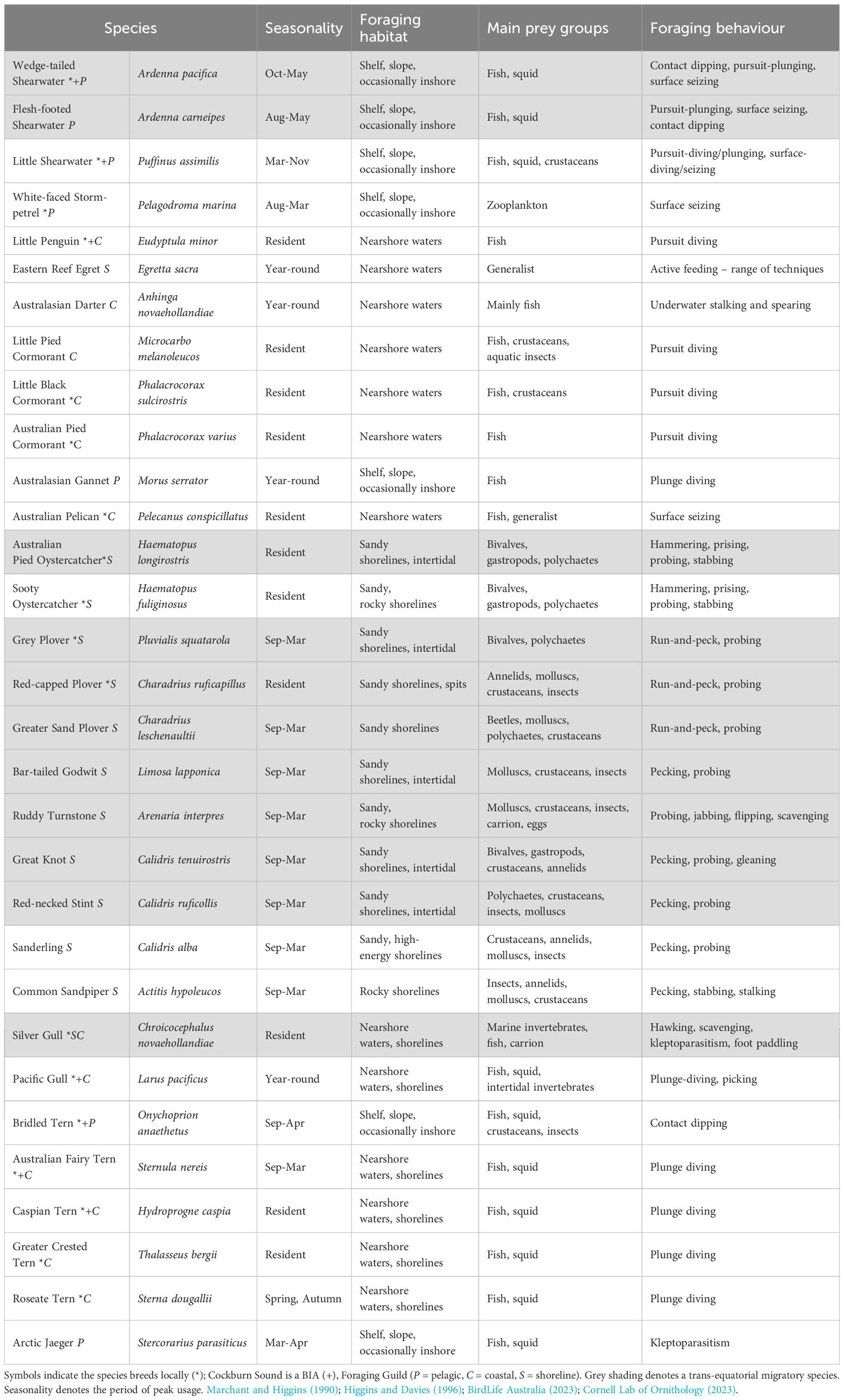
Table 6. Common marine birds of Cockburn Sound and/or those for which Cockburn Sound or adjacent waters are identified as a Biologically Important Area (BIA), i.e. where species of regional significance aggregate for breeding, feeding, resting or migration.
Marine birds in CS utilise different foraging habitats, and feed at various spatial scales (10s to 1,000s kms) and trophic levels, but can generally be classified into three main guilds (Table 6). These guilds are i) pelagic seabirds that primarily feed offshore and spend their entire lives at sea, only returning to land to breed; ii) coastal foraging seabirds that feed in nearshore waters, and except for the Little Penguin, typically return to shore when they are not foraging; and iii) resident and migratory shorebirds that utilise shorelines, salt lakes and/or intertidal areas for foraging. The diets of several species have been examined from marine waters adjacent to CS, which highlight the importance of inshore spawning fishes as a prey source (Dunlop, 1997; Murray et al., 2011; Stockwell et al., 2021). For example, the Australian Fairy Tern and Little Penguin feed predominantly on small, schooling fishes, such as Blue Sprat, Sandy Sprat, and Southern Garfish, although their relative dietary contributions are likely to vary among years, depending on prey abundance (Cannell, 2018; Greenwell et al., 2021). Cockburn Sound also serves as a core foraging habitat for penguins from the GI and nearby Penguin Island (~5 km south) colonies, and the prevalence of foraging fish within CS likely plays a crucial role in sustaining these colonies (Cannell, 2018; Sutton, 2022).
Through their nesting activities marine birds can also significantly alter habitats and impact ecosystem functioning (McKechnie, 2006; Grant et al., 2022). The Great Pied Cormorant (Phalocrocorax varius) is a major producer of guano within CS, with large colonies potentially generating several tonnes each year (Rippey, 2015). The increased abundance of this species has intensified mechanical and chemical stress on island habitats, resulting in significant vegetation damage on Carnac Island and adjacent islands, leading to the possible displacement of other seabirds (Rippey et al., 2002). Marine birds inhabiting CS face a range of persistent and diverse pressures in both their marine and terrestrial habitats, including climate change, severe weather events, introduced predators, human disturbances, coastal development activities, recreational and commercial fishing operations, and eutrophication (Cannell et al., 2016; Greenwell and Dunlop, 2023).
Marine mammals
Pinnipeds
The Australian Sea Lion and New Zealand Fur Seal (Arctocephalus forsteri) are regularly sighted in CS. The former species is endemic, ranging from the Abrolhos Islands (WA) to the Pages in South Australia, and listed as endangered and protected under Commonwealth and State legislation (Table 5). Individuals typically occur in relatively small and widely distributed breeding island colonies, many of which are genetically distinct with females displaying high natal site fidelity. The biological characteristics of Australian Sea Lions, together with an estimated >60% decline in the overall population over the last 40 years (Goldsworthy et al., 2021), makes this species vulnerable to local and population-wide extinction. Cockburn Sound falls within the ‘Houtman Abrolhos to Rottnest Shelf Waters’ Important Marine Mammal Area (IUCN, 2020). This designated area includes WA’s sea lion breeding colonies and associated important haul out sites and foraging habitats, with the nearest breeding colony to CS being Jurien Bay (~300 km north). Male sea lions haul out at Carnac Island year-round and utilise other metropolitan island beaches and GI regularly (Figure 4). Peak numbers at Carnac Island occur outside of breeding periods. Recent counts indicate ≥75 sea lions may haul out on metropolitan islands at any one time (Salgado Kent and D’cruz, 2021), however, the number that use CS is unknown. As Australian Sea Lions spend ~40-60% of their time foraging at sea (Costa and Gales, 2003; Fowler et al., 2006), the numbers using the region are likely to be significantly greater than those counted at haul-out sites. They are benthic foragers and consume a diverse range of prey including cephalopods (i.e. octopus, squid and cuttlefish), teleosts (i.e. leatherjackets, wrasse, flatheads, mullets and whiting) and crustaceans (i.e. crabs, prawns, and rock lobsters) (Goldsworthy, 2018).
New Zealand Fur Seals have a broader distribution including Australia, New Zealand and some subantarctic islands, with 17 breeding colonies recorded in WA, of which Rottnest Island is the most northern (Campbell et al., 2014). Fur seals regularly haul out at a range of locations in CS and share haul-out islands with Australian Sea Lions. In contrast to Australian Sea Lions, many New Zealand Fur Seal breeding sites have grown rapidly since the 1990s, with the WA population estimated to be ~17,200 in 2010/2011 and near carrying capacity (Campbell et al., 2014).
Threats to pinnipeds in the region include interactions and mortalities with fishing gear (mainly rock lobster pots), boat strikes and human disturbance/harassment at haul-out and breeding sites (Mawson and Coughran, 1999; Cannell, 2004). Although regular sea lion haul-out islands are recognised for their importance and fall within marine parks and/or are classed as nature reserves (Figure 4), sea lions experience significant levels of disturbance when resting from people on land, paddlers, vessels (including tour operators) and jet skis which can alter their behaviour (Osterrieder et al., 2016).
Dolphins
The Indo-Pacific Bottlenose Dolphin (Tursiops aduncus) shows a high degree of site fidelity to local areas and appears to reside in relatively small communities within CS and its adjacent waters (Chabanne et al., 2012, 2017a; Nicholson et al., 2021). As apex predators, dolphins are ecologically important in the food web, while also having conservation status as an iconic species that is socially and culturally valued and popular for marine tourism. The species is listed by the IUCN as near-threatened and is protected in WA under Commonwealth and State legislation (Table 5).
Based on photo-identification studies spanning 1993-2015, a resident community of ~75 juvenile and adult dolphins was initially described by Finn (2005), with subsequent studies indicating that the community remained relatively stable (Ham, 2009; Chabanne et al., 2017b). Chabanne et al. (2017a) identified two additional dolphin communities in OA and the SCE. Although the three communities were described as socially and spatially discrete, limited inter-population movements occurred, including with those identified in nearby Shoalwater Bay and Warnbro Sound (Nicholson et al., 2021). However, there has been limited evidence of individual permanent movement, with the SCE community not increasing beyond 23 adults and juveniles over the last two decades. The OA community is thought to be smaller than that of CS (~43 juvenile/adult dolphins; Chabanne et al., 2017a). Despite being limited or temporary, those movements have allowed for continuous gene flow among the communities that were each characterised by highly related individuals (Chabanne et al., 2021).
In addition to OA, the Kwinana Shelf was described as an important foraging and nursery area for dolphins in CS (Figure 4; Finn, 2005). This significance is supported by observations of large feeding aggregations of dolphins and seabirds across the Kwinana Shelf from autumn to spring, alongside seasonal schools of fish (Finn and Calver, 2008). The shallow, productive, and sheltered waters of CS offer unique ecosystem features for dolphins not available elsewhere along the south-western coastline.
