- 1Department of Marine Technology, Norwegian University of Science and Technology, Trondheim, Norway
- 2Washington State Department of Ecology, Lacy, WA, United States
- 3Department of Fisheries and New Biomarine Industry, SINTEF Ocean, Trondheim, Norway
- 4Department of Biology, Norwegian University of Science and Technology, Trondheim, Norway
Both the mainstream media and fisheries industry publications have documented extensive contention over the relatively novel commercial fishery for the planktonic copepod Calanus finmarchicus (Calanus) in Norway. Opposition to the fishery is concentrated among coastal cod and herring fishers, in part due to concerns about bycatch of fish eggs and larvae. Here we report results from a scenario-based experiment embedded in a survey of those fishers (n=184). We tested whether the introduction of technologically-enabled real-time bycatch management, either through onboard sampling, underwater imaging, or environmental DNA, would increase support for, or trust in management of, the Calanus fishery versus a control. We find that deployment of underwater imaging increases trust in Calanus management; however, no treatment increases support, which remains very low. Open-ended rationales for self-reported levels of trust indicate that potential ecosystem effects of fishing the bottom of the food web, and mismatched values between fishers and managers, may be of more concern to our sample than the possibility of bycatch.
1 Introduction
Norway is an ocean-based economy, with significant income from oil and gas, maritime transport, and seafood, and the government has expressed the ambition to continue to grow these sectors (Norwegian Ministry of Trade, Industry and Fisheries, 2019). The Norwegian government has pursued ecosystem-based management of its extensive ocean jurisdictions since the early 2000s and began developing integrated ocean management plans in the mid- to late-2000s (Fasoulis, 2021). Nonetheless, marine and coastal use conflicts continue to occur, especially between wild-capture fisheries and aquaculture (Bailey and Eggereide, 2020; Bergh et al., 2023) or offshore energy (Arbo and Thủy, 2016; Knol-Kauffman et al., 2023).
This study examines one example of use conflicts in Norwegian waters: the controversy over the Fisheries Directorate’s decision to allow fishing for the planktonic copepod Calanus finmarchicus (raudåte in Norwegian; hereinafter Calanus). Both resistance to and justification of the Calanus fishery have featured in the media and industry publications. Many reports of the conflict focus on fears that the Calanus fishery will impact two long-extant, high-value, wild-capture fisheries: Norwegian spring-spawning herring (Clupea harengus L.) and Atlantic cod (Gadus morhua) (Danielsen, 2021; Norges Fiskarlag National Board, 2021). Calanus is super-abundant in the North Atlantic: the Norwegian Institute of Marine Research (IMR) estimates a biomass of 33 million tonnes in the Norwegian Sea (Fiskeridirekteratet, 2016). Within this region, several Calanus species coexist, including C. finmarchicus, C. glacialis, and C. helgolandicus (Choquet et al., 2018). However, distinguishing between these species where their distributions overlap has long posed challenges (Choquet et al., 2018; Lindeque et al., 1999; Unstad and Tande, 1991). Though Calanus fisheries typically target C. finmarchicus, catches may include other Calanus species due to overlapping habitat, similar appearance and difficulties in precise species identification.
Unsurprisingly, and roughly analogous to krill in the Southern Ocean, Calanus plays a key ecological role as prey for multiple marine and bird species. The species also plays a role in the carbon cycle due to annual seasonal vertical migration; Calanus overwinter at depth and migrate to the surface to feed and reproduce when days lengthen and phytoplankton bloom (Pinti et al., 2023). Calanus species can also be used as indicators of climate change (Hays et al., 2005), as shifts in their populations and distribution reflect change to Atlantic water circulation (e.g., Falk-Petersen et al., 2007; Wassmann et al., 2006). Norway opened a commercial fishery for Calanus in 2019. Between 2003 and 2019, Calanus fishing was permitted on an experimental basis, with a single company permitted to catch a small amount annually. That company developed and marketed a human nutritional supplement based on Calanus oil, which is high in Omega-3 fatty acids and other desirable nutrients. However, long-term development of the Calanus fishery is not likely to hinge on such a niche market; instead, Calanus is a potential source of feed for Norwegian aquaculture, primarily salmon.
Norwegian ambitions towards ocean economy growth include the commonly cited goal of realizing a five-fold increase in aquaculture production by 2050 (Falk-Petersen et al., 2007; Wassmann et al., 2006). The potential sustainability impacts of realizing this ambition are considerable. In particular, farmed salmon in Norway are currently fed a primarily plant-based diet, with soy from Brazil making up the largest proportion of feed (Aas et al., 2022). Reliance on imported soy raises concerns about both security and sustainability of the salmon feed supply and significantly increases the climate footprint of farmed salmon (Aas et al., 2022), which is particularly problematic in the context of EU climate regulations. Calanus, high in energy, protein and lipids, is interesting as a potentially more sustainable source of aquaculture feed (Bøgwald et al., 2023; Colombo-Hixson et al., 2013; Olsen et al., 2004). The current commercial Calanus quota follows the precautionary principle (254,000 tonnes, or less than 1% of the estimated biomass), and even at that quota level fishery uptake has been slow and catches very small. Positioning the species as a source of aquaculture inputs will require significant increases in both quota and catch.
As noted above, Calanus fishing has been controversial, and even more so since commercial expansion was proposed. Arguments have played out in the Norwegian news media and industry and research publications. Although reported concerns include actual vs. estimated biomass (Lindbæk, 2020) and ecosystem effects (Eggen, 2017), much of the controversy has focused on the possibility that trawling for Calanus might result in bycatch (Danielsen, 2021; Norges Fiskarlag National Board, 2021). Larger organisms can avoid Calanus nets which, although made with extremely fine mesh, have relatively small mouths and are deployed behind vessels travelling very slowly. However, planktonic organisms including Calanus, but also eggs and larvae of other marine species, are less likely to be able to escape the trawls. The Calanus fishery is not subject to seasonal restrictions but currently operates in the early spring when the copepod returns to surface waters and occurs closer to the coast, where current fishing technologies can more successfully target the species. Other, higher-value fisheries are also generally closed at this time, allowing for effort- and gear-switching. The reproductive cycles of both cod and spring-spawning herring rely, in those same spring months, on the northward-flowing currents along the Norwegian coast to carry planktonic juveniles from more southerly spawning grounds to the more northerly areas where they mature. The co-occurence of this annual cycle with Calanus fishing activity risks bycatch.
IMR, in research in support of the 2016 Calanus management plan, found that that bycatch in the Calanus fishery was within safe levels (Broms et al., 2016). However, anecdotal reports and scoping interviews indicate that cod and herring fishers’ trust in bycatch estimates were dealt an early blow by the discovery of improper onboard sampling procedures on Calanus fishing vessels (Heldahl, 2019). Indeed, suspicion of the Calanus fishery - and particularly the coastal fishery - remains high (Sandnes, 2022) (see also Crosman and Hayes, 2025).
Fishers’ trust in fisheries management is widely seen as both a pinch point and a necessity for smooth, successful management (Ford and Stewart, 2021; Ordoñez-Gauger et al., 2018; Silva et al., 2021). But stakeholder trust in management is a complex concept, with the components, targets or sources of trust not always clear-cut (Stern and Coleman, 2015) (see also Crosman and Hayes, 2025). There are myriad possibilities beyond fishers’ lacking enough (or the correct) information to make trust judgements. When fishers express low trust in management, for instance, are those sentiments grounded in a history of contested agency decisions, rule-making, and enforcement (Bidgood, 2013; Forman, 2023; Associated Press, 2009)? Or perhaps in a mismatch between fishers’ on-the-water observations and the conclusions of fisheries researchers (Lindbæk, 2022a)? As discussed in (Crosman and Hayes, 2025), perceived value similarity (PytlikZillig et al., 2016; Schroeder et al., 2021; Siegrist, 2021), perceived benevolence (PytlikZillig et al., 2016; Siegrist, 2021; Stern and Coleman, 2015), and perceived competence (McEvily and Tortoriello, 2011; Stern and Coleman, 2015) should also all be expected to predict fishers’ trust in management. Low trust in management might also stem from controversy over the way relevant data are collected, as exemplified by the erosion of trust in Calanus fishery bycatch management that occurred with the discovery of improper on-board sampling discussed above.
Since that controversy, on-board sampling procedures have been revised and standardized, and the industry continues to report negligible bycatch (Lindbæk, 2022b). Updated IMR analyses reportedly also show that bycatch remains within safe levels (Jenssen, 2022). Model-based findings agree that, at current levels, fishing for Calanus will have minimal ecosystem effects (Hansen et al., 2021). Nonetheless, in a nod to cod and herring fishers’ concerns, the Fisheries Directorate’s current total quota of 254,000 tonnes of Calanus includes a restriction that only 3000 tonnes may caught along the coast (in waters shallower than 1000m). However, in 2022, while this research was ongoing, the Fisheries Directorate asked the Institute of Marine Research to investigate the possibility of increasing the coastal share of the Calanus quota to 15,000 tonnes of the 254,000 tonne total (Martinussen, 2022).
