- Biological Oceanographic Division, Council of Scientific and Industrial Research (CSIR)-National Institute of Oceanography, Panaji, Goa, India
Coral reefs are important ecosystems that host a variety of life and provide essential services to ecosystems and people. But they are increasingly at risk due to human activities and climate change. In India, Lakshadweep coral reefs support a significant proportion of the local population’s livelihood, being a promising area with a wide range of fish, mollusks, crustaceans and seaweeds. And, in addition to the human activities, the earlier three El Nino events impacted Lakhadweep coral reefs with significant coral mortality. Therefore, it is crucial to continuously and rapidly assess their biodiversity to monitor the health of the reef. Due to its advantages over traditional methods, environmental DNA (eDNA) metabarcoding is an efficient tool for continuous monitoring and is non-invasive. However, there have been limited eDNA studies, and none have been conducted on the Lakshadweep islands in India considering human inhabitation. The present study conducted an eDNA-based biodiversity assessment focusing on the metazoan community using a COI gene fragment amplified with Mico1intF and jgHCO2198 primers targeting approximately 350bp. The study recovered genetic information of key species, 4 Families of Scleractinia, Poritidae, Pocilloporidae, Euphyllidae, and Merulinidae, 9 Species of Echinoderms and 19 species of fish communities. In addition to this, 12 different taxa of Arthropoda, 6 Mollusks and 7 Porifera. In total, 25 different taxa were observed in the Algal community, including micro- and Macroalgal assemblages. A total of 15 phyla were recorded from both human-inhabited and uninhabited Reefs. Fish communities were more abundant in uninhabited reefs, and the number of detected taxa was also higher in uninhabited reef samples. Previous biodiversity assessments in the Lakshadweep archipelago relied on occasional underwater surveys, lacking continuous monitoring. Our study, employing eDNA monitoring, provides a baseline for continuous and rapid biodiversity study in monitoring the status using genetic information with the perspective of human inhabitation in the Lakshadweep coral reefs.
1 Introduction
Coral reefs are incredibly diverse marine ecosystems, consisting of a wide variety of interdependent species, such as the symbiosis between corals and zooxanthellae, and predator-prey interactions (Reaka, 1997; Knowlton, 2001). Despite occupying only 1% of the ocean’s surface area, coral reefs are home to approximately 25% of all marine eukaryotic and prokaryotic species (Mies et al., 2020). However, coral reefs are also one of the most endangered environments globally (Ransome et al., 2017). Projections suggest that as much as one-third of coral species face the threat of extinction within this century (Carpenter et al., 2008; Knowlton, 2001), and that by 2050, around 90% of coral reefs could suffer severe degradation (McLeod et al., 2019). The possible decrease in coral populations will greatly affect many other species that depend on these habitats (Alvarez-Filip et al., 2009). In India, coral reefs span an area of around 2379 km² (Venkataraman, 2011). The Lakshadweep reefs are primarily coral atolls; the atolls span a total reef area of 933.7 square kilometers, with a lagoon area of 510 square kilometers (Bahuguna and Nayak, 1998; Manikandan et al., 2016). The Lakshadweep Sea, rich in fish resources, supports a significant proportion of the local population’s livelihoods, with tuna being the most critical species of commercial interest (Mohan et al., 2021). Furthermore, the Lakshadweep archipelago is regarded as a promising area for ornamental fishes, crustaceans, mollusks, and seaweeds (Silas et al., 1982). Given the importance of these reefs, their conservation is a priority. Earlier, three El Niño events that impacted Lakshadweep coral reefs caused significant coral mortality: 80% in 1998, 44% in 2010, and 31% in 2016 (Yadav et al., 2018). Additionally, human activities have led to the pollution of lagoon waters, increased sedimentation, and sea erosion in the Lakshadweep archipelago (Purvaja et al., 2019). All these threats to the coral reefs necessitate continuous monitoring and delineation of biodiversity from time to time for the Lakshadweep archipelago.
Studies have documented the biodiversity of the coral reefs of the Lakshadweep archipelago (Pillai, 1986; Mohan et al., 2021), fish diversity (Rajan et al., 2021), mollusk diversity (Ravinesh and Kumar, 2015), and macroalgal diversity (Mathil et al., 2023; Rajasuriya et al., 2002). More comprehensive studies are warranted for biomonitoring and to develop effective management measures for reef conservation. Most of the available studies used traditional underwater survey methods to assess the biodiversity of the coral reefs. While these methods have advantages, they also have certain disadvantages, such as assigning taxa based on morphological plasticity and the issues of larval samples or limited visibility underwater. The biodiversity information obtained from conventional methods is thus inherently biased, time-consuming, expensive and labor-intensive. In contrast, eDNA metabarcoding can enable rapid assessment of coral reef biodiversity cost-effectively and efficiently by leveraging the genetic information present in the shed genetic materials of marine organisms (DiBattista et al., 2020; Mathon et al., 2022; Dugal et al., 2023; Levy et al., 2023). eDNA monitoring is also ideal for rapidly assessing the spatial and temporal changes in coral reef environments by capturing genetic information that cannot be biased by morphological plasticity. Thus, eDNA metabarcoding surveys enable efficient documenting of the biodiversity, health, and bio-invasions of coral reef environments (Holman et al., 2019; Rishan et al., 2023).
The Lakshadweep archipelago’s coral reefs are home to a wide variety of organisms, but few recent studies have documented their biodiversity, and no study has surveyed their biodiversity by leveraging genetic information. To address this gap, we conducted a biodiversity assessment using the mitochondrial cytochrome oxidase subunit I (COI) short fragment, established as a method relying on non-invasive sample collection (Leray et al., 2013; Wangensteen et al., 2018). To delineate the potential interaction of human inhabitation with coral reef biodiversity, the current study applied eDNA sampling in both Human-inhabited Islands’ reefs (Agatti and Kavaratti) and Human-Uninhabited or pristine reefs, Chereapani Reef, or Byramgore Reef. Our findings inform the conservation strategies from the perspective of rapid biodiversity assessment and human inhabitation.
2 Methodology
2.1 Sample collection and DNA isolation
To study the biodiversity of the Lakshadweep Islands, considering two groups, human-inhabited and human-uninhabited reef environments. Water samples were collected from two human-inhabited Islands, Agatti and Kavaratti, one site each representing two sites that are inhabited by humans and three sites from the Byramgore or Chereapani Reef represent human-uninhabited or pristine environments (Figure 1).