Whales
Humpback and Southern Right whales are regularly sighted in Perth coastal waters, including CS. Humpback whale populations have recovered strongly since the cessation of whaling (Bejder et al., 2016), with an estimated population of >30,000 in 2008, and an increase of ~10-13% per annum (Salgado Kent et al., 2012). Humpback whales migrate annually along the WA coast from high-latitude summer feeding areas in the Southern Ocean to low-latitude winter breeding grounds (Chittleborough, 1965; Jenner et al., 2001). This migratory corridor, including waters off the Perth metropolitan region, is recognised as an Important Marine Mammal Area (IUCN, 2020), particularly during the southern migration (~August-November) when mothers are travelling with their dependent calves.
Southern Right Whales also experienced near extinction from whaling and are listed as endangered (Table 5). There are two recognised Australian sub-populations, with those recorded off the Perth metropolitan area considered part of the western sub-population comprising ~3,164 whales with a recovery rate estimated at ~6% per annum (Smith et al., 2021). Calving and mating occur in this region between April and September, with mother-calf pairs remaining for at least two months until the calves are strong enough to travel to feeding grounds in the Southern Ocean (Salgado Kent et al., 2022). Although the population remains below its historic size, there is increasing evidence of an expansion in calving grounds and connecting habitats off Australia’s coast, including increased sightings off Perth.
Restoration and enhancement
Recognition of the biological and social importance of CS, and its history of environmental perturbation, has led to numerous attempts to restore and enhance habitats and increase fish stocks. Most notably, for over 30 years various projects have attempted to restore the seagrass meadows that were lost within CS. Initially these projects used manual transplanting of seedlings, sprigs and plugs, however, despite being successful elsewhere in the world these projects had limited success (Kendrick et al., 2025). Further development of mechanical transplanting equipment allowed large sods to be harvested from areas designated for dredging and transplanted elsewhere, and while a range of trials improved the survival of the transplanted seagrasses and some were considered successful, costs were prohibitive (Sinclair et al., 2013). Recent efforts have instead focused on the collection and delivery of seeds to the substrate using community members, i.e. Seeds for Snapper (Sinclair et al., 2021; Kendrick et al., 2025). Floating fruits collected by boaters and divers are taken to an aquaculture facility where dehiscence occurs, after which the negatively buoyant seeds are scattered over restoration areas. Initial results showed seedling establishment was up 10% and the surviving seedlings aggregated, produced multiple shoots, and demonstrated some coalescence after three years (Sinclair et al., 2021). Cost-benefit analysis indicated that seeding 1 ha is 5.6 and 3.7 times cheaper than transplanting using professional and volunteer-based labour, respectively, and that the costs would be recovered in 7 and 11 years, respectively (Rogers et al., 2019). Successful seagrass restoration would likely have other benefits to CS such as increase fish diversity and abundance (Hardinson et al., 2023; Sievers et al., 2024), however, while long term seagrass monitoring efforts have been undertaken, little emphasis has been placed on determining the effectiveness of restoration on associated faunal communities.
Artificial reefs have been used around the world to enhance benthic habitats, either accidentally (e.g. shipwrecks) or deliberately (Ramm et al., 2021). There are three purpose-built artificial reefs within CS and two notable shipwrecks, the D9 Dredge ex Parmelia and the Omeo (Figure 1). The D9 Dredge was a 51 m steel-hulled suction dredge that foundered in CS in 1962 and was towed and sunk on the edge of the Kwinana Shelf. The wreck is a particularly popular recreational fishing location. Omeo was a 65 m long, 789 t clinker-built iron barque that was swept ashore in 1905 after breaking its mooring during a storm (Green, 2006). Although the wreck has deteriorated substantially, its sheltered location ~30 m from shore, makes it a popular tourist snorkelling spot. A variety of different concrete modules were deployed close to Omeo to create the Coogee Maritime Trail, which aimed to provide suitable habitat for rocky reef-associated species. Monitoring of the 70 modules showed they were initially colonised by brown foliose algae that declined over time, during which solitary ascidians established (Hammond et al., 2020). However, while reef-associated fish species were recorded, the modules have not increased species richness or abundance. Another reef was deployed 100 m offshore from C.Y. O’Connor Beach in 2022 consisting of 135,2 m high concrete modules. The primary purpose of this reef, however, is to attenuate wave energy, reduce coastal erosion and minimise the need for sand replenishment (Geldard et al., 2021). The artificial structures within CS are all relatively small. The conventional wisdom is that small artificial reefs primarily act as fish attractors, and a reef volume of 4,000 m3 has been suggested as a minimum size in order to increase fish production (Pickering and Whitmarsh, 1997). It is therefore unlikely that the artificial structures currently in place in CS are causing any meaningful change on fish populations, beyond acting as a focal point for fishing and diving.
Stocking, i.e. the release of hatchery-reared juveniles, is strongly supported by recreational fishers in WA due to their beliefs that such management interventions will lead to increased catches and in the case of Snapper, contribute to recovery of depleted stocks (Obregón et al., 2020; Tweedley et al., 2023). A stocking program for Snapper (‘Snapper Guardians’) was initiated in 2014, where rather than collecting and holding broodstock in a hatchery, this program collects fertilised eggs from wild spawning aggregations in CS, which are cultured for up to 80 days before being released at ~50 mm in length (Partridge et al., 2017). No differences in genetic diversity (i.e. allelic richness and heterozygosity) were detected between wild and hatchery-reared individuals. Annual stocking has occurred since 2016, with >300,000 juveniles released to date. The otoliths of these fish were stained using alizarin complexone to allow the effectiveness of the releases to be assessed in the future (Partridge et al., 2017), although no specific field surveys to evaluate stocking effectiveness were developed. Of the 1,980 juvenile Snapper captured between 2014 and 2023 during Blue Swimmer Crab surveys in CS, only four were hatchery-reared and none have been collected through annual monitoring of adult stocks of Snapper (Fairclough et al., 2025). Releasing hatchery-reared fish can be a successful way to increase the abundance of fish in wild stocks, but it can also fail without clear objectives, field trials and monitoring programs (Molony et al., 2003). For the Atlantic Cod (Gadus morhua), over 100 years of releasing larvae and juveniles failed to have the desired effects on stocks and was deemed uneconomical (Svåsand et al., 2000). Successes are usually most noticeable in closed/semi-closed systems such as lakes and estuaries or in situations where stocks are depleted. In the case of an over-exploited sparid Acanthopagrus butcheri in Blackwood Estuary, hatchery-reared fish dominated subsequent commercial catches and were still present after 16 years (Cottingham et al., 2020). Stocking was also suggested by Johnston et al. (2011) as a potential intervention if stocks of Blue Swimmer Crab did not fully recover following revised management measures, however, only experimental-scale releases have occurred to date and improved hatchery methods are required to increase survival prior to any commercial-scale releases (Jenkins et al., 2017).
Summary
The last hundred years of heavy utilisation mean CS has been substantially modified compared to pre-European settlement. Nevertheless, its environment remains diverse and ‘productive’ (e.g. social amenity, fisheries, aquaculture), despite some major and concerning ecological changes in recent decades (e.g. reduced productivity of the crab fishery, penguin population decline, fish kills and the appearance of invasive species). Management responses to these recent issues have included stricter fisheries controls, greater protection for threatened species, and increased research and monitoring. Detecting a regime shift within a system can take years (deYoung et al., 2008), however, considering how slow the crab fishery has been to recover during the most recent closure, the patchy success of seagrass replanting and seeding, and early signs of tropicalisation, there are a range of early signs. The research synthesized in this review is summarized into a conceptual model of the different environmental and anthropogenic pressures operating on the environment within CS (Figure 5). While capturing some of the key species across different trophic levels, this figure does not attempt to capture the full food web, but rather highlights some of the key pressures and responses, and how they are currently known to impact the system.
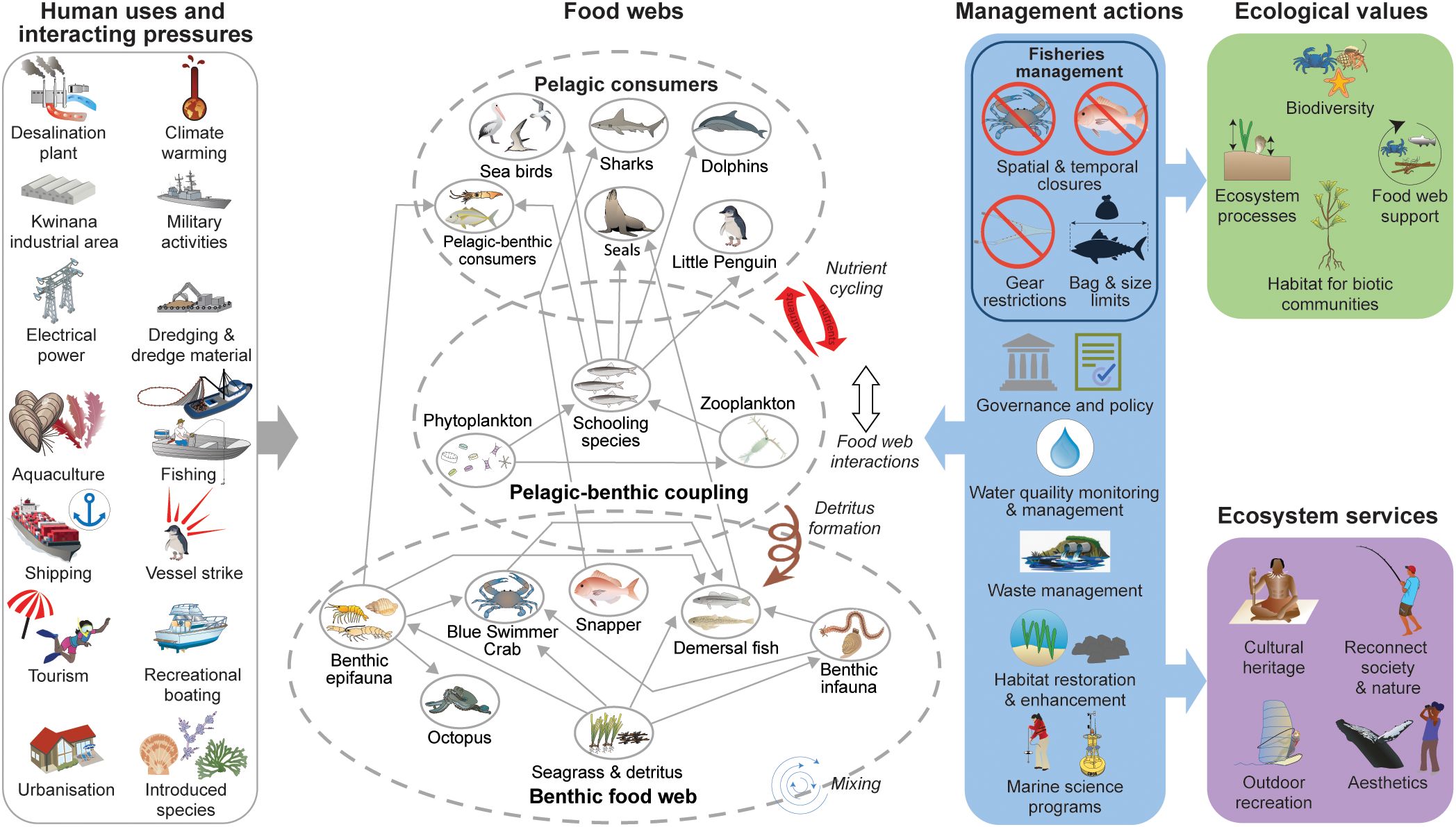
Figure 5. Conceptual model of Cockburn Sound, including the human uses and pressures impacting the food web, and the management responses that have been discussed in the current review.
