- 1Instituto de Acústica, Universidad Austral de Chile, Valdivia, Chile
- 2Programa de Doctorado en Ciencias de la Ingeniería, Pontificia Universidad Católica de Chile, Santiago, Chile
- 3Instituto de Ciencias Naturales Alexander von Humboldt, Facultad de Ciencias del Mar y Recursos Biológicos, Universidad de Antofagasta, Antofagasta, Chile
- 4CETALAB, Laboratory for the Study of Marine Megafauna, Antofagasta, Chile
Fisheries bycatch is one of the main threats for porpoise species worldwide. In the Humboldt Current system of northern Chile, the elusive Burmeister’s porpoise (Phocoena spinipinnis) inhabits the coastal waters of Mejillones Bay and suffers bycatch mortality in purse seine fishing nets and coastal gillnets. In order to mitigate bycatch of this near-threatened species, this study aimed to (1) analyze the diurnal and nocturnal acoustic behaviors of the Burmeister’s porpoise and (2) evaluate the potential of banana pingers as acoustic deterrent tools. For 27 days during austral summer, a full wave form capture porpoise detector was anchored 7 m above sea level in Mejillones Bay where Burmeister’s porpoises are frequently observed. During this time, the detector registered acoustic activity continuously over 13 days, and a banana pinger deployed over 100 m away from the detector emitted high frequency sounds continuously for 14 days. The results show that the number of clicks and detection-positive minutes were significantly higher while the inter-click intervals and high click rate feeding buzz were significantly lower at night, indicating that Burmeister’s porpoises forage mainly nocturnally in this bay. With pingers present, the probability of detection of Burmeister’s porpoise acoustic activity decreased by 20%, suggesting that pingers could be an effective tool to avoid Burmeister’s porpoise mortality in fishing nets. Future studies should implement pingers in artisanal purse seine fishing nets and coastal gillnets in order to mitigate Burmeister’s porpoise bycatch in northern Chile.
1 Introduction
Bycatch (the incidental, unmanaged catch of non-target species during fishing activities) is a prevalent threat to the survival of marine vertebrates (Davies et al., 2009) and occurs in all commercial fishing operations throughout the globe (Read et al., 2006). In fact, annual bycatch estimates of 38,505,242 tons represent 40.4% of global fisheries catch (Davies et al., 2009). Vertebrates like sea birds, sea turtles, sharks and marine mammals are more prone to die in fishing nets because they are secondary consumers or top predators of the marine trophic chain; long lifespans and low reproduction rates, such mortality can have detrimental long-term consequences for their abundance (Read et al., 2006), especially for cetaceans. The International Whaling Commission (2023) estimates that 300,000 cetaceans are captured and killed as bycatch every year worldwide (IWC, 2023). Some countries in the northern hemisphere, such as the United Kingdom, have implemented bycatch quantification and regulation measurements, but such information is limited in the southern hemisphere (Northridge et al., 2016; 2019). In the United States, 3,029 small cetaceans were estimated to be caught between 1990 and 1999 (Read et al., 2006), whereas 2019 bycatch records in the United Kingdom registered 833 harbor porpoises (Phocoena phocoena), 278 short-beaked common dolphins (Delphinus delphis) and 278 seals (Halichoerus grypus) (Kingston et al., 2019).
Porpoises are especially vulnerable to commercial fishing bycatch as they inhabit coastal waters and prey on the same target species than humans. Bycatch mortality is more prone to negatively impact cetacean population sizes due to lower reproductive rates and late age of sexual maturity compared to other marine mammals. Due to the increase of the illegal fishery of the endangered totoaba (Totoaba macdonaldi) fish in the Gulf of California, Mexico, the use of gillnets has resulted in recent dramatic die-offs of the vaquita porpoise (Phocoena sinus). This porpoise is one of the most endangered species in the world, as of 2020, just 10 remaining individuals of this species were recorded (Rojas-Bracho et al., 2022). Less-studied porpoise species, like the Burmeister’s porpoise (Phocoena spinipinnis), are also subject to considerable bycatch pressure. Present in both the Southeast Pacific and Southwest Atlantic, its distribution ranges from northern Peru (ca. 5°S) to Southern Brazil (ca. 28°S) and includes the Falkland Islands (Weir and Rutherford, 2019). Along the coasts of Peru and Chile, the Burmeister’s porpoise is increasingly used as bait for king crab (Lithodes santolla), false king crab (Paralomis granulosa) and shark fisheries. The Burmeister’s porpoise is cataloged as near threatened by the red list of threatened species of the International Union for the Conservation of Nature (Félix et al., 2018; Van Waerebeek et al., 1997; Mangel et al., 2010; Lescrauwaet and Gibbons, 1994). Due to its elusive behavior, few studies have documented the abundance of Burmeister’s porpoise. Most studies available on this species have been conducted on carcasses stranded on the beaches or landed at ports (Van Waerebeek et al., 2018; Santillán, 2022; Garcia-Cegarra et al., 2024), making it difficult to assess the species ecology and its interactions with fisheries activities.
Some studies suggest that high frequency acoustic deterrent devices, also known as pingers, may decrease bycatch of small cetaceans such as porpoises (Kraus et al., 1997). These acoustic alarms are placed along fishing nets and emit high frequency sound pulses that deter porpoises from approaching the net, therefore reducing the risk of entanglement (Dawson and Lusseau, 2013). Pingers appear to be useful tools in reducing harbor porpoise bycatch in the North Atlantic (Dawson and Lusseau, 2013; Kindt-Larsen et al., 2019; McGarry et al., 2020; Brennecke et al., 2022; Königson et al., 2022). Pilot experiments also demonstrate that active pingers decreased the probability of detection of harbor porpoises by 37% in the United Kingdom and 50% in Sweden (Omeyer et al., 2020; Aksoy, 2022). In Peru, Mangel et al. (2013) and Clay et al. (2018) both demonstrated that pingers attached to gillnets reduced the probability of bycatch of Burmeister’s porpoises by 50% and 86%, respectively.
Burmeister’s porpoises have also been observed in Chile, specifically in the western half of Mejillones Bay in northern Chile (García-Cegarra et al., 2024). They are observed throughout the year with an increased presence of mother–calf pairs during austral spring and summer. Abundance estimates in Mejillones Bay show 76 individuals (CV = 25.9%) at a density of 0.45 individuals/km2 (CV = 26%) (Garcia-Cegarra et al., 2024). This porpoise’s distribution overlaps with artisanal and commercial purse seine fishing vessel routes and operations (García and Pacheco, 2019; García-Cegarra et al., 2021, 2024). This leads high rates of mortality via gillnets targeting Chilean silverside (Odontesthes regia) and purse seine nets targeting the Peruvian anchovy (Engraulis ringens), which are the primary forage species for the porpoise (García-Godos et al., 2007; García-Cegarra et al., 2024). In 2022, changes to Chilean fisheries regulation for the Peruvian anchovy led to an increase in the number of artisanal purse seine vessels in Mejillones Bay (from 16 vessels in 2019 to 45 vessels in 2024) (García-Cegarra and Pacheco, 2019; SERNAPESCA, 2023). Because this policy change is likely to augment the bycatch mortality of Burmeister’s porpoise in the area, the use of acoustic deterrent tools such as pingers may help mitigate the impact of increased fishery activity.