Real-time management (also known as dynamic management) relies on rapid transfer and integration of multiple types of data and information to allow managers and/or users to quickly respond to changing conditions (Maxwell et al., 2015). Adaptive management strategies, in which management is tailored to both lessons learned and ecosystem change, have already been widely adopted; real-time management shows potential for significantly shortening adaptation time frames (Maxwell et al., 2015). Technology can enable real-time or near-real-time management response, while supporting ecosystem-based management that pursues multiple, sometimes conflicting goals in systems that undergo rapid and sometimes unpredictable change (e.g., fisheries) (Dunn et al., 2016). Technologically-enabled real-time management can improve efficiency of fisheries management, including bycatch management (Hazen et al., 2018). Rapid genetic analysis of cod caught in Lofoten allowed continuation of the Norwegian fishery for North-East Atlantic cod while avoiding bycatch of Coastal cod over an 11-year study period (Dahle et al., 2018). Model-based tests of real-time closures (‘move-on rules’, in which all fishing vessels leave an area when bycatch is detected) have shown improved efficiency of bycatch reduction when compared to static management in New England (Dunn et al., 2016). And new or refined technologies allow real-time monitoring that would have been inconceivable until relatively recently: for example, underwater camera technology combined with supervised machine learning can now identify harmful algal species as they bloom in the waters surrounding Hong Kong (Guo et al., 2021). Indeed, the introduction of technology including onboard electronic monitoring systems, cloud computing, mobile phone technology, and artificial intelligence could be transformational for both the foundations and speed of management response (Bradley et al., 2019).
Although technology development within sustainable fisheries is a well published area (Honarmand Ebrahimi et al., 2021; Jiang et al., 2024; Lucchetti et al., 2023; Vinuesa et al., 2020), fishers’ perceptions and acceptance of new technologies is less examined. Very recent work shows that fishers’ perceptions of technology that influences fisheries, and thereby fisheries management, rely on transparent policy development and close stakeholder involvement early in the implementation phase (Ahlquist et al., 2025).
The foregoing discussions lead us to the current study. Given the controversy over fishing for Calanus, and specifically the widely-reported controversy over bycatch of cod and herring eggs and larvae; given the potential of real-time management to transform management, including management of bycatch; and given the novel technologies now available to support real-time management, this study seeks to answer two preliminary and one primary research questions. First, how supportive are Norwegian coastal cod and herring fishers of fishing for Calanus, and how much do they trust current management of the Calanus fishery? Secondly, can the introduction of technologically-mediated real-time bycatch management increase trust in fisheries management of a contested fishery?
2 Materials and methods
In order to investigate these questions, we developed a survey instrument to assess Norwegian cod and herring fishers’ overall trust in fisheries management in Norway, their specific trust in current management of the Calanus finmarchicus fishery and their reactions to one of four randomly assigned management scenarios. Results from the portion of the survey dealing with respondents’ trust in Norwegian fisheries management generally, and the predictors of trust in both general fisheries and Calanus management, are reported in more depth in Crosman and Hayes (2025).
2.1 Respondent identification and recruitment
Potential respondents were identified from a publicly available list of fishing vessels maintained by the Norwegian Fisheries Directorate. The list was filtered to include only vessels holding cod and/or spring-spawning herring quota in regions affected by Calanus fishing (i.e., excluding vessels fishing only in the North Sea). The list was filtered again to remove vessels longer than 27,99m (leaving only sizes defined as coastal under Norwegian regulations). The resulting 1598 vessels were cross-referenced with publicly available ownership information to create a list of 1450 individuals with significant ownership shares. We sent the resulting list to Norfakta, an independent polling company, for survey administration. Norfakta secured contact information for 915 potential respondents and successfully contacted and completed anonymous phone surveys with 184 respondents (20% response rate, representing 12,7% of the identified universe of respondents). This somewhat low response rate may reflect the fact that, anecdotally, some Norwegians block calls from known polling organizations such as Norfakta; Norfakta reports that each potential respondent was called at least eight times. The survey was developed in English and administered by phone, in Norwegian, in June 2023. Norfakta provided translation, which was verified with a translation check by a native Norwegian/fluent English speaker prior to administration.
No personal data were collected from respondents and phone calls were not recorded, obviating the need for written consent under the EU General Data Protection Regulation as implemented by Sikt, the Norwegian Agency for Shared Services in Education and Research. However, respondents were informed of their rights and read a short informed consent statement at the commencement of the phone survey (Appendix A).
2.2 Survey
The survey instrument was developed based on the literature discussed above, as well as 14 key informant scoping interviews with experts in digital, data-driven, ocean monitoring, ‘big data’ technologies (e.g., marine autonomy, underwater imaging, environmental DNA, applied artificial intelligence); Calanus ecology, management, and fishing; and Norwegian fisheries management. Interview respondents agreed that while there is currently no use of ‘big data’ technologies in Calanus management, such technologies could substantially improve the management knowledge base. Respondents with knowledge of the controversy over Calanus fishing, including fisheries researchers, fisheries managers, and industry representatives, perceived opposition to be primarily grounded in bycatch concerns. After development, the survey was vetted by key informants to ensure that questions were suitable and salient to potential respondents.
Survey respondents were first asked a series of demographic questions including sample inclusion verification items (vessel ownership, species fished), professional role, education, and age. To measure both demographics and economic stakes, respondents were asked how much of their income derived from the fisheries they participate in. To gauge social/cultural stakes, they were asked how many generations of their family worked as fishermen. In addition to the demographic items included in the survey instrument, we derived additional demographics from public records held by the Norwegian Fisheries Directorate. This included the region of Norway in which the respondent’s primary residence is located, the number of salient fishing vessels owned, the number of companies with which the respondent was associated (and company types), the maximum, minimum and average length of all owned vessels, and the total cod and/or herring quota held across all salient vessels.
After a series of items focused on general trust in Norwegian fisheries management (Crosman and Hayes, 2025), respondents received items targeting their awareness of, and support for, the Norwegian Calanus fishery and trust in Norwegian Calanus management (Table 1). Respondents were then read a randomly assigned management scenario, and survey items related to trust in Calanus management and support for Calanus fishing were repeated to measure any treatment effect on these measures. Trust items both before and after the scenario treatment included open-ended follow-ups (Table 1).
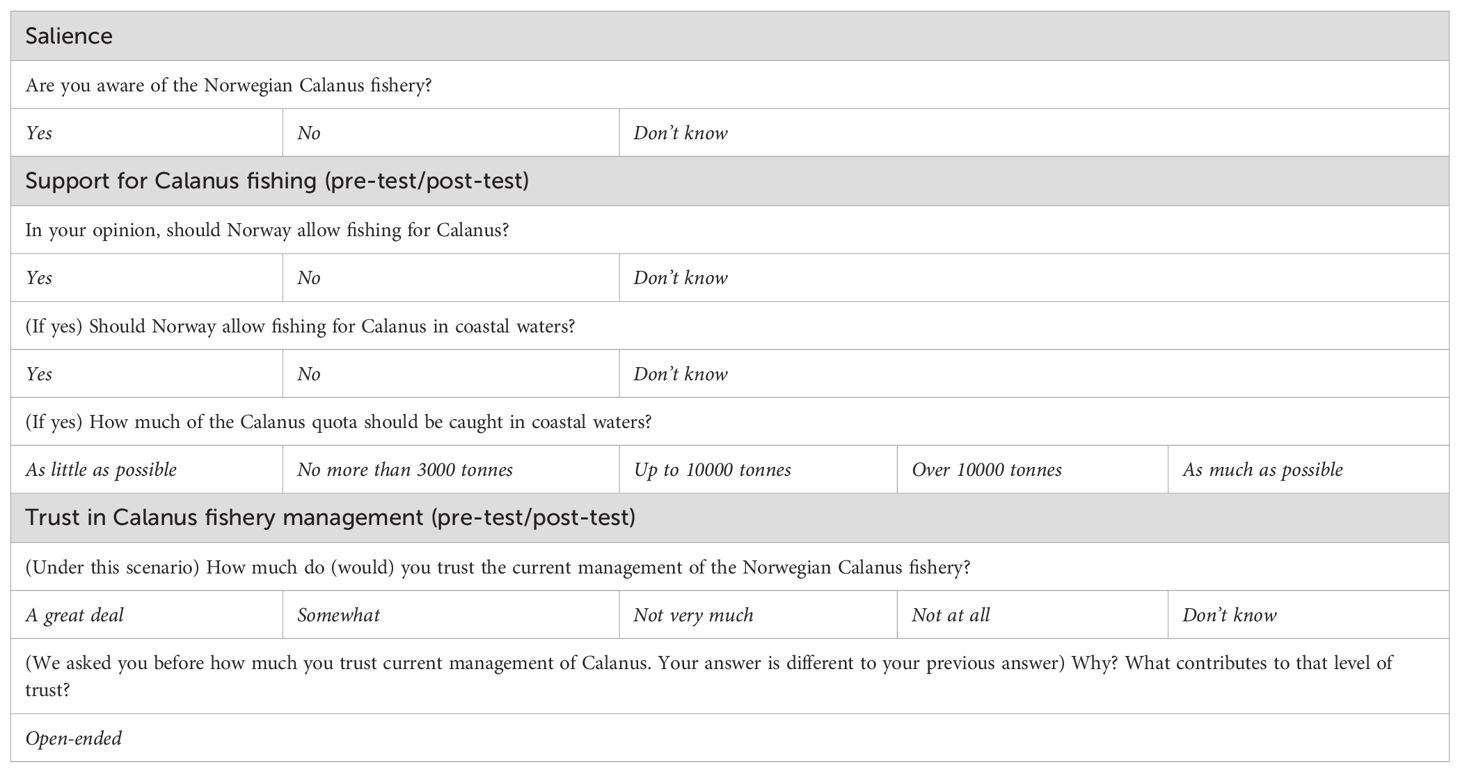
Table 1. Survey items targeting awareness, support, and trust in the Calanus fishery, as well as management policy preferences.