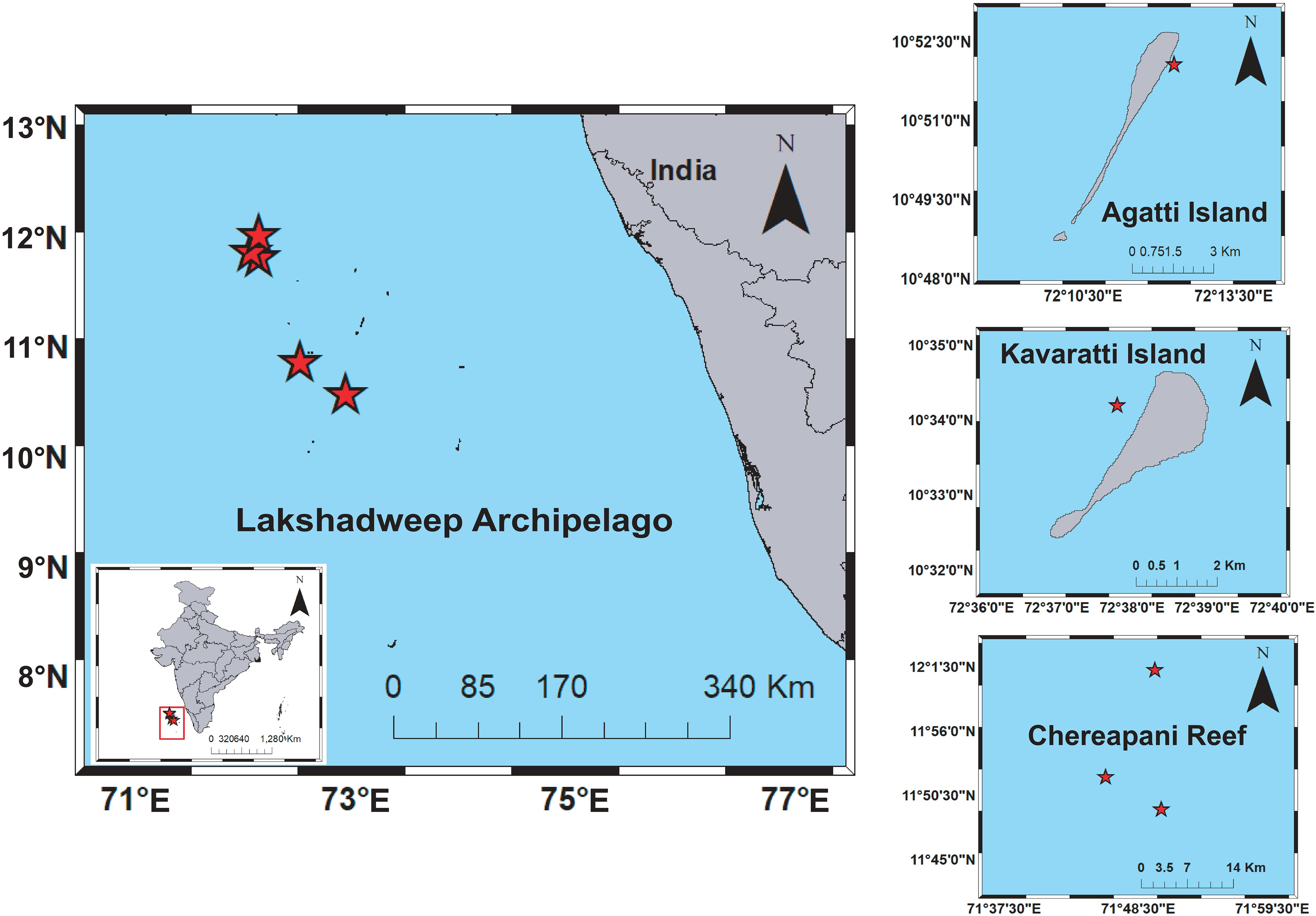
Figure 1. The eDNA Sampling Locations in Lakshadweep Archipelago, Agatti, Kavaratti and Chereapani reef.
A total of 5 liters of water was collected in triplicate per sampling site from a depth of over 1 meter, with water cans sterilized by washing with 4% bleach and ethanol before and between sampling. The water samples were filtered using a sterile syringe connected to a 0.45μm Sterivex™ cartridge. The filter unit was then filled with a Sample Protector for RNA/DNA (Takara Bio Inc.), and the samples were transported to the laboratory for further processing. Controls were not included during the sampling. To extract the DNA, the filter unit was filled with an SDS lysis buffer (1.25% SDS, 0.1M Tris-HCl pH 7.5, 0.05M EDTA pH 8.0, 0.25M NaCl) and incubated at 58°C for approximately 12 hours or overnight. Subsequently, phase separation was carried out using Chloroform: Isoamyl alcohol (24:1) with the lysate. The DNA was then washed with 100% ethanol, air-dried, and resuspended in nuclease-free water. The isolated DNA was stored at −20°C until further processing.
2.2 PCR amplification, library preparation and sequencing
The isolated DNA was amplified for a gene fragment of cytochrome oxidase subunit I (COI) of length 350bp using the Mico1intF/jgHCO2198 primers (Geller et al., 2013; Leray et al., 2013). The amplification was performed using Premix Taq™ DNA polymerase (Ex Taq™ Version 2.0; Takara Bio Inc.) in triplicate for each sample with a negative control. The thermal program consisted of initial denaturation at 95°C for 5 min, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 49°C for 30 seconds, extension at 72°C for 45 seconds, and final extension at 72°C for 10 min. The success of the amplification was confirmed on agarose gel electrophoresis, 2% stained with GelGreen® (Biotium) and the successful amplicon was excised and purified using the GeneJET PCR Purification Kit (Thermo Scientific™).
The purified amplicon was checked for quality on an agarose gel before library preparation. The concentration and purity were determined using the QIAxpert System (QIAGEN Inc.) and Qubit 4 fluorometer (Thermo Scientific™). The NEBNext® Ultra™ DNA Library Prep Kit for Illumina® (New England Biolabs® Inc.) was used to prepare the library following the manufacturer’s procedure. The amplicons were put through a series of enzymatic procedures for end repair and tailing with dA-tail. The tail ends were ligated with adapter sequences, and the adapter-ligated fragments were purified using SPRI beads and a magnetic plate. The clean fragments were indexed with sample-specific barcodes, BC, and Illumina adapters P5 and P7, to generate final libraries. The resulting amplicons were checked for size confirmation with the TapeStation D1000 System (Agilent Technologies, Inc.) and were quantified using the Qubit 4 fluorometer (ThermoScientific®). Based on the quantification, the amplicons were pooled together for equimolar concentration. The final product was then sequenced using the Illumina MiSeq platform with MiSeq Reagent Kit v2 (500-cycles) (Illumina, Inc.) following the manufacturer’s protocol. The library preparation of the purified amplified products and sequencing was carried out at MedGenome Labs Ltd. (Banglore, India).
2.3 Sequence pre-processing and taxonomic assignment
The results of the sequencing were analyzed using Qiime2 (v qiime2-2025.7) (Bolyen et al., 2019). The initial step was to demultiplex the sequences and import them into the Qiime2 pipeline. The imported reads were removed from primer sequences using cutadapt (Martin, 2011) with minimum mismatch of 1bp followed by reads were truncated at the length of 214 bp and 163 bp and passed through dada2 pipeline (Callahan et al., 2016) within qiime2 to denoise, remove chimera and clustering reads to amplicon sequence variants (ASVs). The singleton sequences were removed before taxonomic assignment from the total Amplicon Sequence Variants (ASVs). The filtered sequences were assigned to taxonomy using the preformatted DNA database for COI (MIDORI2_LONGEST_NUC_GB255_CO1_QIIME) (Leray et al., 2022) using consensus-blast with a 97% confidence level. Based on the taxonomic assignment, ASVs assigned to a minimum of the Phylum level were retained for further analysis.
2.4 Community diversity indices and statistics
The diversity within the samples was analyzed using various alpha diversity indices, including Phylogenetic Diversity (Faith pd), Species Diversity (Shannon entropy), Richness (Chao1) and Observed Features. Additionally, beta diversity was calculated using the metrics Bray-Curtis, Jaccard Index and Unifrac distances (unweighted and weighted). All the diversity indices were calculated using the core-metrics plugin within qiime2 (v qiime2-2023.7). A Non-Metric Multidimensional Scaling (nMDS) plot was generated using the Bray-Curtis distance matrix, considering both presence and abundance of ASVs. Kruskal-Wallis test was carried out to compare the groups, human inhabited and uninhabited, using the Bray-Curtis distance matrix.
3 Results
3.1 Sequencing results and taxonomic assignment
A total of 2107439 paired-end reads were generated, with an average of 421488 reads per sample. After removal of primers using cutadapt and denoising, chimaera removal and clustering using dada2, a total of 4323 ASVs were observed from all samples (Supplementary Table S1). After removal of singleton sequences and ASVs not assigned to a minimum to phylum level, 149 ASVs were retained, contributing to Metazoan taxa, excluding Algae (Microalgae and Macroalgae) and 173 ASVs were contributing to Algal taxa. The majority of the sequences were filtered out as singleton sequences and taxonomic assignment with a 97% confidence level, however, the analysis proceeded with the ASVs retained to ensure higher confidence in species identification. In total 6 phyla, Arthropoda, Chordata, Cnidaria, Echinodermata, Mollusca, Porifera, were observed and, 9 phyla of algal community, Bacillariophyta, Chlorophyta, Cryptophyceae, Dinophyceae, Eustigmatophyceae, Hapophyta, Pelagophyceae and Prasinodermophyta were observed when the taxonomic assignment was carried out using the preformatted COI database (MIDORI2_LONGEST_NUC_GB255_CO1_QIIME) (Leray et al., 2022).