The Burmeister’s porpoise’s acoustics have not yet been studied in Chile. Just one study performed in Tierra del Fuego, Argentina indicates that the Burmeister’s porpoise emits sounds at a median centroid frequency of 144 ± 5 [138 157] kHz and peak frequency at 135 ± 2 [133 164] kHz with a wave spectrum of narrow band high frequencies (NBHF) median -3 dB and -10 dB (Reyes et al., 2018). The Burmeister’s porpoise’s acoustics has yet to be studied in Chile, a key first step in attenuating bycatch mortality. In this study, we aim to characterize the diurnal and nocturnal acoustic behaviors of the Burmeister’s porpoise and assess whether pingers may act as an effective deterrent by decreasing the probability of acoustic detection of Burmeister’s porpoise in the vicinity.
2 Methods
2.1 Study area
Mejillones Bay is located in northern Chile’s Antofagasta Region. This bay extends 18 km from Punta Angamos (23°1’42” S, 70°30’31’’ W) to Chacaya Beach (22°57’39’’ S, 70°18’31” W) (Figure 1A). The constant upwelling cells along the Mejillones Peninsula fuels primary production and, consequently, the Humboldt Current system marine trophic chain, of which the Peruvian anchovy is a keystone species. This allows a high biodiversity of cetaceans, sea lions and seabirds in the area (García-Cegarra et al., 2021; Garcia-Cegarra and Martínez-López, 2023). The bay is characterized by the presence of sandy beaches, the city of Mejillones is an industrial port containing nine maritime terminals for the import and export of mining supplies. In addition, the area hosts one of the most important artisanal fishing industries in northern Chile with a total of 45 both artisanal and commercial purse seine fishing vessels as well as 68 artisanal coastal gillnet fishing vessels registered by the National Service of Aquaculture and Fisheries (SERNAPESCA, 2023). The primary fisheries targeted in this region are the Peruvian anchovy and the Pacific bonito (Sarda chilensis).
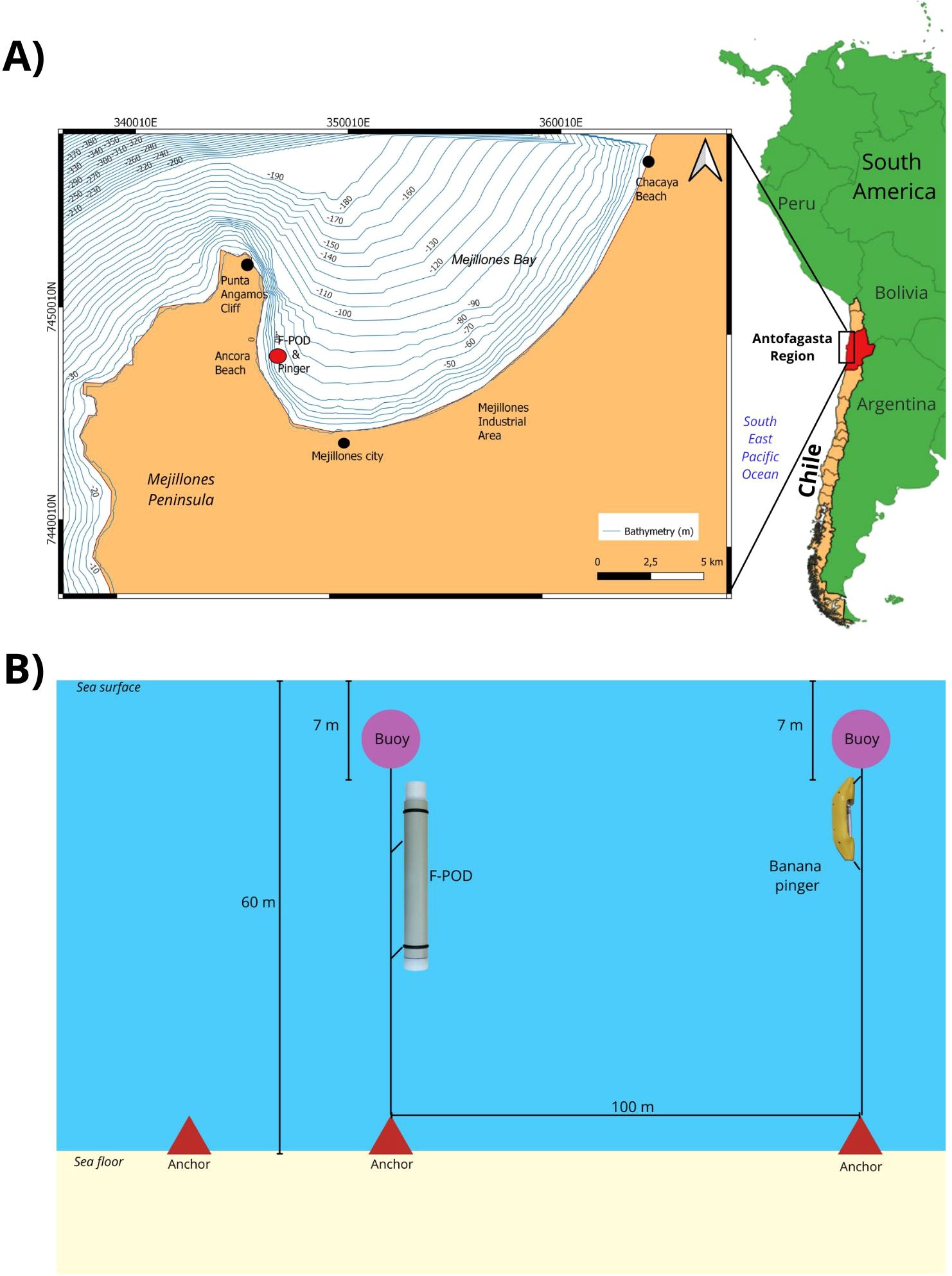
Figure 1. (A) Study area map of Mejillones Bay in Northern Chile and point of installation of F-POD in the western boundary of the bay where Burmeister’s porpoises are frequently observed; (B) pilot study performed with the draw of the pinger and F-POD installation.