2.3 Scenarios
We developed four hypothetical Calanus management scenarios; respondents were randomly assigned one scenario treatment. The primary criterion for the scenarios was that the management they described be technically feasible within the next five years. The scenarios were 1) control/business-as-usual, 2) real-time management with on-board sampling, 3) real-time management using underwater imaging, and 4) real-time management using environmental DNA. Random assignment was used to allow us to draw causal conclusions about the effects of the different scenarios on support for the Calanus fishery and trust in Calanus management. Given the history of controversy over early onboard sampling of bycatch discussed in the Introduction, we selected scenario 2 to test for the effect of real-time management that continued to rely on onboard samples. Scenarios 3 and 4 allowed for the collection of data without relying on Calanus vessels and fishers, and thus real-time management response independent of fishing operations.
All four scenarios started with common language identifying the full Calanus commercial quota (254,000 tonnes at the time of survey administration), the portion that may be fished between the Norwegian baseline and the 1000m depth line (i.e., the coastal quota – 3,000 tonnes at the time of survey administration), and likely time of fishery operations (spring and summer months). The business-as-usual treatment (1) specified that, consistent with current bycatch monitoring methods, Calanus fishers would take physical samples from each trawl haul and subsequently send those samples to the Institute of Marine Research (IMR) for analysis. IMR analysis would include identification of eggs and larvae of fish species of commercial interest, with regulations adjusted on a yearly basis as samples were processed. The real-time treatment (2) specified that trawl samples, rather than being sent to IMR, were to be poured onto an onboard light table and digitally photographed, with images sent to shore and analyzed immediately and temporary closure of the Calanus fishery when high levels of fish eggs and larvae were detected.
The underwater imaging treatment (3) replaced on-board trawl sampling with high-resolution underwater particle cameras mounted on buoys stationed in the northward flowing coastal currents between Trondheim and Lofoten. Digital images were to be collected at regular intervals and immediately analyzed onboard the buoys with the use of image-recognition AI. When high levels of fish eggs and larvae were detected that information was to be sent to shore and the Calanus fishery temporarily closed. Lastly, the real-time eDNA treatment (4) replaced the underwater cameras with eDNA samplers, with AI-driven analysis trained to detect spikes of eDNA consistent with spawning of fish species of commercial interest (i.e., cod and herring). Such a spike would trigger transmission of the information to shore and temporary closure of the Calanus fishery. The text of Scenarios 1 and 2 may be found in Appendix B. The texts of Scenarios 3 and 4, plus fuller discussion of the relevant technological and scientific rationales for those treatments, may be found in Appendices C and D respectively.
2.4 Data handling and analysis
Responses were recoded in R for analysis. Responses to trust items (e.g., How much do you trust current management of the Norwegian Calanus fishery)? were recoded to run from 0 (Not at all) to 3 (A great deal), with Don’t know responses coded as NA. Similarly, questions targeting familiarity with technology (e.g., How familiar are you with camera technology that allows high-resolution underwater imaging of small particles, like plankton)? were recoded from 0 (Not at all familiar/never heard of it) to 3 (Very familiar). Items with yes/no responses (e.g., In your opinion, should Norway allow fishing for Calanus)? were coded as binary (1=Yes), with Don’t know coded as NA. The exception to this was the items measuring support, where Don’t know was treated as intermediate to Yes and No (coded as 0.5).
We ran Fisher’s exact tests to check for control/treatment group balance on pre-treatment items, including familiarity with the Calanus fishery, trust in Calanus fishery management, and support for coastal Calanus fishing. Each of the Fisher’s exact tests resulted in a p-value greater than 0.1, where a lack of statistical significance is interpreted as a failure to reject the null hypothesis that the variable of interest is drawn from the same distribution across the different treatments. In order to test for scenario-specific treatment effects, and as our response variables are ordered categorical, we ran a Stuart-Birch test (Birch, 1965; Stuart, 1955) as implemented in the ‘coin’ R package (Hothorn et al., 2008), which is used to test for marginal homogenity of ordered factors, and in this case to test for differences between pre- and post-treatment responses to management trust and Calanus support items. Data were analyzed using R version 2023.06.1 + 524.
Open-ended responses were translated from Norwegian into English by a native Norwegian speaker and a native English speaker working cooperatively. Responses were qualitatively coded according to a coding scheme derived from peer-reviewed literature on trust (e.g., Cook and Gronke, 2005; Emborg et al., 2020; PytlikZillig et al., 2016; Siegrist, 2021; Siegrist et al., 2000; Stern and Coleman, 2015), and informed by key informant interview responses. For a more in-depth discussion of the relevant trust constructs, as well as the full coding scheme, see Crosman and Hayes, 2025). Codes were added to the coding scheme as distinctly different concepts were encountered in survey responses. Codes were assigned to each proposition in an open-ended response; many responses thus received multiple codes. To check intercoder reliability, an independent coder coded 20% of responses. Average percent agreement between the two coders was 94%, and Cohen’s Kappa, which corrects for the possibility of agreement by chance, was 66% (moderate to substantial agreement) (Altman, 1990; McHugh, 2012).
3 Results
All 184 survey respondents were found to be appropriate for sample inclusion, confirming that they owned a full or part share in at least one fishing vessel fishing for cod and/or herring. Of those, 37 respondents were randomly assigned to the control/business-as-usual group, 35 to the real-time management treatment, 60 to the underwater imaging treatment, and 52 to the eDNA treatment.
Control and treatment groups were well balanced, with Fisher’s exact test results allowing us to reject the null hypotheses that a) treatment group was significantly associated with initial attitudes towards Calanus and its management and b) that treatment groups significantly varied across demographic variables, including species fished, professional role, years employed, generations employed, fisheries-derived income, education, company type, region, number of boats, and cod and herring quotas.
3.1 Demographics
In general, the sample tended towards homogeneity. 176 respondents reported fishing for cod, including 30 who also reported fishing for herring. Eight respondents reported fishing for only herring. 174 respondents reported fishing for other species in addition to cod and herring; of these, two respondents specified that they fished for Calanus. Due to the very small number of women in the potential sample, combined with identifiability concerns, Norfakta declined to directly collect information about the gender of respondents; however, based on Norwegian fisheries statistics it is very likely that our sample was overwhelmingly male.
The majority of respondents (n=172) operated their own vessel. Of the remaining 12 respondents, four specified that they held another onboard role, three were involved only on the business side, and three were silent partners. Two respondents did not specify a role. Respondents reported 29.1 years of employment in their fisheries, on average (minimum 2, maximum 60), and 4.2 generations of family employed in fishing, on average (minimum 0, maximum 99).
Most respondents were highly dependent on their fisheries for income, with 115 deriving more than three-quarters of their household income and 159 deriving more than half. Respondents tended not to hold college degrees: 155 of them left school after grunnskole or videegående skole (roughly equivalent to ages 13 to 16), with an additional 11 having attended some college without receiving a degree. The youngest respondent was 23 and the oldest 74, with an average age of 51.
170 respondents were affiliated with only one company; of those companies, 56 were sole proprietorships and 107 were privately-held limited liability companies. 34 respondents had their residence of record in the Western fjords (the region encompassing Stavanger and Bergen); 36 in mid-Norway (encompassing Ålesund, Trondheim and Frodo); and the remaining 114 in northern Norway (encompassing Bodø/Lofoten, Tromsø, and all the way to the Norwegian/Russian border). 122 of the respondents had full or part ownership of only one registered fishing vessel and 41 had ownership in two (maximum vessels owned = 6, one respondent). The mean average vessel length was 12.7m (minimum 7.92, maximum 27.99). Among those holding cod quota, the average total amount of quota held was 82.4 tonnes (minimum 17.2, maximum 653.2); among those holding herring quota, the average total quota held was 326 tonnes (minimum 72.2, maximum 1045.1).
Our ability to assess sample representativeness is limited by the types of information that are publicly available about Norwegian fishers. However, based on Norwegian fisheries statistics (Norwegian Directorate of Fisheries, 2025, 2023), our sample appears to be a relatively good demographic match with the sampling frame (Table 2). In spite of this finding, it is possible that respondents differed systematically from non-respondents on their opinions of the Calanus fishery or its management. We lack the necessary data to assess this possibility.