Given the significant commercial, recreational, industrial and governmental interests in CS, it is likely that the management of CS will continue to provide significant challenges for regulatory bodies. This is further complicated by government policies that are administered by multiple departments without a holistic framework. Western Australia is not unique in this regard, with similar issues identified (and in part addressed) for other highly utilized coastal areas such as Sydney Harbour (Banks et al., 2016), San Francisco Bay (Cloern and Jassby, 2012) and Thames Estuary (Scarr, 2024). Even with a joined-up approach to management, the range of interacting pressures in CS (Figure 5), that may have cumulative effects on the environment require ever increasingly complex models for effective management (Elliott et al., 2017).
Previous large developments within CS, such as the construction of the causeway, shellsand dredging and the development of a desalination plant, have undertaken extensive monitoring. However, the cumulative impact of the multitude of interacting pressures, highlighted in Figure 5, remains poorly understood. The proposed large-scale port development in CS will have a range of potential immediate impacts during port construction, and longer-term impacts once the port is operational. While some of the impacts are foreseeable, such as the loss of habitat under the immediate footprint of the port, others may be less expected. For example, declining abundance and productivity of some species may be symptoms of a chronically-stressed system which has less resilience to further change. However, it is impossible to predict whether CS could be approaching a tipping point of ecological change, or whether the current communities will continue to display high tolerance.
Much of the data reviewed here were collected as part of one-off projects, with only a limited number of long-term monitoring programs being undertaken (e.g. summer water quality, some fishery stocks, and penguin populations). Adequately managing the environment of CS in the coming decades will likely require an expansion of fisheries-independent monitoring efforts, and a focus on a broader community of species and habitats than has historically been the case. Initial stages of the port development have included an extensive AU$13.5 million research program overseen by the Western Australian Marine Science Institution, to provide data for an environmental impact assessment. The collation of data across this program may address many knowledge gaps identified by the scientific community and stakeholders. However, it is also likely to raise new questions that will require future monitoring to answer, particularly in the context of cumulative anthropogenic and climatic pressures, ecosystem resilience and tipping points.
Author contributions
PM: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. DY: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. KK: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. CG: Writing – original draft, Writing – review & editing. SC: Writing – original draft, Writing – review & editing. DC: Writing – original draft, Writing – review & editing. GH: Writing – original draft, Writing – review & editing. DJ: Writing – original draft, Writing – review & editing. DF: Writing – original draft, Writing – review & editing. CW: Writing – original draft, Writing – review & editing. AC: Writing – original draft, Writing – review & editing. GJ: Writing – original draft, Writing – review & editing. JN: Writing – original draft, Writing – review & editing. MB: Writing – original draft, Writing – review & editing. HL: Visualization, Writing – original draft, Writing – review & editing. CS: Writing – original draft, Writing – review & editing. EC: Writing – original draft, Writing – review & editing. AT: Writing – original draft, Writing – review & editing. JP: Writing – original draft, Writing – review & editing. MM: Writing – original draft, Writing – review & editing. NL: Writing – original draft, Writing – review & editing. JT: Conceptualization, Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors thank the Department of Primary Industries and Regional Development, Murdoch University and Edith Cowan University for providing salaries and access to historical data and the scientific literature. This study was initiated following the commencement of the Western Australian Marine Science Institute (WAMSI) Westport Marine Science Program (https://wamsi.org.au/research/programs/wamsi-westport-marine-science-program/), which was funded by the Western Australian Government, Department of Transport. Symbols in Figures 2 and 5 courtesy of the Integration and Application Network (ian.umces.edu/symbols/).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1563654/full#supplementary-material
References
Adams D. H., Sebastian A., and Paperno R. (2022). Population decline of Gulf pipefish (Syngnathus scovelli) in a subtropical estuary: ecosystem changes and habitat loss. Mar. Biodiversity 52, 57. doi: 10.1007/s12526-022-01289-x
Afrifa-Yamoah E., Taylor S., Desfosses C. J., and Mueller U. A. (2021). Imputation of missing count data of recreational boat retrievals from remote camera surveys in the Perth Metropolitan region, Western Australia (Perth, Australia: Department of Primary Industries and Regional Development).
Allan S. J., O’Connell M. J., Harasti D., Klanten O. S., and Booth D. J. (2022). Space use by the endemic common (weedy) seadragon (Phyllopteryx taeniolatus): influence of habitat and prey. J. Fish Biol. 100, 175–183. doi: 10.1111/jfb.14931
Ammresh, Sherratt E., Thomson V. A., Lee M. S. Y., Dunstan N., Allen L., et al. (2023). Island Tiger Snakes (Notechis scutatus) gain a ‘head start’ in life: How both phenotypic plasticity and evolution underlie skull shape differences. Evolutionary Biol. 50, 111–126. doi: 10.1007/s11692-022-09591-z
Andrys J., Kala J., and Lyons T. J. (2017). Regional climate projections of mean and extreme climate for the southwest of Western Australia, (1970–1999 compared to 2030–2059). Climate Dynamics 48, 1723–1747. doi: 10.1007/s00382-016-3169-5
Aubret F., Bonnet X., Pearson D., and Shine R. (2005). How can blind tiger snakes (Notechis scutatus) forage successfully? Aust. J. Zoology 53, 283–288. doi: 10.1071/ZO05035
Banks J., Hedge L. H., Hoisington C., Strain E. M., Steinberg P. D., and Johnston E. L. (2016). Sydney Harbour: Beautiful, diverse, valuable and pressured. Regional Stud. Mar. Sci, 353–361. doi: 10.1016/j.rsma.2016.04.007
Barbier E. B., Hacker S. D., Kennedy C., Koch E. W., Stier A. C., and Silliman B. R. (2010). The value of estuarine and coastal ecosystem services. Ecol. Monogr. 81, 169–193. doi: 10.1890/10-1510.1
Beck M. W., Brumbaugh R. D., Airoldi L., Carranza A., Coen L. D., Crawford C., et al. (2011). Oyster reefs at risk and recommendations for conservation, restoration, and management. BioScience 61, 107–116. doi: 10.1525/bio.2011.61.2.5
Beck M. W., Heck K. L., Able K. W., Childers D. L., Eggleston D. B., Gillanders B. M., et al. (2001). The identification, conservation, and management of estuarine and marine nurseries for fish and invertebrates. BioScience 51, 633–641. doi: 10.1641/0006-3568(2001)051[0633:TICAMO]2.0.CO;2
Bejder M., Johnston D. W., Smith J., Friedlaender A., and Bejder L. (2016). Embracing conservation success of recovering humpback whale populations: Evaluating the case for downlisting their conservation status in Australia. Mar. Policy 66, 137–141. doi: 10.1016/j.marpol.2015.05.007
Bellchambers L., De Lestang S., Smith K. D., and Thomson A. W. (2006). Catch prediction for the blue swimmer crab (Portunus pelagicus) in Cockburn Sound, Western Australia. Bull. Mar. Sci. 79, 329–339. doi: 10.4027/BMECPCC.2010.06
Bice C. M., Furst D., Lamontagne S., Oliver R. L., Zampatti B. P., and Revill A. S. (2015). The influence of freshwater discharge on productivity, microbiota community structure and trophic dynamics in the Murray Estuary: evidence of freshwater derived trophic subsidy in the Sandy Sprat (Adelaide, South Australia: Goyder Institute for Water Research Technical Report Series).
BirdLife Australia (2023). Birdata. Available online at: https://birdata.birdlife.org.au/home (Accessed June 2 2023).
BMT (2018). Cockburn Sound-Drivers-Pressures-State-Impacts-Responses Assessment 2017 Final Report (Perth, Western Australia: BMT Western Australia Pty Ltd).
BMT Oceanica (2018). Perth metropolitan desalination plant, Cockburn Sound benthic macrofauna community and sediment habitat – Repeat survey 2013 (Perth, Australia: Oceanica Consulting Pty Ltd).
BOM (2024). Climate statistics for Australian locations (Melbourne, Victoria: Bureau of Meteorology). Available at: http://www.bom.gov.au/climate/averages/tables/cw_009021_All.shtml.
Bonnet X., Pearson D., Ladyman M., Lourdais O., and Bradshaw D. (2002). ‘Heaven’ for serpents? A mark–recapture study of tiger snakes (Notechis scutatus) on Carnac Island, Western Australia. Austral Ecol. 27, 442–450. doi: 10.1046/j.1442-9993.2002.01198.x
Botting G., Green B., and Rose T. (2009). Sounding out - a way forward: a journey towards facilitating multiple-use of Cockburn Sound and Owen Anchorage. Stage one. Parts I, II and III (Rockingham, Australia: Cockburn Sound Management Council).
Bourke D. and Chase S. (2009). Study of the contaminants in the waters of Cockburn Sound 2008 (Perth, Western Australia: Report to Cockburn Sound Management Council by PB).