3.2 Insights into the metazoan community
A total of 64 metazoan taxa, representing six phyla, were recorded across various taxonomic levels. Figure 2 illustrates the relative abundance of the metazoan community (excluding algae), based on the number of families observed (Supplementary Table S2). All six phyla were detected in eDNA samples from the Agatti, Kavaratti, and Chereapani reefs. Some taxa appeared only in a subset of samples, likely due to tidal and ocean currents that introduce ephemeral or less commonly observed species (Magurran and Henderson, 2003).
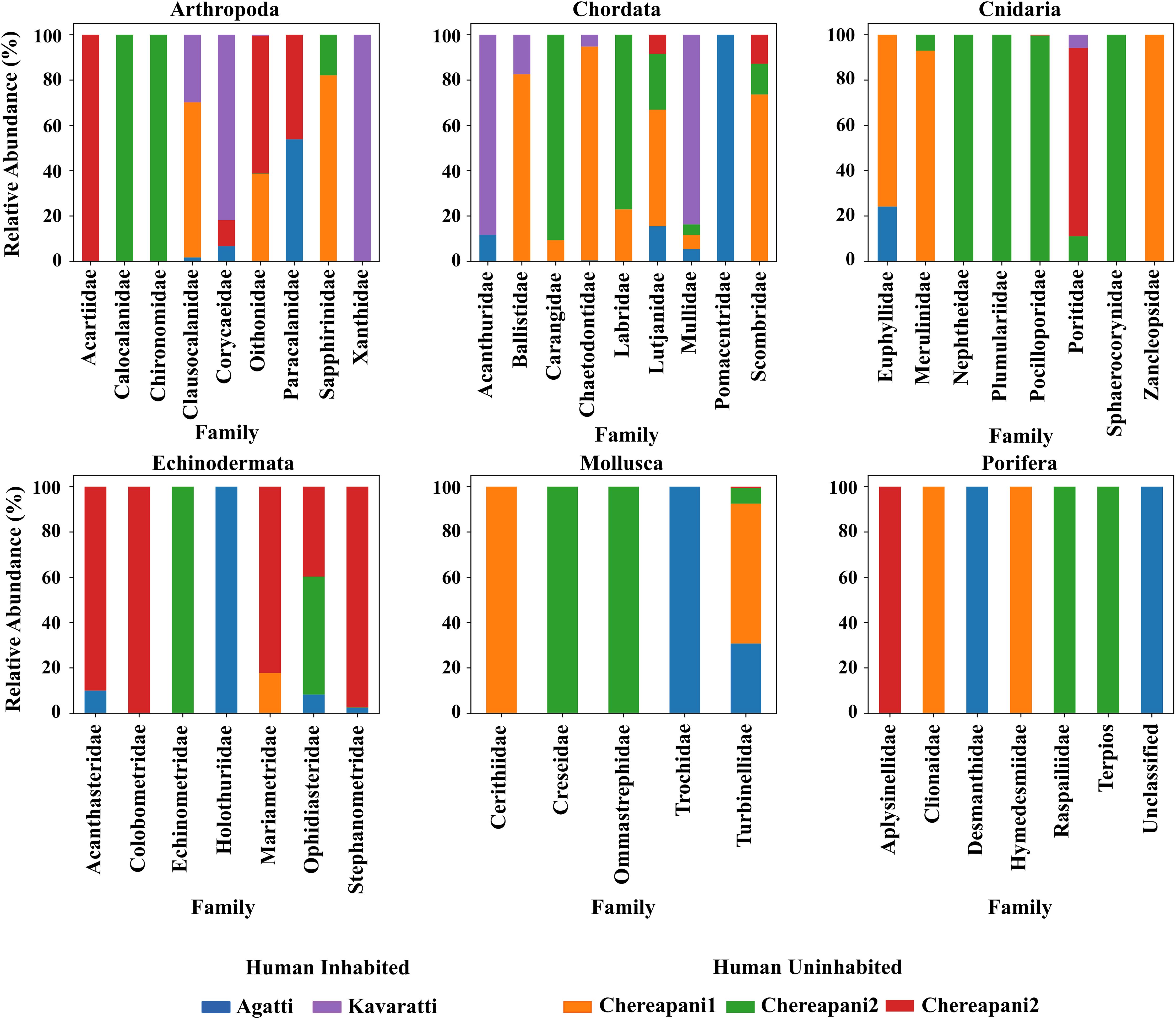
Figure 2. The abundance of each metazoan class (excluding algae) observed in the samples from Agatti, Kavaratti, Chereapani 1, Chereapani 2, and Chereapani 3.
A total of 19 fish species were observed, representing nine families: Acanthuridae, Balistidae, Carangidae, Chaetodontidae, Labridae, Lutjanidae, Mullidae, Pomacentridae, and Scombridae. Herbivorous fishes such as Scarus and Acanthurus play a key role in controlling coral phase shifts and promoting coral resilience. Additionally, nine species of echinoderms were recorded, including Acanthaster planci (the Crown-of-Thorns starfish), which was observed around both inhabited and uninhabited islands. Regarding the algal community, nine phyla comprising 25 taxa were identified (Supplementary Table S3). The presence of herbivorous fish corresponds with the observed algal diversity. Our findings on algal composition are consistent with those of previous studies (Mathil et al., 2023; Figure 3).
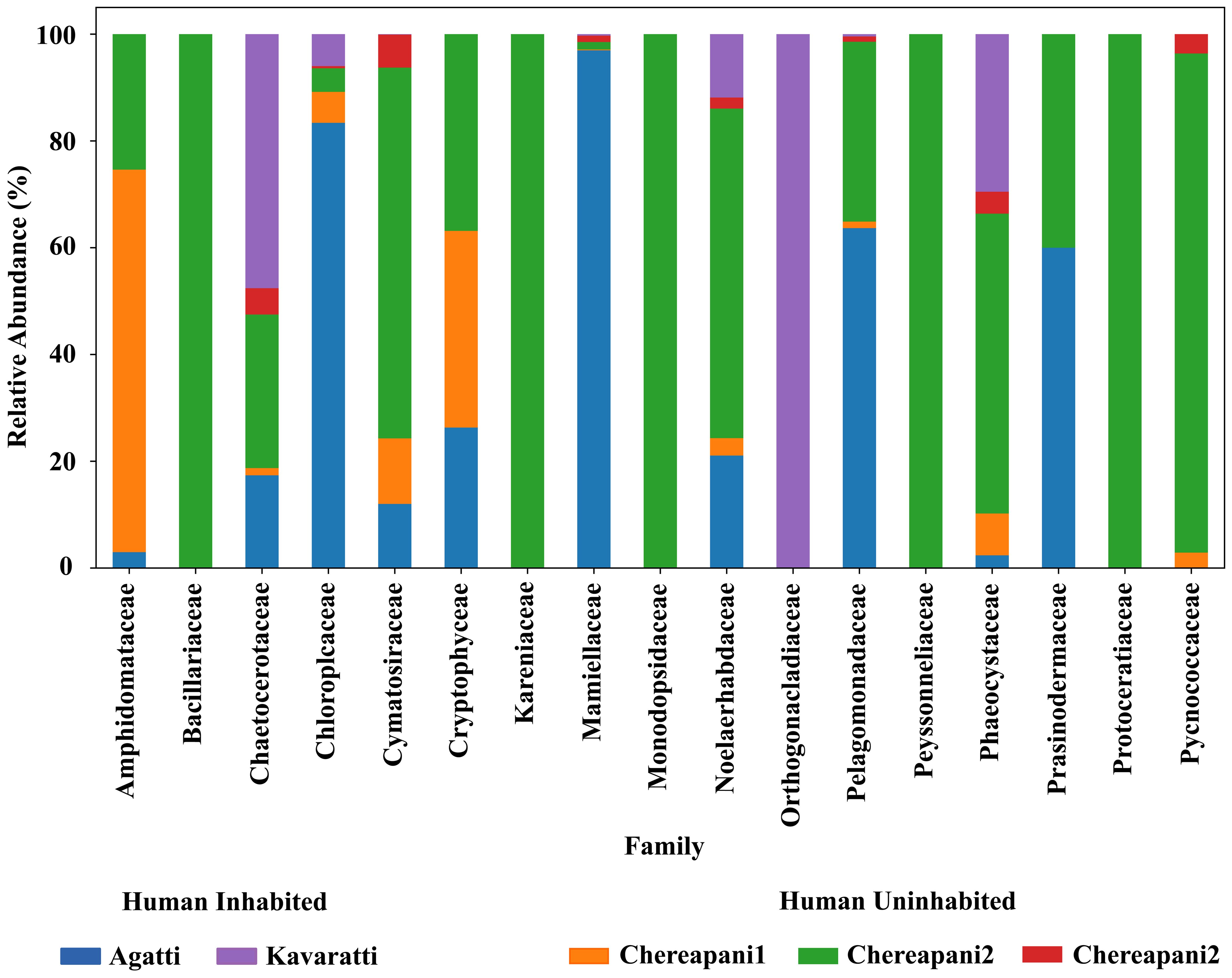
Figure 3. Abundance of algal families observed from eDNA samples collected from the Agatti, Kavaratti, and Chereapani reefs.