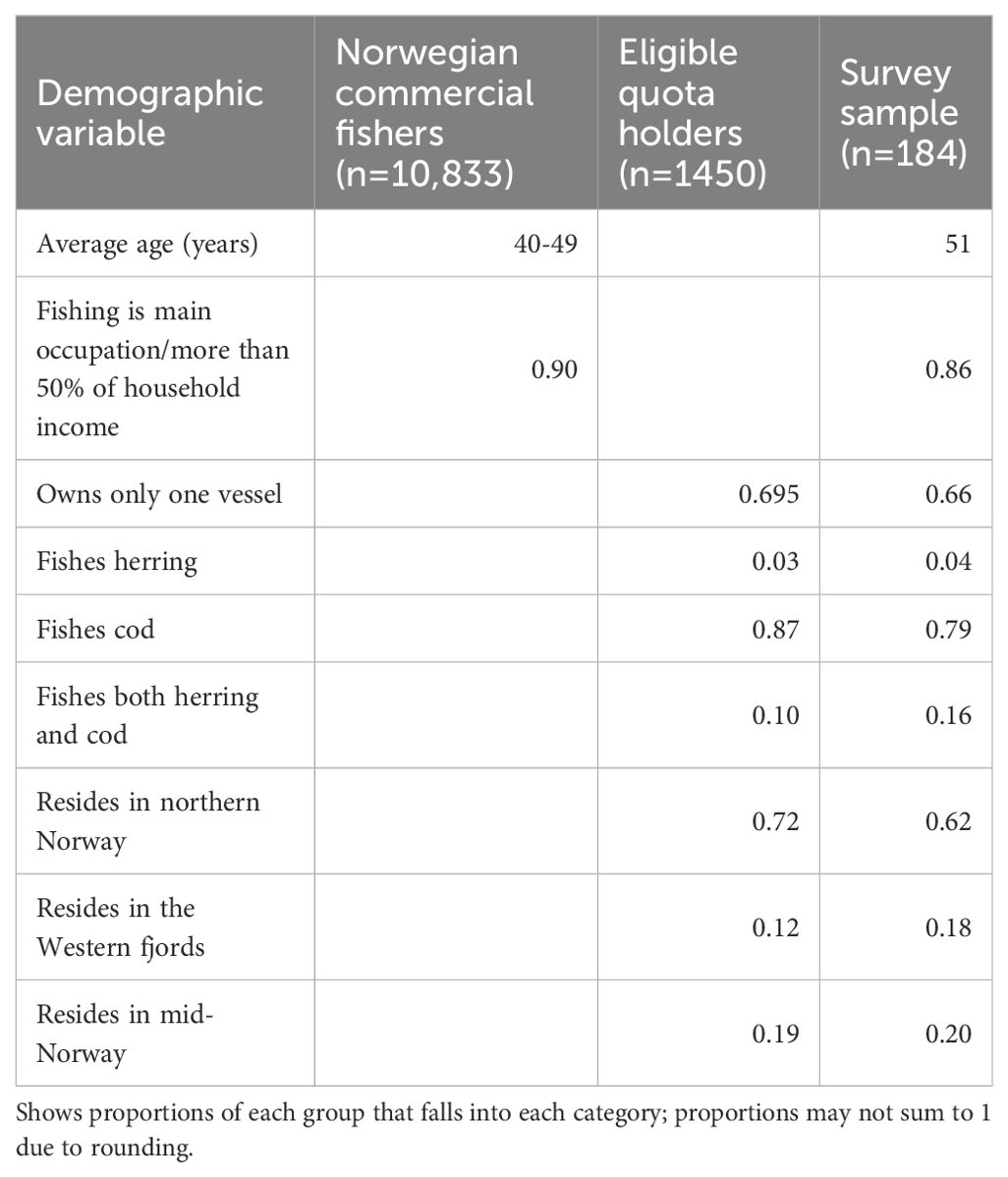
Table 2. Sample representativeness based on publicly available information on Norwegian commercial fishers and registered quota holders who met initial survey eligibility requirements.
Eighty-nine percent (n=164) of the respondents reported familiarity with the Norwegian Calanus fishery. In the underwater imaging treatment group, 26 of 60 reported no familiarity with underwater imaging, while 21 ranked themselves as ‘not very familiar’ and the remaining 13 reported that they were ‘somewhat familiar’. Of the 52 respondents in the eDNA treatment group, 18 reported no familiarity with eDNA, 25 ranked themselves as ‘not very familiar’, and 9 reported that they were ‘somewhat familiar’. Across these two groups, reported familiarity with AI for use in image/pattern recognition was also generally low, with 27 and 17 respondents reporting no familiarity, 17 and 17 rating themselves as ‘not very familiar’, and 16 and 18 responding ‘somewhat familiar’ (disaggregated into eDNA and UWI groups respectively).
3.2 Experimental results - support for fishing for Calanus and trust in Calanus fisheries management
Only 29 respondents (16% of the sample) initially supported fishing for Calanus in Norwegian waters; of those, only six supported fishing for Calanus in coastal waters (three did not provide a response to the follow-up question on coastal fishing). Neither of the two respondents who reported fishing for Calanus supported the fishery. Because support for Calanus fishing was so low, we were unable to further explore predictors of support for coastal fishing or the percentage of the quota allocated to coastal areas. None of the treatments significantly changed the lack of support for the Norwegian Calanus fishery (Table 3, Figure 1). The control/business-as-usual group did show a small, marginally significant (p <.10), increase in support; this result is discussed further below.
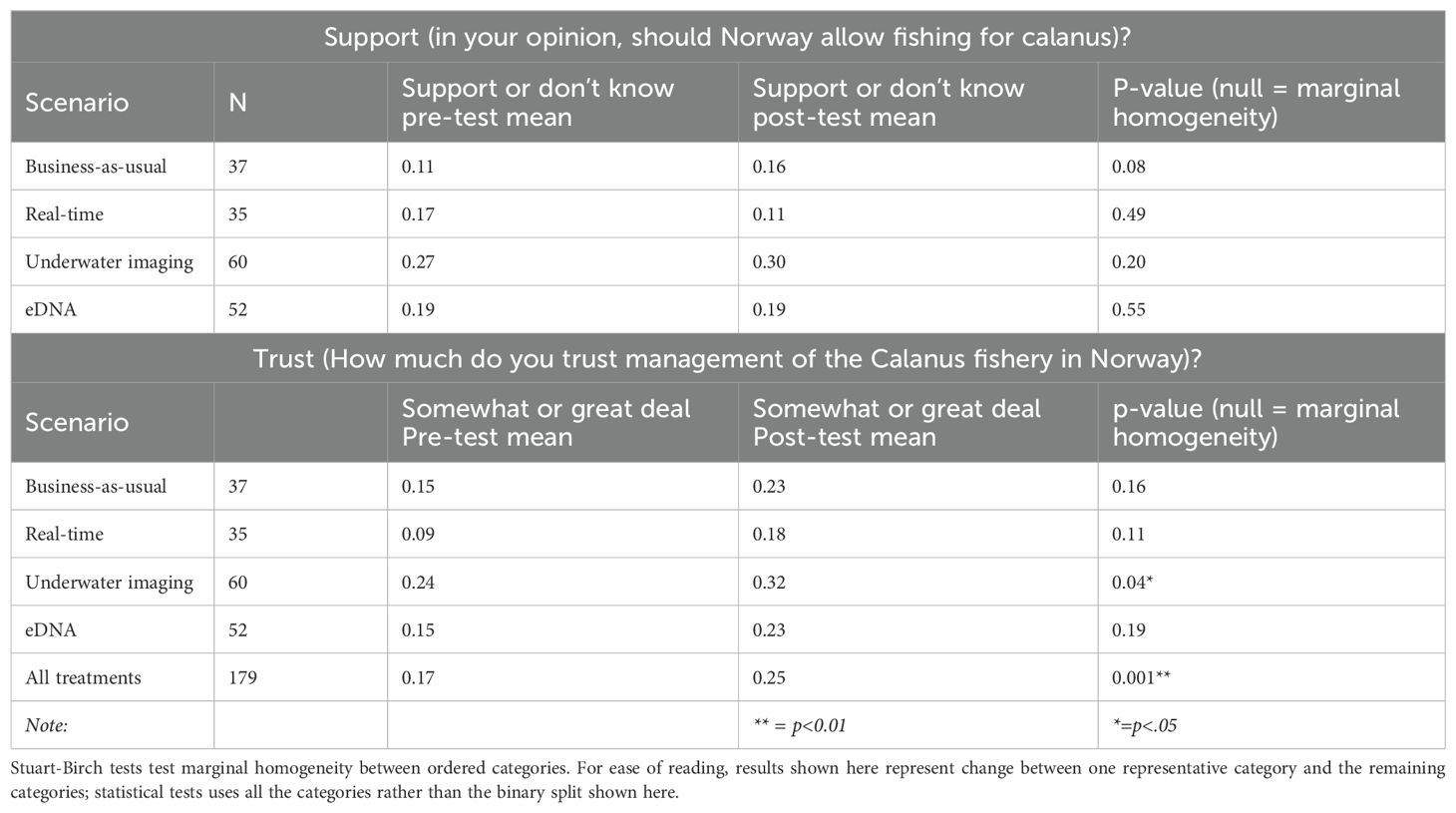
Table 3. Results of Stuart-Birch tests results comparing pre- and post-tests across control and treatment groups for both support of Calanus fishery and trust of Calanus fishery management.
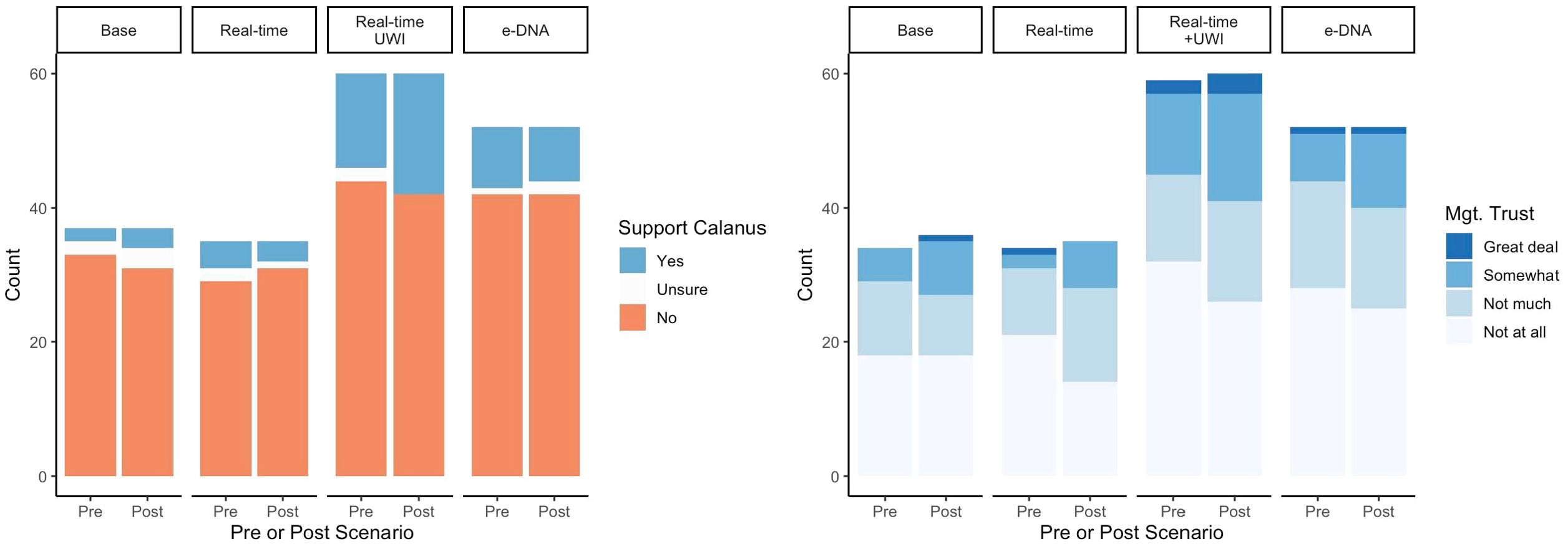
Figure 1. Pre- and post-test support for Calanus fishing (‘In your opinion, should Norway allow fishing for Calanus?’; yes/no/don’t know) (left) and trust in Calanus management (‘How much do you trust management of the Calanus fishery’; 4-point response scale from ‘not at all’ to ‘a great deal’) (right). Mismatched pre- and post-test counts reflect a small number of individuals who answered ‘I don’t know’ to either the initial trust item or the post-treatment follow-up.