Breheny N., Beckley L., and Wakefield C. (2012). Ichthyoplankton assemblages associated with pink snapper (Pagrus auratus) spawning aggregations in coastal embayments of southwestern Australia. J. R. Soc. Western Aust. 95, 103–114. doi: 10.1111/j.1574-6968.2012.02617.x
Cambridge M. L., Chiffings A. W., Brittan C., Moore L., and McComb A. J. (1986). The loss of seagrass in Cockburn Sound, Western Australia. II. Possible causes of seagrass decline. Aquat. Bot. 24, 269–285. doi: 10.1016/0304-3770(86)90062-8
Cambridge M. L. and Hocking P. J. (1997). Annual primary production and nutrient dynamics of the seagrasses Posidonia sinuosa and Posidonia australis in south-western Australia. Aquat. Bot. 59, 277–295. doi: 10.1016/S0304-3770(97)00062-4
Cambridge M. L., How J. R., Lavery P. S., and Vanderklift M. A. (2007). Retrospective analysis of epiphyte assemblages in relation to seagrass loss in a eutrophic coastal embayment. Mar. Ecol. Prog. Ser. 346, 97–107. doi: 10.3354/meps06993
Cambridge M. L. and McComb A. J. (1984). The loss of seagrasses in Cockburn Sound, Western Australia. I. The time course and magnitude of seagrass decline in relation to industrial development. Aquat. Bot. 20, 229–243. doi: 10.1016/0304-3770(84)90089-5
Campbell R., Holley D., Collins P., and Armstrong S. (2014). Changes in the abundance and distribution of the New Zealand fur seal (Arctocephalus forsteri) in Western Australia: are they approaching carrying capacity? Aust. J. Zoology 62, 261–267. doi: 10.1071/ZO14016
Campbell T. I., Tweedley J. R., Johnston D. J., and Loneragan N. R. (2021). Crab diets differ between adjacent estuaries and habitats within a sheltered marine embayment. Front. Mar. Sci. 8, 564695. doi: 10.3389/fmars.2021.564695
Cannell B. L. (2004). Distributions of the major marine fauna found in the Perth metropolitan area (Yanchep to Mandurah) (Perth, Western Australia: Marine Conservation Branch, Department of Conservation and Land Management).
Cannell B. L. (2018). Understanding the toll of consecutive years of warm waters on Little Penguins and refining their capacity as bioindicators of the marine coastal ecosystem. Report Year 2. Report prepared for the City of Rockingham and Fremantle Ports (Perth, Australia: Murdoch University).
Cannell B. L., Campbell K., Fitzgerald L., Lewis J. A., Baran I. J., and Stephens N. S. (2016). Anthropogenic trauma is the most prevalent cause of mortality in Little Penguins, Eudyptula minor, in Perth, Western Australia. Emu - Austral Ornithology 116, 52–61. doi: 10.1071/MU15039
Caputi N., Fletcher W., Pearce A., and Chubb C. (1996). Effect of the Leeuwin Current on the recruitment of fish and invertebrates along the Western Australian coast. Mar. Freshw. Res. 47, 147–155. doi: 10.1071/MF9960147
Caputi N., Jackson G., and Pearce A. (2014). The marine heat wave off Western Australia during the summer of 2010/11: 2 years on (Perth, Australia: Department of Fisheries, Western Australia).
Carter M. and Anderson R. (2010). Cockburn Sound’s World War II anti-submarine boom net: Historical background and site inspections (Perth, Australia: Department of Maritime Archaeology).
Chabanne D. B. H., Allen S. J., Sherwin W. B., Finn H., and Krützen M. (2021). Inconsistency between socio-spatial and genetic structure in a coastal dolphin population. Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.617540
Chabanne D. B. H., Finn H., and Bejder L. (2017a). Identifying the relevant local population for environmental impact assessments of mobile marine fauna. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00148
Chabanne D., Finn H., Salgado-Kent C., and Bedjer L. (2012). Identification of a resident community of bottlenose dolphins (Tursiops aduncus) in the Swan Canning Riverpark, Western Australia, using behavioural information. Pacific Conserv. Biol. 18, 247–262. doi: 10.1071/PC120247
Chabanne D. B. H., Pollock K. H., Finn H., and Bejder L. (2017b). Applying the multistate capture–recapture robust design to characterize metapopulation structure. Methods Ecol. Evol. 8, 1547–1557. doi: 10.1111/mee3.2017.8.issue-11
Chiffings A. W. (1979). Technical report on nutrient enrichment and phytoplankton (Perth, Australia: Department of Conservation and Environment).
Chittleborough R. G. (1965). Dynamics of two populations of the humpback whale, Megaptera novaeangliae (Borowski). Mar. Freshw. Res. 16, 33–128. doi: 10.1071/MF9650033
Clark G., Fischer M., and Hunter C. (2021). Australia state of the environment 2021: Coasts (Canberra, Australia: Independent report to the Australian Government Minister for the Environment, Commonwealth of Australia).
Cloern J. E. and Jassby A. D. (2012). Drivers of change in estuarine-coastal ecosystems: Discoveries from four decades of study in San Francisco Bay. Rev. Geophysics 50, RG4001. doi: 10.1029/2012RG000397
Collard L. and Bracknell C. (2012). Beeliar Boodjar: An introduction to Aboriginal history in the city of Cockburn, Western Australia. Aust. Aboriginal Stud. 1, 86–91.
Connolly R. M., Melville A. J., and Preston K. M. (2002). Patterns of movement and habitat use by leafy seadragons tracked ultrasonically. J. Fish Biol. 61, 684–695. doi: 10.1111/j.1095-8649.2002.tb00904.x
Cornell Lab of Ornithology (2023). Birds of the World. Available online at: https://birdsoftheworld.org/bow/home (Accessed June 2 2023).
Costa D. P. and Gales N. J. (2003). Energetics of a benthic diver: Seasonal foraging ecology of the Australian sea lion, Neophoca cinerea. Ecol. Monogr. 73, 27–43. doi: 10.1890/0012-9615(2003)073[0027:EOABDS]2.0.CO;2
Costanza R., D’arge R., De Groot R., Farber S., Grasso M., Hannon B., et al. (1997). The value of the world’s ecosystems services and natural capital. Nature 387, 253–260. doi: 10.1038/387253a0
Costello C., Springborn M., McAusland C., and Solow A. (2007). Unintended biological invasions: Does risk vary by trading partner? J. Environ. Economics Manage. 54, 262–276. doi: 10.1016/j.jeem.2007.06.001
Cottingham A., Bossie A., Valesini F., Tweedley J. R., and Galimany E. (2023). Quantifying the potential water filtration capacity of a constructed shellfish reef in a temperate hypereutrophic estuary. Diversity 15, 113. doi: 10.3390/d15010113
Cottingham A., Hall N. G., Loneragan N. R., Jenkins G. I., and Potter I. C. (2020). Efficacy of restocking an estuarineresident species demonstrated by long-term monitoring of cultured fish with alizarin complexone-stained otoliths. A case study. Fisheries Res. 227, 105556. doi: 10.1016/j.fishres.2020.105556
Coulson P., Leporati S., Chandler J., Hart A., and Caputi N. (2016). Determining the dynamics of WA squid populations through research and recreational fishing (Perth, Australia: Murdoch University).
Cowley P. D., Tweedley J. R., and Whitfield A. K. (2022). Conservation of estuarine fishes. Fish Fisheries Estuaries, 617–683. doi: 10.1002/9781119705345.ch10
Crain C. M., Halpern B. S., Beck M. W., and Kappel C. V. (2009). Understanding and managing human threats to the coastal marine environment. Ann. New York Acad. Sci. 1162, 39–62. doi: 10.1111/j.1749-6632.2009.04496.x
Crawley K. R. and Hyndes G. A. (2007). The role of different types of detached macrophytes in the food and habitat choice of a surf-zone inhabiting amphipod. Mar. Biol. 151, 1433–1443. doi: 10.1007/s00227-006-0581-0
Crawley K. R., Hyndes G. A., and Ayvazian S. G. (2006). Influence of different volumes and types of detached macrophytes on fish community structure in surf zones of sandy beaches. Mar. Ecol. Prog. Ser. 307, 233–246. doi: 10.3354/meps307233
Cresswell G. R. (1991). The Leeuwin Current- observations and recent models. J. R. Soc. Western Aust. 74, 1–15.
Crisafulli B. M., Fairclough D. V., Keay I. S., Lewis P., How J. R., Ryan K. L., et al. (2019). Does a spatiotemporal closure to fishing Chrysophrys auratus (Sparidae) spawning aggregations also protect individuals during migration? Ocean Tracking Network: Advancing Aquat. Res. Manage. 01, 1171–1185. doi: 10.1139/cjfas-2017-0449@cjfas-otn.issue01
CSCA (2013). Coastal Vulnerability Study: Erosion and inundation hazard assessment (Perth, Western Australia: Cockburn Sound Coastal Alliance).
CSMC (2016). Cockburn Sound Annual Environmental Monitoring Report 2015–2016 (Perth, Australia: Cockburn Sound Management Council).
CSMC (2018). State of Cockburn Sound Marine Area Report 2018 (Perth, Australia: Cockburn Sound Management Council).
CSMC (2020). “Cockburn Sound annual environmental monitoring report 2019-20,” in Assessment against the environmental quality objectives and criteria set in the State Environmental (Cockburn Sound) Policy (Cockburn Sound Management Council, Joondalup, Australia).
CSMC (2022). 2022 State of Cockburn Sound Marine Area Report (Joondalup, Australia: Cockburn Sound Management Council).
Dapson I. (2011). Assessing ecosystem recovery in transplanted Posidonia australis at Southern Flats, Cockburn Sound (Perth, Western Australia: Murdoch University).
Daviot J. R. (2017). The potential for Baited Remote Underwater Video to detect changes in fish communities caused by natural and human-induced events in Cockburn Sound (Murdoch University: Honours).
DCE (1979). Cockburn Sound Environmental Study 1976-1979. Report No. 2 (Perth, Western Australia: Department of Conservation and Environment).
Defeo O. and Elliott M. (2021). The ‘triple whammy’ of coasts under threat – Why we should be worried! Mar. Pollut. Bull. 163, 111832. doi: 10.1016/j.marpolbul.2020.111832
de Lestang S., Bellchambers L. M., Caputi N., Thomson A. W., Pember M. B., Johnston D. J., et al. (2010). “Stock-recruitment-environment relationship in a Portunus pelagicus fishery in Western Australia,” in Biology and Management of Exploited Crab Populations under Climate Change. Eds. Kruse G. H., Eckert G. L., Foy R. J., Lipcius R. N., Sainte-Marie B., Stram D. L., and Woodby D. (Alaska Sea Grant, University of Alaska, Fairbanks, USA).