2.2 Data collection and experiment design
We deployed a passive acoustic logger hydrophone full waveform capture porpoise detector (F-POD; Chelonia Limited, www.chelonia.co.uk) on the western boundary of Mejillones Bay from January 29 to February 11, 2023 at 60 m depth in the area were Burmeister’s porpoises are usually observed at high density (23°4”108’S, 70°29”902’W) (García-Cegarra et al., 2024) (Figure 1A). The F-POD was 67 cm in length and 9 cm in diameter and weighed 3.5 kg. The device contained 10 alkaline cell batteries allowing a maximum autonomous running time of 200–212 days, recording ambient water temperature every minute. Its polypropylene housing with 0.7 kg buoyancy allowed the device to self-orient vertically, permitting a maximum porpoise detection range of 400 m, and reduce surface noise for its 20–160 kHz omni-directional hydrophone. It contained a removable 8 GB SD card to store porpoise click information and digital time domain wave form using 5 μs resolution duration, intensity, band width, frequency and envelope criteria to select possible cetacean clicks in the range of 20–160 kHz. Permits to anchor the F-POD on the floor of the Mejillones Bay for the duration of the study were obtained from the Chilean Army Oceanographic and Hydrographic Service (SHOA R.E. No. 8476-2022) (Figure 1B). During the installation, Burmeister’s porpoises were observed within 1 km of the installation point. The F-POD was installed 7 m below sea surface, and abuoy was placed to indicate its location.
On February 11, 2023, we retrieved the F-POD and its data. Simultaneously, a pinger was deployed 100 m away from the FPOD (23°4032’S, 70° 29.936’W) at the same 60 m depth, and at 7 m below sea surface (Figure 1B). The Banana Pinger (Fishtek Marine, UK) measured 185 x 52 x 42 mm, weighed 229 g, and emitted frequencies of 50–120 kHz with harmonics and a sound level of 145 ± 3dB at 1m. Pings lasted 300 ms with randomized ping intervals every 4–12 s in accordance with the European Legislation EC 812/2004. The pinger was active from February 11–25, 2023. On February 25, we recovered the pinger, F-POD, and remaining instruments on the sea floor.
2.3 Data analysis
Using the F-Pod.exe software (Chelonia Limited, Build 1.0), we analyzed the F-POD audio clip based on frequency peaks of 125–145 kHz and number of clicks in a determined period (termed”click trains”), ranging 15–100 per s. We filtered NBHF between 130 and 160 kHz for porpoise detection clicks. The acoustic response variables we analyzed were: (1) spectrogram and wave form, which describes how pressure varies over time, as well as the click’s envelope attack, sustain and release; (2) number of clicks, or the total amount of clicks detected in a 60 min period; (3) inter-click interval (ICI), or the time between consecutive clicks of a train; (4) detection-positive minutes (DPM), or the amount of min that clicks were detected in a 60 min period; (5) buzzfeed, or the amount of potential high click rate feeding buzzat the end of the prey approach during foraging when the ICI was less than10,000 μs (Todd et al., 2023; Nuuttila et al., 2013); and (6) click train duration, or the duration of a click train independent of the number of clicks that it contains. The software allows selecting these acoustic variables and exporting it in a.csv format to be visualized in Excel (Microsoft Office, Build 2304). We categorized Burmeister’s porpoises as either absent (i.e., when clicks were not recorded) or present (i.e., when clicks were recorded in 1h) for the analysis. Designating day as 08:00 to 19:59 and night as 20:00 to 07:59, we then associated porpoise absence/presence with day and night. We also included the absence/presence of the pinger as a factor in our analysis.
We conducted our statistical analyses in R Studio (R Core Team, 2023). For descriptive analysis, we performed a Shapiro–Wilk normality test to assess data normality distribution. On data that met the normality criteria, we conducted an analysis of variance and designated a p-value < 0.05 as significant when compared to the control (i.e., acoustic response variables among the factors with/without pinger and day/night). We calculated the probability of F-POD acoustic detection of the Burmeister’s porpoise as the probability of a positive acoustic detection within 1 hour, regardless of the total number of detections.