Similarly, trust in management of the Calanus fishery started very low across all groups (overall mean of 0.63, equivalent to between ‘not at all’ and ‘not very much’, in response to the prompt ‘How much do you trust management of the Norwegian Calanus fishery?’, 4-point scale, 0=not at all, 4=a great deal). Trust among all treatment groups increased after being presented with the treatment scenario. While the treatment group presented with the underwater imaging scenario was the only group for which that increase was statistically significant at the 5% level, the combined sample, pooled across all treatment groups, showed a statistically significant increase in trust as well (p = 0.001) (Table 3, Figure 1).
Trust and support are related. 61% of Calanus fishing supporters report trusting current management of the Calanus fishery ‘somewhat’ or ‘a great deal’, as compared to only 8% of those who do not support Calanus fishing. The result is highly significant and the variables are moderately positively correlated (correlation coefficient of 0.48; p > 0.0001).
3.3 Open-ended results
Respondents reported a variety of reasons for their responses to the closed-ended trust in management items, as reflected in the codes most commonly applied to open-ended responses. Code mentions varied with how much trust respondents expressed in Calanus fishery management (Figure 2, Table 4). Respondents who reported low trust (responding ‘not at all’ or ‘not very much’ to the prompt ‘how much do you trust the current management of the Norwegian Calanus fishery?, n=149) mentioned ‘ecosystem/food web effects’ in almost half of their responses (n=64); other commonly mentioned codes included ‘bycatch’ (n=30), ‘research’ (n=30), ‘value [dis]similarity’ (n= 27), ‘risk’ (n=26), ‘distance from shore’ (n=22), and ‘uncertainty’ (n=17), ‘fishing operations’ (n=11), and ‘level of exploitation’ (n=9). Respondents who reported higher trust (‘somewhat’ or ‘a great deal’, n=30) most commonly mentioned ‘research’ (n=10), managerial ‘competence and expertise’ (n=6), and ‘social/dispositional trust’ (n=5) in their responses. Other commonly mentioned codes in this group included ‘ecosystem effects’ and ‘bycatch’ (n=4 for both).
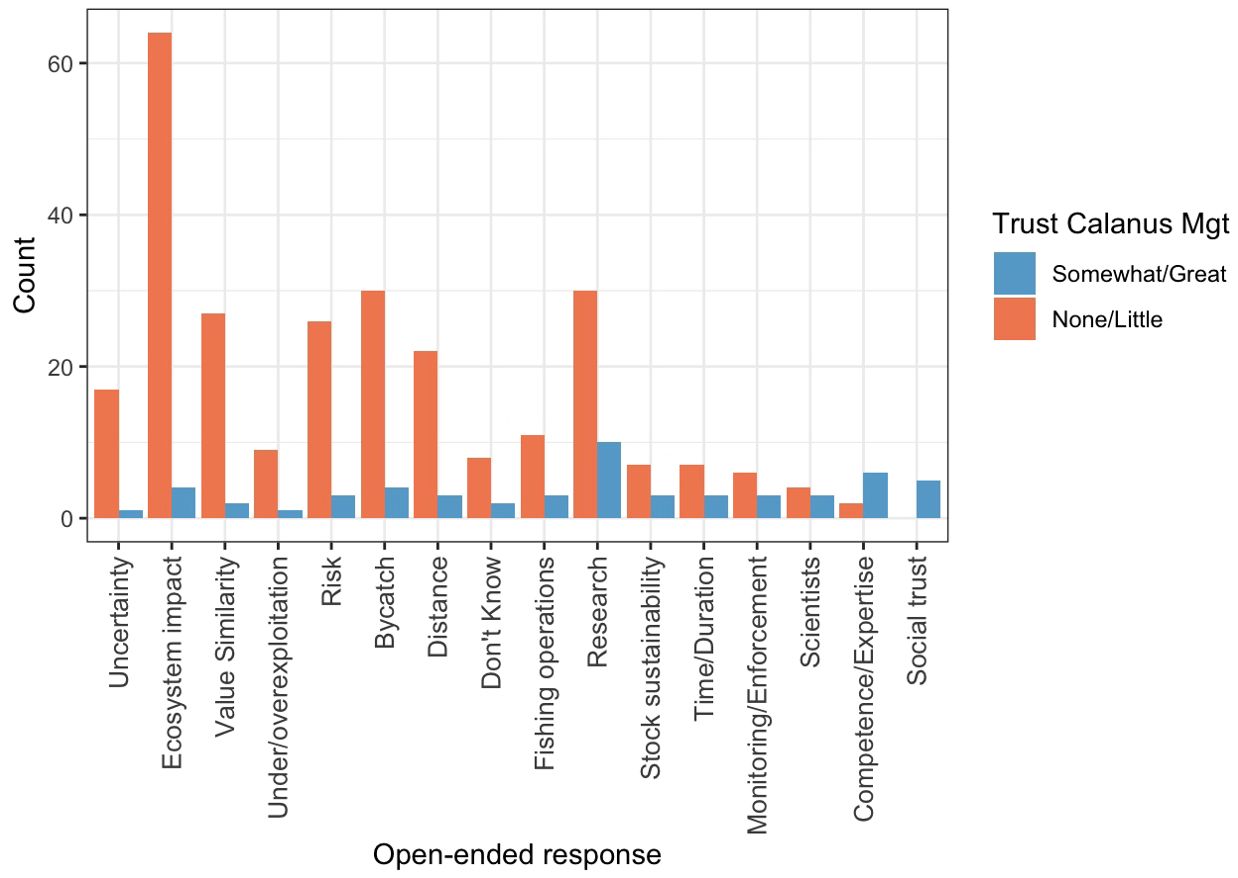
Figure 2. Most common codes mentioned in responses to the follow-up item asking respondents to explain their trust or lack of trust in Calanus management (‘Why? What contributes to that level of trust?’). Responses are disaggregated by those who report higher trust in management (replying ‘somewhat’ or ‘a great deal’ in response to ‘How much do you trust current management of the Calanus fishery’) and those who report lower trust (responding ‘not very much’ or ‘not at all’).
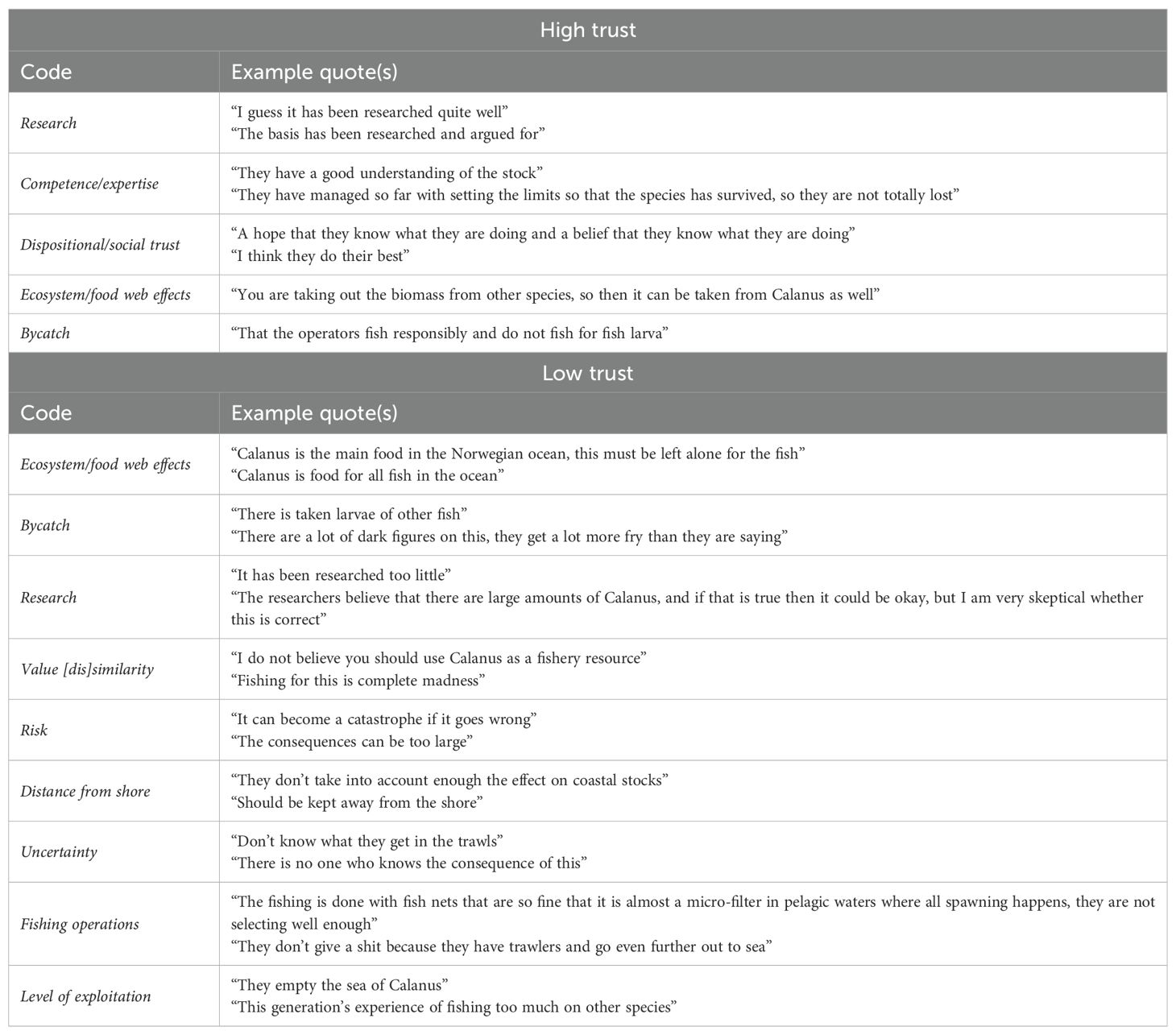
Table 4. Example texts assigned to parent codes commonly mentioned in responses to the prompt: Why? What contributes to that level of trust?