Devaney D. M. (1978). The benthic fauna of Cockburn Sound, Western Australia, Part III: Echinodermata: Ophiuroida (Perth, Australia: Western Australian Museum).
deYoung B., Barange M., Beaugrand G., Harris R., Perry R. I., Scheffer M., et al. (2008). Regime shifts in marine ecosystems: detection, prediction and management. Trends Ecol. Evol. 23, 402–409. doi: 10.1016/j.tree.2008.03.008
Dias P. J., Lukehurst S. S., Simpson T., Rocha R. M., Tovar-Hernández M. A., Wellington C., et al. (2021). Multiple introductions and regional spread shape the distribution of the cryptic ascidian Didemnum perlucidum in Australia: an important baseline for management under climate change. Aquat. Invasions 16, 297–313. doi: 10.3391/ai.2021.16.2.06
DPIRD (2023). Didemnum vexillum (carpet sea squirt) infected sites. Guidance Document (Perth, Western Australia: Department of Primary Industries and Regional Development).
DPIRD (2024). Department of Primary Industries and Regional Development Annual Report 2023-24 (Perth, Western Australia: Department of Primary Industries and Regional Development).
Dunlop J. N. (1997). Foraging range, marine habitat and diet of Bridled Terns breeding in Western Australia. Corella 21, 77–82.
Dunlop J. N. and Greenwell C. N. (2021). Seasonal movements and metapopulation structure of the Australian fairy tern in Western Australia. Pacific Conserv. Biol. 27, 47–60. doi: 10.1071/PC20030
Dunlop J. N. and Storr G. M. (1981). Seabird islands: Carnac island, Western Australia. Corella 5, 71–74.
Elliott M., Burdon D., Atkins J. P., Borja A., Cormier R., de Jonge V. N., et al. (2017). And DPSIR begat DAPSI(W)R(M)!” - A unifying framework for marine environmental management. Mar. pollut. Bull. 118, 27–40. doi: 10.1016/j.marpolbul.2017.03.049
EPA (2015). State Environmental (Cockburn Sound) Policy 2015 (Perth, Western Australia: Environmental Protection Authority).
EPA (2017). Environmental quality criteria reference document for Cockburn Sound (Perth, Western Australia: Environmental Protection Authority).
Fairclough D. V. (2021). Partitioning of marine transition zone reefs among temperate, sub-tropical and tropical fishes is related more to depth and habitat than temperature. Mar. Ecol. Prog. Ser. 672, 175–192. doi: 10.3354/meps13778
Fairclough D. V., Beheregaray L. B., Sandoval-Castillo J., Vornicu D. E., Tweedley J., Cottingham A., et al. (2025). Snapper connectivity and evaluation of juvenile stocking. Prepared for the WAMSI Westport Marine Science Program Vol. 60 (Perth, Western Australia: Western Australian Marine Science Institution).
Feng M., Meyers G., Pearce A., and Wijffels S. (2003). Annual and interannual variations of the Leeuwin Current at 32°S. J. Geophysical Research: Oceans 108, 3355. doi: 10.1029/2002JC001763
Finn H. (2005). Conservation biology of bottlenose dolphins (Tursiops sp.) in Perth metropolitan waters (Perth, Western Australia: PhD, Murdoch University).
Finn H. and Calver M. C. (2008). Feeding aggregations of bottlenose dolphins and seabirds in Cockburn Sound, Western Australia. Western Aust. Nat. 26, 157–172.
Florisson J. H., Tweedley J. R., Walker T. H. E., and Chaplin J. A. (2018). Reef vision: A citizen science program for monitoring the fish faunas of artificial reefs. Fisheries Res. 206, 296–308. doi: 10.1016/j.fishres.2018.05.006
Fowler S. L., Costa D. P., Arnould J. P., Gales N. J., and Kuhn C. E. (2006). Ontogeny of diving behaviour in the Australian sea lion: trials of adolescence in a late bloomer. J. Anim. Ecol. 75, 358–367. doi: 10.1111/j.1365-2656.2006.01055.x
Fraser M. W. and Kendrick G. A. (2017). Belowground stressors and long-term seagrass declines in a historically degraded seagrass ecosystem after improved water quality. Sci. Rep. 7, 14469. doi: 10.1038/s41598-017-14044-1
Gaughan D., Craine M., Stephenson P., Leary T., and Lewis P. (2008). “Regrowth of pilchard (Sardinops sagax) stocks off southern WA following the mass mortality event of 1998/99,” in Final FRDC Report - Project 2000/135, Fisheries Research Report No. 176 (Perth, Western Australia: Western Australia Department of Fisheries).
Gaughan D. J. and Mitchell R. W. D. (2000). The biology and stock assessment of the tropical sardine, Sardinella lemuru, off the mid-west coast of Western Australia (Perth, Australia: Department of Fisheries, Western Australia).
Gaughan D., Neira F., Beckey L., and Potter I. (1990). Composition, seasonality and distribution of the ichthyoplankton in the lower Swan Estuary, south-western Australia. Mar. Freshw. Res. 41, 529–543. doi: 10.1071/MF9900529
Geldard J., Lowe R., Draper S., Ellwood E., Wood A., Roe T., et al. (2021). “Performance of engineered wave attenuating reef structures,” in Australasian Coasts & Ports 2021 Conference, Christchurch, 30 November – 3 December 2021 (Red Hook, New York: Curran Associates, Inc.).
Gibbs M. (2012). Whale catches from the 19th century shore stations in Western Australia. J. Cetacean Res. Manage. 12, 129–135. doi: 10.47536/jcrm.v12i1.599
Goldsworthy S. D. (2018). “Australian sea lion: Neophoca cinerea,” in Encyclopedia of Marine Mammals, 3rd ed. Eds. Würsig B., Thewissen J. G. M., and Kovacs K. M. (Academic Press). doi: 10.1016/B978-0-12-804327-1.00052-2
Goldsworthy S. D., Shaughnessy P. D., Mackay A. I., Bailleul F., Holman D., Lowther A. D., et al. (2021). Assessment of the status and trends in abundance of a coastal pinniped, the Australian sea lion Neophoca cinerea. Endangered Species Res. 44, 421–437. doi: 10.3354/esr01118
Grant M. L., Bond A. L., and Lavers J. L. (2022). The influence of seabirds on their breeding, roosting and nesting grounds: A systematic review and meta-analysis. J. Anim. Ecol. 91, 1266–1289. doi: 10.1111/1365-2656.13699
Green J. N. (2006). Survey of the Port Coogee development area (Perth, Australia: Department of Maritime Archaeology; Western Australian Museum, No. 213).
Greenwell C. N. and Dunlop J. N. (2023). Drivers of colony failure in a vulnerable coastal seabird, the Australian Fairy Tern (Sternula nereis nereis). Pacific Conserv. Biol, 490–502. doi: 10.1071/PC23001
Greenwell C. N., Tweedley J. R., Moore G. I., Lenanton R. C. J., Dunlop J. N., and Loneragan N. R. (2021). Feeding ecology of a threatened coastal seabird across an inner shelf seascape. Estuarine Coast. Shelf Sci. 263, 107627. doi: 10.1016/j.ecss.2021.107627
Greenwood J., Keesing J., Donn M., and McFarlane D. J. (2016). Nitrogen Budget for Cockburn Sound, Western Australia (Perth, Australia: Commonwealth Scientific and Industrial Research Organisation).
Hallett C. S., Hobday A. J., Tweedley J. R., Thompson P. A., McMahon K., and Valesini F. J. (2018). Observed and predicted impacts of climate change on the estuaries of south-western Australia, a Mediterranean climate region. Regional Environ. Change 18, 1357–1373. doi: 10.1007/s10113-017-1264-8
Halpern B. S., Frazier M., Afflerbach J., Lowndes J. S., Micheli F., O’Hara C., et al. (2019). Recent pace of change in human impact on the world’s ocean. Sci. Rep. 9, 11609. doi: 10.1038/s41598-019-47201-9
Halpern B. S., Walbridge S., Selkoe K. A., Kappel C. V., Micheli F., D’agrosa C., et al. (2008). A global map of human impact on marine ecosystems. Science 319, 948–952. doi: 10.1126/science.1149345
Ham G. S. (2009). Population biology of bottlenose dolphins (Tursiops sp.) in Cockburn Sound, Western Australia (Honours Murdoch University).
Hammond M., Bond T., Prince J., Hovey R. K., and McLean D. L. (2020). An assessment of change to fish and benthic communities following installation of an artificial reef. Regional Stud. Mar. Sci. 39, 101408. doi: 10.1016/j.rsma.2020.101408
Hardinson S. B., McGlathery K. J., and Castorani M. C. N. (2023). Effects of seagrass restoration on coastal fish abundance and diversity. Conserv. Biol. 37, e14147. doi: 10.1111/cobi.14147
Hart A. M., Murphy D., Harry A., and Fisher E. A. (2018). Resource Assessment Report Western Australian Octopus Resource (Perth, Australia: Department of Primary Industries and Regional Development).
He Q. and Silliman B. R. (2019). Climate change, human impacts, and coastal ecosystems in the anthropocene. Curr. Biol. 29, R1021–R1035. doi: 10.1016/j.cub.2019.08.042
Hecht M. K., Chaim K., and Hecht B. M. (1974). Distribution of the Yellow-Bellied Sea Snake, Pelamis platurus, and its significance in relation to the fossil record. Herpetologica 30, 387–396.
Hewitt C. L., Campbell M. L., Moore K. M., Murfet N. B., and McEnnulty F. (2000). Introduced species survey, Port of Fremantle and Cockburn Sound, Western Australia (Hobart, Australia: Centre for Research on Introduced Marine Pests).
Higgins P. J. and Davies S. J. (1996). Handbook of Australian, New Zealand & Antarctic birds Vol. 3 (Snipe to pigeons, Melbourne, Australia: Oxford University Press).
Hobday A. J., Oliver E. C. J., Sen Gupta A., Benthuysen J. A., Burrows M. T., Donat M. G., et al. (2018). Categorizing and naming marine heatwaves. Oceanography 31, 162–173. doi: 10.5670/oceanog.2018.205
Hobday A. J. and Pecl G. T. (2014). Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Rev. Fish Biol. Fisheries 24, 415–425. doi: 10.1007/s11160-013-9326-6
Hovey R. K. and Fraser M. W. (2018). Benthic habitat mapping of Cockburn Sound (Prepared for the Department of Environment Regulation and Fremantle Ports, Western Australia on behalf of the Cockburn Sound Management Council).