3 Results
With the F-POD, we registered 27 days of acoustic records, obtaining a total of 639 hours of records, of which 13 days (307 hours) were without pinger and 14 days (332 hours) were with pinger. During the first day of the F-POD installation, a group of eight Burmeister’s porpoises were observed within 50–100 m of the anchoring point, corroborating the presence of Burmeister’s porpoises at the study site. The F-POD registered 102 detections without pinger and 40 detections with pinger. The device registered 118,082 clicks at NBHF, of which 89,634 were without pinger and 28,448 with pinger (Figure 2A). The average registered DPM was 5.94 (± SD = 4.49) without pinger and 4.35 (± SD = 2.99) with pinger (Figure 2B). Clicks recorded with the F-POD had 10 recordings of pressure, allowing the F-POD software to calculate a waveform of 100–200 μs (Supplementary Figure S1). For a total of 5,451 clicks identified by the software, the average peak frequency was 132.12 kHz (± SD = 4.04). The ICI average was 23,582.65 μs (± SD = 28,928.63) and sound pressure level of 150 (dimensions are not defined in the F-POD documentation).
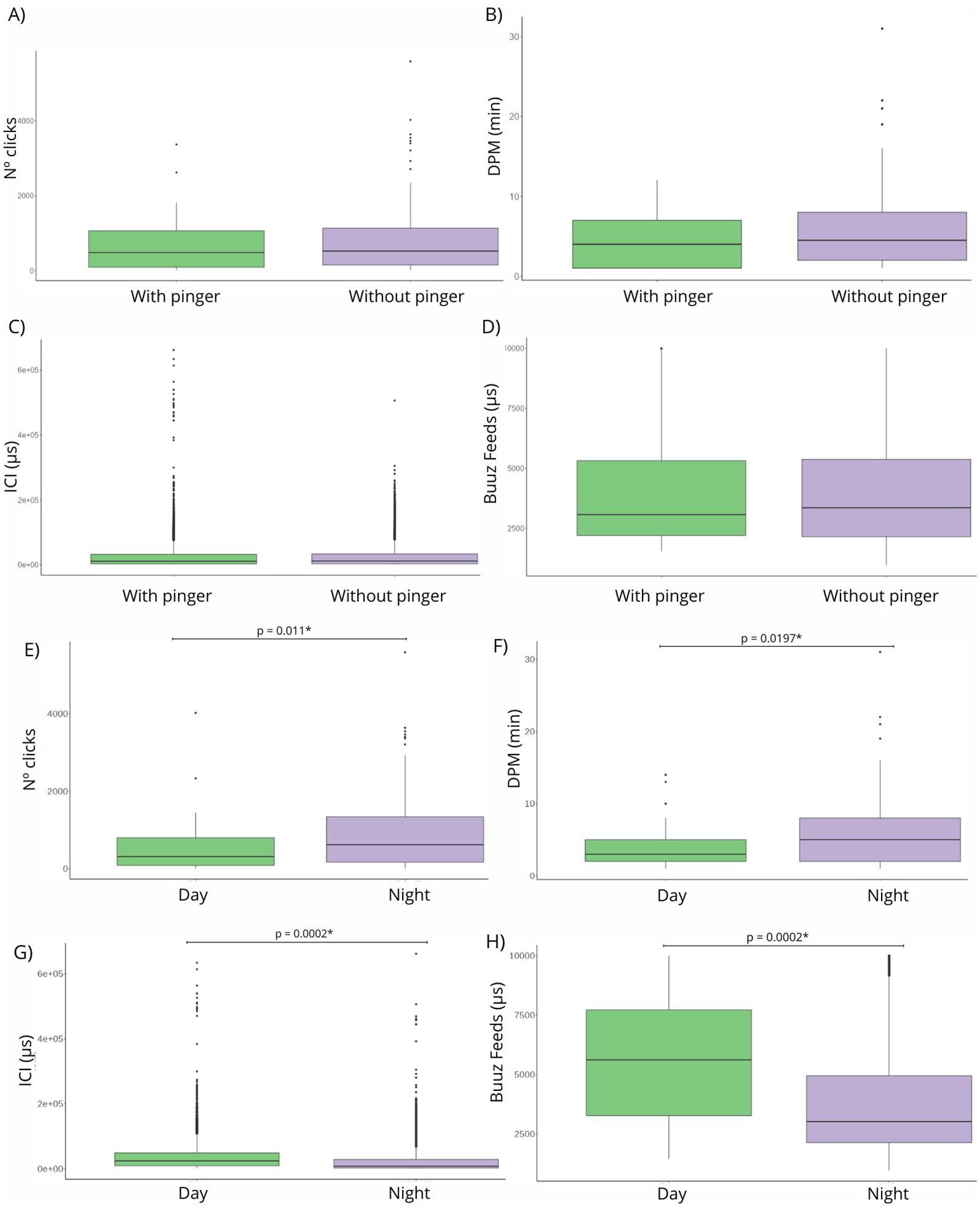
Figure 2. Boxplot showing significant differences (p-value< 0.05) in the acoustic response variables: (A) number of clicks, (B) detection positive minutes (DPM), (C) inter-click interval (μs), and (D) buzzfeeds (μs) and with and without pinger (E) number of clicks, (F) detection positive minutes (DPM), (G) inter-click interval (ICI) (μs), and (H) buzzfeeds (μs) between day and night.
3.1 Burmeister’s porpoise acoustic differences between day and night
Of the total clicks registered, 77% occurred at night. During 24 hours, more clicks were detected between 17:00 and 04:00 (i.e., nighttime) than between 09:00 and 16:00 (i.e., daytime), a difference found to be significant by a Kruskal–Wallis test (Figure 2E). The DPM analysis resulted in an average of 3.94 (± SD = 3.15) during daytime and an average of 5.77 (± SD = 4.62) during nighttime, another difference found to be significant by a Kruskal–Wallis test (Figure 2F). ICI values were significantly lower at night (ICI day average = 32,056.21 μs, ± SD = 30,996.03; ICI night average = 20,337.39 μs, ± SD = 25,258.53) (Figure 2G). Overall, these results indicate that foraging buzzfeeds are performed during night as the inter-click interval is lower in the night. During the day, 27% are buzzfeeds, which significantly increase to 54% at night (Figure 2H). Buzzfeeds were not detected from 7:00 to 11:00 and from 12:00 to 16:00 in Mejillones Bay. Click train duration was higher at night, but not significantly.
3.2 Acoustic differences with and without pingers presence
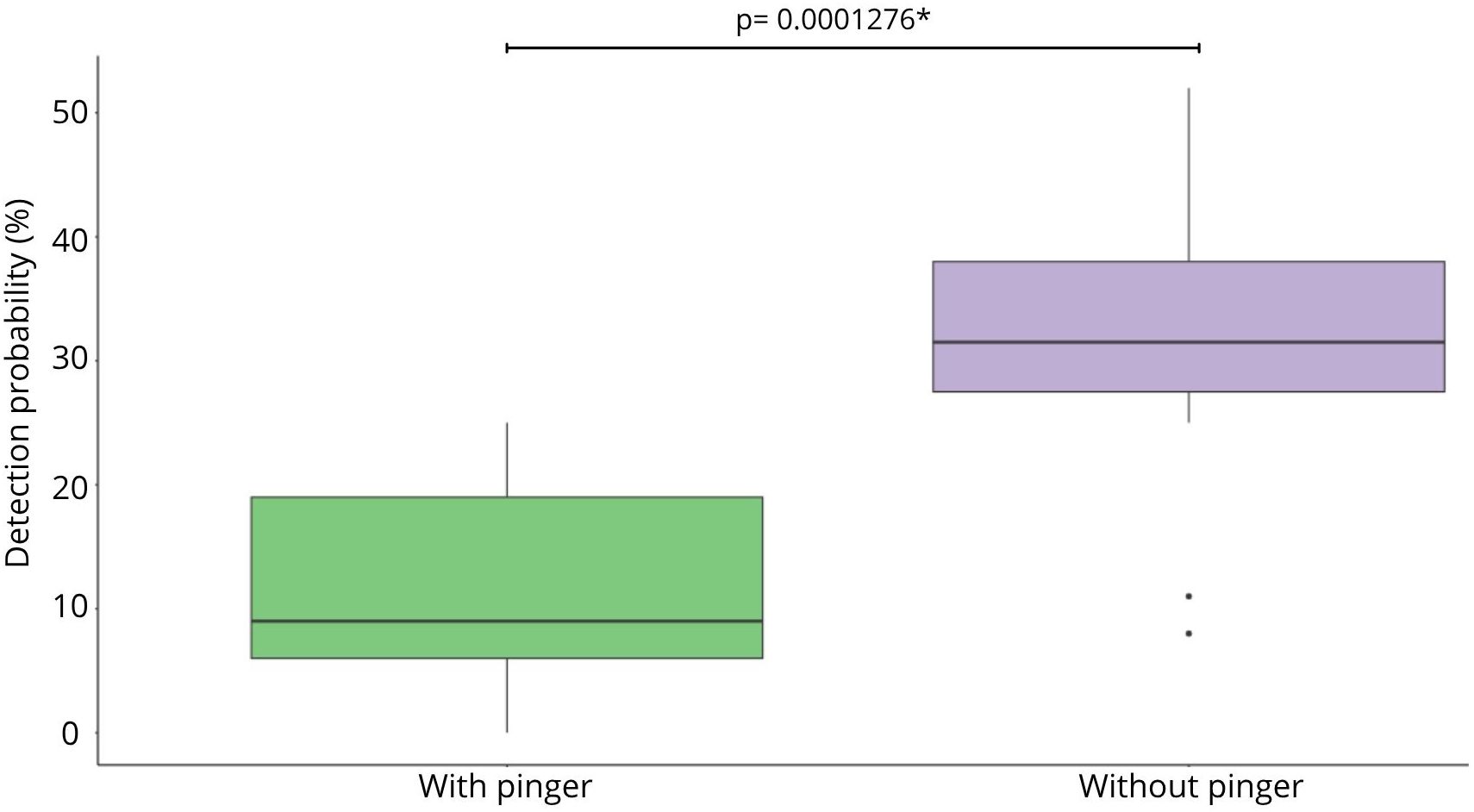
The DPM results were not significantly higher without pinger than with, showing also higher values among 20 and 30 DPM in the pinger’s absence. When the pinger was present, DPM values were lower than 15 DPM. Without pinger, 75% of the clicks occurred at night, and with pinger, 84% of the clicks occurred at night (Figure 3A). Independent of the pinger’s absence/presence, the DPM results are higher at night (Figure 3B) (Supplementary Table 1). The number of clicks detected by the F-POD was higher without pinger, but not significantly. The ICI detected were significantly lower with the pingers present (Figure 3C). Buzzfeeds also decreased with the pinger present, but not significantly, and continued throughout the night, regardless of the pinger’s absence/presence (Figure 3D). Click trains lasted longer with pinger, but not significantly (Supplementary Table 1). A chi-squared test highlighted significant differences among total NBHF sounds detected with and without pinger (Figure 4). Therefore, the probability of detection of acoustic activity without pinger was 32% and, with pinger, it decreased significantly to 12% (Figure 4).