3.3 Community diversity and rarefaction
A rooted phylogenetic tree was constructed using ASVs classified to at least the phylum level, after removing singleton sequences. Alpha diversity metrics—including Phylogenetic Diversity (Faith_PD), Species Diversity (Shannon Entropy), Richness (Chao1), and Observed Features—were calculated based on this tree. Notably, samples from the human-uninhabited reef exhibited higher phylogenetic diversity and Shannon entropy, whereas the human-inhabited reef samples showed higher richness and observed features (Figure 4). An alpha rarefaction analysis demonstrated that the rarefaction curves plateaued at a sequencing depth of 9,984, indicating sufficient depth to capture the ASV diversity in all samples.
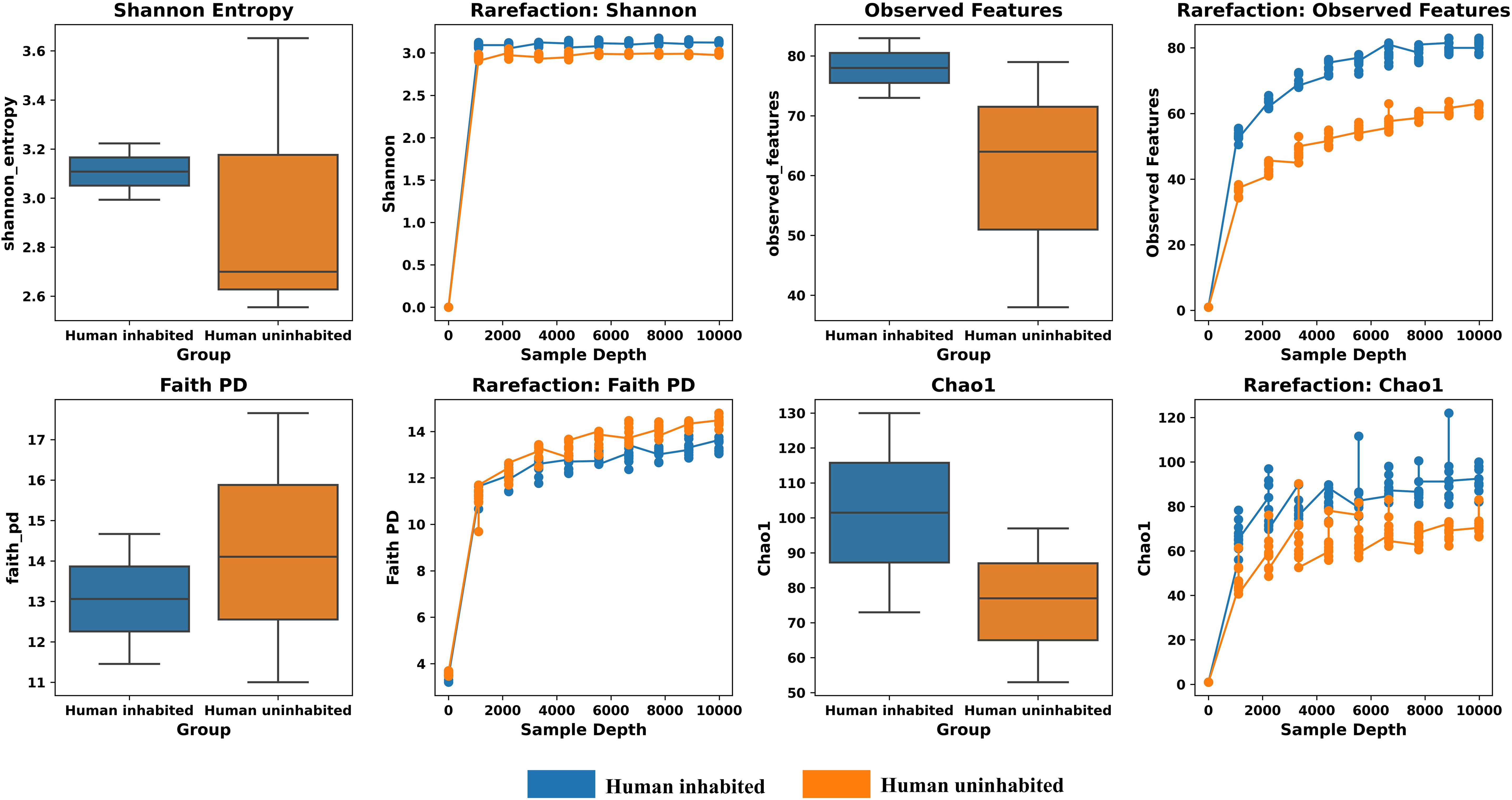
Figure 4. Alpha Diversity Indices, Diversity (Shannon Entropy and Chao 1), Phylogenetic Diversity (Faith-pd) and Observed Features.