4 Discussion
Norwegian cod and herring fishers have publicly expressed opposition to the Norwegian Calanus fishery, particularly the coastal portion (within the Norwegian baseline, less than 1000km from shore). Perceptions of managers, as well as press coverage and public statements from fishers, have grounded this opposition primarily in concerns about bycatch of cod and herring eggs and larvae, which are transported each spring from more southerly spawning grounds by the northward-flowing currents along the Norwegian coast and thus subject to bycatch in Calanus trawls.
In this context, we used a survey experiment (n=184) to test the effects of three randomly assigned treatments, each based in a switch to technologically-facilitated real-time management, on Norwegian coastal cod and herring fishers’ support for the Norwegian Calanus fishery and trust in Calanus fishery management. We also explored the rationales provided for levels of self-reported trust. In a separate work, we tested whether determinants of initial trust in the Calanus fishery followed patterns predicted by theory and confirmed by these same respondents’ self-reported trust in Norwegian fisheries management more generally (Crosman and Hayes, 2025).
Lack of support for the Calanus fishery is high in our sample – 84% of our respondents did not support the Norwegian Calanus fishery prior to treatment, and there was no statistically significant (p<.05) change in support after treatment. The control/business-as-usual group did, however, see a marginally significant (p<.10) increase in support after treatment. A closer look at this change reveals how marginal it is. Among 37 respondents in the control group, only three changed their response between pre- and post-scenario; two went from not supporting to unsure, and one went from unsure to supporting. While it is possible this represents a small but real change in attitude toward the Calanus fishery due to the treatment/business-as usual scenario, none of the other more extensive treatments exhibited the same softening of fisher attitudes toward the Calanus fishery. As a result, we believe this marginal result is not particularly meaningful.
Stakeholder trust in Norwegian Calanus fishery management is likewise low. However, we do see a statistically significant increase in trust following the underwater imaging treatment scenario. While this group represents the only treatment-specific statistically significant increase in trust, the pooled sample across all treatments (including the control/business-as-usual) saw a statistically significant increase in trust as well. This amounted to an 8-percentage point increase in the proportion of the sample indicating ‘somewhat’ or ‘a great deal’ of trust, almost 50% more than in the pre-test, and is fairly consistent across treatment assignments. Although the vast majority (89%) of our respondents reported that they were familiar with the Calanus fishery, they may not have had the details of management front-of-mind. Given the negative public discourse surrounding the fishery, the controversy around bycatch monitoring, and empirical findings that such negative events have disproportionate influence on cognitive assessments (i.e., negativity bias, Rozin and Royzman, 2001), it seems likely that negative associations were more immediately cognitively accessible before treatment. Even a brief reminder of the improved bycatch monitoring protocols currently in place may have alleviated some suspicion grounded in initial assessments driven by immediate negative associations.
The lack of statistical significance found for the eDNA treatment may be attributable to a lack of familiarity with the technology. The proportions of respondents reporting they were ‘somewhat familiar’ with the two technologies were similar (0.17 for eDNA versus 0.22 for underwater imaging). It seems likely, however, that – once introduced in more detail in the full scenario text – underwater imaging was more easily cognitively related to known, similar technologies such as cell phone imaging and internet image searches. eDNA, by contrast, may be more difficult to relate to commonly encountered phenomena.
More critically, some of our results may be attributable to low statistical power due to small sample size. We sought, but were unable to secure, an overall sample that would allow treatment groups of approximately 80 respondents; instead, our treatment groups ranged from 36 (real-time management with onboard sampling) to 60 (underwater imaging). We chose four treatments due to the importance of each, but the group sizes are necessarily low due to the relatively low number of potential respondents in the sampling frame; furthermore, due to Norfakta’s use of simple randomization, group size varied considerably. With a dependent variable measured with non-continuous (ordered categorical) data, we should expect to see an inherently noisy measure compared to a continuous outcome. It may be no coincidence that the group that showed a statistically significant increase in trust was the largest one. Low statistical power especially hinders our ability to draw firm conclusions about null effects. Future work that applies block randomization could be used to secure more even group sizes, although such an approach would also complicate survey administration.
Given that fishers have been slow to join the Calanus fishery (Hogrenning, 2023), and that respondents may think themselves unlikely to become involved in fishing Calanus, the high opposition and low response to treatment has an obvious economic explanation. There is some non-negative probability that the Calanus fishery harms the fishery they participate in while a very low probability (respondents may even perceive a zero probability) that they would benefit from a Calanus fishery. This is consistent with the detected change in trust, where a perceived improvement in bycatch measures improves trust in management but does not change support.
Critically, respondents’ open-ended responses show that reports grounding conflict over Calanus in bycatch concerns miss a large portion of the story, and thus that a management focus on minimizing bycatch may be a red herring. The overwhelming majority of our respondents mentioned ecosystem/food web effects when prompted to provide a rationale for their [lack of] trust in Calanus management. Indeed, concerns about ecosystem effects of Calanus fishing reflect an accurate understanding of the role that the species plays in North Atlantic food webs, and our findings clearly indicate many of the fishers in our sample fundamentally disagree with fishing the bottom of the food web. Based on open-ended responses, this disagreement is at least to some extent grounded in differing values; indeed, responses coded as value (dis)similarity often also included mentions of ecosystem effects (e.g., “Have a completely different opinion about whether it is wise to take Calanus from the sea, it is what the fish is supposed to live off”). The perceived match (or mis-match) between management and resource user values is a well-known antecedent of trust in studies of both natural resource management and risk (PytlikZillig et al., 2016; Schroeder et al., 2021; Siegrist et al., 2000). A companion work to the current study finds that the perception that managers share fishers’ values is a statistically significant predictor of probable trust in Norwegian fisheries management for the same sample discussed here; however, it is only marginally and inconsistently predictive of probable trust in Calanus management (Crosman and Hayes, 2025). Notably, in that study, the modelled shared-value measure was not specific to Calanus and occurred before the topic of Calanus was introduced to the survey. In this study, shared values was a commonly mentioned code in open-ended responses; the most commonly mentioned code, ecosystem/food web effects, may also partially capture a value-driven disagreement over the advisability of fishing for Calanus.
In the face of a basic misunderstanding of the drivers of the conflict and an apparent value mismatch, technology can only take us so far. Our results show that strategic deployment of underwater cameras and image recognition software to facilitate real-time closure of the Calanus fishery in response to high bycatch potential would likely – given uncertainty over how a treatment presented in a hypothetical scenario might translate into a real-world context – increase trust in Calanus management among cod and herring fishers. However, given our larger findings, we caution that this solution could be unnecessarily resource intensive while failing to address the real problem. For fishers who are concerned about bycatch, a reminder of existing bycatch minimization efforts may be an equally effective (and much cheaper) approach to increasing trust, given the marginally significant increase in trust associated with the control/business-as-usual treatment, combined with low statistical power. However, we believe that any approach that simply corrects possible management information deficits also ignores the bigger issue. Our sample’s overall lack of support for the Calanus fishery, and specifically respondents’ concerns that fishing for Calanus runs unacceptable risks to ecosystem integrity, call for frank conversations about uncertainty, risks and values. Such a conversation should be approached from a conflict resolution frame; indeed, technology could plausibly be employed to monitor real-time ecosystem state in the service of conflict resolution. Our findings indicate that absent such a conversation, technocratic solutions are likely to have minimal impact on trust in Calanus management or support for the Calanus fishery.
In closing, we advise a tempered application of the finding that deployment of underwater imaging technology might increase trust in Calanus management. Low and unchanging support, combined with open-ended responses, seem to indicate entrenched opposition, and that bycatch is of less concern (or at least less front-of-mind) than potential ecosystem effects of fishing for Calanus. Addressing the existing conflict between cod and Calanus in Norway will require a more in-depth approach than simply deploying new technology. More broadly, our results indicate that fisheries managers should strive to understand the multi-faceted roots of conflict, in order to ensure that proposed management solutions are well matched to the full set of stakeholder concerns.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Norwegian Agency for Shared Services in Education and Research (Sikt). The studies were conducted in accordance with the local legislation and institutional requirements. Written consent requirements were waived by Sikt.