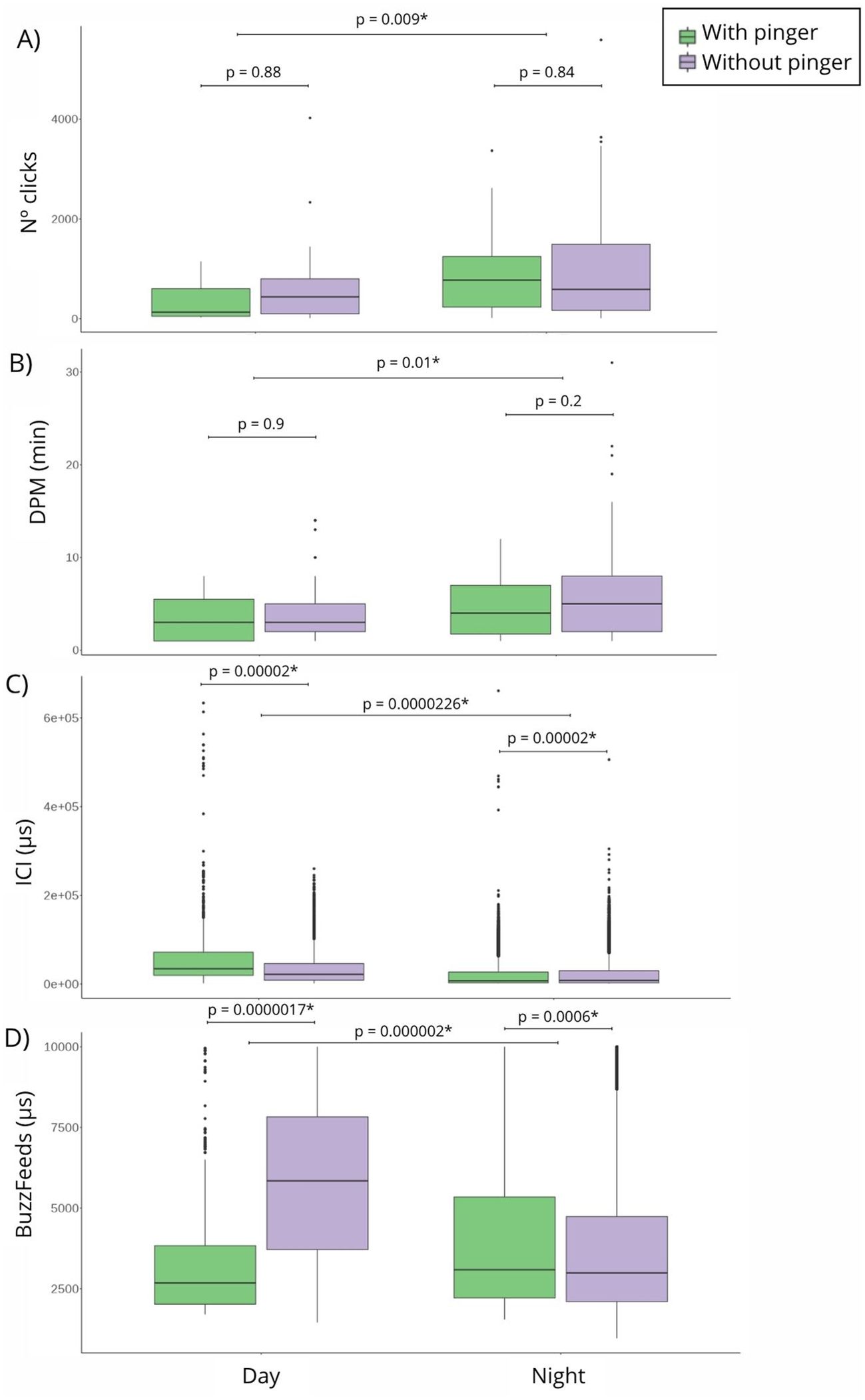
Figure 3. Differences (p-value< 0.05) between day and night with and without pinger in the acoustic response variables: (A) number of clicks, (B) detection-positive minutes, (C) inter-click interval (μs) and (D) buzzfeeds (μs).
4 Discussion
This study characterizes the presence of Burmeister’s porpoises in northern Chile by acoustic identification via deploying an F-POD in the area where the species have been observed in Mejillones Bay. Throughout the study period, acoustic activity during nocturnal foraging was detected by way of analyzing buzzfeeds and ICI. We also piloted the use of a deterrent pinger near the F-POD anchorage point, which significantly decreased the probability of acoustic detection of the animals. These results indicate that Burmeister’s porpoises forage more frequently at night in Mejillones Bay and that the implementation of pingers in artisanal purse seine and gillnets in the bay could be an effective tool to mitigate their bycatch.
4.1 Acoustic behavior in Mejillones Bay
The acoustic wave form detected in this study averaged a frequency of 132.13 kHz, a result comparable to that reported by Reyes et al. (2018) for Burmeister’s porpoises observed in Tierra del Fuego (Argentinean Patagonia) of 135 ± 2 [133 164] kHz. According to the acoustic variables analyzed here, Burmeister’s porpoises in Mejillones Bay emit an average of 5.49 DPM per hour, 40,868.84 μs of ICI and 84.14 buzzfeeds per hour. In Peru, Clay et al. (2018) found a DPM of 0.01 in 21 hour. The stark difference may be due to methodological discrepancies; the effort of Clay et al. (2018) was 30 times lower than ours, and they utilized acoustic devices deployed on boat trips, therefore augmenting the ambient noise levels and potentially deterring animals unknowingly. Aksoy (2022) followed methodology more comparable to ours on harbor porpoises in Sweden, resulting in DPM values similar to ours.