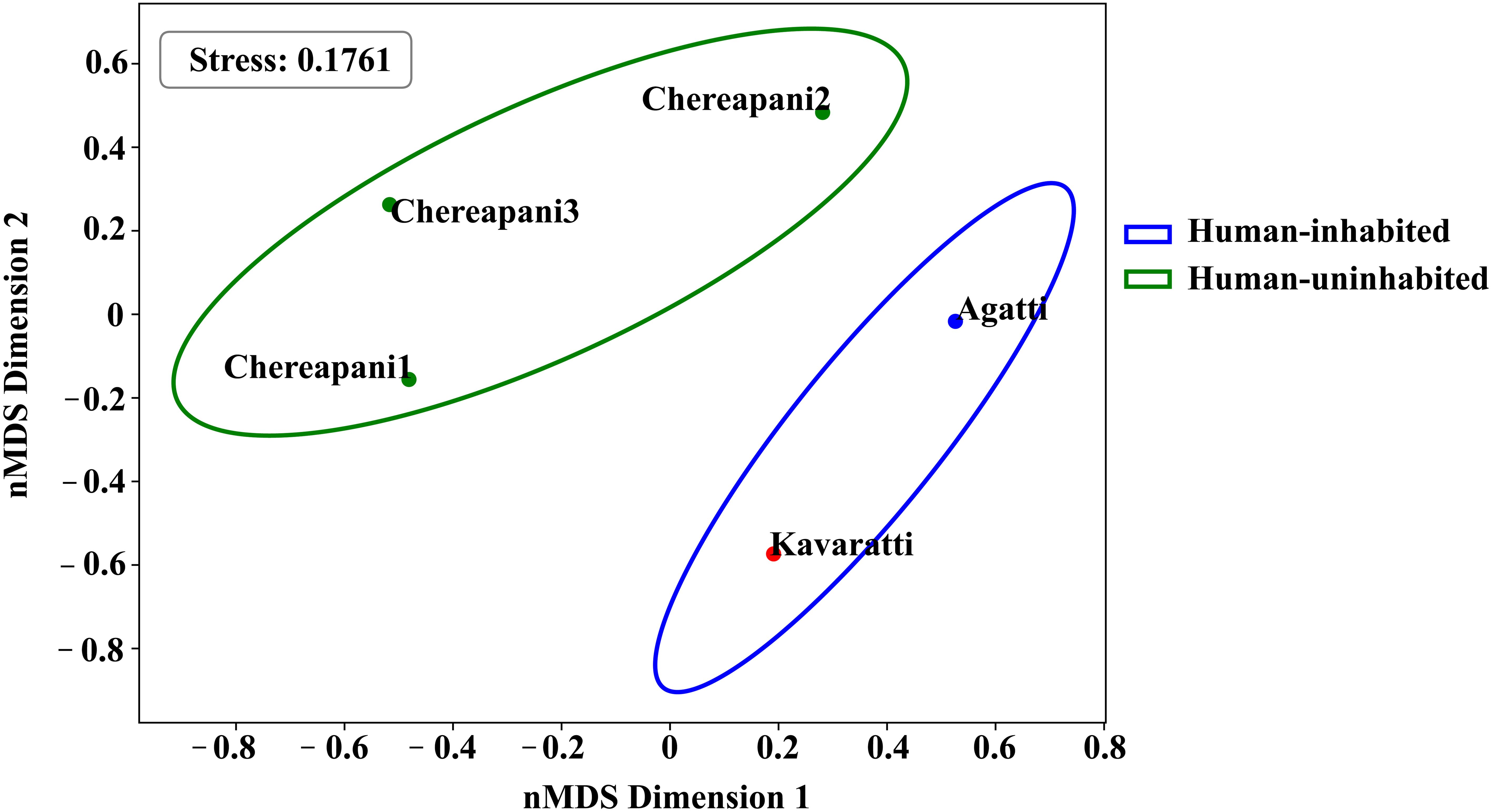
Beta diversity was assessed using Bray-Curtis, Jaccard, Unweighted UniFrac, and Weighted UniFrac distance matrices to evaluate community similarities and dissimilarities. Clear clustering between human-inhabited (Agatti, Kavaratti) and human-uninhabited (Chereapani 1, 2, and 3) sites was evident only in the Unweighted UniFrac analysis (Figure 5). Additionally, an nMDS plot based on Bray-Curtis distances showed intra-group clustering (Figure 6), with a stress value of 0.176, indicating an acceptable ordination.
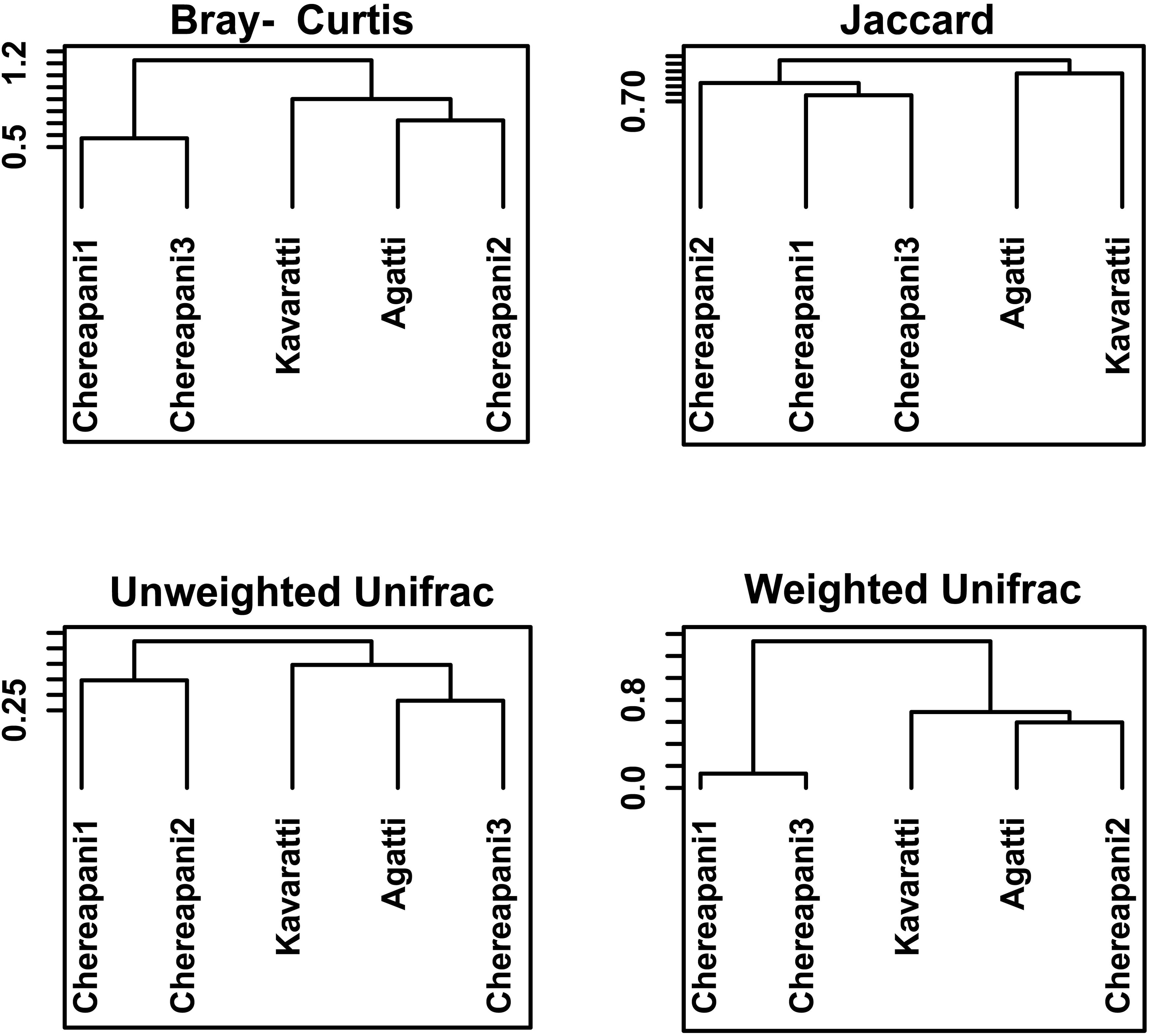
Figure 5. Beta Diversity Indices, Bray-Curtis, Jaccard, Unweighted-Unifrac and Weighted-Unifrac distance dendrogram.
A Kruskal-Wallis H test yielded a test statistic of 0.466 and a p-value of 0.49, suggesting no statistically significant differences in community composition between the groups (p ≥ 0.05). While the current findings are insufficient to reject the null hypothesis, expanding the dataset-particularly across temporal and spatial gradients-will be critical to fully understand the impact of human habitation on coral reef biodiversity.