Author contributions
KC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Methodology, Project administration, Writing – original draft, Writing – review & editing. AH: Formal Analysis, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. ED: Conceptualization, Writing – original draft, Writing – review & editing. SM: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. KMC was supported through funding via the World Economic Forum Hoffmann Fellowship programme, and by SFI Harvest, Norwegian Research Council project number 309661; SM was supported through funding via the Norwegian Research Council project number 315728.
Acknowledgments
We thank Dag Standahl for expert advice on Norwegian fisheries, Signe Annie Sønvisen for help with sample identification, Jan Birger Jørgensen for reviewing the survey instrument, and Trine Aas-Hansen for research assistance. We are very grateful to all the respondents who participated in this survey. The survey instrument was translated and administered by Norfakta.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1572772/full#supplementary-material
References
Aas T. S., Åsgård T., and Ytrestøyl T. (2022). Utilization of feed resources in the production of Atlantic salmon (Salmo salar) in Norway: An update for 2020. Aquacult. Rep. 26, 101316. doi: 10.1016/j.aqrep.2022.101316
Ahlquist I. H., Hatlebrekke H. H., and Tiller R. (2025). Fishing for solutions: Norwegian fishers’ perspectives on the implementation of automatic catch registration for combating IUU fishing. Mar. Pol. 179, 106750. doi: 10.1016/j.marpol.2025.106750
Altman D. G. (1990). Practical Statistics for Medical Research (New York: Chapman and Hall/CRC). doi: 10.1201/9780429258589
Arbo P. and Thủy P. T. T. (2016). Use conflicts in marine ecosystem-based management — The case of oil versus fisheries. Ocean Coast. Manage. 122, 77–86. doi: 10.1016/j.ocecoaman.2016.01.008
Associated Press. (2009). New England fishermen protest new catch rules (NBC News). Available online at: https://www.nbcnews.com/id/wbna33553385 (Accessed October 21, 2024).
Bailey J. L. and Eggereide S. S. (2020). Mapping actors and arguments in the Norwegian aquaculture debate. Mar. Policy 115, 103898. doi: 10.1016/j.marpol.2020.103898
Bergh Ø., Beck A. C., Tassetti A. N., Olsen E., Thangstad T. H., Gonzalez-Mirelis G., et al. (2023). Analysis of spatial conflicts of large scale salmonid aquaculture with coastal fisheries and other interests in a Norwegian fjord environment, using the novel GIS-tool SEAGRID and stakeholder surveys. Aquaculture. 574, 739643. doi: 10.1016/j.aquaculture.2023.739643
Bidgood J. (2013). As Fisheries Struggle, Debate Heats Up Over How to Help (The New York Times). Available online at: https://www.nytimes.com/2013/02/16/us/debate-over-how-to-help-massachusetts-fishing-towns.html (Accessed October 21, 2024).
Birch M. W. (1965). The Detection of partial association, II: the general case. J. Royal Stat. Soc.: Series B (Methodological) 27, 111–124. doi: 10.1111/j.2517-6161.1965.tb00593.x
Bøgwald I., Østbye T.-K. K., Pedersen A. M., Rønning S. B., Dias J., Eilertsen K.-E., et al. (2023). Calanus finmarchicus hydrolysate improves growth performance in feeding trial with European sea bass juveniles and increases skeletal muscle growth in cell studies. Sci. Rep. 13, 12295. doi: 10.1038/s41598-023-38970-5
Bradley D., Merrifield M., Miller K. M., Lomonico S., Wilson J. R., and Gleason M. G. (2019). Opportunities to improve fisheries management through innovative technology and advanced data systems. Fish Fisheries 20, 564–583. doi: 10.1111/faf.12361
Broms C., Strand E., Utne K. R., Hjøllo S., Sundby S., and Melle W. (2016). Vitenskapelig bakgrunnsmateriale for forvaltningsplan for raudåte (Fisken og Havet No. 8/2016) (Havforsknings Instituttet). Available online at: https://hi.no/resources/publikasjoner/fisken-og-havet/2016/fisken_og_havet_nr_8-2016_forvaltningsplan_for_raudate.pdf (Accessed October 21, 2024).
Choquet M., Kosobokova K., Kwaśniewski S., Hatlebakk M., Dhanasiri A. K. S., Melle W., et al. (2018). Can morphology reliably distinguish between the copepods Calanus finmarchicus and C. glacialis, or is DNA the only way? Limnol. Ocean Methods 16, 237–252. doi: 10.1002/lom3.10240
Colombo-Hixson S. M., Olsen R. E., Tibbetts S. M., and Lall S. P. (2013). Evaluation of Calanus finmarchicus copepod meal in practical diets for juvenile Atlantic halibut (Hippoglossus hippoglossus). Aquacult. Nutr. 19, 687–700. doi: 10.1111/anu.12016
Cook T. E. and Gronke P. (2005). The skeptical American: revisiting the meanings of trust in government and confidence in institutions. J. Politics 67, 784–803. doi: 10.1111/j.1468-2508.2005.00339.x
Crosman K. M. and Hayes A. L. (2025). 'It builds on trust': Exploring fishers' trust in management of fisheries in Norway. Front. Mar. Sci. 12. doi: 10.3389/fmars.2025.1572697
Dahle G., Johansen T., Westgaard J.-I., Aglen A., and Glover K. A. (2018). Genetic management of mixed-stock fisheries “real-time”: The case of the largest remaining cod fishery operating in the Atlantic in 2007–2017. Fisheries Res. 205, 77–85. doi: 10.1016/j.fishres.2018.04.006
Danielsen D. J. (2021). Verdens første raudåte-fabrikk åpnet på Sortland – selskapet Zooca står bak (NRK). Available online at: https://www.nrk.no/nordland/verdens-forste-raudate-fabrikk-apnet-pa-sortland-_-selskapet-zooca-star-bak-1.15631628 (Accessed June 15, 2024).
Dunn D. C., Maxwell S. M., Boustany A. M., and Halpin P. N. (2016). Dynamic ocean management increases the efficiency and efficacy of fisheries management. Proc. Natl. Acad. Sci. 113, 668–673. doi: 10.1073/pnas.1513626113
Eggen M. (2017). Ikke rør raudåta! (Tronderbladet). Available online at: https://www.tronderbladet.no/meninger/i/ppPOKw/ikke-ror-raudata (Accessed June 15, 2024).
Emborg J., Daniels S. E., and Walker G. B. (2020). A framework for exploring trust and distrust in natural resource management. Front. Commun. 5. doi: 10.3389/fcomm.2020.00013
Falk-Petersen S., Pavlov V., Timofeev S., and Sargent J. R. (2007). “Climate variability and possible effects on arctic food chains: The role of Calanus,” in Arctic Alpine Ecosystems and People in a Changing Environment. Eds. Ørbæk J. B., Kallenborn R., Tombre I., Hegseth E. N., Falk-Petersen S., and Hoel A. H. (Springer Berlin Heidelberg, Berlin, Heidelberg), 147–166. doi: 10.1007/978-3-540-48514-8_9
Fasoulis I. (2021). Governing the oceans: A study into Norway’s ocean governance regime in the wake of United Nations Sustainable Development Goals. Regional Stud. Mar. Sci. 48, 101983. doi: 10.1016/j.rsma.2021.101983
Fiskeridirekteratet (2016). Forvaltningsplan for raudåte (Fiskeridirektoratet). Available online at: https://www.fiskeridir.no/Yrkesfiske/Dokumenter/Rapporter/2016/Forvaltningsplan-for-raudaate (Accessed June 15, 2024).
Ford E. and Stewart B. D. (2021). Searching for a bridge over troubled waters: An exploratory analysis of trust in United Kingdom fisheries management. Mar. Policy 132, 104686. doi: 10.1016/j.marpol.2021.104686
Forman E. (2023). During protest in harbor, fishermen say fish rules need more leeway (Gloucester Daily Times). Available online at: https://fisherynation.com/archives/124561 (Accessed October 21, 2024).
Guo J., Ma Y., and Lee J. H. W. (2021). Real-time automated identification of algal bloom species for fisheries management in subtropical coastal waters. J. Hydro-environment Res. 36, 1–32. doi: 10.1016/j.jher.2021.03.002
Hansen C., Skogen M., Rong Utne K., Broms C., Strand E., and Sætre Hjøllo S. (2021). Patterns, efficiency and ecosystem effects when fishing Calanus finmarchicus in the Norwegian Sea—using an individual-based model. Mar. Ecol. Prog. Ser. 680, 15–32. doi: 10.3354/meps13942
Hays G., Richardson A., and Robinson C. (2005). Climate change and marine plankton. Trends Ecol. Evol. 20, 337–344. doi: 10.1016/j.tree.2005.03.004
Hazen E. L., Scales K. L., Maxwell S. M., Briscoe D. K., Welch H., Bograd S. J., et al. (2018). A dynamic ocean management tool to reduce bycatch and support sustainable fisheries. Sci. Adv. 4, eaar3001. doi: 10.1126/sciadv.aar3001
Heldahl H.Ø. (2019). Får lov å fiske Raudåte selv etter feil i prøvetakingen (NRK). Available online at: https://www.nrk.no/nordland/far-lov-a-fiske-raudate-selv-etter-feil-i-provetakingen-1.14795544 (Accessed June 15, 2024).
Hogrenning E. (2023). En studie av det norske fisket etter raudåte. Kan aktivitetsnivået i fisket påvirkes av forhold i andre fiskeri? Nofima AS. Available online at: https://nofima.brage.unit.no/nofima-xmlui/handle/11250/3072881 (Accessed October 22, 2024).