Hyndes G. A., Kendrick A. J., Macarthur L. D., and Stewart E. (2003). Differences in the species- and size-composition of fish assemblages in three distinct seagrass habitats with differing plant and meadow structure. Mar. Biol. 142, 1195–1206. doi: 10.1007/s00227-003-1010-2
Hyndes G. A. and Lavery P. S. (2005). Does transported seagrass provide an important trophic link in unvegetated, nearshore areas? Estuarine Coast. Shelf Sci. 63, 633–643. doi: 10.1016/j.ecss.2005.01.008
IUCN (2020). Final Report of the Sixth IMMA Workshop: Important Marine Mammal Area Regional Workshop for Australia-New Zealand and South East Indian Ocean (Perth, Western Australia: Marine Mammal Protected Areas Task Force).
Jakobs S. and Braccini M. (2019). Acoustic and conventional tagging support the growth patterns of grey nurse sharks and reveal their large-scale displacements in the west coast of Australia. Mar. Biol. 166, 150. doi: 10.1007/s00227-019-3594-1
Jenkins G., Michael R., and Tweedley J. R. (2017). Identifying future stock enhancement options for Blue Swimmer Crabs (Portunus armatus) (Perth, Australia: Recreational Fishing Initiatives Fund. Project 2015/09).
Jenner K. C. S., Jenner M. N. M., and McCabe K. A. (2001). Geographical and temporal movements of humpback whales in Western Australian waters. APPEA J. 2001, 749–765. doi: 10.1071/AJ00044
Johnston D., Harris D., Caputi N., and Thomson A. (2011). Decline of a Blue Swimmer Crab (Portunus pelagicus) fishery in Western Australia—History, contributing factors and future management strategy. Fisheries Res. 109, 119–130. doi: 10.1016/j.fishres.2011.01.027
Johnston D. J. and Yeoh D. E. (2021). Temperature drives spatial and temporal variation in the reproductive biology of the blue swimmer crab Portunus armatus A. Milne-Edwards 1861 (Decapoda: Brachyura: Portunidae). J. Crustacean Biol. 41, ruab032. doi: 10.1093/jcbiol/ruab032
Johnston D., Yeoh D., Harris D., Denham A., and Fisher E. (2020). Blue Swimmer Crab (Portunus armatus) Resource in the west coast bioregion, Western Australia. Part 1: Peel-Harvey Estuary, Cockburn Sound and Swan-Canning Estuary (Perth, Australia: Department of Primary Indistrues and Regional Development).
Kajtar J. B., Holbrook N. J., and Hernaman V. (2021). A catalogue of marine heatwave metrics and trends for the Australian region. J. South. Hemisphere Earth Syst. Sci. 71, 284–302. doi: 10.1071/ES21014
Kendrick G. A., Austin R., Ferretto G., van Keulen M., and Verduin J. J. (2025). Lessons learnt from revisiting decades of seagrass restoration projects in Cockburn Sound, southwestern Australia. Restor. Ecology, e70040. doi: 10.1111/rec.70040
Kendrick G. A., Aylward M. J., Hegge B. J., Cambridge M. L., Hillman K., Wyllie A., et al. (2002). Changes in seagrass coverage in Cockburn Sound, Western Australia between 1967 and 1999. Aquat. Bot. 73, 75–87. doi: 10.1016/S0304-3770(02)00005-0
Kendrick A. J. and Hyndes G. A. (2003). Patterns in the abundance and size-distribution of syngnathid fishes among habitats in a seagrass-dominated marine environment. Estuarine Coast. Shelf Sci. 57, 631–640. doi: 10.1016/S0272-7714(02)00402-X
Kendrick A. J. and Hyndes G. A. (2005). Variations in the dietary compositions of morphologically diverse syngnathid fishes. Environ. Biol. Fishes 72, 415–427. doi: 10.1007/s10641-004-2597-y
King C. (2014). Investigating the movement patterns of sharks and the significance of potential shark predation attempts on bottlenose dolphins (Tursiops aduncus) in the waters of south-western Australia (Honours Murdoch University).
Ladyman M., Seubert E., and Bradshaw D. (2020). The origin of tiger snakes on Carnac Island. J. R. Soc. Western Aust. 103, 39–42.
Lemm A. J., Hegge B. J., and Masselink G. (1999). Offshore wave climate, Perth (Western Australia), 1994-96. Mar. Freshw. Res. 50, 95–102. doi: 10.1071/MF98081
Lenanton R. C., Caputi N., Kangas M., and Craine M. (2009). The ongoing influence of the Leeuwin Current on economically important fish and invertebrates off temperate Western Australia - has it changed? J. R. Soc. Western Aust. 92, 111–127.
Lenanton R. C. J., Dowling C. E., Smith K. A., Fairclough D. V., and Jackson G. (2017). Potential influence of a marine heatwave on range extensions of tropical fishes in the eastern Indian Ocean—Invaluable contributions from amateur observers. Regional Stud. Mar. Sci. 13, 19–31. doi: 10.1016/j.rsma.2017.03.005
Lettoof D. C., Aubret F., and von Takach B. (2024). Evidence for a natural population of tiger snakes (Notechis scutatus) on Carnac Island. J. R. Soc. Western Australia. 107, 31–35. doi: 10.70880/001c.126009
MacLachlan I. (2013). Kwinana Industrial Area: agglomeration economies and industrial symbiosis on Western Australia’s Cockburn Sound. Aust. Geographer 44, 383–400. doi: 10.1080/00049182.2013.852505
Marchant S. and Higgins P. J. (1990). Handbook of Australian, New Zealand & Antarctic birds Vol. 1 (Ratites to ducks, Melbourne, Australia: Oxford University Press).
Marks R., Hesp S. A., Denham A., Loneragan N. R., Johnston D., and Hall N. (2021). Factors influencing the dynamics of a collapsed blue swimmer crab (Portunus armatus) population and its lack of recovery. Fisheries Res. 242, 106035. doi: 10.1016/j.fishres.2021.106035
Marsh L. M. (1978a). The benthic fauna of Cockburn Sound, Western Australia, Part II: Coelenterata (Perth, Australia: Western Australian Museum).
Marsh L. M. (1978b). The benthic fauna of Cockburn Sound, Western Australia, Part IV: Echinodermata; Crinoidea, Asteroidea, Echinoidea and Holothuroidea (Perth, Australia: Western Australian Museum).
Masselink G. and Pattiaratchi C. B. (2001). Characteristics of the sea breeze system in Perth, Western Australia, and its effect on the nearshore wave climate. J. Coast. Res. 17, 173–187.
Mawson P. R. and Coughran D. K. (1999). Records of sick, injured and dead pinnipeds in Western Australia 1980-1996. J. R. Soc. Western Aust. 82, 121–128.
McAuley R. B., Bruce B. D., Keay I. S., Mountford S., Pinnell T., and Whoriskey F. G. (2017). Broad-scale coastal movements of white sharks off Western Australia described by passive acoustic telemetry data. Mar. Freshw. Res. 68, 1518–1531. doi: 10.1071/MF16222
McDonald J. I. and Wells F. E. (2009). Results of a 2007 survey of the Swan River region for four introduced marine species (Perth, Australia: Department of Fisheries, Western Australia).
McIlroy J. (1986). Bathers Bay whaling station, Fremantle, Western Australia. Aust. J. Historical Archaeology 4, 43–50.
McKechnie S. (2006). Biopedturbation by an island ecosystem engineer: Burrowing volumes and litter deposition by sooty shearwaters (Puffinus griseus). New Z. J. Zoology 33, 259–265. doi: 10.1080/03014223.2006.9518455
Mellbrand K., Lavery P. S., Hyndes G., and Hambäck P. A. (2011). Linking land and sea: Different pathways for marine subsidies. Ecosystems 14, 732–744. doi: 10.1007/s10021-011-9442-x
Millington P. (1990). Long term management measures for the Cockburn Sound restricted entry fisheries (Perth, Australia: Fisheries Department Western Australia).
Molony B. W., Lenanton R., Jackson G., and Norriss J. (2003) Stock enhancement as a fisheries management tool. Reviews in Fish Biology and Fisheries. 13 (4), 409–432. Available online at: https://link.springer.com/content/pdf/10.1007/s11160-004-1886-z.pdf.
Montelli L. (2010). Non-Indigenous marine species (NIMS) in biofouling on RAN vessels: Threat analysis (Fishermans Bend, Victoria: Maritime Platforms Division).
Montelli L. and Lewis J. (2008). Survey of biofouling on Australian Navy ships: Crustacea; Isopdoda and Amphipoda; Caprellidea (Fishermans Bend, Victoria: Maritime Platforms Division).
Moore A., Schirmer J., Magnusson A., Keller K., Hinten G., Galeano D., et al. (2023). National Social and Economic Survey of Recreational Fishers 2018-2021 (Canberra: Australian Capital Territory).
Moredoundt N. and Kendall S. (2012). Cockburn Coast Cultural Heritage Strategy (Perth, Australia: Town Planning Urban Design and Heritage).
Murray D. C., Bunce M., Cannell B. L., Oliver R., Houston J., White N. E., et al. (2011). DNA-based faecal dietary analysis: A comparison of qPCR and high throughput sequencing approaches. PloS One 6, e25776. doi: 10.1371/journal.pone.0025776
Neira F. J., Potter I. C., and Bradley J. S. (1992). Seasonal and spatial changes in the larval fish fauna within a large temperate Australian estuary. Mar. Biol. 112, 1–16. doi: 10.1007/BF00349721
Newman S. J., Wise B. S., Santoro K. G., and Gaughan D. J. (Eds.) (2023). Status Reports of the Fisheries and Aquatic Resources of Western Australia 2021/22: The State of the Fisheries (Western Australia: Department of Primary Industries and Regional Development).