4 Discussion
This study represents the first eDNA metabarcoding-based biodiversity survey of coral reefs in the Lakshadweep archipelago, highly diverse marine ecosystems comprising complex ecological networks. Historical events such as the 1997-1998 mass bleaching affected 16% of the world’s coral reefs (Hill and Wilkinson, 2004), and multiple El Niño Southern Oscillation events have caused coral mortalities in the Lakshadweep Islands (Yadav et al., 2018). Additionally, anthropogenic impacts, including lagoon water pollution, have increased sedimentation and erosion (Purvaja et al., 2019). These threats underscore the need for consistent reef monitoring to track ecological indicators such as live and dead coral cover, phase shifts, and invasive species presence.
The decline in classical taxonomic expertise (Engel et al., 2021; Mora et al., 2011) has limited traditional monitoring approaches, which often require SCUBA-based visual surveys and are typically biased toward megafauna such as corals, fish, and macroalgae. In contrast, eDNA enables a broader, non-invasive approach that can also detect cryptofauna.
In this study, 2,107,439 sequence reads were generated using the COI gene fragment. Previous research has shown that COI often yields higher OTU counts compared to 18S rRNA markers (Laroche et al., 2020), although the 18S gene can detect taxa absent in COI datasets due to limitations in COI reference libraries (Cristescu, 2014; DiBattista et al., 2020). This highlights the need for multiple gene markers and more comprehensive genetic databases to capture the full range of biodiversity. A large proportion of ASVs remained unassigned even to the phylum level, suggesting the presence of undescribed or genetically unrepresented taxa.
This study explored reef biodiversity using eDNA collected from five sites across the Lakshadweep Islands, including the Agatti, Kavaratti, and Chereapani reefs. Key indicator species were documented, including hard corals, fish communities, and other groups such as Cnidarians and Echinoderms. Notably, the outbreak coral predator, the Crown-of-Thorns starfish, was detected, consistent with previous observations by Senthilnathan et al. (2014). In addition, Terpios sp., a sponge known to pose a threat to live corals, was identified at the uninhabited Chereapani reef, corroborating earlier reports from Das et al. (2020) and Dennis and Senthilnathan (2023). These findings highlight the need for ongoing monitoring of coral predators and invasive species, particularly in human-influenced reef environments. Including human inhabitation in reef assessments is essential, as reefs with minimal local impact tend to show greater resilience (Smith et al., 2016).
The fish communities observed comprised 19 species, consistent with previous reports on the faunal diversity of the Lakshadweep Islands (Anand and Pillai, 2003; Gowri et al., 2016; Rajan et al., 2021). Among these, Scarus and Acanthurus- both herbivorous species—were recorded, aligning with the presence of algal communities documented in this study. Sponges were also detected in the eDNA data, a notable finding given that traditional identification typically requires microscopic examination of spicules. All sponge orders identified in this study have previously been reported by Varsha et al. (2021) through SCUBA-based surveys. Porifera were predominantly recorded from the Chereapani reef, except the family Desmanthidae, which was observed in the Agatti reef sample. Families belonging to Octocorallia and Hydrozoa, including Nephthidae (Octocorallia) and Plumulariidae, Sphaerocorynidae, and Zancleopsidae (Hydrozoa), were exclusively observed in uninhabited reef samples. True corals of the order Scleractinia were represented by four families: Euphyllidae, Merulinidae, Poritidae, and Pocilloporidae. Except for Merulinidae, the other three are considered abundant taxa in the region (Das et al., 2023). Euphyllidae and Poritidae were observed in both inhabited and uninhabited reef samples, whereas Merulinidae and Pocilloporidae were limited to uninhabited reefs. Detection of these taxa could potentially be improved by using Scleractinian-specific markers (Shinzato et al., 2021), as the amplification of eDNA from other groups, particularly algae, may mask the signal from coral species. Similar marker-specific challenges apply to fish (Miya et al., 2015) and other taxonomic groups.
The algal community was dominated by Chlorophyta, consistent with Mathil et al. (2023). The genus Ramicrusta, a known threat to coral larvae (Williams and García-Sais, 2020; Cayemitte et al., 2023), was also detected. Algal proliferation may indicate a phase shift in the reef ecosystem. According to Steneck (1988), algal biomass typically declines with increased herbivory. However, our data indicate substantial algal presence alongside herbivorous fish, suggesting complex interactions or localized impacts. Previous studies (Purvaja et al., 2019) reported coral damage due to sedimentation and anthropogenic pressures. Still, challenges persist in measuring phase shifts across spatial and temporal scales (Done, 1992; McManus and Polsenberg, 2004; Jouffray et al., 2015; Donovan et al., 2018). Notably, phytoplankton blooms may also be linked to sponge decline (Peterson et al., 2006), suggesting interdependencies among algae, sponges, and corals that merit further investigation.
Alpha diversity metrics showed higher species richness (Shannon index) and phylogenetic diversity (Faith PD) in human-uninhabited reef samples (Chereapani) compared to inhabited reefs (Agatti, Kavaratti), suggesting anthropogenic impacts on reef biodiversity. However, the sample size was insufficient to statistically differentiate community compositions, reinforcing the need for expanded spatial and temporal sampling.
While eDNA offers an efficient tool for biodiversity monitoring, accuracy over time, spatial consistency, and quantitative interpretations remain challenges. Ongoing improvements in reference databases and barcoding methods are critical to enhancing taxonomic resolution. Temporal eDNA sampling is especially valuable for distinguishing resident from transient species (Magurran and Henderson, 2003). This pilot study establishes a foundational dataset for future monitoring of Lakshadweep’s reef biodiversity and serves as a baseline for expanding molecular reef assessments.
Further studies incorporating seasonal variation and additional markers (e.g., Shinzato et al., 2021; Miya et al., 2015; Casey et al., 2021) are essential to capture comprehensive biodiversity. Marine biodiversity is declining globally due to anthropogenic pressure, overfishing, pollution, and invasive species (Bongaarts, 2019; Brito-Morales et al., 2020). Yet, less than 10% of marine ecosystems are currently under protection, with only a fraction designated as highly protected marine protected areas (MPAs) (Visalli et al., 2020; Yang et al., 2024). eDNA has emerged as a powerful tool to support ecosystem health monitoring and biodiversity-based management strategies, and it can inform the design of ecologically representative and interconnected MPAs.
5 Conclusion
This study used environmental DNA (eDNA) to assess species diversity across Lakshadweep’s coral reefs, documenting key groups such as Cnidarians, Echinoderms, Fish, Mollusks, and Macroalgae. While no statistically significant difference in community composition was found between human-inhabited and uninhabited reefs, trends in diversity metrics suggest human influence on reef ecosystems. These findings highlight the importance of continued biodiversity monitoring, especially in the context of human disturbance and climate change. As reef ecosystems are vital for ecological function and local livelihoods, future biodiversity assessments should be temporally expanded and incorporate specific molecular markers to improve species detection and support conservation efforts.
Data availability statement
The Next Generation Sequencing datasets generated for this study were submitted to the National Centre for Biotechnology Information (NCBI) Sequence Read Archive, accession numbers: SRR26560965, SRR26560966, SRR26560964, SRR24958384 and SRR24958385.
Author contributions
BA: Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft. RD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work manpower wages were supported by the project no. GAP3484, CSIR-NIO, Goa.
Acknowledgments
The authors gratefully acknowledge the support received from Dr. N Marimuthu, Scientist E, Zoological Survey of India, for assistance with sample collection. Mr. Brandis Carlier, Project Associate, CSIR NIO, for his support with the sampling location map.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmars.2025.1592429/full#supplementary-material
References
Alvarez-Filip L., Dulvy N. K., Gill J. A., Côté I. M., and Watkinson A. R. (2009). Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc. R. Soc. B: Biol. Sci. 276, 3019–3025. doi: 10.1098/rspb.2009.0339
Anand P. and Pillai N. G. K. (2003). Habitat distribution and species diversity of coral reef fishes in the reefslope of the Kavaratti atoll, Lakshadweep, India. J. Marine Biol. Assoc. India 45 (1), 88-98.