Honarmand Ebrahimi S., Ossewaarde M., and Need A. (2021). Smart fishery: A systematic review and research agenda for sustainable fisheries in the age of AI. Sustainability 13, 6037. doi: 10.3390/su13116037
Hothorn T., Hornik K., Wiel M. A., and van de, Zeileis A. (2008). Implementing a Class of Permutation Tests: The coin Package. J Stat. Softw. 28, 1–23. doi: 10.18637/jss.v028.i08
Jenssen E. (2022). Taper én voksen torsk per tonn raudåte (kystogfjord.no). Available online at: https://www.kystogfjord.no/nyheter/i/dw1loo/taper-en-voksen-torsk-per-tonn-raudaate (Accessed October 21, 2024).
Jiang Y., Huang L., Liu Y., and Wang S. (2024). Impact of digital development and technology innovation on the marine fishery economy quality. Fishes 9, 266. doi: 10.3390/fishes9070266
Knol-Kauffman M., Nielsen K. N., Sander G., and Arbo P. (2023). Sustainability conflicts in the blue economy: planning for offshore aquaculture and offshore wind energy development in Norway. Maritime Stud. 22, 47. doi: 10.1007/s40152-023-00335-z
Lindbæk E. (2020). Kysten mangler raudåte – men forskerne har ingen klare indikasjoner på kraftig nedgang (+) (fiskeribladet.no). Available online at: https://www.fiskeribladet.no/nyheter/kysten-mangler-raudate-men-forskerne-har-ingen-klare-indikasjoner-pa-kraftig-nedgang/2-1-846943 (Accessed June 15, 2024).
Lindbæk E. (2022a). Fiskerne opplever «kul på havet» med lodde, men kvoten er minimal (Fiskeribladet). Available online at: https://www.fiskeribladet.no/fiskeri/fiskerne-opplever-kul-pa-havet-med-lodde-men-kvoten-er-minimal/2-1-1180159 (Accessed October 10, 2024).
Lindbæk E. (2022b). Raudåteprodusenten Zooca: Bare 30 kilo bifangst i et trålhal med fem tonn raudåte (fiskeribladet.no). Available online at: https://www.fiskeribladet.no/fiskeri/raudateprodusenten-zooca-bare-30-kilo-bifangst-i-et-tralhal-med-fem-tonn-raudate/2-1-1222008 (Accessed June 15, 2024).
Lindeque P. K., Harris R. P., Jones M. B., and Smerdon G. R. (1999). Simple molecular method to distinguish the identity of Calanus species (Copepoda: Calanoida) at any developmental stage. Mar. Biol. 133, 91–96. doi: 10.1007/s002270050446
Lucchetti A., Melli V., and Brčić J. (2023). Editorial: Innovations in fishing technology aimed at achieving sustainable fishing. Front. Mar. Sci. 10. doi: 10.3389/fmars.2023.1310318
Martinussen T. M. (2022). Direktoratet vil åpne for at mer raudåte kan fiskes kystnært [WWW Document]. fiskeribladet.no. Available online at: https://www.fiskeribladet.no/reguleringer/direktoratet-vil-apne-for-at-mer-raudate-kan-fiskes-kystnart/2-1-1347096 (Accessed October 21, 2024).
Maxwell S. M., Hazen E. L., Lewison R. L., Dunn D. C., Bailey H., Bograd S. J., et al. (2015). Dynamic ocean management: Defining and conceptualizing real-time management of the ocean. Mar. Policy 58, 42–50. doi: 10.1016/j.marpol.2015.03.014
McEvily B. and Tortoriello M. (2011). Measuring trust in organisational research: Review and recommendations. J. Trust Res. 1, 23–63. doi: 10.1080/21515581.2011.552424
McHugh M. L. (2012). Interrater reliability: the kappa statistic. Biochem. Med. (Zagreb) 22, 276–282. doi: 10.11613/BM.2012.031
Norges Fiskarlag National Board (2021). Krever raudåte-stopp. Available online at: https://fiskarlaget.no/krever-raudate-stopp/ (Accessed June 15 2024).
Norwegian Directorate of Fisheries (2023). Fartøyregisteret. Available online at: https://www.fiskeridir.no/registre/fartoyregisteret/fartoy-og-eieropplysninger (Accessed May 24, 2025).
Norwegian Directorate of Fisheries (2025). Fiskermanntallet. Available online at: https://www.fiskeridir.no/statistikk-tall-og-analyse/data-og-statistikk-om-yrkesfiske/fiskere-i-fiskermanntallet (Accessed May 24, 2025).
Norwegian Ministry of Trade, Industry and Fisheries (2019). Blue Opportunities: The Norwegian Government’s updated ocean strategy (Norwegian Ministry of Trade, Industry and Fisheries). Available online at: https://www.regjeringen.no/globalassets/departementene/nfd/dokumenter/strategier/w-0026-e-blue-opportunities_uu.pdf (Accessed June 5, 2024).
Olsen R. E., Henderson R. J., Sountama J., Hemre G. I., Ringø E., Melle W., et al. (2004). Atlantic salmon, Salmo salar, utilizes wax ester-rich oil from Calanus finmarchicus effectively. Aquaculture 240, 433–449. doi: 10.1016/j.aquaculture.2004.07.017
Ordoñez-Gauger L., Richmond L., Hackett S., and Chen C. (2018). It’s a trust thing: Assessing fishermen’s perceptions of the California North Coast marine protected area network. Ocean Coast. Manage. 158, 144–153. doi: 10.1016/j.ocecoaman.2018.03.034
Pinti J., Jónasdóttir S. H., Record N. R., and Visser A. W. (2023). The global contribution of seasonally migrating copepods to the biological carbon pump. Limnol. Oceanogr. 68, 1147–1160. doi: 10.1002/lno.12335
PytlikZillig L. M., Hamm J. A., Shockley E., Herian M. N., Neal T. M. S., Kimbrough C. D., et al. (2016). The dimensionality of trust-relevant constructs in four institutional domains: results from confirmatory factor analyses. J. Trust Res. 6, 111–150. doi: 10.1080/21515581.2016.1151359
Rozin P. and Royzman E. B. (2001). Negativity Bias, Negativity Dominance, and Contagion. Pers. Soc. Psychol. Rev. 5, 296–320. doi: 10.1207/S15327957PSPR0504_2
Sandnes Ø. (2022). – Totalt uakseptabelt at myndighetene tillater raudåtetråling innenfor grunnlinjene (fiskeribladet.no). Available online at: https://www.fiskeribladet.no/debatt/-totalt-uakseptabelt-at-myndighetene-tillater-raudatetraling-innenfor-grunnlinjene/2-1-1339979 (Accessed October 21, 2024).
Schroeder S. A., Landon A. C., Cornicelli L., Fulton D. C., and McInenly L. (2021). Institutional trust, beliefs, and evaluation of regulations, and management of chronic wasting disease (CWD). Hum. Dimensions Wildlife 26, 228–244. doi: 10.1080/10871209.2020.1808915
Siegrist M. (2021). Trust and risk perception: A critical review of the literature. Risk Anal. 41, 480–490. doi: 10.1111/risa.13325
Siegrist M., Cvetkovich G., and Roth C. (2000). Salient value similarity, social trust, and risk/benefit perception. Risk Anal. 20, 353–362. doi: 10.1111/0272-4332.203034
Silva M. R. O., Pennino M. G., and Lopes P. F. M. (2021). Predicting potential compliance of small-scale fishers in Brazil: The need to increase trust to achieve fisheries management goals. J. Environ. Manage. 288, 112372. doi: 10.1016/j.jenvman.2021.112372
Stern M. J. and Coleman K. J. (2015). The multidimensionality of trust: applications in collaborative natural resource management. Soc. Natural Resour. 28, 117–132. doi: 10.1080/08941920.2014.945062
Stuart A. (1955). A test for homogeneity of the marginal distributions in a two-way classification. Biometrika 42, 412–416. doi: 10.2307/2333387
Unstad K. H. and Tande K. S. (1991). Depth distribution of Calanus finmarchicus and C. glacialis in relation to environmental conditions in the Barents Sea. Polar Res. 10, 409–420. doi: 10.3402/polar.v10i2.6755
Vinuesa R., Azizpour H., Leite I., Balaam M., Dignum V., Domisch S., et al. (2020). The role of artificial intelligence in achieving the Sustainable Development Goals. Nat. Commun. 11, 233. doi: 10.1038/s41467-019-14108-y
Keywords: fisheries conflict, fisheries management, real-time management, scenario-based experiment, trust
Citation: Crosman KM, Hayes AL, Davies EJ and Majaneva S (2025) Conflict, cod and Calanus: can technology increase trust in management of a contested fishery? Front. Mar. Sci. 12:1572772. doi: 10.3389/fmars.2025.1572772
Received: 07 February 2025; Accepted: 03 September 2025;
Published: 01 October 2025.
Edited by:
Jianchuan Yin, Guangdong Ocean University, ChinaReviewed by:
Elizabeth Drury O’Neill, Stockholm University, SwedenMargaret W Wilson, University of California, Santa Barbara, United States
Tanguy Sandré, UMS3342 Observatoire des sciences de l’univers de l’UVSQ (OVSQ), France
Copyright © 2025 Crosman, Hayes, Davies and Majaneva. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Katherine M. Crosman, a2F0aGVyaW5lLm0uY3Jvc21hbkBudG51Lm5v
†Present addresses: Katherine M. Crosman, Department of Climate and Environment SINTEF Ocean, Trondheim, Norway
Sanna Majaneva, Akvaplan-niva, Trondheim, Norway
 Katherine M. Crosman
Katherine M. Crosman Adam L. Hayes
Adam L. Hayes Emlyn J. Davies3
Emlyn J. Davies3