Nicholson K., Loneragan N., Finn H., and Bejder L. (2021). Social, spatial and isotopic niche partitioning identify an estuarine community of bottlenose dolphins as a discrete management unit. Aquat. Conservation: Mar. Freshw. Ecosyst. 31, 3526–3542. doi: 10.1002/aqc.3736
Obregón C., Hughes M., Loneragan N. R., Poulton S. J., and Tweedley J. R. (2020). A two-phase approach to elicit and measure beliefs on management strategies: Fishers supportive and aware of trade-offs associated with stock enhancement. Ambio 49, 640–649. doi: 10.1007/s13280-019-01212-y
Osterrieder S. K., Salgado Kent C., and Robinson R. (2016). Responses of Australian sea lions, Neophoca cinerea, to anthropogenic activities in the Perth metropolitan area, Western Australia. Aquat. Conservation: Mar. Freshw. Ecosyst. 27, 414–435. doi: 10.1002/aqc.2668
Partridge G. J., Ginbey B. M., Woolley L. D., Fairclough D. V., Crisafulli B., Chaplin J., et al. (2017). Development of techniques for the collection and culture of wild-caught fertilised snapper (Chrysophrys auratus) eggs for stock enhancement purposes. Fisheries Res. 186, 524–530. doi: 10.1016/j.fishres.2016.08.025
Pattiaratchi C., Lavery P., Wyllie A., and Hick P. (1994). Estimates of water quality in coastal waters using multi-date Landsat Thematic Mapper data. Int. J. Remote Sens. 15, 1571–1584. doi: 10.1080/01431169408954192
Pearce A. and Feng M. (2007). Observations of warming on the Western Australian continental shelf. Mar. Freshw. Res. 58, 914–920. doi: 10.1071/MF07082
Pearce A., Hutchins B., Hoschke A., and Fearns P. (2016). Record high damselfish recruitment at Rottnest Island, Western Australia, and the potential for climate-induced range extension. Regional Stud. Mar. Sci. 8, 77–88. doi: 10.1016/j.rsma.2016.09.009
Pearson D., Shine R., and Williams A. (2005). Spatial ecology of a threatened python (Morelia spilota imbricata) and the effects of anthropogenic habitat change. Austral Ecol. 30, 261–274. doi: 10.1111/j.1442-9993.2005.01462.x
Penn J. W. (1977). Trawl caught fish and crustaceans from Cockburn Sound (Perth, Australia: Department of Fisheries and Wildlife, Western Australia).
Pickering H. and Whitmarsh D. (1997). Artificial reefs and fisheries exploitation: a review of the ‘attraction versus production’ debate, the influence of design and its significance for policy. Fisheries Res. 31, 39–59. doi: 10.1016/S0165-7836(97)00019-2
Plaskett D. and Potter I. C. (1979). Heavy metal concentrations in the muscle tissue of 12 species of teleost from Cockburn Sound, Western Australia. Aust. J. Mar. Freshw. Res. 30, 607–616. doi: 10.1071/MF9790607
Platell M. E. and Potter I. C. (2001). Partitioning of food resources amongst 18 abundant benthic carnivorous fish species in marine waters on the lower west coast of Australia. J. Exp. Mar. Biol. Ecol. 261, 31–54. doi: 10.1016/S0022-0981(01)00257-X
Poh B., Tweedley J. R., Chaplin J. A., Trayler K. M., and Loneragan N. R. (2018). Estimating predation rates of restocked individuals: The influence of timing-of-release on metapenaeid survival. Fisheries Res. 198, 165–179. doi: 10.1016/j.fishres.2017.09.019
Potter I. C., Warwick R. M., Hall N. G., and Tweedley J. R. (2015). “The physico-chemical characteristics, biota and fisheries of estuaries,” in Freshwater Fisheries Ecology. Ed. Craig J. (Wiley-Blackwell).
Prince R. I. T. (2004). Stranding of small juvenile leatherback turtle in Western Australia. Mar. Turtle Newslett. 104, 3–4.
Ramm L., Florisson J. H., Watts S. L., Becker A., and Tweedley J. R. (2021). Artificial reefs in the Anthropocene: A review of geographical and historical trends in their design, purpose, and monitoring. Bull. Mar. Sci. 97, 699–728. doi: 10.5343/bms.2020.0046
Red Map Australia (2023). Available online at: https://www.redmap.org.au/ (Accessed July 23 2023).
Reimann L., Vafeidis A. T., and Honsel L. E. (2023). Population development as a driver of coastal risk: Current trends and future pathways. Cambridge Prisms: Coast. Futures 1, e14. doi: 10.1017/cft.2023.3
Rippey E. (2015). “Change over time on the Shoalwater Islands,” in Natural history and management of the Shoalwater Islands and Marine Park. Point Peron Camp School (Department of Parks and Wildlife), 10–13. doi: 10.1002/aqc.3736
Rippey E., Rippey J. J., and Dunlop J. N. (2002). Increasing numbers of pied cormorants breeding on the islands off Perth, Western Australia and consequences for the vegetation. Corella 26, 61–64.
Robson B. J., Bukaveckas P. A., and Hamilton D. P. (2008). Modelling and mass balance assessments of nutrient retention in a seasonally-flowing estuary (Swan River Estuary, Western Australia). Estuarine Coast. Shelf Sci. 76, 282–292. doi: 10.1016/j.ecss.2007.07.009
Robson N. A., Hetzel Y., Whiting S., Wijeratne S., Pattiaratchi C. B., Withers P., et al. (2017). Use of particle tracking to determine optimal release dates and locations for rehabilitated neonate sea turtles. Front. Mar. Sci. 4. doi: 10.3389/fmars.2017.00173
Rogers A. A., Burton M. P., Statton J., Fraser M., Kendrick G., Sinclair E., et al. (2019). Benefits and costs of alternate seagrass restoration approaches (Report to the National Environmental Science Programme, Marine Biodiversity Hub).
Roque B. M., Salwen J. K., Kinley R., and Kebreab E. (2019). Inclusion of Asparagopsis armata in lactating dairy cows’ diet reduces enteric methane emission by over 50 percent. J. Cleaner Production 234, 132–138. doi: 10.1016/j.jclepro.2019.06.193
Roque B. M., Venegas M., Kinley R. D., de Nys R., Duarte T. L., Yang X., et al. (2021). Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PloS One 16, e0247820. doi: 10.1371/journal.pone.0247820
Rose T. H., Smale D. A., and Botting G. (2012). The 2011 marine heat wave in Cockburn Sound, southwest Australia. Ocean Sci. 8, 545–550. doi: 10.5194/os-8-545-2012
Ryan K. L., Lai E. K. M., and Smallwood C. B. (2022). Boat-based recreational fishing in Western Australia 2020/21 (Perth, Australia: Department of Primary Industries and Regional Development).
Salgado Kent C. P., Burton C., Giroud M., and Elsdon B. (2022). A photo-identification study of southern right whales to update aggregation area classification in the southwest of Australia (Perth, Australia: Report to the National Environmental Science Program. Edith Cowan University).
Salgado Kent C. P. and D’Cruz A. (2021). Conservation management recommendations relating to potential impacts of human disturbance on endangered Australian sea lions in the Perth Metropolitan area, Western Australia (Perth, Australia: Department of Biodiversity, Conservation, and Attractions, Western Australia).
Salgado Kent C., Jenner C., Jenner M. N., Bouchet P., and Rexstad E. (2012). Southern Hemisphere Breeding Stock D humpback whale population estimates from North West Cape, Western Australia. J. Cetacean Res. Manage. 12, 29–38. doi: 10.47536/jcrm.v12i1.588
Sampey A., Fromont J., and Johnston D. J. (2011). Demersal and epibenthic fauna in a temperate marine embayment, Cockburn Sound, Western Australia: determination of key indicator species. J. R. Soc. Western Aust. 94, 1–18.
Scarr A. M. (2024). Is English estuarine environmental management fit for purpose, and if not, what needs to be done? PhD Thesis (United Kingdom: University of London). 274.
Shokri M. R., Gladstone W., and Jelbart J. (2009). The effectiveness of seahorses and pipefish (Pisces: Syngnathidae) as a flagship group to evaluate the conservation value of estuarine seagrass beds. Aquat. Conservation: Mar. Freshw. Ecosyst. 19, 588–595. doi: 10.1002/aqc.1009
Sievers M., Rasmussen J. A., Nielsen B., Steinfurth R. C., Flindt M. R., Melvin S. D., et al. (2024). Restored seagrass rapidly provides high-quality habitat for mobile animals. Restor. Ecol. 33, e14343. doi: 10.1111/rec.14343
Silva L. G. M., Doyle K. E., Duffy D., Humphries P., Horta A., and Baumgartner L. J. (2020). Mortality events resulting from Australia’s catastrophic fires threaten aquatic biota. Global Change Biol. 26, 5345–5350. doi: 10.1111/gcb.v26.10
Simpfendorfer C. A., Goodreid A., and McAuley R. B. (2001). Diet of three commercially important shark species from Western Australian waters. Mar. Freshw. Res. 52, 975–985. doi: 10.1071/MF01017
Simpson M., Morris R. L., Harasti D., and Coleman R. A. (2021). Swimming nets have positive effects on populations of the endangered White’s seahorse Hippocampus whitei. Aquat. Conservation: Mar. Freshw. Ecosyst. 31, 60–73. doi: 10.1002/aqc.3451
Sinclair E. A., Sherman C. D. H., Statton J., Copeland C., Matthews A., Waycott M., et al. (2021). Advances in approaches to seagrass restoration in Australia. Ecol. Manage. Restor. 22, 10–21. doi: 10.1111/emr.12452
Sinclair E. A., Verduin J., Krauss S. L., Hardinge J., Anthony J., and Kendrick G. A. (2013). A genetic assessment of a successful seagrass meadow (Posidonia australis) restoration trial. Ecol. Manage. Restor. 14, 68–71. doi: 10.1111/emr.2013.14.issue-1
Skene D., Ryan D., Brooke B., Smith J., and Radke L. (2005). The geomorphology and sediments of Cockburn Sound (Canberra, Australia: Geoscience Australia).
Smale D. A. and Childs S. (2012). The occurrence of a widespread marine invader, Didemnum perlucidum (Tunicata, Ascidiacea) in Western Australia. Biol. Invasions 14, 1325–1330. doi: 10.1007/s10530-011-0167-8
Smit A., Brearley A., Hyndes G., and Lavery P. (1998). “Shellsand and dredging environmental management programme,” in Project S1: Ecological significance of seagrass. Task 11: trophic structure and linkages. Final report (Prepared for Cockburn Cement Limited).
Smit A. J., Brearley A., Hyndes G. A., Lavery P. S., and Walker D. I. (2005). Carbon and nitrogen stable isotope analysis of an Amphibolis griffithii seagrass bed. Estuarine Coast. Shelf Sci. 65, 545–556. doi: 10.1016/j.ecss.2005.07.002
Smit A. J., Brearley A., Hyndes G. A., Lavery P. S., and Walker D. I. (2006). δ15N and δ13C analysis of a Posidonia sinuosa seagrass bed. Aquat. Bot. 84, 277–282. doi: 10.1016/j.aquabot.2005.11.005
Smith K., Dowling C., Mountford S., Hesp A., Howard A., and Brown J. (2017). Status of southern garfish (Hyporhamphus melanochir) in Cockburn Sound, Western Australia (Perth, Australia: Department of Fisheries, Western Australia).
Smith K. and Grounds G. (2020). “West coast nearshore and estuarine finfish resource status report,” in Status reports of the fisheries and aquatic resources of Western Australia 2018/19: The State of the Fisheries. Eds. Gaughan D. J. and Santoro K. G. (Department of Primary Industries and Regional Development, Perth, Australia).