Bahuguna A. and Nayak S. (1998). Coral reefs of the Indian coast. Scientific Note (SAC/RSA/RSAG/DOD-COS/SN/16/97), Space Application Centre, Ahmedabad, 56p.
Bolyen E., Rideout J. R., Dillon M. R., Bokulich N. A., Abnet C. C., Al-Ghalith G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Bongaarts J. (2019). IPBES, 2019. Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services. Population and Development Review 45, 680–681. doi: 10.1111/padr.12283
Brito-Morales I., Schoeman D. S., Molinos J. G., Burrows M. T., Klein C. J., Arafeh-Dalmau N., et al. (2020). Climate velocity reveals increasing exposure of deep-ocean biodiversity to future warming. Nat. Climate Change 10, 576–581. doi: 10.1038/s41558-020-0773-5
Callahan B. J., McMurdie P. J., Rosen M. J., Han A. W., Johnson A. J. A., and Holmes S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Carpenter K. E., Abrar M., Aeby G., Aronson R. B., Banks S., Bruckner A., et al. (2008). One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563. doi: 10.1126/science.1159196
Casey J. M., Ransome E., Collins A. G., Mahardini A., Kurniasih E. M., Sembiring A., et al. (2021). DNA metabarcoding marker choice skews perception of marine eukaryotic biodiversity. Environ. DNA 3, 1229–1246. doi: 10.1002/edn3.245
Cayemitte K., Aoki N., Ferguson S. R., Mooney T. A., and Apprill A. (2023). Ramicrusta invasive alga causes mortality in Caribbean coral larvae. Front. Marine Sci. 10. doi: 10.3389/fmars.2023.1158947
Cristescu M. E. (2014). From barcoding single individuals to metabarcoding biological communities: towards an integrative approach to the study of global biodiversity. Trends Ecol. Evol. 29, 566–571. doi: 10.1016/j.tree.2014.08.001
Das R. R., Sreeraj C. R., Mohan G., Abhilash K. R., Samuel V. K. D., Ramachandran P., et al. (2020). Incursion of the killer sponge Terpios hoshinota Rützler & Muzik 1993 on the coral reefs of the Lakshadweep archipelago, Arabian Sea. J. Threatened Taxa 12, 17009–17013. doi: 10.11609/jott.5790.12.14.17009-17013
Das R. R., Sreeraj C. R., Mohan G., Simon N. T., Ramachandran P., Ramachandran R., et al. (2023). Evidence of coral diseases, phase shift, and stressors in the atolls of lakshadweep islands, arabian sea—With geographical notes on their occurrence within the Indian EEZ and contiguous international waters. Diversity. 15 (3), 382. doi: 10.3390/d15030382
Dennis A. and Senthilnathan L. (2023). Monitoring of coral-killing sponge (Terpios hoshinota) caused rapid coral tissue loss assessment through environmental parameters in the Palk Bay. Regional Stud. Marine Sci. 66, 103137. doi: 10.1016/j.rsma.2023.103137
DiBattista J. D., Reimer J. D., Stat M., Masucci G. D., Biondi P., De Brauwer M., et al. (2020). Environmental DNA can act as a biodiversity barometer of anthropogenic pressures in coastal ecosystems. Sci. Rep. 10, 8365. doi: 10.1038/s41598-020-64858-9
Done T. J. (1992). Phase shifts in coral reef communities and their ecological significance. Hydrobiologia 247, 121–132. doi: 10.1007/BF00008211
Donovan M. K., Friedlander A. M., Lecky J., Jouffray J.-B., Williams G. J., Wedding L. M., et al. (2018). Combining fish and benthic communities into multiple regimes reveals complex reef dynamics. Sci. Rep. 8, 16943. doi: 10.1038/s41598-018-35057-4
Dugal L., Thomas L., Meenakshisundaram A., Simpson T., Lines R., Colquhoun J., et al. (2023). Distinct coral reef habitat communities characterized by environmental DNA metabarcoding. Coral Reefs 42, 17–30. doi: 10.1007/s00338-022-02301-3
Engel M., Ceriaco L., Daniel G., Dellapé P., Löbl I., Marinov M., et al. (2021). The taxonomic impediment: a shortage of taxonomists, not the lack of technical approaches. Zoological J. Linn. Soc. 193, 381–387. doi: 10.1093/zoolinnean/zlab072
Geller J., Meyer C., Parker M., and Hawk H. (2013). Redesign of PCR primers for mitochondrial cytochrome c oxidase subunit I for marine invertebrates and application in all-taxa biotic surveys. Mol. Ecol. Resour 13, 851–861. doi: 10.1111/men.2013.13.issue-5
Gowri V. S., Pramiladevi I. R. R., and Nammalwar P. (2016). Biodiversity of selected herbivorous coral reef fishes and their role in coral management, India. Int. J. Res. Sci. Innov, 3, 94-10.
Hill J. and Wilkinson C. (2004). Methods for ecological monitoring of coral reefs. Australian Institute of Marine Science, Townsville, AU, 117.
Holman L. E., de Bruyn M., Creer S., Carvalho G. R., Robidart J., and Rius M. (2019). Detection of introduced and resident marine species using environmental DNA metabarcoding of sediment and water. Sci. Rep. 9, 11559. doi: 10.1038/s41598-019-47899-7
Jouffray J.-B., Nyström M., Norström A. V., Williams I. D., Wedding L. M., Kittinger J. N., et al. (2015). Identifying multiple coral reef regimes and their drivers across the Hawaiian archipelago. Philos. Trans. R. Soc. B: Biol. Sci. 370, 20130268. doi: 10.1098/rstb.2013.0268
Knowlton N. (2001). Coral reef biodiversity–habitat size matters. Science 292, 1493–1495. doi: 10.1126/science.1061690
Laroche O., Kersten O., Smith C. R., and Goetze E. (2020). Environmental DNA surveys detect distinct metazoan communities across abyssal plains and seamounts in the western Clarion Clipperton Zone. Mol. Ecol. 29, 4588–4604. doi: 10.1111/mec.v29.23
Leray M., Knowlton N., and Machida R. J. (2022). MIDORI2: A collection of quality controlled, preformatted, and regularly updated reference databases for taxonomic assignment of eukaryotic mitochondrial sequences. Environ. DNA 4, 894–907. doi: 10.1002/edn3.v4.4
Leray M., Yang J. Y., Meyer C. P., Mills S. C., Agudelo N., Ranwez V., et al. (2013). A new versatile primer set targeting a short fragment of the mitochondrial COI region for metabarcoding metazoan diversity: application for characterizing coral reef fish gut contents. Front. Zool 10, 34. doi: 10.1186/1742-9994-10-34
Levy N., Simon-Blecher N., Ben-Ezra S., Yuval M., Doniger T., Leray M., et al. (2023). Evaluating biodiversity for coral reef reformation and monitoring on complex 3D structures using environmental DNA (eDNA) metabarcoding. Sci. Total Environ. 856, 159051. doi: 10.1016/j.scitotenv.2022.159051
Magurran A. E. and Henderson P. A. (2003). Explaining the excess of rare species in natural species abundance distributions. Nature 422, 714–716. doi: 10.1038/nature01547
Manikandan B., Jeyaraman R., Mohan H., Periasamy R., Murali M., and Ingole B. (2016). Community structure and coral health status across the depth gradients of Grande Island, Central west coast of India. Regional Stud. Marine Sci. 7, 150-158. doi: 10.1016/j.rsma.2016.05.013
Martin M. (2011). Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 17, 10–12. doi: 10.14806/ej.17.1.200
Mathil S., S S., and Babu I. (2023). Assessment of the spatial variation in the macro-algal resources of Lakshadweep, India. J. Marine Biol. Assoc. India 65 (1). doi: 10.6024/jmbai.2023.65.1.2285-07
Mathon L., Marques V., Mouillot D., Albouy C., Andrello M., Baletaud F., et al. (2022). Cross-ocean patterns and processes in fish biodiversity on coral reefs through the lens of eDNA metabarcoding. Proc. Biol. Sci. 289, 20220162. doi: 10.1098/rspb.2022.0162
McLeod E., Bruton-Adams M., Förster J., Franco C., Gaines G., Gorong B., et al. (2019). Lessons from the pacific islands – adapting to climate change by supporting social and ecological resilience. Front. Marine Sci. 6. doi: 10.3389/fmars.2019.00289
McManus J. W. and Polsenberg J. F. (2004). Coral–algal phase shifts on coral reefs: Ecological and environmental aspects. Prog. Oceanography 60, 263–279. doi: 10.1016/j.pocean.2004.02.014
Mies M., Francini-Filho R. B., Zilberberg C., Garrido A. G., Longo G. O., Laurentino E., et al. (2020). South atlantic coral reefs are major global warming refugia and less susceptible to bleaching. Front. Marine Sci. 7. doi: 10.3389/fmars.2020.00514
Miya M., Sato Y., Fukunaga T., Sado T., Poulsen J. Y., Sato K., et al. (2015). MiFish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: detection of more than 230 subtropical marine species. R. Soc. Open Sci. 2, 150088. doi: 10.1098/rsos.150088
Mohan G., Goutham S., Simon N., Vijay Kumar D., Robin R., Govindasamy H., et al. (2021). Status of health and conservation classification of tropical coral reefs in Lakshadweep archipelago. Wetlands Ecol. Manage. 29, 653–668. doi: 10.1007/s11273-021-09801-z
Mora C., Tittensor D. P., Adl S., Simpson A. G., and Worm B. (2011). How many species are there on Earth and in the ocean? PloS Biol. 9, e1001127. doi: 10.1371/journal.pbio.1001127
Peterson B. J., Chester C. M., Jochem F. J., and Fourqurean J. W. (2006). Potential role of sponge communities in controlling phytoplankton blooms in Florida Bay. Marine Ecol. Prog. Ser. 328, 93–103. doi: 10.3354/meps328093
Pillai C. S. G. (1986). Status of coral reefs in Lakshadweep. Marine Fisheries Information Service, Technical and Extension Series, 68, 38-41.