With the F-POD’s capacity to continually monitor acoustics, we obtained both diurnal and nocturnal patterns. Porpoises are often known to be nocturnal and demonstrate higher acoustic activity atnight (Osiecka and Wahlberg, 2020). This study represents the first formal documentation to our knowledge that Burmeister’s porpoises forage mainly at night in the Humboldt Current system. We found significant differences for the acoustic variables ICI, DPM, number of clicks and buzzfeeds analyzed between day and night. With a greater number of clicks and lower values of ICI detected at night, our results suggest that foraging buzzfeeds were more present in the night than during the day.
According to Garcia-Godos et al. (2007), the Peruvian anchovy comprise 88.9% of the diet of the Burmeister’s porpoises on the coast of Peru, followed by the Chilean silverside (Odontesthes regia) at 6.5% and Chilean hake (Merlucius gayi) at 0.6%. In Mejillones Bay, the Peruvian anchovy comprise most of the fishing captures of artisanal purse seine vessels for the manufacture of fish meal and fish oil (SERNAPESCA, 2005) while Chilean silverside is usually fished in artisanal coastal gillnets for human consumption (Pupelde, 2004; Dyer, 2000). During 2022, a total of 100,000 metric tons of Peruvian anchovy were caught in Mejillones Bay, dwarfing the one metric ton catch of Chilean silverside (SERNAPESCA, 2023). However, there is also presence of squids in Mejillones Bay and it is known that harbor porpoises in the North Atlantic forage on squids (Leopold, 2015), and the vaquita in the Gulf of Mexico also forages on two squid species (Pérez-Cortés et al., 1996). Squids could be a prey for Burmeister’s porpoises during night time in Mejillones Bay. Further studies should analyze stomach content or stable isotope analyses should understand the species’ diet composition and how it may overlap with fisheries.
Due to the scarce information regarding Burmeister’s porpoise’s presence and ecology in northern Chile, future studies could implement passive acoustic monitoring tools in Mejillones Bay to detect in the species’ presence and its fluctuations. For example harbor porpoises in the United Kingdom (Nuuttila et al., 2017) have been observed to change their habitat preferences and distribution throughout the year, seasons, day movements across the bay or even with the effect of the. Other studies show how porpoises distribution or habitat use may be linked to prey distribution, which could alter acoustic behavior. Kimura et al. (2011) showed a seasonal presence of the Yangtze finless porpoise (Neophocoena asiaorientalis) related to prey fish presence. Paitach et al. (2021) found that, in the Babitonga Bay (Brazil), the acoustic behavior of the franciscana dolphin (Pontoporia blainvillei) was altered (i.e., in click emission rate and feeding buzzfeeds) when the dolphin was in the bay due to prey availability.
Previous work based on systematic abundances studies indicates that, in Mejillones Bay, Burmeister’s porpoises are mainly distributed along the western boundary (90.6 m mean depth) (Garcia-Cegarra and Pacheco, 2019; Garcia-Cegarra et al., 2024). While the western boundary is characterized by the artisanal fishing presence and tourism, the eastern boundary is characterized by the presence of large cargo vessels and industrial mining ports (Garcia-Cegarra and Pacheco, 2019). This may led to an increase of underwater noise in the industrial eastern boundary of Mejillones Bay, which may deter the presence of the Burmeister’s porpoises. One of the limitations of this study is the lack of spatial replication as the study was just performed in the western boundary of the bay and Burmeister’s porpoise’s behavior under a different sonic regime resulting from differing anthropogenic activities remains unknown. We suggest that future studies implement passive acoustic monitoring tools on both boundaries of the bay in order to assess whether industrial underwater noise affects the habitat preferences and distribution of the Burmeister’s porpoise.
4.2 Effectiveness of pingers as acoustic deterrent tools for Burmeister’s porpoises bycatch and conservation management recommendations in Chile
Acoustic response variables analyzed here—DPM, buzzfeeds, number of clicks and click train duration—did not show significant differences between the pinger absence/presence, but seem to be different anecdotally. ICI is lower with the pinger, indicating that Burmeister’s porpoise may emit more foraging acoustic behavior with the pinger present. These non-significant differences in acoustic response variables with and without pinger could be attributed to an aversive response where the porpoises moved away from the pinger and F-POD while the pinger was on, but still performed either social or foraging behaviors. However, the limitation of this study regarding the relatively short pinger testing period of 14 days may interpret the results just in the short term. Burmeister’s porpoise mating behavior and/or foraging ecology could be limited to seasonality as for example the presence of mother–calf pairs have been observed in spring and austral summer seasons in Mejillones Bay. Future studies could repeat the methodology of this pilot study for additional days and conduct another non-pinger session in order to guard against temporal differences that might have occurred by chance during the study period. However, due to the intense fishing effort in the bay and the probability that the F-POD and pingers were caught accidentally in the purse seine fishing nets, we decided perform one month of study during the Peruvian anchovy fishing ban. Despite the short testing period, this study showed that, with the pinger, the acoustic detection probability decreased from 32% to 12%. This reduction in acoustic probability is similar to that obtained by Omeyer et al. (2020) for harbor porpoises in England, whose detection probability decreased to 37% with the presence of apinger. Aksoy (2022) also showed a 50% reduction in DPM with pingers present. In Peru, Mangel et al. (2013) and Clay et al. (2018) showed a significant reduction in acoustic activity of the Burmeister’s porpoise with pingers present on artisanal fishing nets. Overall, these studies show that pingers are effective tools to deter the presence of Burmeister’s porpoises up to 400 m away. Further studies should implement pingers in artisanal purse seine nets and gillnets during fishing operations in order to assess the in situ effectiveness of pingers to deter the presence of Burmeister’s porpoises from the fishing area, especially at night.
The Burmeister’s porpoise is cataloged globally as near threatened by the IUCN red list of threatened species (Félix et al., 2018). International initiatives such as the Agreement on the Conservation of Small Cetaceans of the Baltic, North East Atlantic, Irish and North Seas (ASCOBANS) suggest that cetaceans are animals of interest. However, in Chile, protection tools beyond protocols for incidental catch of cetaceans (Ministry of Economy, Development, and Reconstruction of Chile, 1992) do not yet exist. Conservation objectives have the potential to restore and maintain stocks of small cetaceans to 80% or more of the carrying capacity (ASCOBANS, 1997). Beyond Chilean fisheries and aquaculture law, which prohibits the catch of cetacean species, efforts to reduce bycatch like conservation management plans or research to assess the conservation status of Burmeister’sporpoise does not yet exist in the Southeast Pacific. Recently, Mejillones Bay has been identified as an Important Marine Mammal Area created by the Marine Mammal Protected Areas Task Force program of the International Committee on Marine Mammal Protected Areas, IUCN and World Commission on Protected Areas. These areas are defined as discrete portions of habitat that are crucial to marine mammal species and have the potential to be delineated and managed for conservation. Due to intense bycatch mortality that Burmeister’s porpoises suffer in northern Chile, we strongly recommend the implementation of special conservation management plans in order to decrease such mortality and preserve this near threatened cetacean species.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Comite de Ética Universidad de Antofagasta. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
DD: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. AG-C: Conceptualization, Funding acquisition, Investigation, Supervision, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. Boat surveys were funded by D.D grant for field work by the University Austral de Chile Master Thesis Grants. F-POD and pingers were funded by Chelonia Limited and Fishtek Marine, UK. AG-C is funded by an ANID FONDECYT Iniciacion (N°. 11251002). Diego Díaz was funded by a Master Thesis Grant Field work of the Universidad Austral de Chile.