Smith J. N., Jones D., Travouillon K., Kelly N., Double M., and Bannister J. L. (2021). Monitoring population dynamics of ‘Western’ Right Whales off southern Australia 2018-2021 (Western Australian Museum: National Environmental Science Program, Marine Biodiversity Hub).
Sommerville E., Platell M. E., White W. T., Jones A. A., and Potter I. C. (2011). Partitioning of food resources by four abundant, co-occurring elasmobranch species: relationships between diet and both body size and season. Mar. Freshw. Res. 62, 54–65. doi: 10.1071/MF10164
Spencer T., Adams J., Le Tissier M., Murray A. B., and Splinter K. (2023). Coastal futures: new framings, many questions, some ways forward. Cambridge Prisms: Coast. Futures e32, 1–11. doi: 10.1017/cft.2023.22
Steedman R. K. and Craig P. D. (1983). Wind-driven circulation of Cockburn sound. Mar. Freshw. Res. 34, 187–212. doi: 10.1071/MF9830187
Stockwell S., Greenwell C. N., Dunlop J. N., and Loneragan N. R. (2021). Distribution and foraging by non-breeding Caspian Terns on a large temperate estuary of south-western Australia–preliminary investigations. Pacific Conserv. Biol. 28, 48–56. doi: 10.1071/PC20082_CO
Strydom S., McCallum R., Lafratta A., Webster C. L., O’Dea C. M., Said N. E., et al. (2023). Global dataset on seagrass meadow structure, biomass and production. Earth Syst. Sci. Data 15, 511–519. doi: 10.5194/essd-15-511-2023
Sumner N. and Lai E. (2012). Boat-based Recreational Fishing Catch and Effort in Cockburn Sound and Owen Anchorage during 1996/97, 2001/02 and 2005/06 (Perth, Australia: Department of Fisheries, Western Australia).
Sutton A. (2022). Conceptual population model and knowledge review for Western Australian little penguin populations Vol. 48 (Western Australia: Report prepared for the Department of Biodiversity, Conservation and Attractions).
Sutton A. L. and Shaw J. L. (2019). Literature review and preliminary risk assessment of the marine environment for the Westport Port and Environs Strategy (Perth, Australia: Western Australian Marine Science Institution).
Svåsand T., Kristiansen T. S., Pedersen T., Salvanes A. G. V., Engelsen R., Nævdal G., et al. (2000). The enhancement of cod stocks. Fish Fisheries 1, 173–205. doi: 10.1046/j.1467-2979.2000.00017.x
Talbot V. and Chegwidden A. (1982). Cadmium and other heavy metal concentrations in selected biota from Cockburn Sound, Western Australia. Aust. J. Mar. Freshw. Res. 33, 779–788. doi: 10.1071/MF9820779
Tate A. C., Rudd. L. J., Smallwood C. B., and Ryan K. L. (2024). Metropolitan Monitoring Program 2023 (Perth, Western Australia: Department of Primary Industries and Reional Development).
Tate A. C., Smallwood C. B., and Ryan K. L. (2025). Metropolitan Monitoring Program 2024 (Perth, Western Australia: Department of Primary Industries and Regional Development).
Tinsley D. (1998). “The Thames estuary: a history of the impact of humans on the environment and a description of the current approach to environmental management,” in A Rehabilitated Estuarine Ecosystem: The environment and ecology of the Thames Estuary. Ed. Attrill M. J. (Springer US, Boston, Massachusetts).
Tull M. (1985). The development of the Port of Fremantle, Australia’s western gateway. Great Circle 7, 116–138.
Tweedley J. R., Obregón C., Beukes S. J., Loneragan N. R., and Hughes M. (2023). Selecting from the fisheries managers’ tool-box: recreational fishers’ views of stock enhancement and other management options. Fishes 8, 460. doi: 10.3390/fishes8090460
Valesini F. J., Potter I. C., and Clarke K. R. (2004). To what extent are the fish compositions at nearshore sites along a heterogeneous coast related to habitat type? Estuarine Coast. Shelf Sci. 60, 737–754. doi: 10.1016/j.ecss.2004.03.012
Valesini F. J. and Tweedley J. R. (2015). Effect of the Becher Point boat ramp on the food resources of Little Penguins on Penguin Island (Perth, Western Australia: Murdoch University).
Wakefield C. B. (2010). Annual, lunar and diel reproductive periodicity of a spawning aggregation of snapper Pagrus auratus (Sparidae) in a marine embayment on the lower west coast of Australia. J. Fish Biol. 77, 1359–1378. doi: 10.1111/j.1095-8649.2010.02756.x
Wakefield C. B., Johnston D. J., Harris D. C., and Lewis P. (2009). A preliminary investigation of the potential impacts of the proposed Kwinana Quay development on the commercially and recreationally important fish and crab species in Cockburn Sound (Perth, Australia: Western Australian Department of Fisheries).
Wakefield C. B., Lewis P. D., Coutts T. B., Fairclough D. V., and Langlois T. J. (2013). Fish assemblages associated with natural and anthropogenically-modified habitats in a marine embayment: Comparison of baited videos and opera-house traps. PloS One 8, e59959. doi: 10.1371/journal.pone.0059959
Walker D. I., Denninson W., and Edgar G. J. (1999). “Status of Australian seagrass research and knowledge,” in Seagrass in Australia: Strategic Review and Development of an R & D Plan. Eds. Butler A. and Jernakoff P. (CSIRO Publishing, Melbourne, Australia).
Waltham N. J., Elliott M., Lee S. Y., Lovelock C., Duarte C. M., Buelow C., et al. (2020). UN decade on ecosystem restoration 2021–2030—What chance for success in restoring coastal ecosystems? Front. Mar. Sci. 7, 71. doi: 10.3389/fmars.2020.00071
Water Corporation (2019). Perth Seawater Desalination Plant 2 - Referal. Environmental Review Document (Perth, Australia: Water Corporation).
Waycott M., Duarte C. M., Carruthers T. J. B., Orth R. J., Dennison W. C., Olyarnik S., et al. (2009). Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. 106, 12377–12381. doi: 10.1073/pnas.0905620106
Wells F. E. and Threlfall T. J. (1980). A survey of the soft bottom molluscs of Cockburn Sound, Western Australia. Veliger 23, 131–140.
Whisson G. and Hoschke A. (2021). The Perth Coast Fish Book Identification Guide: Mandurah to Two Rocks (Fremantle, Australia: Aqua Research And Monitoring).
Whitehead P. J. P. (1985). FAO species catalogue. Vol. 7. Clupeoid fishes of the world (suborder Clupeoidei). An annotated and illustrated catalogue of the herrings, sardines, pilchards, sprats, shads, anchovies and wolf-herrings, vol. 125. (FAO (Food and Agriculture Organization of the United Nations) Fisheries Synopsis), 1–303.
Wildsmith M. D., Potter I. C., Valesini F. J., and Platell M. E. (2005). Do the assemblages of the benthic macroinvertebrates in nearshore waters of Western Australia vary among habitat types, zones and seasons? J. Mar. Biol. Assoc. United Kingdom 85, 217–232. doi: 10.1017/S0025315405011100h
Wilson B. R., Kendrick G. W., and Brearley A. (1978). The benthic fauna of Cockburn Sound, Western Australia, Part I: Prosobranch Gastropod & Bivalve Molluscs (Perth, Australia: Western Australian Museum).
Xiao R., Gao G., Feng M., Greenwood J., Keesing J., Yin B., et al. (2022). Three-dimensional numerical simulation of circulation and vertical temperature structure during summer in Cockburn Sound. Regional Stud. Mar. Sci. 51, 102187. doi: 10.1016/j.rsma.2022.102187
Yeoh D., Johnston D., and Harris D. (2021). Squid and cuttlefish resources of Western Australia (Perth, Australia: Department of Primary Industries and Regional Development).
York P. H., Hyndes G. A., Bishop M. J., and Barnes R. S. K. (2018). “Faunal assemblages of seagrass ecosystems,” in Seagrasses of Australia: Structure, Ecology and Conservation. Eds. Larkum A. W. D., Kendrick G. A., and Ralph P. J. (Springer International Publishing, Cham).
Young E. J. (2022). Health and Disease Status of Sea Turtles in Western Australia (PhD, Murdoch University).
Keywords: anthropogenic development, biodiversity, ecosystem change, eutrophication, fisheries, industry, management, restoration
Citation: Mitchell PJ, Yeoh DE, Krispyn KN, Greenwell CN, Cronin-O’Reilly S, Chabanne DBH, Hyndes GA, Johnston D, Fairclough DV, Wellington C, Cottingham A, Jackson G, Norriss JV, Braccini M, Lozano-Montes H, Salgado Kent CP, Clitheroe E, Tate A, Penn JW, Massam M, Loneragan NR and Tweedley JR (2025) Ecological resources of a heavily modified and utilised temperate coastal embayment: Cockburn Sound. Front. Mar. Sci. 12:1563654. doi: 10.3389/fmars.2025.1563654
Received: 20 January 2025; Accepted: 09 May 2025;
Published: 16 June 2025.
Edited by:
Peng Zhang, Guangdong Ocean University, ChinaReviewed by:
Savanna Barry, University of Florida, United StatesMatheus De Barros, Dauphin Island Sea Lab, United States
Copyright © 2025 Mitchell, Yeoh, Krispyn, Greenwell, Cronin-O’Reilly, Chabanne, Hyndes, Johnston, Fairclough, Wellington, Cottingham, Jackson, Norriss, Braccini, Lozano-Montes, Salgado Kent, Clitheroe, Tate, Penn, Massam, Loneragan and Tweedley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peter J. Mitchell, UGV0ZXIuTWl0Y2hlbGxAZHBpcmQud2EuZ292LmF1
†These authors have contributed equally to this work and share first authorship
 Peter J. Mitchell
Peter J. Mitchell Daniel E. Yeoh1†
Daniel E. Yeoh1† Kurt N. Krispyn
Kurt N. Krispyn Delphine B.H. Chabanne
Delphine B.H. Chabanne Glenn A. Hyndes
Glenn A. Hyndes Danielle Johnston
Danielle Johnston David V. Fairclough
David V. Fairclough Claire Wellington
Claire Wellington Alan Cottingham
Alan Cottingham Gary Jackson
Gary Jackson Hector Lozano-Montes
Hector Lozano-Montes Chandra P. Salgado Kent
Chandra P. Salgado Kent Erin Clitheroe
Erin Clitheroe Alissa Tate
Alissa Tate Neil R. Loneragan
Neil R. Loneragan James R. Tweedley
James R. Tweedley