Purvaja R., Yogeswari S., Debasis T., Hariharan G., Raghuraman R., Muruganandam R., et al. (2019). “Chapter 27 - Challenges and Opportunities in the Management of Coral Islands of Lakshadweep, India,” in Coasts and Estuaries. Eds. Wolanski E., Day J. W., Elliott M., and Ramachandran R. (Amsterdam, Netherlands: Elsevier), 461–476.
Rajan R., Rajan P., Mishra S., Shrinivaasu S., and Surendar C. (2021). Fishes of Lakshadweep archipelago: new records, review and a revised checklist. Marine Biodiversity Records 14, 14. A. ND. T. doi: 10.1186/s41200-021-00208-6
Rajasuriya A., Zahir H., Muley E. V., Venkataraman K., Wafar M. V. M., Khan S. M. M., et al. (2002). Status of coral reefs in South Asia: Bangladesh, India, Maldives, Sri Lanka. In Proceedings of the Ninth International Coral Reef Symposium, Bali, 23-27 October 2000, 2, 841-845. doi: 10.1007/1978-1090-1481-2639-1002_1064
Ransome E., Geller J. B., Timmers M., Leray M., Mahardini A., Sembiring A., et al. (2017). The importance of standardization for biodiversity comparisons: A case study using autonomous reef monitoring structures (ARMS) and metabarcoding to measure cryptic diversity on Mo’orea coral reefs, French Polynesia. PloS One 12, e0175066. doi: 10.1371/journal.pone.0175066
Ravinesh R. and Kumar B. (2015). A checklist of the marine molluscs of Lakshadweep, India. J. Aquat. Biol. Fisheries 3, 15–55.
Reaka M. (1997). The global biodiversity of coral reefs: a comparison with rainforests. Biodiversity II: Understanding and protecting our biological resources. 2, 551.
Rishan S., Kline R., and Rahman M. (2023). Applications of environmental DNA (eDNA) to detect subterranean and aquatic invasive species: A critical review on the challenges and limitations of eDNA metabarcoding. Environ. Adv. 12, 100370. doi: 10.1016/j.envadv.2023.100370
Senthilnathan L., Ranith R., Machendiranathan M., Thangaradjou T., Babu I., Choudhury S. B., et al. (2014). Are Lakshadweep Corals Heading Toward COT Outbreak (Washington, DC, USA: Smithsonian Institution Scholarly Press).
Shinzato C., Narisoko H., Nishitsuji K., Nagata T., Satoh N., and Inoue J. (2021). Novel mitochondrial DNA markers for scleractinian corals and generic-level environmental DNA metabarcoding. Front. Marine Sci. 8. doi: 10.3389/fmars.2021.758207
Silas E. G., Pillai P. P., and C. M. F. R. Institute (1982). Resources of tunas and related species and their fisheries in the Indian Ocean. CMFRI bulletin, 32, 1-174.
Smith J. E., Brainard R., Carter A., Grillo S., Edwards C., Harris J., et al. (2016). Re-evaluating the health of coral reef communities: baselines and evidence for human impacts across the central Pacific. Proc. Biol. Sci. 283. doi: 10.1098/rspb.2015.1985
Varsha M. S., Sethulakshmi M., Joshi K. K., Varghese M., Kavungal V., Antony T. P., et al. (2021). 2021 sponge fauna of the lakshadweep. J. Marine Biol. Assoc. India 63, 97–109. S. K R. doi: 10.6024/jmbai.2021.63.1.2274-15
Venkataraman K. (2011). Coral reefs in India. Encyclopedia of modern coral reefs. Ed. Hopley D. (Dordrecht: Springer), 267-275. doi: 10.1007/1978-1090-1481-2639-1002_1064
Visalli M. E., Best B. D., Cabral R. B., Cheung W. W. L., Clark N. A., Garilao C., et al. (2020). Data-driven approach for highlighting priority areas for protection in marine areas beyond national jurisdiction. Marine Policy 122, 103927. doi: 10.1016/j.marpol.2020.103927
Wangensteen O. S., Palacín C., Guardiola M., and Turon X. (2018). DNA metabarcoding of littoral hard-bottom communities: high diversity and database gaps revealed by two molecular markers. Peerj 6, e4705. doi: 10.7717/peerj.4705
Williams S. and García-Sais J. (2020). A potential new threat on the coral reefs of Puerto Rico: The recent emergence of Ramicrusta spp. Marine Ecol. 41. doi: 10.1111/maec.12592
Yadav S., Alcoverro T., and Arthur R. (2018). Coral reefs respond to repeated ENSO events with increasing resistance but reduced recovery capacities in the Lakshadweep archipelago. Coral Reefs 37, 1245–1257. doi: 10.1007/s00338-018-1735-5
Keywords: reef environment, biodiversity assessment, eDNA metabarcoding, coral reef conservation, community structure, Lakshadweep archipelago
Citation: Ammanabrolu BS and Dineshram R (2025) Unveiling diversity in inhabited and uninhabited reefs of the Lakshadweep archipelago, India using eDNA. Front. Mar. Sci. 12:1592429. doi: 10.3389/fmars.2025.1592429
Received: 12 March 2025; Accepted: 19 May 2025;
Published: 19 June 2025.
Edited by:
Josephine Anthony, Meenakshi Academy of Higher Education and Research, IndiaReviewed by:
Jerald Wilson, Madurai Kamaraj University, IndiaRajamanickam Krishnamurthy, Arignar Anna Government Arts and Science College Chennai, India
Copyright © 2025 Ammanabrolu and Dineshram. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ramadoss Dineshram, ZGluZXNoLm5pb0Bjc2lyLnJlcy5pbg==
 Bharath Subramanyam Ammanabrolu
Bharath Subramanyam Ammanabrolu Ramadoss Dineshram
Ramadoss Dineshram