Acknowledgments
We thank Fishtek Marine and Chelonia Limited UK providing the F-POD and pingers. We warmly thank CSIM Limitada divers, especially Luis Aguilar, for their help during the F-POD and pinger anchoring dives.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1582414/full#supplementary-material
References
Aksoy S. B. (2022). Do potentially seal-safe pingers deter harbour porpoises (Phocoena phocoena) in the vicinity of gillnets and thereby reduce bycatch? Master thesis Appplied Ethology and Animal Biology. Department of Physics, Chemistry and Biology, Linköping University, Denmark.
ASCOBANS (1997). Report of the first meeting of the Advisory Committee on incidental catches (bycatch) of small cetaceans. ASCOBANS Secretariat.
Brennecke D., Siebert U., Kindt-Larsen L., Midtiby H., Egemose H., Ortiz S., et al. (2022). The fine-scale behavior of harbor porpoises towards pingers. Fish. Res. 255, 106437. doi: 10.1016/j.fishres.2022.106437
Clay T. A., Mangel J. C., Alfaro-Shigueto J., Hodgson D. J., and Godley B. J. (2018). Distribution and habitat use of a cryptic small cetacean, the Burmeister’s porpoise, monitored from small-scale fishery platform. Front. Mar. Sc. 5. doi: 10.3389/fmars.2018.00220
Davies R. W. D., Cripps S. J., Nickson A., and Porter G. (2009). Defining and estimating global marine fisheries bycatch. Mar. Pol. 33, 661–672. doi: 10.1016/j.marpol.2009.01.003
Dawson S. M. and Lusseau D. M. (2013). Pseudo-replication confounds the assessment of long-distance detection of gillnets by porpoises: Comment on Nielsen et al. Mar. Ecol. Prog. Ser. 478, 301–302. doi: 10.3354/meps10337
Dyer B. (2000). Revisión sistemática de los pejerreyes de Chile (Teleostei, Atheriniformes). Est. Oceanol. 19, 99–127.
Félix F., Alfaro J., Reyes J., Mangel J., Dellabianca N., Heinrich S., et al. (2018). Phocoena spinipinnis. The IUCN Red List of Threatened Species 2018: e.T17029A50370481. doi: 10.2305/IUCN.UK.2018-2.RLTS.T17029A50370481.en
García-Cegarra A. M., Hall A., and Martínez-Lopez E. (2024). Bycatch and pollution are the main threats for Burmeister’s porpoises inhabiting a high-industrialized bay in the Humboldt Current System. Environ. Res. 251, 118621. doi: 10.1016/j.envres.2024.118621, PMID: 38492834
Garcia-Cegarra A. M. and Martínez-López E. (2023). Metal concentrations in feathers of red-legged cormorants (Phalacrocorax gaimardi) and sources of plastic in a nesting colony from nothern Chile. Mar. Pol. Bull. 190, 114817. doi: 10.1016/j.marpolbul.2023.114817, PMID: 36931167
García-Cegarra A. M. and Pacheco A. S. (2019). Collision risk areas between fin and humpback whales with large cargo vessels in Mejillones Bay (23°S), Northern Chile. Mar. Pol. 103, 182–186. doi: 10.1016/j.marpol.2018.12.022
Garcia-Cegarra A. M., Toro F., and Gonzalez-Borasca V. (2021). Citizen science as a tool to assess cetacean diversity in the Atacama Desert coast. Ocean Coast. Manage. 213, 94–102. doi: 10.1016/j.ocecoaman.2021.105858
García-Godos I., Van Waerebeek K., Reyes J., and Alfaro-Shigueto J. (2007). Prey occurrence in the stomach contents of four small cetacean species in Peru. Lat. Amer. J. @ Aqua. Mamm. 6, 171–183. doi: 10.5597/lajam00122
IWC (2023). International Whalling Commission Focus on cetacean bycatch in the western central Pacific Ocean, Western and Central Pacific Fisheries Commission Scientific Committee (Koror, Palau: Western and Central Pacific Fisheries Commission (WCPFC)). August 2023. SC19-EB-WP-10.
Kimura S., Akamatsu T., Li S., Lijun D., Wang D., Wang D., et al. (2011). Seasonal changes in the local distribution of Yangtze finless porpoises related to fish presence. Mar. Mam. Sc. 28, 308–324. doi: 10.1111/j.1748-7692.2011.00490.x
Kindt-Larsen L., Berg C. W., Tougaard J., and Sveegaard S. (2019). Bycatch of harbor porpoise (Phocoena phocoena) in Danish gillnet fisheries. Fish. Res. 214, 94–102.
Kingston A. I., Thomas L., and Northridge S. (2019). UK bycatch monitoring programme report for 2019. Available online at: https://randd.defra.gov.uk/ProjectDetails?ProjectId=19943 (Accessed August 7, 2025).
Königson S., Naddafi R., Hedgärde M., Pettersson A., Östman Ö., Benavente Normann E., et al. (2022). Will harbor porpoises (Phocoena phocoena) be deterred by a pinger that cannot be used as “dinner bell” by seals? Mar. Mamm. Sc. 38, 469–485. doi: 10.1111/mms.12880
Kraus S. D., Read A. J., Solow A., Baldwin K., Spradlin T., and Anderson E. (1997). Acoustic alarms reduce porpoise mortality. Nature 388, 525. doi: 10.1038/41451
Leopold M. F. (2015). Eat and be eaten. Porpoise Diet Studies. (Thesis). Wageningen University, The Netherlands.
Lescrauwaet A. C. and Gibbons J. (1994). Mortality of small cetaceans and the crab bait fishery in the Magallanes area of Chile since 1980. Repo. International Whalling Commission, Special Issue 15, SC/46/SM11.
Mangel J. C., Alfaro-Shigueto J., Van Waerebeek K., Caceres C., Bearhop S., Witt M. J., et al. (2010). Small cetacean captures in Peruvian artisanal fisheries: High despite protective legislation. Biol. Cons. 143, 136–143. doi: 10.1016/j.biocon.2009.09.017
Mangel J. C., Alfaro-Shigueto J., Witt M., Hodgson D. J., and Godley B. (2013). Using pingers to reduce bycatch of small cetaceans in Peru’s small-scale driftnet fishery. Oryx 47 (3), 396–403. doi: 10.1017/S0030605312000658
McGarry T., de Silva R., Canning S., Mendes S., Prior A., Stephenson S., et al. (2020). Evidence bas for application of Acoustic Deterrent Devices (ADDs) as marine mammal mitigation (Version 3). JNCC Report No. 615 (Peterborough: JNCC). Available online at: https://hub.jncc.gov.uk/assets/e2d08d7a-998b-4814-a0ae-4edf5d887a02 (Accessed August 7, 2025).
Ministry of Economy, Development, and Reconstruction of Chile (1992). Decree 430 – Establishes the consolidated, coordinated, and systematized text of Law No. 18892, the General Law on Fisheries and Aquaculture.
Northridge S., Kingston A., and Thomas L. (2016). Annual report on the implementation of council regulation (EC) No 812/2004 during 2015 (Sea Mammal Research Unit, University of St Andrews Scotland: Defra and the European Commission).
Northridge S., Kingston A., and Thomas L. (2019). Annual report on the implementation of council regulation (EC) No 812/2004 during 2018 (Sea Mammal Research Unit, University of St Andrews Scotland: Defra and the European Commision).
Nuuttila H. K., Courtene-Jones W., Baulch S., Simon M., and Evans P. G. H. (2017). Don’t forget the porpoise: acoustic monitoring reveals fine scale temporal variation between bottlenose dolphin and harbour porpoise in Cardigan Bay SAC. Mar. Biol. 164 (11), 179. doi: 10.1007/s00227-017-3081-5, PMID: 28275285
Nuuttila H. K., Meier R., Evans P. G. H., Turner J. R., Bennell J. D., and Hiddink J. G. (2013). Identifying foraging behaviour of wild bottlenose dolphins (Tursiops truncatus) and harbour porpoises (Phocoena phocoena) with static acoustic dataloggers. Aq. Mam. 39, 147–161. doi: 10.1578/am.39.2.2013.147
Omeyer L. C. M., Doherty P. D., Dolman S., Enever R., Reese A., Tregenza N., et al. (2020). Assessing the effects of banana pingers as a bycatch mitigation device for Harbour porpoises (Phocoena phocoena). Front. Mar. Sci. 7. doi: 10.3389/fmars.2020.00285
Osiecka J. O. and Wahlberg M. (2020). The diel pattern in harbour porpoise clicking behaviour is not a response to prey activity. Scient. Rep. 10, 14876. doi: 10.1038/s41598-020-71957-0, PMID: 32913327
Paitach R. L., Amundin M., Teixeira G., and Cremer M. J. (2021). Echolocation variability of franciscana dolphins (Pontoporia blainvillei) between estuarine and open-sea habitats, within sights into foraging patterns. J. Acoust. Soc Am. 150, 3987. doi: 10.1121/10.0007277, PMID: 34852630
Pérez-Cortés M. H., Silver G., and Villa-Ramirez B. (1996). Contribución al conocimiento de la alimentación de la vaquita, Phocoenasinus. INP-SEMARNAT. Ciencia Pesquera 13, 66–72.
Pupelde (2004). Monitoreo y caracterización de artes y aparejos de pesca en la pesquería del Pejerrey Odontesthes regia en aguas interiores de la Décima región. Informe Final Proyecto del COREPA and FEREPA. 87.
R Core Team (2023). R: A language and environmentforstatisticalcomputing (Vienna: R FoundationforStatistical Computing). Available online at: https://www.R-project-org/ (Accessed August 7, 2025).
Read A. J., Drinker P., and Northridge S. (2006). Bycatch of marine mammals in U.S. and global fisheries. Cons. Biol. 20, 163–169. doi: 10.1111/l.1523-1739.2006.00338.x
Reyes V., Marino A., Dellabianca N. A., Hevia M., Torres M., Raya Rey A., et al. (2018). Clicks of wild Burmeister’s porpoises (Phocoena spinipinnis) in Tierra del Fuego, Argentina. Mar. Mamm. Sci. 34 (4), 1070–1081. doi: 10.1111/mms.12500
Rojas-Bracho L., Taylor B. L., and Jaramillo-Legorreta A. (2022). Phocoena sinus. The IUCN Red List of Threatened Species 2022: e.T17028A214541137. doi: 10.2305/IUCN.UK.2022-1.RLTS.T17028A214541137.en
Santillán L. (2022). Observations of Burmeister’s porpoise (Phocoena spinipinnis) in the Northern coast of Peru. Aquat. Mamm. 48, 266–272. doi: 10.1578/AM.48.3.2022.266
SERNAPESCA (2005). Anuario Estadístico de Pesca. Available online at: http://www.sernapesca.cl/index.php?option=com_remositoryandItemid=54andfunc=selectandid=43 (Accessed August 7, 2025).
SERNAPESCA. (2023). Informe de gestión anual 2022. Servicio Nacional de Pesca y Acuicultura, Gobierno de Chile. Available online at: https://www.sernapesca.cl/informes/informe-anual-gestion-2022.
Todd N. R. E., Kavanagh A. S., Rogan E., and Jessopp M. J. (2023). What the F-POD? Comparing the F-POD and C-POD for monitoring of harbour porpoise (Phocoena phocoena). Ecol. Evol. 13, e10186. doi: 10.1002/ece3.10186, PMID: 37304366
Van Waerebeek K., Azapa M., Reyes J., Alfaro-Shigueto J., Santillán L., Barreda E., et al. (2018). “Beach cast small cetaceans bear evidence of continued catches and utilisation in coastal Peru 2000-2017,” in International Whaling Commission Conference Paper SC/67B/HIM/01. (IWC Scientific Committee Paper No. SC/67B/HIM/01).
Van Waerebeek K., Van Bressem M. F., Félix F., Alfaro-Shigueto J., Garcia-Godos A., Chavez-Lisambart L., et al. (1997). Mortality of dolphins and porpoises in coastal fisheries off Peru and Southern Ecuador in 1994. Biol. Cons. 81, 43–49. doi: 10.1016/S0006-3207(96)00152-8
Keywords: acoustic deterrent, F-POD, passive acoustic monitoring, Phocoena spinipinnis, Chile
Citation: Díaz D and García-Cegarra AM (2025) Pingers as a potential deterrent tool to mitigate Burmeister’s porpoise (Phocoena spinipinnis) bycatch while foraging nocturnally in the Humboldt Current System: a pilot study. Front. Mar. Sci. 12:1582414. doi: 10.3389/fmars.2025.1582414
Received: 24 February 2025; Accepted: 25 July 2025;
Published: 28 August 2025.
Edited by:
Zhongchang Song, Xiamen University, ChinaCopyright © 2025 Díaz and García-Cegarra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ana M. García-Cegarra, YW5hbWFyaWEuZ2FyY2lhQHVhbnRvZi5jbA==
 Diego Díaz1,2
Diego Díaz1,2 Ana M. García-Cegarra
Ana M. García-Cegarra